Abstract
Diabetes mellitus (DM) affects bone metabolism and leads to osteoporosis; however, its pathogenetic mechanisms remain unknown. We found that high glucose (HG) conditions induced the production of reactive oxygen species (ROS) and the expression of proteins related to MAPKs [phosphorylated (p)-ERK, p-JNK, and p-p38], NF-κB (NF-κB, p-IκB, and IKK), and NACHT-LRR-PYD domains–containing protein 3 (NALP3) (NLRP3) [apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC), caspase-1, IL-18, IL-1β, and NLRP3] in osteoclasts (OCs) in vitro. Further analysis showed that in HG-induced OCs, ROS is an upstream signal for MAPKs, NF-κB, and the NLRP3 inflammasome. Moreover, MAPKs mediated the activation of NF-κB and NLRP3, whereas NF-κB up-regulated the NLRP3 inflammasome response. Interestingly, HG inducement enhanced the bone resorption of OCs but inhibited their efferocytosis, whereas insulin and lipoxin A4 (4) treatment reversed this phenomenon. In streptozotocin-induced diabetic rats in vivo, the numbers and the bone-resorption capacity of OCs as well as the serum levels of TRACP-5b were significantly increased, and the expression of MAPK-, NF-κB-, and NLRP3 inflammasome–related proteins in the proximal tibia were also significantly elevated; however, treatment with insulin and LXA4 reversed this elevation. Together, these results demonstrated that the activation of ROS/MAPKs/NF-κB/NLRP3 and the inhibition of efferocytosis in OCs are the main causes of osteoporosis in DM.—An, Y., Zhang, H., Wang, C., Jiao, F., Xu, H., Wang, X., Luan, W., Ma, F., Ni, L., Tang, X., Liu, M., Guo, W., Yu, L. Activation of ROS/MAPKs/NF-κB/NLRP3 and inhibition of efferocytosis in osteoclast-mediated diabetic osteoporosis.
Keywords: diabetes mellitus osteoporosis, reactive oxygen species, osteoclasts, infalmmasome
Diabetes mellitus (DM) is a chronic and stubborn epidemic that may cause many complications; among them, osteoporosis is widely recognized as an emerging public health problem (1, 2). Diabetic osteoporosis is a systemic, metabolic bone disease caused by dysfunctions in bone remodeling that cause low bone mass, microstructural changes in the bone tissue, decreased bone strength, and increased bone fragility, leading to a high risk of fractures (3). According to previous reports, diabetic osteoporosis is related to hyperglycemia (4), calcium and phosphorus metabolic disorders (5), and the lack of insulin (6); however, its pathogenetic mechanisms remain to be further studied.
Until now, several factors have been suggested to be implicated in diabetic osteoporosis. Reactive oxygen species (ROS) are a natural byproduct of the normal metabolism of oxygen, and when their concentrations are beyond a certain range, they can disturb the oxidant-antioxidant balance. This disturbance can lead to many inflammatory diseases, such as diabetic osteoporosis (7). ROSs damage cells through a variety of mechanisms, such as protein inactivation and peroxidation of lipid membranes, which lead to DNA damage and the stimulation of some signaling pathways that lead to tissue damage (8). ROS can induce periodontal tissue destruction, which is associated with osteoclastic bone resorption (7). The NACHT-LRR-PYD domains–containing protein 3 (NALP3) inflammasome is a protein complex, including apoptosis-associated speck-like protein containing a caspase activation and recruitment domain (ASC) and caspase-1, and it is important for the maturation of IL-1β and IL-18 (9). It is implicated not only in inflammatory disorders but also in numerous metabolic diseases. The NLRP3 inflammasome can be activated by various host danger-associated molecular patterns, including glucose, in DM (10), and a recent study reported that the NLRP3 inflammasome plays an important role in bone resorption (11, 12). NF-κB is a transcription factor that regulates the expression of multiple genes (13) and governs various cellular functions, including inflammatory signaling (14). In addition, NF-κB has been proposed to be the sensor for oxidative stress that can be activated by ROS (15). MAPKs can control various cellular activities, including gene expression, mitosis, differentiation, and cell survival and apoptosis (16). In particular, MAPKs (ERK, p38, and JNK) have important roles in regulating inflammatory and immune responses (17).
Tissue-resident macrophages are key immune cells that respond to tissue damage by producing cytokines, which play an important role in the recruitment of granulocytes from the blood into the tissue (18). According to the degree of the injury, neutrophils accumulate in the tissue and rapidly undergo apoptosis (19). The process of clearing dead cells (or apoptotic cells) by macrophages is called efferocytosis. Apoptotic neutrophils can inhibit macrophage inflammatory responses in vitro, whereas the failure to clear apoptotic cells exacerbates inflammation in vivo (20, 21), indicating that efferocytosis plays a crucial role in modulating the inflammatory response in macrophages (and other phagocytes) to promote inflammation resolution. An osteoclast (OC) is a tissue-specific polykaryon macrophage that adheres to or near the bone surface and is produced by the differentiation of a monocyte or macrophage precursor cell (22). OCs play an important role in bone remodeling to maintain bone health. Bone mineral density (BMD) loss and osteoporosis are caused by exceeding osteoclastic bone resorption without a parallel quantity of bone formation by osteoblasts (23). At present, the drug treatments for osteoporosis, including estrogens, bisphosphonates, and selective estrogen-receptor modulators, target OC-mediated bone resorption, chiefly retarding BMD loss and thereby decreasing the risk of bone fractures (22, 24).
A recent report suggested that the NLRP3 inflammasome response could cause OC maturation (25). However, to our knowledge, there has been no systemic study on the activation of ROS, MAPKs, NF-κB, and NLRP3 in OCs involved in diabetic osteoporosis. In this study, we address the relationships between diabetic osteoporosis, the inflammatory activation of OCs, and the inhibition of efferocytosis.
MATERIALS AND METHODS
Antibodies and chemicals
The primary antibodies used for Western blot and immunofluorescence (anti–phosphorylated (p)-ERK antibody, anti–p-JNK antibody, anti–p-p38 antibody, anti–NF-κB antibody, anti-IKK antibody, anti–p-IκB antibody, anti-NLRP3 antibody, anti-ASC antibody, anti–caspase-1 antibody, anti–IL-18 antibody, anti–IL-1β antibody, anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody, anti–Histone H3 antibody, and anti-MertK antibody) were all purchased from Cell Signaling Technology (Danvers, MA, USA). Both the horseradish peroxidase–labeled goat anti-rabbit and anti-mouse secondary antibodies were obtained from Beyotime Biotechnology (Haimen, China). The immunofluorescence secondary antibodies were obtained from BioLegend (San Diego, CA, USA). pHrodo Red succinimidyl ester (pHrodo) was purchased from Thermo Fisher Scientific (Waltham, MA, USA). Other chemicals were purchased from Beijing Dingguo Changsheng Biotechnology (Beijing, China). Glucose (purity >99.5%) and streptozotocin (STZ; purity >98%) were purchased from MilliporeSigma (Burlington, MA, USA). Proresolving mediator lipoxin A4 (LXA4) (95% purity) was purchased from Cayman Chemicals (Ann Arbor, MI, USA).
In vitro osteoclastogenesis using purified bone marrow–derived monocytes
Bone marrow cultures were prepared using previously described methods with modifications (26). In brief, whole bone marrow cells were flushed from the long bones of 8-wk-old male Sprague-Dawley rats and plated in 100-mm tissue culture plates in α-minimum essential medium (MEM) containing 10% fetal bovine serum (FBS). Cells were incubated at 37°C with 5% CO2 overnight. Nonadherent cells were harvested and incubated in α-MEM containing 10% FBS plus 25 ng/ml recombinant rat M-CSF (PeproTech, Rocky Hill, NJ, USA) at 37°C with 5% CO2 for 48 h to purify the bone marrow–derived monocytes (BMMs). Then, isolated BMMs were cultured in α-MEM containing 10% FBS plus 25 ng/ml recombinant rat M-CSF (PeproTech) and 50 ng/ml recombinant rat TNF-related activation-induced cytokines (TRANCE)-tumor necrosis factor ligand superfamily member 11 (TNFSF11)-receptor for activation of NF-κB ligand (RANKL) (R&D Systems, Minneapolis, MN, USA). Rat OC (multinucleated, large, spread cells) formation was observed on d 7 of culture. To identify the OCs, cells were fixed and stained for tartrate-resistant acid phosphatase (TRAP) using a Leukocyte Acid Phosphatase Kit (MilliporeSigma). All experiments were performed in BMM-differentiated OCs.
