Abstract
Background:
Determination of the radiosensitivity of a specific tumor is essential to its precision tumor radiotherapy, but the measurement of cellular radiosensitivity with a routine colony forming assay is both labor- and time-consuming. An alternative option allowing rapid and precise prediction of radiosensitivity is necessary.
Methods:
In this study, we exposed 4 in vitro cultured cell lines to various doses of X-rays or carbon ions and then measured their radiosensitivities with a routine colony-forming assay, and monitored the kinetics of cell cycle distribution with routine propidium iodine staining and flow cytometry.
Results:
Based on the results, we correlated cellular radiosensitivity with a dynamic assay of cell cycle distribution, specifically, the negative correlation of cellular radiosensitivity with the accumulated G2/M arrested cells at 48 hours after exposure. The higher the proportion of accumulated G2/M arrested cells at 48 hours after exposure, the lower the radiosensitivity of the cell line, that is, the higher radioresistance of the cell line.
Conclusion:
These findings provide an optional application of regular cell cycle analysis for the prediction of tumor radiosensitivity.
Keywords: ionizing radiation, G2/M block, radiosensitivity, precision medicine, melanoma and squamous cell carcinoma
Introduction
Precision medicine is thought to provide a personalized treatment plan based on the genetic background.1 For tumor radiotherapy, precision treatment depends on the radiosensitivity of a specific tumor to treatment radiation. Currently, the colony forming assay is the classic measurement of cellular radiosensitivity.2 It usually takes approximately 2 weeks for one repeat, and it takes more than 1 month to obtain reliable results. For clinical applications, a rapid assay to determine the radiosensitivity of a tumor is therefore urgently needed.
Cell cycle checkpoint is a highly evolved cellular mechanism by which a cell positively halts progression through the cell cycle until it ensures the orderly and timely progression and completion of critical events such as DNA replication and chromosome segregation .3 It is critical for cells to maintain their genomic stability. Theoretically, any damages to the DNA or cell cycle checkpoint pathway might lead to cell cycle block at a certain phase, and the more severe damage ionizing radiation induces, the more sensitive the cells. Even though radiation-induced cell cycle arrest has been widely studied, the correlation between cellular radiosensitivity and cell cycle arrest is not well-established.
In this study, we conducted quantitative experimental studies with 4 cell lines, 2 melanoma cell lines, C32TG and MeWo, and 2 squamous cell carcinoma cell lines, FaDu and SAS. We measured their radiosensitivities to X-rays and carbon ions with classic colony forming assays, and measured the dynamics of cell cycle distribution with routine propidium iodide (PI) staining and flow cytometry. We established a negative linear correlation of cellular radiosensitivity (D10, SF2) with the accumulated G2/M blocked cells at 48 hours after exposure.
Methods
Cell Culture
Human malignant melanoma cell lines, C32TG and MeWo, and squamous cell carcinoma cell lines, FaDu and SAS, were purchased from the American Type Culture Collection (Manassas, Virginia) and stored by the near-infrared spectroscopy. The cells were cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, Missouri) containing 10% fetal bovine serum (FBS; Hyclone, Logan, Utah), 100 μg/mL streptomycin, and 100 units/mL penicillin in a humidified atmosphere of 95% air and 5% CO2.
Irradiation
Both X-rays and carbon ion irradiation were conducted at the National Institute of Radiological Sciences, Chiba, Japan.4 Briefly, X-ray irradiations were performed with a Pantak-320 S generator (Shimadzu, Tokyo, Japan) operated at 200 kVp and 20 mA with 0.5 mm Al and 0.5 mm Cu filters. The dose rate was 1 Gy/min. Carbon ions with an original energy of 290 MeV/u were modified with filters to obtain a linear energy transfer (LET) of 100 keV/µm generated by a heavy ion medical accelerator at Chiba (HIMAC, Chiba, Japan) with a dose rate of 1 Gy/min. All irradiations were conducted at room temperature.
