Key Points
Question
Is breastfeeding associated with reduction in postpartum relapses in women with multiple sclerosis?
Findings
In this systematic review and meta-analysis of 24 studies that include 2974 women, there was a reduced rate of postpartum multiple sclerosis relapses in women who were breastfeeding compared with those who were not breastfeeding, with a stronger benefit of exclusive rather than nonexclusive breastfeeding. Compared with nonbreastfeeding, breastfeeding was associated with a 43% lower rate of postpartum relapse, although it is not possible to exclude residual confounding.
Meaning
Breastfeeding appears to be protective against postpartum multiple sclerosis relapses, although additional high-quality prospective studies appear to be needed.
Abstract
Importance
Multiple sclerosis (MS) relapses may be increased in the postpartum period, and whether breastfeeding is associated with reduction in the risk of postpartum relapses remains controversial.
Objective
To perform a systematic review and meta-analysis to evaluate whether breastfeeding is associated with reduction in postpartum MS relapses compared with not breastfeeding.
Data Sources
PubMed and Embase were searched for studies assessing the association between breastfeeding and MS disease activity published between January 1, 1980, and July 11, 2018, as well as reference lists of selected articles.
Study Selection
All study designs assessing the association between breastfeeding and postpartum relapses in MS relative to a comparator group were included.
Data Extraction and Synthesis
Study eligibility assessment and extraction of study characteristics, methods, and outcomes, were performed independently by 2 reviewers following PRISMA guidelines. Risk of bias was evaluated by 2 independent reviewers with the ROBINS-I tool for nonrandomized, interventional studies. Findings from studies with data available for the number of women with postpartum relapses in the breastfeeding and nonbreastfeeding groups were combined with a random-effects model.
Main Outcomes and Measures
Postpartum MS relapse.
Results
The search identified 462 unique citations, and 24 (2974 women) satisfied eligibility criteria and were included, of which 16 were included in the quantitative meta-analysis. The pooled summary odds ratio for the association of breastfeeding with postpartum relapses was 0.63 (95% CI, 0.45-0.88; P = .006) compared with a reference of nonbreastfeeding. Pooled adjusted hazard ratio across 4 studies that reported this finding was 0.57 (95% CI, 0.38-0.85; P = .006). There was moderate heterogeneity (I2 = 48%), which was explained by variable prepregnancy relapse rate, postpartum follow-up duration, and the publication year. A stronger association was seen in studies of exclusive rather than nonexclusive breastfeeding, although both demonstrated an association. Studies were rated at moderate and serious risk of bias, with concern for residual confounding, although sensitivity analysis including only moderate quality studies was consistent with a protective outcome of breastfeeding.
Conclusions and Relevance
These findings suggest that breastfeeding is protective against postpartum relapses in MS, although high-quality prospective studies to date are limited and well-designed observational studies that aim to emulate a randomized trial would be of benefit.
This systematic review and meta-analysis examines the occurrence of multiple sclerosis relapses in women who are breastfeeding
Introduction
Multiple sclerosis (MS) commonly occurs in women in childbearing years,1 and although several disease-modifying therapies (DMTs) reduce MS relapses,2 none are recommended in pregnancy or breastfeeding.3,4,5 Multiple sclerosis relapse rates decrease in the third trimester of pregnancy, but increase in the 3 months post partum, with up to 30% of women having a relapse.6 Postpartum relapses are associated with high baseline relapse rate and disability6,7 and may worsen disability.8 Women are often forced to choose whether to breastfeed or forgo breastfeeding to resume DMT post partum. Despite several observational studies, there is no consensus to date on the association between breastfeeding and postpartum relapse control. A meta-analysis performed in 2012 suggested that women who did not breastfeed were almost twice as likely to have a postpartum relapse than those who breastfed.9 However, there was significant heterogeneity, and the investigators did not assess whether prepregnancy disease activity altered study findings or completely separate exclusive breastfeeding, which has different hormonal effects than nonexclusive breastfeeding.10 Additional large studies have been published since 2012.8,10,11,12
We aim to provide an updated summary of the association between breastfeeding and the risk of postpartum MS relapses. We also perform subgroup analyses to examine breastfeeding in studies of women with active and less active MS, and whether any association was limited to studies of exclusive breastfeeding.
Methods
Our systematic review protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO). It was registered prior to performing the review and is publicly available.13 This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guideline.14
Search Strategy and Selection Criteria
In collaboration with a University of California, San Francisco medical librarian experienced with systematic reviews (E. Whitaker), a comprehensive search of PubMed and Embase was performed on July 11, 2018. This search included published manuscripts and abstracts. The PubMed search included the following terms: (multiple sclerosis[MeSH] or multiple sclerosis, relapsing-remitting[MeSH] or demyelinating diseases[MeSH] or multiple sclerosis or MS or relapsing remitting multiple sclerosis or RRMS or relapsing multiple sclerosis or demyelinating disease*) and (postpartum period[MeSH] or peripartum period[MeSH] or breast feeding[MeSH] or postpartum or breast feed* or breastfeed* or peripartum) and (relapse or attack or exacerbation or disease activity or event or disability). An equivalent Embase search was performed. We used Web of Science for forward and backward reference searching of selected articles to identify additional relevant studies. We also consulted with experts in the field (Kerstin Hellwig, MD [Department of Neurology, St Josef Hospital, Ruhr–University Bochum], and Annette Langer-Gould, MD, PhD [Department of Research and Evaluation, Kaiser Permanente Southern California]) to identify additional studies.
Eligibility criteria included studies published on January 1, 1980, or later of women with MS assessing the association between breastfeeding compared with nonexclusive or no breastfeeding on postpartum relapses. All study designs and settings were included. Studies not assessing breastfeeding, not including a control group, or not measuring the relapse outcome were excluded. For inclusion in the meta-analysis portion of the study, studies were required to include point estimates comparing breastfeeding with nonbreastfeeding on the postpartum relapse outcome (odds ratio [OR], risk ratio [RR] or hazard ratio [HR], or data to allow calculation of these values).
Two of us (K.M.K., A.R.) independently screened titles and abstracts of identified articles after removing duplicates. Covidence,15 an internet-based platform, was used to facilitate collaboration between reviewers during the study selection and data abstraction process. These 2 reviewers then independently assessed the full text of potentially relevant articles for inclusion and exclusion criteria. Discrepancies were discussed, and another author (E.W.) was consulted as needed. Agreement between reviewers for study inclusion was assessed with κ.
Data Extraction
A data abstraction form was created and data were abstracted from selected articles independently in duplicate (K.M.K., A.R.). Disagreements were resolved by consensus and with another author (E.W.) as needed. Information collected included study characteristics, including setting and year; design; characteristics of the sample; markers of disease activity, including prepregnancy and pregnancy relapse rates; baseline disability; and use of DMT before, during, and after pregnancy. We also collected the definitions of breastfeeding and nonbreastfeeding groups, including the duration of breastfeeding and whether exclusive breastfeeding was required. We collected definitions for the relapse outcome, the postpartum period during which these relapses were assessed, as well as main study results of the association between breastfeeding and postpartum relapses. In cases where these data were not available, corresponding authors were contacted.
The primary exposure was breastfeeding compared with nonbreastfeeding strategies. Exclusive breastfeeding requires no regular formula feedings (ie, ≤1 formula bottle per day) for the first 2 months post partum. Studies were considered to evaluate exclusive breastfeeding when the breastfeeding group required exclusive breastfeeding, while those who did not breastfeed at all or supplemented breastfeeding with formula were in the nonbreastfeeding group. Otherwise, studies were considered to evaluate nonexclusive breastfeeding. The primary outcome was the proportion of women who relapsed over the follow-up period in the breastfeeding vs comparator group. An MS relapse requires new or worsening neurologic symptoms lasting at least 24 hours in the absence of infection or an alternative cause as determined by the physician.
