Key Points
Question
Does genetic liability to insomnia, hypersomnia, and chronotype differentiate subtypes of bipolar disorder?
Findings
In this case-control study of 4672 participants with bipolar disorder and 5714 control participants, individuals with bipolar disorder I had significantly greater genetic liability to longer sleep duration, whereas individuals with bipolar disorder II had significantly greater genetic liability to insomnia; these findings were replicated in an independent sample. Individuals with bipolar subtypes did not differ in genetic liability to morning or evening chronotype.
Meaning
Associations between polygenic liability to insomnia and hypersomnia and clinical strata within bipolar disorder are shown in this study for the first time, to our knowledge.
This case-control study uses a genome-wide association study and mendelian randomization to ascertain whether polygenic risk scores for sleep traits are associated with individuals with bipolar disorder I or II vs control participants.
Abstract
Importance
Insomnia, hypersomnia, and an evening chronotype are common in individuals with bipolar disorder (BD), but whether this reflects shared genetic liability is unclear. Stratifying by BD subtypes could elucidate this association and inform sleep and BD research.
Objective
To assess whether polygenic risk scores (PRSs) for sleep traits are associated with BD subtypes I and II.
Design, Setting, and Participants
This case-control study was conducted in the United Kingdom and Sweden with participants with BD and control participants. Multinomial regression was used to assess whether PRSs for insomnia, daytime sleepiness, sleep duration, and chronotype are associated with BD subtypes compared with control participants. Affected individuals were recruited from the Bipolar Disorder Research Network. Control participants were recruited from the 1958 British Birth Cohort and the UK Blood Service. Analyses were repeated in an independent Swedish sample from August 2018 to July 2019. All participants were of European ancestry.
Exposures
Standardized PRSs derived using alleles from genome-wide association studies of insomnia, sleep duration, daytime sleepiness, and chronotype. These were adjusted for the first 10 population principal components, genotyping platforms, and sex.
Main Outcomes and Measures
Association of PRSs with BD subtypes, determined by semistructured psychiatric interview and case notes.
Results
The main analysis included 4672 participants with BD (3132 female participants [67.0%]; 3404 with BD-I [72.9%]) and 5714 control participants (2812 female participants [49.2%]). Insomnia PRS was associated with increased risk of BD-II (relative risk [RR], 1.14 [95% CI, 1.07-1.21]; P = 8.26 × 10−5) but not BD-I (RR, 0.98 [95% CI, 0.94-1.03]; P = .409) relative to control participants. Sleep-duration PRS was associated with BD-I (RR, 1.10 [95% CI, 1.06-1.15]; P = 1.13 × 10−5) but not BD-II (RR, 0.99 [95% CI, 0.93-1.06]; P = .818). Associations between (1) insomnia PRS and BD-II and (2) sleep-duration PRS and BD-I were replicated in the Swedish sample of 4366 individuals with BD (2697 female participants [61.8%]; 2627 with BD-I [60.2%]) and 6091 control participants (3767 female participants [61.8%]). Chronotype and daytime-sleepiness PRS were not associated with BD subtypes.
Conclusions and Relevance
Per this analysis, BD subtypes differ in genetic liability to insomnia and hypersomnia, providing further evidence that the distinction between BD-I and BD-II has genetic validity. This distinction will be crucial in selecting participants for future research on the role of sleep disturbance in BD.
Introduction
Bipolar disorder (BD) and sleep have often been linked. First, reduced sleep duration, a symptom of manic episodes, has been implicated as a prodrome and trigger of mania.1,2,3,4,5 Second, insomnia (difficulty initiating and maintaining sleep6) and hypersomnia (prolonged sleep duration or excessive daytime sleepiness7) are commonly reported symptoms of bipolar depression7,8,9 that persist in the interepisode period9,10,11,12,13,14,15,16,17,18 and are associated with significant distress and impairment.10 Third, there is evidence that individuals with BD display greater evening preference for sleep (ie, an evening chronotype) than healthy control participants.19,20,21 At present, sleep interventions for individuals with BD primarily focus on reducing insomnia22,23,24 and stabilizing circadian rhythms.25 Understanding the association between sleep and BD is important and could inform clinical interventions.
Recent genome-wide association studies (GWAS)26,27,28,29,30,31 provide an opportunity to examine the association between sleep and BD at the genomic level. Using summary-level data, some studies have demonstrated a positive genetic correlation between BD and sleep duration.28,30 Other studies, however, have found no significant genetic correlations between BD, chronotype, and insomnia.26,28 These analyses have used summary-level data and therefore may have been limited by a lack of individual bipolar phenotypic and genotypic data. In particular, associations between BD subtypes6 (ie, type 1 [BD-I] and type 2 [BD-II]) and sleep traits have been neglected, despite evidence of heterogeneity between BD subtypes in genetics, illness course, clinical features, and etiologies.32,33,34,35,36 There is also evidence that individuals with BD subtypes differ in sensitivity to sleep loss3 and rates of hypersomnia and insomnia during depressive episodes.37
We therefore aimed to determine whether genetic liability for insomnia, hypersomnia, and chronotype differs in BD-I and BD-II. Given a lack of evidence on whether these sleep traits differ between individuals with BD-I or BD-II in the interepisode period, we had no prior hypothesis about which sleep traits, if any, would be associated with BD subtypes.
To test the associations between sleep and BD phenotypes, we adopted the polygenic risk score (PRS) method to estimate the burden of risk alleles associated with 4 sleep-associated phenotypes (insomnia, sleep duration, excessive daytime sleepiness, and chronotype) in people with BD-I or BD-II and control participants.38,39 In secondary analyses, we conducted a 2-sample mendelian randomization (MR) study to test whether the data were consistent with a causal association between sleep and BD phenotypes.
Method
Sample Recruitment
Individuals With BD
Participants with BD were recruited within the United Kingdom by the Bipolar Disorder Research Network (bdrn.org).35 All participants reported their race as white, were genetically unrelated, were 18 years or older, and had been recruited systematically (eg, via community mental health teams) or nonsystematically (eg, via websites, radio advertisements, or voluntary groups, such as Bipolar UK). Participants were excluded if they had affective illness experienced solely in response to alcohol or substance misuse or secondary to medical illness or medication use.
