Key Points
Question
Is body composition associated with tolerance and adherence to anthracycline and taxane-based chemotherapy and, thus, breast cancer mortality?
Findings
In this cohort study of 1395 patients with nonmetastatic breast cancer, greater adiposity was associated with lower relative dose intensity (ratio of delivered to planned chemotherapy dose). Lower relative dose intensity was associated with increased risk of death after breast cancer and partially mediated the association of greater adiposity with mortality.
Meaning
Although most chemotherapies are dosed according to body surface area, body composition may identify patients at risk for lower relative dose intensity, which could compromise therapeutic efficacy and may be one of multiple pathways through which adiposity is associated with increases in breast cancer mortality.
Abstract
Importance
Although most chemotherapies are dosed on body surface area or weight, body composition (ie, the amount and distribution of muscle and adipose tissues) is thought to be associated with chemotherapy tolerance and adherence.
Objectives
To evaluate whether body composition is associated with relative dose intensity (RDI) on anthracycline and taxane-based chemotherapy or hematologic toxic effects and whether lower RDI mediates the association of adiposity with mortality.
Design, Setting, and Participants
An observational cohort study with prospectively collected electronic medical record data was conducted at Kaiser Permanente Northern California, a multicenter, community oncology setting within an integrated health care delivery system. Participants included 1395 patients with nonmetastatic breast cancer diagnosed between January 1, 2005, and December 31, 2013, and treated with anthracycline and taxane-based chemotherapy. Data analysis was performed between February 25 and September 4, 2019.
Exposures
Intramuscular, visceral, and subcutaneous adiposity as well as skeletal muscle were evaluated from clinically acquired computed tomographic scans at diagnosis.
Main Outcomes and Measures
The primary outcome was low RDI (<0.85), which is the ratio of delivered to planned chemotherapy dose, derived from infusion records; in addition, hematologic toxic effects were defined based on laboratory test values. To evaluate associations with overall and breast cancer–specific mortality, logistic regression models adjusted for age and body surface area were fit as well as Cox proportional hazards models adjusted for age, race/ethnicity, adiposity, Charlson comorbidity index score, and tumor stage and subtype. The mediation proportion was computed using the difference method.
Results
The mean (SD) age at diagnosis of the 1395 women included in the study was 52.8 (10.2) years. Greater visceral (odds ratio [OR], 1.19; 95% CI, 1.02-1.39 per SD) and intramuscular (OR, 1.16; 95% CI, 1.01-1.34 per SD) adiposity were associated with increased odds of RDI less than 0.85. Greater muscle mass was associated with a decreased odds of hematologic toxic effects (OR, 0.84; 95% CI, 0.71-0.98 per SD). Relative dose intensity less than 0.85 was associated with a 30% increased risk of death (hazard ratio, 1.30; 95% CI, 1.02-1.65). Lower RDI partially explained the association of adiposity with breast cancer–specific mortality (mediation proportion, 0.20; 95% CI, 0.05-0.55).
Conclusions and Relevance
Excess adiposity, presenting as larger visceral or intramuscular adiposity, was associated with lower RDI. Lower RDI partially mediated the association of adiposity with worse breast cancer–specific survival. Body composition may help to identify patients likely to experience toxic effects and subsequent dose delays or reductions, which could compromise chemotherapeutic efficacy.
This cohort study evaluates the association between body composition and the risk of death based on the ratio of planned vs administered dose of chemotherapy in women with breast cancer.
Introduction
Improvements in early detection and treatment have improved the prognosis for the more than 3.4 million US women living with breast cancer.1 Most chemotherapy regimens are dosed on body surface area (BSA) or weight. Yet, toxic effects are common during chemotherapy and can result in dose reductions or delays, reducing the efficacy of these life-saving therapies.2 Body composition, including the amount and distribution of muscle and adipose tissues, is one factor associated with both resilience to the physiologic stress of cancer treatment and the pharmacokinetics of certain chemotherapy drugs for breast cancer.3,4,5,6 When the American Society of Clinical Oncology released guidelines to determine doses for patients according to their full BSA,7 the society also acknowledged that real-world data are limited and affirmed the importance of analyses of body composition and adverse events.7
With the substantial variation in body composition at a given BSA or body mass index (BMI; calculated as weight in kilograms divided by height in meters squared),3,8 lower muscle and/or greater adiposity could alter the volume of distribution for chemotherapy agents, thus increasing the risk of toxic effects and resulting in dose delays or reductions. To date, few studies have examined body composition and adherence to chemotherapy among patients with breast cancer; studies that have were limited by small sample sizes and heterogeneous patient populations and study designs.4,5,6,9,10,11,12 Furthermore, none of these previous studies examined mortality outcomes. The literature supports the hypothesis that body composition partially explains heterogeneity in patients’ tolerance for chemotherapy and thus could help to identify patients at high risk of treatment-related toxic effects.4,5,6,9,10,11,12 However, to our knowledge, whether chemotherapy adherence mediates the association between body composition and breast cancer mortality has never been formally examined.
