Key Points
Question
Are soluble lectinlike oxidized low-density lipoprotein receptor-1 (sLOX-1) levels associated with noncalcified coronary burden in patients with psoriasis?
Findings
In this cohort study of 175 consecutive patients with psoriasis, sLOX-1 levels were elevated in patients with psoriasis compared with age- and sex-matched controls and associated with noncalcified coronary burden independent of hyperlipidemia status. At 1 year, in those who had clinical improvement in psoriasis, a reduction in sLOX-1 level was associated with a reduction in noncalcified coronary burden.
Meaning
These findings suggest that reductions of sLOX-1 level at 1 year are associated with improvements in patients with psoriasis.
Abstract
Importance
Psoriasis, a chronic inflammatory skin disease associated with accelerated noncalcified coronary burden (NCB) by coronary computed tomography angiography (CCTA), accelerates lipoprotein oxidation in the form of oxidized modified lipoproteins. A transmembrane scavenger receptor for these oxidized modified lipoproteins is lectinlike oxidized low-density lipoprotein receptor-1 (LOX-1), which has been reported to be associated with coronary artery disease. It is unknown whether this receptor is associated with coronary artery disease in psoriasis.
Objective
To assess the association between soluble LOX-1 (sLOX-1) and NCB in psoriasis over time.
Design, Setting, and Participants
In a cohort study at the National Institutes of Health, 175 consecutive patients with psoriasis were referred from outpatient dermatology practices between January 1, 2013, and October 1, 2017. A total of 138 consecutively recruited patients with psoriasis were followed up at 1 year.
Exposures
Circulating soluble lectinlike oxidized low-density lipoprotein receptor-1 levels were measured blindly by field scientists running undiluted serum using an enzyme-linked immunosorbent assay.
Main Outcomes and Measures
Coronary computed tomography angiography scans were performed to quantify NCB in all 3 major epicardial coronary arteries by a reader blinded to patient demographics, visit, and treatment status.
Results
Among the 175 patients with psoriasis, the mean (SD) age was 49.7 (12.6) years and 91 were men (55%). The cohort had relatively low median cardiovascular risk by Framingham risk score (median, 2.0 [interquartile range (IQR), 1.0-6.0]) and had a mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) suggestive of overweight profiles (29.6 [6.0]). Elevated sLOX-1 levels were found in patients with psoriasis compared with age- and sex-matched controls (median, 210.3 [IQR, 110.9-336.2] vs 83.7 [IQR, 40.1-151.0]; P < .001), and were associated with Psoriasis Area Severity Index (PASI) score (β = 0.23; 95% CI, 0.082-0.374; P = .003). Moreover, sLOX-1 was associated with NCB independent of hyperlipidemia status (β = 0.11; 95% CI, 0.016-0.200; P = .023), an association which persisted after adjusting for traditional cardiovascular risk factors, statin use, and biologic psoriasis treatment (β = 0.10; 95% CI, 0.014-0.193; P = .03). At 1 year, in those who had clinical improvement in PASI (eg, >50% improvement), a reduction in sLOX-1 (median, 311.1 [IQR, 160.0-648.8] vs median, 224.2 [IQR, 149.1 - 427.4]; P = .01) was associated with a reduction in NCB (β = 0.14; 95% CI, 0.028-0.246; P = .02).
Conclusions and Relevance
Soluble lectinlike oxidized low-density lipoprotein receptor-1 levels were elevated in patients with psoriasis and were associated with severity of skin disease. Moreover, sLOX-1 associated with NCB independent of hyperlipidemia status, suggesting that inflammatory sLOX-1 induction may modulate lipid-rich NCB in psoriasis. Improvement of skin disease was associated with a reduction of sLOX-1 at 1 year, demonstrating the potential role of sLOX-1 in inflammatory atherogenesis in psoriasis.
This cohort study examines whether soluble lectinlike oxidized low-density lipoprotein receptor-1 levels are associated with noncalcified coronary burden over time in patients with psoriasis.
