Summary
Background
Carboplatin and paclitaxel administered every 3 weeks is standard-of-care first-line chemotherapy for epithelial ovarian cancer. The Japanese JGOG3016 trial showed a significant improvement in progression-free and overall survival with dose-dense weekly paclitaxel and 3-weekly carboplatin. In this study, we aimed to compare efficacy and safety of two dose-dense weekly regimens to standard 3-weekly chemotherapy in a predominantly European population with epithelial ovarian cancer.
Methods
In this phase 3 trial, women with newly diagnosed International Federation of Gynecology and Obstetrics stage IC–IV epithelial ovarian cancer were randomly assigned to group 1 (carboplatin area under the curve [AUC]5 or AUC6 and 175 mg/m2 paclitaxel every 3 weeks), group 2 (carboplatin AUC5 or AUC6 every 3 weeks and 80 mg/m2 paclitaxel weekly), or group 3 (carboplatin AUC2 and 80 mg/m2 paclitaxel weekly). Written informed consent was provided by all women who entered the trial. The protocol had the appropriate national research ethics committee approval for the countries where the study was conducted. Patients entered the trial after immediate primary surgery, or before neoadjuvant chemotherapy with subsequent planned delayed primary surgery. The trial coprimary outcomes were progression-free survival and overall survival. Data analyses were done on an intention-to-treat basis, and were powered to detect a hazard ratio of 0·75 in progression-free survival. The main comparisons were between the control group (group 1) and each of the weekly research groups (groups 2 and 3).
Findings
Between June 6, 2011, and Nov 28, 2014, 1566 women were randomly assigned to treatment. 72% (365), completed six protocol-defined treatment cycles in group 1, 60% (305) in group 2, and 63% (322) in group 3, although 90% (454), 89% (454), and 85% (437) completed six platinum-based chemotherapy cycles, respectively. Paclitaxel dose intensification was achieved with weekly treatment (median total paclitaxel dose 1010 mg/m2 in group 1; 1233 mg/m2 in group 2; 1274 mg/m2 in group 3). By February, 2017, 1018 (65%) patients had experienced disease progression. No significant progression-free survival increase was observed with either weekly regimen (restricted mean survival time 24·4 months [97·5% CI 23·0–26·0] in group 1, 24·9 months [24·0–25·9] in group 2, 25·3 months [23·9–26·9] in group 3; median progression-free survival 17·7 months [IQR 10·6–not reached] in group 1, 20·8 months [11·9–59·0] in group 2, 21·0 months [12·0–54·0] in group 3; log-rank p=0·35 for group 2 vs group 1; group 3 vs 1 p=0·51). Although grade 3 or 4 toxic effects increased with weekly treatment, these effects were predominantly uncomplicated. Febrile neutropenia and sensory neuropathy incidences were similar across groups.
Interpretation
Weekly dose-dense chemotherapy can be delivered successfully as first-line treatment for epithelial ovarian cancer but does not significantly improve progression-free survival compared with standard 3-weekly chemotherapy in predominantly European populations.
Funding
Cancer Research UK, Medical Research Council, Health Research Board in Ireland, Irish Cancer Society, Cancer Australia.
Introduction
Epithelial ovarian cancer is the most common cause of gynaecological cancer-related death in high-income countries.1 The majority of women with ovarian cancer present with advanced disease and, despite primary multimodality treatment with cytoreductive surgery and chemotherapy, most patients experience disease relapse within 2 years of diagnosis. For more than two decades, platinum–paclitaxel doublet chemotherapy administered every 3 weeks for six to eight cycles has been the reference standard of care first-line treatment for ovarian cancer.2, 3 Chemotherapy is most commonly administered after primary surgery. However, in women for whom complete primary cytoreduction is deemed unlikely, delayed surgery after three to four primary (neoadjuvant) chemotherapy cycles has been widely adopted. This approach follows publication of two randomised trials4, 5, 6 showing that overall survival is not inferior to upfront surgery and is associated with less perioperative morbidity. It is, therefore, important that first-line trials include the option of primary chemotherapy.
Research in context.
Evidence before this study
Carboplatin and paclitaxel administered every 3 weeks has been a standard component of first-line epithelial ovarian cancer treatment for more than 20 years. Preclinical studies showed that metronomic taxane scheduling improved drug delivery, increased tumour apoptosis, and decreased angiogenesis. We searched PubMed for clinical trials published between Jan 1, 1990, and Dec 31, 2018, using the terms “ovarian cancer” AND “chemotherapy” AND “paclitaxel” AND “progression-free survival”, to identify phase 3 trials which evaluated weekly dose-dense paclitaxel scheduling throughout first-line epithelial ovarian cancer treatment. Three relevant trials were identified.
The Japanese JGOG3016 trial randomly assigned women with International Federation of Gynecology and Obstetrics (FIGO) stage II–IV epithelial ovarian cancer to 3-weekly carboplatin–paclitaxel or a regimen incorporating weekly paclitaxel (delivered to a higher total dose), and 3-weekly carboplatin. Clinically significant improvements in progression-free survival and overall survival were reported. However, weekly paclitaxel was associated with increased toxicity, which negatively affected treatment delivery.
MITO-7, a European trial, randomly assigned women with stage IC–IV epithelial ovarian cancer to 3-weekly carboplatin–paclitaxel or both drugs given weekly with the same planned total paclitaxel dose. No progression-free survival difference was reported. Weekly chemotherapy was better tolerated and associated with improved quality of life. However, the lack of paclitaxel dose intensification and weekly carboplatin scheduling might have negatively affected the outcome of the weekly treatment.
GOG-262, an American study, randomly assigned women with stage II-IV epithelial ovarian cancer to 3-weekly carboplatin–paclitaxel or weekly treatment identical to JGOG3016. 84% of GOG-262 participants opted to receive the anti-angiogenic bevacizumab, in addition to chemotherapy. No progression-free survival difference was observed between treatment groups, although weekly paclitaxel was better tolerated than in JGOG3016. However, in the subgroup who did not receive bevacizumab, weekly paclitaxel improved progression-free survival. It is not possible to conclude whether the use of maintenance bevacizumab masked a weekly paclitaxel treatment effect in the whole trial population.
Added value of this study
In ICON8, 1566 women with FIGO stage IC–IV epithelial ovarian cancer were randomly assigned to 3-weekly carboplatin–paclitaxel, 3-weekly carboplatin and weekly dose-dense paclitaxel (replicating the JGOG3016 weekly regimen), or weekly carboplatin with weekly dose-dense paclitaxel. Patients entered ICON8 after immediate primary surgery or received neoadjuvant chemotherapy with delayed primary surgery. Neither weekly regimen improved progression-free survival compared with standard 3-weekly treatment. Although most patients were able to complete six chemotherapy cycles, both weekly treatments were associated with more treatment modifications and a higher incidence of grade 3 or higher toxic effects.
Implications of all the available evidence
Weekly dose-dense paclitaxel should no longer be recommended as a component of first-line epithelial ovarian cancer treatment for women of non-Japanese ethnic origin. Because potential ethnic pharmacogenomic differences might affect the efficacy and toxicity of paclitaxel, dose-dense paclitaxel could remain a first-line treatment option for Japanese women with ovarian cancer.
In the past decade, there has been significant interest in the evaluation of weekly dose-dense paclitaxel scheduling. Preclinical evidence suggests that metronomic taxane administration improves drug delivery, increases tumour cell apoptosis, and reduces angiogenesis.7, 8 Weekly paclitaxel treatment is an efficacious and well tolerated approach in recurrent platinum-resistant ovarian cancer,9, 10, 11 and confers a survival advantage compared with 3-weekly scheduling in both the adjuvant and metastatic settings for breast cancer.12, 13
The JGOG3016 trial14, 15 randomly assigned 637 Japanese women with newly diagnosed epithelial ovarian cancer to receive conventional 3-weekly (ie, once every 3 weeks) carboplatin–paclitaxel chemotherapy or an investigational group in which weekly dose-dense paclitaxel at a dose of 80 mg/m2 was administered with 3-weekly carboplatin. Weekly treatment resulted in an 11-month prolongation of median progression-free survival and a corresponding 38-month improvement in median overall survival. However, weekly dose-dense treatment caused more haematological and neurological toxic effects, which compromised the ability to deliver six cycles of treatment. In contrast, the evaluation of weekly scheduling of both carboplatin and paclitaxel in phase 2 trials showed both excellent tolerability and promising efficacy.16, 17
The Gynecologic Cancer Intergroup (GCIG) International Collaboration on Ovarian Neoplasms 8 (ICON8) trial was designed to evaluate whether the incorporation of dose-dense weekly paclitaxel into first-line treatment of ovarian cancer in a predominantly European patient group would improve survival outcomes, and to determine whether weekly scheduling of carboplatin would reduce haematological toxicity, improve deliverability, and maintain the efficacy of dose-dense paclitaxel.
