Abstract
Metabolic biomarkers have potentially wider use in disease diagnosis and prognosis as well as in monitoring disease response to treatment. While biomarkers such as interleukins, microRNA, and lactate have been proposed for disease surveillance, there are still conflicting results regarding their clinical utility. Treatment of commonly encountered disease of acute care such as sepsis, trauma, and poisoning often relies on clinical diagnosis and therapy guided by use of surrogate markers of illness severity. The measurement of mitochondrial function, including respiration and motility, may offer superior alternatives to such markers. Assessing mitochondrial function in a clinical context has the potential to impact the area of acute care in terms of diagnosis, prognosis, and treatment. The study of mitochondrial bioenergetics has become critical in understanding the pathophysiology and treatment of complex diseases such as diabetes and cardiovascular disorders.
Keywords: mitochondria, sepsis, resuscitation, medical toxicology, bioenergetics, mitochondrial motility
Introduction
The mitochondria are involved in a wide range of clinical diseases, ranging from heart disease and diabetes to acute illnesses such as sepsis, traumatic injuries, and poisoning. As the mitochondria are the center of cellular function and energy production, it is not surprising that these disease processes result in bioenergetic dysfunction.1–3 The availability of highly sensitive instruments coupled with minimally invasive and rapid protocols to obtain key bioenergetic parameters of mitochondrial respiration promises great potential for improving understanding and management of complex diseases.
The measurement of mitochondrial respiration in patients with acute illnesses such as sepsis has the potential to identify those at risk for rapid disease progression and can monitor clinical course and response to treatment in real time, making it an ideal marker for the acute care setting. Other important advantages include the ability to examine different cell types and evaluate their contribution to the clinical disease, fine-tuning understanding of pathophysiology at a subcellular organelle and molecular level. Mitochondrial-directed therapy is currently being explored, and as these treatments become available, this method of measurement will become necessary. The cost and time to obtain these measurements are minimal when compared to more traditional methods of obtaining mitochondrial function in biopsies, making this technology viable. Mitochondrial bioenergetics is very promising in the area of acute care, and while significant research in the area of bioenergetics exists for chronic disease such as obesity, diabetes, and even aging, there is a paucity of research in the area of acute illnesses.
Mitochondrial Background
The mitochondrion (plural mitochondria) is an important organelle that ranges in size from about 8 to 10 μm with a diameter of 0.5 to 1 μm, but considerable variation can be seen in the structure and size of this organelle depending on the cell type. Mitochondria are remarkably mobile and plastic organelles forming a highly dynamic network in the majority of cells where they constantly undergo fission and fusion. The number of mitochondria in a cell can vary widely by organism, tissue, and cell type. The mitochondria consist of a highly specialized double-membrane organization critical for the generation of a proton motive force and energy production.4 Multiple copies of mitochondrial DNA are contained within the matrix, the space enclosed by each organelle’s inner membrane. The primary function of the mitochondria is the production of adenosine triphosphate (ATP), a primary cellular energy substrate. The mitochondria are also involved in calcium regulation, generation of reactive oxygen species (ROS), uncoupling, and play an important role in apoptosis mediated by cytochrome c.5
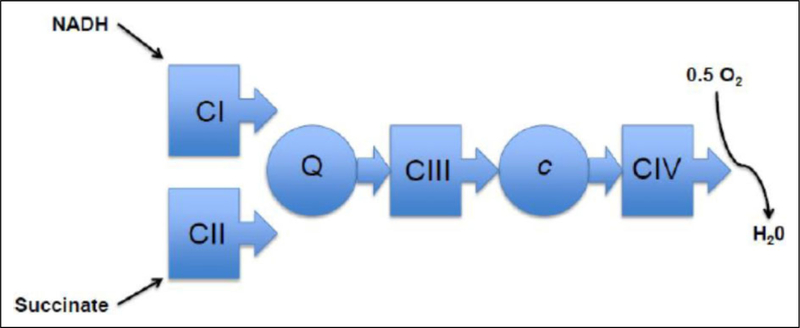
The mitochondria generate energy by oxidizing hydrogen derived from our dietary carbohydrates (TCA cycle) and fats (β-oxidation) with oxygen to generate heat and ATP. The electron transport chain or system (ETS) transfers electrons through a series of redox reactions. Two electrons donated from NADH+ H+ to complex I (NADH dehydrogenase) or from succinate to complex II (succinate dehydrogenase) are passed sequentially to ubiquinone to give ubisemiquinone and then ubiquinol. Ubiquinol transfers its electrons to complex III (ubiquinol: cytochrome c oxidoreductase), which transfers them to cytochrome c. From cytochrome c, the electrons flow to complex IV (cytochrome c oxidase) and finally to oxygen to give H2O. Each of these ETS complexes incorporates multiple electron carriers.6,7 With the exception of complex II, transfer of electrons to the various complexes will generate a proton motive force by pumping protons against a gradient. The electrochemical gradient is then utilized by complex V to produce ATP. Although the term ETS is commonly used, it sometimes may be misinterpreted as exclusively proceeding in one direction. With the exception of complex IV, all reactions are reversible.8 Figure 1 illustrates the series of redox reactions that occur along the ETS.
