Abstract
This study examines the prevalence of autoantibodies against neuronal surface antigens in the sera of individuals with psychotic disorders compared with control participants.
Patients with antibodies against neuronal surface antigens (NSAbs), including the N-methyl-d-aspartate receptor (NMDAR), typically develop autoimmune encephalitis with characteristic neurological and psychiatric symptoms.1 In 4% of individuals with NMDAR encephalitis, isolated psychotic episodes occur without simultaneous neurological involvement.2 This raises the question of whether NSAbs are underdiagnosed in patients with psychotic disorders. Results of studies testing this, however, are contradictory,3,4,5 possibly because of differences in the methods of antibody detection. Previously, in a cohort of 475 patients with schizophrenia, we found no one with NMDAR antibodies after excluding 2 false-positive results by combined immunohistochemistry (IHC) tests and cell-based assays (CBAs) across laboratories.6 In this analysis, we extend these studies to other common NSAbs known to cause encephalitis, aiming to expand the clinical spectrum of psychosis potentially caused by autoantibodies.
Methods
The cohort included individuals with psychotic disorders, who were further divided into those with schizophrenia, schizoaffective disorder, brief psychotic disorder, and first-episode psychosis diagnoses (as defined by the DSM-IV), and control participants (Table). Samples from all participants were screened by IHC testing for immunoreactivity to rat hippocampus, and if results were questionable or positive (grades 1-3), the samples were analyzed by live and fixed CBAs. If samples were graded 3 on IHC testing, they were also tested on live primary rat hippocampal neurons (Figure, A).
Table. Demographics, Diagnostics, and Neuronal Surface Autoantibodies Test Results by Different Methods.
| Characteristic | Control Participantsa | Individuals With Psychotic Disorders | Individuals With Subdiagnoses of Psychotic Disorders, No. (%) | ||||
|---|---|---|---|---|---|---|---|
| Schizophrenia | Schizoaffective Disorder | Brief Psychotic Disorder | First-Episode Psychosis | Other Psychotic Diagnoses | |||
| Total, No.b | 257 | 621 | 476 | 44 | 38 | 45 | 18 |
| Mean (SD) age, y | 44.1 (16.6) | 34.4 (11.9) | 36.6 (11.9) | 31.5 (7.9) | 26.7 (7.9) | 22.2 (5.6) | 32.4 (10.2) |
| Female | 122 (47.5) | 248 (39.9) | 188 (39.5) | 27 (61.4) | 13 (34.2) | 16 (35.6) | 4 (22.2) |
| Grade, No. (%) | |||||||
| 1 | 14 (5.4) | 40 (6.4) | 29 (6.1) | 4 (9.1) | 2 (5.3) | 2 (4.4) | 3 (16.7) |
| 2 | 8 (3.1) | 13 (2.1) | 10 (2.1) | 2 (4.5) | 1 (2.6) | 0 | 0 |
| 3c | 5 (1.9) | 14 (2.3) | 10 (2.1) | 1 (2.3) | 2 (5.3) | 1 (2.2) | 0 |
| Cell-based assaysd | |||||||
| Tested, No. | 27 | 67 | 49 | 7 | 5 | 3 | 3 |
| Caspr2, live | 3 | 3 | 2 | 0 | 1 | 0 | 0 |
| Othere | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Live neurons, No. | |||||||
| Tested | 5 | 14 | 10 | 1 | 2 | 1 | 0 |
| Positive | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
Abbreviations: Caspr2, contactin-associated protein-like 2; IHC, immunohistochemistry.
Control participants consisted of 200 individuals who had donated blood and 57 individuals who were without a psychiatric diagnosis.
All cases with grade 1 to 3 by IHC testing were further tested by cell-based assays to determine the existence of antibodies to known neuronal surface antigens.
All cases with grade 3 by IHC testing were further tested by live neurons to characterize if they target to unknown neuronal surface antigens.