Cell Survival
Cell survival was determined using a conventional colony forming assay. Briefly, the cells were collected by trypsinization and resuspended in RPMI-1640 medium containing 10% FBS immediately after exposure. The cell concentration was determined using a model Z1 cell Coulter counter with a 100 µm aperture tube. The cells were diluted with medium and seeded in 60 mm Petri dishes (Falcon, Germantown, Washington, DC) to obtain 10 to 100 colonies per dish. The dishes were incubated for the appropriate days corresponding to the cell lines, then fixed with 10% formaldehyde for 10 minutes and stained with crystal violet to visualize the colonies. Colonies containing more than 50 cells were counted as survivors. Three parallel dishes were scored each time for every dose.
Cell Cycle Distribution
The cells were harvested and fixed with 70% prechilled ethanol for > 24 hours at −20°C. The fixed cells were washed twice with phosphate-buffered saline, then treated with 100 μg/mL RNase A and 50 μg/mL PI mixed buffer (BD Biosciences, San Jose, California) for 30 minutes at 37°C. The cell cycle distribution was analyzed with Modfit software (Verity Software, Topsham, Maine) from the histogram of the DNA content measured with a flow cytometer (FACScan, Becton Dickinson, Franklin Lakes, New Jersey). The increased G2/M cells (%) were used to quantitate the induction of the G2/M block, which was obtained by subtracting the number of control G2/M cells from those of the irradiated samples.5
Statistical Analysis
All the experiments were repeated independently at least 3 times, and the data are presented as the mean ± standard error (SE). All results were plotted and analyzed with Kaleidagraph (Synergy Software, Philadelphia, Pennsylvania). The survival data were fitted with a linear quadratic model and the dose dependency of the induction of G2/M block was fitted with a linear model.
Results
Different Cell Lines Differ in Radiosensitivity, but Carbon Ion Irradiation Decreases the Difference
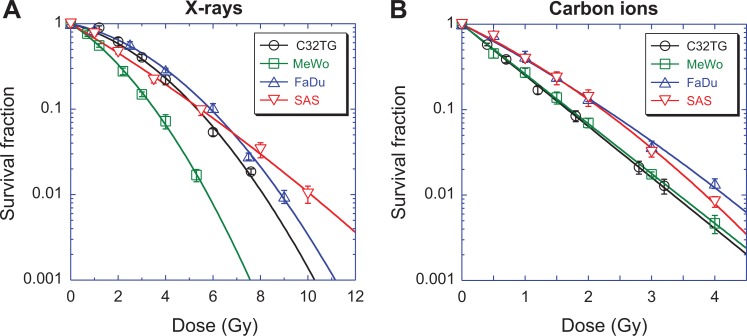
For X-ray irradiation, there were shoulders in the survival curves of C32TG, MeWo, and FaDu cells (Figure 1A). The ratio of α/β for the MeWo cell line was the highest, while that for FaDu was the lowest, which meant that MeWo was the most radiosensitive to X-rays and FaDu was the most radioresistant of the 3 cell lines (Table 1). Different from these 3 cell lines, SAS had a tiny shoulder in its survival curves and thus a high α/β. The sensitivities of the 4 kinds of cell lines to X-rays were diverse.
Figure 1.
Cell survival curves of 4 cell lines. Survival was measured with a routine colony-forming assay. (A) X-ray irradiation, (B) carbon ions with 100 kev/µm. Data are presented as the mean ± standard error. Experimental data were fitted using a linear-quadratic model. All experiments were independently repeated at least 3 times.
Table 1.