Participants provided written informed consent. The study had ethical approval from the West Midlands Multi-Centre Research Ethics Committee, in addition to local research and development approval by UK National Health Service Trusts and Health Boards.
Control Participants
Control participants aged 18 years or older were recruited via the UK Blood Service and the 1958 Birth Cohort. Characteristics and recruitment of this sample has been described previously.40 All control participants reported their race as white.
Measures
Individuals with BD were assessed using the Schedules for Clinical Assessment in Neuropsychiatry interview,41 administered by trained research psychologists or psychiatrists in the research team (A.D.F., L.F., K.G.-S., L.J., N.C., and I.J.). Information from this interview was combined with clinical case note data to make lifetime best-estimate DSM-IV diagnoses. Measures taken to increase reliability of distinguishing BD subtypes are outlined in eAppendix 1 in the Supplement. Interrater reliability for differentiating between a best-estimate lifetime DSM-IV diagnosis of BD-I and BD-II was found to be good (κ, 0.85).
Discovery Data Sets for Sleep Traits
The discovery data sets were GWAS summary statistics for insomnia,27 sleep duration,30 daytime sleepiness,31 and chronotype42 conducted in participants recruited to the UK Biobank.43 Sleep phenotypes were assessed using touchscreen questions. To assess insomnia symptoms, participants were asked, “Do you have trouble falling asleep at night, or do you wake up in the middle of the night?” with the possible responses “never/rarely,” “sometimes,” “usually,” and “prefer not to answer.” The insomnia GWAS was conducted in 236 163 participants who answered “usually” (affected individuals) or “never/rarely” (control participants). We chose to use this relatively extreme GWAS rather than a larger GWAS conducted by the same authors (ie, comparing responses of never or rarely with sometimes or usually for the question on insomnia symptoms) because (1) we considered this to better approximate meaningful insomnia; (2) it identified a larger number of genome-wide significant loci (28 vs 9 in the larger GWAS), suggesting that, despite the smaller sample size, a clearer distinction in phenotype offered better power; and (3) the authors used results of the extremes GWAS, not the larger GWAS, in validation analyses. The sleep-duration GWAS was conducted in 448 609 participants who were asked, “About how many hours sleep do you get in every 24 hours? (Please include naps.)” Responses were in 1-hour increments and were analyzed as a continuous variable. The daytime sleepiness GWAS was conducted in 452 071 participants and assessed using the question, “How likely are you to doze off or fall asleep during the daytime when you don’t mean to? (eg, when working, reading, or driving),” with responses “never/rarely,” “sometimes,” “often,” or “all the time” analyzed on a scale of 1 to 4 points.
The chronotype GWAS consisted of 403 195 individuals who answered the question “Do you consider yourself to be….?” Those who answered “definitely a ‘morning’ person” or “more a ‘morning’ than ‘evening’ person” were coded as affected individuals, and those who answered “more an ‘evening’ than a ‘morning’ person” or “definitely an ‘evening’ person” coded as control participants. Hence positive effect sizes from this GWAS indicate a morning chronotype, whereas negative effect sizes indicate an evening chronotype.
Polygenic Risk Scores
Full details on genotyping, quality control, and imputation are in eAppendix 1 and the eFigure in the Supplement. We generated polygenic risk scores (PRSs) using PLINK version 1.944 in PRSice.45 Imputed genotypes were clumped for linkage disequilibrium (window, 500 kb; r2 = 0.20), and single-nucleotide polymorphisms most significantly associated with sleep traits were retained. Clumping resulted in retaining 92 085, 92 096, and 91 950 single-nucleotide polymorphisms for daytime sleepiness, sleep duration, and insomnia, respectively. After clumping, PRSs for sleep traits were generated using PRSice45 at P value thresholds (PT) P < 1.00, P ≤ .50, P ≤ .20, P ≤ .10, P ≤ .05, P ≤ .01, and P ≤ .001 and converted to z scores. This range of P value thresholds was chosen in the absence of an independent sample that indicated which PRS P value threshold explained the most variance in each of the respective sleep phenotypes.
Statistical Analysis
Data analysis was conducted in R version 3.33 (R Foundation for Statistical Computing). We performed multinomial logistic regression analyses examining associations between PRS for the aforementioned sleep traits (at the range of P value thresholds described) and individuals with BD subtypes (BD-I or BD-II) vs control participants. All analyses were adjusted for sex and 10 population principal components. In sensitivity analyses, we performed direct comparisons between the BD subtype groups by first performing the same multinomial regressions but changing the reference group from control participants to participants with BD-I or BD-II, and then by using logistic regressions that corrected for age, sex, and 10 population principal components. Results are reported at the P value thresholds that showed the most significant results with a false-discovery rate correction applied (using the Benjamini and Hochberg46 approach).
MR Analyses
In cases in which we observed significant associations between sleep phenotype PRS and BD subtypes, we conducted follow-up 2-sample MR studies to test whether sleep phenotypes (exposures) were potentially causally related to BD subtypes (the outcome). Mendelian randomization is a causal inference method that uses genetic variants as instrumental variables for an exposure of interest. It relies on 3 assumptions: (1) genetic variants must be strongly associated with the exposure, (2) genetic variants should not be associated with confounders of the exposure-outcome relationship, and (3) genetic variants should only be associated with the outcome through the exposure in question.47 We used genome-wide significant single-nucleotide polymorphisms as genetic instruments for the sleep phenotypes. Instrument-exposure effects were taken from the sleep-trait GWAS summary statistics, and instrument-outcome effects were taken from BD-I and BD-II GWAS summary statistics.48 Four MR methods were used to assess relationships between sleep phenotypes and BD subtypes: the inverse variance weighted,49 weighted median,50 weighted mode,51 and MR Egger49 regression methods. To test for evidence of pleiotropy, we examined the intercept of MR Egger regressions49 and the Cochran Q and Rücker Q statistics.52,53 Data pruning, harmonization, and analyses were conducted in R version 3.33 using the “TwoSampleMR” package.54
Replication Sample
We sought to replicate the study findings using Swedish individuals with BD (n = 4366) and control participants (n = 6091) recruited via the St Göran Bipolar project55 and the Swedish National Quality Register for Bipolar Affective Disorder (BipoläR).56,57 Full details of the samples, genotyping, quality control, and imputation are in eAppendix 2 in the Supplement.
