Abstract
Background
A secondary contralateral thoracic surgery is a challenging procedure and is rarely indicated. We retrospectively compared the perioperative values to find out whether video-assisted thoracoscopic surgery under spontaneous ventilation is feasible for this surgery.
Material/Methods
Patients were retrospectively collected from January 1, 2015 to December 30, 2018 who underwent secondary contralateral video-assisted thoracoscopic surgeries with mechanical ventilation (MV-VATS group) or spontaneous ventilation (SV-VATS group). A propensity score-matching analysis was used to counterbalance the discrepancies. The primary outcome measures were the values of respiratory mechanics and hemodynamics, and the secondary outcome measures were postoperative recovery and complications.
Results
In the SV-VATS group, the operation and anesthesia times were shorter (P=0.008 and P=0.020, respectively). The peak respiratory pressure value was lower (P<0.001), and there was less use of analgesic drugs during the operation (P<0.001). The vital signs and oxygenation were stable during the operation and in post-anesthesia care unit. The extubation time of laryngeal mask airway, chest-tube duration, and postoperative hospital stay were shorter in the SV-VATS group (P=0.015, P=0.000, P=0.003, respectively), but the duration of intensive care unit stay, the postoperative clinical complications, and chest radiography results were not significantly different between the 2 groups (P>0.05). In the SV-VATS group, postoperative leukocyte count (P<0.001) and neutrophil ratio (P=0.001) were lower and the postoperative value of PaCO2 was slightly higher (P=0.026).
Conclusions
VATS under spontaneous ventilation might be an alternative approach for patients who undergo a secondary contralateral thoracic surgery with intraoperative stable vital signs, and does not increase postoperative complications.
MeSH Keywords: Anesthesia; Anesthesia, Intratracheal; Intubation; Thoracic Surgery, Video-Assisted
Background
With the growing aging population, increased life expectancy, and advancement in diagnostic methods and surgical techniques, it is possible for patients to undergo a secondary thoracic surgery for recurrent thoracic tumors and repeated bullae rupture to improve long-term survival and quality of life [1].
One-lung ventilation under general anesthesia is the criterion standard therapy for thoracic surgery. However, in patients with prior contralateral thoracic surgery, hypoxemia may occur due to the presence of relatively fewer functional alveoli in the residual lung at the dependent side during one-lung ventilation. Moreover, elevated shunt fraction and reduced functional lung parenchyma complicate one-lung ventilation during secondary contralateral lobectomy [2]. Therefore, an appropriate anesthesia method should be selected with meticulous care for a secondary contralateral thoracic surgery due to inadequate cardiopulmonary reserve and unfavorable pathophysiological changes [3]. Recently, anesthesia under spontaneous ventilation has been widely performed in a variety of video-assisted thoracoscopic surgeries (VATS), such as pulmonary nodules resection, bullectomy, and lobectomy [4–6]. VATS under spontaneous ventilation has better ventilation perfusion matching due to maintaining the contractile function of the diaphragm, and accelerates postoperative rehabilitation [4–6]. VATS under spontaneous ventilation also has been proposed for patients with prior contralateral thoracic surgeries [7,8]. Nevertheless, these observations were just case reports, and few systematic comparisons have been published on the feasibility of VATS under spontaneous ventilation for these patients.
Here, through a retrospective observation, we speculate that VATS under spontaneous ventilation is feasible for patients who undergo a secondary contralateral thoracic surgery, and we also speculate that it can improve postoperative recovery in the post-anesthesia care unit (PACU) and general ward, with decreased postoperative complications.
Material and Methods
Study design
This retrospective observation was approved by the Research Ethics Committee of the First Affiliated Hospital of Guangzhou Medical University (2018 No. K-22) and was conducted under an institutional process that obviated the need for patients’ consent. Data were collected from medical records of thoracic patients from January 1, 2015 to December 30, 2018 in our institution. The inclusion criteria were adult patients over 18 years old, with an American Society of Anesthesiologists (ASA) physical status of less than 4, and without serious cardiopulmonary dysfunction, who underwent a 3-port VAST, and all included patients had a history of only one prior contralateral thoracic surgery. The exclusion criteria were thoracotomy and non-pulmonary operation, including mediastinal tumor resection, thoracentesis, or exploration, pericardial window treatment, and esophagus surgery. Demographic variables, intraoperative hemodynamic, ventilation data (at 30 min before pleural closing), and postoperative variables in PACU and general ward were collected. All patients could choose the anesthesia method they received after having them explained by surgeons and anesthetists. The surgeons were senior doctors who were trained in our center.
The included cases were divided into 2 groups. Patients who received general anesthesia with double-lumen intubation under mechanical ventilation composed the MV-VATS group, while those who received laryngeal mask airway (LMA) under spontaneous ventilation composed the SV-VATS group.
Anesthesia procedure
Electrocardiogram (ECG), heart rate (HR), invasive mean arterial pressure (MAP), pulse oxygen saturation (SpO2), partial pressure of end-tidal carbon dioxide (PETCO2), respiratory rate (RR), tidal volume (vt), and bispectral index (BIS) were monitored in all patients.
