This cohort study analyzes ozone exposure levels, smoking history, self-reported respiratory outcomes, and other demographic and clinical data of participants in the Air Pollution Study of the Subpopulations and Intermediate Outcome Measures In COPD Study.
Key Points
Question
What is the association of long-term ambient ozone exposure with health outcomes among individuals with a heavy smoking history and with or without airways obstruction?
Findings
In this cross-sectional study of 1874 current and former smokers with or without chronic obstructive pulmonary disease, higher concentration of 10-year historical ambient ozone exposure was associated with greater computed tomography scan–measured emphysema and gas trapping, worse patient-reported outcomes and functional status, and increased respiratory exacerbations.
Meaning
This study found that long-term ambient ozone exposure was associated with worse respiratory outcomes and increased emphysema and gas trapping, independent of smoking and workplace exposures, in smokers with or at risk for chronic obstructive pulmonary disease.
Abstract
Importance
Few studies have investigated the association of long-term ambient ozone exposures with respiratory morbidity among individuals with a heavy smoking history.
Objective
To investigate the association of historical ozone exposure with risk of chronic obstructive pulmonary disease (COPD), computed tomography (CT) scan measures of respiratory disease, patient-reported outcomes, disease severity, and exacerbations in smokers with or at risk for COPD.
Design, Setting, and Participants
This multicenter cross-sectional study, conducted from November 1, 2010, to July 31, 2018, obtained data from the Air Pollution Study, an ancillary study of SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study). Data analyzed were from participants enrolled at 7 (New York City, New York; Baltimore, Maryland; Los Angeles, California; Ann Arbor, Michigan; San Francisco, California; Salt Lake City, Utah; and Winston-Salem, North Carolina) of the 12 SPIROMICS clinical sites. Included participants had historical ozone exposure data (n = 1874), were either current or former smokers (≥20 pack-years), were with or without COPD, and were aged 40 to 80 years at baseline. Healthy persons with a smoking history of 1 or more pack-years were excluded from the present analysis.
Exposures
The 10-year mean historical ambient ozone concentration at participants’ residences estimated by cohort-specific spatiotemporal modeling.
Main Outcomes and Measures
Spirometry-confirmed COPD, chronic bronchitis diagnosis, CT scan measures (emphysema, air trapping, and airway wall thickness), 6-minute walk test, modified Medical Research Council (mMRC) Dyspnea Scale, COPD Assessment Test (CAT), St. George’s Respiratory Questionnaire (SGRQ), postbronchodilator forced expiratory volume in the first second of expiration (FEV1) % predicted, and self-report of exacerbations in the 12 months before SPIROMICS enrollment, adjusted for demographics, smoking, and job exposure.
Results
A total of 1874 SPIROMICS participants were analyzed (mean [SD] age, 64.5 [8.8] years; 1479 [78.9%] white; and 1013 [54.1%] male). In adjusted analysis, a 5-ppb (parts per billion) increase in ozone concentration was associated with a greater percentage of emphysema (β = 0.94; 95% CI, 0.25-1.64; P = .007) and percentage of air trapping (β = 1.60; 95% CI, 0.16-3.04; P = .03); worse scores for the mMRC Dyspnea Scale (β = 0.10; 95% CI, 0.03-0.17; P = .008), CAT (β = 0.65; 95% CI, 0.05-1.26; P = .04), and SGRQ (β = 1.47; 95% CI, 0.01-2.93; P = .048); lower FEV1% predicted value (β = −2.50; 95% CI, −4.42 to −0.59; P = .01); and higher odds of any exacerbation (odds ratio [OR], 1.37; 95% CI, 1.12-1.66; P = .002) and severe exacerbation (OR, 1.37; 95% CI, 1.07-1.76; P = .01). No association was found between historical ozone exposure and chronic bronchitis, COPD, airway wall thickness, or 6-minute walk test result.
Conclusions and Relevance
This study found that long-term historical ozone exposure was associated with reduced lung function, greater emphysema and air trapping on CT scan, worse patient-reported outcomes, and increased respiratory exacerbations for individuals with a history of heavy smoking. The association between ozone exposure and adverse respiratory outcomes suggests the need for continued reevaluation of ambient pollution standards that are designed to protect the most vulnerable members of the US population.
Introduction
Tropospheric, or ground-level, ozone is generated mainly in and downwind of large urban areas by chemical reactions between multiple pollutants in the presence of sunlight.1 Exposure to ambient concentrations of tropospheric ozone is regulated by the US Environmental Protection Agency Clean Air Act, and data from controlled human exposure studies and observational cohort studies support a causal relationship between ozone exposure and adverse health effects in humans.1 Short-term ozone exposure (which varies from hours to weeks) is associated with adverse respiratory outcomes, including acute changes in lung function,1,2,3 and increases in asthma symptoms2 and respiratory-related emergency department (ED) visits.4 Less is known about the respiratory sequelae of long-term ozone exposure (which varies from months to years). Although previous studies suggest that long-term ozone exposure is associated with increased respiratory-related mortality,5,6,7,8,9 few studies have focused on the implications of ozone exposure for those with a heavy smoking burden who may be at high risk for developing chronic lung disease.10,11 Furthermore, little is known about the association of ozone exposure with respiratory morbidity in individuals with chronic obstructive pulmonary disease (COPD), despite the knowledge that exposure to particles and gases can cause COPD.12 A recent scientific assessment by the Environmental Protection Agency acknowledges the scarcity of epidemiologic studies into the association between long-term ozone exposure and COPD-related health outcomes.1
The goal of this cross-sectional study was to investigate the association between 10-year historical ozone exposure and the respiratory health of current and former smokers with or without airways obstruction. These individuals were enrolled in the Subpopulations and Intermediate Outcome Measures In COPD Study (SPIROMICS), an ongoing multicenter prospective cohort study that aims to identify new COPD subgroups and intermediate markers of disease progression.13 We hypothesized that exposure to higher concentrations of 10-year historical ozone, estimated with fine-scale spatiotemporal modeling, was associated with increased risk of respiratory disease, worse respiratory-related morbidity, and higher exacerbation risk.