Results
Sample Description
Among the individuals with BD, 3132 were female (67.0%), with a median age of 46 (range, 18-89) years. A total of 3404 participants met criteria for BD-I, and 1268 met criteria for BD-II. Among control participants, 2812 of 5714 (49.2%) were female. The Swedish sample consisted of 6091 control participants (3767 female participants [61.8%]) and 4366 affected individuals (2697 female participants [61.8%]), of whom 2627 met criteria for BD-I and 1739 met criteria for BD-II.
Correlations Between PRSs for Sleep Traits
Across all PRS P value thresholds, insomnia PRSs were negatively associated with sleep-duration PRSs (r range, −0.17 to −0.30; P < 1 × 10−4) and positively associated with daytime-sleepiness PRSs (r range, 0.04-0.10; P = .007 to P < 1 × 10−4). Sleep-duration PRSs were negatively associated with daytime-sleepiness PRSs (r range, −0.03 to −0.00), but these associations were not significant across all thresholds (range, P = .028-.916). Morningness PRSs were not significantly associated with PRS for insomnia, sleep duration, or daytime sleepiness.
PRSs for Sleep Traits: Case-Control Analyses
Logistic regression comparing individuals with BD with control participants revealed that, across all PRS P value thresholds, case status was significantly associated with PRSs for sleep duration (odds ratio [OR], 1.07 [95% CI, 1.03-1.12]; P = 5.52 × 10−4; P = 5.52 × 10−4 with adjustment for false-discovery rate [PRS PT < 1.00]) and daytime sleepiness (OR, 1.10 [95% CI, 1.06-1.15]; P = 2.31 × 10−6; P = 1.05 × 10−5 with adjustment for false-discovery rate [PRS PT ≤ .01]) and negatively associated with morning chronotype (OR, 0.91 [95% CI, 0.88-0.95]; P = 1.86 × 10−5; P = 6.26 × 10−5 with adjustment for false-discovery rate [PRS PT ≤ .05]) but not significantly associated with insomnia (OR, 0.98 [95% CI, 0.94-1.02]; P = .39 [PRS PT < 1.00]; eTables 2-5 in the Supplement).
PRS for Sleep Traits by BD Subtypes
Results at the most significant PRS P value thresholds (with corrected P values) are summarized. Results at other PRS P value thresholds (PT) are provided in eAppendix 1 and eTables 18 and 19 in the Supplement.
Insomnia PRS
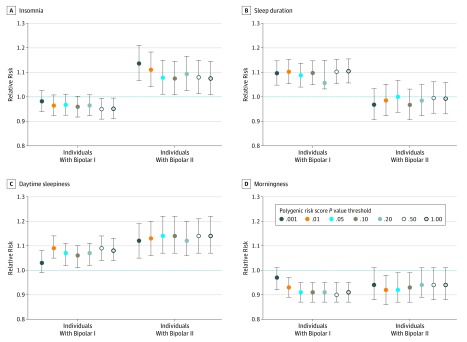
Multinomial regressions comparing individuals with BD subtypes to control participants revealed that insomnia PRS was significantly associated with a decreased risk of BD-I at a PRS PT of P < 1.00 and P ≤ .50, but significant associations were not seen at other P value thresholds (eTable 6 in the Supplement). At all P value thresholds, insomnia PRS was significantly associated with BD-II (relative risk [RR], 1.14 [95% CI, 1.07-1.21]; P = 8.26 × 10−5, P = .001 with false-discovery rate adjustment [PRS PT ≤ .001]). Results at all PT are shown in the Figure, A. In direct tests, insomnia PRS was significantly associated with BD-II compared with BD-I (RR, 1.16 [95% CI, 1.08-1.24]; P = 1.39 × 10−5; P = 1.95 × 10−4 with false-discovery rate adjustment; OR, 1.14 [95% CI, 1.07-1.22]; P = 6.81 × 10−5 [PRS PT ≤ .001]; eTables 10-11 in the Supplement).
Figure. Relative Risk Ratios for Individuals With Bipolar Subtypes vs Control Participants.
Relative risk of insomnia (A), sleep duration (B), daytime sleepiness (C), and morningness (D) for patients with bipolar subtypes compared with control participants, as anticipated based on polygenic risk scores. Error bars indicate 95% CIs.
Sleep-Duration PRS
At all PRS P value thresholds, multinomial regression comparing individuals with BD subtypes with control participants revealed that sleep-duration PRS was associated with BD-I (RR, 1.10 [95% CI, 1.06-1.15]; P = 1.13 × 10−5; P = 1.07 × 10−4 with false-discovery rate adjustment [PRS PT < 1.00]; eTable 7 in the Supplement). Associations between sleep-duration PRS and BD-II were not significant at any PRS P value threshold (eTable 7 in the Supplement). Results at all P value thresholds are shown in the Figure, B. Direct comparisons between the subgroups with BD-I and BD-II revealed that sleep-duration PRS was significantly associated with BD-I (RR, 1.11 [95% CI, 1.04-1.19]; P = 1.69 × 10−3; P = 4.74 × 10−3 with false-discovery rate adjustment; OR, 1.11 [95% CI, 1.04-1.19]; P = .002 [PRS PT < 1.00]; eTables 12-13 in the Supplement).
Daytime-Sleepiness PRS
Compared with the control group, daytime-sleepiness PRS was associated with BD-I and BD-II at all PRS P value thresholds (except PT < .001; eTable 8 in the Supplement). Results at all PRS PT are shown in the Figure, C. Direct comparisons between BD subtypes were not significant after correction for multiple testing (eTable 14-15 in the Supplement).