In-house cell-based assays were used for the following antigens: N-methyl-d-aspartate receptor 1 (alone and GluN1/GluN2B), leucine-rich glioma-inactivated 1, Caspr2, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor, and γ-aminobutyric acid receptor subunit A and B. Human embryonic kidney (HEK) 293 cells were transfected with 4 μg of expression vectors for the respective human sequences. Cells were fixed in formaldehyde, 3.6%, for 10 minutes and permeabilized with Triton-X-100, 0.3%, for 10 minutes. After blocking with bovine serum albumin, 1%, for 1 hour, cells were incubated with human sera diluted 1:40 in albumin, 1%, together with an antibody targeting the according antigen for 1 hour at room temperature. Cover glasses were mounted onto 7 μL of 4′,6-diamidino-2-phenylindole mounting medium and evaluated by 2 observers (of whom 1 was blinded) independently on a BX51 microscope (Olympus) for antibody reactivity. When results were positive, the staining was repeated with serial dilution (1:50 up to 1:3200). Live cell-based assays were performed for all 6 neuronal surface antibodies as described for the fixed cell-based assay, with small modifications. In these cases, HEK293 cells were grown and transfected as described, with the difference that antigens were expressed with fluorescent reporter proteins, if available (leucine-rich glioma-inactivated 1–green fluorescent protein, N-methyl-d-aspartate receptor 1–green fluorescent protein, and Caspr2-mCherry). Human serum was incubated, diluted 1:50 in Dulbecco modified Eagle medium with bovine serum albumin, 1%, and 25mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid at room temperature for 1 hour, and fixed in formaldehyde, 3.6%. The secondary antibodies were incubated without additional permeabilization or blocking steps. Mounting and analysis was done as for the fixed cell-based assays.
Tests in this category included live and fixed cell-based assays for detecting antibodies to N-methyl-d-aspartate receptor, α-amino-3 hydroxy-5-methyl-4-isoxazolepropionic acid receptor, γ-aminobutyric acid receptor subunits A and B, leucine-rich glioma-inactivated 1, and a fixed cell-based assay for antibodies to Caspr2.
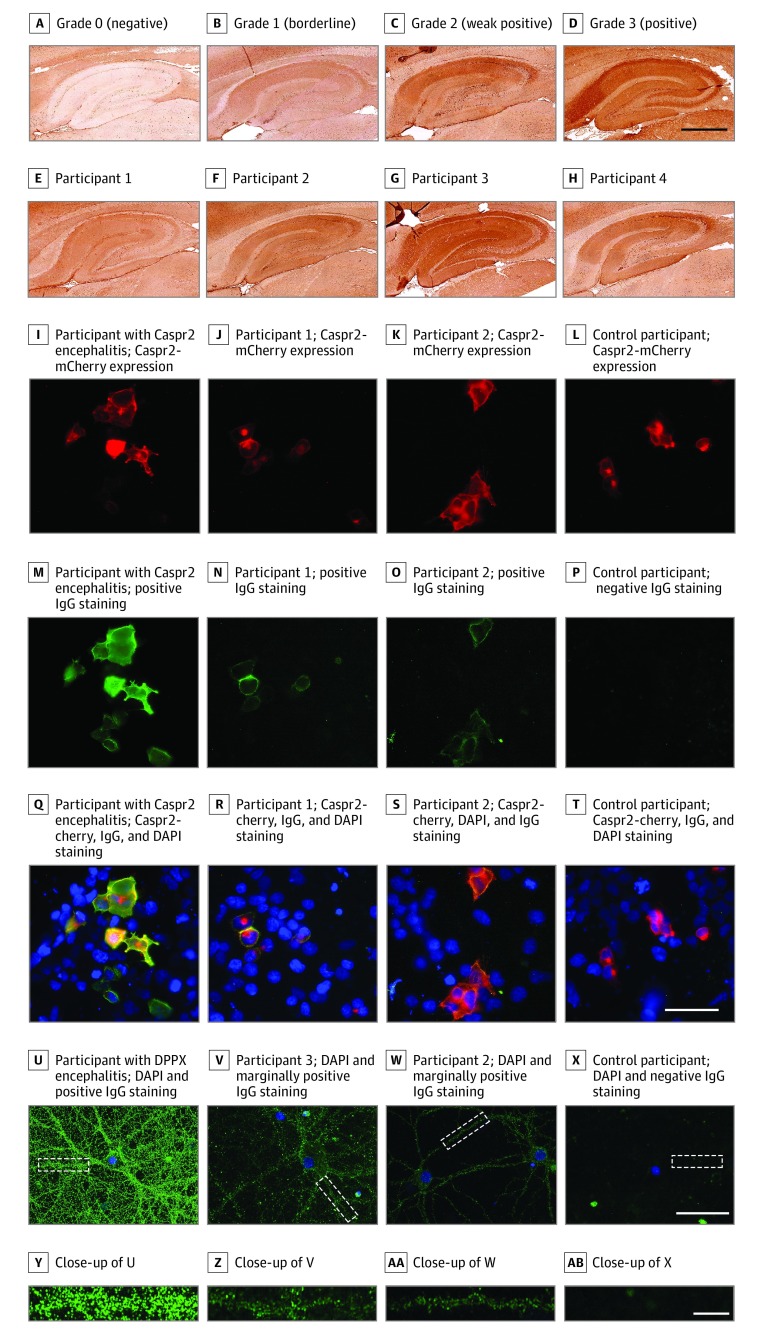
Figure. Autoantibody Detection Strategy and Representative Results.