To address this gap, we studied 1395 Kaiser Permanente Northern California (KPNC) patients receiving anthracycline and taxane-based chemotherapy for nonmetastatic breast cancer whose body composition was assessed from diagnostic computed tomographic (CT) scans. Building on previous work demonstrating that body composition was associated with breast cancer survival,13 our primary objective was to investigate whether body composition was associated with chemotherapy adherence measured as relative dose intensity (RDI) (ie, the ratio of delivered to planned dose intensity, which encompasses dose reductions and treatment delays). Secondarily, we examined hematologic toxic effects and inpatient hospitalizations during chemotherapy. In addition, we investigated whether lower RDI explained the previous observation that greater adiposity was associated with increased mortality after breast cancer.13
Methods
The Breast Cancer, Sarcopenia and Near-term Survival study included all KPNC members diagnosed with nonmetastatic, invasive breast cancer between January 1, 2005, and December 31, 2013, who had an abdominal or pelvic CT scan performed for diagnostic purposes and no history of cancer (n = 3139).13 The present analysis included women who received anthracycline and taxane-based chemotherapy at KPNC (1329 of 1395 [95.3%] of whom who had stage II or III cancer). Median time from definitive surgery to chemotherapy initiation was 1.28 (interquartile range, 0.92-1.83) months. The KPNC Institutional Review Board approved the study with waiver of informed consent.
Body Composition
We quantified body composition from CT scans taken within 6 months of diagnosis but before chemotherapy or radiotherapy (median time from diagnosis to scan, 1.15 [interquartile range, 0.56-1.68] months; range, 5.52-5.23 months). Two centrally trained research assistants blinded to clinical outcomes used SliceOmatic software, version 5.0 (TomoVision)9 to contour the cross-sectional area of each tissue in centimeters squared at the third lumbar vertebra, distinguishing muscle from visceral and subcutaneous adipose tissues using anatomic knowledge and tissue-specific Hounsfield unit ranges.8 The coefficients of variation were 0.66% for muscle, 0.79% for subcutaneous tissue, 6.72% for visceral tissue, and 1.59% for total adipose tissue quantifications between raters. Single-slice third lumbar vertebra tissue areas are well correlated with whole-body tissue volumes.10 To quantify intramuscular adiposity, we computed the average radiation attenuation (also known as radiodensity) of skeletal muscle tissue in Hounsfield units; in multivariable models, this variable was scaled inversely to represent greater intramuscular adiposity (lower muscle radiodensity indicates greater accumulation of intramyocellular triglycerides).14
Covariate and Death Assessment
From KPNC’s comprehensive electronic medical record (EMR) and Cancer Registry we obtained information on demographics (age and race/ethnicity) and tumor characteristics (American Joint Committee on Cancer stage, estrogen/progesterone receptor, and ERBB2 [formerly HER2] status), and cancer treatment (radiotherapy, anthracycline-containing chemotherapy, ERBB2-directed and/or endocrine therapy). The KPNC EMR began in 1996, encapsulates all care within KPNC, and is composed of diagnostic, procedural, laboratory, infusion, and radiographic data from various sources, including, but not limited to, inpatient, outpatient, claims, and referrals. We combined mortality data from KPNC membership records, California death records, and the Social Security Administration to determine patients’ vital status and causes of death. We classified death as breast cancer–specific when breast cancer was listed as the primary or contributing cause of death.
Chemotherapy Treatment
We defined common anthracycline and taxane-based chemotherapy regimens according to the National Comprehensive Cancer Network guidelines at the time of the patient’s diagnosis15 from KPNC infusion records. Regimens of interest included doxorubicin given with cyclophosphamide followed by paclitaxel or docetaxel (taxanes); doxorubicin given with cyclophosphamide followed by a taxane given with trastuzumab; or doxorubicin, cyclophosphamide, and a taxane given together. The first cycle began with the date of chemotherapy initiation and ended with the date of the next chemotherapy administration, provided that the next administration was at least 6 but not more than 90 days after chemotherapy initiation (weeks between cycles ranged from 1 to 3 depending on the drug and regimen; eTable 2 in the Supplement).
Outcomes
Although our main analysis considered the patient’s entire chemotherapy experience, we also present results separately for the anthracycline and taxane treatments in eTable 3 in the Supplement. Per previous publications,16,17 we computed RDI as the delivered dose intensity divided by the expected dose intensity. Relative dose intensity is a composite measure encompassing dose reductions and treatment delays. Early discontinuation of therapy was a separate outcome defined as receiving fewer than 75% of the recommended number of cycles for a given regimen. Delivered dose intensity was calculated as the total dose in milligrams per square meter of BSA administered over the course of therapy divided by the time in weeks to complete chemotherapy. Expected dose intensity was based on the planned course of treatment (National Comprehensive Cancer Network–recommended dose in milligrams per square meter per week). We calculated RDI separately for the doxorubicin with cyclophosphamide and taxane portions of therapy and defined low RDI as a reduction of 15% or more (ie, RDI <0.85) on either doxorubicin with cyclophosphamide or taxane. To identify substantial departures from the planned course of treatment, we defined treatment delays as any chemotherapy cycle received 3 or more days later than the guideline-recommended gap between administrations.
