Key Points
Question
Can tools from computational psychiatry and decision neuroscience be used to understand and assess prospective opioid reuse risk?
Findings
This longitudinal study serially examined computational markers of risky decision-making in individuals who use opioids, studied over 7 months of treatment. An increase in an individual marker of tolerance to ambiguity (a context in which there is limited information about environmental risk) was significantly tied to imminent opioid use (within 1-4 weeks) in a manner statistically independent of standard clinical factors associated with opioid use.
Meaning
In this study, computational markers of risky decision-making capture distinct latent cognitive processes with clinical utility for detecting opioid reuse vulnerability.
This community-based case-control study uses computational psychiatry and decision neuroscience tools to identify changes in decision-making before opioid reuse in individuals being treated for opioid use disorders (vs control participants).
Abstract
Importance
Opioid addiction is a major public health problem. Despite availability of evidence-based treatments, relapse and dropout are common outcomes. Efforts aimed at identifying reuse risk and gaining more precise understanding of the mechanisms conferring reuse vulnerability are needed.
Objective
To use tools from computational psychiatry and decision neuroscience to identify changes in decision-making processes preceding opioid reuse.
Design, Setting, and Participants
A cohort of individuals with opioid use disorder were studied longitudinally at a community-based treatment setting for up to 7 months (1-15 sessions per person). At each session, patients completed a risky decision-making task amenable to computational modeling and standard clinical assessments. Time-lagged mixed-effects logistic regression analyses were used to assess the likelihood of opioid use between sessions (t to t + 1; within the subsequent 1-4 weeks) from data acquired at the current session (t). A cohort of control participants completed similar procedures (1-5 sessions per person), serving both as a baseline comparison group and an independent sample in which to assess measurement test-retest reliability. Data were analyzed between January 1, 2018, and September 5, 2019.
Main Outcomes and Measures
Two individual model-based behavioral markers were derived from the task completed at each session, capturing a participant’s current tolerance of known risks and ambiguity (partially unknown risks). Current anxiety, craving, withdrawal, and nonadherence were assessed via interview and clinic records. Opioid use was ascertained from random urine toxicology tests and self-reports.
Results
Seventy patients (mean [SE] age, 44.7 [1.3] years; 12 women and 58 men [82.9% male]) and 55 control participants (mean [SE] age, 42.4 [1.5] years; 13 women and 42 men [76.4% male]) were included. Of the 552 sessions completed with patients (mean [SE], 7.89 [0.59] sessions per person), 252 (45.7%) directly preceded opioid use events (mean [SE], 3.60 [0.44] sessions per person). From the task parameters, only ambiguity tolerance was significantly associated with increased odds of prospective opioid use (adjusted odds ratio, 1.37 [95% CI, 1.07-1.76]), indicating patients were more tolerant specifically of ambiguous risks prior to these use events. The association of ambiguity tolerance with prospective use was independent of established clinical factors (adjusted odds ratio, 1.29 [95% CI, 1.01-1.65]; P = .04), such that a model combining these factors explained more variance in reuse risk. No significant differences in ambiguity tolerance were observed between patients and control participants, who completed 197 sessions (mean [SE], 3.58 [0.21] sessions per person); however, patients were more tolerant of known risks (B = 0.56 [95% CI, 0.05-1.07]).
Conclusions and Relevance
Computational approaches can provide mechanistic insights about the cognitive factors underlying opioid reuse vulnerability and may hold promise for clinical use.
Introduction
For the first time, drug overdose is the leading cause of unintentional death in the United States. Opioids, including heroin and more recently fentanyl, account for the largest proportion of these lethal intoxications.1,2,3,4 Despite these risks and the availability of evidence-based treatments for opioid use disorder (OUD), high rates of reuse, relapse, and treatment dropout are common,5,6 suggesting that a core cognitive process conferring opioid use vulnerability may be associated with increased tolerance for risk.
Prior studies have indeed identified aspects of risky decision-making relevant to OUD diagnosis and clinical course.7,8,9,10,11,12,13,14,15,16 In cross-sectional studies, individuals who used opioids compared with control participants demonstrate deficits in detecting risk7 and learning from rewards and punishments.9,12,17,18,19,20 Further work has also suggested that risk-taking may be associated with negative long-term outcomes, such as relapse.10,21 Important questions remain unanswered, however, beyond cross-sectional differences in these global constructs. Most notably, it is unknown whether these between-participant findings capture traitlike features of OUD or statelike features associated with fluctuating vulnerability for opioid seeking and use. To resolve the precise association between risky decision-making and opioid use vulnerability, a longitudinal within-participant assessment that parses risk-taking into its constituent components is needed.
Decades of work in psychology and economics has decomposed the global propensity for risk-taking into 2 interconnected but theoretically,22,23 neurobiologically,24,25,26,27 and developmentally28,29 separable components: tolerance for known risks and tolerance for unknown, or ambiguous, risks. More recently, this distinction has also entered clinical neuroscience.30,31 Known-risk tolerance is defined by a willingness to accept courses of action for which the precise odds of a given outcome are known. Ambiguity tolerance is instead defined by a willingness to accept actions for which these odds are not fully known or cannot be estimated. This distinction is relevant for most real-world decisions, in which exact outcome probabilities are rarely fully known23,32; however, its relevance for OUD has not been explicitly tested, because most tasks or analytic approaches commonly used in the clinical literature assess a mixture of these 2 behavioral components.33,34 Tasks or approaches that draw on the economics (and more broadly on decision neuroscience) literature have several theoretical and practical advantages in this regard. By design, these tasks or approaches orthogonalize the contribution of known-risk and ambiguity to the characterization of individual risk-taking, allowing these measures to be independently derived. They also probe preference-based behavior in the absence of a learning requirement (which poses challenges for repeated-measures designs). Finally, although less common in clinical research, as reviewed, these tasks or approaches are well-established in basic science and are growing in use in other fields as well.
Leveraging these tools, we examined whether known-risk tolerance, ambiguity tolerance, or both are associated with episodic opioid use among individuals receiving treatment in a real-world community-based setting. We focused on this population because opioid reuse typically precipitates treatment failure,35 and given potential interactions with maintenance pharmacotherapy (via methadone or buprenorphine), it increases risk for medical complications and overdose.36 We further embedded our assessments within the naturalistic conditions of patients’ treatment trajectories, enabling us to temporally link our measures to opioid use while accounting for other time-varying factors (eg, craving, nonadherence).
