Abstract
The contribution of neurons to growth and refinement of the microvasculature during postnatal brain development is only partially understood. Tissue hypoxia is the physiologic stimulus for angiogenesis by enhancing angiogenic mediators partly through activation of hypoxia-inducible factors (HIFs). Hence, we investigated the HIF oxygen-sensing pathway in postmitotic neurons for physiologic angiogenesis in the murine forebrain during postnatal development by using mice lacking the HIF suppressing enzyme prolyl-4-hydroxylase domain (PHD)2 and/or HIF-1/2α in postmitotic neurons. Perinatal activation or inactivation of the HIF pathway in neurons inversely modulated brain vascularization, including endothelial cell number and proliferation, density of total and perfused microvessels, and vascular branching. Accordingly, several angiogenesis-related genes were up-regulated in vivo and in primary neurons derived from PHD2-deficient mice. Among them, only VEGF and adrenomedullin (Adm) promoted angiogenic sprouting of brain endothelial cells. VEGF and Adm additively enhanced endothelial sprouting through activation of multiple pathways. PHD2 deficiency in neurons caused HIF-α stabilization and increased VEGF mRNA levels not only in neurons but unexpectedly also in astrocytes, suggesting a new mechanism of neuron-to-astrocyte signaling. Collectively, our results identify the PHD-HIF pathway in neurons as an important determinant for vascularization of the brain during postnatal development.—Nasyrov, E., Nolan, K. A., Wenger, R. H., Marti, H. H., Kunze, R. The neuronal oxygen-sensing pathway controls postnatal vascularization of the murine brain.
Keywords: PHD, HIF, VEGF, adrenomedullin, cerebral angiogenesis
Vascularization of the vertebrate CNS begins early during embryogenesis with the formation of the perineural vascular plexus around the neural tube (1). Subsequently, the developing brain and spinal cord are predominantly vascularized by secondary angiogenesis from the perineural vascular plexus, where new vessel sprouts invade the CNS and extend toward the ventricle (1). Upon reaching the ventricle, vessels form new branches that surround the ventricle and reverse their direction toward the pia. Finally, branches anastomose with other branches, giving rise to a rich capillary plexus (1). Although general and CNS-specific mechanisms of developmental angiogenesis throughout embryogenesis have been extensively studied over the past years (1–3), the growth and refinement of the cerebral microvasculature during postnatal CNS development is only poorly understood. Studies on vascularization of the postnatal rodent retina suggest that VEGF, among other angiogenic factors, is of crucial importance for retinal angiogenesis. Although astrocytes and Müller glia are the major source of VEGF in the retina (4–6), conditional gene deletion and cell ablation approaches have clearly demonstrated that VEGF derived from neurons (including retinal ganglion cells, amacrine, and horizontal cells) rather than glial cells is most important for retinal developmental angiogenesis (7, 8). The pivotal importance of postmitotic neurons for precise spatiotemporal vascularization of the embryonic CNS has also been demonstrated in the developing spinal cord, where motor neurons control blood vessel patterning by an autocrine mechanism that titrates motor neuron-derived VEGF via their own expression of Fms-related tyrosine kinase 1 (Flt1), a soluble isoform of VEGF receptor-1 acting as a VEGF trap (9).
Hypoxia is the principal physiologic stimulus that induces angiogenesis and provides a stimulus-response pathway by which all cells are ensured adequate oxygenation (10). Expression of virtually all of the critical angiogenic growth factors is induced by hypoxia either directly or indirectly through hypoxia-inducible factors (HIFs) (10). HIFs are heterodimeric transcription factors that consist of oxygen-regulated HIF-1α or HIF-2α and constitutively expressed HIF-1β subunits that mediate adaptive responses to hypoxia/ischemia in all nucleated cells of metazoan organisms (10). HIF-α subunits are targeted for proteasomal degradation under nonhypoxic conditions through hydroxylation of conserved proline residues by prolyl-4-hydroxylase domain (PHD) enzymes utilizing molecular oxygen and α-ketoglutarate as cosubstrates (10, 11). Prolyl hydroxylation of HIF-α causes binding to the von Hippel-Lindau protein, an E3 ubiquitin ligase, resulting in immediate HIF-1α and HIF-2α ubiquitination and proteasomal degradation (10, 11). Hydroxylation of a conserved asparagine residue of HIF-1α and HIF-2α catalyzed by factor-inhibiting HIF prevents binding to the transcriptional coactivators CREB-binding protein and p300. Consequently, hypoxia-induced inhibition of prolyl and asparaginyl hydroxylase activity results in a rapid increase in HIF-α levels and transcriptional activity (10, 11). Post-translationally stabilized HIF-α translocates to the nucleus, dimerizes with HIF-1β, and binds to hypoxia response elements, which function as cis-acting elements that determine the target genes for activation by HIF (10, 11).
PHD2 is the most abundant PHD isoform in neurons and the adult murine CNS (12, 13). PHD2 has further been reported to be the critical oxygen sensor setting the low steady-state levels of HIF-1α in normoxia (14). However, although previous experimental studies indicate a pivotal role of postmitotic neurons (7–9) and the molecular PHD-HIF axis in developmental brain angiogenesis (15–17), their importance for postnatal cerebrovascular remodeling is still largely unknown. Thus, the present study elucidated whether and how the oxygen-sensing machinery in mature neurons controls physiologic angiogenesis in the murine forebrain during early postnatal development.
MATERIALS AND METHODS
Mice
All transgenic mouse lines were established on a C57Bl/6 background. We used female and male littermate mice that were age-matched between experimental groups. Mice were either newborn [postnatal day (P)0], 1 (P7), or 12 (P84) weeks of age. All animal experiments were approved by the local animal welfare committee (Regierungspräsidium Karlsruhe, Germany, permission number: 35-9185.81/G-103/12, 35-9185.81/G-45/18), conformed to the Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), Bethesda, MD, USA] and were performed in accordance with the recently published Animal Research: Reporting In Vivo Experiments (ARRIVE) guidelines (https://www.nc3rs.org.uk/arrive-guidelines). All mice were housed at constant room temperature (22 ± 2°C) and relative humidity (50–55%) on a controlled 12:12 h light/dark cycle, and they were provided with standard laboratory chow (LASQCdiet Rod16; LASvendi, Soest, Germany) and water ad libitum. Neuron-specific Phd2 (nPhd2 Δ/Δ), Hif1a/Hif2a (nHif1a/Hif2a ΔΔ/ΔΔ), and Phd2/Hif1a/Hif2a (nPhd2/Hif1a/Hif2a ΔΔΔ/ΔΔΔ)-deficient mice were generated as previously described (18–20). Corresponding floxed littermates (Phd2 f/f, Hif1a/Hif2a ff/ff, Phd2/Hif1a/Hif2a fff/fff) were used as control [hereinafter referred to as wild-type (WT) mice]. The mean body weights of all transgenic mouse lines are summarized in Supplemental Table S1. Mice were genotyped using primers (Eurofins Genomics, Ebersberg, Germany) described in Table 1. All mice were randomly allocated to experimental groups. Investigators were blinded for mouse genotype in all experiments and analyses. Evaluation of all read-out parameters was done independently and in a blinded fashion.
TABLE 1.
Primers used to genotype mice
| Sequence, 5′–3′ |
|||
|---|---|---|---|
| Allele | Forward | Reverse | Amlicon size (bp) |
| Camk2a:cre | GGTTCTCCGTTTGCACTCAGGA | GCTTGCAGGTACAGGAGGTAGT | 400 |
| Phd2 floxed | CTCACTGACCTACGCCGTGT | CGCATCTTCCATCTCCATTT | wt: 220 |
| floxed: 386 | |||
| Hif1a floxed | GCAGTTAAGAGCACTAGTTG | GGAGCTATCTCTCTAGACC | wt: 215 |
| floxed: 250 | |||
| Hif2a floxed | GAGAGCAGCTTCTCCTGGAA | TGTAGGCAAGGAAACCAAGG | wt: 182 |
Inspiratory hypoxia
Twelve-week-old C57Bl/6 mice (Charles River Laboratories, Wilmington, MA, USA) were placed in their home cages into an animal chamber (A-chamber, 76 × 51 × 51 cm; BioSpherix, Parish, NY, USA) attached to a ProOx P360 oxygen controller (BioSpherix) displacing air oxygen inside the chamber with nitrogen (normobaric hypoxia). At the beginning of the experiment, oxygen concentration was set to 8%. The initial oxygen concentration in the chamber of roughly 21% (altitude: 110 m above sea level) exponentially declined (after 15 min: 10.0%, after 30 min: 8.6%, after 45 min: 8.4%, after 60 min 8.1%) and reached 8.0% oxygen after 75 min (corresponding to 7100 m above sea level). Mice were kept for a total of 6 or 24 h in the chamber. Control mice were kept at room air pressure (∼21% oxygen). Without reoxygenation, mice were then euthanized by decapitation, the brains were isolated, shock frozen in liquid nitrogen, and stored at −80°C until use. During the entire procedure, mice had free access to food, water, and nesting material used as environmental enrichment.
Evaluation of vascular permeability
Mice were anesthetized by a mixture of 2% isoflurane in 70% N2O and remainder O2. Prewarmed 0.9% NaCl solution containing either 0.4 kD sodium fluorescein (1.2 mg/200 µl, F6377; MilliporeSigma, Burlington, MA, USA), 10 kD FITC-dextran (1 mg/100 µl, FD10S; MilliporeSigma), or 75 kD tetramethylrhodamine-dextran (1 mg/100 µl, T1162; MilliporeSigma) was injected into the tail vein and allowed to circulate for 30 min. Then, mice were transcardially perfused, their brains were harvested, and the amount of fluorescent dyes was quantified by fluorimetry as previously described (21).