Additional, secondary outcomes included hematologic toxic effects and inpatient hospitalization during chemotherapy. From laboratory records available in the EMR, we defined hematologic toxic effects as any of the following: neutropenia (absolute neutrophil count <1000/μL [to convert to ×109 per liter, multiply by 0.001] or International Classification of Diseases, Ninth Revision [ICD-9], diagnosis codes 288.00, 288.03, 288.09, 288.5, 288.50, 288.51, 288.59), anemia (hemoglobin <10 g/dL [to convert to grams per liter, multiply by 10]), and/or thrombocytopenia (platelets <50 × 103/μL [to convert to ×109 per liter, multiply by 1] or ICD-9 codes 287.4, 287.5). We defined inpatient hospitalization as any hospitalization that occurred during the time in which the patient was receiving chemotherapy based on the date of hospital admission from inpatient records drawn from patient encounters across all KPNC facilities.
Medical assistants measured patients’ height and weight at clinical visits. We calculated initial dosing BSA from the patient’s height and weight at the time of the initial chemotherapy administration. We calculated BMI from the height and weight measurements closest to the CT scan.
Statistical Analysis
Data analysis was performed between February 25 and September 4, 2019. We computed descriptive statistics, including means (SDs) and percentages, overall and by RDI less than 0.85. Using separate logistic regression models for each dichotomous outcome, we examined associations of each exposure with our primary outcome (RDI <0.85) and with hematologic toxic effects, inpatient hospitalization, treatment delay, and early discontinuation. All models were adjusted for age and initial dosing BSA. As a sensitivity analysis, we fit models adjusting for the Charlson comorbidity index score (excluding cancer)18 to understand the association of comorbidities and the estimated associations with the body composition variables. We report the linear associations of the continuous body composition exposures per SD unit change. We examined associations in deciles to look for evidence of nonlinearity.
We assessed survival from the last chemotherapy administration date through December 31, 2015, the latest date for which cause-of-death records are available from California. We used Kaplan-Meier survival curves to calculate the probability of overall survival and cumulative incidence curves to calculate the probability of breast cancer–specific mortality. We used Cox proportional hazards regression models to estimate the association of low RDI less than 0.85 with overall mortality and breast cancer mortality. We selected covariates and potential confounders a priori, based on previous scientific knowledge, including age at diagnosis, race/ethnicity, total adiposity, Charlson comorbidity index score, stage of cancer, and estrogen and progesterone receptor and ERBB2 status. We report the cause-specific hazard ratios (HRs) for breast cancer death herein because analyses accounting for competing risks yielded similar results.19,20 To assess whether the association between adiposity and breast cancer mortality was mediated through lower RDI, we computed the mediation proportion in the context of a Cox proportional hazards model using the difference method, which compares estimates from models with and without the hypothesized mediator, and constructed 95% CIs using the Δ method.21 P values <.05 were considered significant. Statistical analyses were performed using SAS for Windows, version 9.4 (SAS Institute Inc).
Results
eTable 1 in the Supplement reports baseline characteristics of the cohort overall and by low RDI less than 0.85. Mean (SD) age at diagnosis was 52.8 (10.2) years, BSA was 1.8 (0.2) m2, and BMI was 28.7 (6.4). Nearly a third (415 [29.7%]) of the patients experienced low RDI less than 0.85 while receiving anthracycline and/or taxane therapy. Patients with low RDI were slightly older (mean [SD] age, 54.5 [10.5] vs 52.0 [10.0] years) and had slightly larger BSA (mean [SD], 1.9 [0.3] m2 vs 1.8 [0.2] m2) but similar comorbidity scores (mean [SD], 0.4 [1.0] vs 0.3 [0.6]) and demographic characteristics (eg, non-Hispanic white, 602 [62.9%] vs 261 [61.3%]) to those with higher RDI. Adverse outcomes, including toxic effects and inpatient hospitalizations, were more common during the taxane than anthracycline portion of therapy.