In the MV-VATS group, a double-lumen endobronchial tube (Medtronics, Minneapolis, USA) was inserted after anesthesia induction. One-lung ventilation was commenced by protective ventilation strategies with tidal volume (vt) of 6 mL·kg−1, positive end-expiratory pressure of 5 cmH2O, and peak respiratory pressure (Ppeak) of under 30 cmH2O. Anesthesia was initiated and maintained by propofol and remifentanil target-controlled infusion (TCI) and sevoflurane inhalation, with intermittent intravenous injection of cisatracurium.
In the SV-VATS group, previously described anesthetic technique [9], in which anesthesia was induced by propofol and remifentanil TCI with target plasma concentrations of 3–4 ug/mL and 2–3 ng/mL respectively, was used. When patients lost consciousness and BIS was lower than 60, LMA (Ambu, Inc., Glen Burnie, USA) was inserted to allow spontaneous breathing. Anesthesia was maintained by propofol (2–2.5 ug/mL), remifentanil (0.5–1.5 ng/mL), and intravenous dexmedetomidine (0.5–1 ug·kg−1·h−1). The BIS was maintained at 40–60 throughout the surgery. The anesthesia depth was adjusted by increasing the concentration of propofol and remifentanil to attenuate the mediastinal movement or cough reflex. Synchronous intermittent mandatory ventilation (SIMV) (vt 4–5 ml/kg, RR 10–12/min) was applied to improve air exchange through a laryngeal mask if SpO2 declined to below 90% or PETCO2 was over 60 mmHg. At the completion of the main surgical procedure, SIMV (vt 6ml/kg, RR 15/min) was administered to eliminate the accumulated CO2.
For all patients, FiO2 was increased to maintain SpO2 ≥90%, and vasoactive drugs (Dopamine or Norepinephrine) were used to maintain mean arterial pressure (MAP) >60 mmHg. All patients were transferred to the PACU, where they were extubated or remained intubated according to their recovery conditions and preoperative evaluation, then they were transferred to the ICU or general ward.
Surgical procedure
The thoracoscopic procedures were similar in patients of MV-VATS and SV-VATS group. In the SV-VATS group, local anesthetic infiltration was performed on the chest wall with 2% lidocaine before skin incision. After the thoracic cavity was explored, the surface of the visceral pleura was sprayed with 2% lidocaine 5 mL, while intercostal and vagus nerve were blocked with 2% lidocaine 2.5 mL and 0.75% ropivacaine 2.5 mL under direct vision for inhibition of pain and coughing reflex. Lung collapse was achieved under atmospheric pressure in SV-VATS patients and by one-lung ventilation in MV-VATS patients. Because most of the lesions were small nodules or ground-glass nodules, lymph node dissection was not routinely performed in wedge resection. At the end of the procedure, the chest tube was removed immediately after lung reexpansion, or inserted to aerofluxus, which was decided by the surgeons according to the operation condition. The chest tube was removed when there was no air leakage after 3 h of tube clamping and drainage less than 200 mL in 24 h.
The blood samples were collected before the operation and immediately after the operation. The postoperative period was defined as the length of postoperative hospital stay. Clinical complications occurred during the postoperative period, such as thoracentesis, dyspnea (shortness of breath, RR >25/min, or SpO2 <94%) and air leakage, were recorded. Readmission was not included in postoperative complications. When compared with those on the first postoperative day, the postoperative chest radiography results (e.g., atelectasis, pulmonary exudation, or pneumonia) on the fourth postoperative day were regarded as radiography complications. Criteria for discharge were stable clinical conditions with SpO2 ≥94% at rest and all chest tubes removed.
Statistical analysis
All statistical analyses were performed using JMP, version 9, for Windows (JMP, Cary, NC). Any missing data on leukocyte count, neutrophil ratio, and hemoglobin values were replaced by series mean. A propensity score-matching analysis was used to counterbalance the discrepancies between the 2 groups. The propensity score model development was done by including age, sex, body mass index, ASA physical status classification, cardiac risk index, comorbidity, surgical sites and types of the prior and secondary thoracic surgery, and median interval between the prior and secondary thoracic surgery in the logistic regression model to predict the MV-VATS group.
Continuous data are presented as means (standard deviation) for normal distribution, or as medians (lower, upper quartiles) for skewness distribution. Dichotomous data are presented as numbers (%). All continuous variables were analyzed through a one-way ANOVA for homogeneity of variance test and a one-sample Kolmogorov-Smirnov test (K-S test) for normal distribution. The between-group differences were analyzed with an independent-samples t test for continuous variables, with homogeneity of variance and normal distribution. The Mann-Whitney U test was used for dichotomous data and skewed distributed data. The changes between the preoperative and postoperative leukocyte count, neutrophil ratio, and hemoglobin values in each group were analyzed by a paired-samples t test and analysis of covariance (ANCOVA). A P value of less than 0.05 was deemed to be statistically significant.
Results
A total of 79 patients in the MV-VATS group and 42 patients in the SV-VATS group met the inclusion criteria. Two comparable patient groups (n=39 for each group) were identified by using propensity score-matching analysis to counterbalance the discrepancies (Tables 1–4).
Table 1.
