Abstract
Abnormal placental development is one of the main etiological factors for intrauterine growth restriction (IUGR). Here, we show that LIN28A and LIN28B are significantly lower and lethal-7 (let-7) microRNAs (miRNAs) significantly higher in term human IUGR vs. normal placentas. We hypothesize that let-7 miRNAs regulate genes with known importance for human placental development [high-mobility group AT-hook 1 (HMGA1), transcriptional regulator Myc-like (c-myc), vascular endothelial growth factor A (VEGF-A), and Wnt family member 1 (WNT1)] by targeting the AT-rich interacting domain (ARID)-3B complex. ACH-3P cells with LIN28A and LIN28B knockout (DKOs) significantly increased let-7 miRNAs, leading to significantly decreased ARID3A, ARID3B, and lysine demethylase 4C (KDM4C). Similarly, Sw.71 cells overexpressing LIN28A and LIN28B (DKIs) significantly decreased let-7 miRNAs, leading to significantly increased ARID3A, ARID3B, and KDM4C. In ACH-3P cells, ARID3A, ARID3B, and KDM4C make a triprotein complex [triprotein complex comprising ARID3A, ARID3B, and KDM4C (ARID3B-complex)] that binds the promoter regions of HMGA1, c-MYC, VEGF-A, and WNT1. ARID3B knockout in ACH-3P cells disrupted the ARID3B-complex, leading to a significant decrease in HMGA1, c-MYC, VEGF-A, and WNT1. DKOs had a significant reduction, whereas DKIs had a significant increase in HMGA1, c-MYC, VEGF-A, and WNT1, potentially due to regulation by the ARID3B-complex. This is the first study showing regulation of let-7 targets in immortalized human trophoblast cells by the ARID3B-complex.—Ali, A., Anthony, R. V., Bouma, G. J., Winger, Q. A. LIN28-let-7 axis regulates genes in immortalized human trophoblast cells by targeting the ARID3B-complex.
Keywords: intrauterine growth restriction, placenta, gene regulation
Intrauterine growth restriction (IUGR) is a pregnancy-associated condition in which the fetuses cannot reach their maximum growth potential. Every year, about 30 million neonates in the world are born intrauterine growth restricted (1, 2). Although there are many risk factors for development of IUGR, placenta-associated conditions such as abnormal uteroplacental vasculature, preeclampsia, avascular villi, single umbilical artery, placental insufficiency, abruptio placenta, and placental infections are the most common etiological factors (3–5). In pregnancies compromised by IUGR, the size of the placenta is ∼24% smaller compared with placentas from normal pregnancies (6). A malfunctioning placenta fails to supply enough nutrients and oxygen to the growing fetus, leading to IUGR and other long-term complications in both mother and fetus (7, 8). Hence, a healthy and properly functioning placenta is essential for normal fetal growth.
During the first trimester of human pregnancy, fetal-derived trophoblast cells proliferate and differentiate into different trophoblast lineages: syncytiotrophoblast and extravillous trophoblast cells (9). The extravillous trophoblast cells invade the maternal uterus and remodel the spiral arteries to ensure proper blood flow to the growing fetus (9). These events are regulated through complex genetic pathways. If any of these steps is compromised, it can result in placental disorders leading to IUGR (10). Therefore, investigating the underlying molecular mechanisms of trophoblast proliferation, invasion, and migration and spiral artery remodeling is necessary to improve diagnosis and treatment of various pregnancy-related disorders.
Oncofetal-placental proteins play a significant role in placental development and maintenance (11). The pluripotency factors LIN28A (lin-28 homolog A) and LIN28B (lin-28 homolog B) are highly conserved oncofetal-placental proteins. LIN28A and LIN28B have also been described as protooncogenes because of their ability to regulate and stabilize oncogenes at the posttranscriptional level in tumor cells (12, 13). Another important function of LIN28 is to inhibit the biogenesis of lethal-7 (let-7) microRNAs (miRNAs) in mammalian cells by binding primary and precursor let-7 (14–19). We have previously shown that, in the first trimester (11.5 wk), human placenta LIN28B is dominant and is localized to cytotrophoblast cells (20). In another study, Canfield et al. (21) showed that, in term human placentas, LIN28B mRNA is 1300-fold higher than LIN28A mRNA, and LIN28B protein level is significantly higher in both cytotrophoblasts and syncytiotrophoblast compared with decidua. Knockdown of LIN28 in mouse and human trophoblast cells increases the level of let-7 miRNAs (20, 22).
Liao et al. (23) demonstrated that let-7 miRNAs modulate their target genes in cancer cells by 2 distinct mechanisms. Either they follow a pathway of directly binding the mRNAs of target genes or they follow a chromatin-dependent pathway involving 4 different proteins: AT-rich interaction domain (ARID)-3A, ARID3B, lysine demethylase 4C (KDM4C), and membrane transporter importin-9 (23). ARID3A and ARID3B are members of the highly conserved family of ARID proteins that regulate gene expression by binding AT-rich DNA in promoter regions of genes and remodeling the chromatin material (24, 25). These 2 paralogs share 89.9% amino acid similarity and bind the same DNA regions (26). ARID3A and ARID3B function together and play a vital role in lymphocyte expansion, proliferation of human hematopoietic progenitors, development of colorectal cancer, cell cycle, embryonic development, and transcriptional regulation of stemness genes (27–31). Arid3a has also been shown to play an important role in development of murine placenta. Arid3a knockout (KO) results in abnormal placental development and embryonic lethality at d 12.5 (32). KO of Arid3b in mice causes developmental abnormalities and is embryonic lethal (32–35). In humans, ARID3B is high in malignant tumor tissues compared with control tissues, suggesting a role in cell proliferation and migration (34). ARID3B is exclusively localized in the nucleus, whereas ARID3A is shuttled between the cytoplasm and nucleus by the membrane transporter importin-9 (23, 36, 37). In the nucleus, ARID3B binds ARID3A by interacting with its REKLES-β domain (23, 37). The ARID3A-ARID3B duplex binds KDM4C to make a triprotein complex comprising ARID3A, ARID3B, and KDM4C (ARID3B-complex) that binds the promoter regions of genes and demethylates H3K9me3 and H3K27me3 (23). Histone demethylation caused by KDM4C leads to structural modifications in chromatin, resulting in increased expression of target genes (23). Let-7 miRNAs regulate their target genes in different cancer cell lines by targeting the ARID3B-complex mRNAs (23). However, existence and functionality of this pathway in placenta is yet to be explored.
Many core targets of let-7 miRNAs identified in cancer studies also play a critical role in early human placental development and are important for rapid proliferation of trophoblast cells, maintaining a population of progenitor cytotrophoblasts, placental angiogenesis, and modification of spiral arteries (38–42). High-mobility group AT-hook 1 (HMGA1), transcriptional regulator Myc-like (c-MYC), vascular endothelial growth factor A (VEGF-A), and Wnt family member 1 (WNT1) are examples of such genes. HMGA1 promotes the invasion of trophoblast cells, and reduced levels of HMGA1 have been linked to pathogenesis of preeclampsia (43, 44). The c-MYC protooncogene has been identified as a proliferation factor in human cytotrophoblast cells. Its amount decreases when cytotrophoblasts differentiate into syncytiotrophoblast (45). VEGF-A plays a critical role in human trophoblast migration and differentiation, spiral artery remodeling, and placental angiogenesis (46–49). Reduced concentration of VEGF-A is one of the factors in the pathogenesis of preeclampsia and IUGR (50). The protooncogene WNT1 is highly expressed in early placentas compared with term placentas. It is important for proliferation of trophoblast cells, and reduced WNT1 is also one of the factors in the pathogenesis of preeclampsia (51, 52). Hence, HMGA1, c-MYC, VEGF-A, and WNT1 are important for placental development, and exploring the mechanisms behind the regulation of these genes will be a step toward diagnosis and management of placenta-associated disorders. The objective of this study is to explore the mechanism for regulation of HMGA1, c-MYC, VEGF-A, and WNT1 by the ARID3B-complex and its correlation with the LIN28–let-7 axis in immortalized first-trimester human trophoblast cells.
MATERIALS AND METHODS
Term human placental tissue samples
Term human placental tissue samples and the clinical characteristics of the patients used in this study have been previously described by Jozwik et al. (53). Briefly, term placentas from normal pregnancies (control) and the IUGR pregnancies were collected immediately postpartum. The collected tissue samples were snap frozen in liquid nitrogen and then stored at −80°C. miRNA and protein were extracted from these samples for further analysis.
Cell lines
Two different first-trimester human trophoblast cell lines, ACH-3P and Sw.71, were used in this study. ACH-3P cells are hybrid cells obtained by fusion of primary first-trimester human trophoblast cells (wk 12 of gestation) with a human choriocarcinoma cell line (AC1-1) (54). Sw.71 cells are immortalized first-trimester human trophoblast cells (wk 7 of gestation) produced by infecting primary trophoblast cells with hTERT (55). Both cell lines were maintained in DMEM–F-12 medium supplemented with 10% heat-inactivated fetal bovine serum and 1× penicillin–streptomycin–amphotericin B solution at 37°C and 5% CO2.
CRISPR/Cas9
KO ACH-3P cells were generated using a clustered regularly interspaced short palindromic repeat (CRISPR)/CRISPR-associated protein 9 (Cas9)–based genome editing technique. Target-specific guide RNAs (gRNAs) for LIN28A, LIN28B, and ARID3B were designed using web-based tools (56, 57) and cloned in lentiCRISPRv2 plasmid (Addgene, Watertown, MA, USA). The lentiCRISPRv2 plasmids with specific gRNAs were used to make second-generation lentiviral particles. The lentiviral particles were used to infect the ACH-3P cells at 70–80% confluency in 1 well of a 12-well plate (Corning, Corning, NY, USA). After 48–72 h, the infected cells were selected using 2–4 ng/µl puromycin. Western blot was performed to confirm gene knockout. To obtain cell lines with complete knockout of the target gene, single-cell colonies were selected. Colonies with depletion of protein of interest were used for further experiments. ACH-3P cells with LIN28A knockout (AKOs), ACH-3P cells with LIN28B knockout (BKOs), ACH-3P cells with LIN28A and LIN28B knockout (DKOs), and ACH-3P cells with ARID3B knockout (ARID3B KOs) were generated. To create DKOs, AKOs were infected with lentiviral particles designed for knockout of LIN28B. Single-cell colonies were selected and tested by Western blot to confirm a double knockout of LIN28A and LIN28B. ACH-3P cells infected with lentiviral particles containing scrambled gRNA were used as scramble control (SC) cells. The gRNA sequences used in this study are listed in Supplemental Table S1.
