Key Points
Question
What morbidities are associated with postnatal cytomegalovirus infection at discharge in infants with very low birth weight?
Findings
In this multicenter cohort study of 304 infants with very low birth weight and postnatal cytomegalovirus infection, increased risks were found for failed hearing screen and bronchopulmonary dysplasia, an increased postnatal age at discharge, and decreased weight for length at discharge compared with control infants without infection.
Meaning
These findings suggest that postnatal cytomegalovirus infection is associated with significant morbidity at discharge; prospective, long-term studies are needed to determine the full effects of postnatal cytomegalovirus infection.
This multicenter cohort study assesses the risks for failed hearing screen, increased postnatal age at discharge, and decreased growth at discharge in infants with very low birth weight and postnatal cytomegalovirus infection.
Abstract
Importance
Studies suggest that postnatal cytomegalovirus (CMV) infection can lead to long-term morbidity in infants with very low birth weight (VLBW; <1500 g), including bronchopulmonary dysplasia (BPD), necrotizing enterocolitis (NEC), and neurodevelopmental impairment. However, to date, the association of postnatal CMV with hearing, growth, and length of stay among VLBW infants is unknown.
Objectives
To determine the risk for failed hearing screen, increased postnatal age at discharge, or decreased growth at discharge in VLBW infants with postnatal CMV infection compared with CMV-uninfected infants and to compare the risk for other major outcomes of prematurity, including BPD and NEC, in infants with and without postnatal CMV infection.
Participants
This multicenter retrospective cohort study included VLBW infants from 302 neonatal intensive care units managed by the Pediatrix Medical Group from January 1, 2002, through December 31, 2016. Infants hospitalized on postnatal day 21 with a diagnosis of postnatal CMV and hearing screen results after a postmenstrual age of 34 weeks were included in the study population. Data were analyzed from December 11, 2017, to June 14, 2019.
Main Outcomes and Measures
Infants with and without postnatal CMV infection were matched using propensity scores. Poisson and linear regression were used to examine the association between postnatal CMV and the risk of failed hearing screen, postnatal age at discharge, growth, BPD, and NEC.
Results
A total of 304 infants with postnatal CMV were identified, and 273 of these infants (89.8%; 155 boys [56.8%]) were matched with 273 infants without postnatal CMV (148 boys [54.2%]). Hearing screen failure occurred in 45 of 273 infants (16.5%) with postnatal CMV compared with 25 of 273 infants (9.2%) without postnatal CMV (risk ratio [RR], 1.80; 95% CI, 1.14 to 2.85; P = .01). Postnatal CMV was also associated with an increased postnatal age at discharge of 11.89 days (95% CI, 6.72 to 17.06 days; P < .001) and lower weight-for-age z score (−0.23; 95% CI, −0.39 to −0.07; P = .005). Analysis confirmed an increased risk of BPD (RR, 1.30; 95% CI, 1.17 to 1.44; P < .001), previously reported on infants from this cohort from 1997 to 2012, but not an increased risk of NEC after postnatal day 21 (RR, 2.00; 95% CI, 0.18 to 22.06; P = .57).
Conclusions and Relevance
These data suggest that postnatal CMV infection is associated with lasting sequelae in the hearing and growth status of VLBW infants and with prolonged hospitalization. Prospective studies are needed to determine the full effects of postnatal CMV infection and whether antiviral treatment reduces the associated morbidity.
Introduction
Cytomegalovirus (CMV) is the leading infectious cause of developmental impairment and the leading nongenetic cause of hearing loss in the developed world.1,2,3,4 Infection occurs in utero (congenital infection) or via mucosal exposure to CMV, frequently through virus shed in breast milk (postnatal infection).5,6,7 Historically, CMV was transmitted to hospitalized infants through blood transfusions, but the use of CMV-seronegative or leukoreduced blood has significantly reduced this mode of transmission.5,8 Exposure to breast milk from CMV-infected mothers is now the most common means of transmission.5 Although harmless in full-term immunocompetent infants, postnatal CMV infection in infants with very low birth weight (VLBW) (<1500 g) can result in a severe sepsislike illness characterized by pneumonitis, enteritis, hepatitis, and thrombocytopenia and may lead to long-term neurological impairment.9,10,11
Historically it was thought that, as in term infants,12 preterm infants who survive postnatal CMV infection have no long-term sequelae. However, more recent studies10,13 suggest that this may not be the case. A prospective study14 that followed up infants with and without a diagnosis of postnatal CMV reported no differences in neurodevelopmental outcomes at 2.0 to 4.5 years of age. However, when the same population was followed up longer, significant differences in neurodevelopment were observed starting at 6 years of age.10 In addition, a study that used the Pediatrix Medical Group Database13 indicated that VLBW infants with postnatal CMV had an increased risk of bronchopulmonary dysplasia (BPD) at a postmenstrual age (PMA) of 36 weeks when compared with matched CMV-uninfected counterparts. Other studies15,16,17,18,19,20 have shown a variable association of postnatal CMV with BPD and neurodevelopmental impairment in preterm infants, with some studies finding a correlation and others not. Finally, several studies5,21,22 report necrotizing enterocolitis (NEC) in preterm infants after postnatal CMV. In these studies, it is unclear whether CMV causes intestinal damage and NEC itself or leads to immunologic or inflammatory changes that make preterm infants more susceptible to NEC.
Whether postnatal CMV infection in preterm infants should be treated or actively prevented remains controversial because the full extent of morbidity and whether treatment reduces morbidity are unknown. The objective of our study was to further define the morbidities associated with recognized postnatal CMV infection of VLBW infants at hospital discharge. Defining the full scope of morbidity associated with postnatal CMV will provide valuable data to support a large-scale clinical trial to determine whether treatment improves outcomes in this population.