Introduction
Chronic inflammatory diseases such as psoriasis have high systemic inflammatory risk and have been reported to be associated with increased myocardial infarction as well as cardiovascular (CV) mortality independent of traditional risk factors1,2 in part through accelerated noncalcified coronary plaque.3 Psoriasis accelerates lipoprotein oxidation in the form of oxidized low-density lipoprotein (ox-LDL),4 which has been shown to promote early atherosclerotic plaque development in animal models.5,6 Lectinlike oxidized low-density lipoprotein receptor-1 (LOX-1), a transmembrane scavenger receptor for ox-LDL, is highly expressed by endothelial cells in states of hyperlipidemia as well as in the presence of pro-inflammatory cytokines.7 The interaction between ox-LDL and LOX-1 is important for the entry of ox-LDL into the arterial wall, induction of inflammatory signaling pathways, and apoptosis of smooth muscle cells, factoring in to formation, progression, and rupture of atherosclerotic plaque.5,6 It has been previously shown that LOX-1 blockade by either genetic inactivation or antibody neutralization suppresses atherosclerosis development in several animal models.5 Lectinlike oxidized low-density lipoprotein receptor-1 is expressed on the cell surface and can be proteolytically cleaved and transformed into serum soluble forms.5 Soluble LOX-1 (sLOX-1) is the measurable serum soluble form of LOX-1 which can be directly measured in the blood by traditional enzyme-linked immunosorbent assay (ELISA).5 Soluble lectinlike oxidized low-density lipoprotein receptor-1 has been shown to be elevated in acute coronary syndromes, stable coronary disease,8 and diabetes,9 in which sLOX-1 distinguished disease severity, monitored response to treatment, and identified coronary artery disease beyond traditional biomarkers such as high-sensitivity C-reactive protein (hs-CRP).8,9 However, it is unknown whether sLOX-1 is elevated in psoriasis and is associated with coronary artery disease over time.
This report used baseline and follow-up visits in the cohort study of patients with psoriasis to examine sLOX-1 behavior in vivo. We hypothesized that sLOX-1 levels would be higher in patients with psoriasis and would be associated with subclinical coronary artery disease independent of hyperlipidemia status. In addition, we explored treatment of psoriasis and sLOX-1 levels over time.
Methods
Study Design and Population
A total of 175 consecutive patients with psoriasis (138 followed up at 1 year) were examined from an ongoing longitudinal cohort study of patients with psoriasis recruited between January 1, 2013, and October 1, 2017 (The Psoriasis, Atherosclerosis, and Cardiometabolic Disease Initiative). To understand sLOX-1 levels in patients without psoriasis, a total of 94 age- and sex-matched healthy controls were included in our analyses. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines were followed for reporting of our findings.10 Study protocols were approved by the institutional review board at the National Institutes of Health. Research was conducted in accordance with the Declaration of Helsinki. All participants included in the study were older than 18 years at the time of recruitment and provided written informed consent after a full explanation of the procedures.
Inclusion and Exclusion Criteria
Participants underwent blood draw and coronary computed tomography angiography (CCTA) imaging at baseline and 1-year follow-up. Participants with psoriasis were required to have a formal diagnosis of plaque psoriasis by a dermatologist and were examined by a certified health care professional to confirm the onset, duration, and severity of skin disease as assessed by the Psoriasis Area Severity Index (PASI) score. Change in PASI score was calculated from baseline to 1 year. The proportion of patients reaching at least 50% improvement in PASI (PASI50) at 1 year was also evaluated.11
Participants were excluded if they had estimated glomerular filtration rate less than 30 mL/min/1.73m2, existing cardiovascular disease, any comorbid condition known to be associated with cardiovascular disease or systemic inflammation, such as uncontrolled hypertension, internal malignancy within 5 years, HIV, active infection within 72 hours of baseline, major surgery within 3 months, and pregnancy or lactation. Age- and sex-matched controls included both men and women who were at least 18 years of age and were enrolled at the MedImmune research specimen collection program. Physician consent was required for donors to enroll in this program, including that they must be healthy per the physician’s standards.
Coronary Artery Characterization
Guidelines implemented by the National Institutes of Health Radiation Exposure Committee were followed. Coronary computed tomography angiography scans were performed with prospective electrocardiogram gating, 100 kV or 120 kV tube potential, and tube current of 100 to 850 mA adjusted to the patient’s body size, with a gantry rotation time of 275 ms. All CCTA scans were performed using similar settings. Images were acquired at a slice thickness of 0.5 mm with a slice increment of 0.25 mm.3,11,12,13 Patients with psoriasis underwent CCTA on the same day as blood draw, using the same CT scanner, 320-detector row (Aquilion ONE VISION). A single reader (blinded to demographics, treatment, and time of scan) evaluated coronary artery disease characteristics across each of the main coronary arteries greater than 2 mm using the dedicated software QAngio CT (Medis).3 Automated longitudinal contouring of the inner lumen and outer wall was performed, and results were manually adjusted when clear deviations were present. Results of the automated contouring were also reviewed on transverse reconstructed cross-sections of the artery on a section-by-section basis at 0.5-mm increments. Lumen attenuation was adaptively corrected using gradient filters and intensity values within the artery. The primary outcome of the study, coronary burden per unit length, was calculated to account for variable coronary artery lengths between patients. Segmental volume (assessed as cubic millimeters) was divided by the corresponding segment length (assessed as millimeters), and was subsequently attenuated for luminal intensity, which yielded noncalcified coronary burden (NCB) and dense-calcified coronary burden. The interreader and intrareader variabilities for scans were less than 5%. Quantitative and qualitative coronary burden evaluations were performed in 98% of the available coronary segments.
