Abstract
The ventral pallidum (VP) is a critical node of the mesocorticolimbic reward circuit and is known to modulate social behaviors in rodents. Arginine vasopressin (AVP) signaling via the V1A receptor (V1AR) within the VP is necessary for the expression of socially motivated affiliative behaviors in monogamous voles. However, whether the VP-AVP system regulates socially motivated behaviors in non-monogamous species remains unknown. Here, we determined the extent of AVP fiber innervation in the VP as well as the involvement of the VP-AVP system in sociosexual motivation in adult male and female rats. We found that males have nearly twice the density of AVP-immunoreactive (AVP-ir) fibers in the VP compared to females, suggesting the possibility that males experience enhanced AVP signaling in the VP. We further found that this sex difference in VP-AVP-ir fiber density likely arises from an observed sex difference (males > females) in the percentage of VP-projecting AVP-ir cell bodies located in the bed nucleus of the stria terminalis and medial amygdala. To determine the behavioral implications of this sex difference, we next blocked AVP signaling in the VP by antagonizing VP-V1ARs in male and female rats and tested their preference to investigate an unfamiliar male rat or unfamiliar estrus female rat confined to corrals located on opposite ends of a three-chamber apparatus. Under vehicle conditions, males showed a significantly greater innate preference to investigate an opposite sex over same sex conspecific than estrus females. Interestingly, VP-V1AR antagonism significantly reduced males’ opposite sex preference, while enhancing estrus females’ opposite sex preference. Importantly, all subjects reliably discriminated between male and female stimulus rats regardless of drug treatment, demonstrating a change in motivational state rather than a perceptual impairment induced by VP-V1AR blockade. These results provide a novel functional link between a sex difference in ventral pallidal AVP fiber density and the sex-specific regulation of a sexually motivated behavior necessary for reproductive success.
Keywords: Ventral Pallidum, Opposite Sex Preference, Sociosexual Motivation, Vasopressin, Sex Differences
1. Introduction
The ventral pallidum (VP) has been implicated in the display of numerous reinforcing behaviors and is a critical component of the mesocorticolimbic reward pathway—a dopaminergic pathway in the brain known to regulate motivation, reinforcement, and reward (for review, see Smith et al., 2009; Cromwell and Berridge, 1993; Berridge, 1996). In this pathway, the VP receives and processes converging inputs from several cortical and limbic structures, including prefrontal, orbitofrontal, and infralimbic cortices, as well as from the nucleus accumbens, ventral tegmental area, extended amygdala, and lateral hypothalamus (Heimer and Wilson, 1975; Reep et al., 1982; Yim and Mogenson, 1983; Grove, 1988; Klitenick et al., 1992; Zaborszky et al., 1997; Mitrovic and Napier 1998; Maurice et al., 1997; Usuda et al., 1998; Fudge et al., 2002). More recently, the VP has been described as a component of the social decision-making network— a collection of brain nuclei that integrates the mesocorticolimbic dopamine system with forebrain and midbrain nuclei known to be important for the display of social behaviors (O’Connell and Hofmann, 2011). Indeed, the VP is a major output source of other nuclei within the social decision-making network, such as the bed nucleus of the stria terminals (BNST) and medial amygdala (MeA). The VP in turn projects to motor centers, making it well positioned to process hedonic/reinforcing aspects of social stimuli, driving the appropriate motivated behavioral response to salient social information (O’Connell and Hofmann, 2011; Otero-Gracia et al., 2013).
Arginine vasopressin (AVP) is a nine amino acid neuropeptide synthesized primarily by neurons in the paraventricular (PVN) and supraoptic nuclei (SON) of the hypothalamus. In addition, AVP-immunoreactive (ir) neurons have also been found in the MeA and BNST (Buijs, 1978, 1980; Sofroniew 1983; van Leeuwen and Café, 1983; Hou-Yu et al., 1986; Rood et al., 2013). AVP signaling in the brain has been shown to be necessary for the display of many species-typical social behaviors including social recognition, aggression, social play, pair bonding and parental care (Winslow et al., 1993; Bamshad et al., 1994; Ferris et al., 1997; Albers et al., 2006; Veenema et al., 2010; Veenema et al., 2012; Veenema et al., 2013). Importantly, AVP-ir fibers and AVP V1A receptors (V1AR) have been found in the VP of several rodent species (De Vries and Buijs, 1983; Wang et al., 1996; Rood and De Vries, 2011; Otero-Garcia et al., 2013; Insel et al., 1991; Berry et al., 2008; Bales et al, 2007a; Young et al., 1999; Lim et al., 2004; Smith et al., 2017).
Much of what is known about AVP signaling in the VP and its effects on social behavior stem from studies in the monogamous prairie vole, Microtus ochrogaster. Previous work in male prairie voles has shown that pharmacological blockade of the V1AR in the VP impairs mating-induced partner preference formation (Lim and Young, 2004), suggesting that V1AR signaling in the VP is necessary for pair bond formation. Furthermore, mating increased Fos expression (a surrogate marker of neural activity) in the VP of male prairie voles in a V1AR-dependent manner (Lim and Young, 2004). In addition, viral vector-induced overexpression of the V1AR in the VP induced partner preference formation in non-monogamous male meadow voles (Lim et al., 2004), and increased the frequency of non-sexual affiliative behaviors toward ovariectomized females (i.e., olfactory investigation, grooming) in male mice (Young et al., 1999). Thus, over the past few decades the focus of AVP signaling in the VP has been placed on studying affiliative/attachment behaviors (e.g. pair bonding) in males and mostly in monogamous species. Therefore, the role of VP-AVP signaling in sexually motivated behaviors in female rodents and in non-monogamous species remains unknown.
Here, we sought to determine the role of the VP-AVP system in opposite sex preference in male and female rats. First, we compared AVP-ir fiber density in the VP of male rats with that of female rats. Next, we used retrograde tract tracing combined with AVP immunohistochemistry to identify the sites of origin of AVP-ir fibers in the VP in both sexes. Lastly, by administering a specific V1AR antagonist into the VP, we determined the role of AVP signaling in the VP on the innate preference to investigate opposite sex over same sex conspecifics (“sociosexual motivation”) in male and female rats. Our findings demonstrate the involvement of AVP signaling in the VP in sociosexual motivation in a non-monogamous species and reveal an intriguing sex difference in which the VP-AVP system facilitates sociosexual motivation in males, while reducing it in females.
2. Materials and Methods
2.1. Animals
Adult male and female Wistar rats (70-day-old) were obtained from Charles River Laboratories and housed under standard laboratory conditions (12-h light/dark cycle, lights on at 7:00 am, food and water available ad libitum, 22°C, 60% humidity) in standard rat cages (26.7 × 48.3 × 20.3 cm) on woodchip bedding for a minimum of two weeks before experiments began. All subject rats were gonad-intact and sexually-naïve/non-maternal and co-housed in same-sex groups of two to three unless otherwise specified. For Experiment 3, sexually-naïve testes-intact adult male and sexually-naïve ovariectomized, estradiol and progesterone-primed adult female rats were used as stimulus animals. All experiments were conducted in accordance with the guidelines of the NIH Guide for the Care and Use of Laboratory Animals, and approved by Boston College Institutional Animal Care and Use Committee (IACUC).
2.2. Experimental Procedures
2.3. Experiment 1: Sex Differences in Ventral Pallidal AVP Fiber Density
2.3.1. Tissue Collection and Immunohistochemistry
In order to determine whether there are sex differences in AVP-ir within the VP, adult male (n=7) and female rats (n=7) were deeply anesthetized under continuous 3% isoflurane vapor and received transcardiac perfusions with 4% paraformaldehyde dissolved in 0.1M borate buffer (pH 9.5). Brains were removed and post-fixed in 4% paraformaldehyde in 0.1M borate buffer (pH 9.5) for 24 h and then cryoprotected in 30% sucrose (dissolved in 0.9% NaCl) for 48 h. Next, brains were flash-frozen in cold 2-methylbutane and stored at −45°C. Coronal (30 μm) sections were collected using a cryostat and stored free-floating in anti-freeze cryoprotectant (0.25 mM polyvinylpyrolidone, 30% sucrose, 30% ethylene glycol in TBS) at −20°C. Tissue sections from each subject were subsequently immunolabeled for AVP.
AVP-ir was visualized using methods identical to DiBenedictis et al., (2017). Briefly, tissue sections were first washed in tris buffered saline (TBS; pH 7.4) for 3-4 hours to remove residual anti-freeze solution. Next, sections were subjected to an antigen retrieval step (0.05M sodium citrate in TBS), blocked in blocking solution (20% normal goat serum [NGS], 0.3% Triton-X, 1% H2O2 in TBS), and incubated overnight at 4°C in mouse anti-AVP (PS41, Dr. Harold Gainer, NINDS; 1:100, 2% NGS, 0.3% Triton-X). Sections were rinsed in TBS and incubated in biotinylated secondary antibody solution (goat anti-mouse [1:500; Vector, Burlingame, CA], 2% NGS, 0.3% Triton-X in TBS) for 1 h. Tissue sections were then incubated in avidin-biotin complex (ABC Elite Kit; Vector) for 1 h and visualized using 3,3’-diaminobenzadine (DAB peroxidase substrate kit; Vector). Tissue sections were then mounted on gelatin-coated slides, rinsed in 50% ethanol, air-dried, and coverslipped using Permount (Fisher Scientific, Pittsburgh, PA). Images were acquired with a 20X objective on an Olympus BX43 microscope with a DP72 digital color camera (Olympus America Inc, Center Valley, PA), and were in all cases analyzed by an experimenter blind to treatment groups.
