Abstract
Variants in the gene encoding the triggering receptor expressed on myeloid cells 2 (TREM2) are known to increase the risk of developing Alzheimer disease and Parkinson’s disease (PD). However, the potential role of TREM2 effect on synucleinopathy has not been characterized. In this study, we investigated whether loss of TREM2 function affects α-synucleinopathy both in vitro and in vivo. In vitro, BV2 microglial cells were exposed to α-synuclein (α-syn) in the presence or absence of TREM2 small interference RNA. For in vivo studies, wild-type controls and TREM2 gene knockout mice were intracranially injected in the substantia nigra with adeno-associated viral vectors expressing human α-syn (AAV-SYN) to induce PD. Our results revealed that knockdown of TREM2 aggravated α-syn–induced inflammatory responses in BV2 cells and caused greater apoptosis in SH-SY5Y cells treated with BV2-conditioned medium. In mice, TREM2 knockout exacerbated dopaminergic neuron loss in response to AAV-SYN. Moreover, both in vitro and in vivo TREM2 deficiency induced a shift from an anti-inflammatory toward a proinflammatory activation status of microglia. These data suggest that impairing microglial TREM2 signaling aggravates proinflammatory responses to α-syn and exacerbates α-syn–induced neurodegeneration by modulating microglial activation state.—Guo, Y., Wei, X., Yan, H., Qin, Y., Yan, S., Liu, J., Zhao, Y., Jiang, F., Lou, H. TREM2 deficiency aggravates α-synuclein–induced neurodegeneration and neuroinflammation in Parkinson’s disease models.
Keywords: microglia, PD, α-syn, inflammation
Parkinson’s disease (PD) is the most common neurodegenerative movement disorder, which is characterized by progressive loss of dopamine-producing neurons of the substantia nigra pars compacta (SNpc) in association with intracellular aggregates of α-synuclein (α-syn) (1). A growing body of evidence suggests that neuroinflammation mediated by microglia, the resident immune cells of the brain, plays a key role in PD pathogenesis (2). However, studies over the past 2 decades have reported both beneficial and detrimental effects of microglia in PD (3–5). Thus, the role of microglia and inflammation in PD onset and progression remains poorly understood.
Triggering receptor expressed on myeloid cells 2 (TREM2) is a V-type Ig domain–containing transmembrane protein that is expressed in mononuclear phagocytes including microglia, osteoclasts, and macrophages (6). TREM2 mutation has been identified as a risk factor for several neurodegenerative diseases, including Nasu-Hakola disease (7, 8), Alzheimer disease (9, 10), and PD (11–13). TREM2 is specifically expressed on microglia in the brain and has been shown to impact microglial activation, inflammation, phagocytosis, proliferation, and survival (14–17). However, the specific molecular effects of TREM2 are still being investigated, but emerging data suggest that TREM2 plays a role in microglia-mediated inflammation (18–21).
Several studies have attempted to investigate the effect of TREM2 on PD pathology. Belloli et al. (22) reported that lack of TREM2 did not modify the loss of dopaminergic neurons following acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) toxicity, but lowered MPTP proinflammatory response, indicating TREM2 may promote activation of microglia and subsequent neuroinflammation in the PD brain. In contrast, our recent study found that overexpression of TREM2 attenuates neurodegeneration and neuroinflammation in MPTP-induced mice model of PD (23). Hence, the role of TREM2 in PD pathogenesis is still controversial. In order to clarify the exact role of TREM2 in PD pathology, it is necessary to determine its effects in an animal model that better replicates human PD pathology with synucleinopathy. Therefore, in this study, we aimed to investigate whether TREM2 affected synucleinopathy and related neuroinflammation and to observe if it also modulated microglial activation state in PD models. We used an established synucleinopathy model of stereotaxic delivering pseudotype 9 recombinant adeno-associated vector to ventral midbrain. This model nicely replicates α-syn aggregates and dystrophic Lewy neuritis (24). Using this model, we observed accelerated α-syn–induced neurodegeneration together with increased microglial activation in mice lacking TREM2. These data suggest that impairing microglial TREM2 signaling may exacerbate α-syn–induced neurodegeneration by modulating microglial activation state.
MATERIALS AND METHODS
Generation of TREM2−/− mice
TREM2+/− mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA) (stock no. 027197). Male and female TREM2+/− mice were crossed to generate TREM2−/− mice. The primers used for genotyping were as follows: forward 5′-AAGCAAGTGGCTGTCTCCTC-3′ and reverse 5′-GTGTACTCACCCTCCAGCA-3′. Genotyping was performed by PCR at 4 wk of age. The expected PCR results were as follows: TREM2−/− (mutant) = 212 bp, TREM2+/− (heterozygote) = 212 and 387 bp, TREM2+/+ [wild type (WT)] = 387 bp (Supplemental Fig. S1).
Adeno-associated viral vectors expressing human α-syn PD model
Male TREM2−/− mice and their WT littermates (8–12 wk) were used for the experiments. Mice were maintained in a pathogen free facility and exposed to a 12-h light-dark cycle with food and water provided ad libitum. Recombinant Adeno-associated viral vectors expressing human α-syn (AAV-SYN; AAV2/9-SYN) and AAV2/9-green fluorescent protein (GFP) were obtained from Hanbio Technology (Shanghai, China). Mice were deeply anesthetized with sodium pentobarbital and unilaterally injected with 2 μl of AAV2/9-GFP or AAV2/9-SYN (1 × 1012 TU/ml diluted in sterile PBS) into the right substantia nigra (SN). Coordinates were anterior-posterior 3.0 mm from bregma, mediolateral 1.2 mm from midline, and dorsoventral 4.3 mm from dura, as described with some modification (25). All research conducted on animals were approved by the Institutional Animal Care and Use Committee at Shandong University.
α-Syn fibrils preparation
Purified human WT recombinant α-syn was purchased from rPeptide (Watkinsville, GA, USA) and dissolved in sterile distilled water to create a stock solution (1 mg/ml). Stock solutions of α-syn were incubated with constant agitation at 37°C for 7 d. This process allowed α-syn to form fibrils (26), which were used in this study.
Cell culture and treatment
The immortalized murine microglial cell line BV2 was maintained at 37°C in a 5% CO2 humidified incubator in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum and 100 U/ml penicillin and streptomycin (Thermo Fisher Scientific). Before each experiment, the medium of BV2 cells was replaced by serum-free DMEM, and cells were then treated with 500 nM recombinant human α-syn (prepared as described above) at the indicated time points. For the induction of M2 phenotype, BV2 cells were treated with recombinant IL-4 (20 ng/ml; Peprotech, Rocky Hill, NJ, USA) for 24 h. Human neuroblastoma SH-SY5Y cells were maintained in DMEM-F12 supplemented with 10% fetal bovine serum.
Transfection of BV2 cells with TREM2 small interference RNA or TREM2 adenoviral vectors
TREM2 small interference RNA (siRNA) (MilliporeSigma, Burlington, MA, USA) was used for knockdown in BV2 cells. TREM2 siRNA consisted of the following sequence: GCGUUCUCCUGAGCAAGUUdTdT, AACUUGCUCAGGAGAACGCdTdT; whereas control (scramble) siRNA consisted of the following sequence: UUCUCCGAACGUGUCACGUTT, ACGUGACACGUUCGGAGAATT. TREM2 cells were transfected for 48 h with either TREM2 siRNA or scramble siRNA. For overexpression of TREM2, cells were transfected for 48 h with either adenoviral particles encoding TREM2 (GeneChem Biotech, Shanghai, China) or the control vectors. Adenoviral particles encoding TREM2was tagged with a flag in the C-terminal. The efficiencies of knockdown and overexpression of TREM2 were confirmed by RT-PCR and Western blot.