Patient’s demographics data before and after matching.
| Variable | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| MV-VATS group | SV-VATS group | P value | MV-VATS group | SV-VATS group | P value | |
| n=79 | n=42 | n=39 | n=39 | |||
|
| ||||||
| Median age (y) | 53.77 (16.32) | 46.57 (18.42) | 0.029 | 45.5 (18.0) | 45.7 (18.7) | 0.956 |
|
| ||||||
| Gender (n,%) | 0.950 | 0.617 | ||||
| Male | 56 (71%) | 30 (71%) | 27 (69%) | 29 (74%) | ||
| Female | 23 (29%) | 12 (29%) | 12 (31%) | 10 (26%) | ||
|
| ||||||
| Body mass index (kg/m2) | 21.73 (3.5) | 21.17 (3.8) | 0.424 | 21.4 (3.5) | 21.3 (3.9) | 0.832 |
|
| ||||||
| ASA physical status class (n, %) | 0.029 | 0.852 | ||||
| I | 21 (27%) | 17 (40%) | 18 (46%) | 17 (44%) | ||
| II | 42 (53%) | 19 (45%) | 16 (41%) | 17 (44%) | ||
| III | 16 (20%) | 6 (15%) | 5 (13%) | 5 (12%) | ||
|
| ||||||
| Comorbidity (n, %) | <0.001 | 0.493 | ||||
| Cardiovascular disease | 6 (8%) | 3 (7%) | 3 (8%) | 3 (8%) | ||
| Pulmonary disease | 5 (6%) | 4 (9%) | 2 (5%) | 3 (8%) | ||
| Neurological disease | 1 (1%) | 0 | 0 | 0 | ||
| Thyropathy | 0 | 2 (5%) | 0 | 0 | ||
| Two kinds of comorbidities | 1 (1%) | 1 (2%) | 0 | 1 (3%) | ||
|
| ||||||
| LVEF (%) | n=62 | n=35 | 0.660 | n=35 | n=35 | 0.487 |
| 71.37 (5.66) | 71.91 (6.08) | 70.3 (5.3) | 71.3 (6.0) | |||
|
| ||||||
| Cardiac risk index (n, %) | 0.852 | 0.559 | ||||
| 1 point | 76 (96%) | 40 (95%) | 38 (97%) | 37 (95%) | ||
| 2 points | 3 (4%) | 2 (5%) | 1 (3%) | 2 (5%) | ||
|
| ||||||
| Types of prior thoracic procedure (n, %) | 0.554 | 0.887 | ||||
| Bullectomy | 27 (34%) | 16 (39%) | 15 (38%) | 15 (38%) | ||
| Wedge resection | 6 (7%) | 3 (7%) | 1 (3%) | 3 (8%) | ||
| Lobectomy | 41 (52%) | 22 (52%) | 23 (59%) | 20 (51%) | ||
| Segmentectomy | 2 (3%) | 1 (2%) | 0 | 1 (3%) | ||
| Lung volume reduction | 3 (4%) | 0 | ||||
|
| ||||||
| Prior surgical site (n, %) | 0.392 | 0.513 | ||||
| Left side | 38 (48%) | 17 (41%) | 18 (46%) | 15 (38%) | ||
| Right side | 40 (51%) | 24 (57%) | 20 (51%) | 23 (59%) | ||
| Both sides | 1 (1%) | 1 (2%) | 1 (3%) | 1 (3%) | ||
|
| ||||||
| Pathology diagnosis of the second surgery (n, %) | 0.465 | 0.798 | ||||
| Bulla | 30 (38%) | 17 (40%) | 18 (46%) | 15 (39%) | ||
| Malignant | 39 (49%) | 23 (55%) | 18 (46%) | 24 (61%) | ||
| Benign | 7 (9%) | 2 (5%) | 2 (5%) | 0 | ||
| Bronchiectasia | 1 (1%) | 0 | 1 (3%) | 0 | ||
| COPD | 2 (3%) | 0 | 0 | 0 | ||
|
| ||||||
| Median interval between prior and secondary thoracic surgery (month) | 0.546 | 0.824 | ||||
| 32.29 (5–48) | 25.57 (6–36) | 15 (8–37) | 16 (6–36) | |||
|
| ||||||
| Preoperative hospital stay (d) | 8 (4–9) | 7 (3–10) | 0.714 | 5 (3–8) | 6 (3–10) | 0.233 |
|
| ||||||
| Pulmonary function tests (n=23, %) | n=53 | n=25 | 0.304 | n=23 | n=23 | 0.996 |
| FVC% predicted | 87.27 (20.64) | 91.98 (13.63) | 0.208 | 91.3 (22.7) | 91.3 (13.4) | 0.801 |
| FEV1% predicted | 77.17 (21.58) | 83.26 (15.14) | 0.536 | 81.1 (20.8) | 82.4 (15.0) | 0.769 |
| FEV1/FVC | 90.08 (15.26) | 92.24 (11.95) | 93.1 (13.9) | 91.9 (12.4) | ||
|
| ||||||
| DLCO% predicted (%) | n=23 | n=16 | 0.070 | n=12 | n=13 | 0.811 |
| 73.9 (18.3) | 83.0 (8.3) | 79.6 (14.0) | 80.7 (7.3) | |||
LVEF – left ventricular ejection fraction; FVC – forced vital capacity; FEV1 – forced expiratory volume in one second; COPD – chronic obstructive pulmonary emphysema; DLCO – carbon monoxide diffusing capacity.
Table 2.