The key elements of study design were assessed and reported for each study. Formal risk of bias assessment was performed with the ROBINS-I tool16 for assessing risk of bias in nonrandomized studies of interventions. This tool allows risk of bias judgment within 7 domains (confounding, selection of participants, classification of interventions, deviations from intended interventions, missing data, measurement of outcomes, and selection of reported result), at low, moderate, serious, or critical risk. These domains were combined to result in an overall risk of bias judgment as low, moderate, serious, or critical. All studies were included in the primary analysis except those with critical risk of bias. Sensitivity analysis including only studies with low or moderate risk of bias was performed. Risk of bias assessment was performed by 2 independent reviewers (K.M.K., A.R.) and disagreements were discussed with another author (E.W.) as necessary.
Statistical Analysis
Studies and results were qualitatively compared and summarized. Studies with data available for the primary outcome of relapses in the breastfeeding and comparator groups over the postpartum follow-up period were included in the quantitative meta-analysis. Study results were statistically combined using summary estimates from the individual studies with random-effects models17 and displayed with forest plots. Several studies only reported ORs, so the primary summary estimate was an OR, with pooling adjusted OR when available. Not all studies reported data to allow pooling of risk ratios, although this was done when possible. Adjusted HR was also statistically combined when reported. Sensitivity analysis including only studies with low or moderate risk of bias was performed.
Clinical heterogeneity was evaluated by examining differences in study design and patient characteristics. Heterogeneity was evaluated by inspecting forest plots, and with tests for heterogeneity by calculating Q statistic and I2 values. To further understand heterogeneity, several preplanned sensitivity and subgroup analyses of the primary outcome were performed. Subgroup analyses included stratification by baseline disease activity defined by relapse rate before pregnancy, with studies divided into 2 groups with the cutoff at the median baseline relapse rate. Meta-regression was performed to determine whether baseline relapse rate was associated with study point estimates.
Subgroup analyses also included stratification by duration of breastfeeding (<2 vs ≥2 months) and whether breastfeeding was exclusive. Sensitivity analyses included evaluating the proportion with relapse at 3 and 6 months post partum including only studies with data available at these specific time points as the outcome of breastfeeding may differ over time. Subgroup analyses were also performed by publication year comparing older (2010 and earlier) vs newer (2011-2019) studies.
Publication bias was evaluated by visually inspecting a funnel plot, and statistical testing with the Begg and Egger tests. Stata, version 15 (metan package; StataCorp) was used for all analyses, and 2-sided α = .05 was used to determine statistical significance.
Results
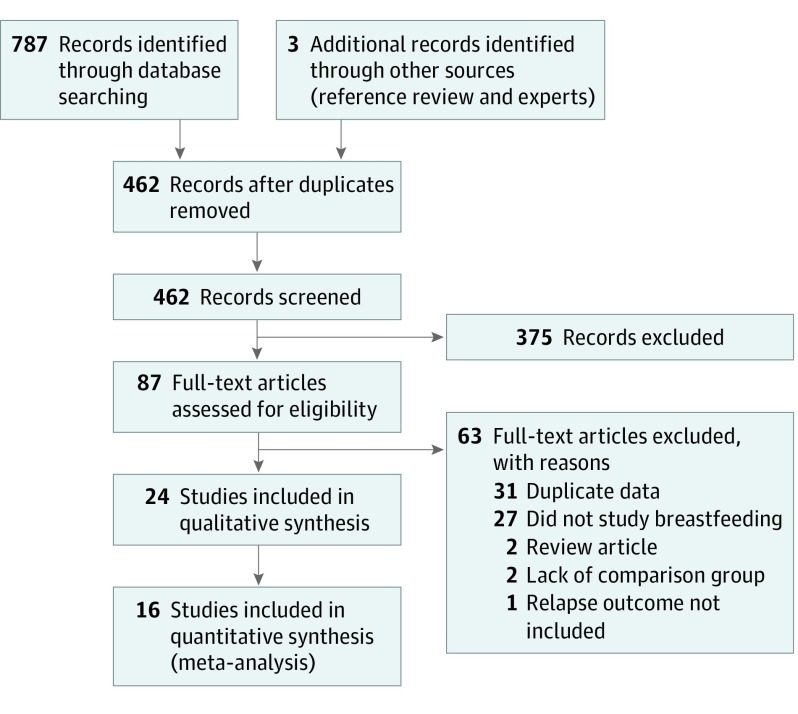
The search strategy identified 787 references (Figure 1) and an additional 3 references were identified through reference review and experts. After removing duplicate records, 462 references were screened for potential relevance through titles and abstracts. This process yielded 87 potentially eligible studies that underwent full-text eligibility review. Of these, 63 studies were excluded (31 duplicate data, 27 not studying breastfeeding, 2 review articles, 2 lacking a comparison group, and 1 not studying relapses) and 24 studies (2974 women) were included in the review.8,10,11,12,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 Some well-known studies of breastfeeding were excluded6,38,39 owing to duplicate data on the same patients as included studies.8,21,35 Of the 24 studies, 16 were included in the quantitative meta-analysis8,10,11,12,20,21,23,24,25,26,27,30,31,32,35,37 because these included the required data or the data were provided by authors. Eight studies were excluded from the meta-analysis owing to lack of data.18,19,22,28,29,33,34,36 Agreement between reviewers for eligibility was noted at both abstract (Cohen κ = 0.90) and full-text (Cohen κ = 0.89) review.
Figure 1. PRISMA Flow Diagram of Study Selection.
Study identification, screening, eligibility review, and selection for this systematic review and meta-analysis. PRISMA indicates Preferred Reporting Items for Systematic Reviews and Meta-analyses.
Characteristics of the 24 studies are presented in the eTable in the Supplement.8,10,11,12,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37 All studies were cohort design, with most including prospective follow-up, although many had retrospective follow-up, with some relying on remote patient recall.23,24,32,33,34 All studies included relapsing-remitting MS. Mean patient age (range, 28-34 years) and disease duration (range, 4-9 years) were similar across studies. Baseline annualized relapse rate (ARR) before pregnancy varied across studies, ranging from 0.02 to 1, although the Expanded Disability Status Scale score was similar with all but one study37 having mean or median Expanded Disability Status Scale score from 1.0 to 2.0, indicating mild disability.
The definition of breastfeeding vs nonbreastfeeding varied across studies, with 2 ways of categorizing this exposure. Nine studies8,10,12,19,23,28,30,31,36 required the breastfeeding group to breastfeed exclusively without regular formula supplementation, generally for at least 2 months, and the nonbreastfeeding group included individuals not breastfeeding at all or with shorter duration or nonexclusive breastfeeding. Other studies included any history of breastfeeding in the breastfeeding group, and the nonbreastfeeding group included women without any breastfeeding. A standard definition of an MS relapse was used across studies, although postpartum relapses were evaluated at variable times, including 3, 6, 9, or 12 months post partum.
Although several studies did not report baseline characteristics in the breastfeeding and nonbreastfeeding groups, in some studies the breastfeeding group had lower prepregnancy or pregnancy ARR and less previous use of DMT. Studies that discussed postpartum DMT cointervention reported higher DMT use in the nonbreastfeeding group.