Methods
Study design and participants
ICON8 was an international, open-label, randomised phase 3, three-arm trial of weekly dose-dense chemotherapy as first-line treatment in patients with histologically confirmed epithelial ovarian, primary peritoneal, or fallopian tube carcinoma (herein collectively termed ovarian cancer). Patients were recruited from 117 sites in the UK, Australia, New Zealand, Mexico, South Korea, and Republic of Ireland (appendix pp 6, 7). All patients were 18 years or older, had International Federation of Gynecology and Obstetrics (FIGO; 1988) stage IC–IV cancer (with mandatory high-risk histological subtype for patients with FIGO 1988 stage IC or IIA disease); had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2; life expectancy longer than 12 weeks; and adequate haematological, renal, and hepatic functions; and be able to start chemotherapy within 8 weeks after IPS. They had not received previous systemic therapy for ovarian cancer and were not planned to receive maintenance treatment after completion of protocol therapy. All patients gave written informed consent to join the trial.
In the UK, ethical approval was granted by the London–Chelsea research ethics committee. The trial also received ethical approval from appropriate national or local institutional review boards in other jurisdictions. The protocol can be found online.
Randomisation and masking
Patients were randomly assigned (1:1:1) to one of three treatment groups: group 1 (control) for 3-weekly carboplatin area under the curve (AUC)5 or AUC6 and 3-weekly paclitaxel 175 mg/m2; group 2 for 3-weekly carboplatin AUC5 or AUC6 and weekly paclitaxel 80 mg/m2; and group 3 for weekly carboplatin AUC2 and weekly paclitaxel 80 mg/m2, given in all groups for six cycles. Patients were randomly assigned using the Medical Research Council Clinical Trials Unit (MRC CTU) at University College London (UCL) randomisation telephone service; the method of minimisation was used with stratification factors of GCIG group, disease stage (FIGO stage IC high grade serous, clear cell, or grade III carcinoma; FIGO stage IIA high grade serous, clear cell, or grade III carcinoma; FIGO stage IIB; FIGO stage IIC; FIGO stage IIIA; FIGO stage IIIB; FIGO stage IIIC; FIGO stage IV), and outcome and timing of surgery (IPS plus FIGO stage IC–III with no visible residual disease; IPS plus FIGO stage IC–III with residual disease ≤1 cm; IPS plus FIGO stage IV or FIGO stage IC–III with residual disease >1 cm; no surgery currently planned; or DPS is planned). Patients and clinicians were not masked to their allocated group.
Procedures
Patients were able to enter the trial after upfront (immediate) primary cytoreductive surgery (IPS) or could receive primary (neoadjuvant) chemotherapy with a plan for delayed primary cytoreductive surgery (DPS) at the decision of the local multidisciplinary gynaecological oncology team. Patients were also eligible for the trial if there was no plan for surgery.
All participants started chemotherapy within 2 weeks of randomisation. Patients in group 1 received carboplatin AUC5 or AUC6 by intravenous infusion over 30–60 min and paclitaxel 175 mg/m2 by intravenous infusion over 3 h on day 1 of a 21-day cycle for six cycles; patients in group 2 received carboplatin as in group 1 and dose-fractionated paclitaxel 80 mg/m2 by intravenous infusion over 1 h on days 1, 8, and 15 of a 21-day cycle for six cycles; and patients in group 3 received carboplatin AUC2 by intravenous infusion over 30–60 min on day 1 and paclitaxel 80 mg/m2 by intravenous infusion over 1 h on days 1, 8, and 15 of a 21-day cycle for six cycles. Carboplatin dose was calculated using the Calvert formula with starting AUC determined by the method used to calculate renal function at trial entry: AUC5 for radioisotopic glomerular filtration rate, measured 24 h urinary creatinine clearance and Wright formula estimation, and AUC6 for modified Cockcroft-Gault or Jelliffe formula estimation.18, 19, 20, 21
In all groups, treatment proceeded on day 1 of each cycle if absolute neutrophil count (ANC) was 1·0 × 109 cells per L or more and platelet count was 75 × 109 cells per L or more. In group 2, paclitaxel was administered at days 8 and 15 if ANC was 0·5 × 109 cells per L or more and platelets 50 × 109 cells per L or more; if these haematological parameters were not met, paclitaxel was omitted. In group 3, day 8 and day 15 weekly carboplatin and paclitaxel were administered if ANC was 1·0 × 109 cells per L or more and platelet count 75 × 109 cells per L or more; both drugs were deferred if these values were not achieved. Protocol-defined dose alterations (delay, reduction, omissions) were allowed for haematological and other toxic effects if deemed clinically necessary. Single-agent carboplatin was accepted as protocol treatment if patients were unable to tolerate paclitaxel. In the event of carboplatin hypersensitivity, trial management guidelines allowed continuation of carboplatin with increased hypersensitivity prophylaxis, the use of a formal carboplatin desensitisation regimen, or a switch to cisplatin for severe or recurrent hypersensitivity.
Surgery was done either as IPS, ie, before randomisation, followed by six cycles of chemotherapy; or as planned DPS, ie three cycles of chemotherapy, followed by surgery, which was subsequently followed by a further three cycles of chemotherapy; however, surgery could be done at a later date if deemed clinically appropriate. Day 15 treatment was omitted from the chemotherapy cycle before DPS to prevent chemotherapy-related toxic effects affecting surgical timing and it was recommended that surgery was done within 32 days of the start of the presurgery chemotherapy cycle.
During chemotherapy, patients were seen 3-weekly before administration of day 1 of each chemotherapy cycle, and an end-of-treatment visit occurred 6 weeks after day 1 of the last cycle of protocol chemotherapy. Thereafter, patients were followed up 6-weekly from the end of treatment visit until 9 months after randomisation, then 3-monthly until 2 years after randomisation, then 6-monthly for a further 4 years, and annually thereafter. After disease progression, patients were followed up 6-monthly until 6 years and annually thereafter.
In all patients, baseline disease evaluation was done by abdominopelvic CT and chest radiograph. CT scans were repeated 6 weeks after the final cycle of protocol chemotherapy in all patients. In those receiving primary chemotherapy, two additional CT scans were done: after three cycles of chemotherapy to allow surgical planning and then 4 weeks after DPS. All imaging was reported using Response Evaluation Criteria in Solid Tumours (RECIST) version 1.1.22 Serum CA125 tumour marker measurements were done at baseline, on day 1 of each treatment cycle, and at each follow-up visit. During follow-up, routine imaging was not mandatory but was triggered by clinical symptoms suggestive of disease progression or when the GCIG criteria for CA125 progression were met.23 Disease progression was defined by RECIST version 1.1 on the basis of radiological, clinical, or symptomatic indicators of disease progression and did not include isolated, asymptomatic CA125 progression. For women in whom CA125 progression occurred in the absence of radiological progression by RECIST version 1.1, repeat imaging was mandated 3-monthly until RECIST progression.
Outcomes
The trial had two co-primary outcomes: progression-free survival and overall survival. For each outcome, two comparisons are made: group 2 versus group 1 and group 3 versus group 1. For both progression-free survival and overall survival, the timing of primary analyses was event-driven and required 602 events in each comparison. It was anticipated that the progression-free survival analyses would occur approximately 2 years before the overall survival analyses. This Article reports the mature progression-free survival outcome and preliminary overall survival results.
Progression-free survival was calculated from the date of randomisation to the date of the first indication of disease progression or death from any cause, whichever occurred first in the intention-to-treat population (ie, all patients). Patients were censored on the date last seen, be that the date of last assessment when patient was confirmed to be alive with no progression, or the date a patient was confirmed to be lost to follow up or withdrawn from the trial.
Overall survival was calculated from date of randomisation to the date of death from any cause in the intention-to-treat population.
