Abstract
Reactive oxygen species (ROS) can act as second messengers in various signaling pathways, and abnormal oxidation contributes to multiple diseases, including cancer. Detecting and quantifying protein oxidation is crucial for a detailed understanding of reduction-oxidation reaction (redox) signaling. We developed an Activated Thiol Sepharose–based proteomic (ATSP) approach to quantify reversible protein oxidation. ATSP can enrich H2O2-sensitive thiol peptides, which are more likely to contain reactive cysteines involved in redox signaling. We applied our approach to analyze hereditary leiomyomatosis and renal cell carcinoma (HLRCC), a type of kidney cancer that harbors fumarate hydratase (FH)-inactivating mutations and has elevated ROS levels. Multiple proteins were oxidized in FH-deficient cells, including many metabolic proteins such as the pyruvate kinase M2 isoform (PKM2). Treatment of HLRCC cells with dimethyl fumarate or PKM2 activators altered PKM2 oxidation levels. Finally, we found that ATSP could detect Src homology region 2 domain–containing phosphatase-2 and PKM2 oxidation in cells stimulated with platelet-derived growth factor. This newly developed redox proteomics workflow can detect reversible oxidation of reactive cysteines and can be employed to analyze multiple physiologic and pathologic conditions.—Xu, Y., Andrade, J., Ueberheide, B., Neel, B. G. Activated Thiol Sepharose–based proteomic approach to quantify reversible protein oxidation.
Keywords: redox regulation, PKM2, HLRCC, ATSP
Over the past 2 decades, increasing evidence has revealed that oxidation of cysteinyl residues is an important post-translational modification that can regulate enzymatic activity, protein localization, and protein-protein interactions (1–3). However, compared with other post-translational modifications, protein oxidation is understudied, at least in part because of technical difficulties in detecting oxidation events (4), such as the instability of some protein oxidation states, postlysis oxidation from ambient oxidants (mainly O2), signal attenuation from surrounding antioxidants, and low signal:noise ratio due to the transience of protein oxidation during signal transduction.
Multiple techniques attempt to overcome these difficulties. Some were designed to specifically detect oxidation of protein tyrosine phosphatases (PTPs), which are particularly sensitive to oxidation. The modified in-gel phosphatase assay was the first to detect oxidation of multiple PTPs simultaneously (5, 6). Subsequently, our group used a specific antibody against the most highly oxidized state of classic PTPs to develop a mass spectrometry (MS)-based approach to globally detect their oxidation (7, 8). However, many other types of protein can be oxidized, and in several cases, such oxidation has important physiologic and pathologic consequences (9–12). More general reduction-oxidation reaction (redox) techniques have been developed to globally detect protein oxidation, such as the isotope-coded affinity tag method (13), the modified cysteinyl-labeling assay (14, 15), and dimedone-dimedone antibody-based approaches (16–19). Each of these approaches has certain technical limitations. For example, the isotope-coded affinity tag method measures the decrease in oxidation signal after cellular stimulation, resulting in an intrinsically low signal:noise ratio (4). Although dimedone analogs directly label oxidized cysteines, they mainly react with sulfenic acids (S-OHs), which are only one of the reversible oxidation states of cysteine. Because the sulfenic acid state is usually labile and rearranges rapidly to a sulfenylamide (S-N) or disulfide (S-S) (20–22), dimedone-dimedone antibody-based approaches might miss important oxidation events, especially in cells under chronic oxidative stress, which is an important feature of many pathologies.
Activated Thiol Sepharose (ATS), which contains a dipyridyl disulfide group that can bind to free thiol–containing proteins (23), is another promising redox probe. The disulfide bond formed between ATS and free thiols is both strong and reversible, enabling harsh purification conditions followed by easy, quantitative elution for MS analysis. First developed for thiol-based affinity chromatography for elongation factor purification from Escherichia coli (24), ATS was subsequently used to define the disulfide proteome in plants (25). Later, Hu et al. (26) used an ATS-based approach to identify >100 disulfide-forming proteins in menadione-treated E. coli. In addition, several other groups have used ATS to assess cysteine oxidation or other cysteine modifications inside cells (27–30). All of those studies featured a protein denaturation step before ATS labeling, which disrupts the local environment of the reactive cysteines (involved in redox signaling), rendering them indistinguishable from structural cysteines (not involved in redox signaling).
We developed an ATS-based proteomic (ATSP) approach that preserves and thus enriches for reactive cysteines. ATSP coupled with MS is specialized for identifying cysteine oxidation sites and for global quantification of the reversible redox proteome, whereas ATSP coupled with immunoblotting (IB) can be used to quantify the oxidation of specific proteins and validate the results from ATSP-MS. We used ATSP-MS to analyze cellular models of fumarate hydratase (FH)-mutated hereditary leiomyomatosis and renal cell carcinoma (HLRCC), a disorder in which increased reactive oxygen species (ROS) levels are thought to contribute to pathogenesis. We identified multiple proteins (especially metabolic enzymes) that were oxidized in these cells, including pyruvate kinase M2 isoform (PKM2) and transketolase (TKT). PKM2 oxidation was confirmed by ATSP-IB and could be modulated by treating cells with dimethyl fumarate (DMF) or PKM2 activators. We also investigated protein oxidation in response to physiologic stimuli to confirm the sensitivity of ATSP. We conclude that ATSP has great potential for detecting the reversible oxidation of reactive cysteines in redox signaling.
MATERIALS AND METHODS
Cell lines and cell culture
Rat1 cells were kindly provided by Dr. Nicholas Tonks (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA). UOK262 FH–wild-type (WT), UOK262 FH-deficient, UOK268 FH-WT, and UOK268 FH-deficient cells were kindly provided by Dr. W. Marston Linehan (National Cancer Institute, Bethesda, MD, USA). All the cells were cultured in DMEM (Corning, Corning, NY, USA). All medium contained 10% fetal bovine serum (Corning) and 100 U/ml penicillin-streptomycin (Thermo Fisher Scientific, Waltham, MA, USA).
ATSP approach
A lysis buffer containing 20 mM Tris-HCl (pH 7.35), 150 mM NaCl, 10% glycerol, 1% NP40 was degassed overnight with vacuum at room temperature and then transferred to a hypoxia workstation (Don Whitley Scientific, Bingley, United Kingdom). N-ethylmaleimide (NEM) (30 mM; MilliporeSigma, Burlington, MA, USA), 0.5 U/μl catalase (MilliporeSigma), 0.125 U/μl superoxide dismutase (MilliporeSigma), and a protease inhibitor cocktail (2 μg/ml antipain, 2 μg/ml pepstatin A, 20 μg/ml leupeptin, and 20 μg/ml aprotinin) were added to the lysis buffer immediately before use. Within the hypoxia workstation, cells were quickly washed once with degassed PBS, immediately lysed, and incubated for 1 h at 4°C in the dark with constant rotation. Homogenates were clarified at 10,000 g for 1 min at 4°C. Supernatants were collected, and the buffer was exchanged by gel filtration (GE Healthcare, Waukesha, WI, USA) into the degassed solution [20 mM HEPES (pH 6.5), 100 mM NaCl, 10% glycerol, 0.05% NP40, and 0.02 U/μl catalase]. Samples were then treated with 1.5 mM DTT and rotated in the dark for 2 h at 37°C. Within the hypoxia workstation, lysates were buffer exchanged again to remove DTT. ATS 4B (GE Healthcare) was added, and the samples were rotated overnight at 4°C in the dark.
For ATSP-IB, ATS-bound proteins were washed 3 times with RIPA buffer and 1 time with 8 M urea and then incubated with 2× Laemmli sample buffer (30 min at 56°C) before resolving on SDS-PAGE. For ATSP-MS, ATS-bound proteins were first washed 3 times with RIPA buffer. ATS-bound proteins were then incubated in a buffer containing 8 M urea and 20 mM iodoacetamide (IAM) [dissolved in 50 mM ammonium bicarbonate (pH 8–8.5)] for 10 min at room temperature in the dark with constant rotation (repeated once). After alkylation, ATS-bound proteins were washed with 50 mM ammonium bicarbonate (pH 8–8.5, 4×) and then digested with 1 μg trypsin (Thermo Fisher Scientific) for 30 min at 37°C with constant rotation. The supernatant after first trypsin digestion was discarded. ATS-bound proteins and peptides were washed once with 50 mM ammonium bicarbonate and again digested with 1 μg trypsin overnight at 37°C with constant rotation. The next day, ATS-bound peptides were washed with 50 mM ammonium bicarbonate (3 times) and eluted with 1 mM tris(2-carboxyethyl)phosphine (TCEP) (Thermo Fisher Scientific) for 30 min at 37°C with constant rotation. The eluates were then alkylated with 10 mM IAM (30 min at room temperature in the dark). The elution and alkylation procedure was repeated 1 more time, and the 2 eluates were combined, acidified with TFA (final pH ∼2), purified using StageTips (Thermo Fisher Scientific), and analyzed by MS.