Overexpression of LIN28A and LIN28B
To overexpress genes in Sw.71 cells, lentiviral gene expression vectors expressing LIN28A (pLV[Exp]-Puro-EF1A > hLIN28A[XM_011542148.1]) or LIN28B (pLV[Exp]-Bsd-EF1A > hLIN28B[NM_001004317.3]) were purchased (VectorBuilder, Chicago, IL, USA). Second-generation lentiviral particles were generated using expression vectors. Lentiviral particles were used to infect Sw.71 cells at 70–80% confluency in 1 well of a 12-well plate (Corning). After 48–72 h, the infected cells were selected using appropriate antibiotics (2–4 ng/µl puromycin for LIN28A-expressing cells and 4–8 ng/µl blasticidin for LIN28B-expressing cells). Successful gene knock-in was confirmed using real-time RT-PCR and Western blot analysis. Sw.71 cells overexpressing LIN28A (AKIs), Sw.71 cells overexpressing LIN28B (BKIs), and Sw.71 cells overexpressing both LIN28A and LIN28B (DKIs) were generated. To create DKIs, LIN28A-overexpressing cells were infected with LIN28B expression lentiviral particles. Sw.71 cells infected with lentiviral expression particles containing stuffer nucleotide sequence were used to generate expression vector control (EVC) Sw.71 cells.
Production of second-generation lentiviral particles
To generate second-generation lentiviral particles, 3 vectors were used, including transfer vector (lentiCRISPR v.2 or expression vector), packaging plasmid (psPAX2 from Addgene), and envelope plasmid (pMD2.G from Addgene). 293FT cells (Thermo Fisher Scientific, Waltham, MA, USA) were cultured in DMEM high-glucose medium supplemented with 10% heat-inactivated fetal bovine serum and 1× penicillin–streptomycin–amphotericin B solution at 37°C and 5% CO2. 8.82 µg transfer vector DNA, 6.66 μg psPAX2 packaging plasmid DNA, and 2.70 μg pMD2.G envelope plasmid DNA were used to make viral particles. The plasmid DNA was mixed with 180 µl of polyfect transfection reagent (Qiagen, Germantown, MD, USA), and the final volume was brought up to 855 µl using DMEM high-glucose medium without any supplements. The plasmids-polyfect mixture was incubated at room temperature for 10 min and then gently mixed in the medium on 293FT cells at 70–80% confluence. Cells were incubated for 4–6 h at 37°C and 5% CO2. After incubation time, the transfection medium was replaced by fresh DMEM high-glucose medium supplemented with 10% heat-inactivated fetal bovine serum and 1× penicillin–streptomycin–amphotericin B solution. After 72 h, the medium containing lentiviral particles was collected and ultracentrifuged over a 20% sucrose cushion at 22,000 rpm for 2 h at 4°C. The pelleted lentiviral particles were resuspended in 1× PBS, measured in aliquots, and stored at −80°C. The frozen viral aliquot was resuspended in 0.5–1 ml of appropriate medium with 8 μg/ml polybrene. The target cells were incubated with lentiviral particles for 24 h at 37°C and 5% CO2. After 24 h of incubation, targeted cells were washed, and fresh medium was put on the cells. After 72 h of culture, cells were selected with appropriate selection antibiotic as previously described.
RNA extraction and real-time RT-PCR
For real-time RT-PCR analysis, mRNA was isolated from human placental tissue and immortalized first-trimester human trophoblast cells using RNeasy Mini Kit (Qiagen) following the manufacturer’s protocol. The mRNA was reverse transcribed to cDNA using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). Real-time RT-PCR reactions were run in triplicate in 384-well plates using 10 µl reaction volume in each well. The reaction volume included 5 µl of 2× Light-Cycler 480 Sybr Green I Master (Roche, Basel, Switzerland), 50 ng cDNA, and 1 µM of target-specific forward and reverse primers. Primer sequences used for real-time RT-PCR are listed in Supplemental Table S2. PCR reactions were incubated in the Light-Cycler 480 PCR machine (Roche) at the following cycling conditions: 95°C for 10 min, 45 cycles of 95°C for 30 s, 55°C for 1 min, and 72°C for 1 min. Relative mRNA levels were normalized using glyceraldehyde 3-phosphate dehydrogenase (GAPDH). For miRNA profiling, total RNA was extracted using an miRNeasy Mini Kit (Qiagen) following the manufacturer’s protocol. Three hundred nanograms total RNA was reverse transcribed to cDNA using an miScript RT II Kit (Qiagen). Real-time RT-PCR reactions were run in triplicate in 384-well plates using 10 µl reaction volume in each well. The reaction volume included 5 µl of 2× QuantiTech Sybr Green Master Mix (Qiagen), 3 ng cDNA, 1× miScript universal primer (Qiagen), and 1× miScript assay for let-7 miRNA (let-7a, let-7b, let-7c, let-7d, let-7e, let-7f, let-7g, and let-7i). These reactions were incubated in the Light-Cycler 480 PCR machine (Roche) at the following cycling conditions: 95°C for 15 min, 45 cycles of 94°C for 15 s, 55°C for 30 s, and 70°C for 30 s. Relative miRNA levels were normalized using small nucleolar RNA, C/D box 48 (SNORD-48).
Protein extraction and Western blot
Western blot analysis was performed using whole-cell lysate to quantify proteins in cells and tissue samples. For protein extraction, cell pellets were resuspended in 200–400 µl RIPA buffer [20 mM Tris, 137 mM NaCl, 10% glycerol, 1% nonidet P-40, 3.5 mM SDS, 1.2 mM sodium deoxycholate, and 1.6 mM EDTA (pH 8)] containing 1× protease-phosphate inhibitor cocktail (MilliporeSigma, Burlington, MA, USA). The whole-cell lysate was incubated on ice for 5 min and then centrifuged at 14,000 g for 5 min to remove cell debris. To extract protein from human placental tissues, the tissue was dipped in liquid nitrogen and ground using mortar and pestle and then homogenized in RIPA buffer. Homogenized samples were sonicated using a Bioruptor Sonication System (Diagenode, Denville, NJ, USA) for 5 cycles of 30 s on and 30 s off. Sonicated samples were centrifuged at 14,000 g for 5 min to remove debris. Protein concentration was measured using the BCA protein assay kit (Thermo Fisher Scientific). Protein was separated in 10% Bis-Tris gels (Bio-Rad) at 90 V for 15 min and 125 V for 60 min and then transferred to a nitrocellulose membrane with a 0.45-μm pore size (Bio-Rad) at 100 V for 2 h at 4°C. The membranes were then blocked in 5% nonfat dry milk solution in Tris-buffered saline with Tween 20 (TBST) [50 mM Tris, 150 mM NaCl, and 0.05% Tween 20 (pH 7.6)] for 1 h at room temperature. After blocking, the membranes were washed 3 times with 1× TBST for 5 min each and then incubated at 4°C overnight with specific primary antibody. After overnight incubation, the membranes were washed 3 times with 1× TBST for 5 min each. After washing, the membranes were incubated with appropriate secondary antibody conjugated to horseradish peroxidase for 1 h at room temperature. After removing the secondary antibody, the membranes were washed following the same procedure and developed using Super Signal WestDura Extended Duration Substrate (Thermo Fisher Scientific) and imaged using the ChemiDoc XRS + Chemiluminescence System (Bio-Rad). The images were quantified using Image-Lab software (Bio-Rad). To normalize protein quantity, β-actin, α-tubulin, or GAPDH was used as loading control. Each experiment was repeated on 3 replicates. The antibodies used and their dilutions are listed in Supplemental Table S3.
Coimmunoprecipitation
Proteins were extracted from cells following the same procedure used for Western blotting. Protein-G Magnetic Beads (Bio-Rad) were used following the manufacturer’s protocol. Briefly, 100 µl of thoroughly resuspended protein-G magnetic beads were conjugated with 2 µg ARID3A or ARID3B antibody. The antibody-conjugated protein-G beads were then incubated with 25–50 µg protein sample. After immunoprecipitation, proteins were eluted using 1× Laemmli buffer and used for Western blot following the same procedure previously described. The membranes were probed using ARID3B and KDM4C antibodies to determine protein-protein interaction. Normal mouse IgG was used as a negative control in this experiment.
Chromatin immunoprecipitation
The chromatin immunoprecipitation (ChIP) assay was performed using Magna ChIP G (MilliporeSigma) following the manufacturer’s protocol. Wild-type cells at 70–80% confluence or ARID3B KO cells were used in this procedure. Five hundred and fifty microliters formaldehyde was added in 19 ml medium for crosslinking and kept on ice for 10 min. Chromatin was extracted from formaldehyde-treated cells and sheared to produce 100–1000 bp strands using Bioruptor Sonication System (Diagenode). Sonication was done at 4°C for 20 cycles: 30 s on and 30 s off. Immunoprecipitation was done using ARID3B or KDM4C antibodies, and immunoprecipitated DNA was eluted and used to run end-point PCR for HMGA1, c-MYC, VEGF-A, or WNT1 using positive primers designed to amplify the promoter region and negative primers designed to amplify the distal enhancer region upstream to the promoter region of the specific gene. The primers used in ChIP assay are listed in Supplemental Table S4. ChIP input DNA was used as positive controls for PCR, and sheared chromatin immunoprecipitated by normal rabbit IgG was used as negative control.
Cell proliferation assay
Cell proliferation was measured using the Quick Cell Proliferation Assay Kit (ab65475; Abcam, Cambridge, MA, USA) following the manufacturer’s protocol. This assay is based on cleavage of tetrazolium salt to formazan by mitochondrial dehydrogenases. SC, LIN28 DKO, and ARID3B KO cells were plated to a density of 2500 cells per 100 µl in 96-well tissue culture plates, with 4 replicates of each cell type. After 24 or 48 h of plating the cells, 10 µl tetrazolium salt reagent was added in each well, followed by incubation for 2 h in standard culture conditions. Absorbance was measured using Cytation 3 Multi-Mode Reader (BioTek Instruments, Winooski, VT, USA) at 440 nm with a reference wavelength of 650 nm.
Statistics
All data were analyzed using Prism 7 Software (GraphPad, La Jolla, CA, USA). To determine significance of mRNAs and proteins, all values were compared between KI vs. EVC Sw.71 cells, KO vs. SC ACH-3P cells, and term human placental tissue from IUGR pregnancies vs. normal pregnancies using a Student’s t test. Treatment effects on miRNA expression were determined by ANOVA followed by Tukey’s honest significant difference post hoc test. Values of P < 0.05 were considered statistically significant. The error bars in the figures indicate sem.