Bone resorption assay in vitro
BMMs were plated in 6-well flat-bottom plates (Wuxi Nest Biotechnology, Jiangsu, China) and treated with 25 ng/ml M-CSF and 50 ng/ml RANKL for 7 d, and part of the cells were fixed and stained for TRAP using a Leukocyte Acid Phosphatase Kit to identify the OCs. The other cells were digested with trypsin and completely removed from the 6-well flat-bottom plates to make a cell suspension, and then added to a 24-well plate (Wuxi Nest Biotechnology), which had bone slices in the wells. Then, the cells were pretreated with 3 inhibitors, insulin (1 U), the caspase-1 inhibitor Ac-YVAD-cmk (40 μM), and LXA4 (50 ng) for 1 h, and then treated with 35 mM glucose (MilliporeSigma) for 36 h. Then, scanning electron microscopy (SEM) (Jeol, Akishima, Japan) was used to analyze the number of resorption pits.
Detection of cytosolic ROS in vitro
For cytosolic ROS detection, dihydrorhodamine (DHR) 123 (MilliporeSigma), which is a fluorescent indicator of cytosolic ROS, was used in this study. Cells were stimulated with high glucose (HG) and then incubated with DHR 123 for 20 min without light. The results were obtained with a spectrofluorophotometer.
Western blot
Cells treated with HG were harvested in cold PBS and centrifuged at 3000 g for 5 min at 4°C. The collected cells were incubated on ice with RIPA lysis buffer (MilliporeSigma) containing 1 mM PMSF (MilliporeSigma) for 10 min. The supernatant of the lysate was obtained by centrifugation at 12,000 g for 10 min at 4°C. A BCA Protein Assay Kit (Beyotime) was used to quantify the concentration of protein. Equal amounts of protein were separated on a 15% polyacrylamide gel and transferred onto PVDF membranes (Beyotime). The membranes were blocked for 2 h in blocking buffer [5% nonfat milk, 0.1% Tween 20, and Tris-buffered saline (TBS)] and incubated with the appropriate primary antibody at 4°C overnight followed by incubation with the corresponding peroxidase-conjugated secondary antibody for 2 h. The corresponding bands were detected using an ECL Detection Kit (Beyotime). The images were collected by a CanoScan LiDE 100 Scanner (Canon, Tokyo, Japan). Protein blots were measured with ImageJ software (National Institutes of, Bethesda, MD, USA). Then, all experimental conditions and NF-κB activation were evaluated by Western blot assay of isolated nuclear fractions.
OC efferocytosis assay in vitro
To exclude the presence of attached but not engulfed apoptotic cells during the determination of the net phagocytosis rate, apoptotic neutrophils were labeled with pHrodo (Thermo Fisher Scientific) for the engulfment assay (27). Briefly, apoptotic neutrophils were washed twice with PBS and resuspended in PBS at a concentration of 106 cells/ml followed by incubation at room temperature for 30 min with 0.1 µg/ml pHrodo. Then, the cells were washed twice in PBS and used for engulfment. pHrodo-labeled apoptotic cells were gated according to their forward and side scatter properties and then used to make a cutoff point for fluorescence emission. After coculture of the apoptotic cells, which were induced with HG, with OCs (10:1) for 36 h, the culture was washed with PBS and the adherent cells were collected for assessment. The samples were analyzed with a Fluorescence-Activated Cell Sorting (FACS) Calibur Flow Cytometer (BD Biosciences, San Jose, CA, USA). The acquired data were analyzed with FlowJo software (Treestar, Ashland, OR, USA).
Animals and the induction of the male diabetic rat model by STZ
Eight-week-old male rats of the Sprague-Dawley strain were unfed overnight, and 24 rats were treated with STZ (60 mg/kg body weight in 0.05 M citrate buffer; pH 4.5; intraperitoneally). Eight control animals received the same volume of the STZ diluent. The diagnosis of diabetes was based on glycosuria and hyperglycemia [blood glucose (starving) >11.1 mM]. All rats subjected to the STZ injection were rendered diabetic, and no animals died during the experimental period. Four days after the injection of STZ, 8 diabetic rats were intraperitoneally injected with insulin or LXA4. The rats had free access to both food and water, which were provided fresh daily. At 21 d after the injection of STZ or the diluent, the blood and femoral and tibial bones were collected under anesthesia after no overnight access to feed (starving). Urine was collected to determine the level of deoxypyridinoline. The sera were used to determine the concentrations of glucose, insulin, Ca, P, tartrate-resistant acid phosphatase 5b (TRACP-5b), cathepsin K, and osteocalcin. After the removal of the muscle and tendons, the femoral bone was used for biochemical analysis and the histologic analysis.
BMD analysis
Measurements of BMD were assessed using dual-energy X-ray absorptiometry (DXA; Hologic, Marlborough, MD, USA). All scans were centrally reviewed and analyzed (Synarc, San Francisco, CA, USA). Whole-body BMDs and bone minerals were measured in diabetic rats and insulin- or LXA4-treated diabetic rats.
Histomorphometric analysis
The proximal tibia was fixed in 4% paraformaldehyde, decalcified in 10% EDTA, and embedded in paraffin. Sections (4 μm) were stained with hematoxylin and eosin (H&E) and then stained for TRAP activity using a Leukocyte Acid Phosphatase Kit (387-A) to visualize the OCs per field. Morphometric measurements of trabecular structure (trabecular bone volume, bone surface, and number) in the H&E-stained sections were measured in the proximal tibia at ×200 magnification/field, and 15–20 fields were counted. The area was measured using ImageJ software (28), and 3 independent observers performed a quantitative evaluation of the same section.
Fluorescence microscopy
The proximal tibia was fixed in 4% paraformaldehyde, decalcified in 10% EDTA, and embedded in paraffin. Sections (4 μm) were used for fluorescence microscopy. Sections were dewaxed in water in order to then perform antigen retrieval after washing 3 times, and blocking was performed with 5% bovine serum albumin for 30 min at room temperature. After washing 3 times, the slides were incubated with a primary antibody at 4°C overnight. After washing 3 times, the sections were incubated with the corresponding secondary antibody at room temperature for 1 h. To label the DNA, the cells were incubated with Hoechst 33342 (MilliporeSigma) (1 μM) for 5 min. Images were collected using a laser-scanning confocal microscope (FluoView FV1000; Olympus, Tokyo, Japan).
Ethics statement
The rats were housed in microisolator cages and received food and water freely. The laboratory temperature was 24 ± 1°C and the relative humidity was 40–80%. The protocol used in this study was approved by the Committee on the Ethics of Animal Care and Use of the National Research Center for Veterinary Medicine (Permit 20160313088). The study was conducted following the Guide for the Care and Use of Animals in Research (People’s Republic of China). All animal studies were performed under isoflurane anesthesia and every effort was made to minimize suffering.
Statistical analysis
Comparisons of mean values from 3 experiments were statistically evaluated by 1-way ANOVA. Differences with 2-tailed values of P < 0.05 were considered statistically significant. All statistical analyses were performed using SPSS software (v.11.5; IBM, Chicago, IL, USA).
RESULTS
HG induced the NOX2-dependent production of cytosolic ROS and the expression of MAPK-, NF-κB–, and NLRP3 inflammasome–related proteins in vitro
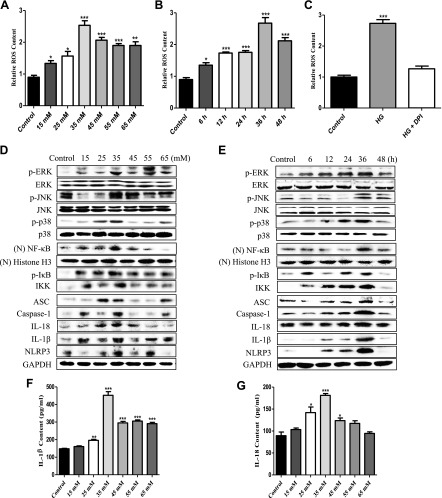
To investigate whether HG induced ROS, we used a fluorescent indicator of cytosolic ROS (DHR 123) (29) in a spectrofluorophotometer assay to detect cytosolic ROS production in HG-induced OCs. OCs were incubated in 24-well flat-bottom plates and treated with HG at concentrations of 15–65 mM for 5 time points (6–48 h). Our results showed that 35-mM HG treatment triggered a large increase in cytosolic ROS production at the 36-h time point (Fig. 1A, B), whereas diphenyleneiodonium (DPI) treatment significantly inhibited the enhancement (Fig. 1C). These results demonstrated that HG could induce the production of ROS and the production was NADPH oxidase 2 (NOX2)-dependent.
