Abstract
The aim of this study was to evaluate the effects of butyrolactone-I (A6) on type 2 diabetes (T2D) in db/db mice because A6 was found to inhibit α-glucosidase activities and TNF-α release, which were associated with improving T2D. Male db/db mice were divided into 6 groups and given an equivalent volume of olive oil, acarbose, or different doses of A6 for 4 wk (n = 8/group). In this study, 11 butenolide derivatives were screened for their α-glucosidase and TNF-α suppressive activity in vitro. A6, an efficient α-glucosidase inhibitor, exerts hypoglycemic and multiple activities in reducing weight, improving glucose tolerance and insulin resistance, increasing short-chain fatty acid (SCFA) levels, activating SCFA-induced increases in glucagon-like peptide 1 and peroxisome proliferator-activated receptor-γ expression, enhancing intestinal mucosal barrier function and mitigating endoxemia in db/db mice. These effects may result from mediation of gut microbiota by A6. Meanwhile, A6, with potent TNF-α–lowering properties, was demonstrated to have multiple salutary effects with excellent structural stability and long-term safety in vivo. A6, an effective α-glucosidase inhibitor with high security and stability, exerted potent antidiabetic effects in vivo. Furthermore, the modulation of gut microbiota of A6 was demonstrated to be one of the mechanisms contributing to anti-inflammation properties and improving endoxemia. Our work confirms that the compound A6 is a prospective drug candidate for T2D.—Wu, W., Liu, L., Zhu, H., Sun, Y., Wu, Y., Liao, H., Gui, Y., Li, L., Liu, L., Sun, F., Lin, H. Butyrolactone-I, an efficient α-glucosidase inhibitor, improves type 2 diabetes with potent TNF-α–lowering properties through modulating gut microbiota in db/db mice.
Keywords: inflammatory cytokines, antidiabetic, anti-inflammation
Diabetes has become a global public health problem. It is estimated that 439 million people worldwide are expected to develop the disease by 2030, for which type 2 diabetes (T2D) accounts for about 90% (1). At present, there are 3 types of drugs used to treat T2D: metformin, acarbose, and thiazolidinedione (2). α-Glucosidase is a well-known target for the research of antidiabetic drugs. Acarbose, an α-glucosidase inhibitor, is preferred for people with only impaired glucose tolerance (3). T2D is a chronic metabolic disease assoicated with peripheral insulin resistance, dyslipidemia, obesity, low-grade systemic inflammation, of which systemic inflammation caused by infection has become one of the acute complications of T2D; the control of inflammation can obviously improve the abnormality of glucose metabolism (4).
Ideal anti-T2D drugs should aim at multiple therapeutic targets and play multiple roles in glycemic control, decreasing plasma lipid levels, losing weight, and improving systemic inflammation. Clinical studies have shown that T2D is often accompanied by an increase in the concentration of various inflammatory cytokines, among which TNF-α is an important indicator of systemic inflammation (5). Ghada et al. found that the expression of cytokine TNF-α was significantly increased in the infected diabetic mice compared with the infected normal mice, and the inflammatory response time to pathogens was significantly prolonged, which may be the reason why patients with diabetes are prone to infection (6). Recently, various researchers have reported that down-regulation of TNF-α could improve systemic inflammation of T2D rats (7, 8).
T2D was the first to be used to study microflora-associated diseases. 16S rRNA gene amplifier sequencing revealed a shifting of the structure of the gut microbiota, showing a selective enrichment of phylum Proteobacteria and a selective down-regulation effect on phylum Firmicutes of patients with T2D (9), implying that the “microbiota-to-host paradigm” will become a new strategy for the therapy of T2D.
Drugs [metformin (10), acarbose (11), pioglitazone (12)] have demonstrated treatment of T2D through modulating of the human gut microbiome, and these alterations were closely associated with the changes of intestinal homeostasis (13). α-Glucosidase inhibitor (10, 14) leads to a large accumulation of undigested carbohydrates in the lower part of the intestine, which alters the composition and distribution of gut microbes (15, 16). An efficient α-glucosidase inhibitor with potent anti-inflammation properties of lowering TNF-α and gut microbiota regulation effect could be an aussichtsreich candidate drug to cure T2D.
Microbial-derived compounds with biologic activity diversity and chemical structure richness are usually identified with multiple effects (17). The fungus Aspergillus terreus is widely present in both marine benthos and terrestrial creatures and was previously reported to be rich in a characteristic metabolites-butenolide (18, 19). The butenolide was widely concerned with its wide range of biologic activities, such as anti-inflammatory (17), antioxidant (20), and α-glucosidase inhibitor activities (21). Herein, 11 butenolides from the marine sponge–derived fungus A. terreus were sifted for their TNF-α and α-glucosidase inhibitory activites in vitro. Compound butyrolactone-I (A6), with high security and excellent stability, showed multiple and effective anti-T2D functions associated with lowered blood glucose, reduction of body weight, and anti-inflammation activities in db/db mice. Furthermore, 16S rRNA gene sequencing analysis, short-chain fatty acid (SCFA) and intestinal mucosal barrier function demonstrated that gut microbiota played an important role in mediating the therapeutic efficacies of A6.
MATERIALS AND METHODS
Inhibition assay of α-glucosidase activity
The preperation of α-glucosidase from mouse small intestinal mucosa was performed as previously described (15). The experiment was executed in triplicate with different concentrations of compounds individually. The formula for calculating the inhibition rate of the compound on α-glucosidase is as follows: the inhibition rate (%) = 1 − [(ODtest − ODtest blank)/(ODcontrol − ODcontrol blank)] × 100.
Measurement of TNF-α secretion by ELISA
RAW264.7 cells were pretreated with different indicated concentrations of A1–A11 for 1 h and then stimulated with LPS (1 μg/ml) for 24 h. The secretion of TNF-α was detected by using the Quantikine ELISA Kit for mouse TNF-α (R&D Systems, Minneapolis, MN, USA) according to the manufacturer’s instructions. Half maximal inhibitory concentration (IC50) values were determined by the inhibition rates of different concentration gradients and calculated by Pad Prism 5.0 (GraphPad Software, La Jolla, CA, USA).
Cytotoxic activity measurement
The effects of A6 on cytotoxic activity were determined using a Cell Counting Kit (CCK)-8 Assay (Dojindo, Kyushu, Japan). Briefly, the cells were seeded in 96-well plates at a density of 2 × 104 cells/well and then incubated at 37°C for 24 h. After being pretreated with different concentrations of A6 for 48 h alone, NCM460 and 16HBE cells were incubated with the CCK-8 solution for 1 h. The absorbance at 450 nm was measured in a full wavelength microplate reader (spectra Max190; Molecular Devices, San Jose, CA, USA). Each group was established in 6 wells and independently performed in triplicate.
Pharmacokinetic determination of A6 by HPLC−mass spectrometry
For the in vivo pharmacokinetic study, 6 healthy male SD rats (6–8 wk old) weighing 210−240 g were orally administrated A6 with doses of 3.0 mg/kg. At 0 (prior to dosing), 0.5, 1, 2, 4, 6, 8, 10, 12, 14, 16, and 18 h after dosing, a blood sample (∼0.2 ml) was collected from each animal via the caudal vein using heparinized 1.5 ml centrifuge tube and stored at −80°C until analysis.
Twenty microliters of cell lysate was added to each 0.2 ml sample, 5 μl of internal standard solution (A6) was added and vortexed for 30 s and placed on ice to lyse for 30 min. Then, the mixture was broken by an ultrasonic crushing instrument for 15 min in the ice bath and centrifuged at 4°C and 10,000 g for 20 min. The supernatant was transferred into another clean 1.5 ml centrifuge tube and concentrated by centrifuging at 4°C, the remaining water freeze drying. Then 300 μl methanol was added for resolubilization. The mixture solution was injected for the HPLC−mass spectrometry (MS) analysis. Chromatography separation was carried out on a YMC-Pack Pro RS column (250 mm × 4.6 mm, 5 μm; Waters, Milford, MA, USA). Gradient elution was performed with a mixture of MeCN (mobile phase A) and deionized water (mobile phase B) as follows: 0−0.5 min 25% A, 0.4−0.41 min 25−80% A, 0.41−2.0 min 80−95% A, 2.0−3.0 min 90% A, 3.0−4.0 min 25% A. The flow rate was kept at a constant 0.3 ml/min, and the total run time was 4.0 min. The temperatures of column were maintained at 25°C. All data were collected and processed by using MassLynx NT 4.1 software with a QuanLynx program (Waters).
Experimental animals
For the long-term safety assay, 6–8 wk C57BL/6 mice were sorted into 2 groups (n = 10 each group) based on body weight, 24 h food intake, blood glucose alanine transaminase (ALT) and aspartate transaminase (AST) level. The treatment group was given 40.0 mg/kg of A6 daily by gavage. The control group was given oral gavage of an equivalent volume of olive oil (pharmaceutic adjuvant; Taihua Reagent, Shanxi, China). Daily treatment continued for 3 mo. Survival, body weight, blood glucose, liver function, and organ coefficients were recorded.