Preplanned secondary outcomes were safety, quality of life, and health economics (latter not reported in this Article). Adverse events were graded according to the National Cancer Institute Common Toxicity Criteria for Adverse Events (CTCAE) version 4, and were collected at baseline and at every chemotherapy cycle. Quality of life was collected using the European Organisation for Research and Treatment of Cancer quality-of-life questionnaire (QLQ)-C30, QLQ-OV28, and EQ5D tools at baseline, all chemotherapy cycles, and at all scheduled follow-up visits.
To evaluate treatment delivery, definitions of completed per-protocol treatment cycles were prespecified: in group 1 the administration of both carboplatin and paclitaxel on day 1; in group 2 the administration of carboplatin on day 1 and at least two doses of weekly paclitaxel in a 3-week cycle; and in group 3 concurrent administration of at least two weekly doses of carboplatin and paclitaxel in a 3-week cycle. In addition, the number of completed platinum cycles, defined as all cycles in which an adequate 3-weekly dose of carboplatin (or cisplatin in those with severe or recurrent carboplatin hypersensitivity) was administered with or without paclitaxel, was recorded. Total dose received and dose intensities for carboplatin and paclitaxel were also calculated for each participant. Allowance was made for presurgery day 15 treatment omission in patients who underwent DPS.
Statistical analysis
ICON8 was powered to detect a hazard ratio [HR] of 0·75 in both progression-free survival and overall survival. For both analyses, the main comparisons are between the control group (group 1) and each of the research groups (ie, group 1 vs group 2 and group 1 vs group 3). Each comparison was analysed using a two-sided 97·5% CI, to take the multiple comparisons into account. To calculate sample size, we estimated that 70% of patients would enter the trial following the IPS pathway with an estimated median progression-free survival of 18 months, and 30% of patients would follow the DPS pathway with an estimated median progression-free survival of 12 months, resulting in an overall median progression-free survival of 16·2 months. Assuming exponential survival and comparing the treatment groups using an unadjusted log-rank test, 602 events were required for each comparison to achieve 90% power, resulting in a total sample size of 1485 patients.
The unadjusted log-rank test was used as the primary test to determine if there was a difference between the Kaplan-Meier survival curves. A Cox proportional hazards model was to be used as the primary estimation of treatment effect if the proportional hazards assumption was satisfied. However, if evidence of non-proportional hazards was found, the restricted mean survival time was to be used as the primary measure of the estimation of the size of treatment effect.
Safety was analysed as the worst grade reported for each event in the intention-to-treat population.
An exploratory analysis with limited power comparing groups 2 and 3 was preplanned, in the event that both research groups were found to significantly improve progression-free survival compared with the control group.
The independent data monitoring committee met annually and had overall oversight of all emerging data within the study. All analyses were done using Stata version 15. The trial is registered on ClinicalTrials.gov (number NCT01654146) and ISRCTN Registry (number ISRCTN10356387).
Role of the funding source
The funders had no role in study design, data collection, data analysis, data interpretation, or in the writing of the report. ECJ, ARC, ADC, and RK had full access to all the data in the study, and the Trial Management Group had final responsibility for the decision to submit for publication.
Results
Between June 6, 2011, and Nov 28, 2014, 1566 patients were recruited into ICON8 (522 were included in group 1, 523 in group 2, and 521 in group 3). Baseline characteristics were well balanced across groups (table 1). The median age was 62 years (IQR 54–68) and 1444 (93%) participants were ECOG performance status 0–1, 1073 (69%) had high grade serous carcinoma, and 1119 (72%) had disease stage IIIC or higher. 746 (48%) of patients underwent IPS followed by chemotherapy, whereas 779 (50%) patients entered the study with a plan to undergo DPS; these proportions are different to the 70:30 ratio expected at trial conception. 41 (3%) patients for whom surgical intervention was not deemed to be appropriate joined the trial (table 1).
Table 1.
Baseline characteristics
| Group 1 (n=522) | Group 2 (n=523) | Group 3 (n=521) | ||
|---|---|---|---|---|
| Age, years | 63 (55–68) | 61 (54–67) | 62 (53–68) | |
| Participating group | ||||
| UK | 465 (89%) | 468 (89%) | 464 (89%) | |
| Korea | 10 (2%) | 12 (2%) | 10 (2%) | |
| Ireland | 9 (2%) | 7 (1%) | 8 (2%) | |
| Australia and New Zealand | 24 (5%) | 23 (4%) | 23 (4%) | |
| Mexico | 14 (3%) | 13 (2%) | 16 (3%) | |
| Origin | ||||
| Ovary (epithelial) | 420 (81%) | 424 (81%) | 433 (83%) | |
| Fallopian tube | 24 (5%) | 27 (5%) | 21 (4%) | |
| Primary peritoneal | 77 (15%) | 70 (13%) | 65 (13%) | |
| Missing data | 1 (<1%) | 2 (<1%) | 2 (<1%) | |
| Histological type | ||||
| High-grade serous carcinoma | 365 (70%) | 346 (66%) | 362 (69%) | |
| Low-grade serous carcinoma | 9 (2%) | 11 (2%) | 8 (2%) | |
| Serous (no grade specified) carcinoma | 8 (2%) | 8 (2%) | 9 (2%) | |
| Clear cell | 32 (6%) | 41 (8%) | 34 (7%) | |
| Endometrioid | 26 (5%) | 19 (4%) | 22 (4%) | |
| Carcinosarcoma | 2 (<1%) | 7 (1%) | 3 (1%) | |
| Mixed or other types | 80 (15%) | 91 (17%) | 83 (16%) | |
| FIGO stage | ||||
| IC or IIA | 56 (11%) | 56 (11%) | 52 (10%) | |
| IIB or IIC | 47 (9%) | 47 (9%) | 37 (7%) | |
| IIIA or IIIB | 43 (8%) | 55 (11%) | 54 (10%) | |
| IIIC | 273 (52%) | 266 (51%) | 272 (52%) | |
| IV | 103 (20%) | 99 (19%) | 106 (20%) | |
| ECOG performance status | ||||
| 0 | 246 (47%) | 250 (48%) | 235 (45%) | |
| 1 | 237 (45%) | 230 (44%) | 246 (47%) | |
| 2 | 37 (7%) | 40 (8%) | 39 (7%) | |
| Missing data | 2 (<1%) | 3 (1%) | 1 (<1%) | |
| Timing of surgery | ||||
| Immediate | 251 (48%) | 247 (47%) | 248 (48%) | |
| Delayed | 257 (49%) | 263 (50%) | 259 (50%) | |
| Inoperable | 14 (3%) | 13 (2%) | 14 (3%) | |
Data are median (IQR), n (%), or n. FIGO=International Federation of Gynecology and Obstetrics. ECOG=Eastern Cooperative Oncology Group.
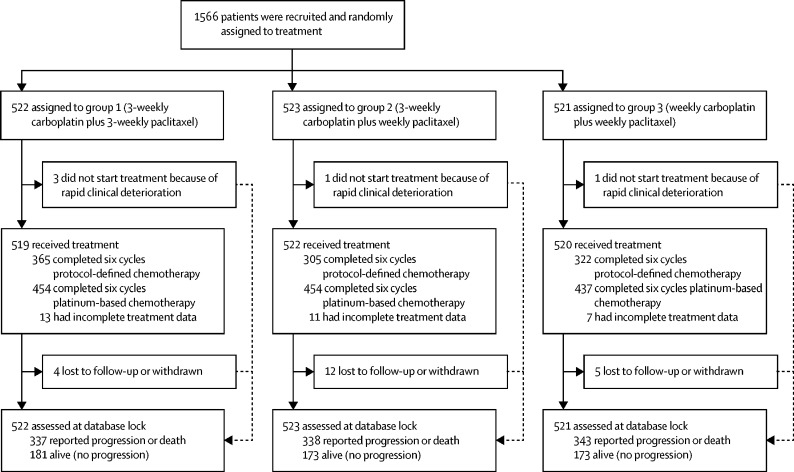
Five patients did not commence protocol treatment because of rapid clinical deterioration (figure 1; appendix p 1). Of the 1561 women who started chemotherapy, complete treatment information is available for 1530. In group 1, 365 (72%) of 506 patients completed six cycles of per-protocol therapy. This proportion was higher than that in groups 2 (305 [60%] of 511) and 3 (322 [63%] of 513). However, more than 85% of all women received six cycles of platinum-based chemotherapy (454 [90%] in group 1; 454 [89%] in group 2; and 437 [85%] in group 3; appendix p 1). The incidence of both dose delays and omissions was higher in groups 2 and 3, although the proportion of patients requiring a dose reduction was similar in all three groups (appendix p 1). To evaluate the effect of weekly dose-dense paclitaxel on treatment delivery, total administered dose and relative dose intensity was calculated for all patients. Of note, although median total carboplatin dose was similar in all groups (AUC30 [IQR 29–35] in group 1 vs AUC30 [29–34] in group 2 vs AUC33 [30–35] in group 3), the median total paclitaxel dose administered was higher in the two weekly dose-dense arms (1010 mg/m2 [840–1050] vs 1233 mg/m2 [1020–1357] vs 1274 mg/m2 [1042–1368]; appendix p 1), indicating that, although more treatment alterations occurred in groups 2 and 3, the paclitaxel dose was intensified with weekly dosing.