Parameters of Cell Survival Curves.
| X Rays | Carbon | RBE | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| α | β | α/β | D50 | D10 | SF2 | α | β | α/β | D50 | D10 | SF2 | 50 | 10 | |
| C32TG | 0.172 | 0.049 | 3.55 | 2.40 | 5.34 | 0.58 | 1.377 | - | - | 0.50 | 1.67 | 0.06 | 4.77 | 3.19 |
| MeWo | 0.421 | 0.065 | 6.48 | 1.36 | 3.54 | 0.33 | 1.342 | - | - | 0.52 | 1.72 | 0.07 | 2.63 | 2.06 |
| FaDu | 0.133 | 0.044 | 3.05 | 2.74 | 5.90 | 0.64 | 0.897 | 0.051 | 17.52 | 0.74 | 2.27 | 0.14 | 3.70 | 2.60 |
| SAS | 0.380 | 0.007 | 51.73 | 1.76 | 5.48 | 0.45 | 0.820 | 0.097 | 8.43 | 0.77 | 2.22 | 0.13 | 2.28 | 2.47 |
Abbreviation: RBE, relative biological effectiveness.
High LET carbon ion irradiation changed the shape of the survival curves dramatically (Figure 1B). The shoulder in the survival curves of C32TG and MeWo disappeared and there was rarely a difference in the survival of these 2 cell lines. The differences between FaDu and SAS also decreased, even though a tiny shoulder remained for both cell lines. Two melanoma cell lines were relatively more sensitive to carbon ions than the 2 squamous cell carcinoma cell lines.
Consistently, carbon ions had a better ability to kill cancer cells. The relative biological effectiveness (RBE) at 10% survival was 3.19 for C32TG, 2.06 for MeWo, 2.60 for FaDu, and 2.47 for SAS.
Both X-Rays and Carbon Ions Induce G2/M Block in a Both Time- and Dose-Dependent Manner
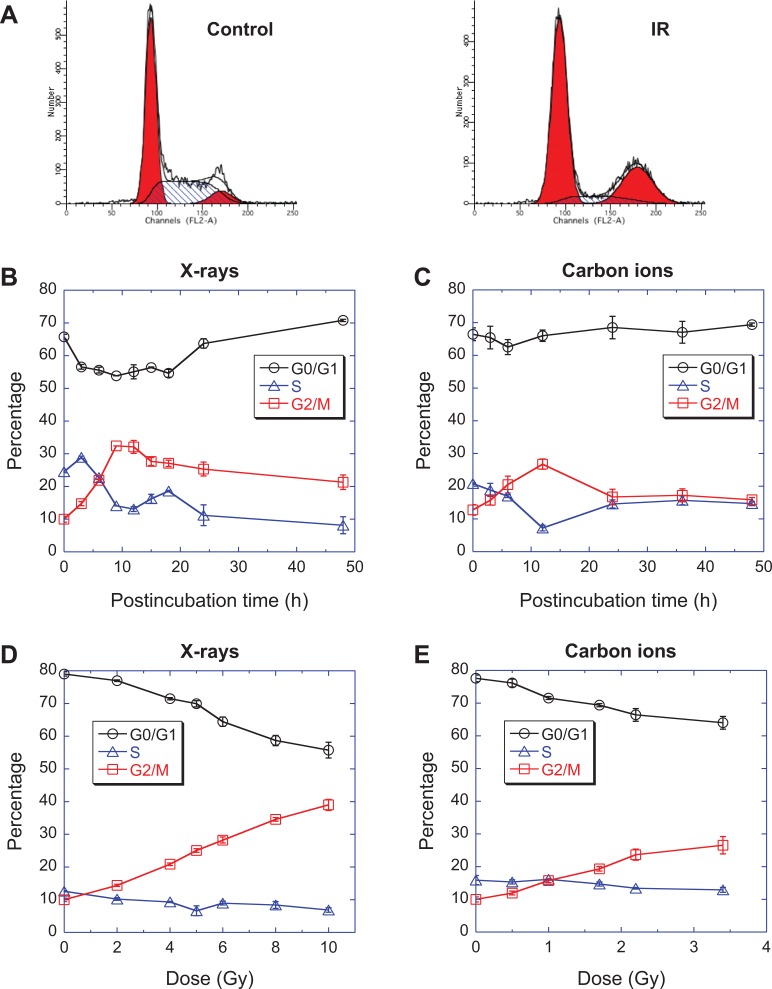
Dynamic studies on the cell cycle distribution using flow cytometry revealed G2/M arrest in all 4 tested cell lines. Figure 2B shows that the proportion of G0/G1 phase of C32TG cells decreased immediately after exposure to 5 Gy of X-rays, while the proportion of S phase cells increased slightly and then decreased in 3 hours. The proportion of G2/M phase cells gradually increased after exposure to X-rays and reached a peak at 9 hours, then decreased at 15 hours. However, the proportion of G2/M phase cells was still higher than the controls at 48 hours. Obviously, a G2/M arrest was induced in G32TG cells by X-rays. In a similar manner, the G2/M arrest was induced by carbon ions and reached a maximum at 12 hours after exposure (Figure 2C).