RESULTS
LIN28A and LIN28B and ARID3A and ARID3B in term human placentas from IUGR pregnancies and normal pregnancies
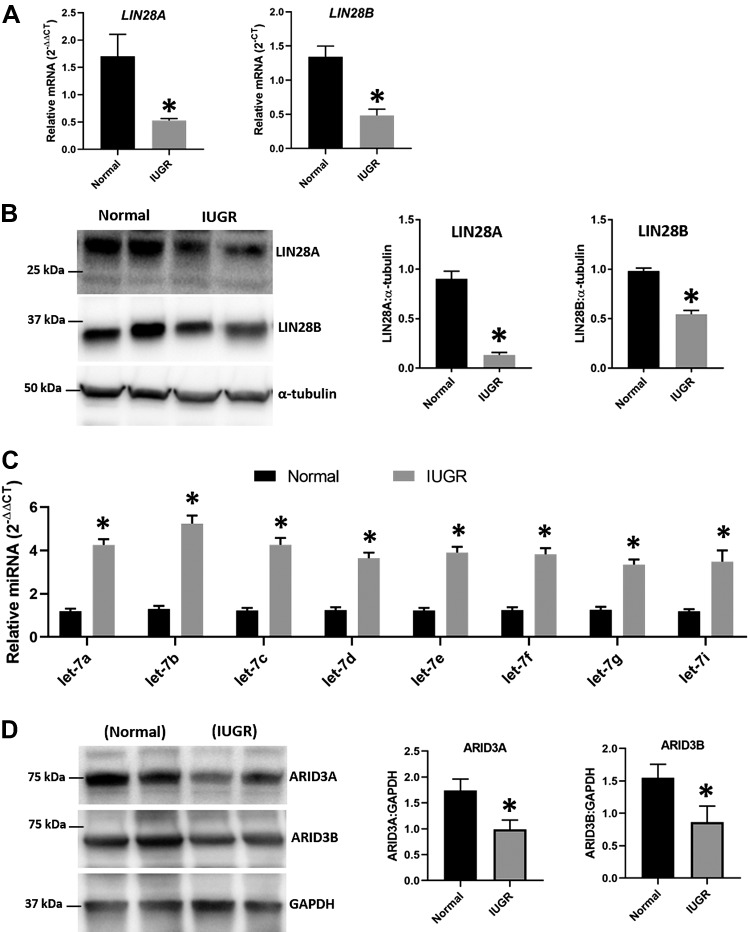
LIN28A and LIN28B were quantified by real-time RT-PCR and Western blot in term human placentas from IUGR pregnancies (n = 8) and normal pregnancies (n = 8). Both LIN28A and LIN28B were significantly reduced in term human placentas from IUGR pregnancies compared with normal pregnancies (Fig. 1A). Densitometric analysis of Western blots showed that LIN28A and LIN28B proteins were significantly reduced in term human placentas from IUGR pregnancies compared with normal pregnancies (Fig. 1B). Real-time RT-PCR data showed that let-7 miRNAs (let-7a–g and -7i) were significantly higher in term human placentas from IUGR pregnancies compared with normal pregnancies (Fig. 1C). Western blot data showed that ARID3A and ARID3B proteins were also significantly reduced in term human placentas from IUGR pregnancies compared with normal pregnancies (Fig. 1D). These results suggest that reduced levels of LIN28A and LIN28B in term human placentas from IUGR pregnancies lead to significantly higher levels of let-7 miRNAs that correlate with reduced levels of ARID3A and ARID3B.
Figure 1.
LIN28A, LIN28B, and let-7 miRNAs in term human placentas from IUGR pregnancies and normal pregnancies. A) LIN28A and LIN28B in term human placentas from IUGR pregnancies (n = 8) compared with normal pregnancies (n = 8). B) Representative immunoblots for LIN28A, LIN28B, and α-tubulin and densitometric analysis of immunoblotting results for LIN28A and LIN28B in term human placentas from IUGR pregnancies (n = 8) compared with normal pregnancies (n = 8). C) Let-7 miRNAs in term human placentas from IUGR pregnancies (n = 8) compared with normal pregnancies (n = 8). D) Representative immunoblots for ARID3A, ARID3B, and GAPDH and densitometric analysis of immunoblotting results for ARID3A and ARID3B in term human placentas from IUGR pregnancies (n = 6) compared with normal pregnancies (n = 6). *P < 0.05 vs. normal placentas.
LIN28A, LIN28B, and let-7 miRNAs in immortalized first-trimester human trophoblast cell lines
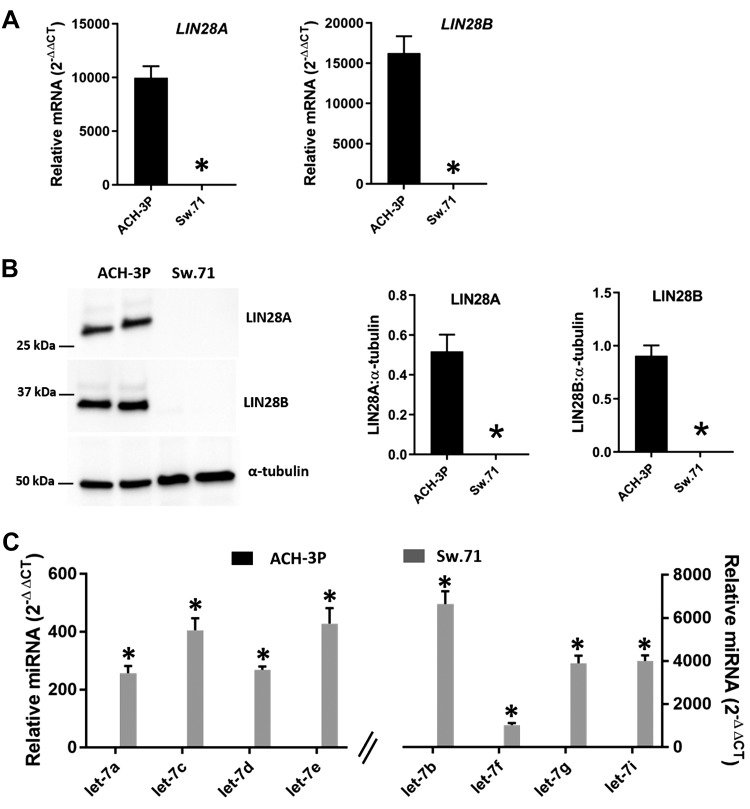
The results from human term placental tissues led us to further investigate the LIN28–let-7 axis and its potential role in early placental development. For this purpose, 2 first-trimester human trophoblast cell lines were used: ACH-3P cells and Sw.71 cells. Real-time RT-PCR data showed that LIN28A was ∼10,000-fold higher and LIN28B about 15,000-fold higher in ACH-3P cells compared with Sw.71 cells (Fig. 2A). Western blot data showed prominent bands for both LIN28A and LIN28B in ACH-3P cells and no detectable proteins in Sw.71 cells (Fig. 2B). Real-time RT-PCR analysis revealed that let-7 miRNAs (let-7a–g and -7i) were significantly higher in Sw.71 cells compared with ACH-3P cells (Fig. 2C). These results suggest that let-7 miRNAs are low in ACH-3P cells and correspond to high LIN28A and LIN28B, whereas let-7 miRNAs are high in Sw.71 cells and correspond to low LIN28A and LIN28B.
Figure 2.
LIN28A, LIN28B, and let-7 miRNAs in ACH-3P cells and Sw.71 cells. A) LIN28A and LIN28B mRNA levels in ACH-3P cells compared with Sw.71 cells (n = 3). B) Representative immunoblots for LIN28A, LIN28B, and α-tubulin and densitometric analysis of immunoblotting results for LIN28A and LIN28B in ACH-3P cells compared with Sw.71 cells (n = 3). C) Let-7 miRNAs in ACH-3P cells compared with Sw.71 cells (n = 3). *P < 0.05 vs. ACH-3P cells.
Knockout of LIN28 in ACH-3P cells
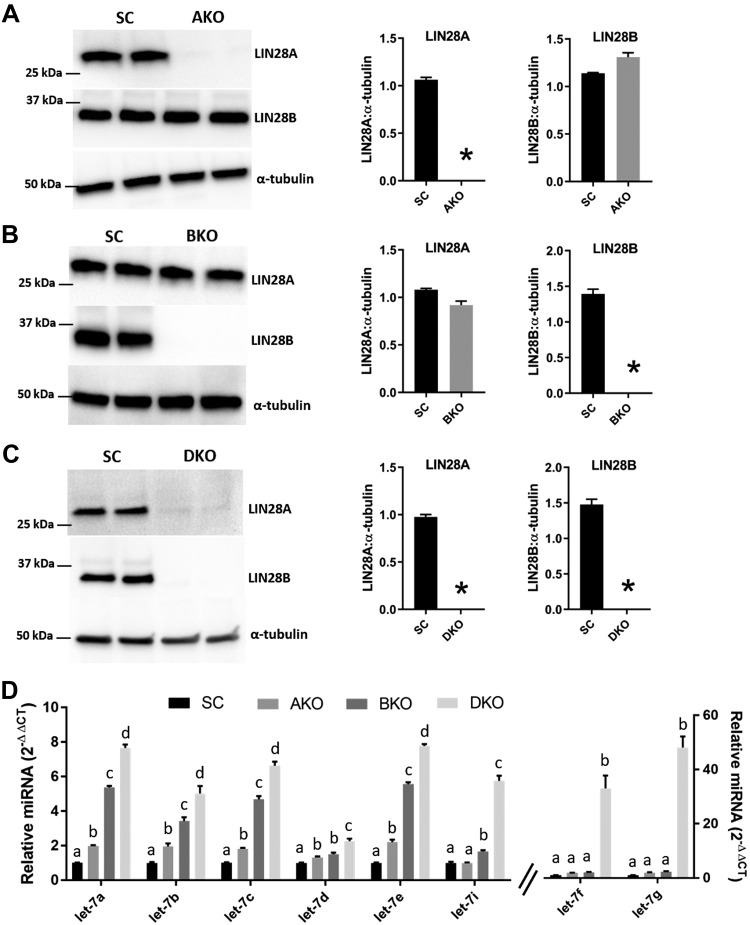
ACH-3P cells had high LIN28A and LIN28B. To see whether reduced levels of LIN28A or LIN28B would affect let-7 miRNA levels, 3 knockout cell lines were generated using CRISPR/Cas9–based genome editing: AKO, BKO, and DKO. Western blot analysis confirmed that AKOs had significant depletion of LIN28A without significant change in LIN28B compared with SC ACH-3P cells (Fig. 3A). BKOs had significant depletion of LIN28B without significant change in LIN28A compared with SC (Fig. 3B). In DKOs, both LIN28A and LIN28B were significantly depleted compared with SC (Fig. 3C). Real-time RT-PCR analysis was done to compare let-7 miRNAs (let-7a–g and -7i) between SC, AKOs, BKOs, and DKOs. AKOs had significantly higher levels of let-7a–e and no significant change in levels of let-7d, -7f, and -7i compared with SC. BKOs had significantly higher levels of let-7a–e and -7i and no change in let-7f and -7g compared with SC. However, compared with AKOs, the levels of let-7a–c, 7e, and 7i significantly increased, whereas the levels of let-7d, -7f, and -7g remained unchanged in BKOs. DKOs had a significant increase in all let-7 miRNAs (let-7a–g, and -7i) compared with SC, AKOs, and BKOs (Fig. 3D). These results show that knockout of LIN28B affects more let-7 miRNAs than knockout of LIN28A, and DKOs had the greatest increase in let-7 miRNAs.
Figure 3.
AKO, BKO, and DKO in ACH-3P cells and its effect on let-7 miRNAs. A–C) Representative immunoblots for LIN28A, LIN28B, and α-tubulin and densitometric analysis of LIN28A and LIN28B in AKOs, BKOs, and DKOs compared with SC (n = 3). D) Comparison of let-7 miRNAs in SC vs. AKO vs. BKO vs. DKO (n = 3). Letters (a–d) indicate a significant difference (P < 0.05) in let-7 miRNA by treatment. *P < 0.05 vs. SC.