Figure 1.
Schematic representation of the 4 complexes and their sequential arrangement in the electron transfer system. The proton motive force generated by CI, CIII, and CIV will allow CV (ATP synthase) to generate ATP. It is important to realize that the electron transport system is largely reversible with the exception of CIV. ATP indicates adenosine triphosphate; C, complex; c, cytochrome; Q, ubiquinone or coenzyme Q.
Mitochondrial Respiration Instruments
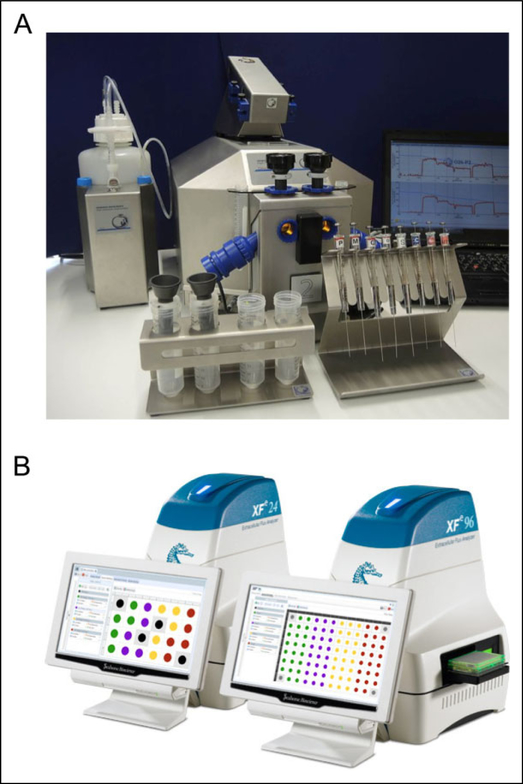
There are a variety of instruments that contain highly sensitive microcathode oxygen electrodes allowing high-resolution measurement of mitochondrial respiration. Major instruments in this area of research include Strathkelvin Instruments, Seahorse XF (Seahorse Biosciences, North Billerica, Massachusetts), and Oroboros O2K (Oroboros Instruments, Innsbruck, Austria) in Figure 2. The latter two instruments have the most applicability to the acute care setting and will be discussed in this section. The instruments developed by Seahorse Biosciences and Oroboros Instruments are similar in that both measure mitochondrial respiration in a wide variety of mitochondrial preparations and also allow for oxygen and temperature control.9
Figure 2.
A, Oroboros O2K fluorometer by OROBOROS INSTRUMENTS—www.oroboros.at. B, The Seahorse XFe24 and XFe96 by Seahorse Bioscience (Agilent Technologies)—www.seahorsebio.com.
Seahorse Biosciences currently have 3 models (XF, XFe, and XFp) available that measure changes to the concentrations of dissolved oxygen (oxygen consumption rate) and pH (extracellular acidification rate) in the media. These instruments are well suited for high-throughput screening. There are 4 injection ports for each well able to use a variety of substrates and reagents. The Seahorse utilizes disposable plastic sensor cartridges and cell plates, where monolayers of cells are seeded. Primary or cell lines can be grown on the plates, while isolated mitochondria and cells in suspension must be adhered with an adhesive such as Cell-Tak or gelatin. Once cell preparation is complete, the experiment can be completed in 1 to 3 hours and is fully automated. Some limitations to the Seahorse XF model include only 4 injection ports making comprehensive titrations with reagents more difficult and increased cost due to the reagent kits, disposable sensor cartridges, and cell plates. Another limitation of the Seahorse XF is that each well is not self-contained and can be affected by adjacent wells that may occur with off-gassing from compounds such as cyanide (CN) at 37°C. Some strengths of the Seahorse include the ability to study adherent cells that may be relevant for understanding cell–cell interactions or cell–substrate interactions in diseases and toxic exposures.
Oroboros O2K is available through Oroboros instruments, and is capable of high-resolution respirometry similar to the Seahorse. The O2K is a modular device that is comprised of 2 Duran glass 2-mL chambers with a magnetic stirrer bar and both are self-contained isolated chambers. The O2K can also measure respiration in a wide variety of preparations similar to the Seahorse but is measured in suspension medium so does not require adherence to the bottom of a well. One of the strengths of the O2K is the ability to perform a variety of titrations with a combination of substrate-uncoupler-inhibitors often referred to as a substrate-uncoupler-inhibitor-titrations (SUIT) protocol. This protocol allows study of the complex interactions of coupling and substrate control in a single assay. More than 20 titration steps can be used in a single assay. Another strength of the O2K is the modular aspect of the instrument that allows a variety of different modules to be applied to measure multiple aspects of mitochondrial physiology.10 Optical sensors are inserted through the front window of the O2K-glass chambers for measurement of hydrogen peroxide production (Amplex red), ATP production (Magnesium green), mt-membrane potential (Safranin, TMRM), and Ca2+ (Calcium green) simultaneously to measurement of mitochondrial respiration.11 One of the limitations of the O2K is the presence of only 2 chambers, making high-throughput testing impractical without multiple O2K units.