All studies were rated as having either moderate or serious risk of bias with the ROBINS-I tool16 (Table). The 7 studies8,10,11,12,21,25,31 judged as having moderate risk of bias generally involved prospective follow-up of pregnancy cohorts with adjustment for confounding, although possible residual confounding could not be excluded. The other studies were judged as having serious risk of bias, mainly owing to a lack of control for confounding, as well as measurement bias in studies relying on recall of breastfeeding and relapses. There was also concern for immortal person-time bias in several studies that required 2 or more months of breastfeeding to enter the breastfeeding group, as this could lead to an immortal period that artificially decreases the breastfeeding relapse rate.
Table. Effect of Breastfeeding on Postpartum MS Relapses in Included Studies.
| Source | Sample Size, No. | ≥1 PP Relapse, No. (%) | PP Relapse in BF vs Non-BF (Reference Non-BF) | Other Summary Measures | Adjustment Variables | Overall Conclusion | Risk of Bias Rating for BF→ PP Relapse Association | |||
|---|---|---|---|---|---|---|---|---|---|---|
| BF | Non-BF | BF | Non-BF | OR (95% CI) | RR (95% CI) | |||||
| Achiron et al,18 2004 | 79 | 29 | NA | NA | NA | NA | NA | None | BF no association with PP relapses | Serious (confounding; NI for measurement bias) |
| Airas et al,19 2010 | 49 | 12 | NA | NA | NA | NA | 0-6 mo: ARR similar (BF 1.02 ± 1.36 vs non-BF 1.50 ± 1.30), P = .36 | None | BF no association with PP relapses; BF less frequent if active MS prepregnancy | Serious (confounding; measurement of intervention: immortal person-time) |
| Benoit et al,20 2016 | 43 | 35 | NA | NA | 0-3 mo Unadjusted: 0.36 (95% CI, 0.12-1.12),a P = .08 | NA | NA | None | BF no association with PP relapses | Serious (confounding) |
| Confavreux et al,21 1998 | 122 | 87 | NA | NA | 0-3 mo Adjusted: 0.8 (95% CI, 0.4-1.5),a P = .51 | NA | 0-3 mo: ARR similar (BF 1.2; 95% CI, 0.9-1.4 vs non-BF 1.3; 95% CI, 1.0-1.6) | Age, disease duration, relapses year before and in pregnancy | BF no association with PP relapses | Moderate |
| De Las Heras et al,22 2007 | 18 | 44 | NA | NA | NA | NA | NA | None | BF no association with PP relapses | Serious (confounding) |
| Fernández Liguori et al,24 2009 | 79 | 24 | Overall (0-12 mo): 32 (40.5%); 0-3 mo: 14 (17.7%) | Overall (0-12 mo): 6 (25%); 0-3 mo: 2 (8.3%) | Overall (0-12 mo) unadjusted: 2.04 (95% CI, 0.75-5.53),a P = .17; 0-3 mo unadjusted: 2.37 (95% CI, 0.55-NA), P = .27 | Overall (0-12 mo) unadjusted: 1.62 (95% CI, 0.77-3.41); 0-3 mo unadjusted: 2.13 (95% CI, 0.52-8.71) | NA | None | BF no association with PP relapses | Serious (confounding, selection, measurement of intervention and outcome: recall bias) |
| Fernández Liguori et al,23 2012 [abstract] | 14 | 26 | 0-6 mo: 5 (35.7%) | 0-6 mo: 4 (15.4%) | 0-6 mo Unadjusted: 3.06 (95% CI, 0.71-13.23), P = .14; 0-6 mo adjusted: 2.47 (95% CI, 0.42-14.53),a P = .32 | 0-6 mo Unadjusted: 2.32 (95% CI, 0.74-7.28), P = .14 | NA | Relapses in 1 y prepregnancy and pregnancy | BF no association with PP relapses with trend to more PP relapses in BF group | Serious (confounding, selection, measurement of intervention and outcome: recall bias, immortal person-time) |
| Gulick and Halper,25 2002 | 140 | 35 | Overall (0-12 mo): 47 (33.6%); 0-6 mo: 35 (25%) | Overall (0-12 mo): 22 (62.9%); 0-6 mo: 18 (51.4%) | Overall (0-12 mo) Unadjusted: 0.30 (95% CI, 0.14-0.64),a P = .002; 0-6 mo unadjusted: 0.31 (95% CI, 0.15-0.67), P = .002 | 0-12 mo Unadjusted: 0.53 (95% CI, 0.38-0.75); 0-6 mo unadjusted: 0.49 (95% CI, 0.32-0.75) | 0-3 mo Adjusted: OR, 0.63 (95% CI, 0.41-0.97) for majority BF (reference, stopped or BF reduced), non-BF excluded; 0-3 mo: lower ARR BF (0.49) vs non-BF (1.71), P < .001 | Age, disease duration, educational level, DMT use, relapse year before and in pregnancy | Fewer PP relapses in BF than non-BF 0-6 and 0-12 mo PP; increased percentage feeding by breast decreased relapses in 3-mo PP in BF group | Moderate |
| Haas and Hommes,26 2007 | 139 | 24 | Overall (0-3 mo): 28 (20.1%); only BF≥3 mo: 9/91 (9.9%); only BF<3 mo: 19/48 (39.6%) | Overall (0-3 mo): 7 (29.2%) | Overall (0-3 mo): Unadjusted: 0.61 (95% CI, 0.24-1.58),a P = .32; limit to ≥3 mo vs non-BF: unadjusted: 0.27 (95% CI, 0.09-0.79), P = .015 | Overall (0-3 mo): Unadjusted: 0.69 (95% CI, 0.34-1.40); limit to ≥3 mo vs non-BF: unadjusted: 0.34 (95% CI, 0.14-0.82) | NA | None | Fewer PP relapses in BF ≥3 mo vs non-BF but not for BF <3 mo | Serious (confounding, measurement of intervention: immortal person-time) |
| Hanulíková et al,27 2013 | 49 | 24 | 0-6 mo: 9 (18.4%) | 0-6 mo: 8 (33.3%) | 0-6 mo Unadjusted: 0.45 (95% CI, 0.15-1.37),a P = .24 | 0-6 mo Unadjusted: 0.55 (95% CI, 0.24-1.25) | NA | None | BF no association with PP relapses | Serious (confounding) |
| Hellwig et al,28 2009 [correspondence] | 151 | 62 | NA | NA | NA | NA | 0-3 mo: Lower ARR BF (0.7) vs non-BF (1.6), P < .02; 4-6 mo: BF and non-BF similar ARR | None | BF exclusively decreased PP relapse in first 3 mo | Serious (confounding, measurement of intervention: immortal person-time) |