Male db/db and C57BL/6 mice (6–8 wk old) were purchased from Shanghai SLAC Laboratory Animal Co., Ltd (Shanghai, China). Animals were housed in the animal center of Ningbo University Medicine College (Ningbo, China). Every 5 mice were housed in a cage and maintained under a 12-h light/dark cycle (lights on at 08:00 am) in temperature- and humidity-controlled environments (21 ± 2°C and ∼60%, respectively) with free access to food and water. All mice were allowed to habituate to the housing environment for 3 d prior to gavage administration. All experimental procedures were performed in accordance with the health guide for the care and use of laboratory animals of national institutes, and animal protocols were approved by the ethical committee of animal use and protection.
In assays with A6, male C57BL/6 mice of the control group were only given an equivalent volume of olive oil. A total of 40 db/db male mice were divided into 5 groups (n = 8) and placed in clear plastic cages with a mesh top under specific pathogen-free conditions. The experimental groups were designed as follows: the mice of the model group were inoculated by mouth with equivalent volume of olive oil; the acarbose-treated group was treated with 13.5 mg/kg acarbose; and A6-treated groups were treated with A6 of different concentrations, which were accordingly called low-dose group (0.25 mg/kg), medium-dose group (1.0 mg/kg), and high-dose group (4.0 mg/kg).
For antibiotics mice study, 6–8 wk male db/db mice were fed with gentamycin (100 mg/L), erythromycin (10 mg/L), neomycin (0.5 g/L), vancomycin (0.5 g/L), and ampicillin (1 g/L) in drinking water for 3 wk. Cecum observation and anaerobic cultivation of fecal samples confirmed successful depletion of gut microbiota after the antibiotic treatment (1).
In the antibiotic experiments, 16 male db/db mice and 16 antibiotics-treated male db/db mice were sorted into 4 groups (n = 8 each) based on their body weight, 24 h food intake, blood glucose, and insulin resistance levels. The treatment group of antibiotic db/db mice was given 4.0 mg/kg/d of A6 by oral gavage. The control group of antibiotic db/db mice was given an equivalent volume of olive oil by mouth. All treatments continued for 4 wk.
Twenty-four hour food intake measurement
Groups of mice were placed in a clean cage with a known amount of food. Twenty-four hours later, the amount of remaining food was measured, with the difference viewed as food intake per 24 h. Error bars reflect sem of 3 measurements made 1 wk apart.
Free diet blood glucose
Mice were placed in a clean cage and unfed for 2 h. Blood glucose concentration was then determined using a Roche glucometer (Basel, Switzerland) and expressed in mM.
Glucose and insulin tolerance test
C57BL/6 and db/db mice were treated with A6 or acarbose for 4 wk. After being left unfed for 5 h, baseline blood glucose levels were measured using a Roche glucometer and expressed in mM. Mice were then injected intraperitoneally with 2 g of glucose/kg in sterile PBS or with 0.5 U insulin/kg (MilliporeSigma, Burlington, MA, USA), and blood glucose levels were measured 30, 60, 90, and 120 min after injection, as previously described (22).
Biochemical measurement
Levels of serum ALT and AST levels followed the manufacturer’s instructions (23).
Molecular docking
Molecular docking was performed with the aim of revealing the interaction between A6 and α-glucosidase at an atomic level. The docking of A6 into α-glucosidase [Protein Data Bank (PDB; http://www.rcsb.org/) ID: 3W37] was carried out by AutoDock program. The AutoDockTools 1.5.6 package was employed to generate the docking input files, PyMol 1.6 as the graphical user interface for 3D structure visualization, and Ligplus as the 2D structure-protein interaction visualizer to show the hydrogen bonds and hydrophobic contacts. The docking center has been defined as center_x: 1.147, center_y: −14.365, and center_z: −24.699. Grid points of 40 40 40 with 0.375 Å spacing were calculated around the docking area for all the ligand atom types using AutoGrid4. One hundred separate docking calculations were performed using the Lamarckian genetic algorithm local search method. The docking results from each of the 100 calculations were clustered on the basis of root-mean-square deviation (rmsd = 2.0 Å) between the Cartesian coordinates of the ligand atoms and were ranked on the basis of the free energy of binding. In terms of the above parameters, a redocking process was performed as well and it presented an excellent similarity to the ligand in 3W37 Crystal Structure, revealing that the method we developed was reliable.
Analysis of cytokine levels
Serum samples were collected through centrifugation and stored at −80°C prior to further analysis. The levels of 6-cell cytokines were determined using the BD Cytometric Bead Array mouse inflammatory Cytokine Kit (BD BioSciences, San Jose, CA, USA) according to the manufacturer’s instructions. Samples and standards were analyzed on a flow Cytometer Calibur Flow Cytometer (BD BioSciences). The concentrations were assessed using FCAP Array software (BD Biosciences).
Quantification of fecal inflammatory marker by ELISA
For quantification of fecal/serum lipocalin-2 (Lcn-2), flagellin C (Flic), LPS by ELISA, frozen fecal samples were reconstituted in PBS containing 0.1% Tween 20 to a final concentration of 100 mg/ml and vortexed for 20 min to get a homogenous fecal suspension. These samples were then centrifuged at 10,000 g and 4°C for 10 min. Clear supernatants were collected and stored at −20°C until analysis. Lcn-2, Flic, and LPS levels were estimated in the supernatants using a Duoset Murine Lcn-2 ELISA Kit (R&D Systems), Flic ELISA Kit (Thermo Fisher Scientific, Waltham, MA, USA), and LPS ELISA Kit (Huamei, Wuhan, China) following the manufacturer’s instructions.
In vivo epithelial barrier permeability
In vivo assay of intestinal barrier function was performed using an FITC-labeled dextran method, as previously described. Mice were deprived of food and water for 4 h, and were then gavaged with 15 mg of permeability tracer FITC-labeled dextran 4 kDa (MillieporeSigma). Blood was collected after 3 h, and fluorescence intensity was measured in the serum (excitation, 490 nm; emission, 520 nm; Fluorescence Spectrophotometer; BioTek Instruments, Winooski, VT, USA). FITC-dextran concentrations were determined using a standard curve generated by serial dilution of FITC-dextran in mice serum.
Western blot analysis
Intestinal tissue of mice was washed twice with ice-cold PBS and groudn by Tenbroeck Tissue Grinders (Voxin, Wuxi, China) for 10 min on ice. The homogenates were centrifuged at 10,000 rpm for 10 min at 4°C. Protein concentration was determined using the Bio-Rad DC Protein assay reagent (Bio-Rad, Hercules, CA, USA) according to the manufacturer’s instructions (24).
Proteins (30 μg) in the nuclear extracts or in the whole-tissue homogenate were separated by 10% SDS-PAGE, transferred to polyvinylidene fluoride membranes, and probed with antibodies against Krüppel-like factor 4 (Klf4), mucoprotein 2 (Muc-2), and NF-κB p65 antibodies (Santa Cruz Biotechnology, Dallas, TX, USA), glucagon-like peptide 1 (GLP-1), and peroxisome proliferator-activated receptor-γ (PPAR-γ) (Cell Signaling Technology, Danvers, MA, USA), followed by appropriate horseradish peroxidase–linked secondary antibodies (Cell Signaling Technology). The proteins were detected by WesternBright ECL (Advansta, San Jose, CA, USA).
Histologic analysis
To grade the severity of liver histopathology, the liver tissues were dissected, washed with 4% paraformaldehyde, embedded in paraffin, cut into 5-μm-thick sections, and stained with hematoxylin and eosin. The histologic grade was blindly determined by some veterinary pathologists. The liver severity was quantified using drug-induced liver injury–pathologic scroring system (DILI-PSS) (25).
Immunohistochemical analyses
For immunocytochemistry, the colonic tissues were fixed with 4% paraformaldehyde in PBS, embedded in paraffin and cut into 5-μm-thick sections. The tissues were treated with 1% bovine serum albumin (Nacalai Tesque, Kyoto, Japan) at room temperature for 60 min. The following antibodies were used in the present study, including anti-TNF-α antibody (1:200, 8242; Cell Signaling Technology). Secondary antibodies used were rabbit anti-mouse IgG (PV-9005; ZSGB-BIO, Beijing, China). Nonimmunized mouse IgG at equal concentrations was used as the negative controls for each primary antibody. Images were taken using the Leitz Dialux 22 Microscope (Leica Microsystems, Wetzlar, Germany) and Qicam Fast 1394 camera (Teledyne QImaging, Surrey, BC, Canada), and they were analyzed by Image-Pro Plus 6.0 software (Media Cybernetics UK, Marlow, United Kingdom). The immunologic histologic chemistry indexes for IL-6 and TNF-α were determined as average integral optical densities [AIOD; AIOD = (positive area/total area) × IOD].