Methods
Study Population
A retrospective cohort study using a clinical database of infants from neonatal intensive care units (NICUs) managed by the Pediatrix Medical Group from January 1, 2002, through December 31, 2016, was conducted. Physicians generate infant data daily from admission history and physical examinations, progress notes, and discharge summaries with administered medications, limited dosing information, and diagnoses available. Data are extracted and consolidated into the Pediatrix BabySteps Clinical Data Warehouse.23 This study was approved by the Duke University institutional review board, who waived informed consent because all data were deidentified. The study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Infants included weighed less than 1500 g at birth, were hospitalized on postnatal day 21, and had hearing screen results after PMA of 34 weeks. Infants were considered to have congenital CMV and excluded from the study if they had detection of CMV by culture or polymerase chain reaction analysis or diagnosis of CMV infection, acquired CMV infection, or congenital CMV infection before postnatal day 21. The study population included infants who did not undergo testing and those who had negative test results for CMV.
Postnatal CMV Group
Postnatal CMV was defined as a diagnosis of CMV infection, congenital CMV, acquired CMV infection, or detection of CMV by culture or polymerase chain reaction analysis of blood, urine, cerebrospinal fluid, or respiratory tract secretions on or after postnatal day 21.13 Infants with a diagnosis of congenital CMV after postnatal day 21 but without positive virologic test results before postnatal day 21 were included in the analysis in the postnatal CMV group. We assumed these infants were misclassified as having congenital CMV because postnatal CMV is not a diagnosis code. These infants were later excluded from the postnatal CMV group in the sensitivity analysis. Infants were excluded from the postnatal CMV group if they had a diagnosis of intracranial calcifications or microcephaly, treatment with ganciclovir sodium, valganciclovir hydrochloride, cidofovir, or foscarnet sodium before postnatal day 21, or transaminitis (aspartate aminotransferase levels >150 U/L and alanine aminotransferase levels >90 U/L [to convert to microkatals per liter, multiply by 0.0167]) before postnatal day 21. These criteria were selected to eliminate infants with probable congenital rather than postnatal CMV.
Non–Postnatal CMV Group
Infants without postnatal CMV were selected from all VLBW infants hospitalized on postnatal day 21 who met the inclusion and exclusion study criteria but did not meet criteria for postnatal CMV. This group includes infants who did not undergo testing and those who had test findings negative for CMV.
Propensity Score–Matched Groups
Because the outcomes measured are affected by many different factors, propensity score matching was used to obtain similar populations with similar exposures for comparison.13 Comorbidities, surrogates for severity of illness, and exposures were assessed on postnatal day 21. In addition, risk factors for hearing loss, NEC, and BPD were assessed. The following variables were included in a logistic regression model to generate propensity scores: gestational age, birth weight, small for gestational age status, race/ethnicity, discharge year, NICU site, use of antenatal corticosteroids, days of breast milk exposure from postnatal days 15 to 21, days of aminoglycoside (gentamicin sulfate, tobramycin, and amikacin sulfate) and loop diuretic (furosemide and bumetanide) exposure, NEC, grade 3 or 4 intraventricular hemorrhage, patent ductus arteriosus, sepsis occurring on or before postnatal day 21, vasopressor medications (amrinone lactate, dobutamine hydrochloride, dopamine hydrochloride, epinephrine, milrinone lactate, and norepinephrine bitartrate), type of respiratory support, and fraction of inspired oxygen on postnatal day 21.24 Discharge year and NICU site were included as categorical variables to account for changes and differences in clinical practice and outcomes. Infants were matched 1:1 on the propensity score using nearest-neighbor matching, provided the score difference was less than 0.01.25
Definitions of Propensity Score Covariates
Small for gestational age status was defined as birth weight below the tenth percentile for gestational age based on Olsen growth curves.26 Race/ethnicity was assigned as entered by the treating physician. Sepsis was defined as isolation of at least 1 bacterial or fungal pathogen from blood. Likely bacterial contaminants, including nonspeciated streptococci, Bacillus species, Corynebacterium species, and Micrococcus species, were excluded. We included definite and probable coagulase-negative staphylococcus infections as previously described.27
Primary Outcomes
Failed Hearing Screen
The final hearing screen result after PMA of 34 weeks was used to define hearing screen status (ie, if an infant failed and then passed the test, the result was counted as passed). If 2 hearing screen results from the same day had disparate results, the passing result was taken.
Postnatal Age at Discharge
Postnatal age at discharge was defined as the age (in days) on the day of first discharge from the NICU. Postnatal age at discharge was used as a surrogate for length of stay (LOS) because LOS (discharge age minus admission age) is falsely low for infants born elsewhere and transferred for ongoing care. Postmenstrual age at discharge was defined as PMA on the day of first discharge from the NICU. Discharge could include transfer or death that occurred after 34 weeks of PMA.
Anthropomorphic Measurements
Weight (in kilograms), recumbent length (in centimeters), and head circumference (in centimeters) were measured at 40 weeks’ PMA or discharge, whichever occurred first. If a length or head circumference was not available for that day, the value for the previous day was used. Growth parameters were converted to z scores based on the Olsen growth curves.26
Secondary Outcomes
Bronchopulmonary dysplasia was defined in infants with a gestational age of less than 32 weeks as receiving respiratory support (nasal cannula, continuous positive airway pressure, or mechanical ventilation) continuously from PMA of 36 weeks and 0 days to 36 weeks and 6 days and in infants with a gestational age of at least 32 weeks receiving supplemental oxygen or respiratory support from a postnatal age of 28 to 34 days.28 Necrotizing enterocolitis was defined as a diagnosis of medical or surgical NEC by the physician. Necrotizing enterocolitis that occurred before postnatal day 21 was included as a covariate in the propensity score.
