Key Points
Question
Is fenfluramine safe and effective for treating patients with Dravet syndrome who have frequent seizures despite taking a stiripentol-inclusive antiepileptic drug regimen?
Findings
Oral fenfluramine (0.4 mg/kg/d; maximum 17 mg/d) provided a 54.0% greater reduction in mean monthly convulsive seizure frequency than placebo in patients with Dravet syndrome who were taking stiripentol-containing antiepileptic drug regimens; a significantly greater proportion of patients who were taking fenfluramine (vs placebo) experienced a clinically meaningful (≥50%) or profound (≥75%) reduction in monthly convulsive seizure frequency. The most common adverse events included decreased appetite, pyrexia, fatigue, and diarrhea; no patient developed valvular heart disease or pulmonary hypertension.
Meaning
Adjunctive fenfluramine may be a safe, effective new treatment option for patients with Dravet syndrome with seizures that are not controlled by a regimen including stiripentol.
This randomized clinical trial investigates whether fenfluramine reduced monthly convulsive seizure frequency relative to placebo in patients with Dravet syndrome who were already taking stiripentol-inclusive regimens.
Abstract
Importance
Fenfluramine treatment may reduce monthly convulsive seizure frequency in patients with Dravet syndrome who have poor seizure control with their current stiripentol-containing antiepileptic drug regimens.
Objective
To determine whether fenfluramine reduced monthly convulsive seizure frequency relative to placebo in patients with Dravet syndrome who were taking stiripentol-inclusive regimens.
Design, Setting, and Participants
This double-blind, placebo-controlled, parallel-group randomized clinical trial was conducted in multiple centers. Eligible patients were children aged 2 to 18 years with a confirmed clinical diagnosis of Dravet syndrome who were receiving stable, stiripentol-inclusive antiepileptic drug regimens.
Interventions
Patients with 6 or more convulsive seizures during the 6-week baseline period were randomly assigned to receive fenfluramine, 0.4 mg/kg/d (maximum, 17 mg/d), or a placebo. After titration (3 weeks), patients’ assigned dosages were maintained for 12 additional weeks. Caregivers recorded seizures via a daily electronic diary.
Main Outcomes and Measures
The primary efficacy end point was the change in mean monthly convulsive seizure frequency between fenfluramine and placebo during the combined titration and maintenance periods relative to baseline.
Results
A total of 115 eligible patients were identified; of these, 87 patients (mean [SD], age 9.1 [4.8] years; 50 male patients [57%]; mean baseline frequency of seizures, approximately 25 convulsive seizures per month) were enrolled and randomized to fenfluramine, 0.4 mg/kg/d (n = 43) or placebo (n = 44). Patients treated with fenfluramine achieved a 54.0% (95% CI, 35.6%-67.2%; P < .001) greater reduction in mean monthly convulsive seizure frequency than those receiving the placebo. With fenfluramine, 54% of patients demonstrated a clinically meaningful (≥50%) reduction in monthly convulsive seizure frequency vs 5% with placebo (P < .001). The median (range) longest seizure-free interval was 22 (3.0-105.0) days with fenfluramine and 13 (1.0-40.0) days with placebo (P = .004). The most common adverse events were decreased appetite (19 patients taking fenfluramine [44%] vs 5 taking placebo [11%]), fatigue (11 [26%] vs 2 [5%]), diarrhea (10 [23%] vs 3 [7%]), and pyrexia (11 [26%] vs 4 [9%]). Cardiac monitoring demonstrated no clinical or echocardiographic evidence of valvular heart disease or pulmonary arterial hypertension.
Conclusions and Relevance
Fenfluramine demonstrated significant improvements in monthly convulsive seizure frequency in patients with Dravet syndrome whose conditions were insufficiently controlled with stiripentol-inclusive antiepileptic drug regimens. Fenfluramine was generally well tolerated. Fenfluramine may represent a new treatment option for Dravet syndrome.
Trial Registration
ClinicalTrials.gov identifier: NCT02926898
Introduction
Dravet syndrome (DS) is a severe developmental epileptic encephalopathy with an incidence of 1:15 700 to 1:40 900.1 Dravet syndrome typically presents in the first year of life with frequent, pharmacoresistant convulsive seizures,1,2 which may contribute to intellectual disability and impairments in motor control, behavior, and cognition.3 Most patients (70%-85%) have sodium voltage-gated channel alpha subunit 1 gene (SCN1A) sodium channel alterations, although diagnosis is solely based on electroclinical criteria.1,2,4 Patients with DS who are younger than 18 years have increased risk of sudden unexpected death in epilepsy, with a mortality rate of 7% to 18%. Frequency of generalized tonic-clonic seizures is a major risk factor for this outcome.5,6,7
Even with polypharmacy, seizures remain poorly controlled in most patients with DS. In a survey of 274 patients, 45% receiving multitreatment modalities continued to experience more than 4 tonic-clonic seizures per month.8 Until the recent approval of cannabidiol and stiripentol by the US Food and Drug Administration, stiripentol in combination with valproate and clobazam remained the only approved treatment for DS outside of the United States. Despite treatment with stiripentol-inclusive antiepileptic drug (AED) regimens, 38% of patients were reported to present with weekly seizures (>3 per month).8,9 Given the risks of sudden unexpected death in epilepsy, cognitive deficits, and other neurological consequences putatively caused at least in part by poorly controlled seizures, there remains an urgent unmet need for treating DS.9,10,11
Results from a recent phase 3, placebo-controlled randomized clinical study (Study 1 [NCT02682927; NCT02826863])12 demonstrated that fenfluramine provided a significantly greater reduction in monthly convulsive seizure frequency (MCSF) vs placebo. Patients receiving stiripentol were excluded from Study 1 because pharmacokinetic data were not yet available to evaluate dosage modifications needed to compensate for an expected fenfluramine-stiripentol drug interaction. Given the widespread use of stiripentol for DS, it is important to assess the benefit and tolerability of add-on fenfluramine in stiripentol-containing AED regimens. A phase 1 pharmacokinetic study and earlier physiologically based pharmacokinetic modeling were used to select a fenfluramine dose of 0.4 mg/kg/d (maximum, 17 mg/d) added to stiripentol-valproate-clobazam triple therapy.13 Here, we evaluate efficacy and safety of add-on fenfluramine in patients with DS receiving stiripentol-inclusive AED regimens.
