Abstract
Neoantigen vaccines involving multi-peptides and poly-epitope-encoding RNA or DNA have undergone early phase clinical testing with modest reported antitumor effects [ 1]. The less-than-expected activity of these neoantigenic vaccines may correspond with the development of immune escape mechanisms. One permutation on neoantigen vaccines, which may counter or prevent these adaptive immune escape mechanisms, are ‘personalized’ oncolytic viruses that encode one or more tumor-specific transgenes. Herein, positive therapeutic effects for MY-NEOVAX™, personalized neoantigen-enhanced oncolytic adenoviruses, are described for two heavily pretreated end-stage patients, one with high-grade metastatic neuroendocrine carcinoma of the pancreas and the other with colorectal cancer metastatic to the brain, liver and lungs. To date, treatment benefit has exceeded 12 months without dose-limiting toxicities or related serious adverse events and with documented radiologic stabilization and improved performance status.
INTRODUCTION
Neoantigen-based peptide, DNA, RNA and dendritic cell vaccines have been used in the clinic, mostly in melanoma, with mixed results. This less-than-expected efficacy has been attributed to immune escape mechanisms [2], which include low neoantigen burden, the immunosuppressive tumor microenvironment and rapid epitope loss. Oncolytic viruses are an active form of immunotherapy that are armed (or not) with therapeutic transgenes such as granulocyte-macrophage colony stimulating factor or interleukin (IL)-12 whose protein products are intended to amplify antitumor immunity [3].
MY-NEOVAX™ is the proprietary name for patient-specific replication conditional oncolytic adenovirus, which are engineered and manufactured by EpicentRx, Inc., to carry up to 50 neoantigenic transgenes identified on next generation sequencing (NGS). Unlike other neoantigen vaccines, these personalized viruses have the potential to circumvent immune escape mechanisms such as low antigen burden, epitope loss and tumor-mediated immunosuppression because (i) every time the virus replicates it also copies the therapeutic transgenes, which leads to their overexpression and presumably drives tumor-specific cytotoxic CD8+ T-cell responses against the neoantigens even if the mutational load is low, (ii) insurance against loss of one epitope multiple neoantigens are carried and expressed by the virus and (iii) viral infection stimulates the release of cytokines (IL-1, IL-6, IL-12, IL-18, IFN-γ and TNF-α) and chemokines (RANTES, MIP-1α/β) that counteract local and systemically induced immunosuppression and facilitate the recruitment of effector T cells for the generation antitumor immunity [4]. A simplified mechanism of neoantigen-armed oncolytic viruses is illustrated in Fig. 1.
Figure 1.
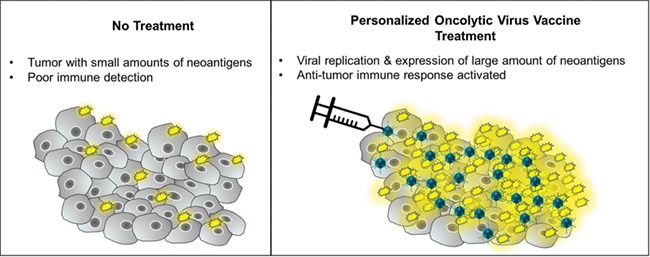
Simplified mechanism of oncolytic viruses armed with neoantigens.
Two heavily pretreated end-stage patients, JM, a 30-year-old male with high-grade metastatic neuroendocrine carcinoma of the pancreas, and WB, a 64-year-old male with colorectal cancer metastatic to the brain, liver and lungs, were treated with these personalized oncolytic viruses i.e. MY-NEOVAX™ under separate Food and Drug Administration- and Institutional Review Board-approved compassionate use protocols. As described below, both patients have demonstrated long-lasting disease stabilization > 12 months in the absence of any dose-limiting toxicities or related serious adverse events. The specific transportation, handling and administration requirements for MY-NEOVAX, which are easily and routinely managed, have been described in a separate publication [5].
CASE REPORTS
Case 1
The first patient, JM, is a 30-year-old male with high-grade neuroendocrine carcinoma of the pancreas metastatic to the retroperitoneum and cervical lymph nodes. Previous treatments included cisplatin/etoposide to which he was refractory, the experimental agent, RRx-001, to which he responded for over a year and which primed a subsequent response to previously refractory platinum etoposide, ipilimumab and nivolumab, to which he was non-responsive, and a third retrial of platinum etoposide that was ineffective. At the time of enrollment on the MY-NEOVAX™ compassionate use protocol, his tumors were rapidly progressing, and his functional performance status was declining. Based on NGS profiling, a virus was Good Manufacturing Practice (GMP)-manufactured to carry several neoantigens including the mutated gene for adenopolyposis coli (APC).
MY-NEOVAX™ has been administered intratumorally (IT) to JV in an interventional radiology (IR) suite at a dose of 1 × 1012 in the retroperitoneum and cervical lymph nodes once every 2 weeks. Following the first dose of virus, immediately after which he experienced fever, myalgia, chills for 24 h, high levels of virus were detected in the blood presumably from leakage through injured tumor vasculature and/or from extrusion via the injection track once the syringe was removed. This viremia was cleared within 48 h; however, 1 week later a secondary viremia was observed presumably due to propagation of the viral infection, with lysis and release of progeny viruses from the infected tumor cells.