Statistical Analysis
Data were analyzed from December 11, 2017, to June 14, 2019. Poisson regression with a sandwich variance estimator (categorical variables)29 or linear regression (continuous variables) conditioning on the matched infant pairs was used to determine the association between postnatal CMV and the risk of outcomes in the propensity score–matched cohort. The choice of Poisson regression, as opposed to logistic regression, was appropriate because the odds ratio obtained using logistic regression is an upwardly biased estimate of the risk ratio (RR) when the outcome is not rare (>10%), as was the case for our outcomes of failed hearing screen and BPD.30 Histograms for each outcome were generated to confirm a normal distribution. We evaluated the balance of the 2 matched groups by comparing counts and percentages for covariates used to determine the propensity scores. We calculated the standardized percentage bias, defined as the percentage difference of the sample means in the postnatal CMV and non–postnatal CMV groups, as a percentage of the square root of the average of the sample variances in the CMV and non–postnatal CMV groups.25 We performed a sensitivity analysis excluding infants given the diagnosis of congenital CMV after postnatal day 21, before propensity score matching. Statistical analyses were performed using Stata, version 15.1 (StataCorp, LLC). All statistical tests were 2-sided, and P < .05 was considered statistically significant.
Results
Study Population
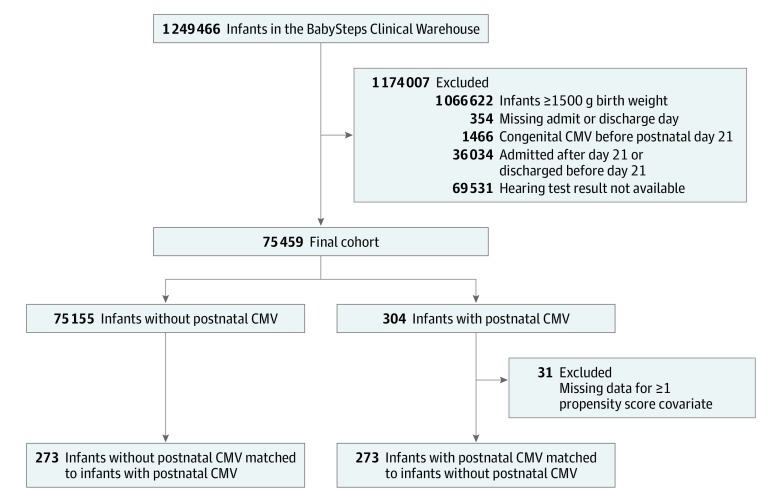
We identified 75 459 VLBW infants from 302 sites who met the inclusion criteria; 304 (0.4%) met criteria for postnatal CMV infection (Figure). In total, 229 (75.3%) met criteria based on virologic testing results and diagnosis coding; 15 (4.9%), based on virologic testing alone; and 56 (18.4%), based on diagnostic coding alone. Infants meeting criteria on diagnostic coding alone could have had missing laboratory data or a positive sample type not included in our analysis. The specimens with positive test results for CMV included urine (211 [85.1%]), blood (14 [5.7%]), tracheal (7 [2.8%]), cerebrospinal fluid (4 [1.6%]), and nasopharyngeal (12 [4.8%]). We matched 273 infants with postnatal CMV infection (89.8%; 155 boys [56.8%] and 118 girls [43.2%]) with 273 infants without postnatal CMV (148 boys [54.2%] and 125 girls [45.8%]). The other 31 infants with postnatal CMV were excluded owing to missing data for at least 1 covariate for the propensity score. The 546 infants in the propensity-matched cohort were identified at 66 NICU sites and had similar baseline characteristics (Table 1 and eFigure in the Supplement). One infant died in the postnatal CMV group (1 of 273 [0.4%]), and no infants died in the non–postnatal CMV group.
Figure. Study Cohort Flow Diagram.
CMV indicates cytomegalovirus.
Table 1. Covariates Used for Propensity Score Matching of Preterm Infants.
| Covariate | Infant Groupa | |
|---|---|---|
| Non–Postnatal CMV (n = 273) | Postnatal CMV (n = 273) | |
| Use of antenatal corticosteroids | 242 (88.6) | 243 (89.0) |
| Gestational age, wk | ||
| ≤24 | 99 (36.3) | 104 (38.1) |
| 25-28 | 164 (60.1) | 154 (56.4) |
| 29-31 | 9 (3.3) | 14 (5.1) |
| ≥32 | 1 (0.4) | 1 (0.4) |
| Birth weight, g | ||
| <500 | 16 (5.9) | 22 (8.1) |
| 500-749 | 127 (46.5) | 126 (46.2) |
| 750-999 | 96 (35.2) | 89 (32.6) |
| ≥1000 | 34 (12.5) | 36 (13.2) |
| Male | 148 (54.2) | 155 (56.8) |
| Small for gestational age | 44 (16.1) | 51 (18.7) |
| Race/ethnicity | ||
| White | 112 (41.0) | 107 (39.2) |
| African American | 72 (26.4) | 69 (25.3) |
| Hispanic | 58 (21.3) | 66 (24.2) |
| Other | 31 (11.4) | 31 (11.4) |
| Received diuretic | 86 (31.5) | 98 (35.9) |
| Received >7 d of aminoglycoside therapy | 148 (54.2) | 147 (53.9) |
| Discharge year | ||
| 2002-2005 | 26 (9.5) | 24 (8.8) |
| 2006-2009 | 68 (24.9) | 72 (26.4) |
| 2010-2013 | 118 (43.2) | 112 (41.0) |
| 2014-2016 | 61 (22.3) | 65 (23.8) |
| Comorbidities on or before postnatal day 21 | ||
| NEC | 1 (0.4) | 2 (0.7) |
| PDA | 202 (74.0) | 196 (71.8) |
| Grade 3 or 4 IVH | 26 (9.5) | 28 (10.3) |
| Sepsis | 47 (17.2) | 37 (13.6) |
| Pressor support on postnatal day 21 | 13 (4.8) | 9 (3.3) |
| Respiratory support on postnatal day 21 | ||
| Room air | 6 (2.2) | 6 (2.2) |
| Oxygen, CPAP, or NIPPV | 106 (38.8) | 101 (37.0) |
| Conventional ventilator | 98 (35.9) | 102 (37.4) |
| High-frequency ventilator | 63 (23.1) | 64 (23.4) |
| Fio2 on postnatal day 21, % | ||
| 21 | 55 (20.2) | 48 (17.6) |
| 22-50 | 193 (70.7) | 192 (70.3) |
| 51-100 | 25 (9.2) | 33 (12.1) |
| Breast milk intake from days 15 to 21, d | ||
| 0 | 103 (37.7) | 103 (37.7) |
| 1-6 | 106 (38.8) | 106 (38.8) |
| 7 | 64 (23.4) | 64 (23.4) |
Abbreviations: CMV, cytomegalovirus; CPAP, continuous positive airway pressure; Fio2, fraction of inspired oxygen; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; NIPPV, noninvasive positive pressure ventilation; PDA, patent ductus arteriosus.