Blood Analyses
Circulating sLOX-1 was measured blindly at the National Institutes of Health laboratory by field scientists running undiluted serum using an ELISA-based assay (MedImmune) developed with Meso Scale Discovery (Meso Scale Discovery) platform. The Meso Scale Discovery high bind plates were coated with 5 μg/mL of an in-house (MedImmune) generated anti-LOX-1 monoclonal antibody overnight, and blocked for 1 hour, and samples (25 μL/well, no dilution) were added along with recombinant human LOX-1 as standard for 2 hours. Plates were washed using Meso Scale Discovery Tris wash buffer 3 times after each incubation step. Human LOX-1/OLR1 antibody (AF1798 from R&D Systems) was tagged using Sulfo-Tag (Meso Scale Discovery) conjugation kit (R31AA-2) to generate detection antibody. The tagged detection antibody was added and incubated for 1 hour. A (2×) Meso Scale Discovery read buffer was used to read plates on Meso Scale Discovery machine. The sLOX-1 levels from the samples were interpolated from standard curve values using Meso Scale Discovery workbench software (Meso Scale Discovery). Assay was tested for matrix effects and no interference. The interassay and intraassay variations from our analysis were less than 10%.
Covariates
Patients were asked to complete survey-based questionnaires regarding smoking, previous CVD, family history of CVD, and previously established diagnoses of hypertension and diabetes. Patient responses were then confirmed during history and physical examination by the health care professional. Cardiovascular disease included acute coronary syndrome comprising both myocardial infarction and unstable angina pectoris, angina pectoris, cerebrovascular event, transient ischemic attack, and peripheral vascular disease and revascularization procedures including both coronary artery bypass grafting and percutaneous interventional procedures. Diabetes was defined as fasting glucose greater than or equal to 125.95 mg/dL (to convert to millimoles per liter, multiply by 0.0555), glycated hemoglobin A1c greater than 6.5%, or use of diabetic medication. Hypertension was defined as systolic blood pressure greater than or equal to 140 mm Hg, diastolic blood pressure greater than or equal to 90 mm Hg, or use of antihypertensive medication. Hyperlipidemia was defined as total cholesterol levels greater than 200 mg/dL, low-density lipoprotein levels greater than or equal to 160 mg/dL, or high-density lipoprotein levels greater than or equal to 40 mg/dL (to convert to millimoles per liter, multiply by 0.0259). Hypertriglyceridemia however was not included. Cholesterol efflux capacity was measured in duplicate using a validated cell-based ex vivo assay.4
Clinical Data and Laboratory Measurements
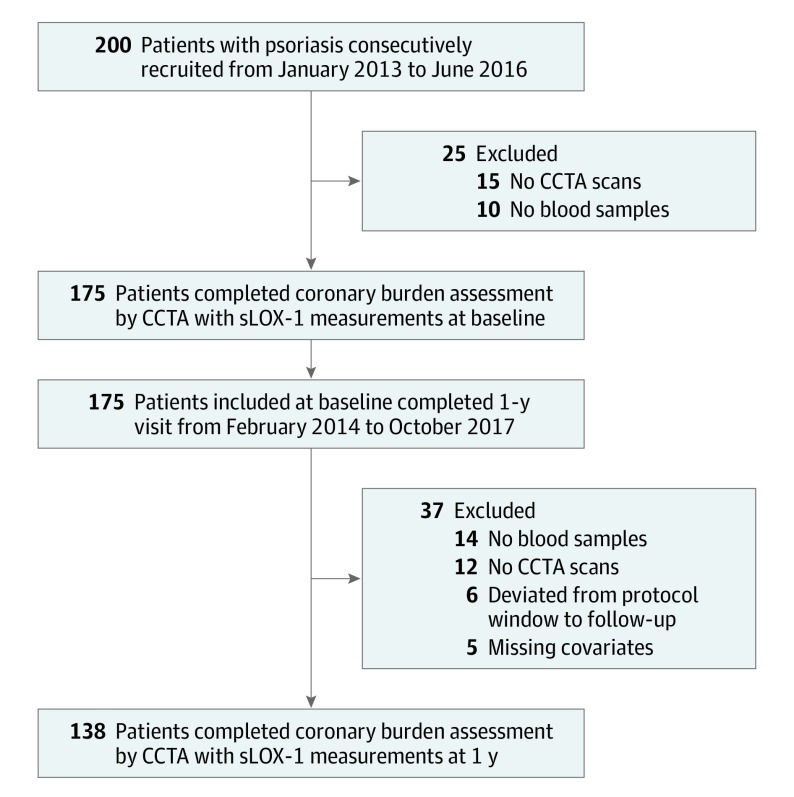