Immunofluorescence staining, image acquisition, and analysis
For evaluation of perfused cerebrovasculature, 100 µl 2% fluorescent Evans Blue (EB) dye was intravenously applied to anesthetized adult 12-wk-old (P84) mice. After 30 min of systemic circulation, mice were euthanized by cervical dislocation. Brains were harvested from EB-perfused P84 mice as well as naive newborn (P0) and juvenile (P7) mice. Brains were embedded into Tissue-Tek (Sakura Finetek, Staufen, Germany), and coronal tissue sections (10-µm thickness; P0/P7: +1.1 mm relative to Bregma, P84: +3.6, +1.1, +0.5, −0.5, −1.8, and −2.6 mm relative to Bregma) were prepared using a Leica CM1520 cryostat (Leica Biosystems, Wetzlar, Germany) at a constant temperature of −15°C. Prior to immunofluorescence staining, native images were acquired of EB-perfused tissue sections as explained below. Immunofluorescence staining was performed as previously described (20). Antibodies are summarized in Table 2. Stained brain sections were scanned in a 20-fold magnification with a Plan-Apochromat ×20/0.8 objective (Carl Zeiss Microscopy, Thornwood, NY, USA) mounted on a Zeiss Axiovert 200 Microscope (Carl Zeiss Microscopy). Whole-section image acquisition was performed with an Orca Flash 4.0 camera (Hamamatsu Photonics, Hamamatsu City, Japan), and captured images were processed by TissueFAXS slides 4.2 software (TissueGnostics, Vienna, Austria) into TIFF files. Acquired images were analyzed using Fiji software (NIH). Advanced macros were designed and optimized for automated, user-independent signal segmentation, colocalization and counting. Anatomic sub regions were defined according to the Allen mouse brain atlases (https://mouse.brain-map.org/static/atlas; http://developingmouse.brain-map.org/static/atlas). Vascular phenotyping was performed independently in each anatomic subregion using the following parameters: 1) density of EB-perfused, functional microvasculature, 2) density of CD31+ vessel segments, 3) endothelial cell number (CD31+/DAPI+ nuclei), and 4) number of branching points of CD31+ vascular structures. Endothelial cell proliferation was determined by quantification of Ki67+/CD31+ nuclei. Cerebrovascular pericyte coverage was defined by evaluating the ratio of desmin-positive/CD31+ area relative to total CD31+ area.
TABLE 2.
Antibodies used for immunoblotting and immunofluorescence staining
| Antibody type | Immunogen | Host species | Clonality | Conjugate | Supplier | Catalog no. |
|---|---|---|---|---|---|---|
| Primary | Hu β-tubulin | Rb | Polyclonal | — | Abcam | ab6046 |
| Primary | Ms CD-31 | Rt | Monoclonal | — | BD Biosciences (San Jose, CA, USA) | 557355 |
| Primary | Hu desmin | Rb | Polyclonal | — | Dianova (Barcelona, Spain) | DLN-13732 |
| Primary | Rt ERK1/2 | Rb | Polyclonal | — | Cell Signaling Technology (Danvers, MA, USA) | 9102 |
| Primary | Hu phospho-ERK1/2 | Rb | Polyclonal | — | Cell Signaling Technology | 9101 |
| Primary | Hu HIF-1α | Rb | Polyclonal | — | Novus Biologicals (Centennial, CO, USA) | NB100-449 |
| Primary | Ms HIF-2α | Gt | Polyclonal | — | R&D Systems | AF2997 |
| Primary | Hu Ki67 | Rb | Polyclonal | — | Abcam | ab15580 |
| Primary | Ms PHD2 | Rb | Polyclonal | — | Novus Biologicals | NB100-2219 |
| Primary | Hu TBP | Ms | Monoclonal | — | Abcam | ab818 |
| Secondary | Rb IgG | Gt | Polyclonal | HRP | Thermo Fisher Scientific | 31460 |
| Secondary | Gt IgG | Rb | Polyclonal | HRP | R&D Systems | R-401-C-ABS |
| Secondary | Ms IgG | Gt | Polyclonal | HRP | Thermo Fisher Scientific | 31432 |
| Secondary | Rt IgG | Gt | Polyclonal | Cy2 | Dianova | 112-225-143 |
| Secondary | Rt IgG | Dk | Polyclonal | Cy3 | Dianova | 712-166-150 |
| Secondary | Rb IgG | Gt | Polyclonal | Cy3 | Dianova | 111-165-003 |
Dk, donkey; Gt, goat; HRP, horseradish peroxidase; Hu, human; Ms, mouse; phospho, phosphorylated; Rb, rabbit; Rt, rat; TBP, TATA-box-binding protein.
RNA FISH
The RNAscope Fluorescent Multiplex Kit and customized target probes (320850; Advanced Cell Diagnostics, Newark, CA, USA) were used to detect Vegfa, solute carrier family 1, member 3 (Slc1A3; astrocyte marker), and discs large MAGUK scaffold protein 4 (Dlg4; neuronal marker) transcripts in RNAscope Protease IV-pretreated, fresh frozen coronal brain sections (10-µm thickness; +1.1 mm relative to Bregma) according to the manufacturer’s instructions. Tissue sections were counterstained with 0.02% DAPI for 30 s and covered with ProLong Gold Antifade Mountant (P36930; Thermo Fisher Scientific, Waltham, MA, USA). The Zeiss Axio Scan.Z1 Slide Scanner (Carl Zeiss Microscopy) was used to digitally capture images using an Orca Flash 4.0 Camera (Hamamatsu Photonics) at a 40-fold magnification. Czi-files were converted into TIFF format and were further analyzed using Fiji software. Quantification of transcript levels was performed using advanced macros, allowing for signal counting on a cellular level. On average, 50,000 cells were analyzed per section of each P84 brain, and 80,000 cells were analyzed per section of P7 brains. Cells with high Slc1A3 and low Dlg4 mRNA levels were defined as astrocytes, whereas cells with low Slc1A3 and high Dlg4 mRNA levels were defined as neurons. The mean expression level of Vegfa in astrocytes and neurons was evaluated based on the average cellular count of mRNA dot signals in either cell type.
Organotypic hippocampal slice culture
Organotypic slice cultures from mouse hippocampus were prepared and maintained for 1 wk as previously reported (22).
Primary cell cultures
Primary mixed glial cell cultures were prepared from the brains of newborn mice (P0–2) as previously described (23). After 5 d, residual microglial cells were eliminated by treatment with 50 mM l-leucine methyl ester (L1002; MilliporeSigma) for 1 h, and microglia-free astrocytes were cultured for a further 7–9 d. Cortical neurons from neonatal mice (P0-1) were prepared and maintained as previously described (24). Neurons cultured for 8 d in vitro were exposed to 6 h of hypoxia (1% O2) in an Invivo2 Plus Hypoxia Workstation (Ruskinn Technology, Bridgend, United Kingdom) flooded with a humidified gas mixture consisting of 5% CO2 and 94% N2 at 37°C. Neurons cultured for 8 d in vitro were treated with either 0.1, 0.5, or 1 mM dimethyloxalylglycine (DMOG, D3695; MilliporeSigma) in 0.1% DMSO for 6 h. Control cells were treated with 0.1% DMSO.
In vitro angiogenesis assay
Endothelial cell spheroids of defined cell number from immortalized mouse cerebral endothelial cells (bEnd.3) (25) were generated as previously described (26). Following embedding into collagen gels (26), spheroids were treated with recombinant human (rh)VEGF-A165 (293-VE-010, 50 ng/ml; R&D Systems, Minneapolis, MN, USA) and/or rh-adrenomedullin (Adm; A2327, 200 nM; MilliporeSigma), recombinant mouse (rm)-osteopontin (Opn, 441-OP-050, 2 µg/ml; R&D Systems), and rh-erythropoietin (Epo, 287-TC-500, 5 U/ml; R&D Systems), respectively, for 24 h. For certain experiments, PD98059 (513000, 20 µM; MilliporeSigma), PP2 (ab120308, 5 µM; Abcam, Cambridge, MA, USA), wortmannin (681675, 0.2 µM; MilliporeSigma), or N(γ)-nitro-l-arginine methyl ester (L-NAME, 80210, 5 mM; Cayman Chemicals, Ann Arbor, MI, USA) were applied to endothelial spheroids alone or in combination 1 h prior to costimulation with rhVEGF and rhAdm. In vitro angiogenesis was evaluated by measuring the length and number of the sprouts (calculated as cumulative sprout length) that had grown out of each spheroid (×10 objective magnification) using cellSens imaging software (Olympus, Tokyo, Japan) analyzing ≥10 spheroids per experimental group and experiment (26).
Real-time quantitative RT-PCR analysis
RNA isolation from tissue samples or cells, cDNA synthesis, and real-time quantitative PCR were performed as recently described (27). Primer sequences are listed in Table 3.
TABLE 3.