A-H, Representative results of rat-brain immunohistochemistry testing using human sera. Autoantibody reactivity was shown by using a biotinylated antihuman IgG-specific secondary antibody, streptavidin-conjugated peroxidase and diaminobenzidine. The grading of rat-brain immunohistochemistry was based on 4 staining intensities of the hippocampus (A, grade 0, from a healthy control participant; B, grade 1, and C, grade 2, from the sera of the affected cohort; and D, grade 3, from the serum of an patient with autoimmune encephalitis and anti–contactin-associated protein-like 2 [Caspr2] antibodies). E-H, Samples that were also reactive on cell-based assays or neuronal staining. Scale bar = 500 μm (D). I-T, Results from live cell-based assays for Caspr2. Human sera were incubated on live human embryonic kidney 293 cells transfected with Caspr2 (with mCherry tag [red], I-L) and stained with antihuman IgG Alexa-488 (green). The presented results include a positive result from a participant with anti-Caspr2 encephalitis (M and Q; the same serum as in D) and individuals with psychosis (N and R) or from the control group (O and S) and a negative sample from a control participant (P and T). Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI; blue). Q-T, Images show mCherry, DAPI, and positive IgG staining. Scale bar = 50 μm (T). U-X, Staining on rat hippocampal primary live neurons. Neurons were incubated with human serum samples and subsequently with antihuman IgG Alexa-488 and DAPI. Each image represents 1 or 2 neurons (scale bar = 50 μm [X]), and underneath, the zoomed-in image of the area identified with a white outline (scale bar = 10 μm [AB]). U, A positive result is represented by serum from a patient with encephalitis and autoantibodies against dipeptidyl-peptidase-like protein-6 (DPPX); V, sera of a patient with schizophrenia (the same individual as in G) and an individual from the control cohort (the same individual as in H), both tested with low reactivity on neurons, and the control participant with a negative result was represented by serum from a healthy individual. All sera were diluted 1:50. Nuclei were stained with DAPI.
Approval for this study was obtained from the review boards of Erasmus Medical Centre, University Medical Centre Utrecht, and Katholieke Universiteit Leuven. Written consent was obtained from patients and control participants. Images of rat-brain IHC were taken by the iScan HT slide scanner (Ventana; ×20 objective) and visually graded 0 to 3 (Ventana Image Viewer) for the hippocampal immunoreactivity of sera based on the intensity and contrast of the staining. Statistical analyses were done in SPSS version 23.0 for Windows (IBM). Data were collected from February 2016 to February 2018.