Methods
Study Population
The current cross-sectional study, conducted from November 1, 2010, to July 31, 2018, is an analysis of data collected at the SPIROMICS enrollment visit as part of the SPIROMICS Air Pollution Study (SPIROMICS AIR), an ancillary study that included 2382 participants enrolled at 7 (New York City, New York; Baltimore, Maryland; Los Angeles, California; Ann Arbor, Michigan; San Francisco, California; Salt Lake City, Utah; and Winston-Salem, North Carolina) of the 12 SPIROMICS clinical sites.14 A total of 1874 SPIROMICS AIR participants (648 smokers without airways obstruction and 1226 smokers with COPD) had available 10-year historical ozone exposure data. SPIROMICS was approved by the institutional review board at each of the 12 clinical centers throughout the United States. All study participants provided written informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Participants in SPIROMICS were 40 to 80 years of age at baseline and were current or former smokers (≥20 pack-years) with or without evidence of obstructive lung disease.13 Healthy persons with a smoking history of 1 or more pack-years were not included in the present analysis. Spirometry was performed before and after the administration of a bronchodilator.15 Current and former smokers were categorized as having either no spirometric evidence of airflow obstruction or having COPD (postbronchodilator forced expiratory volume in the first second of expiration/forced vital capacity [FEV1/FVC] < 70%).
Ambient Ozone Exposure Assessment
Residential address at baseline and over the previous 10 years was obtained and geocoded with a geographic information system (ArcGIS 10.3; ESRI). Two-week mean outdoor concentrations of ozone outside each participant’s home were predicted using spatiotemporal modeling methods.14,16,17 Models incorporated cohort-focused monitoring conducted by SPIROMICS AIR; pollutant concentrations previously measured by the MESA (Multi-Ethnic Study of Atherosclerosis and Air Pollution) Air Study in Baltimore, New York City, Los Angeles, and Winston-Salem; monitoring by the New York City Community Air Study; and regulatory agencies in all SPIROMICS AIR communities.18,19 The 2-week mean concentration predictions were calculated to obtain a long-term historical ozone concentration for the 10 years prior to the baseline visit that was specific to the residential history reported. Ozone estimates were unavailable for 239 individuals with at least 1 address outside the geographic areas covered by the prediction models and for 269 individuals whose addresses could not be geocoded accurately.
Participant Characterization
Trained staff at the clinical sites collected demographic and clinical data. Smoking history was defined as lifetime cumulative pack-years and as a binary indication (yes or no) of smoking within the past month. Occupational exposure to vapors, gas, dust, or fumes (VGDF) at the longest-held job was ascertained by interview.20,21,22 Educational attainment was defined as high school or less or more than high school, and personal income was categorized by yearly income of less than $15 000, $15 000 to $34 999, $35 000 to $49 999, $50 000 to $74 999, or more than $75 000. Neighborhood median household income was obtained using 2010 federal census data at the tract level.23 Participants reported the usual number of hours spent outside per day per season; mean seasonal values were calculated to obtain a yearly estimate.
Respiratory Outcomes
Respiratory-specific quality of life was ascertained using the St. George’s Respiratory Questionnaire (SGRQ),24 and health status was identified with the COPD Assessment Test (CAT).25 Dyspnea was assessed with the modified Medical Research Council (mMRC) Dyspnea Scale,26 and functional exercise capacity was evaluated with the 6-minute walk test (6MWT).27 Chronic bronchitis was defined by a positive response to either the classic or alternative SGRQ definition.28 Exacerbations were based on report of antibiotic and/or steroid use, unscheduled physician visits, ED visits and hospitalizations for COPD, and frequency of these instances over the past year. Severe exacerbations were defined as events requiring an ED visit or hospitalization.
Participants underwent whole-lung multidetector helical computed tomography (CT) scans.29 Percentage of emphysema was defined as percent of total voxels in the field less than −950 HU (Hounsfield units) at total lung capacity, and percentage of air trapping was defined as percent of total voxels in the field less than −856 HU at residual volume. Pi10 (the square root of the wall area of a theoretical airway with a lumen perimeter of 10 mm) was used as a measure of airway wall thickness.30
Statistical Analysis
From May 1, 2018, to September 1, 2019, we performed multivariable regression analyses to examine the association between 10-year historical ozone exposure concentration and outcomes assessed at the enrollment visit. A minimally adjusted model accounted for age, race/ethnicity (white vs non-white), sex, and study site, and a moderately adjusted model added personal and neighborhood income. Fully adjusted models accounted for body mass index, current smoking status, smoking pack-years, VGDF exposure, and educational status in addition to the covariates included in the moderately adjusted model.
For continuous outcomes, including CT metrics and disease severity outcomes (ie, respiratory symptoms, lung function, and functional status), we used least-squares regression modeling to estimate the change in the predicted level of outcomes for a 5-ppb (parts per billion) increase in 10-year historical ozone concentration. For dichotomous outcomes, including COPD diagnosis, chronic bronchitis diagnosis, and exacerbations (event of reporting any exacerbation in the past 12 months), we used logistic regression models to estimate the odds ratio (OR) of the event for a 5-ppb increase in 10-year historical ozone concentration. We conducted standard regression analyses, including checking linearity assumption using a fractional polynomial model approach as well as evaluating residual distribution for normality and heteroscedasticity as appropriate (eFigure 1 in the Supplement). In addition, we used the fractional polynomial approach to explore the functional shape of the exposure-response associations.31
Sensitivity analyses included the addition of 10-year historical concentration of particulates with a diameter of 2.5 μm (PM2.5) or less to the fully adjusted model, 1- and 5-year mean ambient ozone concentration as the main exposure variable, and modeled ozone concentration limited to the warm season (April-September) as the exposure. In separate analyses, to explore effect modification given a priori hypotheses, we ran fully adjusted 2-way interaction models, examining the interaction between COPD status, smoking pack-years, current smoking status, sex, and time spent outdoors with ozone exposure. For the COPD effect modification analysis, COPD was not included as an outcome.
All analyses were performed with StataMP software, version 15.1 (StataCorp LLC). The threshold of statistical significance for the main associations and interaction terms was a 2-sided P < .05.
Results
This cross-sectional study analyzed 1874 participants, with a mean (SD) age of 64.5 (8.8) years and among whom 1479 (78.9%) were white and 1013 (54.1%) were male. More than a third of participants (n = 688 [36.7%]) reported current smoking, and the mean (SD) pack-years reported were 49.8 (28.9). Mean (SD) postbronchodilator FEV1 was 74.7% (25.8%) predicted, and 1226 participants (65.4%) had a COPD diagnosis. Almost half of participants (904 [48.6%]) reported VGDF exposure. Over the course of the year, the median (interquartile range [IQR]) time spent outdoors was 3.3 (2.0-5.5) hours per day (Table 1).
