This multi-institutional phase 1 clinical trial assesses the safety and tolerability of adding a course of immunotherapy after completion of chemoradiotherapy in patients with node-positive cervical cancer.
Key Points
Question
Is immunotherapy after chemoradiotherapy in node-positive cervical cancer tolerable?
Findings
In this multi-institutional phase 1 trial, 21 patients with node-positive cervical cancer received chemoradiotherapy followed by ipilimumab therapy at a maximum tolerated dose of 10 mg/kg. Two patients experienced self-limiting grade 3 toxic effects.
Meaning
This study’s findings suggest that treatment with anti–cytotoxic T-lymphocyte antigen 4 after chemoradiotherapy is tolerable for patients with node-positive cervical cancer.
Abstract
Importance
Despite standard chemoradiotherapy (CRT), most women with lymph node (LN)–positive cervical cancer experience disease recurrence. Immunotherapy is being investigated in the up-front treatment setting.
Objectives
To assess the safety of sequential immunotherapy after CRT and to investigate human papillomavirus (HPV) genotype and HLA allele status on survival and programmed cell death 1 (PD-1) expression before and after CRT and sequential immunotherapy.
Design, Setting, and Participants
This prospective phase 1 trial conducted in 29 Gynecology Oncology Cooperative Group member institutions enrolled participants from December 18, 2012, to August 31, 2016, with a 14.8-month median follow-up and translational end points. Thirty-four women with International Federation of Gynecology and Obstetrics stage IB2 to IVA cervical cancer with positive pelvic LNs, para-aortic LNs, or both were enrolled; 13 did not receive ipilimumab and were excluded from the analysis. Data were analyzed from January 21 to April 4, 2018.
Interventions
Treatment consisted of 6 weekly doses of cisplatin, 40 mg/m2, concurrent with radiotherapy. After completion of chemotherapy, sequential ipilimumab was given every 21 days for 4 doses. Two dosage levels of ipilimumab, 3 mg/kg and 10 mg/kg, were studied to identify the maximum tolerated dose.
Main Outcomes and Measures
The primary end point was safety, and the secondary end points were overall survival and progression-free survival. Exploratory end points included HPV genotype, HLA allele status, and PD-1 expression measured in peripheral blood.
Results
The median age of the 32 participants included in the intent-to-treat analysis was 50 (range, 26-61) years, and 22 patients (69%) were white. Of the 21 patients who received ipilimumab, all had positive pelvic LN, and 6 (29%) had positive para-aortic LNs. All patients completed CRT, and of the 21 patients who received at least 2 cycles of ipilimumab, 18 (86%) completed 4 cycles of ipilimumab, and 3 (14%) completed 2 cycles. The maximum tolerated dose was 10 mg/kg. Two of the 21 patients (9.5%) who received ipilimumab had self-limiting grade 3 toxic effects (lipase increase; dermatitis). The 12-month overall survival was 90%, and progression-free survival was 81%. Human papillomavirus genotype and HLA subtype were not associated with progression-free survival or overall survival. T cells expressing PD-1 increased after CRT, and levels were sustained with ipilimumab.
Conclusions and Relevance
This study’s findings suggest that the use of immunotherapy after CRT for curative-intent treatment of patients with cervical cancer is tolerable and effective. The results indicated that PD-1 was upregulated after CRT and sustained with sequential ipilimumab therapy. These immune findings may help guide future therapies to harness the activated T-cell phenotype in patients with node-positive cervical cancer.
Introduction
More than 2 decades ago, the National Institutes of Health released a consensus statement recommending the addition of chemotherapy to radiotherapy (chemoradiotherapy) (CRT) for the curative-intent treatment of locally advanced cervical cancer.1 However, despite achieving a 4-year progression-free survival (PFS) of 51% and overall survival (OS) of 55%, standard treatment with CRT does not cure patients with stage III or IV disease.2 Patients with para-aortic lymph node (PALN) metastasis also continue to have a poor prognosis, with a 3-year PFS rate of 34% and OS rate of 39%3,4; these low survival rates constitute a significant unmet medical need. The development of novel therapeutic strategies, such as the use of immunotherapy, is therefore important. Cervical cancer has been identified to be a direct consequence of viral infections by specific high-risk subtypes, most prevalently human papillomavirus genotype 16 (HPV-16) and HPV-18.5,6,7 Some studies report that the presence of HPV-18 with or without multiple genotypes is associated with disease recurrence and poor survival compared with infection with other single-type HPV genotypes.8,9,10,11 In addition, certain major histocompatibility genes may influence disease prognosis given that recognition of tumor cells by the immune system depends on peptide presentation on major histocompatibility complex alleles to T cells. The HLA-A*0201–restricted immune response is the most common subtype for which certain HPV E6 and E7 peptides have been defined as immunogenic.12 Despite an initial antitumor host immune response, cancer often develops owing to mechanisms such as local immune suppression, immune evasion, induction of dysfunctional T-cell mechanisms, and immune tolerance.13
Multimodality treatment options, including immune checkpoint blockade or immune checkpoint blockade with radiotherapy (RT), have the potential to enhance immune-mediated antitumor activity.14 Preclinical data exist regarding the combination of radiation and immune checkpoint blockade. In a study looking at immune-mediated inhibition of metastasis in a mouse model, a significant increase in survival was associated with a combination of RT and anti–cytotoxic T-lymphocyte antigen 4 (anti–CTLA-4) treatment.15 Recent clinical data show promise for combining RT and anti–CTLA-4 in the treatment of melanoma, prostate cancer, and metastatic lung and liver lesions from various primary cancers.16,17,18,19 Early data suggest that the immune cells, in particular CD8+ T cells, play a key role in tumor cell death within a radiation field.20,21 Radiotherapy causes migration of dendritic cells and neoantigen release, which can result in T-cell activation and proliferation.14 Radiotherapy has also been reported to increase expression of programmed cell death 1 (PD-1), leading to immune exhaustion.22,23 Furthermore, RT increases the density of lymphocytes infiltrating a tumor, likely by extravasation of these lymphocytes into the extracellular space of tumors and the activation of chemokines.14,24
In this phase 1 study, we examined the clinical tolerability and outcomes, including 1-year progression-free survival (PFS) and overall survival (OS) rates, associated with CRT and sequential ipilimumab therapy. We evaluated the association of HPV subtype and HLA genotype with outcomes. Furthermore, we investigated PD-1 expression at baseline, after CRT, and after sequential immunotherapy to understand the immune regulation of curative-intent therapy as a platform for developing novel strategies in the treatment of cervical cancer.