Infants in the non–postnatal CMV group could have negative test results for CMV or never underwent testing. Infants in the postnatal CMV group were positive for CMV by diagnosis or virologic test result after postnatal day 21. Unless otherwise indicated, data are expressed as number (percentage) of infants. Percentages have been rounded and may not total 100.
Primary Outcomes
In this matched cohort, 45 of 273 infants (16.5%) with postnatal CMV failed their hearing screen, compared with only 25 of 273 (9.2%) without postnatal CMV (RR, 1.80; 95% CI, 1.14-2.85; P = .01) (Table 2). In addition, postnatal CMV was associated with an increased postnatal age at discharge of 11.89 days (95% CI, 6.72-17.06 days; P < .001) and increased PMA at discharge of 1.52 days (95% CI, 0.82-2.22 days; P < .001) (Table 3). Infants with postnatal CMV also had lower weight-for-age z scores (−0.23; 95% CI, −0.39 to −0.07; P = .005) at PMA of 40 weeks or discharge, but not length-for- age (0.07; 95% CI, −0.32 to 0.46; P = .70) or head circumference–for-age (−0.26 95% CI, −0.62 to 0.11; P = .17) z scores (Table 3). In a sensitivity analysis excluding infants given a diagnosis of congenital CMV after postnatal day 21 (n = 102), the results were similar (eTables 1 and 2 in the Supplement).
Table 2. Categorical Outcomes of VLBW Infants in Propensity Score–Matched Cohort.
| Outcome | Infant Group, No. (%)a | RR (95% CI)b | |
|---|---|---|---|
| Non–Postnatal CMV (n = 273) | Postnatal CMV (n = 273) | ||
| Failed hearing screen | 25 (9.2) | 45 (16.5) | 1.80 (1.14-2.85) |
| BPDc | 166 (61.9) | 218 (79.9) | 1.30 (1.17-1.44) |
| NEC | 1 (0.4) | 2 (0.7) | 2.00 (0.18-22.06) |
Abbreviations: BPD, bronchopulmonary dysplasia; CMV, cytomegalovirus; NEC, necrotizing enterocolitis; RR, risk ratio; VLBW, very low birth weight.
Infants in the non–postnatal CMV group could have negative test results for CMV or never underwent testing. Infants in the postnatal CMV group were positive for CMV by diagnosis or virologic test result after postnatal day 21.
Calculated using conditional Poisson regression.
Diagnosis could not be obtained owing to missing data in 5 infants in the non–postnatal CMV group.
Table 3. Continuous Outcomes of VLBW Infants, Propensity Score-Matched Cohort.
| Outcome | Infant Group, Median (IQR)a | Difference (95% CI)b | |
|---|---|---|---|
| Non–Postnatal CMV | Postnatal CMV | ||
| LOS, d | 102 (85 to 124) | 113 (93 to 133) | 11.89 (6.72 to 17.06) |
| Postmenstrual age, wk | 39.9 (37.9 to 42.6) | 41.1 (39.0 to 43.8) | 1.52 (0.82 to 2.22) |
| Weight, kg | 2.85 (2.42 to 3.14) | 2.82 (2.48 to 3.12) | NA |
| Weight-for-age z score | −0.91 (−1.47 to −0.38) | −1.08 (−1.89 to −0.57) | −0.23 (−0.39 to −0.07) |
| Length, cm | 46.0 (44.0 to 47.5) | 46.0 (44.5 to 48.0) | NA |
| Length-for-age z score | −1.39 (−2.12 to −0.87) | −1.46 (−2.78 to −0.90) | 0.07 (−0.32 to 0.46) |
| Head circumference, cm | 33.0 (32.0 to 34.0) | 33.0 (32.0 to 34.5) | NA |
| Head circumference for age z score | −0.57 (−1.10 to −0.17) | −0.69 (−1.31 to −0.03) | −0.26 (−0.62 to 0.11) |
Abbreviations: CMV, cytomegalovirus; LOS, length of stay; NA, not applicable; VLBW, very low birth weight.
Infants in the non–postnatal CMV group could have negative test results for CMV or never underwent testing. Infants in the postnatal CMV group were positive for CMV by diagnosis or virologic test result after postnatal day 21.
Calculated using conditional linear regression.
Secondary Outcomes
Confirming the previous report,13 postnatal CMV was significantly associated with BPD, including 218 of 273 (79.8%) of those with postnatal CMV and 166 of 268 (61.9%) of those without postnatal CMV (RR, 1.30; 95% CI, 1.17-1.44; P < .001) (Table 2). There was no significant association between postnatal CMV and NEC, which occurred in 1 infant in the non–postnatal CMV group (0.4%) and 2 in the postnatal CMV group (0.7%) (RR, 2.00; 95% CI, 0.18-22.06; P = .57) (Table 2). The results were similar in the sensitivity analysis (eTables 1 and 2 in the Supplement).