Real-time RT-PCR
Total mRNA was isolated from BV2 cells or mice brain tissues using Trizol reagent (Roche, Basel, Switzerland). The mRNA levels were analyzed by real-time RT-PCR using a Bio-Rad iCycler system (Bio-Rad, Hercules, CA, USA). The sequences of the specific primers for target genes are listed below. TREM2: forward (5′-CTGGAACCGTCACCATCACTC-3′) and reverse (5′-CGAAACTCGATGACTCCTCGG-3′); TNF-α: forward (5′-CAGGCGGTGCCTATGTCTC-3′) and reverse (5′-CGATCACCCCGAAGTTCAGTAG-3′); IL-1β: forward (5′-GAAATGCCACCTTTTGACAGTG-3′) and reverse (5′-TGGATGCTCTCATCAGGACAG-3′); iNOS: forward (5′-ACATCGACCCGTCCACAGTAT-3′) and reverse (5′-CAGAGGGGTAGGCTTGTCTC-3′); cyclooxygenase-2 (COX2): forward (5′-TGCACTATGGTTACAAAAGCTGG-3′) and reverse (5′-TCAGGAAGCTCCTTATTTCCCTT-3′), arginase 1 (ARG1): forward (5′-TCACCTGAGCTTTGATG TCG-3′) and reverse (5′-CTGAAAGGAGCCCTGTCTTG-3′); YM1: forward (5′-CAGGGTAATGAGTGGGTTGG-3′) and reverse (5′-CACGGCACCTCCTAAATTGT-3′); glyceraldehyde 3-phosphate dehydrogenase (GAPDH): forward (5′-GAGTCCACTGGCGTCTTCAC-3′) and reverse (5′-TGAGTCCTTCCACGATACCAA-3′). The data of real-time PCR were analyzed using the value 2−ΔΔCt. GAPDH was used as the internal control.
Conditioned medium preparation, measurement of cell viability, and cell apoptosis in SH-SY5Y cells
BV2 microglial cells were transfected with either scramble siRNA or TREM2 siRNA for 48 h. Cells were then treated with 500 nM α-syn for 4 h. The supernatants were collected as the conditioned medium (CM) and were filtered through 0.45 μm filters. The microglial CM was added to SH-SY5Y cells, and the 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay (for viability) and annexin V-FITC/propidium iodide (PI) double staining assay (for cell apoptosis) were performed 24 h later. For MTT assay, SH-SY5Y cells were incubated with 5 mg/ml of MTT solution at 37°C for 4 h. The medium was then carefully removed, the formazan crystals that had formed after cell exposure to MTT were dissolved in 150 μl of DMSO, and the absorbance was measured at 570 nm on a plate reader (Varioskan Flash; Thermo Fisher Scientific, Waltham, MA, USA). Alternatively, cell apoptosis was detected with an Annexin V-FITC/PI Double Staining Kit (BD Biosciences, San Jose, CA, USA) according to the manufacturer’s instruction. Briefly, cells were treated with CM collected from α-syn–treated BV2 cell cultures for 24 h, and then 1 × 106 cells were harvested, washed twice with ice-cold PBS, and evaluated for apoptosis by double staining with annexin V-FITC and PI in binding buffer using a Cytoflex Flow Cytometer (Beckman Coulter Life Sciences, Indianapolis, IN, USA).
Western blot analysis
Protein extracts were prepared from BV2 cells or brain tissues by homogenization in RIPA buffer (150 mM NaCl, 0.5% sodium deoxycholate, 0.1% SDS, 1% NP40, 1 mM EDTA, and 50 mM Tris pH 8.0), containing protease inhibitors and Halt phosphatase inhibitor mixture (Thermo Fisher Scientific). Samples were sonicated for 5 s and particulates were eliminated by centrifugation at 14,000 g for 10 min at 4°C. Protein concentration was determined by bicinchonininc acid method. Samples were separated on SDS-PAGE and transferred to nitrocellulose membranes. Immunoblots were incubated with primary antibody in 5% milk. The primary antibodies used in this study included the following: TREM2 (Ab 95470; Abcam, Cambridge, MA, USA), ARG1 (sc-271430; Santa Cruz Biotechnology, Dallas, TX, USA), phosphorylated (p)-STAT1 (9167; Cell Signaling Technology, Danvers, MA, USA), total STAT1 (9172; Cell Signaling Technology), p-STAT6 (BS187; Bioworld Technology, St. Louis Park, MN, USA), total STAT6 (9362; Cell Signaling Technology), COX2 (BS1076; Bioworld Technology), tyrosine hydroxylase (TH) (MAB318; MilliporeSigma), iNOS (N7782; MilliporeSigma) and β-actin (A2228; MilliporeSigma). Densitometry analysis was performed using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Histology and immunofluorescence
Animals were anesthetized with sodium pentobarbital, transcardially perfused with 0.9% normal saline, followed by 4% paraformaldehyde in 0.1 M PBS (pH 7.4). Brains were dissected, postfixed in 4% paraformaldehyde for 24 h, and cryopreserved in 30% sucrose for 48 h. Frozen brains were coronally sectioned at 20 μm on a cryomicrotome and sections were mounted on slides. Using an antibody against TH, dopaminergic neurons were identified with biotinylated secondary antibody and streptavidin ABC solution (Vector Laboratories, Burlingame, CA, USA). Immunostaining was visualized after diaminobenzidine staining (Vector Laboratories) using bright field microscopy (Olympus, Tokyo, Japan).
For immunofluorescence staining, sections were incubated with appropriate combinations of the following antibodies: mouse anti-TH (MAB318; MilliporeSigma), mouse anti-glial fibrillary acidic protein (GFAP; MAB360; MilliporeSigma), and rabbit anti- ionized calcium binding adaptor molecule 1 (Iba1; 109041-AP; Proteintech, Rosemont, IL, USA) at 4°C overnight, followed by incubation with Alexa Fluor-488 or Alexa Fluor-546–conjugated secondary antibody (Invitrogen, Carlsbad, CA, USA) at room temperature for 2 h.
For TH neuron quantification, we used unbiased stereology; the total number of TH+ cells in the SNpc was counted using the optical fractionator probe (MBS Bioscience, Williston, VT, USA) by investigators blinded to the experimental design. Every fifth section, covering the entire extent of these regions, was included in the procedure. The data were expressed as a percentage of the corresponding area from the noninjected, intact side. Immunoreactivity for GFAP and IBA1 were measured using ImageJ software. The mean values were obtained by averaging the counts from 3 coronal sections for each animal.
Statistical analysis
Statistical analysis and graphs were generated with Prism 6.0 software (GraphPad Software, La Jolla, CA, USA). Statistical significance between multiple groups was examined by 1-way ANOVA followed by Tukey post hoc testing. All data are expressed as means ± sem, with P < 0.05 considered statistically significant.
