Abstract
The commensal microbiota is one of the environmental triggers of rheumatoid arthritis (RA). Recent studies have identified the characteristics of the gut microbiota in patients with RA. However, it is still unclear how the microbiota can be modulated to slow down disease progression. In the present study, berberine, a modulator of gut microbiota with substantial anti-RA effect, was chosen to explore the mechanisms by which the microbiota modulators ameliorate RA. The results showed that oral administration of berberine alleviated collagen-induced arthritis (CIA) in rats in a gut microbiota-dependent manner. Berberine down-regulated the diversity and richness of the gut bacteria, reduced the abundance of Prevotella, and elevated the abundance of butyrate-producing bacteria in CIA rats as determined by the 16S rRNA gene sequence, which might function through limiting the generation of nitrate and stabilizing the physiologic hypoxia in the intestine. Moreover, berberine treatment significantly increased the intestinal butyrate level and promoted the expression and activity of butyryl-CoA:acetate-CoA transferase (BUT). The coadministration of a BUT inhibitor largely diminished the adjustment of intestinal environment and the antiarthritic effect of berberine. In conclusion, modulators of the gut microbiota might serve as therapeutic agents for RA by inducing the butyrate generation through promoting the expression and activity of BUT.—Yue, M., Tao, Y., Fang, Y., Lian, X., Zhang, Q., Xia, Y., Wei, Z., Dai, Y. The gut microbiota modulator berberine ameliorates collagen-induced arthritis in rats by facilitating the generation of butyrate and adjusting the intestinal hypoxia and nitrate supply.
Keywords: rheumatoid arthritis, BUT, butyrate synthesis
Rheumatoid arthritis (RA) is a multifactorial autoimmune disease that can lead to cartilage and bone damage as well as disability. The etiology of RA involves genetic and environmental factors. The high heritability and high concordance rates of RA in twins implicate the importance of genetic factors (1, 2). Environmental triggers such as commensal microbiota and smoking are also crucial in the development and progression of RA. Rheumatoid factor and anticitrullinated protein antibodies are hallmarks of RA (3, 4). The evidence that the serum levels of RA-related autoantibodies increase during a period that averages 3–5 yr prior to the onset of joint symptoms suggests that the autoimmune dysfunction is initiated outside of the joints. Furthermore, clinical data showing that patients with early RA specifically express mucosal-related antibodies such as IgA-anticitrullinated protein antibodies raise the hypothesis that the autoimmune response might be initiated at the mucosal sites, including the oral, lung, and gastrointestinal mucosa tissues (5). The gastrointestinal tract, which is the mucosal tissue with the largest area, maintains the homeostasis of gut function through commensal microbiota (6). The composition of the gut microbiota differs between individuals with RA and healthy controls, and the abundance of several pathogenic bacteria, including Prevotella and Segmental filamentous bacilli, is reported to be correlated with the severity of RA (7, 8). However, it is still unclear how the microbiota might be regulated to slow down RA progression.
Improvements in the throughput of DNA sequencing have led to an auspicious time for the study of microbiome. The ability to identify microbial markers and to distinguish the microbiota in patients from that of healthy people offers a promising means for early diagnosis and prognosis (9–11). Moreover, success in alleviating disease symptoms by modulating the gut microbiota in several animal models (12, 13) and the positive results of fecal microbiota transplant and probiotic therapies have shed new light on the therapeutics for diseases (14–16). Based on the current understandings, colonized bacteria influence the preservation of health and the progression of diseases through direct or indirect interaction. A noteworthy example for the indirect interaction is the involvement of short-chain fatty acids (SCFAs), the fermentation products of gut bacteria, in the modulation of a plethora of physiologic functions ranging from energy balance to mental health (17). In patients with type 1 diabetes, for example, plasma and fecal levels of acetate and propionate were negatively correlated with glycemic and inflammatory parameters (18); furthermore, acetate- and butyrate-producing diets provided a high degree of protection against development of type 1 diabetes in nonobese diabetic mice by inhibiting the ability of B cells to expand autoreactive T cells and by boosting the number and function of regulatory T cells (19). In murine models of RA, the repressive effect of SCFAs has also been reported (20). Based on these findings, promotion of SCFA generation might be an effective means to ameliorate RA.
As the researches on microbiota developed, some clinically effective drugs with previously uncharacterized target proteins have been shown to be modulators of gut microbiota. For example, metformin, a biguanide derivative that is widely used as the first-line medication for type 2 diabetes and obesity, was recently shown to alter the composition of the gut microbiota by increasing the abundance of Escherichia and Bifidobacterium and decreasing the abundance of Intestinibacter, thereby increasing production of SCFAs (21, 22). Several natural compounds have also been shown to be gut microbiota modulators (23). Among these, berberine, an isoquinoline alkaloid that has been used for decades for the treatment of gastrointestinal disorders, has received the most attention. The evidence indicated that orally administered berberine showed an extremely low bioavailability (<1%) and a dominant gut accumulation (24) and that the positive charge on the nitrogen atom in the berberine molecule promotes its binding to bacteria where it exerts an antibacterial effect. Recent studies indicated that berberine lowered hyperlipidemia and modulated bile acid metabolism by altering the gut microbiota and the SCFA production (25–29). Moreover, our recently published data showed that berberine exerted an antiarthritic effect in a gut-dependent manner (30). In this study, berberine was chosen as a tool to explore the possibility that microbiota modulators alleviate RA and to understand the underlying mechanisms.
MATERIALS AND METHODS
Reagents
Berberine (purity ≥98%) was purchased from Zelang Pharmaceutical Technology Co., Ltd. (Nanjing, China). Leflunomide was purchased from Cinkate Pharmaceutical Corp. (Suzhou, China). Chicken CII, vancomycin hydrochloride, gentamycin sulfate, metronidazole, and heptanoyl CoA were purchased from MilliporeSigma (Burlington, MA, USA). Mycobacterium butyricum was purchased from Becton Dickinson (San Diego, CA, USA). QIAamp DNA Microbiome Kit was purchased from Qiagen (Germantown, MD, USA). NP-40 buffer was purchased from Beyotime (Nantong, China). Antibodies against phosphorylated signal transducer and activator of transcription 3 (p-STAT3), total STAT3, inducible NOS (iNOS), peroxisome proliferator-activated receptor γ (PPARγ), hypoxia-inducible factor-1α (HIF-1α), and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were purchased from Bioworld Technology (St. Louis Park, MN, USA). HiScript Q RT SuperMix and AceQ quantitative PCR (qPCR) SYBR Green Master Mix were purchased from Vazyme Biotech Co (Piscataway, NJ, USA). Other chemical products used were of the highest analytical grade available. Berberine and leflunomide were suspended in 0.5% sodium carboxy methyl cellulose for in vivo studies. Vancomycin hydrochloride, gentamycin sulfate, and metronidazole were dissolved in drinking water for in vivo studies.
Animals
Female Wistar rats, weighing 130–150 g, were purchased from the Comparative Medicine Centre of Yangzhou University (Yangzhou, China). The animal experiments were conducted with the approval of the Animal Ethics Committee of China Pharmaceutical University and conformed to the National Institutes of Health (Bethesda, MD, USA) guidelines on the ethical use of animals. Animals were housed under a 12-h light/dark cycle (22°C ± 2°C) and fed with a standard chow diet and water. The cages and water bottles were sterilized every 2 d. Rats in each group were housed in the same cage, and various groups of rats were placed in different cages and fed with different bottles (SYXK 2016-0011).
Collagen-induced arthritis and treatment
Collagen-induced arthritis (CIA) was established in rats with reference to the method previously described (31). Briefly, CII was emulsified in complete Freund’s adjuvant (1 mg/ml of Mycobacterium butyricum). On d 0, CII emulsion was intradermally injected at the base of the rat’s tail with a volume of 0.2 ml. On d 7, a booster immunization was conducted with a volume of 0.1 ml at the base of the rat’s tail, avoiding the primary injection sites. Rats were observed daily for clinical signs of arthritis, and each paw was scored on a scale of 0–4 by an experienced pathologist blinded to the experimental group: 0 = no swelling or erythema, 1 = slight swelling and or erythema, 2 = low to moderate edema, 3 = pronounced edema with limited joint usage, and 4 = excess edema with joint rigidity. The total score for each rat was calculated as an arthritis index with a maximum value of 16. The volumes of hind paws were determined by a paw volume plethysmometer.
On d 14, rats were divided into different groups according to the articular index scores and orally dosed with berberine or leflunomide from d 14 to 28. Heptanoyl CoA was dissolved with saline and administered at a dose of 0.3 mg/kg by inserting a teflon cannula into the anus of 8 cm. Rats in normal and model groups were given an equal volume of vehicle on the same schedule.
Tissue distribution of berberine
Normal and CIA rats were orally dosed with berberine (200 mg/kg) for 14 d. At 0, 0.25, 0.5, 1, 1.5, 2, 3, 4, 8, 12, and 24 h after the last administration, the blood samples (0.2 ml) were collected into heparinized tubes from the oculi chorioidea vein. After centrifugation at 8000 rpm for 10 min, the supernatant was extracted with ethyl acetate, vacuum swing dried, and redissolved with methanol for analysis. The tissue samples (200 mg) were collected at 0, 1, 2, 4, 8, 12, and 24 h after the last administration. After extraction with methanol-water (1:2, v/v) solution (5 ml/g) and centrifugation at 12,000 rpm for 10 min, the supernatant was extracted with ethyl acetate, vacuum swing dried, and redissolved with methanol for analysis.