IB
Whole-cell lysate (WCL; 10–50 μg) and ATS-bound proteins, supernatant, or both were resolved by SDS-PAGE and analyzed by IB. Antibodies included: anti-PGK1/2, anti-FH, and anti-ERK2 antibodies from Santa Cruz Biotechnology (Dallas, TX, USA); anti–glyceraldehyde 3-phosphate dehydrogenase (GAPDH) antibody from MilliporeSigma; anti-HIF1α antibody from BD Biosciences (San Jose, CA, USA); anti–phosphoglucomutase 1 and anti-TKT antibodies from Abcam (Cambridge, United Kingdom); and anti–phosphoglycerate dehydrogenase (PHGDH), anti–glucose-6-phosphate dehydrogenase (G6PD), anti-PKM2 antibodies from Cell Signaling Technology (Danvers, MA, USA).
PKM2 succination assay
A lysis buffer containing 20 mM Tris-HCl (pH 7.35), 100 mM NaCl, 10% glycerol, and 1% NP40 was degassed overnight at room temperature and then transferred to a hypoxia workstation (Don Whitley Scientific). Immediately before its use, 30 mM NEM, 0.5 U/μl catalase, 0.125 U/ml superoxide dismutase, and a protease inhibitor cocktail (2 μg/ml antipain, 2 μg/ml pepstatin A, 20 μg/ml leupeptin, and 20 μg/ml aprotinin) were added to the lysis buffer. Within the hypoxia workstation, cells were washed quickly with degassed PBS, immediately lysed in the above buffer, and incubated for 1 h at 4°C in the dark with constant rotation. Homogenates were clarified at 10,000 g for 1 min at 4°C, supernatants were collected, and SDS was added to a final concentration of 1%. Samples were then boiled at 95°C for 10 min; allowed to cool at room temperature for ≥30 min; and then diluted to 0.1% SDS with a buffer containing 50 mM HEPES (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1% NP40, and the protease inhibitor cocktail. Protein (1 mg) was immunoprecipitated with anti-PKM2 antibody at 4°C overnight. The next day, Protein A Sepharose (GE Healthcare) was added and incubated for another 3 h at 4°C. Immunoprecipitates were washed 4 times in lysis buffer and 5 times in rinsing buffer [50 mM ammonium bicarbonate (pH 8–8.5)] and then digested with trypsin on the beads. Samples were purified by using StageTips before MS analysis.
Liquid chromatography–tandem MS
The digested sample was loaded onto an Acclaim PepMap trap column (Thermo Fisher Scientific) in line with an Easy-Spray PepMap analytical column (Thermo Fisher Scientific) by using the autosampler of the EASY-nLC 1200 HPLC (Thermo Fisher Scientific) with solvent A (consisting of 2% acetonitrile in 0.5% acetic acid) and solvent B (consisting of 80% acetonitrile in 0.5% acetic acid). Peptides were gradient eluted into the Orbitrap Fusion Lumos Mass Spectrometer (Thermo Fisher Scientific). MS1 scans were acquired in the Orbitrap, and MS2 scans were acquired in the ion trap using a data-dependent acquisition scheme. All acquired MS2 spectra were searched against the UniProt reference proteome database (https://www.uniprot.org/) using Sequest within Proteome Discoverer (Thermo Fisher Scientific). The search parameters were as follows: precursor mass tolerance ±10 ppm, fragment mass tolerance ±0.4 Da, trypsin cleavage with 2 missed cleavages allowed, variable modification of oxidation of methionine, carbamidomethyl, NEM, dioxidation, trioxidation, and succination modification of cysteine, phosphorylation of serine, threonine, or tyrosine, and deamidation of glutamine and asparagine. Peptides were filtered to better than 1% false discovery rate using a target-decoy database strategy. Label-free quantification of MS1 was obtained by using Skyline (v.4.1.0.11796; MacCoss Lab Software, Seattle, WA, USA).
ROS measurements
Cells were treated as indicated, incubated 30 min with 500 nM 2′,7′-dichlorofluorescin diacetate (DCF-DA) (Thermo Fisher Scientific) at 37°C in phenol red–free medium, trypsinized, washed with cold PBS, and analyzed by CytoFlex Flow Cytometer (Beckman Coulter, Brea, CA, USA).
Statistical analysis
Data are presented as means ± sem. Statistical significance was determined using the unpaired Student’s t test. Statistical analyses were performed using Prism 7 (GraphPad Software, La Jolla, CA, USA), with a value of P < 0.05 considered significant.
RESULTS
Development of ATSP
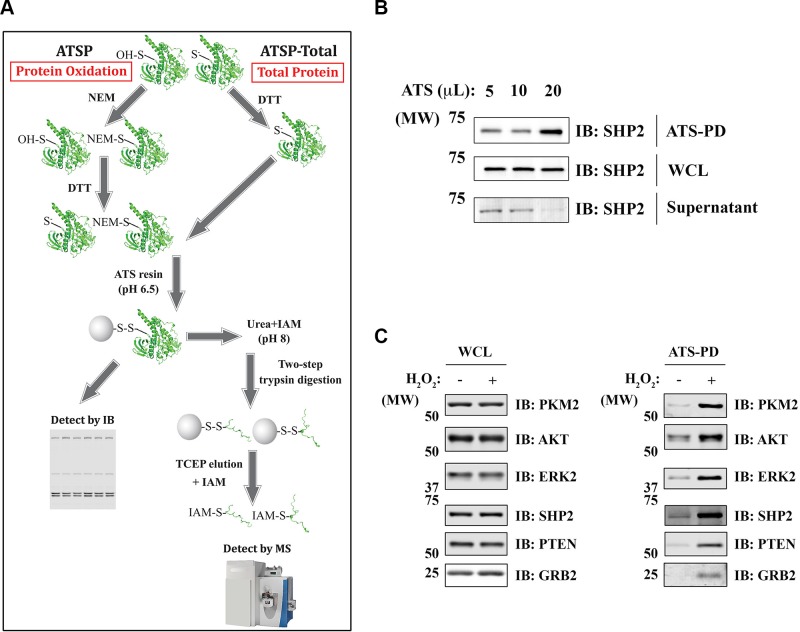
Reactive cysteines usually have lower pKas than typical cysteines (pKa ∼8.5). Consequently, reactive cysteines exist as thiolate anions (S−) at physiologic pH, rendering them more chemically reactive to substrates, ROS, and other chemicals (31). For ATSP, cells were lysed in degassed, nondenaturing lysis buffer containing NEM; under these conditions, reduced (but not oxidized) cysteinyl residues react with NEM. Reversibly oxidized (reactive) cysteinyl residues that escaped alkylation were then reduced with DTT, incubated with ATS resin, placed in sample buffer for IB (ATSP-IB), or processed for MS (ATSP-MS) (Fig. 1A and Materials and Methods). To enhance the specificity of labeling, the ATS incubation was performed at acidic pH with a limited amount of ATS resin (32, 33) (see below). For ATSP-MS analysis, ATS-bound proteins were washed in denaturing wash buffer, subjected to 2-step on-resin trypsin digestion, eluted with TCEP, and alkylated by IAM. ATSP can be modified to quantify total protein (ATSP-total) levels by leaving out the initial NEM alkylation step and using a sufficient amount of ATS resin (Fig. 1A and Materials and Methods). The minimal amount of ATS resin for ATSP was determined empirically for each cell system. For example, as shown in Fig. 1B, a given amount of Rat1 cell lysate was processed by ATSP-total, with sequentially increased amounts of ATS resin. As for the readout, we used Src homology region 2 domain-containing phosphatase-2 (SHP2), which, as a member of PTP superfamily, has a very reactive catalytic cysteinyl residue. The lowest amount of ATS (20 μl in this case) that can deplete SHP2 from supernatant was used for ATSP (Fig. 1B).
Figure 1.