Methods
Women with histologically confirmed cervical cancer (squamous cell carcinoma, adenocarcinoma, or adenosquamous) with International Federation of Gynecology and Obstetrics stages IB2 or IIA with positive PALNs or stages IB, IIIB, or IVA with positive pelvic lymph nodes, PALNs, or both were eligible for inclusion.25 All patients were required to undergo screening with positron-emission tomography and computed tomography and computed tomography of the chest, abdomen, and pelvis. Patients were enrolled from December 18, 2012, to August 31, 2016, and data were analyzed from January 21 to April 4, 2018. The following NRG Oncology/Gynecology Oncology Group (GOG) member institutions participated in this study and provided institutional review board approval: Augusta University Medical Center, Augusta, Georgia; University of Oklahoma Health Sciences Center, Oklahoma City; University of Southern California, Los Angeles; University of Mississippi Medical Center, Jackson; Thomas Jefferson University Hospital, Philadelphia, Pennsylvania; University of California, Davis Comprehensive Cancer Center, Sacramento; Fred Hutchinson Cancer Research Center, Seattle, Washington; Virginia Commonwealth University, Richmond; and Memorial Sloan Kettering Cancer Center, New York, New York. Patients provided written informed consent in compliance with institutional, state, and federal guidelines.
Treatment
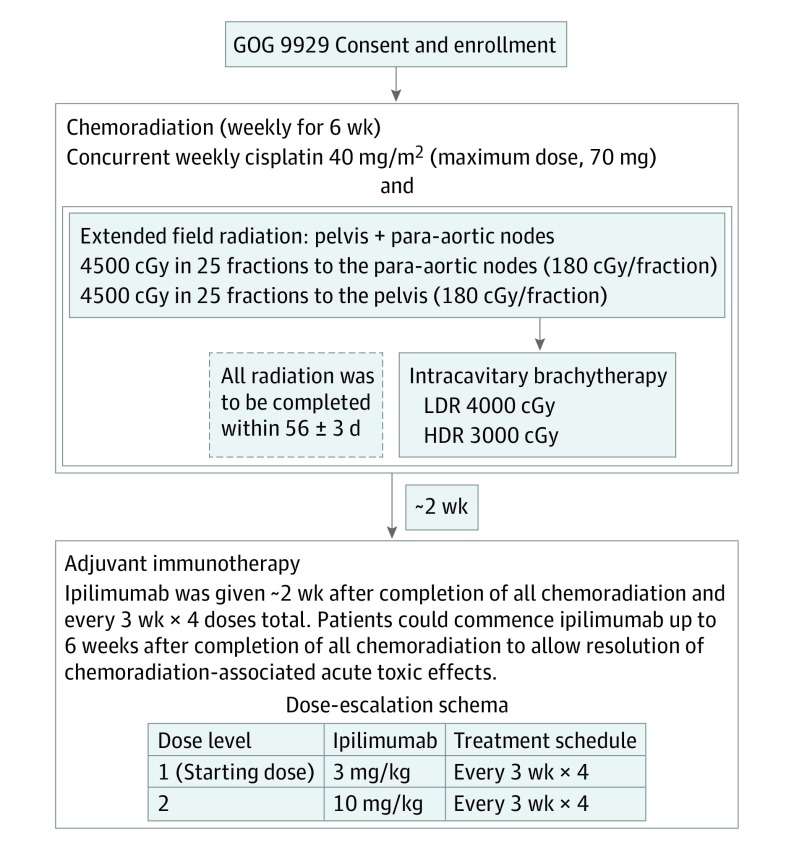
Patients were treated with 6 weekly doses of cisplatin, 40 mg/m2, concurrent with extended-field RT to treat the pelvic lymph nodes and PALNs to 4500 cGy with an RT boost and brachytherapy as shown in Figure 1. External beam RT was delivered using a linear accelerator with a photon energy of 10 mV or greater. Intensity-modulated radiotherapy (IMRT) was not permitted. Radiotherapy needed to be completed within 57 days; if necessary, 3 extra days were allowed. Two to 6 weeks after completion of CRT, tumor response was evaluated by examination and computed tomographic imaging. If there was no evidence of progression, ipilimumab was administered every 21 days for 4 doses. Two dose levels of ipilimumab, 3 mg/kg and 10 mg/kg, were studied to identify the maximum tolerated dose. Responses were defined by immune Response Evaluation Criteria in Solid Tumors 1.1.26 Complete and partial responses required confirmation at 4 or more weeks from initial documentation.
