Abstract
Background
Tourniquet-related complications are a common clinical problem. In the present study, we compared the effects of dexmedetomidine vs. oxycodone in patients undergoing limb ischemia-reperfusion.
Material/Methods
Fifty-four patients undergoing unilateral lower-extremity surgery under combined spinal and epidural anesthesia were randomly assigned to a control (ischemia-reperfusion, I/R) group, a dexmedetomidine (Dex) group, and an oxycodone (Oxy) group. Tourniquet-induced hemodynamic parameters changes among groups were compared. The serum concentration of malondialdehyde (MDA), superoxide dismutase (SOD), tumor necrosis factor-a (TNF-α), interleukin-6 (IL-6), fatty acid binding protein 3 (FABP3), endothelin-1 (ET-1), and brain-derived neurotrophic factor (BDNF) were measured using ELISA before anesthesia and at 30 min and at 6 h after tourniquet release.
Results
In the control group, tourniquet use caused significant increases in systolic arterial pressure (SAP), mean arterial pressure (MAP), diastolic arterial pressure (DAP), and rate-pressure product. Compared with Oxy, Dex significantly decreased heart rate (HR). Both Dex and Oxy lowered SAP compared with the control group. No significant difference was observed in DAP between Dex and Oxy. The levels of MDA, TNF-α, IL-6, FABP3, and ET-1 were significantly higher, while the SOD and BDNF were significantly lower compared to baseline in the I/R group, but the variation range of those agents was significantly smaller in the Dex and Oxy groups, and the measured values were comparable between the 2 groups.
Conclusions
Compared with Dex, Oxy was not inferior in mitigating tourniquet-induced hyperdynamic response, ameliorating the inflammatory reaction, and protecting remote multiple organs in lower-extremity surgery patients.
MeSH Keywords: Dexmedetomidine; Oxycodone; Reperfusion Injury; Surgery, Plastic; Tourniquets
Background
In vascular or orthopedic surgeries, tourniquet placement is a conventional technique used to create bloodless operating fields [1]. However, tourniquet use can lead to significant acute limb ischemia-reperfusion (I/R) injury, characterized by inflammation, tissue edema, muscle necrosis, microvascular perfusion deficits [2], and even remote multiple-organ injuries [3]. In addition to I/R injury, tourniquet inflation can induce a hyperdynamic response [4]. Although tourniquet-induced I/R injury and hyperdynamic response have been recognized for several years, the complex pathophysiologic mechanism and therapeutic interventions are not completely understood.
It is generally agreed that postischemic reperfusion injury involves generation of reactive oxygen species (ROS) and inflammatory cytokines [5]. Furthermore, there is growing evidence that remote multiple-organ injury is correlated with systemic inflammatory response [6]. Therefore, exploring effective strategies to minimize the generation of ROS and ameliorate tourniquet-induced hyperdynamic and inflammatory response could enhance the recovery of patients undergoing limb ischemia-reperfusion.
Dexmedetomidine (Dex) is a new type of highly selective a2 adrenergic receptor agonist. It is an agent that provides sedative, anxiolytic, and analgesic effects, which has been used on surgical patients as an adjuvant anesthetic. Several studies have shown that Dex has a protective effect against I/R injury in multiple organs [7–9], and previous studies revealed that the effect of Dex in attenuating inflammation is associated with vagal-dependent mechanisms [10]. However, whether Dex influences tourniquet-induced I/R injury remains unclear.
Oxycodone (Oxy), a μ and κ opioid receptor agonist, is increasingly used worldwide to treat a variety of painful disorders [11]. Recently, several studies suggested that Oxy not only provides potent analgesia but also relieves the inflammatory response [12,13]. To date, no study has investigated the anti-inflammatory effects of Oxy in patients undergoing limb ischemia-reperfusion.
The aim of the present study was to evaluate the effects of Dex and Oxy on the inflammatory response in patients undergoing lower-limb I/R, to compare hyperdynamic response of patients receiving Dex or Oxy, and to test and compare whether Dex or Oxy can attenuate the tourniquet-induced I/R injury.
Material and Methods
Participants
This prospective, randomized, double-blinded study protocol was approved by the Ethics Committee of the Third Hospital of Hebei Medical University (2018-016-1) and was conducted in the hospital between September 2018 and May 2019. This trial was registered at chictr.org.cn (ChiCTR1800018510).
A biostatistician, who was independent of data management and statistical analyses, generated random numbers using SPSS17.0 software (SPSS, Inc., IL, USA).
Based on a previous study (expected difference in means, 13; expected standard deviation, 9.5) [14], the sample size of the study was estimated with an alpha of 0.05 and power set at 80%, and analysis showed that a sample size of 18 patients in each group was sufficient to acquire statistical significance of between-group differences. Taking into account a loss to follow-up rate of about 6%, we planned to enroll 60 patients. A trained study staff member was responsible for obtaining written informed consent from all recruited patients.
Inclusion criteria of the present trial were as follows: cooperative patients aged 20–75 years, ASA (American Society of Anesthesiologists) physical status I–II, elective orthopedic lower-limb surgeries, and tourniquet duration for 60–90 min. We excluded patients if they had a history of habitual use of anxiolytics or other drugs that affect the central nervous system. In addition, if they refused spinal and epidural anesthesia or refused to cooperate, patients were excluded from the study (Figure 1).
Figure 1.
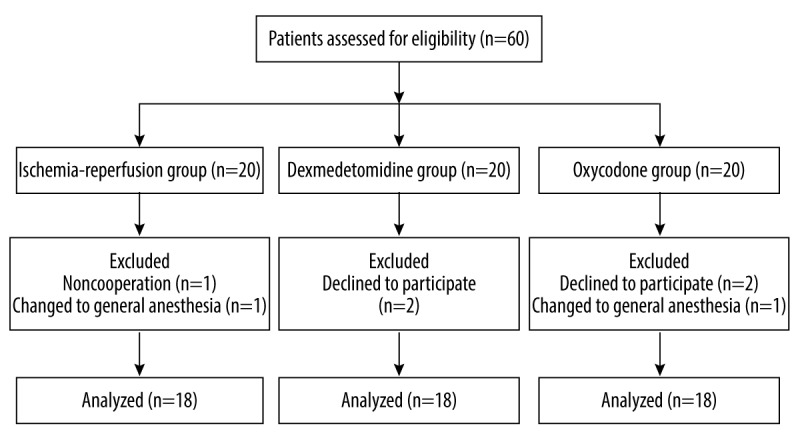
Flowchart of the present study.