Development of ATSP to detect protein oxidation. A) schematic of ATSP. To detect protein oxidation (ATSP), reduced cysteinyl residues are sequestered by NEM, and the oxidized cysteinyl residues are reduced by DTT before adding ATS. ATS-bound proteins are subjected to sequential on-resin trypsin digestion, eluted with TCEP, alkylated by IAM, and analyzed by MS. For detection of total proteins (ATSP-total), all procedures are the same except for the initial alkylation step. B) determination of the amount of ATS resin used for ATSP. Rat1 cells were processed by ATSP-total, WCL, and ATS-bound proteins [pull down, (PD)], and the supernatants were immunoblotted for SHP2. C) Oxidized proteins detected by ATSP. Rat1 cells were treated with 1 mM H2O2 (4 min) or left untreated. After processing for ATSP, WCL and the ATS-bound proteins were analyzed by IB. GRB, growth factor receptor-bound protein; OH-S, sulfuric acid; PTEN, phosphatase and tensin homolog.
We used ATSP-IB to assess protein oxidation in Rat1 cells treated with H2O2 (Fig. 1C). Multiple previously reported ROS-sensitive proteins were oxidized upon H2O2 treatment, including PTPs (SHP2 and phosphatase and tensin homolog), kinases [protein kinase B (AKT) and extracellular regulated kinase 2 (ERK2)], an adaptor protein (growth factor receptor-bound protein 2), and a glycolytic enzyme (PKM2). We also processed the same H2O2-treated samples by ATSP-MS. A total of ∼3300 unique cysteine-containing peptides comprising ∼1300 distinct proteins were detected by MS (Table 1 and Supplemental Table S1). As expected, ATSP highly enriched for cysteine-containing peptides, with >98% of all the peptides identified by MS containing a thiol group (Table 1). In addition, of the ∼3300 cysteine-containing peptides, ∼1800 (representing ∼1000 proteins) showed significantly increased oxidation after H2O2 treatment; these proteins are likely to contain low-pKa reactive cysteines (Supplemental Table S1).
TABLE 1.
The total number of peptides and number of IAM-modified peptides in H2O2-treated Rat1 cells detected by ATSP
| Treatment | IAM-modified peptides | Total peptides | Percentage of IAM-modified peptides |
|---|---|---|---|
| No treatment | 2479 ± 88 | 2517 ± 90 | 98.48 ± 0.24 |
| H2O2 | 3283 ± 189 | 3340 ± 188 | 98.27 ± 0.12 |
Rat1 cells were treated with 1 mM H2O2 or left untreated. After processing for ATSP-MS, the number of total peptides and the cysteine-containing peptides (IAM modified) were listed (means ± sem; n = 3).
Metabolic proteins were highly oxidized in FH-deficient HLRCC cells
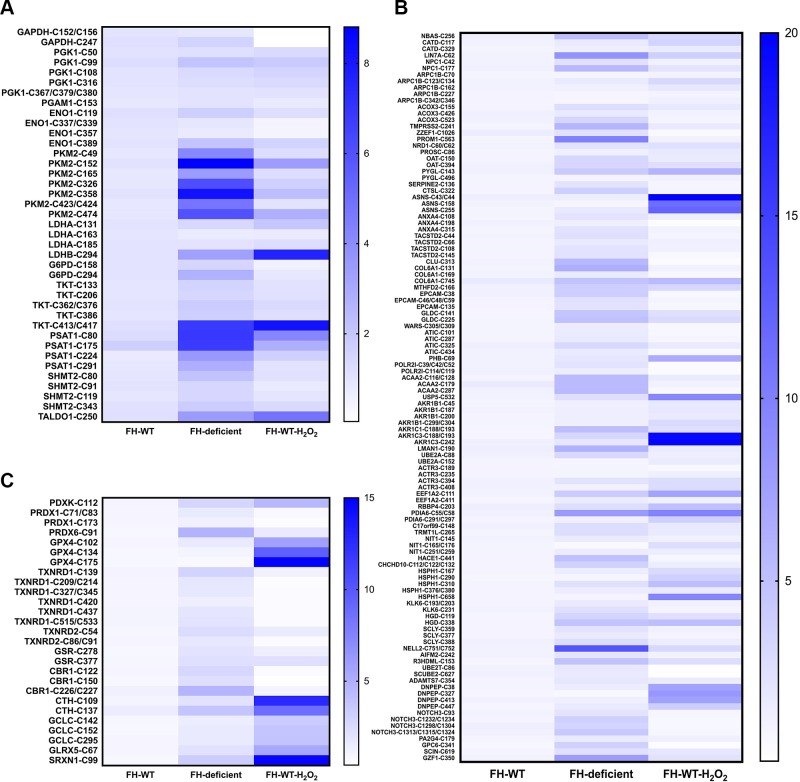
HLRCC is an inherited kidney cancer syndrome associated with inactivating mutations in FH, which encodes a tricarboxylic acid cycle enzyme (34). Loss of FH function results in fumarate accumulation, and this excess fumarate inactivates the glutathione (GSH) antioxidant system, resulting in increased ROS (35, 36). To assess protein oxidation in HLRCC cells, we applied ATSP to the patient-derived HLRCC cell line UOK262 (FH deficient) compared with cells in which WT FH had been reintroduced (UOK262 FH-WT) (37). Similar to the results seen in H2O2-treated Rat1 cells, ∼96% of the MS-recovered peptides from UOK262 cells contained cysteinyl residues (Table 2 and Supplemental Table S2). Furthermore, multiple proteins were highly oxidized in FH-deficient cells, including many metabolic enzymes (Fig. 2A), antioxidant proteins (Fig. 2B), and various other proteins such as those involved in cytoskeleton, translation, and chaperones (Fig. 2C). Among the oxidized metabolic enzymes, multiple cysteines on PKM2 (from the glycolysis pathway), TKT (from the pentose phosphate pathway), and phosphoserine aminotransferase 1 (from the serine synthesis pathway) had the highest oxidation levels in FH-deficient cells (Fig. 2A). Intriguingly, some proteins showed decreased oxidation when treating FH-WT cells with H2O2, including GAPDH and peroxiredoxin 1 (Fig. 2A, B). This paradoxical finding probably reflects the high sensitivity of these proteins to ROS, which results in hyperoxidation of their cysteinyl residues to the irreversible sulfinic (SO2H) or sulfonic (SO3H) acid states (38, 39), which cannot be detected by ATSP.
TABLE 2.
The total number of peptides and number of IAM-modified peptides in UOK262 cells detected by ATSP
| Treatment | IAM-modified peptides | Total peptides | Percentage of IAM-modified peptides |
|---|---|---|---|
| FH-WT | 3416 ± 165 | 3535 ± 162 | 96.64 ± 0.30 |
| FH-deficient | 3380 ± 275 | 3522 ± 289 | 95.97 ± 0.38 |
| H2O2 | 4182 ± 16 | 4326 ± 25 | 96.66 ± 0.19 |
UOK262 FH-WT, FH-deficient, and FH-WT-H2O2 (1 mM; 4 min) cells were processed for ATSP-MS. The number of total peptides and the cysteine-containing peptides (IAM modified) were listed (means ± sem; n = 3).
Figure 2.
Protein oxidation in FH-deficient HLRCC cells detected by ATSP-MS. UOK262 (FH-WT or FH-deficient) cells were processed for ATSP-MS. Metabolic proteins (A), antioxidant proteins (B), and other proteins (C) are shown. Data were analyzed by label-free quantification of peptides from each identified protein (n = 2–3). H2O2 1 mM for 4 min.