Table 1. Baseline Participant Characteristics .
| Variable | Valuea |
|---|---|
| Age, mean (SD), y | 64.5 (8.8) |
| Male sex | 1013 (54.1) |
| White race/ethnicity | 1479 (78.9) |
| BMI, mean (SD) | 28.0 (5.3) |
| Pack-years, mean (SD) | 49.8 (28.9) |
| Current smoker | 688 (36.7) |
| 10-y mean of 2-wk ozone concentration, median (IQR), ppb | 25.1 (6.3) |
| 10-y mean of PM2.5 concentration, median (IQR), μg/m3 | 11.2 (3.6) |
| Time spent outdoors annually, median (IQR), h/d | 3.3 (3.5) |
| Exposure to VGDF at longest job | 904 (48.6) |
| Census tract median household income, in thousands, mean (SD) | 59 (28) |
| Income, US $ | |
| <15 000 | 288 (15.4) |
| 15 000-34 999 | 342 (18.2) |
| 35 000-49 999 | 250 (13.3) |
| 50 000-74 999 | 308 (16.4) |
| ≥75 000 | 330 (17.6) |
| Declined to answer | 356 (19.0) |
| High school education or lower | 679 (36.3) |
| COPD diagnosis | 1226 (65.4) |
| Chronic bronchitis | 802 (42.9) |
| Postbronchodilator FEV1% predicted, mean (SD) | 74.7 (25.8) |
| 6MWT distance, mean (SD), m | 401.3 (106.9) |
| mMRC Dyspnea Scale score, mean (SD) | 1.01 (0.97) |
| CAT score, mean (SD) | 13.4 (8.0) |
| SGRQ score, mean (SD) | 31.3 (19.8) |
| Prevalence of total exacerbations in the 12 mo before baseline visit | 419 (22.4) |
| Prevalence of severe exacerbations in the 12 mo before baseline visit | 195 (10.4) |
Abbreviations: 6MWT, 6-minute walk test; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in the first second of expiration; IQR, interquartile range; mMRC, modified Medical Research Council Dyspnea Scale; ppb, parts per billion; PM2.5, particulate matter with an aerodynamic diameter of 2.5 microns or less; SGRQ, St. George Respiratory Questionnaire; VGDF, vapors, gas, dust, or fumes.
Data presented as number (percentage) unless otherwise specified.
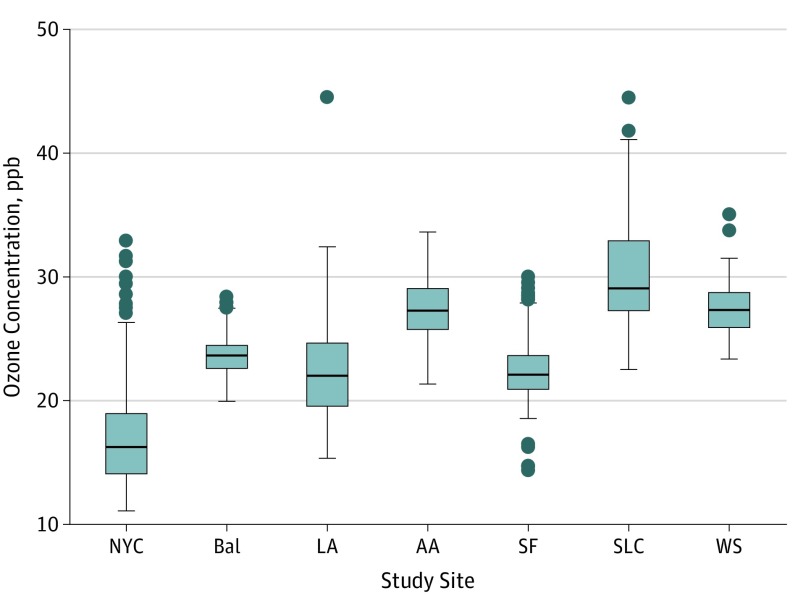
The median (IQR) of the 10-year mean of modeled 2-week ozone concentrations was 25.1 (21.8-28.1) ppb. Ozone concentrations varied by site of enrollment (P < .001), ranging from a median (IQR) of 16.3 (14.0-19.0) ppb in New York City to 29.1 (27.2-33.0) ppb in Salt Lake City (Figure 1). Compared with individuals without available ozone data, participants with estimated ozone concentrations were older (mean age, 62.2 years vs 64.5 years; P < .001), were less likely to be current smokers (45.8% [n = 230] vs 37.3% [n = 688]; P < .001), were less likely to be in the lowest income category of less than $15 000 (24.8% vs 15.4%; P < .001), and tended to have higher neighborhood mean income ($57 000 vs $61 000; P = .003). No differences were observed in race/ethnicity, sex, smoking pack-years, VGDF exposure, COPD status, time spent outdoors, or body mass index in those with or without available ozone data. For the outcomes of percentage of emphysema, FEV1% predicted, and 6MWT, the fractional polynomial models suggested that the association between historical ozone exposure and outcome may be other than linear (eFigure 2 in the Supplement). For all outcomes, results of the minimally and moderately adjusted model are included in eTable 1 in the Supplement.
Figure 1. Distribution of 10-Year Historical Ozone Concentration by Study Site .
The boxes indicate the interquartile range (IQR); the lower hinge (bottom of box), the 25th percentile value; the center line, the median concentration; and the upper hinge (top of box), the 75th percentile value. The upper whisker represents the maximum observation that falls within the upper limit (75th percentile + 1.5 × IQR); the lower whisker, the minimum observation that falls within the lower limit (25th percentile − 1.5 × IQR). The circles represent outlier values.
AA indicates Ann Arbor, Michigan; Bal, Baltimore, Maryland; LA, Los Angeles, California; NYC, New York City, New York; ppb, parts per billion; SF, San Francisco, California; SLC, Salt Lake City, Utah; WS, Winston-Salem, North Carolina.