Figure 1.
Trial profile
Information on surgery was available for 708 (91%) of 779 patients who entered the trial with planned DPS. 602 (77%) underwent interval cytoreductive surgery and complete cytoreduction was achieved in 331 (56%) patients of this cohort (appendix p 2). Neither the proportion of patients who proceeded to DPS nor surgical outcome, as measured by cytoreductive status and incidence of perioperative complications, differed between the three groups.
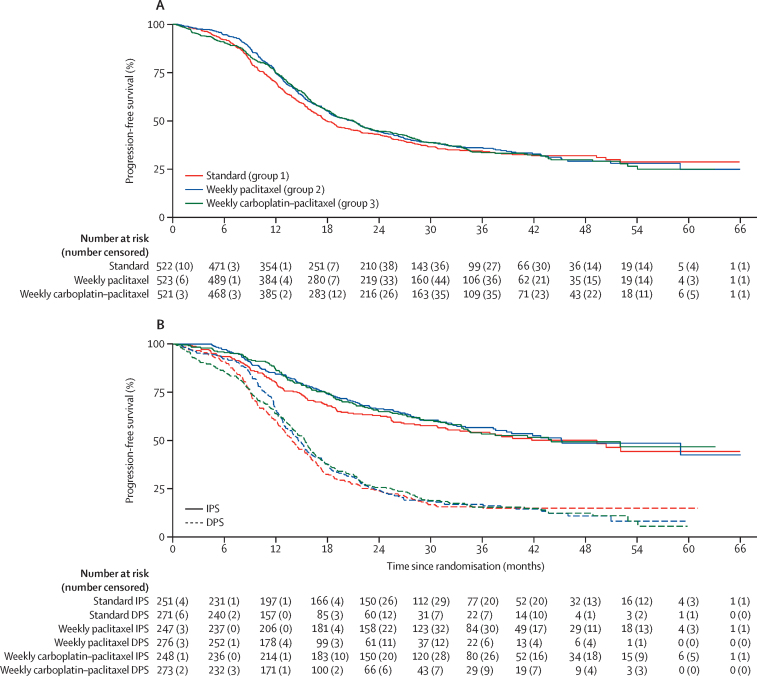
On Feb 20, 2017, after a median follow-up of 36·8 months, 1018 patients had disease progression or had died (337 in group 1, 338 in group 2, and 343 in group 3; figure 1). Evidence of non-proportional hazards was observed (group 1 vs group 2 p=0·011; group 1 vs group 2 p=0·051; figure 2), which, therefore, means that hazard ratios are not robust over time, and so the restricted mean survival time (RMST) is the most appropriate primary estimate of treatment effect. RMST for progression was 24·5 months (97·5% CI 23·0–26·0) in group 1, 24·9 months (24·0–25·9) in group 2, and 25·4 months in group 3 (23·9–26·9). There was no statistically significant difference for progression-free survival for either group (group 1 vs group 2 log-rank p=0·35; group 1 vs group 3 log-rank p=0·51; figure 2). Median progression-free-survival was 17·7 months (IQR 10·6–not reached) for group 1, 20·8 months (11·9–59·0) for group 2, and 21·0 months (12·0–54·0) for group 3.
Figure 2.
Progression-free survival
(A) Overall. (B) Split by immediate and delayed surgery. IPS=primary cytoreductive surgery. DPS=delayed primary cytoreductive surgery.
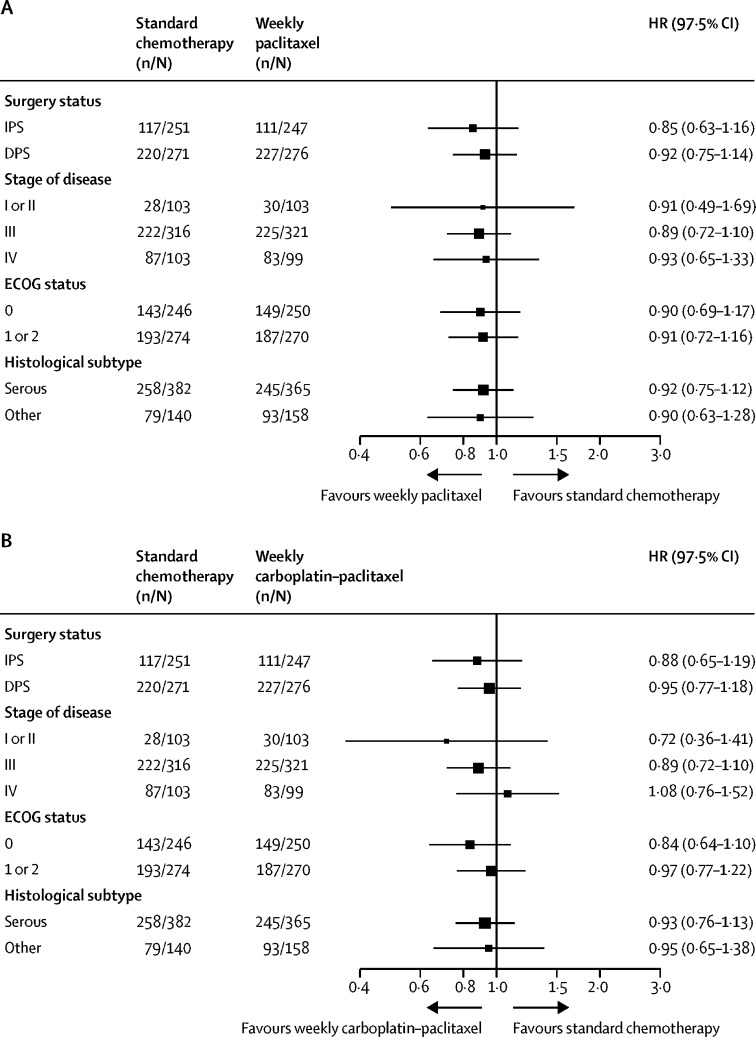
A preplanned subgroup analysis was done to evaluate the effect of weekly chemotherapy on progression-free survival in patients who had undergone IPS and those with a plan for DPS separately (figure 2B). In the IPS cohort, RMST for progression-free survival was 32·6 months (97·5% CI 30·7–34·5) in group 1, 33·0 months (31·5–34·5) in group 2, and 33·3 months (31·5–35·1) in group 3, whereas in those patients planned for DPS, RMST was 18·6 (17·0–20·2), 19·1 (18·0–20·3), and 19·6 months (18·0–21·2). Therefore, no interaction between surgical approach and weekly dose-dense treatment was detected. The superior progression-free survival observed for patients who underwent IPS reflects the different FIGO stage profile in this group compared with patients who entered with a plan for DPS (appendix p 3). Further exploratory analyses did not detect any heterogeneity of treatment effect in subgroups based on disease stage, ECOG performance status, or histological subtype (figure 3).
Figure 3.
Subgroup analysis of progression-free survival.
(A) Group 1 versus group 2. (B) Group 1 versus group 3. ECOG=Eastern Cooperative Oncology Group.
At the time of the primary progression-free survival analysis, 539 deaths had been reported (190 in group 1, 178 in group 2, and 171 in group 3; 61% and 60% of total events required for the final overall survival analysis of each comparison in group 1 vs group 2 and group 1 vs group 3, respectively). Estimated 2-year survival was 80% (95% CI 76–83) in group 1, 82% (78–85) in group 2, and 78% (74–81) in group 3 (appendix p 5). A further six deaths have since been identified as occurring before Feb 20, 2017, but these events are not included in the analysis because they were not reported before the database lock.