Primers used for real-time quantitative RT-PCR
| Sequence, 5′–3′ |
||
|---|---|---|
| Gene | Forward | Reverse |
| Adm | ATTCCTGGACGAGCAGAACA | GCCGTCCTTGTCTTTGTCTG |
| Ang1 | TCCTGACTCAGCACCATGAC | TCTGTAAGGGCTTCCATTCG |
| Angpt1 | AGGCTTGGTTTCTCGTCAGA | TGTCCATGAGCTCCAGTTGT |
| Angpt2 | ACACTGACCTTCCCCAACTC | CTGCCATCTTCTCGGTGTTG |
| Cxcl12 | GCTCTGCATCAGTGACGGTA | TAATTTCGGGTCAATGCACA |
| Epo | CCACCCTGCTGCTTTTACTC | CTCAGTCTGGGACCTTCTGC |
| Epor | CCCAAGTTTGAGAGCAAAGC | TGCAGGCTACATGACTTTCG |
| Fgf2 | ATGAAGGAAGATGGACGGCT | CAGTTCGTTTCAGTGCCACA |
| Hif1a exon 2 | TGCTCATCAGTTGCCACTTC | CGGCATCCAGAAGTTTTCTC |
| Hif2a exon 2 | TGAGTTGGCTCATGAGTTGC | GGAGCTTATGTGTCCGAAGG |
| Igfbp2 | CATCCCCAACTGTGACAAGC | TCCTGCTGCTCGTTGTAGAA |
| Nov | CCCCACAACACCAAAACCAT | TGCTTGTCTTCAGCTCCAGA |
| Nrp1 | GCTCTGAAGACCTGGCAATG | GCAGGTTAAAGCTGCAAGGT |
| Opn | ATGCCACAGATGAGGACCTC | AGCTGACTTGACTCATGGCT |
| Phd2 exon 2 | TCACGCATCTTCCATCTCCA | GGCAACGGAACAGGCTATG |
| Plgf | AGAAGTGGAAGTGGTGCCTT | ACTCAGAAGGACACAGGACG |
| Rps12 | GAAGCTGCCAAAGCCTTAGA | AACTGCAACCAACCACCTTC |
| Vegfa | GTACCTCCATGCCAAGT | ACTCCAGGGCTTCATCGTTA |
| Vegfb | CCAGCCACCAGAAGAAAGTG | TTGTTTGACCACATTGCCCA |
| Flt1 | TGAGGAGCTTTCACCGAACT | TATCTTCATGGAGGCCTTGG |
| Flk1 | GGCGGTGGTGACAGTATCTT | GTCACTGACAGAGGCGATGA |
Ang1, angiogenin-1; Angpt, angiopoietin; Fgf2, fibroblast growth factor-2; Igfbp2, insulin-like growth factor binding protein-2; Nov, nephroblastoma overexpressed gene; Nrp1, neuropilin-1; Plgf, placental growth factor; Rps12, ribosomal protein S12.
Immunoblotting
Isolation of total, cytosolic, and nuclear protein fractions from brain tissue and cells, SDS-PAGE and immunoblotting were conducted as previously reported (18, 22). The antibodies used are given in Table 2.
Statistical analysis
If not indicated otherwise, all results are expressed as means + sd. Differences between 2 independent experimental groups were analyzed by a 2-tailed Student’s t test. Differences of 1 parameter among 3 or more independent experimental groups were analyzed by using a 1-way ANOVA followed by a Holm-Sidak’s multiple comparisons test. Differences of 2 parameters among 2 or more independent experimental groups were analyzed by a 2-way ANOVA followed by a Holm-Sidak’s multiple comparisons test. A probability value of P < 0.05 was considered statistically significant. Data plotting and statistical analyses were done with Prism 6 (GraphPad Software, La Jolla, CA, USA).
RESULTS
Neuronal PHD2 deficiency increases HIF-α protein levels in neurons and nontargeted cells of the postnatal murine brain
Neuron-specific gene knockout mice were generated by crossing animals harboring 2 floxed alleles of the targeted genes with transgenic mice expressing Cre recombinase under control of the calcium/calmodulin-dependent protein kinase II α (Camk2a) gene promoter. We and others provided experimental evidence that Camk2a gene promotor-driven Cre/loxP recombination is restricted to postmitotic forebrain neurons and starts just before birth (19, 28).
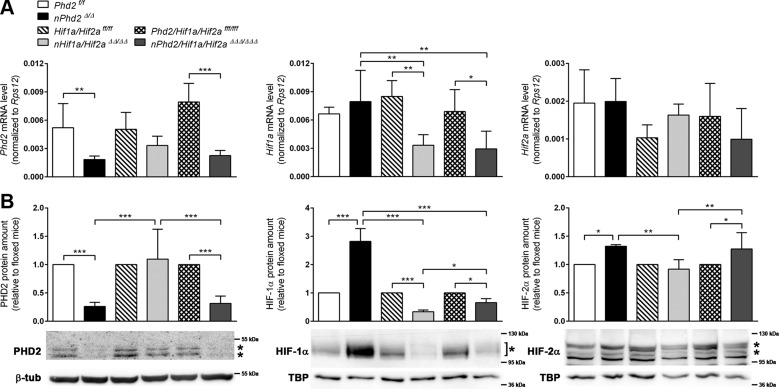
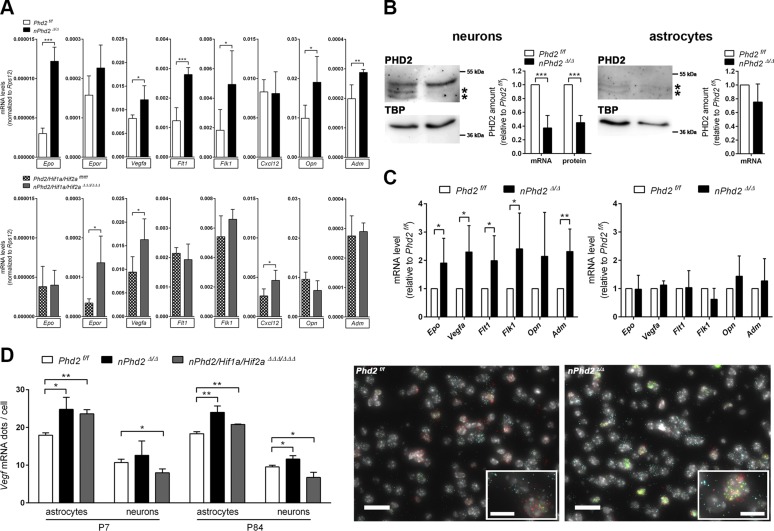
Neuron-specific Phd2 gene ablation reduced total PHD2 mRNA and protein levels in the forebrain of 1-wk-old mice by about 65 and 75%, respectively (Fig. 1). Accordingly, HIF-1α and HIF-2α protein, but not mRNA levels, were significantly increased by 140 and 25%, respectively (Fig. 1). Genetic inactivation of both Hif1a and Hif2a restricted to neurons resulted in a significant reduction of HIF-1α mRNA (−60%) and protein (−65%), whereas the amount of HIF-2α remained unchanged in the forebrain of juvenile mice (Fig. 1). These data suggest that neurons mainly express HIF-1α but not HIF-2α during early postnatal brain development, in accordance with our previous observations in adult mice deficient for either Hif1a or Hif2a (18). Interestingly, HIF-1α and HIF-2α protein levels in Phd2/Hif1a/Hif2a-deficient mice were 110 and 80%, respectively, higher than those in Hif1a/Hif2a-deficient mice, whereas the corresponding transcript levels were comparable (Fig. 1). As compared to corresponding WT littermates, the HIF-1α protein level in Phd2/Hif1a/Hif2a-deficient mice was significantly reduced by 34%, whereas the HIF-2α protein amount was significantly increased by 27% (Fig. 1). This suggests that neuronal loss of PHD2 not only increases HIF-α in the targeted neurons but also stabilizes HIF-α in nontargeted brain cells, such as astrocytes, oligodendrocytes, microglia, or endothelial cells, through a currently unknown mechanism.
Figure 1.
Neuronal PHD2 deficiency increases HIF-α protein levels in neurons and nontargeted cells of the postnatal murine brain. RNA (A) and nuclear/cytosolic proteins (B) were prepared from forebrains of 1-wk-old Phd2f/f, nPhd2Δ/Δ, Hif1a/Hif2aff/ff, nHif1a/Hif2aΔΔ/ΔΔ, Phd2/Hif1a/Hif2afff/fff, and nPhd2/Hif1a/Hif2aΔΔΔ/ΔΔΔ mice. A) Phd2, Hif1a and Hif2a transcript levels were determined by quantitative real-time RT-PCR. Values are normalized to ribosomal protein S12 (Rps12) (means ± sd; n = 5/group; 2-way ANOVA with Holm-Sidak’s multiple comparisons test). *P < 0.05, **P < 0.01, ***P < 0.001. B) Cytosolic PHD2 and nuclear HIF-α protein levels were quantified by Western blotting (protein bands used for densitometric analysis are marked with an asterisk). Values are normalized to β-tubulin (PHD2) or TATA-box-binding protein [TBP (HIF-α)] and expressed as fold change to Phd2f/f, Hif1a/Hif2aff/ff, or Phd2/Hif1a/Hif2afff/fff, respectively (mean ± sd; n = 5/group; 2-way ANOVA with Holm-Sidak’s multiple comparisons test). *P < 0.05, **P < 0.01, ***P < 0.001.