2.3.2. Quantitative Fiber Density Analysis
AVP fiber density analysis was conducted by manually thresholding gray-scale images of AVP-ir fibers in the VP using ImageJ (NIH; imagej.nih.gov/ij; see DiBenedictis et al., 2017). Brain images were acquired under a 20X objective, cropped to 500 X 500 μm2, and pixel density was averaged from three non-overlapping sections in an evenly spaced series (90μm apart) within the VP (Fig. 1A–B). This analysis resulted in an estimate of the fractional area occupied by AVP immunolabeled elements. Background staining levels were sufficient to discern tissue features, and thus ventral pallidal images were acquired based on anatomical landmarks specific to the VP, such as the size, relative location, and morphology of the anterior commissure, horizontal diagonal band of Broca, and lateral ventricles using the Rat Brain Atlas (Paxinos and Watson 2007) as a guide (Fig. 2B). In some cases, sections were compared against reference thionin-stained tissues from rats of the same sex to ensure that regional sampling was comparable across subjects. Fiber density data are reported in number of pixels, with a greater number of pixels indicating a larger proportion of the area occupied by AVP-ir fibers.
Figure 1. Sex difference in vasopressin immunoreactive (AVP-ir) fiber density in the ventral pallidum (VP).
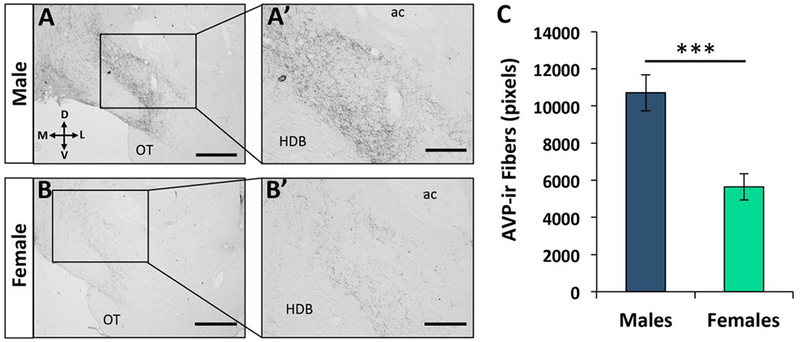
(A-B) Low (A, B; 10X; scale bar = 600μm) and high magnification (A’, B’; 20X; scale bar = 200 μm) photomicrographs depict a high density of AVP-ir fibers present in the VP [+0.60mm from Bregma according to Paxinos and Watson (2007)] of adult male rats (A-A’), compared to adult female rats (B-B’). (C) Quantification of AVP-ir fiber density in the VP (averaged over three 30-μm sections per rat) shows higher AVP-ir fiber density in adult male rats (n=7) compared to adult female rats (n=7). Data are represented as the mean ± SEM, two-tailed t-test; ***p<0.005. ac = anterior commissure; OT = olfactory tubercle; HDB = horizontal diagonal band of Broca.
Figure 2. Vasopressin (AVP) fibers innervating the ventral pallidum (VP) originate from cell bodies in the posterior bed nucleus of the stria terminalis (pBNST) and posterodorsal medial amygdala (MePD) in adult male and female rats.
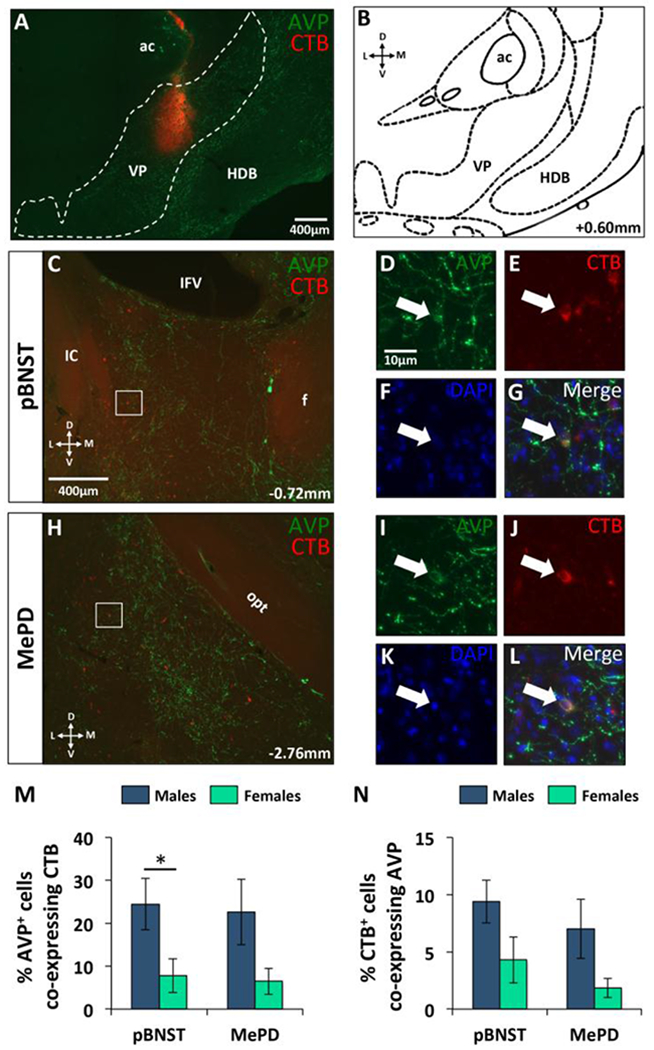
(A) Representative epifluorescent photomicrograph depicting the location of the retrograde tracer (CTB) injection site (red) in the VP, immunolabeled for AVP (green fibers in the VP) in an adult male. (B) Coronal diagram showing the location of the VP used for CTB targeting, adapted from Paxinos and Watson (2007). (C) Overlapping back-labeled CTB+ (red) and AVP-immunoreactive (AVP-ir; green) cell bodies in the pBNST. (D-F) Example of a pBNST cell from the white boxed region in C, positive for AVP (D; green), CTB (E; red), and DAPI (F; blue). (G) Example of a triple-labeled (merged) cell from D-F. (H) Overlapping back-labeled CTB+ (red) and AVP-ir (green) cell bodies in the MePD. (I-K) Example of a MePD cell from the white boxed region in H, positive for AVP (I; green), CTB (J; red), and DAPI (K; blue). (L) Example of a triple-labeled (merged) cell from I-K. (M) Percentage of AVP cells coexpressing CTB in males (n=5) and females (n=5). (N) Percentage of CTB-labeled (VP-projecting) cells co-expressing AVP in males (n=5) and females (n=5). Data are represented as the mean ± SEM, two-tailed t-test; *p<0.05. ac = anterior commissure; f = fornix; HDB = horizontal diagonal band of Broca; IC = internal capsule; IVF = interventricular foramen; opt = optic tract; VP = ventral pallidum.
2.4. Experiment 2: Source of Origin of AVP Fibers in the Ventral Pallidum
To determine the source of the sex difference in AVP innervation of the VP, male and female rats received an injection of a retrograde tracer [cholera toxin-β subunit (CTB)] into the VP and immunohistochemistry was performed to determine co-labeling of AVP with the retrograde tracer in AVP synthesizing brain nuclei.
2.4.1. Retrograde tracer injections
Adult male (n=5) and female (n=5) rats were anesthetized with isoflurane (2-3%; Henry Schein, Dublin, OH) and mounted on a stereotaxic frame with the incisor bar set to −4.5mm, and a heating pad was used to maintain body temperature throughout the surgery. Next, a 1μl, 7000 series Hamilton syringe (Hamilton, Reno, NV) was attached to a motorized stereotaxic injector system (Stoelting, Wood Dale, IL) and a 0.20μl unilateral injection of CTB conjugated to a fluorescent fluorophore (Alexa Fluor 594, Molecular Probes, dissolved in 0.1M PBS, resulting in a 1% CTB solution) was directed at the VP at a rate of 0.10μl/min using the following coordinates: 0.6mm (males) or 0.9mm (females) anterior to Bregma, ±2.6mm lateral to the midline, and 8.5mm ventral to the surface of the skull. Coordinates were chosen to target the center of the VP where we observed the highest concentration of AVP-ir fibers (Fig 1A–B’), corresponding with Bregma 0.6mm of the Rat Brain Atlas (Paxinos and Watson, 2007). Injections were made at a 10° from the midsagittal plane to avoid damage to the sagittal sinus. The needle was left in place for 10 min following the injection to allow time for tissue uptake of the tracer. After allowing seven days for the tracer to be taken up by axon terminals and transported back to the cell bodies of origin, rats were sacrificed, perfused, and brain tissue was immunolabeled for AVP.