The toxic effects experienced by patients during trial chemotherapy are given in detail in table 2 and appendix (p 4). Grade 3 or 4 toxic effects were reported in 213 (42%) patients in group 1, 320 (62%) patients in group 2, and 269 (53%) patients in group 3. The major contributor to the higher incidence of grade 3 or higher events in both weekly treatment groups was uncomplicated neutropenia, which occurred in 76 (15%) of 508 patients in group 1, 181 (35%) of 513 patients in group 2, and 152 (30%) of 510 patients in group 3. This increase might partly be explained by the requirement for weekly full blood count evaluations in groups 2 and 3 compared with every 3 weeks evaluations in group 1. The incidence of febrile neutropenia was low across all three groups with 21 (4%) in group 1, 31 (6%) in group 2, and 16 (3%) in group 3, with no significant difference between groups 2 or 3 and group 1 (group 1 vs group 2 difference 2% [95% CI −0·7 to 4·7], p=0·14; group 1 vs group 3 difference −1% [–3·3 to 1·3], p=0·39). However, the incidence of grade 3 or higher anaemia was significantly higher in patients assigned to group 2 compared with those receiving standard 3-weekly chemotherapy (25 [5%] in group 1 vs 65 [13%]in group 2, difference 8% [4·5–11·5]; p<0·0001]) but not in patients assigned to group 3 (24 [5%], difference 0% [–2·7 to 2·7]; p=1·00).
Table 2.
Adverse events of any grade reported by 10% or more patients in any treatment group
|
Group 1 (n=508) |
Group 2 (n=513) |
Group 3 (n=510) |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | Grade 1 | Grade 2 | Grade 3 | Grade 4 | Grade 5 | |
| Blood and lymphatic system disorders | |||||||||||||||
| Anaemia | 178 (34%) | 134 (26%) | 24 (5%) | 1 (<1%) | 0 | 101 (19%) | 268 (51%) | 65 (12%) | 0 | 0 | 173 (33%) | 183 (35%) | 24 (5%) | 0 | 0 |
| Gastrointestinal disorders | |||||||||||||||
| Constipation | 258 (49%) | 101 (19%) | 4 (<1%) | 2 (<1%) | 0 | 236 (45%) | 86 (16%) | 2 (<1%) | 0 | 0 | 235 (45%) | 91 (17%) | 6 (1%) | 0 | 0 |
| Diarrhoea | 135 (26%) | 34 (7%) | 11 (2%) | 0 | 0 | 164 (31%) | 53 (10%) | 10 (2%) | 0 | 0 | 147 (28%) | 62 (12%) | 14 (3%) | 0 | 0 |
| Mucositis oral | 123 (24%) | 23 (4%) | 1 (<1%) | 0 | 0 | 160 (31%) | 27 (5%) | 0 | 0 | 0 | 138 (26%) | 36 (7%) | 1 (<1%) | 0 | 0 |
| Nausea | 225 (43%) | 94 (18%) | 13 (2%) | 0 | 0 | 226 (43%) | 81 (15%) | 13 (2%) | 0 | 0 | 232 (45%) | 69 (13%) | 5 (<1%) | 0 | 0 |
| Vomiting | 112 (21%) | 41 (8%) | 16 (3%) | 1 (<1%) | 0 | 103 (20%) | 44 (8%) | 18 (3%) | 0 | 0 | 83 (16%) | 32 (6%) | 6 (1%) | 0 | 0 |
| Other | 86 (16%) | 36 (7%) | 7 (1%) | 2 (<1%) | 0 | 120 (23%) | 30 (6%) | 5 (<1%) | 0 | 0 | 118 (23%) | 35 (7%) | 4 (<1%) | 1 (<1%) | 0 |
| General disorders and administration site conditions | |||||||||||||||
| Fatigue | 255 (49%) | 182 (35%) | 15 (3%) | 0 | 0 | 233 (45%) | 205 (39%) | 26 (5%) | 0 | 0 | 252 (48%) | 192 (37%) | 17 (3%) | 0 | 0 |
| Pain | 179 (34%) | 83 (16%) | 17 (3%) | 0 | 0 | 178 (34%) | 78 (15%) | 9 (2%) | 0 | 0 | 177 (34%) | 70 (13%) | 12 (2%) | 0 | 0 |
| Immune system disorders | |||||||||||||||
| Allergic reaction | 15 (3%) | 37 (7%) | 7 (1%) | 1 (<1%) | 0 | 20 (4%) | 24 (5%) | 4 (<1%) | 2 (<1%) | 0 | 27 (5%) | 57 (11%) | 4 (<1%) | 2 (<1%) | 0 |
| Infections and infestations | |||||||||||||||
| All | 37 (7%) | 64 (12%) | 18 (3%) | 3 (<1%) | 0 | 40 (8%) | 97 (19%) | 25 (5%) | 0 | 0 | 51 (10%) | 96 (18%) | 18 (3%) | 4 (<1%) | 0 |
| Injury, poisoning and procedural complications | |||||||||||||||
| All | 6 (1%) | 6 (1%) | 1 (<1%) | 0 | 0 | 5 (<1%) | 4 (<1%) | 2 (<1%) | 0 | 0 | 8 (2%) | 10 (2%) | 2 (<1%) | 0 | 0 |
| Investigations | |||||||||||||||
| ALT or AST elevation | 101 (19%) | 9 (2%) | 4 (<1%) | 0 | 0 | 132 (25%) | 22 (4%) | 4 (<1%) | 0 | 0 | 127 (24%) | 16 (3%) | 5 (<1%) | 1 (<1%) | 0 |
| Creatinine increased | 45 (9%) | 5 (<1%) | 3 (<1%) | 0 | 0 | 66 (13%) | 8 (2%) | 1 (<1%) | 0 | 0 | 30 (6%) | 3 (<1%) | 1 (<1%) | 0 | 0 |
| Neutrophil count decreased | 107 (20%) | 65 (12%) | 59 (11%) | 17 (3%) | 0 | 76 (15%) | 119 (23%) | 146 (28%) | 35 (7%) | 0 | 76 (15%) | 114 (22%) | 133 (26%) | 19 (4%) | 0 |
| Platelet count decreased | 116 (22%) | 26 (5%) | 17 (3%) | 4 (<1%) | 0 | 144 (28%) | 47 (9%) | 43 (8%) | 5 (<1%) | 0 | 108 (21%) | 23 (4%) | 11 (2%) | 5 (<1%) | 0 |
| Weight loss | 54 (10%) | 17 (3%) | 0 | 0 | 0 | 49 (9%) | 16 (3%) | 1 (<1%) | 0 | 0 | 63 (12%) | 15 (3%) | 1 (<1%) | 0 | 0 |
| White blood cells decreased | 143 (27%) | 68 (13%) | 20 (4%) | 2 (<1%) | 0 | 116 (22%) | 148 (28%) | 71 (14%) | 9 (2%) | 0 | 116 (22%) | 139 (27%) | 69 (13%) | 2 (<1%) | 0 |
| Other investigations | 52 (10%) | 14 (3%) | 8 (2%) | 1 (<1%) | 0 | 69 (13%) | 26 (5%) | 16 (3%) | 3 (<1%) | 0 | 66 (13%) | 16 (3%) | 10 (2%) | 0 | 0 |
| Metabolism and nutrition disorders | |||||||||||||||
| Anorexia | 91 (17%) | 43 (8%) | 6 (1%) | 0 | 0 | 80 (15%) | 36 (7%) | 2 (<1%) | 0 | 0 | 87 (17%) | 33 (6%) | 3 (<1%) | 0 | 0 |
| Other metabolism and nutrition disorders | 47 (9%) | 12 (2%) | 4 (<1%) | 0 | 0 | 57 (11%) | 15 (3%) | 11 (2%) | 1 (<1%) | 0 | 46 (9%) | 10 (2%) | 9 (2%) | 1 (<1%) | 0 |
| Musculoskeletal and connective tissue disorders | |||||||||||||||
| Arthralgia | 109 (21%) | 63 (12%) | 7 (1%) | 0 | 0 | 93 (18%) | 14 (3%) | 2 (<1%) | 0 | 0 | 79 (15%) | 16 (3%) | 0 | 0 | 0 |
| Muscle weakness | 52 (10%) | 11 (2%) | 0 | 0 | 0 | 50 (10%) | 7 (1%) | 3 (<1%) | 0 | 0 | 49 (9%) | 9 (2%) | 0 | 0 | 0 |
| Myalgia | 98 (19%) | 41 (8%) | 6 (1%) | 0 | 0 | 78 (15%) | 15 (3%) | 1 (<1%) | 0 | 0 | 81 (16%) | 16 (3%) | 0 | 0 | 0 |
| Nervous system disorders | |||||||||||||||
| Peripheral motor neuropathy | 57 (11%) | 20 (4%) | 0 | 0 | 0 | 59 (11%) | 20 (4%) | 3 (<1%) | 0 | 0 | 45 (9%) | 18 (3%) | 1 (<1%) | 0 | 0 |
| Peripheral sensory neuropathy | 255 (49%) | 132 (25%) | 11 (2%) | 0 | 0 | 226 (43%) | 105 (20%) | 21 (4%) | 0 | 0 | 239 (46%) | 109 (21%) | 8 (2%) | 0 | 0 |
| Other nervous system disorders | 52 (10%) | 10 (2%) | 4 (<1%) | 0 | 0 | 45 (9%) | 12 (2%) | 3 (<1%) | 0 | 0 | 36 (7%) | 13 (2%) | 2 (<1%) | 0 | 0 |
| Renal and urinary disorders | |||||||||||||||
| All | 34 (7%) | 23 (4%) | 2 (<1%) | 0 | 0 | 34 (7%) | 27 (5%) | 3 (<1%) | 0 | 0 | 54 (10%) | 23 (4%) | 5 (<1%) | 0 | 0 |
| Respiratory, thoracic, and mediastinal disorders | |||||||||||||||
| All | 82 (16%) | 13 (2%) | 11 (2%) | 1 (<1%) | 0 | 99 (19%) | 33 (6%) | 4 (<1%) | 1 (<1%) | 0 | 94 (18%) | 24 (5%) | 7 (1%) | 1 (<1%) | 0 |