Experimental protocols
During the study period, the patients underwent routine preoperative preparation. An anesthesiologist who did not participate in the rest of the study was involved only in preparing and dispensing the study drugs according to the randomization results. After entering the operating room, we used standard monitoring devices to assess electrocardiography (ECG), heart rate (HR), noninvasive blood pressure (NIBP), and peripheral capillary oxygen saturation (SpO2).
Combined spinal and epidural block anesthesia were performed at L3–L4 with 2.5 ml of 0.375% bupivacaine. Prior to tourniquet inflation, patients in the Dex group received a loading dose of Dex (0.8 μg/kg), followed by continuous infusion of Dex (0.4 μg/kg/h) until the end of surgery, while in the Oxy and control groups, oxycodone (0.05 mg/kg) and an equal amount of 0.9% saline were administered, respectively, followed by continuous infusion of 0.9% saline until the end of surgery. During the surgery, the block level was maintained at approximately T8–T10. Furthermore, to maintain hemodynamics stability, patients were given ringer lactate solution and hydroxyethyl starch injection.
Heart rate (HR), systolic arterial pressure (SAP), mean arterial pressure (MAP), diastolic arterial pressure (DAP), and HR×SAP (RPP) were recorded at the following time points: before anesthesia (T0), 1 min before tourniquet inflation (T1), 15 min intervals from the beginning of tourniquet inflation (T2, T3, T4, T5, and T6, respectively), and 1 min after tourniquet deflation (T7). At the end of surgery, we recorded operation and tourniquet duration, loss of blood, fluids administered, and urine volume. Beyond that, blood samples were obtained from a peripheral vein in the upper extremities before anesthesia (T0) and at 30 min (T8) and 6 h (T9) after tourniquet release. Plasma was separated by centrifugation at 3000 rpm for 5 min and stored at −80°C for further analysis.
For quantification the serum concentration by enzyme-linked immunosorbent assay (ELISA), commercially available ELISA kits for measuring human tumor necrosis factor-α (TNF-α, Proteintech), interleukin-6 (IL-6, Proteintech), brain-derived neurotrophic factor (BDNF, Proteintech), fatty acid binding protein 3 (FABP3, Elabscience), and endothelin-1 (ET-1, Elabscience) were used. Superoxide dismutase (SOD) and malondialdehyde (MDA) were tested to determine oxidative stress using a human assay kit (Jiancheng Co., Nanjing, China).
Statistical Analysis
SPSS17.0 (SPSS, Inc, IL, USA) was used for statistical analysis. All hemodynamic, I/R, and immune parameters at all time points and in all groups are expressed as median, minimum, maximum, and percentiles. The Kolmogorov-Smirnov test was used to test variables for normal distribution. For clinical characteristics of the patients, data are expressed as frequency and mean±standard deviation. The chi-square test was used to analyze discrete variables, and continuous variables were analyzed with one-way analysis of variance (ANOVA). For all hemodynamic, I/R, and immune parameters, repeated-measures analysis of variance with Bonferroni corrections was performed to determine statistical significance among different time points and groups. Statistical significance was considered as P<0.05.
Results
Clinical Characteristics of Patients
The chi-square test and one-way analysis of variance (ANOVA) were used to analyze clinical characteristics of the patients. No significant differences were observed among groups in terms of sex, age, ASA physical status, BMI, type of surgery, operation time, tourniquet duration, or fluid balance (except for urine volume) (Table 1). The urine volume in the Dex group was higher than in the control and Oxy groups (p<0.05).
Table 1.
Clinical characteristics of the patients.
| Control group | Dexmedetomidine group | Oxycodone group | |
|---|---|---|---|
| Sex (M/F) | 9/9 | 10/8 | 9/9 |
| Age (y) | 55±15 | 54±14 | 54±15 |
| ASA physical status (I/II) | 10/8 | 11/7 | 9/9 |
| BMI (kg·m−2) | 24±2 | 23±3 | 25±2 |
| Type of surgery | |||
| ORIF | 6 | 5 | 5 |
| Arthroscopy | 4 | 5 | 6 |
| TKR | 8 | 8 | 7 |
| Operation time (min) | 102±10 | 104±12 | 105±13 |
| Tourniquet duration (min) | 84±6 | 83±6 | 84±7 |
| Loss of blood (ml) | 91±39 | 89±45 | 88±44 |
| Crystalloids (ml) | 871±142 | 872±187 | 874±158 |
| Colloids (ml) | 591±136 | 594±165 | 589±158 |
| Urine volume (ml) | 474±100 | 761±172* | 476±105# |
ASA – American society of anesthesiologists; BMI – body mass index; ORIF – open reduction and internal fixation of lower limb; TKR – total knee replacement. Data are expressed as mean±SD (n=18 in each group).
P<0.05 vs. control;
P<0.05 vs. dexmedetomidine.
Perioperative variables of patients
Descriptive statistics were applied for perioperative variables of hemodynamic, I/R, and immune parameters, as shown in Tables 2 and 3, and all data are expressed as median, minimum, maximum, and percentiles.
Table 2.
Perioperative variables of hemodynamic parameters.