Figure 1.
HG induced the production of NOX2-dependent cytosolic ROS and the expression of MAPK-, NF-κB–, and NLRP3 inflammasome–related proteins in vitro. A, B) For the quantification of cytosolic ROS, OCs were incubated in 24-well plates (2 × 105 cells/well) and then treated with HG at concentrations from 15 to 65 mM (A) for 5 time intervals (6–48 h) (B). C) OCs were pretreated with DPI (50 µM) for 1 h and then treated with 35 mM HG for 36 h. Cytosolic ROSs were labeled by DHR 123 (1 µM) and detected by a spectrofluorophotometer. The data were analyzed with SPSS software. ***P < 0.001 compared with the control groups in the same cell line. The data are representative of 3 experiments with similar results. D, E) Activation (phosphorylation) of MAPK- (p-ERK, p-JNK, and p-p38), (N) NF-κB–, p-IκB–, IKK–, and NLRP3 inflammasome (ASC, caspase-1, IL-18, IL-1β, and NLRP3)–related proteins, in OCs treated with different doses (15–65 mM) (D) of glucose for different times (6–48 h) (E). Western blot was used to detect the expression of p-ERK, p-JNK, p-p38, NF-κB, p-IκB, IKK, ASC, caspase-1, IL-18, IL-1β, and NLRP3. GAPDH and Histone H3 were used as an internal reference to ensure equal loading. (N) NF-κB, nuclear NF-κB; (N) Histone H3, nuclear Histone H3. F, G) Quantitative detection of IL-1β (F) and IL-18 (G) secretion was performed by ELISA. ***P < 0.001 compared with the control groups in the same cell line. The data are representative of 3 experiments with similar results.
To further clarify the pathways of HG-induced inflammation in OCs, Western blot was used to assay the expression levels of proteins involved in MAPK signaling (p-ERK, p-JNK, and p-p38), NF-κB signaling (NF-κB, p-IκB, and IKK) and the NLRP3 inflammasome (ASC, caspase-1, IL-18, IL-1β, and NLRP3) in HG-induced OCs. OCs were incubated in 6-well flat-bottom plates and treated with HG at concentrations of 15–65 mM for 5 time points (6–48 h). The results showed that the expression of these proteins reached its peak when cells were induced with 35 mM HG (Fig. 1D), and at the time point of 36 h, the expression of these proteins was significantly increased in 35 mM HG-induced OCs compared with untreated OCs (Fig. 1E). In addition, the secretion of IL-1β and IL-18 was significantly increased by 35-mM HG treatment for 36 h (Fig. 1F, G).
In order to detect the effect of early time points on the expression levels of proteins involved in MAPKs, nuclear NF-κB, p-IκB, IKK, and NLRP3 related, OCs were incubated in 6-well flat-bottom plates and treated with HG for 5 time points (0–3 h); the early time points did not induce the expression of MAPKs, nuclear NF-κB, p-IκB, IKK, and NLRP3-related proteins (Supplemental Fig. S1A, B)
Together, these data confirmed that HG could induce the activation of MAPKs, NF-κB, and the NLRP3 inflammasome in OCs in vitro.
NLRP3 inflammasome response induced by HG was ROS/MAPKs/NF-κB–dependent in vitro
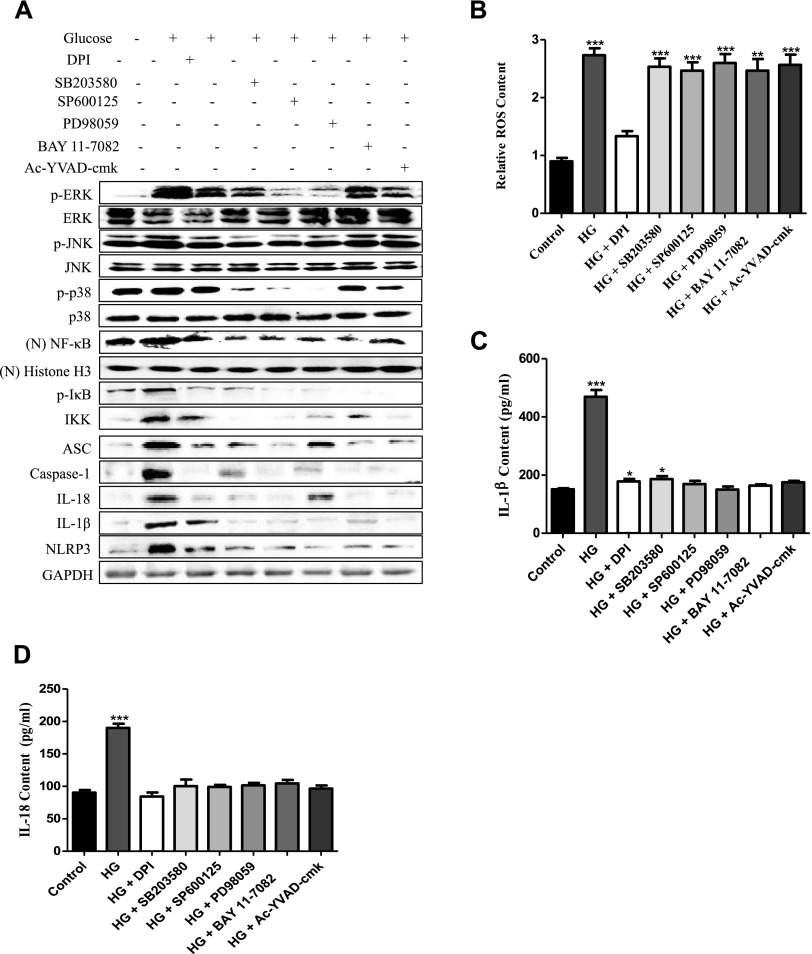
To analyze the possible mechanism of the inflammatory response induced by HG in vitro, we used specific inhibitors to corroborate the correlations between ROS, MAPKs, nuclear NF-κB, and the NLRP3 inflammasome in HG-induced OCs. Our Western blot analysis showed that the ROS inhibitor DPI markedly abolished the expression of MAPK- (p-ERK, p-JNK, and p-p38), nuclear NF-κB–, p-IκB–, IKK-, and NLRP3 inflammasome (ASC, caspase-1, IL-18, IL-1β, and NLRP3)–related proteins (Fig. 2A), and the inhibitors of ERK (PD98059), JNK (SP600125), p38 (SB203580), NF-κB (BAY 11-7082), and caspase-1 (Ac-YVAD-cmk) had no effect on the production of ROS (Fig. 2B). These data indicated that ROS was upstream of MAPKs/NF-κB/NLRP3 and regulated their expression.
Figure 2.
The NLRP3 inflammasome response induced by HG was ROS/MAPKs/NF-κB–dependent in vitro. OCs were pretreated with DPI (50 µM), PD98059 (40 μM), SP600125 (10 μM), SB203580 (10 μM), BAY 11-7082 (10 μM), or Ac-YVAD-cmk (40 μM) for 1 h and then treated with 35 mM HG for 36 h. A) Western blot was used to detect the expression of p-ERK, p-JNK, p-p38, (N) NF-κB, p-IκB, IKK, ASC, caspase-1, IL-18, IL-1β, and NLRP3. GAPDH and Histone H3 were used as an internal reference to ensure equal loading. (N) NF-κB, nuclear NF-κB; (N) Histone H3 nuclear Histone H3 B) Cytosolic ROS were labeled by DHR 123 (1 µM) and detected by a spectrofluorophotometer. The data were analyzed with SPSS software. ***P < 0.001 compared with the control groups in the same cell line. The data are representative of 3 experiments with similar results. C, D) Quantitative detection of IL-1β (C) and IL-18 (D) secretion was performed by ELISA. ***P < 0.001 compared with the control groups in the same cell line. The data are representative of 3 experiments with similar results.