Methods
Trial Design, Ethics, and Oversight
In this double-blind, parallel-group, placebo-controlled, phase 3 randomized clinical study, patients with DS with seizures that were poorly controlled with current AED regimens, which had to include stiripentol plus clobazam or valproic acid, were enrolled at approximately 28 sites in Canada, France, Germany, the Netherlands, Spain, the United Kingdom, and the United States (NCT02926898; eFigure 1 in Supplement 1; trial protocol in Supplement 2).
The study complied with current International Council for Harmonisation Good Clinical Practice guidelines, as described in International Council for Harmonisation Topic E6 Guidelines.14 The protocol was approved by applicable regulatory authorities and an independent ethics committee or institutional review board at each participating institution. All patients or their legal representatives provided written informed consent before enrollment.
Patients and Eligibility Criteria
Patients were male or female; aged 2 to 18 years (inclusive); receiving a stable, stiripentol-inclusive AED regimen; and free of cardiovascular disease on an echocardiogram, electrocardiogram, or physical examination. The diagnosis of DS was validated by a central committee, the Epilepsy Study Consortium (eTable 1 in Supplement 1). Key exclusion criteria were pulmonary arterial hypertension (PAH) or a current condition or history of cardiovascular or cerebrovascular disease (eg, cardiac valvulopathy, myocardial infarction, stroke) and concomitant treatment with modulators of serotonergic activity, AEDs with sodium channel antagonist activity, or cannabinoid products.
Trial Procedures
This study evaluated the safety and efficacy of twice-daily fenfluramine (administered as a fenfluramine hydrochloride oral solution containing 2.2 mg/mL of fenfluramine) added to a stiripentol-inclusive AED regimen (plus valproate or clobazam, at a minimum) in children and young adults with DS (eFigure 1 in Supplement 1). In a drug-interaction study (Cohort 1 [NCT02926898]), fenfluramine was administered with clobazam and valproate and with and without stiripentol. Data from this study and a phase 1 study in healthy adults15 were used in an early version of a physiologically based pharmacokinetic model to help select fenfluramine dose for this study (Study 1504, Cohort 2 [NCT02926898]; 0.4 mg/kg/d; maximum dosage, 17 mg/d).
After a 6-week period to establish baseline seizure frequency, patients were randomized (1:1, via interactive web response system) to fenfluramine or matching placebo groups in a double-blind manner, using sequentially numbered bottles for treatments. Randomization was stratified across ages (<6 years vs ≥6 years) to ensure balance across treatments. The fenfluramine starting dosage was 0.2 mg/kg/d in 2 equal doses, with a gradual blinded titration to 0.4 mg/kg/d (maximum, 17 mg/d) over 3 weeks (eTable 2 in Supplement 1). Patients maintained their use of fenfluramine or placebo for an additional 12 weeks at a stable dosage, then either continued treatment in an open-label extension study or discontinued treatment with a blinded, downward dose–tapering protocol (eTable 2 in Supplement 1). Caregivers recorded doses, any rescue medication, and the number and type of seizures in handheld electronic diaries.
Safety
Treatment-emergent adverse events among the placebo and fenfluramine subgroups were recorded throughout study duration. Standardized color Doppler echocardiography assessed cardiac valve function and structure and any evidence of PAH at study baseline (prior to participants’ receiving any study drugs; days −42 to −21), during the maintenance period (days 40 to 54), at the end of study (between days 90 and 113), and during cardiac follow-up visits (3 and 6 months posttreatment; care was extended up to 24 months in some countries). Primary assessment was done to detect the presence of valvular regurgitation on the aortic and mitral valve, although all valves were assessed. Assessments were graded as absent, trace, mild, moderate, or severe. Blinded core laboratory readers from eResearchTechnology Inc (ERT) Clinical evaluated all echocardiograms for presence and grade of cardiac valve regurgitation and PAH. Electrocardiograms were also evaluated by core laboratory readers (ERT Clinical) for any abnormality.
Efficacy End Points
The primary efficacy end point was a comparison of the difference between fenfluramine and placebo on the change in mean MCSF from baseline to the combined titration and maintenance (T + M) periods. The key secondary efficacy end points were the proportion achieving 50% or greater reduction from baseline levels in MCSF and the duration of the longest seizure-free interval. Other secondary end points included the proportion of patients with 0 seizures or 1 seizure during the T + M periods, the proportion with 25% or more, 75% or more, and 100% reduction in seizures from baseline, change from baseline in clinical global impression ratings by both parents or caregivers and investigators, days of rescue medication use per 28 days, and total seizure frequency (including nonconvulsive seizure types) per 28 days. Patient quality of life and executive function were assessed by the Quality of Life in Childhood Epilepsy Scale,16 Pediatric Quality of Life Inventory,17 and Behavior Rating Inventory of Executive Function instruments.
Statistical Analyses
Sample size was based on results from previous placebo-controlled randomized clinical studies in patients with DS.8,9,18,19,20,21,22 An SD of 55% was assumed for the primary analysis. Using a 2-sided test with α = .05 as a threshold, a sample size of at least 80 total patients (40 patients per treatment group) affords 90% power to detect a 40–percentage point difference in mean change from baseline. The Statistical Analysis Plan is in Supplement 3.
Analysis Population
Safety analyses were performed on all randomized patients who received 1 or more doses of fenfluramine or placebo. The primary end point analysis and the key secondary analyses performed on the modified intent-to-treat population included all randomized patients who received 1 or more doses of fenfluramine or placebo with 1 week or more of seizure diary data.