16S rRNA gene sequence analysis
The genomic DNA was extracted from each cecum content sample of db/db mice treated with A6 according to previous reports (26). The microbiota V3–V4 hypervariable regions of 16 S rRNA gene were amplified by PCR as previously described (27). In our study, the primer sequences were forward 5′-CCTACGGGRSGCAGCAG-3′ and reverse 5′-GGACTACVVGGGTATCTAATC-3′. Sequences were classified taxonomically using the Greengenes reference database (28). Mothur software packages (v.1.30.1; https://www.mothur.org/) were used to calculate the value of Chao1, Ace, Simpson and Shannon index for evaluation of the community richness and community diversity (29). To compare the structure of different microbial communities, we performed the Hcluster analysis, principal component analysis (PCA), and principal coordinate analysis (PCoA). The 16S rRNA gene sequence data were processed using linear discriminant analysis effect size (LEfSe).
SCFA composition analysis
Intestinal contents were collected from animals after the treatment with A6. Each sample was lyophilized and then ground using a mortar. An amount of 100 mg of the homogenic powders was dissolved with 100 μl of 15% phosphoric acid. These mixtures were added to 400 μl aether and 100 μl of 50 μg/ml isocaproic acid as an internal standard. After vortexing, the samples were centrifuged at 12,000 rpm at 4°C and 10 min, the supernatants were used for GC-MS analysis. GC-MS was performed on the Agilent HP-InnoWax Capillary Column (30 m, 0.25 mm ID* 0.25 μ). Oven temperature was programmed from 90 to 120°C at 10°C/min, with a 1-min hold; to 150°C at 5°C/min, with a 6 min hold; to 250°C at 25°C/min, with a 2-min hold. Injection of a 1-μl sample was performed at 250°C. Helium, at a flow of 1.0 ml/min, was the carrier gas. Electronic energy was recorded at 70 eV.
Statistical analysis
Data were performed using the SPSS software, v.16.0 (IBM SPSS, Chicago, IL, USA). The results were expressed as means ± sem. Differences in cytokine levels, the expression of TNF-α, the level of AST, ALT, and AIOD of various inflammatory cytokines among different groups were analyzed using a post hoc Fisher’s least significant difference (LSD) test after a 1-way ANOVA. A value of P < 0.05 was considered statistically significant. The Levene test was used to verify the homogeneity of variance. A PCoA of overall microbial diversity based on an unweighted UniFrac metric was conducted using Bray-Curtis distance to compare all of the samples. The abundance and diversity of the operational taxonomic units (OTUs; β-diversity) were examined with unweighted UniFrac analysis. LEfSe differences among biologic groups were tested for significance using a nonparametric factorial Kruskal-Wallis sum-rank test followed by Wilcoxon’s rank-sum test. These analyses were performed using the prediction analysis for microarrays package within R software (https://www.r-project.org/).
RESULTS
α-Glucosidase and TNF-α inhibitory activities of A1–A11
As to the inhibitory activity against α-glucosidase, compounds A1–A8 showed much stronger activity than acarbose. Compounds A1–A11 inhibited TNF-α with IC50 in the range from 12.31 to 69.68 μM. A6, which displayed the strongest activities against both α-glucosidase (being 5.2-fold stronger than acarbose) and TNF-α, was selected for researching in vivo study (Fig. 1 and Table 1).
Figure 1.
Structures of compounds A1–A11.
TABLE 1.
α-Glucosidase and TNF-α inhibitory activities of A1–A11
| Compound/control | α-Glucosidase inhibition (IC50, µM) | TNF-α inhibition (IC50, µM) |
|---|---|---|
| A1 | 1.52 ± 0.03 | 48.78 ± 7.65 |
| A2 | 1.71 ± 0.03 | 52.36 ± 5.15 |
| A3 | 6.62 ± 0.47 | 69.62 ± 5.83 |
| A4 | 7.47 ± 2.41 | 55.53 ± 7.48 |
| A5 | 7.34 ± 0.02 | 69.68 ± 6.66 |
| A6 | 1.52 ± 0.01 | 12.31 ± 1.12 |
| A7 | 5.69 ± 0.16 | 15.38 ± 1.52 |
| A8 | 6.98 ± 2.62 | 37.45 ± 1.75 |
| A9 | 8.23 ± 1.34 | 24.36 ± 2.46 |
| A10 | 9.01 ± 1.16 | 26.25 ± 2.68 |
| A11 | 30.13 ± 3.62 | 28.49 ± 3.73 |
| Positive control | ||
| Acarbose | 7.84 ± 0.22 | |
| Pomalidomide | 0.39 ± 0.22 |
α-Glucosidase inhibition, small intestinal mucosa from mouse; TNF-α inhibition, RAW264.7 cell (means ± sem).
A6 showed high security and excellent stability
In chemical stability assay, compound A6 showed a good chemical stability when exposed under room temperature for 1 mo (Supplemental Fig. S1A). Then, we further explored whether A6 exhibit toxicity effects in cell viability assay. A6 almost showed no cytotoxicity to NCM460 and 16HBE cells (IC50 >100 μM, individually, Supplemental Fig. S1B, C). Compound A6 was also confirmed to be safe by a long-term safety assay (Supplemental Fig. S2). Daily oral administration of 40.0 mg/kg of A6 for 90 d showed no negative effects on body weight, 24 h food intake, free diet blood glucose, and organ coefficients (Supplemental Fig. S2A–F). At last, we performed histologic analysis of liver tissue sections (Supplemental Fig. S2G, H). The A6-treated (at 40.0 mg/kg) group indicated the morphology of hepatic lobules and hepatocytes was normal, without fatty degeneration and other lesions, and there was no inflammatory cell infiltration in the portal tract (Supplemental Fig. S2G). These results were supported by the DILI-PSS scores, which were not significantly different compared with the C57BL/6 group with A6 group (P > 0.05; Supplemental Fig. S2H).
In vivo pharmacokinetic profile of A6
Compound A6 was further investigated for the plasma concentration profile of A6 (with 3.0 mg/kg). The mean plasma concentration−time curve of A6 after intragastric administration in rats is shown in Supplemental Fig. S3. The relevant pharmacokinetic parameters of A6 showed a half-life of 2.5 h, a mean area under curve (AUC) of 306.3 (μg/L) · h, a Cmax = 58.6 μg/L, and Tmax =7.3 h (Supplemental Table S1).
The hypoglycemic effects of A6 in db/db mice
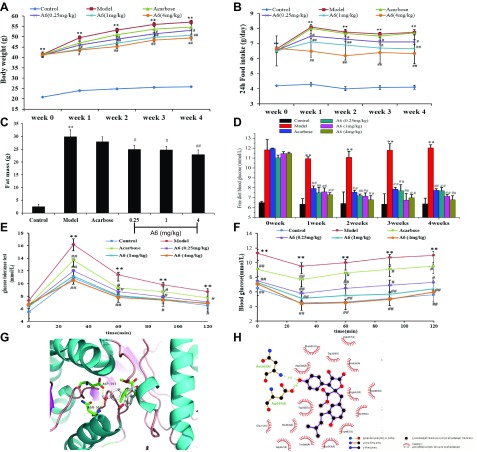
We used db/db diabetic mice to evaluate the hypoglycemic effects for A6. Comparison of the body weight at different time points by repeated measures ANOVA revealed significant within-subject effects [F (1,42) = 40.184, P < 0.01] and between-subject effects [F (1,42) = 2.937, P = 0.018]. One-way ANOVA and Fisher’s LSD analysis on the data from wk 4 (F (5,47) = 5.135, P < 0.01) revealed that the body weight was significantly different. Interestingly, the body weights of db/db mice from the 0.25, 1.0, and 4.0 mg/kg of A6 groups were markedly lower than that of the model group at wk 4, which decreased by about 7.1, 12.3, and 14.1%, respectively (Fig. 2A). Moreover, the 24 h food intake at wk 4 in the compound A6-treated group (4.0 mg/kg) was decreased to 81.8% of the model group (Fig. 2B). The fat mass of the A6 (4.0 mg/kg) treatment group was decreased by 23.3%, compared with the model group mice (Fig. 2C). In addition, free diet blood glucose levels were determined at 2 h following the diet (Fig. 2D). Glucose levels in acarbose- and A6-stimulated group were significantly dereased compared with the model group. Whereas the positive control, acarbose, showed weak effects on weight loss, 24 h food intake, and fat mass.
Figure 2.
Effects of A6 on body weight, food intake, blood glucose, insulin resistance, and docking of A6 with α-glucosidase. A–F) Body weight (A), 24 h food intake (B), fat mass (C), free diet blood glucose in db/db mice (D), glucose intolerance test on the 26th day of treatment in db/db mice (E), insulin resistance test on the 27th day of treatment in db/db mice (F). Control, C57BL/6 mice control; Model, db/db diabetic model mice; Acarbose, db/db mice treated with 13.5 mg/kg acarbose. Repeated measures ANOVA was used to measure differences between the groups for A, B, E, F. One-way ANOVA followed by the 1-way ANOVA after post hoc test was used (C, D). Data are presented as means ± sem. *P < 0.05, **P < 0.05 compared with the control group; #P < 0.05, ##P < 0.01 compared with the model group. G) Docked pose of A6 with α-glucosidase (PDB code: 3W37). H) The interaction between A6 and α-glucosidase (PDB code: 3W37) on a 2D diagram.