Furthermore, to explore the order of precedence of MAPKs, nuclear NF-κB, and NLRP3 in the inflammatory response induced by HG, specific inhibitors of p38 (SB203580), ERK (PD98059), and JNK (SP600125) were used to pretreat OCs before the induction with HG. Among them, SP600125, PD98059, and SB203580 significantly inhibited the production of nuclear NF-κB, p-IκB, IKK, and NLRP3 components (ASC, caspase-1, IL-18, IL-1β, and NLRP3) (Fig. 2A) and the secretion of IL-1β and IL-18 (Fig. 2C, D). These results showed that p-JNK, p-ERK1/2, and p-p38 mediated the activation of nuclear NF-κB–related proteins and the NLRP3 inflammasome by HG in OCs.
Additionally, we used the NF-κB inhibitor BAY 11-7082 and the caspase-1 selective inhibitor Ac-YVAD-cmk to explore the relationship between MAPKs, NF-κB, and the NLRP3 inflammasome. Our Western blot results showed that the inhibition of NF-κB–related proteins by BAY 11-7082 abrogated the NLRP3 inflammasome response, but there was no effect on the expression of p-ERK, p-JNK, and p-p38. In particular, Ac-YVAD-cmk did not affect the HG-induced expression of MAPKs, NF-κB–signaling components (Fig. 2B), or cytosolic ROS production (Fig. 2A). These results indicated that MAPKs mediated the activation of NF-κB and NLRP3, whereas the NF-κB–signaling pathway up-regulated the NLRP3 inflammasome response. Together, these data demonstrated that the ROS/MAPKs/NF-κB pathway regulated the HG-induced NLRP3 inflammasome response in vitro.
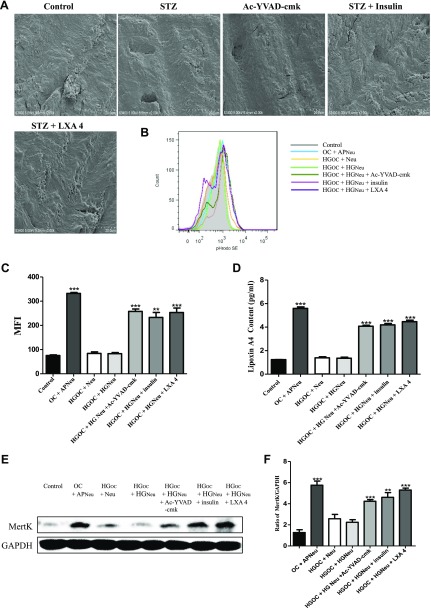
HG inducement increased the bone resorption capacity of OCs in vitro
To verify whether HG can induce bone resorption by OCs, we seeded OCs on slices of bovine bone and treated them with 35 mM glucose for 36 h with or without pretreatment with the caspase-1 inhibitor Ac-YVAD-cmk, insulin, or LXA4. Then, SEM was used to analyze the number of resorption pits. Our results showed that HG-treated cells produced larger, deeper, and more plentiful bone resorption pits than control cells. Interestingly, the presence of Ac-YVAD-cmk, insulin and LXA4 each led to shallower and smaller resorption pits, and the number of pits was also significantly decreased (Fig. 3A).
Figure 3.
HG conditions induced increased bone resorption capacity and inhibited the occurrence of efferocytosis in OCs. A) OCs were seeded on slices of bovine bone and induced with 35 mm glucose for 36 h with or without Ac-YVAD-cmk, insulin, and LXA4 pretreatment. SEM was then used to analyze the numbers of resorption pits. B, C) OCs were cocultured with apoptotic or nonapoptotic neutrophils labeled with pHrodo dye for 30 min. The fluorescence intensity of pHrodo in OCs cocultured with apoptotic neutrophils was detected by flow cytometry. The results are presented as the mean fluorescence intensities (MFIs). ***P < 0.001 compared with the control groups of OCs cocultured with neutrophils (nonapoptotic). The data are representative of 3 experiments with similar results. The histogram figure represents the comparison of the 35-mm HG–induced group and the control group. D) Quantitative detection of cell supernatant LXA4 secretion was performed by ELISA. ***P < 0.001 compared with the control groups. E) Western blot was conducted to assess the level of MertK. F) The ratio of MertK to GAPDH was calculated. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control groups in the same cell line. The data are representative of 3 experiments with similar results. Control, untreated OCs; OC + APNeu, OCs and apoptotic neutrophil coincubation; HGOC + Neu, HG-treated OCs incubated with normal neutrophils; HGOC + HGNeu, OCs and neutrophils treated with HG and then coincubated; HGOC + HGNeu + Ac-YVAD-cmk, OCs and neutrophils treated with HG and then coincubated with Ac-YVAD-cmk; HGOC + HGNeu + insulin, OCs and neutrophils treated with HG and then coincubated with insulin; HGOC + HGNeu + LXA4, OCs and neutrophils treated with HG and then coincubated with LXA4.
HG induces inflammation by inhibiting the occurrence of efferocytosis in vitro
Efferocytosis is the process of phagocytizing apoptotic cells and releasing anti-inflammatory factors to reduce the occurrence of phagocytic inflammation (30, 31). We cocultured OCs with neutrophils and then induced them with 35 mM glucose for 36 h with or without Ac-YVAD-cmk, insulin, or LXA4 pretreatment. The FACS results showed that, compared with the control treatment, HG can induce neutrophil apoptosis and inhibit the occurrence of OC efferocytosis (Fig. 3B, C). However, insulin, the caspase-1 inhibitor Ac-YVAD-cmk, and the proresolving mediator LXA4 (an efferocytosis enhancer) reversed the above phenomenon and enhanced the occurrence of efferocytosis (Fig. 3B, C). An ELISA showed that the concentration of LXA4 was significantly increased in the supernatant of the OC-neutrophil coculture when OC efferocytosis occurred, but the increase was inhibited by HG. In particular, insulin and Ac-YVAD-cmk pretreatment could increase the secretion of LXA4 that was inhibited by HG (Fig. 3D). In addition, our Western blot showed that HG inhibited the expression of MertK, a major macrophage apoptotic cell receptor, and this phenomenon was alleviated by Ac-YVAD-cmk, insulin, and LXA4 pretreatment (Fig. 3E, F). Together, these results suggest that HG induces inflammation by inhibiting the occurrence of efferocytosis.
Insulin and LXA4 treatment alleviated characteristic signs in STZ-induced male diabetic rats in vivo
Based on the above results in vitro, we further studied the related pathogenetic mechanisms of osteoporosis in an STZ-induced male diabetic rat model in vivo. The injection of STZ increased the serum glucose level to >11.1 mM and decreased the serum insulin level in diabetic rats (Table 1), and the body weight (final) was significantly lower in the diabetic rats than in the normal rats. In addition, the weights of the femur and tibia were significantly decreased in the diabetic rats, although no significant differences were observed in the lengths of the femur or tibia. Notably, treatment with insulin and LXA4 significantly restored the serum levels of glucose, insulin, body weight, and the weights of the femur and tibia in the diabetic rats. However, there were no significant differences in the levels of serum Ca and P in the diabetic rats compared with the normal rats (Table 1).
TABLE 1.
Effects of diabetes on water intake, food intake, and serum levels of glucose, insulin, Ca, P, body weight, length, and weight of femur tibia and proximal tibia
| Variable | Control | Diabetes | Insulin | LXA4 |
|---|---|---|---|---|
| Water intake (ml/d) | 80 ± 10 | 120 ± 10a | 100 ± 10a,b | 95 ± 10a,b |
| Food intake (g/d) | 23 ± 1.5 | 31 ± 2.3a | 27 ± 2.1a,b | 28 ± 3.8a,b |
| Serum | ||||
| Glucose (mM) | 4.5 ± 0.5 | 21.3 ± 4.54a | 7.2 ± 3.5a,b | 6.9 ± 4.2a,b |
| Insulin (ng/ml) | 4.65 ± 0.58 | 0.11 ± 0.04a | 2.83 ± 0.63a,b | 2.63 ± 0.93a,b |
| Ca (mg/dl) | 9.87 ± 0.64 | 9.93 ± 0.39 | 9.71 ± 0.71 | 9.64 ± 0.85 |
| P (mg/dl) | 5.90 ± 0.78 | 6.71 ± 0.18 | 6.18 ± 0.52 | 6.53 ± 0.32 |
| Body weight (g) | ||||
| Before fasting | 210 ± 3.4 | 207 ± 5.3 | 209 ± 4.5 | 205 ± 7.8 |
| Final | 300 ± 7 | 180 ± 13a | 220 ± 16a,b | 226 ± 15a,b |
| Bone length (mm) | ||||
| Femur | 32.8 ± 0.4 | 32.5 ± 0.6 | 32.3 ± 0.8 | 32.1 ± 0.6 |
| Tibia | 36.7 ± 0.23 | 36.2 ± 0.2 | 36.1 ± 0.3 | 36.0 ± 0.4 |
| Bone weight (g) | ||||
| Femur | 0.687 ± 0.018 | 0.625 ± 0.018a | 0.674 ± 0.006b | 0.669 ± 0.008b |
| Tibia | 0.543 ± 0.013 | 0.469 ± 0.020a | 0.521 ± 0.005b | 0.512 ± 0.004b |
| Proximal tibia | 0.252 ± 0.010 | 0.202 ± 0.003a | 0.238 ± 0.011b | 0.242 ± 0.008b |
STZ (45 mg/kg body weight in 0.05 M citrate buffer) (diabetes) or the buffer (control) was intraperitoneally injected into rats unfed overnight. Moreover, 3 wk after the STZ injection, water intake and food intake were measured and blood (starving) and femoral and tibial bone were collected. ELISA was used to determine the levels of glucose, insulin, Ca, and P. The length, weight of the femur, tibia, and the weight of the proximal tibia were measured. Values are the means ± sem for 8 rats.