Figure 1. Study Schema.
After the maximum tolerated dose was estimated, the expansion cohort started treatment. GOG indicates Gynecology Oncology Group; HDR, high dose rate brachytherapy; and LDR, low dose rate brachytherapy.
Evaluation of Toxic Effects, Dose Modifications, and Assessment of Response
Toxic effects throughout the study were graded according to Common Terminology Criteria for Adverse Events, version 4.0.27 Dose-limiting toxic effects (DLTs) were defined by drug-related adverse events that meet these criteria, unless clearly unrelated to the study drug (eg, disease progression). The DLT period was defined during the initial 2 cycles of sequential ipilimumab therapy for 2 dose levels, 3 mg/kg and 10 mg/kg, and during 4 ipilimumab cycles in the expansion cohort. A DLT was defined as hematologic, immune-related, or non–immune-related grade 3 adverse effects; grade 4 adverse effects; or grade 5 adverse effects. Any immune-related event that required immunosuppressive treatment or systemic corticosteroid therapy was considered a DLT except when it occurred among patients receiving treatment with systemic corticosteroids for less than 2 weeks. The maximum tolerated dose was determined by the maximum dose level achieved at which not more than 1 patient (among 6) experienced a DLT. Dose-limiting toxic effects and other toxic effects were discussed by the study team in biweekly conference calls. Cisplatin was withheld if the patient’s absolute neutrophil count was less than 1500/μL and the platelet count was less than 100 × 103/μL for a maximum of 3 weeks. (To convert the neutrophil count to cells ×109/L, multiply by 0.001; conversion of the platelet count to cells ×109/L is 1:1.)
Viral and Immune Correlates
Cervical cells were collected via cervical swab prior to any treatment using a cervical specimen collection kit (Digene Cervical Sampler; Qiagen). DNA extraction used the QIAamp MinElute Media Kit (Qiagen). Human papillomavirus genotyping on genomic DNA was performed using the INNO-LiPA HPV Genotyping Extra kit (Innogenetics). Low-resolution DNA typing for HLA-A2 was performed for all patients using standard end point polymerase chain reaction. For patients with positive test results for HLA-A2, high-resolution genotyping was performed by polymerase chain reaction to provide allele-level typing at the HLA-A2 locus with the A*02 SSP UniTray Kit (ThermoFisher Scientific). Anticoagulated blood samples were collected at baseline, after CRT, and after ipilimumab treatment. Peripheral blood mononuclear cells were isolated over a hydrophilic polysaccharide density gradient (Ficoll; Sigma-Aldrich) and cryopreserved for batch testing. After thawing, the peripheral blood mononuclear cells were stained with antibodies for CD3, CD4, CD8, and PD-1 (BioLegend, Inc) for multicolor flow cytometric analysis acquired on a cytometer (FACSCanto II; BD Biosciences) and analyzed with FlowJo software, version 10.5 (BD Biosciences).
Statistical Analysis
This study was designed to evaluate the maximum tolerated dose and feasibility of the study regimens by assessing the tolerability through DLTs. The study had 2 phases: a dose-escalation phase to determine the maximum tolerated dose of sequential ipilimumab therapy based on DLTs in the first and second cycles and a feasibility phase in which the feasibility was examined by evaluating DLTs across 4 cycles. Secondary outcomes included the 1-year PFS and OS by using the Kaplan-Meier method. Exploratory end points included HPV subtype and HLA genotyping on PFS and OS by using Cox proportional hazards regression models.28 For PD-1 expression on peripheral blood mononuclear cells, the percentage of expression on T-cell subsets at the 3 time points was compared using a repeated-measures 1-way analysis of variance followed by a Tukey multiple comparisons test comparing each column (level of significance, P < .05). Prism (version 8; GraphPad Software Inc) and SAS (version 9.4; SAS Institute Inc) statistical software were used for statistical calculations.