| Skin and subcutaneous tissue disorders | |||||||||||||||
| Alopecia | 36 (7%) | 428 (82%) | 0 | 0 | 0 | 62 (12%) | 404 (77%) | 0 | 0 | 0 | 66 (13%) | 382 (73%) | 0 | 0 | 0 |
| Rash | 84 (16%) | 19 (4%) | 1 (<1%) | 0 | 0 | 116 (22%) | 50 (10%) | 3 (<1%) | 1 (<1%) | 0 | 120 (23%) | 43 (8%) | 5 (<1%) | 0 | 0 |
ALT=alanine transaminase. AST=aspartate transaminase.
194 (12%) trial participants experienced a chemotherapy-related hypersensitivity reaction during treatment (appendix p 4). Although the incidence of reactions to paclitaxel was not affected by weekly scheduling (patients experiencing at least one hypersensitivity reaction 57 [11%] in group 1 vs 36 [7%] in group 2 vs 47 [9%] in group 3), the incidence of carboplatin hypersensitivity reactions was significantly higher in group 3 (3 [1%] vs 4 [1%] vs 40 [8%]). Most carboplatin hypersensitivity reactions were mild (61 [92%] grade 1 or 2; 4 [6%] grade 3; 1 [2%] grade 4) and could be managed using increased hypersensitivity prophylaxis, slower chemotherapy infusion rates, or formal desensitisation protocols according to the ICON8 clinical guidance document. However, more patients in group 3 discontinued carboplatin because of hypersensitivity than in groups 1 and 2.
The toxicity profiles were otherwise similar in all three groups. Of note, despite the higher dose of paclitaxel in groups 2 and 3, there were no significant differences in the incidence of grade 2 or higher sensory neuropathy between treatment groups (143 [27%] vs 126 [24%] vs 117 [22%]).
The primary quality-of-life outcome endpoint was the global score, an overall measure of health and quality of life. Repeated measures longitudinal analysis of the period from randomisation to 9 months found significantly improved quality of life with 3-weekly treatment (group 1 vs group 2 p=0·043; group 1 vs group 3 p=0·0018). However, cross-sectional analysis 9 months after randomisation showed similar quality of life in all three groups at that time point (group 1 vs group 2 p=0·094; group 1 vs group 3 p=0·61). Detailed quality of life analysis will be reported separately.
Discussion
The results of the ICON8 trial show that it is feasible to deliver weekly dose-dense paclitaxel in combination with either 3-weekly or weekly carboplatin in the first-line treatment of high-risk ovarian cancer. However, neither of these regimens is associated with an improvement in survival outcomes compared with standard 3-weekly carboplatin–paclitaxel treatment in the predominantly European population treated on this trial.
ICON8 has many strengths. It was a large, international study that included both patients who received upfront primary surgery and neoadjuvant chemotherapy with delayed primary surgery, consistent with current standard practice. Chemotherapy completion was high, with over 85% of all patients receiving at least six cycles of platinum-based chemotherapy. Notably, although delivery of weekly dose-dense chemotherapy resulted in more dose delays and omissions compared with 3-weekly treatment, a significant increase in paclitaxel dose-density was achieved in both weekly treatment groups without any negative effect on carboplatin dose. This result means that the key treatment goal of the trial was met, reinforcing the applicability of the trial results.
The recruitment of women with early stage high-risk ovarian cancer to the same trial as those with bulky inoperable stage III and stage IV disease could be considered a possible weakness of the design. However, subset analyses based on stage and surgical timing did not identify any heterogeneity in survival outcomes, supporting our eligibility criteria. It should also be noted that, although ICON8 had participation from five international trials groups, almost 90% of recruitment took place in the UK, meaning that it is not possible to explore any potential differences in the efficacy of dose-dense paclitaxel among different ethnic groups within this trial.
The ICON8 trial was conceived following the JGOG3016 trial,14, 15 which found that in Japanese women with newly-diagnosed ovarian cancer, weekly dose-dense paclitaxel at a dose of 80 mg/m2 administered with 3-weekly carboplatin was more efficacious than conventional 3-weekly carboplatin–paclitaxel chemotherapy with clinically significant improvements in both progression-free survival and overall survival albeit at the expense of greater treatment-related toxicity (table 3). Although we were unable to show a similar survival benefit from weekly dose-dense paclitaxel in combination with either 3-weekly or weekly carboplatin in ICON8, both dose-dense regimens were better tolerated in our predominantly European population with 85% of patients in all our study groups receiving six cycles of platinum-based chemotherapy compared with only 62% of those allocated to weekly chemotherapy in JGOG3016. Notably, the reported incidence of key grade 3 or higher chemotherapy-related toxic effects was lower in both the 3-weekly and weekly dose-dense treatment groups in ICON8. The incidence of febrile neutropenia in group 2 (3-weekly carboplatin and weekly paclitaxel) of ICON8 was 6% compared with 9% in the identical treatment group in JGOG3016. Similar trends were observed for grade 3 or higher peripheral sensory neuropathy (4% vs 7%) and grade 3 or higher anaemia (13% vs 69%) indicating tolerability of carboplatin–paclitaxel is different between the two study populations.
Table 3.
Summary of progression-free survival outcomes in phase 3 trials evaluating weekly first-line chemotherapy in epithelial ovarian cancer
|
Number of patients |
HR (95% CI) |
Number of patients |
HR (95% CI) | |||
|---|---|---|---|---|---|---|
| 3-weekly carboplatin and paclitaxel | 3-weekly carboplatin and weekly paclitaxel | 3-weekly carboplatin and paclitaxel | Weekly carboplatin and paclitaxel | |||
| ICON8* | 522 | 523 | 0·9 (0·77–1·05) | 522 | 521 | 0·93 (0·78–1·08) |
| JGOG 3016 | 319 | 312 | 0·65 (0·53–0·80) | ·· | ·· | ·· |
| GOG-0262† | 57 | 55 | 0·62 (0·4–0·95) | ·· | ·· | ·· |
| MITO-7 | ·· | ·· | ·· | 404 | 406 | 0·96 (0·8–1·16) |
HR=hazard ratio.
For the purposes of comparison, 95% CIs are used in this table; however, 97·5% CIs were used for the main analysis because of multiple testing.
Patients were given the option to receive bevacizumab in addition to randomised treatments; only patients who did not receive bevacizumab are included in this table.