Methods
Participants
Participants were individuals with OUD receiving medication-assisted outpatient treatment and sociodemographically similar control participants. We primarily recruited patients having recently initiated treatment (within ≤1 month) who were expected to concomitantly use opioids within 6 months35; those with more than 1 month in treatment reporting frequent use were also recruited. All participants provided written informed consent in accordance with procedures approved by the New York University School of Medicine institutional review board.
Inclusion criteria were 18 years or older and the ability to read and understand English. Patients additionally needed to have an expressed goal of achieving opioid abstinence. Exclusion criteria were (1) primary substance use other than opioids; (2) a history of head trauma, loss of consciousness longer than 30 minutes, or neurologic disease; (3) untreated or unstable medical conditions, including liver enzyme levels more than 3 times the normal range and CD4 counts less than 500 cells/μL (AIDS at stage 2 or greater); (4) untreated or unstable psychiatric conditions, including a current manic episode, active psychosis, or suicidal ideation; and (5) a failure to understand or comply with study procedures. Control participant–specific exclusion criteria were (6) a history of substance abuse (except for nicotine and alcohol use restricted to college or military service); and (7) a urine drug screening with positive results. Inclusion and exclusion criteria were ascertained during a comprehensive interview including the Addiction Severity Index37 and by consulting clinic records.
Fifteen patients were excluded; 11 did not understand the task, as evidenced by self-report or poor-quality data (eMethods in the Supplement), 1 later disclosed no desire to achieve abstinence, and 3 had an exclusionary result of a neurologic history. In addition, 4 control participants were excluded; 3 did not understand the task, and 1 tested positive for cocaine at session 2.
Study Timeline and Procedures
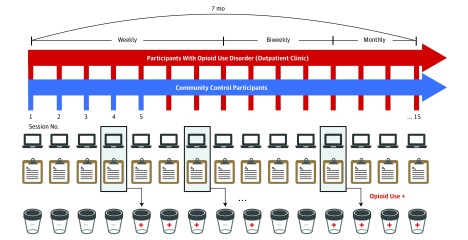
Data were collected between March 5, 2015, and August 29, 2017. Patients completed up to 15 sessions over 7 months (Figure 1). Because the period of early recovery is associated with highest relapse and overdose risk,36 we sampled this period most densely: the first 8 sessions were scheduled weekly, the following 4 sessions biweekly, and the final 3 monthly. Control participants completed up to 5 weekly sessions. This allowed us to investigate most clinical events of interest in patients, as well as assess parameter test-retest reliability in control participants. The number of sessions completed differed across participants because of scheduling conflicts, treatment dropout or transfer, and loss to follow-up (eMethods and eFigures 1-3 in the Supplement).
Figure 1. Longitudinal Study Design and Procedures.
Participants with opioid use disorder completed up to 15 study sessions over 7 months. Control participants completed up to 5 sessions. Each session was made up of the decision-making task and clinical assessments of anxiety, craving, withdrawal, and adherence to treatment. Illicit opioid use was ascertained by random urine toxicology tests and self-reports.
At each session, participants completed clinical assessments and a risky decision-making task. Participants additionally completed a temporal-discounting task as part of a separate study that will be reported elsewhere.
Risky Decision-making Task and Computational Modeling
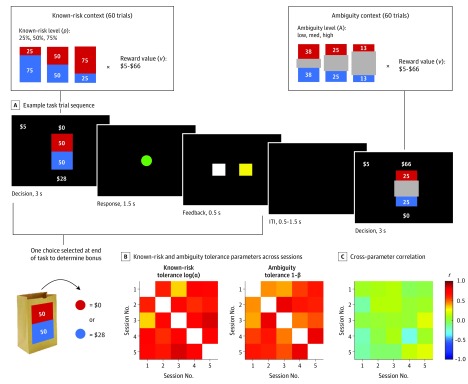
Individual known-risk and ambiguity tolerance were estimated on a well-validated task.25,38 On each trial, participants chose between a guaranteed $5 and a lottery (Figure 2A). Each lottery had 2 possible outcomes: v or $0, where v ranged from $5 to $66. On half of the trials, v could be received with 1 of 3 known probabilities (p: 25%, 50%, or 75%); on the other half, the probability information was occluded and thus incompletely known (the ambiguity context). We used 3 levels of ambiguity, termed A: low (24% unknown), medium (50% unknown), and high (74% unknown). Each amount v appeared with each p and A level once in random order over 4 blocks of 30 trials (for 120 trials). So-called catch trials offering a guaranteed $5 vs a 50% chance of $4 were also included at the start of each block.
Figure 2. Decision-making Task and Model-Derived Parameter Test-Retest Reliability.
A, Example task trial sequence. In known-risk trials, participants chose between a guaranteed $5 and a lottery that was defined by an explicit probability (p) (either 25%, 50%, or 75%) of receiving $5 to $66 (v). In ambiguity trials, the lotteries were defined by a partially unknown probability (ambiguity level, A), where either 24%, 50%, or 74% of the probability information was occluded and thus unknown. In the example shown, the probability of receiving $66 is anywhere between 25% and 75%. In reality, the true underlying probability in ambiguity trials was fixed at 50%. At the end of the task, a single trial was selected at random and played out to determine a participant’s bonus for the session completed, either $5 (if the guaranteed option was chosen on the selected trial) or by playing the lottery, which involved drawing a chip from the corresponding lottery bag to earn either v or $0 instead. No outcomes were shown during the task. B, Both model-derived known-risk and ambiguity tolerance parameters exhibited good test-retest reliability in control participants tested up to 5 times approximately 1 week apart. C, The 2 parameters were largely uncorrelated with each other across sessions. ITI indicates intertrial interval.