At the time of recruitment, a health care professional collected data on patient demographics, clinical history, physical examination, and anthropometric measurements. Blood samples were collected after an overnight fast and analyzed for basic chemistry, complete lipid profile, insulin, and hs-CRP at the National Institutes of Health Clinical Center. Baseline psoriasis treatment was patient-reported and defined by use of any of the following in the 3 months before their baseline visit: systemic therapy (steroids or methotrexate), biologic therapy (adalimumab, etanercept, ustekinumab, secukinumab, and ixekizumab), statins, light therapy (psoralen plus UV or UV-B), and topical treatments. Most of the cohort underwent intensification of psoriasis treatment at 1 year and the same variables were recorded again at 1 year follow-up. Clinical parameters, including blood pressure, height, weight, and waist and hip circumferences, were measured. Laboratory parameters including fasting blood glucose, fasting lipid panel, complete blood count, and systemic inflammatory markers, including hs-CRP, were evaluated in a clinical laboratory. Study recruitment is depicted in Figure 1.
Figure 1. Recruitment Scheme of Patients With Psoriasis.
CCTA indicates coronary computed tomography angiography; sLOX-1, soluble lectinlike oxidized low-density lipoprotein receptor-1.
Data were reported as mean (SD) and 95% CIs for parametric variables, median (IQR) for nonparametric variables, and percentages for categorical variables. In baseline analyses, parametric variables were compared between the 2 groups using t test and nonparametric variables were compared between the 2 groups using Mann-Whitney test. In longitudinal analyses, parametric variables were analyzed using paired t test and nonparametric variables using Wilcoxon signed rank test. Dichotomous variables were analyzed using the χ2 test. In multivariable linear regression analyses, the potential confounding variables were determined and added to the base model by purposeful selection. Standardized β values and 95% CIs from these analyses were reported, which indicate number of SD change in the outcome variable per SD change in the predicting outcome. A 2-sided P value less than .05 was considered statistically significant. All statistical analyses were performed using Stata, version 12 (Stata Corp) by National Institutes of Health staff, blinded to clinical demographics and imaging characteristics. We hypothesized a 20% difference in sLOX-1 between the psoriasis group and the control group. Thus, our sample size had more than 90% power to detect this difference at an α of .05.
Results
Characteristics of Patients With Psoriasis at Baseline
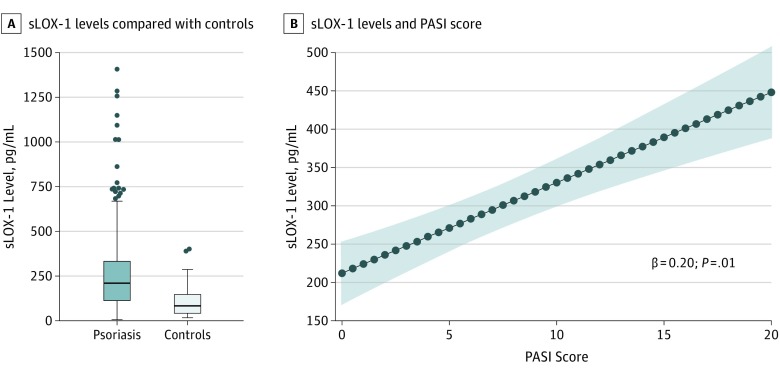
The psoriasis cohort included 175 patients (mean [SD] age, 49.7 (12.6) years; 91 men [55%]). The cohort had relatively low median CV risk by Framingham risk score (median, 2.0 [IQR, 1.0-6.0]) and had a mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) suggestive of overweight profiles (29.6 [6.0]). The demographic and clinical characteristics of patients with psoriasis are summarized in the eTable in the Supplement. Additionally, patients with psoriasis had moderate to severe skin disease (PASI score, median, 5.6 [IQR, 3.0-9.2]), with 21% of the cohort (37 of 175) receiving biologic psoriasis therapy for skin disease management at baseline. Moreover, patients with psoriasis had significantly elevated sLOX-1 compared with age- and sex-matched controls (psoriasis vs controls; sLOX-1, median, 210.3 [IQR, 110.9-336.2] vs median, 83.7 [IQR, 40.1-151.0]; P < .001]) (Figure 2A).