Figure 2.
Dose- and time-dependency of the cell cycle distribution of C32TG cells exposed to ionizing radiation. (A) the cell cycle distribution measured with propidium iodide staining and flow cytometry, (B) dynamics of cell cycle distribution after exposure to 5 Gy of X-rays, (C) dynamics of cell cycle distribution after exposure to 1.7 Gy of carbon ions, (D) the cell cycle distribution 48 hours after exposure to various doses of X-rays, (E) the cell cycle distribution 48 hours after exposure to various doses of carbon ions. Data are presented as the mean ± standard error. All experiments were independently repeated at least 3 times.
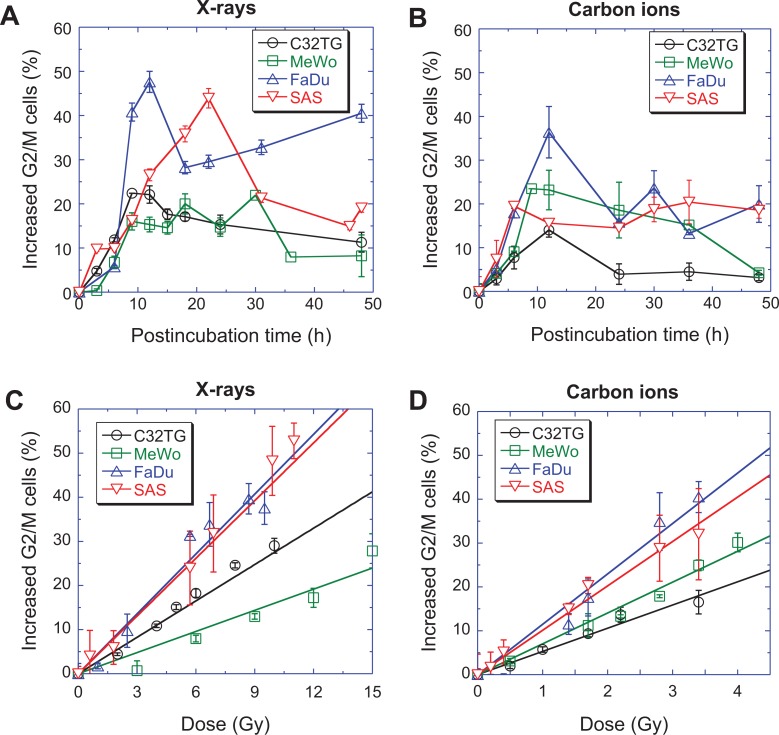
However, the dynamics of the G2/M arrest induced by ionizing radiation differed among cell lines. Figure 3A and B show that the G2/M arrest of different cell lines reached a maximum value at different postincubation times; C32TG and MeWo at 9 hours, FaDu at 12 hours, and SAS at 24 hours after exposure to X-rays. For carbon ions, all cell lines reached their maximum G2/M block much faster than X-ray exposure; SAS at 6 hours, MeWo at 9 hours, and C32TG and FaDu at 12 hours (Figure S1).
Figure 3.