Statistical Methods
Continuous variables were summarized using descriptive statistics (means with SDs, medians with interquartile or full ranges, and 95% CIs). Categorical variables were summarized with frequencies and percentages. The distribution of baseline characteristics across treatment groups was assessed using Wilcoxon rank sum for continuous variables (age, body mass index [calculated as weight in kilograms divided by height in meters squared], and baseline MCSF), Fisher exact test for dichotomous variables (age group and sex), and Freeman-Halton test for categorical variables (race/ethnicity and number of concomitant AEDs). The primary efficacy end point was analyzed using an analysis of covariance model with treatment group and age group (<6 years and ≥6 years) as factors and baseline frequency as a covariate. The primary analysis compared the fenfluramine group with the placebo group with a 2-sided test (with an α = .05 significance threshold). Efficacy analyses used a serial gatekeeper approach with a hierarchy of significance tests to maintain a type I error rate with α = .05 across analyses. The testing order was (1) change in MCSF from baseline, (2) the proportion of patients achieving 50% MCSF reduction or greater from baseline, and (3) the longest convulsive seizure–free interval.
The proportion of patients achieving 50% reduction in MCSF or greater during the T + M period was analyzed using a logistic regression model that incorporates the same factors and covariate as the analysis of covariance in the primary analysis. For the longest interval between convulsive seizures during the T + M period, treatment groups were compared using a Wilcoxon rank sum test. For assessing clinical global impression improvement, patients rated as showing any improvement (scores ≤3) or clinically meaningful improvement (≤2) vs no improvement (scores ≥4 or ≥3, respectively) were compared using the Cochran-Mantel-Haenszel test (fenfluramine vs placebo).
Frequency of treatment-emergent adverse events and serious adverse events were presented by treatment group using the Preferred Term from the Medical Dictionary for Regulatory Activities (MedDRA). Results of electrocardiogram and Doppler echocardiogram analyses were summarized by treatment group using descriptive statistics.
Results
Patient Disposition
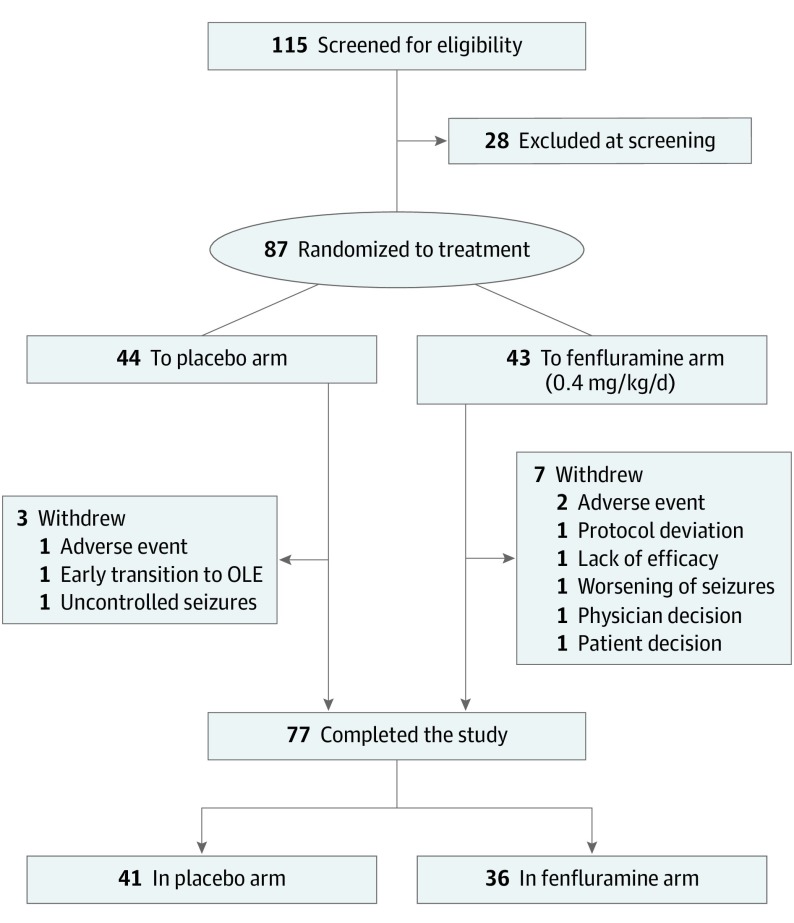
A total of 115 patients were screened for eligibility, with 87 patients randomized to treatment (44 to placebo and 43 to fenfluramine); 77 completed the study (41 who received placebo and 36 who received fenfluramine; Figure 1). Most patients who failed screening did not meet the randomization criteria (26 of 28 patients [93%]), including meeting baseline seizure frequency, echocardiogram requirements, and compliance with daily seizure diary; additionally, 1 patient elected to withdraw during screening, and 1 withdrew because of use of a prohibited medication. Of those randomized, 3 in the placebo group and 7 in the fenfluramine group withdrew early (Figure 1).
Figure 1. Patient Disposition.
OLE indicates open-label extension.
Patient Baseline Characteristics
Most patients were male (placebo group, 27 of 44 [61%]; fenfluramine group, 23 of 43 [54%]; Table 1) and between 6 and 19 years old (placebo group, 32 of 44 [73%]; fenfluramine group, 31 of 43 [72%]). Baseline MCSF was comparable in both groups (mean [SD]: placebo group, 21.6 [27.7] seizures; fenfluramine group, 27.9 [36.9] seizures). Most patients were receiving either 3 concomitant AEDs (placebo group, 26 of 44 [59%]; fenfluramine group, 19 of 43 [44%]) or 4 concomitant AEDs (placebo group, 16 of 44 [36%]; fenfluramine group, 16 of 43 [37%]). Besides the protocol-specified stiripentol, the most frequent AEDs were clobazam, levetiracetam, topiramate, and valproate (all forms) (Table 1).