CT scans at 2 months after the initial treatment were read as stable disease, but significant necrosis was detectable, likely indicative of the direct oncolytic effect along with an approximately 18% tumor shrinkage (Fig. 2).
Figure 2.
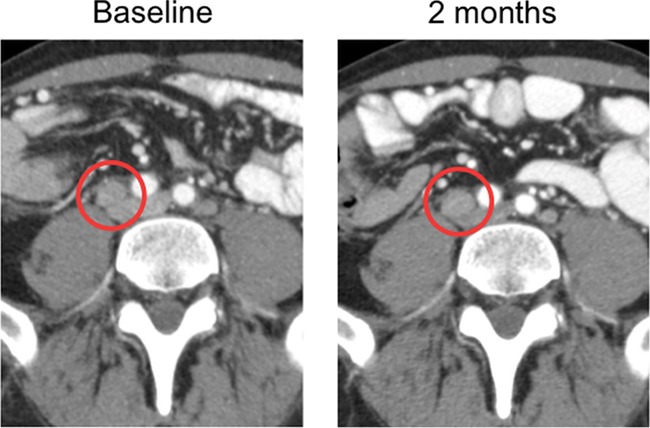
Shrinkage of a retroperitoneal mass circled in red with evidence of necrosis on a 2-month CT scan.
In addition, the patient has demonstrated a significantly improved performance status, having been able to train for and participate in long-distance ruck marches and marathon bike rides. One serious adverse event (SAE), a fractured femur, occurred when the all-terrain vehicle he was riding overturned and landed on his leg. During open reduction and internal fixation surgery, no tumor was found in the femur.
Case 2
The second patient is a 64-year-old male, WB, with colorectal cancer metastatic to the lungs, liver and brain. Previous therapies included FOLFIRI/Erbitux, Xelox, Xeloda/Avastin, Regorafenib, Erlotinib for brain metastases and FOLFIRI/Avastin. From the FOLFIRI/Avastin, on which he progressed, WB experienced Grade 4 neutropenia, severe diarrhea and rapid deterioration prior to starting treatment with MY-NEOVAX™. From the brain metastases, cognitive dysfunction was present. Since Avastin may predispose to hemorrhagic complications for several days after its discontinuation, the decision was made to inject WB subcutaneously with the virus at a dose of 5 × 1011, which was GMP-manufactured to also carry the mutated gene for adenopolyposis coli (APC) based on NGS, in the inguinal region remote from any tumor.
Similar to the first patient, his CT scans were also read as stable and his performance status markedly approved to the point that he went from cognitively impaired and bedbound to cognitively normal and fully participatory in tae-kwon-do classes, where he recently earned a blue belt, which is two below a black belt. He was treated for over 4 months with mild arthralgias as the only toxicity. A slight interval enlargement of his lung tumors was seen on CT scan; although this enlargement was attributed to pseudoprogression, it led to his discontinuation from the trial; however, over 12 months later, after having been restarted on chemotherapy, he remains largely stable.
DISCUSSION
The expected survival of last-line treatment refractory colorectal cancer [6] and high-grade neuroendocrine carcinoma is less than 6 months [7]. The present results with these two patients suggest that MY-NEOVAX as monotherapy circumvented immunologic tolerance. A Phase 1 study is planned in combination with other immunotherapies. Biopsies have been performed, and when their analysis is complete, results will be reported.
ACKNOWLEDGEMENTS
None.
Conflict of Interest
The authors declare that EpicentRx, Inc., funds research of MY-NEOVAX.
FUNDING
No funding was received for the writing of this manuscript.
Ethical Approval
The research was conducted in accordance with the Helsinki declaration, and the relevant Institutional Review Boards approved the research.
Consent
Written informed consent was obtained from both patients referred to in this case report.
References
- 1. van der Burg SH , Correlates of immune and clinical activity of novel cancer vaccines. Semin Immunol. 2018. Oct;39:119–136. doi: 10.1016/j.smim.2018.04.001. [DOI] [PubMed] [Google Scholar]
- 2. Chu Y, Liu Q, Wei J, Liu B. Personalized cancer neoantigen vaccines come of age. Theranostics 2018;8:4238–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Larson C, Oronsky B, Scicinski J, Fanger GR, Stirn M, Oronsky A, et al. . Going viral: a review of replication-selective oncolytic adenoviruses. Oncotarget. 2015 Aug 21;6:19976–89Review. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Guidotti LG, Chisari FV. Noncytolytic control of viral infections by the innate and adaptive immune response. Annu Rev Immunol. 2001; 19:65–91. [DOI] [PubMed] [Google Scholar]
- 5. Larson C, Oronsky B, Varner G, Caroen S, Burbano E, Insel E, et al. . Practical guide to the handling and administration of personalized transcriptionally attenuated oncolytic adenoviruses (PTAVs). Oncoimmunology. 7:e1478648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Cutsem EV, Sobrero AF, Siena S, Falcone A, Ychou M, Humblet Y, et al. . Phase III CORRECT trial of regorafenib in metastatic colorectal cancer (mCRC). J Clin Oncol 201230:15_suppl, 3502–3502 [Google Scholar]
- 7. Oronsky B, Ma PC, Morgensztern D, Carter CA, Nothing But NET. A review of neuroendocrine tumors and carcinomas. Neoplasia (New York, NY) 2017;19:991–1002. [DOI] [PMC free article] [PubMed] [Google Scholar]