Overexpression of LIN28 in Sw.71 cells
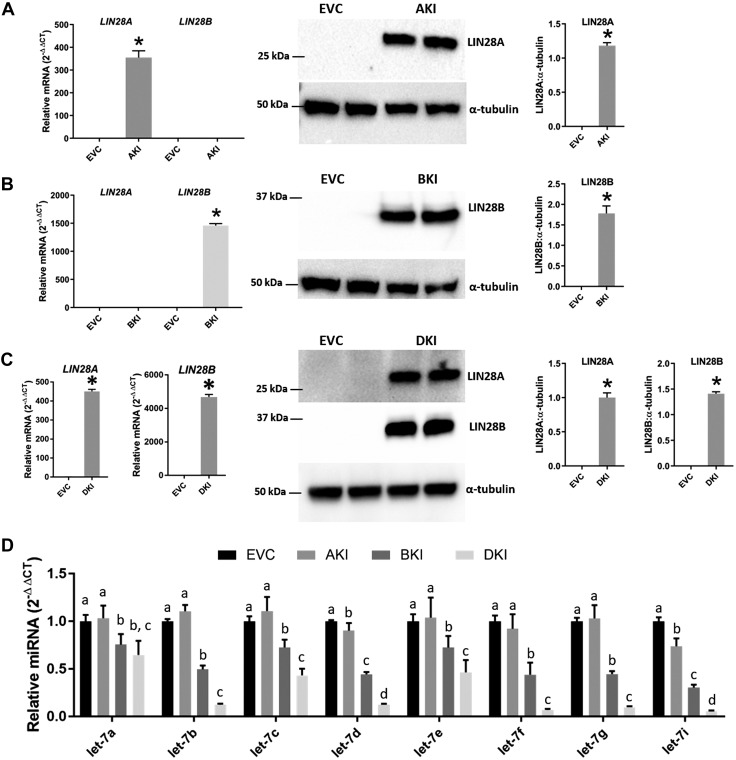
We further assessed the regulation of let-7 miRNAs by LIN28 by overexpressing one or both paralogs of LIN28 in Sw.71 cells, which have low expression of LIN28A and LIN28B. For this purpose, 3 knock-in cell lines were generated: AKIs, BKIs, and DKIs. Real-time RT-PCR and Western blot analysis confirmed that AKIs had significantly higher LIN28A and no change in LIN28B compared with EVC Sw.71 cells (Fig. 4A). BKIs had significantly higher LIN28B and no change in LIN28A compared with EVC (Fig. 4B). DKIs had a significant increase in both LIN28A and LIN28B compared with EVC (Fig. 4C). Real-time RT-PCR analysis was done to compare let-7 miRNAs (let-7a–g and -7i) between these cell lines. AKIs had significant decreases in let-7d and -7i, whereas let-7a–c, and -7e, let-7f, and -7g were unchanged compared with EVC. BKIs had significant decreases in all let-7 miRNAs (let-7a–g, and -7i) compared with EVC as well as AKIs. DKIs had significant reductions in all let-7 miRNAs (let-7a–g, and -7i) compared with EVC and AKIs, whereas let-7b–g, and -7i significantly increased and let-7a remained unchanged compared with BKIs (Fig. 4D). These results indicate that overexpression of LIN28B has a greater impact on let-7 miRNAs compared with overexpression of LIN28A, whereas overexpression of both LIN28A and LIN28B results in a significant reduction in let-7 miRNAs compared with overexpression of LIN28A or LIN28B alone.
Figure 4.
Knock-in of LIN28A (AKI), LIN28B (BKI), and LIN28A-B (DKI) in Sw.71 cells and its effect on let-7 miRNAs. A–C) Detection of LIN28A and LIN28B mRNA and protein in AKIs, BKIs, and DKIs compared with EVC (n = 3). D) Comparison of let-7 miRNAs in EVC vs. AKI vs. BKI vs. DKIs (n = 3). *P < 0.05 vs. EVC.
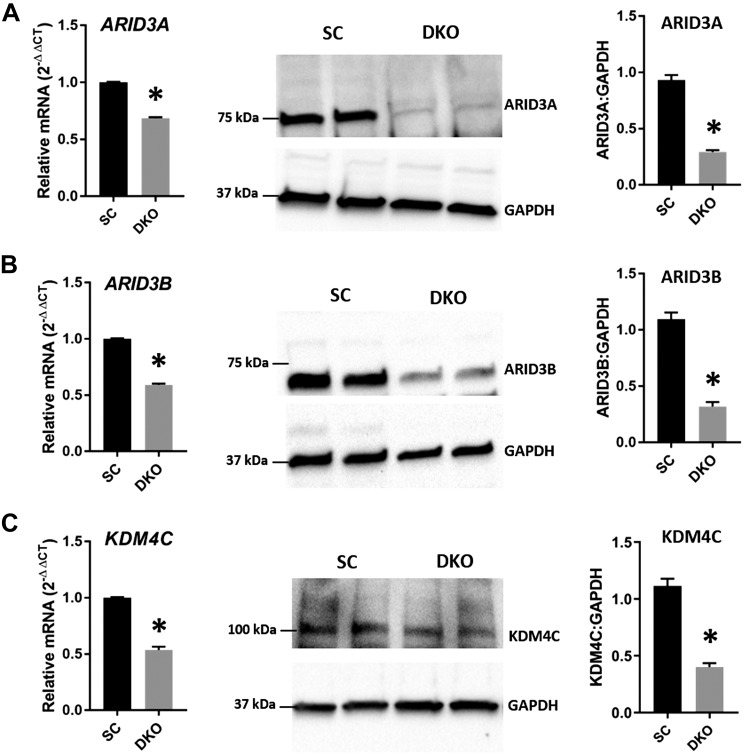
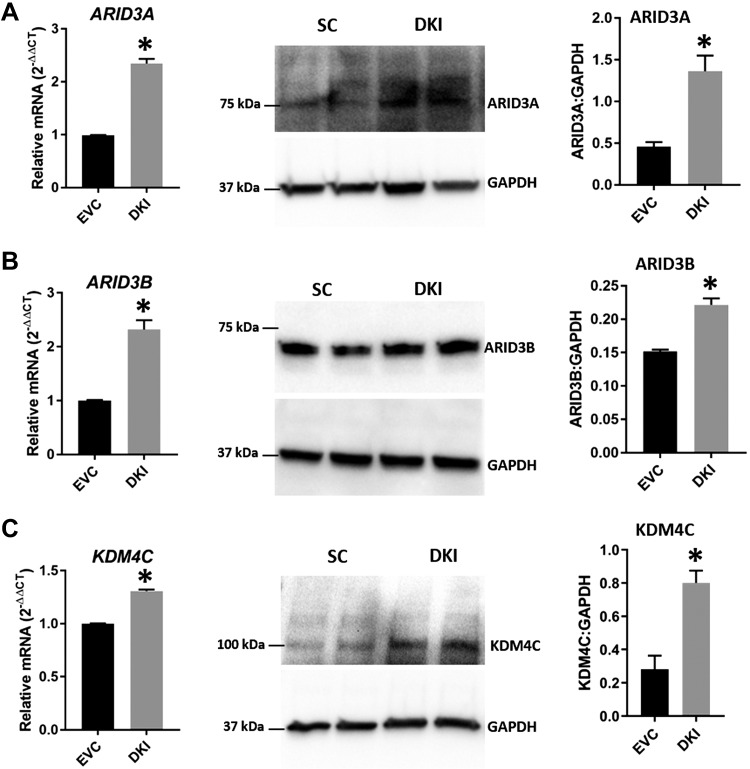
ARID3A, ARID3B, and KDM4C in DKOs and DKIs
To determine whether a change in let-7 miRNAs affects the components of the chromatin-based gene regulation pathway (ARID3A, ARID3B, and KDM4C), the mRNA and protein levels of these genes were analyzed in DKOs and DKIs. Real-time RT-PCR and Western blot data showed that ARID3A, ARID3B, and KDM4C were significantly reduced in DKOs compared with SC (Fig. 5A–C). These results indicate that elevated let-7 miRNAs correlate with reduced ARID3A, ARID3B, and KDM4C in DKOs. Similarly, real-time RT-PCR and Western blot data showed significant increases in ARID3A, ARID3B, and KDM4C in DKIs compared with EVC (Fig. 6A–C), suggesting that reduced let-7 miRNAs correlate with increased ARID3A, ARID3B, and KDM4C in DKIs.
Figure 5.
ARID3A, ARID3B, and KDM4C in DKOs. Detection of ARID3A (A), ARID3B (B), and KDM4C (C) mRNA and protein in LIN28 DKOs compared with SC (n = 3). *P < 0.05 vs. SC.
Figure 6.
ARID3A, ARID3B, and KDM4C in DKIs. Detection of ARID3A (A), ARID3B (B), and KDM4C (C) mRNA and protein in DKIs compared with EVC (n = 3). *P < 0.05 vs. EVC.