Table 1. Patient Baseline Characteristics.
| Characteristic | Patients, No. (%) | P Valuea | ||
|---|---|---|---|---|
| Receiving Fenfluramine | Receiving Placebo | Total | ||
| No. | 43 | 44 | 87 | NA |
| Age, mean (SD) [range], y | 8.8 (4.6) [2-18] | 9.4 (5.1) [2-19] | 9.1 (4.8) [2-19] | .57 |
| Patients <6 y | 12 (28) | 12 (27) | 24 (28) | >.99 |
| Male | 23 (53) | 27 (61) | 50 (57) | .52 |
| Race | ||||
| White | 23 (53) | 29 (66) | 52 (60) | .66 |
| Black/African American | 1 (2) | 2 (5) | 3 (3) | |
| Asian | 2 (5) | 1 (2) | 3 (3) | |
| Other | 3 (7) | 1 (2) | 4 (5) | |
| Not reported or missingb | 13 (30) | 11 (25) | 24 (28) | |
| Unknown | 1 (2) | 0 (0) | 1 (1) | |
| BMI, mean (SD) | 17.3 (2.7) | 19.1 (4.9) | 18.2 (4.0) | .11 |
| Convulsive seizure frequency per 28 d | ||||
| Median (range) | 14.0 (3-213) | 10.7 (3-163) | NA | .62 |
| Mean (SD) | 27.9 (36.9) | 21.6 (27.6) | NA | |
| No. of concomitant AEDs at baseline | ||||
| 2 | 1 (2) | 1 (2) | 2 (2) | .10 |
| 3 | 19 (44) | 26 (59) | 45 (52) | |
| 4 | 16 (37) | 16 (36) | 32 (37) | |
| 5 | 7 (16) | 1 (2) | 8 (9) | |
| Other antiepileptic treatments in ≥10% of subgroupc,d | ||||
| Stiripentol | 43 (100) | 44 (100) | 87 (100) | NC |
| Clobazam | 40 (93) | 42 (96) | 82 (94) | |
| Valproate | 38 (88) | 39 (89) | 77 (89) | |
| Topiramate | 14 (33) | 7 (16) | 21 (24) | |
| Levetiracetam | 6 (14) | 5 (11) | 11 (13) | |
Abbreviations: AED, antiepileptic drug; BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; NC, not calculated.
P values (fenfluramine vs placebo) were calculated by Wilcoxon rank sum test (age, BMI, and baseline convulsive seizure frequency per 28 days), Fisher exact test (age group and sex), and Freeman-Halton test (race and number of concomitant AEDs) with statistical significance set at P less than .05.
Not reported or missing: privacy laws in some regions and countries preclude disclosure of certain personal information.
Concomitant medications in less than 10% of patients included acetazolamide, clonazepam, diazepam, ethosuximide, felbamate, gamma-aminobutyric acid, lorazepam, phenobarbital, pregabalin, and zonisamide.
The number of patients following a ketogenic diet was 4 (5%) in the overall population. The number of patients with vagal nerve stimulator implantation was 5 (6%) overall.
Efficacy
The study achieved its primary efficacy objective: patients randomized to fenfluramine achieved an estimated 54.0% (95% CI, 35.6%-67.2%) greater reduction in mean MCSF between the baseline and T + M periods than those who received placebo (P < .001; Table 2). Significantly more patients in the fenfluramine group than the placebo group experienced a clinically meaningful (≥50%) reduction in mean MCSF (fenfluramine group, 23 of 43 [54%] vs placebo group, 2 of 44 [5%]; P < .001) and significantly longer seizure-free intervals (median [range], 22.0 [3.0-105.0] days vs 13.0 [1.0-40.0] days; P = .004).
Table 2. Efficacy End Points in the Combined Titration and Maintenance Period.
| End Point | Patients Receiving Fenfluramine (n = 43) | Patients Receiving Placebo (n = 44) | P Value |
|---|---|---|---|
| Primary and Key Secondary End Points | |||
| Estimated difference from placebo in convulsive seizure frequency per 28 d, % (95% CI)a | 54.0 (35.6-67.2) | 1 [Reference] | <.001 |
| Reduction in MCSF from baselineb | |||
| ≥50%, No. (%) | 23 (54) | 2 (5) | <.001 |
| Odds ratio (95% CI) | 26.0 (5.5-123.2) | 1 [Reference] | |
| Longest convulsive seizure-free interval, db | |||
| Mean (SD) | 29.7 (27.3) | 13.4 (7.5) | .004 |
| Median (range) | 22.0 (3.0-105.0) | 13.0 (1.0-40.0) | |
| Other Secondary End Points | |||
| Reduction in mean MCSF from baselinec | |||
| ≥25% | |||
| No. (%) | 30 (70) | 12 (27) | <.001 |
| Odds ratio (95% CI) | 6.4 (2.5-16.5) | 1 [Reference] | |
| ≥75% | |||
| No. (%) | 15 (35) | 1 (2) | .003 |
| Odds ratio (95% CI) | 23.7 (2.9-191.8) | 1 [Reference] | |
| Seizure freedom or near seizure freedom, No. (%) [95% CI] | |||
| 0 Convulsive seizures | 1 (2) [0.1-12.3] | 0 (0) [0.0-8.0] | .49 |
| ≤1 Convulsive seizure | 5 (12) [3.9-25.1] | 0 (0) [0.0-8.0] | .03 |
| Percentage change from baseline in convulsive seizure frequency per 28 d, median (range) | −63.1 (−100.0 to 115.0) | −1.1 (−82.8 to 435.1) | <.001 |
| Nonconvulsive seizure frequency per 28 dd | |||
| No. of patients (%) | 17 (40) | 22 (50) | NA |
| Percentage change from baseline per 28 d, median (range) | −0.5 (−100.0 to 611.2) | −49.7 (−100.0 to 529.4) | .18 |
| Percentage change from baseline in total seizure frequency per 28 d, median (range) | −41.1 (−100.0 to 133.2) | −5.9 (−73.8 to 375.6) | .003 |
| No. of days of rescue medication use per 28 d | |||
| Baseline, mean (SD) | 2.1 (2.6) | 1.4 (2.5) | NA |
| Combined titration and maintenance periods, mean (SD) | 1.4 (2.2) | 1.2 (2.6) | .25 |
| Nonseizure Outcomes, No. (%) | |||
| Clinical global impression of improvemente | |||
| Very much improved or much improved | |||
| Parent/caregiver rating | 14 (33) | 9 (21) | .14 |
| Investigator rating | 19 (44) | 7 (16) | .008 |
| Any improvementf | |||
| Parent/caregiver rating | 26 (61) | 16 (36) | .009 |
| Investigator rating | 31 (72) | 14 (32) | <.001 |
Abbreviations: MCSF, monthly convulsive seizure frequency; NA, not applicable.
