Abstract
Adipose tissue–secreted extracellular vesicles (EVs) containing microRNAs (miRNAs) convey intercellular message signaling. The biogenesis of EV-miRNAs from perivascular adipose tissue (PVAT) and their roles in intercellular communication in response to obesity-associated inflammation have not yet been fully explored. By feeding mice a high-fat diet for 16 wk, we established obesity-associated, chronic low-grade inflammation in PVAT, characterized as hypertrophy of perivascular adipocytes, decreased adipogenesis, and proinflammatory macrophage infiltration. We show that PVAT-derived EVs and their encapsulated miRNAs can be taken up into vascular smooth muscle cells (VSMCs) in vivo and in vitro. miR-221-3p is one of the highly enriched miRNAs in obese PVAT and PVAT-derived EVs. Transfer and direct overexpression of miR-221-3p dramatically enhances VSMC proliferation and migration. Peroxisome proliferator–activated receptor γ coactivator 1α is identified as a miR-221-3p target in VSMC phenotypic modulation. Obese mice secrete abundant miRNA-containing EVs, evoking inflammatory responses in PVAT and vascular phenotypic switching in abdominal aorta of lean mice. Local delivery of miR-221-3p mimic in femoral artery causes vascular dysfunction by suppressing the contractile genes in the arterial wall. Our findings provide an EV–miR-221-3p–mediated mechanism by which PVAT triggers an early-stage vascular remodeling in the context of obesity-associated inflammation.—Li, X., Ballantyne, L. L., Yu, Y., Funk, C. D. Perivascular adipose tissue–derived extracellular vesicle miR-221-3p mediates vascular remodeling.
Keywords: exosome, microRNAs, smooth muscle cell, inflammation, obesity
Obesity is a substantial public health issue because it gives rise to multifaceted complications and consequently exacerbates the development of diabetes, vasculopathies, and heart disease. Obesity-associated chronic low-grade inflammation leads to progressive accumulation of immune cells in adipose tissue, including perivascular adipose tissue (PVAT). PVAT is the fat tissue surrounding blood vessels, such as the small mesenteric and femoral arteries or large abdominal aorta (AA), and plays wide-ranging physiologic roles beyond a supporting scaffold (1). PVAT differs between species and anatomic location and is generally classified as white adipose tissue (lipid-storing adipocytes), brown adipose tissue (thermoactive adipocytes), and beige adipose tissue (brown-like adipocytes) (2). PVAT is now considered a metabolically active organ that mediates communication with vascular cells in both autocrine and paracrine fashion via production of a variety of adipokines (3, 4).
Research into the messengers conveying the communication between a blood vessel and its surrounding PVAT has, for the most part, been limited to the roles of cytokines and chemokines. Apart from these secretory factors, adipocytes are also known to secrete various types of extracellular vesicles (EVs) (5, 6), including exosomes and microvesicles. Emerging evidence demonstrates the important role of EVs in intercellular communication by selective packaging of proteins, lipids, and nucleic acids, including microRNAs (miRNAs) (7, 8). Moreover, these secreted EVs can be taken up into neighboring or distant cells to modulate the recipient’s function (9, 10). Visceral adipose tissue–derived exosomes from obese animals are proatherosclerotic by regulating macrophage function (11). Recently, both visceral and subcutaneous adipose tissues were verified to constitute an important source of circulating exosomal miRNAs, which exert local or distant regulatory functions (12). Several studies reported association of changes in the exosomal miRNAs with the prognosis of vascular remodeling (13, 14). Most recently, EV-mediated cargo exchange (protein and lipid) between adipocytes and endothelial cells (15)/macrophages (16) has revealed novel information conveying networks.
The possibility that PVAT-derived EVs act as a mode of intercellular communication has not yet been explored. We aim to provide evidence that PVAT or PVAT-derived adipocytes from obese animals secrete EVs containing miRNAs that can be taken up into vascular smooth muscle cells (VSMCs), leading to VSMC proliferation, migration, and eventually phenotypic switching. We also identify the most dysregulated miRNA that serves as an extracellular mediator to modulate vascular function in the context of obesity.
MATERIALS AND METHODS
Cell lines
3T3-L1 cells [CL-173; American Type Culture Collection (ATCC), Manassas, VA, USA] were maintained in DMEM/F-12 basal medium containing 10% fetal bovine serum (FBS), penicillin-streptomycin, and glutamine. Differentiation of adipocytes was performed using a previously described protocol (17). Briefly, 3T3-L1 cells were induced with a differentiation cocktail consisting of 0.5 mM 3-isobutyl-1-methylxanthine, 1 µM dexamethasone, 10 µg/ml insulin, 0.2 mM indomethacin, and 1 µM rosiglitazone for 7–10 d. 3T3-L1–derived adipocytes were designated as 3T3-Ad. Differentiation efficiency was validated by Oil Red O staining.
Mouse aortic smooth muscle cells (SMCs) immortalized with SV40 large T antigen (MOVAS, CRL-2797; ATCC) and were cultured with DMEM basal medium containing 0.2 mg/ml G-418 (11811-023; Thermo Fisher Scientific, Waltham, MA, USA) and 10% FBS.
Primary mouse VSMC isolation
Primary mouse VSMCs were cultured from 6- to 8-wk-old mice (male or female) as previously described (18). In brief, aortas were aseptically excised and placed in cold PBS. Adhering fat and connective tissues were carefully removed under a dissecting microscope, and endothelial cells were scraped with forceps. The vessels were then cut into 2–3 small pieces (∼5 mm each), transferred into 60-mm dishes, and covered with a coverslip. The vessel pieces were cultured for 7–10 d in DMEM/F-12 supplemented with 1% penicillin-streptomycin solution, and 20% FBS at 37°C in a humidified atmosphere containing 5% CO2. Every batch of VSMCs was tested by immunofluorescence staining of SMC marker smooth muscle α-actin (SMαA) (FITC-conjugated antibody, 1:200, F3777; MilliporeSigma, Burlington, MA, USA) to ensure that the purity of VSMCs was above 95%. Cultured aortic SMCs were routinely used between passages 2 and 3.
Primary preadipocyte isolation and adipocyte differentiation
Mesenteric preadipocytes derived from stromal vascular cells were obtained by collagenase digestion. The 16- to 28-wk-old mice (male or female) to be used for mesenteric adipose tissue (MAT) isolation were perfused through the heart at the left ventricle with cold PBS for 5 min to remove blood from the tissue. MAT was gently dissected and rinsed 3 times in cold PBS, then minced. The minced tissues were digested with 100 U/ml collagenase IV (LS004188; Worthington Biochemical, Lakewood, NJ, USA) in PBS in a water bath with shaking at 37°C for 45 min. The digested tissues were filtered through a 100-µm filter and centrifuged at 50 g for 5 min to remove the floating cell layer (primary mature adipocytes). The stromal vascular fraction cells were pelleted by centrifugation at 500 g for 5 min and incubated in red blood cell lysis buffer for 5 min at room temperature. Finally, mesenteric preadipocytes were isolated through filtration (40-µm filter) and centrifugation at 500 g for 5 min.
For adipocyte differentiation, preadipocytes were first cultured to confluence in DMEM/F-12 medium containing 10% FBS, penicillin-streptomycin, and glutamine and then induced with a differentiation cocktail as previously described (17), consisting of 0.5 mM 3-isobutyl-1-methylxanthine, 1 µM dexamethasone, 10 µg/ml insulin, 0.2 mM indomethacin, and 1 µM rosiglitazone. After 3 d, the medium was replaced with DMEM containing 10% FBS, penicillin-streptomycin, glutamine, 10 µg/ml insulin, and 1 µM rosiglitazone every other day. Differentiated primary adipocytes were designated as MAT-Ad.
Isolation of EVs from MAT
Differential ultracentrifugation was used to isolate EVs from ex vivo tissue explants, as previously described (5, 19). For MAT-derived EVs (MAT-EVs), 16- to 28-wk-old mice (male or female) to be used for MAT-EVs isolation were perfused through the heart at the left ventricle with cold PBS for 5 min to remove blood from the tissue. The MATs from 5 to 6 mice were combined and minced gently to avoid rupturing cells. Minced pieces were weighed and incubated with DMEM/F-12 medium (250 mg wet tissue/ml) containing 10% exosome-depleted FBS (EXO-FBS-250A-1; System Biosciences, Palo Alto, CA, USA) and 1% penicillin-streptomycin. After incubation for 24 h, the minced tissues were filtered through a 100-µm filter and centrifuged at 500 g for 5 min to remove debris and dead cells in the medium. The medium was then subjected to centrifugation at 2000 g for 20 min at 4°C. The resulting supernatant was then filtered through a 0.2-µm syringe-driven filter to remove any contaminating large microvesicles and any remaining cellular debris. Samples were then centrifuged in a fixed angle rotor at 100,000 g (70.1 Ti Rotor, XL-100K; Beckman Coulter, Brea, CA, USA) for 1.5 h to pellet EVs. The pellet was resuspended in cold PBS and centrifuged again at 100,000 g for 30 min. The final EV-containing pellet was resuspended in PBS. The amount of EVs released from MATs was quantified using a Bradford assay relative to wet tissue weight.
Isolation of EVs from cell culture supernatants
3T3-Ad and differentiated primary adipocytes (MAT-Ad) were cultured in DMEM/F-12 medium supplemented with 10% exosome-depleted FBS. Prior to isolation of EVs, cells (3 × 107) were challenged with LPS (1 µg/ml; MilliporeSigma) or PBS control for 24 h. EVs were isolated from the supernatant of both 3T3-Ad and MAT-Ad cells according to a combination of methods and using miRCURY exosome isolation kit (76743; Qiagen, Hilden, Germany) (20). Briefly, after the ultracentrifugation step described above, the top seven-eighths portion of supernatant was discarded. The remaining one-eighth portion was precipitated overnight at 4°C and centrifuged at 20°C at 3200 g for 30 min to pellet EVs. The EV pellet was resuspended in 100 μl of resuspension buffer. EVs isolated from 3T3-Ad or MAT-Ad are designated as 3T3-Ad-EVs and MAT-Ad-EVs, respectively.
Transmission electron microscopy
To assess the presence of EVs in culture-conditioned medium (CM), a standard transmission electron microscopy procedure was used (12, 16). Briefly, resuspended EVs (5 µl) were loaded to 400-mesh Formvar and carbon-coated copper grids (Electron Microscopy Sciences, Hatfield, PA, USA) and were allowed to settle by incubating at room temperature for 20 min and then negatively stained (21) and the grids examined with a Hitachi H-7000 transmission electron microscope system (Hitachi High-Technologies, Schaumburg, IL, USA) operated at 80 kV.
EV trafficking assay
To monitor EV trafficking, EVs isolated from MAT explant/cell culture supernatants were labeled with PKH26 fluorescent dye using the PKH26 fluorescent cell linker kit (PKH26GL-1KT; MilliporeSigma). After PKH26 staining, an equal volume of 1% bovine serum albumin was added to allow binding of excess dye, and the labeled EVs were washed in PBS and collected by ultracentrifugation (100,000 g for 20 min) at 4°C. Finally, PKH26-labeled EVs were resuspended in PBS, applied to recipient cells (50 µg/ml), and incubated for 18 h. The trafficking assays of EVs were carried out in 2 different settings: uptake of labeled 3T3-EVs in primary mouse VSMCs and uptake of labeled MAT-EVs in primary mouse VSMCs. The uptake of labeled 3T3-EVs in primary mouse VSMCs was performed in 4-well CultureSlides (BD Falcon; Corning Life Sciences, Durham, NC, USA). PKH26-labeled EVs were incubated with 4% paraformaldehyde–fixed VSMCs or nonfixed VSMCs for 18 h. Live VSMCs were then fixed and immunolabeled by FITC-conjugated SMαA antibody in addition to DAPI staining. Cellular internalization of 3T3-L1–derived EVs into murine VSMCs, shown as punctate labeling, was visualized with a fluorescent microscope (Leica Microsystems, Buffalo Grove, IL, USA). During the uptake of labeled MAT-EVs in primary mouse VSMCs, nonlabeled EVs (50 µg/ml) and PKH26 dye (1 × 10−6 M) alone were used as controls.
Western blot analysis
The characterization of EVs was confirmed by measuring expression of EV-associated protein markers ALIX, tumor susceptibility gene 101 (TSG101), CD63, and CD9 by Western blot analysis (17). The adipocyte marker perilipin (PLIN) was also detected (15, 22). Mouse peritoneal resident macrophages (R-MΦ) and primary endothelial cells were isolated and cultured by using our established protocols (23). Whole-cell lysates were used as positive control for macrophage marker CD68 and endothelial cell marker CD31, respectively. Isolated lysates of EVs were prepared in RIPA buffer supplemented with protease and phosphatase inhibitors (Thermo Fisher Scientific). Equal amounts of lysate proteins from each biologic replicate were subjected to Western blotting. Blots were probed with rabbit anti-ALIX (ab186429; Abcam, Cambridge, MA, USA), rabbit anti-CD63 (ab216130; Abcam), rabbit anti-CD9 (ab92726; Abcam), rabbit anti-TSG101 (ab125011; Abcam), mouse anti-CD31 (ab24590; Abcam), mouse anti-CD68 (ab31630; Abcam), and mouse monoclonal antibody for PLIN (10R-2478; Fitzgerald Industries International, Acton, MA, USA) followed by washing and incubation with the respective secondary antibodies. MAT, PVAT, and AA tissues were homogenized with T-PER (Thermo Fisher Scientific) and peroxisome proliferator–activated receptor γ coactivator 1α (PGC-1α) was detected with rabbit anti–PGC-1α (ab54481; Abcam). Immunodetection was visualized with Clarity (or Clarity Max) Western ECL substrate (Bio-Rad, Hercules, CA, USA). We normalized protein expression to β-actin or α-tubulin bands.