RESULTS
TREM2 expression is dynamically regulated by various PD stimuli
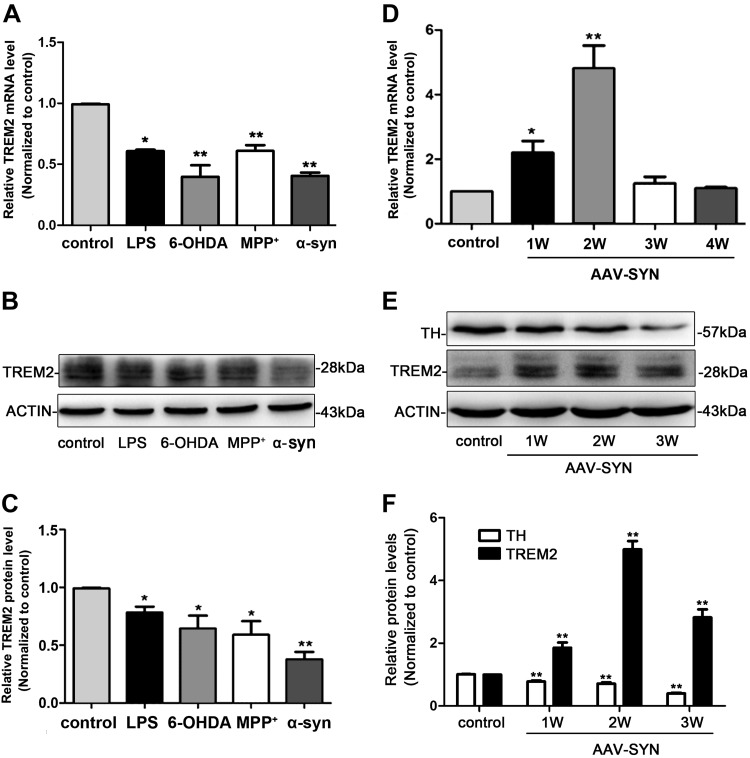
We first assessed the expression pattern of TREM2 in BV2 cells. Our results revealed that both the mRNA and protein levels of TREM2 in BV2 cells were down-regulated following exposure to LPS (100 ng/ml), 1-methyl-4-phenylpyridinium (MPP+) (50 μM), 6-hydroxydopamine (6-OHDA) (75 μM), and α-syn (500 nM) (Fig. 1A–C). We next observed the expression of TREM2 in vivo in the AAV-SYN PD mouse model. In contrast to the observations in vitro, TREM2 mRNA level was up-regulated in the SN of AAV-SYN PD mice model (Fig. 1D). Consistent with the mRNA expression, TREM2 protein levels showed a similar pattern (Fig. 1E, F). In addition, we characterized the expression profile of TREM2 in a 6-OHDA PD mice model and found that TREM2 showed similar expression pattern as those observed in AAV-SYN mice (Supplemental Fig. S2). Taken together, the results demonstrated that in vitro, various PD inflammatory stimuli decreased TREM2 expression, whereas in in vivo PD animal models, inflammatory stimuli increased TREM2 expression. Our results were consistent with previous reports. Some studies showed that in vitro, application of classically proinflammatory molecules (TNF-α, IL-1β, reactive oxygen species, IFN-γ, TLR agonists, including LPS) decreased TREM2 expression (27–30), whereas others showed that in vivo, inflammation and different disease states consistently increased TREM2 expression (12, 31–33).
Figure 1.
The expression pattern of TREM2 in BV2 microglial cells and AAV-SYN–induced PD mice models. A) BV2 microglial cells were exposed to LPS (100 ng/ml), MPP+ (50 μM), 6-OHDA (75 μM), and α-syn (500 nM) for 24 h; the mRNA level of TREM2 was analyzed with real-time RT-PCR. B, C) Representative immunoblot documents and summarized data showing the protein level of TREM2 in BV2 microglial cells after being exposed to LPS (100 ng/ml), MPP+ (50 μM), 6-OHDA (75 μM), and α-syn (500 nM) for 24 h. D) Real-time RT-PCR analysis of TREM2 mRNA level in the SN at different time points after AAV-SYN injection in mice. E, F) Representative immunoblot documents and summarized data showing the protein levels of TREM2 and TH in the striatum at different time points after AVV-SYN injection in mice. All data are presented as the mean ± sem of triplicate independent experiments. *P < 0.05, **P < 0.01 vs. control.
TREM2 deletion aggravates AAV-SYN–associated neurodegeneration in vivo
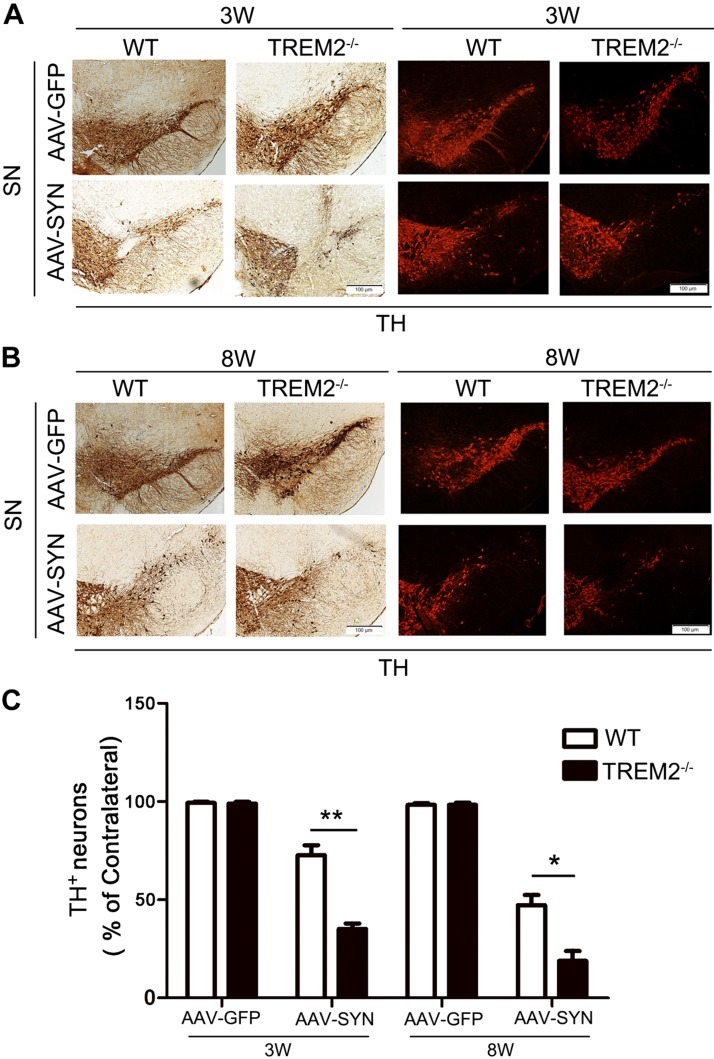
To investigate the role of TREM2 on neurodegeneration, WT and TREM2−/− mice were injected with AAV-GFP and AAV-SYN. The efficiency of GFP and α-syn transduction upon adeno-associated virus vector delivery in mice SN was shown in Supplemental Fig. S3. At 3 and 8 wk after virus transduction, brains were collected and stained for TH. TH+ neurons were counted using unbiased stereology to assess α-syn–induced neurodegeneration (Fig. 2A, B). We observed that TH+ neurons were partially lost at 3 wk after AAV-SYN transduction. For WT mice, we noted ∼28% loss of TH+ neurons that progressed through 8 wk to ∼53% loss of TH+ neurons. More importantly, TH+ neuron loss was much greater in TREM2−/− mice, where we observed a more profound loss of TH+ neurons in AAV-SYN–injected animals (∼65% loss at 3 wk, and ∼81% loss at 8 wk) (Fig. 2C). These data suggest that TREM2−/− mice are more prone to AAV-SYN–induced neurodegeneration than their WT littermates.
Figure 2.
TREM2 knockout aggravates AAV-SYN–associated neurodegeneration in vivo. AAV-GFP and AAV-SYN were unilaterally injected in the SN of WT and TREM2−/− mice. Brains were collected at 3 and 8 wk after virus injection; immunohistochemical and immunofluorescent staining for TH were performed in the SN. A, B) Representative image of TH immunoreactive neurons in the injected SN at 3 and 8 wk after virus injection. C) Quantification of TH+ neurons in the SN of WT and TREM2−/− mice using unbiased stereology at 3 and 8 wk after virus injection. Data are presented as means ± sem, n = 4–6 mice/group. *P < 0.05, **P < 0.01.