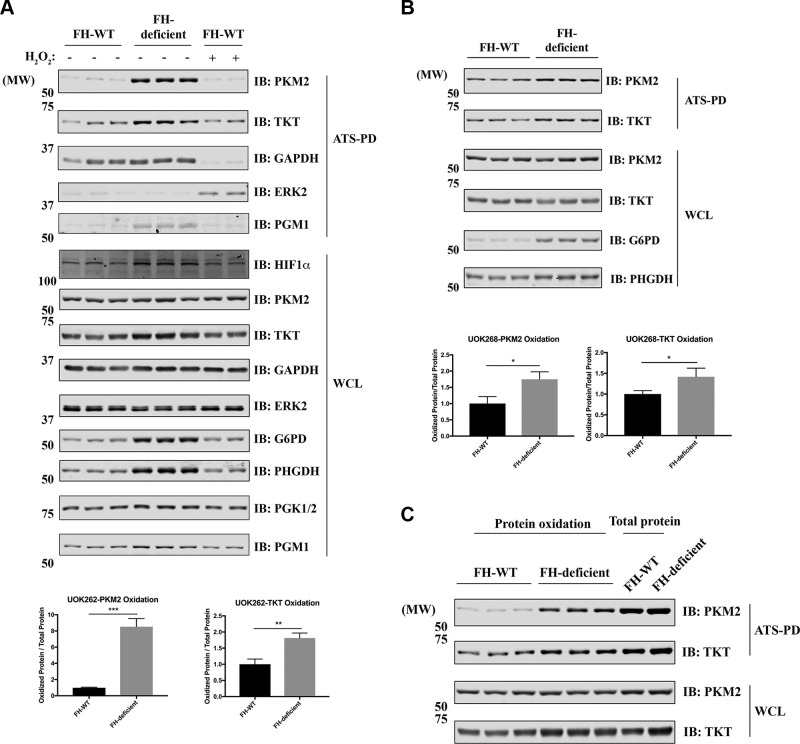
We used ATSP-IB to validate some of the ATSP-MS results. As predicted by ATSP-MS, PKM2 and TKT were oxidized in FH-deficient compared with FH-WT cells (Fig. 3A). The total amounts of G6PD and PHGDH, which are involved in the pentose phosphate pathway and serine synthesis pathway, respectively, were increased significantly in FH-deficient cells. By contrast, TKT and PKM2 showed only little or no increase in expression in FH-deficient cells (Fig. 3A). Similar results were obtained by using another patient-derived HLRCC cell line, UOK268, again comparing FH-deficient cells with FH-WT cells (Fig. 3B). To assess the stoichiometry of PKM2 oxidation, we compared the amount of oxidized PKM2 (using ATSP) with the total amount of PKM2 (using ATSP-total). On average, ∼20–30% of PKM2 was oxidized in UOK262 FH-deficient cells (Fig. 3C).
Figure 3.
PKM2 is oxidized in FH-deficient cells. A, B) UOK262 (A) and UOK268 (B) (FH-WT and FH-deficient) cells were processed for ATSP. WCL and ATS-bound proteins were analyzed by IB with the indicated antibodies. The relative amounts of PKM2 and TKT oxidation are quantified below. Data represent means ± sem (n = 6), unpaired, 2-tailed Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001. C) UOK262 (FH-WT and FH-deficient) cells were processed for ATSP or ATSP-total. WCL and ATS-bound proteins were analyzed by IB with the indicated antibodies. HIF1α, hypoxia-inducible factor 1 α; PD, pull down; PGK, phosphoglycerate kinase; PGM, phosphoglycerate mutase.
PKM2 is not heavily succinated in UOK262 FH-deficient cells
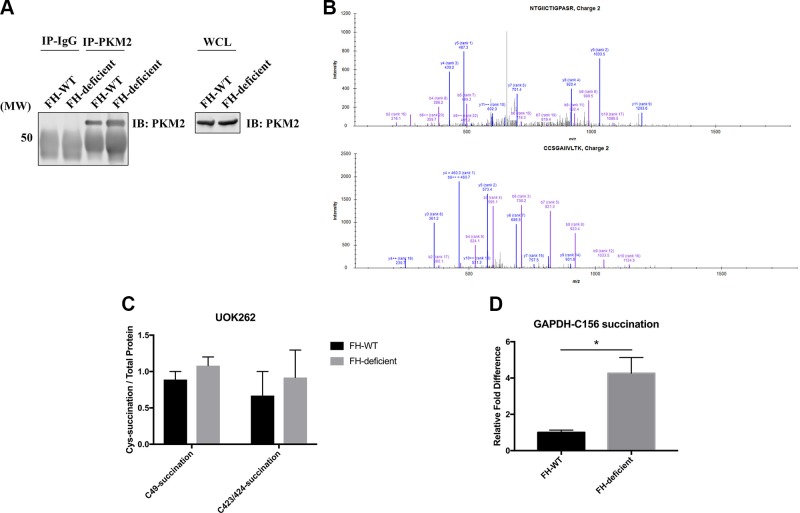
Excess fumarate resulting from FH inactivation also can directly couple to reactive cysteines, altering protein function (40, 41). Because PKM2 is highly oxidized in FH-deficient cells, we investigated whether PKM2 is also heavily succinated in these cells. PKM2 can be effectively precipitated under denaturing conditions (Fig. 4A), so we developed an MS-based assay to measure PKM2 succination levels (Materials and Methods). As shown in Fig. 4B, 2 succinated PKM2 peptides were detected by MS, but neither peptide was more abundant in FH-deficient cells compared with FH-WT cells (Fig. 4C). We also did not detect PKM2 succination and reversible oxidation on the same tryptic peptide using ATSP-MS (Supplemental Table S2). By contrast, coexisting succination and reversible oxidation were found on GAPDH tryptic peptides, and the level of GAPDH succination was significantly higher in FH-deficient cells than FH-WT cells (Fig. 4D and Supplemental Table S2). These findings are consistent with a previous report that GAPDH can be easily succinated (41) and suggest that in FH-deficient cells, PKM2 is mainly oxidized by excessive ROS rather than succinated by excess fumarate.
Figure 4.
PKM2 is not heavily succinated in UOK262 FH-deficient cells. A–C) UOK262 (FH-WT and FH-deficient) cell lysates were denatured in 1% SDS buffer, diluted to 0.1% SDS, and immunoprecipitated using anti-PKM2 antibody. A) Cell lysates and immunoprecipitates were immunoblotted with anti-PKM2 antibody. B) MS spectra of 2 succinated PKM2 peptides. C) Relative amounts of succinated peptides in FH-WT and FH-deficient cells. D) UOK262 (FH-WT or FH-deficient) cells were processed for ATSP-MS. The relative level of GAPDH succination was quantified by label-free quantification. Cys, cysteine; IP, immunoprecipitation. Data represent means ± sem (n = 3), unpaired, 2-tailed Student’s t test. *P < 0.05.
DMF and PKM2 activators can modulate PKM2 oxidation
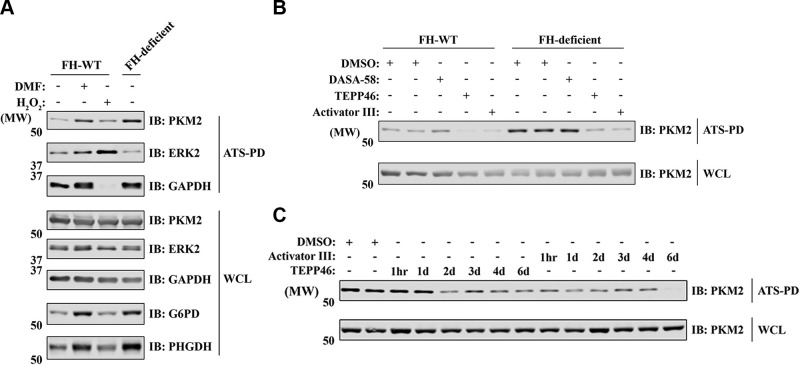
To test whether PKM2 oxidation is related to intracellular accumulation of fumarate, we treated UOK262 FH-WT cells with DMF, a cell-permeable fumarate analog. Long-term DMF treatment of FH-WT cells induced PKM2 oxidation and G6PD and PHGDH up-regulation to levels similar to those in FH-deficient cells, indicating that the observed protein oxidation and overexpression is likely a direct consequence of the accumulation of fumarate in FH-deficient cells (Fig. 5A).
Figure 5.
DMF and PKM2 activators can modulate PKM2 oxidation. A) UOK262 cells were treated with DMF (100 μM, 7 d) or H2O2 (1 mM, 4 min) and processed for ATSP. WCL and ATS-bound proteins were analyzed by IB with indicated antibodies. B) UOK262 cells were treated with various PKM2 activators (DASA-58, 20 μM for 7 d; TEPP-46, 20 μM for 7 d; activator III, 8 μM for 7 d) and processed for ATSP. WCL and ATS-bound proteins were analyzed by IB with anti-PKM2 antibody. C) FH-deficient UOK262 cells were treated with PKM2 activators (20 μM TEPP-46 or 8 μM Activator III) for the indicated time points and processed for ATSP. WCL and ATS-bound proteins were analyzed by IB with anti-PKM2 antibody. DASA-58, PKM2 activator drug; PD, pull down.