Primary outcome.
Key secondary outcome.
Odds ratios are calculated for comparisons with outcomes in the placebo group. An age-adjusted logistic regression model was used to estimate all odds ratios, except for those comparing fenfluramine with placebo at the 25% and 75% seizure-reduction response levels, for which the age adjustment was eliminated because of potential model instability. Note that an odds ratio larger than 1.00 can be much larger than the corresponding relative risk.
Not all patients had nonconvulsive seizures.
P values from the Cochran-Mantel-Haenszel test controlling for age group.
Includes the responses “very much improved,” “much improved,” and “minimally improved.”
The median percentage reduction from baseline in MCSF in the fenfluramine group was 63.1% (range, −100.0% to 115.0%), compared with 1.1% (range, −82.8% to 435.1%) in the placebo group during the T + M periods (P < .001; Table 2). The median number of convulsive seizure–free days was significantly higher in patients treated with fenfluramine than placebo (median [range]: fenfluramine group, 24.4 [1.9-28.0] days vs placebo group, 20.3 [0.0-26.4] days; P = .001).
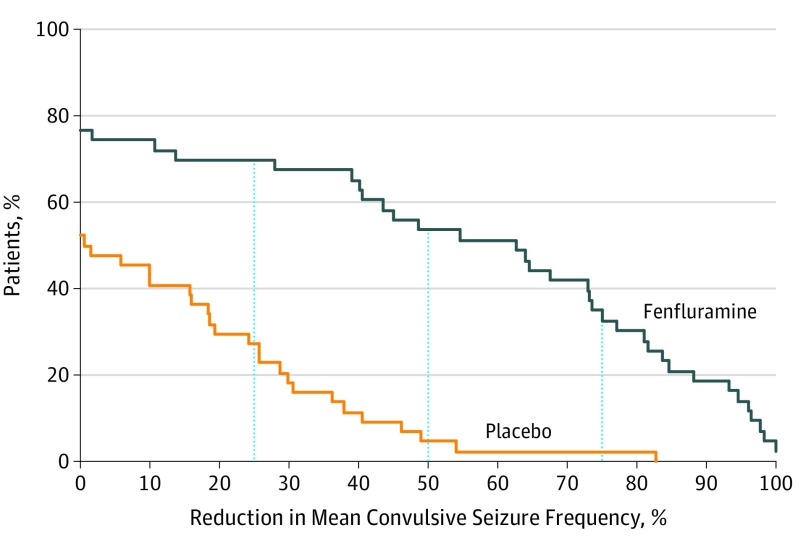
A significantly greater proportion of patients achieved a 25% or greater, 50% or greater, or 75% or greater reduction in MCSF in the fenfluramine group than placebo (fenfluramine group: 30 of 43 [70%], 23 of 43 [54%], and 15 of 43 [35%] vs placebo group, 12 of 44 [27%], 2 of 44 [5%], and 1 of 44 [2%], respectively; Figure 2). There was no difference in the number of patients experiencing complete seizure freedom (1 of 43 [2%] in the fenfluramine group vs 0 in the placebo group); however, 5 of 43 patients treated with fenfluramine (12%) experienced no more than a single convulsive seizure during the T + M periods (compared with 0 patients treated with placebo; P = .03). The significant reduction in MCSF vs placebo was apparent at the first assessment (maintenance week 2; T + M week 5) and was consistently maintained throughout the 15-week combined T + M periods (eFigure 2 in Supplement 1). Patients treated with fenfluramine achieved a median 41.1% (range, −100.0% to 133.2%) decrease from baseline in total seizure frequency vs 5.9% (range, −73.8% to 375.6%) for those receiving placebo (P = .003) (Table 2).
Figure 2. Cumulative Response Curves for Percent Reduction in Monthly Convulsive Seizure Frequency From Baseline.
Results are plotted for combined titration and maintenance periods. Vertical dashed lines represent 25%, 50%, and 75% reduction in monthly convulsive seizure frequencies; percentages correspond to the proportion of patients in the fenfluramine or placebo groups who met or exceeded each response level. Twelve of 44 patients (27%) in the placebo group and 30 of 43 (70%) in the fenfluramine group experienced 25% or greater reductions (P < .001); 2 of 44 patients (5%) in the placebo group and 23 of 43 (54%) in the fenfluramine group experienced 50% or greater reductions (P < .001); and 1 of 44 (2%) in the placebo group and 15 of 43 (35%) in the fenfluramine group experienced 75% reductions (P = .003). The P values are vs placebo and are estimated by logistic regression (as per Table 2).
Days of rescue medication use were low in both treatment groups at baseline. Patients in the fenfluramine treatment group experienced a numerically greater (although statistically insignificant) reduction in days of rescue medication use (Table 2).
At the end of the T + M period, 19 of 43 investigators (44%) and 14 of 43 caregivers (33%) rated patients in the fenfluramine treatment group as much improved or very much improved vs 7 of 44 (16%) and 9 of 44 (21%), respectively, in the placebo group (P = .008 and P = .14) (eFigure 3 in Supplement 1). Significantly more patients receiving fenfluramine than placebo were rated as having any improvement (including being minimally improved) by both investigators (fenfluramine group, 31 of 43 [72.1%] vs placebo group, 14 of 44 [31.8%]; P < .001) and caregivers (fenfluramine group, 26 of 43 [60.5%] vs placebo group, 16 of 44 [36.4%]; P = .009). There were no significant differences noted between groups on the Quality of Life in Childhood Epilepsy Scale, Pediatric Quality of Life Inventory, and Behavior Rating Inventory of Executive Function.