Neuron-specific activation or inactivation of the HIF signaling pathway inversely modulates vascularization of the adult murine CNS
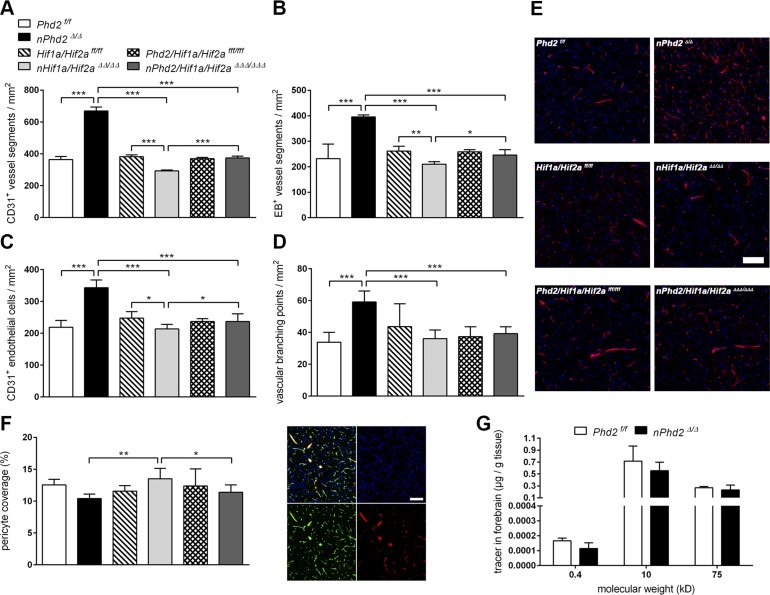
The stabilization of HIF-α subunits by neuronal Phd2 deletion (Fig. 1) was associated with significantly increased overall density of both microvascular vessel segments (+83%) and perfused, functional vasculature (+55%) in the forebrain of adult mice (Fig. 2A, B, E). Conversely, neuron-specific Hif1a and Hif2a deficiency resulted in significant reduction of total vascular density (−23%) and perfused microvessels (−20%) (Fig. 2A, B, E). Accordingly, neuronal Phd2 deficiency led to significantly increased endothelial cell density (+57%) and vessel branching points (+80%), whereas the density of brain microvascular endothelial cells was significantly decreased by 14% in Hif1a/Hif2a-deficient mice as compared with age-matched WT littermates (Fig. 2C, D). Vascular parameters did not differ between Phd2/Hif1a/Hif2a-deficient mice and corresponding WT animals (Fig. 2A–E). In accordance with the increased HIF-1α and HIF-2α protein levels as compared with Hif1a/Hif2a-deficient mice (Fig. 1), age-matched Phd2/Hif1a/Hif2a-deficient mice showed significantly increased density of total (+27%) and perfused (+17%) brain microvessels as well as endothelial cell numbers (+11%) (Fig. 2A–C, E). Thus, our histologic analyses suggest that HIF signaling in nontargeted cells of Phd2/Hif1a/Hif2a-deficient mice might activate compensatory vascularization. Overall, vascular changes were present in all studied subregions but were most pronounced in forebrain areas originating from the cortical plate (cortex, striatum, olfactory bulb) and less distinct in midbrain regions (hypothalamus, thalamus), the hippocampal region, and white matter (Supplemental Fig. S1). Moreover, vascular pericyte coverage and leakage of differently sized tracer molecules into the forebrain parenchyma were comparable between adult neuron-specific Phd2-deficient mice and WT littermates indicating proper microvascular maturity (Fig. 2F, G).
Figure 2.
Neuron-specific activation or inactivation of the HIF signaling pathway inversely modulates vascularization of the adult murine CNS. A–D) The density of vessel segments (A), perfused vessel segments (B), endothelial cells (C), and vascular branching points (D) was determined by CD31 immunofluorescence staining of EB-perfused brain sections from 12-wk-old Phd2f/f, nPhd2Δ/Δ, Hif1a/Hif2aff/ff, nHif1a/Hif2aΔΔ/ΔΔ, Phd2/Hif1a/Hif2afff/fff, and nPhd2/Hif1a/Hif2aΔΔΔ/ΔΔΔ mice (means ± sd; n = 6/group; 2-way ANOVA with Holm-Sidak’s multiple comparisons test; *P < 0.05, **P < 0.01, ***P < 0.001. E) Representative microphotographs: CD31 (red), DAPI (blue). Scale bar, 100 µm. F) Vascular pericyte coverage was analyzed through quantification of desmin+/CD31+ area related to total CD31+ area (means ± sd; n = 6/group; 2-way ANOVA with Holm-Sidak’s multiple comparisons test). *P < 0.05, **P < 0.01, ***P < 0.001. Representative microphotographs: CD31 (green), desmin (red), DAPI (blue). Scale bar, 50 µm. G) Fluorescent dyes of different molecular sizes were applied intravenously to 12-wk-old Phd2f/f and nPhd2Δ/Δ mice. The amount of tracer molecules extravasated into brain parenchyma was quantified by fluorimetry (means ± sd; n = 5/group; Student’s t test). *P < 0.05, **P < 0.01, ***P < 0.001.
The PHD2-HIF-α axis in neurons controls vessel growth in the murine CNS during early postnatal development
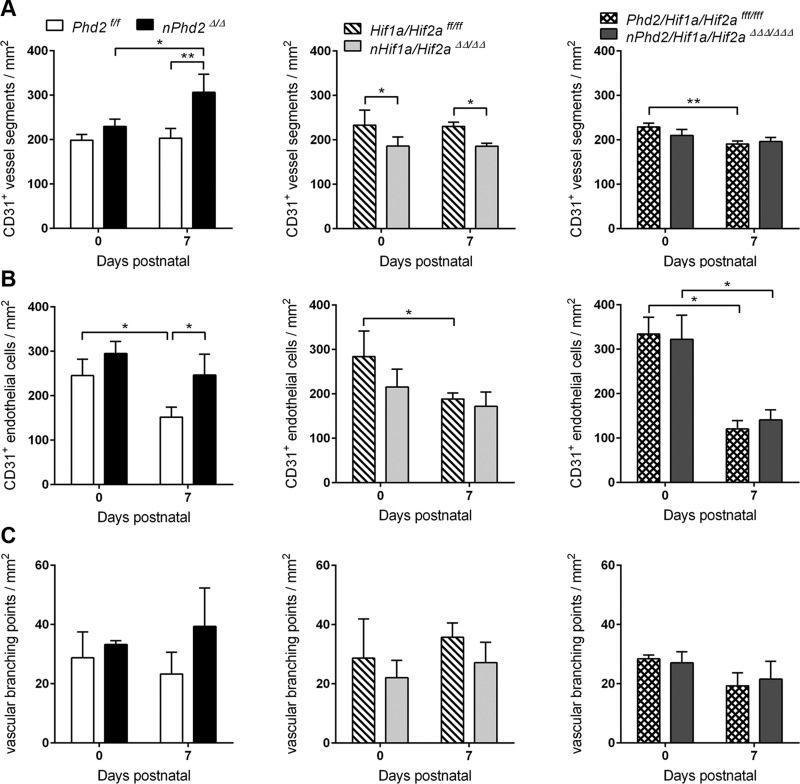
In newborn mice, the cerebral microvasculature was widely comparable irrespective of the mouse genotype (Fig. 3 and Supplemental Fig. S2), which is in accordance with the assumed onset of Cre recombinase expression under control of the Camk2a gene promoter around the time of birth (19, 28). Only 1 wk after birth, however, total vessel density (+51%) and endothelial cell number (+62%) were significantly increased in Phd2-deficient mice as compared with age-matched WT littermates (Fig. 3A, B). The increase in endothelial cell number and vessel density as well as vascular branching was most pronounced in the striatum (Supplemental Fig. S2A–C). Hif1a/Hif2a-deficient mice of the same age showed a significantly decreased microvessel density across the cortex, striatum, and white matter (Supplemental Fig. S2A), resulting in a significant overall reduction of the cerebral vessel density by 20% (Fig. 3A). It is also worth mentioning that relative increase or decrease of vascular density that is due to neuronal loss of PHD2 or HIF-α, respectively, was quite comparable in the juvenile (P7) and adult (P84) mouse brain (Figs. 3A and 2A). Additional loss of PHD2 prevented the disruption of early postnatal brain vascularization resulting from the absence of neuronal HIF signaling in 1-wk-old mice (Fig. 3A and Supplemental Fig. S2A).
Figure 3.
The PHD2-HIF-α axis in neurons controls vessel growth in the murine CNS during early postnatal development. The density of vessel segments (A), endothelial cells (B), and vascular branching points (C) was determined by CD31 immunofluorescence staining in brain sections from 0 to 1-wk-old Phd2f/f, nPhd2Δ/Δ, Hif1a/Hif2aff/ff, nHif1a/Hif2aΔΔ/ΔΔ, Phd2/Hif1a/Hif2afff/fff, and nPhd2/Hif1a/Hif2aΔΔΔ/ΔΔΔ mice (means ± sd; n = 3/group; 2-way ANOVA with Holm-Sidak’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001.
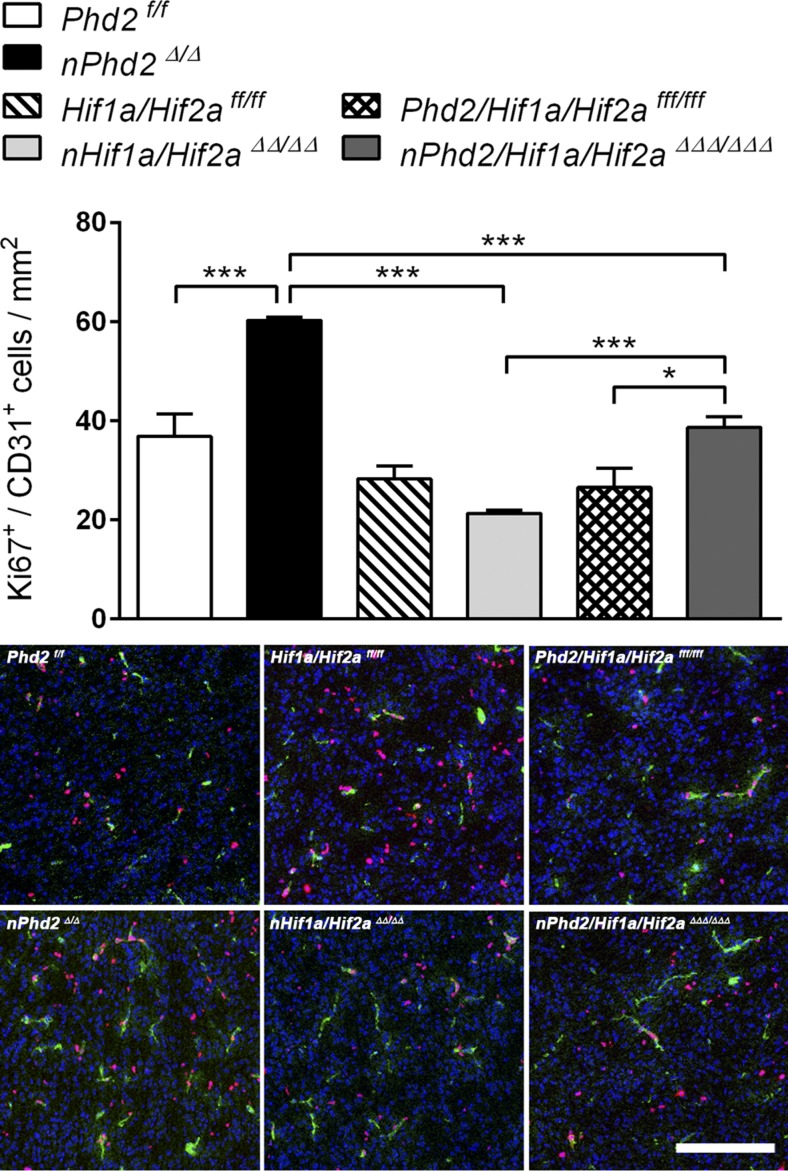
Endothelial cell proliferation in the brain of juvenile (P7) Phd2-deficient mice was significantly enhanced by 63%, as estimated by Ki67 immunofluorescent detection. Hif1a/Hif2a-deficient mice exhibited a nonsignificant (P = 0.06) reduction by 25% as compared with WT littermates (Fig. 4). In accordance with the abovementioned histologic results, proliferation of endothelial cells in P7 brains of Phd2/Hif1a/Hif2a-deficient mice was significantly increased by 81 and 46% as compared with Hif1a/Hif2a-deficient mice and WT littermates, respectively (Fig. 4).