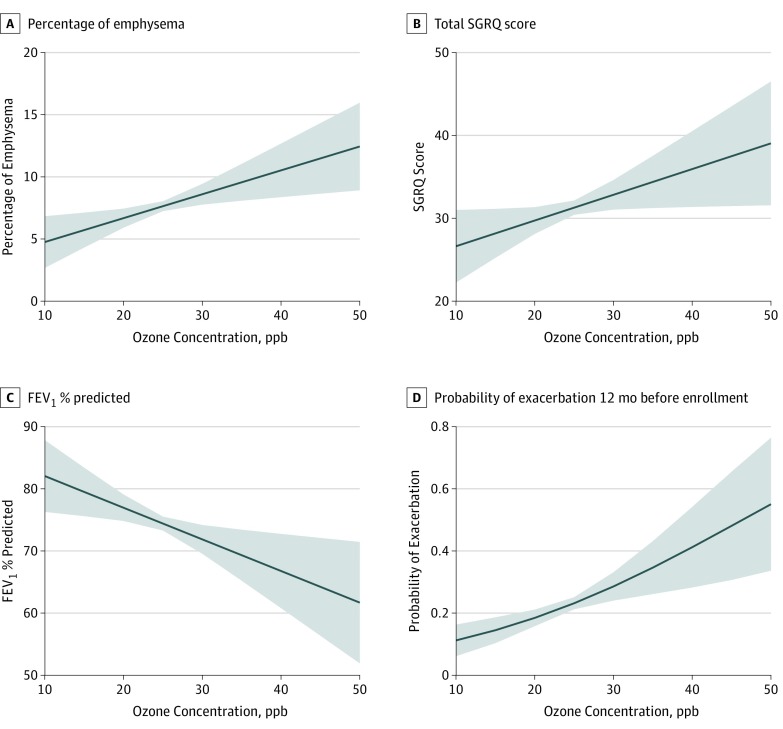
In the fully adjusted model, a 5-ppb increase in historical ozone exposure concentration was associated with a 0.94 (95% CI, 0.25-1.64; P = .007) increase in percentage of emphysema and a 1.60 (95% CI, 0.16-3.04; P = .03) increase in percentage of air trapping (Figure 2A). No association was observed between ozone exposure and chronic bronchitis (OR, 1.14; 95% CI, 0.96-1.35; P = .14), COPD (OR, 1.16; 95% CI, 0.97-1.40; P = .10), or Pi10 (effect estimate, 0.0001; 95% CI, −0.005 to 0.006; P = .87) (Table 2).
Figure 2. Adjusted Associations of 10-Year Historical Ozone Concentration With Selected Outcomes of Interest.
FEV1 indicates forced expiratory volume in the first second of expiration; ppb, parts per billion; SGRQ, St. George Respiratory Questionnaire. Shaded areas represent 95% CIs.
Table 2. Association Between 10-Year Ozone Concentration and Health Outcomes in Current and Former Smokers .
| Estimated Change in Outcome per 5-ppb Increase in 10-y Mean Ozone Concentration | Minimally Adjusted Modela | Fully Adjusted Modelb | ||
|---|---|---|---|---|
| Effect Estimate (95% CI) | P Value | Effect Estimate (95% CI) | P Value | |
| Respiratory disease risk | ||||
| COPD, OR | 1.06 (0.90 to 1.25) | .46 | 1.16 (0.97 to 1.40) | .10 |
| Chronic bronchitis, OR | 1.01 (0.87 to 1.18) | .86 | 1.14 (0.96 to 1.35) | .14 |
| CT-measured outcomes | ||||
| Pi10 | −0.001 (−0.007 to 0.004) | .57 | 0.0001 (−0.005 to 0.006) | .87 |
| Percentage of emphysema | 0.93 (0.22 to 1.63) | .01 | 0.94 (0.25 to 1.64) | .007 |
| Percentage of air trapping | 1.22 (−0.26 to 2.70) | .11 | 1.60 (0.16 to 3.04) | .03 |
| Respiratory morbidity | ||||
| SGRQ score | −0.41 (−1.85 to 1.03) | .58 | 1.47 (0.01 to 2.93) | .048 |
| CAT score | −0.10 (−0.69 to 0.49) | .74 | 0.65 (0.05 to 1.26) | .04 |
| mMRC Dyspnea Scale score | 0.03 (−0.04 to 0.10) | .45 | 0.10 (0.03 to 0.17) | .008 |
| FEV1% predicted | −0.99 (−2.84 to 0.86) | .29 | −2.50 (−4.42 to −0.59) | .01 |
| 6MWT distance | 3.57 (−4.13 to 11.27) | .36 | −7.65 (−15.57 to 0.27) | .06 |
| Exacerbations | ||||
| Any exacerbation in 12 mo before enrollment, OR | 1.27 (1.06 to 1.52) | .009 | 1.37 (1.12 to 1.66) | .002 |
| Severe exacerbation in 12 mo before enrollment, OR | 1.29 (1.03 to 1.63) | .03 | 1.37 (1.07 to 1.76) | .01 |
Abbreviations: 6MWT, 6-minute walk test; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); CAT, COPD Assessment Test; COPD, chronic obstructive pulmonary disease; CT, computed tomography; FEV1, forced expiratory volume in the first second of expiration; mMRC, modified Medical Research Council Dyspnea Scale; OR, odds ratio; Pi10, the square root of the wall area of a theoretical airway with a lumen perimeter of 10 mm; ppb, parts per billion; SGRQ, St. George Respiratory Questionnaire; VGDF, vapors, gas, dust, or fumes.
Adjusted for age, race/ethnicity, sex, and study site.
Adjusted for age, race/ethnicity, sex, study site, BMI, current smoking status, pack-years, VGDF exposure, educational level, and personal and neighborhood income.
When accounting for confounders in the fully adjusted model, higher concentration of 10-year ozone exposure was associated with worse quality of life, functional status, and dyspnea. Specifically, a 5-ppb increase in ozone was associated with a 1.47-point increase in SGRQ score (95% CI, 0.01-2.93; P = .048), a 0.65-point increase in CAT score (95% CI, 0.05-1.26; P = .04), and a 0.10-point increase in mMRC Dyspnea Scale score (95% CI, 0.03-0.17; P = .008) (Figure 2B). Greater 10-year ozone exposure concentration was associated with a −2.50% lower FEV1% predicted value (95% CI, −4.42 to −0.59; P = .01) (Figure 2C). No association was observed between historical ozone exposure and 6MWT result (Table 2).
A total of 419 participants (22.4%) reported any exacerbation in the past 12 months, and 195 (10.4%) reported severe exacerbation that required an ED visit or hospitalization. In the fully adjusted model, a higher concentration of 10-year ozone exposure was associated with increased odds of reporting both any exacerbation (OR, 1.37; 95% CI, 1.12-1.66; P = .002) and severe exacerbation requiring an ED visit or hospitalization (OR, 1.37; 95% CI, 1.07-1.76; P = .01). Specifically, a 5-ppb increase in mean 10-year ozone exposure concentration was associated with a 37% increased odds of reporting both any exacerbation and severe exacerbation (Table 2; Figure 2D).