| Time | Sham | Dex | Oxy | |
|---|---|---|---|---|
| HR, median (Min, Max)(IQR) beats.min-1 | T0 | 69 (60, 89) (66–77) | 73 (60, 86) (61–81) | 70 (52, 85) (63–79) |
| T1 | 69 (55, 78) (64–73) | 60 (49, 70) (52–64) | 66 (54, 80) (56–73) | |
| T2 | 70 (56, 80) (66–72) | 60 (50, 71) (55–66) | 67 (52, 83) (57–71) | |
| T3 | 68 (55, 81) (64–70) | 59 (51, 71) (55–64) | 65 (53, 80) (58–71) | |
| T4 | 68 (58, 83) (65–70) | 60 (47, 72) (55–66) | 68 (56, 77) (61–72) | |
| T5 | 69 (55, 84) (67–71) | 60 (49, 70) (55–66) | 69 (61, 85) (65–74) | |
| T6 | 71 (60, 81) (68–76) | 61 (47, 76) (55–67) | 70 (53, 86) (67–76) | |
| T7 | 76 (63, 87) (68–81) | 66 (52, 78) (63–72) | 77 (65, 93) (71–81) | |
| SAP, median (Min, Max)(IQR) mmHg | T0 | 144 (129, 153) (138–149) | 139 (109, 169) (135–146) | 139 (123, 164) (130–146) |
| T1 | 127 (115, 138) (122–129) | 130 (109, 157) (119–139) | 130 (105, 164) (121–136) | |
| T2 | 130 (120, 142) (127–134) | 131 (102, 156) (121–142) | 128 (115, 137) (122–130) | |
| T3 | 133 (128, 148) (130–139) | 127 (100, 162) (118–142) | 123 (117, 135) (120–128) | |
| T4 | 136 (115, 148) (133–144) | 127 (100, 167) (117–135) | 124 (100, 138) (120–129) | |
| T5 | 139 (114, 148) (136–142) | 127 (98, 151) (112–133) | 127 (113, 136) (124–131) | |
| T6 | 142 (121, 150) (137–144) | 125 (105, 158) (115–132) | 134 (118, 145) (128–139) | |
| T7 | 136 (120, 150) (132–141) | 115 (95, 140) (106–127) | 128 (116, 140) (122–131) | |
| MAP, median (Min, Max)(IQR) mmHg | T0 | 94 (82, 108) (90–99) | 92 (83, 118) (90–98) | 91 (82, 110) (88–99) |
| T1 | 84 (78, 92) (83–89) | 88 (77, 109) (85–92) | 90 (72, 101) (81–93) | |
| T2 | 87 (81, 93) (85–89) | 90 (75, 115) (87–94) | 88 (75, 98) (85–93) | |
| T3 | 90 (83, 96) (85–92) | 89 (73, 111) (85–91) | 86 (78, 97) (83–88) | |
| T4 | 92 (74, 97) (88–94) | 88 (72, 114) (84–94) | 84 (74, 98) (81–86) | |
| T5 | 92 (75, 99) (89–96) | 89 (69, 108) (84–90) | 87 (78, 94) (83–88) | |
| T6 | 94 (78, 99) (92–96) | 87 (71, 114) (85–90) | 92 (82, 96) (89–93) | |
| T7 | 87 (84, 93) (85–91) | 81 (67, 99) (74–84) | 84 (77, 90) (81–85) | |
| DAP, median (Min, Max)(IQR) mmHg | T0 | 69 (58, 86) (63–76) | 70 (60, 92) (68–77) | 69 (60, 90) (65–73) |
| T1 | 64 (59, 73) (61–69) | 68 (58, 89) (65–73) | 69 (50, 80) (63–74) | |
| T2 | 65 (58, 75) (61–68) | 70 (62, 94) (65–73) | 70 (55, 82) (64–75) | |
| T3 | 69 (59, 74) (63–70) | 69 (60, 86) (65–72) | 66 (57, 85) (62–70) | |
| T4 | 68 (53, 77) (66–71) | 70 (57, 87) (66–73) | 63 (57, 79) (61–67) | |
| T5 | 69 (55, 78) (64–73) | 68 (54, 87) (66–71) | 66 (57, 75) (62–70) | |
| T6 | 70 (57, 76) (67–73) | 69 (52, 92) (64–73) | 69 (61, 75) (67–72) | |
| T7 | 63 (60, 70) (61–67) | 60 (50, 78) (58–65) | 62 (55, 66) (60–64) | |
| RPP, median (Min, Max)(IQR) | T0 | 9945 (8220, 13617) (9529–10948) | 9486 (7680, 13520) (8419–11297) | 9860 (7332, 11730) (9280–10465) |
| T1 | 8568 (6820, 10062) (7865–9412) | 7418 (5700, 9928) (6834–8621) | 8665 (5775, 10064) (7859–9383) | |
| T2 | 8967 (7056, 10640) (8462–9625) | 7815 (5406, 10608) (6815–8887) | 8273 (6604, 10956) (7434–8975) | |
| T3 | 8973 (7336, 10368) (8466–9624) | 7620 (5700, 10206) (6621–8793) | 8074 (6519, 10480) (7018–8604) | |
| T4 | 9338 (6670, 11288) (8806–10063) | 7467 (5311, 10521) (6588–8870) | 8404 (6100, 10626) (7464–8865) | |
| T5 | 9862 (7410, 11340) (9163–10024) | 7899 (5390, 9060) (6507–8145) | 8732 (6954, 11424) (8113–9720) | |
| T6 | 9973 (7623, 11232) (9227–10819) | 7605 (5170, 9954) (6773–8359) | 9174 (6254, 12470) (8757–10517) | |
| T7 | 10170 (8448, 12750) (9110–10958) | 7868 (5500, 9933) (6768–8300) | 9532 (7540, 13020) (8965–10670) |
Sham – control (ischemia-reperfusion, I/R) group; Dex – dexmedetomidine group; Oxy – oxycodone group; Min – minimum; Max – maximum; IQR – interquartile range.
Table 3.
Perioperative variables of I/R and immune parameters.
| Time | Sham | Dex | Oxy | |
|---|---|---|---|---|
| MDA, median (Min, Max) (IQR) μmol·L−1 | T0 | 4.16 (2.91, 4.69) (3.92–4.43) | 4.05 (3.02, 5.09) (3.76–4.42) | 4.12 (3.32, 5.38) (3.51–4.83) |
| T8 | 4.66 (3.81, 5.84) (4.25–5.09) | 4.25 (3.03, 5.25) (4.02–4.69) | 4.19 (3.39, 5.18) (3.94–4.63) | |
| T9 | 5.08 (3.94, 6.87) (4.77–5.62) | 4.47 (3.25, 7.00) (3.96–5.00) | 4.55 (3.16, 6.03) (4.23–5.03) | |
| SOD, median (Min, Max)(IQR) U·mL−1 | T0 | 73.18 (53.80, 83.09) (64.36–79.56) | 75.15 (53.65, 88.27) (69.55–77.01) | 70.12 (51.07, 92.25) (64.10–75.01) |
| T8 | 60.14 (44.58, 65.52) (53.54–62.71) | 63.32 (44.40, 82.72) (58.27–70.35) | 64.80 (50.35, 88.10) (59.34–67.89) | |
| T9 | 51.08 (34.25, 61.35) (45.89–54.43) | 56.93 (47.92, 70.84) (50.88–61.43) | 55.54 (50.09, 66.31) (50.73–61.50) | |