Figure 2. Soluble Lectinlike Oxidized Low-Density Lipoprotein Receptor-1 (sLOX-1) Levels in Psoriasis and Controls and Psoriasis Severity.
A, sLOX-1 levels (pg/mL) are increased in psoriasis when compared with age- and sex-matched controls. The horizontal line in the middle of each box indicates the median, while the top and bottom borders of the box mark the 75th and 25th percentiles, respectively. The whiskers above and below the box mark the 90th and 10th percentiles. The points beyond the whiskers indicate outliers. B, sLOX-1 levels (pg/mL) are positively associated with Psoriasis Area Severity Index (PASI) score independent of traditional risk factors (β = 0.20; 95% CI, 0.047-0.350; P = .01). The blue dots indicate predicted value, and the shaded area indicates the 95% CI.
sLOX-1, Psoriasis Severity, and NCB
We found a linear dependent association between sLOX-1 and psoriasis skin disease severity (β = 0.23; 95% CI, 0.082-0.374; P = .003) which persisted after adjustment for traditional risk factors including Framingham risk score, body mass index, statin use, and biologic psoriasis treatment (β = 0.20; 95% CI, 0.05-0.35; P = .01) (Figure 2B). Additionally, sLOX-1 was found to be associated with NCB independent of hyperlipidemia status in psoriasis (β = 0.11; 95% CI, 0.02-0.20; P = .02). Furthermore, this association persisted in the fully adjusted model (β = 0.10; 95% CI, 0.01-0.19; P = .03).
Psoriasis Treatment and sLOX-1
Given the association between sLOX-1 and psoriasis severity, we hypothesized that patients who had a clinically meaningful improvement in their skin disease severity by at least 50% (PASI50) would also have improvement in sLOX-1 levels. After 1 year, 53% (25 0f 45) who began biologic psoriasis therapy experienced PASI50 whereas only 17% (16 of 93) did not have a PASI50 response after biologic therapy initiation. At 1 year, 45 of 138 patients (33%) had an improvement in psoriasis skin disease severity by at least 50% (median, 6.8 [IQR, 4.1-10.8] vs 1.9 [IQR, 0.8-3.6]; P < .001), whereas 93 of 138 patients (67%) had either a less than 50% improvement in psoriasis severity or worsening of their psoriasis severity, as indicated by their PASI score (median score, 4.0 [IQR, 2.6-7.1] vs 4.5 [IQR, 2.6-7.2]; P = .84). The hs-CRP levels decreased significantly only in PASI50 responders at 1 year (median, 2.1 [IQR, 0.8-7.1] vs 1.1 [IQR, 0.6-3.2]; P = .01) as did sLOX-1 (median, 311.1 [IQR, 160.0-648.8] vs 224.2 [IQR, 149.1-427.4]; P = .01) and NCB (mean [SD]; 1.13 [0.45]; 95% CI, 1.05-1.22; vs 1.07 [0.45]; 95% CI, 0.98-1.15; P = .01) (Table). Furthermore, improvement in psoriasis skin disease severity at 1 year was associated with decreased sLOX-1, independent of Framingham risk score, measure of obesity, statin use, and biologic psoriasis therapy (β = 0.40; 95% CI, 0.220-0.570; P < .001). A decrease in circulating sLOX-1 had a linear association with improvement in NCB even in fully adjusted analyses (β = 0.14; 95% CI, 0.028-0.246; P = .02).
Table. Characteristics of Patients With Psoriasis at 1 Yeara.