Results
A total of 621 affected individuals (mean [SD] age, 34.4 [11.9] years; 248 female participants [39.9%]) and 257 control participants (mean [SD] age, 44.1 [16.6] years; 112 female participants [47.5%]) were included. Overall, 40 of 878 serum samples (4.6%) were IHC positive (grades 2-3), with no difference between groups (Table). Representative stainings with grade 0 to 3 are shown in the Figure, as well as staining patterns of 4 cases that also tested positive on live CBA or neurons. Further analyses of the IHC-reactive sera for known NSAbs revealed that 6 of 94 samples (6.4%) had contactin-associated protein-like 2 (Caspr2) autoantibodies detected by a live CBA but not a fixed CBA; 3 were from individuals with psychotic disorders and 3 from control participants (Figure). However, of these, only 3 had patterns similar to Caspr2 on IHC testing (1 from an individual with schizophrenia and 2 from control participants). One serum sample from a person with schizophrenia and 1 from a control participant (at 1:50 dilution) had weakly positive results on live neurons (Figure). Sera graded 3 by IHC testing or found to be positive by any CBA (n = 25) were retested in a reference diagnostic laboratory according to established diagnostic procedures for autoimmune encephalitis. No known autoantibodies by IHC testing, fixed CBA, or staining on live neurons (at 1:200 dilution) were detected there.
Discussion
Recent findings with live CBA tests showed that NMDAR autoantibodies were present in 3% of patients with first-episode psychosis but not in control participants; other NSAbs (including anti-Caspr2) were not disease specific.5 In contrast, we did not find any individuals who were anti-NMDAR positive in the subcohort with first-episode psychosis. The Caspr2 antibodies detected by live CBA were not confirmed by other methods. Antibodies that are only detectable by live CBA are thus unlikely to be relevant, highlighting the importance of careful laboratory procedures and standardization regarding autoantibody measurements.
Although brain-reactive autoantibodies were found by IHC testing in both the group with disorder and the control group, no individual sample showed a known NSAbs pattern, as confirmed by the fixed CBA results and the reference laboratory results. Only 1 individual with a disorder and 1 control participant had unidentified NSAbs by live-neuron staining. These results indicate despite extensive laboratory testing that the levels of autoantibodies did not differ between affected individuals and control participants within the study population.
Limitations of this study are that cerebrospinal fluid samples were unavailable and positive samples could have been missed by IHC testing (eg, because of species differences). In light of the cross-sectional nature of the study and the relatively small number of individuals in each diagnostic group, it is conceivable that autoantibodies might be found in studies with larger sample sizes and/or longitudinal samples collected at different times during the course of the illness.
References
- 1.Dalmau J, Armangué T, Planagumà J, et al. An update on anti-NMDA receptor encephalitis for neurologists and psychiatrists: mechanisms and models [published online July 17, 2019]. Lancet Neurol. doi: 10.1016/S1474-4422(19)30244-3 [DOI] [PubMed] [Google Scholar]
- 2.Kayser MS, Titulaer MJ, Gresa-Arribas N, Dalmau J. Frequency and characteristics of isolated psychiatric episodes in anti–N-methyl-D-aspartate receptor encephalitis. JAMA Neurol. 2013;70(9):1133-1139. doi: 10.1001/jamaneurol.2013.3216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Endres D, Perlov E, Baumgartner A, et al. Immunological findings in psychotic syndromes: a tertiary care hospital’s CSF sample of 180 patients. Front Hum Neurosci. 2015;9:476-476. doi: 10.3389/fnhum.2015.00476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bergink V, Armangue T, Titulaer MJ, Markx S, Dalmau J, Kushner SA. Autoimmune encephalitis in postpartum psychosis. Am J Psychiatry. 2015;172(9):901-908. doi: 10.1176/appi.ajp.2015.14101332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lennox BR, Palmer-Cooper EC, Pollak T, et al. ; PPiP study team . Prevalence and clinical characteristics of serum neuronal cell surface antibodies in first-episode psychosis: a case-control study. Lancet Psychiatry. 2017;4(1):42-48. doi: 10.1016/S2215-0366(16)30375-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Witte LD, Hoffmann C, van Mierlo HC, Titulaer MJ, Kahn RS, Martinez-Martinez P; European Consortium of Autoimmune Mental Disorders (CAIMED) . Absence of N-methyl-D-aspartate receptor IgG autoantibodies in schizophrenia: the importance of cross-validation studies. JAMA Psychiatry. 2015;72(7):731-733. doi: 10.1001/jamapsychiatry.2015.0526 [DOI] [PubMed] [Google Scholar]