Oxidation of PKM2 on C358 has been reported in diamide-treated lung cancer cell lines; moreover, C358 oxidation inhibits tetramer formation and impairs its glycolytic activity (42). Pretreatment with PKM2 activators, which can promote tetramer formation, can protect reduced PKM2 from diamide-induced oxidation (42). There were ≥7 different cysteine sites on PKM2 that were oxidized in HLRCC cells, including C358 (Fig. 2A), so we asked whether different PKM2 activators can reverse PKM2 oxidation. After a 7-d incubation, 2 out of 3 PKM2 activators (TEPP-46 and activator III) significantly reduced PKM2 oxidation in UOK262 cells, and both PKM2 activators reduced PKM2 oxidation levels in FH-deficient cells to those seen in FH-WT cells (Fig. 5B). These findings suggest that most oxidized cysteine sites on PKM2 are directly or indirectly involved in PKM2 tetramer formation. Although it took TEPP-46 1–2 d to reduce PKM2 in FH-deficient cells, activator III can reverse PKM2 oxidation within 1 h of treatment (Fig. 5C), indicating that the redox state of PKM2 is quite dynamic.
PKM2 activators have limited effects on FH-deficient cell proliferation
We next asked whether preventing or reversing PKM2 oxidation affects cell proliferation. Both PKM2 activators (TEPP-46 and activator III) had mild growth inhibitory effects on FH-deficient compared with FH-WT cells, and this effect was only seen after long-term PKM2 activator treatment (Fig. 6). These results indicate that PKM2 oxidation plays a minor role in cell proliferation of FH-deficient cells, at least under these in vitro conditions.
Figure 6.
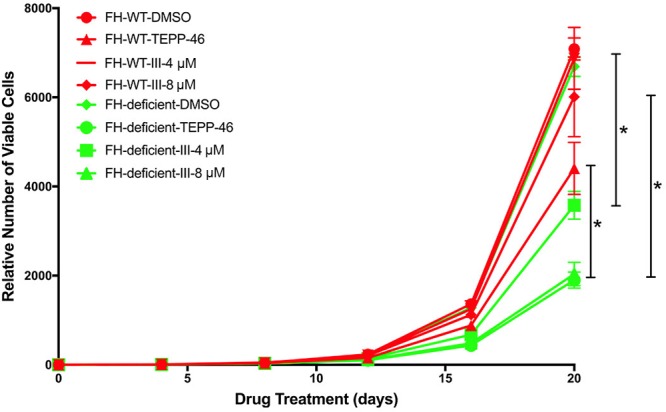
Effects of PKM2 activators on the proliferation of UOK262 cells. UOK262 (FH-WT and FH-deficient) cells were treated with PKM2 activators or DMSO control for the indicated time points. The relative number of viable cells was counted after drug treatments. Data represent means ± sem (n = 3), unpaired, 2-tailed Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
ATSP detects platelet-derived growth factor–induced protein oxidation
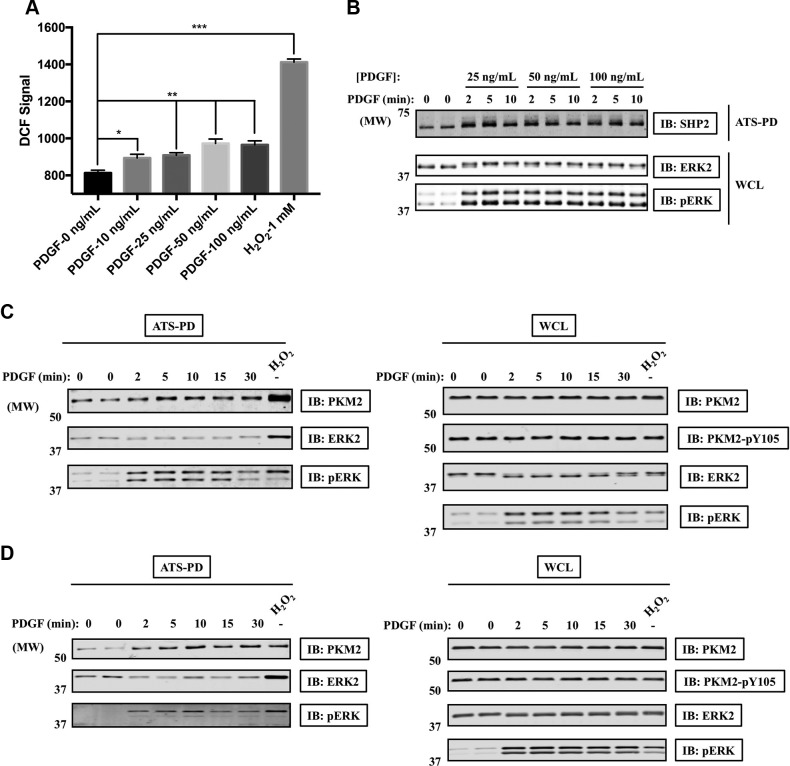
Finally, we asked whether ATSP could detect protein oxidation in response to a physiologic stimulus. Platelet-derived growth factor (PDGF) stimulation induces ROS generation and oxidation of various signaling proteins (5, 43, 44). Consistent with previous results, PDGF evoked ROS generation in Rat1 cells to a level lower than that induced by H2O2 (Fig. 7A). Furthermore, by using ATSP-IB, we could detect SHP2 oxidation in these cells (Fig. 7B). Although PKM2 reportedly is phosphorylated at Y105 and inactivated in response to fibroblast growth factor receptor 1 activation (45), we did not detect PDGF-induced PKM2 tyrosyl phosphorylation. Instead, PKM2 was oxidized following PDGF stimulation (Fig. 7C, D). These results imply that oxidation might be an important mechanism for regulating PKM2 activity in growth factor signaling, which merits further investigation.
Figure 7.
SHP2 and PKM2 are oxidized in response to PDGF stimulation. A) Rat1 cells were starved overnight and then treated with PDGF-BB or H2O2 for 10 min. The relative amount of ROS was measured using DCF-DA and flow cytometry (n = 3); unpaired, 2-tailed Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001. B, C) Rat1 cells were starved overnight, stimulated with PDGF-BB, and then processed for ATSP-IB. Cell lysates or ATS-bound samples were immunoblotted with the indicated antibodies. D) Swiss 3T3 cells were starved overnight, stimulated with 50 ng/ml PDGF-BB, and then processed for ATSP-IB. Cell lysates or ATS-bound samples were immunoblotted with the indicated antibodies. PD, pull down; pERK, phosphorylation of extracellular signal–regulated kinase.
DISCUSSION
Redox signaling is involved in a variety of signaling pathways and diseases, and understanding its roles requires sensitive and specific techniques to detect oxidized proteins. We developed ATSP to monitor and quantify the reversible oxidation of functional thiol-containing proteins. Because reactive cysteines are only a subset of all cysteinyl residues and lack canonical consensus sequence motifs, it is quite difficult to directly identify them and study their regulation (33). ATSP capitalizes on the low pKa of such cysteines (46, 47) by: 1) lysing cells in nondenaturing conditions to preserve the structures that confer the low pKas of these reactive cysteines, 2) using a relatively acidic labeling buffer to reduce the labeling of nonreactive cysteines (32, 5), and 3) minimizing the concentration of redox probes to label reactive cysteines, whose labeling is less sensitive to the redox probe concentrations than that of nonreactive cysteines (33).
ATSP includes 2 separate workflows: ATSP-MS and ATSP-IB. ATSP-MS is useful for identifying specific cysteinyl oxidation sites and globally assessing protein-thiol oxidation. Hence, ATSP-MS is suitable for screening for reactive cysteine oxidation during redox signaling. ATSP-IB can be used to validate the results from ATSP-MS because it is more cost-effective. Moreover, some low-abundance proteins are sometimes difficult to quantify by MS because their recovery can be quite variable; ATSP-IB circumvents this limitation.