Mitochondrial Respiration Parameters
The Oroboros O2K and Seahorse XF measure mitochondrial respiration in real time, and key parameters can be obtained with the use of established inhibitors and uncouplers that serve as sensitive indicators of response to mitochondrial stress. The following sequential injections of select compounds are carried out in a SUIT protocol that can be used to assess mitochondrial response to disease processes such as sepsis or hemorrhagic shock. Figure 3 illustrates a typical mitochondrial respiration tracing from a SUIT protocol in intact cells. The following terms are important in the interpretation of mitochondrial respiration12:
Routine respiration: Routine respiration, also known as basal respiration, is a measure of oxygen consumption due to the combination of ATP production and proton leak. This represents energy demand under steady-state conditions. Changes in routine respiration in patients with disease compared to control can indicate altered mitochondrial function and should be interpreted in the context of the other following mitochondrial parameters.
Proton leak: After the measurement of routine respiration, cells are exposed to oligomycin, an inhibitor of complex V. The remaining mitochondrial respiration after injection of oligomycin is attributable to proton leak. Although some proton leak is expected under physiologic conditions, significant proton leak can be an indication of mitochondrial membrane and/or complex damage. The use of oligomycin also allows an estimate of oxygen consumption secondary to ATP production, often referred to as ATP-linked respiration.
Maximal respiration: The addition of a mitochondrial uncoupler such as dinitrophenol or carbonyl cyanide-4-(trifluoromethoxy) phenylhydrazone stimulates maximal respiration by mimicking a physiological energy demand leading to an increase in oxygen consumption. The difference between maximal respiration and routine respiration represents the cell’s spare respiratory capacity (SRC). The SRC indicates the cell’s ability to respond to energetic stress and is a measure of a cell’s fitness. A decrease in the SRC may limit the cell’s ability to handle a stressor, resulting in mitochondrial dysfunction.
Residual oxygen consumption: The addition of mitochondrial inhibitors such as the combination of rotenone (complex I) and antimycin (complex III) will completely inhibit the ETS. The remaining oxygen consumption is due to nonmitochondrial respiration in the form of oxidases and other cellular enzymes. Residual oxygen consumption may be increased in the presence of a stress response indicating increased inflammation.
Figure 3.
Representative tracing of oxygen consumption measured in intact peripheral blood mononuclear cells. The solid gray line shows the rate of oxygen consumption. After measuring routine oxygen consumption, the adenosine triphosphate (ATP)-synthase inhibitor oligomycin is added to obtain LEAK. The uncoupler carbonyl cyanide m-chlorophenylhydrazone (CCCP) allows maximal oxygen consumption stimulating maximal respiration assuming all required substrates are present. Finally, rotenone (complex I inhibitor) and antimycin A (complex III inhibitor) will completely prevent oxygen consumption through the electron respiratory chain. The remaining residual oxygen consumption is attributed from other nonmitochondrial sources such as oxidases. Adenosine triphosphate-linked oxygen consumption is calculated as routine respiration minus leak and spare respiratory capacity is calculated as maximal respiration minus routine oxygen consumption.
There are many other SUIT protocols that can be used to assess specific complex-linked respiration in cells that are permeabilized. Cell permeabilization is commonly carried out with the use of a mild detergent such as digitonin or saponin. These agents permeabilize plasma membranes selectively due to their high cholesterol content. Higher doses of these agents will affect the mitochondrial membranes so the correct dose is required to avoid mitochondrial membrane permeabilization.9
Methods
The authors conducted a scientific review of all available literature published over the past 20 years. Our primary objective was to evaluate the measurement of mitochondrial respiration in areas of acute care that includes sepsis, trauma, and poisoning. We initiated a PubMed database search using the MESH terms “mitochondria bioenergetics,” “sepsis,” “cyanide,” “carbon monoxide,” “poisoning,” and “mitochondrial motility.” Articles were selected and agreed upon by the authors based on relevance and impact. Effort was made to include both positive and negative studies where appropriate. Emphasis was placed on well-conducted experimental data, case studies, and controlled trials when possible. Studies were only excluded due to redundancy. After analysis of the available data, this article concludes with recommendations based on the existing scientific evidence.