Morphology and immunostaining of adipose tissue
Following euthanization by CO2 asphyxiation and perfusion with cold PBS through the heart, MATs, femoral arteries, and AA with intact PVAT were dissected. Tissues were fixed in 10% buffered-formalin for 24 h, processed routinely, and embedded in paraffin for staining with hematoxylin and eosin. For quantitative analysis of adipocyte area, tissue sections were captured with Improvision OpenLab Imaging system (PerkinElmer, Waltham, MA, USA) on an inverted microscope (DM IRB; Leica Microsystems) and were quantified via Image-Pro Plus software (Media Cybernetics, Rockville, MD, USA). In abdominal aortic and femoral arterial cross sections, medial and adventitial area analysis was performed as previously described (24). All specimens were analyzed by an investigator blinded to the study design.
During the immunohistochemical staining, paraffin-embedded tissue sections were deparaffinized, rehydrated, and subjected to antigen retrieval. After permeabilization and preincubation in goat serum and peroxidase blocking solution, the sections were incubated overnight at 4°C with a mouse monoclonal anti-CD68 antibody (ab31630; Abcam) at a 1:100 dilution. After washing, sections were incubated with a goat anti-mouse horseradish peroxidase–conjugated secondary antibody at a 1:200 dilution. The immunohistochemical reaction was accomplished using an ImmPACT DAB peroxidase substrate kit (SK-4105; Vector Laboratories, Burlingame, CA, USA). On some slides, CD68 was stained fluorescently. To visualize the arterial intima and media layers, immunofluorescence staining of AA and the surrounding PVAT was performed by incubation of sections with primary antibodies directed against mouse CD31 (FITC conjugated, 1:200, 102506; BioLegend, San Diego, CA, USA), and SMαA (FITC conjugated, 1:200, F3777; MilliporeSigma), as previously described (25).
For the localization of CD63 in adipose tissue, immunostaining of MAT was performed to reveal the positive staining for EV marker CD63 and the adipocyte marker PLIN. Both Alexa Fluor 488 (green) anti-rabbit and Alexa Fluor 594 (red) anti-mouse antibodies (Thermo Fisher Scientific) were used as secondary antibodies. Microscopy was conducted with an Olympus IX81 inverted microscope (Olympus, Center Valley, PA, USA) using a Quorum WaveFX-X1 spinning disk confocal system (Quorum Technologies, Lewes, United Kingdom). Images were recorded with a Hamamatsu EM-CCD camera (09100-13; Hamamatsu Photonics, Hamamatsu, Japan), and the resultant images were further processed and analyzed with MetaMorph software (Molecular Devices, San Jose, CA, USA).
RNA extraction and real-time quantitative PCR
Total cellular RNAs were extracted from cells/tissues using the Direct-zol RNA MiniPrep Kit (Zymo Research, Irvine, CA, USA). The EV-containing RNAs were extracted by the Direct-zol RNA MicroPrep Kit. RNA quantity was assessed using a Nanodrop N-1000 spectrophotometer (Nano Drop Technologies, Wilmington, DE, USA), and the quality of the isolated RNA was then checked with Agilent RNA 6000 Nano Assay Kit reagents using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA).
Synthesis of cDNA and real-time quantitative PCR (qPCR) were performed as previously outlined (26). Oligonucleotide primers are listed in Table 1 and miRNA assays are listed in Table 2. Primary miR-221-3p was determined by qPCR (model 7500; Thermo Fisher Scientific) using a TaqMan Pri-miRNA Assay (Mm03307181_pri). For detection of mature miRNA expression, TaqMan Advanced miRNA cDNA Synthesis Kit (A25576; Thermo Fisher Scientific) was used to synthesize cDNA, and Fast Advanced Master Mix (444557) was used for the qPCR assays according to the manufacturer’s instructions. Both cellular and EV-containing miR-221-3p (mmu481005_mir) were detected. Cellular and EV–miR-221-3p expression data were normalized to levels of endogenous miR-361-5p (478056_mir) and miR-320a (478594_mir), respectively.
TABLE 1.
Primers used in real-time qPCR reactions
| Sequence, 5′–3′ |
|||
|---|---|---|---|
| Gene | Protein | Forward | Reverse |
| Mir221 | Pre-miR-221-3p | GAAGAAATGATTCCAGGTAGC | TGAACATCCAGGTCTGGGGCA |
| Gapdh | GAPDH | ATGGCCTTCCGTGTTCCTA | ATGCCTGCTTCACCACCTTCT |
| Adipoq | ADIPOQ | CAAGGCCGTTCTCTTCACCT | CCCCATACACTTGGAGCCAG |
| Ucp1 | UCP1 | AGGCTTCCAGTACCATTAGGT | CTGAGTGAGGCAAAGCTGATTT |
| Cidea | CIDEA | ATCACAACTGGCCTGGTTACG | TACTACCCGGTGTCCATTTCT |
| Pparg | PPARγ | TGCTGCAGGCCCTGGAACTC | AGCACCTTGGCGAACAGCTGG′ |
| Lep | LEP | GTGCTGAGAGGACTTTGTGGGCC | CCTTCGCTCCAGACTGTGGCAC |
| Ccl2 | CCL2, MCP-1 | TTAAAAACCTGGATCGGAACCA | GCATTAGCTTCAGATTTACGGGT |
| Ccl3 | CCL3, MIP1α | CTGCAACCAAGTCTTCTCAGC | CTGCCTCCAAGACTCTCAGG |
| Il1b | IL-1β | TGAAGTTGACGGACCCCAAA | TGATGTGCTGCTGCGAGATT |
| Il6 | IL-6 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| Tnf | TNF-α | TGGCCTCCCTCTCATCAGTT | GGTGGTTTGCTACGACGTGG |
| Ptgs2 | COX-2 | GCTCACGAAGGAACTCAG | GGATTGGAACAGCAAGGAT |
| Itgax | ITGAX, CD11c | ACGTCAGTACAAGGAGATGTTGGA | TCCTATTGCAGAATGCTTCTTTACC |
| Acta2 | ACTA2, SMαA | TCCTGACGCTGAAGTATCCGAT | GGCCACACGAAGCTCGTTATAG |
| Tagln | TAGLN, SM22α | GATATGGCAGCAGTGCAGAG | AGTTGGCTGTCTGTGAAGTC |
| Cnn1 | CNN1 | GCGTCACCTCTATGATCCCAA | CCCAGACCTGGCTCAAAGAT |
| Myh11 | MYH11 | AAGCAGCCAGCATCAAGGAG | AGCTCTGCCATGTCCTCCAC |
| Smtn | SMTN | GGGCAGTATCTTCGACCGAG | GGCAGACTCTGTGCCTTCAT |
| Icam1 | ICAM1 | ACCACGGAGCCAATTTCTCAT | TGGAAGATCGAAAGTCCGGAG |
| Vcam1 | VCAM1 | TTGGCTGTGACTCCCCTTCTT | AGAGCTCAACACAAGCGTGGA |
| Mmp2 | MMP2 | GGCTGACATCATGATCAACTTTGG | GCCATCAGCGTTCCCATACTTTAC |
| Mmp9 | MMP9 | GAGAACACCACCGAGCTATCCACT | AGAGAGGAGTCTGGGGTCTGGTTT |
| Ppargc1a | PPARGC1A, PGC-1α | GTCACCACCGAAATCCTTAT | ATCTACTGCCTGGGGACCTT |
ADIPOQ, adiponectin; LEP, leptin.
TABLE 2.
Assays used in real-time qPCR reactions
| miRNA | Assay Identification | Catalog no. |
|---|---|---|
| miR-221-3p | mmu481005_mir | A25576 |
| Pri-miR-221-3p | Mm03307181_pri | 4427012 |
| miR-361-5p | mmu481127_mir | A25576 |
| miR-320a | 478594_mir | A25576 |
Assay manufacturer: Thermo Fisher Scientific.
miRNA mimic or inhibitor transfection
mirVana miRNA mimic (4464067), Cy3-labeled miRNA mimic (sense: 5′-AACCCAGCAGACAAUGUAGCUTT-3′; antisense: 5′-AGCUACAUUGUCUGCUGGGUUUC-3′; SKU: 4464073) or miRNA inhibitor (4464085) and respective negative controls were purchased from Thermo Fisher Scientific. miRNA and a control miRNA (25 nM, final concentration) were transfected into recipient cells with Lipofectamine RNAiMax reagent (Thermo Fisher Scientific). After 24 h, the transfection efficiencies were validated by qPCR, and transfected cells were used for further experiments.
Coculture assay for EV transportation
Cocultivation of adipocytes and SMCs was investigated in 12 transwell plates (C3401; Corning, Corning, NY, USA). The adipocytes treated with negative control miRNA mimic (4464059; Thermo Fisher Scientific) were used in the control group. In the EV transport assay, after transfection of Cy3-labeled miR-221-3p, adipocytes were seeded onto the 0.4-µm inserts, which allow transport of EVs but not cells. In parallel, SMCs at a ratio of 1:1 were seeded in the lower well, cultured in starvation medium containing 0.5% exosome-depleted FBS for 16 h. After washing with PBS twice and refreshing with FluoroBrite DMEM (Thermo Fisher Scientific), the appearance of Cy3 red fluorescence on SMCs in the lower chamber was examined. The transport of miR-221-3p–containing EVs through the transwell from transfected adipocytes to SMCs was also detected by qPCR analysis for the miRNA expression in SMCs. To inhibit EV secretion, transfected adipocytes were pretreated with GW4869 (an inhibitor of neutral sphingomyelinase, 10 µM, 13127; Cayman Chemical, Ann Arbor, MI, USA) for 24 h. These adipocytes were used to coculture with SMCs for another 16 h. As control, cells were treated with vehicle (DMSO).
Functional assays for MOVAS
All functional assays were conducted as described in previous studies (18, 27). These experiments included proliferation assays-cell counting and 5-bromo-2’-deoxy-uridine (BrdU) incorporation; migration assays–wound healing (scratch model), and transwell migration (insert membrane pore = 8 µm). MOVAS cells were subjected to CM treatment, MAT-EV treatment, cocultivation with transfected 3T3-Ad (insert membrane pore = 0.4 µm), or direct transfection of various miRNAs.
CM treatment of MOVAS
MAT explant CM was collected (as previously described in MAT-EV isolation section), and fresh CM was used. MOVAS cells were plated (5 × 104/well) and cultured in 12-well plates. Cells were allowed to grow to 80–90% confluence and subjected to serum starvation by culture in DMEM containing 0.5% serum for 24 h. The starved cells were subjected to various treatments for another 16 h prior to functional assays (cell counting and wound healing).
EV treatment of MOVAS
In previous studies, EVs from obese mice at a dose of 10 ∼ 100 µg/ml were used for in vitro experiments (5). In our in vitro treatment, 50 µg/ml of MAT-EVs (on the basis of protein measurement) were added to the starved recipient cells. After 16 h, functional assays (cell counting and wound healing) were performed.
Coculture of MOVAS with transfected 3T3-Ad
3T3-Ad cells were transfected with miR-221-3p mimics or negative control as indicated in the figure legends. Transfected cells in 0.4-µm inserts were cocultured with starved MOVAS cells for 16 h prior to functional assays (cell counting and wound healing).
Direct miRNA transfection in MOVAS
MOVAS cells transfected with miRNA mimic, inhibitor, or respective control were subjected to various treatments for another 24 h prior to functional assays (BrdU incorporation, transwell assay, cell counting, and wound healing).
Cell counting
MOVAS cells were plated (7 × 104 per well) and cultured in 12-well plates. Cells were allowed to grow to 80–90% confluence and transfected with miR-221-3p mimic, miR-221-3p inhibitor, or respective negative controls as indicated in the figures. Transfected cells were cultured overnight and subjected to serum starvation by culture in DMEM containing 0.5% serum for 24 h. After starvation, the cells were treated with 10% serum for 18–24 h before trypsinizing and manually counting the cells using a hemocytometer.
BrdU incorporation assay
MOVAS cells were transfected as described above, and were recultured (5 × 104 per well) in 4-well culture slides overnight, followed by serum starvation for 24 h. Starved VSMCs were restimulated with 10% FBS or 10 ng/ml platelet-derived growth factor (PDGF-BB, PMG0045; Thermo Fisher Scientific), respectively, for 24 h. Cell proliferation was evaluated using BrdU Labeling and Detection Kit II (11299964001; Roche, Basel, Switzerland) according to the manufacturer’s instructions. Briefly, cells were incubated with BrdU at a final concentration of 10 μM for 1 h before staining. BrdU-positive cell number/field was counted by ImageJ, fold change relative to control group representing cell proliferation ability.
Wound healing (scratch model)
In brief, MOVAS cells were cultured in 12-well plates and transfected as described above. Transfected cells were allowed to grow to ∼100% confluence and then were subjected to serum starvation for 24 h. The cells were scratched and rinsed with PBS 3 times to remove cell debris. Denuded cell surface area of each wound at 0 h (A0) and 16 h (A16) was obtained by photomicrographic images and measured with Image-Pro Plus software by an experienced investigator who was blinded to the treatments. The percentage of closures (closure %) were calculated as (A0−A16)/A0 * 100.