TREM2 deletion worsens AAV-SYN–induced microgliosis and astrogliosis in PD animal model
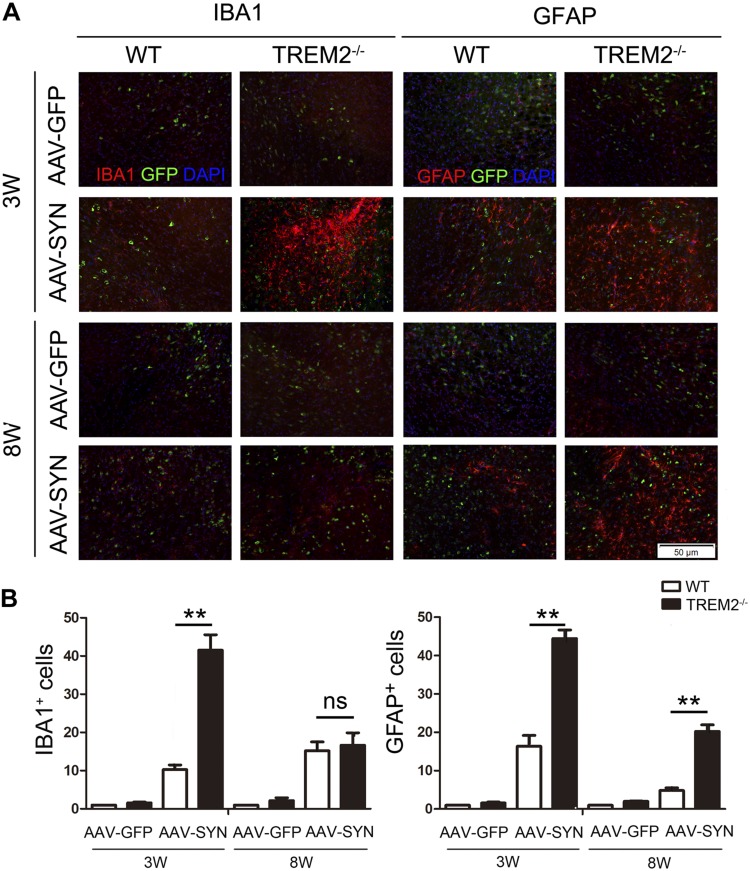
A distinctive hallmark of PD is the presence of low-grade chronic inflammation characterized by microgliosis and astrocytosis at the basal ganglia. Therefore, we next evaluated if TREM2 might modulate gliosis triggered by α-syn overexpression in vivo. To this end, IBA1 immunostaining showed that α-syn overexpression significantly increased microgliosis in the ipsilaterally lesioned side of the WT and TREM2−/− mice 3 wk after injection and returned to basal levels by 8 wk. Importantly, this α-syn–induced microgliosis was more significant in TREM2−/− mice compared to that in WT mice. GFAP immunofluorescence also confirmed significantly more astrogliosis in the ipsilateral ventral midbrain of TREM2−/− mice compared to WT mice at 3 and 8 wk after virus injection (Fig. 3). Taken together, these data suggest that TREM2 deletion accelerates dopaminergic neurodegeneration in the AAV-SYN PD mice models by increasing microgliosis and astrogliosis.
Figure 3.
TREM2 knockout exacerbates AAV-SYN–induced microgliosis and astrogliosis in vivo. AAV-GFP and AAV-SYN were unilaterally injected in the SN of WT and TREM2−/− mice. Mice were euthanized at 3 and 8 wk after virus injection. A) Immunofluorescence for IBA1-positive microglia and GFAP-positive astrocytes (red) in ipsilateral side of the SN. B) Quantification of immunofluorescence data showed that TREM2 knockout exacerbated AAV-SYN–induced microgliosis and astrogliosis. ns, no significance. Data are presented as means ± sem, n = 6 mice/group. **P < 0.01.
TREM2 knockout induces a shift from anti- to proinflammatory microglial activation state in AAV-SYN PD mice
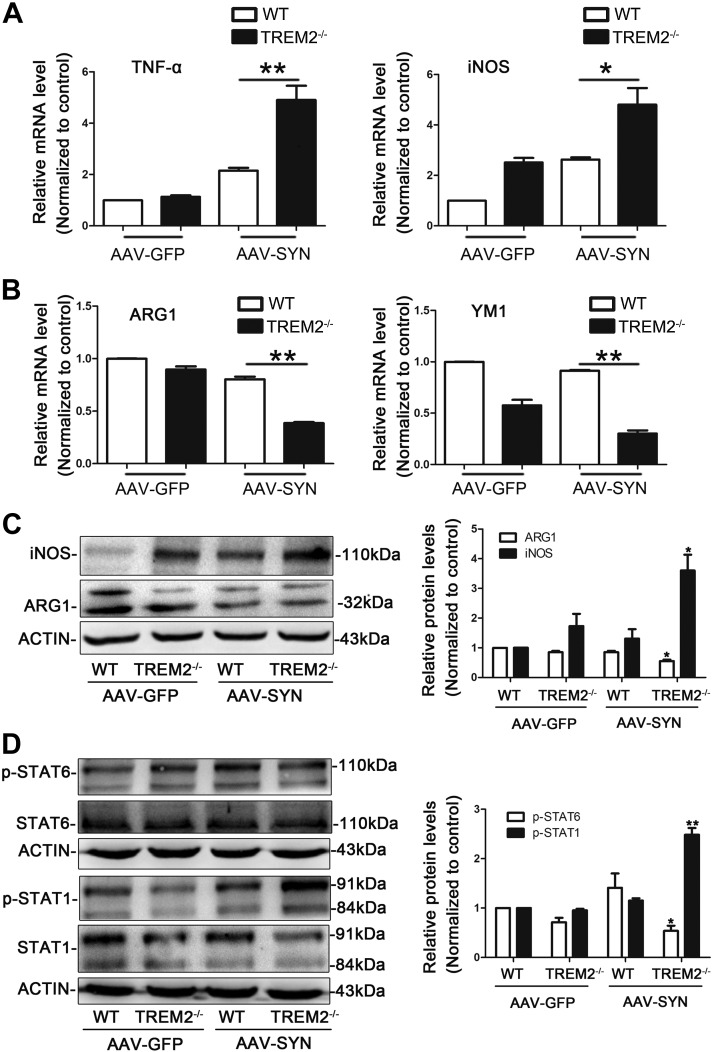
Microglial activation state can be distinguished by the expression of characteristic surface markers. By comparing the mRNA levels of these markers between WT and TREM2−/− mice, we showed that the mRNA levels of the proinflammatory M1 markers (TNF-α and iNOS) were significantly increased in the SN of TREM2−/− mice compared to WT mice following AAV-SYN transduction (Fig. 4A), whereas the levels of M2 markers (ARG1 and YM1) were significantly decreased (Fig. 4B). Western blot analysis of the ipsilateral SN also showed that TREM2 deletion increased the protein expression of iNOS while decreasing the expression of ARG1 (Fig. 4C). These findings suggest that TREM2 knockout promoted the switch of microglial phenotype from anti-inflammatory M2 to proinflammatory M1 in the pathogenesis of PD.
Figure 4.
TREM2 knockout induces a shift from anti- to proinflammatory microglial activation state in AAV-SYN PD mice. AAV-GFP and AAV-SYN were unilaterally injected in the SN of WT and TREM2−/− mice. Mice were euthanized at 3 wk after virus injection. A, B) The expression of the proinflammatory marker TNF-α, iNOS (A), and anti-inflammatory marker ARG1, YM1 (B) in the ipsilateral SN were detected by real-time RT-PCR. *P < 0.05, **P < 0.01. C) Representative immunoblot and quantitative analysis of iNOS and ARG1 protein levels in the ipsilateral SN of WT and TREM2−/− mice. D) Representative immunoblot and quantitative analysis of p-STAT1 and p-STAT6 protein levels in the ipsilateral SN of WT and TREM2−/− mice. Data are presented as means ± sem, n = 4 animals/group. *P < 0.05, **P < 0.01 vs. WT + AAV-SYN group.
It was reported that many members of the STAT family, including STAT1 and STAT6, are involved in phenotypic switching of microglia and macrophages (34). For example, STAT1 increases M1 polarization (35), and STAT6 is positively related to M2 marker expression (36). Therefore, we next measured if TREM2 affected the expression of both proteins in the SN of WT and TREM2−/− mice. We found that TREM2−/− mice had a marked increase in phosphorylated STAT1 and a decrease in phosphorylated STAT6 compared with WT mice after AAV-SYN transduction (Fig. 4D). The results suggest that TREM2 deficiency appears to induce a shift from anti- to proinflammatory microglial activation state, possibly via regulating STAT1 and STAT6 phosphorylation.