Induction of the G2/M block in 4 cell lines by ionizing radiation. (A) Dynamics of G2/M phase cells after exposure to 5 Gy of X-rays, (B) dynamics of G2/M phase cells after exposure to 1.7 Gy of carbon ions, (C) the dose-dependent induction of the G2/M block 48 hours after X-ray exposure, (D) the dose-dependent induction of the G2/M block 48 hours after carbon ion exposure. The data are presented as the mean ± standard error. All experiments were independently repeated at least 3 times.
The maximum of G2/M blocked cells differed among the cell lines (Table 2). For X-ray irradiation, the maximal G2/M arrests were 32.11%, 30.01%, 57.61%, and 53.94% for C32TG, MeWo, FaDu, and SAS, respectively. For carbon ion irradiation, the maximal G2/M arrests were 24.01%, 33.46%, 46.37%, and 30.45% for C32TG, MeWo, FaDu, and SAS, respectively. Because different cell lines reached their maximum values at different postincubation times, it was not easy to compare the maximums of G2/M arrests of various cell lines.
Table 2.
Parameters of G2/M Block Induction.
| Cells | X Rays | Carbon | RBE | ||||
|---|---|---|---|---|---|---|---|
| Maximum | At 48 Hours | Slope | Maximum | At 48 Hours | Slope | ||
| C32TG | 32.11 ± 1.96 | 21.33 ± 2.24 | 3.04 | 24.01 ± 1.57 | 13.14 ± 0.59 | 5.19 | 1.71 |
| MeWo | 30.01 ± 2.27 | 18.24 ± 4.73 | 1.85 | 33.46 ± 0.00 | 14.29 ± 0.56 | 7.39 | 4.00 |
| FaDu | 57.61 ± 0.00 | 50.51 ± 0.00 | 5.04 | 46.37 ± 5.88 | 29.98 ± 4.20 | 12.97 | 2.57 |
| SAS | 53.94 ± 0.00 | 29.07 ± 0.00 | 4.82 | 30.45 ± 4.98 | 28.56 ± 1.85 | 9.74 | 2.02 |
Abbreviation: RBE, relative biological effectiveness.
Consistent G2/M Blocked Cells at 48 Hours After Exposure Might be an Indicator of Cellular Radiosensitivity
There was a dose-dependent accumulation of G2/M blocked cells 48 hours after exposure (Figure 2D and E). With increasing irradiation doses, the proportion of G0/G1 phase cells gradually decreased, while the proportion of G2/M phase cells gradually increased, and the proportion of S phase cells showed minimal changes. A similar dose-dependent accumulation of G2/M blocked cells was observed with carbon ions (Figure 2C).
The dose-dependent accumulation of G2/M blocked cells was also correlated with cell type (Figure 3C and D). A linear dependency of accumulated G2/M cells with X-ray irradiation doses was shown for FaDu, C32TG, and MeWo cells. MeWo was the most resistant cell line, while FaDu was the most sensitive cell line among the 3 cell lines. The accumulation of G2/M SAS cells at 48 hours after exposure was slightly different from other cell lines.
The dose-dependent accumulation of G2/M blocked cells was correlated with the type of radiation. Carbon ion irradiation was better at blocking cells in G2/M phase even 48 hours after exposure, so the RBE was higher than 1, 1.71, 4.00, 2.57, and 2.20 for C32TG, MeWo, FaDu, and SAS, respectively (Figure S2).