Chronotype PRS
Polygenic risk score for morningness was associated with a reduced relative risk of BD-I in affected individuals compared with the control participants (RR, 0.90 [95% CI, 0.86-0.95]; P = 1.06 × 10−5; P = 1.11 × 10−4 with false-discovery rate adjustment [PRS PT ≤ .50] at all PRS P value thresholds except PT less than .001. In individuals with BD compared with control participants, morningness PRS was associated with a reduced risk of BD-II, but this finding was not significant across most PRS P value thresholds (eTable 9 in the Supplement). Results at all PRSs are shown in the Figure. Direct comparisons between BD subtypes were not significant (eTable 16-17 in the Supplement).
MR Analyses
Across all MR methods, we did not find evidence of a potential causal relationship between insomnia and BD-II or sleep duration and BD-I. However, the direction of effect was consistent when assessing the effect of insomnia with BD-II (Table). Although MR Egger intercepts were not significantly different from zero for analyses of BD-I and BD-II , the Cochran Q and Rücker Q statistics indicated significant heterogeneity in effect estimates (insomnia: Rücker Q35 = 53.33; P = .024; Cochran Q36 = 54.03; P = .027; sleep duration: Rücker Q54 = 126.92; P = 8.33 × 10−8; Cochran Q55 = 128.91; P = 7.18 × 10−8; Table), possibly because of horizontal pleiotropy.
Table. Results of 2-Sample Mendelian Randomization Studies.
| Mendelian Randomization Method | Insomnia in Bipolar Disorder IIa | Sleep Duration in Bipolar Disorder Ib | ||||
|---|---|---|---|---|---|---|
| log(Odds Ratio) or Q Statistic | SE or df | P Value | log(Odds Ratio) or Q Statistic | SE or df | P Value | |
| Inverse variance weightedc | 0.256 | 0.149 | .087 | 0.396 | 0.209 | .059 |
| Weighted medianc | 0.355 | 0.183 | .052 | 0.245 | 0.219 | .264 |
| Weighted modec | 0.410 | 0.300 | .180 | 0.005 | 0.361 | .990 |
| MR Eggerc | 0.577 | 0.498 | .255 | -0.274 | 0.757 | .719 |
| Rücker Qd | 53.33 | 35 | .024 | 126.92 | 54 | 8.33 × 10−8 |
| Cochran Qd | 54.03 | 36 | .027 | 128.91 | 55 | 7.18 × 10−8 |
37 Single-nucleotide polymorphisms.
56 Single-nucleotide polymorphisms.
Log(odds ratio) and SEs are presented.
Q statistics and df are presented.
Replication Sample
In the Swedish sample, insomnia PRS was significantly associated with BD-II (RR, 1.07 [95% CI, 1.01-1.13]; P = .013 [PRS PT ≤ .001]) compared with control participants, whereas the association with BD-I was not significant. Sleep-duration PRS was associated with a significant increased relative risk of BD-I compared with control participants (RR, 1.11 [95% CI, 1.06-1.16]; P = 1.72 × 10−5 [PRS PT < 1.00]). The association between sleep-duration PRS and BD-II was marginally significant (RR, 1.06 [95% CI, 1.00-1.12]; P = .042 [PRS PT <1.00]).
Discussion
Bipolar disorder is heterogeneous in symptom presentation and most likely in the mechanisms that underlie these presentations. Genetics can help refine diagnostic groups that share similar etiologies.58 In this study, we provide what is to our knowledge the first evidence that genetic liability to insomnia and longer sleep duration differs according to BD subtype.
Genetic liability to insomnia as indexed by PRS was associated with increased relative risk of BD-II compared with control participants and those with BD-I. The stronger association between insomnia PRS and BD-II may explain nonsignificant genetic correlations between BD and insomnia in previous research,26,28 because individuals with BD-II are usually underrepresented in BD GWAS (eg, only 11% in a recent study59). Future research should explore possible reasons for this association.
Hypersomnia in BD populations has remained relatively underresearched, but researchers have recently called for increased efforts to understand its underlying biology and role in BD.16,60 This is because of its high prevalence and recurrence across bipolar depressive episodes7,37 in addition to high interepisode prevalence and association with relapse.17,61 We used sleep-duration and daytime-sleepiness PRS as proxies for genetic liability to hypersomnia. Sleep-duration PRS was associated with increased relative risk of BD-I but not BD-II and was significantly more strongly associated with BD-I than BD-II in a direct comparison. In contrast, daytime-sleepiness PRS was not significantly associated with BD subtypes. Daytime-sleepiness PRS may be a proxy for insomnia, in that daytime sleepiness can be induced by insomnia,62 and we observed significant positive correlations between insomnia and daytime-sleepiness PRS. These results support existing research on the importance of hypersomnia in individuals with BD16,60 (and BD-I in particular37) and provide further evidence that hypersomnia is not a unitary construct.63,64
The results of the MR analyses do not support potentially causal relationships between insomnia and BD-II or sleep duration and BD-I. However, we observed significant heterogeneity in the genetic instruments, thereby violating the third assumption of MR and potentially biasing the results. Therefore, while insomnia and sleep duration could be useful clinical stratifiers, there is currently insufficient evidence to support a causal inference. Further research is needed to elucidate the biological mechanisms underpinning the genetic association between BD-I and longer sleep duration.
Implications
Clinical and biological heterogeneity, combined with a classification that is not grounded in biology, are obstacles to advancing BD research. We provide some evidence of heterogeneity in genetic propensity to some sleep traits within BD (specifically insomnia and sleep duration), highlighting differences in the way some sleep-associated genetic factors are associated with BD subtypes. This adds to previously published work on stratification in BD65 and work suggesting that different factors may influence the 2 conditions.32,33,34
These results suggest that clinical trials of sleep interventions should stratify participants by clinical subtype and genetic liability to insomnia or hypersomnia. Future work should explore which factors drive the differences in genetic liability for insomnia/sleep duration between BD subtypes.