An Agilent 1260 HPLC-MS/MS System (Agilent Technologies, Santa Clara, CA, USA) consisting of a quaternary pump system, autosampler, and an Agilent 6400 Triple Quadrupole Mass Spectrometer were employed for sample analysis. Chromatographic separation was achieved on an Agilent Zorbax Extend-C18 Column (50 × 2.1 mm, 1.8 μm). The mobile phase was water containing 0.1% formic acid-acetonitrile (85:15, v/v) at a flow rate of 0.2 ml/min. The parameters for positive electrospray ionization analysis were as follows: nebulizer gas (N2) pressure, 40 psi; drying gas (N2) flow, 10.0 L/min; drying gas temperature, 350°C. The quantification was carried out using multiple reaction monitoring modes. The m/z transitions were 336.1→320.0 for berberine and 272.2→171.0 for the internal standard (dextromethorphan).
Histopathological examination
Rats were euthanized with an excess dose of chloral hydrate hydrochloride at the termination of the experiment on d 28. The ankle joints were taken and fixed in 10% neutral buffered formalin for 48 h, decalcified in EDTA, embedded in paraffin, and sliced into 5-mm-thick sections. Hematoxylin and eosin (H&E) staining was performed, and histologic changes in joints were determined by a pathologist blinded to the experimental group and scored according to the degree of hyperplasia inflammatory cell infiltration, synovial hyperplasia and congestion, fibrous tissue hyperplasia, bone erosion, and regeneration. These histologic changes were scored on a scale of 0–4: 0 = normal, 1 = slight change, 2 = moderate change, 3 = severe change, 4 = extremely severe change. The total scores were determined by summing the scores of 4 features.
qPCR assay
Total RNAs from rat tissues were extracted with Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and reverse transcribed into cDNA. The gene expression was analyzed with AceQ qPCR SYBR Green Master Mix and normalized to GAPDH. The primer sequences were shown in Table 1. The results were evaluated using the comparative threshold cycle (2−ΔΔCt) method (32).
TABLE 1.
Primers used in qPCR
| Sequence, 5′−3′ |
||
|---|---|---|
| Target genes | Forward | Reverse |
| TNF-α | GTCTGTGCCTCAGCCTCTTC | GAGCCCATTTGGGAACTTCT |
| IL-6 | AGTTGCCTTCTTGGGACTGA | CCTCCGACTTGTGAAGTGAAGTGGT |
| IL-1β | CAGCAGCATCTCGACAAGAG | AAAGAAGGTGCTTGGGTCCT |
| IL-17A | TCCAGCAAGAGATCCTGGTC | TGGCGGACAATAGAGGAAAC |
| IL-17F | CGTCTCTTTGCGTTAGATGATG | GCACTTCATTGAGCTCTACAAG |
| IL-21 | AACTTCTAACAGCTCCACAAGA | GTGCCTCTGTTTATTTCCTGTC |
| IL-22 | GATAACAACACAGATGTCAGGC | CCTGCTTCATCAAGTAGCATTG |
| RORC | GAAGGCAAATACGGTGGTGT | AGAGGGCAATCTCATCCTCA |
| NOS2 | AGACACATACTTTACGCCACTA | TCAAAGACCTCTGGATCTTGAC |
| PPARγ | GCTACTGCATGTGATCAAGAAG | CGACTGGGACTTTTCTGCTAAT |
| Angptl4 | CAGCAGCAGAGATACCTATCAA | CTTGTCTACCCCATTGTCTAGG |
| Keratin20 | GACACTGAACGCCAGAACCAAGAG | TGCCGTCTACCACTTCCTCCAC |
| Adora2B | CCTGCTTCGTGCTGGTGCTC | CCTCTTGCTCGTGTTCCAGTGAC |
| BNIP3 | CAGCGTTCCAGCTTCCGTCTC | AGCTGCTTCGTCCAGATTCATGC |
| BNIP3L | CATGAACAGCAGCAATGGCAACG | TGGATGGAAGACGAGGAAGGAGCG |
| LDHA | CAATCTGGATTCGGCTCGGTTCC | CGGCGACATTCACACCACTCC |
| SLC2A1 | GCTGTGGCTGGCTTCTCTAACTG | AGCAGCACCGTGAAGATGATGAAG |
Western blotting assay
The tissue lysates were prepared using NP-40 buffer. The concentration of protein was determined using the Bradford assay (Beyotime, Nantong, China). The tissue lysates were separated by 10% SDS-PAGE and transferred to NC membranes. The membranes were blocked with 5% nonfat milk at room temperature for 2 h and incubated with different antibodies at 4°C overnight. After washing, the membranes were incubated with secondary antibodies at 37°C for 2 h, and the proteins were detected by the Odyssey Infrared Imaging System (Li-Cor Biosciences, Lincoln, NE, USA). All blots were stripped and incubated with GAPDH antibody to ascertain equal protein loading.
Gut microbiota analysis
At the termination of the experiment on d 28, cecal content samples were freshly collected in germ-free tubes and stored at −80°C. Genomic DNA was extracted using QiAmp DNA Microbiome Kit according to the manufacturer’s protocol. The sequencing of 16S rRNA genes (V3-V4 region) was performed with Illumina MiSeq (San Diego, CA, USA). A total of 734,897 tags were generated, with an average of 40,827 tags per sample. These clean tags were clustered to operational taxonomic units (OTUs) at a similarity level of 97% and performed bioinformatics with USEARCH (v.7.0.1090; https://omictools.com/usearch-tool) (33) and QIIME (v.1.80; http://qiime.org/) (34).
SCFA quantification
At the termination of the experiment on d 28, cecal content samples were freshly collected and stored at −80°C. The samples (100 mg) were dissolved in ddH2O and mixed with 2-methylbutyric acid. After centrifugation at 12,000 rpm for 20 min, the supernatant was processed with isopropanol-pyridine (3:2, v/v) solution and platelet cytotoxic factor solution for derivatization, and then extracted with n-hexane for analysis.
An Agilent 7890A/5975C GC-MS system was employed for sample analysis. Chromatographic separation was achieved on an Agilent HP-5 capillary column (30 m × 0.25 mm, 0.25 μm) in the split mode (10:1). The helium carrier flow was 1.0 ml/min. The injector temperature was 280°C. The oven temperature was initially 60°C for 5 min and was gradually increased to 250°C at a rate of 10°C/min.
Cultivation of cecal and colonic bacteria
Samples were collected directly from the cecum and colon. Each sample was taken from the center of the stool, weighed, suspended in sterile distilled water, and homogenized for 3 min. The suspension was filtered through a 1-mm sieve. The growth medium was as follows: arabinogalactan (1 g/L), pectin (2 g/L), xylan (1 g/L), starch (4.2 g/L), glucose (0.4 g/L), yeast extract (3 g/L), peptone (1 g/L), mucin (4 g/L), and cysteine (0.5 g/L) (35).
Nitrate measurement
Nitrate measurement was performed as previously described (36). Briefly, the mucus layer of intestine was scraped, homogenized in PBS, and filter sterilized. An adapted Griess assay was applied. Griess reagent A was added to sample solution. After incubation for 10 min, equal volumes of Griess reagent B were added. The absorbance at wavelength of 540 nm was measured. After incubation for 8 h, the absorbance at a wavelength of 540 nm was measured again. The nitrate concentration was determined by subtracting the initial absorbance from the absorbance 8 h later.
Enzyme assay
The activities of butyrate kinase (BUK) and butyryl-CoA:acetate-CoA transferase (BUT) were determined according to a previous description (37). BUK activity was determined by detecting butyrohydroxamic acid formation in butyryl-phosphate forming. Briefly, samples were added to the reaction mixture containing Tris/HCl (pH 7.4), ATP, MgCl2, 6% hydroxylamine hydrochloride, and sodium butyrate. The reaction mixtures were incubated for 15 min and stopped by adding 10% trichloroacetic acid. After adding 2.5% FeCl3 solution, the absorbance at wavelength of 540 nm was measured. BUT activity was determined by detecting acetyl-CoA formation using acetate and butyryl-CoA as the substrates. The concentration of acetyl-CoA was determined by using an Acetyl-CoA Detection Kit (Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instruction.
Statistical analysis
Data were analyzed with the Prism 5.0 software (GraphPad, La Jolla, CA, USA). The results are presented as the means ± sem. Significant differences were assessed through a 1-way ANOVA followed by a post hoc Tukey’s test. A value of P < 0.05 was considered statistically significant.
RESULTS
Berberine exerted antiarthritis effect through modulation of gut microbiota
It is well known that oral berberine is poorly absorbed and exhibits an extremely low plasma exposure and a high gut accumulation (24–29). To determine whether berberine exerted a local action in the gut under arthritis status, we compared the pharmacokinetics and tissue distribution of berberine in normal and CIA rats after 14 d of oral administration at a dose of 200 mg/kg. The data obtained from an LC-MS/MS analysis showed that the plasma concentrations of berberine were very low (peak concentration: 13.15 ng/ml). Similar to the pharmacokinetic characteristics in normal rats, berberine significantly accumulated in the intestines in CIA rats (Supplemental Fig. S1). These findings suggested that the gut might be the site for the action of berberine in arthritis, and berberine could serve as a tool for the exploration of the antiarthritic effect of gut microbiota modulators.