Results
Patient Characteristics
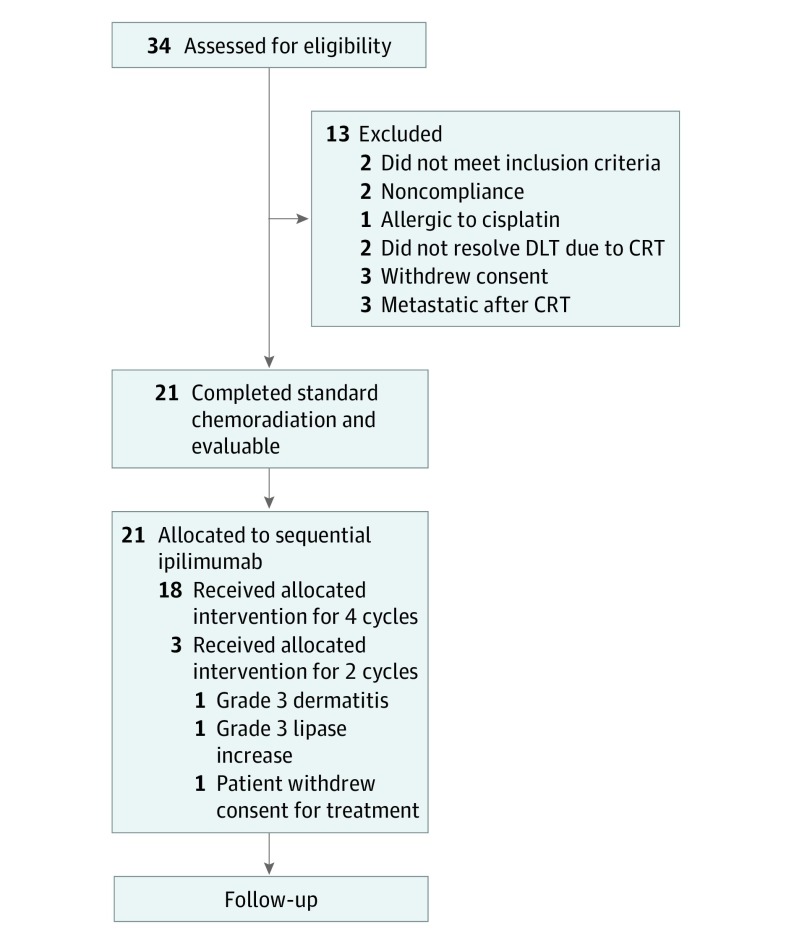
The median age of the 32 participants was 50 years (range, 26-61 years), and 22 (69%) were white. Thirty-four patients were enrolled from December 18, 2012, to August 31, 2016, with a median follow-up of 14.8 months. Two patients did not meet the inclusion criteria; therefore, 32 patients were included in the intent-to-treat analysis. All 32 had positive pelvic lymph nodes, and 9 (29%) also had PALN disease; 29 cancers (90%) were of the squamous cell histologic type. Thirteen patients did not receive any ipilimumab and were excluded from the analysis because they did not meet the inclusion criteria (2 patients), because they did not adhere to treatment (2 patients), or owing to a cisplatin allergy (1 patient), unresolved toxic effects due to CRT (2 patients), withdrawal of consent (3 patients), or progression of disease after CRT (3 patients) as shown in the CONSORT diagram (Figure 2). Baseline characteristics of the 32 patients in the intent-to-treat analysis overall and by dosage level are presented in the Table. Twenty-one patients were allocated to receive sequential ipilimumab therapy and were therefore evaluable for study end points having at least 2 cycles of ipilimumab.
Figure 2. Gynecology Oncology Group 9929 Study CONSORT Diagram.
DLT indicates dose-limiting toxic effects; CRT, chemoradiotherapy.
Table. Baseline Characteristics of All Eligible Intent-to-Treat Patients.
| Characteristic | Dosage of Ipilimumab, No. (%) | ||
|---|---|---|---|
| All (N = 32) | 3 mg/kg Every 3 wk (n = 3) | 10 mg/kg Every 3 wk (n = 29) | |
| Age group, y | |||
| 20-29 | 1 (3) | 0 | 1 (3) |
| 30-39 | 8 (25) | 0 | 8 (28) |
| 40-49 | 8 (25) | 3 (100) | 5 (17) |
| 50-59 | 14 (44) | 0 | 14 (48) |
| 60-69 | 1 (3) | 0 | 1 (3) |
| Ethnicity | |||
| Hispanic or Latino | 8 (25) | 2 (67) | 6 (21) |
| Non-Hispanic | 24 (75) | 1 (33) | 23 (79) |
| Race | |||
| White | 22 (69) | 3 (100) | 19 (66) |
| Black or African American | 5 (16) | 0 | 5 (17) |
| Asian | 2 (6) | 0 | 2 (7) |
| American Indian or Alaskan Native | 1 (3) | 0 | 1 (3) |
| Other | 1 (3) | 0 | 1 (3) |
| Unknown | 1 (3) | 0 | 1 (3) |
| Performance statusa | |||
| 0 | 20 (63) | 1 (33) | 19 (66) |
| 1 | 12 (38) | 2 (67) | 10 (34) |
| Cell type | |||
| Adenocarcinoma, unspecified | 3 (9) | 1 (33) | 2 (7) |
| Adenosquamous | 1 (3) | 1 (33) | 0 |
| Squamous cell carcinoma | 28 (88) | 1 (33) | 27 (93) |
| Cancer stage | |||
| 1B2 | 4 (13) | 0 | 4 (14) |
| 2A | 4 (13) | 0 | 4 (14) |
| 2B | 16 (50) | 1 (33) | 15 (52) |
| 3B | 7 (22) | 2 (67) | 5 (17) |
| 4A | 1 (3) | 0 | 1 (3) |
| Lymph nodes | |||
| Negative | 1 (3) | 0 | 1 (3) |
| Only pelvic positive | 19 (59) | 2 (67) | 17 (59) |
| Only para-aortic positive | 2 (6) | 0 | 2 (7) |
| Both pelvic and para-aortic positive | 9 (28) | 0 | 9 (31) |
| Not evaluated | 1 (3) | 1 (33) | 0 |
| Measurable disease | 32 (100) | 3 (100) | 29 (100) |
Performance status was defined according to the Eastern Oncology Cooperative Group Performance Scale, in which scores range from 0 to 2. A score of 0 indicates fully active, able to carry on all predisease performance without restriction; 1, restricted in physically strenuous activity but ambulatory and able to carry out work of a light or sedentary nature (eg, light housework, office work); and 2, ambulatory and capable of all self-care but unable to carry out any work activities; up and about more than 50% of waking hours.