The inhibitory effect of A6 on α-glucosidase was confirmed by the glucose tolerance test (GTT) at wk 4 (Fig. 2E). Treatment of A6 and acarbose induced a significant decrease of blood glucose levels at 30, 60, 90, and 120 min when compared with that of the model group.
Following exogenous insulin (0.8 U/kg intraperitoneally) at wk 4, blood glucose declined just 15.9% in the model mice at 30 min (Fig. 2F). The most obvious decline of the glucose level was 38.6% at 30 min in A6-treated (4.0 mg/kg) mice. Compound A6 treatment could improve glucose tolerance and insulin resistance.
Docking of A6 with α-glucosidase
To investigate the structure-function relationship for the binding of A6 and α-glucosidase, docking analysis of A6 within the α-glucosidase active site was performed using AutoDockTools v.1.5.6. In our docking result (Fig. 2G, H), A6 was docked conformation in the lowest energy, and formed 2 hydrogen bonds with α-glucosidase. A phenolic group formed 2 hydrogen bonds with α-glucosidase at ASN 569 and ASP 597 with distance of 3.02 and 2.45 Å, respectively.
A6 profoundly changed the structure of the gut microbiota in db/db mice
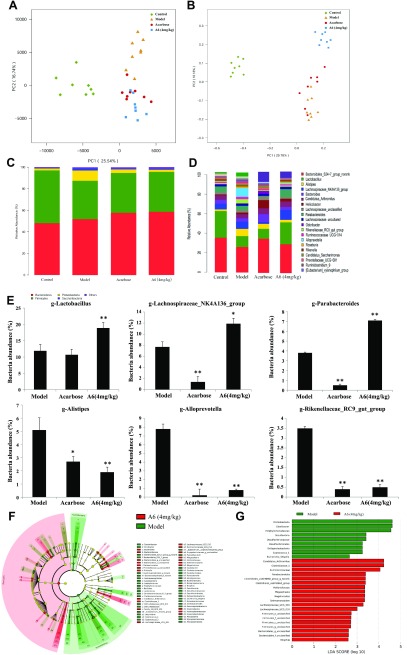
To evaluate the influence of compound A6 on intestinal microbiota, we investigated the abundance and composition of gut microbiota by high-throughput sequencing of the V3–V4 hypervariable region of the 16S rDNA genes from the cecum contents of mice and performed comparative analysis among the control-, model-, acarbose-, and A6-treated groups (at 4.0 mg/kg). The coverage of more than 99.8% indicates an adequate sequencing depth for all samples (Supplemental Table S2). After quality control, a total of 1,580,319 high-quality reads were recovered for downstream analysis. Based on these data, sequences with ≥97% similarity were combined into OTUs and an average of 442, 371, 379, and 399 OTUs were, respectively, identified in control, model, acarbose, and A6 (4.0 mg/kg) group (Supplemental Table S2). To estimate bacterial richness and diversity of the gut microbial community, we calculated the Chao, Ace, Shannon and Simpson indices. Interestingly, we observed a significantly increase in bacterial richness after A6 treatment compared with model-treated db/db mice (Supplemental Table S3). These preliminary results suggest that intake of A6 could change the composition of the gut microbiota.
To verify the above hypothesis, we performed the PCA and PCoA. PCA and PCoA visualized the relative similarity of gut microbiota composition and abundance, showed that A6-treated db/db mice significantly differed from that of model group mice (Fig. 3A, B), these results confirmed the modulatory effects of compound A6 on gut community of db/db mice.
Figure 3.
Structural modulation of gut microbiota by compound A6 in db/db mice. PCA score plot of the gut microbiota (A), PCoA score plot of the gut microbiota based on unweighted UniFrac metrics (B), response of gut microbiota at the phylum levels to different dose of A6 treatment (C), response of gut microbiota at the phylum levels to different dose of A6 treatment (D), comparison of the taxonomic abundance among the indicated groups (E) (n = 8/group). Data are presented as the means ± sem. The statistical significance in bacterial abundance among the different samples was assessed by the 1-way ANOVA after a post hoc Fisher’s LSD test. *P < 0.05, **P < 0.01. F) LEfSe taxonomic cladogram, different colors suggest enrichment of certain taxa in model group (green) and A6 (4 mg/kg, red). G) LDA score, an LDA score greater than 2 indicated a higher relative abundance in the corresponding group than in the other 2 groups.
A6 modulated gut microbiota at different taxonomic levels
To illustrate the kinds of bacteria that were affected by intake of compound A6, we analyzed the composition of the gut microbiota at both the phylum and genus levels. At the phylum level, the abundance of saccharibacteria of A6 and acarbose group were almost unchanged compared with the model group. As for Proteobacteria, significant decreases in its population were observed in response to A6 (at 4.0 mg/kg) and acarbose treatment, whereas, in contrast, an increase of Firmicutes and Bacteroidetes abundance was noted after A6 and acarbose treatment (Fig. 3C and Table 2).
TABLE 2.
Effects of A6 on the relative abundance (%) at the phylum level of gut microbiota according to taxon-based analysis
| Phylum | Bacteroidetes | Firmicutes | Proteobacteria | Saccharibacteria |
|---|---|---|---|---|
| Control | 48.52 ± 6.2 | 46.99 ± 2.3 | 1.99 ± 0.7 | 0.71 ± 0.3 |
| Model | 51.79 ± 5.9 | 32.62 ± 1.7** | 9.55 ± 1.9** | 1.74 ± 0.6 |
| Acarbose | 54.66 ± 8.0 | 41.01 ± 5.2# | 3.29 ± 1.3# | 0.60 ± 0.2 |
| A6 (4.0 mg/kg) | 54.17 ± 6.5 | 42.94 ± 3.0## | 1.41 ± 0.5## | 0.41 ± 0.1 |
P < 0.01 compared with that of the control group; #P < 0.05, ##P < 0.01 compared with that in the model group (means ± sem).
At the genus level, acarbose and A6 (at 4.0 mg/kg) showed a similar shifting of the structure of the gut microbiota, a selective decreasing of Alistipes, Alloprevotella, and Rikenellaceae_RC9_gut_group (Fig. 3D, E) compared with the model group. It is worth noting that A6 significantly increased the populations of Lachnospiraceae_NK4A136_group and Lactobacillus (Fig. 3D), the potent SCFA-producing and anti-inflammatory commensal bacterium in the gut, which is the only species in its phylum Firmicutes (15, 30). However, acarbose demonstrated a selective down-regulation effect on Parabacteroides and Lachnospiraceae_NK4A136_group compared with the model group.
To fully dissect the effects of A6 on gut microbiota, we further analyzed the intestinal bacterial communities at different taxonomic levels using the LEfSe algorithm with significance at P < 0.05. As shown in the taxonomic cladograms, LEfSe analysis visualized and confirmed the modulatory effects of A6 (4.0 mg/kg) on the gut microbiota in db/db mice (Fig. 3F). Specifically, A6-untreated increased the abundance of commensal bacteria belonging to the phylum of Proteobacteria, including Rikenellaceae, Alistipes, Rikenella, Alloprevotella, prevotellaceae, and Desulfovibrionaceae (Fig. 3F, G). Among the intestinal microbes that were enriched by A6 (4.0 mg/kg), Veillonellaceae, Clostridiaceae, Firmicutes_o_unclassified, and Lachnospiraceae stood out, most of which belonged to the phylum of Firmicutes and Bacteroidetes (Fig. 3F, G).
Compound A6 improves intestinal mucosal barrier funcition, endotoxemia, and SCFA in the db/db mice
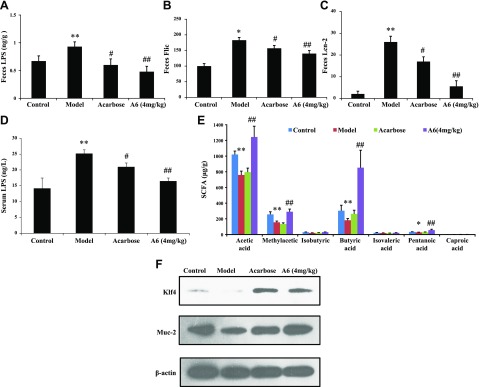
In accordance with the above analysis, we found that the abundance of proinflammatory bacteria in the genus of Alistipes, Alloprevotella, and Desulfovibrio (31, 32) was significantly decreased in the cecum content of A6-treated mice. Thus, we measured the capacity of feces from model and A6-treated mice to proinflammatory marker (LPS, Flic, and Lcn-2) expression. Exposure to A6 significantly reduced fecal levels of LPS, Flic, and Lcn-2 in db/db mice (P < 0.05, Fig. 4A–C).
Figure 4.
Compound A6 improves intestinal mucosal barrier funcition, endotoxemia, and SCFA in db/db mice. A) The level of the inflammatory marker LPS in feces. B) The level of the inflammatory marker Flic in feces. C) The level of the inflammatory marker Lcn2 in feces. D) The level of LPS in serum (n = 8). E) The level of SCFA in feces. F) Protein expression level of Klf4, Muc-2 in the gut. Data are presented as the mean ± sem. Significance was determined using 1-way ANOVA after post hoc test. *P < 0.05; **P < 0.01 compared with the control group; #P < 0.05, ##P < 0.01 compared with the model group.