Transwell migration assay
MOVAS cells were cultured and transfected as described. Transfected cells were harvested by trypsinization. An aliquot (50,000 cells/200 μl) in serum-free DMEM was dispensed into the transwell inserts (8-μm pore size, 07-200-150; Corning) and DMEM with 10% serum or 10 ng/ml PDGF-BB was placed in the lower chamber. The transwell plates were incubated at 37°C in a 5% CO2 incubator for 12 ∼ 18 h. Nonmigrated cells in the top insert were carefully removed with a cotton swab, and the migrated cells in the bottom side were stained with Giemsa stain. Images were captured at 5 fixed locations (right, bottom, left, up, and center), and migrated cells were counted by an experienced investigator blinded to the treatments.
Argonaute-RNA coimmunoprecipitation assay
Twenty-four hours after miR-221-3p overexpression in MOVAS cells, Argonaute protein immunoprecipitation and purification of total RNAs associated with Argonaute were performed with the miRNA Target IP Kit (25500; Active Motif, Carlsbad, CA, USA) (17).
Nanostring analysis
Total cellular RNA or EV-RNA was extracted as described above, and the quality of the isolated RNA was verified with Agilent RNA 6000 Nano Assay Kit reagents using an Agilent 2100 Bioanalyzer (Agilent Technologies). Cellular RNA samples with an RNA Integrity Number of 8.0 or higher were used for Nanostring analysis. The RNA samples were quantified using a standard Nano Drop ND-1000 spectrophotometer (Nano Drop Technologies, Wilmington, DE, USA). The RNA concentrations were then adjusted to 33 ng/µl. RNA (100 ng/3 µl) was used to determine the levels of gene expression. Mouse v.1.5 miRNA Expression Assay Kit was distributed by Nanostring Technologies (Seattle, WA, USA). Procedures were performed according to the manufacturer’s protocol (Nanostring Technologies). Collected data were imported into nSolver 3.0 software. Grouped data were then normalized to the geometric means of spiked-in positive controls (controls for assay efficiency) and spiked-in negative controls (normalized for background). Internal negative control probes included in each assay were used to determine a background threshold for each sample. Positive controls were included to normalize for any differences in preparation, hybridization, and processing efficiency. Housekeeping genes, including β-actin, β2-microglobulin (B2M), glyceraldehyde 3-phosphate dehydrogenase (GAPDH), and ribosomal protein L19 (Rpl19) exhibit stable expression across samples, and were also used during normalization in the nCounter system (Nanostring Technologies). A combination of miR-186, 191, 26a, 320a, 361, and 423-3p reference genes was validated as optimal for miRNA expression.
Nanostring miRNA expression counts were then analyzed with nSolver v.3.0 software to identify significantly regulated probes. A minimum threshold of 3-fold change was set to select the differentially regulated probes/genes in high-fat diet (HFD) tissues or LPS-challenged cells, relative to the normal chow diet (NCD) tissues or PBS-treated cells, respectively. Significantly regulated gene probes were sorted by using a Venn diagram generator (http://bioinfogp.cnb.csic.es/tools/venny). Heatmaps representing the expression of probes across groups were generated and hierarchically clustered based on the default parameters of the software. The data discussed in this manuscript have been deposited in the National Center for Biotechnology Information (NCBI) Gene Expression Omnibus and are accessible through the Gene Expression Omnibus series accession number GSE126808.
In vivo EV treatment
Fed a normal diet, recipient age-matched male and female mice (C57BL/6J, 4 mice/gender/treatment, 12 wk old) were randomly assigned NCD-EVs or HFD-EVs. Previous studies have shown that the quantity of circulating exosomes in obese mice is ∼30 µg/mouse (5, 17). Therefore, for in vivo treatment, obese or lean MAT-derived EVs (30 µg) were adoptively transferred into recipient mice via intraperitoneal injection once a week for 4 wk. Prior to injection, the isolated EVs were labeled with the PKH26 red fluorescent dye using a commercially available PKH26 fluorescent cell linker kit (MilliporeSigma) according to the manufacturer’s protocol. Mouse AA and the surrounding PVATs were separated, snap frozen in liquid nitrogen, and stored at −80°C for future RNA assay. Part of the AA with intact PVAT was dissected for histologic and immunostaining studies. Two AAs from each group were pooled, and total RNAs (including small RNAs) were extracted (Direct-zol RNA MicroPrep Kit; Zymo Research) for qRT-PCR analysis of miR-221-3p and mRNAs of genes of interest.
Perivascular delivery of miR-221-3p mimic to femoral PVAT
Fed a normal diet, male and female adult mice (C57BL/6J, 4 mice/gender/treatment, 12 to 16 wk old) were randomly assigned miR-221-3p mimic or negative control mimic. The day before the surgery, 30% pluronic gel was prepared under constant stirring overnight at 4°C, followed by filtration (0.2-µm syringe filter) and kept on ice until use. Analgesia was administered subcutaneously [20 mg/kg tramadol (Ultram; Chiron AS, Trondheim, Norway)] at least 1 h prior to surgery. Mice were then anesthetized with 1% isoflurane by nose cone. Prior to skin incision, 30 µl of local anesthetics lidocaine 20 mg/ml and bupivacaine 5 mg/ml (1:1) was injected. The left femoral artery was exposed with intact PVAT and miRNAs (2.5 nmol in 100 μl gel) applied perivascularly as described in a previous study (27). After surgery, the skin was closed and sutured, followed by subcutaneous administration of 1.0 ml of warm normal saline solution. Mice were returned to their cages once fully mobile. The entire procedure was performed on a heated pad. Postoperative care, including subcutaneous administration of meloxicam (first dose, 2 mg/kg; second dose, 1 mg/kg), separate caging, and continuous monitoring, was provided. After a month, the left femoral artery and surrounding PVAT were harvested for RNA extraction. Two femoral arteries and associated PVAT from each group were pooled, and total RNAs (including small RNAs) were extracted (Direct-zol RNA MicroPrep Kit) for qRT-PCR analysis of miR-221-3p and mRNAs of genes of interest.
Diet-induced obesity
Male C57BL/6J mice were fed a NCD or a HFD (60% fat; D12492; Research Diets, New Brunswick, NJ, USA) ad libitum from 4 wk of age to 20 wk. Mouse MAT, AA, and the surrounding PVAT was snap frozen with liquid nitrogen and stored at −80°C for future RNA or protein assays.
Animal study approval
Wild-type C57BL/6J breeder mice were received from The Jackson Laboratory (Bar Harbor, ME, USA) and bred in house. All mice were maintained on a 12-h light/dark cycle. All procedures for animal experimentation were undertaken in accordance with the principles and guidelines of the Canadian Council on Animal Care and approved by the Queen’s University Animal Care Committee (2017-1799).
Quantification and statistical analysis
Results are expressed as means ± sem. A Student’s t test and 1-way ANOVA analysis were used for normally distributed variables. Nonparametric Kruskal-Wallis tests were used for non-normally distributed variables. Each data point derived from qPCR assays represents the mean of technical duplicates. All cell line culture data are representatives of 3 independent replicated experiments. For primary cell culture experiments, the n value corresponds to a cell preparation from separate mice. For all mouse studies, the n value corresponds to individual mice of a given treatment. Values of P < 0.05 were considered statistically significant. The statistical analysis was performed with Prism 8.0 software (GraphPad Software, La Jolla, CA, USA). Repeated measures of postbaseline body weight were analyzed by use of a General Linear Model in IBM SPSS Statistics 19 software (IBM SPSS, Chicago, IL, USA).
RESULTS
PVAT inflammation initiates an early-stage VSMC phenotype switching
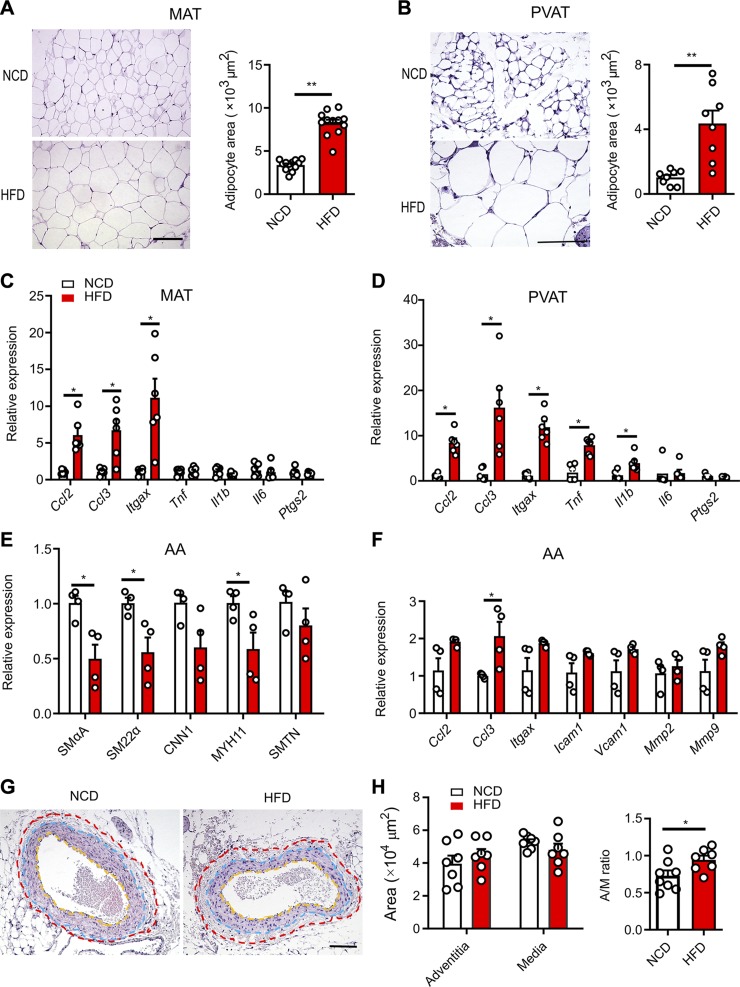
Fed an HFD for 16 wk, mice increased body weight and preferentially accumulated MAT (Supplemental Fig. S1A, B), compared with their NCD-fed counterparts. Next, we demonstrated the impacts of HFD on adipogenesis and inflammation in MAT and AA-PVAT, which are predominantly composed of white adipose and beige adipose tissue, respectively (2). HFD-fed mice showed a substantial increase in adipocyte size in MAT (Fig. 1A) and AA-PVAT (Fig. 1B). Adipocyte-derived anti-inflammatory adiponectin (Adipoq) transcript abundance was lower in HFD mice. Meanwhile, the thermogenesis-associated gene uncoupling protein-1 (Ucp1) and adipogenesis regulators CIDEA (Cidea, cell death inducing DFFA like effector a) and peroxisome proliferator–activated receptor γ (PPARγ, Pparg) were also decreased in HFD-MAT (Supplemental Fig. S1C) but not in HFD AA-PVAT (Supplemental Fig. S1D). Conversely, leptin transcript expression was significantly enhanced by HFD both in MAT and AA-PVAT (Supplemental Fig. S1C, D). These findings confirm that perivascular adipocytes display hypertrophic expansion and impaired adipogenesis, especially in white MAT. Consequently, expression of proinflammatory genes, such as C-C motif chemokine ligand 2 and 3 (Ccl2 and Ccl3), and Itgax (CD11c), was increased by HFD compared with NCD in both MAT and AA-PVAT (Fig. 1C, D). These findings indicate a remarkably higher state of proinflammatory cytokines/chemokines, consistent with previous reports (1, 28). The number of CD68-positive crown-like structure features, formed by infiltrating macrophages around adipocytes (29, 30), was markedly enhanced in MAT from HFD-fed mice (Supplemental Fig. S1E). Higher transcript expression of proinflammatory genes was also found in MAT-Ad and in 3T3-Ad challenged with bacterial LPS (1 µg/ml) as compared with PBS control (Supplemental Fig. S1F, G). Taken together, these findings confirm that perivascular adipocytes of mice experience a chronic inflammation state while on a long-term HFD.
Figure 1.
Obesity initiates PVAT inflammation, leading to phenotypic switching in arterial SMCs. A) Hematoxylin and eosin (HE)–stained MAT and mean area of adipocytes (n = 12). Scale bar, 100 µm. B) HE-stained AA-PVAT and mean area of adipocytes (n = 8). Scale bar, 100 µm. C, D) Quantification of real-time qPCR for relative mRNA expression of proinflammatory genes in MAT (C) and AA-PVAT (D). E) Relative mRNA expression of selective contractile marker genes including SMαA, SM22α, CNN1, SM-MYH11, and SMTN in AA (n = 4). F) Relative mRNA expression of selective “synthetic” genes including Ccl2, Ccl3, Itgax, Icam1, Vcam1, Mmp2, and Mmp9 in AA (n = 4). G) HE-stained AAs. The adventitial area is shown within the red and blue dotted lines and medial area is between the blue and yellow dotted lines. Scale bar, 100 µm. H) Quantification of AA adventitial area, medial area, and adventitia-to-media (A/M) ratio. Some unflushed blood remained in the lumen. All data are presented as means ± sem. *P < 0.05, **P < 0.01 vs. corresponding NCD value (unpaired, 2-tailed Student’s t test).
Over the course of HFD feeding, AA SMC-selective contractile marker genes, such as SMαA, smooth muscle 22α (SM22α), and smooth muscle myosin heavy chain (SM-MYH11), were significantly down-regulated, whereas smooth muscle calponin 1 (CNN1), and smoothelin (SMTN) showed a trend to decrease in HFD-fed mice as compared with NCD-fed mice (Fig. 1E). Conversely, the opposite effects were seen with “synthetic” genes including Ccl2, Ccl3, Itgax, Icam1, Vcam1, Mmp2, and Mmp9, showing a consistent trend to increase (Fig. 1F). These data suggest that gradually exacerbated inflammation in PVAT could induce an impairment of contractility in arterial SMCs, within which there is a phenotypic switching from a contractile to synthetic phenotype. SMαA immunostaining did not reveal clear migration of SMC and neointimal hyperplasia (Supplemental Fig. S1H). However, further histologic analysis revealed a greater adventitia-to-media ratio in the AA of HFD mice than in NCD mice (Fig. 1G, H), suggesting that the AAs from obese mice undergo an early-stage adventitial remodeling (24, 31).