2.4.2. Tissue Collection and Immunohistochemistry
AVP-ir fibers and cell bodies were visualized using the same procedure as described in Experiment 1, with the exception that a fluorescent-conjugated secondary antibody (goat anti-mouse Alexa 488, 1:500; Invitrogen, Carlsbad, CA) was used along with fluorescent microscopy. This allowed for simultaneous visualization of AVP-ir (green) and back-labeled CTB+ fibers and cell bodies (red). Tissue sections were then mounted on gelatin-coated slides, rinsed in 50% ethanol, air-dried, and coverslipped with Vectashield HardSet Mounting Medium with 4′,6-diamidino-2-phenylindole (DAPI) (H-1500; Vector Labs, Burlingame, CA, USA).
2.4.3. Quantitative analysis of CTB/AVP cells
The number of CTB+, AVP-ir, and CTB/AVP co-labeled neurons were counted using ImageJ (NIH; imagej.nih.gov/ij). Images were acquired using an Axiolmager fluorescence microscope with ApoTome attachment (Carl Zeiss Microscopy GmbH, Jena, Germany), Orca-R2 camera (Hamamatsu, Bridgewater, NJ, USA) and Zen software (Carl Zeiss Microscopy GmbH, Jena, Germany). Tiled confocal fluorescent images (2 × 2mm; 20μm Z-plane) encompassing the posterior bed nucleus of the stria terminalis (pBNST) and posterodorsal medial amygdala (MePD) were Z-stacked, and separated into red, green, and yellow channels using the ImageJ split channel function. CTB+ neurons were counted using the cell counter function in ImageJ from the red channel, AVP-ir neurons were counted from the green channel, and colabeled neurons were quantified from the yellow channel. The total number of colabeled cells in each image was then averaged across three non-overlapping sections in an evenly spaced series (90pm apart) for both the pBNST and MePD. Many AVP-ir cells were observed in the paraventricular (PVN) and supraoptic (SON) nuclei of the hypothalamus, however in no cases were any back-labeled CTB+ cell bodies co-expressing AVP observed in these regions. Thus, these regions were not further analyzed.
2.5. Experiment 3: Effects of Ventral Pallidal V1AR Antagonism on Opposite Sex Preference
To determine the role of AVP signaling in the VP in sociosexual motivation, sexually naïve testes-intact adult male and ovary-intact estrus female rats were tested for the effects of V1AR blockade in the VP on the preference to investigate an opposite sex conspecific over a same sex conspecific. Conspecific stimulus animals consisted of sexually naïve testes-intact adult male rats and ovariectomized, estradiol and progesterone-primed adult female rats (Fig. 3A–C).
Figure 3. Sex differences in ventral pallidum (VP) V1A receptor antagonism (V1ARa) on opposite sex preference in adult rats.
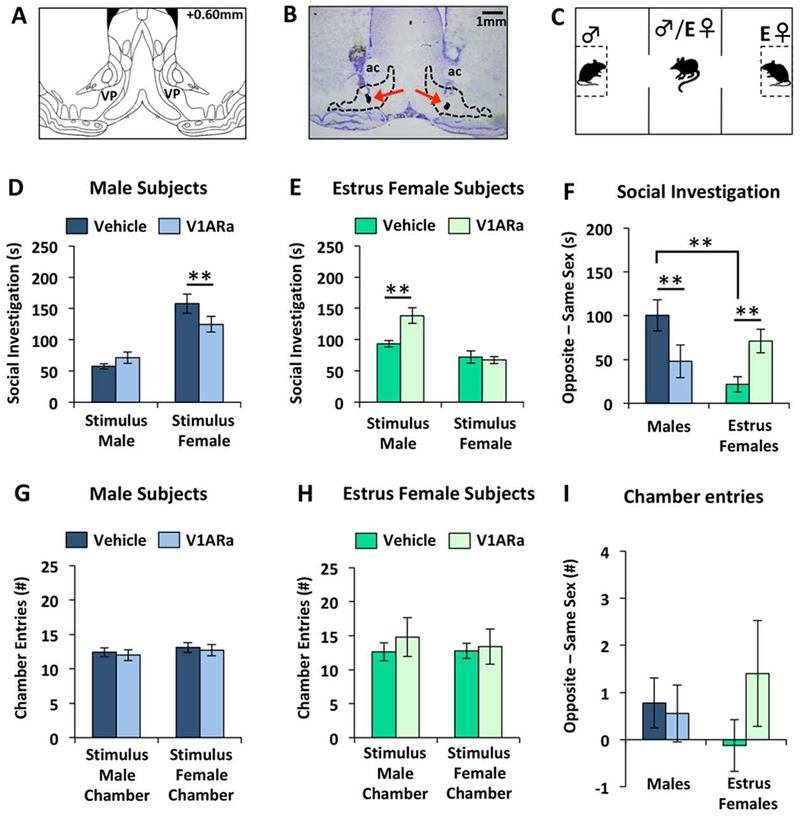
(A) Coronal diagram showing the location of the VP, adapted from Paxinos and Watson (2007). (B) Representative photomicrograph of a thionin-stained coronal section showing the bilateral location of injection cannulae (using charcoal as marker) targeting the VP. (C) Illustration of the three chamber opposite sex preference paradigm. All stimulus rats were novel to subject rats. (D-E) Amount of time (D) males (n=9) or (E) estrus females (Estrus Female + Vehicle, n=8; Estrus Female + V1ARa, n=5) spent investigating either a testes-intact male stimulus rat (“Stimulus Male”) or an ovariectomized, estradiol and progesterone-primed female rat (“Stimulus Female”). (F) Social investigation difference score, calculated by the amount of time males and estrus females spent investigating the opposite sex minus the time spent investigating the same sex. (G-H) Number of entries of males (G) and estrus females (H) into the testes-intact male stimulus chamber (“Stimulus Male Chamber”) and the ovariectomized, ovariectomized, estradiol and progesterone-primed female stimulus chamber (“Stimulus Female Chamber”). (I) Chamber entry difference score, calculated by the number of entries of males and estrus females into the opposite sex chamber minus the number of entries into the same sex chamber. Data are represented as the mean ± SEM, t-test; **p<0.01. VP = ventral pallidum.
2.5.1. Cannulations
After 5 days of daily handling, subject adult male (n=9) and female (n=16) rats were anesthetized with isoflurane (2-3%; Henry Schein, Dublin, OH), and mounted on a stereotaxic frame as described above (see section 2.4.1). Guide cannulae (9mm, 22 gauge, Plastics One, Roanoke, VA) were bilaterally implanted 2mm dorsal to the VP using the following coordinates: 0.6mm (males) or 0.9mm (females) anterior to Bregma, ±2.6mm lateral to the midline, and 6.4mm ventral to the surface of the skull, at an angle of 10° in order to avoid damage to the sagittal sinus. Coordinates were chosen in order to target the posteromedial region of the VP since we observed the highest density of AVP-ir fibers in adult male and female rats in this region (Fig 1A–B), corresponding with Bregma 0.60mm of the Rat Brain Atlas (Paxinos and Watson, 2007). Guide cannulae were fixed to the skull with three stainless steel screws and dental acrylic adhesive, and were closed with dummy cannulae (28 gauge; Plastics One). Following surgery, subject rats were individually housed in standard rat cages (26.7 × 48.3 × 20.3 cm), and given daily Rimadyl injections for 3 days. Subjects were handled daily following surgery (including removing and replacing dummy cannulae to habituate subjects to injection procedures), and were tested for opposite sex preference starting four days after surgery.
2.5.2. Bilateral ovariectomy and estrus induction of stimulus female rats
Manipulation of stimulus female rats’ estrous cycle was necessary to ensure that stimulus females were in behavioral estrus during opposite sex preference testing, such that they were thus maximally attractive to subject male rats. Female stimulus rats were anesthetized using continuous 3% isoflurane (Butler Schein Animal Health, Dublin, OH), and 3 cm bilateral incisions were made just ventral to the base of the ribcage through which the ovaries were extracted and removed. Following excision, muscle and skin were sutured closed using absorbable collagen sutures (CP Medical, Norcross, GA) and subcutaneous injections of Rimadyl were administered once daily for four days following surgery. Stimulus females were individually housed for one week following surgery, and later pair housed with another ovariectomized female for four weeks prior to use in behavioral testing. Behavioral estrus was induced in ovariectomized stimulus females prior to testing. This was accomplished by first administering a 0.05ml subcutaneous injection of Estradiol benzoate (EB; 10 μg in 0.05ml peanut oil) 48 hours before testing followed by a 0.05ml subcutaneous injection of Progesterone (P; 500μg in 0.05ml peanut oil) 3-4 hours prior to testing. Vaginal smears were performed on all stimulus females prior to behavioral testing to confirm estrus induction resulting from the above hormone regimen based on vaginal cell characteristics according to Goldman et al., 2007.