Participants were instructed that each lottery corresponded to a physical bag containing 100 red and blue chips. The bags were always nearby, and participants could inspect them after the experiment if they wished. For known-risk lotteries, the precise number of red and blue chips in the corresponding bags was specified. For ambiguous lotteries, only partial information was provided, but to eliminate bias, the number of red and blue chips were made equal in the ambiguous displays. To incentivize participants to choose according to their true preferences, compensation was $10 per session plus a variable bonus. At the conclusion of each session, 1 trial was randomly selected for realization, and the choice made on that trial determined the bonus ($5 or the lottery amount; Figure 2A). No bonus was received for missed responses.
As in prior work,25,28,30,31 we modeled participants’ choice data with a power utility model that separately parameterizes known-risk and ambiguity tolerance.39 Here, the expected utility (EU) of each option (guaranteed or lottery) on each trial is given by
 |
where v is the winning amount, p is the probability of winning, A is the fraction of p that is unknown, α is a participant-specific known-risk tolerance parameter, and β is a participant-specific ambiguity tolerance parameter. An α of 1 indicates risk neutrality, α less than 1 indicates risk aversion, and α greater than 1 indicates risk seeking. A β of 0 indicates ambiguity neutrality, a β greater than 0 indicates ambiguity aversion (pessimism about the hidden probability of winning v), and a β less than 0 indicates ambiguity seeking (optimism about the hidden probability of winning v). To estimate α and β using maximum-likelihood estimation in MATLAB version R2018a (MathWorks), we fit a probabilistic choice function to the trial-by-trial data:
 |
where Pr is the probability the lottery is chosen, EU(lottery) and EU(safe) are the expected utilities (from the first equation) of the lottery and guaranteed options, respectively, and μ is a third participant-specific parameter capturing choice stochasticity. For ease of interpretation, we report 1 − β such that higher values indicate higher ambiguity tolerance. We also report natural log-transformed values of α and μ, because these data were nonnormally distributed.
Clinical Variables Collected at Each Session
At each session, anxiety was assessed with the State-Trait Anxiety Inventory,40 and withdrawal was assessed with the Subjective Opiate Withdrawal Scale.41 Opioid craving was assessed with a visual analog scale for the period spanning the past 7 days, including the session date. Treatment adherence was quantified as the proportion of confirmed dispensed medication doses in the period since the last study session (while nonadherence was defined as 1 − adherence; eMethods in the Supplement in the details).
Opioid Use
Positive use was defined as any illicit opioid use self-reported on a timeline followback42 completed at each session or a positive or scheduled but refused or not completed urine toxicology test in the period between study sessions. Random (approximately weekly) urine tests were administered by the clinic; results of these tests were obtained from clinic records. For control participants, a urine test was administered at each session (eMethods in the Supplement for details).
Statistical Analysis
Data were analyzed between January 1, 2018, and September 5, 2019. Our primary analysis was time-lagged mixed-effects logistic regression, with the presence or absence of opioid use between sessions t and t + 1 as the outcome variable, and the model-derived decision-making parameters at session t as independent variables (Figure 3A shows illustrative examples of how the data were parsed for each participant). This analysis estimates the likelihood of opioid use after sessions when an individual is more tolerant of known risks or ambiguity compared with when the same individual is less tolerant of known risks or ambiguity. Extensions included the time-varying clinical variables (anxiety, craving, withdrawal, nonadherence, and recent use) as independent variables. In exploratory analyses, we examined diagnostic group differences in decision-making. Because groups differed in education, race/ethnicity, income, and baseline depression and anxiety levels (Table 1), these variables were included as nuisance covariates. All models were estimated in MATLAB version R2018a (MathWorks) using fitglme and included random intercepts for participant and session. Except for urine drug screenings, where scheduled but refused or not completed tests were considered to have positive results, we did not impute missing data. Missing data were censored (eMethods in the Supplement).
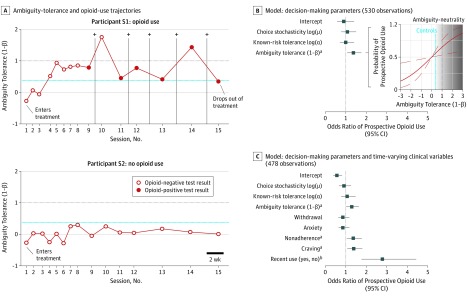
Figure 3. Time-Lagged Association With Prospective Opioid Use.
A, Raw data showing the ambiguity tolerance and opioid-use trajectories of 2 participants with opioid use disorder, illustrating how sessions were parsed in the group-level time-lagged analysis assessing prospective opioid use. B, Standardized odds ratios from the decision-making parameters only model as factors associated with prospective opioid use and expected probability of opioid use as a function of ambiguity tolerance level (based on the fitted parameter estimates from Table 2), showing opioid use was more likely than not to occur when a patient’s ambiguity tolerance surpassed the level observed in control participants and prior to ambiguity neutrality. The blue line in A and B represents the mean ambiguity tolerance observed in control participants; the line where ambiguity tolerance equals 1 on the y-axis represents ambiguity neutrality. The dark gray zone in B, demarcates an anticipated high opioid use–risk state. C, Standardized odds ratios from the full model, including the decision-making parameters and time-varying clinical variables as factors associated with prospective opioid use (Table 2; combined model).
Table 1. Demographic and Clinical Characteristics of the Study Sample.