Significantly different from control value (P < 0.05).
Significantly different from diabetes (P < 0.05).
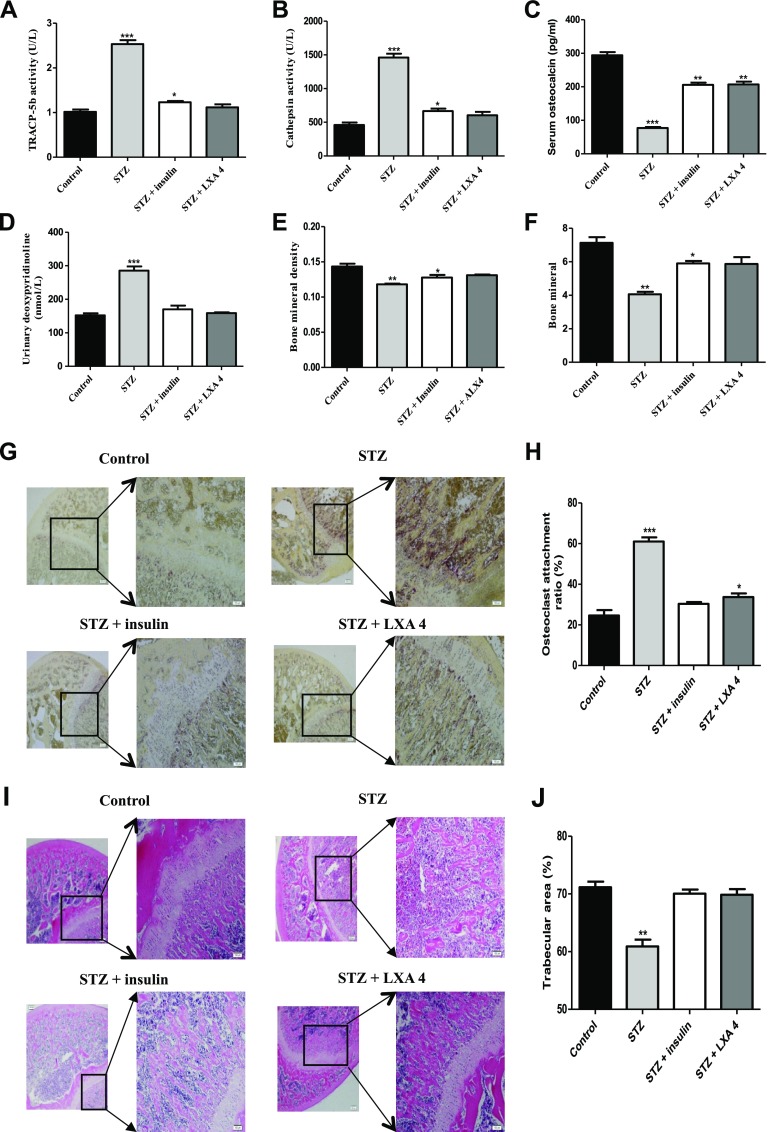
Insulin and LXA4 treatment improved the indicators of osteoporosis in STZ-induced diabetic rats in vivo
Next, we assayed the changes in various indicators of osteoporosis in STZ-induced male diabetic rats compared with normal rats. First, we determined the serum concentrations of bone resorption markers, including TRACP-5b, cathepsin K (a marker of OCs), osteocalcin, and urinary deoxypyridinoline. In the STZ-induced diabetic rats compared with the normal rats, the levels of osteocalcin in the serum were significantly decreased; on the contrary, the activity levels of TRACP-5b, cathepsin K, and urinary deoxypyridinoline were significantly increased (Fig. 4A–D). These changed values recovered to normal-rat levels after treatment with insulin and LXA4. Furthermore, DXA was used to assay the changes in BMD and bone mineral content. The results showed that in diabetic rats compared with normal rats, the BMD and bone mineral content were significantly decreased, but insulin or LXA4 treatment suppressed this decrease (Fig. 4E, F). Moreover, histochemical staining of the proximal tibia for TRAP, a marker of OCs, showed that diabetic rats had more TRAP-positive cells in the metaphysic of the proximal tibia than normal rats (Fig. 4G, H). Further H&E staining showed that the trabecular bone thickness and number of OCs were significantly decreased in the diabetic rats compared with the normal rats (Fig. 4I, J), and treatment with insulin and LXA4 restored the trabecular bone index and the number of OCs in diabetic rats to the control level.
Figure 4.
Insulin and LXA4 treatment improved the indicators of osteoporosis in STZ-induced diabetic rats in vivo. A–D) The levels of TRAP (A), cathepsin K (B), and osteocalcin in the serum (C), and deoxypyridinoline in the urine (D) were detected by ELISA. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control groups. The data are representative of 8 rat experiments with similar results. E, F) DXA was used to assay the BMD (E) and bone mineral content (F). *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control groups. G) Paraformaldehyde-fixed, decalcified, and paraffin-embedded proximal tibias obtained from the control, diabetic, insulin-, or LXA4-supplemented diabetic rats were processed for TRAP staining. TRAP-positive cells appear red (original magnification, ×20). H) The percentage of OC attachment ratio was calculated. *P < 0. 05, ***P < 0.001 compared with the control groups. I) The bone tissue was stained with H&E (original magnification, ×20). J) The percentage of trabecular area was calculated by ImageJ. **P < 0.01 compared with the control groups. The data are representative of 8 rat experiments with similar results.
The expression of proteins involved in MAPK signaling, NF-κB signaling, and the NLRP3 inflammasome in OC-mediated osteoporosis in STZ-induced diabetic rats in vivo
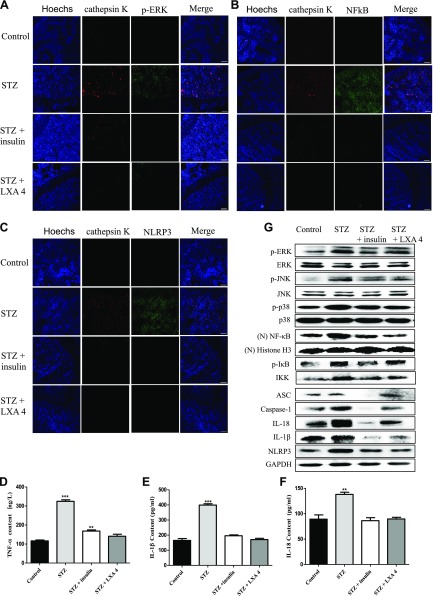
Additionally, we verified whether there was consistency in the expression of proteins involved in MAPK signaling, NF-κB signaling, the NLRP3 inflammasome and the inhibition of efferocytosis in OC-mediated osteoporosis between HG treatment in vitro and STZ-induced male diabetic rats in vivo. First, immunofluorescence was used to analyze the proximal tibia for cathepsin K (anti–cathepsin K antibody, a marker of OCs) colocalization with p-ERK (anti–p-ERK antibody), NF-κB (anti–NF-κB antibody), and NLRP3 (anti-NLRP3 antibody). Our results showed that the number of OCs was significantly increased in the diabetic rats, and the protein levels of p-ERK (Fig. 5A), NF-κB (Fig. 5B), and NLRP3 (Fig. 5C) colocalized with cathepsin K were increased. However, treatment with insulin and the proresolving mediator LXA4 (an efferocytosis enhancer) reversed this phenomenon.