| Parameter | ≥50% Improvement in Psoriasis | <50% Improvement or Worsening in Psoriasis | ||||
|---|---|---|---|---|---|---|
| At Baseline (n = 45) | At 1 y (n = 45) | P Value | At Baseline (n = 93) | At 1 y (n = 93) | P Value | |
| Demographic and clinical characteristics | ||||||
| Age, mean (SD) [95% CI], y | 50.9 (11.3) [47.44-54.37] | 52.0 (11.3) [48.55-55.49] | <.001 | 49.4 (12.0) [46.88-51.89] | 50.4 (12.1) [47.90-52.92] | <.001 |
| Men, No. (%) | 32 (71) | 32 (71) | >.99 | 47 (51) | 47 (51) | >.99 |
| Hypertension, No. (%) | 10 (22) | 10 (22) | >.99 | 31 (33) | 31 (33) | >.99 |
| Hyperlipidemia, No. (%) | 19 (42) | 20 (44) | .32 | 42 (45) | 42 (45) | >.99 |
| Type 2 diabetes, No. (%) | 4 (9) | 4 (9) | >.99 | 10 (11) | 12 (13) | .16 |
| BMI, mean (SD) [95% CI] | 30.5 (6.5) [28.48-32.51] | 30.3 (6.3) [28.40-32.26] | .25 | 29.2 (5.5) [27.99-30.31] | 28.9 (5.5) [27.55-29.90] | .17 |
| Current smoker, No. (%) | 3 (7) | 1 (2) | .16 | 9 (10) | 9 (10) | >.99 |
| Statin use, No. (%) | 13 (29) | 13 (29) | >.99 | 29 (31) | 29 (31) | >.99 |
| Clinical and laboratory values | ||||||
| Cholesterol, mean (SD) [95% CI], mg/dL | ||||||
| Total | 184.3 (35.0) [173.39-195.23] | 179.2 (38.3) [167.24-191.10] | .20 | 182.8 (36.6) [175.09-190.53] | 183.8 (39.0) [175.59-192.03] | .62 |
| HDL | 54.1 (16.6) [48.88-59.22] | 53.1 (16.2) [48.11-58.18] | .27 | 55.1 (16.9) [51.55-58.65] | 57.7 (21.3) [53.25-62.21] | .03 |
| LDL | 104.6 (28.3) [95.71-113.56] | 103.3 (36.7) [91.72-114.91] | .38 | 102.4 (29.6) [96.10-108.73] | 99.1 (31.7) [92.29-105.82] | .14 |
| Triglycerides, mean (SD) [95% CI], mg/dL | 121.7 (85.2) [95.12-148.21] | 118.4 (62.5) [98.96-137.90] | .40 | 126.0 (78.4) [109.50-142.55] | 135.8 (76.1) [119.78-151.85] | .09 |
| Framingham risk score, median (IQR) | 4.0 (1.0-6.0) | 4.5 (1.0-7.0) | .88 | 2.0 (1.0-5.0) | 2.0 (1.0-4.0) | .09 |
| High sensitivity c-reactive protein, median (IQR), mg/L | 2.1 (0.8-7.1) | 1.1 (0.6-3.2) | .01 | 2.0 (0.7-4.1) | 1.8 (0.8-4.1) | .22 |
| Soluble LOX-1, median (IQR), pg/mL | 311.1 (160.0-648.8) | 224.2 (149.1-427.4) | .01 | 198.9 (84.8-290.3) | 262.9 (127.2-427.9) | .001 |
| Psoriasis severity | ||||||
| PASI score, median (IQR) | 6.8 (4.1-10.8) | 1.9 (0.8-3.6) | <.001 | 4.0 (2.6-7.1) | 4.5 (2.6-7.2) | .84 |
| Treatment, No. (%) | ||||||
| Biologic | 5 (11) | 29 (64) | <.001 | 22 (24) | 38 (41) | <.001 |
| Topical | 30 | 26 | .41 | 59 | 57 | .83 |
| UV light | 9 | 5 | .16 | 13 | 13 | >.99 |
| Coronary characterization, mm2 (×100) | ||||||
| NCB, mean (SD) [95% CI] | 1.13 (0.45) [1.05-1.22] | 1.07 (0.45) [0.98-1.15] | .01 | 1.10 (0.46) [1.04-1.16] | 1.15 (0.57) [1.07-1.23] | .046 |
| Dense-calcified coronary burden, median (IQR) | 0.020 (0.005-0.052) | 0.020 (0.006-0.075) | .16 | 0.019 (0.006-0.064) | 0.016 (0.002-0.062) | .002 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HDL, high-density lipoprotein; IQR, interquartile range; LDL, low-density lipoprotein; LOX-1, lectinlike oxidized low-density lipoprotein receptor-1; NCB, noncalcified coronary burden; PASI, Psoriasis Area Severity Index.
SI conversion factors: To convert total cholesterol, HDL, and LDL to millimoles per liter, multiply by 0.0259; triglycerides to millimoles per liter, multiply by 0.0113; C-reactive protein to nanomoles per liter, multiply by 9.524.
Values reported in the table as mean (SD) [95% CI] or median (IQR) for continuous data and No. (%) for categorical data. P value less than .05 was deemed significant. P values were derived from a single paired t test for parametric variables and Wilcoxon signed rank test for nonparametric variables.