Intraoperative variables.
| Variables | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| MV-VATS group | SV-VATS group | P value | MV-VATS group | SV-VATS group | P value | |
| n=79 | n=42 | n=39 | n=39 | |||
|
| ||||||
| Secondary surgical site (n,%) | 0.583 | 0.497 | ||||
| Left lung | 41 (52%) | 24 (57%) | 20 (51%) | 23 (59%) | ||
| Right lung | 38 (48%) | 18 (43%) | 19 (49%) | 16 (41%) | ||
|
| ||||||
| Types of secondary thoracic procedure (n, %) | 0.158 | 1.0 | ||||
| Bullectomy | 30 (38%) | 16 (38%) | 16 (41%) | 16 (41%) | ||
| Wedge resection | 26 (33%) | 23 (55%) | 21 (54%) | 21 (54%) | ||
| Lobectomy | 13 (16%) | 3 (7%) | 2 (5%) | 2 (5%) | ||
| Lung volume reduction | 2 (3%) | 0 | 0 | 0 | ||
| Segmentectomy | 8 (10%) | 0 | 0 | 0 | ||
|
| ||||||
| Operation time (min) | 90 (60–135) | 60 (45–90) | 0.009 | 85 (60–135) | 60 (45–90) | 0.008 |
|
| ||||||
| Anesthesia time (min) | 201.8 (82.8) | 151.1 (53.7) | <0.001 | 185.7 (74.0) | 150.4 (55.7) | 0.020 |
|
| ||||||
| Blood loss (ml) | 20 (10–50) | 5 (5–10) | <0.001 | 20 (10–50) | 5 (5–10) | <0.001 |
|
| ||||||
| The amount of liquid infusion (ml) | 1563.2 (428.7) | 1112.8 (390.2) | <0.001 | 1475.6 (430.9) | 1095.9 (394.7) | <0.001 |
|
| ||||||
| Urine volume (ml) | 400 (250–650) | 300 (137–563) | 0.064 | 400 (200–600) | 300 (100–550) | 0.302 |
|
| ||||||
| One-lung ventilation variables | ||||||
| Vt (L) | 0.35 (0.05) | 0.30 (0.08) | <0.001 | 0.34 (0.06) | 0.30 (0.08) | 0.005 |
| Ppeak (cmH2O) | 22 (19–24) | 2 (2–8) | <0.001 | 21.0 (19–24) | 2.0 (2–9) | <0.001 |
| SpO2 (%) | 99 (96–100) | 99 (97–100) | 0.282 | 99 (96–100) | 99 (96–100) | 0.448 |
| PETCO2 (mmHg) | 35.5 (4.2) | 48.8 (9.4) | <0.001 | 35.9 (3.9) | 49.4 (9.5) | <0.001 |
| FiO2 (%) | 69.9 (15.1) | 84.1 (13.1) | <0.001 | 70.7 (16.0) | 83.7 (12.9) | 0.027 |
|
| ||||||
| Vital signs during operation | ||||||
| HR (bpm) | 70.4 (8.8) | 73.3 (8.8) | 0.091 | 69.5 (7.1) | 73.4 (8.5) | 0.031 |
| MAP (mmHg) | 78.1 (8.6) | 77.1 (8.8) | 0.585 | 77.5 (8.7) | 77.2 (9.1) | 0.909 |
| BIS | 46.6 (4.9) | 47.6 (5.7) | 0.284 | 46.2 (4.4) | 47.5 (5.6) | 0.262 |
|
| ||||||
| Intraoperative anesthetic concentration | ||||||
| Propofol (ug/ml) | 0.8 (0.5–1.0) | 1.9 (1.6–2.0) | <0.001 | 0.8 (0.5–1.0) | 1.8 (1.6–2.0) | <0.001 |
| Sevoflurane (vol%) | 1.5 (1.3–1.7) | 0 | <0.001 | 1.5 (1.2–1.6) | 0 | <0.001 |
| Remifentanil (ug/kg/min) | 0.09 (0.08–0.1) | 0.03 (0.03–0.05) | <0.001 | 0.09 (0.08–0.1) | 0.03 (0.03–0.05) | <0.001 |
| Dexmedetomidine (ug) | 0 (0–54) | 82.9 (60–114.5) | <0.001 | 0 (0–54) | 82.5 (60–117) | <0.001 |
| Cisatracurium (mg) | 10 (6–12) | 0 | <0.001 | 9 (4–12) | 0 | <0.001 |
|
| ||||||
| Intraoperative vasoactive drugs (n, %) | 0.277 | 0.009 | ||||
| Yes | 19 (24%) | 14 (33%) | 8 (21%) | 14 (36%) | ||
| No | 60 (76%) | 28 (67%) | 31 (79%) | 25 (64%) | ||
Ppeak – peak respiratory pressure; FiO2 – fraction inspired oxygen concentration.
Table 3.
Early recovery in PACU.
| Variables | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| MV-VATS group | SV-VATS group | P value | MV-VATS group | SV-VATS group | P value | |
| n=79 | n=42 | n=39 | n=39 | |||
| Extubation time (min) | 20 (15–30) | 15 (10–21) | 0.001 | 20 (15–25) (Double-lumen tube) | 15 (10–22) (Laryngeal mask airway) | 0.015 |
| Consciousness recovery time (min) | 30 (25–35) | 20 (15–31) | 0.001 | 30 (25–35) | 20 (15–35) | 0.339 |
|
| ||||||
| Vital signs before leaving PACU | ||||||
| HR (bpm) | 79.8 (10.9) | 75.3 (11.2) | 0.033 | 78.4 (9.1) | 75.5 (11.4) | 0.025 |
| MAP (mmHg) | 93.2 (11.6) | 88.6 (9.7) | 0.029 | 92.3 (11.8) | 88.3 (9.7) | 0.108 |
| SpO2 (%) | 99 (98–100) | 100 (98–100) | 0.329 | 99 (98–100) | 100 (98–100) | 0.201 |
|
| ||||||
| Patient controlled infusion intravenous analgesia (n, %) | 55 (70%) | 29 (69%) | 0.948 | 31 (79%) | 28 (72%) | 0.432 |
|
| ||||||
| Mean PACU stay (min) | 87 (60–110) | 75 (60–105) | 0.433 | 75 (50–110) | 75 (60–105) | 0.907 |
PACU – Post Anesthesia Care Unit.