Discussion
In this study of preterm infants with postnatal CMV infection, we found that VLBW infants with postnatal CMV have an 80% relative increase in the risk of having a failed hearing screen. In addition, these infants had an increased postnatal age at discharge of almost 2 weeks compared with their matched CMV-uninfected counterparts. Moreover, we found that infants with postnatal CMV had lower weight at PMA of 40 weeks. Finally, this cohort supported the findings of an increased risk for BPD in preterm infants diagnosed with postnatal CMV. To our knowledge, this study is the first to show an association between postnatal CMV and hearing loss, postnatal age at discharge, or growth among preterm infants. Low weight at 40 weeks of PMA and BPD are risk factors for neurodevelopmental impairment, suggesting that postnatal CMV could be an important factor in the neurodevelopmental outcome of preterm infants,31,32,33,34 elevating the importance of understanding the extent that CMV infection is tied to long-term health and development for premature infants.
The incidence of postnatal CMV in this study (0.4%) is much lower than that estimated in a recent meta-analysis,9 which found an infection rate of 6.5%, with 1.4% developing a sepsislike syndrome. In most NICUs, infants are not frequently tested or screened for postnatal CMV. Infants with postnatal CMV in this cohort were likely tested because they developed symptoms consistent with infection. Therefore, the incidence of postnatal CMV in this cohort is likely a portion of those with symptomatic postnatal CMV, may underestimate the true overall incidence, and may only be applicable to symptomatic infections.
Preterm infants are at high risk for hearing impairment and could be at increased risk because of postnatal CMV.35 Congenital CMV is the leading nongenetic cause of sensorineural hearing loss.2 The mechanism of hearing loss after congenital CMV infection is not fully understood, but it is thought that direct viral cytopathic effects and the host immune/inflammatory response play a role in the developing fetus and infant.36,37,38,39,40 Preterm infants with postnatal CMV could be at increased risk of hearing loss by the same mechanisms, especially VLBW infants, who are immunocompromised and have an immature blood-brain barrier.41,42,43 Two small studies found no association between postnatal CMV and hearing loss; however, these studies were limited by small size (22 and 49 infants with postnatal CMV infection), and infants in both studies were identified by prospective screening, not because they were symptomatic.14,20 The findings in the present cohort suggest that hearing impairment could be a sequelae of postnatal CMV that is more likely to occur in infants with symptomatic disease. Infants with symptomatic congenital CMV are also more likely to have long-term sequelae, but most are asymptomatic at birth, so most sensorineural hearing loss in congenital CMV occurs in asymptomatic infants.3,44,45 Future studies are needed to confirm this association and determine whether treatment of postnatal CMV reduces the risk.
Importantly, a failed hearing screen does not necessarily correlate with permanent hearing loss, and we do not have long-term outcomes for these infants. In term infants, the rate of newborn hearing screen failure is approximately 2% to 4%, but less than 1% of all infants will develop hearing loss.46 On the other hand, some infants who pass the newborn hearing screen are subsequently found to have sensorineural hearing loss.47,48 Congenital CMV, in particular, is known to cause progressive hearing loss, and VLBW infants are known to be at a higher risk for progressive hearing loss.35,45,48 Although the high incidence of a failed hearing screen does not directly identify children who will develop hearing failure, the two have a strong association.49
With the clinical symptoms observed in preterm infants with postnatal CMV, it is not surprising these infants have an increased postnatal age and PMA at discharge. In fact, a recent study in California50 found that infants with CMV (without differentiation between postnatal and congenital infection) had increased LOS and mortality. In addition, it is expected that infants will have poor growth during a critical illness, but the decreased weight in these infants was found at discharge. These significant differences are likely multifactorial. Preterm infants with postnatal CMV can develop a severe sepsislike illness that requires increased cardiorespiratory support. This alone can lead to an increased LOS and decreased growth but can also contribute to the development of BPD, which can further increase LOS and decrease growth.51,52,53 In addition, CMV-induced enteritis may lead to further feeding intolerance, poor weight gain, and increased LOS. An increased LOS is not only associated with increased complications and a burden to families,54,55 but it can increase the health care cost by tens of thousands of dollars.56
Several studies13,16,57 have shown an association between postnatal CMV and BPD and suggest that, as with hearing impairment, BPD could be a complication of symptomatic postnatal CMV. This association could be secondary to direct CMV cytopathic damage to the lungs or increased respiratory support required during the acute illness. Postnatal CMV infection is also known to cause enteritis, and small case studies have linked it to NEC in VLBW infants.22,58 We did not find an association between postnatal CMV and NEC. However, the incidence of NEC in this study was very low (0.4% in the non–postnatal CMV group and 0.7% in the postnatal CMV group), compared with an estimated prevalence of 5% to 7% in VLBW infants.59,60 Infants were matched on demographics, events, and risk factors in the first 21 postnatal days, including NEC and many risk factors for NEC. In addition, a significant portion of NEC in VLBW infants happens before postnatal day 21.61 Postnatal CMV alone may not have a strong association with NEC, or the results may be biased toward the null hypothesis because of the matching criteria used and the small number of infants with NEC.
Strengths and Limitations
The strengths of this study include the large database used that incorporated data from multiple sites across the country and allowed us to use propensity scoring to match clinically similar infants. There are several limitations to this study. First, it is a retrospective study with data only available for infants while they were hospitalized. Infants were not routinely screened for congenital or postnatal CMV. Thus, this cohort likely represents infants with symptomatic postnatal CMV disease, and the control group may include infants with CMV who never received testing. Moreover, some infants with congenital CMV may have inadvertently been classified as having postnatal CMV. We attempted to prevent this by excluding infants with symptoms or diagnoses consistent with congenital CMV (eg, microcephaly). The head circumference of infants positive for postnatal CMV in this study was not different than those without postnatal CMV, supporting a diagnosis of postnatal rather than congenital CMV in our cases. In a sensitivity analysis excluding infants given the diagnosis of congenital CMV after postnatal day 21, and therefore possibly misclassified, the results were similar.