When we applied ATSP-MS to H2O2-treated Rat1 cells, we found that more than one-half of the detected peptides (∼1800) showed a significant increase in oxidation. Because H2O2 is a relatively mild oxidant species and mainly reactive cysteines are ROS-sensitive under low to mild oxidative stress, our results suggest that the number of potentially reactive protein-thiols might be higher than previously appreciated. Many proteins have been reported to be oxidized by ROS under various physiologic or pathologic conditions. For example, ATM oxidation reportedly results in its activation in DNA damage repair–deficient cells (10), whereas oxidation of AKT2 inhibits its kinase activity during PDGF signaling (16). KEAP1 oxidation disrupts the Kelch-like enoyl CoA hydratase (ECH)-associated protein 1 (KEAP1)-cullin 3 ubiquitination system, leading to nuclear factor erythroid 2–related factor 2 stabilization and nuclear factor erythroid 2–related factor 2–mediated transcription of antioxidant genes, an important mechanism by which cancer cells overcome oxidative stress (48). Oxidation of multiple mitochondrial and endoplasmic reticulum proteins, as well as oxidation of different enzymes (e.g., deubiquitinases, glycolytic proteins, and PTPs), also have been reported. The ability of ATSP to identify so many oxidized cysteinyl peptides and proteins makes it useful for studying the pleiotropic changes that occur in response to oxidative stress. It is important to note, however, that although H2O2 is a relatively mild oxidant, it was used at levels far higher than those achieved following PDGF stimulation (as assessed by DCF-DA staining). Therefore, many of the proteins identified by ATSP-MS in H2O2-stimulated cells could be relevant mainly for understanding oxidative stress as opposed to normal signaling.
By applying ATSP-MS to FH-mutated HLRCC cell lines, we found multiple metabolic proteins were highly oxidized, including ≥7 cysteine sites on PKM2. Although only C358 has been reported to regulate PKM2 tetramer formation, other cysteine sites might be also directly or indirectly involved in tetramer formation because PKM2 activators can fully reverse PKM2 oxidation. Similar results have been reported for skeletal-type ryanodine receptor (RyR1). Although RyR1-C3635 is selectively nitrosylated, 6 additional cysteines on RyR1 may also contribute to the redox regulation of the channel (49, 50). In addition, PKM2 plays important roles in multiple cancers (51), and PKM2 oxidation has been implicated in protecting lung cancer cells from oxidative stress (42). Perhaps surprisingly, then, treatment with either of the 2 PKM2 activators had little differential effect on the proliferation of FH-deficient compared with FH-WT HLRCC cells. These results do not, however, exclude the possibility that PKM2 oxidation plays a more important role in the proliferation and survival of such cells under the more challenging environment of a tumor. Additional studies are needed to evaluate this possibility.
In addition to FH deficiency, ATSP also was able to detect PDGF-induced protein oxidation in Rat1 cells. Intriguingly, in addition to SHP2 oxidation, which has been reported previously by Meng et al. (5), we also detected PDGF-induced PKM2 oxidation. Because inhibiting PKM2 helps to shunt metabolites into metabolic pathways that support macromolecule biosynthesis, our results suggest that localized PKM2 oxidation or inactivation might be involved in growth factor–induced cell growth. Notably, PKM2 reportedly is inactivated by Y105 phosphorylation in fibroblast growth factor receptor signaling (45), but we did not detect this modification in PDGF-stimulated cells. Conceivably, PKM2 inactivation could be a general feature of growth factor signaling, but might be accomplished by distinct mechanisms in response to different growth factors. Future studies should address these important issues.
We also noticed that oxidation induced by H2O2 and FH deficiency is not the same (Fig. 2). This discrepancy might be due to several reasons: 1) H2O2 treatment is relatively short (4 min), whereas FH-deficiency evokes a state of chronic ROS stress for the cells; hence, protein oxidation due to FH-deficiency likely reflects a steady state of oxidative stress countered by long-term cellular adaption. 2) H2O2 addition is an extrinsic ROS stress and might be expected to have greater effects on proteins at or near the cell membrane because H2O2 would be inactivated as it diffuses into cells; by contrast, FH deficiency results in intrinsic, intracellular ROS stress that can alter the GSH:glutathione disulfide ratio, so it might be expected to affect proteins that rely on GSH as the redox buffer or on proteins residing in cellular compartments with higher basal ROS. 3) Different reactive cysteines might be hyperoxidized (e.g., to the sulfinic or sulfonic acid states) in H2O2-treated cells and FH-deficient cells. Because ATSP only detects reversibly oxidized proteins, different extents of irreversible oxidation could account for the different redox proteomes observed.
There are several potential drawbacks of ATSP. First, ATS might not fit into all molecules with reactive cysteines. We used ATS-4B in all of the above experiments, but we have also tested another commercially available ATS, ATS-6B. ATS-6B worked well for detecting the specific proteins that we investigated by ATS-IB (unpublished results), but potential differences between ATS-6B and ATS-4B in the ATS-MS implementation warrants future investigation. Second, some ATS-bound proteins can be detected by IB but not by MS. This finding indicates that additional work is needed to increase the sensitivity of MS detection; for example, sample fractionation or using a different protease (some cysteines might be contained in tryptic peptides that are refractory to analysis by liquid chromatigraphy–MS). Third, we used DTT to reduce reversibly oxidized proteins in the current ATSP format; however, DTT does not distinguish between different forms of reversible oxidation such as nitrosylation, glutathionylation, sulfenylation, and disulfide bonds. To circumvent this problem, other specific reducing reagents could be used. For example, ascorbate only reduces nitrosylated thiols, NH2OH can convert S-acylation to free thiols, and arsenite can reduce sulfenic acids but not disulfides (30, 52, 53). Lastly, ATSP cannot detect irreversibly oxidized proteins. Therefore, if a certain protein does not show increased reversible oxidation using ATSP, we cannot exclude the possibility that it might be irreversibly oxidized. This phenomenon is illustrated by GAPDH oxidation (Figs. 2A and 3A), in which GAPDH is heavily irreversibly oxidized by H2O2, so the H2O2-induced GAPDH oxidation as assessed by ATS actually appeared to decrease.
As mentioned before, besides disulfide bridges, cysteine glutathionylation is also an important reversible oxidation. For example, in response to oxidative stressors such as ischemia-reperfusion in the heart, glutathionylation of mitochondrial complex I is likely to induce conformational changes that reduce its enzymatic activity and lower ROS generation (54). Glutathionylation of many other proteins, such as actin, tubulin, RAS, annexin, and isocitrate dehydrogenase, has also been reported. Several techniques have been developed to directly detect protein glutathionylation, including radiolabeled GSH, biotinylated GSH, and anti-GSH antibodies. Coupling ATSP with these approaches might give a more comprehensive view of protein-reversible oxidation in different cellular systems.
In summary, we developed a novel redox workflow (ATSP) to globally detect reversible protein oxidation. ATSP is suitable for detecting oxidation of reactive cysteines, which are more likely to be involved in redox signaling. We applied ATSP to HLRCC cells that have high ROS levels, and we found that multiple metabolic enzymes were oxidized in these cells, including PKM2. ATSP also detected PDGF-induced SHP2 and PKM2 oxidation. Therefore, ATSP has great potential to be applied to other ROS-related physiologic and pathologic conditions and help to better understand redox signaling.
ACKNOWLEDGMENTS
The authors thank Dr. Nicholas Tonks (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY, USA) for providing Rat1 cells, and Dr. W. Marston Linehan [U.S. National Institutes of Health (NIH) National Cancer Institute (NCI)] for providing HLRCC cells. This work was supported by NIH/NCI Grant R01CA49152 (to B.G.N.) and the Princess Margaret Cancer Foundation. The mass spectrometric analysis is, in part, supported by the Laura and Isaac Perlmutter Cancer Center Support Grant P30CA016087 from the NCI, and a shared instrumentation grant from the NIH Office of the Director (1S10OD010582-01A1) for the purchase of an Orbitrap Fusion Lumos Mass Spectrometer. B.G.N. is a cofounder of, receives consulting fees from, and holds equity in Northern Biologics (Toronto, ON, Canada) and Navire Pharma (San Francisco, CA, USA). He also is an expert witness for Johnson and Johnson (New Brunswick, NJ, USA). His spouse holds equity in Mirati Therapeutics (San Diego, CA, USA) and Array BioPharma (Boulder, CO, USA). Y.X. is an employee of Geneseeq. The remaining authors declare no conflicts of interest.