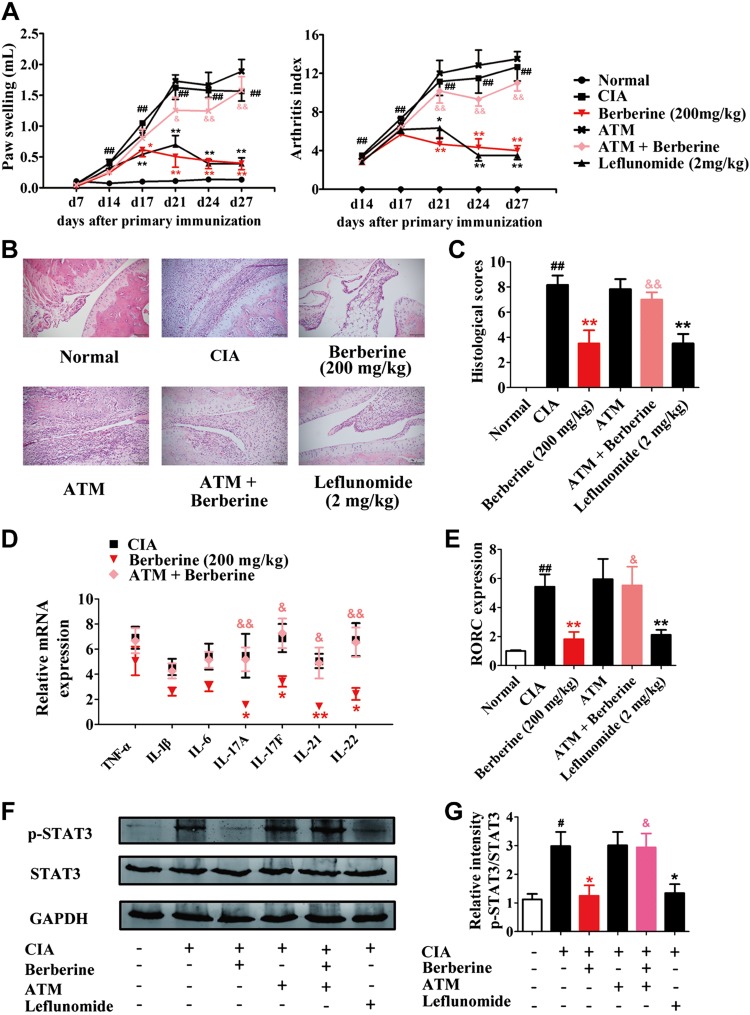
Oral administration of either berberine (200 mg/kg) or leflunomide (2 mg/kg) markedly reduced the arthritis indices and paw swelling in CIA rats (Fig. 1A). The results of the examination of the pathology showed that berberine and leflunomide could reduce the infiltration of inflammatory cells, decrease the synovial hyperplasia, and ameliorate the destruction of bone and cartilage in the ankle joints of rats (Fig. 1B, C). Moreover, berberine down-regulated the splenic level of proinflammatory cytokines, especially the T helper (Th)17 cell-related cytokines IL-17A, IL-17F, IL-21, and IL-22. The expression of RAR-related orphan receptor γT (RORγt; the transcription factor in Th17 cells) and the phosphorylation of STAT3 (the key factor of Th17 cell differentiation) were also significantly reduced by berberine treatment (Fig. 1D–G).
Figure 1.
Treatment with broad-spectrum antibiotics diminished the antiarthritic effect of berberine. Rats were intragastrically dosed with berberine or leflunomide and dosed with ATM by adding broad-spectrum ATMs (0.5 g/L vancomycin hydrochloride, 1 g/L gentamycin sulfate, 1 g/L metronidazole) in drinking water of CIA rats from d 14 to 28. A) The arthritis indices and paw swelling were determined. B) Histopathological changes of right ankle joints in each group were determined by H&E staining (original magnification, ×200). C) The histologic scores of each group were calculated. D) The mRNA expression of TNF-α, IL-1β, IL-6, IL-17A, IL-17F, IL-21, and IL-22 were determined by qPCR assay. E) The mRNA expression of RORγt was determined by qPCR assay. F) The p-STAT3 was evaluated by Western blotting assay. G) The relative intensities of p-STAT3/total STAT3 were calculated. Leflunomide was taken as a positive control. Data were expressed as means ± sem, n = 6. #P < 0.05, ##P < 0.01 vs. normal group; *P < 0.05, **P < 0.01 vs. CIA group; &P < 0.05, &&P < 0.01 vs. berberine group.
To evaluate the importance of the gut microbiota in the antiarthritic effect of berberine, broad-spectrum antimicrobials (ATMs, 0.5 g/L vancomycin hydrochloride, 1 g/L gentamycin sulfate, 1 g/L metronidazole) were added to the drinking water of CIA rats from d 14 to establish a pseudo-germ-free model. The ATM itself did not affect CIA in rats, but it markedly diminished the antiarthritic effect of berberine (Fig. 1), which suggested that the gut microbiota was deeply involved in the effect of berberine. Conversely, ATM did not influence the antiarthritic effect of leflunomide, indicating that gut microbiota was not related to the effect of leflunomide, and the antiarthritic mechanism of berberine might be distinct from that of leflunomide (Supplemental Fig. S2).
Berberine altered the gut microbiota composition in CIA rats
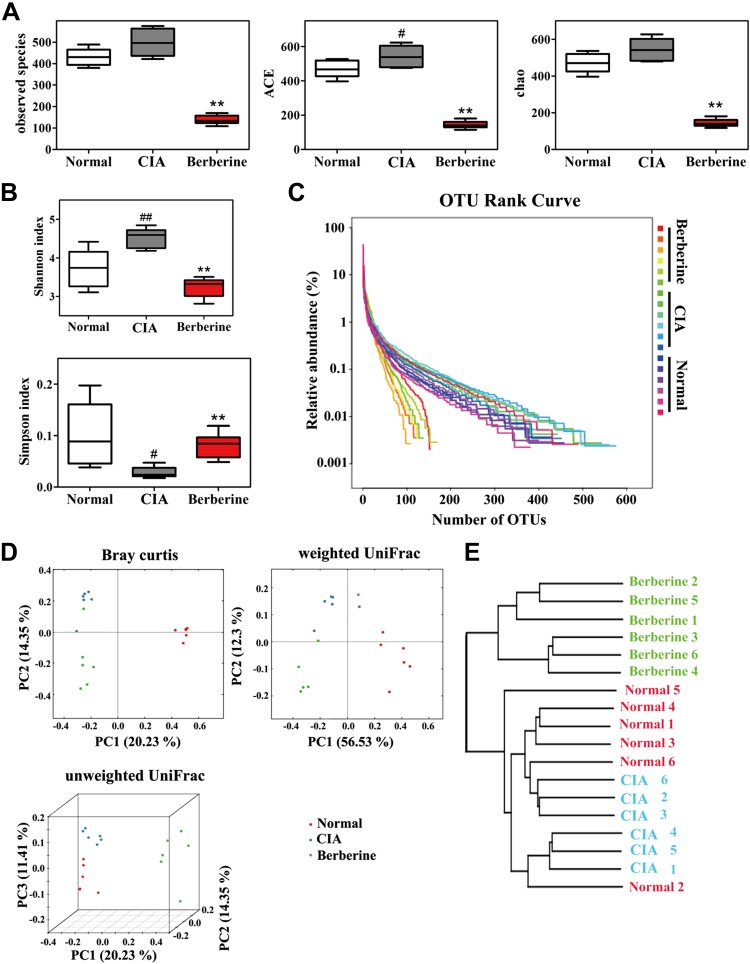
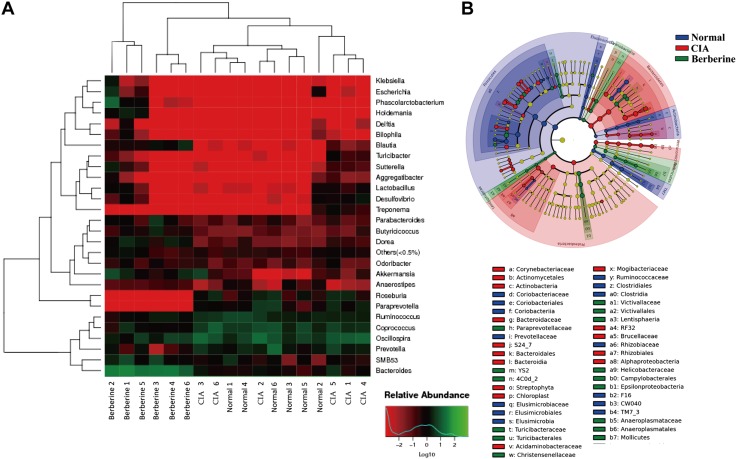
To gain insight into the changes in the gut microbiota after administration of berberine in CIA rats, the abundance and composition of microbiota were analyzed by high-throughput sequencing the V3 and V4 regions of 16S rRNA gene in the cecal contents. The results showed that the microbial diversity and richness were markedly increased in CIA rats compared with normal rats (Fig. 2A–C), and the distinct clustering of microbiota in normal and CIA rats are shown in Fig. 2D, E. As a natural antibacterial agent (38, 39), berberine significantly down-regulated the diversity and richness of gut microbiota in CIA rats, and the distinct microbiota clustering of berberine- or vehicle-treated rats is shown in Fig. 2. Furthermore, the relative abundance of the taxa was calculated and expressed as a heatmap (Fig. 3A). According to these results, the abundance of genera positively related to the production of SCFAs, including Blautia, Butyricicoccus, and Parabacteroides, were up-regulated in rats treated with berberine. In contrast, the abundance of genera negatively related to the production of SCFAs, including Prevotella, Paraprevotella, and Coprococcus, were down-regulated by berberine. The results of linear discriminant analysis effect size (LEfSe) analysis showed that berberine treatment reversed the Proteobacteria predominance in the gut microbiota of CIA rats (Fig. 3B).