Strengths
This study was conducted on the world’s largest single cohort of BD with genotypic and phenotypic data. Phenotypic data were collected using face-to-face semistructured interviews and case notes with high interrater reliability. We were therefore able to explore genetic associations using individual-level genetic data, which provided more granularity than summary statistics. In addition, we were able to replicate the results for insomnia PRS and BD-II and sleep-duration PRS and BD-I in an independent sample.
Limitations
This study has several limitations. First, potential recruitment bias in our BD sample may have reduced its representativeness and influenced the results.66 Second, we were unable to adjust for additional variables (eg, age, education), because these were unavailable for control participants. Third, the index of hypersomnia is imprecise because the available GWAS summary statistics measured sleep duration as total hours slept30; previous work suggests that hypersomnia is better characterized by total time in bed.17,64,67 Fourth, there is evidence that 5% to 17% of patients with BD-II convert to BD-I,68,69 which could have resulted in some individuals with BD-II being misclassified in this sample. However, this would have reduced power to observe differences between the 2 subtypes rather than resulted in positive results we observe for insomnia and sleep duration. Finally, variants associated with insomnia or hypersomnia at ages 40 to 69 years (the age of the UK Biobank sample43) may differ from those associated in childhood or early adulthood. This may have increased our type-2 error rate, because most patients with BD experience the first onset of impairing symptoms in adolescence or early adulthood.70 Genetic risk for insomnia or hypersomnia that manifests during or prior to early adulthood may be more strongly associated with BD than those associated with midlife insomnia. These results should be replicated using future sleep trait GWAS in younger samples of sufficient size for PRS analysis.
Conclusions
To our knowledge, this is the first study to explore whether genetic liability for sleep traits is associated with clinical strata of individuals with BD. Future work should explore potential mechanisms underlying differences between the BD subtypes in genetic liability for sleep traits.
eAppendix 1. Bipolar Diagnoses, Genotyped Data, Additional Analyses and Replication Sample
eTable 1. Description of genotyping platforms for the BD and control samples. SNP N is before QC and imputation. Wave 1 of the BDRN data is part of the International Cohort Collection for Bipolar Disorder (ICCBD).
eFigure. Principal component analysis of imputed genotype data, after exclusion of variants showing platform frequency differences.
eTable 2. Logistic regressions between polygenic risk scores for insomnia and bipolar disorder cases compared to controls
eTable 3. Logistic regressions between polygenic risk scores for sleep duration and bipolar disorder cases compared to controls
eTable 4. Logistic regressions between polygenic risk scores for daytime sleepiness and bipolar disorder cases compared to controls
eTable 5. Logistic regressions between polygenic risk scores for morningness and bipolar disorder cases compared to controls
eTable 6. Multinomial regressions between polygenic risk scores for insomnia and bipolar subtypes compared to controls.
eTable 7. Multinomial regressions between polygenic risk scores for sleep duration and bipolar subtypes compared to controls.
eTable 8. Multinomial regressions between polygenic risk scores for daytime sleepiness and bipolar subtypes compared to controls.
eTable 9. Multinomial regressions between polygenic risk scores for morningness and bipolar subtypes compared to controls.
eTable 10. Sensitivity analyses: Multinomial regressions of insomnia polygenic risk scores and clinical status using bipolar I disorder as the reference group.
eTable 11. Logistic regressions between polygenic risk scores for insomnia and odds of bipolar II disorder compared to bipolar I disorder.
eTable 12. Sensitivity analyses: Multinomial regressions of sleep duration polygenic risk scores and clinical status using bipolar II disorder as the reference group.
eTable 13. Logistic regressions between polygenic risk scores for sleep duration and odds of bipolar I disorder compared to bipolar II disorder.
eTable 14. Sensitivity analyses: Multinomial regressions of daytime sleepiness polygenic risk scores and clinical status using bipolar I disorder as the reference group.
eTable 15. Logistic regressions between polygenic risk scores for daytime sleepiness and odds of bipolar II disorder compared to bipolar I disorder.
eTable 16. Sensitivity analyses: Multinomial regressions of morningness polygenic risk scores and clinical status using bipolar II disorder as the reference group.
eTable 17. Logistic regressions between polygenic risk scores for morningness and odds of bipolar I disorder compared to bipolar II disorder.
eTable 18. Frequencies of cases and controls in Swedish replication sample by genotyping wave.
eAppendix 2. Replication Sample - Materials and methods
eTable 19. Frequencies of controls, bipolar I disorder cases, and bipolar II disorder cases (by sex).
eReferences.