| Hellwig et al,10 2015 | 120 | 81 | Overall (0-6 mo): 29 (24.2%); 0-12 mo: 56 (46.7%) | Overall (0-6 mo): 31 (38.3%); 0-12 mo: 38 (46.9%) | Overall (0-6 mo) Unadjusted: 0.51 (95% CI, 0.28-0.95), P = .032; overall (0-6 mo) adjusted (PS): 0.56 (95% CI 0.32-0.98),a P = .04; 0-12 mo unadjusted: 0.99 (95% CI, 0.56-1.74), P = .97 | Overall (0-6 mo) Unadjusted: 0.63 (95% CI, 0.41-0.96); 0-12 mo unadjusted: 0.99 (95% CI, 0.74-1.34) | Unadjusted HR: 0.56 (95% CI 0.33-0.92), P = .02 (reference, non-BF); adjusted HR: 0.59 (95% CI, 0.35-0.98),a P = .04 (reference, non-BF) | HR: age, relapse frequency 2 y prepregnancy, relapse in pregnancy; tested but did not meet their definition of confounder: disease duration, DMT prepregnancy, DMT at conception; OR: all of the above adjusted with PS | Those who intended to BF exclusively for ≥2 mo had lower relapse in 6 mo PP than no or non-exclusive BF, but once regular feedings introduced, disease activity returns | Moderate (only owing to cannot exclude residual confounding and outcome assessors not blinded), otherwise low risk of bias |
| Horvat Ledinek et al,29 2013 [abstract] | NA | NA | NA | NA | NA | NA | NA | None | BF no association with PP relapse | Serious (confounding; NI for measurement bias) |
| Iorio et al,30 2009 [correspondence] | 8 | 15 | 0-12 mo: 2 (25%) | 0-12 mo: 8 (53.3%) | 0-12 mo Unadjusted: 0.29 (95% CI, 0.05-1.77),a P = .19 | 0-12 mo Unadjusted: 0.47 (95% CI, 0.13-1.70) | New T2 lesions brain or spinal cord 1 y PP: BF 4/8 vs non-BF 10/15, P = .74 | None | BF no association with PP relapses but sample size small | Serious (confounding; measurement of intervention: immortal person-time) |
| Jesus-Ribeiro et al,11 2017 | 72 | 39 | 0-12 mo: 23 (31.9%) | 0-12 mo: 20 (51.3%) | 0-12 mo Unadjusted: 0.45 (95% CI, 0.20-0.99),a P = .046; adjusted OR: BF not associated with PP relapses 0-12 mo (OR not provided) | 0-12 mo Unadjusted: 0.62 (95% CI, 0.40-0.98) | 0-12 mo: slightly lower ARR BF (0.5 ± 0.9) vs non-BF (0.6 ± 0.6), P = .17 | Age at MS onset, age at pregnancy, disease duration, baseline EDSS, relapses in years before and during pregnancy, previous DMT | BF no association with PP relapses | Moderate |
| Langer-Gould et al,31 2009 | 14 | 15 | 0-12 mo: 5 (35.7%) | 0-12 mo: 13 (86.7%) | 0-12 mo Unadjusted: 0.09 (95% CI, 0.02-0.50),a P = .0047 | 0-12 mo Unadjusted: 0.41 (95% CI, 0.20-0.86) | Unadjusted HR: 0.2 (95% CI, 0.07-0.59), P = .003 (reference, non-BF); adjusted HR: 0.14 (95% CI, 0.04-0.48),a P = .002 (reference, non-BF) | Age, disease duration, relapse frequency 2 y prepregnancy, prepregnancy DMT | Exclusive BF and suppression of menses reduce risk of PP relapses | Moderate (measurement of intervention: immortal person-time but similar findings restricted to those BF not influenced by worsening MS symptoms) |
| Langer-Gould et al,12 2019 [abstract] | 167 | 299 | NA | NA | NA | NA | Adjusted HR: 0.58 (95% CI, 0.38-0.88),a,b P = .01 (reference non-BF) | Measures of disease severity | Exclusive BF reduced rate of PP relapse | Moderate (measurement of intervention: immortal person-time) |
| Nelson et al,32 1988 | 96 | 95 | 0-9 mo: 36 (37.5%) | 0-9 mo: 29 (30.5%) | 0-9 mo Unadjusted: 1.37 (95% CI, 0.75-2.48),a P = .31 | 0-9 mo Unadjusted: 1.23 (95% CI, 0.82-1.83) | Similar mean time to PP relapse (BF 3 mo vs non-BF 3.1 mo) | None | BF no association with PP relapses | Serious (confounding, measurement of intervention and outcome: recall bias) |
| Nikseresht et al,33 2017 [abstract] | NA | NA | NA | NA | NA | NA | NA | None | BF no association with PP relapses or MRI gadolinium enhancing lesions | Serious (confounding, measurement of intervention and outcome: recall bias) |
| Perez-Sanchez et al,34 2014 [abstract] | 53 | 26 | NA | NA | NA | NA | NA | None | BF no association with PP relapses | Serious (confounding, selection, measurement of intervention and outcome: recall bias) |
| Portaccio et al,8 2014 | 121 | 229 | 0-12 mo: 43 (35.5%) | 0-12 mo: 105 (45.9%) | 0-12 mo Unadjusted: 0.65 (95% CI, 0.41-1.02),a P = .06 | 0-12 mo Unadjusted: 0.78 (95% CI, 0.59-1.02) | Adjusted HR: 0.78 (95% CI, 0.53-1.13),a P = .18 (reference, non-BF) | Age, disease duration, baseline EDSS, number relapses in y before and in pregnancy, prior DMT, early vs delayed DMT PP, smoking, alcohol, toxin exposure during pregnancy | BF no association with PP relapses | Moderate (measurement of intervention: immortal person-time) |
| Runia et al,35 2015 | 28 (Only 25 ≥ 2 mo) | 15 | 0-3 mo: 7 (25%) | 0-3 mo: 4 (26.7%) | 0-3 mo Unadjusted: 0.92 (95% CI, 0.23-3.59),a P = .91 | 0-3 mo Unadjusted: 0.94 (95% CI, 0.33-2.70) | NA | None | BF no association with PP relapses | Serious (confounding) |
| Sahebi Vaighan et al,36 2017 [abstract] | 27 | 12 | NA | NA | NA | NA | 0-6 mo: Unadjusted HR 0.05 (95% CI, 0.006-0.38) (reference, non-BF); 0-36 mo: unadjusted HR 0.39 (95% CI, 0.19-0.84) (reference, non-BF) | None | Exclusive BF reduced rate of PP relapses | Serious (confounding, measurement of intervention: immortal person-time) |
| Worthington et al,37 1994 | 8 | 7 (4 No BF, 3 stopped) | 0-6 mo: 3 (37.5%) | 0-6 mo: 3 (42.8%) | 0-6 mo Unadjusted: 0.80 (95% CI, 0.11-5.74),a P = .83 | 0-6 mo Unadjusted: 0.88 (95% CI, 0.25-3.02) | NA | None | BF no association with PP relapses | Serious (confounding) |
Abbreviations: ARR, annualized relapse rate; BF, breastfeeding; DMT, disease-modifying therapy; EDSS, Expanded Disability Status Scale; HR, hazard ratio; MRI, magnetic resonance imaging; MS, multiple sclerosis; NA, not available; NI, no information; OR, odds ratio; PP, postpartum; PS, propensity score; RR, risk ratio.
The primary point estimates reported in each study that were included in the quantitative meta-analysis.
The 95% CI was approximated based on P value because 95% CI was not reported in the abstract.