| Characteristics | Mean (SE) | Test | P Value | |
|---|---|---|---|---|
| Participants With OUD (n = 70) | Control Participants (n = 55) | |||
| Demographic | ||||
| Age, y | 44.7 (1.3) | 42.36 (1.5) | t123 = 1.17 | .24 |
| Sex, No. | ||||
| Male | 58 | 42 | χ21 = 0.81 | .37 |
| Female | 12 | 13 | ||
| Race, No. | ||||
| African American | 20 | 28 | χ22 = 9.13 | .01 |
| White | 48 | 23 | ||
| Other | 2 | 4 | ||
| Ethnicity, No. | ||||
| Hispanic | 24 | 10 | χ21 = 4.03 | .045 |
| Non-Hispanic | 46 | 45 | ||
| Education, ya | 12.00 (0.24) | 14.61 (0.27) | t113 = 7.10 | <.001 |
| Nonverbal IQ scoreb | 92.47 (1.88) | 95.08 (2.56) | t94 = 0.81 | .42 |
| Numeracy scorec | 3.78 (0.12) | 3.75 (0.20) | t112 = 0.14 | .89 |
| Income per mo, median (range), $d | 650 (0-20 600) | 1000 (0-8000) | z = 2.26 | .02 |
| Psychiatric Attributes and Substance Use | ||||
| Beck Depression Inventory–II scoree | 15.55 (1.42) | 4.69 (0.74) | t101 = 6.44 | <.001 |
| Beck Anxiety Inventory scoref | 17.61 (1.52) | 3.27 (0.71) | t102 = 8.05 | <.001 |
| Fägerstrom Test for Nicotine Dependence scoreg | 3.83 (0.30) | 2.00 (0.75) | t60 = 2.27 | .03 |
| Lifetime use, y | ||||
| Alcoholh | 20.92 (1.79) | NA | NA | NA |
| Cocainei | 14.22 (1.66) | NA | NA | NA |
| Benzodiazepinej | 6.13 (1.25) | NA | NA | NA |
| Opioid | 14.66 (1.43) | NA | NA | NA |
| Medication, No. | ||||
| Methadone | 61 | NA | NA | NA |
| Buprenorphine | 9 | NA | NA | NA |
| Methadone dose, median (range), mg | 90 (14-230) | NA | NA | NA |
| Intravenous use, % | 43 | NA | NA | NA |
| History of overdose, % | 31 | NA | NA | NA |
| Hepatitis C positive status, % | 34 | NA | NA | NA |
| HIV positive status, % | 3 | NA | NA | NA |
Abbreviations: NA, not applicable; OUD, opioid use disorder.
Education data are missing for 9 participants with OUD and 1 control participant.
Kaufman Brief Intelligence Test: normative data suggest that scaled scores between 85 and 115 constitute the mean nonverbal IQ of the population. These data are missing for 23 participants with OUD and 6 control participants.
Numeracy module of the Health and Human Services Survey, with a range of 0 to 6 points; numeracy data are missing for 11 participants with OUD.
Income data are missing for 11 participants with OUD and 1 control participant.
Beck Depression Inventory (range, 0-63 points). Depression severity cutoff scores for the inventory are as follows: 0 to 13 points, minimal depression; 14 to 19 points, mild depression; 20 to 28 points, moderate depression; and 29 to 63 points, severe depression. These data are missing for 15 participants with OUD and 7 control participants.
Beck Anxiety Inventory (range, 0-63 points). Anxiety severity cutoff scores are as follows: 0 to 9 points, minimal anxiety; 10 to 16 points, mild anxiety; 17 to 29 points, moderate anxiety; and 30 to 63 points, severe anxiety. These data are missing for 14 participants with OUD and 7 control participants.
Fägerstrom Test for Nicotine Dependence: data shown are for current smokers: 53 participants with OUD and 9 control participants.
56 Participants.
47 Participants.
38 Participants.
Results
Table 1 provides demographic and clinical information on the final sample. Eighty-five individuals with OUD and 59 control participants were recruited. After exclusions, 70 patients (mean [SE] age, 44.7 [1.3] years; 12 women and 58 men [82.9% male]) and 55 control participants (mean [SE] age, 42.4 [1.5] years; 13 women and 42 men [76.4% male]) were included in analyses. Of the patients recruited, 55 (79%) had recently initiated treatment (within ≤1 month). Of the sessions completed, following data-quality exclusions (eMethods in the Supplement), a total of 749 sessions (89.6%) were retained for analysis (552 for patients; mean [SE], 7.89 [0.59] sessions per person; 197 sessions for control participants; mean [SE], 3.58 [0.21] sessions per person).
Both known-risk and ambiguity tolerance demonstrated good test-retest reliability in control participants. Figure 2B shows pairwise correlations for each parameter across sessions (r range, 0.31-0.82; mean [SE] r, 0.62 [0.03]). The 1 − k intraclass correlation coefficient for known-risk tolerance, computed for the 25 control participants with data for all 5 sessions, was 0.87 (95% CI, 0.77-0.94), and for ambiguity tolerance, it was 0.89 (95% CI, 0.81-0.95). Importantly, as previously reported,25,28,30,31 the 2 parameters were largely uncorrelated (r range, −0.30 to 0.19; mean [SE] r, 0.01 [0.02]; Figure 2C), confirming they captured distinct aspects of participants’ risk-taking behavior.
Time-Lagged Association of Decision-making Parameters With Prospective Opioid Use
By contrast, the task parameters in patients demonstrated higher variability as captured by lower, though still moderate, intraclass correlation coefficients (eResults in the Supplement), perhaps reflecting in part fluctuating vulnerability in this group. Indeed, of 552 sessions completed with patients, 271 sessions (49.1%) involved positive results for recent opioid use (mean [SE], 3.87 [0.43] sessions per person) and 252 sessions (45.7%) involved positive results for prospective (immediate future) use (mean [SE], 3.60 [0.44] sessions per person); a determination regarding prospective use could not be made in 22 sessions (eMethods in the Supplement).
To examine if we could identify when opioid use was likely to occur, we performed time-lagged analyses with the 2 risk-taking parameters and (for completeness) choice stochasticity as independent variables. We found a significant effect for ambiguity tolerance but not known-risk tolerance or choice stochasticity, such that holding the other factors constant, a 1 − SD increase in ambiguity tolerance was associated with a 37% increase in the odds of prospective use (adjusted odds ratio, 1.37 [95% CI, 1.07-1.76]; Table 2; Figure 3B). This association was present at the shortest timescale examined (within the first 8 sessions; eTable 1 in the Supplement); it was also robust to potential medication confounds (eTable 2 in the Supplement) and our opioid use–labeling scheme (eTable 3 in the Supplement).