References
- 1.Wehr TA, Sack DA, Rosenthal NE. Sleep reduction as a final common pathway in the genesis of mania. Am J Psychiatry. 1987;144(2):201-204. [DOI] [PubMed] [Google Scholar]
- 2.Wehr TA. Sleep loss: a preventable cause of mania and other excited states. J Clin Psychiatry. 1989;50(12)(suppl):8-16. [PubMed] [Google Scholar]
- 3.Lewis KS, Gordon-Smith K, Forty L, et al. Sleep loss as a trigger of mood episodes in bipolar disorder: individual differences based on diagnostic subtype and gender. Br J Psychiatry. 2017;211(3):169-174. doi: 10.1192/bjp.bp.117.202259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leibenluft E, Albert PS, Rosenthal NE, Wehr TA. Relationship between sleep and mood in patients with rapid-cycling bipolar disorder. Psychiatry Res. 1996;63(2-3):161-168. doi: 10.1016/0165-1781(96)02854-5 [DOI] [PubMed] [Google Scholar]
- 5.Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disord. 2006;8(3):271-274. doi: 10.1111/j.1399-5618.2006.00330.x [DOI] [PubMed] [Google Scholar]
- 6.American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders. 5th ed Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 7.Kaplan KA, Harvey AG. Hypersomnia across mood disorders: a review and synthesis. Sleep Med Rev. 2009;13(4):275-285. doi: 10.1016/j.smrv.2008.09.001 [DOI] [PubMed] [Google Scholar]
- 8.Forty L, Smith D, Jones L, et al. Clinical differences between bipolar and unipolar depression. Br J Psychiatry. 2008;192(5):388-389. doi: 10.1192/bjp.bp.107.045294 [DOI] [PubMed] [Google Scholar]
- 9.Harvey AG, Talbot LS, Gershon A. Sleep disturbance in bipolar disorder across the lifespan. Clin Psychol (New York). 2009;16(2):256-277. doi: 10.1111/j.1468-2850.2009.01164.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kanady JC, Soehnera AM, Harvey AG. A retrospective examination of sleep disturbance across the course of bipolar disorder. J Sleep Disord Ther. 2015;4(2):318-328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harvey AG, Schmidt DA, Scarnà A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. Am J Psychiatry. 2005;162(1):50-57. doi: 10.1176/appi.ajp.162.1.50 [DOI] [PubMed] [Google Scholar]
- 12.Geoffroy PA, Scott J, Boudebesse C, et al. Sleep in patients with remitted bipolar disorders: a meta-analysis of actigraphy studies. Acta Psychiatr Scand. 2015;131(2):89-99. doi: 10.1111/acps.12367 [DOI] [PubMed] [Google Scholar]
- 13.Plante DT, Winkelman JW. Sleep disturbance in bipolar disorder: therapeutic implications. Am J Psychiatry. 2008;165(7):830-843. doi: 10.1176/appi.ajp.2008.08010077 [DOI] [PubMed] [Google Scholar]
- 14.Harvey AG, Soehner AM, Kaplan KA, et al. Treating insomnia improves mood state, sleep, and functioning in bipolar disorder: a pilot randomized controlled trial. J Consult Clin Psychol. 2015;83(3):564-577. doi: 10.1037/a0038655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng TH, Chung KF, Ho FYY, Yeung WF, Yung KP, Lam TH. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: a systematic review and meta-analysis. Sleep Med Rev. 2015;20:46-58. doi: 10.1016/j.smrv.2014.06.006 [DOI] [PubMed] [Google Scholar]
- 16.Kaplan KA, Williams R. Hypersomnia: an overlooked, but not overestimated, sleep disturbance in bipolar disorder. Evid Based Ment Health. 2017;20(2):59. doi: 10.1136/eb-2016-102433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaplan KA, Gruber J, Eidelman P, Talbot LS, Harvey AG. Hypersomnia in inter-episode bipolar disorder: does it have prognostic significance? J Affect Disord. 2011;132(3):438-444. doi: 10.1016/j.jad.2011.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ritter PS, Marx C, Lewtschenko N, et al. The characteristics of sleep in patients with manifest bipolar disorder, subjects at high risk of developing the disease and healthy controls. J Neural Transm (Vienna). 2012;119(10):1173-1184. doi: 10.1007/s00702-012-0883-y [DOI] [PubMed] [Google Scholar]
- 19.Kim KL, Weissman AB, Puzia ME, et al. Circadian phase preference in pediatric bipolar disorder. J Clin Med. 2014;3(1):255-266. doi: 10.3390/jcm3010255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Melo MCA, Abreu RLC, Linhares Neto VB, de Bruin PFC, de Bruin VMS. Chronotype and circadian rhythm in bipolar disorder: a systematic review. Sleep Med Rev. 2017;34:46-58. doi: 10.1016/j.smrv.2016.06.007 [DOI] [PubMed] [Google Scholar]
- 21.Takaesu Y. Circadian rhythm in bipolar disorder: a review of the literature. Psychiatry Clin Neurosci. 2018;72(9):673-682. doi: 10.1111/pcn.12688 [DOI] [PubMed] [Google Scholar]
- 22.Sheaves B, Freeman D, Isham L, et al. Stabilising sleep for patients admitted at acute crisis to a psychiatric hospital (OWLS): an assessor-blind pilot randomised controlled trial. Psychol Med. 2018;48(10):1694-1704. doi: 10.1017/S0033291717003191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaplan KA, Harvey AG. Behavioral treatment of insomnia in bipolar disorder. Am J Psychiatry. 2013;170(7):716-720. doi: 10.1176/appi.ajp.2013.12050708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaplan KA, Talavera DC, Harvey AG. Rise and shine: a treatment experiment testing a morning routine to decrease subjective sleep inertia in insomnia and bipolar disorder. Behav Res Ther. 2018;111(111):106-112. doi: 10.1016/j.brat.2018.10.009 [DOI] [PubMed] [Google Scholar]
- 25.Frank E, Kupfer DJ, Thase ME, et al. Two-year outcomes for interpersonal and social rhythm therapy in individuals with bipolar I disorder. Arch Gen Psychiatry. 2005;62(9):996-1004. doi: 10.1001/archpsyc.62.9.996 [DOI] [PubMed] [Google Scholar]
- 26.Lane JM, Liang J, Vlasac I, et al. Genome-wide association analyses of sleep disturbance traits identify new loci and highlight shared genetics with neuropsychiatric and metabolic traits. Nat Genet. 2017;49(2):274-281. doi: 10.1038/ng.3749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lane JM, Jones SE, Dashti HS, et al. ; HUNT All In Sleep . Biological and clinical insights from genetics of insomnia symptoms. Nat Genet. 2019;51(3):387-393. doi: 10.1038/s41588-019-0361-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jansen PR, Watanabe K, Stringer S, et al. ; 23andMe Research Team . Genome-wide analysis of insomnia in 1,331,010 individuals identifies new risk loci and functional pathways. Nat Genet. 2019;51(3):394-403. doi: 10.1038/s41588-018-0333-3 [DOI] [PubMed] [Google Scholar]