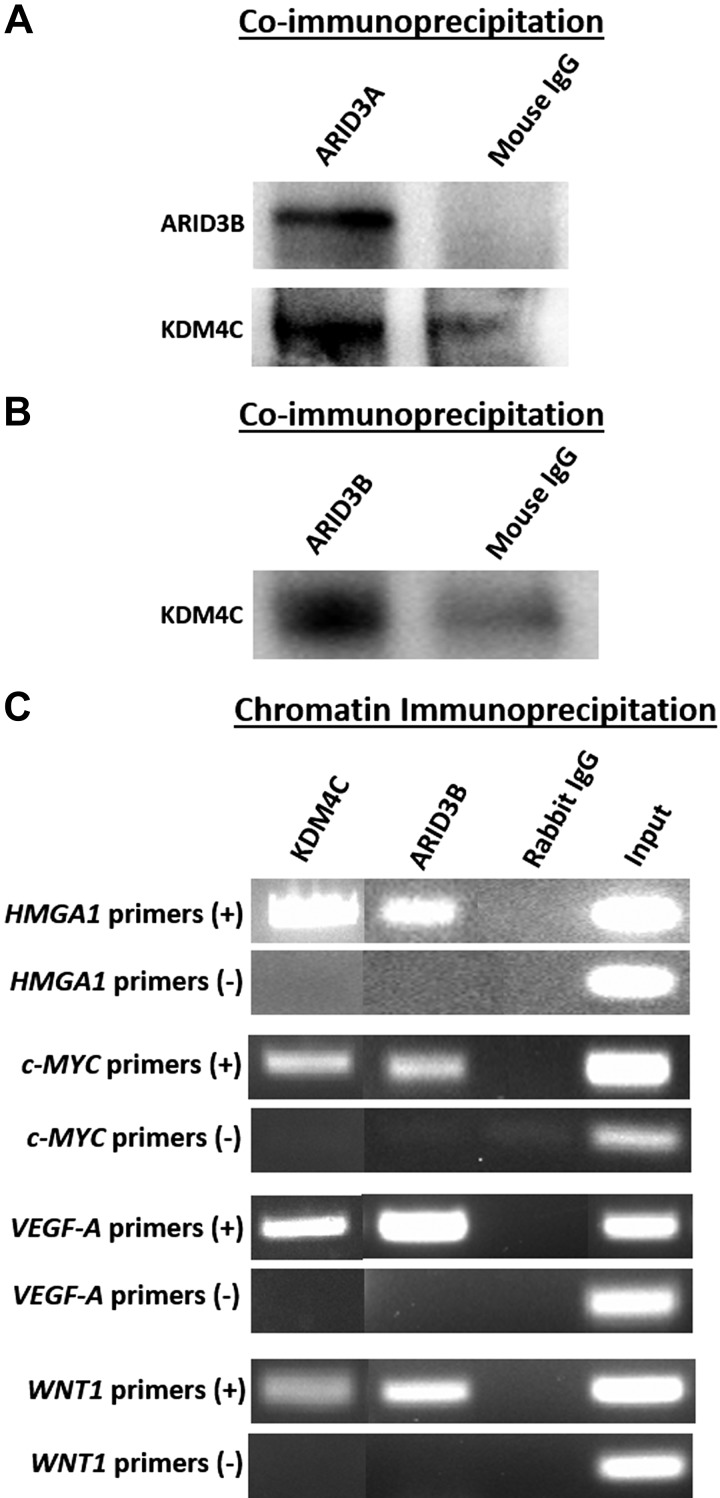
ARID3A, ARID3B, and KDM4C co-occupy promoter regions in HMGA1, c-MYC, VEGF-A, and WNT1
To determine whether ARID3A, ARID3B, and KDM4C regulate let-7 miRNA target genes with known importance for placental development (HMGA1, c-MYC, VEGF-A, and WNT1), coimmunoprecipitation (co-IP) and ChIP assays were performed using ACH-3P cells. Co-IP using ARID3A antibody indicated that ARID3A is complexed with ARID3B and KDM4C in ACH-3P cells (Fig. 7A). Furthermore, co-IP using ARID3B antibody confirmed complex formation between ARID3B and KDM4C in ACH-3P cells (Fig. 7B). These results indicate the existence of an ARID3B-complex composed of ARID3A, ARID3B, and KDM4C in ACH-3P cells. The ChIP assay confirmed co-occupancy of ARID3B and KDM4C in promoter regions of HMGA1, c-MYC, VEGF-A, and WNT1 (Fig. 7C). These data suggest that the ARID3B-complex is involved in regulation of HMGA1, c-MYC, VEGF-A, and WNT1 expression by binding their promoter regions in immortalized first-trimester human trophoblast cells.
Figure 7.
ARID3B complex in ACH-3P cells. A) Co-IP assay using ARID3A and normal mouse IgG for immunoprecipitation and ARID3B and KDM4C for immunoblotting. B) Co-IP using ARID3B and normal mouse IgG for immunoprecipitation and KDM4C for immunoblotting. C) ChIP by KDM4C, ARID3B, and normal rabbit IgG using sheared chromatin from ACH-3P cells and end-point PCR for HMGA1, c-MYC, VEGF-A, and WNT1.
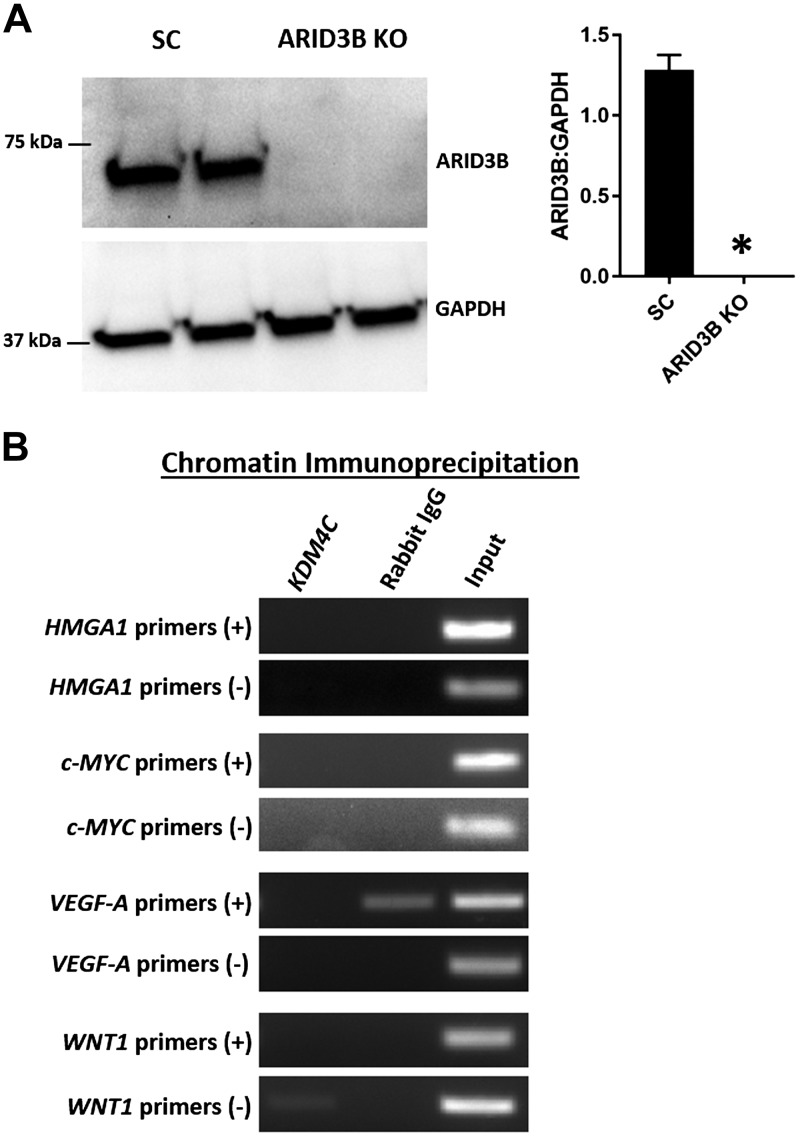
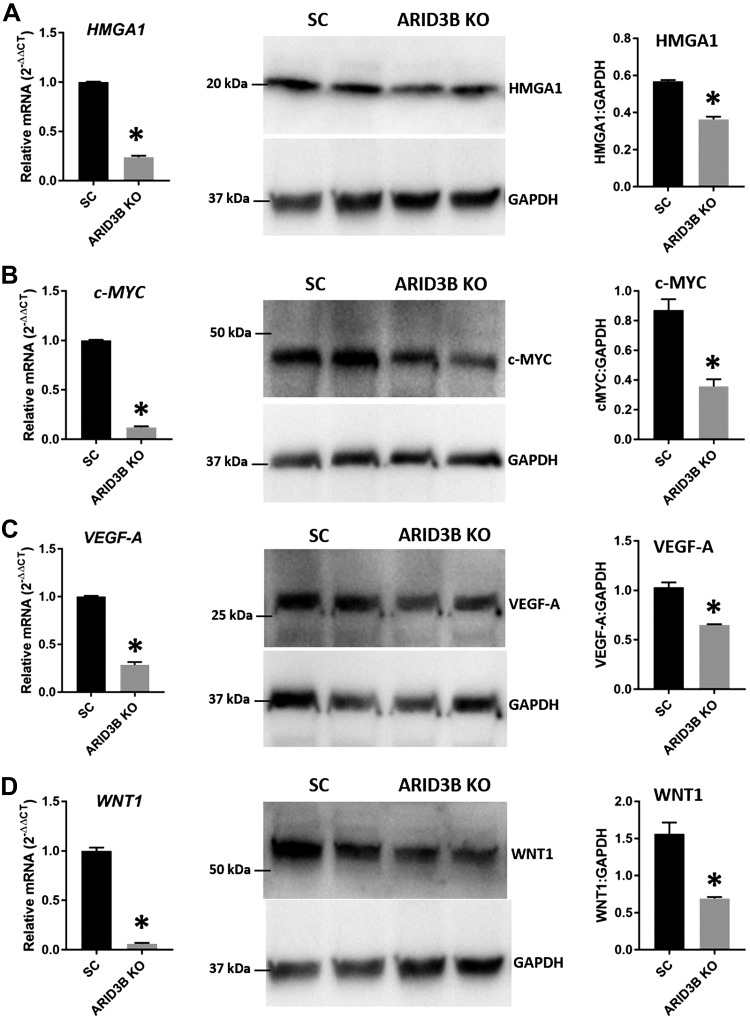
HMGA1, c-MYC, VEGF-A, and WNT1 in ARID3B KO cells
To test the role of the ARID3B-complex in regulation of HMGA1, c-MYC, VEGF-A, and WNT1 in immortalized first-trimester human trophoblast cells, this complex was disrupted by knocking out ARID3B in ACH-3P using CRISPR/Cas9–based genome editing. Western blot analysis confirmed significant depletion of ARID3B in ARID3B KO cells compared with SC (Fig. 8A). A ChIP assay performed using KDM4C antibody revealed that KDM4C could not bind to the promoter regions of HMGA1, c-MYC, VEGF-A, and WNT1 in ARID3B KO cells (Fig. 8B). These data confirm that KDM4C binds the promoter regions of HMGA1, c-MYC, VEGF-A, and WNT1 only in the presence of ARID3B. Because KDM4C could not bind the promoter regions of HMGA1, c-MYC, VEGF-A, and WNT1 in ARID3B KO cells, we determined its effect on the expression of these genes. Real-time RT-PCR and Western blot analysis showed a significant reduction in HMGA1, c-MYC, VEGF-A, and WNT1 in ARID3B KO cells compared with SC (Fig. 9), indicating that the disruption of the ARID3B-complex is correlated with reductions in HMGA1, c-MYC, VEGF-A, and WNT1 in ACH-3P cells.
Figure 8.
Chromatin binding of KDM4C in ARID3B KO cells. A) Representative immunoblots for ARID3B and GAPDH and densitometric analysis of immunoblotting results for ARID3B in ARID3B KO cells compared with SC (n = 3). B) ChIP by KDM4C and normal rabbit IgG using sheared chromatin from ARID3B KO cells and end-point PCR for HMGA1, c-MYC, VEGF-A, and WNT1. *P < 0.05 vs. SC.
Figure 9.
HMGA1, c-MYC, VEGF-A, and WNT1 in ARID3B KO cells. Detection of HMGA1 (A), c-MYC (B), VEGF-A (C), and WNT1 (D) mRNA and protein in ARID3B KO cells compared with SC (n = 3). *P < 0.05 vs. SC.