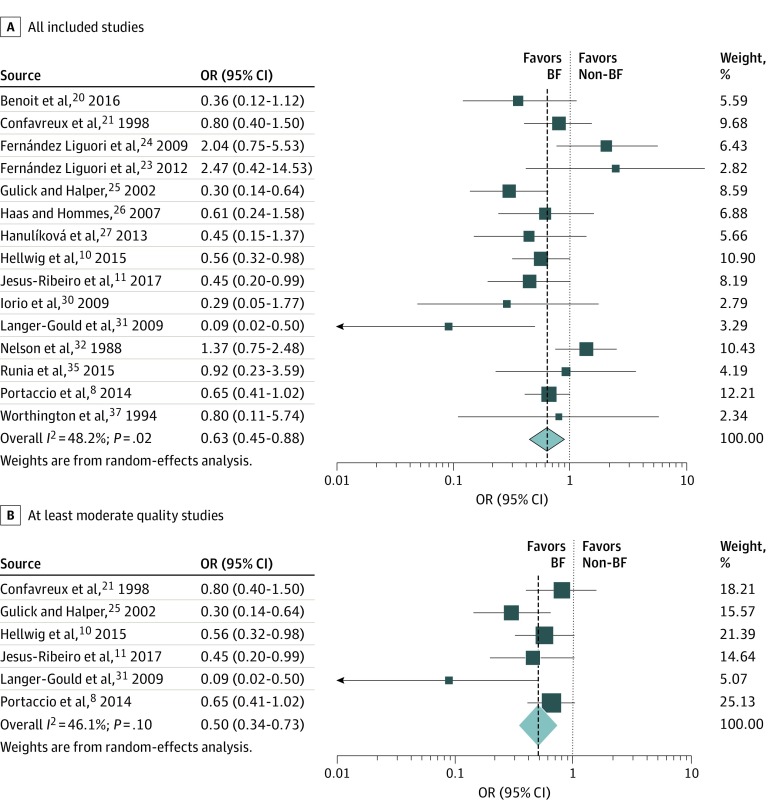
Findings for the association between breastfeeding and nonbreastfeeding with postpartum relapses are reported in the Table and Figure 2. The summary estimate from 15 studies for the OR for breastfeeding vs nonbreastfeeding on postpartum relapses was 0.63 (95% CI, 0.45-0.88; P = .006) (Figure 2A). Women who breastfed had 37% lower odds of a postpartum relapse compared with those who did not breastfeed. There was moderate heterogeneity with I2 = 48.2% (P = .02). When restricting analysis to the 6 studies with moderate risk of bias with data available to pool, findings were similar, with a summary OR of 0.50 (95% CI, 0.34-0.73; P < .001) (Figure 2B). Heterogeneity remained moderate, although all point estimates suggested a benefit of breastfeeding (I2 = 46.1%; P = .10).
Figure 2. Association Between Breastfeeding and Postpartum Multiple Sclerosis Relapses.
Odds ratios across all included studies (A) and in those at least moderate in quality (B). BF indicates breastfeeding.
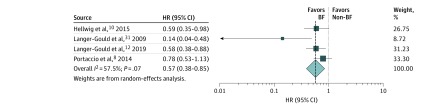
Thirteen studies reported data to calculate unadjusted RR, and the summary RR was 0.75 (95% CI, 0.60-0.94; P = .01). Four studies with moderate risk of bias8,10,12,31 reported adjusted HR, also suggesting that breastfeeding was protective with a summary HR of 0.57 (95% CI, 0.38-0.85; P = .006) (Figure 3), although there was heterogeneity (I2 = 57.5%; P = .07). The ARR post partum in breastfeeding and nonbreastfeeding groups was reported in only 5 studies and ranged from 0.49 to 1.2 in the breastfeeding group, with lower ARR in the breastfeeding than nonbreastfeeding group with absolute ARR difference ranging from 0.1 to 1.2 (Table). There was no evidence for publication bias, as visual inspection of the funnel plot (eFigure 1 in the Supplement) did not show asymmetry, and there was no statistically significant evidence for publication bias (Begg test z = −0.54; P = .59; Egger intercept value = −0.49; P = .60).
Figure 3. Association Between Breastfeeding and Postpartum Relapses in Studies Reporting Hazard Ratio.
BF indicates breastfeeding.
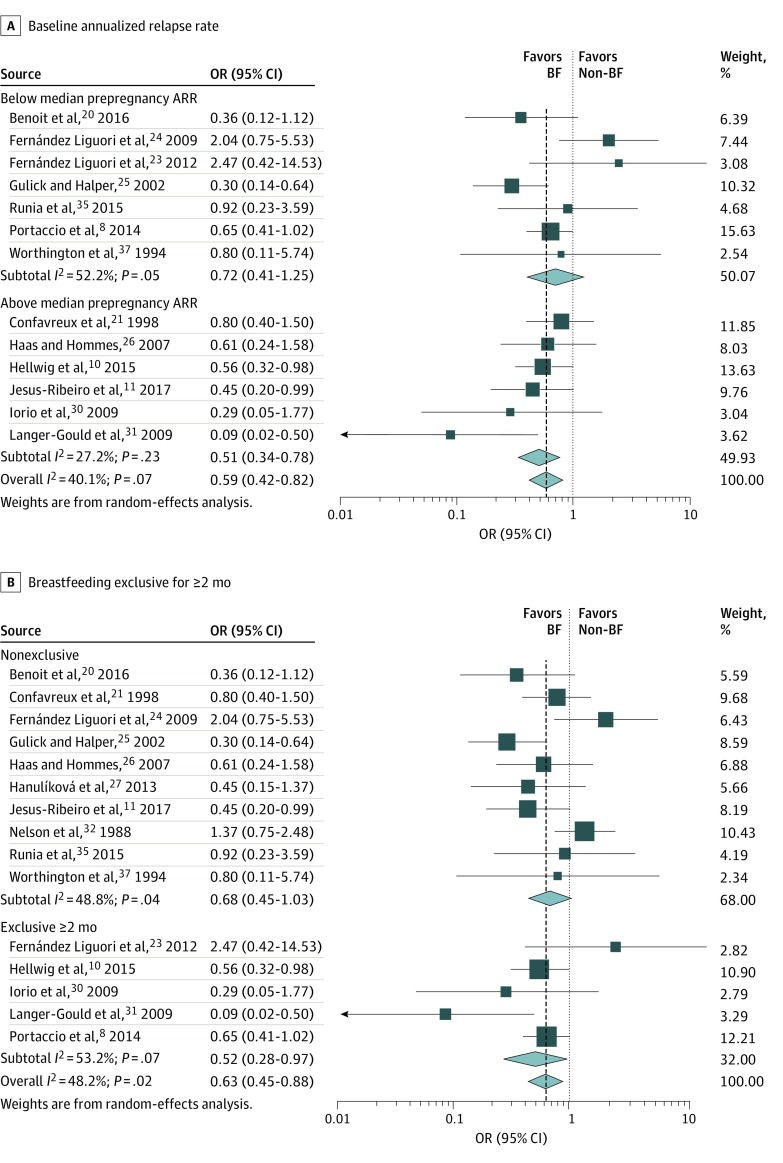
To explain the heterogeneity, several preplanned subgroup analyses were performed. The meta-analysis was stratified by prepregnancy ARR below and above the median of 0.58. There was less heterogeneity (I2 = 27.2%; P = .23) and more benefit of breastfeeding in studies with higher pre-pregnancy ARR (summary OR, 0.51; 95% CI, 0.34-0.78; P = .002) than those with lower pre-pregnancy ARR (I2 = 52.2%; P = .05; summary OR, 0.72; 95% CI, 0.41-1.25; P = .24) (Figure 4A). However, meta-regression did not reveal a statistically significant association between prepregnancy ARR and ORs of the studies, although there was somewhat more suggested benefit of breastfeeding in studies with higher baseline ARR (P = .07).
Figure 4. Stratified Data on Association of Breastfeeding (BF) With Postpartum Multiple Sclerosis Relapses.
Odds ratios (ORs) are stratified by baseline annualized relapse rate (A) and whether BF was exclusive for 2 or more months (B).
The association between breastfeeding and postpartum relapses appeared stronger in studies of exclusive breastfeeding for 2 months or more (summary OR, 0.52; 95% CI, 0.28-0.97; P = .04) compared with studies including any breastfeeding in the breastfeeding group (summary OR, 0.68; 95% CI, 0.45-1.03; P = .07) (Figure 4B), although summary point estimates suggested benefit in studies of both exclusive and nonexclusive breastfeeding. However, the heterogeneity across studies was not explained by these 2 definitions of breastfeeding as heterogeneity remained when restricting to studies with exclusive or nonexclusive breastfeeding separately.