Adverse Events and Outcomes
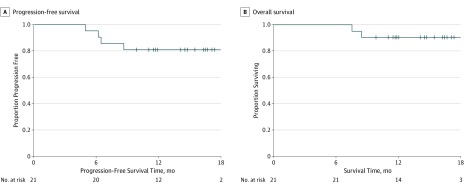
All patients completed CRT, with 21 patients completing at least 2 cycles of ipilimumab: 18 (86%) completed 4 cycles of ipilimumab and 3 (14%) completed 2 cycles, and there were no dose reductions. The reasons that the 3 patients completed 2 cycles were grade 3 dermatitis, grade 3 lipase increase, and withdrawal of consent for treatment (1 each). No DLTs occurred in 3 patients receiving ipilimumab at a dose of 3 mg/kg every 3 weeks, and none were observed in 5 patients receiving ipilimumab at a dose of 10 mg/kg every 3 weeks; the latter dose was then identified as the maximum tolerated dose. Two DLTs occurred in 2 of the 13 patients in the feasibility portion: a grade 3 lipase increase and a grade 3 dermatitis in cycle 2 of ipilimumab. Both DLTs were self-limiting without further complications. One patient had a delay in cycle 4 of ipilimumab owing to a decreased absolute neutrophil count, which was self-limiting. Most of the acute toxic effects were grade 1 or 2 diarrhea, dermatitis, and endocrinopathies, with 1 grade 3 gastrointestinal tract toxic effect of abdominal pain. One patient receiving a dose of 3 mg/kg experienced a grade 3 cognitive disturbance after 1 cycle of ipilimumab, with negative results on magnetic resonance imaging of the brain, that resolved within 1 week. With a median follow-up of 14.8 months, there were no grade 4 or 5 adverse events. Grade 3 and 4 adverse events occurring during the ipilimumab therapy are listed in eTable 1 in the Supplement. Based on Kaplan-Meier estimates, 12-month PFS was 81% (Figure 3A) and 12-month OS was 90% (Figure 3B); medians of neither end point have been reached.
Figure 3. Progression-Free and Overall Survival in Patients Receiving 2 or More Cycles of Ipilimumab.
Cervical swab samples for DNA testing were collected from each patient before any treatment was administered. Twenty of the 21 patient samples (95%) were confirmed to be HPV positive, and 1 HPV evaluation was inconclusive. All samples were genotyped; of the 21 samples, 11 (52%) were HPV-16, 3 (14%) were HPV-18, and 7 (34%) were determined to be another high-risk HPV type. Six patients tested positive for more than 1 HPV genotype, including 2 low-risk genotypes. Major histocompatibility HLA-A*0201 allele status was determined at baseline. Eight patients (38%) carried the HLA-A*0201 allele, which is consistent with published frequencies of this particular allele in the general population. In addition, an analysis relating the HPV and HLA genotype was performed to both PFS and OS using Cox proportional hazards regression.29 There was no statistically significant difference in PFS (eTable 2 in the Supplement) or OS (eTable 3 in the Supplement).
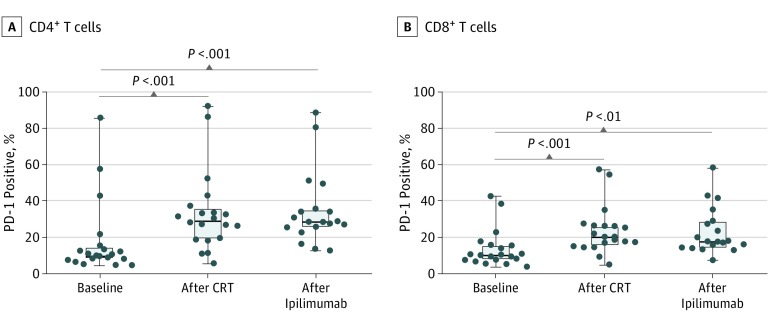
Multicolor flow cytometry on peripheral lymphocytes revealed a significant increase in PD-1 expression on both CD4+ and CD8+ T cells after CRT (median CD4 expression, 29.1; 95% CI, 22.7-43.5; median CD8 expression, 20.1; 95% CI, 17.1-29.3) compared with baseline (median CD4 expression, 10.0; 95% CI, 7.8-27.3; median CD8 expression, 9.9; 95% CI, 8.5-18.2), and this increase was sustained throughout ipilimumab treatment (median CD4 expression, 28.3; 95% CI, 24.8-44.4; median CD8 expression, 17.5; 95% CI, 16.8-29.3), as shown in Figure 4. This expression did not correlate with survival outcomes (eTable 4 and eTable 5 in the Supplement).
Figure 4. Expression of Programmed Cell Death 1 (PD-1) After Chemoradiotherapy (CRT) and Ipilimumab Administration.
Peripheral blood lymphocytes were phenotyped by multicolor flow cytometry for T-cell activation markers. Both CD4+ and CD8+ T cells were associated with significantly increased expression of PD-1 compared with baseline, and the percentage of PD-1–positive cells was sustained throughout the 12 weeks of ipilimumab treatment. Boxplots show 25th to 75th percentiles, with median (horizontal line in the box) PD-1 expression in all patients (solid circles) with evaluable data. Whiskers indicate minimum and maximum values.
Discussion
To our knowledge, this phase 1 study is the first to show tolerability with a signal of efficacy of an immune checkpoint inhibitor, anti–CTLA-4, as a part of the definitive treatment of locally advanced cervical cancer. Our data suggest that sequential ipilimumab treatment after standard CRT was feasible with few grade 3 adverse effects, all of which were self-limiting. This study used extended-field RT, 3-dimensional conformal RT, which encompasses higher bowel volume and dose than pelvic RT or contemporary intensity-modulated RT.30 Furthermore, our study highlights the immune regulation of CRT for patients with cervical cancer. We found that PD-1 expression was increased during CRT and sustained with sequential immunotherapy, serving as a translational platform for future strategies.