- 29.Jones SE, Tyrrell J, Wood AR, et al. Genome-wide association analyses in 128,266 individuals identifies new morningness and sleep duration loci. PLoS Genet. 2016;12(8):e1006125. doi: 10.1371/journal.pgen.1006125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dashti HS, Jones SE, Wood AR, et al. GWAS in 446,118 European adults identifies 78 genetic loci for self-reported 2 habitual sleep duration supported by accelerometer-derived estimates. bioRxiv. https://www.biorxiv.org/content/biorxiv/early/2018/04/19/274977.full.pdf. Published 2018. doi: 10.1101/274977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H, Lane JM, Jones SE, et al. Genome-wide association analysis of excessive daytime sleepiness identifies 42 loci that suggest phenotypic subgroups. bioRxiv. https://www.biorxiv.org/content/10.1101/454561v1.full. Published 2018. Accessed 8, 2019. doi: 10.1101/454561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Baek JH, Park DY, Choi J, et al. Differences between bipolar I and bipolar II disorders in clinical features, comorbidity, and family history. J Affect Disord. 2011;131(1-3):59-67. doi: 10.1016/j.jad.2010.11.020 [DOI] [PubMed] [Google Scholar]
- 33.Caseras X, Murphy K, Lawrence NS, et al. Emotion regulation deficits in euthymic bipolar I versus bipolar II disorder: a functional and diffusion-tensor imaging study. Bipolar Disord. 2015;17(5):461-470. doi: 10.1111/bdi.12292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Caseras X, Lawrence NS, Murphy K, Wise RG, Phillips ML. Ventral striatum activity in response to reward: differences between bipolar I and II disorders. Am J Psychiatry. 2013;170(5):533-541. doi: 10.1176/appi.ajp.2012.12020169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jones L, Metcalf A, Gordon-Smith K, et al. Gambling problems in bipolar disorder in the UK: prevalence and distribution. Br J Psychiatry. 2015;207(4):328-333. doi: 10.1192/bjp.bp.114.154286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Charney AW, Ruderfer DM, Stahl EA, et al. Evidence for genetic heterogeneity between clinical subtypes of bipolar disorder. Transl Psychiatry. 2017;7(1):e993. doi: 10.1038/tp.2016.242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Steinan MK, Scott J, Lagerberg TV, et al. Sleep problems in bipolar disorders: more than just insomnia. Acta Psychiatr Scand. 2016;133(5):368-377. doi: 10.1111/acps.12523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wray NR, Lee SH, Mehta D, Vinkhuyzen AAE, Dudbridge F, Middeldorp CM. Research review: Polygenic methods and their application to psychiatric traits. J Child Psychol Psychiatry. 2014;55(10):1068-1087. doi: 10.1111/jcpp.12295 [DOI] [PubMed] [Google Scholar]
- 39.Dudbridge F. Power and predictive accuracy of polygenic risk scores. PLoS Genet. 2013;9(3):e1003348. doi: 10.1371/journal.pgen.1003348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wellcome Trust Case Control Consortium Genome-wide association study of 14,000 cases of seven common diseases and 3,000 shared controls. Nature. 2007;447(7145):661-678. doi: 10.1038/nature05911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wing JK, Babor T, Brugha T, et al. ; Schedules for Clinical Assessment in Neuropsychiatry . SCAN: schedules for clinical assessment in neuropsychiatry. Arch Gen Psychiatry. 1990;47(6):589-593. doi: 10.1001/archpsyc.1990.01810180089012 [DOI] [PubMed] [Google Scholar]
- 42.Jones SE, Lane JM, Wood AR, et al. Genome-wide association analyses of chronotype in 697,828 individuals provides insights into circadian rhythms. Nat Commun. 2019;10(1):343. doi: 10.1038/s41467-018-08259-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sudlow C, Gallacher J, Allen N, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12(3):e1001779. doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang CC, Chow CC, Tellier LCAM, Vattikuti S, Purcell SM, Lee JJ. Second-generation PLINK: rising to the challenge of larger and richer datasets. Gigascience. 2015;4(1):7. doi: 10.1186/s13742-015-0047-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Euesden J, Lewis CM, O’Reilly PF. PRSice: polygenic risk score software. Bioinformatics. 2015;31(9):1466-1468. doi: 10.1093/bioinformatics/btu848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc B. 1995;57(1):289-300. doi: 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 47.Zheng J, Baird D, Borges M-C, et al. Recent developments in mendelian randomization studies. Curr Epidemiol Rep. 2017;4(4):330-345. doi: 10.1007/s40471-017-0128-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stahl E, Forstner A, McQuillin A, et al. Genome-wide association study identifies 30 loci associated with bipolar disorder. bioRxiv. https://www.biorxiv.org/content/10.1101/173062v. Published 2017. Accessed November 8, 2019. doi: 10.1101/173062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bowden J, Davey Smith G, Burgess S. Mendelian randomization with invalid instruments: effect estimation and bias detection through Egger regression. Int J Epidemiol. 2015;44(2):512-525. doi: 10.1093/ije/dyv080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. 2016;40(4):304-314. doi: 10.1002/gepi.21965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data Mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. 2017;46(6):1985-1998. doi: 10.1093/ije/dyx102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bowden J, Del Greco M F, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data Mendelian randomization. Stat Med. 2017;36(11):1783-1802. doi: 10.1002/sim.7221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greco M FD, Minelli C, Sheehan NA, Thompson JR. Detecting pleiotropy in Mendelian randomisation studies with summary data and a continuous outcome. Stat Med. 2015;34(21):2926-2940. doi: 10.1002/sim.6522 [DOI] [PubMed] [Google Scholar]
- 54.Hemani G, Zheng J, Elsworth B, et al. The MR-Base platform supports systematic causal inference across the human phenome. Elife. 2018;pii:e34408. doi: 10.7554/eLife.34408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jakobsson J, Zetterberg H, Blennow K, Johan Ekman C, Johansson AGM, Landén M. Altered concentrations of amyloid precursor protein metabolites in the cerebrospinal fluid of patients with bipolar disorder. Neuropsychopharmacology. 2013;38(4):664-672. doi: 10.1038/npp.2012.231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Karanti A, Bobeck C, Osterman M, et al. Gender differences in the treatment of patients with bipolar disorder: a study of 7354 patients. J Affect Disord. 2015;174:303-309. doi: 10.1016/j.jad.2014.11.058 [DOI] [PubMed] [Google Scholar]