Figure 2.
Berberine down-regulated the diversity and richness of gut microbiota in CIA rats. Rats were intragastrically dosed with berberine from d 14 to 28. Cecal content samples were freshly collected on d 28. The sequencing of 16S rRNA genes (V3-V4 region) was performed with the Illumina MiSeq. A) Observed species, abundance-based coverage estimator (ACE) indices, and Chao indices were calculated. B) Shannon indices and Simpson indices were calculated after rarefying to an equal number of sequence reads for all samples. C) The relative abundance of each OTU was calculated, and the OTU rank curve was drawn. D) The β-diversity analysis (PCoA) of gut microbiota was conducted. E) The multiple sample similarity tree was calculated. Data were expressed as means ± sem, n = 6 (A, B). PCoA, principal coordinates analysis. #P < 0.05 vs. normal group; **P < 0.01 vs. CIA group.
Figure 3.
Berberine modulated the taxa relative abundance of gut microbiota in CIA rats. Rats were intragastrically dosed with berberine from d 14 to 28. Cecal content samples were freshly collected on d 28. The sequencing of 16S rRNA genes (V3-V4 region) was performed with the Illumina MiSeq. A) The relative abundance of bacteria in genus level and the sample similarity of microbota in each sample were calculated and the heatmap clustering analysis was conducted. B) LEfSe analysis was conducted, and the taxonomic cladogram was shown.
Berberine limited the generation of nitrate and stabilized physiologic hypoxia in the intestine of CIA rats
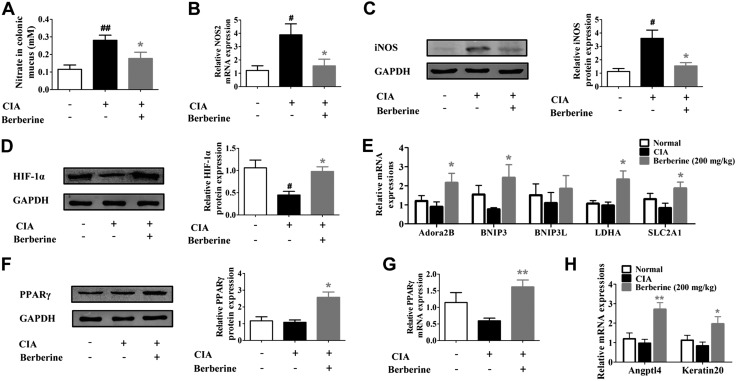
The expansion of Proteobacteria is a common marker of gut dysbiosis. When the anoxia circumstance in the gut lumen is disturbed or the host produces excessive nitrate because of inflammation, facultative anaerobic Proteobacteria respire nitrate as an alternative electron acceptor and become the dominant bacteria (40, 41). The LEfSe analysis showed that berberine treatment reversed the Proteobacteria predominance in the gut microbiota of CIA rats. We wondered whether berberine treatment reshaped the generation of host-derived nitrate and intestinal hypoxia to break the predominance of Proteobacteria and regain the gut microbiota homeostasis that was disturbed by the pathologic changes of arthritis. To address this issue, the availability of nitrate and the expression of HIF-1α, the key indicator of hypoxia, were evaluated. As shown in Fig. 4A–C, the levels of nitrate, NOS2 mRNA, and iNOS protein in the intestinal mucus were significantly elevated in CIA rats. Berberine treatment markedly reduced the abnormally elevated nitrate and suppressed the expressions of NOS2 mRNA and iNOS protein. Moreover, the physiologic hypoxia condition, which was disturbed in CIA rats, was also rescued by berberine treatment. The expression of HIF-1α was decreased in the intestine of CIA rats, and berberine treatment up-regulated the expression of HIF-1α (Fig. 4D) and related target genes (Fig. 4E). Moreover, berberine was shown to significantly enhance the expression and activation of PPARγ (Fig. 4F–H), a metabolic sensor that is highly expressed in gut tissues (42). These results indicated that berberine could down-regulate the generation of host-derived nitrate, rescue the disturbed intestinal hypoxia, and restore the impaired balance of energy supply in the gut of CIA rats.
Figure 4.
Berberine limited nitrate generation and restored HIF-1α expression in intestines of CIA rats. Rats were intragastrically dosed with berberine from d 14 to 28. A) The nitrate concentration in colonic mucus was determined. B) The mRNA expression of NOS2 in intestinal tissues was determined by qPCR assay. C) The protein level of iNOS in intestinal tissues was evaluated by Western blotting assay, and the relative intensity of iNOS/GAPDH was calculated. D) The protein level of HIF-1α in intestinal tissues was evaluated by Western blotting assay, and the relative intensity of HIF-1α/GAPDH was calculated. E) The mRNA expression of HIF-1α target genes, including adenosine a2B receptor (Adora2B), Bcl-2–interacting protein 3 (BNIP3), Bcl-2–interacting protein 3–like (BNIP3L), lactate dehydrogenase (ALDHA), and solute carrier family 2 member (1SLC2A1), in intestinal tissues was determined by qPCR assay. F) The protein level of PPARγ in intestinal tissues was evaluated by Western blotting assay, and the relative intensity of PPARγ/GAPDH was calculated. G) The mRNA expression of PPARγ in intestinal tissues was determined by qPCR assay. H) The mRNA expression of PPARγ target genes, including angiopoietin-like 4 (Angptl4) and Keratin20, in intestinal tissues was determined by qPCR assay. Data were expressed as means ± sem, n = 6. #P < 0.05, ##P < 0.01 vs. normal group; *P < 0.05, **P < 0.01 vs. CIA group.
Berberine up-regulated the synthesis of butyrate in the gut of CIA rats by promoting the expression and activity of BUT
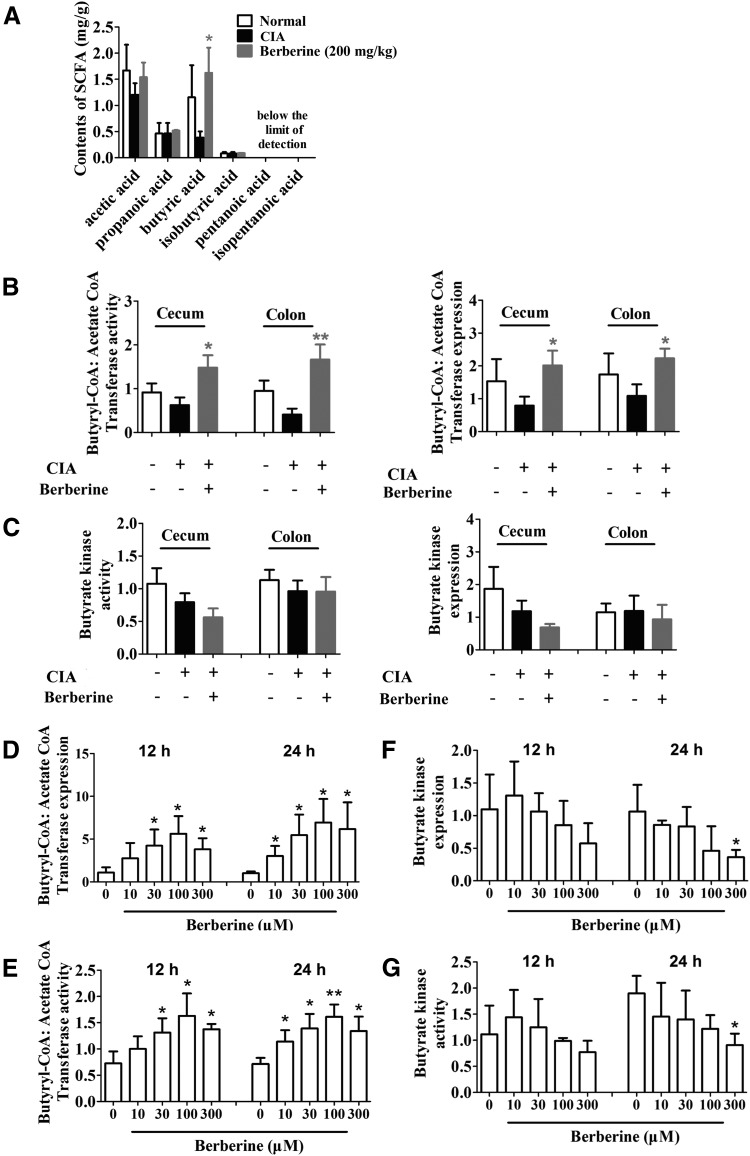
The data presented in Fig. 3 showed that the relative abundance of SCFA-producing bacteria in the gut microbiota of CIA rats was up-regulated by berberine treatment. To determine whether the generation of SCFAs was also affected, we further evaluated the levels of SCFAs in the cecal contents using GC-MS. Among the 6 types of SCFAs we tested, the level of butyrate and its proportion within all the SCFAs declined in the cecal contents of CIA rats. Berberine treatment significantly increased the content and proportion of butyrate (Fig. 5A and Supplemental Fig. S3). The current knowledge on the synthetic pathways for butyrate indicates that the acetyl-CoA pathway is the most prevalent (79.7%). In this scenario, the final step from butyryl-CoA to butyrate is catalyzed by either BUK or BUT (43). To shed light on the mechanism by which berberine elevated the level of butyrate, we evaluated the expression and activity of BUK and BUT in the intestinal contents of CIA rats. The results showed that the expression and activity of BUT in both cecal and colonic contents were significantly increased by berberine treatment (Fig. 5B). In contrast, the expression and activity of BUK were not affected by berberine (Fig. 5C). We next incubated the isolated gut bacteria to determine whether the effect of berberine on BUT was dependent on host intestinal environment. The results showed that berberine substantially enhanced the expression and activity of BUT in vitro (Fig. 5D, E). In contrast to the in vivo results, berberine slightly reduced the in vitro expression and activity of BUK (Fig. 5F, G). These findings revealed that berberine could directly up-regulate the expression and activity of BUT in bacteria and enhance the production of butyrate from the gut microbiota.