TREM2 suppresses α-syn–induced proinflammatory responses in BV2 microglial cells
In order to examine the role of TREM2 specifically in the responses of microglia to fibrillary α-syn, we used α-syn fibrils made from recombinant WT human α-syn as activators of microglial cells in vitro. Two hours after adding α-syn fibrils (500 nM), BV2 microglial cells appeared to be activated and increased expression of inflammatory markers, as measured by RT-PCR and Western blot. The inflammatory responses peaked 4 h after adding α-syn fibrils (Supplemental Fig. S4). Therefore, we chose this time point to observe the effects of TREM2 on α-syn–induced inflammatory responses in the following experiment.
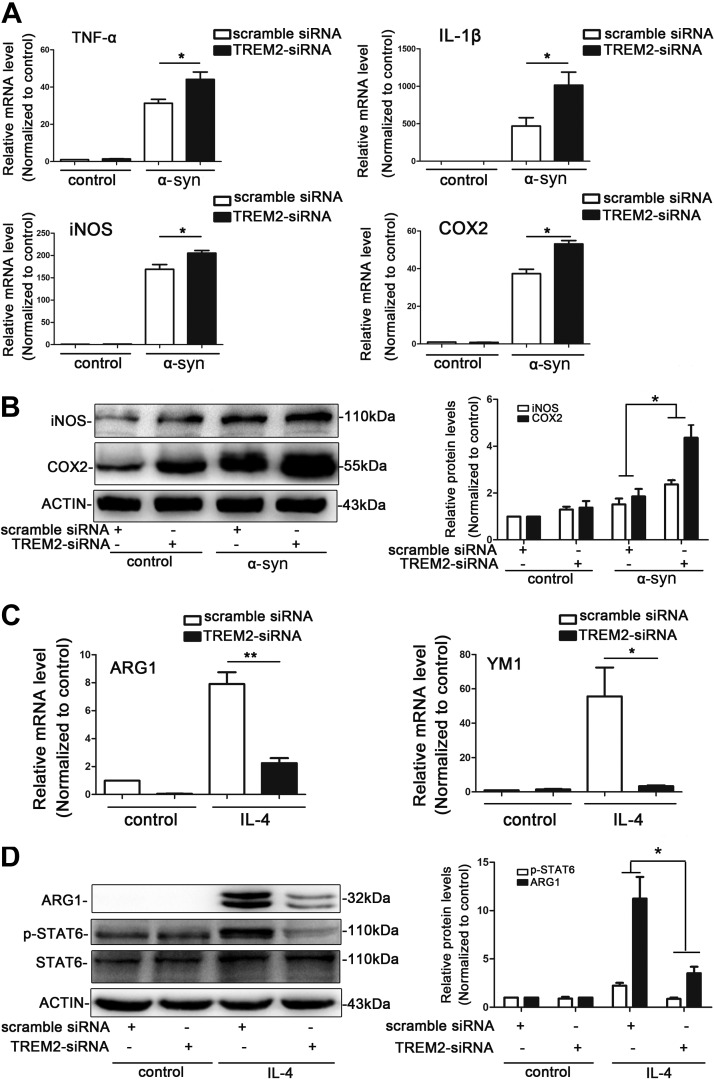
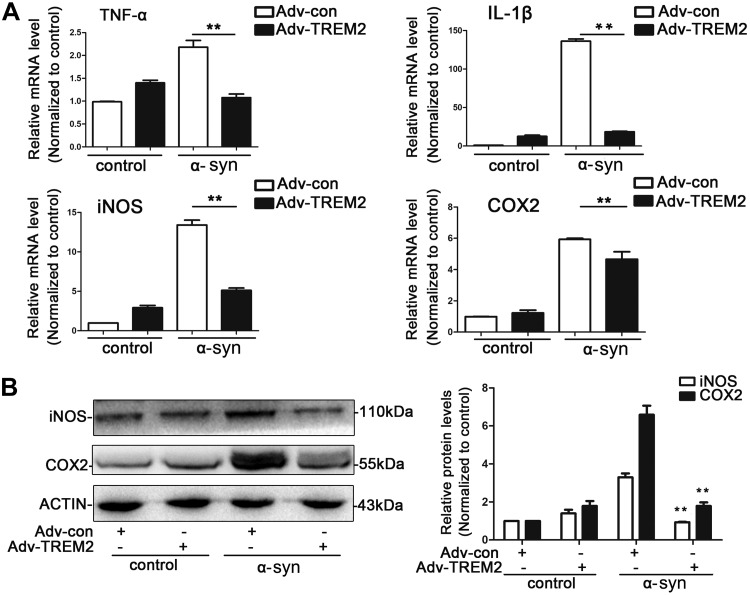
To further assess the effects of TREM2 on microglial activation state, we manipulated the expression of TREM2 in BV2 cells by either knockdown of TREM2 with specific siRNA or overexpression of TREM2 via adenoviral vectors. The efficiencies of knockdown and overexpression were verified by real-time RT-PCR and Western blot analysis [TREM2 siRNA can knock down ∼75% of TREM2 mRNA and protein expression compared with scramble siRNA (Supplemental Fig. S5)]. As can be seen in Fig. 5A, mRNA expression of several proinflammatory M1 marker genes, including TNF-α, IL-1β, iNOS, and COX2, were significantly increased in TREM2 knockdown cells as compared to control following α-syn exposure. Consistently, Western blots revealed significant more iNOS and COX2 expression after TREM2 knockdown compared to control cells treated with scrambled siRNA following α-syn exposure (Fig. 5B). Conversely, TREM2 overexpression remarkably inhibited α-syn–induced proinflammatory responses in BV2 cells (Fig. 6). These data suggest that TREM2 may induce a phenotype shift in microglial cells away from the M1 type at least in vitro.
Figure 5.
TREM2 deficiency leads to enhanced proinflammatory gene expression in BV2 microglial cells. A, B) TREM2 knockdown increased the expression of proinflammatory markers. Relative mRNA levels of TNF-α, IL-1β, iNOS, and COX2 were detected by real-time RT-PCR in control and TREM2 siRNA-treated BV2 cells after exposure to α-syn for 4 h (A). Representative immunoblot documents and summarized data showing the protein levels of iNOS and COX2 in control and TREM2 siRNA-treated BV2 cells after exposure to α-syn for 4 h (B). C, D) TREM2 knockdown decreased the expression of related anti-inflammatory markers in BV2 cells. Relative mRNA levels of ARG1 and YM1 were detected by real-time RT-PCR in control and TREM2 siRNA-treated BV2 cells after exposure to IL-4 for 24 h (C). The protein levels of ARG1 and p-STAT6 in control and TREM2 siRNA-treated BV2 cells after exposure to IL-4 for 24 h were detected by Western blot analysis (D). Data are presented as the mean ± sem of triplicate independent experiments. *P < 0.05, **P < 0.01.
Figure 6.
TREM2 overexpression suppresses α-syn–induced proinflammatory responses in BV2 microglial cells. Cells were transfected with TREM2 vector or a control vector for 48 h, followed by treatment with α-syn for another 4 h. Cells were then collected for the following analysis. A) mRNA levels of TNF-α, IL-1β, COX-2, and iNOS were measured by real-time RT-PCR. **P < 0.01. B) Protein levels of COX-2 and iNOS were detected by Western blot analysis. All data are presented as the mean ± sem of triplicate independent experiments. **P < 0.01 vs. adenoviral particles encoding control vectors + α-syn group.