Figure 4.
The PHD2-HIFα axis in neurons regulates endothelial cell proliferation in the early postnatal mouse brain. Endothelial cell proliferation was evaluated by immunofluorescent detection of Ki67+/CD31+ nuclei in brain sections of 1-wk-old Phd2f/f, nPhd2Δ/Δ, Hif1a/Hif2aff/ff, nHif1a/Hif2aΔΔ/ΔΔ, Phd2/Hif1a/Hif2afff/fff, and nPhd2/Hif1a/Hif2aΔΔΔ/ΔΔΔ mice (means ± sd; n = 3/group; 2-way ANOVA with Holm-Sidak’s multiple comparisons test). *P < 0.05, **P < 0.01, ***P < 0.001. Representative microphotographs: CD31 (green), Ki67 (magenta), DAPI (blue). Scale bar, 50 µm.
Neuronal PHD2 deficiency induces a proangiogenic transcriptional response in neurons and astrocytes in a HIF-dependent manner
It is well known that numerous proangiogenic factors are up-regulated during tissue hypoxia mainly through HIF-mediated transcriptional activation (10). Accordingly, several genes related to angiogenic processes, including Vegfa, Epo, Opn, Adm, Flt1, and fetal liver kinase 1 (Flk1) encoding for VEGF receptor-1 and 2, respectively, were increasingly expressed in cerebral tissue of juvenile (P7) Phd2-deficient mice (Fig. 5A and Supplemental Fig. S3A). In the CNS of age-matched Phd2/Hif1a/Hif2a-deficient mice, Vegfa mRNA levels were also significantly increased, whereas Epo, Opn, Adm, Flt, or Flk1 transcript levels were not altered as compared with WT littermates (Fig. 5A and Supplemental Fig. S3A). Moreover, Epor (Epo receptor) and Cxcl12 (C-X-C motif chemokine-12) mRNA levels were significantly up-regulated in the CNS of juvenile Phd2/Hif1a/Hif2a-deficient mice but not in Phd2-deficient animals (Fig. 5A).
Figure 5.
Neuron-specific PHD2 deficiency induces a proangiogenic response in neurons and astrocytes. A) RNA was extracted from forebrains of 1-wk-old Phd2f/f, nPhd2Δ/Δ, Phd2/Hif1a/Hif2afff/fff, and nPhd2/Hif1a/Hif2aΔΔΔ/ΔΔΔ mice. Proangiogenic factor mRNA levels were estimated by real-time RT-PCR and normalized to ribosomal protein S12 (Rps12) (means ± sd; n = 5/group; Student’s t test). *P < 0.05, **P < 0.01, ***P < 0.001. B) Real-time RT-PCR and immunoblotting were used to determine Phd2 transcript levels and nuclear PHD2 protein levels (protein bands used for densitometric analysis are marked with an asterisk), respectively, in primary neurons and astrocytes isolated from brains of neonatal Phd2 f/f and nPhd2 Δ/Δ mice [means ± sd; n = 5/5 (neurons), n = 4/4 (astrocytes); Student’s t test]. *P < 0.05, **P < 0.01, ***P < 0.001. C) mRNA levels were quantified by real-time RT-PCR [means ± sd; n = 5/5 (neurons), n = 4/4 (astrocytes); Student’s t test]. *P < 0.05, **P < 0.01, ***P < 0.001. Values are normalized to Rps12 (mRNA) or TATA-box-binding protein [TBP (protein)] and expressed as fold change to Phd2f/f. D) Multiplex RNA FISH was applied to detect Vegfa mRNA in neurons and astrocytes in coronal brain sections of juvenile (P7) and adult (P84) Phd2f/f, nPhd2Δ/Δ, and nPhd2/Hif1a/Hif2aΔΔΔ/ΔΔΔ mice (means ± sd; n = 3/group; Student’s t test). *P < 0.05, **P < 0.01, ***P < 0.001. Neurons and astrocytes were identified according to Dlg4 and Slc1a3 transcript levels exclusively expressed in neurons and astrocytes, respectively. Representative microphotographs: Vegfa (green), Slc1a3 (red), Dlg4 (cyan), DAPI (white). Scale bar, 20 µm. Inlays show the same micrographs at higher magnification (scale bars, 10 µm).
To exclude that those genes are up-regulated in nontargeted vascular structures, organotypic hippocampal slices extracted from 1-wk-old Phd2-deficient mice and WT littermates were cultured ex vivo for 7 d, leading to the regression of functional microvasculature, as previously reported (29). Similarly to the entire brain tissue from Phd2-deficient mice, in hippocampal slice cultures, which lost functional endothelial cells, Epor and Cxcl12 mRNA was not changed, whereas Vegfa, Epo, Opn, Adm, Flt1, and Flk1 transcript levels were still significantly increased (Supplemental Fig. S3B), pointing to the neuronal origin of these angiogenic factors.
To further address the neuronal origin, neurons were isolated from neonatal Phd2-deficient and WT mice and then cultured in vitro. Cells exhibited significantly reduced PHD2 transcript and protein levels (Fig. 5B), which was accompanied by significant up-regulation of Vegfa, Epo, Adm, Flt1, and Flk1 (Fig. 5C). By contrast, Phd2, Vegfa, Epo, Adm, Flt11, and Flk1 mRNA levels in astrocytes originating from newborn Phd2-deficient mice did not differ significantly from those in astrocytes derived from WT littermates (Fig. 5B, C). In accordance with previous results (30), PHD2 protein levels in astrocytes were at the lower detection limit of immunoblotting, irrespective of the genotype (Fig. 5B). Given that increased vascularization, HIF-α, and Vegfa levels in Phd2-deficient brains are not fully abrogated by additional deletion of Hif1a and Hif2a, induction of HIF-VEGF signaling in nontargeted cells may also contribute to enhanced brain angiogenesis resulting from neuronal Phd2 gene ablation. Hence, we applied high-resolution multiplex RNA FISH to determine Vegfa mRNA abundance in neurons and astrocytes of the juvenile (P7) and adult (P84) mouse brain. Astrocytes (Slc1a3+/Dlg4− cells) showed almost twice as many Vegfa mRNA dots per cell as neurons (Dlg4+/Slc1a3− cells) (Fig. 5D). In juvenile and adult mice with neuron-specific Phd2 knockout, both astrocytes and neurons exhibited significantly increased Vegfa mRNA levels as compared with age-matched WT littermates (Fig. 5D). Additional neuron-specific loss of Hif1a and Hif2a lowered Vegfa mRNA in neurons and prevented up-regulation by Phd2 deletion but did not affect Vegfa up-regulation in astrocytes (Fig. 5D).
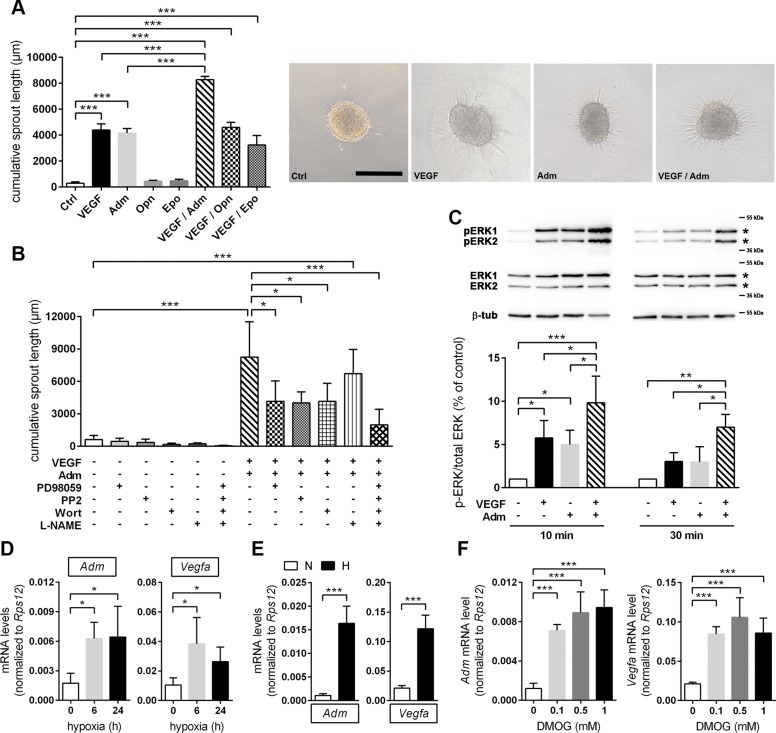
VEGF and Adm additively promote angiogenic sprouting of the cerebral microvasculature
We next analyzed the angiogenic potential of the growth factors that were increased by Phd2 deficiency (Fig. 5). Three-dimensional spheroid cultures of murine cerebrovascular endothelial bEnd.3 cells showed significantly increased angiogenic sprouting upon treatment with rhVEGF-A165 or rhAdm, whereas rhEpo and rmOpn did not promote endothelial sprouting (Fig. 6A). Interestingly, when applied together with VEGF-A, Adm additively enhanced endothelial sprouting, whereas Opn and Epo did not further increase the proangiogenic stimulation by VEGF-A.