PVAT produces and secretes EVs containing miRNAs
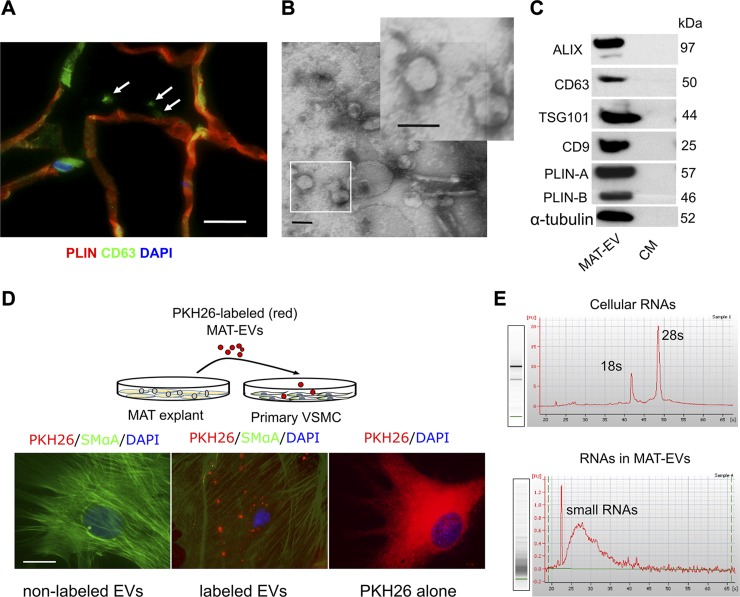
A number of reports have indicated that miRNAs are readily packaged in EVs and actively secreted from adipocytes, suggesting roles as intercellular signaling molecules (6, 12, 32). We carried out several assays to determine whether MAT or MAT-Ad produce and release EVs. Immunostaining of MAT revealed positive staining for EV marker CD63 in the interstitial space, adjacent to neighboring adipocytes (Fig. 2A), in agreement with previous findings in subcutaneous white adipose tissue (15). Next, EVs were isolated from the CM of MAT explants and culture supernatants of MAT-Ad and 3T3-Ad, and characterized as cup-shaped in morphology with a size of 50–150 nm in diameter (Fig. 2B and Supplemental Fig. S2A). These EVs are morphologically heterogeneous, especially as shown in MAT explant–derived EVs in electron microscopy studies. Western blot analysis revealed the presence of the EV-related protein markers ALIX (97 kDa), CD63 (50 kDa), TSG101 (44 kDa), and CD9 (25 kDa) and the adipocyte marker PLIN-A and PLIN-B (Fig. 2C and Supplemental Fig. S2B). Further Western blot analysis demonstrated that MAT-derived EVs exhibited very strong adipocyte marker expression of PLIN-A/B, minimal evidence of macrophage-derived CD68, and no expression of endothelial cell marker CD31 (Supplemental Fig. S2C), indicating these secreted EVs were mainly generated from perivascular adipocytes.
Figure 2.
Adipose tissue secretes EVs containing miRNAs. A) Immunostaining for the EV marker CD63 (green) and the adipocyte marker PLIN (red). The white arrows indicate extracellular CD63 stainings by confocal imaging. Scale bar, 15 µm. B) Electron microscopy analysis of EVs secreted by MAT explants, showing a size of ∼50–150 nm in diameter. The inset shows higher magnification of EVs. Scale bars, 100 nm. C) EV-related protein markers ALIX (97 kDa), CD63 (50 kDa), TSG101 (44 kDa), and CD9 (25 kDa) as well as adipocyte marker PLIN-A/B (57/46 kDa) were detected by Western blot analysis in MAT-EVs or supernatant CM. Blots are representative of 3 independent experiments. D) Uptake of EVs: Isolated MAT-EVs were labeled with PKH26 (red) and incubated (50 µg/ml) with primary VSMCs for 18 h. Middle, PKH26-labeled MAT-EVs. Nonlabeled MAT-EVs and PKH26 dye (1 × 10−6 M) alone were used as controls. Scale bar, 25 µm. E) Total RNA from MAT (cellular) or MAT-EVs was analyzed with a bioanalyzer. Both gels and electropherograms are shown. Y axis of the electropherogram is arbitrary fluorescence unit intensity, and x axis is migration time in seconds.
Moreover, these adipose tissue- or adipocyte-derived EVs, labeled with the fluorescent dye PKH26, can be subsequently taken up by primary VSMCs, as indicated by the punctate presence of red fluorescence staining in live cells cocultured with PKH26-labeled EVs (Fig. 2D, middle), but not in cells cocultured with nonlabeled EVs (Fig. 2D, left). Lipophilic PKH26 dye was able to label whole cells by incorporating in membranes when applied alone (Fig. 2D, right). Further studies revealed that efficient uptake of labeled 3T3-EVs could only occur in nonfixed (live) cells rather than in in prefixed (dead) SMCs (Supplemental Fig. S2E, F). Total RNA purified from tissue- or cell-derived EVs barely contains rRNA (18s and 28s) but does contain large amounts of small RNAs, including miRNAs, compared with that of cellular RNA of MAT or MAT-Ad (Fig. 2E and Supplemental Fig. S2D).
Obesity and inflammation up-regulate miR-221-3p expression in the artery and PVAT
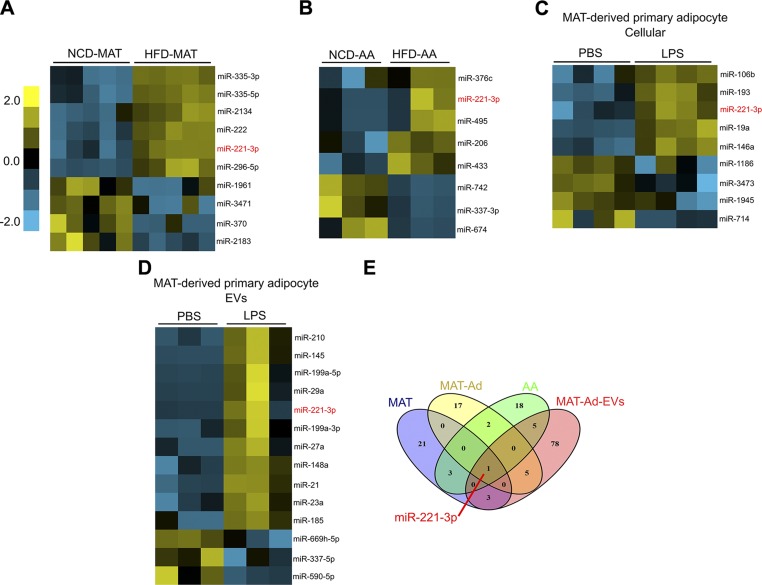
To profile the differentially expressed miRNAs in obesity or inflammation, an integrative miRNA analysis (578 mouse miRNAs) was performed using Nanostring technology in MAT, MAT-Ad, AA, and MAT-Ad-EVs. Hierarchical clustering analysis performed on the most significantly regulated miRNAs (HFD vs. NCD in tissues; LPS challenge vs. PBS in cells) showed a set of miRNAs that are differentially expressed in obese vs. lean MAT or AA tissues (Fig. 3A, B), and in LPS-challenged vs. PBS-treated MAT-Ads (Fig. 3C). A heatmap representing the dysregulated miRNAs in MAT-Ad-EVs was also generated, which included up- and down-regulated candidates induced by prolonged exposure to LPS (Fig. 3D). Significantly dysregulated miRNAs were then sorted by Venn diagram analysis (Fig. 3E), showing the overlapping miRNAs. miR-221-3p was identified as an obesity-associated inflammatory pathway among the 4 profiled conditions. Our findings are consistent with previous reports in which miR-221-3p was found to influence adipocyte differentiation and lipid metabolism (33, 34) as well as to promote white adipose tissue inflammation and impair insulin sensitivity in obesity (35).
Figure 3.
Obesity and inflammation lead to changes in miRNA profiles determined by Nanostring technology. A) Differential expression level of miRNAs between MATs of mice fed with an NCD or HFD (n = 5). B) Hierarchical clustering analysis of miRNAs in AA. Two to 3 AA samples from the same groups were pooled during total RNA extraction (n = 3). C, D) Differential expression level of miRNAs in MAT-Ad exposed to PBS or LPS (1 µg/ml) for 24 h (C) and MAT-Ad-EVs (D). E) Identification of commonly dysregulated miRNAs. Venn diagram shows the number of differentially expressed (fold change >3) miRNAs induced by obesity in MAT and AA or challenged by LPS in MAT-Ad and MAT-Ad-EVs. miR-221-3p is identified as the commonly up-regulated miRNA with greater abundance in obese tissues/inflamed cells.
Adipocyte-derived miR-221-3p is transported to adjacent aorta
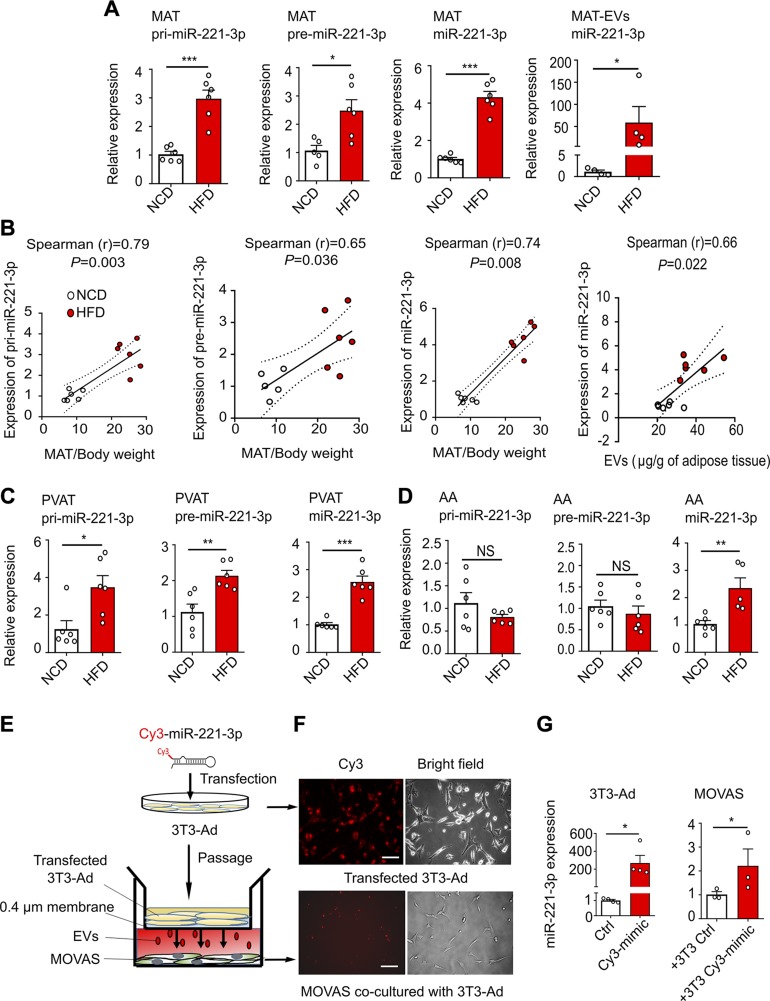
The identified miR-221-3p was chosen as the prime candidate for further study. Our results show that MAT from obese mice expresses ∼3-fold greater intracellular levels of primary (pri-) miR-221-3p and ∼2-fold greater levels of precursor (pre-)miR-221-3p, which results in ∼4-fold increased levels of mature miR-221-3p, and further leads to a significant increase of miR-221-3p in the MAT-EVs (Fig. 4A). Of note, the miR-221-3p expression level is significantly correlated with MAT weight relative to body weight, at the primary, precursor, and mature transcript levels (Fig. 4B). Moreover, mature miR-221-3P enrichment in MAT is positively correlated with the secreted EV amount from MAT explants (Fig. 4B). Qualitatively similar results of elevated miR-221-3p expression were seen in AA-PVAT from HFD mice (Fig. 4C), in MAT-Ad (Supplemental Fig. S3A), and in 3T3-Ad (Supplemental Fig. S3B), both challenged by LPS for 24 h. These data demonstrate that miR-221-3p biogenesis is enhanced in MAT and PVAT during obesity-associated inflammation. miR-221-3p is also one of the players in regulating VSMC phenotype (36–38). Consistent with these findings, mature miR-221-3p expression was markedly increased in AA from obese mice compared with AA from lean mice (Fig. 4D). Interestingly, pri- and pre-miR-221-3p levels in AA from both obese and lean mice are comparable (Fig. 4D), indicating that the biosynthetic source of mature miR-221-3p is not AA, but most likely produced from neighboring PVAT and then transported into AA via miRNA-containing EVs.
Figure 4.