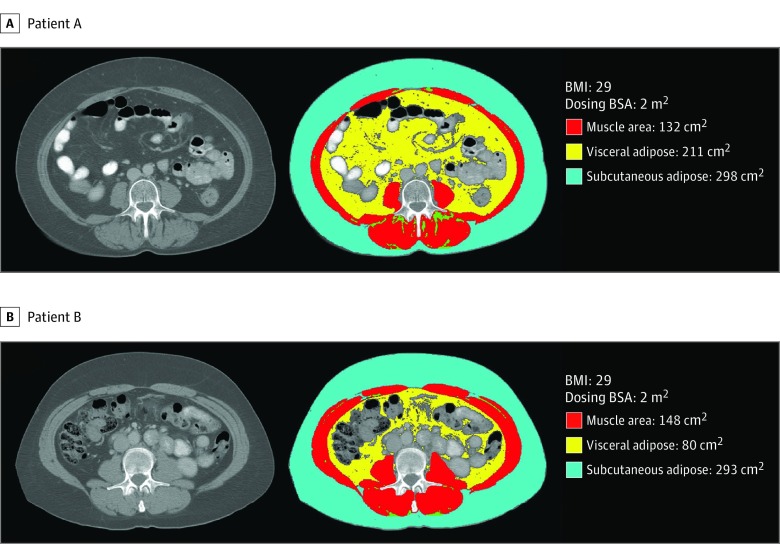
As shown in Figure 1, there were large differences in body composition among patients with the same BSA. Overall, BSA explained substantial variation in subcutaneous adiposity (R2 = 0.65), but only limited variation in visceral adiposity (R2 = 0.41), intramuscular adiposity (R2 = 0.15), or muscle tissue area (R2 = 0.48).
Figure 1. Two Patients With Identical Body Surface Area (BSA) and Body Mass Index (BMI), but Different Body Composition.
Although both patients have a dosing BSA of 2 m2 and BMI of 29 (calculated as weight in kilograms divided by height in meters squared), patient A may have a higher risk of toxic effects and dose reduction than patient B. Patient A has greater visceral (211 cm2) and intramuscular (muscle radiodensity 34 Hounsfield units [HU]) adiposity and smaller muscle area (132 cm2) than patient B (visceral adipose area 80 cm2, muscle radiodensity 50 HU, and muscle area 148 cm2).
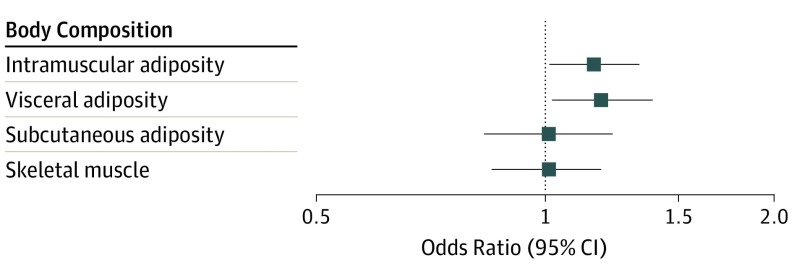
Figure 2 shows the association of body composition exposures with low RDI less than 0.85, adjusted for age and BSA. Patients with greater visceral and intramuscular adiposity were at greater risk of receiving less than the planned dose of chemotherapy, with odds ratios (ORs) for low RDI less than 0.85 of 1.19 (95% CI, 1.02-1.39) per SD for visceral adiposity and 1.16 (95% CI, 1.01-1.34) per SD for intramuscular adiposity. As reported in eTable 3 in the Supplement, all forms of adiposity were associated with increased risk of discontinuing chemotherapy early (visceral: OR, 1.16; 95% CI, 0.94-1.43 per SD; intramuscular: OR, 1.14; 95% CI, 0.91-1.44 per SD; subcutaneous: OR, 1.28; 95% CI, 0.96-1.70 per SD). Patients with greater visceral (OR, 1.17; 95% CI, 0.95-1.43 per SD) and intramuscular (OR, 1.09; 95% CI, 0.91-1.32 per SD) adiposity also had an elevated risk of inpatient hospitalization during chemotherapy. In contrast, greater muscle mass was associated with decreased odds of hematologic toxic effects (OR, 0.84; 95% CI, 0.71-0.98 per SD muscle area).
Figure 2. Risk of Low Relative Dose Intensity of Anthracycline and Taxane-Based Chemotherapy by SD of Body Composition Exposures.
Odds ratios are per SD exposure from logistic regression models adjusted for age at diagnosis and initial dosing body surface area. Greater intramuscular and visceral adiposity are associated with a higher risk of low relative dose intensity less than 0.85.
Adjustment for comorbidities and race/ethnicity made little difference to the estimated effect of body composition exposures; thus, these covariates were excluded from final models. For example, the risk of low RDI per SD moved from OR of 1.19 (95% CI, 1.02-1.39) to OR of 1.19 (95% CI, 1.01-1.40) for visceral adiposity and from OR of 1.16 (95% CI, 1.01-1.34) to OR of 1.17 (95% CI, 1.02-1.35) for intramuscular adiposity after including the Charlson comorbidity index score in addition to age and BSA.