Table 4.
Later recovery in the ward (n=39).
| Variables | Before matching | After matching | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| MV-VATS group | SV-VATS group | P value | MV-VATS group | SV-VATS group | P value | |
| n=79 | n=42 | n=39 | n=39 | |||
| Mean ICU stay (d) | 1 (0–1) | 0 (0–1) | 0.012 | 1.0 (0–1) | 0 (0–1) | 0.066 |
|
| ||||||
| Postoperative clinical complication (n, %) | 0.020 | 0.052 | ||||
| Without complication | 66 (83%) | 41 (98%) | 33 (84%) | 38 (97%) | ||
| Thoracentesis | 5 (6%) | 0 | 2 (5%) | 0 | ||
| Dyspnea | 2 (3%) | 0 | 1 (3%) | 0 | ||
| Air leakage in chest tube | 3 (4%) | 1 (2%) | 2 (5%) | 1 (3%) | ||
| Arrhythmia | 1 (1%) | 0 | 1 (3%) | 0 | ||
| Mechanical ventilation | 2 (3%) | 0 | 0 | 0 | ||
|
| ||||||
| Chest-tube duration (d) | 2 (2–3) | 1 (0–2) | <0.001 | 2 (2–3) | 1 (0–2) | <0.001 |
|
| ||||||
| Postoperative hospital stay (d) | 6 (4–8) | 3 (2–4) | <0.001 | 5 (3–8) | 3 (2–4) | 0.003 |
|
| ||||||
| Chest radiography results on the fourth postoperative day (n, %) | 0.037 | 0.212 | ||||
| Normal | 56 (71%) | 37 (88%) | 30 (76%) | 34 (87%) | ||
| Pulmonary exudation | 10 (13%) | 1 (2%) | 2 (5%) | 1 (3%) | ||
| Pneumothorax or atelectasis | 8 (10%) | 4 (10%) | 4 (10%) | 4 (10%) | ||
| Pleural effussion | 1 (1%) | 0 | 1 (3%) | 0 | ||
| Pneumonia | 3 (4%) | 0 | 1 (3%) | 0 | ||
| Two kinds of radiography results | 1 (1%) | 0 | 1 (3%) | 0 | ||
Variables comparison during operation
When compared with those in the MV-VATS group, the mean operation and anesthesia time in the SV-VATS group were significantly shorter (P=0.008, P=0.020) and there were less blood loss and liquid infusion (P<0.001). Although the PETCO2 and FiO2 were higher (P<0.001, P=0.027), the Ppeak was significantly lower in the SV-VATS group (P<0.001), and the difference in SpO2 between the 2 groups was insignificant (P=0.448). HR was higher (P=0.031) and more vasoactive agents were used in the SV-VATS group (P=0.009). Comparisons showed that higher concentration of sedatives, including propofol and dexmedetomidine, and lower concentration of analgesics were used in the SV-VATS group (P<0.001) (Table 2).
Variables comparison in PACU
In the PACU, the time of LMA extubation was shorter in the SV-VATS group (P=0.015), but the comparisons of consciousness recovery time, mean PACU stay and the incidence of intravenous analgesia did not show any between-group differences (P=0.339, P=0.907, P=0.432). Vital signs of MAP and SpO2 in the 2 groups were similar (P=0.108, P=0.201), while HR was slightly lower in the SV-VATS group (P=0.025) (Table 3).
Variables comparison in the ward
In the general ward, comparison of the 2 groups did not yield any significant differences in the mean ICU stay, postoperative clinical complications, or chest radiography results (P=0.066, P=0.052, P=0.212, respectively). However, the mean chest-tube duration and postoperative hospital stay were shorter in the SV-VATS group (P<0.001, P=0.003) (Table 4).