Figure 5.
The expression of proteins involved in MAPK signaling, NF-κB signaling, and the NLRP3 inflammasome in OC-mediated osteoporosis was assessed in STZ-induced diabetic rats in vivo. A–C) Immunofluorescence was used to analyze the proximal tibia for cathepsin K colocalization with p-ERK (A), NF-κB (B), and NLRP3 (C). The images were obtained by laser-scanning confocal microscopy with an original magnification, ×20 objective lens. Scale bars, 50 μm. D–F) The levels of TNF-α (D), IL-1β (E), and IL-18 (F) in rat serum were detected by ELISA. *P < 0.05, **P < 0.01, ***P < 0.001 compared with the control groups. The data are representative of 8 rat experiments with similar results. G) MAPK/NF-κB/NLRP3 inflammasome–related protein levels were assessed in the proximal tibia. Total protein was extracted from the proximal tibia of rats 3 wk after the STZ injection. The protein levels of p-ERK, p-JNK, nuclear NF-κB, p-IκB, IKK, ASC, caspase-1, IL-18, IL-1β, and NLRP3 were determined by Western blot and are expressed relative to the internal standard, GAPDH, and Histone H3 The data are representative of 8 rat experiments with similar results.
Furthermore, we tested the levels of TNF-α, IL-1β, and IL-18 in serum from STZ-induced male diabetic rats and control rats. Our results showed that significant increases in the TNF-α, IL-1β, and IL-18 levels were observed in the diabetic rats, and the increase was inhibited by insulin and LXA4 (Fig. 5D–F) treatment. Using Western blot, we also investigated the levels of p-ERK1/2, p-JNK, p-p38, NF-κB, and NLRP3 in the proximal tibia of STZ-induced diabetic rats and normal rats. The results showed that the levels of p-PEK1/2, p-JNK, p-p38, NF-κB, IKK, p-IκB, ASC, caspase-1, IL-1β, IL-18, and NLRP3 in the proximal tibia of diabetic rats were significantly increased; in contrast, insulin and LXA4 (an efferocytosis enhancer) significantly inhibited the expression of these proteins (Fig. 5G). Thus, all of these results demonstrated that, consistent with the earlier in vitro results, HG could induce the occurrence of OC inflammation mediated by MAPKs/NF-κB/NLRP3 inflammasome and the inhibition of efferocytosis in OCs in vivo, which may be the main causes of diabetic osteoporosis.
DISCUSSION
The coexistence of DM and osteoporosis, including bone loss and bone mineral metabolism, has been established by many researchers, both in diabetic patients and in experimental animal models (32). Diabetic osteopenia exerts seriously unfavorable effects on the health and quality of life of diabetic patients; it may cause an increased risk of bone fractures and a delay in the healing of bone fractures (33). Bone resorption is mediated by OCs, which are derived from hematopoietic cells, specifically the monocyte-macrophage lineage originating in the bone marrow (34). The number and resorptive efficacy of mature OCs is determined by the proliferation and differentiation of OC precursors (35). M-CSF and RANKL are necessary for osteoclastic differentiation, which eventually leads to the expression of TRAP, multinucleation, and bone-resorbing activity (22, 36). In our study, we used M-CSF and RANKL to induce bone marrow–derived macrophages to become TRAP-expressing, multinucleated mature OCs with good bone-resorption activity. Interestingly, HG induced enhanced bone resorption by OCs (Fig. 3A).
At present, epidemiologic studies support the theory that hyperglycemia, diabetes, and osteoporosis are associated with increased oxidative stress (37, 38). Previous studies showed that oxidative stress or overproduction of ROS could disrupt the balance between oxidation and antioxidant defense systems, which can lead to bone loss by facilitating lipid peroxidation, lowering the levels of antioxidant enzymes, inhibiting bone formation, and inducing apoptosis of osteoblasts but promoting bone resorption by OCs (7, 39). Until now, the following 2 types of mechanisms involved in OC inflammation have been reported: NOX2 dependent and NOX2 independent. Our results showed that HG-induced OC inflammation was NOX2 dependent (Fig. 1C) because the NOX2 inhibitor DPI inhibited the inflammatory response induced by HG in vitro in this study.
MAPKs are a family of serine-threonine protein kinases that mediate fundamental biologic processes reacting to external stress signals (40), and they are considered to be important modulators of inflammatory responses and innate immune responses. In response to inflammatory signals, the MAPKs cascade is activated through the phosphorylation of p38, ERK1/2, and JNK (41). Recent studies have demonstrated that the MAPK-signaling pathway is involved in NF-κB activation. Normally, NF-κB is located in the cytoplasm (42), but once it is stimulated by cytokines or exogenous inducers such as LPS, NF-κB dissociates from the cytoplasmic complex and translocates to the nucleus to regulate the expression of inflammatory mediators (43). Therefore, the inhibition of the MAPK and NF-κB pathways is responsible for the protection from inflammatory responses. In our study, we found that HG can significantly induce the expression of MAPKs and NF-κB–related proteins in OCs (Fig. 1D, E). In addition, we demonstrated that treatment with the ERK inhibitor PD98059, JNK inhibitor SP600125, and p38 inhibitor SB203580 inhibited the expression of NF-κB– and NLRP3 inflammasome–related proteins (Fig. 2B) in OCs in vitro. Additionally, NF-κB inhibitor BAY 11-7082 treatment significantly decreased the levels of NLRP3 inflammasome components induced by HG (Fig. 2B).
NLRP3 belongs to the nucleotide-binding oligomerization domain-like receptor family of pattern recognition receptors. NLRP3 is critical for the activation of caspase-1 and the secretion of IL-1β in response to bacterial and endogenous stimuli (44). It is implicated not only in inflammatory disorders but also in numerous metabolic diseases, some of which are caused by specific endogenous components (45). Currently, researchers have found that the NLRP3 inflammasome is activated by various host danger-associated molecular patterns, such as glucose in type 2 diabetes, cholesterol crystals in atherosclerosis, and fatty acids in obesity. Notably, it was recently reported that the loss of NLRP3 attenuates the osteopenia associated with aging in mice (11). Moreover, Alippe et al. (12) think that the NLRP3 inflammasome plays an important role in bone resorption in conditions of low-grade inflammation, although the underlying cellular mechanisms were not studied in detail. In particular, our results also indicated that HG can induce the expression of NLRP3 inflammasome–related proteins in OCs (Fig. 1D, E), accompanied by a significantly enhanced bone-resorption capacity of OCs (Fig. 3A). Therefore, these results showed that the activation of the NLRP3 inflammasome enhances bone resorption by OCs, which was consistent with previous reports.
Efferocytosis is a process of clearing dead cells (or apoptotic cells), which is essential to maintaining tissue homeostasis. In addition, efferocytosis is mediated through the interaction between phagocytic receptors (e.g., MertK, scavenger receptor class B type 1, and lipoprotein receptor-related protein 1) and apoptotic cell ligands (e.g., “eat-me” signals, phosphatidylserine, and apolipoprotein E) with the assistance of bridging molecules (46, 47). If the efferocytotic process is defective, such as in atherosclerosis, the apoptotic cells will accumulate and then undergo secondary necrosis (48). Phagocytic oxidative stress can lead to defective efferocytosis, which inhibits the ability of phagocytes to engulf apoptotic cells (49). However, interestingly, administering specialized proresolving mediators, such as resolvin D1, resolvin D2, maresin 1, or LXA4, which play roles as efferocytosis enhancers, promotes improved efferocytosis and reduces the incidence of phagocytic inflammation (50–52). In the current study, we found that HG induced defective efferocytosis in OCs in vitro, and the proresolving mediator LXA4 and an inhibitor of the NLRP3 inflammasome reversed the defective efferocytosis and decreased the inflammatory response (Fig. 3B–F). In addition, LXA4 treatment slowed the occurrence of osteoporosis by reducing inflammation in OCs in STZ-induced male diabetic rats in vivo (Fig. 4G–J) and reduced the expression of proteins involved in MAPK signaling, NF-κB signaling, and the NLRP3 inflammasome in vivo (Fig. 5). However, the complex mechanisms for efferocytosis and inflammation still await further study.