The duration of postpartum follow-up, which ranged from 3 to 12 months, explained heterogeneity across study findings. When restricting to outcome assessment at 3 and 6 months post partum separately, there was no important heterogeneity (3 months I2 0%; P = .64; 6 months I2 21.5%; P = .28). Four studies evaluated the association of breastfeeding with relapses in the 3 months post partum, with a summary OR of 0.67 (95% CI, 0.42 to 1.05; P = .08), while 5 studies evaluated the association of breastfeeding with relapses in the 6 months post partum, with a summary OR of 0.52 (95% CI, 0.32 to 0.84; P = .008) (eFigure 2 in the Supplement).
Preplanned subgroup analysis by publication year showed that while there was significant heterogeneity in publications in 2010 and earlier (I2 = 67.2%; P = .003), there was no relevant heterogeneity in publications after 2010 (I2 = 0%; P = .60). Publications before and after 2010 both showed a benefit of breastfeeding on postpartum relapses (≤2010: OR, 0.63; 95% CI, 0.34-1.16; P = .14; >2010: OR, 0.59; 95% CI, 0.44-0.78; P < .001).
There were 8 studies18,19,22,28,29,33,34,36 excluded from the meta-analysis owing to lack of data availability, and 6 of these18,19,22,29,33,34 reported no effect of breastfeeding on postpartum relapses but did not provide raw data or measures of association. All of these excluded studies were at serious risk of bias, and 4 studies29,33,34,36 were reported only in abstract form.
Discussion
This systematic review and meta-analysis suggests that breastfeeding is protective against postpartum relapses in women with MS. Those who breastfed had 37% lower odds of postpartum relapse compared with those who did not breastfeed or did not exclusively breastfeed post partum. There is concern for residual confounding in these summary estimates, as many studies did not adjust for relevant confounders, such as prior MS disease activity. However, the strongest studies8,10,12,31 reported adjusted HRs, with a 43% lower rate of postpartum relapses in those who breastfed, consistent with a beneficial effect.
There was moderate heterogeneity between studies regarding the association between breastfeeding and postpartum relapses, even when limiting to higher-quality studies. This heterogeneity was most explained by the variable duration of follow-up post partum, ranging from 3 to 12 months, as there was no relevant heterogeneity when restricting to studies evaluating 3- and 6-month postpartum relapses. This heterogeneity by postpartum follow-up duration was expected because the effect of breastfeeding on postpartum relapses may vary over the postpartum year. Year of publication also explained heterogeneity, with no relevant heterogeneity when restricted to studies published after 2010, with more consistent benefit of breastfeeding demonstrated in recent studies.
Another factor contributing to heterogeneity was relapse rate before pregnancy. Among studies with a baseline relapse rate higher than the median, there appeared to be greater benefit with breastfeeding and no relevant heterogeneity. However, there was no statistically significant association between prepregnancy ARR and the OR with meta-regression, and higher-quality studies tended to have higher baseline ARR. In addition, in studies with lower prepregnancy ARR, postpartum relapse rate may be intrinsically lower, making it more difficult to detect an association with breastfeeding. Thus, it is unclear whether higher baseline relapse activity is associated with a greater benefit of breastfeeding.
The benefit of breastfeeding was stronger in studies requiring exclusive breastfeeding for at least 2 months, although benefit appeared to be present even in some studies of nonexclusive breastfeeding. Although the benefit may not be limited to exclusive breastfeeding, it is possible that individuals who exclusively breastfed could have driven the benefit in studies that allowed nonexclusive breastfeeding, as partial and exclusive breastfeeding were grouped together. In addition, the methodologically sound study by Hellwig et al10 demonstrated that women who partially breastfed experienced relapse risk similar to the risk in those who did not breastfeed at all, suggesting that partial breastfeeding may not be beneficial. Most studies in this meta-analysis did not separately evaluate partial breastfeeding. Given this, conclusions on the benefit of partial breastfeeding on postpartum relapses cannot be made with the available data.
Overall, these findings are similar to those of a previous meta-analysis,9 demonstrating an apparent benefit of breastfeeding on postpartum MS relapses, particularly for exclusive breastfeeding. The overall estimate of heterogeneity in our meta-analysis (48%) was somewhat less than in the previous meta-analysis (63%), likely owing to less heterogeneity among more-recent studies. We provide an updated meta-analysis with additional large cohort studies included and systematic assessment of bias. We also include a pooled summary adjusted HR, which we believe is a more appropriate measure of association for this research question and addresses confounding.
It is hypothesized that exclusive breastfeeding may be beneficial in reducing postpartum MS relapses, potentially owing to hormonal changes associated with breastfeeding, which include high prolactin levels and suppression of pulsatile gonadotropin-releasing hormone and luteinizing hormone, with resulting lactational amenorrhea.40,41,42,43,44 When breastfeeding is reduced and supplemental feedings are introduced, the prolactin level decreases, ovarian activity resumes, and menses return,42 which may explain the loss of the protective effect of breastfeeding on MS relapses when supplementation begins later in the postpartum year.10
Limitations of identified studies include the potential for bias, with residual confounding the largest concern. Only 7 of 24 studies adjusted for confounders, with key confounders being markers of earlier disease activity, as those with higher baseline ARR may defer breastfeeding to resume DMT and are also more likely to experience relapses post partum. This confounding could explain an observed apparently protective effect of breastfeeding, although a protective effect remained in pooled adjusted HRs, suggesting that confounding did not explain the entire observed protective effect. Immortal person-time bias45 could also explain an observed protective effect, given the requirement in many studies for at least 2 months of breastfeeding to enter the breastfeeding group. For example, if 2 months of breastfeeding is required to be in the breastfeeding group and an individual stops breastfeeding at 1 month owing to a relapse, she would be assigned to the nonbreastfeeding group. This grouping can artificially decrease the number of women with relapse in the breastfeeding group and bias toward a protective effect. This issue was overcome in the well-designed study by Hellwig et al,10 as they assigned breastfeeding exposure groups at baseline based on individuals’ intent to breastfeed exclusively for 2 months or longer, thus avoiding an immortal period.
There is also potential for error in measurement of breastfeeding status, as mothers may overreport breastfeeding owing to social desirability, which is particularly a concern in studies requiring recall. Relapse outcomes were generally confirmed by neurologists, although they were not blinded to breastfeeding status. A few studies relied on recall of both breastfeeding and postpartum relapses, which may result in dependent and differential measurement error that could bias away from the null. Most studies included prospective pregnancy registries with fairly complete follow-up or retrospective reports of all pregnant women with MS at a center, with low concern for selection bias. However, retrospective studies that required response to participate may have introduced selection bias. An additional source of bias includes differential cointervention, as several studies reported the nonbreastfeeding group was more likely to receive DMT post partum. Although we cannot separate or compare benefits of breastfeeding and DMT on postpartum relapses with available data, the increased likelihood of the nonbreastfeeding group to receive DMT post partum could potentially mask a protective effect. However, this differential cointervention would not be expected to induce a spurious protective effect and the benefit of breastfeeding may be even greater than estimated in this meta-analysis.