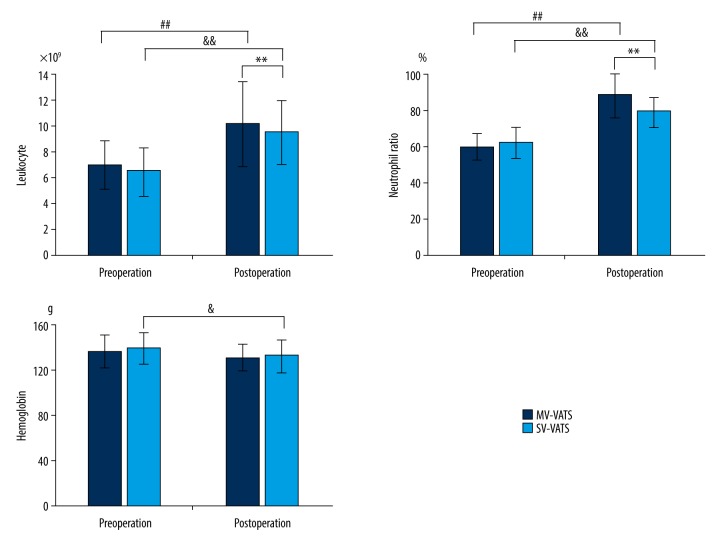
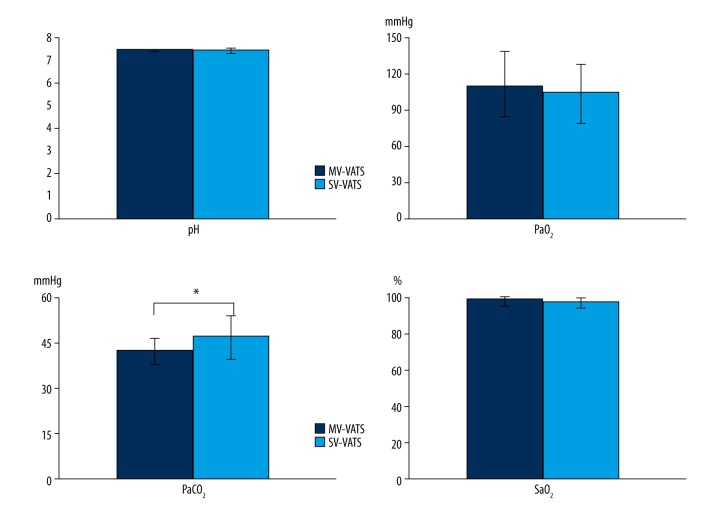
The postoperative leukocyte count and neutrophil ratio in the SV-VATS group were lower than those in the MV-VATS group (P<0.001, P=0.001). In both groups, the postoperative leukocyte count and neutrophil ratio were higher than preoperative values (P<0.001 in the MV-VATS group and P<0.001 in the SV-VATS group) (Figure 1). Insignificant differences were observed in the postoperative values of pH, PaO2, and SaO2 between the 2 groups (P=0.659, P=0.578, P=0.499, respectively), but PaCO2 was slightly higher in the SV-VATS group (P=0.026) (Figure 2).
Figure 1.
Blood cells analysis change in the MV-VATS group and SV-VATS group before and after the operation. # P values present the comparison of pre-operation and post-operation in the MV-VATS group. & P values present the comparison of pre-operation and post-operation in the SV-VATS group. * P values present the postoperative comparison between the MV-VATS group and SV-VATS group.
Figure 2.
Postoperative blood gas values change in the MV-VATS group and SV-VATS group. * P values present the postoperative comparison between the MV-VATS group and SV-VATS group.
Discussion
The successful debut of spontaneous ventilation for VATS was reported by Pompeo in 2004 [4], and has been widely performed in a variety of VATS procedures [4–6]. It can avoid ventilation-induced lung injury and decrease the incidence of pulmonary complications for high-risk patients [10–13]. From 2011 to present, we have accumulated experience with more than 3000 patients, even in the cases of secondary contralateral thoracic surgeries.
The present observational study analyzed the perioperative outcomes of patients undergoing secondary contralateral thoracic surgeries with spontaneous ventilation. Our results indicated that VATS under spontaneous ventilation is feasible and safe for these patients, with satisfactory outcomes and postoperative results similar to those under general anesthesia with one-lung ventilation. In the SV-VATS group, the vital signs were stable during the operation and in the PACU. The operation and anesthesia times were significantly shorter, which resulted in less intraoperative blood loss and liquid infusion.
Oxygenation challenge and critical hypercapnia are likely to be more prevalent in repeated surgery due to the lack of functional parenchyma, less vascular territory, and altered lung mechanics after prior contralateral lobectomy [2,14]. The loss of lung function in patients with a prior lobe resection is approximately 24% [15]. Even 5 or more years after pneumonectomy, lung capacity is only 10–15% greater than before [16]. Thus, hypoxemia and hypercapnia may occur during total one-lung collapse [14]. Selective lobar blockade can be a specific technique to improve intraoperative oxygenation in a secondary contralateral pulmonary resection [17,18]. However, bronchial blockage is easily shifted due to the surgical traction or the bronchial angle [14], and partial lobar ventilation may also affect pulmonary tissue separation. Other methods, such as high-frequency jet ventilation and extracorporeal support, are seldom used owing to the difficulty in eliminating PaCO2 [19], or are limited to carefully selected cases [20]. In contrast, VATS under spontaneous ventilation can preserve the mediastinal and diaphragm motion with a smaller decrease of functional residual capacity [21], conserve the pulmonary compliance, and reduce atelectasis in the dependent lung [22,23], which are favorable for ventilation to perfusion matching in lateral decubitus, with less risk of hypoxemia. In addition, SIMV was applied to ensure oxygenation when SpO2 decreased to lower than 90%. Therefore, in our observation, oxygenation level could be maintained over 94% during the operation and in the PACU, and the postoperative values of PaO2 and SaO2 were the same in both groups. Moreover, mild hypercapnic acidosis may reduce the severity of lung injury [24]. The concentration of intraoperative PETCO2 and postoperative PaCO2 in the SV-VATS group showed no significant carbon dioxide retention during and after the operation, which suggests that mild hypercapnia can be compensated and returned to normal level when VATS is performed under spontaneous ventilation.