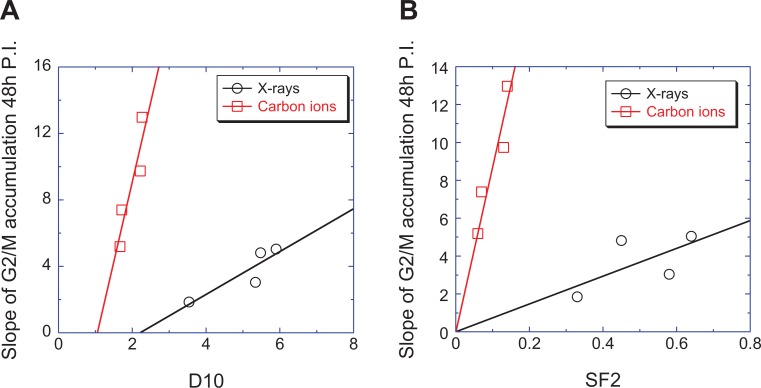
An obvious correlation between cellular radiosensitivity and G2/M block was seen via the shape of the survival curves shown in Figure 1A and B, and the accumulation of G2/M arrested cells shown in Figure 3C and D. To quantitate the correlations, we plotted the accumulations of G2/M arrested cells at 48 hours versus SF2 and D10 (Figure 4). Positive correlations were found (with D10, R2 = 0.79 for X-rays and 0.84 for carbon ions; with SF2, R2 = 0.39 for X-rays and 0.88 for carbon ions).
Figure 4.
Correlation between the G2/M block and cellular radiosensitivity. Based on the dose-dependency of the G2/M block as shown in Figure 3C and 3D, we fitted the data with a linear mode to obtain the slope. The slopes of the dose-dependent induction of G2/M blocked cells were plotted versus D10 (A), the dose for 10% survival, and SF2 (B), the survival level induced by 2 Gy of ionizing radiation. The slope of the accumulation in G2/M arrested cells at 48 hours after exposure was linearly correlated with D10 or SF2.
Discussion
Cell cycle checkpoints are well-conserved, self-protective processes for cells to ensure proper and intact genetic materials to segregate into 2 daughter cells.6 Ionizing radiation induces DNA damages and induces DNA repair of cell cycle checkpoints.7 The more severe the ionizing radiation induces DNA damages, the longer the cells were blocked in a specific cell phase. Interference of the cell cycle with chemicals, small interfering RNA, or inhibitors to cell cycle kinases usually sensitizes tumor cells to ionizing radiation8-12 so there must be a correlation between cell cycle block and cellular radiosensitivity. In the present study, we conducted quantitative studies with 4 cell lines, to determine the relationship between cellular radiosensitivity and cell cycle block, and to establish a negative correlation between cellular radiosensitivity and the accumulation of G2/M arrested cells at 48 hours after exposure. Even though the peak of the G2/M induction was correlated with cellular radiosensitivity, it was hardly detected because different cell lines reached their peaks at different times after exposure. The linear dependent accumulation on irradiation dose and its positive correlation with SF2/D10 made the increase of G2/M arrested cells at 48 hours a better candidate indicator of cellular radiosensitivity.
In general, for all cell lines, those cells with minimal damage and efficient repair already finish their DNA damage repair and reenter the cell cycle in 48 hours after irradiation. However, if there is anything abnormal in the cells, such as nonrepair, misrepaired DNA damages, or strict cell cycle checking ability, the cells are likely to be blocked at the G2/M phase for an extended period of time. For severely damaged cells with minimal repair capabilities, apoptosis is initiated with resultant cell death. For those cells with incomplete repair, they are very likely blocked at the G2/M phase for extended periods of time. The proportion of these cells is dependent on irradiation dose, as confirmed by the present study. Proliferative death is probably the fate of these cells, so the higher the proportion of death, the more radiosensitive the cell line, which explains (1) the negative dependency of cell survival on irradiation dose and (2) the negative correlation of cell cycle block with cellular radiosensitivity.