2.5.3. Opposite sex preference test
One day prior to testing, all subject and stimulus rats were moved to the behavioral testing room for 3-4 h, and subsequently placed in the testing apparatus wherein rats were free to explore the testing arena for 10 min in order to acclimate to the behavioral apparatus. An opposite sex preference test was conducted on the first and second days of behavioral testing, following a 3-4hr habituation to the behavioral testing room. Briefly, opposite sex preference tests were conducted in a behavioral apparatus consisting of three Plexiglas chambers (40 × 27 × 40 cm) that were separated by Plexiglas partitions with rectangular openings (10 x 10.2 cm) to allow passage between chambers (Fig. 3C). A testes-intact male and an ovariectomized, estradiol and progesterone-primed female stimulus rat were each placed in their respective lateral chambers, confined to Plexiglas corrals (18 × 10 × 21 cm) with translucent Plexiglas bars (0.6 cm wide, spaced 1.75 cm apart). This restricted movement of the stimulus rats, while still allowing for social investigation by the subject rat. Locations of the stimulus animals within the testing arena were switched between tests to account for possible bias towards a particular chamber by each subject, and all stimulus rats were novel to subject rats across all tests. Twenty minutes following microinjection of either vehicle or V1AR antagonist (counterbalanced, within-subjects), the subject rat was placed in the center chamber and had free movement throughout the three chambers wherein the subject was allowed to investigate the testes-intact male and ovariectomized, estradiol and progesterone-primed female stimulus rats for 10 min. Behavior was recorded with a webcam positioned above the three chamber apparatus (Logitech HD Pro Webcam C910), and later scored by an experimenter blind to experimental treatment using JWatcher software (http://www.jwatcher.ucla.edu). Upon completion of behavioral testing each day, free-cycling subject female rats received vaginal swabs to determine estrous cycle stage (estrous or anestrous, as determined by vaginal cytology; see Goldman et al., 2007). Female subject rats were subsequently grouped according to estrous cycle stage for statistical analysis. The total amount of time male and female subject rats spent investigating each stimulus rat in the presence or absence of the V1AR antagonist was measured and averaged for each group. An opposite sex preference was defined as the subject rat spending significantly more time investigating the opposite sex stimulus animal vs. the same sex stimulus animal. In addition, a difference score (time spent investigating opposite sex conspecific minus time spent investigating same sex conspecific) was calculated in order to directly compare the effect of drug treatment (V1AR antagonist vs. vehicle) on opposite sex preference. A difference score was also calculated for number of chamber entries in order to directly assess the effect of drug treatment on locomotor behavior. Locomotor activity was measured by counting the number of times the subject rat entered each of the lateral arms of the three chamber apparatus (i.e. stimulus animal chambers) and was compared across drug treatment conditions.
2.5.4. Sex discrimination (habituation/dishabituation) test
The sex discrimination (habituation/dishabituation) test was conducted on the third and fourth days of behavioral testing, following a 3-4 h habituation each day to the behavioral testing room. The purpose of this test was to determine whether VP-V1AR antagonism had an effect on subjects’ ability to perceive the difference between male and ovariectomized, estradiol and progesterone-primed female stimulus rats. Twenty minutes following microinjection of either vehicle or V1AR antagonist (counterbalanced, within-subjects), subject rats were placed within a single Plexiglas chamber (40 × 27 × 40 cm), and were subsequently exposed for 2 min to stimuli (with a one minute inter-trial interval) in the following order: two consecutive exposures to the same empty Plexiglas corral, followed by three consecutive exposures to the same ovariectomized, estradiol and progesterone-primed female confined to a Plexiglas corral, followed by three exposures to the same testes-intact male confined to a Plexiglas corral, totaling eight 2-min trials (Fig. 4A). Sex discrimination was defined by the occurrence of a significant dishabituation response (indexed by increased investigation time) to the first presentation of a novel animal as compared to the third presentation of a familiar animal or second presentation of an empty corral. Because this was a test of perceptual (rather than motivational) ability, subject females were free cycling and were tested at random stages of their estrous cycle; they were not grouped according to estrous cycle stage for subsequent statistical analysis. The total amount of time subject male and female rats spent investigating each stimulus rat in the presence or absence of the V1AR antagonist was measured and averaged for each group.
Figure 4. Ventral pallidum (VP) V1A receptor antagonism (V1ARa) did not alter the ability to discriminate between same sex and opposite sex rats.
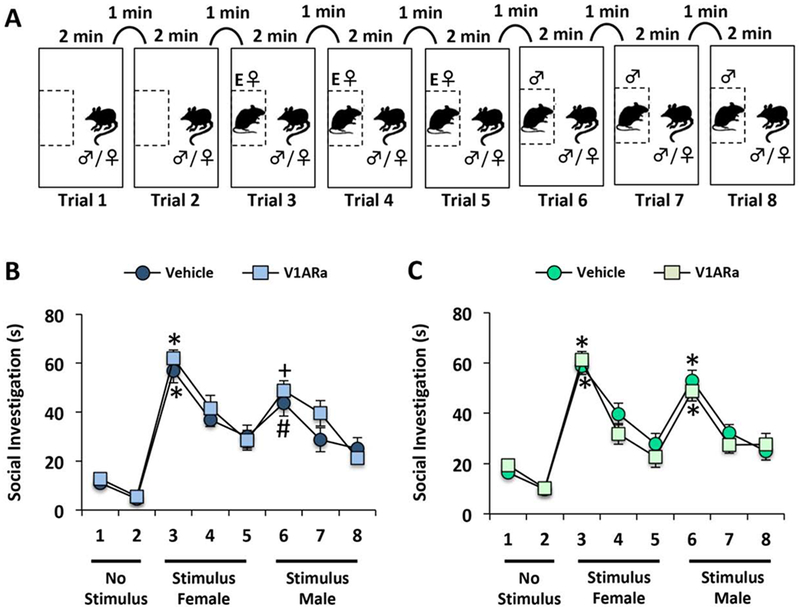
(A) Illustration of the habituation-dishabituation behavioral paradigm used to determine whether male and female rats could discriminate between same sex and opposite sex rats after V1ARa treatment in the VP. Following two presentations of an empty corral, stimulus rats (ovariectomized, estradiol and progesterone-primed female followed by testes-intact male) were presented three consecutive times for two minutes, with a one-minute inter-trial interval. All stimulus rats were novel to subject rats. (B-C) V1ARa treatment did not have an effect on the ability of males (n=9) (B) or females (n=16) (C) to discriminate between testes-intact male (“Stimulus Male”) and ovariectomized, estradiol and progesterone-primed female stimulus rats (“Stimulus Female”). Data are represented as the mean ± SEM, paired t-tests comparing the second presentation of the empty corral (trial 2) with the first presentation of the ovariectomized, estradiol and progesterone-primed female stimulus rat (trial 3), and the first presentation of the intact male stimulus rat (trial 6) with the third presentation of the ovariectomized, estradiol and progesterone-primed female rat (trial 5); *p<0.001: trial 3 vs. 2 in vehicle and V1Ra treated male and female rats, trial 6 vs. 5 in vehicle and V1aRa treated females; +p<0.01: trial 6 vs. 5 in vehicle treated males; #p<0.05: trial 6 vs. 5 in V1ARa treated males.
2.5.5. Microinjections
Injection systems consisted of polyethylene tubing connected to an injector cannula and a 10μl Hamilton syringe. The injector cannula (28 gauge, Plastics One) extended 2mm beyond the guide cannula. Subjects received bilateral microinjections of either a V1AR antagonist (d(CH2)5[Tyr(Me)2]AVP; 8ng/0.5μl; Manning et al., 2008) or vehicle (aCSF; 0.5 μl) 20 minutes prior to the start of behavioral testing. The injector system was kept in place for approximately 30 seconds after the injection to ensure tissue uptake of the infusion, and dummy cannulae were then replaced. Drug treatments were given in a counter-balanced order, such that each subject received V1AR antagonist treatment on the first day of behavioral testing followed by vehicle treatment on the second day of behavioral testing or vice-versa. This experimental design was used for both the opposite sex preference test (testing days 1 and 2) and the sex discrimination test (testing days 3 and 4), with the same subject rats used in both tests, totaling four days of behavioral testing for each subject rat, and order of drug treatment was randomized for each of the two behavioral tests. Following behavioral testing, subject rats were euthanized with CO2, and charcoal was injected through the guide cannula to confirm proper cannula placement on thionin-stained coronal brain sections (Fig 3B).