Limitations
Limitations of this meta-analysis include the use of pooled OR, as this was the most commonly reported measure. Odds ratios do not approximate RRs when the outcome is common, and although mathematically correct, ORs are difficult to interpret. Thus, we reported summary RR and HR as well. As expected, the association with risk is lower than the association with odds of postpartum relapse. Adjusted HRs are the more appropriate measure to use to evaluate the association between breastfeeding and the rate of postpartum MS relapses, although adjusted HRs were reported in only 4 studies. In addition, there was moderate heterogeneity, although this was explained by the duration of postpartum follow-up, year of publication, and baseline ARR. There did not appear to be publication bias, and we attempted to avoid publication bias by including data in abstract form. Furthermore, we could not determine the length of time that breastfeeding may be beneficial, as this was not evaluated by most available studies. Although some studies evaluating relapse risk throughout the postpartum year showed benefit of breastfeeding, the high-quality study by Hellwig et al10 found a return of disease activity when supplemental feedings began, with greater benefit of breastfeeding noted in the first 6 months post partum. In addition, individual patient data were not available to pool. Without individual patient data, we could not perform additional sensitivity analyses that would be helpful, such as limiting analysis to women not receiving DMT, evaluating partial breastfeeding, or conducting sophisticated analyses to control for confounding by baseline features.
Conclusions
There appears to be evidence for an association between breastfeeding and lower postpartum MS relapses, which may be greater with higher baseline disease activity and exclusive breastfeeding. Despite the potential sources of bias, findings were consistent across sensitivity analyses, including limiting to studies at the lowest risk of bias and restricting to studies reporting adjusted HRs. We believe the consistency of the findings strengthens our conclusion for a protective association between breastfeeding and postpartum relapses.
Given the variable findings and inability to conduct a randomized trial, it would be beneficial to replicate the findings of Hellwig et al10 evaluating intent to breastfeed exclusively in an independent cohort, with blinded adjudication of relapses. Until these data become available, it is reasonable to educate women about the potential protective effect of breastfeeding on postpartum relapses and support their decision to breastfeed for benefit to both the mother and newborn as recommended by the World Health Organization.46 Despite reduction in postpartum relapses with breastfeeding, ARRs remained fairly high postpartum, highlighting the need to identify additional strategies to prevent postpartum relapses. We believe further study is required to evaluate the duration of benefit associated with breastfeeding and the safety of breastfeeding during treatment with DMTs, because treatment appears to be a key barrier to breastfeeding for women with MS.
eTable. Characteristics of Included Studies
eFigure 1. Funnel Plot Evaluating for Publication Bias With No Publication Bias Demonstrated
eFigure 2. Stratified Data on Association of Breastfeeding With Postpartum Multiple Sclerosis Relapses in Studies Reporting 3-Month and 6-Month Postpartum Relapse Outcomes
eReferences
References
- 1.Bove R, Chitnis T. The role of gender and sex hormones in determining the onset and outcome of multiple sclerosis. Mult Scler. 2014;20(5):520-526. doi: 10.1177/1352458513519181 [DOI] [PubMed] [Google Scholar]
- 2.Wingerchuk DM, Carter JL. Multiple sclerosis: current and emerging disease-modifying therapies and treatment strategies. Mayo Clin Proc. 2014;89(2):225-240. doi: 10.1016/j.mayocp.2013.11.002 [DOI] [PubMed] [Google Scholar]
- 3.Bove R, Alwan S, Friedman JM, et al. Management of multiple sclerosis during pregnancy and the reproductive years: a systematic review. Obstet Gynecol. 2014;124(6):1157-1168. doi: 10.1097/AOG.0000000000000541 [DOI] [PubMed] [Google Scholar]
- 4.Lu E, Wang BW, Guimond C, Synnes A, Sadovnick D, Tremlett H. Disease-modifying drugs for multiple sclerosis in pregnancy: a systematic review. Neurology. 2012;79(11):1130-1135. doi: 10.1212/WNL.0b013e3182698c64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wundes A, Pebdani RN, Amtmann D. What do healthcare providers advise women with multiple sclerosis regarding pregnancy? Mult Scler Int. 2014;2014:819216. doi: 10.1155/2014/819216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vukusic S, Hutchinson M, Hours M, et al. ; Pregnancy In Multiple Sclerosis Group . Pregnancy and multiple sclerosis (the PRIMS study): clinical predictors of post-partum relapse. Brain. 2004;127(pt 6):1353-1360. doi: 10.1093/brain/awh152 [DOI] [PubMed] [Google Scholar]
- 7.Hughes SE, Spelman T, Gray OM, et al. ; MSBase study group . Predictors and dynamics of postpartum relapses in women with multiple sclerosis. Mult Scler. 2014;20(6):739-746. doi: 10.1177/1352458513507816 [DOI] [PubMed] [Google Scholar]
- 8.Portaccio E, Ghezzi A, Hakiki B, et al. ; MS Study Group of the Italian Neurological Society . Postpartum relapses increase the risk of disability progression in multiple sclerosis: the role of disease modifying drugs. J Neurol Neurosurg Psychiatry. 2014;85(8):845-850. doi: 10.1136/jnnp-2013-306054 [DOI] [PubMed] [Google Scholar]
- 9.Pakpoor J, Disanto G, Lacey MV, Hellwig K, Giovannoni G, Ramagopalan SV. Breastfeeding and multiple sclerosis relapses: a meta-analysis. J Neurol. 2012;259(10):2246-2248. doi: 10.1007/s00415-012-6553-z [DOI] [PubMed] [Google Scholar]
- 10.Hellwig K, Rockhoff M, Herbstritt S, et al. Exclusive breastfeeding and the effect on postpartum multiple sclerosis relapses. JAMA Neurol. 2015;72(10):1132-1138. doi: 10.1001/jamaneurol.2015.1806 [DOI] [PubMed] [Google Scholar]
- 11.Jesus-Ribeiro J, Correia I, Martins AI, et al. Pregnancy in multiple sclerosis: a Portuguese cohort study. Mult Scler Relat Disord. 2017;17:63-68. doi: 10.1016/j.msard.2017.07.002 [DOI] [PubMed] [Google Scholar]
- 12.Langer-Gould A, Smith J, Albers K, et al. Pregnancy-related relapses in a large, contemporary multiple sclerosis cohort: no increased risk in the postpartum period [abstract]. Neurology. 2019;92(15 suppl):S6.007. [Google Scholar]
- 13.National Institute for Health Research https://www.crd.york.ac.uk/PROSPERO/. Accessed August 16, 2018.
- 14.Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred Reporting Items for Systematic Reviews and Meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264-269, W64. doi: 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- 15.Covidence. World-class systematic review management http://www.covidence.org. Accessed July 19, 2018.