To determine if TREM2 may affect anti-inflammatory M2 polarization, BV2 cells were converted into M2 cells by IL-4 treatment for 24 h. As shown in Fig. 5C, expression of M2 markers ARG1 and YM1 were markedly increased after exposure of BV2 cells to IL-4. By comparing control and TREM2 knockdown cells, we were able to confirm significant reduction of anti-inflammatory M2 marker genes in TREM2 siRNA-treated cells. TREM2 knockdown also significantly decreased ARG1 protein levels. After IL-4 treatment, TREM2 knockdown markedly impaired signaling pathways that promote M2 polarization, as measured by p-STAT6 immunoblot (Fig. 5D). Cumulatively, the data suggest that TREM2 deficiency decreases anti-inflammatory M2 microglial activation in vitro.
Suppressing microglial TREM2 expression is detrimental to cultured neurons
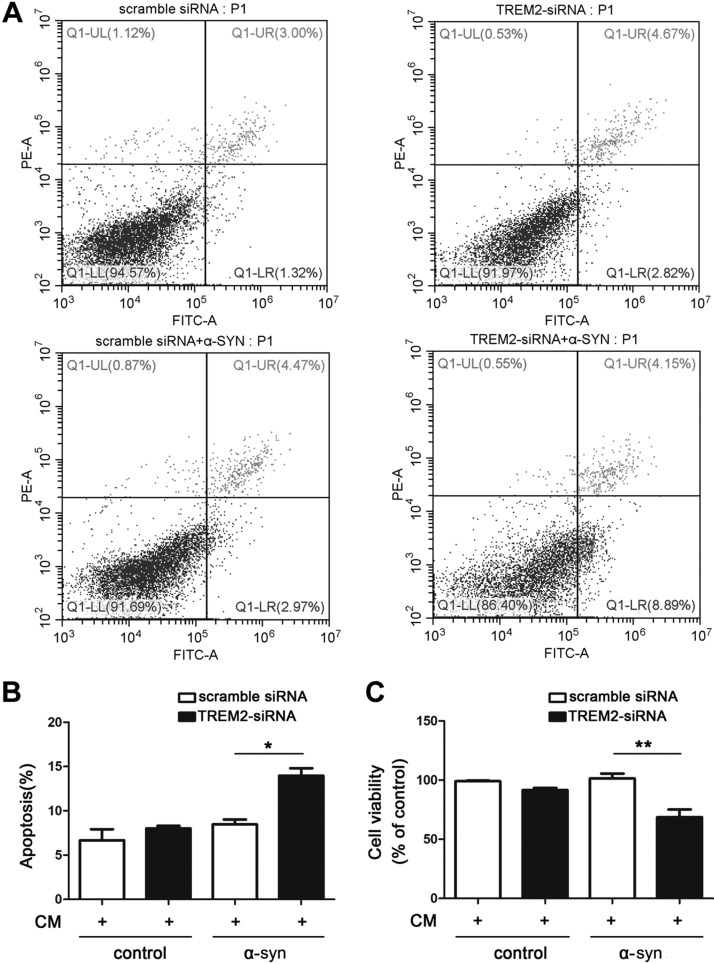
Microglial activation and the subsequent secretion of inflammatory cytokines in the CNS could cause neuronal apoptosis and synaptic damage. We have observed that TREM2 deficiency potentiates α-syn–induced inflammatory responses and induces a shift from anti- to proinflammatory microglial activation state, we then asked whether suppressing TREM2 expression might affect neuronal viability through neuron-microglia interactions. To address this, we cultured SH-SY5Y neuronal cells with CM from BV2 microglia treated with α-syn in the presence of TREM2 siRNA or scrambled siRNA. SH-SY5Y survival and apoptosis were quantified after CM exposure using flow cytometry (Fig. 7A, B) and MTT assay (Fig. 7C), respectively. We observed that CM from TREM2-knockdown BV2 cells induced significant SH-SY5Y cell death and apoptosis compared with CM from siRNA-treated BV2 cells, likely resulting from higher proinflammatory cytokines levels in CM from TREM2-knockdown BV2 cells (Supplemental Fig. S6). Taken together, the data strongly suggest that suppressing TREM2 expression in microglia reduces viability of neuronal cells by shifting the microglial activation state.
Figure 7.
Suppression of TREM2 in microglia is detrimental to cultured neurons. The control and TREM2-knockdown BV2 cells were treated with α-syn for 4 h. CM was then harvested and added into SH-SY5Y neuron culture for another 24 h. A, B) The apoptosis of SH-SY5Y cells were assessed by Annexin V-PI staining using flow cytometry (A), and the apoptotic rates were shown in graph (B). C) The cell viability of SH-SY5Y cells were detected using MTT assay. Data are presented as the mean ± sem of triplicate independent experiments. *P < 0.05, **P < 0.01.
DISCUSSION
Here, we examined the role of TREM2 in α-syn–induced neurodegeneration and neuroinflammation. Using in vivo models where α-syn was overexpressed; we found that mice with TREM2 knockout had significantly increased inflammatory responses to α-syn producing subsequent neurodegeneration as compared to our findings in WT mice. In vitro, TREM2 suppresses α-syn–induced proinflammatory responses in BV2 microglial cells. Moreover, TREM2 deficiency induced a shift from anti- to proinflammatory microglial activation state both in vivo and in vitro. The results of this study provide compelling data regarding how a loss of TREM2 function impacts α-syn–associated pathology and related neurodegeneration in PD brains.
TREM2 is known to play a key role in the regulation of several neuroinflammatory disorders (37–39). Furthermore, numerous findings connect TREM2 with PD, even though the precise contribution of TREM2 to PD requires additional study. TREM2 gene mutation is associated with increased PD risk (11–13). And although Belloli et al. (22) report that TREM2 knockout mice have less of a proinflammatory response to MPTP, our prior study shows that TREM2 overexpression suppresses neuroinflammation in the MPTP PD model (23). These contradictory findings suggest that there is a level of complexity associated with TREM2 and α-syn that contributes to pathology in PD. Until now, there was no direct evidence supporting a role for TREM2 in synucleinopathy. With the AAV-SYN PD mice model, we demonstrated that TREM2 deletion aggravated AAV-SYN–associated dopaminergic neuron loss. TREM2 knockout mice also exhibited increased microglial and astrocyte activation that elicits inflammatory responses. Together, our new findings provide compelling support suggesting that both TREM2 and α-syn play significant roles in PD pathology.
Accumulating evidence suggests that chronic neuroinflammation plays a critical role in dopaminergic neurodegeneration in PD. Activation of SNpc microglia is also observed in patients with PD (40, 41). This implies that microglial activation is initiated directly or indirectly by α-syn misfolding, pathogens, or environmental toxins. Numerous studies have shown that aggregated α-syn is released from dying or dead dopaminergic neurons, which could shift microglia toward a proinflammatory phenotype (26, 42). Similar to macrophages, microglia can adopt different activation states, formerly distinguished into a proinflammatory M1 and an alternative, anti-inflammatory activation state M2, as defined in macrophages (43). Recently, instead of this overly simplistic model, a concept of multiple or mixed microglial activation states was proposed (44–46). Our in vitro data revealed that deleting TREM2 stimulated inflammatory microglial responses to aggregated α-syn by shifting microglia from a protective toward a proinflammatory phenotype. In the AAV-SYN PD mice model, TREM2 knockout also aggravated α-syn–induced neuroinflammation in the nigrostriatal pathway, likely by shifting microglial activation from an anti-inflammatory to a proinflammatory status, which further increased the vulnerability of dopaminergic neurons to α-syn–induced inflammatory challenges. This strongly suggests that modulating TREM2 function in microglia is a novel therapeutic target for PD and other synucleinopathies. In accordance with our findings, work by others also confirmed that TREM2 modulates microglial phenotypes in vitro, and that TREM2 is up-regulated in MPTP PD mice (47). However, those studies failed to directly measure the role of TREM2 in an in vivo animal model of PD. In contrast, we provide compelling in vitro and in vivo data defining important effects of a role for TREM2 in synucleinopathy.