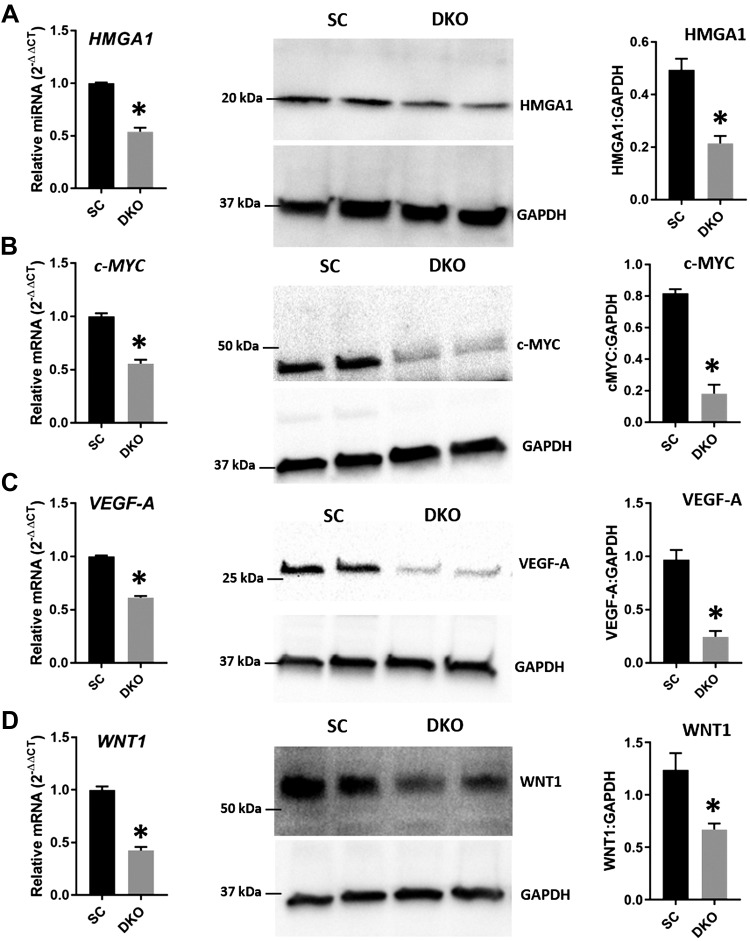
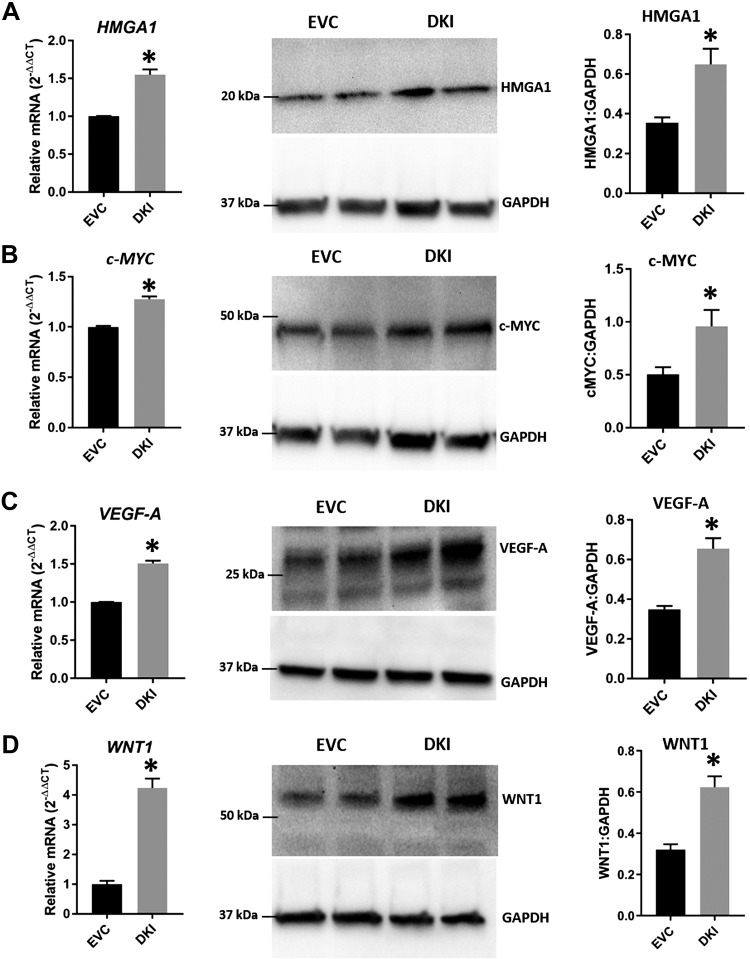
HMGA1, c-MYC, VEGF-A, and WNT1 in DKOs and DKIs
The ARID3B-complex regulates HMGA1, c-MYC, VEGF-A, and WNT1, and because levels of ARID3A, ARID3B, and KDM4C decreased in LIN28 DKOs and increased in DKIs, we measured the levels of HMGA1, c-MYC, VEGF-A, and WNT1 in these cells. Real-time RT-PCR and Western blot data revealed that HMGA1, c-MYC, VEGF-A, and WNT1 were significantly reduced in DKOs compared with SC (Fig. 10) and significantly increased in DKIs compared with EVC (Fig. 11). These results suggest that HMGA1, c-MYC, VEGF-A, and WNT1 are regulated by the ARID3B-complex and let-7 miRNAs.
Figure 10.
HMGA1, c-MYC, VEGF-A, and WNT1 in DKOs. Detection of HMGA1 (A), c-MYC (B), VEGF-A (C), and WNT1 (D) mRNA and protein in DKOs compared with SC (n = 3). *P < 0.05 vs. SC.
Figure 11.
HMGA1, c-MYC, VEGF-A, and WNT1 in DKIs. Detection of HMGA1 (A), c-MYC (B), VEGF-A (C), and WNT1 (D) mRNA and protein in DKIs compared with EVC (n = 3). *P < 0.05 vs. EVC.
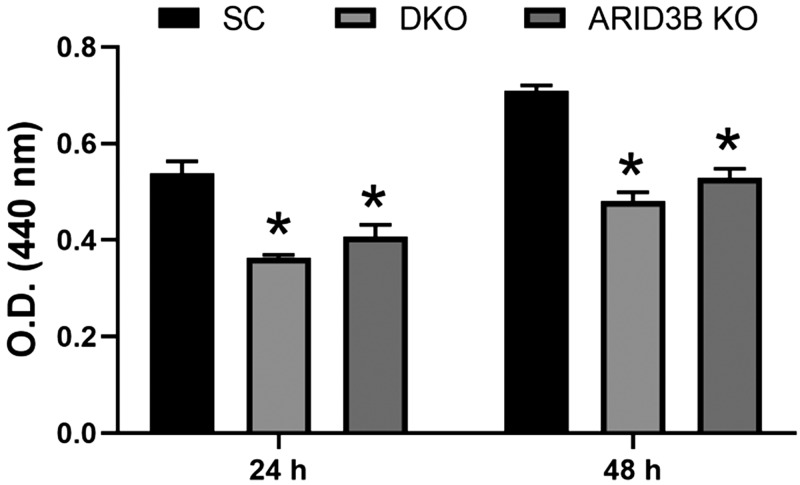
Proliferation of LIN28 DKO and ARID3B KO cells
To determine the effect of LIN28 DKO or ARID3B KO on the functionality of ACH-3P cells, proliferation was measured after 24 and 48 h. Proliferation of LIN28 DKO and ARID3B KO cells was significantly reduced compared with SC (Fig. 12).
Figure 12.
Cell proliferation of SC, LIN28 DKO, and ARID3B KO cells measured after 24 and 48 h using the Quick Cell Proliferation Assay Kit (n = 3). *P < 0.05 vs. SC.
DISCUSSION
The pluripotency factors LIN28A and LIN28B have both been detected in human placenta and potentially regulate trophoblast cell proliferation (20, 21). The LIN28 proteins are known to inhibit the maturation of let-7 miRNAs, which regulate expression of many genes posttranscriptionally by binding their mRNAs. According to a recent study by Canfield et al. (21), LIN28B is the predominant LIN28 paralog and is significantly reduced in term human placentas from pregnancies with preeclampsia without IUGR compared with placentas from normal pregnancies, whereas there was no change in LIN28A. They further reported that there was no change in the level of let-7g miRNAs, which may suggest a compensatory role of LIN28A. However, Canfield et al. (21) did not report levels of other let-7 miRNAs. In this study, we compared levels of LIN28 and let-7 miRNAs in term human placentas from IUGR pregnancies vs. normal pregnancies. The results showed that both LIN28A and LIN28B were significantly reduced and levels of let-7 miRNAs were significantly increased in placentas from IUGR pregnancies. Let-7 miRNAs target genes with known importance for placental development, including ARID3A and ARID3B. Understanding gene regulation by the LIN28–let-7 axis in human placentas during the first trimester of pregnancy can provide more insight about its role in placental growth and placenta-associated disorders.
Although both ACH-3P and Sw.71 cells are immortalized first-trimester human trophoblast cell lines, they were created using different methods. ACH-3P cells were obtained by fusion of primary first-trimester human trophoblast cells (wk 12 of gestation) with a human choriocarcinoma cell line (AC1-1) (54). Sw.71 cells are immortalized first-trimester human trophoblast cells (wk 7 of gestation) produced by expressing hTERT in primary trophoblast cells (55). We compared the expression of LIN28 and let-7 miRNAs in these cell lines. Surprisingly, ACH-3P cells have higher LIN28A and LIN28B levels and lower let-7 miRNAs compared with Sw.71 cells. The contrasting levels of LIN28 and let-7 miRNAs in these cells is interesting and suggests a potential difference in let-7 gene regulation in these different cell lines. The detection of let-7 miRNA target genes HMGA1, c-MYC, VEGF-A, and WNT1 protein by Western blot was lower in Sw.71 compared with ACH-3P cells, suggesting targeting of mRNA by let-7 miRNA. Knockout of LIN28 in ACH-3P cells leads to a significant increase in levels of let-7 miRNAs and a significant reduction in HMGA1, c-MYC, VEGF-A, and WNT1 compared with SC. Similarly, overexpression of LIN28 in Sw.71 cells lead to a significant decrease in levels of let-7 miRNAs and significant increases in HMGA1, c-MYC, VEGF-A, and WNT1 compared with EVC. These results suggest that let-7 miRNAs target genes in immortalized first-trimester human trophoblast cells and are important regulators of gene expression during placental development.
In cancer cells, the ARID3B-complex binds the promoter regions of genes and regulates their expression through the histone demethylation action of KDM4C (23). Although HMGA1, c-MYC, VEGF-A, and WNT1 have been shown as targets of let-7 miRNAs, this is the first study describing regulation of these genes through the ARID3B-complex in immortalized first-trimester human trophoblast cell lines. Co-IP data confirmed complex formation among ARID3A, ARID3B, and KDM4C in ACH-3P cells, and a ChIP assay showed that the ARID3B-complex binds the promoter regions of HMGA1, c-MYC, VEGF-A, and WNT1. ChIP using ARID3B knockout ACH-3P cells showed that, with the loss of ARID3B, KDM4C could no longer bind the promoter regions of HMGA1, c-MYC, VEGF-A, and WNT1, suggesting that ARID3B-complex formation is required for the binding of KDM4C to the promoter regions of these genes. This inability of KDM4C to bind to the promoter region and produce histone demethylation potentially resulted in the significant reduction in levels of HMGA1, c-MYC, VEGF-A, and WNT1 detected in ARID3B KO cells. Although the ARID3B-complex is important for activation of HMGA1, c-MYC, VEGF-A, and WNT1, the presence of low levels of HMGA1, c-MYC, VEGF-A, and WNT1 in ARID3B KO cells indicates the potential involvement of other pathways in the regulation of these genes.