Conclusions
Our results highlight the potential sequelae of postnatal CMV, including a potential risk for hearing loss, increased LOS, BPD, and decreased weight at discharge in this population already at high risk for long-term neurodevelopmental impairment. We believe a large-scale, multicenter prospective study is needed to fully define the morbidity associated with postnatal CMV and determine whether antiviral treatment of the infant reduces the effects of infection.
eTable 1. Secondary Outcomes of VLBW Infants, Propensity Score–Matched Cohort
eTable 2. Primary Outcomes of VLBW Infants, Propensity Score–Matched Cohort
eFigure. Standardized Percentage Bias for Propensity Score Covariates
References
- 1.Cannon MJ, Davis KF. Washing our hands of the congenital cytomegalovirus disease epidemic. BMC Public Health. 2005;5:70. doi: 10.1186/1471-2458-5-70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith RJH, Bale JF Jr, White KR. Sensorineural hearing loss in children. Lancet. 2005;365(9462):879-890. doi: 10.1016/S0140-6736(05)71047-3 [DOI] [PubMed] [Google Scholar]
- 3.Manicklal S, Emery VC, Lazzarotto T, Boppana SB, Gupta RK. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev. 2013;26(1):86-102. doi: 10.1128/CMR.00062-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kenneson A, Cannon MJ. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol. 2007;17(4):253-276. doi: 10.1002/rmv.535 [DOI] [PubMed] [Google Scholar]
- 5.Josephson CD, Caliendo AM, Easley KA, et al. Blood transfusion and breast milk transmission of cytomegalovirus in very low-birth-weight infants: a prospective cohort study. JAMA Pediatr. 2014;168(11):1054-1062. doi: 10.1001/jamapediatrics.2014.1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yasuda A, Kimura H, Hayakawa M, et al. Evaluation of cytomegalovirus infections transmitted via breast milk in preterm infants with a real-time polymerase chain reaction assay. Pediatrics. 2003;111(6, pt 1):1333-1336. doi: 10.1542/peds.111.6.1333 [DOI] [PubMed] [Google Scholar]
- 7.Capretti MG, Lanari M, Lazzarotto T, et al. Very low birth weight infants born to cytomegalovirus-seropositive mothers fed with their mother’s milk: a prospective study. J Pediatr. 2009;154(6):842-848. doi: 10.1016/j.jpeds.2008.12.046 [DOI] [PubMed] [Google Scholar]
- 8.de Cates CR, Gray J, Roberton NR, Walker J. Acquisition of cytomegalovirus infection by premature neonates. J Infect. 1994;28(1):25-30. doi: 10.1016/S0163-4453(94)94037-1 [DOI] [PubMed] [Google Scholar]
- 9.Lanzieri TM, Dollard SC, Josephson CD, Schmid DS, Bialek SR. Breast milk-acquired cytomegalovirus infection and disease in VLBW and premature infants. Pediatrics. 2013;131(6):e1937-e1945. doi: 10.1542/peds.2013-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brecht KF, Goelz R, Bevot A, Krägeloh-Mann I, Wilke M, Lidzba K. Postnatal human cytomegalovirus infection in preterm infants has long-term neuropsychological sequelae. J Pediatr. 2015;166(4):834-839.e1. doi: 10.1016/j.jpeds.2014.11.002 [DOI] [PubMed] [Google Scholar]
- 11.Lombardi G, Garofoli F, Manzoni P, Stronati M. Breast milk-acquired cytomegalovirus infection in very low birth weight infants. J Matern Fetal Neonatal Med. 2012;25(suppl 3):57-62. doi: 10.3109/14767058.2012.712345 [DOI] [PubMed] [Google Scholar]
- 12.Kumar ML, Nankervis GA, Jacobs IB, et al. Congenital and postnatally acquired cytomegalovirus infections: long-term follow-up. J Pediatr. 1984;104(5):674-679. doi: 10.1016/S0022-3476(84)80942-7 [DOI] [PubMed] [Google Scholar]
- 13.Kelly MS, Benjamin DK, Puopolo KM, et al. Postnatal cytomegalovirus infection and the risk for bronchopulmonary dysplasia. JAMA Pediatr. 2015;169(12):e153785. doi: 10.1001/jamapediatrics.2015.3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vollmer B, Seibold-Weiger K, Schmitz-Salue C, et al. Postnatally acquired cytomegalovirus infection via breast milk: effects on hearing and development in preterm infants. Pediatr Infect Dis J. 2004;23(4):322-327. doi: 10.1097/00006454-200404000-00009 [DOI] [PubMed] [Google Scholar]
- 15.Sawyer MH, Edwards DK, Spector SA. Cytomegalovirus infection and bronchopulmonary dysplasia in premature infants. AJDC. 1987;141(3):303-305. doi: 10.1001/archpedi.1987.04460030081030 [DOI] [PubMed] [Google Scholar]
- 16.Mukhopadhyay S, Meyer SA, Permar SR, Puopolo KM. Symptomatic postnatal cytomegalovirus testing among very low-birth-weight infants: indications and outcomes. Am J Perinatol. 2016;33(9):894-902. doi: 10.1055/s-0036-1581080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nijman J, de Vries LS, Koopman-Esseboom C, Uiterwaal CSPM, van Loon AM, Verboon-Maciolek MA. Postnatally acquired cytomegalovirus infection in preterm infants: a prospective study on risk factors and cranial ultrasound findings. Arch Dis Child Fetal Neonatal Ed. 2012;97(4):F259-F263. doi: 10.1136/archdischild-2011-300405 [DOI] [PubMed] [Google Scholar]
- 18.Neuberger P, Hamprecht K, Vochem M, et al. Case-control study of symptoms and neonatal outcome of human milk-transmitted cytomegalovirus infection in premature infants. J Pediatr. 2006;148(3):326-331. doi: 10.1016/j.jpeds.2005.09.030 [DOI] [PubMed] [Google Scholar]