VATS under spontaneous ventilation can reduce the dosage of the analgesic drugs during the operation and in the PACU, thus enabling a faster and improved earlier recovery, as confirmed by previous studies [10,13]. Furthermore, VATS under spontaneous ventilation for secondary contralateral thoracic surgeries does not increase postoperative complications or abnormal chest radiograph results. The duration of chest-drainage tube was shorter and the postoperative leukocyte count and neutrophil ratio were lower in the SV-VATS group, which indicated a low degree of airway inflammation, in agreement with the results of Liu [25].
In addition to effective local anesthesia, great emphasize must be placed on several aspects during spontaneous ventilation surgery. First, the depth of anesthesia should be consistent with the intensity of stimulation. The concentration of remifentanil or propofol should be increased to deepen anesthesia during insertion of the laryngeal mask airway, invasive arteriovenous puncture, the endoscope entering the thoracic cavity, and excessive traction of the lung tissue or fluid irrigation of the thoracic cavity. Generally, cough and laryngospasm can be resolved by increasing the concentration of inhaled oxygen, preemptive vagus nerve block and lidocaine spay on the lung surface, and intravenous remifentanil 50–100 ug or propofol 30–50 mg to deepen anesthesia. In case of intractable cough or laryngospasm, especially those persisting even after use of above-mentioned management techniques, muscle relaxants must be used and SIMV should be instituted. Second, PETCO2 and SpO2 should be monitored closely. The SIMV or manually-assisted ventilation can be applied to assist CO2 discharge when PETCO2 is over 60 mmHg and/or when SpO2 is less than 90%. Although the ratio of instituting SIMV in the SV-VATS group was about 21%, low ventilation pressure and high breathing frequency in SIMV can avoid pulmonary over-expansion in the non-dependent lung and will not interfere with the operation. Third, special attention should be paid to the adverse effect of mediastinal oscillation on hemodynamics. Dopamine or norepinephrine can be given to maintain stable hemodynamics when necessary. Fourth, for leisions in the lower lobe of the lung, it is recommended that the endoscopic hole can be placed in the sixth or fifth intercostal space to avoid diaphragm elevation resulting from surgical stimulation. In case of diaphragm elevation, unilateral phrenic nerve block is not recommended because more obvious elevation may occur. Fifth, vigilance against the possibility of reflux and aspiration should be emphasized. Preoperative exclusion of patients with history of gastric reflux is of paramount importance to spontaneous ventilation surgery, and effective resolution includes passage of a suction catheter into the stomach through the esophageal lumen of LMA to aspirate the upper esophagus. When checking for air leakage, setting an inflation pressure ceiling of 25 cmH2O is helpful to reduce reflux and aspiration. Once aspiration occurs, airway irrigation under fiberoptic bronchoscope or tracheal intubation are required.
The present study has certain limitations. First, this was a single-center retrospective analysis, and propensity analysis was performed to ameliorate the selection bias. Second, the results of blood gas analysis and pulmonary function examination could not be assessed for each patient, which may be unfavorable for the evaluation of pulmonary gas exchange. Third, the major thoracic procedures in this study, such as bullectomy and pulmonary wedge resection, are relatively simple, and further research is needed for more complex thoracic procedures.
Conclusions
VATS under spontaneous ventilation is a suitable option for patients who undergo a secondary contralateral thoracic surgery, and may accelerate rehabilitation. However, this method may be most suitable for simple thoracic procedures with shorter operative time, and the feasibility and safety in more complicated and longer procedures remain to be explored.
Acknowledgement
We are grateful for the help of Dr. Huai Chen for analysis of the chest radiography results and to Professor Xiaohui Wen for help with collecting clinical data.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Toufektzian L, Patris V, Potaris K, Konstantinou M. Is it safe and worthwhile to perform pulmonary resection after contralateral pneumonectomy? Interact Cardiovasc Thorac Surg. 2015;20:265–69. doi: 10.1093/icvts/ivu385. [DOI] [PubMed] [Google Scholar]
- 2.Durkin C, Lohser J. Oxygenation and ventilation strategies for patients undergoing lung resection surgery after prior lobectomy or pneumonectomy. Curr Anesthesiol Rep. 2016;6:135–41. [Google Scholar]
- 3.Liu Y, Cui P, Yang Z, et al. Right lower lobectomy 8 years after left pneumonectomy for a second primary lung cancer. J Cardiothoracic Surg. 2013;8:46. doi: 10.1186/1749-8090-8-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pompeo E, Mineo D, Rogliani P, et al. Feasibility and results of awake thoracoscopic resection of solitary pulmonary nodules. Ann Thorac Surg. 2004;78:1761–68. doi: 10.1016/j.athoracsur.2004.05.083. [DOI] [PubMed] [Google Scholar]