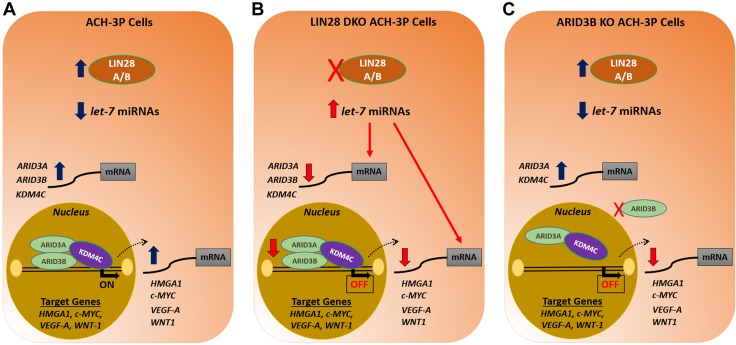
To our knowledge, the LIN28–let-7–ARID3B pathway has not previously been described as a mechanism for gene regulation in immortalized first-trimester human trophoblast cells (Fig. 13). Knockout of LIN28A and LIN28B in ACH-3P cells not only significantly increased amounts of let-7 miRNAs but also led to significant reduction in ARID3B-complex proteins, potentially because of increased targeting by let-7 miRNAs. Hence, significant reduction in HMGA1, c-MYC, VEGF-A, and WNT1 in DKOs can be both due to increased targeting of their mRNAs by let-7 miRNAs and reduced activation of these genes by the ARID3B-complex. Similarly, overexpression of LIN28A and LIN28B in Sw.71 cells not only significantly reduced the levels of let-7 miRNAs but also increased the expression of ARID3B-complex proteins. The significant increase in HMGA1, c-MYC, VEGF-A, and WNT1 in DKIs could be due to less targeting of their mRNAs by let-7 miRNAs and increased activation of these genes by the ARID3B-complex.
Figure 13.
Graphical abstract. A) In ACH-3P cells, let-7 miRNAs are low because of high LIN28A and LIN28B. Because of low levels of let-7 miRNAs, ARID3A, ARID3B, and KDM4C are higher. The ARID3B complex binds the promoter regions of HMGA1, c-MYC, VEGF-A, and WNT1 and turns on these genes by chromatin modification. B) In LIN28 DKOs, let-7 miRNAs are higher. Elevated let-7 miRNAs target HMGA1, c-MYC, VEGF-A, and WNT1, leading to reduced expression of these genes. In addition, the elevated let-7 miRNAs also target ARID3A, ARID3B, and KDM4C. This results in less binding of the ARID3B complex to promoter regions of HMGA1, c-MYC, VEGF-A, and WNT1, leading to reduced expression of these genes. C) In ARID3B KO cells, the ARID3B complex is disrupted. KDM4C cannot bind to DNA in the absence of ARID3B, which turns off this pathway of chromatin modification, and hence, HMGA1, c-MYC, VEGF-A, and WNT1 are reduced.
During early placental development in humans, a reserve of progenitor cytotrophoblast cells is maintained while these cells differentiate to different lineages. Complex molecular mechanisms are involved in maintaining this balance, many of which are yet to be explored. This study not only extends the understanding of the LIN28–let-7 axis in immortalized first-trimester human trophoblast cells but also highlights the importance of the ARID3B-complex in regulation of genes with known roles in placental health and disease. Rapidly proliferating cytotrophoblast cells play a critical role in placental development. Our results showed that knockout of LIN28A and LIN28B or ARID3B significantly reduced HMGA1, c-MYC, VEGF-A, and WNT1 and decreased proliferation of immortalized first-trimester human trophoblast cells. We suggest that decreased expression of LIN28A and LIN28B or ARID3A and ARID3B in human placentas can result in reduced proliferation of progenitor cytotrophoblast cells, potentially leading to development of IUGR. Further studies need to be done to explore other genes regulated by let-7 miRNAs and the role of the ARID3B-complex in the regulation of non–let-7 target genes in the placenta.
ACKNOWLEDGMENTS
The authors thank Dr. Marcin Jozwik (University of Warmia and Mazury, Olsztyn, Poland) and Dr. Maciej Jozwik (Medical University of Bialystok, Bialystok, Poland) for providing the human placental tissue samples (53). This project was supported by Agriculture and Food Research Initiative Competitive Grant 2017-67015-26460 from the U.S. Department of Agriculture (USDA) National Institute of Food and Agriculture. The authors thank the Institute of International Education (IIE) and United States Education Foundation in Pakistan (USEFP) for funding the doctoral studies of A.A. at Colorado State University through the Foreign Fulbright Scholarship Program. The authors declare no conflicts of interest.
Glossary
- AKI
Sw.71 cell overexpressing LIN28A
- AKO
ACH-3P cell with LIN28A knockout
- ARID
AT-rich interaction domain
- ARID3B-complex
triprotein complex comprising ARID3A, ARID3B, and KDM4C
- ARID3B KO
ACH-3P cell with ARID3B knockout
- BKI
Sw.71 cell overexpressing LIN28B
- BKO
ACH-3P cell with LIN28B knockout
- c-myc
transcriptional regulator Myc-like
- Cas9
CRISPR-associated protein 9
- ChIP
chromatin immunoprecipitation
- co-IP
coimmunoprecipitation
- CRISPR
clustered regularly interspaced short palindromic repeat
- DKI
Sw.71 cell overexpressing LIN28A and LIN28B
- DKO
ACH-3P cell with LIN28A and LIN28B knockout
- EVC
expression vector control
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- gRNA
guide RNA
- HMGA1
high-mobility group AT-hook 1
- IUGR
intrauterine growth restriction
- KDM4C
lysine demethylase 4C
- let-7
lethal-7
- miRNA
microRNA
- SC
scramble control
- TBST
Tris-buffered saline with Tween 20
- WNT1
Wnt family member 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
A. Ali performed research, analyzed the data, and wrote the manuscript; A. Ali and Q. A. Winger designed the research; R. V. Anthony and G. J. Bouma helped in research design; and R. V. Anthony, G. J. Bouma, and Q. A. Winger contributed new reagents and analytic tools and also edited the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Beune I. M., Bloomfield F. H., Ganzevoort W., Embleton N. D., Rozance P. J., van Wassenaer-Leemhuis A. G., Wynia K., Gordijn S. J. (2018) Consensus based definition of growth restriction in the newborn. J. Pediatr. 196, 71–76.e1 [DOI] [PubMed] [Google Scholar]
- 2.Sharma D., Shastri S., Farahbakhsh N., Sharma P. (2016) Intrauterine growth restriction - part 1. J. Matern. Fetal Neonatal. Med. 29, 3977–3987 [DOI] [PubMed] [Google Scholar]
- 3.Djakovic A., Rieger L., Wirbelauer J., Kalla J., Dietl J. (2007) [Severe foetal growth retardation in a patient with uterus bicornis, velamentous insertion and partial placental abruption in the 26th week of gestation--a case report]. Z. Geburtshilfe Neonatol. 211, 169–173 [DOI] [PubMed] [Google Scholar]
- 4.Wilkins-Haug L., Quade B., Morton C. C. (2006) Confined placental mosaicism as a risk factor among newborns with fetal growth restriction. Prenat. Diagn. 26, 428–432 [DOI] [PubMed] [Google Scholar]
- 5.Yu K. M. (1992) [Relation between placental morphometry and fetal growth]. Zhonghua Fu Chan Ke Za Zhi 27, 217–219, 250 [PubMed] [Google Scholar]
- 6.Heinonen S., Taipale P., Saarikoski S. (2001) Weights of placentae from small-for-gestational age infants revisited. Placenta 22, 399–404 [DOI] [PubMed] [Google Scholar]
- 7.Lyons P., ed. (2006) Obstetrics in Family Medicine: A Practical Guide, Humana Press, Totowa, NJ, USA: [Google Scholar]
- 8.Barker D. J. P. (1999) Fetal origins of cardiovascular disease. Ann. Med. 31 (Suppl 1), 3–6 [PubMed] [Google Scholar]
- 9.Norwitz E. R. (2007) Defective implantation and placentation: laying the blueprint for pregnancy complications. Reprod. Biomed. Online 14, 101–109 [DOI] [PubMed] [Google Scholar]
- 10.Scifres C. M., Nelson D. M. (2009) Intrauterine growth restriction, human placental development and trophoblast cell death. J. Physiol. 587, 3453–3458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devor E. J., Reyes H. D., Santillan D. A., Santillan M. K., Onukwugha C., Goodheart M. J., Leslie K. K. (2014) Placenta-specific protein 1: a potential key to many oncofetal-placental OB/GYN research questions. Obstet. Gynecol. Int. 2014, 678984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma W., Ma J., Xu J., Qiao C., Branscum A., Cardenas A., Baron A. T., Schwartz P., Maihle N. J., Huang Y. (2013) Lin28 regulates BMP4 and functions with Oct4 to affect ovarian tumor microenvironment. Cell Cycle 12, 88–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng C., Neumeister V., Ma W., Xu J., Lu L., Bordeaux J., Maihle N. J., Rimm D. L., Huang Y. (2012) Lin28 regulates HER2 and promotes malignancy through multiple mechanisms. Cell Cycle 11, 2486–2494 [DOI] [PubMed] [Google Scholar]
- 14.Piskounova E., Polytarchou C., Thornton J. E., LaPierre R. J., Pothoulakis C., Hagan J. P., Iliopoulos D., Gregory R. I. (2011) Lin28A and Lin28B inhibit let-7 microRNA biogenesis by distinct mechanisms. Cell 147, 1066–1079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hagan J. P., Piskounova E., Gregory R. I. (2009) Lin28 recruits the TUTase Zcchc11 to inhibit let-7 maturation in mouse embryonic stem cells. Nat. Struct. Mol. Biol. 16, 1021–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rybak A., Fuchs H., Smirnova L., Brandt C., Pohl E. E., Nitsch R., Wulczyn F. G. (2008) A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat. Cell Biol. 10, 987–993 [DOI] [PubMed] [Google Scholar]
- 17.Newman M. A., Thomson J. M., Hammond S. M. (2008) Lin-28 interaction with the Let-7 precursor loop mediates regulated microRNA processing. RNA 14, 1539–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heo I., Joo C., Kim Y.-K., Ha M., Yoon M.-J., Cho J., Yeom K.-H., Han J., Kim V. N. (2009) TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138, 696–708 [DOI] [PubMed] [Google Scholar]
- 19.Heo I., Joo C., Cho J., Ha M., Han J., Kim V. N. (2008) Lin28 mediates the terminal uridylation of let-7 precursor microRNA. Mol. Cell 32, 276–284 [DOI] [PubMed] [Google Scholar]
- 20.West R. C., McWhorter E. S., Ali A., Goetzman L. N., Russ J. E., Anthony R. V., Bouma G. J., Winger Q. A. (2019) HMGA2 is regulated by LIN28 and BRCA1 in human placental cells. Biol. Reprod. 100, 227–238 [DOI] [PubMed] [Google Scholar]