Figure 6.
VEGF and Adm cooperatively promote angiogenic sprouting of brain microvascular endothelial cells through multiple signaling pathways. A) Collagen gel-embedded spheroids of murine cerebrovascular endothelial bEnd.3 cells were treated with 50 ng/ml rhVEGF-A165 and/or 200 nM rhAdm, 2 µg/ml rmOpn, and 5 U/ml rhEpo, respectively, for 24 h. Untreated spheroids served as control (Ctrl). The length and number of sprouts (calculated as cumulative sprout length) that had grown out of each spheroid (≥10 spheroids/group and experiment) were determined using cellSens imaging software (means ± sd; n = 3/group; 1-way ANOVA with Holm-Sidak’s multiple comparisons test). *P < 0.05, **P < 0.01, ***P < 0.001. The top-right panel shows representative microphotographs, scale bar, 200 µm. B) Spheroids were treated with 20 µM PD98059, 5 µM PP2, 0.2 µM wortmannin, and 5 mM L-NAME alone or in combination for 1 h. Subsequently, spheroids were costimulated with 50 ng/ml rhVEGF-A165 and 200 nM rhAdm or were left untreated. After 24 h, cumulative sprout length was calculated as previously described (means ± sd; n = 3/group; 1-way ANOVA with Holm-Sidak’s multiple comparisons test. *P < 0.05, **P < 0.01, ***P < 0.001. C) bEnd.3 cell monolayer was treated with 50 ng/ml rhVEGF-A165 and/or 200 nM rhAdm for 10 or 30 min. Total protein was isolated, and immunoblotting was applied to quantify total and phosphorylated ERK1/2 protein levels (protein bands used for densitometric analysis are marked with an asterisk). Values are normalized to β-tubulin and expressed as fold change to control (means ± sd; n = 4/group; 1-way ANOVA with Holm-Sidak’s multiple comparisons test). *P < 0.05, **P < 0.01, ***P < 0.001. Representative Western blot images are shown above the bar graph. D) Adm and Vegf mRNA levels were quantified in forebrain tissue lysates of adult C57Bl/6 mice kept for 6 or 24 h either at normobaric hypoxia (8% O2) or room air pressure (∼21% O2) using real-time RT-PCR. Values are normalized to ribosomal protein S12 (Rps12) (means ± sd; n = 4–6/group; 1-way ANOVA with Holm-Sidak’s multiple comparisons test). *P < 0.05, **P < 0.01, ***P < 0.001. E, F) Primary neurons isolated from brains of neonatal C57Bl/6 mice were either exposed to 6 h hypoxia (1% O2) (E) or treated with 0.1, 0.5, or 1 mM DMOG for 6 h (F). Control cells were cultured under normoxic conditions or were treated with 0.1% DMSO for 6 h, respectively. Gene expression was analyzed by real-time RT-PCR. Values are normalized to Rps12 [means ± sd; n = 6; 1-way ANOVA with Holm-Sidak’s multiple comparisons test (E) and n = 4; Student’s t test (E)]. *P < 0.05, **P < 0.01, ***P < 0.001.
To elucidate signaling pathways that underlie the additive proangiogenic effect of VEGF-A and Adm, bEnd.3 cells were pretreated with PD98059, PP2, wortmannin, and L-NAME to inhibit MAPKK 1 and 2 (MAP2K1/2), Src family kinases, PI3K, and eNOS, respectively, which are all known to control endothelial cell proliferation and migration (31). Pharmacological inhibition of MAP2K1/2, Src, and PI3K significantly reduced the cooperative proangiogenic effect of VEGF-A and Adm, whereas eNOS inhibition did not markedly affect endothelial cell sprouting induced by VEGF-A and Adm (Fig. 6B). Cotreatment with all pharmacological inhibitors further decreased sprouting angiogenesis in response to VEGF-A and Adm (Fig. 6B), suggesting that the targeted signaling pathways independently affect angiogenic sprouting. In accordance with the pronounced blockage of endothelial cell sprouting through MAP2K1/2 inhibition, combined short-term treatment with VEGF-A and Adm significantly increased the phosphorylation of ERK 1 and 2, direct downstream targets of MAP2K1/2, as compared with either untreated, VEGF-treated, or Adm-treated endothelial cells (Fig. 6C).
Our abovementioned results point to a HIF-dependent regulation of Adm expression in neurons. Accordingly, exposure to inspiratory hypoxia increased Adm and Vegfa transcript levels in the adult murine CNS to a comparable extent (Fig. 6D). Similarly, Adm, Vegfa, Epo, Flt1, and Flk1 mRNA levels in primary mouse neurons were significantly enhanced in response to hypoxia (Fig. 6E and Supplemental Fig. S3C) and treatment with the pan-PHD inhibitor DMOG (Fig. 6F and Supplemental Fig. S3D).
DISCUSSION
VEGF is an important factor for CNS angiogenesis (32). However, it currently remains unclear which cell types provide the crucial cues for postnatal vessel growth in the brain. Here, we demonstrate that the hypoxia pathway in neurons is a central determinant of postnatal vascularization of the brain. Furthermore, we discover neuron-derived Adm in concert with VEGF of neuronal and astrocytic origin as major angiogenic factors responsible for the cerebral vascular response.
Adm, a 52 aa peptide originally isolated as hypotensive factor from human pheochromocytoma, is a pleiotropic multifaceted molecule that is synthesized in a variety of cell and tissue types (33). The Adm receptor consists of a heterodimer formed by calcitonin receptor-like receptor and receptor activity–modifying protein (RAMP) 2 or 3 (33). In the rodent CNS, Adm is mainly released by cerebrovascular endothelial cells but also neurons and astrocytes (34, 35). Gain- and loss-of-function studies in the rodent CNS showed that Adm is involved in various physiologic brain functions, such as drinking, salt intake and eating behavior (36), anxiety, locomotion, motor coordination (37), and pain sensitivity (38). Previous studies demonstrated that the vascular endothelial Adm-RAMP2 system is crucial for integrity and homeostasis of the peripheral vasculature (33, 39). We here show for the first time that Adm—in concert with other proangiogenic factors such as VEGF—also promotes the growth of the cerebral microvasculature in the CNS, as stimulation of the hypoxic pathway in postmitotic neurons increases the abundance of Adm in the early postnatal brain.
Previous reports suggested astrocytes as the main source of VEGF. Astrocytes display the strongest VEGF expression around the growing vascular plexus (40). However, there is evidence against a major role of the PHD2-HIF-VEGF axis in astrocytes. PHD2, the main prolyl-hydroxylase in the brain (12), is absent in astrocytes, whereas HIF-1α stability is clearly PHD2-regulated in neurons (30). Furthermore, loss of hypoxia signaling or VEGF expression in astrocytes by deleting either both HIF-α subunits or VEGF, respectively, did not impair normal development of retinal vasculature (6, 41), suggesting that other cell types, such as neurons, compensate for VEGF production. From a physiologic point of view, neurons are most suitable for oxygen sensing within the brain parenchyma. Neurons are among the cells most sensitive to hypoxia (42). They have a high energy turnover, rely on aerobic metabolism, and thus mainly determine the local tissue pO2 (43). Because neurons largely depend on continuous oxygen delivery by diffusion, a dense vascular network resulting in short diffusion distances is of critical importance. As a consequence, local hypoxia should be immediately sensed by neurons and result in an appropriate adaptive response. Indeed, we here show that activation of the hypoxic pathway in neurons by deletion of PHD2 results in a strong angiogenic reaction in the brain. On the other hand, inactivation of the hypoxic pathway reduced vascular density, further confirming the pivotal importance of neuronal oxygen-sensing mechanisms for proper postnatal brain vascularization. Earlier work already suggested neurons to be essential for the regulation of vascular density in the CNS. VEGF dosage in neural progenitor cells critically regulated the density of the vascular plexus in the developing CNS (44), and VEGF produced by the embryonic neuroectoderm was required for the vascularization of the brain (32). Moreover, neuroretina-specific deletion of HIF-1α impaired the retinal vascular development (16). Taken together, these data suggest that neurons—but not astrocytes—act as primary oxygen sensors for the regulation of vascular density in the brain.
Unexpectedly, deletion of the oxygen-sensing PHD2 in postmitotic neurons caused HIF-α stabilization and transcriptional activation of VEGF-A not only in neurons but also in astrocytes of the juvenile murine CNS. This effect was independent of neuronal HIF-α. Additional loss of PHD2 in HIF-α–deficient neurons prevented disruption of vascularization and resulted in an enhanced endothelial cell proliferation. In this regard, astrocytic VEGF-A up-regulation may compensate for the loss of VEGF-A in neurons lacking HIF-α and might consecutively counterbalance a decrease in microvessel density. The neuronal hypoxic pathway might lead to HIF-α stabilization and up-regulation of proangiogenic VEGF-A in astrocytes, which contributes to the postnatal brain vascularization. The mechanism for this neuron-to-astrocyte signaling remains currently unknown. Today, it is well established that a dynamic bidirectional communication between astrocytes and neurons contributes to brain information processing (45). Indeed, astrocytes are equipped to sense and integrate neuronal information through ionic channels, neurotransmitter receptors and transporters, and intracellular signaling pathways (46). Thus, as part of the tripartite synapse, the structure consisting of pre- and postsynaptic elements of the synapse and an associated astrocytic process, astrocytes cooperate with neurons on several levels, including neurotransmitter trafficking, uptake, and recycling (45, 46).