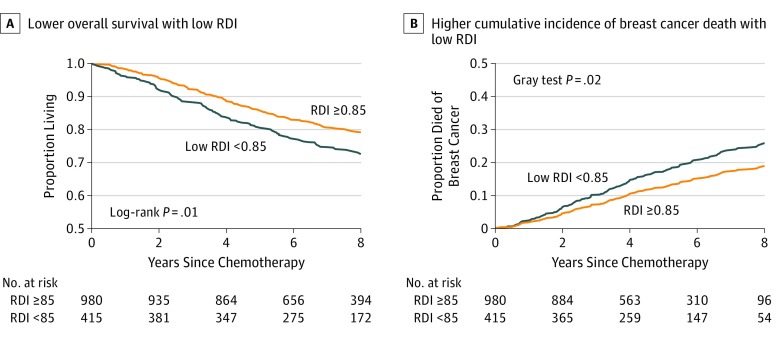
Over a median of 7.3 years of follow-up (maximum, 12 years), 318 women (22.8%) died, including 201 women (63.2%) from breast cancer. Patients with low RDI less than 0.85 had shorter overall survival (Figure 3A) (log-rank P = .02) and higher incidence of breast cancer–specific death (Figure 3B) (Gray test P = .02). Our multivariable models adjusted for age at diagnosis, age, race/ethnicity, adiposity, Charlson comorbidity index score, stage of cancer, and hormone receptor and ERBB2 status. Compared with women who received higher RDI, women with low RDI less than 0.85 had a 30% increased risk of dying from any cause (HR, 1.30; 95% CI, 1.02-1.65) and a 38% increased risk of dying from breast cancer (HR, 1.38; 95% CI, 1.02-1.85). Lower RDI explained 20% of the association of adiposity with breast cancer–specific mortality; however, 95% CIs were consistent with a large range of mediation effect sizes (the proportion mediated by continuous RDI was 0.22 [95% CI, 0.05-0.58] for overall mortality and 0.20 [95% CI, 0.05-0.55] for breast cancer–specific mortality).
Figure 3. Overall Survival and Cumulative Incidence of Breast Cancer Death by Relative Dose Intensity (RDI) on Anthracycline and Taxane-Based Chemotherapy.
Lower overall survival (A) and higher cumulative incidence of breast cancer death associated with low RDI (B). Multivariable models were adjusted for age at diagnosis, race/ethnicity, total adiposity, Charlson comorbidity index score, stage of cancer, and estrogen and progesterone receptor and ERBB2 (formerly HER2) status. Compared with women who received higher RDI, women with low RDI less than 0.85 had a 30% higher risk of dying from any cause (hazard ratio [HR], 1.30; 95% CI, 1.02-1.65) and a 38% higher risk of dying from breast cancer (HR, 1.38; 95% CI, 1.02-1.85).
Discussion
To our knowledge, this study is the largest to date of body composition and adherence to anthracycline and taxane-based chemotherapy for breast cancer. We found that greater adiposity was associated with an increased risk of low RDI less than 0.85 independent of age and BSA. Lower RDI was associated with an increased risk of death and partially explained the association of adiposity with breast cancer mortality. Our findings suggest that consideration of patient body composition could help optimize chemotherapy dosing or target supportive interventions to mitigate toxic effects. Decreasing dose reductions and delays is crucial to optimize chemotherapeutic efficacy.
Previous research on body composition and chemotherapy has examined a variety of regimens with relatively small sample sizes.4,5,6,9,10,11,12 The 2 largest and most recent studies examined associations between body composition and chemotherapy tolerance in patients with early-stage breast cancer. With findings similar to ours, Shachar and colleagues9 examined 151 patients receiving anthracycline and taxane-based chemotherapy and noted that skeletal muscle gauge (a composite measure incorporating both muscle area and radiodensity) was associated with greater toxic effects, whereas BSA was not. Associations with adiposity were in the same direction as those in our study. Another recent study of 172 patients with nonmetastatic breast cancer from the Netherlands also found that higher absolute levels of fat mass and lower relative levels of lean mass (measured by dual-energy x-ray absorptiometry) increased the risk of toxic effect–related dose modifications.10
There are several possible explanations for our findings. Despite BSA-based dosing, many chemotherapy agents still have significant interpatient variability in drug pharmacokinetics; the addition of other parameters, such as body composition, could decrease that variability and better estimate the probability of drug toxic effects.22,23,24,25 This hypothesis is supported by a small body of literature on breast cancer.4,5,6 For example, in a study of 84 Asian women, greater visceral adiposity predicted greater doxorubicin exposure and hematologic toxic effects, while BSA and muscle mass did not.5 Other studies have found that patients with lower muscle mass and breast cancer who were receiving epirubicin6 or capecitabine4 were more likely to experience toxic effects compared with those with greater muscle mass and that body composition was an independent predictor of epirubicin pharmacokinetics.6 Finally, patients with obesity who were receiving BSA-based dosing of doxorubicin had higher systemic drug exposure compared with lean controls, implying that adipose tissue may be an important predictor of drug clearance.26 The underlying hypothesis is that greater adiposity and/or lower muscle mass alter the tissue volume available for the distribution of hydrophilic compounds, such as doxorubicin, thereby altering drug clearance and increasing the risk of dose-limiting toxic effects.4,5,6,22,23,24,25,26 Meanwhile, lipophilic drugs, such as docetaxel or paclitaxel, could accumulate in adipose tissue, risking late release and increasing toxic effects.27 Because the regimens examined in this study were composed of multiple agents with different pharmacokinetics and toxic effects, future research should examine the role of body composition in chemotherapy tolerance by specific drug type.