Figure 5.
Berberine up-regulated butyrate synthesis in CIA rats by promoting the expression and activity of BUT. Rats were intragastrically dosed with berberine from d 14 to 28. A) The content of SCFAs, including acetic acid, propanoic acid, butyric acid, isobutyric acid, pentanoic acid, and isopentanoic acid, in cecal content was determined by GC-MS. B) The activity and expression of butyryl-CoA:acetate-CoA transferase in cecal content were determined. C) The activity and expression of butyrate kinase in cecal content were determined. D–G) Gut bacteria were separated and treated with berberine in vitro. The expression (D) and activity (E) of butyryl-CoA:acetate-CoA transferase were determined. Besides, the expression (F) and activity (G) of butyrate kinase were determined. Data were expressed as means ± sem, n = 6. *P < 0.05, **P < 0.01 vs. CIA group in (A–C), *P < 0.05, **P < 0.01 vs. berberine (0 μM) group (D–G).
Inhibition of BUT prevented the rescue of the intestinal microenvironment by berberine
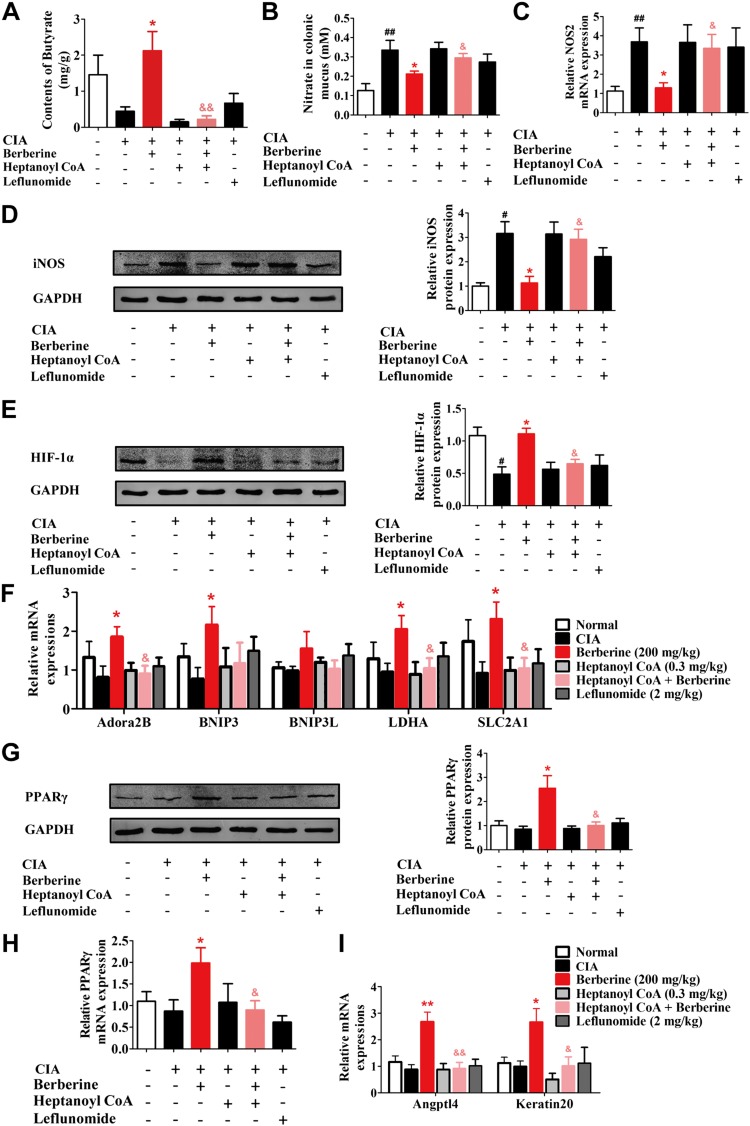
To determine whether the berberine-induced activation of BUT and the resulting increase in butyrate contributed to the recovery of nitrate and oxygen homeostasis in the host intestines, heptanoyl CoA, a competitive inhibitor of BUT (44), was coadministered with berberine in CIA rats. It was shown that heptanoyl CoA markedly decreased the cecal concentration of butyrate and nearly completely eliminated the elevation of butyrate level caused by berberine treatment (Fig. 6A). Heptanoyl CoA also strikingly restored the berberine-mediated reduction of nitrate generation, prevented the berberine-induced suppression of NOS2 mRNA and iNOS protein expression, and inhibited the enhancement of the expression of HIF-1α and its downstream target genes that was induced by berberine (Fig. 6B–F). Furthermore, after coadministration with heptanoyl CoA, berberine failed to enhance the expression and activation of PPARγ (Fig. 6G–I). Taken together, these findings indicated that berberine restored the homeostasis of nitrate and oxygen in the gut of CIA rats by activating BUT and enhancing butyrate generation.
Figure 6.
Rectal gavage of heptanoyl CoA reversed the effect of berberine on intestinal nitrate consumption and HIF-1α expression. Rats were intragastrically dosed with berberine or leflunomide, and they were dosed with heptanoyl CoA by inserting a teflon cannula into the anus of 8 cm from d 14 to 28. Heptanoyl CoA was administered 30 min before the administration of vehicle or berberine. A) The content of butyrate in cecal content was determined by GC-MS. B) The nitrate concentration in colonic mucus was determined. C) The mRNA expression of NOS2 in intestinal tissues was determined by qPCR assay. D) The protein level of iNOS in intestinal tissues was evaluated by Western blotting assay, and the relative intensity of iNOS/GAPDH was calculated. E) The protein level of HIF-1α in intestinal tissues was evaluated by Western blotting assay, and the relative intensity of HIF-1α/GAPDH was calculated. F) The mRNA expression of HIF-1α target genes, including Adora2B (adenosine a2B receptor), BNIP3 (Bcl-2–interacting protein 3), BNIP3L(Bcl-2–interacting protein 3–like), LDHA (lactate dehydrogenase A), and SLC2A1 (solute carrier family 2 member 1) in intestinal tissues was evaluated by Western blotting assay, and the relative intensity of PPARγ/GAPDH was calculated. H) The mRNA expression of PPARγ in intestinal tissues was determined by qPCR assay. I) The mRNA expression of PPARγ target genes, including Angptl4 and Keratin20, in intestinal tissues was determined by qPCR assay. Leflunomide was taken as a positive control. Data were expressed as means ± sem, n = 6. #P < 0.05, ##P < 0.01 vs. normal group; *P < 0.05, **P < 0.01 vs. CIA group; &P < 0.05, &&P < 0.01 vs. berberine group.