Diabetes is associated with, and may be promoted by, low-grade inflammation (33, 34). We assessed the levels of 6 inflammatory cytokines in serum samples of db/db mice (Table 3). The levels of TNF-α, IL-6, and monocyte chemotactic protein-1 were elevated in the model group compared with the control group (P < 0.01) but were markedly decreased on the TNF-α level observed in the serum of the A6- and acarbose-treated group compared with the model group (P < 0.05). Meanwhile, our results showed that acarbose and compound A6 treatment at doses of 4.0 mg/kg led to 16.3 and 34.8% reduction of serum LPS in db/db mice (Fig. 4D) as compared with that in the model group, which was correlated with reduced gut permeability.
TABLE 3.
The levels of 6 inflammatory cytokines in serum of db/db mice (pg/ml)
| Group | IL-6 | IL-10 | MCP-1 | IFN-γ | TNF-α | IL-12p70 |
|---|---|---|---|---|---|---|
| Control | 3.2 ± 0.8 | 13.6 ± 2.3 | 24.5 ± 0.5 | 2.2 ± 0.2 | 4.6 ± 0.7 | 3.5 ± 0.5 |
| Model | 13.0 ± 2.2** | 16.8 ± 4.2 | 45.4 ± 5.8** | 2.1 ± 0.5 | 54.7 ± 5.6** | 4.2 ± 0.5 |
| Acarbose | 11.4 ± 1.5 | 15.3 ± 2.7 | 45.1 ± 2.8 | 2.0 ± 0.5 | 38.2 ± 2.7# | 4.1 ± 1.3 |
| A6 (4 mg/kg) | 2.9 ± 0.4## | 16.6 ± 2.1 | 43.3 ± 1.5 | 1.9 ± 0.3 | 4.2 ± 1.1## | 3.3 ± 0.4 |
MCP-1, monocyte chemotactic protein-1. **P < 0.01 compared with that of the control group; #P < 0.05, ##P < 0.01 compared with that in the model group (means ± sem).
In previous reports, members of Lachnospiraceae were to generate SCFA (30), which increased the expression of mucin in the gut thought to reflect recovery of intestinal injury. Thus, we analyzed the SCFA of mouse feces by GC-MS in A6/acarbose-treated db/db mice (Fig. 4E). We observed an increase of 1.37-fold, 1.87-fold, 2.11-fold, and 2.03-fold in acetic acid, methylacetic acid, butyric acid, and pentanoci acid, respectively, in the A6-treated (4.0 mg/kg) group compared with the model group. We investigated the effects of A6 on expression of mucin proteins and mucosal barrier function in db/db mice. Figure 4F showed that treatment with A6 (4.0 mg/kg) increased the expression of mucin (Muc-2 and Klf4) in the gut, which correlated with decreased levels of serum LPS and inflammatory cytokine.
Overall, compound A6 exposure to db/db mice results in decreasing proinflammatory marker (LPS, Flic, and Lcn-2) expression and improvement of the protective function of the mucus.
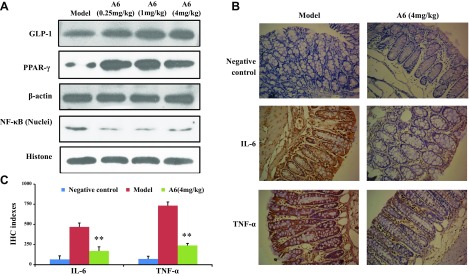
A6 activated GLP-1 signaling pathway, inhibiting IL-6 and TNF-α expression
SCFAs can activate the GLP-1 signaling pathway (35). Furthermore, we found that compound A6 increased SCFA of mouse feces. Therefore, we performed Western blotting assays to determine whether A6 could enhance GLP-1 signaling pathway in the small intestines of db/db mice. As shown in Fig. 5A, treatment with A6 increased the expression of GLP-1 and PPAR-γ and decreased the level of the NF-κB in the small intestine at a dose of 4.0 mg/kg. We also examined the effects of A6 on TNF-α and IL-6 expression by immunohistochemistry in the colonic lumen of db/db mice (Fig. 5B, C). The results showed that the significant down-regulation was observed in the A6-treated group, which showed a 2.76-fold decrease in IL-6 levels and a 3.11-fold down-regulation in TNF-α levels compared with the model group (P < 0.01).
Figure 5.
Compound A6 enhances the SCFA-stimulated activation of GLP-1 and NF-κB signal pathway. A) The effect of A6 on the expression of GLP-1, PPAR-γ, and NF-κB. B) The impact of A6 on the secretion of IL-6 and TNF-α in db/db mice colons. Original magnification, ×200. C) The immunologic histologic chemistry indexes for IL-6 and TNF-α. **P < 0.01 compared with model group.
The effect of A6 on antibiotics-induced germ-free mice
Antibiotic-induced germ-free mice were generated by oral application of some broad-spectrum antibiotics (vancomycin, erythromycin, ampicillin, gentamycin, and neomycin) to db/db mice for 3 wk (Supplemental Fig. S4). In nonantibiotic pretreatment assay, the db/db mice treated with 4.0 mg/kg of A6 showed a 22.5, 19.8, 45.5, 20.0, 19.1, and 40.0% reduction in body weight, 24 h food intake, free diet blood glucose, AUC of impairing GTT (IGTT), AUC of insulin tolerance test, and serum LPS, respectively, compared with that of the model group. In a separate experiment with antibiotics-induced germ-free db/db mice, there is no significant difference in the reduction of body weight, 24 h food intake, and serum LPS, impairing glucose tolerance, and insulin tolerance between the A6-treated group and the model group (Supplemental Fig. S4A, B, D–F). However, the free diet blood glucose levels were significantly down-regulated in A6-treated (4.0 mg/kg) antibiotics-induced germ-free db/db mice compared with that of the vehicle-treated group (P < 0.01, Supplemental Fig. S4C). Based on the above, these results showed that the therapeutic efficacy of A6 was largely dependent on its regulation of gut microbiota.
DISCUSSION
The marine sponge–derived fungus A. terreus is an abundant source of butyrolactone derivatives, which are formed by a 5-membered lactone ring and 2 benzene rings (36). A6, isolated from A. terreus var. africanus IFO 8835, was first described in 1977 (37, 38). Furthermore, the idea of A6 as a quorum sensing (QS) molecule has been certified (39). The supplement of A6 to the A. terreus cultures was found to switch on secondary metabolism and morphologic development (40, 41). Furthermore, Raina et al. (41) demonstrated that A6 interfered with the QS system by regulating the expression of a large number of related genes, including transcription factors, membrane, and ribosomal proteins. Although QS has only recently been discovered in A. terreus, the molecular mechanism by which A6 regulates the production of secondary metabolites is unclear.
A6 has been found to exhibit various activities, including anti-inflammation (17), anti-tumor (42), and antioxidant activities (20). As show in Table 1, A6 showed the most powerful anti-inflammation activity compared with the others, causing speculation that the prenyl and dihydropyran ring moieties may increase the anti-inflammation activity. A6–A9, possessing a 3-phenyl-4-benzyl substituent, turned out to be much more active than A1–A5 with 2-benzyl-3-phenyl, which suggests that the substituted pattern is a crucial factor for the anti-inflammation activity. Research showes that A6, as a naturally selective inhibitor of cyclin-dependent kinases (CDKs)-1/2, participates in the progression from G1 to S phase and from G2 to M phase in HuH-7 or Hela cell, respectively (38). Furthermore, inhibitors of CDKs could clear potentially injurious inflammatory cells and enhance the resolution of inflammation by both limiting cell proliferation and promoting cell apoptosis (43, 44). But in our cell viability assay, we found that A6 had no effect on cell proliferation by CCK8 in normal cells NCM460 and 16HBE. These results indicated that the anti-inflammatory effect of A6 was not related to inhibitory activity of CDKs. Rencently, Zhang et al. (17) indicated that A6 could prevent the translocation of NF-κB to the nucleus, reducing the inflammatory response, which may be the potential molecular mechanism underlying the anti-inflammatory of A6. Meanwhile, A6 exhibited a stronger activity compared with A7, which was in accordance with a previous report that found that the prenyl chain makes critical contributions to the α-glucosidase inhibitory effect (45). In this present study, A6 exhibited the strongest α-glucosidase and TNF-α inhibitory activity, which were associated with diabetes. Thus, we researched the improving T2D effect of A6 in db/db mice.