- 21.Canfield J., Arlier S., Mong E. F., Lockhart J., VanWye J., Guzeloglu-Kayisli O., Schatz F., Magness R. R., Lockwood C. J., Tsibris J. C. M., Kayisli U. A., Totary-Jain H. (2019) Decreased LIN28B in preeclampsia impairs human trophoblast differentiation and migration. FASEB J. 33, 2759–2769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seabrook J. L., Cantlon J. D., Cooney A. J., McWhorter E. E., Fromme B. A., Bouma G. J., Anthony R. V., Winger Q. A. (2013) Role of LIN28A in mouse and human trophoblast cell differentiation. Biol. Reprod. 89, 95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liao T.-T., Hsu W.-H., Ho C.-H., Hwang W.-L., Lan H.-Y., Lo T., Chang C.-C., Tai S.-K., Yang M.-H. (2016) let-7 modulates chromatin configuration and target gene repression through regulation of the ARID3B complex. Cell Rep. 14, 520–533 [DOI] [PubMed] [Google Scholar]
- 24.Wilsker D., Probst L., Wain H. M., Maltais L., Tucker P. W., Moran E. (2005) Nomenclature of the ARID family of DNA-binding proteins. Genomics 86, 242–251 [DOI] [PubMed] [Google Scholar]
- 25.Kortschak R. D., Tucker P. W., Saint R. (2000) ARID proteins come in from the desert. Trends Biochem. Sci. 25, 294–299 [DOI] [PubMed] [Google Scholar]
- 26.Raney B. J., Cline M. S., Rosenbloom K. R., Dreszer T. R., Learned K., Barber G. P., Meyer L. R., Sloan C. A., Malladi V. S., Roskin K. M., Suh B. B., Hinrichs A. S., Clawson H., Zweig A. S., Kirkup V., Fujita P. A., Rhead B., Smith K. E., Pohl A., Kuhn R. M., Karolchik D., Haussler D., Kent W. J. (2011) ENCODE whole-genome data in the UCSC genome browser (2011 update). Nucleic Acids Res. 39, D871–D875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wood J. J., Boyne J. R., Paulus C., Jackson B. R., Nevels M. M., Whitehouse A., Hughes D. J. (2016) ARID3B: a novel regulator of the Kaposi’s Sarcoma-associated herpesvirus lytic cycle. J. Virol. 90, 9543–9555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bobbs A., Gellerman K., Hallas W. M., Joseph S., Yang C., Kurkewich J., Cowden Dahl K. D. (2015) ARID3B directly regulates ovarian cancer promoting genes. PLoS One 10, e0131961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roy L., Samyesudhas S. J., Carrasco M., Li J., Joseph S., Dahl R., Cowden Dahl K. D. (2014) ARID3B increases ovarian tumor burden and is associated with a cancer stem cell gene signature. Oncotarget 5, 8355–8366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ratliff M. L., Mishra M., Frank M. B., Guthridge J. M., Webb C. F. (2016) The transcription factor ARID3a is important for in vitro differentiation of human hematopoietic progenitors. J. Immunol. 196, 614–623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Habir K., Aeinehband S., Wermeling F., Malin S. (2017) A role for the transcription factor Arid3a in mouse B2 lymphocyte expansion and peritoneal B1a generation. Front. Immunol. 8, 1387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rhee C., Edwards M., Dang C., Harris J., Brown M., Kim J., Tucker H. O. (2017) ARID3A is required for mammalian placenta development. Dev. Biol. 422, 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Takebe A., Era T., Okada M., Martin Jakt L., Kuroda Y., Nishikawa S. (2006) Microarray analysis of PDGFR alpha+ populations in ES cell differentiation culture identifies genes involved in differentiation of mesoderm and mesenchyme including ARID3b that is essential for development of embryonic mesenchymal cells. Dev. Biol. 293, 25–37 [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi K., Era T., Takebe A., Jakt L. M., Nishikawa S. (2006) ARID3B induces malignant transformation of mouse embryonic fibroblasts and is strongly associated with malignant neuroblastoma. Cancer Res. 66, 8331–8336 [DOI] [PubMed] [Google Scholar]
- 35.Casanova J. C., Uribe V., Badia-Careaga C., Giovinazzo G., Torres M., Sanz-Ezquerro J. J. (2011) Apical ectodermal ridge morphogenesis in limb development is controlled by Arid3b-mediated regulation of cell movements. Development 138, 1195–1205 [DOI] [PubMed] [Google Scholar]
- 36.Webb C. F., Bryant J., Popowski M., Allred L., Kim D., Harriss J., Schmidt C., Miner C. A., Rose K., Cheng H.-L., Griffin C., Tucker P. W. (2011) The ARID family transcription factor bright is required for both hematopoietic stem cell and B lineage development. Mol. Cell. Biol. 31, 1041–1053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim D., Probst L., Das C., Tucker P. W. (2007) REKLES is an ARID3-restricted multifunctional domain. J. Biol. Chem. 282, 15768–15777 [DOI] [PubMed] [Google Scholar]
- 38.Hua Z., Lv Q., Ye W., Wong C.-K. A., Cai G., Gu D., Ji Y., Zhao C., Wang J., Yang B. B., Zhang Y. (2006) MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS One 1, e116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jin B., Wang W., Meng X. X., Du G., Li J., Zhang S. Z., Zhou B. H., Fu Z. H. (2016) Let-7 inhibits self-renewal of hepatocellular cancer stem-like cells through regulating the epithelial-mesenchymal transition and the Wnt signaling pathway. BMC Cancer 16, 863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahman M. M., Qian Z. R., Wang E. L., Sultana R., Kudo E., Nakasono M., Hayashi T., Kakiuchi S., Sano T. (2009) Frequent overexpression of HMGA1 and 2 in gastroenteropancreatic neuroendocrine tumours and its relationship to let-7 downregulation. Br. J. Cancer 100, 501–510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wagner S., Ngezahayo A., Murua Escobar H., Nolte I. (2014) Role of miRNA let-7 and its major targets in prostate cancer. Biomed. Res. Int. 2014, 376326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang X., Cai H., Liang Y., Chen L., Wang X., Si R., Qu K., Jiang Z., Ma B., Miao C., Li J., Wang B., Gao P. (2015) Inhibition of c-Myc by let-7b mimic reverses mutidrug resistance in gastric cancer cells. Oncol. Rep. 33, 1723–1730 [DOI] [PubMed] [Google Scholar]
- 43.Uchikura Y., Matsubara K., Matsubara Y., Mori M. (2015) P34. Role of high-mobility group A1 protein in trophoblast invasion. Pregnancy Hypertens. Int. J. Womens Cardiovasc. Health 5, 243 [Google Scholar]
- 44.Uchikura Y., Matsubara K., Muto Y., Matsubara Y., Fujioka T., Matsumoto T., Sugiyama T. (2017) Extranuclear translocation of high-mobility group A1 reduces the invasion of extravillous trophoblasts involved in the pathogenesis of preeclampsia: new aspect of high-mobility group A1. Reprod. Sci. 24, 1630–1638 [DOI] [PubMed] [Google Scholar]
- 45.Kumar P., Luo Y., Tudela C., Alexander J. M., Mendelson C. R. (2013) The c-Myc-regulated microRNA-17~92 (miR-17~92) and miR-106a~363 clusters target hCYP19A1 and hGCM1 to inhibit human trophoblast differentiation. Mol. Cell. Biol. 33, 1782–1796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lala N., Girish G. V., Cloutier-Bosworth A., Lala P. K. (2012) Mechanisms in decorin regulation of vascular endothelial growth factor-induced human trophoblast migration and acquisition of endothelial phenotype. Biol. Reprod. 87, 59 [DOI] [PubMed] [Google Scholar]
- 47.Li Y., Zhu H., Klausen C., Peng B., Leung P. C. K. (2015) Vascular endothelial growth factor-A (VEGF-A) mediates activin A-induced human trophoblast endothelial-like tube formation. Endocrinology 156, 4257–4268 [DOI] [PubMed] [Google Scholar]
- 48.Pang V., Bates D. O., Leach L. (2017) Regulation of human feto-placental endothelial barrier integrity by vascular endothelial growth factors: competitive interplay between VEGF-A165a, VEGF-A165b, PIGF and VE-cadherin. Clin. Sci. (Lond.) 131, 2763–2775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu K., Liu F., Wu W., Chen Y., Wu H., Zhang W. (2019) Long non-coding RNA HOX transcript antisense RNA (HOTAIR) suppresses the angiogenesis of human placentation by inhibiting vascular endothelial growth factor A expression. Reprod. Fertil. Dev. 31, 377–385 [DOI] [PubMed] [Google Scholar]
- 50.Liu F., Wu K., Wu W., Chen Y., Wu H., Wang H., Zhang W. (2018) miR-203 contributes to pre-eclampsia via inhibition of VEGFA expression. Mol. Med. Rep. 17, 5627–5634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sonderegger S., Husslein H., Leisser C., Knöfler M. (2007) Complex expression pattern of Wnt ligands and frizzled receptors in human placenta and its trophoblast subtypes. Placenta 28 (Suppl A), S97–S102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang X., Zhang Z., Zeng X., Wang J., Zhang L., Song W., Shi Y. (2018) Wnt/β-catenin signaling pathway in severe preeclampsia. J. Mol. Histol. 49, 317–327 [DOI] [PubMed] [Google Scholar]
- 53.Jozwik M., Pietrzycki B., Jozwik M., Anthony R. V. (2009) Expression of enzymes regulating placental ammonia homeostasis in human fetal growth restricted pregnancies. Placenta 30, 607–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hiden U., Wadsack C., Prutsch N., Gauster M., Weiss U., Frank H.-G., Schmitz U., Fast-Hirsch C., Hengstschläger M., Pötgens A., Rüben A., Knöfler M., Haslinger P., Huppertz B., Bilban M., Kaufmann P., Desoye G. (2007) The first trimester human trophoblast cell line ACH-3P: a novel tool to study autocrine/paracrine regulatory loops of human trophoblast subpopulations--TNF-alpha stimulates MMP15 expression. BMC Dev. Biol. 7, 137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Straszewski-Chavez S. L., Abrahams V. M., Alvero A. B., Aldo P. B., Ma Y., Guller S., Romero R., Mor G. (2009) The isolation and characterization of a novel telomerase immortalized first trimester trophoblast cell line, Swan 71. Placenta 30, 939–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bae S., Park J., Kim J.-S. (2014) Cas-OFFinder: a fast and versatile algorithm that searches for potential off-target sites of Cas9 RNA-guided endonucleases. Bioinformatics 30, 1473–1475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Park J., Bae S., Kim J.-S. (2015) Cas-Designer: a web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 31, 4014–4016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.