However, there is another possible fate for those cells with incompletely repaired DNA damages that reenter the cell cycle with damaged DNA if their cell cycle machinery is either abnormal or damaged and their cell cycle checkpoints do not strictly work. High LET radiations such as carbon ions not only damage genetic materials but also probably damage cell cycle checkpoint machinery, which impairs the monitoring efficiency of cell cycle checkpoints and results in cell cycle suspension,13 implying (1) the high RBE of carbon ions in inducing the accumulation of G2/M blocked cells at 48 hours after exposure and (2) the high radiosensitivity of cells to carbon ions. When cells reenter the cell cycle with incompletely repaired DNA damage, there is a negative correlation between G2/M block at 48 hours after exposure and cellular radiosensitivity, which no longer exists. This is the reason why we could not exclude exceptions, for example, the 92-1 and OCM-1 melanoma cell lines. As we previously reported, OCM-1 cells are extremely resistant to both X-rays and carbon ions, whereas 92-1 cells are extremely sensitive even though both are melanoma cell lines.14
Cellular radiosensitivity has a critical impact on clinical prognoses. Our data on cellular radiosensitivity has shown that the sensitivities of different cell lines were diverse, implying that for radiotherapy with X-rays, it is essential to consider the radiosensitivity of a specific tumor. However, consistent with other reports that the difference in cellular radiosensitivity decreases along with increasing LET,15 our results also showed that carbon ion irradiation diminished the difference in radiosensitivities between cell lines, implying that for carbon ion therapy, the difference in radiosensitivities among individuals are minimal, especially for the same kind of tumor.
However, the time taken for the establishment and culturing of skin fibroblasts and for the clonogenic assay of cell killing currently precludes the use of fibroblasts in a predictive assay. Clonogenic assay is still not applicable to other cell lines that are difficult to form clones. Attention has, therefore, focussed on the use of unrepaired DSB, rH2AX, micronuclei, chromosomal aberrations, cell cycle which are more readily available. A potentially more rapid (< 3 day) involves quantifying radiation-induced cells damage.16 In order to assess the applicability of the micronucleus (MN) and G2 assays as biomarkers of in vitro radiosensitivity and cancer susceptibility, De Ridder et al17 investigated the inter- and intraindividual variation of these end points. Multiple blood sampling may be necessary to draw reliable conclusions. G2 and MN assay can be used in a confident way as biomarkers of individual radiosensitivity or cancer susceptibility. Vral et al18 assessed the chromosomal radiosensitivity with the G2 and the G0-micronucleus assay. The results demonstrated that the group of patients with breast cancer with a known or putative genetic predisposition was on the average more radiosensitive than a population of healthy women, and this with the G2 as well as with the high dose rate and low dose rate MN assay. With the G2 assay, 43% of the patients were found to be radiosensitive. A higher proportion of the patients were radiosensitive with the MN assay (45% with high dose rate and 61% with low dose rate). For us, we hope to develop cell cycle as a biomarker of cell radiosensitivity. This method can reflect radiation damage to cells or tissues more quickly.
Determination of cellular radiosensitivity is important for precision treatment of patients with cancer who require radiotherapy, especially photon treatment, as suggested by the present study. Overall, we have shown that a cell cycle assay with routine PI staining and flow cytometry can determine the cellular radiosensitivity in 3 days, which might be optional for clinical applications.
Supplemental Material
Supplementary_Figures for The Cell Cycle G2/M Block Is an Indicator of Cellular Radiosensitivity by Chang Liu, Jing Nie, Rensheng Wang and Weidong Mao in Dose-Response
Acknowledgments
The authors are grateful to Dr Mizuho Aoki and Ms Akiko Uzawa of the National Institute of Radiological Sciences, Chiba, Japan, for their kind help in sample preparation and radiation. This work was supported by the National Key R&D Program of China (nos. 2018YFC0115704 and 2018YFC0115705).