Safety
The most common treatment-emergent adverse events in the fenfluramine group included decreased appetite, pyrexia, fatigue, and diarrhea (Table 3). Treatment-emergent adverse events–associated discontinuations occurred in 3 patients. Two patients (4.5%) receiving placebo and 9 patients (20.9%) receiving fenfluramine experienced weight decreases of 7% or more from baseline. Of these patients, 5 were also receiving topiramate (another AED with anorectic properties).
Table 3. Most Common (≥10%) Noncardiovascular Treatment-Emergent Adverse Events in Any Treatment Group.
| Outcome | Patients, No. (%) | |
|---|---|---|
| Receiving Fenfluramine (n = 43) | Receiving Placebo (n = 44) | |
| Patients with ≥1 treatment-emergent adverse event. | 42 (98) | 42 (96) |
| Patients with ≥1 serious treatment-emergent adverse event. | 6 (14) | 7 (16) |
| Treatment-emergent adverse events in ≥10% of patients in any treatment group | ||
| Decreased appetite | 19 (44) | 5 (11) |
| Pyrexia | 11 (26) | 4 (9) |
| Fatigue | 11 (26) | 2 (5) |
| Diarrhea | 10 (23) | 3 (7) |
| Nasopharyngitis | 7 (16) | 15 (34) |
| Blood glucose decreased | 6 (14) | 2 (5) |
| Lethargy | 6 (14) | 2 (5) |
| Bronchitis | 5 (12) | 2 (5) |
| Seizure | 2 (5) | 7 (16) |
No cases of valvular heart disease (VHD) or PAH were observed in any patient at any time during the study. Echocardiographic monitoring demonstrated that all patients were within the normal physiologic range at all points for mitral and aortic valve function, as well as pulmonic and tricuspid valve function. No abnormalities in valve structure were reported. Observed valve regurgitation in the T + M periods was limited to trace mitral regurgitation and 1 echocardiogram finding of mild mitral regurgitation in a patient who also had a screening echocardiogram finding of mild mitral regurgitation at baseline. Trace mitral regurgitation is not a pathologic finding; rather, it is a normal physiologic finding on echocardiogram commonly seen in normal, healthy children.23 Observations of trace mitral regurgitation were largely sporadic. Of the 12 patients who had trace mitral regurgitation at any point (including 9 receiving fenfluramine and 3 receiving placebo), 6 patients had trace mitral regurgitation findings at week 8 that were not present at week 14; 5 patients had trace mitral regurgitation findings at week 14 only; and 1 patient had trace mitral regurgitation at both weeks 8 and 14.
Discussion
This study achieved its primary objective, demonstrating that adding fenfluramine to stiripentol-containing AED regimens provided significant reduction in MCSF in patients with DS. A significant reduction in MCSF was achieved at the earliest point measured (week 2 of the maintenance period) and was maintained throughout the 15-week T + M period. In addition, a significantly greater proportion of patients achieved a 50% or greater or 75% or greater reduction in MCSF in the fenfluramine vs placebo groups. Also, a significantly greater proportion of patients treated with fenfluramine experienced no more than 1 convulsive seizure during the entire T + M period vs placebo. Investigators reported that significantly more patients in the fenfluramine group had clinically meaningful improvements in clinical global impression scores (ie, they were rated much improved or very much improved). Both caregivers and parents and investigators reported statistically significant differences between treatment groups in any improvement (ie, those rated minimally improved or better).
Stiripentol, used since the early 2000s, has been approved in Europe, Japan, and Canada, and as per labeled instructions, it must be coprescribed with clobazam and valproate. Stiripentol was approved by the US FDA in 2018, and per US label, it must be coprescribed with clobazam. Prior controlled trials have reported stiripentol efficacy in reducing seizure frequency when used in combination with clobazam and valproate-containing regimens; however, most patients continue to experience seizures.8,9,18,19,20 In 1 study,9 despite treatment with stiripentol and 3 to 4 other AEDs, 79% of patients with DS continued to experience weekly or monthly seizures at follow-up a median of 8 years in length.
Similarly, patients entering the current study were experiencing high numbers of seizures at baseline, as high as 1 seizure per day (or more in some patients). Thus, patients enrolled in this study experienced significant and clinically meaningful improvements with fenfluramine added to their current stiripentol-containing AED regimen (54.0% [95% CI, 35.6%-67.2%] greater reduction in mean MCSF vs patients treated with placebo). The magnitude of treatment outcome is further highlighted by the fact that significantly more patients with added fenfluramine vs placebo experienced reductions in MCSF that were clinically meaningful (≥50%; 54% vs 5%) and profound (≥75%; 35% vs 2%), which are magnitudes of response not commonly seen in DS.
Fenfluramine was generally well tolerated. Importantly, no patient developed valvular heart disease or PAH, and all echocardiograms in all patients demonstrated normal valve function without observation of abnormal valve morphology. Fenfluramine as an anorectic agent for adults was removed from the market in 1997 based on reports of increased risk of valvular heart disease when prescribed in higher dosages (60-120 mg/d) and (most often) when prescribed with phentermine.24 In addition, cases of PAH were also reported.25 In the patient population of adults with obesity, higher dosages (>40-60 mg daily) than those required for efficacy in the current study (≤17 mg/d) were reported.26,27,28,29,30 Importantly, an intensive, prospectively defined cardiovascular monitoring program was used, with color Doppler echocardiographic examinations before and during the trial to identify functional changes in cardiac valves and signs of PAH, and all echocardiograms were interpreted by 2 reviewers blinded to treatment group. There was no valvular heart disease or PAH observed in any patient at any time during the study. Only findings of intermittent, trace mitral regurgitation were observed, which are physiologic findings also observed in children with normal health.23 This study supports previous reports of the cardiovascular safety in patients with DS at the lower dosages used for seizure management in short-term and long-term trials relative to the higher dosages for adults with obesity.12,31,32 Weight loss occurred but at rates commensurate with previous clinical studies,22 and none of the patients experienced weight loss that resulted in trial discontinuation.