| TNF-α, median (Min, Max)(IQR) pg·mL−1 | T0 | 23.08 (15.34, 36.72) (19.79–27.78) | 24.94 (15.68, 31.48) (22.55–28.51) | 22.58 (15.94, 30.09) (20.17–26.70) |
| T8 | 29.79 (18.48, 40.24) (25.96–31.82) | 27.48 (19.07, 35.08) (26.03–30.63) | 23.75 (15.14, 35.33) (18.76–28.35) | |
| T9 | 36.95 (24.52, 47.02) (34.92–41.34) | 30.43 (18.48, 36.35) (25.45–33.11) | 26.81 (12.51, 42.91) (19.78–35.92) | |
| IL-6, median (Min, Max)(IQR) pg·mL−1 | T0 | 6.19 (3.91, 8.53) (4.52–7.51) | 6.43 (4.33, 8.00) (5.28–7.26) | 5.99 (4.08, 7.31) (5.18–6.66) |
| T8 | 8.12 (5.11, 11.71) (6.39–9.88) | 7.15 (6.25, 11.67) (6.65–8.60) | 5.57 (4.64, 9.46) (5.21–6.87) | |
| T9 | 28.34 (19.79, 39.17) (23.11–31.05) | 21.42 (17.73, 27.96) (19.97–25.65) | 18.19 (13.11, 30.80) (14.17–22.65) | |
| FABP3, median (Min, Max)(IQR) ng·mL−1 | T0 | 1.84 (0.95, 2.72) (1.50–2.17) | 1.83 (1.09, 2.66) (1.53–2.27) | 1.93 (1.22, 2.68) (1.65–2.14) |
| T8 | 2.38 (1.51, 3.30) (2.02–2.67) | 2.13 (1.44, 2.83) (1.72–2.53) | 2.10 (1.43, 2.96) (1.89–2.52) | |
| T9 | 2.53 (1.17, 3.72) (2.03–2.92) | 2.29 (1.62, 3.16) (1.69–2.70) | 2.21 (1.62, 2.77) (1.96–2.44) | |
| ET-1, median (Min, Max)(IQR) pg·mL−1 | T0 | 21.82 (15.13, 36.67) (18.77–28.30) | 23.08 (13.57, 41.90) (18.67–28.06) | 23.78 (17.63, 35.16) (19.91–27.91) |
| T8 | 27.59 (19.62, 44.63) (22.91–33.82) | 26.18 (19.32, 41.49) (21.74–29.75) | 28.72 (21.83, 36.80) (25.83–32.31) | |
| T9 | 37.81 (26.18, 50.83) (27.98–45.09) | 27.94 (19.27, 38.47) (23.43–31.27) | 30.22 (20.60, 35.78) (26.92–31.77) | |
| BDNF, median (Min, Max)(IQR) pg·mL−1 | T0 | 217.91 (156.55, 277.28) (203.55–240.34) | 222.34 (150.64, 277.46) (210.32–241.76) | 217.38 (185.56, 267.47) (201.62–239.39) |
| T8 | 219.73 (152.22, 273.12) (195.36–241.60) | 218.01 (181.64, 289.71) (205.81–232.21) | 198.13 (151.64, 251.31) (180.29–208.28) | |
| T9 | 177.03 (132.10, 252.68) (156.91–204.66) | 200.15 (161.17, 304.65) (171.78–227.52) | 174.86 (121.77, 213.91) (164.78–193.38) |
Sham – control (ischemia-reperfusion, I/R) group; Dex – dexmedetomidine group; Oxy – oxycodone group; Min – minimum; Max – maximum; IQR – interquartile range.
Hemodynamic changes among groups
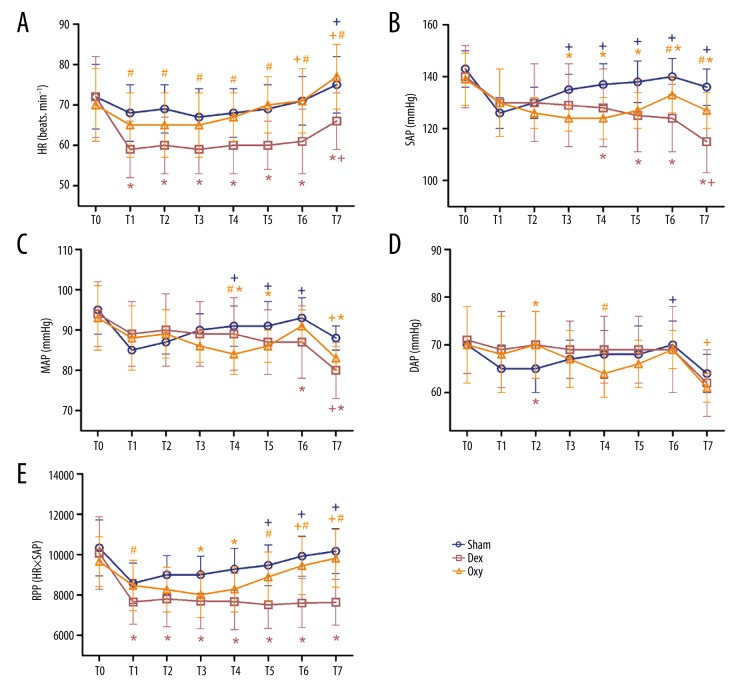
Repeated-measures analysis of variance was performed to analyze hemodynamic parameters among groups. For baseline hemodynamic data of HR, SAP, MAP, DAP, and RPP, no significant difference was found among groups (Figure 2A–2E).
Figure 2.
Hemodynamic changes among groups. We recorded HR (A), SAP (B), MAP (C), DAP (D), and RPP (E) at the following time points: before anesthesia (T0), 1 min before tourniquet inflation (T1), 15 min intervals from the beginning of tourniquet inflation (T2, T3, T4, T5, and T6, respectively), and 1 min after tourniquet deflation (T7). Sham – control (ischemia-reperfusion, I/R) group; Dex – dexmedetomidine group; Oxy – oxycodone group. Data are expressed as mean±SD. + P<0.05 vs. T1; * P<0.05 vs. sham; # P<0.05 vs. Dex.
No significant difference was observed between the control and Oxy groups in HR values, while HR values in the Dex group measured at T1, T2, T3, T4, T5, T6, and T7 were significantly lower than in the 2 other groups (p<0.05) (Figure 2A).
Tourniquet use caused significant increases in SAP in the control group, as the SAP values recorded at T3, T4, T5, T6, and T7 were significantly higher than at T1 (p<0.05) (Figure 2B). Dex or Oxy loading caused variations of SAP, MAP, and DAP values by time interaction effects. The SAP values measured at T4, T5, T6, and T7 in the control group were significantly higher than those in the Dex and Oxy groups (p<0.05). In addition, SAP values recorded at T6 and T7 in the Dex group were significantly lower than in the Oxy group (p<0.05) (Figure 2B).
As shown in Figure 2, tourniquet use caused significant increases in MAP in the control group, as the MAP values recorded at T4, T5, and T6 were significantly higher than at T1 (p<0.05) (Figure 2C). Furthermore, MAP values measured at T6 and T7 in the control group were significantly higher than in the Dex group (p<0.05). MAP values observed at T4, T5, and T7 were significantly lower in the Oxy group than in the control group (p<0.05), and MAP values measured at T4 were significantly lower in the Oxy group than in the Dex group (p<0.05) (Figure 2C).
In contrast to SAP, tourniquet use caused significant increases in DAP in the control group only at T6 (p<0.05), and no significant change was observed in DAP between groups except at T2 and T4. The value of DAP in the control group recorded at T2 was significantly lower than in the Dex and Oxy groups (p<0.05). At T4, DAP values in the Oxy group were significantly lower than in the Dex group (p<0.05) (Figure 2D).