Authors’ Note: Chang Liu and Jing Nie contributed equally to this work.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Chang Liu  https://orcid.org/0000-0002-7047-4901
https://orcid.org/0000-0002-7047-4901
Weidong Mao  https://orcid.org/0000-0002-6880-7148
https://orcid.org/0000-0002-6880-7148
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Kohane IS. Ten things we have to do to achieve precision medicine. Science. 2015;349(6243):37–38. [DOI] [PubMed] [Google Scholar]
- 2. Oppitz U, Baier K, Wulf J, et al. The in vitro colony assay: a predictor of clinical outcome. Int J Rad Biol. 2001;77(1):105–110. [DOI] [PubMed] [Google Scholar]
- 3. Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274(5293):1664–1772. [DOI] [PubMed] [Google Scholar]
- 4. Zhou GM, Kawata T, Furusawa Y, et al. Protective effects of melatonin against low- and high-LET irradiation. J Rad Res. 2006;47(2):175–181. [DOI] [PubMed] [Google Scholar]
- 5. Pei H, Hu W, Guo Z, et al. Long noncoding RNA CRYBG3 blocks cytokinesis by directly binding G-actin. Can Rese. 2018;78(16):4563–4572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li XT, Nicklas RB. Mitotic forces control a cell-cycle checkpoint. Nature. 1995; 373(6515):630–632. [DOI] [PubMed] [Google Scholar]
- 7. Weinert T. Cell cycle—a DNA damage checkpoint meets the cell cycle engine. Science. 1997;277(5331):1450–1451. [DOI] [PubMed] [Google Scholar]
- 8. Macha MA, Rachagani S, Qazi AK, et al. Afatinib radiosensitizes head and neck squamous cell carcinoma cells by targeting cancer stem cells. Oncotarget. 2017;8(13):20961–20973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sarcar B, Kahali S, Prabhu AH, et al. Targeting radiation-induced G(2) checkpoint activation with the Wee-1 inhibitor MK-1775 in glioblastoma cell lines. Mole Can Therap. 2011;10(12):2405–2414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kriegs M, Kasten-Pisula U, Riepen B, et al. Radiosensitization of HNSCC cells by EGFR inhibition depends on the induction of cell cycle arrests. Oncotarget. 2016;7(29):45122–45133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lu S, Ke Y, Wu CY, et al. Radiosensitization of clioquinol and zinc in human cancer cell lines. BMC Cancer. 2018;18(1): 448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhao D, Sun SY, Lu H, et al. Enhanced radiosensitivity and G2/M arrest were observed in radioresistant esophageal cancer cells by knocking down RPA expression. Cell Biochem Biophys. 2014;70(2):887–891. [DOI] [PubMed] [Google Scholar]
- 13. He JP, Li JH, Ye CY, et al. Cell cycle suspension a novel process lurking in G(2) arrest. Cell Cycle. 2011;10(9):1468–1476. [DOI] [PubMed] [Google Scholar]
- 14. Ye C, Zhang X, Wan J, et al. Radiation-induced cellular senescence results from a slippage of long-term G2 arrested cells into G1 phase. Cell Cycle. 2013;12(9):1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ding N, Pei HL, He JP, et al. Simulated studies on the biological effects of space radiation on quiescent human fibroblasts. Adv Space Res. 2013;52(7):1314–1319. [Google Scholar]
- 16. Jones LA, Scott D, Cowan R, et al. Abnormal radiosensitivity of lymphocytes from breast-cancer patients with excessive normal tissue-damage after radiotherapy—chromosome-aberrations after low dose-rate irradiation. Int J Radiat Biol. 1995;67(5):519–528. [DOI] [PubMed] [Google Scholar]
- 17. Vral A, Thierens H, Baeyens A, De Ridder L. Chromosomal aberrations and in vitro radiosensitivity: intra-individual versus inter-individual variability. Toxicol Lett. 2004;149(1-3):345–352. [DOI] [PubMed] [Google Scholar]
- 18. Baeyens A, Thierens H, Claes K, et al. Chromosomal radiosensitivity in breast cancer patients with a known or putative genetic predisposition. Brit J Cancer. 2002;87(12):1379–1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary_Figures for The Cell Cycle G2/M Block Is an Indicator of Cellular Radiosensitivity by Chang Liu, Jing Nie, Rensheng Wang and Weidong Mao in Dose-Response