Two further large phase 3 randomised studies, done during the lifetime of the ICON8 study, have also investigated weekly chemotherapy administration in first-line ovarian cancer treatment and are summarised in table 3. The European MITO-7 trial24 evaluated the concept of weekly chemotherapy scheduling without dose intensification. It recruited 822 women with FIGO stage IC–IV epithelial ovarian cancer who received conventional 3-weekly carboplatin–paclitaxel or weekly scheduling of both carboplatin (AUC2) and paclitaxel (60 mg/m2) for 18 weeks. No difference in efficacy was observed between the two treatment groups, although patients allocated to weekly treatment experienced less toxicity and had superior quality of life during treatment. In particular, incidence of febrile neutropenia (0·5% vs 3%) and grade 2 or higher sensory neuropathy (6% vs 17%) were significantly lower in patients receiving weekly chemotherapy. Notably, quality of life, measured weekly using the Functional Assessment of Cancer Therapy-Ovarian Trial Outcome Index, deteriorated transiently 1 week after each treatment cycle, during standard 3-weekly chemotherapy. This pattern was not observed during weekly scheduling and the treatment-by-time interaction favoured weekly chemotherapy in all quality-of-life analyses indicating that weekly carboplatin (AUC2) and paclitaxel (60 mg/m2) might be an attractive alternative to 3-weekly scheduling for selected patients. In parallel, the Gynecologic Oncology Group 262 trial enrolled 692 women with stage II–IV epithelial ovarian cancer who were randomly assigned to 3-weekly carboplatin–paclitaxel or 3-weekly carboplatin and weekly intensified paclitaxel (80 mg/m2).25 Concurrent and maintenance bevacizumab was permitted in both groups at patient or investigator choice and was administered to 84% of trial participants. Intention-to-treat analysis did not show any improvement in efficacy with weekly paclitaxel chemotherapy nor indicate any significant reduction in treatment tolerability or increased toxicity. In contrast to MITO-7, a small statistically significant detriment in quality of life was observed in patients receiving weekly intensified paclitaxel, albeit at a level considered unlikely to have clear clinical relevance. However, in the subgroup of patients who did not receive bevacizumab (n=112), weekly paclitaxel administration was associated with a 4-month median progression-free survival improvement (HR 0·62 [95% CI 0·40–0·95]; p=0·03). Although it is possible that this improvement was a true treatment effect of weekly dose-dense paclitaxel that is masked in the whole trial population by the effect of maintenance bevacizumab, the small size of the no bevacizumab subgroup means that this result must be interpreted with caution. Caution is particularly relevant given the lack of benefit observed with weekly dose-dense paclitaxel in over 1000 women in the ICON8 trial.
It is possible that ethnic pharmacogenomic differences underlie the discordant outcomes between ICON8 and JGOG3016, and that weekly dose-dense paclitaxel treatment could still be considered as a first-line treatment option for Japanese women with epithelial ovarian cancer. An analysis of parallel US and Japanese phase 3 trials evaluating 3-weekly carboplatin–paclitaxel in advanced lung cancer showed superior survival outcomes and higher proportions of haematological toxicity in Japanese patients with paclitaxel-based chemotherapy that on exploratory analysis were associated with ethnically distributed single nucleotide polymorphisms (SNPs) CYP3A4*1B and ERCC2K751Q.26 SNPs near AIPL1 and BCR have also been associated with paclitaxel-induced cytotoxicity in an Asian population.27 Of note, the longer survival and reduced tolerability of both 3-weekly chemotherapy and weekly dose-dense paclitaxel in the JGOG3016 patient group compared with that observed in ICON8 support the potential for relevant pharmacogenomic differences that might affect treatment outcomes in patients with epithelial ovarian cancer and identifies an area for further research.
In conclusion, the results of the ICON8 trial provide evidence that weekly dose-dense paclitaxel should not be incorporated as a standard-of-care component of first-line multimodality treatment of epithelial ovarian cancer for women who are not of Japanese ethnic origin.
Data sharing
Data will be shared according to the Medical Research Council Clinical Trials Unit controlled access approach, based on the following principles: no data should be released that would compromise an ongoing trial or study; there must be a strong scientific or other legitimate rationale for the data to be used for the requested purpose; investigators who have invested time and effort into developing a trial or study should have a period of exclusivity in which to pursue their aims with the data, before key trial data are made available to other researchers; the resources required to process requests should not be underestimated, particularly successful requests that lead to preparing data for release, thus adequate resources must be available to comply in a timely manner or at all, and the scientific aims of the study must justify the use of such resources; and data exchange complies with Information Governance and Data Security Policies in all the relevant countries. Researchers wishing to access data from the ICON8 should contact mrcctu.icon8and8b@ucl.ac.uk in the first instance.
Acknowledgments
Acknowledgments
We thank the women who participated in the trial and their families.
Contributors
ARC, IAM, AD, J-WK, DMO, JH, CC, SBl, JDB, RN, TP, AMS, MP, and JAL led and coordinated the study design. ARC, IAM, AD, J-WK, DMO, JH, CC, SBl, JDB, RN, TP, HG, RL, GD, HME, MH, SBa, RMG, RJ, SW, and JAL contributed to patient recruitment and data collection. ECJ, ADC, and SS analysed data, which were interpreted by all co-authors. ARC was the chief investigator of the trial, and AD, J-WK, and DMO were principal investigators for the international groups. ARC, ECJ, IAM, AD, J-WK, DMO, JH, SBl, JDB, RN, TP, ADC, GSG, RK, and JAL are members of the trial management group. ARC and ECJ drafted the manuscript, the final version of which was approved by all co-authors.
Declaration of interests
ARC reports grants from Cancer Research UK, during the conduct of the study; grants, personal fees, and non-financial support from AstraZeneca; non-financial support from Clovis Oncology; and personal fees and non-financial support from Roche, all outside the submitted work. CC reports personal fees from Pfizer UK, outside the submitted work. DMO reports grants from Cancer Trials Ireland, during the conduct of the study; and financial support for travel and accommodation from AstraZeneca, outside the submitted work. HG is an employee of AstraZeneca and Imperial College London. IAM reports personal fees from Clovis Oncology Takeda, Tesaro, and personal fees and grants from AstraZeneca, outside the submitted work. JDB reports personal fees from and was co-founder and consultant of Inivata, grants from Aprea, non-financial support from Clovis Oncology, and personal fees from Bayer and AstraZeneca, outside the submitted work. JH reports grants from Cancer Research UK, during the conduct of the study. JAL reports grants and personal fees from AstraZeneca and Merck Sharpe & Dohme, and personal fees from Roche, Clovis Oncology, and Tesaro, outside the submitted work. ECJ reports grants from Cancer Research UK, during the conduct of the study. RJ reports personal fees from CLOVIS Advisory Board, Tesaro Advisory Board, and Amgen Advisory Board, and non-financial support from AstraZeneca and Tesaro, outside the submitted work. RL reports personal fees for travel grants, conference costs, and advisory work from AstraZeneca and Tesaro, outside the submitted work. RMG reports personal fees, non-financial support, and other support (for national trial coordinating investigator) from AstraZeneca and Tesaro; personal fees and other support (for site principal investigator for a trial) from Clovis Oncology, Roche, Immunogen; personal fees from Sotio; grants from Boehringer Ingelheim; and grants and other support (for site principal investigator for a trial) from Lilly/Ignyta, all outside the submitted work. SSt reports grants from Cancer Research UK, during the conduct of the study. SSu reports honorarium for attendance at one advisory board meeting from AstraZeneca, outside the submitted work. SBa reports personal fees from Roche, AstraZeneca, Tesaro, Clovis, Pharmamar, Seattle Genetics, Merck Serono, and Gamamabs; grants from AstraZeneca and Tesaro; and travel support from Nucana, all outside the submitted work. TP reports personal fees and non-financial support from Merck Sharp & Dohme, and non-financial support from Roche and IGEA Medical, outside the submitted work. ADC, AMS, GSG, GD, HME, J-WK, AD, MH, MP, RN, RK, SBl, and SW declare no competing interests.