Clinical Implications
Our findings reinforce the notion that body composition may affect chemotherapy tolerance and thereby outcomes. The association of body composition with lower RDI is clinically significant because it reduces the efficacy of life-saving chemotherapy. Although our study is one of only a few that have examined RDI and survival among patients with early-stage breast cancer,28,29,30,31,32 nearly all previous studies showed that lower RDI on anthracycline and/or taxane-based chemotherapy was associated with worse survival.28,29,30,32 For what we believe to be the first time, we found that lower RDI partially mediated the association of adiposity with breast cancer mortality.
Currently, chemotherapy dosing is based on BSA and/or weight. However, BSA and weight do not fully capture adiposity or other aspects of body composition. As highlighted by the American Society of Clinical Oncology statement on chemotherapy for adults with obesity,7 research on body composition and chemotherapy dosing is needed. In the immediate term, interventions such as aerobic exercise and resistance training are effective for building muscle and reducing adiposity, are safe in patients with breast cancer (including those receiving active treatment), and could improve chemotherapy completion.33,34,35 In the long-term, while tailoring chemotherapy doses to body composition has the potential to improve chemotherapy tolerance, research must first explore the differing association of specific adipose and muscle depots with the pharmacokinetics and pharmacodynamics of specific agents. To change practice, prospective dose optimization studies are required. Already, such efforts have been undertaken. For example, an ongoing trial compares cisplatin dosing based either on standard BSA or on lean mass among patients with advanced lung cancer.36
Information on body composition may prove useful in identifying patients at risk for chemotherapy toxic effects and treatment disruptions to be targeted for therapeutic interventions. For many patients with breast cancer receiving chemotherapy, CT scans are part of the diagnostic workup, and automated methods exist to accurately assess body composition from these scans. For other patients, the incorporation of additional measures of body shape, such as waist circumference (a proxy for visceral adiposity) might be considered. Although this study included only women with breast cancer, findings are likely generalizable to other cancers where patients receive anthracycline and/or taxane-based chemotherapy and thus could be of high clinical significance, especially for cancers in which CT imaging is routine, such as ovarian cancer.
Strengths and Limitations
Defining chemotherapy regimens and toxic effects from EMR data afforded us what was, to our knowledge, the largest sample size to date and enhanced generalizability through inclusion of a racially and ethnically diverse population seen in community oncology practice. Furthermore, this study appears to be the first to demonstrate an association between body composition and chemotherapy adherence while also linking low RDI with breast cancer mortality.
Important caveats should be noted. In contrast to a trial in which dose, timing, and duration are protocol defined and toxic effects are monitored and documented, in EMR data, the decision to modify chemotherapy dosing, while often due to toxic effects, may also be based on patient or clinician choice. Patients with early-stage breast cancer were less likely to have CT scans (performed in 34% of patients with stage II and 13% of those with stage I cancer). However, results were similar when we repeated our analyses restricting them to women with stage III breast cancer for whom CT imaging is standard of care. Furthermore, women with vs without scans were similar with respect to age, race/ethnicity, BMI, and hormone receptor status.
Conclusions
Greater adiposity was associated with lower RDI in women receiving anthracycline and taxane-based chemotherapy, which may partially explain the association of adiposity with breast cancer mortality. Body composition determined with clinically acquired CT scans provides information beyond age and BSA that may help identify patients who need supportive interventions to mitigate toxic effects. Future chemotherapy trials should incorporate measures of body composition to better understand optimal chemotherapy dosing.
eTable 1. Participant Characteristics Overall and by Relative Dose Intensity
eTable 2. Anthracycline and Taxane-Based Chemotherapy Regimens
eTable 3. Outcomes During Anthracycline and Taxane-Based Chemotherapy by Standard Deviation of Body Composition Exposures
References
- 1.National Cancer Institute Surveillance Epidemiology and End Results Cancer stat facts: female breast cancer. Cancer Statistics 2015; https://seer.cancer.gov/statfacts/html/breast.html. Accessed September 19, 2018.