2.6. Statistics
Statistical analyses were conducted using PASW/SPSS Statistics software (Version 22; IBM, Armonk, NY). In Experiment 1, male and female AVP fiber densities in the VP were calculated and compared using two-tailed independent sample t-tests. In Experiment 2, a two-tailed independent sample t-test was used to compare the percentage of co-labeled neurons within the pBNST between males and females (% CTB+ cells coexpressing CTB and % AVP+ cells coexpressing CTB). A separate two-tailed independent sample t-test was used to compare the percentage of co-labeled neurons within the MePD between males and females (% CTB+ cells coexpressing CTB and % AVP+ cells coexpressing CTB). No co-localization was observed in the PVN or SON, thus no statistical analyses were performed in these brain regions. In Experiment 3, the amount of time (in seconds) subject rats spent investigating stimulus rats, as well as the number of entries into each lateral chamber was recorded, and vehicle and V1AR antagonist treatments were compared using paired t-tests for males and two-tailed independent sample t-tests for females (because female rats were free cycling for opposite sex preference testing and grouped according to estrous cycle stage). Difference scores in social investigation (defined by time spent investigating opposite sex stimulus rat minus time spent investigating same sex stimulus rat) and difference scores in chamber entries were analyzed using a two-way ANOVA (sex x V1AR antagonist treatment) followed by two tailed independent sample t-tests when a significant interaction effect was found. For the sex discrimination (habituation/dishabituation) test, paired t-tests were used to compare investigation times (in seconds) for each group (Vehicle and V1AR antagonist): the second presentation of the empty corral versus the first presentation of the ovariectomized, estradiol and progesterone-primed female stimulus, and the third presentation of the ovariectomized, estradiol and progesterone-primed female stimulus versus the first presentation of the testes-intact male stimulus. Statistical significance was set at p < 0.05.
3. Results
3.1. Experiment 1: Sex Differences in Ventral Pallidal AVP Fiber Density
A sex difference in AVP-ir fiber density was found in the VP such that adult males displayed a significantly higher density of AVP-ir fibers in the VP compared to adult females (t(12) = −4.22; p < 0.005; Fig. 1A–C). Of note was the presence of what appeared to be en passant synaptic puncta sprouting off of AVP-ir axons coursing through the VP, suggesting the possibility of synaptic connections between AVP-ir axonal fibers and neurons in this region.
3.2. Experiment 2: Source of Origin of AVP Fibers in the Ventral Pallidum
Co-localization of CTB and AVP were observed in the pBNST and MePD following CTB injections in the VP (Fig. 2A–L), indicating that AVP-ir cell bodies in the BNST and MePD send monosynaptic projections to the VP. Males showed a significantly greater percentage of AVP+ cells co-expressing CTB compared to females in the pBNST (t(8) = 2.33; p < 0.05), and showed a trend toward a significant difference in the MePD (t(8) = 1.97; p = 0.08; Fig. 2M). However, we observed no sex differences in the percentage of CTB+ cells co-expressing AVP in either the pBNST or MePD (pBNST: t(8) = 1.86; p = 0.10; MePD: t(8) = 1.90; p = 0.09; Fig. 2N). While there was a high density of AVP+ cells in the PVN and SON, in no instances did we observe AVP+ cells co-expressing CTB in these two regions (data not shown).
3.3. Experiment 3: Effects of Ventral Pallidal V1AR Antagonism on Sociosexual Motivation
3.3.1. Opposite Sex Preference Test
Males administered with a V1AR antagonist into the VP spent significantly less time investigating ovariectomized, estradiol and progesterone-primed female stimulus rats compared to vehicle treatment (t(8) = −3.70; p < 0.01), but spent a similar amount of time investigating male stimulus rats regardless of treatment (t(8) = 1.38; p = 0.21; Fig. 3D). In contrast, estrus females given a V1AR antagonist into the VP spent significantly more time investigating male stimulus rats compared to vehicle treated estrus females (t(11) = −3.78; p < 0.005), but spent a similar amount of time investigating ovariectomized, estradiol and progesterone-primed female stimulus rats regardless of treatment (t(11) = 0.34; p = 0.74; Fig. 3E). These opposing effects of V1aR antagonist treatment on opposite sex preference in males vs. females were also seen when calculating a difference score based on time spent investigating the opposite sex minus time spent investigating the same sex (Sex x V1AR antagonist treatment interaction: F(1,27) = 9.12; p < 0.005; Fig 3F). Specifically, V1AR antagonist treated males displayed a reduced preference for the opposite sex (ovariectomized, estradiol and progesterone-primed female) as indexed by a significant reduction in the difference in investigation times of the opposite sex vs. same sex (t(8) = −3.23; p < 0.01), while V1AR antagonist treated estrus females showed an increased preference for the opposite sex (testes-intact male; t(11) = −3.24; p < 0.01). Interestingly, this difference score also revealed that vehicle treated males displayed a greater preference for the opposite sex than vehicle treated estrus females (t(15) = 3.80; p < 0.005) and that V1AR antagonist treatment eliminated this sex difference (V1AR antagonist treated males vs. V1aR antagonist treated estrus females: t(12) = −0.85; p = 0.41; Fig. 3F).
In contrast to estrus females, anestrus females did not spend significantly more time investigating male vs. ovariectomized, estradiol and progesterone-primed female stimulus rats under vehicle conditions (male investigation: 91.4±12.6s; ovariectomized, estradiol and progesterone-primed female investigation: 99.1±12.8s; t(17) = 0.38; p = 0.72). However, similar to estrus females, anestrus females given a V1AR antagonist into the VP spent significantly more time investigating male vs. ovariectomized, estradiol and progesterone-primed female stimulus rats (male investigation: 97.5±8.7s; ovariectomized, estradiol and progesterone-primed female investigation: 67.7±8.6s; t(17) = −3.08; p < 0.05).
In a subset of males with inadvertent off target cannula placements (unilateral hits only, n=4; or bilateral misses in most cases centered caudal to the VP in a region including the lateral preoptic area; n=2), V1AR antagonist treatment resulted in significantly more time investigating the ovariectomized, estradiol and progesterone-primed female stimulus rats than the male stimulus rats (ovariectomized, estradiol and progesterone-primed female investigation, 160±12.2s; male investigation, 66.1±10.5s; t(5) = −5.82; p < 0.0001). This suggests that the effect of the V1AR antagonist treatment observed in male rats was specific to the VP.
There was no significant effect of V1AR antagonist treatment on locomotor activity as reflected by the number of male and ovariectomized, estradiol and progesterone-primed female chamber entries in subject males and estrus females (for males: male chamber entries, t(8) = −0.37; p = 0.36; ovariectomized, estradiol and progesterone-primed female chamber entries, t(8) = −1.04; p = 0.33; for females: male chamber entries, t(11) = −0.78; p = 0.45; estrus female chamber entries, t(11) = −0.27; p = 0.80; Fig. 3G–H). Furthermore, there were no main effects on the difference in the number of male chamber entries minus ovariectomized, estradiol and progesterone-primed female chamber entries (Sex effect: F(1.27) = 0.00; p = 0.97; V1AR antagonist effect: F(1.27) = 1.00; p = 0.33; Sex x V1AR antagonist effect: F(1.27) = 1.80; p = 0.19; Fig 3I).
3.3.2. Sex Discrimination (Habituation/Dishabituation) Test
VP-V1AR antagonist treatment did not have an effect on the ability to discriminate between the sexes in either male (Fig. 4B) or female (Fig. 4C) subject animals. In detail, both vehicle and V1AR antagonist treated males spent more time investigating an ovariectomized, estradiol and progesterone-primed female than the empty corral (second “No Stimulus” versus first “Stimulus Female”; vehicle: t(8) = 10.7; p < 0.001; V1AR antagonist: t(8) = 17.2; p < 0.001), and spent more time investigating a testes-intact male upon first exposure compared to an ovariectomized, estradiol and progesterone-primed female upon third exposure (vehicle: t(8) = 2.73; p < 0.05; V1AR antagonist: t(8) = 3.40; p < 0.01; Fig 4B). Similarly, both vehicle and V1AR antagonist treated females spent more time investigating an ovariectomized, estradiol and progesterone-primed female than the empty corral (second “No Stimulus” versus first “Stimulus Female”; vehicle: t(15) = 14.2; p < 0.001; V1AR antagonist: t(15) = 15.8; p < 0.001), and spent more time investigating a testes-intact male upon first exposure compared to an ovariectomized, estradiol and progesterone-primed female upon third exposure (vehicle: t(15) = 4.75; p < 0.001; V1AR antagonist: t(15) = 6.15; p < 0.001; Fig. 4C).
4. Discussion
We report that adult male rats had nearly twice the density of AVP-ir fibers in the VP than adult female rats. This sex difference in VP-AVP-ir fiber density likely arises from cell bodies located in the BNST and MeA. Specifically, we found that male rats had a significantly greater percentage of VP- projecting AVP-ir cells in the BNST and a similar, but non-significant trend was observed in the MeA. We further showed that pharmacological V1AR blockade in the VP resulted in a reduced preference for the opposite sex in males, and an increased preference for the opposite sex in estrus females. Importantly, all subjects were still able to perceive the difference between male and female stimulus rats and showed normal levels of locomotor activity regardless of drug treatment. These results point to an important role for VP-AVP signaling in sociosexual motivation in a non-monogamous rodent species.