And even though our study had limitations, we are now planning studies to validate our findings on the role of TREM2 in PD using microglia-specific TREM2 knockout mice. Such studies will allow us to better describe the underlying molecular mechanisms by which TREM2 regulates microglial activation.
CONCLUSIONS
Our findings demonstrate that suppressing TREM2 expression aggravates α-syn–induced inflammatory responses in BV2 microglial cells and exacerbates dopaminergic neuron loss in AAV-SYN PD mice. Moreover, TREM2 deficiency induced a shift from anti-inflammatory toward proinflammatory microglial activation states both in vitro and in vivo. These findings further support the view that TREM2 appears to regulate α-syn–induced neuroinflammation and neurodegeneration by modulating microglial activation states and immune pathogenesis of PD. Further understanding the mechanisms of TREM2-mediated microglial activation may identify new PD therapeutics.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors are thankful to Dr. Ruth G. Perez (Texas Tech University Health Science Center, El Paso, TX, USA) for editorial assistance with the manuscript. This work was supported by grants from the Shandong Natural Science Foundation (ZR2018MH043) and the Shandong Province Science and Technology Program (2017GSF18187). The authors declare no conflicts of interest.
Glossary
- α-syn
α-synuclein
- ARG1
arginase 1
- AAV-SYN
adeno-associated viral vectors expressing human α-synuclein
- CM
conditioned medium
- COX2
cyclooxygenase-2
- GFAP
glial fibrillary acidic protein
- GFP
green fluorescent protein
- Iba1
ionized calcium binding adaptor molecule 1
- MPP+
1-methyl-4-phenylpyridinium
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- MTT
3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2-H-tetrazolium bromide
- OHDA
hydroxydopamine
- PD
Parkinson’s disease
- PI
propidium iodide
- siRNA
small interference RNA
- SN
substantia nigra
- SNpc
substantia nigra pars compacta
- TH
tyrosine hydroxylase
- TREM2
triggering receptor expressed on myeloid cells 2
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Guo and H. Lou designed the study; Y. Guo, X. Wei, Y. Qin, S. Yan, J. Liu, and Y. Zhao performed the experiments and collected the data; H. Yan and H. Lou analyzed the data; F. Jiang and H. Lou drafted this article; and all authors read and approved the final manuscript.
REFERENCES
- 1.Dickson D. W. (2018) Neuropathology of Parkinson disease. Parkinsonism Relat. Disord. 46 (Suppl 1), S30–S33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Perry V. H. (2012) Innate inflammation in Parkinson’s disease. Cold Spring Harb. Perspect. Med. 2, a009373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jha M. K., Lee W. H., Suk K. (2016) Functional polarization of neuroglia: implications in neuroinflammation and neurological disorders. Biochem. Pharmacol. 103, 1–16 [DOI] [PubMed] [Google Scholar]
- 4.Moehle M. S., West A. B. (2015) M1 and M2 immune activation in Parkinson’s disease: foe and ally? Neuroscience 302, 59–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Le W., Wu J., Tang Y. (2016) Protective microglia and their regulation in Parkinson’s disease. Front. Mol. Neurosci. 9, 89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Colonna M., Wang Y. (2016) TREM2 variants: new keys to decipher Alzheimer disease pathogenesis. Nat. Rev. Neurosci. 17, 201–207 [DOI] [PubMed] [Google Scholar]
- 7.Paloneva J., Mandelin J., Kiialainen A., Bohling T., Prudlo J., Hakola P., Haltia M., Konttinen Y. T., Peltonen L. (2003) DAP12/TREM2 deficiency results in impaired osteoclast differentiation and osteoporotic features. J. Exp. Med. 198, 669–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paloneva J., Manninen T., Christman G., Hovanes K., Mandelin J., Adolfsson R., Bianchin M., Bird T., Miranda R., Salmaggi A., Tranebjaerg L., Konttinen Y., Peltonen L. (2002) Mutations in two genes encoding different subunits of a receptor signaling complex result in an identical disease phenotype. Am. J. Hum. Genet. 71, 656–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guerreiro R., Wojtas A., Bras J., Carrasquillo M., Rogaeva E., Majounie E., Cruchaga C., Sassi C., Kauwe J. S., Younkin S., Hazrati L., Collinge J., Pocock J., Lashley T., Williams J., Lambert J. C., Amouyel P., Goate A., Rademakers R., Morgan K., Powell J., St George-Hyslop P., Singleton A., Hardy J.; Alzheimer Genetic Analysis Group (2013) TREM2 variants in Alzheimer’s disease. N. Engl. J. Med. 368, 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P. V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A. I., Lah J. J., Rujescu D., Hampel H., Giegling I., Andreassen O. A., Engedal K., Ulstein I., Djurovic S., Ibrahim-Verbaas C., Hofman A., Ikram M. A., van Duijn C. M., Thorsteinsdottir U., Kong A., Stefansson K. (2013) Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 368, 107–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benitez B. A., Cruchaga C.; US-Spain PD Research Group (2013) TREM2 and Parkinson’s disease. N. Engl. J. Med. 369, 1567–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu G., Liu Y., Jiang Q., Jiang Y., Feng R., Zhang L., Chen Z., Li K., Liu J. (2016) Convergent genetic and expression datasets highlight TREM2 in Parkinson’s disease susceptibility. Mol. Neurobiol. 53, 4931–4938 [DOI] [PubMed] [Google Scholar]
- 13.Rayaprolu S., Mullen B., Baker M., Lynch T., Finger E., Seeley W. W., Hatanpaa K. J., Lomen-Hoerth C., Kertesz A., Bigio E. H., Lippa C., Josephs K. A., Knopman D. S., White C. L., III, Caselli R., Mackenzie I. R., Miller B. L., Boczarska-Jedynak M., Opala G., Krygowska-Wajs A., Barcikowska M., Younkin S. G., Petersen R. C., Ertekin-Taner N., Uitti R. J., Meschia J. F., Boylan K. B., Boeve B. F., Graff-Radford N. R., Wszolek Z. K., Dickson D. W., Rademakers R., Ross O. A. (2013) TREM2 in neurodegeneration: evidence for association of the p.R47H variant with frontotemporal dementia and Parkinson’s disease. Mol. Neurodegener. 8, 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ulrich J. D., Ulland T. K., Colonna M., Holtzman D. M. (2017) Elucidating the role of TREM2 in Alzheimer’s disease. Neuron 94, 237–248 [DOI] [PubMed] [Google Scholar]
- 15.Zheng H., Jia L., Liu C. C., Rong Z., Zhong L., Yang L., Chen X. F., Fryer J. D., Wang X., Zhang Y. W., Xu H., Bu G. (2017) TREM2 promotes microglial survival by activating Wnt/β-Catenin pathway. J. Neurosci. 37, 1772–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cantoni C., Bollman B., Licastro D., Xie M., Mikesell R., Schmidt R., Yuede C. M., Galimberti D., Olivecrona G., Klein R. S., Cross A. H., Otero K., Piccio L. (2015) TREM2 regulates microglial cell activation in response to demyelination in vivo. Acta Neuropathol. 129, 429–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y., Cella M., Mallinson K., Ulrich J. D., Young K. L., Robinette M. L., Gilfillan S., Krishnan G. M., Sudhakar S., Zinselmeyer B. H., Holtzman D. M., Cirrito J. R., Colonna M. (2015) TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell 160, 1061–1071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Painter M. M., Atagi Y., Liu C. C., Rademakers R., Xu H., Fryer J. D., Bu G. (2015) TREM2 in CNS homeostasis and neurodegenerative disease. Mol. Neurodegener. 10, 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y., Ulland T. K., Ulrich J. D., Song W., Tzaferis J. A., Hole J. T., Yuan P., Mahan T. E., Shi Y., Gilfillan S., Cella M., Grutzendler J., DeMattos R. B., Cirrito J. R., Holtzman D. M., Colonna M. (2016) TREM2-mediated early microglial response limits diffusion and toxicity of amyloid plaques. J. Exp. Med. 213, 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jay T. R., von Saucken V. E., Landreth G. E. (2017) TREM2 in neurodegenerative diseases. Mol. Neurodegener. 12, 56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Leyns C. E. G., Ulrich J. D., Finn M. B., Stewart F. R., Koscal L. J., Remolina Serrano J., Robinson G. O., Anderson E., Colonna M., Holtzman D. M. (2017) TREM2 deficiency attenuates neuroinflammation and protects against neurodegeneration in a mouse model of tauopathy. Proc. Natl. Acad. Sci. USA 114, 11524–11529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belloli S., Pannese M., Buonsanti C., Maiorino C., Di Grigoli G., Carpinelli A., Monterisi C., Moresco R. M., Panina-Bordignon P. (2017) Early upregulation of 18-kDa translocator protein in response to acute neurodegenerative damage in TREM2-deficient mice. Neurobiol. Aging 53, 159–168 [DOI] [PubMed] [Google Scholar]