- 2.Muss HB, Berry DA, Cirrincione C, et al. ; Cancer and Leukemia Group B Experience . Toxicity of older and younger patients treated with adjuvant chemotherapy for node-positive breast cancer: the Cancer and Leukemia Group B Experience. J Clin Oncol. 2007;25(24):3699-3704. doi: 10.1200/JCO.2007.10.9710 [DOI] [PubMed] [Google Scholar]
- 3.Prado CM. Body composition in chemotherapy: the promising role of CT scans. Curr Opin Clin Nutr Metab Care. 2013;16(5):525-533. doi: 10.1097/MCO.0b013e328363bcfb [DOI] [PubMed] [Google Scholar]
- 4.Prado CM, Birdsell LA, Baracos VE. The emerging role of computerized tomography in assessing cancer cachexia. Curr Opin Support Palliat Care. 2009;3(4):269-275. doi: 10.1097/SPC.0b013e328331124a [DOI] [PubMed] [Google Scholar]
- 5.Wong AL, Seng KY, Ong EM, et al. Body fat composition impacts the hematologic toxicities and pharmacokinetics of doxorubicin in Asian breast cancer patients. Breast Cancer Res Treat. 2014;144(1):143-152. doi: 10.1007/s10549-014-2843-8 [DOI] [PubMed] [Google Scholar]
- 6.Prado CM, Lima IS, Baracos VE, et al. An exploratory study of body composition as a determinant of epirubicin pharmacokinetics and toxicity. Cancer Chemother Pharmacol. 2011;67(1):93-101. doi: 10.1007/s00280-010-1288-y [DOI] [PubMed] [Google Scholar]
- 7.Griggs JJ, Mangu PB, Anderson H, et al. ; American Society of Clinical Oncology . Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2012;30(13):1553-1561. doi: 10.1200/JCO.2011.39.9436 [DOI] [PubMed] [Google Scholar]
- 8.Prado CM, Cushen SJ, Orsso CE, Ryan AM. Sarcopenia and cachexia in the era of obesity: clinical and nutritional impact. Proc Nutr Soc. 2016;75(2):188-198. doi: 10.1017/S0029665115004279 [DOI] [PubMed] [Google Scholar]
- 9.Shachar SS, Deal AM, Weinberg M, et al. Body composition as a predictor of toxicity in patients receiving anthracycline and taxane-based chemotherapy for early-stage breast cancer. Clin Cancer Res. 2017;23(14):3537-3543. doi: 10.1158/1078-0432.CCR-16-2266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berg MMGA, Kok DE, Posthuma L, et al. Body composition is associated with risk of toxicity-induced modifications of treatment in women with stage I-IIIB breast cancer receiving chemotherapy. Breast Cancer Res Treat. 2019;173(2):475-481. doi: 10.1007/s10549-018-5014-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzuca F, Onesti CE, Roberto M, et al. Lean body mass wasting and toxicity in early breast cancer patients receiving anthracyclines. Oncotarget. 2018;9(39):25714-25722. doi: 10.18632/oncotarget.25394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gouérant S, Leheurteur M, Chaker M, et al. A higher body mass index and fat mass are factors predictive of docetaxel dose intensity. Anticancer Res. 2013;33(12):5655-5662. [PubMed] [Google Scholar]
- 13.Caan BJ, Cespedes Feliciano EM, Prado CM, et al. Association of muscle and adiposity measured by computed tomography with survival in patients with nonmetastatic breast cancer. JAMA Oncol. 2018;4(6):798-804. doi: 10.1001/jamaoncol.2018.0137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aubrey J, Esfandiari N, Baracos VE, et al. Measurement of skeletal muscle radiation attenuation and basis of its biological variation. Acta Physiol (Oxf). 2014;210(3):489-497. doi: 10.1111/apha.12224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goetz MP, Gradishar WJ, Anderson BO, et al. Breast cancer, version 3.2018: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw. 2019;17(2):118-126. doi: 10.6004/jnccn.2019.0009 [DOI] [PubMed] [Google Scholar]
- 16.Hryniuk WM, Goodyear M. The calculation of received dose intensity. J Clin Oncol. 1990;8(12):1935-1937. doi: 10.1200/JCO.1990.8.12.1935 [DOI] [PubMed] [Google Scholar]
- 17.Griggs JJ, Sorbero ME, Stark AT, Heininger SE, Dick AW. Racial disparity in the dose and dose intensity of breast cancer adjuvant chemotherapy. Breast Cancer Res Treat. 2003;81(1):21-31. doi: 10.1023/A:1025481505537 [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 19.Lin G, So Y, Johnston G Analyzing survival data with competing risks using SAS software. SAS Global Forum 2012. https://support.sas.com/resources/papers/proceedings12/344-2012.pdf. Accessed October 22, 2019.