Mitochondrial Bioenergetics in Sepsis
Interest in the use of biomarkers to diagnose patients with sepsis, identify severity of illness, and assess treatment effectiveness is growing.13 The systemic inflammatory response due to an acute infection can dramatically alter mitochondrial function due to local tissue hypoxia, production of excessive nitric oxide and radical oxygen species, hormonal alterations, and downregulation of mitochondrial gene transcription.14
Mitochondrial dysfunction, specifically alterations in mitochondrial respiration and oxygen metabolism, has also been implicated as a major factor in the decreased response of immune cells in the later stages of sepsis.15 Peripheral blood mononuclear cells (PBMCs), including monocytes and lymphocytes, are the most widely studied cells due to their major role in sepsis. Fortunately, there are well-defined procedures to obtain and isolate PBMCs from whole blood that make the measurement of mitochondrial respiration accessible in both the adult and pediatric population.
The mitochondrial dysfunction observed in sepsis is linked with impairment of electron flow through the ETS and also injury to the complexes.16,17 There is also increased proton conductance through the inner membrane or leak, which may indicate mitochondrial damage. This will result in an alteration of oxidative phosphorylation leading to energy failure and cell death. In PBMCs, a decrease in ATP synthesis is from the reduction in mitochondrial oxygen consumption. One study demonstrated a reduction in the content of the F1F0 ATP synthase complex. In the same study, the reduction in oxygen consumption was related to increased organ failure and hospital mortality.18
Spare respiratory capacity is another critical measure of mitochondrial respiration that can be dramatically affected in the patient with sepsis. The SRC is a measure of mitochondrial reserve available for cells to produce ATP in response to a stress-induced increase in metabolic demand such as sepsis. It is calculated as maximal respiration minus routine respiration. Spare respiratory capacity indicates how close to its bioenergetic limit a cell is operating.19 A decrease in SRC indicates mitochondrial dysfunction that may not be apparent until there is a stress with a demand for ATP. Spare respiratory capacity is thought to represent a cell’s reserve and thus their “fitness.” Cells that are faced with overwhelming metabolic demands may not have the bioenergetic reserve to meet these needs, leading to organ dysfunction.20,21
An alternative theory is that PMBCs can increase maximal respiration in the setting of sepsis compared to healthy individuals.22 The differences in study may be due to differences in methodological procedures. For example, this study cells obtained were resuspended in plasma that may have different substrates for mitochondrial respiration that may explain the differences seen in other studies.
Mitochondrial Bioenergetics in Trauma
Trauma is a leading cause of morbidity and mortality in industrialized nations, and injury-related complaints account for approximately 30% of emergency department visits in the United States each year.21 Hemorrhagic shock results in early mortality after acute injury, however, multiorgan failure (MOF) is the leading cause of postresuscitation death in traumatic injury.23 This process is multifactorial and includes a series of intracellular reactions leading to MOF that is independent of successful initial resuscitation. Central to MOF is the development of mitochondrial bioenergetic failure, resulting in a reduction in ATP production and increased ROS generation and cellular dysfunction.24 In addition to this direct injury, mitochondrial dysfunction is thought to contribute to destruction of immune cells and immunosuppression after trauma.25 In fact, animal studies and clinical observations have demonstrated an association between mitochondrial dysfunction, MOF, and mortality or poor outcomes in hemorrhagic shock.
Increased delivery of oxygen to tissues, though targeted in early resuscitation, may later be harmful in the setting of underlying mitochondrial dysfunction during reperfusion. Additionally, ischemia–reperfusion and sepsis are considered to be leading causes of mortality in surgical intensive care unit (ICU) patients.26 Early detection of mitochondrial dysfunction in hemorrhagic shock may provide an opportunity to identify at-risk patients and improve goal-directed treatment in manner similar to current efforts in the management of sepsis.
Current markers to assess impaired aerobic metabolism such as lactate and base deficit are neither specific nor sensitive for degree of metabolic derangement or efficacy of therapeutic intervention, prompting a need for a more appropriate assay to assess mitochondrial function. Several methods of detecting bioenergetic failure in the setting of hemorrhage have been studied. A noninvasive measure of mitochondrial function, near-infrared spectroscopy as previously demonstrated evidence of mitochondrial injury in human patients with MOF after hemorrhagic shock. This technique, however, has been criticized for being technically challenging and has been limited to the research setting. Investigators are currently utilizing PBMCs in severe hemorrhage to study mitochondrial dysfunction in a manner similar to that used in sepsis with varied success.