- 5.Pompeo E, Tacconi F, Frasca L, Mineo TC. Awake thoracoscopic bullaplasty. Eur J Cardiothorac Surg. 2011;39:1012–17. doi: 10.1016/j.ejcts.2010.09.029. [DOI] [PubMed] [Google Scholar]
- 6.Chen JS, Cheng YJ, Hung MH, et al. Nonintubated thoracoscopic lobectomy for lung cancer. Ann Surg. 2011;254:1038–43. doi: 10.1097/SLA.0b013e31822ed19b. [DOI] [PubMed] [Google Scholar]
- 7.Lu YF, Hung MH, Hsu HH, Chen JS. Non-Intubated thoracoscopic segmentectomy for second primary lung cancer in a patient with previous contralateral lobectomy and emphysematous bullae. J Cardiothorac Vasc Anesth. 2016;30:1639–40. doi: 10.1053/j.jvca.2015.12.024. [DOI] [PubMed] [Google Scholar]
- 8.Galvez C, Navarro-Martinez J, Bolufer S, et al. Benefits of awake uniportal pulmonary resection in a patient with a previous contralateral lobectomy. Ann Transl Med. 2014;2:93. doi: 10.3978/j.issn.2305-5839.2014.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lan L, Cen Y, Zhang C, et al. A propensity score-matched analysis for non-intubated thoracic surgery. Med Sci Monit. 2018;24:8081–87. doi: 10.12659/MSM.910605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guarracino F, Gemignani R, Pratesi G, et al. Awake palliative thoracic surgery in a high-risk patient: One-lung, non-invasive ventilation combined with epidural blockade. Anaesthesia. 2008;63:761–63. doi: 10.1111/j.1365-2044.2008.05443.x. [DOI] [PubMed] [Google Scholar]
- 11.Kiss G, Claret A, Desbordes J, Porte H. Thoracic epidural anaesthesia for awake thoracic surgery in severely dyspnoeic patients excluded from general anaesthesia. Interact Cardiovasc Thorac Surg. 2014;19:816–23. doi: 10.1093/icvts/ivu230. [DOI] [PubMed] [Google Scholar]
- 12.Mineo TC. Epidural anesthesia in awake thoracic surgery. Eur J Cardiothorac Surg. 2007;32:13–19. doi: 10.1016/j.ejcts.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 13.Tacconi F, Pompeo E, Mineo TC. Duration of air leak is reduced after awake nonresectional lung volume reduction surgery. Eur J Cardiothorac Surg. 2009;35:822–28. doi: 10.1016/j.ejcts.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 14.Ruiz P, Kovarik G. Lung mechanics and gas exchange in one lung ventilation following contralateral resection. Can J Anesth. 2005;52:986–89. doi: 10.1007/BF03022063. [DOI] [PubMed] [Google Scholar]
- 15.McGlade DP, Slinger PD. The elective combined use of a double lumen tube and endobronchial blocker to provide selective lobar isolation for lung resection following contralateral lobectomy. Anesthesiology. 2003;99:1021–22. doi: 10.1097/00000542-200310000-00040. [DOI] [PubMed] [Google Scholar]
- 16.Laros CD, Westermann CJ. Dilatation, compensatory growth, or both after pneumonectomy during childhood and adolescence. A thirty-year follow-up study. J Thorac Cardiovasc Surg. 1987;93:570–76. [PubMed] [Google Scholar]
- 17.Campos JH, Ledet C, Moyers JR. Improvement of arterial oxygen saturation with selective lobar bronchial block during hemorrhage in a patient with previous contralateral lobectomy. Anesth Analg. 1995;81:1095–96. doi: 10.1097/00000539-199511000-00036. [DOI] [PubMed] [Google Scholar]
- 18.Campos JH. Update on selective lobar blockade during pulmonary resections. Curr Opin Anaesthesiol. 2009;22:18–22. doi: 10.1097/ACO.0b013e32831a437a. [DOI] [PubMed] [Google Scholar]
- 19.Misiolek H, Knapik P, Swanevelder J, et al. Comparison of double-lung jet ventilation and one-lung ventilation for thoracotomy. Eur J Anaesthesiol. 2008;25:15–21. doi: 10.1017/S0265021507000701. [DOI] [PubMed] [Google Scholar]
- 20.Ishikawa N, Sato H, Hiranuma C, Takizawa M. A surgical intervention using percutaneous cardiopulmonary support for contralateral pneumothorax following pneumonectomy. Ann Thorac Cardiovasc Surg. 2001;7:235–36. [PubMed] [Google Scholar]
- 21.Noda M, Okada Y, Maeda S, Kondo T. Successful thoracoscopic surgery for intractable pneumothorax after pneumonectomy under local and epidural anesthesia. J Thorac Cardiovasc Surg. 2011;141:1545–47. doi: 10.1016/j.jtcvs.2010.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Liu YJ, Hung MH, Hsu HH, et al. Effects on respiration of nonintubated anesthesia in thoracoscopic surgery under spontaneous ventilation. Ann Transl Med. 2015;3:107. doi: 10.3978/j.issn.2305-5839.2015.04.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hung MH, Hsu HH, Cheng YJ, Chen JS. Nonintubated thoracoscopic surgery: State of the art and future directions. J Thorac Dis. 2014;6:2–9. doi: 10.3978/j.issn.2072-1439.2014.01.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinclair SE, Kregenow DA, Lamm WJ, et al. Hypercapnic acidosis is protective in an in vivo model of ventilator-induced lung injury. Am J Respir Crit Care Med. 2002;166:403–8. doi: 10.1164/rccm.200112-117OC. [DOI] [PubMed] [Google Scholar]
- 25.Liu J, Cui F, Li S, et al. Nonintubated video-assisted thoracoscopic surgery under epidural anesthesia compared with conventional anesthetic option: A randomized control study. Surg Innov. 2015;22:123–30. doi: 10.1177/1553350614531662. [DOI] [PubMed] [Google Scholar]