Glossary
- ATS
Activated Thiol Sepharose
- ATSP
ATS-based proteomic
- DCF-DA
2′,7′-dichlorofluorescin diacetate
- DMF
dimethyl fumarate
- FH
fumarate hydratase
- G6PD
glucose-6-phosphate dehydrogenase
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSH
glutathione
- HLRCC
hereditary leiomyomatosis and renal cell carcinoma
- IAM
iodoacetamide
- IB
immunoblotting
- Keap1
Kelch-like enoyl CoA hydratase (ECH)-associated protein 1
- MS
mass spectrometry
- NEM
N-ethylmaleimide
- PDGF
platelet-derived growth factor
- PHGDH
phosphoglycerate dehydrogenase
- PKM2
pyruvate kinase M2 isoform
- PTP
protein tyrosine phosphatase
- redox
reduction-oxidation reaction
- ROS
reactive oxygen species
- RyR1
skeletal-type ryanodine receptor
- SHP2
Src homology region 2 domain-containing phosphatase-2
- TCEP
tris(2-carboxyethyl)phosphine
- TKT
transketolase
- WCL
whole-cell lysate
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Xu and B. G. Neel designed the research; Y. Xu performed the research; Y. Xu and B. G. Neel analyzed the data and wrote the paper; and J. Andrade and B. Ueberheide analyzed the mass spectrometry data.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Ostman A., Frijhoff J., Sandin A., Böhmer F. D. (2011) Regulation of protein tyrosine phosphatases by reversible oxidation. J. Biochem. 150, 345–356 [DOI] [PubMed] [Google Scholar]
- 2.Dinkova-Kostova A. T., Holtzclaw W. D., Cole R. N., Itoh K., Wakabayashi N., Katoh Y., Yamamoto M., Talalay P. (2002) Direct evidence that sulfhydryl groups of Keap1 are the sensors regulating induction of phase 2 enzymes that protect against carcinogens and oxidants. Proc. Natl. Acad. Sci. USA 99, 11908–11913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu H., Nishitoh H., Ichijo H., Kyriakis J. M. (2000) Activation of apoptosis signal-regulating kinase 1 (ASK1) by tumor necrosis factor receptor-associated factor 2 requires prior dissociation of the ASK1 inhibitor thioredoxin. Mol. Cell. Biol. 20, 2198–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karisch R., Neel B. G. (2013) Methods to monitor classical protein-tyrosine phosphatase oxidation. FEBS J. 280, 459–475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Meng T. C., Fukada T., Tonks N. K. (2002) Reversible oxidation and inactivation of protein tyrosine phosphatases in vivo. Mol. Cell 9, 387–399 [DOI] [PubMed] [Google Scholar]
- 6.Meng T. C., Buckley D. A., Galic S., Tiganis T., Tonks N. K. (2004) Regulation of insulin signaling through reversible oxidation of the protein-tyrosine phosphatases TC45 and PTP1B. J. Biol. Chem. 279, 37716–37725 [DOI] [PubMed] [Google Scholar]
- 7.Karisch R., Fernandez M., Taylor P., Virtanen C., St-Germain J. R., Jin L. L., Harris I. S., Mori J., Mak T. W., Senis Y. A., Östman A., Moran M. F., Neel B. G. (2011) Global proteomic assessment of the classical protein-tyrosine phosphatome and “Redoxome”. Cell 146, 826–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu Y., Taylor P., Andrade J., Ueberheide B., Shuch B., Glazer P. M., Bindra R. S., Moran M. F., Linehan W. M., Neel B. G. (2018) Pathologic oxidation of PTPN12 underlies ABL1 phosphorylation in hereditary leiomyomatosis and renal cell carcinoma. Cancer Res. 78, 6539–6548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frijhoff J., Dagnell M., Godfrey R., Ostman A. (2014) Regulation of protein tyrosine phosphatase oxidation in cell adhesion and migration. Antioxid. Redox Signal. 20, 1994–2010 [DOI] [PubMed] [Google Scholar]
- 10.Guo Z., Kozlov S., Lavin M. F., Person M. D., Paull T. T. (2010) ATM activation by oxidative stress. Science 330, 517–521 [DOI] [PubMed] [Google Scholar]
- 11.Ishii T., Sunami O., Nakajima H., Nishio H., Takeuchi T., Hata F. (1999) Critical role of sulfenic acid formation of thiols in the inactivation of glyceraldehyde-3-phosphate dehydrogenase by nitric oxide. Biochem. Pharmacol. 58, 133–143 [DOI] [PubMed] [Google Scholar]
- 12.Lee J. G., Baek K., Soetandyo N., Ye Y. (2013) Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells. Nat. Commun. 4, 1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sethuraman M., McComb M. E., Huang H., Huang S., Heibeck T., Costello C. E., Cohen R. A. (2004) Isotope-coded affinity tag (ICAT) approach to redox proteomics: identification and quantitation of oxidant-sensitive cysteine thiols in complex protein mixtures. J. Proteome Res. 3, 1228–1233 [DOI] [PubMed] [Google Scholar]
- 14.Boivin B., Zhang S., Arbiser J. L., Zhang Z. Y., Tonks N. K. (2008) A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc. Natl. Acad. Sci. USA 105, 9959–9964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boivin B., Yang M., Tonks N. K. (2010) Targeting the reversibly oxidized protein tyrosine phosphatase superfamily. Sci. Signal. 3, pl2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wani R., Qian J., Yin L., Bechtold E., King S. B., Poole L. B., Paek E., Tsang A. W., Furdui C. M. (2011) Isoform-specific regulation of Akt by PDGF-induced reactive oxygen species. Proc. Natl. Acad. Sci. USA 108, 10550–10555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paulsen C. E., Truong T. H., Garcia F. J., Homann A., Gupta V., Leonard S. E., Carroll K. S. (2011) Peroxide-dependent sulfenylation of the EGFR catalytic site enhances kinase activity. Nat. Chem. Biol. 8, 57–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tsutsumi R., Harizanova J., Stockert R., Schröder K., Bastiaens P. I. H., Neel B. G. (2017) Assay to visualize specific protein oxidation reveals spatio-temporal regulation of SHP2. Nat. Commun. 8, 466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia F. J., Carroll K. S. (2016) An immunochemical approach to detect oxidized protein tyrosine phosphatases using a selective C-nucleophile tag. Mol. Biosyst. 12, 1790–1798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Claiborne A., Miller H., Parsonage D., Ross R. P. (1993) Protein-sulfenic acid stabilization and function in enzyme catalysis and gene regulation. FASEB J. 7, 1483–1490 [DOI] [PubMed] [Google Scholar]
- 21.Salmeen A., Andersen J. N., Myers M. P., Meng T. C., Hinks J. A., Tonks N. K., Barford D. (2003) Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature 423, 769–773 [DOI] [PubMed] [Google Scholar]
- 22.Lee S. R., Yang K. S., Kwon J., Lee C., Jeong W., Rhee S. G. (2002) Reversible inactivation of the tumor suppressor PTEN by H2O2. J. Biol. Chem. 277, 20336–20342 [DOI] [PubMed] [Google Scholar]
- 23.Templeton D. J., Aye M. S., Rady J., Xu F., Cross J. V. (2010) Purification of reversibly oxidized proteins (PROP) reveals a redox switch controlling p38 MAP kinase activity. PLoS One 5, e15012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Caldas T. D., El Yaagoubi A., Kohiyama M., Richarme G. (1998) Purification of elongation factors EF-Tu and EF-G from Escherichia coli by covalent chromatography on thiol-sepharose. Protein Expr. Purif. 14, 65–70 [DOI] [PubMed] [Google Scholar]
- 25.Lee K., Lee J., Kim Y., Bae D., Kang K. Y., Yoon S. C., Lim D. (2004) Defining the plant disulfide proteome. Electrophoresis 25, 532–541 [DOI] [PubMed] [Google Scholar]
- 26.Hu W., Tedesco S., McDonagh B., Bárcena J. A., Keane C., Sheehan D. (2010) Selection of thiol- and disulfide-containing proteins of Escherichia coli on activated thiol-Sepharose. Anal. Biochem. 398, 245–253 [DOI] [PubMed] [Google Scholar]
- 27.Victor K. G., Rady J. M., Cross J. V., Templeton D. J. (2012) Proteomic profile of reversible protein oxidation using PROP, purification of reversibly oxidized proteins. PLoS One 7, e32527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu M., Hou J., Huang L., Huang X., Heibeck T. H., Zhao R., Pasa-Tolic L., Smith R. D., Li Y., Fu K., Zhang Z., Hinrichs S. H., Ding S. J. (2010) Site-specific proteomics approach for study protein S-nitrosylation. Anal. Chem. 82, 7160–7168 [DOI] [PubMed] [Google Scholar]