Table 2. Time-Lagged Association of Task and Clinical Variables With Prospective Opioid Usea.
| Model | B (SE) [95% CI] | t Statistic | P Value | Standardized Odds Ratiob |
|---|---|---|---|---|
| Decision-making Parameters | ||||
| No. of observationsc | 530 | NA | NA | NA |
| df | 526 | NA | NA | NA |
| AIC | 636.6 | NA | NA | NA |
| BIC | 662.3 | NA | NA | NA |
| Log-likelihood | –312.3 | NA | NA | NA |
| Intercept | –0.262 (0.367) [–0.984 to 0.459] | –0.714 | .48 | 0.898 |
| Known-risk tolerance: log(α) | –0.004 (0.237) [–0.469 to 0.461] | –0.016 | .99 | 0.997 |
| Ambiguity tolerance: 1–β | 0.629 (0.256) [0.127-1.132] | 2.461 | .01 | 1.369 |
| Choice stochasticity: log(μ) | 0.096 (0.224) [–0.344 to 0.537] | 0.429 | .67 | 1.076 |
| Decision-making Parameters and Time-Varying Clinical Variables | ||||
| No. of observationsc | 478 | NA | NA | NA |
| df | 469 | NA | NA | NA |
| AIC | 562.4 | NA | NA | NA |
| BIC | 608.2 | NA | NA | NA |
| Log-likelihood | –270.2 | NA | NA | NA |
| Intercept | –1.079 (0.536) [–2.133 to –0.026] | –2.014 | .045 | 0.556 |
| Known-risk tolerance: log(α) | 0.081 (0.224) [–0.359 to 0.522] | 0.363 | .72 | 1.064 |
| Ambiguity tolerance: 1 – β | 0.504 (0.248) [0.016-0.992] | 2.029 | .04 | 1.289 |
| Choice stochasticity: log(μ) | –0.128 (0.222) [–0.565 to 0.309] | –0.575 | .57 | 0.906 |
| State-Trait Anxiety Inventory | –0.009 (0.011) [–0.031 to 0.012] | –0.891 | .37 | 0.867 |
| Craving score via visual analog scale–opioids | 0.107 (0.045) [0.019-0.195] | 2.383 | .02 | 1.384 |
| Subjective Opioid Withdrawal Scale | –0.015 (0.016) [–0.047 to 0.016] | –0.949 | .34 | 0.853 |
| Nonadherence, % | 1.619 (0.732) [0.179-3.058] | 2.210 | .03 | 1.362 |
| Recent opioid use | 1.025 (0.239) [0.556-1.493] | 4.294 | <.001 | 2.786 |
Abbreviations: AIC, Akaike information criterion; BIC, bayesian information criterion; NA, not applicable.
Results of time-lagged linear mixed-effects logistic regressions, including random intercepts for participants and sessions and the listed variables as fixed effects.
Unstandardized odds ratios can be computed from the regression coefficient B as Exp(B). Standardized values provided are from the same model with z-scored continuous variables.
The number of observations reflects the total number of task sessions available for analysis (552) minus 22 sessions that were censored because of unknown prospective opioid use status (decision-making task parameters model) and an additional 52 sessions with missing data on any of the clinical variables listed (combined model; eFigure 3 in the Supplement).
Time-Lagged Association of Clinical Variables With Prospective Opioid Use
It is possible that ambiguity tolerance covaries with another variable, explaining its association with prospective use. We therefore repeated our analysis, adding anxiety, craving, withdrawal, recent treatment compliance (nonadherence), and recent opioid use to the model. However, as shown in Table 2 and Figure 3C, the coefficient for ambiguity tolerance in this extended model remained significant (adjusted odds ratio, 1.29 [95% CI, 1.01-1.65]) and was only modestly reduced in magnitude. Recent opioid use (adjusted odds ratio, 2.79 [95% CI, 1.74-4.45]), opioid craving (adjusted odds ratio, 1.38 [95% CI. 1.06-1.81]), and nonadherence (adjusted odds ratio, 1.36 [95% CI, 1.03-1.79]) were also significant. This indicates that, while the contribution of ambiguity tolerance to the identification of prospective opioid use risk was somewhat lower, although comparable, with that of well-established clinical associated factors (eg, craving), it was independent of these factors. Expressed another way, the full model, including ambiguity tolerance alongside these clinical factors, relative to one without ambiguity tolerance, accounted for more variance explained in reuse risk and emerged as the better model in a formal theoretical likelihood ratio test (likelihood ratio test, 4.19; P = .04; eTable 4 in the Supplement). The same comparison with a reduced, so-called best model (made up only of craving, nonadherence, and recent use) also showed an advantage of including ambiguity tolerance, although the likelihood ratio statistic was reduced to nonsignificance (eTable 5 in the Supplement).
Diagnostic Group Differences in Decision-making Parameters
Finally, we examined aggregate-level differences between groups. Individuals who used substances are reported to be more risk-taking than control participants,7,8,9,10,11,12,13,14,15,16 but it is unknown which component underlies this difference and whether the behavioral phenotypes distinguishing individuals who use from control participants are those also contributing to ongoing drug-use vulnerability. Although most participants were risk averse and ambiguity averse (eFigure 4 in the Supplement), patients had a generally higher known-risk tolerance than control participants (B = 0.56 [95% CI, 0.05-1.07]). Patients also tended to have more stochastic choice behavior, but no significant differences were observed in ambiguity tolerance (eTable 6 in the Supplement).
Discussion
Individuals who continue opioid use during treatment face many health risks, as well as risk of treatment failure. We reasoned that measurable changes in risk tolerance could signal reuse vulnerability and approached this by sampling the decision-making behavior of individual patients over several months of treatment. By applying a decision neuroscience framework that experimentally and computationally dissociates known risk and ambiguity, we aimed to link these measures to opioid use on a clinically actionable week-to-week timescale.