The patients with evaluable data had a high risk of recurrence because all had pelvic lymph nodes and 29% had PALNs. The patients whose cancer recurred within 1 year experienced widespread disease. With historical 1-year PFS of 63% and OS of 80% in the GOG 0125 study, a study of patients with PALN-positive cervical cancer similar to our patient population, the 1-year PFS rate of 81% and OS rate of 90% seen in the present study are promising. Because the patients in the GOG 0125 study were all PALN positive, we would anticipate that our outcome results may be better. Furthermore, based on historical survival curves in cervical cancer,2 most treatment failures occur within the first 1 to 2 years after initiation of therapy, suggesting that, with longer follow-up, our data could show potential durable responses in these patients that are characteristic of therapy with immune checkpoint inhibitors.28
Although this study is the first prospective clinical trial, to our knowledge, reported for locally advanced cervical cancer using CRT and anti–CTLA-4 therapy, data have been reported for immunotherapy in patients with metastatic cervical cancer. A phase 1/2 study evaluated ipilimumab therapy among patients with recurrent and metastatic cervical cancer and showed that, among 34 patients with evaluable data, 3 (9%) had a partial response, 8 (24%) had stable disease, and 23 (67%) had progressive disease, with a median PFS of 2.5 months (95% CI, 2.3-3.2 months).31 In addition to anti–CTLA-4 studies in cervical cancer, anti–PD-1 immunotherapy for cervical cancer has demonstrated efficacy in metastatic disease. Results of the cervical cancer cohort in the phase 2 KEYNOTE-158 (ClinicalTrials.gov identifier: NCT01711515) study showed an overall response rate of 13.3% (95% CI, 7.3%-21.6%), with 3 patients exhibiting complete response and 10 exhibiting partial response to treatment.32 All responses were in programmed cell death 1 ligand (PD-L1)–positive tumors, with an overall response rate in this group of 16.0% (95% CI, 8.8%-25.9%).32 Median PFS was 2.1 months and mean OS was 9.4 months.32 Treatment-related adverse effects occurred in 65.3% of patients, of whom 11.2% had treatment-related grade 3 or 4 adverse effects.32
There are limited data surrounding the optimal RT dose and fractionation schedule to evoke an ideal immune response when combined with anti–CTLA-4 immunotherapy. Radiotherapy and immunotherapy have promise for synergistic function to decrease tumor burden and potentially add to a long-term durable response.33 Ionizing radiation acts on the immune system by altering the microenvironment, inducing immunogenic cell death, increasing effector T-cell homing and priming, and allowing for the personalized antitumor vaccine effect via the release of neoantigens during radiotherapy.33,34,35
We evaluated the HPV genotype of our patients as well as HLA-A*0201 status to investigate whether these tumor characteristics were associated with clinical response. Although 67% (14 of 21) of our patient population was either HPV-16 positive or HPV-18 positive, we did not observe any statistical correlation between HPV genotype or HLA-A*0201 status with PFS or OS, likely owing to the low number of patients whose disease progressed within the 1-year observation period. The PD-1 expression that was increased on T cells during CRT suggests immune activation with radiotherapy. This finding is hypothesis generating and may not correlate with PD-1/PD-L1 expression in the tumor, which we were not able to assess in this study because there was no requirement for biopsy either before treatment or after CRT. In a recent report by Lheureux et al,31 patients with metastatic cervical cancer treated with ipilimumab after measurable disease or progression after platinum chemotherapy had an increase in PD-1 expression on peripheral lymphocyte levels during treatment. In our study, the PD-1 expression seen after CRT and ipilimumab therapy could contribute to immune exhaustion or treatment resistance, which could be a potential target for novel therapies in node-positive cervical cancer. The small number of patients whose disease progressed during this phase 1 study precludes the ability to correlate peripheral blood PD-1 expression with outcomes. Based on the findings of increased PD-1 expression in the frontline curative setting of cervical cancer, the NRG Oncology Group is exploring the PD-1 and PD-L1 axis in Atezolizumab Before and/or With Chemoradiotherapy in Immune System Activation in Patients With Node Positive Stage IB2, II, IIIB, or IVA Cervical Cancer (NCT03738228), an ongoing study of CRT in definitive cervical cancer with atezolizumab as an immune primer or concurrently with CRT.
Limitations
The study limitations include a high attrition rate, all owing to reasons unrelated to and occurring before administration of the study drug. Although immune checkpoint blockade efficacy has been shown in various disease sites, characteristics of patients with cervical cancer who are likely to respond to anti–CTLA-4 immune checkpoint immunotherapy remain largely unknown and need further study. Furthermore, at the time of the design of the study, IMRT was not permitted owing to an inability to compare RT treatment with historical GOG data, difficulty in pretreatment IMRT radiation quality assurance, and concern for potential toxic effect outcome differences between subgroups treated with IMRT. Since that time, with the input of NRG leadership and the NRG RT and cervical cancer committees, IMRT is now a standard offering on definitive cervical cancer trials with a predetermined workflow for quality assurance and RT plan review. Additional detailed analyses on the peripheral blood immune phenotypes, immune regulation, and viral antigen–specific T-cell responses from this study are forthcoming.