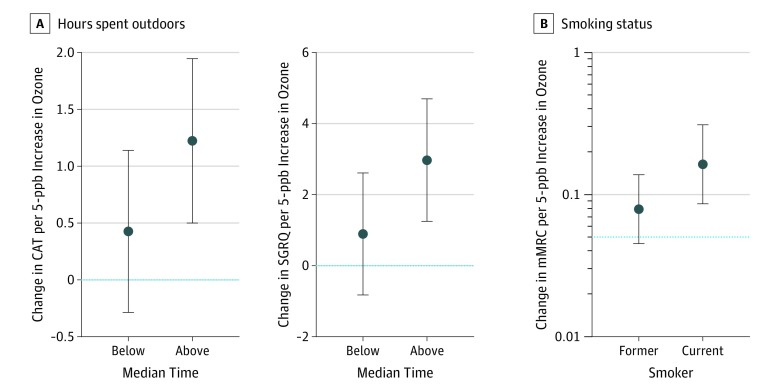
Effect Modification by Time Spent Outdoors, Smoking, Sex, and COPD Status
The association between historical ozone exposure and CAT score was modified by the amount of time spent outdoors such that adverse ozone association with CAT score was significantly amplified for participants who spent more (vs less) than the median time outdoors. For example, a 5-ppb increase in historical ozone exposure concentration was associated with a 1.22-point increase (95% CI, 0.49-1.94; P = .001) in CAT score for those who spent more than the median time outdoors; no associated increase was found among participants who spent less than the median time outdoors (β = 0.42; 95% CI, −0.29 to 1.13; P = .25; P for interaction = 0.03). Similarly, evidence of effect modification was observed by time spent outdoors for the outcome of SGRQ score (above median time: β = 2.97 [95% CI, 1.24-4.69; P = .001]; below median time: β = 0.89 [95% CI, −0.83 to 2.61; P = .31; P for interaction = 0.02]). No further evidence of effect modification was found by time spent outdoors and other outcomes, nor for smoking pack-years, sex, or COPD case status for any of the measured outcomes. Evidence of effect modification was found by smoking status only for the outcome of mMRC Dyspnea Scale score. Specifically, a 5-ppb increase in historical ozone exposure concentration was associated with a 0.17-point increase (95% CI, 0.08-0.26; P < .001) in mMRC Dyspnea Scale score in current smokers; no associated increase was observed in former smokers (β = 0.07; 95% CI, −0.01 to 0.15; P = .11; P for interaction = 0.02) (Figure 3).
Figure 3. Effect Estimate of Ozone on Outcomes by Time Spent Outdoors and Smoking Status.
CAT indicates COPD (chronic obstructive pulmonary disease) Assessment Test; mMRC, modified Medical Research Council Dyspnea Scale; ppb, parts per billion; SGRQ, St. George Respiratory Questionnaire. Error bars represent 95% CIs. P for interaction <.05 for analyses.
Sensitivity Analyses
Inclusion of both ozone and PM2.5 concentration in the same model introduced a modest collinearity issue (correlation when accounting for study site = −0.71), resulting in an increase in the variability of effect estimates for ozone concentration. However, in general, the associations between ozone and COPD outcomes were consistent in models including PM2.5. Specifically, when including PM2.5 in the model, the association between ozone and air trapping, emphysema, mMRC, and lung function remained statistically significant. Although the association with ozone remained in the same direction, a statistically significant association was no longer observed between ozone and SGRQ, CAT, or exacerbations (eTable 2 and eFigure 3 in the Supplement). When considering alternate mean times of ozone exposure, the association between historical ozone exposure and outcomes was similar when using 1- and 5-year historical ozone concentrations (eTable 3 in the Supplement). Similarly, the association between historical ozone exposure and outcomes did not meaningfully vary when ozone predictions were limited to the warm season (eTable 4 in the Supplement).
Discussion
This cross-sectional study of a multicenter cohort of heavy smokers with or at risk for COPD demonstrated an adverse association of historical ozone exposure with COPD and emphysema risk as well as with several markers of respiratory morbidity, including patient-reported outcomes and exacerbations. We found that exposure to a higher concentration of 10-year historical ozone was associated with lower lung function, as well as with more emphysema and air trapping on CT scan, even after accounting for smoking history. These findings support the role of ambient ozone exposure in COPD morbidity, the fourth leading cause of death in the United States and which is often attributed to tobacco exposure in developed countries.32 Ten-year cumulative ozone exposure was also associated with greater symptom burden, worse quality of life and functional status, and more frequent exacerbations among SPIROMICS participants with or without COPD. These results take into account additional occupational and environmental exposures, smoking history, and individual and neighborhood socioeconomic factors. Thus, they highlight the association of long-term ozone exposure with individual-level health outcomes in smokers with or without COPD.