We found increased ambiguity tolerance was associated with prospective opioid use. By contrast, despite evidence that known-risk tolerance was elevated in patients relative to control participants, we did not find evidence that it was associated with opioid reuse at the current temporal resolution. These findings refine our understanding of the role of risky decision-making in addiction7,8,9,10,11,12,13,14,15,16 by identifying subtypes of risk that map onto different OUD features. While ambiguity tolerance might track discrete changes underlying ongoing opioid use vulnerability, known-risk tolerance might reflect traitlike features that confer vulnerability at longer timescales. Indeed, extensive data indicate known-risk attitudes are remarkably stable,43,44 while ambiguity attitudes may be more malleable and susceptible to changes in physiological arousal and other cognitive or emotional states.45
Theoretical model–derived measures present some advantages over simpler behavioral summaries in that, by design, they yield lower construct measurement error, a highly desirable feature for clinical translation. Our 2 specific measures have been previously validated in diverse populations as separable components of an individual’s global propensity for risk-taking.25,28,30,31,46 This specificity allows us to make more precise interpretations regarding the latent processes involved here. For example, it is easy to imagine an individual who is even momentarily more tolerant of ambiguity may enter situations where the risk of substance use is ambiguous or may use substances because the risk of treatment failure is ambiguous. Indeed, it is almost impossible to precisely estimate the odds of these and most other real-world negative outcomes, and previous work indicates that even healthy individuals exhibit a so-called optimism bias in this regard.47
We also found that both known-risk and ambiguity tolerance exhibited good test-retest reliability in a control cohort without changes in opioid use vulnerability. These data provide further confidence that the observed changes across sessions in patients are likely attributable to factors other than measurement noise. As expected, intraclass correlation coefficients were lower in patients but still indicated moderate to good reliability and hence not very high within-participant variability. Thus, for guiding future research, both parameters could be considered to have acceptable levels of reliability, while also being sensitive to both stable and fluctuating OUD features.
In addition to providing novel mechanistic insights, we note our general approach may also prove practically useful. Our initial findings suggest that the contribution of ambiguity tolerance to prospective opioid reuse assessment was comparable with that of well-established clinical factors, such as craving, but was statistically independent of these factors. Combining the 2 thus explained more variance in reuse risk. This suggests that, with further testing, these measures could be combined into improved clinical-risk assessment tools.
Limitations
While this study is first, to our knowledge, to perform this many repeated measures of decision-making in a real-world OUD population, making it uniquely possible (relative to cross-sectional designs) to examine time-lagged associations with fluctuating clinical events, we achieved a long follow-up by reducing sampling frequency. To better define the timescale at which ambiguity tolerance and other measures best capture ongoing vulnerability, more finely sampled data will be required. Translating these measures to mobile platforms that permit real-time assessment will address this goal, as already demonstrated for other cognitive and affective processes in OUD.48 These efforts could also be used to test whether other factors, such as stress exposure and fluctuating mood (including depression), that are known to affect patient outcomes,49,50 are captured by or associated with changes in decision-making. Second, our objective in this study was to capture concomitant opioid use under naturalistic conditions; as such, given design and power considerations, we were unable to formally assess other relevant outcomes (eg, treatment response) or generalizability to other types of substance use. Future studies could use more structured cohort designs and inclusion criteria to address associations with these additional outcomes.
Conclusions
Identifying factors robustly associated with opioid reuse that can provide mechanistic insights regarding the underlying cognitive process involved is important for more targeted intervention and reducing reliance on postevent responding (eg, following reuse), which may come too late in the treatment failure cascade. Our initial data suggest that 1 component of risk-taking, ambiguity tolerance, may provide clinically useful information regarding this prospective risk.
eMethods. Evaluating Behavioral Data Quality and Clinic Records and Study Definitions of Treatment Adherence and Illicit Opioid Use
eResults. Intraclass Correlation Coefficients (ICCs) for the Decision-Making Task Parameters in Opioid Use Disorder Participants
eFigure 1. Examples of Acceptable and Poor-Quality Behavioral Data
eFigure 2. Participants Remaining in the Study by Session Number
eFigure 3. CONSORT Diagram of Sessions Excluded and Retained for Analysis
eFigure 4. Distribution of Decision-Making Task Parameters by Diagnostic Group
eTable 1. Time-Lagged Association of Task Variables with Prospective Opioid Use, First 8 Study Sessions Only
eTable 2. Time-Lagged Association of Task Variables with Prospective Opioid Use, Controlling for Medication Type and Dose
eTable 3. Time-Lagged Association of Task Variables with Prospective Opioid Use, Without Positive Sessions Based on Urine Test Samples “Scheduled but Refused or Not Completed”
eTable 4. Comparison of “Full” Clinical Model to the Same Model Including Ambiguity Tolerance in the Time-Lagged Association with Prospective Opioid Use
eTable 5. Comparison of “Best” Clinical Model to the Same Model Including Ambiguity Tolerance in the Time-Lagged Association with Prospective Opioid Use
eTable 6. Diagnostic Group Differences in Decision-Making Task Parameters
References
- 1.Jones CM, Mack KA, Paulozzi LJ. Pharmaceutical overdose deaths, United States, 2010. JAMA. 2013;309(7):657-659. doi: 10.1001/jama.2013.272 [DOI] [PubMed] [Google Scholar]
- 2.Kerlikowske RG. National drug control strategy: data supplement. https://obamawhitehouse.archives.gov/sites/default/files/ondcp/policy-and-research/ndcs_2013.pdf. Published 2013. Accessed November 25, 2019.
- 3.National Institute on Drug Abuse Trends & statistics. https://www.drugabuse.gov/related-topics/trends-statistics#costs. Published April 2017. Accessed November 26, 2019.