In an animal model, Villarroel et al demonstrated significant PBMC mitochondrial dysfunction as measured by basal, maximal, and ATP-linked respiration following hemorrhage. Additionally, impairment in oxygen consumption was observed when healthy control PBMCs were incubated in plasma from animals in hemorrhagic shock. Conversely, PBMCs with mitochondrial impairment showed marked improvement in oxygen consumption after exposure to healthy plasma, suggesting both a reversible process and a role of plasma factors in observed mitochondrial dysfunction.27 Peripheral blood mononuclear cells have also been investigated as a surrogate for individual organ dysfunction in hemorrhagic shock.28 Unfortunately, a uniform and linear relationship between vital organ (kidney, heart, liver) and PBMC mitochondrial response to hemorrhage was not observed. Additionally, PBMC mitochondrial oxygen consumption did not correlate with the degree of shock in this study. Such findings limit applicability of PBMCs as a proxy for tissue-specific mitochondrial dysfunction after severe hemorrhage.
Peripheral blood mononuclear cell mitochondrial function and its relationship to immunosuppression in hemorrhagic shock has also been studied. In an animal model of hemorrhagic shock, PMBCs showed a decrease in stimulated tumor necrosis factor α secretion and elevated interleukin 6 levels, which correlated with a decrease in mitochondrial function. These findings suggest promise for use of PBMCs as a marker for impaired immune function after trauma.27
Mitochondrial Bioenergetics in Toxicology
Acute toxicologic poisoning, such as drug overdose and exposure to environmental toxins, is the leading cause of injury-related fatalities in the United States. It is also the leading cause of cardiac arrest in patients younger than 40 years, now surpassing motor vehicle collisions.29–31 According to published data from the US Centers for Disease Control and Prevention, nationwide poisoning mortality has tripled over the past 30 years. Poisonings are responsible for more than 80 deaths and more than 2000 emergency department visits per day, generating a significant economic burden related to the care of the poisoned patient.32,33 Mitochondrial toxins are implicated in a large proportion of acute poisonings resulting in cardiac arrest and shock, with a conservative estimated 5000 deaths per year and 20 000 injuries in the United States alone.34,35 Important mitochondrial inhibitors of public health concern are cyanide, hydrogen sulfide, and carbon monoxide.36
The mitochondria play a central role in the clinical toxicity of these agents, however, the exact mechanism of injury that occurs is not fully elucidated. Mitochondrial toxicity involves inhibition of the complexes within the ETS with subsequent reduction in ATP production and secondary damage from ROS generation leading to ischemia–reperfusion injuries. The use of available biomarkers and drug concentrations in management of patients exposed to mitochondrial toxins is limited in that they correlate poorly with disease outcome and response to treatment, necessitating a more sensitive measure. The ability to measure mitochondrial respiration in cells obtained from human blood similar to patients with sepsis and traumatic shock offers a potentially superior assay to detect bioenergetic dysfunction, particularly for agents that inhibit the mitochondria. At this time, there are very limited studies that explore the measurement of mitochondrial respiration in acute toxicologic poisoning.
To demonstrate the utility of the measurement of mitochondrial respiration in toxicology, we exposed healthy donor PBMCs from intact whole blood to CN (100 mmol/L). This was approved by the institutional review board of the University of Pennsylvania, and written informed consent was obtained from all participants. Cyanide is a well-known complex IV inhibitor and mitochondrial toxin. Shown in Figure 4 is the change in SRC following CN exposure. The SRC is obtained by the difference of maximal respiration from routine respiration and measures the reserve capacity or total fitness of a cell after a stress response. This illustrates the negative effect CN has on the SRC. A corresponding decrease in ATP-linked respiration was also found (not shown). There is a recent study that demonstrates the measurement of mitochondrial respiration compared to lactate in an ex vivo study of CN exposure to blood cells.37
Figure 4.
The spare respiratory capacity (SRC) in 4 patient samples where PBMCs were isolated using a Ficoll gradient and mitochondrial respiration was obtained at baseline and after 100 mmol/L cyanide was added (Oroboros O2K). This illustrates that cyanide (complex IV inhibitor) decreases SRC. Mean data (n = 4) were plotted with +standard error of the mean. *P < .05. All study protocols for collection and handling of human samples were reviewed and approved by the institutional review board, University of Pennsylvania.
Mitochondrial Dynamics
The use of mitochondrial bioenergetics has been applied to many areas of acute care such as sepsis and most recently in the area of toxicologic poisoning. In addition to energy production, the mitochondria are also dynamic organelles that play an important role in living cells and have the ability to make dynamic changes in response to environmental stresses or mutations.38 Dynamic processes include mitochondrial motility and the process of fusion/fission.39 The mobile mitochondrial subpopulation can move in different directions. Directed long-range transport occurs both away from the nucleus (anterograde) and toward the center of the cell and nucleus (retrograde). Fission (mitochondria divide) is essential for growing and dividing cells to populate them with adequate numbers of mitochondria.40 Fission can also occur when there is a significant mitochondrial damage that will allow the cell to segregate the damaged portion.41 Fusion (mitochondria fuse) is also critical as it can rescue 2 mitochondria with defects by cross-completing one another. Fusion can therefore maximize oxidative capacity in response to toxic stress. Healthy cells often exhibit characterized localization of mitochondria to high-energy areas within the cell. Diseased cells such as those obtained from patients with underlying mitochondrial disorders often exhibit abnormal localization of mitochondria when compared to healthy cells, and the measurable reduced amplitude of mitochondrial movement in such conditions can be used as an index of cell health.