- 19.Ehrenkranz RA, Walsh MC, Vohr BR, et al. ; National Institutes of Child Health and Human Development Neonatal Research Network . Validation of the National Institutes of Health consensus definition of bronchopulmonary dysplasia. Pediatrics. 2005;116(6):1353-1360. doi: 10.1542/peds.2005-0249 [DOI] [PubMed] [Google Scholar]
- 20.Gunkel J, de Vries LS, Jongmans M, et al. Outcome of preterm infants with postnatal cytomegalovirus infection. Pediatrics. 2018;141(2):e20170635. doi: 10.1542/peds.2017-0635 [DOI] [PubMed] [Google Scholar]
- 21.Tengsupakul S, Birge ND, Bendel CM, et al. Asymptomatic DNAemia heralds CMV-associated NEC: case report, review, and rationale for preemption. Pediatrics. 2013;132(5):e1428-e1434. doi: 10.1542/peds.2013-0087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goelz R, Hamprecht K, Klingel K, Poets CF. Intestinal manifestations of postnatal and congenital cytomegalovirus infection in term and preterm infants. J Clin Virol. 2016;83:29-36. doi: 10.1016/j.jcv.2016.08.289 [DOI] [PubMed] [Google Scholar]
- 23.Thorp JA, Jones PG, Peabody JL, Knox E, Clark RH. Effect of antenatal and postnatal corticosteroid therapy on weight gain and head circumference growth in the nursery. Obstet Gynecol. 2002;99(1):109-115. [DOI] [PubMed] [Google Scholar]
- 24.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Stürmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163(12):1149-1156. doi: 10.1093/aje/kwj149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33-38. doi: 10.1080/00031305.1985.10479383 [DOI] [Google Scholar]
- 26.Olsen IE, Groveman SA, Lawson ML, Clark RH, Zemel BS. New intrauterine growth curves based on United States data. Pediatrics. 2010;125(2):e214-e224. doi: 10.1542/peds.2009-0913 [DOI] [PubMed] [Google Scholar]
- 27.Hornik CP, Benjamin DK, Becker KC, et al. Use of the complete blood cell count in late-onset neonatal sepsis. Pediatr Infect Dis J. 2012;31(8):803-807. doi: 10.1097/INF.0b013e31825691e4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trembath A, Hornik CP, Clark R, Smith PB, Daniels J, Laughon M; Best Pharmaceuticals for Children Act—Pediatric Trials Network . Comparative effectiveness of surfactant preparations in premature infants. J Pediatr. 2013;163(4):955-960.e1. doi: 10.1016/j.jpeds.2013.04.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zou G. A modified Poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702-706. doi: 10.1093/aje/kwh090 [DOI] [PubMed] [Google Scholar]
- 30.Zhang J, Yu KF. What’s the relative risk? a method of correcting the odds ratio in cohort studies of common outcomes. JAMA. 1998;280(19):1690-1691. doi: 10.1001/jama.280.19.1690 [DOI] [PubMed] [Google Scholar]
- 31.Anderson PJ, Doyle LW. Neurodevelopmental outcome of bronchopulmonary dysplasia. Semin Perinatol. 2006;30(4):227-232. doi: 10.1053/j.semperi.2006.05.010 [DOI] [PubMed] [Google Scholar]
- 32.Belfort MB, Rifas-Shiman SL, Sullivan T, et al. Infant growth before and after term: effects on neurodevelopment in preterm infants. Pediatrics. 2011;128(4):e899-e906. doi: 10.1542/peds.2011-0282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Latal-Hajnal B, von Siebenthal K, Kovari H, Bucher HU, Largo RH. Postnatal growth in VLBW infants: significant association with neurodevelopmental outcome. J Pediatr. 2003;143(2):163-170. doi: 10.1067/S0022-3476(03)00243-9 [DOI] [PubMed] [Google Scholar]
- 34.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253-1261. doi: 10.1542/peds.2005-1368 [DOI] [PubMed] [Google Scholar]
- 35.Cristobal R, Oghalai JS. Hearing loss in children with very low birth weight: current review of epidemiology and pathophysiology. Arch Dis Child Fetal Neonatal Ed. 2008;93(6):F462-F468. doi: 10.1136/adc.2007.124214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woolf NK, Koehrn FJ, Harris JP, Richman DD. Congenital cytomegalovirus labyrinthitis and sensorineural hearing loss in guinea pigs. J Infect Dis. 1989;160(6):929-937. doi: 10.1093/infdis/160.6.929 [DOI] [PubMed] [Google Scholar]
- 37.Davis GL, Spector GJ, Strauss M, Middlekamp JN. Cytomegalovirus endolabyrinthitis. Arch Pathol Lab Med. 1977;101(3):118-121. [PubMed] [Google Scholar]
- 38.Rivera LB, Boppana SB, Fowler KB, Britt WJ, Stagno S, Pass RF. Predictors of hearing loss in children with symptomatic congenital cytomegalovirus infection. Pediatrics. 2002;110(4):762-767. doi: 10.1542/peds.110.4.762 [DOI] [PubMed] [Google Scholar]
- 39.Boppana SB, Fowler KB, Pass RF, et al. Congenital cytomegalovirus infection: association between virus burden in infancy and hearing loss. J Pediatr. 2005;146(6):817-823. doi: 10.1016/j.jpeds.2005.01.059 [DOI] [PubMed] [Google Scholar]
- 40.Noyola DE, Demmler GJ, Williamson WD, et al. ; Congenital CMV Longitudinal Study Group . Cytomegalovirus urinary excretion and long term outcome in children with congenital cytomegalovirus infection. Pediatr Infect Dis J. 2000;19(6):505-510. doi: 10.1097/00006454-200006000-00003 [DOI] [PubMed] [Google Scholar]