Inhibition of BUT reversed the antiarthritic effects of berberine
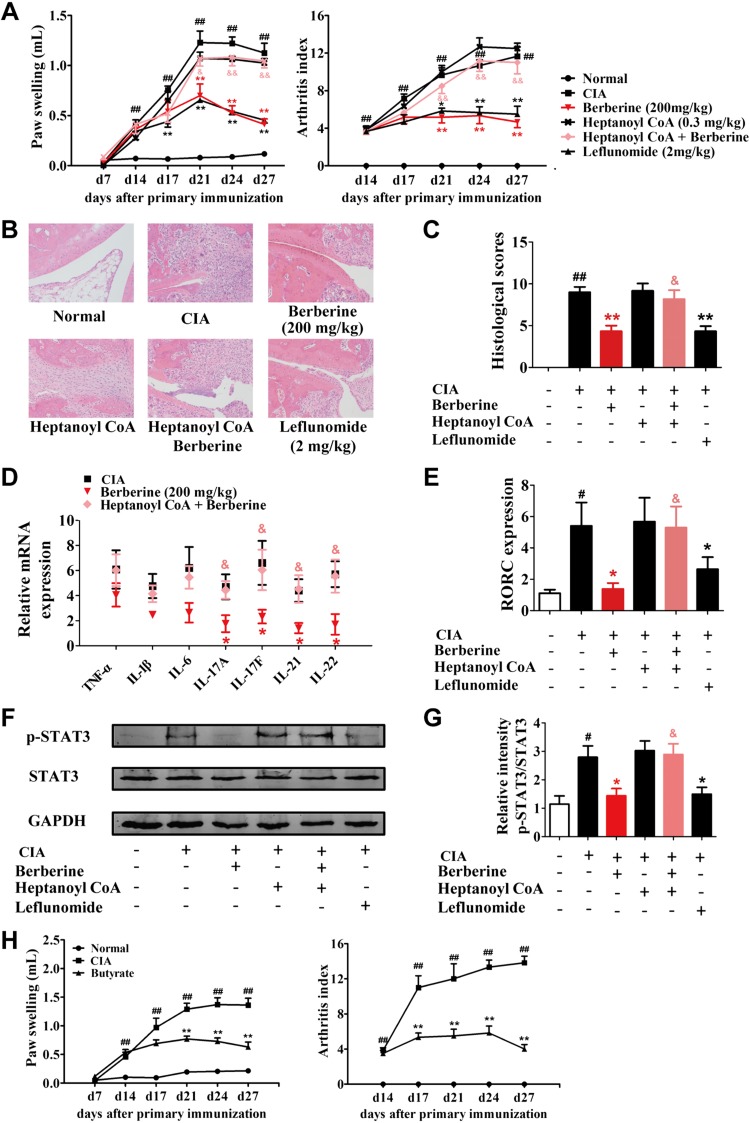
We further examined the influence of heptanoyl CoA on the antiarthritic effect of berberine. After 14 d of coadministration, heptanoyl CoA markedly diminished the antiarthritic effect of berberine as indicated by the enhanced arthritis indices, worsened paw swelling (Fig. 7A), more serious pathologic changes (Fig. 7B, C), increased splenic levels of proinflammatory cytokines (Fig. 7D), and elevated RORγt expression and STAT3 phosphorylation in the spleen (Fig. 7E–G). To confirm the involvement of butyrate, heptanoyl CoA and butyrate were coinfused rectally. The results showed that heptanoyl CoA failed to diminish the antiarthritic effect of berberine when it was coadministered with butyrate (Supplemental Fig. S4). Furthermore, when butyrate alone was infused rectally, the arthritis in rats was significantly alleviated (Fig. 7H). These findings indicate that the activation of BUT and resultant butyrate generation by the gut microbiota played crucial roles in the antiarthritic effect of berberine, especially in the suppression of Th17 cell response.
Figure 7.
Involvement of BUT and butyrate in the antiarthritic effect of berberine. Rats were intragastrically dosed with berberine or leflunomide and dosed with heptanoyl CoA by inserting a teflon cannula into the anus of 8 cm from d 14 to 28. Heptanoyl CoA was administered 30 min before the administration of vehicle or berberine. A) The arthritis indices and paw swelling were determined. B) Histopathological changes of right ankle joints in each group were determined by H&E staining (original magnification, ×200). C) The histologic scores of each group were calculated. D) The expressions of TNF-α, IL-1β, IL-6, IL-17A, IL-17F, IL-21, and IL-22 were determined by qPCR assay. E) The expression of RORγt was determined by qPCR assay. F) The p-STAT3 was evaluated by Western blotting assay. G) The relative intensity of p-STAT3/total STAT3 was calculated. Leflunomide was taken as a positive control. H) Rats were given with butyrate (50 mg/kg) by inserting a teflon cannula into the anus of 8 cm from d 14 to 28. The arthritis indices and paw swelling were determined. Data were expressed as means ± sem, n = 6. #P < 0.05, ##P < 0.01 vs. normal group; *P < 0.05, **P < 0.01 vs. CIA group; &P < 0.05, &&P < 0.01 vs. berberine group.
DISCUSSION
The theory that bacteria are involved in the initiation and progression of RA dates back to the 19th century when the relationship of RA and tuberculosis was noticed, and RA was suggested to be caused by the infection of Mycobacterium (45, 46). Recently, the gut-joint-axis hypothesis has been supported by the findings that 20% of patients with inflammatory bowel disease have recurrent episodes of peripheral arthritis. However, these observations have not yet resulted in practical pharmaceutical development (47). In this study, using berberine as a tool, we illustrated how gut microbiota modulators ameliorate RA and explored a possible new avenue for the development of antiarthritic medication based on the gut-joint-axis hypothesis.
On the basis that the overlap between microbial genes in rats and humans is larger than between mice and humans and that 97% of the functional pathways in the human catalog are present in the rat catalog (48), we here established an experimental arthritis model in rats rather than mice to obtain more convincing conclusions. According to the results of metagenomic shotgun sequencing and a metagenome-wide association study, patients with RA exhibit gut microbiota dysbiosis (7, 8, 49). Similar to the structural differences between patients with RA and healthy people, our sequencing results also showed a distinct microbiota structure clustering between normal and CIA rats. Furthermore, the observation that the relative abundance of Lactobacillus is higher in patients with early RA (50, 51) was also shown to be significant in gut microbiota of CIA rats. Another acknowledged characteristic of gut microbiota in patients with RA is the increased abundance of Prevotella (52). However, we did not notice a significant increase in the abundance of this genus in the gut microbiota of CIA rats. In general, though not identically matched, the changes in the gut microbiota of CIA rats are similar to patients with RA. Thus, our primary exploration of antiarthritic effects of gut microbiota modulators was conducted on the animal model of rat CIA.
Herein, we provided data that showed that berberine failed to alleviate symptoms of arthritis once the gut microbiota was disturbed by broad-spectrum ATMs, indicating that the antiarthritic effect of berberine was dependent on a modulation of the gut microbiota. The fact that broad-spectrum ATMs failed to influence the antiarthritic effect of leflunomide indicated that leflunomide, distinct from berberine, functions in a manner that is independent of the gut microbiome. Interestingly, although both ATMs and berberine could down-regulate the richness of the gut microbiota, the ATMs failed to suppress arthritis in rats. This may be related to the differences in the antibacterial spectrum. In the current study, the ATMs treatment consisted of vancomycin, gentamycin, and metronidazole, which are common antibiotics that inhibit the growth of Gram-positive, Gram-negative, and anaerobic bacteria, respectively. Because vancomycin and gentamycin show low oral absorption, supplementing the drinking water with a solution of the combined antibiotics could guarantee a broad-spectrum antibiotic effect on gut microbiota. However, berberine showed a selective ATM effect, and bacteria such as Butyricicoccus were not inhibited by berberine. Consistent with the opinions of other investigators (53), the selective inhibition of gut bacteria, rather than a broad-spectrum inhibition, might be beneficial for arthritis.
In addition, we found that the abundances of some bacteria that are closely associated with SCFA production, especially butyrate production, were altered by berberine treatment, and berberine selectively promoted the generation of butyrate among the 6 SCFAs tested. The bacteria-modulation profiles of berberine are consistent with the previous findings in ovariectomized-periodontitis and high-fat diet-induced hyperlipidemia models (26, 54), which suggested that the butyrate-generation effect of berberine does not rely on the pathologic status of RA. Butyrate has long been recognized as an immunosuppressive agent and an intestinal microenvironment modulator. In animal models of inflammatory diseases, including RA, butyrate treatment was reported to reduce the expression of proinflammatory cytokines and reduce the percentage of Th17 cells in the spleen (55–58). In the intestinal epithelium, butyrate was shown to augment barrier function and maintain the stability of intestinal microenvironment (40, 41). We showed here that after berberine treatment, the pathologic alleviation in CIA rats was similar to the features of butyrate-treated animals: suppressed pathologic changes, down-regulated proinflammatory cytokines, especially Th17-related cytokines, and stabilized intestinal microenvironment. These phenomena give us a clue that the modulating agents of gut microbiota might be able to ameliorate RA through enhancing the generation of butyrate.
Butyrate is a 4-carbon SCFA that is synthesized via pyruvate and acetyl-CoA, mostly by breaking down complex polysaccharides that escape digestion in the upper gastrointestinal tract (59). The generation of butyrate is predominantly due to the acetyl-CoA pathway, which converts acetyl-CoA to butyryl-CoA in a 4-step process and then produces butyrate by catalyzing the decomposition and transformation of butyryl-CoA with BUK or BUT (43, 60). According to our findings, berberine up-regulated the expression and activity of BUT rather than BUK in both cecal contents of CIA rats and the isolated gut bacteria. More interestingly, coadministration of the BUT inhibitor not only blocked the berberine-induced elevation of butyrate generation in cecal content of CIA rat but also reversed the antiarthritic effects. These results suggested that berberine alleviated arthritis in rats by activating BUT, and the novel therapeutic agents that target BUT might be promising for the management of RA.
According to the abovementioned findings, we noticed that the abundance of butyrate-producing communities in the microbiota and the activity of BUT were elevated, both of which could explain the enhanced butyrate generation. We wondered whether and how these 2 phenomena are related and why berberine, a natural product with broad-spectrum antibiotic effect, caused relative enrichment of butyrate-producing bacteria in the gut microbiota. Our further studies indicated that berberine adjusted the host intestinal environment to a condition that is more conducive to the growth of butyrate-producing bacterial by limiting the generation of nitrate and stabilizing the local physiologic hypoxia in intestine (40, 41), and this adjustment was nearly completely reversed by inhibiting the activity of BUT. It was suggested that the enrichment of butyrate-producing bacteria was probably primarily caused by the activation of BUT and optimized intestinal environment.