- 23.Ren M., Guo Y., Wei X., Yan S., Qin Y., Zhang X., Jiang F., Lou H. (2018) TREM2 overexpression attenuates neuroinflammation and protects dopaminergic neurons in experimental models of Parkinson’s disease. Exp. Neurol. 302, 205–213 [DOI] [PubMed] [Google Scholar]
- 24.Oliveras-Salvá M., Van der Perren A., Casadei N., Stroobants S., Nuber S., D’Hooge R., Van den Haute C., Baekelandt V. (2013) rAAV2/7 vector-mediated overexpression of alpha-synuclein in mouse substantia nigra induces protein aggregation and progressive dose-dependent neurodegeneration. Mol. Neurodegener. 8, 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lastres-Becker I., García-Yagüe A. J., Scannevin R. H., Casarejos M. J., Kügler S., Rábano A., Cuadrado A. (2016) Repurposing the NRF2 activator dimethyl fumarate as therapy against synucleinopathy in Parkinson’s disease. Antioxid. Redox Signal. 25, 61–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang W., Wang T., Pei Z., Miller D. S., Wu X., Block M. L., Wilson B., Zhang W., Zhou Y., Hong J. S., Zhang J. (2005) Aggregated alpha-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 19, 533–542 [DOI] [PubMed] [Google Scholar]
- 27.Zheng H., Liu C. C., Atagi Y., Chen X. F., Jia L., Yang L., He W., Zhang X., Kang S. S., Rosenberry T. L., Fryer J. D., Zhang Y. W., Xu H., Bu G. (2016) Opposing roles of the triggering receptor expressed on myeloid cells 2 and triggering receptor expressed on myeloid cells-like transcript 2 in microglia activation. Neurobiol. Aging 42, 132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Owens R., Grabert K., Davies C. L., Alfieri A., Antel J. P., Healy L. M., McColl B. W. (2017) Divergent neuroinflammatory regulation of microglial TREM expression and involvement of NF-κB. Front. Cell. Neurosci. 11, 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turnbull I. R., Gilfillan S., Cella M., Aoshi T., Miller M., Piccio L., Hernandez M., Colonna M. (2006) Cutting edge: TREM-2 attenuates macrophage activation. J. Immunol. 177, 3520–3524 [DOI] [PubMed] [Google Scholar]
- 30.Gawish R., Martins R., Böhm B., Wimberger T., Sharif O., Lakovits K., Schmidt M., Knapp S. (2015) Triggering receptor expressed on myeloid cells-2 fine-tunes inflammatory responses in murine Gram-negative sepsis. FASEB J. 29, 1247–1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saber M., Kokiko-Cochran O., Puntambekar S. S., Lathia J. D., Lamb B. T. (2017) Triggering receptor expressed on myeloid cells 2 deficiency alters acute macrophage distribution and improves recovery after traumatic brain injury. J. Neurotrauma 34, 423–435 [DOI] [PubMed] [Google Scholar]
- 32.Poliani P. L., Wang Y., Fontana E., Robinette M. L., Yamanishi Y., Gilfillan S., Colonna M. (2015) TREM2 sustains microglial expansion during aging and response to demyelination. J. Clin. Invest. 125, 2161–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kawabori M., Kacimi R., Kauppinen T., Calosing C., Kim J. Y., Hsieh C. L., Nakamura M. C., Yenari M. A. (2015) Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J. Neurosci. 35, 3384–3396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sica A., Bronte V. (2007) Altered macrophage differentiation and immune dysfunction in tumor development. J. Clin. Invest. 117, 1155–1166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Qin H., Holdbrooks A. T., Liu Y., Reynolds S. L., Yanagisawa L. L., Benveniste E. N. (2012) SOCS3 deficiency promotes M1 macrophage polarization and inflammation. J. Immunol. 189, 3439–3448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sheldon K. E., Shandilya H., Kepka-Lenhart D., Poljakovic M., Ghosh A., Morris S. M., Jr (2013) Shaping the murine macrophage phenotype: IL-4 and cyclic AMP synergistically activate the arginase I promoter. J. Immunol. 191, 2290–2298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang T., Yu J. T., Zhu X. C., Tan M. S., Gu L. Z., Zhang Y. D., Tan L. (2014) Triggering receptor expressed on myeloid cells 2 knockdown exacerbates aging-related neuroinflammation and cognitive deficiency in senescence-accelerated mouse prone 8 mice. Neurobiol. Aging 35, 1243–1251 [DOI] [PubMed] [Google Scholar]
- 38.Jiang Y., Li Z., Ma H., Cao X., Liu F., Tian A., Sun X., Li X., Wang J. (2018) Upregulation of TREM2 ameliorates neuroinflammatory responses and improves cognitive deficits triggered by surgical trauma in Appswe/PS1dE9 Mice. Cell. Physiol. Biochem. 46, 1398–1411 [DOI] [PubMed] [Google Scholar]
- 39.Zhai Q., Li F., Chen X., Jia J., Sun S., Zhou D., Ma L., Jiang T., Bai F., Xiong L., Wang Q. (2017) Triggering receptor expressed on myeloid cells 2, a novel regulator of immunocyte phenotypes, confers neuroprotection by relieving neuroinflammation. Anesthesiology 127, 98–110 [DOI] [PubMed] [Google Scholar]
- 40.McGeer P. L., Itagaki S., Boyes B. E., McGeer E. G. (1988) Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology 38, 1285–1291 [DOI] [PubMed] [Google Scholar]
- 41.Gerhard A., Pavese N., Hotton G., Turkheimer F., Es M., Hammers A., Eggert K., Oertel W., Banati R. B., Brooks D. J. (2006) In vivo imaging of microglial activation with [11C](R)-PK11195 PET in idiopathic Parkinson’s disease. Neurobiol. Dis. 21, 404–412 [DOI] [PubMed] [Google Scholar]
- 42.Allen Reish H. E., Standaert D. G. (2015) Role of α-synuclein in inducing innate and adaptive immunity in Parkinson disease. J. Parkinsons Dis. 5, 1–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Arcuri C., Mecca C., Bianchi R., Giambanco I., Donato R. (2017) The pathophysiological role of microglia in dynamic surveillance, phagocytosis and structural remodeling of the developing CNS. Front. Mol. Neurosci. 10, 191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ransohoff R. M. (2016) A polarizing question: do M1 and M2 microglia exist? Nat. Neurosci. 19, 987–991 [DOI] [PubMed] [Google Scholar]
- 45.Joers V., Tansey M. G., Mulas G., Carta A. R. (2017) Microglial phenotypes in Parkinson’s disease and animal models of the disease. Prog. Neurobiol. 155, 57–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Butovsky O., Weiner H. L. (2018) Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 19, 622–635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang Y., Feng S., Nie K., Li Y., Gao Y., Gan R., Wang L., Li B., Sun X., Wang L., Zhang Y. (2018) TREM2 modulates microglia phenotypes in the neuroinflammation of Parkinson’s disease. Biochem. Biophys. Res. Commun. 499, 797–802 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.