Our results show that the BMD of the whole body significantly decreased in the diabetic rats, which is in agreement with a previous study (53). We found that insulin and LXA4 reversed the change in bone loss observed in diabetic rats (Fig. 4E, F). The present study showed that hyperglycemia was capable of impairing the mechanical integrity of the bones in diabetic rats. In addition to the BMD, the bone microarchitecture is another factor related to bone quality. We also found that the microarchitecture of the trabecular bone was partially destroyed in diabetic rats, which might contribute to the deterioration of bone strength under diabetic conditions. In our results, we found that BMD and bone microarchitecture were significantly improved in insulin- or LXA4-treated diabetic rats (Fig. 4I, J). In addition, we found that the number of OCs was significantly increased in the diabetic rats, whereas insulin or LXA4 reduced this content (Fig. 4G, H) and alleviated bone loss (Fig. 4E, F). This may be evidence that the number of OCs and their activity are responsible for diabetic osteoporosis. Therefore, we infer that insulin or LXA4 plays an important role in enhancing bone strength in diabetic rats; at least in part, they can improve BMD and bone microarchitecture.
Overall, this study demonstrates that the activation of ROS/MAPKs/NF-κB/NLRP3 in OCs and the inhibition of efferocytosis are the main causes of osteoporosis in diabetes. This study lays a foundation for deeper research on the pathogenesis and effective control of diabetic osteoporosis.
ACKNOWLEDGMENTS
The authors thank Dr. Yang Wang (Jilin University) for her help in manuscript revision. This work was supported by the Project of Science and Technology Department of Jilin Province (20180414036GH); the Education Department of Jilin Province (2016444); the State Key Project of Research and Development Plan (2017YFD0502200 and 2016YFD0501302); the National Nature Science Foundation of China (31172364 and 81801972); the Fund for Science and Technology Development of Jilin Province (20150101108JC); the Important National Science and Technology Specific Projects (2012ZX10003002); the Program for New Century Excellent Talents in University (NCET-09-0434); and the Science, Technology and Innovation Commission of Shenzhen Municipality (JCYJ2016031100720906 and JSGG20160301100442775). The authors declare no conflicts of interest.
Glossary
- ASC
apoptosis-associated speck-like protein containing a caspase activation and recruitment domain
- BMD
bone mineral density
- DHR
dihydrorhodamine
- DM
diabetes mellitus
- DPI
diphenyleneiodonium
- DXA
dual-energy X-ray absorptiometry
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- H&E
hematoxylin and eosin
- HG
high glucose
- LXA4
lipoxin A4
- NALRP3
NACHT-LRR-PYD domains–containing protein 3
- NOX2
NADPH oxidase 2
- OC
osteoclast
- pHrodo
pHrodo Red succinimidyl ester
- RANKL
receptor for activation of NF-κB ligand
- ROS
reactive oxygen species
- SEM
scanning electron microscopy
- STZ
streptozotocin
- TRACP-5b
tartrate-resistant acid phosphatase 5b
- TRAP
tartrate-resistant acid phosphatase
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
W. Guo and L. Yu designed research; Y. An and H. Zhang performed research; Y. An wrote the paper; C. Wang, H. Xu, and L. Ni analyzed data; and F. Jiao, X. Wang, W. Luan, F. Ma, X. Tang, and M. Liu contributed new reagents or analytic tools.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Bain S., Ramamurthy N. S., Impeduglia T., Scolman S., Golub L. M., Rubin C. (1997) Tetracycline prevents cancellous bone loss and maintains near-normal rates of bone formation in streptozotocin diabetic rats. Bone 21, 147–153 [DOI] [PubMed] [Google Scholar]
- 2.Hie M., Yamazaki M., Tsukamoto I. (2009) Curcumin suppresses increased bone resorption by inhibiting osteoclastogenesis in rats with streptozotocin-induced diabetes. Eur. J. Pharmacol. 621, 1–9 [DOI] [PubMed] [Google Scholar]
- 3.Saito M., Marumo K. (2010) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos. Int. 21, 195–214 [DOI] [PubMed] [Google Scholar]
- 4.Lu H., Kraut D., Gerstenfeld L. C., Graves D. T. (2003) Diabetes interferes with the bone formation by affecting the expression of transcription factors that regulate osteoblast differentiation. Endocrinology 144, 346–352 [DOI] [PubMed] [Google Scholar]
- 5.Vestergaard P. (2009) Bone metabolism in type 2 diabetes and role of thiazolidinediones. Curr. Opin. Endocrinol. Diabetes Obes. 16, 125–131 [DOI] [PubMed] [Google Scholar]
- 6.Irwin R., Lin H. V., Motyl K. J., McCabe L. R. (2006) Normal bone density obtained in the absence of insulin receptor expression in bone. Endocrinology 147, 5760–5767 [DOI] [PubMed] [Google Scholar]
- 7.Toker H., Ozdemir H., Balcı H., Ozer H. (2012) N-acetylcysteine decreases alveolar bone loss on experimental periodontitis in streptozotocin-induced diabetic rats. J. Periodontal Res. 47, 793–799 [DOI] [PubMed] [Google Scholar]
- 8.Nassar H., Kantarci A., van Dyke T. E. (2007) Diabetic periodontitis: a model for activated innate immunity and impaired resolution of inflammation. Periodontol. 2000 43, 233–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schroder K., Tschopp J. (2010) The inflammasomes. Cell 140, 821–832 [DOI] [PubMed] [Google Scholar]
- 10.Lukens J. R., Gross J. M., Kanneganti T. D. (2012) IL-1 family cytokines trigger sterile inflammatory disease. Front. Immunol. 3, 315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Youm Y. H., Grant R. W., McCabe L. R., Albarado D. C., Nguyen K. Y., Ravussin A., Pistell P., Newman S., Carter R., Laque A., Münzberg H., Rosen C. J., Ingram D. K., Salbaum J. M., Dixit V. D. (2013) Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell Metab. 18, 519–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alippe Y., Wang C., Ricci B., Xiao J., Qu C., Zou W., Novack D. V., Abu-Amer Y., Civitelli R., Mbalaviele G. (2017) Bone matrix components activate the NLRP3 inflammasome and promote osteoclast differentiation. Sci. Rep. 7, 6630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang T., Zhang X., Li J. J. (2002) The role of NF-kappaB in the regulation of cell stress responses. Int. Immunopharmacol. 2, 1509–1520 [DOI] [PubMed] [Google Scholar]
- 14.Baichwal V. R., Baeuerle P. A. (1997) Activate NF-kappa B or die? Curr. Biol. 7, R94–R96 [DOI] [PubMed] [Google Scholar]
- 15.Li N., Karin M. (1999) Is NF-kappaB the sensor of oxidative stress? FASEB J. 13, 1137–1143 [PubMed] [Google Scholar]
- 16.Dickinson R. J., Keyse S. M. (2006) Diverse physiological functions for dual-specificity MAP kinase phosphatases. J. Cell Sci. 119, 4607–4615 [DOI] [PubMed] [Google Scholar]
- 17.Ajizian S. J., English B. K., Meals E. A. (1999) Specific inhibitors of p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways block inducible nitric oxide synthase and tumor necrosis factor accumulation in murine macrophages stimulated with lipopolysaccharide and interferon-gamma. J. Infect. Dis. 179, 939–944 [DOI] [PubMed] [Google Scholar]
- 18.Soehnlein O., Lindbom L. (2010) Phagocyte partnership during the onset and resolution of inflammation. Nat. Rev. Immunol. 10, 427–439 [DOI] [PubMed] [Google Scholar]
- 19.Fredman G., Li Y., Dalli J., Chiang N., Serhan C. N. (2012) Self-limited versus delayed resolution of acute inflammation: temporal regulation of pro-resolving mediators and microRNA. Sci. Rep. 2, 639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Poon I. K., Lucas C. D., Rossi A. G., Ravichandran K. S. (2014) Apoptotic cell clearance: basic biology and therapeutic potential. Nat. Rev. Immunol. 14, 166–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Green D. R., Oguin T. H., Martinez J. (2016) The clearance of dying cells: table for two. Cell Death Differ. 23, 915–926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boyle W. J., Simonet W. S., Lacey D. L. (2003) Osteoclast differentiation and activation. Nature 423, 337–342 [DOI] [PubMed] [Google Scholar]