In summary, gut microbiota modulators such as berberine could ameliorate rat CIA by enhancing the generation of butyrate via promoting the expression and activity of BUT. BUT and other bacterial proteins are potential therapeutic targets in RA.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81673441) and the “Double First-Class” University Project (CPU2018GY10). The authors declare no conflicts of interest.
Glossary
- ATM
antimicrobial
- BUK
butyrate kinase
- BUT
butyryl-CoA:acetate-CoA transferase
- CIA
collagen-induced arthritis
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- H&E
hematoxylin and eosin
- HIF-1α
hypoxia-inducible factor-1α
- iNOS
inducible NOS
- LEfSe
linear discriminant analysis effect size
- OTU
operational taxonomic units
- PPARγ
peroxisome proliferator-activated receptor γ
- qPCR
quantitative PCR
- RA
rheumatoid arthritis
- RORγt
RAR-related orphan receptor γ T
- SCFA
short-chain fatty acid
- STAT3
signal transducer and activator of transcription 3
- Th
T helper
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
M. Yue, Z. Wei, and Y. Dai conceptualized and designed the experiments; M. Yue performed the experiments and analyzed the data; Y. Tao, Y. Fang, X. Lian, and Q. Zhang helped with partial experiments; Y. Xia contributed some of the reagents and materials; and M. Yue and Y. Dai wrote the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Smolen J. S., Aletaha D., McInnes I. B. (2016) Rheumatoid arthritis. Lancet 388, 2023–2038 [DOI] [PubMed] [Google Scholar]
- 2.McInnes I. B., Schett G. (2011) The pathogenesis of rheumatoid arthritis. N. Engl. J. Med. 365, 2205–2219 [DOI] [PubMed] [Google Scholar]
- 3.Darrah E., Andrade F. (2018) Rheumatoid arthritis and citrullination. Curr. Opin. Rheumatol. 30, 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chatzidionisyou A., Catrina A. I. (2016) The lung in rheumatoid arthritis, cause or consequence? Curr. Opin. Rheumatol. 28, 76–82 [DOI] [PubMed] [Google Scholar]
- 5.Demoruelle M. K., Deane K. D., Holers V. M. (2014) When and where does inflammation begin in rheumatoid arthritis? Curr. Opin. Rheumatol. 26, 64–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang F., Roy S. (2017) Gut homeostasis, microbial dysbiosis, and opioids. Toxicol. Pathol. 45, 150–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scher J. U., Sczesnak A., Longman R. S., Segata N., Ubeda C., Bielski C., Rostron T., Cerundolo V., Pamer E. G., Abramson S. B., Huttenhower C., Littman D. R. (2013) Expansion of intestinal Prevotella copri correlates with enhanced susceptibility to arthritis. eLife 2, e01202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teng F., Klinger C. N., Felix K. M., Bradley C. P., Wu E., Tran N. L., Umesaki Y., Wu H. J. (2016) Gut microbiota drive autoimmune arthritis by promoting differentiation and migration of Peyer’s patch T follicular helper cells. Immunity 44, 875–888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dubin K., Callahan M. K., Ren B., Khanin R., Viale A., Ling L., No D., Gobourne A., Littmann E., Huttenhower C., Pamer E. G., Wolchok J. D. (2016) Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat. Commun. 7, 10391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schirmer M., Smeekens S. P., Vlamakis H., Jaeger M., Oosting M., Franzosa E. A., Horst R. T., Jansen T., Jacobs L., Bonder M. J., Kurilshikov A., Fu J., Joosten L. A. B., Zhernakova A., Huttenhower C., Wijmenga C., Netea M. G., Xavier R. J. (2016) Linking the human gut microbiome to inflammatory cytokine production capacity. Cell 167, 1897 [DOI] [PubMed] [Google Scholar]
- 11.Villéger R., Lopès A., Veziant J., Gagnière J., Barnich N., Billard E., Boucher D., Bonnet M. (2018) Microbial markers in colorectal cancer detection and/or prognosis. World J. Gastroenterol. 24, 2327–2347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fei N., Zhao L. (2013) An opportunistic pathogen isolated from the gut of an obese human causes obesity in germfree mice. ISME J. 7, 880–884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Grander C., Adolph T. E., Wieser V., Lowe P., Wrzosek L., Gyongyosi B., Ward D. V., Grabherr F., Gerner R. R., Pfister A., Enrich B., Ciocan D., Macheiner S., Mayr L., Drach M., Moser P., Moschen A. R., Perlemuter G., Szabo G., Cassard A. M., Tilg H. (2018) Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67, 891–901 [DOI] [PubMed] [Google Scholar]
- 14.Weingarden A., González A., Vázquez-Baeza Y., Weiss S., Humphry G., Berg-Lyons D., Knights D., Unno T., Bobr A., Kang J., Khoruts A., Knight R., Sadowsky M. J. (2015) Dynamic changes in short- and long-term bacterial composition following fecal microbiota transplantation for recurrent Clostridium difficile infection. Microbiome 3, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivan A., Corrales L., Hubert N., Williams J. B., Aquino-Michaels K., Earley Z. M., Benyamin F. W., Lei Y. M., Jabri B., Alegre M. L., Chang E. B., Gajewski T. F. (2015) Commensal Bifidobacterium promotes antitumor immunity and facilitates anti-PD-L1 efficacy. Science 350, 1084–1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J., Sung C. Y., Lee N., Ni Y., Pihlajamäki J., Panagiotou G., El-Nezami H. (2016) Probiotics modulated gut microbiota suppresses hepatocellular carcinoma growth in mice. Proc. Natl. Acad. Sci. USA 113, E1306–E1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dinan T. G., Cryan J. F. (2017) Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 595, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Groot P. F., Belzer C., Aydin Ö., Levin E., Levels J. H., Aalvink S., Boot F., Holleman F., van Raalte D. H., Scheithauer T. P., Simsek S., Schaap F. G., Olde Damink S. W. M., Roep B. O., Hoekstra J. B., de Vos W. M., Nieuwdorp M. (2017) Distinct fecal and oral microbiota composition in human type 1 diabetes, an observational study. PLoS One 12, e0188475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mariño E., Richards J. L., McLeod K. H., Stanley D., Yap Y. A., Knight J., McKenzie C., Kranich J., Oliveira A. C., Rossello F. J., Krishnamurthy B., Nefzger C. M., Macia L., Thorburn A., Baxter A. G., Morahan G., Wong L. H., Polo J. M., Moore R. J., Lockett T. J., Clarke J. M., Topping D. L., Harrison L. C., Mackay C. R. (2017) Gut microbial metabolites limit the frequency of autoimmune T cells and protect against type 1 diabetes. Nat. Immunol. 18, 552–562; erratum: 951; 1271 [DOI] [PubMed] [Google Scholar]
- 20.Mizuno M., Noto D., Kaga N., Chiba A., Miyake S. (2017) The dual role of short fatty acid chains in the pathogenesis of autoimmune disease models. PLoS One 12, e0173032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Song M., Chan A. T. (2019) Environmental factors, gut microbiota, and colorectal cancer prevention. Clin. Gastroenterol. Hepatol. 17:275–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu H., Esteve E., Tremaroli V., Khan M. T., Caesar R., Mannerås-Holm L., Ståhlman M., Olsson L. M., Serino M., Planas-Fèlix M., Xifra G., Mercader J. M., Torrents D., Burcelin R., Ricart W., Perkins R., Fernàndez-Real J. M., Bäckhed F. (2017) Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858 [DOI] [PubMed] [Google Scholar]
- 23.Gao Z., Li Q., Wu X., Zhao X., Zhao L., Tong X. (2017) New insights into the mechanisms of Chinese herbal products on diabetes: a focus on the “bacteria-mucosal immunity-inflammation-diabetes” axis. J. Immunol. Res. 2017, 1813086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y. T., Hao H. P., Xie H. G., Lai L., Wang Q., Liu C. X., Wang G. J. (2010) Extensive intestinal first-pass elimination and predominant hepatic distribution of berberine explain its low plasma levels in rats. Drug Metab. Dispos. 38, 1779–1784 [DOI] [PubMed] [Google Scholar]
- 25.Wang Y., Tong Q., Shou J. W., Zhao Z. X., Li X. Y., Zhang X. F., Ma S. R., He C. Y., Lin Y., Wen B. Y., Guo F., Fu J., Jiang J. D. (2017) Gut microbiota-mediated personalized treatment of hyperlipidemia using berberine. Theranostics 7, 2443–2451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Y., Shou J. W., Li X. Y., Zhao Z. X., Fu J., He C. Y., Feng R., Ma C., Wen B. Y., Guo F., Yang X. Y., Han Y. X., Wang L. L., Tong Q., You X. F., Lin Y., Kong W. J., Si S. Y., Jiang J. D. (2017) Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism 70, 72–84 [DOI] [PubMed] [Google Scholar]