- 29.Guo J., Gaffrey M. J., Su D., Liu T., Camp D. G., II, Smith R. D., Qian W. J. (2014) Resin-assisted enrichment of thiols as a general strategy for proteomic profiling of cysteine-based reversible modifications. Nat. Protoc. 9, 64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forrester M. T., Hess D. T., Thompson J. W., Hultman R., Moseley M. A., Stamler J. S., Casey P. J. (2011) Site-specific analysis of protein S-acylation by resin-assisted capture. J. Lipid Res. 52, 393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Winterbourn C. C. (2013) The biological chemistry of hydrogen peroxide. Methods Enzymol. 528, 3–25 [DOI] [PubMed] [Google Scholar]
- 32.Lee S. R., Kwon K. S., Kim S. R., Rhee S. G. (1998) Reversible inactivation of protein-tyrosine phosphatase 1B in A431 cells stimulated with epidermal growth factor. J. Biol. Chem. 273, 15366–15372 [DOI] [PubMed] [Google Scholar]
- 33.Weerapana E., Wang C., Simon G. M., Richter F., Khare S., Dillon M. B., Bachovchin D. A., Mowen K., Baker D., Cravatt B. F. (2010) Quantitative reactivity profiling predicts functional cysteines in proteomes. Nature 468, 790–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tomlinson I. P., Alam N. A., Rowan A. J., Barclay E., Jaeger E. E., Kelsell D., Leigh I., Gorman P., Lamlum H., Rahman S., Roylance R. R., Olpin S., Bevan S., Barker K., Hearle N., Houlston R. S., Kiuru M., Lehtonen R., Karhu A., Vilkki S., Laiho P., Eklund C., Vierimaa O., Aittomäki K., Hietala M., Sistonen P., Paetau A., Salovaara R., Herva R., Launonen V., Aaltonen L. A.; Multiple Leiomyoma Consortium (2002) Germline mutations in FH predispose to dominantly inherited uterine fibroids, skin leiomyomata and papillary renal cell cancer. Nat. Genet. 30, 406–410 [DOI] [PubMed] [Google Scholar]
- 35.Sudarshan S., Sourbier C., Kong H. S., Block K., Valera Romero V. A., Yang Y., Galindo C., Mollapour M., Scroggins B., Goode N., Lee M. J., Gourlay C. W., Trepel J., Linehan W. M., Neckers L. (2009) Fumarate hydratase deficiency in renal cancer induces glycolytic addiction and hypoxia-inducible transcription factor 1alpha stabilization by glucose-dependent generation of reactive oxygen species. Mol. Cell. Biol. 29, 4080–4090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adam J., Hatipoglu E., O’Flaherty L., Ternette N., Sahgal N., Lockstone H., Baban D., Nye E., Stamp G. W., Wolhuter K., Stevens M., Fischer R., Carmeliet P., Maxwell P. H., Pugh C. W., Frizzell N., Soga T., Kessler B. M., El-Bahrawy M., Ratcliffe P. J., Pollard P. J. (2011) Renal cyst formation in Fh1-deficient mice is independent of the Hif/Phd pathway: roles for fumarate in KEAP1 succination and Nrf2 signaling. Cancer Cell 20, 524–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y., Valera V. A., Padilla-Nash H. M., Sourbier C., Vocke C. D., Vira M. A., Abu-Asab M. S., Bratslavsky G., Tsokos M., Merino M. J., Pinto P. A., Srinivasan R., Ried T., Neckers L., Linehan W. M. (2010) UOK 262 cell line, fumarate hydratase deficient (FH-/FH-) hereditary leiomyomatosis renal cell carcinoma: in vitro and in vivo model of an aberrant energy metabolic pathway in human cancer. Cancer Genet. Cytogenet. 196, 45–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dalla Rizza J., Randall L. M., Santos J., Ferrer-Sueta G., Denicola A. (2019) Differential parameters between cytosolic 2-Cys peroxiredoxins, PRDX1 and PRDX2. Protein Sci. 28, 191–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maller C., Schröder E., Eaton P. (2011) Glyceraldehyde 3-phosphate dehydrogenase is unlikely to mediate hydrogen peroxide signaling: studies with a novel anti-dimedone sulfenic acid antibody. Antioxid. Redox Signal. 14, 49–60 [DOI] [PubMed] [Google Scholar]
- 40.Blatnik M., Frizzell N., Thorpe S. R., Baynes J. W. (2008) Inactivation of glyceraldehyde-3-phosphate dehydrogenase by fumarate in diabetes: formation of S-(2-succinyl)cysteine, a novel chemical modification of protein and possible biomarker of mitochondrial stress. Diabetes 57, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Blatnik M., Thorpe S. R., Baynes J. W. (2008) Succination of proteins by fumarate: mechanism of inactivation of glyceraldehyde-3-phosphate dehydrogenase in diabetes. Ann. N. Y. Acad. Sci. 1126, 272–275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Anastasiou D., Poulogiannis G., Asara J. M., Boxer M. B., Jiang J. K., Shen M., Bellinger G., Sasaki A. T., Locasale J. W., Auld D. S., Thomas C. J., Vander Heiden M. G., Cantley L. C. (2011) Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sundaresan M., Yu Z. X., Ferrans V. J., Irani K., Finkel T. (1995) Requirement for generation of H2O2 for platelet-derived growth factor signal transduction. Science 270, 296–299 [DOI] [PubMed] [Google Scholar]
- 44.Kwon J., Lee S. R., Yang K. S., Ahn Y., Kim Y. J., Stadtman E. R., Rhee S. G. (2004) Reversible oxidation and inactivation of the tumor suppressor PTEN in cells stimulated with peptide growth factors. Proc. Natl. Acad. Sci. USA 101, 16419–16424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hitosugi T., Kang S., Vander Heiden M. G., Chung T. W., Elf S., Lythgoe K., Dong S., Lonial S., Wang X., Chen G. Z., Xie J., Gu T. L., Polakiewicz R. D., Roesel J. L., Boggon T. J., Khuri F. R., Gilliland D. G., Cantley L. C., Kaufman J., Chen J. (2009) Tyrosine phosphorylation inhibits PKM2 to promote the Warburg effect and tumor growth. Sci. Signal. 2, ra73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis C. T., Seyer J. M., Carlson G. M. (1989) Cysteine 288: an essential hyperreactive thiol of cytosolic phosphoenolpyruvate carboxykinase (GTP). J. Biol. Chem. 264, 27–33 [PubMed] [Google Scholar]
- 47.Knowles J. R. (1976) The intrinsic pKa-values of functional groups in enzymes: improper deductions from the pH-dependence of steady-state parameters. CRC Crit. Rev. Biochem. 4, 165–173 [DOI] [PubMed] [Google Scholar]
- 48.Kansanen E., Kuosmanen S. M., Leinonen H., Levonen A. L. (2013) The Keap1-Nrf2 pathway: mechanisms of activation and dysregulation in cancer. Redox Biol. 1, 45–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sun J., Xin C., Eu J. P., Stamler J. S., Meissner G. (2001) Cysteine-3635 is responsible for skeletal muscle ryanodine receptor modulation by NO. Proc. Natl. Acad. Sci. USA 98, 11158–11162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Voss A. A., Lango J., Ernst-Russell M., Morin D., Pessah I. N. (2004) Identification of hyperreactive cysteines within ryanodine receptor type 1 by mass spectrometry. J. Biol. Chem. 279, 34514–34520 [DOI] [PubMed] [Google Scholar]
- 51.Dayton T. L., Jacks T., Vander Heiden M. G. (2016) PKM2, cancer metabolism, and the road ahead. EMBO Rep. 17, 1721–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Raju K., Doulias P. T., Tenopoulou M., Greene J. L., Ischiropoulos H. (2012) Strategies and tools to explore protein S-nitrosylation. Biochim. Biophys. Acta 1820, 684–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ehring R., Colowick S. P. (1969) The two-step formation and inactivation of acylphosphatase by agents acting on glyceraldehyde phosphate dehydrogenase. J. Biol. Chem. 244, 4589–4599 [PubMed] [Google Scholar]
- 54.Hurd T. R., Requejo R., Filipovska A., Brown S., Prime T. A., Robinson A. J., Fearnley I. M., Murphy M. P. (2008) Complex I within oxidatively stressed bovine heart mitochondria is glutathionylated on Cys-531 and Cys-704 of the 75-kDa subunit: potential role of CYS residues in decreasing oxidative damage. J. Biol. Chem. 283, 24801–24815 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.