- 4.Rudd RA, Aleshire N, Zibbell JE, Gladden RM. Increases in drug and opioid overdose deaths—United States, 2000-2014. MMWR Morb Mortal Wkly Rep. 2016;64(50-51):1378-1382. doi: 10.15585/mmwr.mm6450a3 [DOI] [PubMed] [Google Scholar]
- 5.Hser YI, Saxon AJ, Huang D, et al. Treatment retention among patients randomized to buprenorphine/naloxone compared to methadone in a multi-site trial. Addiction. 2014;109(1):79-87. doi: 10.1111/add.12333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Connery HS. Medication-assisted treatment of opioid use disorder: review of the evidence and future directions. Harv Rev Psychiatry. 2015;23(2):63-75. doi: 10.1097/HRP.0000000000000075 [DOI] [PubMed] [Google Scholar]
- 7.Brand M, Roth-Bauer M, Driessen M, Markowitsch HJ. Executive functions and risky decision-making in patients with opiate dependence. Drug Alcohol Depend. 2008;97(1-2):64-72. doi: 10.1016/j.drugalcdep.2008.03.017 [DOI] [PubMed] [Google Scholar]
- 8.Gowin JL, Sloan ME, Ramchandani VA, Paulus MP, Lane SD. Differences in decision-making as a function of drug of choice. Pharmacol Biochem Behav. 2018;164:118-124. doi: 10.1016/j.pbb.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petry NM, Bickel WK, Arnett M. Shortened time horizons and insensitivity to future consequences in heroin addicts. Addiction. 1998;93(5):729-738. doi: 10.1046/j.1360-0443.1998.9357298.x [DOI] [PubMed] [Google Scholar]
- 10.Verdejo-Garcia A, Chong TTJ, Stout JC, Yücel M, London ED. Stages of dysfunctional decision-making in addiction. Pharmacol Biochem Behav. 2018;164:99-105. doi: 10.1016/j.pbb.2017.02.003 [DOI] [PubMed] [Google Scholar]
- 11.Biernacki K, McLennan SN, Terrett G, Labuschagne I, Rendell PG. Decision-making ability in current and past users of opiates: a meta-analysis. Neurosci Biobehav Rev. 2016;71:342-351. doi: 10.1016/j.neubiorev.2016.09.011 [DOI] [PubMed] [Google Scholar]
- 12.Ahn WY, Vasilev G, Lee SH, et al. Decision-making in stimulant and opiate addicts in protracted abstinence: evidence from computational modeling with pure users. Front Psychol. 2014;5:849. doi: 10.3389/fpsyg.2014.00849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yan WS, Li YH, Xiao L, Zhu N, Bechara A, Sui N. Working memory and affective decision-making in addiction: a neurocognitive comparison between heroin addicts, pathological gamblers and healthy controls. Drug Alcohol Depend. 2014;134:194-200. doi: 10.1016/j.drugalcdep.2013.09.027 [DOI] [PubMed] [Google Scholar]
- 14.Baldacchino A, Balfour DJK, Passetti F, Humphris G, Matthews K. Neuropsychological consequences of chronic opioid use: a quantitative review and meta-analysis. Neurosci Biobehav Rev. 2012;36(9):2056-2068. doi: 10.1016/j.neubiorev.2012.06.006 [DOI] [PubMed] [Google Scholar]
- 15.Biernacki K, Terrett G, McLennan SN, Labuschagne I, Morton P, Rendell PG. Decision-making, somatic markers and emotion processing in opiate users. Psychopharmacology (Berl). 2018;235(1):223-232. doi: 10.1007/s00213-017-4760-0 [DOI] [PubMed] [Google Scholar]
- 16.Verdejo-García AJ, Perales JC, Pérez-García M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2007;32(5):950-966. doi: 10.1016/j.addbeh.2006.06.032 [DOI] [PubMed] [Google Scholar]
- 17.Upton DJ, Kerestes R, Stout JC. Comparing the Iowa and Soochow gambling tasks in opiate users. Front Neurosci. 2012;6:34. doi: 10.3389/fnins.2012.00034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gowin JL, Mackey S, Paulus MP. Altered risk-related processing in substance users: imbalance of pain and gain. Drug Alcohol Depend. 2013;132(1-2):13-21. doi: 10.1016/j.drugalcdep.2013.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myers CE, Rego J, Haber P, et al. Learning and generalization from reward and punishment in opioid addiction. Behav Brain Res. 2017;317:122-131. doi: 10.1016/j.bbr.2016.09.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Myers CE, Sheynin J, Balsdon T, et al. Probabilistic reward- and punishment-based learning in opioid addiction: Experimental and computational data. Behav Brain Res. 2016;296:240-248. doi: 10.1016/j.bbr.2015.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Passetti F, Clark L, Mehta MA, Joyce E, King M. Neuropsychological predictors of clinical outcome in opiate addiction. Drug Alcohol Depend. 2008;94(1-3):82-91. doi: 10.1016/j.drugalcdep.2007.10.008 [DOI] [PubMed] [Google Scholar]
- 22.Ellsberg D. Risk, ambiguity, and the savage axioms. Q J Econ. 1961;75:643-669. doi: 10.2307/1884324 [DOI] [Google Scholar]
- 23.Camerer C, Weber M. Recent developments in modeling preferences—uncertainty and ambiguity. J Risk Uncertain. 1992;5(4):325-370. doi: 10.1007/BF00122575 [DOI] [Google Scholar]
- 24.Krain AL, Wilson AM, Arbuckle R, Castellanos FX, Milham MP. Distinct neural mechanisms of risk and ambiguity: a meta-analysis of decision-making. Neuroimage. 2006;32(1):477-484. doi: 10.1016/j.neuroimage.2006.02.047 [DOI] [PubMed] [Google Scholar]
- 25.Levy I, Snell J, Nelson AJ, Rustichini A, Glimcher PW. Neural representation of subjective value under risk and ambiguity. J Neurophysiol. 2010;103(2):1036-1047. doi: 10.1152/jn.00853.2009 [DOI] [PubMed] [Google Scholar]
- 26.Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49(5):765-775. doi: 10.1016/j.neuron.2006.01.024 [DOI] [PubMed] [Google Scholar]
- 27.Levy I. Neuroanatomical substrates for risk behavior. Neuroscientist. 2017;23(3):275-286. doi: 10.1177/1073858416672414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tymula A, Rosenberg Belmaker LA, Roy AK, et al. Adolescents’ risk-taking behavior is driven by tolerance to ambiguity. Proc Natl Acad Sci U S A. 2012;109(42):17135-17140. doi: 10.1073/pnas.1207144109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Blankenstein NE, Crone EA, van den Bos W, van Duijvenvoorde AC. Dealing with uncertainty: testing risk- and ambiguity-attitude across adolescence. Dev Neuropsychol. 2016;41(1-2):77-92. doi: 10.1080/87565641.2016.1158265 [DOI] [PubMed] [Google Scholar]