- 41.Sharma AA, Jen R, Butler A, Lavoie PM. The developing human preterm neonatal immune system: a case for more research in this area. Clin Immunol. 2012;145(1):61-68. doi: 10.1016/j.clim.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Melville JM, Moss TJM. The immune consequences of preterm birth. Front Neurosci. 2013;7:79. doi: 10.3389/fnins.2013.00079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saunders NR, Dreifuss J-J, Dziegielewska KM, et al. The rights and wrongs of blood-brain barrier permeability studies: a walk through 100 years of history. Front Neurosci. 2014;8:404. doi: 10.3389/fnins.2014.00404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lanzieri TM, Chung W, Flores M, et al. ; Congenital Cytomegalovirus Longitudinal Study Group . Hearing loss in children with asymptomatic congenital cytomegalovirus infection. Pediatrics. 2017;139(3):e20162610. doi: 10.1542/peds.2016-2610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fowler KB, McCollister FP, Dahle AJ, Boppana S, Britt WJ, Pass RF. Progressive and fluctuating sensorineural hearing loss in children with asymptomatic congenital cytomegalovirus infection. J Pediatr. 1997;130(4):624-630. doi: 10.1016/S0022-3476(97)70248-8 [DOI] [PubMed] [Google Scholar]
- 46.Korres S, Nikolopoulos TP, Peraki EE, et al. Outcomes and efficacy of newborn hearing screening: strengths and weaknesses (success or failure?). Laryngoscope. 2008;118(7):1253-1256. doi: 10.1097/MLG.0b013e31816d726c [DOI] [PubMed] [Google Scholar]
- 47.Johnson JL, White KR, Widen JE, et al. A multicenter evaluation of how many infants with permanent hearing loss pass a two-stage otoacoustic emissions/automated auditory brainstem response newborn hearing screening protocol. Pediatrics. 2005;116(3):663-672. doi: 10.1542/peds.2004-1688 [DOI] [PubMed] [Google Scholar]
- 48.Dedhia K, Kitsko D, Sabo D, Chi DH. Children with sensorineural hearing loss after passing the newborn hearing screen. JAMA Otolaryngol Head Neck Surg. 2013;139(2):119-123. doi: 10.1001/jamaoto.2013.1229 [DOI] [PubMed] [Google Scholar]
- 49.Yousefi J, Ajalloueyan M, Amirsalari S, Hassanali Fard M. The specificity and sensitivity of transient otoacustic emission in neonatal hearing screening compared with diagnostic test of auditory brain stem response in Tehran hospitals. Iran J Pediatr. 2013;23(2):199-204. [PMC free article] [PubMed] [Google Scholar]
- 50.Tran C, Bennett MV, Gould JB, Lee HC, Lanzieri TM. Cytomegalovirus infection among infants in neonatal intensive care units, California, 2005 to 2016 [published online March 20, 2019]. Am J Perinatol. doi: 10.1055/s-0039-1683958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stroustrup A, Trasande L. Epidemiological characteristics and resource use in neonates with bronchopulmonary dysplasia: 1993-2006. Pediatrics. 2010;126(2):291-297. doi: 10.1542/peds.2009-3456 [DOI] [PubMed] [Google Scholar]
- 52.Lee SM, Kim N, Namgung R, Park M, Park K, Jeon J. Prediction of postnatal growth failure among very low birth weight infants. Sci Rep. 2018;8(1):3729. doi: 10.1038/s41598-018-21647-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schehr LK, Johnson TS. Concept analysis of growth failure in preterm infants in the NICU. J Obstet Gynecol Neonatal Nurs. 2017;46(6):870-877. doi: 10.1016/j.jogn.2017.09.005 [DOI] [PubMed] [Google Scholar]
- 54.Sekar KC. Iatrogenic complications in the neonatal intensive care unit. J Perinatol. 2010;30(S1)(suppl):S51-S56. doi: 10.1038/jp.2010.102 [DOI] [PubMed] [Google Scholar]
- 55.Argus BM, Dawson JA, Wong C, Morley CJ, Davis PG. Financial costs for parents with a baby in a neonatal nursery. J Paediatr Child Health. 2009;45(9):514-517. doi: 10.1111/j.1440-1754.2009.01551.x [DOI] [PubMed] [Google Scholar]
- 56.Rogowski J. Cost-effectiveness of care for very low birth weight infants. Pediatrics. 1998;102(1, pt 1):35-43. doi: 10.1542/peds.102.1.35 [DOI] [PubMed] [Google Scholar]
- 57.Whitley RJ, Brasfield D, Reynolds DW, Stagno S, Tiller RE, Alford CA. Protracted pneumonitis in young infants associated with perinatally acquired cytomegaloviral infection. J Pediatr. 1976;89(1):16-22. doi: 10.1016/S0022-3476(76)80919-5 [DOI] [PubMed] [Google Scholar]
- 58.Cheong JLY, Cowan FM, Modi N. Gastrointestinal manifestations of postnatal cytomegalovirus infection in infants admitted to a neonatal intensive care unit over a five year period. Arch Dis Child Fetal Neonatal Ed. 2004;89(4):F367-F369. doi: 10.1136/adc.2003.032821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Patel BK, Shah JS. Necrotizing enterocolitis in very low birth weight infants: a systemic review. ISRN Gastroenterol. 2012;2012:562594. doi: 10.5402/2012/562594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Horbar JD, Carpenter JH, Badger GJ, et al. Mortality and neonatal morbidity among infants 501 to 1500 grams from 2000 to 2009. Pediatrics. 2012;129(6):1019-1026. doi: 10.1542/peds.2011-3028 [DOI] [PubMed] [Google Scholar]
- 61.Gordon PV, Clark R, Swanson JR, Spitzer A. Can a national dataset generate a nomogram for necrotizing enterocolitis onset? J Perinatol. 2014;34(10):732-735. doi: 10.1038/jp.2014.137 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Secondary Outcomes of VLBW Infants, Propensity Score–Matched Cohort
eTable 2. Primary Outcomes of VLBW Infants, Propensity Score–Matched Cohort
eFigure. Standardized Percentage Bias for Propensity Score Covariates