Conclusions
Our findings show promise for the use of immunotherapy in the definitive setting of locally advanced, node-positive cervical cancer; patients with this cancer historically have a poor prognosis with standard therapy alone. The use of ipilimumab therapy sequentially after CRT was tolerable and effective. Programmed cell death 1 expression was increased after CRT and sustained with ipilimumab therapy. Furthermore, the use of an immune checkpoint inhibitor could stimulate the antitumor activity of tumor-specific cytotoxic T cells and augment radiation-induced neoantigen load. Our clinical data suggest a high PFS and OS at 1 year for these high-risk patients. Our study is potentially transformative in the standard treatment schema of locally advanced cervical cancer, with the prospect for immuno-oncology to add durable survival in patients with node-positive disease, a current unmet oncologic need.36
eTable 1. Grade ≥3 Treatment-Related Adverse Events During Ipilimumab
eTable 2. Analysis Relating HPV Genotype and HLA-A*0201 Status Variables to PFS
eTable 3. Analysis Relating HPV Genotype and HLA-A*0201 Status Variables to OS
eTable 4. Analysis Relating PBMC PD-1 Expression Change From Baseline to Post-CRT to PFS
eTable 5. Analysis Relating PBMC PD-1 Expression Change From Baseline to Post-CRT to OS
References
- 1.Cancer C. Cervical cancer. NIH Consens Statement. 1996;14(1):1-38. [PubMed] [Google Scholar]
- 2.Rose PG, Bundy BN, Watkins EB, et al. Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med. 1999;340(15):1144-1153. doi: 10.1056/NEJM199904153401502 [DOI] [PubMed] [Google Scholar]
- 3.Varia MA, Bundy BN, Deppe G, et al. Cervical carcinoma metastatic to para-aortic nodes: extended field radiation therapy with concomitant 5-fluorouracil and cisplatin chemotherapy: a Gynecologic Oncology Group study. Int J Radiat Oncol Biol Phys. 1998;42(5):1015-1023. doi: 10.1016/S0360-3016(98)00267-3 [DOI] [PubMed] [Google Scholar]
- 4.Randall LM, Monk BJ, Darcy KM, et al. Markers of angiogenesis in high-risk, early-stage cervical cancer: a Gynecologic Oncology Group study. Gynecol Oncol. 2009;112(3):583-589. doi: 10.1016/j.ygyno.2008.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88(1):63-73. doi: 10.1038/sj.bjc.6600688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muñoz N, Bosch FX, de Sanjosé S, et al. ; International Agency for Research on Cancer Multicenter Cervical Cancer Study Group . Epidemiologic classification of human papillomavirus types associated with cervical cancer. N Engl J Med. 2003;348(6):518-527. doi: 10.1056/NEJMoa021641 [DOI] [PubMed] [Google Scholar]
- 7.Cogliano V, Baan R, Straif K, Grosse Y, Secretan B, El Ghissassi F; WHO International Agency for Research on Cancer . Carcinogenicity of human papillomaviruses. Lancet Oncol. 2005;6(4):204. doi: 10.1016/S1470-2045(05)70086-3 [DOI] [PubMed] [Google Scholar]
- 8.Munagala R, Donà MG, Rai SN, et al. Significance of multiple HPV infection in cervical cancer patients and its impact on treatment response. Int J Oncol. 2009;34(1):263-271. [PubMed] [Google Scholar]
- 9.Bachtiary B, Obermair A, Dreier B, et al. Impact of multiple HPV infection on response to treatment and survival in patients receiving radical radiotherapy for cervical cancer. Int J Cancer. 2002;102(3):237-243. doi: 10.1002/ijc.10708 [DOI] [PubMed] [Google Scholar]
- 10.Kaliff M, Sorbe B, Mordhorst LB, Helenius G, Karlsson MG, Lillsunde-Larsson G. Findings of multiple HPV genotypes in cervical carcinoma are associated with poor cancer-specific survival in a Swedish cohort of cervical cancer primarily treated with radiotherapy. Oncotarget. 2018;9(27):18786-18796. doi: 10.18632/oncotarget.24666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burger RA, Monk BJ, Kurosaki T, et al. Human papillomavirus type 18: association with poor prognosis in early stage cervical cancer. J Natl Cancer Inst. 1996;88(19):1361-1368. doi: 10.1093/jnci/88.19.1361 [DOI] [PubMed] [Google Scholar]
- 12.Qin Y, Ekmekcioglu S, Forget M-A, et al. Cervical cancer neoantigen landscape and immune activity is associated with human papillomavirus master regulators. Front Immunol. 2017;8:689. . doi: 10.3389/fimmu.2017.00689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366(26):2443-2454. doi: 10.1056/NEJMoa1200690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharabi AB, Lim M, DeWeese TL, Drake CG. Radiation and checkpoint blockade immunotherapy: radiosensitisation and potential mechanisms of synergy. Lancet Oncol. 2015;16(13):e498-e509. doi: 10.1016/S1470-2045(15)00007-8 [DOI] [PubMed] [Google Scholar]
- 15.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11(2, pt 1):728-734. [PubMed] [Google Scholar]