Biogenesis and transport of miR-221-3p. A) Real-time qPCR-determined relative expression of pri-miR-221-3p, pre-miR-221-3p, and mature miR-221-3p in MAT, and miR-221-3p in MAT explant–derived EVs (MAT-EVs). B) Spearman’s rank correlation analyses were carried out to characterize the relationships between the ratio of MAT/body weight and pri-miR-221-3p, pre-miR-221-3p, and miR-221-3p in NCD and HFD mice. The last panel shows Spearman’s rank correlation between the relative expression of miR-221-3P and the secreted EV amount from MAT explants. C, D) Expression levels of pri-miR-221-3p, pre-miR-221-3p, and miR-221-3p in mouse PVAT (C) and the AA (D). NS indicates no significance. E) A transwell coculture assay was performed with 3T3-Ad transfected with a Cy3-labeled miR-221-3p mimics in the upper chamber, MOVAS cells in the bottom well, and a 0.4-µm porous membrane between the 2 wells inhibiting cell-cell contact. F) Representative images of 3T3-Ad cells transfected with Cy3-labeled miR-221-mimics (Cy3-mimic) (upper panel); images of recipient MOVAS cells cocultured with transfected 3T3-Ad cells for 24 h (lower panel). Control (ctrl) images are not shown. Scale bar, 100 µm. G) Relative expression of miR-221-3p was measured in transfected 3T3-Ad or recipient MOVAS cells. The pri-miR-221-3p and pre-miR-221-3p data were normalized to levels of endogenous GAPDH, whereas the cellular and EV–miR-221-3p expression data were normalized with miR-361-5p and miR-320a, respectively. All data are presented as means ± sem, *P < 0.05, **P < 0.01, ***P < 0.001 vs. corresponding NCD or ctrl value (unpaired, 2-tailed Student’s t test).
To further determine the EV-mediated intercellular transportation of miR-221-3p from adipocytes to VSMCs, a transwell coculture assay was established. As illustrated in Fig. 4E and Supplemental Fig. S3C, the primary MAT-Ad or 3T3-Ad were transfected with Cy3-labeled mimics of miR-221-3p and then passaged onto the upper chamber of the transwell, with the recipient primary VSMCs or MOVAS cells in the lower chamber. After overnight coculture, adipocyte-derived Cy3-labeled miR-221-3p expression was observed in the SMCs (Fig. 4F and Supplemental Fig. S3D). Moreover, the real-time qPCR data revealed a more than 200-fold increase of miR-221-3p expression in the 3T3-Ad transfected cells with Cy3-miR-221-3p compared with those transfected with control mimic, which resulted in ∼2-fold greater levels of miR-221-3p in the MOVAS cells receiving Cy3-miR-221-3p secreted from the transfected 3T3-Ad (Fig. 4G).
It is well known that EV, especially exosomal formation and release, are modulated by neutral sphingomyelinase (39), which is inhibited by GW4869. Thus, pretreatment (24 h) with GW4869 in transfected adipocytes blocked EV production and delivery of miR-221-3p from adipocytes into SMCs (Supplemental Fig. S3E, F), showing that adipocytes secrete extracellular miRNAs predominantly in an EV-dependent manner. Overall, our results show that PVAT adipocytes can secrete miRNA-containing EVs, which are then efficiently transported into neighboring SMCs.
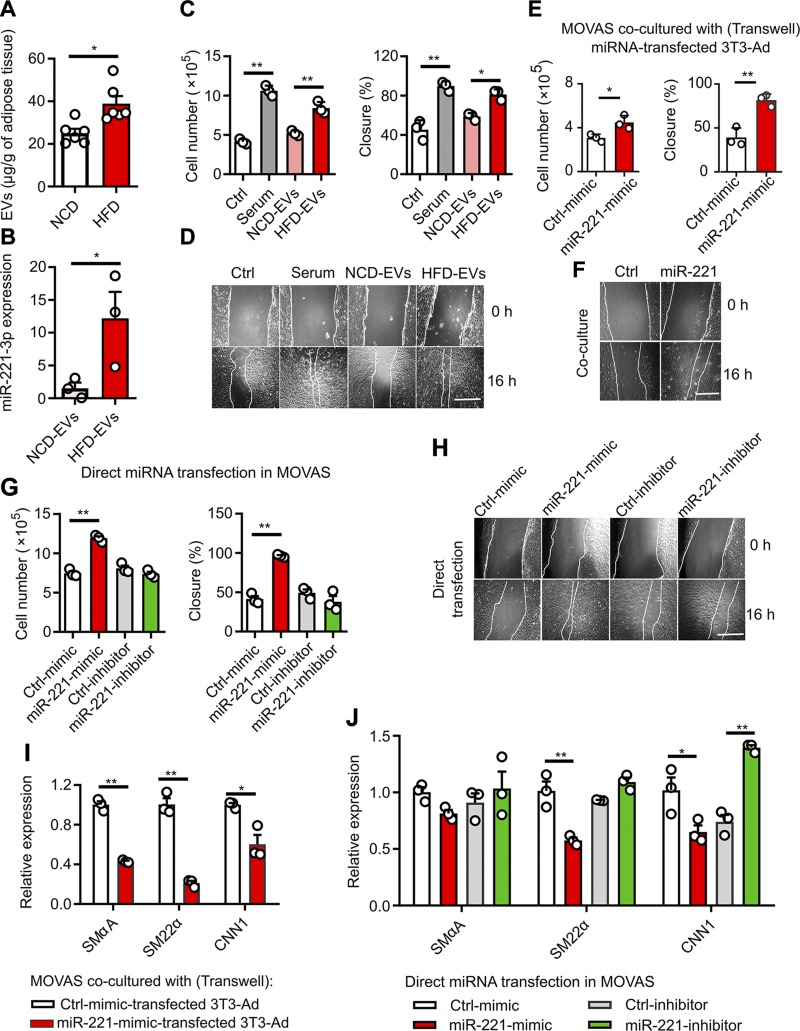
miR-221-3p–containing EVs promote VSMC proliferation, migration, and phenotype switching in vitro
To explore the potential of miRNA-containing EVs in VSMC dysfunction, we examined in vitro models of VSMC proliferation, migration, and phenotype switching. Firstly, we found that more EVs were released in ex vivo MAT cultures of age-matched mice fed an HFD over 16 wk when compared with NCD-fed mice (Fig. 5A). The increased release of EVs is one of the features of obesity-induced adipocyte hypertrophy (5, 11). Moreover, HFD-EVs contain a high abundance of miR-221-3p, which in turn can be taken up by MOVAS cells, leading to ∼10-fold greater intracellular levels of miR-221-3p (Fig. 5B). HFD-EVs significantly promote MOVAS proliferation and migration as demonstrated by cell counting and wound-healing assays (Fig. 5C, D). To further investigate whether MAT-derived EVs are crucially involved in this paracrine action, we then treated MOVAS cells with CM containing EVs or CM depleted of EVs, prepared from obese (HFD-CM) and lean (NCD-CM) MAT explants. MOVAS cell number and wound closure (%) were significantly increased by HFD-CM containing EVs, relative to those of NCD-CM containing EVs (Supplemental Fig. S4A, B). Depletion of EVs in CM by sequential ultracentrifugation partially abrogated the proliferative and promigrating effects (Supplemental Fig. S4C, D). It is important to note that VSMC proliferation, growth, and migration were dramatically enhanced by miR-221-3p overexpression in transwell cocultures (Fig. 5E, F) or in directly transfected MOVAS cells (Fig. 5G, H and Supplemental Fig. S4E), as determined by cell counting, wound healing, BrdU incorporation, and transwell migration assays. In the presence of PDGF-BB (10 ng/ml), a proliferative factor of VSMC (31, 40), miR-221’s effects to enhance SMC growth and migration became more potent (Supplemental Fig. S4F). Real-time qPCR data showed that miR-221-3p expression in 3T3-Ad and MOVAS cells was significantly up-regulated by miR-221-3p mimics and down-regulated by the miR-221-3p inhibitor (Supplemental Fig. S4G, H). The expression of 3 VSMC genes (SmαA, SM22α, and CNN1) was subsequently decreased in MOVAS cells overexpressing miR-221-3p (Fig. 5I, J).
Figure 5.
miR-221-3p modulates VSMC proliferation, migration, and phenotype switching. A) MAT explants isolated from mice fed HFD or NCD for 16 wk were cultured for 24 h. The amounts of secreted EVs were quantified by Bradford protein assay. B) Abundance of miR-221-3p transported via EVs in MOVAS cells. Followed by 24-h serum starvation, 50 µg/ml of NCD- or HFD-EVs, on the basis of protein measurement, were added to the cells for another 24 h. C) After 24 h of serum starvation, MOVAS cells were treated with control (ctrl; 0.5% serum), 20% serum, NCD- or HFD-EVs (50 µg/ml) for an additional 24 h, followed by cell counting and wound-healing assays. D) Representative photographs showing wound healing progression from 0 to 16 h upon treatment with EVs. E) After transfection with miR-221-3p mimics or negative control mimic (Ctrl-mimic), 3T3-Ad cells were cocultured with starved MOVAS cells in the transwell system (membrane pore = 0.4 µm) for 24 h. MOVAS cell growth (cell counting) and migration (wound healing) were carried out. F) Representative photographs showing wound-healing progression from 0 to 16 h in cocultivated MOVAS cells. G) Cell counting and migration assays (wound healing) in MOVAS cells directly transfected with miR-221-3p mimics, miR-221-3p inhibitor, or respective control miRNAs. H) Representative photographs showing wound healing progression from 0 to 16 h in MOVAS cells after direct transfection. I) Relative mRNA expression of selective contractile marker genes including SMαA, SM22α, and CNN1 in MOVAS cells cocultured with 3T3-Ad cells, which were transfected with miR-221-3p mimics or ctrl-mimic. J) Relative mRNA expression of SMαA, SM22α, and CNN1 in MOVAS cells directly transfected with miR-221-3p mimics, miR-221-3p inhibitor, or respective ctrl miRNAs. In wound-healing assays, the percentage of cell closure (%) was calculated. Data are averaged representatives from at least 3 independent experiments. Scale bar, 500 µm (D, F, H). *P < 0.05, **P < 0.01 vs. orresponding NCD or ctrl value (unpaired, 2-tailed Student’s t test or 1-way ANOVA).
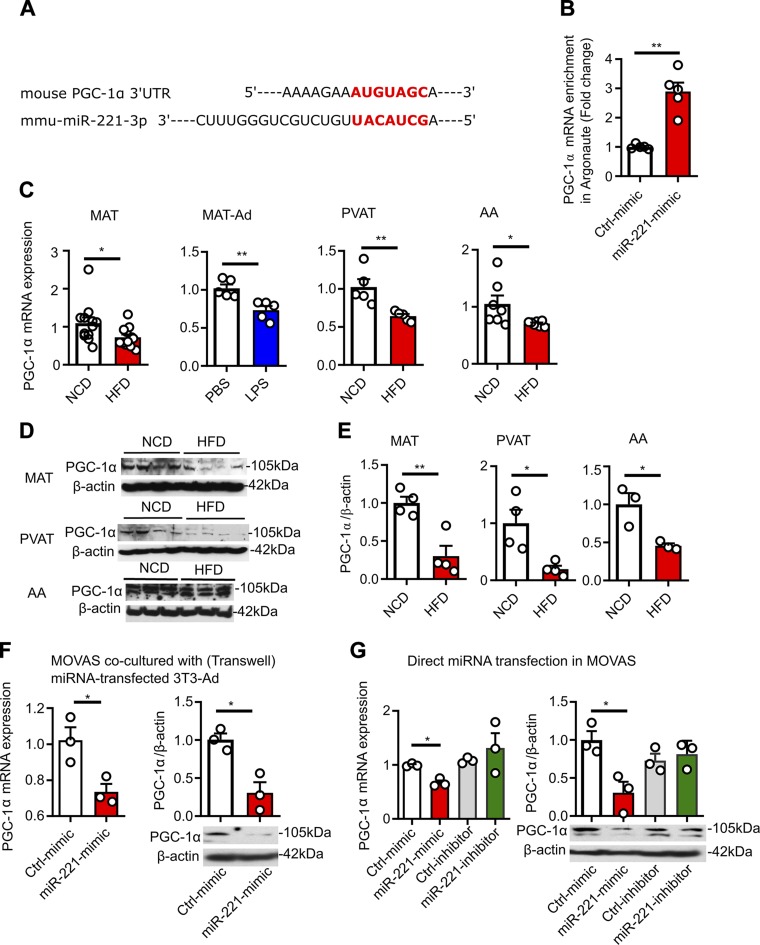
PGC-1α is a functional target gene of miR-221-3p in adipocytes and VSMCs
A conserved miR-221-3p binding site was found within the PGC-1α mRNA 3′-UTR using TargetScan (http://www.targetscan.org/vert_72/) (Fig. 6A). miR-221-3p reportedly influences the progression of atherosclerosis through direct suppression of PGC-1α (41), a master regulator of mitochondrial function (42). To further show that PGC-1α is a direct target of miR-221-3p, we immunoprecipitated Argonaute (Ago), a protein bound with miRNAs, to recruit their target mRNA into the RNA-induced silencing complex. qPCR analysis shows that overexpression of miR-221-3p results in an increase in the amount of PGC-1α bound in the RNA-induced silencing complex, specifically to Ago (Fig. 6B). Data of qPCR and Western blot analyses showed both PGC-1α mRNA (Fig. 6C) and protein expression (Fig. 6D, E) are decreased in HFD-MAT, LPS-challenged MAT-Ads, PVAT, and AA. Similarly, in vitro data show that PGC-1α mRNA and protein expression were down-regulated transcriptionally and translationally by miR-221-3p overexpression, either in the transwell cocultures (Fig. 6F) or in MOVAS cells transfected with miR-221-3p (Fig. 6G). These data indicate that miR-221-3p targets PGC-1α, leading to mitochondrial dysfunction and metabolic disorders in SMCs (42, 43).