- 20.Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496-509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 21.Nevo D, Liao X, Spiegelman D. Estimation and inference for the mediation proportion [published online September 20, 2017]. Int J Biostat. 2017;13(2):20170006.https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28930628&dopt=Abstract doi: 10.1515/ijb-2017-0006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baker SD, Verweij J, Rowinsky EK, et al. Role of body surface area in dosing of investigational anticancer agents in adults, 1991-2001. J Natl Cancer Inst. 2002;94(24):1883-1888. doi: 10.1093/jnci/94.24.1883 [DOI] [PubMed] [Google Scholar]
- 23.Gurney H. Dose calculation of anticancer drugs: a review of the current practice and introduction of an alternative. J Clin Oncol. 1996;14(9):2590-2611. doi: 10.1200/JCO.1996.14.9.2590 [DOI] [PubMed] [Google Scholar]
- 24.Miller AA. Body surface area in dosing anticancer agents: scratch the surface! J Natl Cancer Inst. 2002;94(24):1822-1823. doi: 10.1093/jnci/94.24.1822 [DOI] [PubMed] [Google Scholar]
- 25.Ratain MJ. Body-surface area as a basis for dosing of anticancer agents: science, myth, or habit? J Clin Oncol. 1998;16(7):2297-2298. doi: 10.1200/JCO.1998.16.7.2297 [DOI] [PubMed] [Google Scholar]
- 26.Rodvold KA, Rushing DA, Tewksbury DA. Doxorubicin clearance in the obese. J Clin Oncol. 1988;6(8):1321-1327. doi: 10.1200/JCO.1988.6.8.1321 [DOI] [PubMed] [Google Scholar]
- 27.Sparreboom A, Wolff AC, Mathijssen RHJ, et al. Evaluation of alternate size descriptors for dose calculation of anticancer drugs in the obese. J Clin Oncol. 2007;25(30):4707-4713. doi: 10.1200/JCO.2007.11.2938 [DOI] [PubMed] [Google Scholar]
- 28.Abdel-Rahman O. Outcomes of early-stage breast cancer patients treated with sequential anthracyclines-taxanes in relationship to relative dosing intensity: a secondary analysis of a randomized controlled trial. Clin Transl Oncol. 2019;21(2):239-245. doi: 10.1007/s12094-018-1915-3 [DOI] [PubMed] [Google Scholar]
- 29.Liutkauskiene S, Grizas S, Jureniene K, Suipyte J, Statnickaite A, Juozaityte E. Retrospective analysis of the impact of anthracycline dose reduction and chemotherapy delays on the outcomes of early breast cancer molecular subtypes. BMC Cancer. 2018;18(1):453. doi: 10.1186/s12885-018-4365-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandy J, Della-Fiorentina S. Relative dose intensity in early stage breast cancer chemotherapy: a retrospective analysis of incidence, risk factors and outcomes at a south-west Sydney cancer clinic. Asia Pac J Clin Oncol. 2013;9(4):365-372. doi: 10.1111/ajco.12093 [DOI] [PubMed] [Google Scholar]
- 31.Schraa SJ, Frerichs KA, Agterof MJ, Hunting JCB, Los M, de Jong PC. Relative dose intensity as a proxy measure of quality and prognosis in adjuvant chemotherapy for breast cancer in daily clinical practice. Eur J Cancer. 2017;79:152-157. doi: 10.1016/j.ejca.2017.04.001 [DOI] [PubMed] [Google Scholar]
- 32.Zhang L, Yu Q, Wu XC, et al. Impact of chemotherapy relative dose intensity on cause-specific and overall survival for stage I-III breast cancer: ER+/PR+, HER2- vs. triple-negative. Breast Cancer Res Treat. 2018;169(1):175-187. doi: 10.1007/s10549-017-4646-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Courneya KS, Segal RJ, Mackey JR, et al. Effects of aerobic and resistance exercise in breast cancer patients receiving adjuvant chemotherapy: a multicenter randomized controlled trial. J Clin Oncol. 2007;25(28):4396-4404. doi: 10.1200/JCO.2006.08.2024 [DOI] [PubMed] [Google Scholar]
- 34.Cheema BS, Kilbreath SL, Fahey PP, Delaney GP, Atlantis E. Safety and efficacy of progressive resistance training in breast cancer: a systematic review and meta-analysis. Breast Cancer Res Treat. 2014;148(2):249-268. doi: 10.1007/s10549-014-3162-9 [DOI] [PubMed] [Google Scholar]
- 35.ClinicalTrials.gov. Focus on Reducing Dose-limiting Toxicities in Colon Cancer With Resistance Exercise Study (FORCE). NCT03291951. https://clinicaltrials.gov/ct2/show/NCT03291951. Accessed July 26, 2019.
- 36.ClinicalTrials.gov. A Study Comparing Chemotherapy Dosing Based on Either Standard Body Surface Area or Lean Body Mass in Patients With Advanced Lung Cancer. NCT01624051. https://clinicaltrials.gov/ct2/show/NCT01624051?term=NCT01624051&rank=1. Accessed July 26, 2019.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Participant Characteristics Overall and by Relative Dose Intensity
eTable 2. Anthracycline and Taxane-Based Chemotherapy Regimens
eTable 3. Outcomes During Anthracycline and Taxane-Based Chemotherapy by Standard Deviation of Body Composition Exposures