4.1. A sexually dimorphic BNST/MeA→VP AVP circuit
We demonstrated that adult male rats possessed on average nearly twice as many AVP-ir fibers in the VP compared to adult female rats. To the best of our knowledge, we are the first to report this sex difference in VP-AVP fiber density in a non-monogamous rodent, though a similar sex difference in VP-AVP-ir fiber density was reported in monogamous prairie voles (Lim et al., 2004). Sex differences (males > females) in AVP-ir fiber density have been previously reported in the lateral septum (LS) and lateral habenular nucleus in many rodent species including rats, mice, and prairie voles (De Vries et al., 1981; Miller et al., 1992; Szot & Dorsa, 1993; Bamshad, Novak, & De Vries, 1993; Rood et al., 2013; DiBenedictis et al., 2017). This sex difference in AVP-ir fiber density likely originates from sex differences in the number of AVP-ir cells in the BNST and MeA (De Vries et al., 1981; Miller et al., 1992; Szot & Dorsa, 1993). In support, BNST lesions resulted in a dramatic decrease in AVP-ir fiber density in the LS, as did horizontal cuts made under the LS (De Vries and Buijs, 1983), suggesting that the LS is a major termination point of BNST AVP-ir fibers. We now demonstrate that AVP-ir cell bodies in both the BNST and MeA also send direct projections to the VP. In no instances did we observe VP-projecting AVP-ir neurons in other brain regions known to contain AVP-ir populations, such as the PVN and SON. We further found that males had a significantly greater percentage of VP-projecting AVP-ir cells in the BNST, with a similar, but non-significant trend observed in the MeA. Thus, the sex difference in VP-AVP-ir fibers reported here likely reflects a sex difference in the percentage of AVP-ir neurons in the BNST and MeA that send projections to the VP. Furthermore, we observed the presence of what we believe are possible en passant synaptic puncta on AVP-ir fibers coursing through the VP. If the presence of synaptic puncta were to be definitively demonstrated, this would strengthen the suggestion that these fibers are making synaptic contacts and potentially releasing AVP in the VP. Based on these findings, we speculate that AVP-ir fibers originating from cell bodies in the BNST and MeA likely traverse the VP and adjacent diagonal band of Broca before terminating in the LS (De Vries and Buijs, 1983). Further research is required to test this hypothesis. Taken together, the higher percentage of AVP-ir neurons in the BNST/MeA combined with a greater density of AVP-ir fibers in the VP in males versus females suggest the possibility of greater AVP release in the male VP, which may have relevance for the sex-specific regulation of social behavior. Alternatively, however, we cannot rule out the possibility that a greater density of VP-AVP-ir fibers present in males may reflect excess storage and lower AVP release (or in females, that a lower density of VP-AVP-ir fibers may reflect high secretory activity and therefore greater AVP release). All of these interpretations have been previously suggested (Wang et al., 1994; Landgraf & Neumann, 2004; Koolhaas et al., 2010). Future studies should investigate dynamic changes in VP-AVP release using microdialysis or similar techniques to answer this question.
Our neuroanatomical findings raise the question of the mechanism underlying the observed increase in AVP-ir neurons and fibers in the BNST/MeA → VP pathway in males compared to females. Gonadal steroid hormones are critical for the maintenance of the BNST/MeA → LS circuit in males. Castration of male rats drastically decreased AVP-ir in the BNST/MeA → LS circuit; an effect that was reversed by treating castrates with testosterone (De Vries et al., 1984; De Vries et al., 1986; Miller et al., 1989). This AVP circuit is masculinized perinatally and maintained by male gonadal steroid hormones in adulthood (De Vries et al., 1983). Of note, however, both ovariectomy of adult female rats and castration of adult male rats resulted in a similar overall reduction in AVP-ir fiber density in the LS (De Vries et al., 1984). In support, we recently found that adult female rats possessed a greater density of AVP-ir fibers in the LS and a greater number of AVP-ir cells in the BNST and MeA compared to juvenile female rats (DiBenedictis et al., 2017). These findings suggest that circulating gonadal steroid hormones are also important for the maintenance of AVP-ir expression in adult females. Future work should explore the precise mechanisms through which gonadal steroid hormones maintain AVP-ir fiber/cell body populations in the BNST/MeA → VP pathway in both sexes to determine whether the hormonal mechanisms are similar to those that support sex differences in the BNST/MeA → LS pathway.
4.2. Sex-specific role of the VP-AVP system in sociosexual motivation
Vehicle treated males showed a stronger opposite sex preference than vehicle treated females. This sex difference in opposite sex preference was eliminated by VP-V1AR antagonist treatment. In detail, we showed that acute pharmacological blockade of the V1AR in the VP reduced the innate preference for the opposite sex in adult male rats, while increasing opposite sex preference in adult female rats. Critically, this effect occurred independently of any changes in time spent investigating the same sex or changes in locomotor behavior, the latter indexed by the total number of chamber entries in vehicle versus V1AR antagonist-treated groups. This suggests that the observed V1AR antagonist-induced change in opposite sex preference (decrease in males, increase in females) resulted primarily from a change in the amount of time subject rats spent investigating opposite sex conspecifics and was not due to a generalized lack of motivation to approach and investigate stimulus animals. These results raise the possibility that endogenous AVP signaling in the VP positively regulates sociosexual motivation in male rats and negatively regulates sociosexual motivation in females.
Our results are in line with previous studies that have investigated brain-wide central effects of AVP on sociosexual behavior. For example, intracerebroventricular (ICV) infusion of AVP reduced female, but not male, sexual behavior in rats (Sodersten et al., 1983). Furthermore, ICV infusion of AVP antiserum blocked the AVP-induced decrement in sexual behavior (i.e., lordosis) in estradiol-primed female rats (Sodersten et al., 1983). Likewise, ICV infusion of AVP significantly decreased, while ICV infusion of a V1AR antagonist increased, lordosis behavior as well as hop-dart proceptive sociosexual behavior in estradiol and progesterone-primed female rats (Pendersen and Boccia, 2006). From a functional perspective, Fos expression in AVP-ir neurons in the MeA was augmented in male mice that were allowed to investigate an inaccessible estrus female (Hari Dass and Vyas, 2014), and mating augmented Fos expression in the monogamous male prairie vole VP (Lim and Young, 2004). Based on these and our findings, we predict that sociosexual behavior is associated with an increased activation of the BNST/MeA → VP AVP pathway in males, while in estrus females, this pathway may be inactivated or inhibited. The opposing role of the AVP system in the regulation of male and female rodent sociosexual behavior may also extend to humans. Indeed, it has been shown that AVP is secreted in the sexual arousal phase and is linked to sex drive in men (Murphy et al., 1987), suggesting that AVP may play an evolutionarily conserved role in male sociosexual motivation. However, parallel studies in women are currently lacking. Taken together, these data suggest that AVP signaling via the V1AR is an important regulator of sociosexual behavior in rodents of both sexes, and our study is the first to identify the sociosexual effects of the AVP system to the VP in the non-monogamous rat.
Our results relate to previous studies conducted in monogamous male prairie voles showing that V1AR blockade in the VP impairs mating-induced partner preference formation (Lim and Young, 2004), while viral vector-mediated overexpression of the V1AR in the VP induced partner preference formation in non-monogamous male meadow voles (Lim et al., 2004). Partner preference in prairie voles and opposite sex preference in rats both rely on the ability to discriminate between individuals/sexes. The VP integrates both sensory and motivation-related signals from upstream nuclei in the mesocorticolimbic pathway (e.g., the nucleus accumbens) in order to drive the appropriate behavioral response (Richard et al., 2016). Given this role of the VP in integrating sensory information, it is possible that the effects of VP-V1AR blockade on opposite sex preference in the present study resulted from sensory deficits, such as an inability to discriminate between same sex and opposite sex individuals, rather than from VP-V1AR antagonist-induced changes in the animal’s motivational state. However, both male and female rats were able to perceive the difference between same sex and opposite sex individuals even when treated with a V1AR antagonist. This was indexed by a robust increase in investigation time (dishabituation response) toward the first presentation of a male stimulus rat, as well as a dishabituation response toward the first presentation of an ovariectomized, estradiol and progesterone-primed female stimulus rat. This demonstrates that the observed VP-V1AR antagonist-induced decrease in opposite sex preference in males and increase in opposite sex preference in estrus females most likely reflect changes in motivational state, rather than changes in sensory perception.