These results are in line with findings of a general population study of 5780 individuals that demonstrated a long-term association between higher ozone concentration and increased emphysema9 and a study of 300 individuals with α1-antitrypsin deficiency that showed an association between higher concentration of 1-year ozone exposure and emphysema severity.33 The magnitude of the association between long-term ozone exposure and percentage of emphysema was similar to that seen with several other inhalational exposures, such as occupational exposures in the longest held job.34 In addition, we found that those with higher concentration of historical ozone exposure had significantly lower FEV1% predicted value, supporting previous studies that demonstrated an association between ozone exposure and decreased lung function in respiratory diseases, including both asthma2,35 and COPD.9 Furthermore, although the data in the present study support the existing literature describing the association between long-term ozone exposure and respiratory outcomes in asthma,36 this study fills a substantial knowledge gap by demonstrating that exposure to higher concentrations of long-term ambient ozone may also be a factor in increased respiratory symptoms and burden in adults at risk for chronic lung disease owing to smoking and among those with COPD, which is a leading driver of hospitalizations in the United States.37
The effect sizes found in this analysis translate to meaningful health outcomes that have important implications for assessing the burden of disease in COPD. Specifically, ozone was associated with worse quality of life (as measured by SGRQ) and health status (as measured by CAT) such that living in areas with a 15-ppb higher concentration of historical 10-year ozone was associated with decrements of 4.0 points in SGRQ and 2.0 points in CAT that met the minimal clinically important difference. In addition, long-term ozone exposure was associated with worse dyspnea (according to the mMRC Dyspnea Scale), which, along with CAT score and exacerbation frequency, is a particularly important measure of disease severity and is currently used in the COPD Global Initiative for Chronic Obstructive Lung Disease guidelines to assess symptoms and risk of exacerbations.38 This assessment, along with the degree of airflow limitation based on FEV1% predicted value, provides a composite of disease severity and guides medical management. A 5-ppb increase in 10-year mean ozone concentration was associated with an increase in the odds of total and severe exacerbations (OR, 1.37 for both) in the 12 months before the baseline visit. According to data from the Centers for Disease Control and Prevention,39 approximately 7 million COPD-related ED visits occurred in 2015. A 5-ppb reduction in 10-year historical ozone concentration could potentially decrease ED visits by 27% to 5.1 million, resulting in an impressive reduction in the existing burden of health care resources spent on COPD.40
Indoor concentrations of ozone are substantially lower than outdoor levels41; thus, it is not surprising that the association of ozone exposure with COPD-related health status (CAT score) and quality of life (SGRQ score) tended to be greater in individuals who spent more time outdoors. Future research is warranted to address whether public health messaging concerning poor air quality may influence activity patterns and health outcomes in populations with COPD. Such health alerts on high-outdoor-pollution days should take into consideration the known adverse health implications of poor indoor air quality for COPD outcomes independent of outdoor air quality.42
The present study highlighted that ozone exposure had adverse implications for COPD risk and respiratory morbidity despite heavy smoking exposure (at least 20 pack-years) among all study participants. No effect modification of ozone by cumulative smoking history was found, such that the adverse outcomes of ozone were consistent across pack-years of exposure, suggesting that the association of ozone exposure may be independent of smoking intensity. Current smokers were more susceptible to the implications of ozone exposure only for the outcome of mMRC Dyspnea Scale; for most outcomes, ozone exposure was associated with increased morbidity regardless of smoking status. In addition, the adverse implications of ozone for respiratory outcomes were persistent despite adjustments for multiple confounders, including job exposures, which have been linked with poor outcomes in the SPIROMICS population.34,43 Furthermore, these results remained largely consistent with consideration of historical exposure to PM2.5, a pollutant that has been implicated in both the development and worsening of COPD.44,45,46 The modest collinearity found between ozone and PM2.5 increases the uncertainty in the estimates of ozone outcomes. However, the general consistency in the patterns of associations between both models suggests an independent association between ozone and respiratory outcomes that is robust to the inclusion of additional pollutants. These results are among the first to highlight the distinct association of ozone with important disease outcomes in COPD.
Epidemiologic and controlled chamber studies showed adverse health outcomes after exposure to relatively low concentrations of ozone,47 and the present study provides additional evidence of adverse outcomes associated with ozone throughout the observed concentration range, suggesting the absence of a safe threshold of exposure concentration. The 2015 US National Ambient Air Quality Standards (NAAQS) limited the maximum 8-hour mean concentration to 70 ppb, and no current long-term ozone standard exists.1 Although the mean time used in the current study did not allow for direct comparison with the NAAQS limit, 8-hour peaks are correlated with long-term mean concentrations,17 and more than a third of the US population lives in areas that exceed the current short-term ozone exposure limit.48 Since 2000, ozone concentrations have decreased the least as compared with other criteria air pollutants under NAAQS (ie, 16% decrease from 2000 to 2018, compared with a 39% decrease in PM2.5 concentration over the same period).49 The relative flattening of ozone reduction demonstrates that the US population has been exposed to relatively fixed concentrations of ozone over the past several decades. Results of this study support recent epidemiologic evidence that associated these long-term exposures, in addition to adverse short-term exposures, with health outcomes.8,9,50 We believe that further consideration of adopting the long-term ozone standard of the NAAQS is a public health necessity.
Limitations and Strengths
This study has some limitations. We were unable to establish outdoor behavior patterns over time, and participant report of outdoor activity at the baseline visit may differ from reports in previous years. In addition, generalizability beyond the study sites with available spatiotemporal ozone data may be limited. The potential nonlinear association between ozone and percentage of emphysema, FEV1% predicted, and 6MWT results is intriguing, and the underlying structure of these associations deserves further investigation. In addition, SPIROMICS comprises volunteer participants and thus is not a population-based study, which may introduce self-selection bias.
This study also has several strengths. Previous studies often relied on pollution data gathered from local and national regulatory agencies, which limited the studies’ ability to capture participants’ actual exposure near their home, as local meteorological conditions, emissions, and geographic features can influence ozone levels, resulting in a large amount of spatial variability in ozone concentration.1 The pollution prediction models we used included additional fine-scale spatial information not available from existing public data sets. In addition, the extensive clinical phenotyping of SPIROMICS participants allowed us to examine the association of ozone exposure with several respiratory-specific outcomes in a vulnerable population of current and former heavy smokers.
Conclusions
This cross-sectional study found that long-term exposure to ozone was associated with worse outcomes in smokers with or at risk for COPD. Specifically, long-term ozone exposure was associated with lower lung function and a greater percentage of emphysema and air trapping on CT scan. Furthermore, the significant clinical association of ozone exposure with several measures of respiratory morbidity, including worse patient-reported outcomes, functional status, and higher exacerbation risk, provides additional evidence that long-term ozone exposure has a substantial association with a range of respiratory outcomes. We believe these findings support continued reexamination of ambient pollution standards that are designed to protect the health of the most vulnerable members of the US population.
eFigure 1. Regression Diagnostics. Distribution of Residual Based on Fully Adjusted Regression Model (A); Scatter Plot of Residual Vs 10-Year Ozone (B)
eFigure 2. Fractional Polynomial Approach to Explore the Shape of the Relationship Between Historical Ozone Concentration and FEV1% Predicted (A); % Emphysema (B); and 6 MWT (C)
eFigure 3. Effect Size of Relationship Between 5 ppb Increase in 10-Year Ozone Concentration and Health Outcomes Without Inclusion of 10-Year PM2.5 Concentration (A); With Inclusion of PM2.5 in Model (B)
eTable 1. Relationship Between 10-Year Ozone Concentration and Health Outcomes in Current and Former Smokers (n = 1874)
eTable 2. Relationship Between 10-Year Ozone Concentration and Health Outcomes in Current and Former Smokers (n = 1874) With and Without Inclusion of 10-Year PM2.5 Concentration
eTable 3. Relationship Between Historical Ozone Concentration and Health Outcomes in Current and Former Smokers (n = 1874) With Varying Exposure Averaging Times
eTable 4. Relationship Between Warm Season 10-Year Ozone Concentration (April-September) and Health Outcomes in Current and Former Smokers (n = 1874)
References
- 1.US Environmental Protection Agency . Integrated Science Assessment (ISA) of Ozone and Related Photochemical Oxidants. Final Report. Washington, DC: US Environmental Protection Agency; 2013.