Figure 6.
miR-221-3p targets PGC-1α in adipocytes and VSMCs. A) The predicted miR-221-3p binding site (highlighted in red) within PGC-1α 3ʹ-UTR by TargetScan. B) The abundance of PGC-1α mRNA bound with Argonaute protein in MOVAS cells after overexpression of miR-221-3p. Following the immunoprecipitation via Ago 1, 2, and 3 antibodies, the PGC-1α mRNA transcripts enriched in the RISC components were purified and amplified by qPCR. C) Quantification of relative mRNA expression of PGC-1α in MAT, MAT-Ad, PVAT and AA. D, E) Representative immune-blots (D) and quantification (E) of PGC-1α protein expression in MAT, PVAT, and AA. F) PGC-1α mRNA and protein expression in MOVAS cells cocultured with miRNA-transfected 3T3-Ad cells. G) PGC-1α mRNA and protein expression in MOVAS cells directly transfected with miR-221 mimic, a miR-221 inhibitor, or respective control (ctrl) miRNAs. *P < 0.05, **P < 0.01 vs. corresponding NCD or ctrl value (unpaired, 2-tailed Student’s t test or 1-way ANOVA).
miR-221-3p causes vascular dysfunction in AA and femoral artery
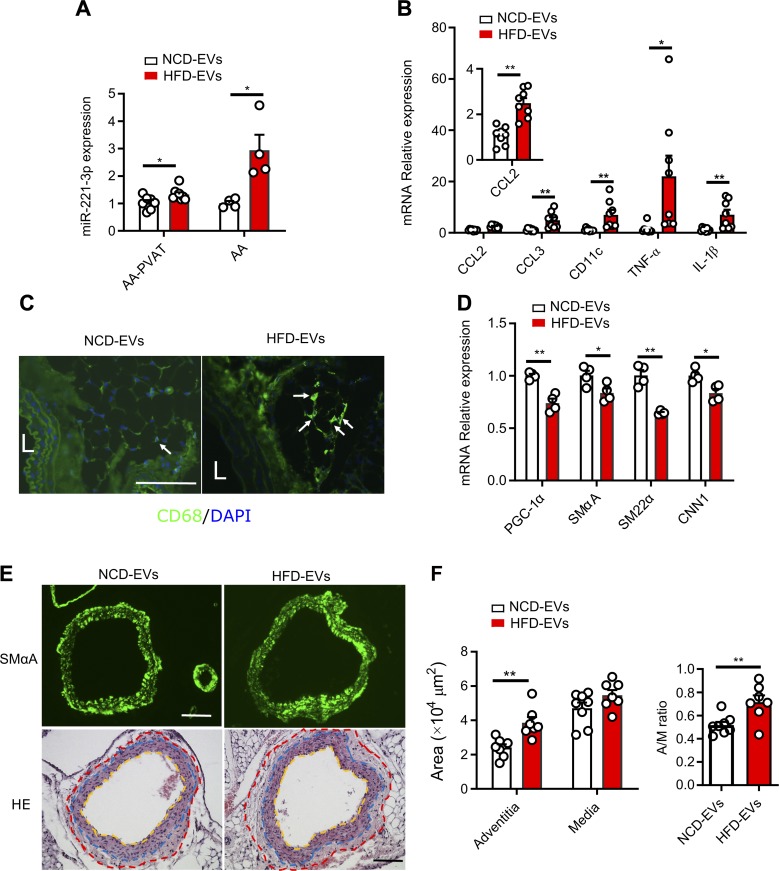
Given the detrimental effect of EVs from obese mice and miR-221-3p on vascular function in vitro, we next evaluated whether MAT-EVs from obese mice could enhance vascular phenotypic switching in lean recipient animals. We confirmed an efficient in vivo tissue uptake of PKH26-labeled EVs when administered intraperitoneally, as measured by the appearance of PKH26 fluorescence in fresh MAT of lean mice (Supplemental Fig. S5A), without differences in fluorescence levels between HFD-EVs and NCD-EVs. Further observation revealed that PKH26-labeled EVs were localized in AA in embedded and processed tissues (Supplemental Fig. S5B). Treatment with EVs, whether obtained from obese or lean mice, had no differential effect on body weight over the course of 4 wk (Supplemental Fig. S5C). As predicted from our in vitro results, a 4-wk treatment with HFD-EVs led to nearly 3-fold greater intracellular levels of miR-221-3p in AA and ∼1.3-fold in abdominal PVAT (Fig. 7A). EV treatment did not change the intercellular expression level of miR-143-5p and miR-155-5p (Supplemental Fig. S5D), further indicating that miR-221-3p was an important cargo in EVs. In addition, lean mice taking up obese HFD-EVs show increased expression of proinflammatory genes in PVAT (Fig. 7B) and enhanced macrophage (CD68-labeled) infiltration in abdominal PVAT (Fig. 7C). The ability of HFD-EVs to enhance vascular dysfunction was further confirmed in vivo, as shown by decreased expression of contractile markers (SmαA, SM22α, and CNN1) and target gene PGC-1α in AA (Fig. 7D). In agreement with previous HFD-induced vascular phenotypic switching, a 4-wk treatment with HFD-EVs did not appear to cause neointimal hyperplasia as determined by SMαA immunostaining at an early stage of vascular dysfunction (Fig. 7E, upper panel). Detailed vascular histopathological analysis indicated that HFD-EVs resulted in a comparable medial area in lean mice treated with NCD-EVs or HFD-EVs, whereas the adventitial area in HFD-EV–treated mice was remarkably greater compared with NCD-EV–treated mice, which was accompanied with an increased adventitia-to-media ratio (Fig. 7E, lower panel and Fig. 7F).
Figure 7.
MAT-derived EVs from obese mice causes arterial phenotypic switching in vivo. A) Intraperitoneal delivery of HFD-EVs from obese mice induced higher expression level of miR-221-3p in lean recipient AA and PVAT. Total RNA was harvested from arteries and surrounding PVATs before undergoing real-time qPCR analyses. B) Quantification of real-time qPCR data for relative mRNA expression of proinflammatory genes in abdominal PVAT. C) Immunohistochemical staining for the macrophage marker CD68 (green) and the nuclei (DAPI, blue). The white arrows indicate CD68-labeled cells. Scale bar, 100 µm. L, lumen. D) Relative mRNA expression of selective contractile marker genes including SMαA, SM22α, and CNN1 and target gene PGC-1α in AA. E) Upper panel, immunohistochemical staining for SMαA of AA. Lower panel, Hematoxylin and eosin (HE)-stained AAs and their PVATs. The adventitial area is shown within the red and blue dotted lines, and the medial area is between the blue and yellow dotted lines. Scale bar, 100 µm. F) Quantification of AA adventitial area, medial area, and adventitia-to-media (A/M) ratio. Data represent means ± sem. Two AAs were pooled for each RNA purification. *P < 0.05, **P < 0.01 vs. corresponding NCD-EV value (unpaired, 2-tailed Student’s t test).
To further determine the potential causal role of EV–miR-221 pathway in arterial remodeling, 2.5 nmol of miR-221-3p mimic or mimic negative control miRNA was perivascularly applied to femoral arteries with intact PVAT. In comparison with tissues treated with mimic control miRNA, both arteries and femoral PVATs treated with miR-221-3p mimics displayed significantly increased expression of miR-221-3p (Supplemental Fig. S5E) and decreased expression of VSMC genes and target PGC-1α gene (Supplemental Fig. S5F). Consistently, direct overexpression of miR-221-3p in the femoral artery resulted in adventitial thickening and a higher adventitia-to-media ratio (Supplemental Fig. S5G, H). These findings suggest that direct or indirect overexpression of miR-221-3p in the PVAT significantly reduced the expression of its target gene, and subsequently caused VSMCs to undergo phenotypic switching and adventitial remodeling in femoral arteries and AAs. As depicted in Supplemental Fig. S5I, we propose a novel EV-miRNA–mediated intercellular communication mechanism between SMCs and their surrounding PVAT.
DISCUSSION
Here, we clearly have shown that PVAT produces miRNA-containing EVs, which are subsequently taken up by neighboring SMCs, leading to arterial remodeling and phenotypic switching, both in vitro and in vivo. Moreover, the biogenesis and enrichment with miR-221-3p in the EVs are regulated by obesity-induced low-grade inflammation. Mechanistically, the EV-mediated miRNA-221-3p transport suppresses downstream target PGC-1α, a known metabolic modulator of SMCs.
PVAT during inflammation secretes miR-221-3p–containing EVs
Adipose tissues are highly heterogeneous tissues that play various roles in the dialogue with their neighboring organs. Surrounding both arteries and veins, PVAT actively modulates vascular functions in normal health and various inflammation-related cardiovascular diseases (1). PVAT inflammation potentially triggers an inward cascade from adventitia to endothelium, which in turn exacerbates endothelial dysfunction and atherosclerosis (44). In rodents, the mesenteric, carotid, and femoral arteries are surrounded by white adipose, whereas the thoracic aorta is surrounded by brown adipose tissue and the AA by beige PVAT (mixture of white and brown adipocytes) (2). Anatomically separated adipose tissue depots appear to be functionally diverse, depending on their cellular composition (28, 45). In our present study, we did not choose thoracic PVAT, which is brown adipose tissue and resistant to diet-induced obesity/inflammation (28, 45). As shown in our data, mesenteric MAT is more sensitive to the HFD challenge, where the adipose “browning” genes are dramatically down-regulated (46). However, white mesenteric and abdominal PVATs are similarly prone to the expansion of adipocytes and HFD-induced inflammation. To evaluate accurately the adipose tissue weight and to obtain a greater tissue yield for downstream EV generation, we used MAT as our main source to derive primary adipocytes and isolate EVs in ex vivo tissue culture.
We have isolated and characterized a pool of PVAT (MAT) or perivascular adipocyte-derived EVs that traffic between adipocytes and VSMCs to communicate proatherogenic signals. Based on the size and biogenesis, EVs are broadly characterized as exosomes and microvesicles. The protocols used in this study did not experimentally distinguish between these 2 types of EVs because of their overlap of size, similar morphology, and biochemical properties (15). Despite their morphologic heterogeneity, they are characterized as small EVs with a cup shape and a size of 50–150 nm in diameter in transmission electron microscopy observations. Although the MAT explant EVs are likely a product of EV secretion from multiple cell types, we further confirmed the presence of adipocyte-specific marker PLIN in MAT-EVs (22, 47) and showed evidence that EVs are released from MAT-Ad and 3T3-Ad. Moreover, these secreted EVs, along with their cargos (herein miRNAs), can be taken up into SMCs, mimicking the transport of EV messenger molecules from adventitial adipocytes to adjacent medial SMCs.
The present study demonstrates that PVAT inflammation increases the generation of EVs, originating mainly from hypertrophic adipocytes. These EVs were recently determined to contain a variety of lipids, revealing a form of intercellular communication with adipose tissue macrophages (16). We also uncovered a positive correlation between miR-221-3p–associated recruitment in these EVs and adipose tissue accumulation, as indicated in HFD-MAT and LPS-challenged MAT-Ad derived EV–miR-221-3p expression detected by both Nanostring technology and qPCR. VSMCs can also produce EVs, harboring exosomal markers CD9 and CD63 (48). However, in our experimental setting, VSMCs acted as recipients in the perivascular microenvironment to accept miR-221-3p containing EVs. In recent reports, VSMCs could internalize EVs derived from endothelial cells (49) or macrophages (14). However, the exact loading mechanism of miR-221-3p into EVs was not addressed in this study and remains to be explored.
Role of miR-221-3p in inflammation and vascular remodeling
In overweight and obesity settings, adipocytes undergo hypertrophy, which leads to increased biogenesis and secretion of an array of miRNAs, including miR-221 (33), miR-155 (32), miR-143 (50), and miR-130b (51, 52). These miRNAs are involved in various cellular responses during vascular remodeling, such as smooth muscle de-differentiation, migration, and proliferation (53–56). miR-221-3p has been primarily identified as an oncogenic miRNA, targeting the tumor suppressors p27 (Kip1) and p57 (Kip2) (57–59), and recently as a regulator of innate immune memory (60). During VSMC phenotype switching, it was demonstrated that miR-221-3P enhances VSMC growth in vitro and neointimal hyperplasia in balloon-injured carotid arteries in vivo (36, 37). By making use of the miRNA gain- and loss-of-function approaches, we demonstrated that miR-221-3p suppresses VSMC contractile gene expression and promotes VSMC proliferation and migration. In addition to direct transfection of VSMCs, we further showed that adding miR-221-3p–containing EVs to VSMCs or coculture of adipocytes overexpressing miR-221-3p with VSMCs are both efficacious ways to induce phenotypic switching.
The expression of miR-221-3p is fine tuned by multiple factors in a context-dependent manner. The delicate processing of pri-miR-221 and pre-miR-221 by Drosha or Dicer (the key enzymes controlling miRNA biogenesis) activity may differ in distinct cell types (61). Here we showed that at an early stage of vascular dysfunction and adventitial remodeling, the biogenesis of miR-221-3p in AAs from obese mice was not recognizably different from the lean counterparts, as indicated by the comparable expression level of pri-miR-221 and pre-miR-221. In contrast, VSMCs were prone to uptake the exogenous EV–miR-221-3p, mainly generated and secreted from adventitial adipocytes. In addition to various in vitro models, we showed that the EV–miR-221-3p pathway can result in thickening of arterial adventitia and furthermore can switch contractile SMCs to a synthetic phenotype in femoral arteries and AAs. This phenomenon would become more prominent with the progression of disease because miR-221 reportedly modulates vascular remodeling in the cardiovascular complications of diabetes (62, 63).