A particularly intriguing finding in our study was that V1AR blockade in the VP had opposite effects on sociosexual motivation in male versus female rats. Why would AVP signaling drive sociosexual motivation in males, while reducing it in females? One potential clue may be found in the non-maternal status of the female rats in the current study. Previous work has shown that the brain AVP system becomes upregulated around parturition and lactation in rats (Caldwell et al., 1987; Landgraf et al., 1991; Walker et al., 2001; Bosch and Neumann, 2008; 2010). Moreover, several studies have pointed to a critical role for AVP in facilitating the onset and maintenance of maternal behavior. Specifically, ICV infusion of AVP accelerated, while ICV infusion of AVP antiserum delayed the onset of maternal behavior in ovariectomized, estradiol-primed virgin female rats (Pedersen et al., 1982; Pedersen et al., 1985). Likewise, in lactating rats, ICV infusion of AVP increased the frequency of maternal behavior displays, while ICV infusion of a V1AR antagonist reduced the frequency of ongoing maternal behavior displayed towards pups (Bosch and Neumann, 2008). Moreover, ICV infusion of a V1AR antagonist reduced maternal aggression and the overall amount of maternal care (indexed by nursing posture, pup retrieval and pup contact) displayed by lactating rats (Bosch and Neumann, 2008; Bosch and Neumann, 2010). One brain region wherein the AVP system has effects on maternal behavior is the medial preoptic area (MPOA). For example, antisense-induced downregulation of the V1AR in the MPOA decreased, while viral vector-induced upregulation of the V1AR in the MPOA increased the frequency of arched back nursing in lactating rats (Bosch and Neumann, 2008). Based on these and our findings, we hypothesize that while an upregulated brain AVP system ensures that females tend to their offspring, it may also ensure that females are not simultaneously sexually receptive. Accordingly, low AVP levels may facilitate female sociosexual motivation while high AVP levels may facilitate maternal behavior—two behaviors that are generally considered to be mutually exclusive (Alfonso et al., 2007; Numan, 2006). Future work should test the role of the VP-AVP system in the expression of maternal behavior.
5. Conclusion
We showed for the first time that there is a sex difference (males > females) in AVP fiber density in the VP of a non-monogamous species, and that this sex difference arises from a sexually dimorphic population of VP-projecting AVP cells in the BNST and MeA. We further showed that this sex difference has important functional consequences in that pharmacological blockade of AVP signaling (by antagonizing V1ARs) in the VP reduced sociosexual motivation in adult male rats, while enhancing this behavior in estrus females. Thus, here we provide a functional link between a sex difference in VP-AVP fiber density and the sex-specific regulation of a sexually motivated behavior necessary for reproduction.
Highlights.
AVP fibers in the ventral pallidum (VP) are denser in adult male than female rats
AVP cells in the BNST and MeA project to the VP in both males and females
Males have a greater percentage of VP-projecting AVP cells in the BNST and MeA
Blocking VP-AVP signaling reduced male, but increased female sociosexual motivation
Acknowledgements
We would like to thank Dr. Maurice Manning (University of Toledo) for providing the V1AR antagonist and Dr. Hal Gainer (NIMH) for providing the AVP primary antibody. We would also like to thank all members of the Veenema lab for their critical reading of the manuscript.
Funding and disclosure
This research was supported by NIH R15MH102807 and NIH R01MH102456 to AHV.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest statement
The authors declare no conflicts of interest.
References
- Albers HE, Dean A, Karom MC, Smith D, Huhman KL, 2006. Role of V1a vasopressin receptors in the control of aggression in Syrian hamster. Brain Res. 16, 425–430. [DOI] [PubMed] [Google Scholar]
- Afonso VM, Sison M, Lovic V, Fleming AS, 2007. Medial prefrontal cortex lesions in the female rat affect sexual and maternal behavior and their sequential organization. Behav. Neurosci 121, 515–526. [DOI] [PubMed] [Google Scholar]
- Bales KL, Plotsky PM, Young LJ, Lim MM, Grotte N, Ferrer E, Carter CS, 2007. Neonatal oxytocin manipulations have long-lasting, sexually dimorphic effects on vasopressin receptors, Neurosci. 144,38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, De Vries GJ, 1993. Sex and species differences in he vasopressin innervation of sexually naïve and parental prairie voles, Microtus ochrogaster and meadow voles, Microtus pennsylvanicus. J Neuroendocrinol. 5, 247–255. [DOI] [PubMed] [Google Scholar]
- Bamshad M, Novak MA, de Vries GJ, 1994. Cohabitation alters vasopressin innervation and paternal behavior in prairie voles (Microtus ochrogaster). Physiol. Behav 56, 751–758. [DOI] [PubMed] [Google Scholar]
- Beery AK, Lacey EA, Francis DD, 2008. Oxytocin and vasopressin receptor distributions in a solitary and a social species of tuco-tuco (Ctenomys haigi and Ctenomys sociabilis). J. Comp. Neurol 507, 1847–1859. [DOI] [PubMed] [Google Scholar]
- Berridge KC, 1996. Food reward: brain substrates of wanting and liking. Neurosci. Biobehav. Rev 20, 1–25. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID, 2008. Brain vasopressin is an important regulator of maternal behavior independent of dams’ trait anxiety. Proc. Natl. Acad. Sci. U. S. A 105, 17139–17144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch OJ, Neumann ID, 2010. Vasopressin released within the central amygdala pro- motes maternal aggression. Eur. J. Neurosci 31, 883–891. [DOI] [PubMed] [Google Scholar]
- Bosch OJ, Pfortsch J, Beiderbeck DI, Landgraf R, Neumann ID, 2010. Maternal behaviour is associated with vasopressin release in the medial preoptic area and bed nucleus of the stria terminalis in the rat. J. Neuroendocrinol 22, 420–429. [DOI] [PubMed] [Google Scholar]
- Buijs RM, 1978. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways the to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 192, 423–435. [DOI] [PubMed] [Google Scholar]
- Buijs RM, 1980. Immunocytochemical demonstration of vasopressin and oxytocin in the rat brain by light and electron. J. Histochem. Cytochem 28, 357–60. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Greer ER, Johnson MF, Prange AJ Jr., Pedersen CA, 1987. Oxytocin and vasopressin immunoreactivity in hypothalamic and extrahypothalamic sites in late pregnant and postpartum rats. Neuroendocrinology 46, 39–47. [DOI] [PubMed] [Google Scholar]
- Cromwell HC, Berridge KC, 1993. Where does damage lead to enhanced food aversion: the ventral pallidum/substantia innominata or lateral hypothalamus? Brain Res. 624, 1–10. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, 1983. The origin of the vasopressinergic and oxytocinergic innervation of the rat brain with special reference to the lateral septum. Brain Res. 273, 307–317. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Sluiter AA, 1984. Gonadal hormone actions on the morphology of the vasopressinergic innervation of the adult rat brain. Brain Res. 298, 141–145. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Buijs RM, Swaab DF, 1981. Ontogeny of the vasopressinergic neurons of the suprachiasmatic nucleus and their extrahypothalamic projections in the rat brain – presence of a sex difference in the lateral septum. Brain Res. 218 67–78. [DOI] [PubMed] [Google Scholar]
- De Vries GJ, Duetz W, Buijs RM, van Heerikhuize J, Vreeburg JT, 1986. Effects of andorgens and estrogens on the vasopressin and oxytocin innervation of the adult rat brain. Brain Res. 399, 296–302. [DOI] [PubMed] [Google Scholar]
- DiBenedictis BT, Nussbaum ER, Cheung HK, Veenema AH, 2017. Quantitative mapping reveals age and sex differences in vasopressin, but not oxytocin, immunoreactivity in the rat social behavior neural network. J. Comp. Neurol 525, 2549–2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris CF, Melloni RH, Koppel G, Perry KW, Fuller RW, Delville Y, 1997. Vasopressin/serotonin interactions in the anterior hypothalamus control aggressive behavior in golden hamsters. J. Neurosci. 17, 4331–4340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fudge JL, Kunishio K, Walsh P, Richard C, Haber SN, 2002. Amygdaloid projections to ventromedial striatal subterritories in the primate. Neurosci. 110, 257–275. [DOI] [PubMed] [Google Scholar]
- Goldman JM, Murr AS, Cooper RL, 2007. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res. B. Dev. Reprod. Toxicol 80, 84–87. [DOI] [PubMed] [Google Scholar]
- Grove EA, 1988. Neural associations of the substantia innominata in the rat: afferent connections. J. Comp. Neurol 277, 315–346. [DOI] [PubMed] [Google Scholar]
- Hari Dass SA, Vyas A, 2014. Copulation or sensory cues from the female augment Fos expression in arginine vasopressin neurons of the posterodorsal medial amygdala of male rats. Front. Zool 11, 42–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heimer L, Wilson RD, 1975. The subcortical projections of the allocortex: similarities in the neural associations of the hippocampus, the periform cortex and the neocortex, in: Santini M, (Ed.). Gogli centennial symposium proceedings. Raven Press, New York, pp. 173–193. [Google Scholar]