Study Limitations
Outcomes associated with fenfluramine (eg, appetite suppression, efficacy in MCSF reduction) could theoretically lead to functional unblinding, but post hoc analyses found no evidence of this. The study duration was short, and longer-term observations will be necessary to fully characterize long-term effectiveness and safety, including developmental end points and an cardiovascular safety profile. However, 1 open-label study30 (treatment up to 30 years in Belgium) and an open-label extension to the phase 3 studies33,34 (treatment up to 3 years) have reported continued, significant improvement in MCSF reduction without development of valvular heart disease or PAH.
Conclusions
Fenfluramine demonstrated statistically significant and clinically meaningful efficacy in this phase 3 trial in patients with DS currently receiving a stiripentol-containing AED regimen without the development of valvular heart disease or PAH. Fenfluramine may represent an important, effective new treatment option for patients with DS and seizures inadequately controlled on stiripentol-inclusive AED regimens.
eFigure 1. Study design
eFigure 2. Greater Percentage Change in MCSF Over Time in Fenfluramine vs Placebo Treatment Groups
eFigure 3. Clinical Global Impression of Improvement
eTable 1. Diagnostic Criteria for Inclusion in Trial
eTable 2. Titration and Tapering Algorithms
Trial Protocol.
Statistical Analysis Plan.
Data Sharing Statement.
References
- 1.Wirrell EC, Laux L, Donner E, et al. Optimizing the diagnosis and management of Dravet syndrome: recommendations from a North American consensus panel. Pediatr Neurol. 2017;68:18-34.e3. doi: 10.1016/j.pediatrneurol.2017.01.025 [DOI] [PubMed] [Google Scholar]
- 2.Wu YW, Sullivan J, McDaniel SS, et al. Incidence of Dravet syndrome in a US population. Pediatrics. 2015;136(5):e1310-e1315. doi: 10.1542/peds.2015-1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dravet C, Bureau M, Oguni H, Fukuyama Y, Cokar O. Severe myoclonic epilepsy in infancy: Dravet syndrome. Adv Neurol. 2005;95:71-102. [PubMed] [Google Scholar]
- 4.Depienne C, Trouillard O, Saint-Martin C, et al. Spectrum of SCN1A gene mutations associated with Dravet syndrome: analysis of 333 patients. J Med Genet. 2009;46(3):183-191. doi: 10.1136/jmg.2008.062323 [DOI] [PubMed] [Google Scholar]
- 5.Skluzacek JV, Watts KP, Parsy O, Wical B, Camfield P. Dravet syndrome and parent associations: the IDEA League experience with comorbid conditions, mortality, management, adaptation, and grief. Epilepsia. 2011;52(suppl 2):95-101. doi: 10.1111/j.1528-1167.2011.03012.x [DOI] [PubMed] [Google Scholar]
- 6.Cooper MS, Mcintosh A, Crompton DE, et al. Mortality in Dravet syndrome. Epilepsy Res. 2016;128:43-47. doi: 10.1016/j.eplepsyres.2016.10.006 [DOI] [PubMed] [Google Scholar]
- 7.Harden C, Tomson T, Gloss D, et al. Practice guideline summary: sudden unexpected death in epilepsy incidence rates and risk factors: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology and the American Epilepsy Society. Epilepsy Curr. 2017;17(3):180-187. doi: 10.5698/1535-7511.17.3.180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aras LM, Isla J, Mingorance-Le Meur A. The European patient with Dravet syndrome: results from a parent-reported survey on antiepileptic drug use in the European population with Dravet syndrome. Epilepsy Behav. 2015;44:104-109. doi: 10.1016/j.yebeh.2014.12.028 [DOI] [PubMed] [Google Scholar]
- 9.De Liso P, Chemaly N, Laschet J, et al. Patients with Dravet syndrome in the era of stiripentol: a French cohort cross-sectional study. Epilepsy Res. 2016;125:42-46. doi: 10.1016/j.eplepsyres.2016.05.012 [DOI] [PubMed] [Google Scholar]
- 10.Harden C, Tomson T, Gloss D, et al. Practice guideline summary: Sudden unexpected death in epilepsy incidence rates and risk factors: report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology. 2017;88(17):1674-1680. doi: 10.1212/WNL.0000000000003685 [DOI] [PubMed] [Google Scholar]
- 11.Chiron C, Helias M, Kaminska A, et al. Do children with Dravet syndrome continue to benefit from stiripentol for long through adulthood? Epilepsia. 2018;59(9):1705-1717. doi: 10.1111/epi.14536 [DOI] [PubMed] [Google Scholar]
- 12.Lagae L, Sullivan J, Helen Cross J, et al. ZX008 (fenfluramine HCl oral solution) in Dravet syndrome: results of a phase 3, randomized, double-blind, placebo-controlled trial. Lancet. in press. [Google Scholar]
- 13.Rubino C, Boyd B, Zhang L, Ismail M, Trang M, Farfel GM. ZX008 (fenfluramine oral solution) as adjunctive therapy for Dravet syndrome seizures: a pharmacometric approach to quantify potential drug-drug interactions to support phase 3 dose selection. Annual Meeting of the America Epilepsy Society; December 1-5, 2017; Washington, DC. [Google Scholar]
- 14.U.S. Department of Health and Human Services Food and Drug Administration . Center for Drug Evaluation and Research; Center for Biologics Evaluation and Research. E6(R2) Good clinical practice: integrated addendum to ICH E6(R1) guidance for industry. https://www.fda.gov/media/93884/download. Published March 2018. Accessed October 28, 2019.