PGC-1α is a target of miR-221-3p in VSMCs
Our study here is the first to reveal an inverse relationship between the expression levels of miR-221-3p and its target gene PGC-1α both in vivo and in vitro. Previous studies have shown that PGC-1α functions as a transcriptional regulator that can modulate mitochondrial biogenesis and function by targeting mitochondrial genes during adaptive thermogenesis (42, 64). Recent studies demonstrate that PGC-1α acts as a master regulator of vascular disease progression, particularly in endothelial dysfunction and VSMC proliferation, through effects on oxidative stress, apoptosis, inflammation, and cell proliferation. Up-regulation of PGC-1α mitigates these parameters and can reduce atherosclerotic lesions (65–69). PGC-1α is readily induced by exercise (70) and caloric restriction (71); in contrast, it is down-regulated by obesity (72). miR-221-3p, along with miR-19b-3p and miR-222-3p, located in the intima of atherosclerotic vessels, could posttranscriptionally regulate PGC-1α protein expression, exerting their roles in the pathogenesis of atherosclerosis by modulating endothelial cell apoptosis (41). In this study, we provide more evidence that PGC-1α is a target gene of miR-221-3p–mediated VSMC phenotype modulation. We specifically demonstrate that miR-221-3p transcriptionally and posttranscriptionally inhibits PGC-1α by using miRNA target immunoprecipitation, qPCR, and Western blot analysis. The inhibition of PGC-1α expression was observed in obese MAT, PVAT, and AA as well as in SMCs overexpressing miR-221-3p.
We recognize there are some potential limitations to the conclusions that can be drawn from the current studies. First, we chose white adipose tissue MAT to generate EVs, which are heterogeneous populations. In our preparations, there may have been some minor macrophage contamination, as evidenced by weak CD68 immunoblot staining. An affinity-based EV purification, or a nanoscale flow cytometry separation could sort distinct subpopulations of EVs from specific sources and with variable functions (73). Another limitation is that we did not deliver anti-miR221-3p to the MAT explants prior to isolating EVs/coculture to block miR-221-3p functions. To further validate and increase the translational feasibility of our current findings, delivery of anti-miR221-3p in vivo and extending these studies to a hyperlipidemia- or wire-induced atherogenic animal model would substantiate a therapeutic potential of anti–miR-221-3p in obesity- and chronic inflammation-related diseases.
Our data thus uncovers the existence of an EV-mediated PVAT-to-VSMC signaling axis, which becomes evident in the context of vascular inflammation. PVAT-derived EVs, accumulating in the PVAT in an inflammatory context are readily taken up by proliferating SMCs. Encapsulated miR-221-3p acts as a form of adipokine, exerting paracrine regulation. At an early preatherosclerotic stage, VSMCs exhibit a low proliferative rate and maintain a stable phenotype. Early intervention or down-regulation of miR-221-3p, as in the use of miRNA inhibitors, could be a potential treatment to prevent or slow obesity-induced vascular dysfunction.
ACKNOWLEDGMENTS
The authors thank Brooke Ring-Snetsinger for the technical assistance in NanoString analysis, and Xiaohu Yan for help with electron microscopy (both from Queen’s University). This work was supported by Canadian Institutes of Health Research (CIHR) Grant MOP142476. C.D.F. was supported by a Tier 1 Canada Research Chair in Molecular, Cellular, and Physiological Medicine. The authors declare no conflicts of interest.
Glossary
- 3T3-Ad
3T3-L1–derived adipocyte
- AA
abdominal aorta
- BrdU
5-bromo-2’-deoxy-uridine
- CM
conditioned medium
- CNN1
calponin 1
- EV
extracellular vesicle
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- HFD
high-fat diet
- MAT
mesenteric adipose tissue
- MAT-Ad
MAT-derived adipocyte
- miRNA
microRNA
- NCD
normal chow diet
- PDGF
platelet-derived growth factor
- PGC-1α
peroxisome proliferator–activated receptor γ coactivator 1α
- PLIN
perilipin
- PVAT
perivascular adipose tissue
- qPCR
quantitative PCR
- SMαA
smooth muscle α-actin
- SM22α
smooth muscle 22α
- SMC
smooth muscle cell
- TSG101
tumor susceptibility gene 101
- VSMC
vascular SMC
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X. Li and L. L. Ballantyne performed the experiments; X. Li, Y. Yu, and C. D. Funk designed the experiments, discussed the project, and wrote the manuscript; and all authors reviewed the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Chatterjee T. K., Stoll L. L., Denning G. M., Harrelson A., Blomkalns A. L., Idelman G., Rothenberg F. G., Neltner B., Romig-Martin S. A., Dickson E. W., Rudich S., Weintraub N. L. (2009) Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ. Res. 104, 541–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brown N. K., Zhou Z., Zhang J., Zeng R., Wu J., Eitzman D. T., Chen Y. E., Chang L. (2014) Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler. Thromb. Vasc. Biol. 34, 1621–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajsheker S., Manka D., Blomkalns A. L., Chatterjee T. K., Stoll L. L., Weintraub N. L. (2010) Crosstalk between perivascular adipose tissue and blood vessels. Curr. Opin. Pharmacol. 10, 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van de Voorde J., Pauwels B., Boydens C., Decaluwé K. (2013) Adipocytokines in relation to cardiovascular disease. Metabolism 62, 1513–1521 [DOI] [PubMed] [Google Scholar]
- 5.Deng Z. B., Poliakov A., Hardy R. W., Clements R., Liu C., Liu Y., Wang J., Xiang X., Zhang S., Zhuang X., Shah S. V., Sun D., Michalek S., Grizzle W. E., Garvey T., Mobley J., Zhang H. G. (2009) Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 58, 2498–2505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogawa R., Tanaka C., Sato M., Nagasaki H., Sugimura K., Okumura K., Nakagawa Y., Aoki N. (2010) Adipocyte-derived microvesicles contain RNA that is transported into macrophages and might be secreted into blood circulation. Biochem. Biophys. Res. Commun. 398, 723–729 [DOI] [PubMed] [Google Scholar]
- 7.Van Niel G., D’Angelo G., Raposo G. (2018) Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 [DOI] [PubMed] [Google Scholar]
- 8.Valadi H., Ekström K., Bossios A., Sjöstrand M., Lee J. J., Lötvall J. O. (2007) Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat. Cell Biol. 9, 654–659 [DOI] [PubMed] [Google Scholar]
- 9.Bang C., Batkai S., Dangwal S., Gupta S. K., Foinquinos A., Holzmann A., Just A., Remke J., Zimmer K., Zeug A., Ponimaskin E., Schmiedl A., Yin X., Mayr M., Halder R., Fischer A., Engelhardt S., Wei Y., Schober A., Fiedler J., Thum T. (2014) Cardiac fibroblast-derived microRNA passenger strand-enriched exosomes mediate cardiomyocyte hypertrophy. J. Clin. Invest. 124, 2136–2146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sun X., Lin J., Zhang Y., Kang S., Belkin N., Wara A. K., Icli B., Hamburg N. M., Li D., Feinberg M. W. (2016) MicroRNA-181b improves glucose homeostasis and insulin sensitivity by regulating endothelial function in white adipose tissue. Circ. Res. 118, 810–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xie Z., Wang X., Liu X., Du H., Sun C., Shao X., Tian J., Gu X., Wang H., Tian J., Yu B. (2018) Adipose-derived exosomes exert proatherogenic effects by regulating macrophage foam cell formation and polarization. J. Am. Heart Assoc. 7, e007442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomou T., Mori M. A., Dreyfuss J. M., Konishi M., Sakaguchi M., Wolfrum C., Rao T. N., Winnay J. N., Garcia-Martin R., Grinspoon S. K., Gorden P., Kahn C. R. (2017) Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 542, 450–455; 545, 252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tang Y., Yu S., Liu Y., Zhang J., Han L., Xu Z. (2017) MicroRNA-124 controls human vascular smooth muscle cell phenotypic switch via Sp1. Am. J. Physiol. Heart Circ. Physiol. 313, H641–H649 [DOI] [PubMed] [Google Scholar]
- 14.Sharma H., Chinnappan M., Agarwal S., Dalvi P., Gunewardena S., O’Brien-Ladner A., Dhillon N. K. (2018) Macrophage-derived extracellular vesicles mediate smooth muscle hyperplasia: role of altered miRNA cargo in response to HIV infection and substance abuse. FASEB J. 32, 5174–5185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crewe C., Joffin N., Rutkowski J. M., Kim M., Zhang F., Towler D. A., Gordillo R., Scherer P. E. (2018) An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 175, 695–708.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Flaherty S. E., III, Grijalva A., Xu X., Ables E., Nomani A., Ferrante A. W., Jr (2019) A lipase-independent pathway of lipid release and immune modulation by adipocytes. Science 363, 989–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying W., Riopel M., Bandyopadhyay G., Dong Y., Birmingham A., Seo J. B., Ofrecio J. M., Wollam J., Hernandez-Carretero A., Fu W., Li P., Olefsky J. M. (2017) Adipose tissue macrophage-derived exosomal miRNAs can modulate in vivo and in vitro insulin sensitivity. Cell 171, 372–384.e12 [DOI] [PubMed] [Google Scholar]
- 18.Liu G., Gong Y., Zhang R., Piao L., Li X., Liu Q., Yan S., Shen Y., Guo S., Zhu M., Yin H., Funk C. D., Zhang J., Yu Y. (2018) Resolvin E1 attenuates injury-induced vascular neointimal formation by inhibition of inflammatory responses and vascular smooth muscle cell migration. FASEB J. 32, 5413–5425 [DOI] [PubMed] [Google Scholar]
- 19.Kranendonk M. E., Visseren F. L., van Balkom B. W., Nolte-’t Hoen E. N., van Herwaarden J. A., de Jager W., Schipper H. S., Brenkman A. B., Verhaar M. C., Wauben M. H., Kalkhoven E. (2014) Human adipocyte extracellular vesicles in reciprocal signaling between adipocytes and macrophages. Obesity (Silver Spring) 22, 1296–1308 [DOI] [PubMed] [Google Scholar]
- 20.Bidarimath M., Khalaj K., Kridli R. T., Kan F. W., Koti M., Tayade C. (2017) Extracellular vesicle mediated intercellular communication at the porcine maternal-fetal interface: a new paradigm for conceptus-endometrial cross-talk. Sci. Rep. 7, 40476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jia L., Zhou X., Huang X., Xu X., Jia Y., Wu Y., Yao J., Wu Y., Wang K. (2018) Maternal and umbilical cord serum-derived exosomes enhance endothelial cell proliferation and migration. FASEB J. 32, 4534–4543 [DOI] [PubMed] [Google Scholar]
- 22.Eguchi A., Lazic M., Armando A. M., Phillips S. A., Katebian R., Maraka S., Quehenberger O., Sears D. D., Feldstein A. E. (2016) Circulating adipocyte-derived extracellular vesicles are novel markers of metabolic stress. J. Mol. Med. (Berl.) 94, 1241–1253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Seta F., Chung A. D., Turner P. V., Mewburn J. D., Yu Y., Funk C. D. (2009) Renal and cardiovascular characterization of COX-2 knockdown mice. Am. J. Physiol. Regul. Integr. Comp. Physiol. 296, R1751–R1760 [DOI] [PubMed] [Google Scholar]
- 24.Police S. B., Putnam K., Thatcher S., Batifoulier-Yiannikouris F., Daugherty A., Cassis L. A. (2010) Weight loss in obese C57BL/6 mice limits adventitial expansion of established angiotensin II-induced abdominal aortic aneurysms. Am. J. Physiol. Heart Circ. Physiol. 298, H1932–H1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X., Ballantyne L. L., Che X., Mewburn J. D., Kang J. X., Barkley R. M., Murphy R. C., Yu Y., Funk C. D. (2015) Endogenously generated omega-3 fatty acids attenuate vascular inflammation and neointimal hyperplasia by interaction with free fatty acid receptor 4 in mice. J. Am. Heart Assoc. 4, e001856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X., Mazaleuskaya L. L., Yuan C., Ballantyne L. L., Meng H., Smith W. L., FitzGerald G. A., Funk C. D. (2018) Flipping the cyclooxygenase (Ptgs) genes reveals isoform-specific compensatory functions. J. Lipid Res. 59, 89–101; 2035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang F., Chen Q., He S., Yang M., Maguire E. M., An W., Afzal T. A., Luong L. A., Zhang L., Xiao Q. (2018) miR-22 is a novel mediator of vascular smooth muscle cell phenotypic modulation and neointima formation. Circulation 137, 1824–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Padilla J., Jenkins N. T., Vieira-Potter V. J., Laughlin M. H. (2013) Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304, R543–R552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A. S., Obin M. S. (2005) Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 46, 2347–2355 [DOI] [PubMed] [Google Scholar]