- Hou-Yu A, Lamme AT, Zimmerman EA, Silverman AJ, 1986. Comparative distribution of vasopressin and oxytocin neurons in the rat brain using a double-label procedure. Neuroendocrinology 44, 235–246. [DOI] [PubMed] [Google Scholar]
- Insel TR, Gelhard R, Shapiro LE (1991) The comparative distribution of forebrain receptors for neurohypophyseal peptides in monogamous and polygamous mice, Neurosci. 43, 623–30. [DOI] [PubMed] [Google Scholar]
- Klitenick MA, Deutch AY, Churchill L, Kalivas PW, 1992. Topography and functional role of dopaminergic projections from the ventral mesencephalic tegmentum to the ventral pallidum. Neurosci. 50, 371–386. [DOI] [PubMed] [Google Scholar]
- Koolhaas JM, de Boer SF, Coppens CM, Buwalda B, 2010. Neuroendocrinology of coping styles: Towards understanding the biology of individual variation. Front, Neuroendocrinol. 31, 307–321. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann ID, 2004. Vasopressin and oxytocin release within the brain: a dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol 25, 150–176. [DOI] [PubMed] [Google Scholar]
- Landgraf R, Neumann I, Pittman QJ, 1991. Septal and hippocampal release of vaso- pressin and oxytocin during late pregnancy and parturition in the rat. Neuroendocrinol. 54, 378–383. [DOI] [PubMed] [Google Scholar]
- Lim MM, Young LJ., 2004. Vasopressin-dependent neural circuits underlying pair bond formation in the monogamous prairie vole. Neurosci. 125, 35–45. [DOI] [PubMed] [Google Scholar]
- Lim MM, Wang Z, Olazabal DE, Ren X, Terwilliger EF, Young LJ, 2004. Enhanced partner preference in a promiscuous species by manipulating the expression of a single gene. Nature 429, 754–757. [DOI] [PubMed] [Google Scholar]
- Manning M, Stoev S, Chini B, Durroux T, Mouillac B, Guillon G, 2008. Peptide and non-peptide agonists and antagonists for the vasopressin and oxytocin V1a, V1b, V2 and OT receptors: research tools and potential therapeutic agents. Prog. Brain Res 170, 473–512. [DOI] [PubMed] [Google Scholar]
- Maurice N, Deniau JM, Menetrey A, Glowinski J, Thierry AM, 1997. Position of the ventral pallidum in the rat prefrontal cortex-basal ganglia circuit. Neurosci. 80, 523–534. [DOI] [PubMed] [Google Scholar]
- Miller MA, DeVries GJ, al-Shamma HA, Dorsa DM, 1992. Decline of vasopressin immunoreactivity and mRNA levels in the bed nucleus of the stria terminalis following castration. J. Neurosci 12, 2881–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MA, Urban JH, Dorsa DM, 1989. Sex differences in vasopressin neurons in the bed nucleus of the stria terminalis by in situ hybridization, Peptides 10, 615–619. [DOI] [PubMed] [Google Scholar]
- Mitrovic I, Napier TC, 1998. Substance P attenuates and DAMGO potentiates amygdala glutamatergic neurotransmission within the ventral pallidum. Brain Res. 792, 193–206. [DOI] [PubMed] [Google Scholar]
- Murphy MR, Seckl JR, Burton S, Checkley SA, Lightman SL, 1987. Changes in oxytocin and vasopressin secretion during sexual activity in men. J. Clin. Endocrinol. Metab 65, 738–741. [DOI] [PubMed] [Google Scholar]
- Numan M, 2006. Hypothalamic neural circuits regulating maternal responsiveness towards infants. Behav. Cogn. Neurosci. Rev 4, 163–190. [DOI] [PubMed] [Google Scholar]
- O’Connell LA, Hofmann HA, 2011. The vertebrate mesolimbic reward system and social behavior network: a comparative synthesis. J. Comp. Neurol 519, 3599–3639. [DOI] [PubMed] [Google Scholar]
- Otero-Garcia M, Martin-Sanchez A, Fortes-Marco L, Martinez-Ricos J, Agustin-Pavon C, Lanuza E, Martinez-Garcia F, 2013. Extending the socio-sexual brain: arginine-vasopressin immunoreactive circuits in the telencephalon of mice. Brain Struct. Funct 3, 1055–1081. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C, 2007. The rat brain atlas in stereotaxic coordinates Academic Press, Elsevier Inc., Oxford. [Google Scholar]
- Pedersen CA, Ascher JA, Monroe YL, Prange AJ Jr., 1982. Oxytocin induces maternal behavior in virgin female rats. Science 216, 648–650. [DOI] [PubMed] [Google Scholar]
- Pendersen CA, Boccia ML, 2006. Vasopressin interactions with oxytocin in the control of female sexual behavior. Neurosci. 139, 843–851. [DOI] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Johnson MF, Fort SA, Prange AJ Jr., 1985. Oxytocin antiserum delays onset of ovarian steroid-induced maternal behavior. Neuropeptides 6, 175–182. [DOI] [PubMed] [Google Scholar]
- Reep RL,Winans SS, 1982. Efferent connections of dorsal and ventral agranular insular cortex in the hamster, Mesocricetus auratus. Neurosci. 7, 2609–2635. [DOI] [PubMed] [Google Scholar]
- Richard JM, Ambroggi F, Janak PH, Fields HL, 2016. Ventral pallidum neurons encode incentive value and promote cue-elicited instrumental actions. Neuron 90, 1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, de Vries GJ, 2011. Vasopressin innervation of the mouse (Mus musculus) brain and spinal cord, J. Comp. Neurol 519, 2434–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rood BD, Stott RT, You S, Smith CJ, Woodbury ME, De Vries GJ, 2013. Site of origin and of and sex differences in the vasopressin innervation of the mouse (mus musculus) brain. J. Comp. Neurol 521, 2321–2358. [DOI] [PubMed] [Google Scholar]
- Smith CJ, Poehlmann ML, Li S, Ratnaseelan AM, Bredewold R, Veenema AH, 2017. Age and sex differences in oxytocin and vasopressin V1a receptor binding densities in the rat brain: focus on the social decision-making network. Brain Struct. Funct 222, 981–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KS, Tindell AJ, Aldridge JW, Berridge KC, 2009. Ventral pallidum roles in reward and motivation. Behav. Brain Res 196, 155–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Södersten P, Henning M, Melin P, Ludin S, 1983. Vasopressin alters female sexual behaviour by acting on the brain independently of alterations in blood pressure. Nature 301, 608–610. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, 1983. Morphology of vasopressin and oxytocin neurons and their central and vascular projections. Prog. Brain Res 60, 101–14. [DOI] [PubMed] [Google Scholar]
- Szot P, Dorsa DM, 1993. Expression of vasopressin mRNA in extrahypothalamic nuclei of the homozygous Brattleboro rat is not modulated by testosterone. Neuroendocrinology 58, 381–387. [DOI] [PubMed] [Google Scholar]
- Usuda I, Tanaka K, Chiba T, 1998. Efferent projections of the nucleus accumbens in the rat with special reference to subdivision of the nucleus: biotinylated dextran amine study. Brain Res. 797, 73–93. [DOI] [PubMed] [Google Scholar]
- Van Leeuwen F, Caffe R, 1983. Vasopressin-immunoreactive cell bodies in the bed nucleus of the stria terminalis of the rat. Cell Tissue Res. 228, 525–524. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Beiderbeck DI, Lukas M, Neuman ID, 2010. Distinct correlations of vasopressin release within the lateral septum and the bed nucleus of the stria terminalis with the display of intermale aggression. Horm. Behav 58, 273–281. [DOI] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ, 2012. Vasopressin regulates social recognition in juvenile and adult rats of both sexes, but in sex- and age-specific ways. Horm. Behav 61, 50–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veenema AH, Bredewold R, De Vries GJ, 2013. Sex-specific modulation of juvenile social play by vasopressin. Psychoneuroendocrinology 38, 2554–2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CD, Toufexis DJ, Burlet A, 2001. Hypothalamic and limbic expression of CRF and vasopressin during lactation: implications for the control of ACTH secretion and stress hyporesponsiveness. Prog. Brain Res 133, 99–110. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zhou L, Hulihan TJ, Insel TR, 1996. Immunoreactivity of central vasopressin and oxytocin pathways in microtine rodents: A quantitative comparative study. J. Comp. Neurol 366, 726–737. [DOI] [PubMed] [Google Scholar]
- Wang Z, Smith W, Major DE, De Vries GJ, 1994. Sex and species differences in the effects of cohabitation on vasopressin messenger RNA expression in the bed nucleus of the stria terminalis in prairie voles (Microtus ochrogcister) and meadow voles (Microtus penmylvcmicus). Brain Res. 650, 212–218. [DOI] [PubMed] [Google Scholar]
- Winslow JT, Hastings N, Carter CS, Harbaugh CR, Insel TR, 1993. A role for central vasopressin in pair bonding in monogamous prairie voles. Nature 365, 545–548. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ, 1983. Response of ventral pallidal neurons to amygdala stimulation and its modulation by dopamine projections to nucleus accumbens. J. Neurophysiol 50, 148–161. [DOI] [PubMed] [Google Scholar]
- Young LJ, Nilsen R, Waymire KG, MacGregor GR, Insel TR, 1999. Increased affiliative response to vasopressin in mice expressing the V1a receptor from a monogamous vole. Nature 400, 766–768. [DOI] [PubMed] [Google Scholar]
- Young LJ, Toloczko D, Insel TR, 1999. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J Neuroendocrinol, 11(4), 291–297. [DOI] [PubMed] [Google Scholar]
- Zaborszky L, Gaykema RP, Swanson DJ, Cullinan WE, 1997. Cortical input to the basal forebrain. Neurosci. 79, 1051–1078. [DOI] [PubMed] [Google Scholar]