The magnitude of changes in RPP was significantly different among groups. Those variance values in the control group indicated that tourniquet use also caused RPP increases, as the RPP values measured at T5 and T6 were significantly higher than at T1 (p<0.05) (Figure 2E). Consistent with HR, the RPP values in the Dex group at T1, T2, T3, T4, T5, T6, and T7 were significantly lower than in the control group (p<0.05). RPP values in the Oxy group were significantly lower than in the control group only at T3 and T4 (p<0.05), and although no statistically significant difference was observed at other time point, the RPP values were slightly lower compared with the control group. RPP values measured at T1, T5, T6, and T7 in the Oxy group were significantly higher than in the Dex group (p<0.05) (Figure 2E).
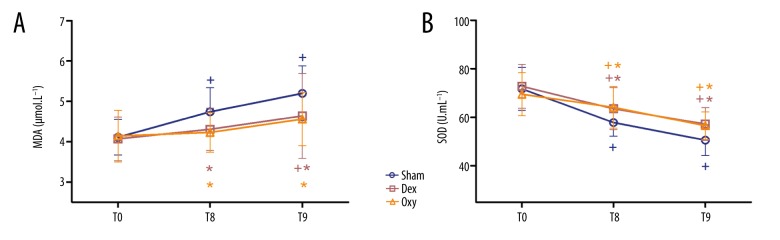
MDA and SOD levels
Repeated-measures analysis of variance was performed to compare the levels of MDA and SOD among groups. Compared to baseline, the levels of MDA were significantly higher in the control group at T8 and T9 (p<0.05). In contrast, MDA levels in the Oxy group measured at T8 and T9 were comparable to those measured at T0, while MDA levels in the Dex group measured at T8 were comparable to those measured at T0. Furthermore, compared with the control group, the level of MDA in the other 2 group at T8 and T9 were significantly lower (p<0.05) (Figure 3A). In all groups, SOD levels at T8 and T9 were significantly lower compared with T0 (p<0.05), and SOD levels in the Dex and Oxy groups at T8 and T9 were higher than in the control group (p<0.05) (Figure 3B).
Figure 3.
Plasma MDA (A) and SOD (B) levels. The serum concentration of malondialdehyde (MDA) and superoxide dismutase (SOD) were measured before anesthesia (T0) and at 30 min (T8) and 6 h after tourniquet release (T9). Sham – control (ischemia-reperfusion, I/R) group; Dex – dexmedetomidine group; Oxy – oxycodone group. Data are expressed as mean±SD. + P<0.05 vs. T0; * P<0.05 vs. sham; # P<0.05 vs. Dex.
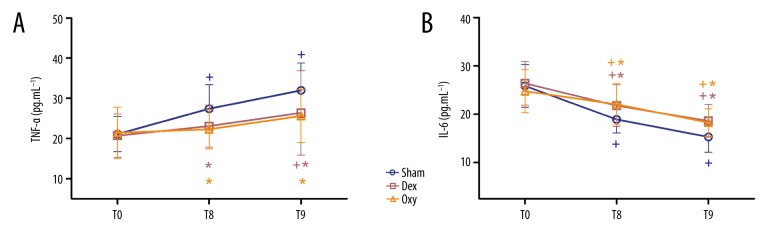
TNF-α and IL-6 levels
Repeated-measures analysis of variance was performed to compare the levels of TNF-α and IL-6 among groups. Plasma TNF-α and IL-6 levels were significantly higher in the control group at T8 and T9 compared with baseline, while in the Dex and Oxy groups, significantly increased levels of TNF-α and IL-6 were observed only at T9 (p<0.05) (Figure 4). As shown in Figure 4, compared to the control group, plasma TNF-α and IL-6 levels in the Dex and Oxy groups at T9 were significantly lower (p<0.05) (Figure 4), and the level of TNF-α in the Oxy group at T8 was significantly lower than in the control and Dex groups (p<0.05) (Figure 4A). In terms of plasma IL-6 levels, no significant difference was observed between the Dex group and Oxy group at T8, while significantly lower IL-6 levels were observed in the Oxy group at T9 (p<0.05) (Figure 4B).
Figure 4.
Plasma TNF-a (A) and IL-6 (B) levels. The serum concentration of tumor necrosis factor-a (TNF-a) and interleukin-6 (IL-6) were tested before anesthesia (T0) and at 30 min (T8) and 6 h after tourniquet release (T9). Sham – control (ischemia-reperfusion, I/R) group; Dex – dexmedetomidine group; Oxy – oxycodone group. Data are expressed as mean±SD. + P<0.05 vs. T0; * P<0.05 vs. sham; # P<0.05 vs. Dex.
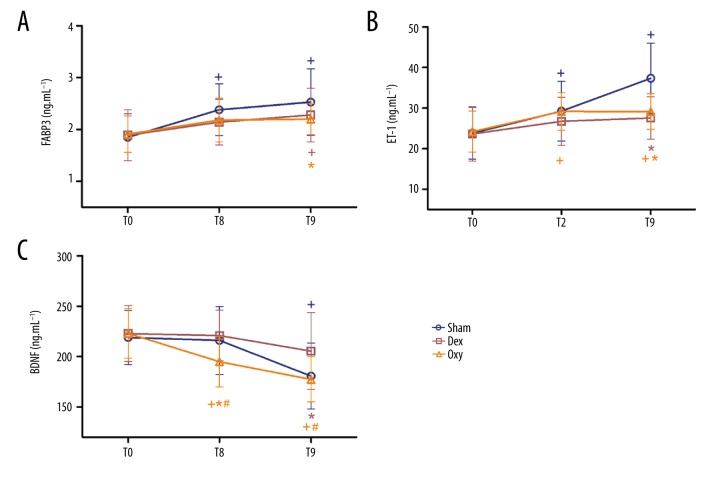
FABP3, ET-1, and BDNF levels
Repeated-measures analysis of variance was performed to compare the levels of FABP3, ET-1, and BDNF among groups. As shown in Figure 5, plasma FABP3 levels were significantly increased in the control group at T8 and T9 compared with baseline, while significantly increased of FABP3 levels were observed only at T9 in the Dex group (p<0.05). In the Oxy group, no significant difference was observed among the 3 time points. Compared to the control group, FABP3 levels in the Oxy group at T9 were significantly lower (p<0.05) (Figure 5A).
Figure 5.