- 30.Hill D. A., Lim H. W., Kim Y. H., Ho W. Y., Foong Y. H., Nelson V. L., Nguyen H. C. B., Chegireddy K., Kim J., Habertheuer A., Vallabhajosyula P., Kambayashi T., Won K. J., Lazar M. A. (2018) Distinct macrophage populations direct inflammatory versus physiological changes in adipose tissue. Proc. Natl. Acad. Sci. USA 115, E5096–E5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang Z. B., Ruan C. C., Lin J. R., Xu L., Chen X. H., Du Y. N., Fu M. X., Kong L. R., Zhu D. L., Gao P. J. (2018) Perivascular adipose tissue-derived PDGF-D contributes to aortic aneurysm formation during obesity. Diabetes 67, 1549–1560 [DOI] [PubMed] [Google Scholar]
- 32.Zhang Y., Mei H., Chang X., Chen F., Zhu Y., Han X. (2016) Adipocyte-derived microvesicles from obese mice induce M1 macrophage phenotype through secreted miR-155. J. Mol. Cell Biol. 8, 505–517 [DOI] [PubMed] [Google Scholar]
- 33.Meerson A., Traurig M., Ossowski V., Fleming J. M., Mullins M., Baier L. J. (2013) Human adipose microRNA-221 is upregulated in obesity and affects fat metabolism downstream of leptin and TNF-α. Diabetologia 56, 1971–1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mysore R., Ortega F. J., Latorre J., Ahonen M., Savolainen-Peltonen H., Fischer-Posovszky P., Wabitsch M., Olkkonen V. M., Fernández-Real J. M., Haridas P. A. N. (2017) MicroRNA-221-3p regulates angiopoietin-like 8 (ANGPTL8) expression in adipocytes. J. Clin. Endocrinol. Metab. 102, 4001–4012 [DOI] [PubMed] [Google Scholar]
- 35.Peng J., Zhou Y., Deng Z., Zhang H., Wu Y., Song T., Yang Y., Wei H., Peng J. (2018) miR-221 negatively regulates inflammation and insulin sensitivity in white adipose tissue by repression of sirtuin-1 (SIRT1). J. Cell. Biochem. 119, 6418–6428 [DOI] [PubMed] [Google Scholar]
- 36.Davis B. N., Hilyard A. C., Nguyen P. H., Lagna G., Hata A. (2009) Induction of microRNA-221 by platelet-derived growth factor signaling is critical for modulation of vascular smooth muscle phenotype. J. Biol. Chem. 284, 3728–3738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu X., Cheng Y., Zhang S., Lin Y., Yang J., Zhang C. (2009) A necessary role of miR-221 and miR-222 in vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ. Res. 104, 476–487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nie X., Chen Y., Tan J., Dai Y., Mao W., Qin G., Ye S., Sun J., Yang Z., Chen J. (2019) MicroRNA-221-3p promotes pulmonary artery smooth muscle cells proliferation by targeting AXIN2 during pulmonary arterial hypertension. Vascul. Pharmacol. 116, 24–35 [DOI] [PubMed] [Google Scholar]
- 39.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 [DOI] [PubMed] [Google Scholar]
- 40.Kingsley K., Huff J. L., Rust W. L., Carroll K., Martinez A. M., Fitchmun M., Plopper G. E. (2002) ERK1/2 mediates PDGF-BB stimulated vascular smooth muscle cell proliferation and migration on laminin-5. Biochem. Biophys. Res. Commun. 293, 1000–1006 [DOI] [PubMed] [Google Scholar]
- 41.Xue Y., Wei Z., Ding H., Wang Q., Zhou Z., Zheng S., Zhang Y., Hou D., Liu Y., Zen K., Zhang C. Y., Li J., Wang D., Jiang X. (2015) MicroRNA-19b/221/222 induces endothelial cell dysfunction via suppression of PGC-1α in the progression of atherosclerosis. Atherosclerosis 241, 671–681 [DOI] [PubMed] [Google Scholar]
- 42.Wu Z., Puigserver P., Andersson U., Zhang C., Adelmant G., Mootha V., Troy A., Cinti S., Lowell B., Scarpulla R. C., Spiegelman B. M. (1999) Mechanisms controlling mitochondrial biogenesis and respiration through the thermogenic coactivator PGC-1. Cell 98, 115–124 [DOI] [PubMed] [Google Scholar]
- 43.Qu A., Jiang C., Xu M., Zhang Y., Zhu Y., Xu Q., Zhang C., Wang X. (2009) PGC-1alpha attenuates neointimal formation via inhibition of vascular smooth muscle cell migration in the injured rat carotid artery. Am. J. Physiol. Cell Physiol. 297, C645–C653 [DOI] [PubMed] [Google Scholar]
- 44.Britton K. A., Fox C. S. (2011) Perivascular adipose tissue and vascular disease. Clin. Lipidol. 6, 79–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fitzgibbons T. P., Kogan S., Aouadi M., Hendricks G. M., Straubhaar J., Czech M. P. (2011) Similarity of mouse perivascular and brown adipose tissues and their resistance to diet-induced inflammation. Am. J. Physiol. Heart Circ. Physiol. 301, H1425–H1437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hou N., Liu Y., Han F., Wang D., Hou X., Hou S., Sun X. (2016) Irisin improves perivascular adipose tissue dysfunction via regulation of the heme oxygenase-1/adiponectin axis in diet-induced obese mice. J. Mol. Cell. Cardiol. 99, 188–196 [DOI] [PubMed] [Google Scholar]
- 47.Connolly K. D., Wadey R. M., Mathew D., Johnson E., Rees D. A., James P. E. (2018) Evidence for adipocyte-derived extracellular vesicles in the human circulation. Endocrinology 159, 3259–3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kapustin A. N., Chatrou M. L., Drozdov I., Zheng Y., Davidson S. M., Soong D., Furmanik M., Sanchis P., De Rosales R. T., Alvarez-Hernandez D., Shroff R., Yin X., Muller K., Skepper J. N., Mayr M., Reutelingsperger C. P., Chester A., Bertazzo S., Schurgers L. J., Shanahan C. M. (2015) Vascular smooth muscle cell calcification is mediated by regulated exosome secretion. Circ. Res. 116, 1312–1323 [DOI] [PubMed] [Google Scholar]
- 49.Togliatto G., Dentelli P., Rosso A., Lombardo G., Gili M., Gallo S., Gai C., Solini A., Camussi G., Brizzi M. F. (2018) PDGF-BB carried by endothelial cell-derived extracellular vesicles reduces vascular smooth muscle cell apoptosis in diabetes. Diabetes 67, 704–716 [DOI] [PubMed] [Google Scholar]
- 50.Jordan S. D., Krüger M., Willmes D. M., Redemann N., Wunderlich F. T., Brönneke H. S., Merkwirth C., Kashkar H., Olkkonen V. M., Böttger T., Braun T., Seibler J., Brüning J. C. (2011) Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat. Cell Biol. 13, 434–446 [DOI] [PubMed] [Google Scholar]
- 51.Prats-Puig A., Ortega F. J., Mercader J. M., Moreno-Navarrete J. M., Moreno M., Bonet N., Ricart W., López-Bermejo A., Fernández-Real J. M. (2013) Changes in circulating microRNAs are associated with childhood obesity. J. Clin. Endocrinol. Metab. 98, E1655–E1660 [DOI] [PubMed] [Google Scholar]
- 52.Wang Y. C., Li Y., Wang X. Y., Zhang D., Zhang H., Wu Q., He Y. Q., Wang J. Y., Zhang L., Xia H., Yan J., Li X., Ying H. (2013) Circulating miR-130b mediates metabolic crosstalk between fat and muscle in overweight/obesity. Diabetologia 56, 2275–2285 [DOI] [PubMed] [Google Scholar]
- 53.Wei Y., Schober A., Weber C. (2013) Pathogenic arterial remodeling: the good and bad of microRNAs. Am. J. Physiol. Heart Circ. Physiol. 304, H1050–H1059 [DOI] [PubMed] [Google Scholar]
- 54.Ji R., Cheng Y., Yue J., Yang J., Liu X., Chen H., Dean D. B., Zhang C. (2007) MicroRNA expression signature and antisense-mediated depletion reveal an essential role of MicroRNA in vascular neointimal lesion formation. Circ. Res. 100, 1579–1588 [DOI] [PubMed] [Google Scholar]
- 55.Cheng Y., Liu X., Yang J., Lin Y., Xu D. Z., Lu Q., Deitch E. A., Huo Y., Delphin E. S., Zhang C. (2009) MicroRNA-145, a novel smooth muscle cell phenotypic marker and modulator, controls vascular neointimal lesion formation. Circ. Res. 105, 158–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cordes K. R., Sheehy N. T., White M. P., Berry E. C., Morton S. U., Muth A. N., Lee T. H., Miano J. M., Ivey K. N., Srivastava D. (2009) miR-145 and miR-143 regulate smooth muscle cell fate and plasticity. Nature 460, 705–710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Galardi S., Mercatelli N., Giorda E., Massalini S., Frajese G. V., Ciafrè S. A., Farace M. G. (2007) miR-221 and miR-222 expression affects the proliferation potential of human prostate carcinoma cell lines by targeting p27Kip1. J. Biol. Chem. 282, 23716–23724 [DOI] [PubMed] [Google Scholar]
- 58.Le Sage C., Nagel R., Egan D. A., Schrier M., Mesman E., Mangiola A., Anile C., Maira G., Mercatelli N., Ciafrè S. A., Farace M. G., Agami R. (2007) Regulation of the p27(Kip1) tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 26, 3699–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fornari F., Gramantieri L., Ferracin M., Veronese A., Sabbioni S., Calin G. A., Grazi G. L., Giovannini C., Croce C. M., Bolondi L., Negrini M. (2008) MiR-221 controls CDKN1C/p57 and CDKN1B/p27 expression in human hepatocellular carcinoma. Oncogene 27, 5651–5661 [DOI] [PubMed] [Google Scholar]
- 60.Seeley J. J., Baker R. G., Mohamed G., Bruns T., Hayden M. S., Deshmukh S. D., Freedberg D. E., Ghosh S. (2018) Induction of innate immune memory via microRNA targeting of chromatin remodelling factors. Nature 559, 114–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liu X., Cheng Y., Yang J., Xu L., Zhang C. (2012) Cell-specific effects of miR-221/222 in vessels: molecular mechanism and therapeutic application. J. Mol. Cell. Cardiol. 52, 245–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lightell D. J., Jr., Moss S. C., Woods T. C. (2018) Upregulation of miR-221 and -222 in response to increased extracellular signal-regulated kinases 1/2 activity exacerbates neointimal hyperplasia in diabetes mellitus. Atherosclerosis 269, 71–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Coleman C. B., Lightell D. J., Jr., Moss S. C., Bates M., Parrino P. E., Woods T. C. (2013) Elevation of miR-221 and -222 in the internal mammary arteries of diabetic subjects and normalization with metformin. Mol. Cell. Endocrinol. 374, 125–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puigserver P., Wu Z., Park C. W., Graves R., Wright M., Spiegelman B. M. (1998) A cold-inducible coactivator of nuclear receptors linked to adaptive thermogenesis. Cell 92, 829–839 [DOI] [PubMed] [Google Scholar]
- 65.Lehman J. J., Barger P. M., Kovacs A., Saffitz J. E., Medeiros D. M., Kelly D. P. (2000) Peroxisome proliferator-activated receptor gamma coactivator-1 promotes cardiac mitochondrial biogenesis. J. Clin. Invest. 106, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Arany Z., He H., Lin J., Hoyer K., Handschin C., Toka O., Ahmad F., Matsui T., Chin S., Wu P. H., Rybkin I. I., Shelton J. M., Manieri M., Cinti S., Schoen F. J., Bassel-Duby R., Rosenzweig A., Ingwall J. S., Spiegelman B. M. (2005) Transcriptional coactivator PGC-1 alpha controls the energy state and contractile function of cardiac muscle. Cell Metab. 1, 259–271 [DOI] [PubMed] [Google Scholar]
- 67.Finck B. N., Kelly D. P. (2007) Peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1) regulatory cascade in cardiac physiology and disease. Circulation 115, 2540–2548 [DOI] [PubMed] [Google Scholar]
- 68.Zhu L., Sun G., Zhang H., Zhang Y., Chen X., Jiang X., Jiang X., Krauss S., Zhang J., Xiang Y., Zhang C. Y. (2009) PGC-1alpha is a key regulator of glucose-induced proliferation and migration in vascular smooth muscle cells. PLoS One 4, e4182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Haemmerle G., Moustafa T., Woelkart G., Büttner S., Schmidt A., van de Weijer T., Hesselink M., Jaeger D., Kienesberger P. C., Zierler K., Schreiber R., Eichmann T., Kolb D., Kotzbeck P., Schweiger M., Kumari M., Eder S., Schoiswohl G., Wongsiriroj N., Pollak N. M., Radner F. P., Preiss-Landl K., Kolbe T., Rülicke T., Pieske B., Trauner M., Lass A., Zimmermann R., Hoefler G., Cinti S., Kershaw E. E., Schrauwen P., Madeo F., Mayer B., Zechner R. (2011) ATGL-mediated fat catabolism regulates cardiac mitochondrial function via PPAR-α and PGC-1. Nat. Med. 17, 1076–1085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Handschin C., Spiegelman B. M. (2008) The role of exercise and PGC1alpha in inflammation and chronic disease. Nature 454, 463–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Pardo R., Vilà M., Cervela L., de Marco M., Gama-Pérez P., González-Franquesa A., Statuto L., Vilallonga R., Simó R., Garcia-Roves P. M., Villena J. A. (2019) Calorie restriction prevents diet-induced insulin resistance independently of PGC-1-driven mitochondrial biogenesis in white adipose tissue. FASEB J. 33, 2343–2358 [DOI] [PubMed] [Google Scholar]
- 72.Pérez S., Rius-Pérez S., Finamor I., Martí-Andrés P., Prieto I., García R., Monsalve M., Sastre J. (2019) Obesity causes PGC-1α deficiency in the pancreas leading to marked IL-6 upregulation via NF-κB in acute pancreatitis. J. Pathol. 247, 48–59 [DOI] [PubMed] [Google Scholar]
- 73.Morales-Kastresana A., Telford B., Musich T. A., McKinnon K., Clayborne C., Braig Z., Rosner A., Demberg T., Watson D. C., Karpova T. S., Freeman G. J., DeKruyff R. H., Pavlakis G. N., Terabe M., Robert-Guroff M., Berzofsky J. A., Jones J. C. (2017) Labeling extracellular vesicles for nanoscale flow cytometry. Sci. Rep. 7, 1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.