Discussion
First, sLOX-1 was found to be elevated in psoriasis and associated with psoriasis severity. Second, sLOX-1 associated with lipid-rich NCB independent of hyperlipidemia status in psoriasis. Finally, a clinically meaningful reduction of psoriasis (PASI50) was associated with a reduction in sLOX-1 in fully adjusted models at 1 year.
sLOX-1 in Psoriasis Associated With Psoriasis Severity
Inflammation is critical in the pathogenesis of psoriasis as well as psoriasis associated atherothrombosis.3 Pro-inflammatory cytokines such as tumor necrosis factor-α, interleukin 1β, and interleukin 6, which are elevated in psoriasis, also activate LOX-1 in a dynamic and self-propagating manner leading to the uptake of ox-LDL by the arterial wall.14 Oxidized low-density lipoprotein, when internalized in endothelial cells, facilitates monocyte adhesion to the endothelial lining and leads to stiffening and senescence of the endothelial cell layer.15 In addition, LOX-1 facilitates uptake of ox-LDL in vascular smooth muscle cells, monocytes, and macrophages, resulting in the formation of foam cells and smooth muscle cell proliferation followed by neointima formation.6 In psoriasis, we found elevated sLOX-1 which also was associated with psoriasis severity supporting inflammatory induction of LOX-1 in psoriasis. While in vitro analysis has showed that ox-LDL increases keratinocyte migration and LOX-1 expression, studies to examine LOX-1 expression in lesional psoriatic skin have not been done to date.5
sLOX-1 and NCB in Psoriasis
Psoriasis is a systemic inflammatory disease associated with a disproportionate upregulation of CV events likely owing to intersection between lipid dysregulation and systemic inflammation.2 Compared with patients with hyperlipidemia who were 10 years older, patients with psoriasis had accelerated NCB, the type of plaque which is associated with rupture and incident myocardial infarction.3 The source of LOX-1 is not known. Lectinlike oxidized low-density lipoprotein receptor-1 expression is inducible in endothelial cells in times of metabolic, lipid, and oxidative stress and has been found in atherosclerotic plaque in humans suggesting endothelial cell production.5 The pro-inflammatory state in psoriasis is associated with elevated sLOX-1 levels. Additionally, sLOX-1 levels are associated with lipid-rich noncalcified coronary plaque burden independent of hyperlipidemia status in patients with psoriasis, supporting a role for sLOX-1 in modulation of lipid-rich plaque in psoriasis.
Psoriasis Treatment and sLOX-1
The Canakinumab Anti-inflammatory Thrombosis Outcomes Study (CANTOS) trial reported that mitigation of residual inflammation with anti-inflammatory therapy, an interleukin 1β inhibitor, was associated with CV risk reduction independent of lipid-level lowering.1 Research has also shown that anti-inflammatory systemic or biologic treatment of psoriasis was associated with a lower incidence of myocardial infarction over time.16 A study recently reported an association between biologic therapy in severe psoriasis and favorable modulation of NCB.17 In the present study, psoriasis improvement was associated with a reduction in sLOX-1 levels. These findings suggest that reduction in in vivo inflammation may be associated with benefits in sLOX-1 over time.
Limitations
Our study has limitations. This was an observational study subject to potential for measured confounders and selection bias. This observational study permits association analyses between psoriasis severity, sLOX-1, and NCB. Cohort studies cannot establish causation and further in vitro and larger in vivo studies are required. The study population comprised mostly patients with moderate psoriasis and therefore results may not be generalizable beyond this group. Additionally, we did not study mechanisms of how sLOX-1 modulation changes in vitro behaviors, but these studies are ongoing. We also did not include hard CV events as outcome in our study population owing to a younger age group.
Conclusions
In this study, circulating sLOX-1 was found to be elevated in psoriasis and to be associated with psoriasis skin disease severity. Soluble lectinlike oxidized low-density lipoprotein receptor-1 was found to be associated with lipid-rich NCB independent of hyperlipidemia status in psoriasis. A reduction in psoriasis skin severity appeared to be associated with a reduction in sLOX-1 levels. Our findings suggest a need for further studies evaluating sLOX-1 in chronic inflammation associated with cardiometabolic disease.