Plasma FABP3 (A), ET-1 (B), and BDNF (C) levels. The serum concentration of fatty acid binding protein 3 (FABP3), endothelin-1 (ET-1), and brain-derived neurotrophic factor (BDNF) were measured before anesthesia (T0) and at 30 min (T8) and 6 h after tourniquet release (T9). Sham – control (ischemia-reperfusion, I/R) group; Dex – dexmedetomidine group; Oxy – oxycodone group. Data are expressed as mean±SD. + P<0.05 vs. T0; * P<0.05 vs. sham; # P<0.05 vs. Dex.
Compared with baseline, the levels of ET-1 in the control and Oxy groups were significantly higher at T8 and T9 (p<0.05). In contrast, ET-1 levels in the Dex group measured at T8 and T9 were comparable to those measured at T0. Compared to the control group, ET-1 levels in the Dex and Oxy groups at T9 were significantly lower (p<0.05) (Figure 5B).
Plasma BDNF levels in the control group were decreased at T8 and T9, but these decreases were statistically significant only at T9 compared with baseline (p<0.05). For the Dex group, there was no significant difference among the 3 time points. Furthermore, compared to the control group, BDNF levels in the Dex group at T9 were significantly higher (p<0.05). For the Oxy group, the levels of BDNF were significantly lower at T8 and T9 than at T0, while the BDNF levels at T8 and T9 were significantly lower than in the Dex group (p<0.05). In addition, the BDNF levels of the Oxy group at T8 were significantly lower than in the control group (p<0.05), while similar levels were observed at T9 (p>0.05) (Figure 5C).
Discussion
Although tourniquet use plays a pivotal role in vascular or orthopedic surgeries, there are -several local and systemic complications related to this device, such as hyperdynamic response, nerve palsy, skeletal muscle damage, and even remote multiple-organ injury [3,4]. Therefore, exploring effective strategies to minimize the damage of tourniquet-induced acute limb ischemia-reperfusion injury can significantly improve outcomes for this large group of patients.
Previous studies confirmed that tourniquet inflation caused a hyperdynamic response, and an augmented sympathetic outflow contributed to increases of epinephrine and norepinephrine in plasma has been proved to be an essential mechanism in this response [15].
Our data revealed that Dex can relieve the tourniquet-caused hyperdynamic response, as shown by decreases of HR and SAP compared to the control group. Furthermore, we found that the Dex effectively reduced SAP, and this effect was gradually amplified with the extension of tourniquet inflation time. Meanwhile, for DAP, we found that Dex had no significant effects, which differs from the findings of Lao [14]. Some studies have shown that patients with more stable perioperative hemodynamics had lower postoperative mortality [16,17]. So, a gradual decrease of Dex dosage rather than a fixed dosage should be considered with the extension of tourniquet inflation time.
Similar to Dex, Oxy also was found to decrease SAP compared with the control group, but had no significant effects on HR and DAP. Our data also revealed that the efficacy of Oxy in reducing SAP gradually decreased with the extension of tourniquet inflation time, which was in contrast to the effect of Dex. The mechanisms underlying these observed differences remain to be elucidated. Although urine volume must be considered, there is no doubt that the pharmacokinetics and pharmacodynamics of Dex and Oxy result in their unique effects, which we plan to investigate in future research.
Beyond the primary hemodynamic parameters, we also calculated the rate-pressure product (RPP) (HR×SAP) because RPP predicts myocardial oxygen consumption [18]. As shown in Figure 2, Dex and Oxy decrease RPP, which means these 2 drugs can reduce intraoperative myocardial oxygen consumption.
Extremity surgery with tourniquet use is associated with the generation of ROS, such as H2O2. These ROS can lead to cellular components damage and initiate the lipid peroxidation process. Malondialdehyde (MDA) is often used to indicate ROS levels [19]. The human body has a defense system against ROS, the first-line defense is antioxidant enzymes such as superoxide dismutase (SOD); these enzymes can accelerate the conversion of ROS into less reactive species and propagate the free radical chain reactions [20–22].
Our study confirmed that tourniquet inflation caused the generation of ROS, because increased MDA levels and decreased SOD levels were observed with respect to baseline. Compared to the control group, MDA levels in the Dex and Oxy groups were significantly lower, while SOD levels were significantly higher. Furthermore, these 2 investigated parameters were comparable between the Dex and Oxy groups. According to our results, Dex and Oxy may offers advantages by suppressing lipid peroxidation and renovating the antioxidant defense system.
In the present study, we showed that tourniquet-induced acute limb ischemia-reperfusion can lead to a systemic inflammatory response, as shown by increased levels of TNF-a and IL-6.
As one of the most sensitive and earliest-released inflammation factors, TNF-a plays a central role in initiating the inflammatory reaction of the innate immune system [23]. Previous studies indicated that the blood concentration of IL-6 was correlated with an increasing incidence of complications, and could be used as a predictor of tissue damage severity [24,25]. In our study, TNF-a and IL-6 levels were significantly elevated after tourniquet release in the control group, but the increases in the Dex and Oxy groups were relatively less. Compared to Dex, Oxy has more obvious advantages in relieving inflammatory response.
Fatty acid binding protein 3 (FABP3) is a cytosolic protein that binds fatty acids for cellular metabolism in muscle cells, and is expressed at higher concentrations in muscle tissues [26,27]. When myofibers are damaged, serum levels of the biomarker increase. In addition, the magnitude of the biomarker is correlated with histopathological alterations. Because of the greater relative expression of FABP3 in heart and in skeletal muscle, FABP3 has been used as a serological biomarker of skeletal muscle or cardiac injury [28,29].
Previous studies have revealed that tourniquet use can lead to significant acute local skeletal muscle injury and even remote cardiac injury [30,31]. Data from this study showed that FABP3 levels were significantly elevated after tourniquet release in the control group, while FABP3 concentrations in the Dex and Oxy groups were slightly lower. Our findings suggest that Dex or Oxy administration can attenuate tourniquet-induced skeletal muscle and cardiac injury.
As a biologically active substance, endothelin-1 (ET-1) is a potent vasoconstrictive and mitogenic peptide mainly produced by endothelium [32], and its pleiotropic effects have been implicated in the regulation inflammation and vascular physiology [33,34]. A previous study confirmed that elevated ET-1 level is associated with endothelial dysfunction [35], suggesting that ET-1 may be used as a surrogate for endothelial function. In addition, increased risk of morbidity and mortality have also been associated with ET-1 levels in patients with or without cardiovascular disease [36]. The present study shows that the concentration of ET-1 was significantly higher after tourniquet removal, and Dex and Oxy could observably mitigated these effects.
Evidence suggests that systemic inflammation (SI) after surgery can induce cognitive complications via immune interacts with the central nervous system [37]. In clinical practice, this condition can translate into prolonged length of hospital stay, increased health care costs, and higher mortality [38]. Although the precise mechanisms are unclear, mounting evidence suggests that neuroinflammation is a key pathogenetic element in a number of cognitive disorders [39].