- 2.Mortimer KM, Neas LM, Dockery DW, Redline S, Tager IB. The effect of air pollution on inner-city children with asthma. Eur Respir J. 2002;19(4):699-705. doi: 10.1183/09031936.02.00247102 [DOI] [PubMed] [Google Scholar]
- 3.Chan CC, Wu TH. Effects of ambient ozone exposure on mail carriers’ peak expiratory flow rates. Environ Health Perspect. 2005;113(6):735-738. doi: 10.1289/ehp.7636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tolbert PE, Klein M, Peel JL, Sarnat SE, Sarnat JA. Multipollutant modeling issues in a study of ambient air quality and emergency department visits in Atlanta. J Expo Sci Environ Epidemiol. 2007;17(suppl 2):S29-S35. doi: 10.1038/sj.jes.7500625 [DOI] [PubMed] [Google Scholar]
- 5.Jerrett M, Burnett RT, Pope CA III, et al. Long-term ozone exposure and mortality. N Engl J Med. 2009;360(11):1085-1095. doi: 10.1056/NEJMoa0803894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Turner MC, Jerrett M, Pope CA III, et al. Long-term ozone exposure and mortality in a large prospective study. Am J Respir Crit Care Med. 2016;193(10):1134-1142. doi: 10.1164/rccm.201508-1633OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zanobetti A, Schwartz J. Ozone and survival in four cohorts with potentially predisposing diseases. Am J Respir Crit Care Med. 2011;184(7):836-841. doi: 10.1164/rccm.201102-0227OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lim CC, Hayes RB, Ahn J, et al. Long-term exposure to ozone and cause-specific mortality risk in the U.S [published online May 3, 2019]. Am J Respir Crit Care Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang M, Aaron CP, Madrigano J, et al. Association between long-term exposure to ambient air pollution and change in quantitatively assessed emphysema and lung function. JAMA. 2019;322(6):546-556. doi: 10.1001/jama.2019.10255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Woodruff PG, Barr RG, Bleecker E, et al. ; SPIROMICS Research Group . Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374(19):1811-1821. doi: 10.1056/NEJMoa1505971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Regan EA, Lynch DA, Curran-Everett D, et al. ; Genetic Epidemiology of COPD (COPDGene) Investigators . Clinical and radiologic disease in smokers with normal spirometry. JAMA Intern Med. 2015;175(9):1539-1549. doi: 10.1001/jamainternmed.2015.2735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization . Global Status Report on Noncommunicable Diseases 2010. Geneva, Switzerland: World Health Organization; 2011.
- 13.Couper D, LaVange LM, Han M, et al. ; SPIROMICS Research Group . Design of the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS). Thorax. 2014;69(5):491-494. doi: 10.1136/thoraxjnl-2013-203897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hansel NN, Paulin LM, Gassett AJ, et al. Design of the subpopulations and intermediate outcome measures in COPD (SPIROMICS) AIR study. BMJ Open Respir Res. 2017;4(1):e000186. doi: 10.1136/bmjresp-2017-000186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159(1):179-187. doi: 10.1164/ajrccm.159.1.9712108 [DOI] [PubMed] [Google Scholar]
- 16.Sampson PD, Szpiro AA, Sheppard L, Lindström J, Kaufman JD. Pragmatic estimation of a spatio-temporal air quality model with irregular monitoring data. Atmos Environ. 2011;45(36):6593-6606. doi: 10.1016/j.atmosenv.2011.04.073 [DOI] [Google Scholar]
- 17.Wang M, Keller JP, Adar SD, et al. Development of long-term spatiotemporal models for ambient ozone in six metropolitan regions of the United States: the MESA Air study. Atmos Environ (1994). 2015;123(A):79-87. doi: 10.1016/j.atmosenv.2015.10.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spalt EW, Curl CL, Allen RW, et al. Time-location patterns of a diverse population of older adults: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air). J Expo Sci Environ Epidemiol. 2016;26(4):349-355. doi: 10.1038/jes.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matte TD, Ross Z, Kheirbek I, et al. Monitoring intraurban spatial patterns of multiple combustion air pollutants in New York City: design and implementation. J Expo Sci Environ Epidemiol. 2013;23(3):223-231. doi: 10.1038/jes.2012.126 [DOI] [PubMed] [Google Scholar]
- 20.Blanc PD, Eisner MD, Balmes JR, Trupin L, Yelin EH, Katz PP. Exposure to vapors, gas, dust, or fumes: assessment by a single survey item compared to a detailed exposure battery and a job exposure matrix. Am J Ind Med. 2005;48(2):110-117. doi: 10.1002/ajim.20187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mehta AJ, Miedinger D, Keidel D, et al. ; SAPALDIA Team . Occupational exposure to dusts, gases, and fumes and incidence of chronic obstructive pulmonary disease in the Swiss Cohort Study on Air Pollution and Lung and Heart Diseases in Adults. Am J Respir Crit Care Med. 2012;185(12):1292-1300. doi: 10.1164/rccm.201110-1917OC [DOI] [PubMed] [Google Scholar]
- 22.Sadhra S, Kurmi OP, Sadhra SS, Lam KB, Ayres JG. Occupational COPD and job exposure matrices: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:725-734. doi: 10.2147/COPD.S125980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.US Census Bureau . American community survey. US Department of Commerce, Economics and Statistics Administration. https://www.census.gov/programs-surveys/acs/. Accessed January 10, 2019.