Fluorescent imaging techniques are used intensively to analyze several important functional and morphological characteristics of mitochondria in living cells.42 Imaging approaches permit both spatial and temporal study of mitochondrial morphology and networks, complex patterns of mitochondrial dynamics, as well as various functional defects and are also the most direct means of visualizing the intracellular heterogeneity of mitochondria. Several mitochondria-specific markers specifically targeted at the mitochondria can be used in combination with high-resolution imaging.43 While currently being applied to patients with underlying mitochondrial disorders, the same techniques that have been used to evaluate mitochondrial motion in cells subjected to varying degrees of cytoarchitectural impairment can be leveraged to samples obtained from poisoned patients, in particular, mitochondrial inhibitors such as CN and CO. Our preliminary work suggests that a failure in bioenergetic function as evidenced with specific cell lines decreased mitochondrial respiration from CN results in a decrease in mitochondrial localization and an increase in perinuclear clustering of mitochondria with increased fission events (Figure 5). In the near future, this will provide invaluable information on both the physiology and structure of the mitochondria in patients who are critically ill as this technique is being explored in human blood cells as well.
Figure 5.
Whole cell imaging and respiration graphs demonstrate the changes in both mitochondrial dynamics and bioenergetics with exposure to cyanide (100 mmol/L) in the rhabdomyosarcoma cell line (RD). 5 (A) Normal mitochondrial distribution in control cells obtained with Cell Light 2.0 green fluorescent protein (GFP) and 4′,6-diamidino-2-phenylindole (DAPI) when compared to the cell exposed to cyanide which show abnormal mitochondrial distribution that is perinuclear with decreased motility. B, The corresponding mitochondrial respiration tracing for both control and cyanide exposed cells with a decrease in overall respiration for the RD cells exposed to cyanide.
Discussion
Metabolic biomarkers have potentially wider use in disease diagnosis and prognosis as well as in monitoring disease response to treatment. Although biomarkers such as interleukins, microRNA, and lactate44,45 have been proposed for disease surveillance, there are still conflicting results regarding their clinical utility. Treatment of commonly encountered disease of acute care such as sepsis, trauma, and poisoning often relies on clinical diagnosis and therapy guided by the use of surrogate markers of illness severity. The measurement of mitochondrial function, including respiration and motility, may offer superior alternatives to such markers. Assessing mitochondrial function in a clinical context has the potential to impact the area of acute care in terms of diagnosis, prognosis, and treatment.
At this time, the use of instruments to measure mitochondrial respiration in critically ill patients in the ICU is feasible, with recent studies demonstrating their potential utility.46 To date, human studies support the presence of mitochondrial dysfunction in sepsis in a variety of human cells such as platelets and PBMCs; however, there are several questions that remains. What is the relationship between the mitochondrial dysfunction seen in blood cells and the patient’s clinical status and prognosis? Another question is the timing of when mitochondrial dysfunction occurs. A third question is whether the measurement of mitochondrial respiration in blood cells is a good reflection of organ dysfunction. Future studies can assess the response of measuring mitochondrial respiration in response to treatment. The current technology is readily available in many laboratories that study mitochondrial physiology but requires some knowledge and training to properly utilize. As research in this rapidly expanding area continue to grow, this will encourage more clinicians to pursue this line of translational work with the potential to impact patient care from a wide variety of acute illnesses.
The study of mitochondrial bioenergetics has become critical in understanding the pathophysiology and treatment of complex diseases such as diabetes and cardiovascular disorders.47 A growing body of evidence supports the importance of mitochondrial function in these disease states and provides evidence that mitochondrial dysfunction increases with the progression of disease. The mitochondria are sensitive to physiologic stresses and respond dynamically. For instance, in states of inflammation and oxidative stress, the mitochondria and the respiratory chain complex may be damaged, resulting in compromised mitochondrial bioenergetic function and subsequent worsening of the disease process.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The project described was supported by award K12 HL109009 from the National Heart, Lung, and Blood Institute (David H. Jang) and award N000141612100 from the Office of Naval Research (David M. Eckmann).