Several studies proved that brain-derived neurotrophic factor (BDNF), a member of the neurotrophin family, can exert anti-neuroinflammation effects [40]. Our study demonstrated that plasma BDNF in the control group remained little changed at T8, but was significantly lower at T9, which suggests an increased risk of cognitive complications. Compared to the control group, this neurotrophin was significantly higher at 6 h after tourniquet release in the Dex group, suggesting that treatment with Dex can decrease the prevalence of cognitive disorders. This result is consistent with a study by Su et al. [41], who found Dex could prevent the incidence of delirium in elderly patients after non-cardiac surgery.
Compared with the control and Dex groups, lower BDNF levels were observed in the Oxy group at 30 min after tourniquet release. In addition, the BDNF level in the Oxy group at 6 h after tourniquet release was also significantly lower than in the Dex group (p<0.05). These unexpected findings suggest that treatment with Oxy may increases risk of cognitive complications. Several studies suggested that Oxy can relieve the inflammatory response [12,13], so we hypothesized that Oxy could reduce inflammatory reaction and improve postoperative cognitive function. However, our study data contradict this hypothesis. We speculate that inflammation-dependent mechanisms exist, and more research is needed to clarify these underlying mechanisms.
There are some limitations to our study. First, the number of patients was relatively small. Second, we only detected BDNF, and the cognitive function of patients was not assessed, so the effects of Dex and Oxy in this aspect should be investigated in future studies. Third, the exact mechanism underlying the regulation of tourniquet-induced acute limb ischemia-reperfusion injury by Dex and Oxy were not explored. Last, we did not evaluate the prolonged benefits of Dex and Oxy, and further investigations are needed to assess the long-term effects of these treatments on both local limb and remote organs following tourniquet-induced I/R injury.
Conclusions
The present study indicated that patients undergoing lower-extremity surgery with tourniquet use showed a hyperdynamic response and elevated proinflammatory cytokines, and even suffered from remote multiple-organ injury. Compared with Dex, Oxy was not inferior in mitigating these effects. As a convenient intervention, both Dex and Oxy appear to be safe for clinical use.
Footnotes
Conflicts of interest
None.
Source of support: This study was financially supported by the National Natural Science Foundation of China (81371231) and the Natural Science Foundation of HeBei Province (H2018206260)
References
- 1.Corrick RM, Tu H, Zhang D, et al. Dexamethasone protects against tourniquet-induced acute ischemia-reperfusion injury in mouse hindlimb. Front Physiol. 2018;9:244. doi: 10.3389/fphys.2018.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaisdell FW. The pathophysiology of skeletal muscle ischemia and the reperfusion syndrome: A review. Cardiovasc Surg. 2002;10(6):620–30. doi: 10.1016/s0967-2109(02)00070-4. [DOI] [PubMed] [Google Scholar]
- 3.Mo Y, Chen S, Yang L, et al. The effect of transcutaneous electrical acupoint stimulation on inflammatory response in patients undergoing limb ischemia-reperfusion. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/8369737. 8369737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaufman RD, Walts LF. Tourniquet-induced hypertension. Br J Anaesth. 1982;54(3):333–36. doi: 10.1093/bja/54.3.333. [DOI] [PubMed] [Google Scholar]
- 5.Turan R, Yagmurdur H, Kavutcu M, Dikmen B. Propofol and tourniquet induced ischaemia reperfusion injury in lower extremity operations. Eur J Anaesthesiol. 2007;24(2):185–89. doi: 10.1017/S0265021506001347. [DOI] [PubMed] [Google Scholar]
- 6.Lin LN, Wang LR, Wang WT, et al. Ischemic preconditioning attenuates pulmonary dysfunction after unilateral thigh tourniquet-induced ischemia-reperfusion. Anesth Analg. 2010;111(2):539–43. doi: 10.1213/ANE.0b013e3181e368d2. [DOI] [PubMed] [Google Scholar]
- 7.Wang SL, Duan L, Xia B, et al. Dexmedetomidine preconditioning plays a neuroprotective role and suppresses TLR4/NF-kappaB pathways model of cerebral ischemia reperfusion. Biomed Pharmacother. 2017;93:1337–42. doi: 10.1016/j.biopha.2017.06.051. [DOI] [PubMed] [Google Scholar]
- 8.Yoshitomi O, Cho S, Hara T, et al. Direct protective effects of dexmedetomidine against myocardial ischemia-reperfusion injury in anesthetized pigs. Shock. 2012;38(1):92–97. doi: 10.1097/SHK.0b013e318254d3fb. [DOI] [PubMed] [Google Scholar]
- 9.Gu J, Sun P, Zhao H, et al. Dexmedetomidine provides renoprotection against ischemia-reperfusion injury in mice. Crit Care. 2011;15(3):R153. doi: 10.1186/cc10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu YJ, Peng K, Meng XW, Ji FH. Attenuation of neuroinflammation by dexmedetomidine is associated with activation of a cholinergic anti-inflammatory pathway in a rat tibial fracture model. Brain Res. 2016;1644:1–8. doi: 10.1016/j.brainres.2016.04.074. [DOI] [PubMed] [Google Scholar]
- 11.Schmidt-Hansen M, Bennett MI, Arnold S, et al. Oxycodone for cancer-related pain. Cochrane Database Syst Rev. 2017;8:CD003870. doi: 10.1002/14651858.CD003870.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang PP, Yeh GC, Huang EY, et al. Effects of dextromethorphan and oxycodone on treatment of neuropathic pain in mice. J Biomed Sci. 2015;22:81. doi: 10.1186/s12929-015-0186-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ye J, Yan H, Xia Z. Oxycodone ameliorates the inflammatory response induced by lipopolysaccharide in primary microglia. J Pain Res. 2018;11:1199–207. doi: 10.2147/JPR.S160659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lao HC, Tsai PS, Su JY, et al. Dexmedetomidine attenuates tourniquet-induced hyperdynamic response in patients undergoing lower limb surgeries: a randomized controlled study. Journal of surgical research. 2013;179(1):e99–e106. doi: 10.1016/j.jss.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Zalunardo MP, Serafino D, Szelloe P, et al. Preoperative clonidine blunts hyperadrenergic and hyperdynamic responses to prolonged tourniquet pressure during general anesthesia. Anesth Analg. 2002;94(3):615–18. doi: 10.1097/00000539-200203000-00025. table of contents. [DOI] [PubMed] [Google Scholar]