In the hypoglycemic assay, treatment with A6 or acarbose in the intragastric administration marked improved glucose intolerance and insulin resistance in db/db mice. Weights were significantly different at the time of the GTT in the 4.0 mg/kg A6-treated (49.36 ± 0.96 g) and model-group mice (57.08 ± 1.3 g), indicating an association between glucose intolerance and weight in the A6-treated db/db mice. The effect of A6 on glucose homeostasis is attributable to ameliorate glucose intolerance and insulin resistance, although a direct effect on β-cell secretion or insulin production could not be ruled out. Further investigation is required to determine precisely the functional responses of β cells to glucose stimulation following A6 and the longer-term effect on β-cell mass of exposure to A6. Overall, compound A6 has been demonstrated so far to be a potential drug candidate with multiple therapeutic effects, including the decrease of body weight and blood glucose as well as the improvement of glucose intolerance and insulin resistance. Compound A6 exhibited more potency than acarbose in reducting body weight and exhibited similar efficacies as that of acarbose in improving hyperglycemia.
Docking is a theoretical and powerful method used for the discovery of interactions between proteins and molecules (46). In our studies, a total of 100 random docking calculations were performed for A6 in the receptor hydrophobic cavity through molecular docking. In the best molecular interaction pose of A6 and α-glucosidase, 2 hydrogen bonds were formed with Asn569 and Asp597, both of which are amino acid residues of α-glucosidase. The binding energy was with high negative values (−110.37 kcal/mol), indicating strong interactions between A6 and α-glucosidase. The energy profile suggests that A6 formed strong interactions with active site residues of α-glucosidase that are due to hydrogen bonds. This study clearly indicated that A6 might be a good inhibitor of α-glucosidase.
α-Glucosidase inhibitor induced an increased concentration of multiple saccharides in the lower parts of the gut (15), which may alter microbial fermentation in the distal intestine. Concomitant with the improved hyperglycemia status, LEfSe analysis also indentified specific gut bacteria whose abundances were altered by treatment of A6. A previous preliminary study has demonstrated that acarbose could increase the relative abundance of Lactobacillus and Bifidobacterium in the gut microbiota and decrease Bacteroides in patients with diabetes (11). In our study, these results also suggest that there are some common trends between the A6 and acarbose treatment effects on the microbiome but also some significant differences. A6 as a powerful α-glucosidase inhibitor increased the relative abundance of Lachnospiraceae, Parabacteroides, and Lactobacillus, which play a vital role in SCFA production and anti-inflammation (15, 30). In contrast, Lachnospiraceae and Parabacteroides were significantly decreased in the acarbose-treament group, which may be the reason why acarbose could not increase the level of SCFA in feces. It is worth noting that the same modulatory effect of A6 and acarbose decreased the abundance of LPS-producing bacteria in the genus of Allorevotella and the proinflammatory bacteria in the genus of Rikenellaceae and Alistipes. This may be one of the reasons that both acarbose and A6 could reduce the proinflammatory marker in the feces of db/db mice.
Increases in LPS-producing gut bacteria or the occurrence of gut dysbiosis can trigger the state of metabolic endotoxemia (47). Metabolic endotoxemia (low-grade elevation in plasma LPS) has been regarded as the major cause for the initiation of diabetes and diabetes-related dysfunctions (48). Lcn-2, LPS, and Flic are sensitive and broadly dynamic markers of intestinal inflammation in mice (49). Thus, we measured these inflammatory markers levels from model, acarbose-, and A6-treated mice. Exposure to A6 and acarbose decreased fecal LPS, Lcn-2, and Flic levels in db/db mice (Fig. 4) and is correlated with the decrease of Allorevotella and Alistipes (22).
Members of Lactobacillus and Lachnospiraceae were reported to generate SCFAs from polysaccharides by fermentation, and to promote the repair of intestinal mucosa to reduce the growth of harmful bacteria in order to maintain a stable intestinal environment (30). SCFA produced by intestinal bacteria plays a key role in stimulating mucin release (22), dampening inflammation (50), and improving the energy metabolism (51). Meanwhile, treatment with A6 down-regulated the expression of serum proinflammatory cytokine IL-6 and TNF-α, the reduction of which can promote fat decomposition and lead to decreased body weight, improving insulin resistance, and hyperinsulinemia (52).
Furthermore, SCFA can regulate intestinal immunity and maintain gut homeostasis of the host (53). It promotes expansion of gram-positive bacterium via SCFA-activated GLP-1 signaling. SCFAs interact with the intestinal cell surface free fatty acid receptor 3 to promote intestinal GLP-1 secretion (54). GLP-1 can promote the insulin secretion and enhance the body’s sensitivity to insulin and then enhance the mitochondrial function, increase energy consumption, and activate the PPAR-γ. PPAR-γ, by interacting with NF-κB, prevents the binding of NF-κB to the homologous cis-elements in the promoter region of the inflammatory factor gene, regulates the inflammatory response, and maintains the stability of the intestinal environment (55). Thus, the increased level of acetic acid, methylacetic acid, butyric acid, and pentanoci acid by A6 that is due to the increase of Lachnospiraceae and Lactobacillus in gut microbiota is conducive to improving gut barrier function and inhibiting the expansion of Rikenellaceae, Alistipes, and Alloprevotella.
At last, we executed assays with antibiotics-induced germ-free db/db mice to further confirm whether the gut microbiota was involved in the regulating effects of A6. The therapeutic effects of A6 (4.0 mg/kg) were largely weakened on reduction of body weight and 24 h food intake, ameliorating blood glucose metabolic disorders, and endotoxemia in antibiotics-treated db/db mice (Supplemental Fig. S4). Interestingly, antibiotic treatment could not completely abrogate the hypoglycemic effects of A6. A6 (4.0 mg/kg) significantly decreased the plasma free diet blood glucose level compared with the antibiotic-induced model group (Supplemental Fig. S4), which could be attributed to the antagonistic action of A6 inhibiting α-glucosidase. The role of gut microbiota in metabolizing A6 and the metabolites of A6 have been reported in rats (56). An et al. found that intestinal bacteria could metabolize A6 into aspernolide E, butyrolactone VI, 7ʹR-methoxy-8ʹS-hydroxy-aspernolide E, 7ʹS-acetyl-8ʹR-hydroxy-aspernolide E, 7ʹR-acetyl-8ʹS-hydroxy-aspernolide E, 7ʹR, 8ʹS-dihydroxy-aspernolide E, 7ʹS, 8ʹR-dihydroxy-aspernolide E, butyrolactone V, 7ʹR-hydroxy-9ʹ-ene-butyrolactone-I, and 7ʹS-hydroxy-9ʹ-ene-butyrolactone-I by hydroxylation, cyclization, and isomerization reactions. Furthermore, the hydroxylation sites were usually at α-position of the double bond of the isopentenyl or the benzyl position. Acetylation and methylation occurred in 7ʹ-OH mostly owing to the smaller steric hindrance of the tetrahydropyran ring moiety (56).
CONCLUSIONS
As previously mentioned, A6, an effective α-glucosidase inhibitor with high security and good stability, exerted potent antidiabetic effects and was firstly demonstrated in this study. It showed multiple activities in glycemic control, including weight reduction, improvement of intestinal mucosal barrier function, and endoxemia relief. It was certified that A6 employed its therapeutic effects through regulation of gut microbiota. The changed gut microbiota increased the level of gut SCFAs (acetic acid, methylacetic acid, butyric acid, and pentanoic acid) and enhanced intestinal mucosal barrier function, which led to an improvement in metabolic endotoxemia. The worldwide epidemic of diabetes and the related metabolic diseases have become a grievous public health problem. This study affirms that A6 is a promising drug candidate for treatment of T2D.
ACKNOWLEDGMENTS
This study was supported by the National Key Research and Development Program of China (2018YFC0310900) and National Natural Science Foundation of China Grants/Awards: U1605221, 81502936, and 21502113. The authors declare no conflicts of interest.