- 27.Li M., Shu X., Xu H., Zhang C., Yang L., Zhang L., Ji G. (2016) Integrative analysis of metabolome and gut microbiota in diet-induced hyperlipidemic rats treated with berberine compounds. J. Transl. Med. 14, 237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo Y., Zhang Y., Huang W., Selwyn F. P., Klaassen C. D. (2016) Dose-response effect of berberine on bile acid profile and gut microbiota in mice. BMC Complement. Altern. Med. 16, 394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sun R., Yang N., Kong B., Cao B., Feng D., Yu X., Ge C., Huang J., Shen J., Wang P., Feng S., Fei F., Guo J., He J., Aa N., Chen Q., Pan Y., Schumacher J. D., Yang C. S., Guo G. L., Aa J., Wang G. (2017) Orally administered berberine modulates hepatic lipid metabolism by altering microbial bile acid metabolism and the intestinal FXR signaling pathway. Mol. Pharmacol. 91, 110–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yue M., Xia Y., Shi C., Guan C., Li Y., Liu R., Wei Z., Dai Y. (2017) Berberine ameliorates collagen-induced arthritis in rats by suppressing Th17 cell responses via inducing cortistatin in the gut. FEBS J. 284, 2786–2801 [DOI] [PubMed] [Google Scholar]
- 31.Kokkola R., Li J., Sundberg E., Aveberger A. C., Palmblad K., Yang H., Tracey K. J., Andersson U., Harris H. E. (2003) Successful treatment of collagen-induced arthritis in mice and rats by targeting extracellular high mobility group box chromosomal protein 1 activity. Arthritis Rheum. 48, 2052–2058 [DOI] [PubMed] [Google Scholar]
- 32.Livak K. J., Schmittgen T. D. (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25, 402–408 [DOI] [PubMed] [Google Scholar]
- 33.Ozkan J., Willcox M., Wemheuer B., Wilcsek G., Coroneo M., Thomas T. (2019) Biogeography of the human ocular microbiota. Ocul. Surf. 17, 111–118 [DOI] [PubMed] [Google Scholar]
- 34.Breban M., Tap J., Leboime A., Said-Nahal R., Langella P., Chiocchia G., Furet J. P., Sokol H. (2017) Faecal microbiota study reveals specific dysbiosis in spondyloarthritis. Ann. Rheum. Dis. 76, 1614–1622 [DOI] [PubMed] [Google Scholar]
- 35.Wang L. L., Guo H. H., Huang S., Feng C. L., Han Y. X., Jiang J. D. (2017) Comprehensive evaluation of SCFA production in the intestinal bacteria regulated by berberine using gas-chromatography combined with polymerase chain reaction. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 1057, 70–80 [DOI] [PubMed] [Google Scholar]
- 36.Rivera-Chávez F., Lopez C. A., Zhang L. F., García-Pastor L., Chávez-Arroyo A., Lokken K. L., Tsolis R. M., Winter S. E., Bäumler A. J. (2016) Energy taxis toward host-derived nitrate supports a salmonella pathogenicity island 1-independent mechanism of invasion. MBio 7, e00960-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu Q., Liu T., Zhu L., Huang H., Jiang L. (2017) Insights from the complete genome sequence of Clostridium tyrobutyricum provide a platform for biotechnological and industrial applications. J. Ind. Microbiol. Biotechnol. 44, 1245–1260 [DOI] [PubMed] [Google Scholar]
- 38.Yao J., Kong W., Jiang J. (2015) Learning from berberine: treating chronic diseases through multiple targets. Sci. China Life Sci. 58, 854–859 [DOI] [PubMed] [Google Scholar]
- 39.Qiu S., Sun H., Zhang A. H., Xu H. Y., Yan G. L., Han Y., Wang X. J. (2014) Natural alkaloids: basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 12, 401–406 [DOI] [PubMed] [Google Scholar]
- 40.Byndloss M. X., Olsan E. E., Rivera-Chávez F., Tiffany C. R., Cevallos S. A., Lokken K. L., Torres T. P., Byndloss A. J., Faber F., Gao Y., Litvak Y., Lopez C. A., Xu G., Napoli E., Giulivi C., Tsolis R. M., Revzin A., Lebrilla C. B., Bäumler A. J. (2017) Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kelly C. J., Zheng L., Campbell E. L., Saeedi B., Scholz C. C., Bayless A. J., Wilson K. E., Glover L. E., Kominsky D. J., Magnuson A., Weir T. L., Ehrentraut S. F., Pickel C., Kuhn K. A., Lanis J. M., Nguyen V., Taylor C. T., Colgan S. P. (2015) Crosstalk between microbiota-derived short-chain fatty acids and intestinal epithelial HIF augments tissue barrier function. Cell Host Microbe 17, 662–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J. H., Song J., Park K. W. (2015) The multifaceted factor peroxisome proliferator-activated receptor γ (PPARγ) in metabolism, immunity, and cancer. Arch. Pharm. Res. 38, 302–312 [DOI] [PubMed] [Google Scholar]
- 43.Vital M., Howe A. C., Tiedje J. M. (2014) Revealing the bacterial butyrate synthesis pathways by analyzing (meta)genomic data. MBio 5, e00889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schulman M., Valentino D. (1976) Kinetics and catalytic properties of coenzyme A transferase from Peptostreptococcus elsdenii. J. Bacteriol. 128, 372–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Whitehouse M. W. (1986) Rheumatoid arthritis and tuberculosis. Lancet 2, 688–689 [DOI] [PubMed] [Google Scholar]
- 46.Holoshitz J., Klajman A., Drucker I., Lapidot Z., Yaretzky A., Frenkel A., van Eden W., Cohen I. R. (1986) T lymphocytes of rheumatoid arthritis patients show augmented reactivity to a fraction of mycobacteria cross-reactive with cartilage. Lancet 2, 305–309 [DOI] [PubMed] [Google Scholar]
- 47.Jethwa H., Abraham S. (2017) The evidence for microbiome manipulation in inflammatory arthritis. Rheumatology (Oxford) 56, 1452–1460 [DOI] [PubMed] [Google Scholar]
- 48.Pan H., Guo R., Zhu J., Wang Q., Ju Y., Xie Y., Zheng Y., Wang Z., Li T., Liu Z., Lu L., Li F., Tong B., Xiao L., Xu X., Li R., Yuan Z., Yang H., Wang J., Kristiansen K., Jia H., Liu L. (2018) A gene catalogue of the Sprague-Dawley rat gut metagenome. Gigascience 7 (abstr.) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gilbert J. A., Quinn R. A., Debelius J., Xu Z. Z., Morton J., Garg N., Jansson J. K., Dorrestein P. C., Knight R. (2016) Microbiome-wide association studies link dynamic microbial consortia to disease. Nature 535, 94–103 [DOI] [PubMed] [Google Scholar]
- 50.Lee N., Kim W. U. (2017) Microbiota in T-cell homeostasis and inflammatory diseases. Exp. Mol. Med. 49, e340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu X., Zou Q., Zeng B., Fang Y., Wei H. (2013) Analysis of fecal Lactobacillus community structure in patients with early rheumatoid arthritis. Curr. Microbiol. 67, 170–176 [DOI] [PubMed] [Google Scholar]
- 52.Maeda Y., Takeda K. (2017) Role of gut microbiota in rheumatoid arthritis. J. Clin. Med. 6, E60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Nieuwenhuis E. E., Visser M. R., Kavelaars A., Cobelens P. M., Fleer A., Harmsen W., Verhoef J., Akkermans L. M., Heijnen C. J. (2000) Oral antibiotics as a novel therapy for arthritis: evidence for a beneficial effect of intestinal Escherichia coli. Arthritis Rheum. 43, 2583–2589 [DOI] [PubMed] [Google Scholar]
- 54.Jia X., Jia L., Mo L., Yuan S., Zheng X., He J., Chen V., Guo Q., Zheng L., Yuan Q., Xu X., Zhou X. (2019) Berberine ameliorates periodontal bone loss by regulating gut microbiota. J. Dent. Res. 98, 107–116 [DOI] [PubMed] [Google Scholar]
- 55.Kim D. S., Kwon J. E., Lee S. H., Kim E. K., Ryu J. G., Jung K. A., Choi J. W., Park M. J., Moon Y. M., Park S. H., Cho M. L., Kwok S. K. (2018) Attenuation of rheumatoid inflammation by sodium butyrate through reciprocal targeting of HDAC2 in osteoclasts and HDAC8 in T cells. Front. Immunol. 9, 1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mathewson N. D., Jenq R., Mathew A. V., Koenigsknecht M., Hanash A., Toubai T., Oravecz-Wilson K., Wu S. R., Sun Y., Rossi C., Fujiwara H., Byun J., Shono Y., Lindemans C., Calafiore M., Schmidt T. M., Honda K., Young V. B., Pennathur S., van den Brink M., Reddy P. (2016) Gut microbiome-derived metabolites modulate intestinal epithelial cell damage and mitigate graft-versus-host disease. Nat. Immunol. 17, 505–513; erratum: 1235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin H. V., Frassetto A., Kowalik E. J., Jr., Nawrocki A. R., Lu M. M., Kosinski J. R., Hubert J. A., Szeto D., Yao X., Forrest G., Marsh D. J. (2012) Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS One 7, e35240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang X., He G., Peng Y., Zhong W., Wang Y., Zhang B. (2015) Sodium butyrate alleviates adipocyte inflammation by inhibiting NLRP3 pathway. Sci. Rep. 5, 12676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Louis P., Flint H. J. (2009) Diversity, metabolism and microbial ecology of butyrate-producing bacteria from the human large intestine. FEMS Microbiol. Lett. 294, 1–8 [DOI] [PubMed] [Google Scholar]
- 60.Vital M., Karch A., Pieper D. H. (2017) Colonic butyrate-producing communities in humans: an overview using omics data. mSystems 2, e00130-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.