- 16.Aronson S, Dyke CM, Levy JH, et al. Does perioperative systolic blood pressure variability predict mortality after cardiac surgery? An exploratory analysis of the ECLIPSE trials. Anesth Analg. 2011;113(1):19–30. doi: 10.1213/ANE.0b013e31820f9231. [DOI] [PubMed] [Google Scholar]
- 17.Aronson S, Stafford-Smith M, Phillips-Bute B, et al. Cardiothoracic Anesthesiology Research Endeavors. Intraoperative systolic blood pressure variability predicts 30-day mortality in aortocoronary bypass surgery patients. Anesthesiology. 2010;113(2):305–12. doi: 10.1097/ALN.0b013e3181e07ee9. [DOI] [PubMed] [Google Scholar]
- 18.Gobel FL, Norstrom LA, Nelson RR, et al. The rate-pressure product as an index of myocardial oxygen consumption during exercise in patients with angina pectoris. Circulation. 1978;57(3):549–56. doi: 10.1161/01.cir.57.3.549. [DOI] [PubMed] [Google Scholar]
- 19.Spirlandeli AL, Deminice R, Jordao AA. Plasma malondialdehyde as biomarker of lipid peroxidation: effects of acute exercise. Int J Sports Med. 2014;35(1):14–18. doi: 10.1055/s-0033-1345132. [DOI] [PubMed] [Google Scholar]
- 20.McCord JM. The evolution of free radicals and oxidative stress. Am J Med. 2000;108(8):652–59. doi: 10.1016/s0002-9343(00)00412-5. [DOI] [PubMed] [Google Scholar]
- 21.Granger DN. Role of xanthine oxidase and granulocytes in ischemia-reperfusion injury. Am J Physiol. 1988;255(6 Pt 2):H1269–75. doi: 10.1152/ajpheart.1988.255.6.H1269. [DOI] [PubMed] [Google Scholar]
- 22.Valko M, Leibfritz D, Moncol J, et al. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol. 2007;39(1):44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Kharbanda RK, Peters M, Walton B, et al. Ischemic preconditioning prevents endothelial injury and systemic neutrophil activation during ischemia-reperfusion in humans in vivo. Circulation. 2001;103(12):1624–30. doi: 10.1161/01.cir.103.12.1624. [DOI] [PubMed] [Google Scholar]
- 24.Asensi V, Valle E, Meana A, et al. In vivo interleukin-6 protects neutrophils from apoptosis in osteomyelitis. Infect Immun. 2004;72(7):3823–28. doi: 10.1128/IAI.72.7.3823-3828.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muehlstedt SG, Richardson CJ, Lyte M, Rodriguez JL. Systemic and pulmonary effector cell function after injury. Crit Care Med. 2002;30(6):1322–26. doi: 10.1097/00003246-200206000-00029. [DOI] [PubMed] [Google Scholar]
- 26.Burch PM, Greg Hall D, Walker EG, et al. Evaluation of the relative performance of drug-induced skeletal muscle injury biomarkers in rats. Toxicol Sci. 2016;150(1):247–56. doi: 10.1093/toxsci/kfv328. [DOI] [PubMed] [Google Scholar]
- 27.Bodie K, Buck WR, Pieh J, et al. Biomarker evaluation of skeletal muscle toxicity following clofibrate administration in rats. Exp Toxicol Pathol. 2016;68(5):289–99. doi: 10.1016/j.etp.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Kochansky CJ, Lyman MJ, Fauty SE, et al. Administration of fenofibrate markedly elevates Fabp3 in rat liver and plasma and confounds its use as a preclinical biomarker of cardiac and muscle toxicity. Lipids. 2018;53(10):947–60. doi: 10.1002/lipd.12110. [DOI] [PubMed] [Google Scholar]
- 29.Pritt ML, Hall DG, Recknor J, et al. Fabp3 as a biomarker of skeletal muscle toxicity in the rat: Comparison with conventional biomarkers. Toxicol Sci. 2008;103(2):382–96. doi: 10.1093/toxsci/kfn042. [DOI] [PubMed] [Google Scholar]
- 30.Bastounis E, Hadjinikolaou L, Pikoulis M, et al. Free radical related myocardial mitochondrial damage following limb ischaemia-reperfusion. Cardiovasc Res. 1994;28(12):1868–71. doi: 10.1093/cvr/28.12.1868. [DOI] [PubMed] [Google Scholar]
- 31.Foster AD, Vicente D, Sexton JJ, et al. Administration of FTY720 during tourniquet-induced limb ischemia reperfusion injury attenuates systemic inflammation. Mediators Inflamm. 2017;2017 doi: 10.1155/2017/4594035. 4594035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;344(8926):852–54. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- 33.Speed JS, Pollock DM. Endothelin, kidney disease, and hypertension. Hypertension. 2013;61(6):1142–45. doi: 10.1161/HYPERTENSIONAHA.113.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Idris-Khodja N, Ouerd S, Trindade M, et al. Vascular smooth muscle cell peroxisome proliferator-activated receptor gamma protects against endothelin-1-induced oxidative stress and inflammation. J Hypertens. 2017;35(7):1390–401. doi: 10.1097/HJH.0000000000001324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Amiri F, Virdis A, Neves MF, et al. Endothelium-restricted overexpression of human endothelin-1 causes vascular remodeling and endothelial dysfunction. Circulation. 2004;110(15):2233–40. doi: 10.1161/01.CIR.0000144462.08345.B9. [DOI] [PubMed] [Google Scholar]
- 36.Yokoi K, Adachi H, Hirai Y, et al. Plasma endothelin-1 level is a predictor of 10-year mortality in a general population: The Tanushimaru study. Circ J. 2012;76(12):2779–84. doi: 10.1253/circj.cj-12-0469. [DOI] [PubMed] [Google Scholar]
- 37.Kawano T, Eguchi S, Iwata H, et al. Impact of preoperative environmental enrichment on prevention of development of cognitive impairment following abdominal surgery in a rat model. Anesthesiology. 2015;123(1):160–70. doi: 10.1097/ALN.0000000000000697. [DOI] [PubMed] [Google Scholar]
- 38.Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. JAMA. 2010;304(16):1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hovens IB, Schoemaker RG, van der Zee EA, et al. Postoperative cognitive dysfunction: Involvement of neuroinflammation and neuronal functioning. Brain Behav Iimmun. 2014;38:202–10. doi: 10.1016/j.bbi.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 40.Jiang Y, Wei N, Lu T, et al. Intranasal brain-derived neurotrophic factor protects brain from ischemic insult via modulating local inflammation in rats. Neuroscience. 2011;172:398–405. doi: 10.1016/j.neuroscience.2010.10.054. [DOI] [PubMed] [Google Scholar]
- 41.Su X, Meng ZT, Wu XH, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: A randomised, double-blind, placebo-controlled trial. Lancet. 2016;388(10054):1893–902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]