- 57.Tidemalm D, Haglund A, Karanti A, Landén M, Runeson B. Attempted suicide in bipolar disorder: risk factors in a cohort of 6086 patients. PLoS One. 2014;9(4):e94097. doi: 10.1371/journal.pone.0094097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Craddock N, O’Donovan MC, Owen MJ. Psychosis genetics: modeling the relationship between schizophrenia, bipolar disorder, and mixed (or “schizoaffective”) psychoses. Schizophr Bull. 2009;35(3):482-490. doi: 10.1093/schbul/sbp020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sklar P, Ripke S, Scott L, et al. Large-scale genome-wide association analysis of bipolar disorder identifies a new susceptibility locus near ODZ4. 2011;43(10):977-983. doi: 10.1038/ng.943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Plante DT. Hypersomnia in mood disorders: a rapidly changing landscape. Curr Sleep Med Rep. 2015;1(2):122-130. doi: 10.1007/s40675-015-0017-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kaplan KA, McGlinchey EL, Soehner A, et al. Hypersomnia subtypes, sleep and relapse in bipolar disorder. Psychol Med. 2015;45(8):1751-1763. doi: 10.1017/S0033291714002918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.American Academy of Sleep Medicine The International Classification of Sleep Disorders: Diagnostic and Coding Manual. 2nd ed Rochester, MN: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 63.Ohayon MM, Dauvilliers Y, Reynolds CF III. Operational definitions and algorithms for excessive sleepiness in the general population: implications for DSM-5 nosology. Arch Gen Psychiatry. 2012;69(1):71-79. doi: 10.1001/archgenpsychiatry.2011.1240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nofzinger EA, Thase ME, Reynolds CF III, et al. Hypersomnia in bipolar depression: a comparison with narcolepsy using the multiple sleep latency test. Am J Psychiatry. 1991;148(9):1177-1181. doi: 10.1176/ajp.148.9.1177 [DOI] [PubMed] [Google Scholar]
- 65.Allardyce J, Leonenko G, Hamshere M, et al. Association between schizophrenia-related polygenic liability and the occurrence and level of mood-incongruent psychotic symptoms in bipolar disorder. JAMA Psychiatry. 2018;75(1):28-35. doi: 10.1001/jamapsychiatry.2017.3485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Munafò MR, Tilling K, Taylor AE, Evans DM, Davey Smith G. Collider scope: when selection bias can substantially influence observed associations. Int J Epidemiol. 2018;47(1):226-235. doi: 10.1093/ije/dyx206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Billiard M, Dolenc L, Aldaz C, Ondze B, Besset A. Hypersomnia associated with mood disorders: a new perspective. J Psychosom Res. 1994;38(1)(suppl 1):41-47. doi: 10.1016/0022-3999(94)90134-1 [DOI] [PubMed] [Google Scholar]
- 68.Coryell W, Endicott J, Maser JD, Keller MB, Leon AC, Akiskal HS. Long-term stability of polarity distinctions in the affective disorders. Am J Psychiatry. 1995;152(3):385-390. doi: 10.1176/ajp.152.3.385 [DOI] [PubMed] [Google Scholar]
- 69.Alloy LB, Urošević S, Abramson LY, et al. Progression along the bipolar spectrum: a longitudinal study of predictors of conversion from bipolar spectrum conditions to bipolar I and II disorders. J Abnorm Psychol. 2012;121(1):16-27. doi: 10.1037/a0023973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tondo L, Lepri B, Cruz N, Baldessarini RJ. Age at onset in 3014 Sardinian bipolar and major depressive disorder patients. Acta Psychiatr Scand. 2010;121(6):446-452. doi: 10.1111/j.1600-0447.2009.01523.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Bipolar Diagnoses, Genotyped Data, Additional Analyses and Replication Sample
eTable 1. Description of genotyping platforms for the BD and control samples. SNP N is before QC and imputation. Wave 1 of the BDRN data is part of the International Cohort Collection for Bipolar Disorder (ICCBD).
eFigure. Principal component analysis of imputed genotype data, after exclusion of variants showing platform frequency differences.
eTable 2. Logistic regressions between polygenic risk scores for insomnia and bipolar disorder cases compared to controls
eTable 3. Logistic regressions between polygenic risk scores for sleep duration and bipolar disorder cases compared to controls
eTable 4. Logistic regressions between polygenic risk scores for daytime sleepiness and bipolar disorder cases compared to controls
eTable 5. Logistic regressions between polygenic risk scores for morningness and bipolar disorder cases compared to controls
eTable 6. Multinomial regressions between polygenic risk scores for insomnia and bipolar subtypes compared to controls.
eTable 7. Multinomial regressions between polygenic risk scores for sleep duration and bipolar subtypes compared to controls.
eTable 8. Multinomial regressions between polygenic risk scores for daytime sleepiness and bipolar subtypes compared to controls.
eTable 9. Multinomial regressions between polygenic risk scores for morningness and bipolar subtypes compared to controls.
eTable 10. Sensitivity analyses: Multinomial regressions of insomnia polygenic risk scores and clinical status using bipolar I disorder as the reference group.
eTable 11. Logistic regressions between polygenic risk scores for insomnia and odds of bipolar II disorder compared to bipolar I disorder.
eTable 12. Sensitivity analyses: Multinomial regressions of sleep duration polygenic risk scores and clinical status using bipolar II disorder as the reference group.
eTable 13. Logistic regressions between polygenic risk scores for sleep duration and odds of bipolar I disorder compared to bipolar II disorder.
eTable 14. Sensitivity analyses: Multinomial regressions of daytime sleepiness polygenic risk scores and clinical status using bipolar I disorder as the reference group.
eTable 15. Logistic regressions between polygenic risk scores for daytime sleepiness and odds of bipolar II disorder compared to bipolar I disorder.
eTable 16. Sensitivity analyses: Multinomial regressions of morningness polygenic risk scores and clinical status using bipolar II disorder as the reference group.
eTable 17. Logistic regressions between polygenic risk scores for morningness and odds of bipolar I disorder compared to bipolar II disorder.
eTable 18. Frequencies of cases and controls in Swedish replication sample by genotyping wave.
eAppendix 2. Replication Sample - Materials and methods
eTable 19. Frequencies of controls, bipolar I disorder cases, and bipolar II disorder cases (by sex).
eReferences.