ATP might be such a neuron-to-astrocyte signal and could link neuronal activity and metabolism to astrocytic function, as purinergic signaling is crucial for integrating functional activity between neurons, glial, and vascular cells in the CNS (47). In neurons, ATP is present in synaptic vesicles and can be either co-stored and co-released with other neurotransmitters or act as a sole transmitter (47), and neuronal electrical activity can determine the rate of ATP release (48). In astrocytes, activation of the ATP receptor P2X7, an ATP-gated cation channel, increased HIF-1α protein stability and promoted transactivation of the HIF target gene, Epo (30, 49). Thus, synaptic processes could confer the hypoxic signal to neighboring cells. Indeed, a recent study provided experimental evidence that PHD2 controls synaptic transmission by regulation of dendritic spine plasticity via HIF-independent modification of the actin cross-linker filamin A (13). However, the precise molecular mechanism linking oxygen sensing in neurons to HIF signaling in astrocytes and possibly further non-neuronal cells is currently unknown.
Taken together, we provide strong evidence that hypoxia sensing, specifically in neurons, controls vascularization and vascular density in the early postnatal and adult brain. Our finding that neuronal PHD2 depletion or general PHD inhibition is able to increase vascular density may have implications for the clinic. PHD inhibitors have recently been approved for the treatment of renal anemia (50). PHD inhibitors may also have beneficial effects for brain oxygenation (by inducing vascular growth) and for the treatment of vascular dementia. Indeed, we and others have previously shown that induction of HIF and HIF-targets, such as VEGF or Epo, are able to improve cognitive function in murine models of hypoperfusion or Alzheimer’s disease (51–53). These factors act in a pleiotropic way, either by a direct neuroprotective action or—as shown here—indirectly by providing better tissue oxygenation through increased vascular growth.
ACKNOWLEDGMENTS
The authors thank Dr. Caroline Arnold (Heidelberg University) for help with the angiogenic sprouting assay. The expert technical assistance of Nadine Heselmaier, Maria Harlacher, and Inge Keller (all from Heidelberg University) is gratefully acknowledged. E.N. was supported by a travel grant of the Boehringer Ingelheim Fonds. R.H.W. was supported by the National Centre of Competence in Research (NCCR) Kidney.CH. K.A.N. was supported by the European Union’s Seventh Framework Programme for Research, Technological Development, and Demonstration (Grant 608847). The authors declare no conflicts of interest.
Glossary
- Adm
adrenomedullin
- Camk2a
calcium/calmodulin-dependent protein kinase II α
- Cxcl12
C-X-C motif chemokine-12
- Dlg4
discs large MAGUK scaffold protein 4
- DMOG
dimethyloxalylglycine
- EB
Evans Blue
- Epo
erythropoietin
- Epor
Epo receptor
- Flk1
fetal liver kinase 1
- Flt1
Fms-related tyrosine kinase 1
- HIF
hypoxia-inducible factor
- L-NAME
N(γ)-nitro-l-arginine methyl ester
- Opn
osteopontin
- PHD
prolyl-4-hydroxylase domain
- RAMP
receptor activity–modifying protein
- rh
recombinant human
- rm
recombinant mouse
- Slc1A3
solute carrier family 1, member 3
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
E. Nasyrov, K. A. Nolan, R. H. Wenger, H. H. Marti, and R. Kunze conceived and designed the experiments; E. Nasyrov, H. H. Marti, and R. Kunze wrote the manuscript with input from all authors; E. Nasyrov and R. Kunze performed the experiments and analyzed the data; H. H. Marti and R. Kunze conceived and directed the study; and all authors read and approved the final manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Paredes I., Himmels P., Ruiz de Almodóvar C. (2018) Neurovascular communication during CNS development. Dev. Cell 45, 10–32 [DOI] [PubMed] [Google Scholar]
- 2.Tata M., Ruhrberg C., Fantin A. (2015) Vascularisation of the central nervous system. Mech. Dev. 138, 26–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wälchli T., Wacker A., Frei K., Regli L., Schwab M. E., Hoerstrup S. P., Gerhardt H., Engelhardt B. (2015) Wiring the vascular network with neural cues: a CNS perspective. Neuron 87, 271–296 [DOI] [PubMed] [Google Scholar]
- 4.Bai Y., Ma J. X., Guo J., Wang J., Zhu M., Chen Y., Le Y. Z. (2009) Müller cell-derived VEGF is a significant contributor to retinal neovascularization. J. Pathol. 219, 446–454 [DOI] [PubMed] [Google Scholar]
- 5.Stone J., Itin A., Alon T., Pe’er J., Gnessin H., Chan-Ling T., Keshet E. (1995) Development of retinal vasculature is mediated by hypoxia-induced vascular endothelial growth factor (VEGF) expression by neuroglia. J. Neurosci. 15, 4738–4747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weidemann A., Krohne T. U., Aguilar E., Kurihara T., Takeda N., Dorrell M. I., Simon M. C., Haase V. H., Friedlander M., Johnson R. S. (2010) Astrocyte hypoxic response is essential for pathological but not developmental angiogenesis of the retina. Glia 58, 1177–1185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sapieha P., Sirinyan M., Hamel D., Zaniolo K., Joyal J. S., Cho J. H., Honoré J. C., Kermorvant-Duchemin E., Varma D. R., Tremblay S., Leduc M., Rihakova L., Hardy P., Klein W. H., Mu X., Mamer O., Lachapelle P., Di Polo A., Beauséjour C., Andelfinger G., Mitchell G., Sennlaub F., Chemtob S. (2008) The succinate receptor GPR91 in neurons has a major role in retinal angiogenesis. Nat. Med. 14, 1067–1076 [DOI] [PubMed] [Google Scholar]
- 8.Usui Y., Westenskow P. D., Kurihara T., Aguilar E., Sakimoto S., Paris L. P., Wittgrove C., Feitelberg D., Friedlander M. S., Moreno S. K., Dorrell M. I., Friedlander M. (2015) Neurovascular crosstalk between interneurons and capillaries is required for vision. J. Clin. Invest. 125, 2335–2346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Himmels P., Paredes I., Adler H., Karakatsani A., Luck R., Marti H. H., Ermakova O., Rempel E., Stoeckli E. T., Ruiz de Almodóvar C. (2017) Motor neurons control blood vessel patterning in the developing spinal cord. Nat. Commun. 8, 14583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rey S., Semenza G. L. (2010) Hypoxia-inducible factor-1-dependent mechanisms of vascularization and vascular remodelling. Cardiovasc. Res. 86, 236–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaelin W. G., Jr., Ratcliffe P. J. (2008) Oxygen sensing by metazoans: the central role of the HIF hydroxylase pathway. Mol. Cell 30, 393–402 [DOI] [PubMed] [Google Scholar]
- 12.Rabie T., Kunze R., Marti H. H. (2011) Impaired hypoxic response in senescent mouse brain. Int. J. Dev. Neurosci. 29, 655–661 [DOI] [PubMed] [Google Scholar]
- 13.Segura I., Lange C., Knevels E., Moskalyuk A., Pulizzi R., Eelen G., Chaze T., Tudor C., Boulegue C., Holt M., Daelemans D., Matondo M., Ghesquière B., Giugliano M., Ruiz de Almodovar C., Dewerchin M., Carmeliet P. (2016) The oxygen sensor PHD2 controls dendritic spines and synapses via modification of filamin A. Cell Rep. 14, 2653–2667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berra E., Benizri E., Ginouvès A., Volmat V., Roux D., Pouysségur J. (2003) HIF prolyl-hydroxylase 2 is the key oxygen sensor setting low steady-state levels of HIF-1alpha in normoxia. EMBO J. 22, 4082–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ding K., Scortegagna M., Seaman R., Birch D. G., Garcia J. A. (2005) Retinal disease in mice lacking hypoxia-inducible transcription factor-2alpha. Invest. Ophthalmol. Vis. Sci. 46, 1010–1016 [DOI] [PubMed] [Google Scholar]
- 16.Nakamura-Ishizu A., Kurihara T., Okuno Y., Ozawa Y., Kishi K., Goda N., Tsubota K., Okano H., Suda T., Kubota Y. (2012) The formation of an angiogenic astrocyte template is regulated by the neuroretina in a HIF-1-dependent manner. Dev. Biol. 363, 106–114 [DOI] [PubMed] [Google Scholar]
- 17.Takeda K., Cowan A., Fong G. H. (2007) Essential role for prolyl hydroxylase domain protein 2 in oxygen homeostasis of the adult vascular system. Circulation 116, 774–781 [DOI] [PubMed] [Google Scholar]
- 18.Barteczek P., Li L., Ernst A. S., Böhler L. I., Marti H. H., Kunze R. (2017) Neuronal HIF-1α and HIF-2α deficiency improves neuronal survival and sensorimotor function in the early acute phase after ischemic stroke. J. Cereb. Blood Flow Metab. 37, 291–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kunze R., Zhou W., Veltkamp R., Wielockx B., Breier G., Marti H. H. (2012) Neuron-specific prolyl-4-hydroxylase domain 2 knockout reduces brain injury after transient cerebral ischemia. Stroke 43, 2748–2756 [DOI] [PubMed] [Google Scholar]