eTable. Characteristics of Psoriasis Patients at Baseline
References
- 1.Ridker PM, Everett BM, Thuren T, et al. ; CANTOS Trial Group . Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119-1131. doi: 10.1056/NEJMoa1707914 [DOI] [PubMed] [Google Scholar]
- 2.Gelfand JM, Neimann AL, Shin DB, Wang X, Margolis DJ, Troxel AB. Risk of myocardial infarction in patients with psoriasis. JAMA. 2006;296(14):1735-1741. doi: 10.1001/jama.296.14.1735 [DOI] [PubMed] [Google Scholar]
- 3.Lerman JB, Joshi AA, Chaturvedi A, et al. Coronary plaque characterization in psoriasis reveals high-risk features that improve after treatment in a prospective observational study. Circulation. 2017;136(3):263-276. doi: 10.1161/CIRCULATIONAHA.116.026859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sorokin AV, Kotani K, Elnabawi YA, et al. Association between oxidation-modified lipoproteins and coronary plaque in psoriasis. Circ Res. 2018;123(11):1244-1254. doi: 10.1161/CIRCRESAHA.118.313608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pothineni NVK, Karathanasis SK, Ding Z, Arulandu A, Varughese KI, Mehta JL. LOX-1 in Atherosclerosis and myocardial ischemia: biology, genetics, and modulation. J Am Coll Cardiol. 2017;69(22):2759-2768. doi: 10.1016/j.jacc.2017.04.010 [DOI] [PubMed] [Google Scholar]
- 6.Akhmedov A, Rozenberg I, Paneni F, et al. Endothelial overexpression of LOX-1 increases plaque formation and promotes atherosclerosis in vivo. Eur Heart J. 2014;35(40):2839-2848. doi: 10.1093/eurheartj/eht532 [DOI] [PubMed] [Google Scholar]
- 7.Mehta JL, Sanada N, Hu CP, et al. Deletion of LOX-1 reduces atherogenesis in LDLR knockout mice fed high cholesterol diet. Circ Res. 2007;100(11):1634-1642. doi: 10.1161/CIRCRESAHA.107.149724 [DOI] [PubMed] [Google Scholar]
- 8.Lubrano V, Del Turco S, Nicolini G, Di Cecco P, Basta G. Circulating levels of lectin-like oxidized low-density lipoprotein receptor-1 are associated with inflammatory markers. Lipids. 2008;43(10):945-950. doi: 10.1007/s11745-008-3227-9 [DOI] [PubMed] [Google Scholar]
- 9.Chen M, Nagase M, Fujita T, Narumiya S, Masaki T, Sawamura T. Diabetes enhances lectin-like oxidized LDL receptor-1 (LOX-1) expression in the vascular endothelium: possible role of LOX-1 ligand and AGE. Biochem Biophys Res Commun. 2001;287(4):962-968. doi: 10.1006/bbrc.2001.5674 [DOI] [PubMed] [Google Scholar]
- 10.von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP; STROBE Initiative . The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147(8):573-577. doi: 10.7326/0003-4819-147-8-200710160-00010 [DOI] [PubMed] [Google Scholar]
- 11.Carlin CS, Feldman SR, Krueger JG, Menter A, Krueger GGA. A 50% reduction in the Psoriasis Area and Severity Index (PASI 50) is a clinically significant endpoint in the assessment of psoriasis. J Am Acad Dermatol. 2004;50(6):859-866. doi: 10.1016/j.jaad.2003.09.014 [DOI] [PubMed] [Google Scholar]
- 12.Kwan AC, May HT, Cater G, et al. Coronary artery plaque volume and obesity in patients with diabetes: the factor-64 study. Radiology. 2014;272(3):690-699. doi: 10.1148/radiol.14140611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salahuddin T, Natarajan B, Playford MP, et al. Cholesterol efflux capacity in humans with psoriasis is inversely related to non-calcified burden of coronary atherosclerosis. Eur Heart J. 2015;36(39):2662-2665. doi: 10.1093/eurheartj/ehv339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sawamura T, Kume N, Aoyama T, et al. An endothelial receptor for oxidized low-density lipoprotein. Nature. 1997;386(6620):73-77. doi: 10.1038/386073a0 [DOI] [PubMed] [Google Scholar]
- 15.Witztum JL, Steinberg D. Role of oxidized low density lipoprotein in atherogenesis. J Clin Invest. 1991;88(6):1785-1792. doi: 10.1172/JCI115499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu JJ, Poon KY, Channual JC, Shen AY. Association between tumor necrosis factor inhibitor therapy and myocardial infarction risk in patients with psoriasis. Arch Dermatol. 2012;148(11):1244-1250. doi: 10.1001/archdermatol.2012.2502 [DOI] [PubMed] [Google Scholar]
- 17.Elnabawi YA, Dey AK, Goyal A, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115(4):721-728. doi: 10.1093/cvr/cvz009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Characteristics of Psoriasis Patients at Baseline