Glossary
- A6
butyrolactone-I
- AIOD
average integral optical density
- ALT
alanine transaminase
- AST
aspartate transaminase
- AUC
area under curve
- CCK
Cell Counting Kit
- CDK
cyclin-dependent kinase
- Flic
flagellin C
- GLP-1
glucagon-like peptide 1
- GTT
glucose tolerance test
- IC50
half maximal inhibitory concentration
- Klf4
Krüppel-like factor 4
- Lcn-2
lipocalin-2
- LEfSe
linear discriminant analysis effect size
- LSD
least significant difference
- Muc-2
mucoprotein 2
- OTU
operational taxonomic unit
- PCA
principal component analysis
- PCoA
principal coordinate analysis
- PPAR-γ
peroxisome proliferator-activated receptor-γ
- QS
quorum sensing
- SCFA
short-chain fatty acid
- T2D
type 2 diabetes
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
W. Wu, L. Liu, and H. Zhu performed the experiments; W. Wu, H. Zhu, Y. Wu, H. Liao, Y. Gui, L. Li, and L. Liu drafted the manuscript; W. Wu, F. Sun, and H. Lin conceived and designed the research; Y. Sun provided the compounds; F. Sun and H. Lin edited and revised the manuscript; and all authors read and approved the final manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Shaw J. E., Sicree R. A., Zimmet P. Z. (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 87, 4–14 [DOI] [PubMed] [Google Scholar]
- 2.Rutledge P. J., Challis G. L. (2015) Discovery of microbial natural products by activation of silent biosynthetic gene clusters. Nat. Rev. Microbiol. 13, 509–523 [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim M., Tuomilehto J., Aschner P., Beseler L., Cahn A., Eckel R. H., Fischl A. H., Guthrie G., Hill J. O., Kumwenda M., Leslie R. D., Olson D. E., Pozzilli P., Weber S. L., Umpierrez G. E. (2018) Global status of diabetes prevention and prospects for action: a consensus statement. Diabetes Metab. Res. Rev. 34, e3021 [DOI] [PubMed] [Google Scholar]
- 4.Frydrych L. M., Bian G., O’Lone D. E., Ward P. A., Delano M. J. (2018) Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 104, 525–534 [DOI] [PubMed] [Google Scholar]
- 5.Anuradha R., Saraswati M., Kumar K. G., Rani S. H. (2014) Apoptosis of beta cells in diabetes mellitus. DNA Cell Biol. 33, 743–748 [DOI] [PubMed] [Google Scholar]
- 6.Naguib G., Al-Mashat H., Desta T., Graves D. T. (2004) Diabetes prolongs the inflammatory response to a bacterial stimulus through cytokine dysregulation. J. Invest. Dermatol. 123, 87–92 [DOI] [PubMed] [Google Scholar]
- 7.Han L. P., Li C. J., Sun B., Xie Y., Guan Y., Ma Z. J., Chen L. M. (2016) Protective effects of celastrol on diabetic liver injury via TLR4/MyD88/NF-κB signaling pathway in type 2 diabetic rats. J. Diabetes Res. 2016, 2641248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo C., Yang H., Tang C., Yao G., Kong L., He H., Zhou Y. (2015) Kaempferol alleviates insulin resistance via hepatic IKK/NF-κB signal in type 2 diabetic rats. Int. Immunopharmacol. 28, 744–750 [DOI] [PubMed] [Google Scholar]
- 9.Qin J., Li Y., Cai Z., Li S., Zhu J., Zhang F., Liang S., Zhang W., Guan Y., Shen D., Peng Y., Zhang D., Jie Z., Wu W., Qin Y., Xue W., Li J., Han L., Lu D., Wu P., Dai Y., Sun X., Li Z., Tang A., Zhong S., Li X., Chen W., Xu R., Wang M., Feng Q., Gong M., Yu J., Zhang Y., Zhang M., Hansen T., Sanchez G., Raes J., Falony G., Okuda S., Almeida M., LeChatelier E., Renault P., Pons N., Batto J. M., Zhang Z., Chen H., Yang R., Zheng W., Li S., Yang H., Wang J., Ehrlich S. D., Nielsen R., Pedersen O., Kristiansen K., Wang J. (2012) A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 490, 55–60 [DOI] [PubMed] [Google Scholar]
- 10.Liu G., Bei J., Liang L., Yu G., Li L., Li Q. (2018) Stachyose improves inflammation through modulating gut microbiota of high-fat diet/streptozotocin-induced type 2 diabetes in rats. Mol. Nutr. Food Res. 62, e1700954 [DOI] [PubMed] [Google Scholar]
- 11.Gu Y., Wang X., Li J., Zhang Y., Zhong H., Liu R., Zhang D., Feng Q., Xie X., Hong J., Ren H., Liu W., Ma J., Su Q., Zhang H., Yang J., Wang X., Zhao X., Gu W., Bi Y., Peng Y., Xu X., Xia H., Li F., Xu X., Yang H., Xu G., Madsen L., Kristiansen K., Ning G., Wang W. (2017) Analyses of gut microbiota and plasma bile acids enable stratification of patients for antidiabetic treatment. Nat. Commun. 8, 1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang B., Sun Y., Sang Y., Liu X., Liang J. (2018) Comparison of dipeptidyl peptidase-4 inhibitors and pioglitazone combination therapy versus pioglitazone monotherapy in type 2 diabetes: a system review and meta-analysis. Medicine (Baltimore) 97, e12633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maury J., Issad T., Perdereau D., Gouhot B., Ferré P., Girard J. (1993) Effect of acarbose on glucose homeostasis, lipogenesis and lipogenic enzyme gene expression in adipose tissue of weaned rats. Diabetologia 36, 503–509 [DOI] [PubMed] [Google Scholar]
- 14.DiNicolantonio J. J., Bhutani J., O’Keefe J. H. (2015) Acarbose: safe and effective for lowering postprandial hyperglycaemia and improving cardiovascular outcomes. Open Heart 2, e000327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang K., Bao L., Zhou N., Zhang J., Liao M., Zheng Z., Wang Y., Liu C., Wang J., Wang L., Wang W., Liu S., Liu H. (2018) Structural modification of natural product ganomycin I leading to discovery of a α-glucosidase and HMG-CoA reductase dual inhibitor improving obesity and metabolic dysfunction in vivo. J. Med. Chem. 61, 3609–3625 [DOI] [PubMed] [Google Scholar]
- 16.Su B., Liu H., Li J., Sunli Y., Liu B., Liu D., Zhang P., Meng X. (2015) Acarbose treatment affects the serum levels of inflammatory cytokines and the gut content of bifidobacteria in Chinese patients with type 2 diabetes mellitus. J. Diabetes 7, 729–739 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y. Y., Zhang Y., Yao Y. B., Lei X. L., Qian Z. J. (2018) Butyrolactone-I from coral-derived fungus Aspergillus terreus attenuates neuro-inflammatory response via suppression of NF-κB pathway in BV-2 cells. Mar. Drugs 16, E202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Da Silva I. P., Brissow E., Kellner Filho L. C., Senabio J., de Siqueira K. A., Vandresen Filho S., Damasceno J. L., Mendes S. A., Tavares D. C., Magalhães L. G., Junior P. A., Januário A. H., Soares M. A. (2017) Bioactive compounds of Aspergillus terreus-F7, an endophytic fungus from Hyptis suaveolens (L.) Poit. World J. Microbiol. Biotechnol. 33, 62 [DOI] [PubMed] [Google Scholar]
- 19.Goutam J., Sharma G., Tiwari V. K., Mishra A., Kharwar R. N., Ramaraj V., Koch B. (2017) Isolation and characterization of “terrein” an antimicrobial and antitumor compound from endophytic fungus Aspergillus terreus (JAS-2) associated from Achyranthus aspera Varanasi, India. Front. Microbiol. 8, 1334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang C., Guo L., Hao J., Wang L., Zhu W. (2016) α-Glucosidase inhibitors from the marine-derived fungus Aspergillus flavipes HN4-13. J. Nat. Prod. 79, 2977–2981 [DOI] [PubMed] [Google Scholar]
- 21.Sun Y., Liu J., Li L., Gong C., Wang S., Yang F., Hua H., Lin H. (2018) New butenolide derivatives from the marine sponge-derived fungus Aspergillus terreus. Bioorg. Med. Chem. Lett. 28, 315–318; erratum: 3281–3282 [DOI] [PubMed] [Google Scholar]
- 22.Chassaing B., Koren O., Goodrich J. K., Poole A. C., Srinivasan S., Ley R. E., Gewirtz A. T. (2015) Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 519, 92–96; erratum: 536, 238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Úbeda M., Lario M., Muñoz L., Borrero M. J., Rodríguez-Serrano M., Sánchez-Díaz A. M., Del Campo R., Lledó L., Pastor Ó., García-Bermejo L., Díaz D., Álvarez-Mon M., Albillos A. (2016) Obeticholic acid reduces bacterial translocation and inhibits intestinal inflammation in cirrhotic rats. J. Hepatol. 64, 1049–1057 [DOI] [PubMed] [Google Scholar]
- 24.Wei W., Feng W., Xin G., Tingting N., Zhanghe Z., Haimin C., Xiaojun Y. (2016) Enhanced effect of κ-carrageenan on TNBS-induced inflammation in mice. Int. Immunopharmacol. 39, 218–228 [DOI] [PubMed] [Google Scholar]
- 25.Zuo Y. N., He X. L., Shi X. N., Wei S. H., Yin H. L. (2017) [The correlation between microRNAs in serum and the extent of liver injury [in Chinese]]. Sichuan Da Xue Xue Bao Yi Xue Ban 48, 368–372 [PubMed] [Google Scholar]