Supplementary Material
References
- 1.Jayson GC, Kohn EC, Kitchener HC, Ledermann JA. Ovarian cancer. Lancet. 2014;384:1376–1388. doi: 10.1016/S0140-6736(13)62146-7. [DOI] [PubMed] [Google Scholar]
- 2.Stuart GCE, Kitchener H, Bacon M. 2010 Gynecologic Cancer InterGroup (GCIG) consensus statement on clinical trials in ovarian cancer: report from the Fourth Ovarian Cancer Consensus Conference. Int J Gynecol Cancer. 2011;21:750–755. doi: 10.1097/IGC.0b013e31821b2568. [DOI] [PubMed] [Google Scholar]
- 3.Bookman MA, Okamoto A, Stuart G. Harmonising clinical trials within the Gynecologic Cancer InterGroup: consensus and unmet needs from the Fifth Ovarian Cancer Consensus Conference. Ann Oncol. 2017;28(suppl 8):viii30–viii35. doi: 10.1093/annonc/mdx449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kehoe S, Hook J, Nankivell M. Primary chemotherapy versus primary surgery for newly diagnosed advanced ovarian cancer (CHORUS): an open-label, randomised, controlled, non-inferiority trial. Lancet. 2015;386:249–257. doi: 10.1016/S0140-6736(14)62223-6. [DOI] [PubMed] [Google Scholar]
- 5.Vergote I, Tropé CG, Amant F. Neoadjuvant chemotherapy or primary surgery in stage IIIC or IV ovarian cancer. N Engl J Med. 2010;363:943–953. doi: 10.1056/NEJMoa0908806. [DOI] [PubMed] [Google Scholar]
- 6.Vergote I, Coens C, Nankivell M. Neoadjuvant chemotherapy versus debulking surgery in advanced tubo-ovarian cancers: pooled analysis of individual patient data from the EORTC 55971 and CHORUS trials. Lancet Oncol. 2018;19:1680–1687. doi: 10.1016/S1470-2045(18)30566-7. [DOI] [PubMed] [Google Scholar]
- 7.Klauber N, Parangi S, Flynn E, Hamel E, D'Amato RJ. Inhibition of angiogenesis and breast cancer in mice by the microtubule inhibitors 2-methoxyestradiol and taxol. Cancer Res. 1997;57:81–86. [PubMed] [Google Scholar]
- 8.Kamat AA, Kim TJ, Landen CN., Jr Metronomic chemotherapy enhances the efficacy of antivascular therapy in ovarian cancer. Cancer Res. 2007;67:281–288. doi: 10.1158/0008-5472.CAN-06-3282. [DOI] [PubMed] [Google Scholar]
- 9.Markman M, Hall J, Spitz D. Phase II trial of weekly single-agent paclitaxel in platinum/paclitaxel-refractory ovarian cancer. J Clin Oncol. 2002;20:2365–2369. doi: 10.1200/JCO.2002.09.130. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg P, Andersson H, Boman K. Randomized trial of single agent paclitaxel given weekly versus every three weeks and with peroral versus intravenous steroid premedication to patients with ovarian cancer previously treated with platinum. Acta Oncol. 2002;41:418–424. doi: 10.1080/028418602320404998. [DOI] [PubMed] [Google Scholar]
- 11.McNeish IA, Ledermann JA, Webber L. A randomised, placebo-controlled trial of weekly paclitaxel and saracatinib (AZD0530) in platinum-resistant ovarian, fallopian tube or primary peritoneal cancer. Ann Oncol. 2014;25:1988–1995. doi: 10.1093/annonc/mdu363. [DOI] [PubMed] [Google Scholar]
- 12.Mauri D, Kamposioras K, Tsali L. Overall survival benefit for weekly vs. three-weekly taxanes regimens in advanced breast cancer: a meta-analysis. Cancer Treat Rev. 2010;36:69–74. doi: 10.1016/j.ctrv.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 13.Sparano JA, Wang M, Martino S. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358:1663–1671. doi: 10.1056/NEJMoa0707056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Katsumata N, Yasuda M, Takahashi F. Dose-dense paclitaxel once a week in combination with carboplatin every 3 weeks for advanced ovarian cancer: a phase 3, open-label, randomised controlled trial. Lancet. 2009;374:1331–1338. doi: 10.1016/S0140-6736(09)61157-0. [DOI] [PubMed] [Google Scholar]
- 15.Katsumata N, Yasuda M, Isonishi S. Long-term results of dose-dense paclitaxel and carboplatin versus conventional paclitaxel and carboplatin for treatment of advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer (JGOG 3016): a randomised, controlled, open-label trial. Lancet Oncol. 2013;14:1020–1026. doi: 10.1016/S1470-2045(13)70363-2. [DOI] [PubMed] [Google Scholar]
- 16.Safra T, Menczer J, Bernstein RM. Combined weekly carboplatin and paclitaxel as primary treatment of advanced epithelial ovarian carcinoma. Gynecol Oncol. 2009;114:215–218. doi: 10.1016/j.ygyno.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 17.Pignata S, Breda E, Scambia G. A phase II study of weekly carboplatin and paclitaxel as first-line treatment of elderly patients with advanced ovarian cancer. A Multicentre Italian Trial in Ovarian cancer (MITO-5) study. Crit Rev Oncol Hematol. 2008;66:229–236. doi: 10.1016/j.critrevonc.2007.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Calvert AH, Newell DR, Gumbrell LA. Carboplatin dosage: prospective evaluation of a simple formula based on renal function. J Clin Oncol. 1989;7:1748–1756. doi: 10.1200/JCO.1989.7.11.1748. [DOI] [PubMed] [Google Scholar]
- 19.Marx GM, Blake GM, Galani E. Evaluation of the Cockroft-Gault, Jelliffe and Wright formulae in estimating renal function in elderly cancer patients. Ann Oncol. 2004;15:291–295. doi: 10.1093/annonc/mdh079. [DOI] [PubMed] [Google Scholar]
- 20.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 21.Jelliffe RW. Letter: Creatinine clearance: bedside estimate. Ann Intern Med. 1973;79:604–605. doi: 10.7326/0003-4819-79-4-604. [DOI] [PubMed] [Google Scholar]
- 22.Eisenhauer EA, Therasse P, Bogaerts J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1) Eur J Cancer. 2009;45:228–247. doi: 10.1016/j.ejca.2008.10.026. [DOI] [PubMed] [Google Scholar]
- 23.Vergote I. Re: new guidelines to evaluate the response to treatment in solid tumors [ovarian cancer]. Gynecologic Cancer Intergroup. J Natl Cancer Inst. 2000;92:1534–1535. doi: 10.1093/jnci/92.18.1534. [DOI] [PubMed] [Google Scholar]
- 24.Pignata S, Scambia G, Katsaros D. Carboplatin plus paclitaxel once a week versus every 3 weeks in patients with advanced ovarian cancer (MITO-7): a randomised, multicentre, open-label, phase 3 trial. Lancet Oncol. 2014;15:396–405. doi: 10.1016/S1470-2045(14)70049-X. [DOI] [PubMed] [Google Scholar]
- 25.Chan JK, Brady MF, Penson RT. Weekly vs. every-3-week paclitaxel and carboplatin for ovarian cancer. N Engl J Med. 2016;374:738–748. doi: 10.1056/NEJMoa1505067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gandara DR, Kawaguchi T, Crowley J. Japanese–US common-arm analysis of paclitaxel plus carboplatin in advanced non-small-cell lung cancer: a model for assessing population-related pharmacogenomics. J Clin Oncol. 2009;27:3540–3546. doi: 10.1200/JCO.2008.20.8793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Komatsu M, Wheeler HE, Chung S. Pharmacoethnicity in paclitaxel-induced sensory peripheral neuropathy. Clin Cancer Res. 2015;21:4337–4346. doi: 10.1158/1078-0432.CCR-15-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be shared according to the Medical Research Council Clinical Trials Unit controlled access approach, based on the following principles: no data should be released that would compromise an ongoing trial or study; there must be a strong scientific or other legitimate rationale for the data to be used for the requested purpose; investigators who have invested time and effort into developing a trial or study should have a period of exclusivity in which to pursue their aims with the data, before key trial data are made available to other researchers; the resources required to process requests should not be underestimated, particularly successful requests that lead to preparing data for release, thus adequate resources must be available to comply in a timely manner or at all, and the scientific aims of the study must justify the use of such resources; and data exchange complies with Information Governance and Data Security Policies in all the relevant countries. Researchers wishing to access data from the ICON8 should contact mrcctu.icon8and8b@ucl.ac.uk in the first instance.