- 24.Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation. The St. George’s Respiratory Questionnaire. Am Rev Respir Dis. 1992;145(6):1321-1327. doi: 10.1164/ajrccm/145.6.1321 [DOI] [PubMed] [Google Scholar]
- 25.Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648-654. doi: 10.1183/09031936.00102509 [DOI] [PubMed] [Google Scholar]
- 26.Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, Wedzicha JA. Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax. 1999;54(7):581-586. doi: 10.1136/thx.54.7.581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J. 2014;44(6):1428-1446. doi: 10.1183/09031936.00150314 [DOI] [PubMed] [Google Scholar]
- 28.Kim V, Crapo J, Zhao H, et al. ; COPDGene Investigators . Comparison between an alternative and the classic definition of chronic bronchitis in COPDGene. Ann Am Thorac Soc. 2015;12(3):332-339. doi: 10.1513/AnnalsATS.201411-518OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sieren JP, Newell JD Jr, Barr RG, et al. ; SPIROMICS Research Group . SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194(7):794-806. doi: 10.1164/rccm.201506-1208PP [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim WJ, Silverman EK, Hoffman E, et al. ; NETT Research Group . CT metrics of airway disease and emphysema in severe COPD. Chest. 2009;136(2):396-404. doi: 10.1378/chest.08-2858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Royston P, Altman DG. Regression using fractional polynomials of continuous covariates: parsimonious parametric modelling. J R Stat Soc Ser C Appl Stat. 1994;43(3):429-453. doi: 10.2307/2986270 [DOI] [Google Scholar]
- 32.Heron M. Deaths: leading causes for 2016. Natl Vital Stat Rep. 2018;67(6):1-77. [PubMed] [Google Scholar]
- 33.Wood AM, Harrison RM, Semple S, Ayres JG, Stockley RA. Outdoor air pollution is associated with disease severity in α1-antitrypsin deficiency. Eur Respir J. 2009;34(2):346-353. doi: 10.1183/09031936.00087908 [DOI] [PubMed] [Google Scholar]
- 34.Paulin LM, Smith BM, Koch A, et al. Occupational exposures and computed tomographic imaging characteristics in the SPIROMICS Cohort. Ann Am Thorac Soc. 2018;15(12):1411-1419. doi: 10.1513/AnnalsATS.201802-150OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen CH, Chan CC, Chen BY, Cheng TJ, Leon Guo Y. Effects of particulate air pollution and ozone on lung function in non-asthmatic children. Environ Res. 2015;137:40-48. doi: 10.1016/j.envres.2014.11.021 [DOI] [PubMed] [Google Scholar]
- 36.Meng Y-Y, Rull RP, Wilhelm M, Lombardi C, Balmes J, Ritz B. Outdoor air pollution and uncontrolled asthma in the San Joaquin Valley, California. J Epidemiol Community Health. 2010;64(2):142-147. doi: 10.1136/jech.2009.083576 [DOI] [PubMed] [Google Scholar]
- 37.Perera PN, Armstrong EP, Sherrill DL, Skrepnek GH. Acute exacerbations of COPD in the United States: inpatient burden and predictors of costs and mortality. COPD. 2012;9(2):131-141. doi: 10.3109/15412555.2011.650239 [DOI] [PubMed] [Google Scholar]
- 38.Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2019 Report. https://goldcopd.org/wp-content/uploads/2018/11/GOLD-2019-v1.7-FINAL-14Nov2018-WMS.pdf. Accessed March 23, 2019. [Google Scholar]
- 39.Centers for Disease Control and Prevention . National hospital ambulatory medical care survey: 2015 emergency department summary tables. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2015_ed_web_tables.pdf. Accessed April 18, 2019
- 40.Soler-Cataluña JJ, Martínez-García MÁ, Román Sánchez P, Salcedo E, Navarro M, Ochando R. Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax. 2005;60(11):925-931. doi: 10.1136/thx.2005.040527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Geyh AS, Xue J, Ozkaynak H, Spengler JD. The Harvard Southern California Chronic Ozone Exposure Study: assessing ozone exposure of grade-school-age children in two Southern California communities. Environ Health Perspect. 2000;108(3):265-270. doi: 10.1289/ehp.00108265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansel NN, McCormack MC, Belli AJ, et al. In-home air pollution is linked to respiratory morbidity in former smokers with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2013;187(10):1085-1090. doi: 10.1164/rccm.201211-1987OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paulin LM, Diette GB, Blanc PD, et al. ; SPIROMICS Research Group . Occupational exposures are associated with worse morbidity in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191(5):557-565. doi: 10.1164/rccm.201408-1407OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Guo C, Zhang Z, Lau AKH, et al. Effect of long-term exposure to fine particulate matter on lung function decline and risk of chronic obstructive pulmonary disease in Taiwan: a longitudinal, cohort study. Lancet Planet Health. 2018;2(3):e114-e125. doi: 10.1016/S2542-5196(18)30028-7 [DOI] [PubMed] [Google Scholar]
- 45.Hansel NN, McCormack MC, Kim V. The effects of air pollution and temperature on COPD. COPD. 2016;13(3):372-379. doi: 10.3109/15412555.2015.1089846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.The Lancet . Air pollution: a major threat to lung health. Lancet. 2019;393(10183):1774. doi: 10.1016/S0140-6736(19)30992-4 [DOI] [PubMed] [Google Scholar]
- 47.Alexis NE, Lay JC, Zhou H, et al. The glutathione-S-transferase mu 1 (GSTM1) null genotype and increased neutrophil response to low-level ozone (0.06 ppm). J Allergy Clin Immunol. 2013;131(2):610-612. doi: 10.1016/j.jaci.2012.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.American Lung Association . The State of the Air. 2019. https://www.lung.org/about-us/media/top-stories/state-of-the-air-2019.html. Accessed July 17, 2019.
- 49.US Environmental Protection Agency. National air quality: status and trends of key air pollutants. https://www.epa.gov/air-trends. Accessed August 29, 2019.
- 50.Balmes JR. Long-term exposure to ozone and cardiopulmonary mortality: epidemiology strikes again [published June 11, 2019]. Am J Respir Crit Care Med. doi: 10.1164/rccm.201906-1105ED [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Regression Diagnostics. Distribution of Residual Based on Fully Adjusted Regression Model (A); Scatter Plot of Residual Vs 10-Year Ozone (B)
eFigure 2. Fractional Polynomial Approach to Explore the Shape of the Relationship Between Historical Ozone Concentration and FEV1% Predicted (A); % Emphysema (B); and 6 MWT (C)
eFigure 3. Effect Size of Relationship Between 5 ppb Increase in 10-Year Ozone Concentration and Health Outcomes Without Inclusion of 10-Year PM2.5 Concentration (A); With Inclusion of PM2.5 in Model (B)
eTable 1. Relationship Between 10-Year Ozone Concentration and Health Outcomes in Current and Former Smokers (n = 1874)
eTable 2. Relationship Between 10-Year Ozone Concentration and Health Outcomes in Current and Former Smokers (n = 1874) With and Without Inclusion of 10-Year PM2.5 Concentration
eTable 3. Relationship Between Historical Ozone Concentration and Health Outcomes in Current and Former Smokers (n = 1874) With Varying Exposure Averaging Times
eTable 4. Relationship Between Warm Season 10-Year Ozone Concentration (April-September) and Health Outcomes in Current and Former Smokers (n = 1874)