- 26.Miller D. N., Bryant J. E., Madsen E. L., Ghiorse W. C. (1999) Evaluation and optimization of DNA extraction and purification procedures for soil and sediment samples. Appl. Environ. Microbiol. 65, 4715–4724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jiang C. Y., Dong L., Zhao J. K., Hu X., Shen C., Qiao Y., Zhang X., Wang Y., Ismagilov R. F., Liu S. J., Du W. (2016) High-throughput single-cell cultivation on microfluidic streak plates. Appl. Environ. Microbiol. 82, 2210–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shang Q., Sun W., Shan X., Jiang H., Cai C., Hao J., Li G., Yu G. (2017) Carrageenan-induced colitis is associated with decreased population of anti-inflammatory bacterium, Akkermansia muciniphila, in the gut microbiota of C57BL/6J mice. Toxicol. Lett. 279, 87–95 [DOI] [PubMed] [Google Scholar]
- 29.Ling Z., Liu X., Jia X., Cheng Y., Luo Y., Yuan L., Wang Y., Zhao C., Guo S., Li L., Xu X., Xiang C. (2014) Impacts of infection with different toxigenic Clostridium difficile strains on faecal microbiota in children. Sci. Rep. 4, 7485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meehan C. J., Beiko R. G. (2014) A phylogenomic view of ecological specialization in the Lachnospiraceae, a family of digestive tract-associated bacteria. Genome Biol. Evol. 6, 703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shen F., Zheng R. D., Sun X. Q., Ding W. J., Wang X. Y., Fan J. G. (2017) Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 16, 375–381 [DOI] [PubMed] [Google Scholar]
- 32.Andoh A., Nishida A., Takahashi K., Inatomi O., Imaeda H., Bamba S., Kito K., Sugimoto M., Kobayashi T. (2016) Comparison of the gut microbial community between obese and lean peoples using 16S gene sequencing in a Japanese population. J. Clin. Biochem. Nutr. 59, 65–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mohammad M. K., Morran M., Slotterbeck B., Leaman D. W., Sun Y., Grafenstein H., Hong S. C., McInerney M. F. (2006) Dysregulated Toll-like receptor expression and signaling in bone marrow-derived macrophages at the onset of diabetes in the non-obese diabetic mouse. Int. Immunol. 18, 1101–1113 [DOI] [PubMed] [Google Scholar]
- 34.Shi H., Kokoeva M. V., Inouye K., Tzameli I., Yin H., Flier J. S. (2006) TLR4 links innate immunity and fatty acid-induced insulin resistance. J. Clin. Invest. 116, 3015–3025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christiansen C. B., Gabe M. B. N., Svendsen B., Dragsted L. O., Rosenkilde M. M., Holst J. J. (2018) The impact of short-chain fatty acids on GLP-1 and PYY secretion from the isolated perfused rat colon. Am. J. Physiol. Gastrointest. Liver Physiol. 315, G53–G65 [DOI] [PubMed] [Google Scholar]
- 36.Lepoittevin J. P., Berl V., Giménez-Arnau E. (2009) Alpha-methylene-gamma-butyrolactones: versatile skin bioactive natural products. Chem. Rec. 9, 258–270 [DOI] [PubMed] [Google Scholar]
- 37.Kiriyama N., Nitta K., Sakaguchi Y., Taguchi Y., Yamamoto Y. (1977) Studies on the metabolic products of Aspergillus terreus. III. metabolites of the strain IFO 8835. (1). Chem. Pharma. Bull. 25, 2593–2601 [Google Scholar]
- 38.Niu X., Dahse H. M., Menzel K. D., Lozach O., Walther G., Meijer L., Grabley S., Sattler I. (2008) Butyrolactone I derivatives from Aspergillus terreus carrying an unusual sulfate moiety. J. Nat. Prod. 71, 689–692 [DOI] [PubMed] [Google Scholar]
- 39.Palonen E. K., Neffling M. R., Raina S., Brandt A., Keshavarz T., Meriluoto J., Soini J. (2014) Butyrolactone I quantification from lovastatin producing Aspergillus terreus using tandem mass spectrometry-evidence of signalling functions. Microorganisms 2, 111–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schimmel T. G., Coffman A. D., Parsons S. J. (1998) Effect of butyrolactone I on the producing fungus, Aspergillus terreus. Appl. Environ. Microbiol. 64, 3707–3712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Raina S., De Vizio D., Palonen E. K., Odell M., Brandt A. M., Soini J. T., Keshavarz T. (2012) Is quorum sensing involved in lovastatin production in the filamentous fungus Aspergillus terreus? Process. Biochem. 47, 843–852 [Google Scholar]
- 42.Nishio K., Ishida T., Arioka H., Kurokawa H., Fukuoka K., Nomoto T., Fukumoto H., Yokote H., Saijo N. (1996) Antitumor effects of butyrolactone I, a selective cdc2 kinase inhibitor, on human lung cancer cell lines. Anticancer Res. 16, 3387–3395 [PubMed] [Google Scholar]
- 43.Tsou Y. C., Wang H. H., Hsieh C. C., Sun K. H., Sun G. H., Jhou R. S., Lin T. I., Lu S. Y., Liu H. Y., Tang S. J. (2016) Down-regulation of BNIP3 by olomoucine, a CDK inhibitor, reduces LPS- and NO-induced cell death in BV2 microglial cells. Neurosci. Lett. 628, 186–193 [DOI] [PubMed] [Google Scholar]
- 44.Rossi A. G., Sawatzky D. A., Walker A., Ward C., Sheldrake T. A., Riley N. A., Caldicott A., Martinez-Losa M., Walker T. R., Duffin R., Gray M., Crescenzi E., Martin M. C., Brady H. J., Savill J. S., Dransfield I., Haslett C. (2006) Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat. Med. 12, 1056–1064; erratum: 1434 [DOI] [PubMed] [Google Scholar]
- 45.Zhang L. H., Feng B. M., Zhao Y. Q., Sun Y., Liu B., Liu F., Chen G., Bai J., Hua H. M., Wang H. F., Pei Y. H. (2016) Polyketide butenolide, diphenyl ether, and benzophenone derivatives from the fungus Aspergillus flavipes PJ03-11. Bioorg. Med. Chem. Lett. 26, 346–350 [DOI] [PubMed] [Google Scholar]
- 46.Lin X., Chai L., Liu B., Chen H., Zheng L., Liu Q., Lin C. (2016) Synthesis, biological evaluation, and docking studies of a novel sulfonamido-based gallate as pro-chondrogenic agent for the treatment of cartilage. Molecules 22, E3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cani P. D., Bibiloni R., Knauf C., Waget A., Neyrinck A. M., Delzenne N. M., Burcelin R. (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 [DOI] [PubMed] [Google Scholar]
- 48.Manco M., Putignani L., Bottazzo G. F. (2010) Gut microbiota, lipopolysaccharides, and innate immunity in the pathogenesis of obesity and cardiovascular risk. Endocr. Rev. 31, 817–844 [DOI] [PubMed] [Google Scholar]
- 49.Chassaing B., Srinivasan G., Delgado M. A., Young A. N., Gewirtz A. T., Vijay-Kumar M. (2012) Fecal lipocalin 2, a sensitive and broadly dynamic non-invasive biomarker for intestinal inflammation. PLoS One 7, e44328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Furusawa Y., Obata Y., Fukuda S., Endo T. A., Nakato G., Takahashi D., Nakanishi Y., Uetake C., Kato K., Kato T., Takahashi M., Fukuda N. N., Murakami S., Miyauchi E., Hino S., Atarashi K., Onawa S., Fujimura Y., Lockett T., Clarke J. M., Topping D. L., Tomita M., Hori S., Ohara O., Morita T., Koseki H., Kikuchi J., Honda K., Hase K., Ohno H. (2013) Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature 504, 446–450; erratum: 506, 254 [DOI] [PubMed] [Google Scholar]
- 51.Li Z., Yi C. X., Katiraei S., Kooijman S., Zhou E., Chung C. K., Gao Y., van den Heuvel J. K., Meijer O. C., Berbée J. F. P., Heijink M., Giera M., Willems van Dijk K., Groen A. K., Rensen P. C. N., Wang Y. (2018) Butyrate reduces appetite and activates brown adipose tissue via the gut-brain neural circuit. Gut 67, 1269–1279 [DOI] [PubMed] [Google Scholar]
- 52.Ziegler T. R., Luo M., Estívariz C. F., Moore D. A., III, Sitaraman S. V., Hao L., Bazargan N., Klapproth J. M., Tian J., Galloway J. R., Leader L. M., Jones D. P., Gewirtz A. T. (2008) Detectable serum flagellin and lipopolysaccharide and upregulated anti-flagellin and lipopolysaccharide immunoglobulins in human short bowel syndrome. Am. J. Physiol. Regul. Integr. Comp. Physiol. 294, R402–R410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Byndloss M. X., Olsan E. E., Rivera-Chávez F., Tiffany C. R., Cevallos S. A., Lokken K. L., Torres T. P., Byndloss A. J., Faber F., Gao Y., Litvak Y., Lopez C. A., Xu G., Napoli E., Giulivi C., Tsolis R. M., Revzin A., Lebrilla C. B., Bäumler A. J. (2017) Microbiota-activated PPAR-γ signaling inhibits dysbiotic Enterobacteriaceae expansion. Science 357, 570–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morrison D. J., Preston T. (2016) Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 7, 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Priyadarshini M., Wicksteed B., Schiltz G. E., Gilchrist A., Layden B. T. (2016) SCFA receptors in pancreatic β cells: novel diabetes targets? Trends Endocrinol. Metab. 27, 653–664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.An X., Feng B. M., Chen G., Chen S. F., Bai J., Hua H. M., Wang H. F., Pei Y. H. (2017) Isolation and identification of phase I metabolites of butyrolactone I in rats. Xenobiotica 47, 236–244 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.