- 16.Sterne JA, Hernán MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177-188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 18.Achiron A, Kishner I, Dolev M, et al. Effect of intravenous immunoglobulin treatment on pregnancy and postpartum-related relapses in multiple sclerosis. J Neurol. 2004;251(9):1133-1137. doi: 10.1007/s00415-004-0495-z [DOI] [PubMed] [Google Scholar]
- 19.Airas L, Jalkanen A, Alanen A, Pirttilä T, Marttila RJ. Breast-feeding, postpartum and prepregnancy disease activity in multiple sclerosis. Neurology. 2010;75(5):474-476. doi: 10.1212/WNL.0b013e3181eb5860 [DOI] [PubMed] [Google Scholar]
- 20.Benoit A, Durand-Dubief F, Amato M-P, et al. History of multiple sclerosis in 2 successive pregnancies: a French and Italian cohort. Neurology. 2016;87(13):1360-1367. doi: 10.1212/WNL.0000000000003036 [DOI] [PubMed] [Google Scholar]
- 21.Confavreux C, Hutchinson M, Hours MM, Cortinovis-Tourniaire P, Moreau T; Pregnancy in Multiple Sclerosis Group . Rate of pregnancy-related relapse in multiple sclerosis. N Engl J Med. 1998;339(5):285-291. doi: 10.1056/NEJM199807303390501 [DOI] [PubMed] [Google Scholar]
- 22.De Las Heras V, De Andrés C, Téllez N, Tintoré M; EMPATIE Study Group . Pregnancy in multiple sclerosis patients treated with immunomodulators prior to or during part of the pregnancy: a descriptive study in the Spanish population. Mult Scler. 2007;13(8):981-984. doi: 10.1177/1352458507077896 [DOI] [PubMed] [Google Scholar]
- 23.Fernández Liguori N, Klajn D, Saladino ML, Silva B, Cáceres F, Garcea O. Breastfeeding and risk of postpartum relapses in women with multiple sclerosis. Mult Scler. 2012;18(12):1825-1826. doi: 10.1177/1352458512466528 [DOI] [Google Scholar]
- 24.Fernández Liguori N, Klajn D, Acion L, et al. Epidemiological characteristics of pregnancy, delivery, and birth outcome in women with multiple sclerosis in Argentina (EMEMAR study). Mult Scler. 2009;15(5):555-562. doi: 10.1177/1352458509102366 [DOI] [PubMed] [Google Scholar]
- 25.Gulick EE, Halper J. Influence of infant feeding method on postpartum relapse of mothers with MS. Int J MS Care. 2002;4(4):183-191. doi: 10.7224/1537-2073-4.4.183 [DOI] [Google Scholar]
- 26.Haas J, Hommes OR. A dose comparison study of IVIG in postpartum relapsing-remitting multiple sclerosis. Mult Scler. 2007;13(7):900-908. doi: 10.1177/1352458506075654 [DOI] [PubMed] [Google Scholar]
- 27.Hanulíková P, Vlk R, Meluzínová E, Rob L. Pregnant women with multiple sclerosis at the Motol Hospital Prague 2007-2011: outcomes analysis. Actual Gyn. 2013;5(1):27-32. [Google Scholar]
- 28.Hellwig K, Haghikia A, Agne H, Beste C, Gold R. Protective effect of breastfeeding in postpartum relapse rate of mothers with multiple sclerosis. Arch Neurol. 2009;66(12):1580-1581. doi: 10.1001/archneurol.2009.281 [DOI] [PubMed] [Google Scholar]
- 29.Horvat Ledinek A, Sega Jazbec S, Rot U, Pirecnik Noc A. Effect of intravenous immunoglobulin treatment on postpartum-related relapses in multiple sclerosis [abstract]. Mult Scler. 2013;19(11):469-470. [Google Scholar]
- 30.Iorio R, Nociti V, Frisullo G, Patanella AK, Tonali PA, Batocchi AP. Breastfeeding and multiple sclerosis. Arch Neurol. 2009;66(12):1580. doi: 10.1001/archneurol.2009.280 [DOI] [PubMed] [Google Scholar]
- 31.Langer-Gould A, Huang SM, Gupta R, et al. Exclusive breastfeeding and the risk of postpartum relapses in women with multiple sclerosis. Arch Neurol. 2009;66(8):958-963. doi: 10.1001/archneurol.2009.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nelson LM, Franklin GM, Jones MC. Risk of multiple sclerosis exacerbation during pregnancy and breast-feeding. JAMA. 1988;259(23):3441-3443. doi: 10.1001/jama.1988.03720230051029 [DOI] [PubMed] [Google Scholar]
- 33.Nikseresht A, Rezaiean Jahromi F, Sharifian Dorche M. Brain MRI activity in multiple sclerosis(MS) before and after pregnancy [abstract]. Mult Scler. 2017;23(3):718. [Google Scholar]
- 34.Perez-Sanchez S, Eichau-Madueño S, Rus-Hidalgo M, Navarro-Mascarell G, Izquierdo G. The role of breastfeeding in multiple sclerosis patients [abstract]. Mult Scler. 2014;20(1):441. [Google Scholar]
- 35.Runia TF, Neuteboom RF, de Groot CJM, de Rijke YB, Hintzen RQ. The influence of vitamin D on postpartum relapse and quality of life in pregnant multiple sclerosis patients. Eur J Neurol. 2015;22(3):479-484. doi: 10.1111/ene.12594 [DOI] [PubMed] [Google Scholar]
- 36.Sahebi Vaighan N, Abadi A, Ghozat R, Delavar Kasmaei H, Gharagozli K. The effect of exclusive breastfeeding on postpartum multiple sclerosis relapses [abstract]. Mult Scler. 2017;23(3):705-706. [Google Scholar]
- 37.Worthington J, Jones R, Crawford M, Forti A. Pregnancy and multiple sclerosis—a 3-year prospective study. J Neurol. 1994;241(4):228-233. doi: 10.1007/BF00863773 [DOI] [PubMed] [Google Scholar]
- 38.Portaccio E, Ghezzi A, Hakiki B, et al. ; MS Study Group of the Italian Neurological Society . Breastfeeding is not related to postpartum relapses in multiple sclerosis. Neurology. 2011;77(2):145-150. doi: 10.1212/WNL.0b013e318224afc9 [DOI] [PubMed] [Google Scholar]
- 39.Neuteboom RF, Hintzen RQ. Breast-feeding, postpartum and prepregnancy disease activity in multiple sclerosis. Neurology. 2011;76(17):1532. doi: 10.1212/WNL.0b013e318210e908 [DOI] [PubMed] [Google Scholar]
- 40.Howie PW, Mcneilly AS. Breast-feeding and postpartum ovulation. IPPF Med Bull. 1982;16(2):1-3. [PubMed] [Google Scholar]
- 41.Howie PW, McNeilly AS, Houston MJ, Cook A, Boyle H. Fertility after childbirth: infant feeding patterns, basal PRL levels and post-partum ovulation. Clin Endocrinol (Oxf). 1982;17(4):315-322. doi: 10.1111/j.1365-2265.1982.tb01596.x [DOI] [PubMed] [Google Scholar]
- 42.Howie PW, McNeilly AS, Houston MJ, Cook A, Boyle H. Fertility after childbirth: post-partum ovulation and menstruation in bottle and breast feeding mothers. Clin Endocrinol (Oxf). 1982;17(4):323-332. doi: 10.1111/j.1365-2265.1982.tb01597.x [DOI] [PubMed] [Google Scholar]
- 43.Glasier A, McNeilly AS, Howie PW. Pulsatile secretion of LH in relation to the resumption of ovarian activity post partum. Clin Endocrinol (Oxf). 1984;20(4):415-426. doi: 10.1111/j.1365-2265.1984.tb03437.x [DOI] [PubMed] [Google Scholar]
- 44.Glasier A, McNeilly AS, Howie PW. The prolactin response to suckling. Clin Endocrinol (Oxf). 1984;21(2):109-116. doi: 10.1111/j.1365-2265.1984.tb03449.x [DOI] [PubMed] [Google Scholar]
- 45.Hernán MA, Sauer BC, Hernández-Díaz S, Platt R, Shrier I. Specifying a target trial prevents immortal time bias and other self-inflicted injuries in observational analyses. J Clin Epidemiol. 2016;79:70-75. doi: 10.1016/j.jclinepi.2016.04.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.World Health Organization Breastfeeding. https://www.who.int/topics/breastfeeding/en/. Accessed April 22, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable. Characteristics of Included Studies
eFigure 1. Funnel Plot Evaluating for Publication Bias With No Publication Bias Demonstrated
eFigure 2. Stratified Data on Association of Breastfeeding With Postpartum Multiple Sclerosis Relapses in Studies Reporting 3-Month and 6-Month Postpartum Relapse Outcomes
eReferences