Footnotes
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Chance B, Williams GR. Respiratory enzymes in oxidative phosphorylation. I. Kinetics of oxygen utilization. J Biol Chem 1955; 217(1):383–393. [PubMed] [Google Scholar]
- 2.Brown GC. Control of respiration and ATP synthesis in mammalian mitochondria and cells. Biochem J 1992;284(pt 1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brown GC. Nitric oxide regulates mitochondrial respiration and cell functions by inhibiting cytochrome oxidase. FEBS Lett 1995; 369(2–3):136–139. [DOI] [PubMed] [Google Scholar]
- 4.Gabaldón T, Huynen MA. Shaping the mitochondrial proteome. Biochim Biophys Acta 2004;1659(2–3):212–220. [DOI] [PubMed] [Google Scholar]
- 5.Cadenas E, Davies KJ. Mitochondrial free radical generation, oxidative stress, and aging. Free Radical Biol Med 2000;29(3–4):222–230. [DOI] [PubMed] [Google Scholar]
- 6.Turrens JF, Alexandre A, Lehninger AL. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys 1985;237(2):408–414. [DOI] [PubMed] [Google Scholar]
- 7.Wallace DC. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu Rev Genet 2005;39:359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moser CC, Page CC, Farid R, Dutton PL. Biological electron transfer. J Bioenerg Biomembr 1995;27(3):263–274. [DOI] [PubMed] [Google Scholar]
- 9.Pesta D, Gnaiger E. High-resolution respirometry: OXPHOS protocols for human cells and permeabilized fibers from small biopsies of human muscle. Methods Mol Biol 2012; 810:25–58. [DOI] [PubMed] [Google Scholar]
- 10.Krumschnabel G, Eigentler A, Fasching M, Gnaiger E. Use of safranin for the assessment of mitochondrial membrane potential by high-resolution respirometry and fluorometry. Methods Enzymol 2014;542:163–181. [DOI] [PubMed] [Google Scholar]
- 11.Krumschnabel G, Fontana-Ayoub M, Sumbalova Z, et al. Simultaneous high-resolution measurement of mitochondrial respiration and hydrogen peroxide production. Methods Mol Biol. 2015;1264:245–261. [DOI] [PubMed] [Google Scholar]
- 12.Chacko BK, Kramer PA, Ravi S, et al. Methods for defining distinct bioenergetic profiles in platelets, lymphocytes, monocytes, and neutrophils, and the oxidative burst from human blood. Lab Invest 2013;93(6):690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singer M, Deutschman CS, Seymour CW, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA 2016;315(8):801–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carré JE, Orban JC, Re L, et al. Survival in critical illness is associated with early activation of mitochondrial biogenesis. Am J Respir Crit Care Med 2010;182(6):745–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brealey D, Brand M, Hargreaves I, et al. Association between mitochondrial dysfunction and severity and outcome of septic shock. Lancet 2002;360(9328):219–223. [DOI] [PubMed] [Google Scholar]
- 16.Callahan LA, Supinski GS.Sepsis induces diaphragm electron transport chain dysfunction and protein depletion. Am J Respir Crit Care Med 2005;172(7):861–868. [DOI] [PubMed] [Google Scholar]
- 17.d’Avila JC, Santiago AP, Amâncio RT, et al. Sepsis induces brain mitochondrial dysfunction. Crit Care Med 2008;36(6): 1925–1932. [DOI] [PubMed] [Google Scholar]
- 18.Japiassú AM, Santiago AP, d’Avila JC, et al. Bioenergetic failure of human peripheral blood monocytes in patients with septic shock is mediated by reduced F1Fo adenosine-5’-triphosphate synthase activity. Crit Care Med 2011;39(5):1056–1063. [DOI] [PubMed] [Google Scholar]
- 19.Brand MD, Nicholls DG. Assessing mitochondrial dysfunction in cells. Biochem J 2011;435(2):297–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weiss SL, Selak MA, Tuluc F, et al. Mitochondrial dysfunction in peripheral blood mononuclear cells in pediatric septic shock. Pediatr Crit Care Med 2015;16(1):e4–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Web site. Emergency Department Visits. http://www.cdc.gov/nchs/fastats/emergency-department.htm. Accessed September 28, 2010.