- 15.Boyd B, Smith S, Gammaitoni A, Galer BS, Farfel GM. A phase I, randomized, open-label, single-dose, 3-period crossover study to evaluate the drug-drug interaction between ZX008 (fenfluramine HCl oral solution) and a regimen of stiripentol, clobazam, and valproate in healthy subjects. Int J Clin Pharmacol Ther. 2019;57(1):11-19. doi: 10.5414/CP203276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sabaz M, Lawson JA, Cairns DR, et al. Validation of the quality of life in childhood epilepsy questionnaire in American epilepsy patients. Epilepsy Behav. 2003;4(6):680-691. doi: 10.1016/j.yebeh.2003.08.012 [DOI] [PubMed] [Google Scholar]
- 17.Varni JW, Seid M, Kurtin PS. PedsQL 4.0: reliability and validity of the Pediatric Quality of Life Inventory version 4.0 generic core scales in healthy and patient populations. Med Care. 2001;39(8):800-812. doi: 10.1097/00005650-200108000-00006 [DOI] [PubMed] [Google Scholar]
- 18.Ziobro J, Eschbach K, Sullivan JE, Knupp KG. Current treatment strategies and future treatment options for Dravet syndrome. Curr Treat Options Neurol. 2018;20(12):52. doi: 10.1007/s11940-018-0537-y [DOI] [PubMed] [Google Scholar]
- 19.Chiron C. Stiripentol for the treatment of Dravet syndrome. Orphan Drugs Res Rev. 2014;4:29-38. doi: 10.2147/ODRR.S47619 [DOI] [Google Scholar]
- 20.Chiron C, Marchand MC, Tran A, et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO study group. Lancet. 2000;356(9242):1638-1642. doi: 10.1016/S0140-6736(00)03157-3 [DOI] [PubMed] [Google Scholar]
- 21.European Medicines Agency . Diacomit: EPAR—scientific discussion. https://www.ema.europa.eu/en/documents/scientific-discussion/diacomit-epar-scientific-discussion_en.pdf. Published January 29, 2009. Accessed February 28, 2019.
- 22.Devinsky O, Cross JH, Laux L, et al. ; Cannabidiol in Dravet Syndrome Study Group . Trial of cannabidiol for drug-resistant seizures in the Dravet syndrome. N Engl J Med. 2017;376(21):2011-2020. doi: 10.1056/NEJMoa1611618 [DOI] [PubMed] [Google Scholar]
- 23.Webb RH, Gentles TL, Stirling JW, Lee M, O’Donnell C, Wilson NJ. Valvular regurgitation using portable echocardiography in a healthy student population: implications for rheumatic heart disease screening. J Am Soc Echocardiogr. 2015;28(8):981-988. doi: 10.1016/j.echo.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 24.Bachorik L. FDA announces withdrawal fenfluramine and dexfenfluramine (Fen-Phen). https://wayback.archive-it.org/7993/20170723090512/https://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm179871.htm. Published 1997. Accessed May 29, 2018.
- 25.Abenhaim L, Moride Y, Brenot F, et al. ; International Primary Pulmonary Hypertension Study Group . Appetite-suppressant drugs and the risk of primary pulmonary hypertension. N Engl J Med. 1996;335(9):609-616. doi: 10.1056/NEJM199608293350901 [DOI] [PubMed] [Google Scholar]
- 26.Dahl CF, Allen MR, Urie PM, Hopkins PN. Valvular regurgitation and surgery associated with fenfluramine use: an analysis of 5743 individuals. BMC Med. 2008;6:34. doi: 10.1186/1741-7015-6-34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopkins PN, Polukoff GI. Risk of valvular heart disease associated with use of fenfluramine. BMC Cardiovasc Disord. 2003;3:5. doi: 10.1186/1471-2261-3-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jick H, Vasilakis C, Weinrauch LA, Meier CR, Jick SS, Derby LE. A population-based study of appetite-suppressant drugs and the risk of cardiac-valve regurgitation. N Engl J Med. 1998;339(11):719-724. doi: 10.1056/NEJM199809103391102 [DOI] [PubMed] [Google Scholar]
- 29.Rich S, Rubin L, Walker AM, Schneeweiss S, Abenhaim L. Anorexigens and pulmonary hypertension in the United States: results from the surveillance of North American pulmonary hypertension. Chest. 2000;117(3):870-874. doi: 10.1378/chest.117.3.870 [DOI] [PubMed] [Google Scholar]
- 30.Schoonjans A, Paelinck BP, Marchau F, et al. Low-dose fenfluramine significantly reduces seizure frequency in Dravet syndrome: a prospective study of a new cohort of patients. Eur J Neurol. 2017;24(2):309-314. doi: 10.1111/ene.13195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lai WW, Pringsheim M, Farfel G, et al. Long-term cardiovascular safety of Fintepla® (fenfluramine HCl oral solution) in the treatment of Dravet syndrome: interim analysis of an open-label safety extension study [poster]. American Epilepsy Society (AES) Annual Meeting; November 30–December 4, 2018; New Orleans, LA. [Google Scholar]
- 32.Schoonjans AS, Marchau F, Paelinck BP, et al. Cardiovascular safety of low-dose fenfluramine in Dravet syndrome: a review of its benefit-risk profile in a new patient population. Curr Med Res Opin. 2017;33(10):1773-1781. doi: 10.1080/03007995.2017.1355781 [DOI] [PubMed] [Google Scholar]
- 33.Ceulemans B, Boel M, Leyssens K, et al. Successful use of fenfluramine as an add-on treatment for Dravet syndrome. Epilepsia. 2012;53(7):1131-1139. doi: 10.1111/j.1528-1167.2012.03495.x [DOI] [PubMed] [Google Scholar]
- 34.Ceulemans B, Schoonjans AS, Marchau F, Paelinck BP, Lagae L. Five-year extended follow-up status of 10 patients with Dravet syndrome treated with fenfluramine. Epilepsia. 2016;57(7):e129-e134. doi: 10.1111/epi.13407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure 1. Study design
eFigure 2. Greater Percentage Change in MCSF Over Time in Fenfluramine vs Placebo Treatment Groups
eFigure 3. Clinical Global Impression of Improvement
eTable 1. Diagnostic Criteria for Inclusion in Trial
eTable 2. Titration and Tapering Algorithms
Trial Protocol.
Statistical Analysis Plan.
Data Sharing Statement.