- 16.Slovin SF, Higano CS, Hamid O, et al. Ipilimumab alone or in combination with radiotherapy in metastatic castration-resistant prostate cancer: results from an open-label, multicenter phase I/II study. Ann Oncol. 2013;24(7):1813-1821. doi: 10.1093/annonc/mdt107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang C, Welsh JW, de Groot P, et al. Ipilimumab with stereotactic ablative radiation therapy: phase I results and immunologic correlates from peripheral T cells. Clin Cancer Res. 2017;23(6):1388-1396. doi: 10.1158/1078-0432.CCR-16-1432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kiess AP, Wolchok JD, Barker CA, et al. Stereotactic radiosurgery for melanoma brain metastases in patients receiving ipilimumab: safety profile and efficacy of combined treatment. Int J Radiat Oncol Biol Phys. 2015;92(2):368-375. doi: 10.1016/j.ijrobp.2015.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kwon ED, Drake CG, Scher HI, et al. ; CA184-043 Investigators . Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700-712. doi: 10.1016/S1470-2045(14)70189-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stone HB, Peters LJ, Milas L. Effect of host immune capability on radiocurability and subsequent transplantability of a murine fibrosarcoma. J Natl Cancer Inst. 1979;63(5):1229-1235. [PubMed] [Google Scholar]
- 21.Lee Y, Auh SL, Wang Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114(3):589-595. doi: 10.1182/blood-2009-02-206870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dovedi SJ, Lipowska-Bhalla G, Beers SA, et al. Antitumor efficacy of radiation plus immunotherapy depends upon dendritic cell activation of effector CD8+ T cells. Cancer Immunol Res. 2016;4(7):621-630. doi: 10.1158/2326-6066.CIR-15-0253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Deng L, Liang H, Burnette B, et al. Irradiation and anti–PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124(2):687-695. doi: 10.1172/JCI67313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hallahan D, Kuchibhotla J, Wyble C. Cell adhesion molecules mediate radiation-induced leukocyte adhesion to the vascular endothelium. Cancer Res. 1996;56(22):5150-5155. [PubMed] [Google Scholar]
- 25.Pecorelli S. Revised FIGO staging for carcinoma of the vulva, cervix, and endometrium. Int J Gynaecol Obstet. 2009;105(2):103-104. doi: 10.1016/j.ijgo.2009.02.012 [DOI] [PubMed] [Google Scholar]
- 26.Nishino M, Giobbie-Hurder A, Gargano M, Suda M, Ramaiya NH, Hodi FS. Developing a common language for tumor response to immunotherapy: immune-related response criteria using unidimensional measurements. Clin Cancer Res. 2013;19(14):3936-3943. doi: 10.1158/1078-0432.CCR-13-0895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE), Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf. Published November 7, 2017. Accessed March 09, 2018.
- 28.Sharma P, Allison JP. The future of immune checkpoint therapy. Science. 2015;348(6230):56-61. doi: 10.1126/science.aaa8172 [DOI] [PubMed] [Google Scholar]
- 29.Cox DR. Regression models and life tables (with discussion). J R Stat Soc B. 1972;34(2):187-219. [Google Scholar]
- 30.Mell LK, Sirák I, Wei L, et al. ; INTERTECC Study Group . Bone marrow-sparing intensity modulated radiation therapy with concurrent cisplatin for stage IB-IVA cervical cancer: an international multicenter phase II clinical trial (INTERTECC-2). Int J Radiat Oncol Biol Phys. 2017;97(3):536-545. doi: 10.1016/j.ijrobp.2016.11.027 [DOI] [PubMed] [Google Scholar]
- 31.Lheureux S, Butler MO, Clarke B, et al. Association of ipilimumab with safety and antitumor activity in women with metastatic or recurrent human papillomavirus-related cervical carcinoma. JAMA Oncol. 2018;4(7):e173776. doi: 10.1001/jamaoncol.2017.3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chung HC Ros W, Delord J-P, et al. Pembrolizumab treatment of advanced cervical cancer: updated results from the phase 2 KEYNOTE-158 study [abstract 5522]. J Clin Oncol. 2018;36(15)(suppl):5522. doi: 10.1200/JCO.2018.36.15_suppl.5522 [DOI] [Google Scholar]
- 33.Smith CA, Freeman ML. Preclinical advances in combined-modality cancer immunotherapy with radiation therapy. Int J Radiat Oncol Biol Phys. 2016;94(1):11-14. doi: 10.1016/j.ijrobp.2015.07.2282 [DOI] [PubMed] [Google Scholar]
- 34.Demaria S, Golden EB, Formenti SC. Role of local radiation therapy in cancer immunotherapy. JAMA Oncol. 2015;1(9):1325-1332. doi: 10.1001/jamaoncol.2015.2756 [DOI] [PubMed] [Google Scholar]
- 35.Formenti SC. The pace of progress in radiation and immunotherapy. Int J Radiat Oncol Biol Phys. 2016;95(4):1257-1258. doi: 10.1016/j.ijrobp.2016.02.063 [DOI] [PubMed] [Google Scholar]
- 36.Ben-Aharon O, Magnezi R, Leshno M, Goldstein DA. Association of immunotherapy with durable survival as defined by value frameworks for cancer care. JAMA Oncol. 2018;4(3):326-332. doi: 10.1001/jamaoncol.2017.4445 doi: 10.1001/jamaoncol.2017.4445 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Grade ≥3 Treatment-Related Adverse Events During Ipilimumab
eTable 2. Analysis Relating HPV Genotype and HLA-A*0201 Status Variables to PFS
eTable 3. Analysis Relating HPV Genotype and HLA-A*0201 Status Variables to OS
eTable 4. Analysis Relating PBMC PD-1 Expression Change From Baseline to Post-CRT to PFS
eTable 5. Analysis Relating PBMC PD-1 Expression Change From Baseline to Post-CRT to OS