- 23.Jaghsi S., Hammoud T., Haddad S. (2018) Relation between circulating vitamin K1 and osteoporosis in the lumbar spine in Syrian post-menopausal women. Open Rheumatol. J. 12, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lacey D. L., Timms E., Tan H. L., Kelley M. J., Dunstan C. R., Burgess T., Elliott R., Colombero A., Elliott G., Scully S., Hsu H., Sullivan J., Hawkins N., Davy E., Capparelli C., Eli A., Qian Y. X., Kaufman S., Sarosi I., Shalhoub V., Senaldi G., Guo J., Delaney J., Boyle W. J. (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93, 165–176 [DOI] [PubMed] [Google Scholar]
- 25.Wang C., Qu C., Alippe Y., Bonar S. L., Civitelli R., Abu-Amer Y., Hottiger M. O., Mbalaviele G. (2016) Poly-ADP-ribosylation-mediated degradation of ARTD1 by the NLRP3 inflammasome is a prerequisite for osteoclast maturation. Cell Death Dis. 7, e2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Suzuki K., Zhu B., Rittling S. R., Denhardt D. T., Goldberg H. A., McCulloch C. A., Sodek J. (2002) Colocalization of intracellular osteopontin with CD44 is associated with migration, cell fusion, and resorption in osteoclasts. J. Bone Miner. Res. 17, 1486–1497 [DOI] [PubMed] [Google Scholar]
- 27.Aziz M., Yang W. L., Wang P. (2013) Measurement of phagocytic engulfment of apoptotic cells by macrophages using pHrodo succinimidyl ester. Curr. Protoc. Immunol. Chapter 14, Unit 14.31 [DOI] [PubMed] [Google Scholar]
- 28.Jeong D. W., Kim E. Y., Kim J. H., Lee B., Hong S., Park J. H., Jung H. S., Sohn Y. (2019) Lycopus lucidus turcz inhibits the osteoclastogenesis in RAW 264.7 cells and bone loss in ovariectomized rat model. Evid. Based Complement. Alternat. Med. 2019, 3231784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Douda D. N., Khan M. A., Grasemann H., Palaniyar N. (2015) SK3 channel and mitochondrial ROS mediate NADPH oxidase-independent NETosis induced by calcium influx. Proc. Natl. Acad. Sci. USA 112, 2817–2822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brancaleone V., Gobbetti T., Cenac N., le Faouder P., Colom B., Flower R. J., Vergnolle N., Nourshargh S., Perretti M. (2013) A vasculo-protective circuit centered on lipoxin A4 and aspirin-triggered 15-epi-lipoxin A4 operative in murine microcirculation. Blood 122, 608–617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morimoto K., Janssen W. J., Fessler M. B., McPhillips K. A., Borges V. M., Bowler R. P., Xiao Y. Q., Kench J. A., Henson P. M., Vandivier R. W. (2006) Lovastatin enhances clearance of apoptotic cells (efferocytosis) with implications for chronic obstructive pulmonary disease. J. Immunol. 176, 7657–7665 [DOI] [PubMed] [Google Scholar]
- 32.Schwartz A. V. (2003) Diabetes mellitus: does it affect bone? Calcif. Tissue Int. 73, 515–519 [DOI] [PubMed] [Google Scholar]
- 33.Herskind A. M., Christensen K., Nørgaard-Andersen K., Andersen J. F. (1992) Diabetes mellitus and healing of closed fractures. Diabete Metab. 18, 63–64 [PubMed] [Google Scholar]
- 34.Manolagas S. C. (2000) Birth and death of bone cells: basic regulatory mechanisms and implications for the pathogenesis and treatment of osteoporosis. Endocr. Rev. 21, 115–137 [DOI] [PubMed] [Google Scholar]
- 35.Harada S., Rodan G. A. (2003) Control of osteoblast function and regulation of bone mass. Nature 423, 349–355 [DOI] [PubMed] [Google Scholar]
- 36.Asagiri M., Takayanagi H. (2007) The molecular understanding of osteoclast differentiation. Bone 40, 251–264 [DOI] [PubMed] [Google Scholar]
- 37.Rains J. L., Jain S. K. (2011) Oxidative stress, insulin signaling, and diabetes. Free Radic. Biol. Med. 50, 567–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Evans J. L., Goldfine I. D., Maddux B. A., Grodsky G. M. (2002) Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr. Rev. 23, 599–622 [DOI] [PubMed] [Google Scholar]
- 39.Pisoschi A. M., Pop A. (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur. J. Med. Chem. 97, 55–74 [DOI] [PubMed] [Google Scholar]
- 40.Ghonime M. G., Shamaa O. R., Das S., Eldomany R. A., Fernandes-Alnemri T., Alnemri E. S., Gavrilin M. A., Wewers M. D. (2014) Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J. Immunol. 192, 3881–3888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mendis E., Kim M. M., Rajapakse N., Kim S. K. (2008) Suppression of cytokine production in lipopolysaccharide-stimulated mouse macrophages by novel cationic glucosamine derivative involves down-regulation of NF-kappaB and MAPK expressions. Bioorg. Med. Chem. 16, 8390–8396 [DOI] [PubMed] [Google Scholar]
- 42.Lawrence T., Bebien M., Liu G. Y., Nizet V., Karin M. (2005) IKKalpha limits macrophage NF-kappaB activation and contributes to the resolution of inflammation. Nature 434, 1138–1143 [DOI] [PubMed] [Google Scholar]
- 43.Tak P. P., Firestein G. S. (2001) NF-kappaB: a key role in inflammatory diseases. J. Clin. Invest. 107, 7–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Franchi L., Eigenbrod T., Muñoz-Planillo R., Nuñez G. (2009) The inflammasome: a caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat. Immunol. 10, 241–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Masters S. L., Simon A., Aksentijevich I., Kastner D. L. (2009) Horror autoinflammaticus: the molecular pathophysiology of autoinflammatory disease (*). Annu. Rev. Immunol. 27, 621–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garbin U., Baggio E., Stranieri C., Pasini A., Manfro S., Mozzini C., Vallerio P., Lipari G., Merigo F., Guidi G., Cominacini L., Fratta Pasini A. (2013) Expansion of necrotic core and shedding of Mertk receptor in human carotid plaques: a role for oxidized polyunsaturated fatty acids? Cardiovasc. Res. 97, 125–133 [DOI] [PubMed] [Google Scholar]
- 47.Tabas I., Bornfeldt K. E. (2016) Macrophage phenotype and function in different stages of atherosclerosis. Circ. Res. 118, 653–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moore K. J., Tabas I. (2011) Macrophages in the pathogenesis of atherosclerosis. Cell 145, 341–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thorp E., Vaisar T., Subramanian M., Mautner L., Blobel C., Tabas I. (2011) Shedding of the Mer tyrosine kinase receptor is mediated by ADAM17 protein through a pathway involving reactive oxygen species, protein kinase Cδ, and p38 mitogen-activated protein kinase (MAPK). J. Biol. Chem. 286, 33335–33344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fredman G., Kamaly N., Spolitu S., Milton J., Ghorpade D., Chiasson R., Kuriakose G., Perretti M., Farokzhad O., Tabas I. (2015) Targeted nanoparticles containing the proresolving peptide Ac2-26 protect against advanced atherosclerosis in hypercholesterolemic mice. Sci. Transl. Med. 7, 275ra20; erratum: 277er2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viola J. R., Lemnitzer P., Jansen Y., Csaba G., Winter C., Neideck C., Silvestre-Roig C., Dittmar G., Döring Y., Drechsler M., Weber C., Zimmer R., Cenac N., Soehnlein O. (2016) Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ. Res. 119, 1030–1038 [DOI] [PubMed] [Google Scholar]
- 52.Martinez R. M., Fattori V., Saito P., Melo C. B. P., Borghi S. M., Pinto I. C., Bussmann A. J. C., Baracat M. M., Georgetti S. R., Verri W. A., Jr., Casagrande R. (2018) Lipoxin A4 inhibits UV radiation-induced skin inflammation and oxidative stress in mice. [E-pub ahead of print] J. Dermatol. Sci. [DOI] [PubMed] [Google Scholar]
- 53.Liang W., Luo Z., Ge S., Li M., Du J., Yang M., Yan M., Ye Z., Luo Z. (2011) Oral administration of quercetin inhibits bone loss in rat model of diabetic osteopenia. Eur. J. Pharmacol. 670, 317–324 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.