- 30.Pushkarskaya H, Tolin D, Ruderman L, et al. Decision-making under uncertainty in obsessive-compulsive disorder. J Psychiatr Res. 2015;69:166-173. doi: 10.1016/j.jpsychires.2015.08.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruderman L, Ehrlich DB, Roy A, Pietrzak RH, Harpaz-Rotem I, Levy I. Posttraumatic stress symptoms and aversion to ambiguous losses in combat veterans. Depress Anxiety. 2016;33(7):606-613. doi: 10.1002/da.22494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Knight FH. Risk, Uncertainty and Profit. Boston, MA: Hart, Schaffner & Marx; Houghton Mifflin Co; 1921. [Google Scholar]
- 33.Schonberg T, Fox CR, Poldrack RA. Mind the gap: bridging economic and naturalistic risk-taking with cognitive neuroscience. Trends Cogn Sci. 2011;15(1):11-19. doi: 10.1016/j.tics.2010.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ekhtiari H, Victor TA, Paulus MP. Aberrant decision-making and drug addiction—how strong is the evidence? Curr Opin Behav Sci. 2017;13:25-33. doi: 10.1016/j.cobeha.2016.09.002 [DOI] [Google Scholar]
- 35.Soyka M, Zingg C, Koller G, Kuefner H. Retention rate and substance use in methadone and buprenorphine maintenance therapy and predictors of outcome: results from a randomized study. Int J Neuropsychopharmacol. 2008;11(5):641-653. doi: 10.1017/S146114570700836X [DOI] [PubMed] [Google Scholar]
- 36.Sordo L, Barrio G, Bravo MJ, et al. Mortality risk during and after opioid substitution treatment: systematic review and meta-analysis of cohort studies. BMJ. 2017;357:j1550. doi: 10.1136/bmj.j1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199-213. doi: 10.1016/0740-5472(92)90062-S [DOI] [PubMed] [Google Scholar]
- 38.Levy I, Rosenberg Belmaker L, Manson K, Tymula A, Glimcher PW. Measuring the subjective value of risky and ambiguous options using experimental economics and functional MRI methods. J Vis Exp. 2012;(67):e3724. doi: 10.3791/3724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gilboa I, Schmeidler D. Maxmin expected utility with non-unique prior. J Math Econ. 1989;18(2):141-153. doi: 10.1016/0304-4068(89)90018-9 [DOI] [Google Scholar]
- 40.Spielsberger CD, Gorsuch RL, Lushene R, Vagg PR, Jacobs GA. Manual for the State-Trait Anxiety Inventory. Palo Alto, CA: Consulting Psychologists Press; 1983. [Google Scholar]
- 41.Handelsman L, Cochrane KJ, Aronson MJ, Ness R, Rubinstein KJ, Kanof PD. Two new rating scales for opiate withdrawal. Am J Drug Alcohol Abuse. 1987;13(3):293-308. doi: 10.3109/00952998709001515 [DOI] [PubMed] [Google Scholar]
- 42.Cervantes EA, Miller WR, Tonigan JS. Comparison of timeline follow-back and averaging methods for quantifying alcohol consumption in treatment research. Assessment. 1994;1(1):23-30. doi: 10.1177/1073191194001001004 [DOI] [PubMed] [Google Scholar]
- 43.Sokol-Hessner P, Raio CM, Gottesman SP, Lackovic SF, Phelps EA. Acute stress does not affect risky monetary decision-making. Neurobiol Stress. 2016;5:19-25. doi: 10.1016/j.ynstr.2016.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frey R, Pedroni A, Mata R, Rieskamp J, Hertwig R. Risk preference shares the psychometric structure of major psychological traits. Sci Adv. 2017;3(10):e1701381. doi: 10.1126/sciadv.1701381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.FeldmanHall O, Glimcher P, Baker AL, Phelps EA. Emotion and decision-making under uncertainty: physiological arousal predicts increased gambling during ambiguity but not risk. J Exp Psychol Gen. 2016;145(10):1255-1262. doi: 10.1037/xge0000205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tymula A, Rosenberg Belmaker LA, Ruderman L, Glimcher PW, Levy I. Like cognitive function, decision making across the life span shows profound age-related changes. Proc Natl Acad Sci U S A. 2013;110(42):17143-17148. doi: 10.1073/pnas.1309909110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sharot T. The optimism bias. Curr Biol. 2011;21(23):R941-R945. doi: 10.1016/j.cub.2011.10.030 [DOI] [PubMed] [Google Scholar]
- 48.Bertz JW, Epstein DH, Preston KL. Combining ecological momentary assessment with objective, ambulatory measures of behavior and physiology in substance-use research. Addict Behav. 2018;83:5-17. doi: 10.1016/j.addbeh.2017.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Martins SS, Fenton MC, Keyes KM, Blanco C, Zhu H, Storr CL. Mood and anxiety disorders and their association with non-medical prescription opioid use and prescription opioid-use disorder: longitudinal evidence from the National Epidemiologic Study on Alcohol and Related Conditions. Psychol Med. 2012;42(6):1261-1272. doi: 10.1017/S0033291711002145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Preston KL, Epstein DH. Stress in the daily lives of cocaine and heroin users: relationship to mood, craving, relapse triggers, and cocaine use. Psychopharmacology (Berl). 2011;218(1):29-37. doi: 10.1007/s00213-011-2183-x [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Evaluating Behavioral Data Quality and Clinic Records and Study Definitions of Treatment Adherence and Illicit Opioid Use
eResults. Intraclass Correlation Coefficients (ICCs) for the Decision-Making Task Parameters in Opioid Use Disorder Participants
eFigure 1. Examples of Acceptable and Poor-Quality Behavioral Data
eFigure 2. Participants Remaining in the Study by Session Number
eFigure 3. CONSORT Diagram of Sessions Excluded and Retained for Analysis
eFigure 4. Distribution of Decision-Making Task Parameters by Diagnostic Group
eTable 1. Time-Lagged Association of Task Variables with Prospective Opioid Use, First 8 Study Sessions Only
eTable 2. Time-Lagged Association of Task Variables with Prospective Opioid Use, Controlling for Medication Type and Dose
eTable 3. Time-Lagged Association of Task Variables with Prospective Opioid Use, Without Positive Sessions Based on Urine Test Samples “Scheduled but Refused or Not Completed”
eTable 4. Comparison of “Full” Clinical Model to the Same Model Including Ambiguity Tolerance in the Time-Lagged Association with Prospective Opioid Use
eTable 5. Comparison of “Best” Clinical Model to the Same Model Including Ambiguity Tolerance in the Time-Lagged Association with Prospective Opioid Use
eTable 6. Diagnostic Group Differences in Decision-Making Task Parameters