- 22.Sjövall F, Morota S, Persson J, et al. Patients with sepsis exhibit increased mitochondrial respiratory capacity in peripheral blood immune cells. Crit Care 2013;17(4):R152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Minei JP, Cuschieri J, Sperry J, et al. The changing pattern and implication of multiple organ failure after blunt injury with hemorrhagic shock. Crit Care Med 2012;40(4): 1129–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cairns CB, Moore FA, Haenel JB, et al. Evidence for early supply independent mitochondrial dysfunction in patients developing multiple organ failure after trauma. J Trauma 1997;42(3): 532–536. [DOI] [PubMed] [Google Scholar]
- 25.Kozlov AV, Bahrami S, Calzia E, et al. Mitochondrial dysfunction and biogenesis: do ICU patients die from mitochondrial failure? Ann Intensive Care 2011;1(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tschoeke SK, Ertel W. Immunoparalysis after multiple trauma. Injury 2007;38(12):1346–1357. [DOI] [PubMed] [Google Scholar]
- 27.Villarroel JP, Guan Y, Werlin E, Selak MA, Becker LB, Sims CA. Hemorrhagic shock and resuscitation are associated with peripheral blood mononuclear cell mitochondrial dysfunction and immunosuppression. J Trauma Acute Care Surg 2013;75(1): 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karamercan MA, Weiss SL, Villarroel JP, et al. Can peripheral blood mononuclear cells be used as a proxy for mitochondrial dysfunction in vital organs during hemorrhagic shock and resuscitation? Shock 2013;40(6):476–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paulozzi L, Crosby A, Ryan G. Increases in age- group-specific injury mortality–United States, 1999–2004. MMWR Morb Mortal Wkly Rep 2007;56(49):1281–1284. [PubMed] [Google Scholar]
- 30.Warner M, Chen LH, Makuc DM, Anderson RN, Minino AM. Drug Poisoning Deaths in the United States, 1980–2008. National Center for Health Statistics Data Brief, December 2011. http://www.cdc.gov/nchs/products/databriefs/db81.htm. Updated March 1, 2016. Accessed December 1, 2014. [PubMed] [Google Scholar]
- 31.Rockett IR, Regier MD, Kapusta ND, et al. Leading causes of unintentional and intentional injury mortality: United States, 2000–2009. Am J Public Health 2012;102(11):e84–e92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McCaig LF, Burt CW. Poisoning-related visits to emergency departments in the United States, 1993–1996. J Toxicol Clin Toxicol 1999;37(7):817–826. [DOI] [PubMed] [Google Scholar]
- 33.Centers for Disease Control and Prevention. Web-based Injury Statistics Query and Reporting System (WISQARS) [Online]. National Center for Injury Prevention and Control, Centers for Disease Control and Prevention (producer). Web site www.cdc.gov/ncipc/wisqars. 2003, Updated March 1, 2016. Accessed December 1, 2014. [Google Scholar]
- 34.Mowry JB, Spyker DA, Cantilena LR Jr, Bailey JE, Ford M. 2012 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 30th Annual Report. Clin Toxicol (Phila) 2013;51(10):949–1229. [DOI] [PubMed] [Google Scholar]
- 35.Mowry JB, Spyker DA, Cantilena LR Jr, McMillan N, Ford M. 2013 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 31st Annual Report. Clin Toxicol (Phila) 2014;52(10):949–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grabowska T, Skowronek R, Nowicka J, et al. Prevalence of hydrogen cyanide and carboxyhaemoglobin in victims of smoke inhalation during enclosed-space fires: a combined toxicological risk. Clin Toxicol (Phila) 2012;50(8):759–763. [DOI] [PubMed] [Google Scholar]
- 37.Jang DH, Shofer FS, Weiss SL, et al. Impairment of mitochondrial respiration following ex-vivo cyanide exposure in peripheral blood mononuclear cells. Clin Tox 2016;54(4):303–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giedt RJ, Pfeiffer DR, Matzavinos A, Kao CY, Alevriadou BR. Mitochondrial dynamics and motility inside living vascular endothelial cells: role of bioenergetics. Ann Biomed Eng 2012; 40(9):1903–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Benard G, Bellance N, James D, et al. Mitochondrial bioenergetics and structural network organization. J Cell Sci 2007; 120(pt 5):838–848. [DOI] [PubMed] [Google Scholar]
- 40.Frederick RL, Shaw JM. Moving mitochondria: establishing distribution of an essential organelle. Traffic 2007;8(12): 1668–1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Giedt RJ, Yang C, Zweier JL, Matzavinos A, Alevriadou BR. Mitochondrial fission in endothelial cells after simulated ischemia/reperfusion: role of nitric oxide and reactive oxygen species. Free Radic Biol Med 2012;52(2):348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song W, Bossy B, Martin OJ, et al. Assessing mitochondrial morphology and dynamics using fluorescence wide-field microscopy and 3D image processing. Methods 2008;46(4): 295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kandel J, Chou P, Eckmann DM, et al. Automated detection of whole-cell mitochondrial motility and its dependence on cytoarchitectural integrity. Biotechnol Bioeng 2015;112(7): 1395–1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikkelsen ME, Miltiades AN, Gaieski DF, et al. Serum lactate is associated with mortality in severe sepsis independent of organ failure and shock. Crit Care Med 2009;37(5):1670–1677. [DOI] [PubMed] [Google Scholar]
- 45.How CK, Hou SK, Shih HC, et al. Expression profile of microRNAs in gram-negative bacterial sepsis. Shock 2015;43(2):121–127. [DOI] [PubMed] [Google Scholar]
- 46.Puskarich MA, Kline JA, Watts JA, Shirey K, Hosler J, Jones AE. Early alterations in platelet mitochondrial function are associated with survival and organ failure in patients with septic shock. J Crit Care 2016;31(1):63–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wallace DC. Mitochondrial energetics and therapeutics. Annu Rev Pathol 2010;5:297–348. [DOI] [PMC free article] [PubMed] [Google Scholar]