- 20.Li L., Saliba P., Reischl S., Marti H. H., Kunze R. (2016) Neuronal deficiency of HIF prolyl 4-hydroxylase 2 in mice improves ischemic stroke recovery in an HIF dependent manner. Neurobiol. Dis. 91, 221–235 [DOI] [PubMed] [Google Scholar]
- 21.Schoch H. J., Fischer S., Marti H. H. (2002) Hypoxia-induced vascular endothelial growth factor expression causes vascular leakage in the brain. Brain 125, 2549–2557 [DOI] [PubMed] [Google Scholar]
- 22.Lin-Holderer J., Li L., Gruneberg D., Marti H. H., Kunze R. (2016) Fumaric acid esters promote neuronal survival upon ischemic stress through activation of the Nrf2 but not HIF-1 signaling pathway. Neuropharmacology 105, 228–240 [DOI] [PubMed] [Google Scholar]
- 23.Regen T., van Rossum D., Scheffel J., Kastriti M. E., Revelo N. H., Prinz M., Brück W., Hanisch U. K. (2011) CD14 and TRIF govern distinct responsiveness and responses in mouse microglial TLR4 challenges by structural variants of LPS. Brain Behav. Immun. 25, 957–970 [DOI] [PubMed] [Google Scholar]
- 24.Zhang S. J., Steijaert M. N., Lau D., Schütz G., Delucinge-Vivier C., Descombes P., Bading H. (2007) Decoding NMDA receptor signaling: identification of genomic programs specifying neuronal survival and death. Neuron 53, 549–562 [DOI] [PubMed] [Google Scholar]
- 25.Montesano R., Pepper M. S., Möhle-Steinlein U., Risau W., Wagner E. F., Orci L. (1990) Increased proteolytic activity is responsible for the aberrant morphogenetic behavior of endothelial cells expressing the middle T oncogene. Cell 62, 435–445 [DOI] [PubMed] [Google Scholar]
- 26.Heiss M., Hellström M., Kalén M., May T., Weber H., Hecker M., Augustin H. G., Korff T. (2015) Endothelial cell spheroids as a versatile tool to study angiogenesis in vitro. FASEB J. 29, 3076–3084 [DOI] [PubMed] [Google Scholar]
- 27.Ernst A. S., Böhler L. I., Hagenston A. M., Hoffmann A., Heiland S., Sticht C., Bendszus M., Hecker M., Bading H., Marti H. H., Korff T., Kunze R. (2019) EphB2-dependent signaling promotes neuronal excitotoxicity and inflammation in the acute phase of ischemic stroke. Acta Neuropathol. Commun. 7, 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Casanova E., Fehsenfeld S., Mantamadiotis T., Lemberger T., Greiner E., Stewart A. F., Schütz G. (2001) A CamKIIalpha iCre BAC allows brain-specific gene inactivation. Genesis 31, 37–42 [DOI] [PubMed] [Google Scholar]
- 29.Peeters C., Hoelen D., Groenendaal F., van Bel F., Bär D. (2003) Deferoxamine, allopurinol and oxypurinol are not neuroprotective after oxygen/glucose deprivation in an organotypic hippocampal model, lacking functional endothelial cells. Brain Res. 963, 72–80 [DOI] [PubMed] [Google Scholar]
- 30.Hirayama Y., Koizumi S. (2017) Hypoxia-independent mechanisms of HIF-1α expression in astrocytes after ischemic preconditioning. Glia 65, 523–530 [DOI] [PubMed] [Google Scholar]
- 31.Simons M., Gordon E., Claesson-Welsh L. (2016) Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 17, 611–625 [DOI] [PubMed] [Google Scholar]
- 32.Raab S., Beck H., Gaumann A., Yüce A., Gerber H. P., Plate K., Hammes H. P., Ferrara N., Breier G. (2004) Impaired brain angiogenesis and neuronal apoptosis induced by conditional homozygous inactivation of vascular endothelial growth factor. Thromb. Haemost. 91, 595–605 [DOI] [PubMed] [Google Scholar]
- 33.Shindo T., Tanaka M., Kamiyoshi A., Ichikawa-Shindo Y., Kawate H., Yamauchi A., Sakurai T. (2019) Regulation of cardiovascular development and homeostasis by the adrenomedullin-RAMP system. Peptides 111, 55–61 [DOI] [PubMed] [Google Scholar]
- 34.Kis B., Kaiya H., Nishi R., Deli M. A., Abrahám C. S., Yanagita T., Isse T., Gotoh S., Kobayashi H., Wada A., Niwa M., Kangawa K., Greenwood J., Yamashita H., Ueta Y. (2002) Cerebral endothelial cells are a major source of adrenomedullin. J. Neuroendocrinol. 14, 283–293 [DOI] [PubMed] [Google Scholar]
- 35.Serrano J., Uttenthal L. O., Martínez A., Fernández A. P., Martínez de Velasco J., Alonso D., Bentura M. L., Santacana M., Gallardo J. R., Martínez-Murillo R., Cuttitta F., Rodrigo J. (2000) Distribution of adrenomedullin-like immunoreactivity in the rat central nervous system by light and electron microscopy. Brain Res. 853, 245–268 [DOI] [PubMed] [Google Scholar]
- 36.Igarashi K., Sakurai T., Kamiyoshi A., Ichikawa-Shindo Y., Kawate H., Yamauchi A., Toriyama Y., Tanaka M., Liu T., Xian X., Imai A., Zhai L., Owa S., Koyama T., Uetake R., Ihara M., Shindo T. (2014) Pathophysiological roles of adrenomedullin-RAMP2 system in acute and chronic cerebral ischemia. Peptides 62, 21–31 [DOI] [PubMed] [Google Scholar]
- 37.Fernández A. P., Serrano J., Tessarollo L., Cuttitta F., Martínez A. (2008) Lack of adrenomedullin in the mouse brain results in behavioral changes, anxiety, and lower survival under stress conditions. Proc. Natl. Acad. Sci. USA 105, 12581–12586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fernández A. P., Serrano J., Martínez-Murillo R., Martínez A. (2010) Lack of adrenomedullin in the central nervous system results in apparently paradoxical alterations on pain sensitivity. Endocrinology 151, 4908–4915 [DOI] [PubMed] [Google Scholar]
- 39.Koyama T., Sakurai T., Kamiyoshi A., Ichikawa-Shindo Y., Kawate H., Shindo T. (2015) Adrenomedullin-RAMP2 system in vascular endothelial cells. J. Atheroscler. Thromb. 22, 647–653 [DOI] [PubMed] [Google Scholar]
- 40.Gerhardt H., Golding M., Fruttiger M., Ruhrberg C., Lundkvist A., Abramsson A., Jeltsch M., Mitchell C., Alitalo K., Shima D., Betsholtz C. (2003) VEGF guides angiogenic sprouting utilizing endothelial tip cell filopodia. J. Cell Biol. 161, 1163–1177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scott A., Powner M. B., Gandhi P., Clarkin C., Gutmann D. H., Johnson R. S., Ferrara N., Fruttiger M. (2010) Astrocyte-derived vascular endothelial growth factor stabilizes vessels in the developing retinal vasculature. PLoS One 5, e11863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bickler P. E., Donohoe P. H. (2002) Adaptive responses of vertebrate neurons to hypoxia. J. Exp. Biol. 205, 3579–3586 [DOI] [PubMed] [Google Scholar]
- 43.Andreone B. J., Lacoste B., Gu C. (2015) Neuronal and vascular interactions. Annu. Rev. Neurosci. 38, 25–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Haigh J. J., Morelli P. I., Gerhardt H., Haigh K., Tsien J., Damert A., Miquerol L., Muhlner U., Klein R., Ferrara N., Wagner E. F., Betsholtz C., Nagy A. (2003) Cortical and retinal defects caused by dosage-dependent reductions in VEGF-A paracrine signaling. Dev. Biol. 262, 225–241 [DOI] [PubMed] [Google Scholar]
- 45.Araque A., Carmignoto G., Haydon P. G., Oliet S. H., Robitaille R., Volterra A. (2014) Gliotransmitters travel in time and space. Neuron 81, 728–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Durkee C. A., Araque A. (2019) Diversity and specificity of astrocyte-neuron communication. Neuroscience 396, 73–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Burnstock G. (2007) Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 87, 659–797 [DOI] [PubMed] [Google Scholar]
- 48.Fields R. D., Stevens B. (2000) ATP: an extracellular signaling molecule between neurons and glia. Trends Neurosci. 23, 625–633 [DOI] [PubMed] [Google Scholar]
- 49.Hirayama Y., Ikeda-Matsuo Y., Notomi S., Enaida H., Kinouchi H., Koizumi S. (2015) Astrocyte-mediated ischemic tolerance. J. Neurosci. 35, 3794–3805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semenza G. L. (2019) Pharmacologic targeting of hypoxia-inducible factors. Annu. Rev. Pharmacol. Toxicol. 59, 379–403 [DOI] [PubMed] [Google Scholar]
- 51.Gruneberg D., Montellano F. A., Plaschke K., Li L., Marti H. H., Kunze R. (2016) Neuronal prolyl-4-hydroxylase 2 deficiency improves cognitive abilities in a murine model of cerebral hypoperfusion. Exp. Neurol. 286, 93–106 [DOI] [PubMed] [Google Scholar]
- 52.Maurice T., Mustafa M. H., Desrumaux C., Keller E., Naert G., de la C García-Barceló M., Rodríguez Cruz Y., Garcia Rodríguez J. C. (2013) Intranasal formulation of erythropoietin (EPO) showed potent protective activity against amyloid toxicity in the Aβ25–35 non-transgenic mouse model of Alzheimer’s disease. J. Psychopharmacol. 27, 1044–1057 [DOI] [PubMed] [Google Scholar]
- 53.Religa P., Cao R., Religa D., Xue Y., Bogdanovic N., Westaway D., Marti H. H., Winblad B., Cao Y. (2013) VEGF significantly restores impaired memory behavior in Alzheimer’s mice by improvement of vascular survival. Sci. Rep. 3, 2053 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.