Abstract
The Hippo pathway is an emerging signaling pathway that plays important roles in organ size control, tissue homeostasis, tumorigenesis, metastasis, drug resistance, and immune response. Although many regulators of the Hippo pathway have been reported, the extracellular stimuli and kinase regulators of the Hippo pathway remain largely unknown. To identify novel regulars of the Hippo pathway, in this study we created the first ultra-bright NanoLuc biosensor (BS) to monitor the activity of large tumor suppressor (LATS) kinase 1, a central player of the Hippo pathway. We show that this NanoLuc BS achieves significantly advanced sensitivity and stability both in vitro using purified proteins and in vivo in living cells and mice. Using this BS, we perform the first kinome-wide screen and identify many kinases regulating LATS and its effectors yes-associated protein (YAP) and transcriptional co-activator with PDZ- binding motif (TAZ). We also show for the first time that activation of receptor tyrosine kinase anaplastic lymphoma kinase (ALK) by its extracellular ligand family with sequence similarity (FAM)150 activates Hippo effector YAP/TAZ by increasing their nuclear translocation. Significantly, we show that constitutively active ALK induces tumorigenic phenotypes, such as increased cancer cell proliferation/colony formation via YAP/TAZ and elevated immune evasion via YAP/TAZ–programmed death-ligand 1 in breast and lung cancer cells. In summary, we have developed a new LATS BS for cancer biology and therapeutics research and uncovered a novel ALK-LATS-YAP/TAZ signaling axis that may play important roles in cancer and possibly other biologic processes.—Nouri, K., Azad, T., Lightbody, E., Khanal, P., Nicol, C. J., Yang, X. A kinome-wide screen using a NanoLuc LATS luminescent biosensor identifies ALK as a novel regulator of the Hippo pathway in tumorigenesis and immune evasion.
Keywords: PD-L1, kinases, lung cancer, immunotherapy, breast cancer
The Hippo signaling pathway was originally discovered in Drosophila and later in mammals as a novel regulator of animal size and cell growth (1–3). Since its initial discovery, the Hippo pathway has been overwhelmingly confirmed to play critical roles in tumorigenesis, metastasis, tissue homeostasis, development, immune response, and drug resistance (2, 4–8). Therefore, elucidating functions of the Hippo pathway in regenerative medicine, cancer biology, and cancer therapeutics has become one of the most attractive areas in biomedical research.
Briefly, activation of the Hippo pathway is initiated by upstream regulators, such as cell-cell contact and nutrient stress, leading mammalian STE20-like (MST)1/2 (mammalian homologs of Drosophila Hippo) serine/threonine kinases to phosphorylate/activate adaptor proteins hMOB1 and WW45 as well as large tumor suppressor kinase (LATS)1/2 , which subsequently phosphorylate and inactivate transcriptional coactivator yes-associated protein (YAP) and its paralog transcriptional co-activator with PDZ- binding motif (TAZ). Phosphorylated (p)S127-YAP (YAP-pS127) and TAZ-pS89 bind to cytoplasmic protein 14-3-3 and are prevented from interacting with TEA domain family member transcription factors in the nucleus to regulate cell proliferation and survival through transactivation of downstream genes (CTGF, Cyr61, FGF).
Despite the tremendous interest in the Hippo pathway, there are currently no tools for measuring the dynamics and activity of the Hippo signaling components in a quantitative, real-time, high-throughput, and noninvasive manner both in vitro and in vivo. To solve this issue, we recently used a Firefly luciferase (FLuc) split luciferase complementation assay (SLCA) approach to develop the first LATS-FLuc-biosensor (BS) that faithfully monitors LATS kinase activity in living cells and a xenograft mouse model (9, 10). However, the breadth of applications for FLuc is limited because of their relatively large sizes, low stability under common cell culture conditions (37°C), and short half-lives. Recently, a new small luciferase (19 kDa) named NanoLuc (NLuc) was engineered from a small luciferase subunit of deep-sea shrimp, Oplophorus gracilirostris (11). This smallest and brightest ATP-independent luciferase enzyme utilizes an optimally designed substrate, furimazine, to emit bioluminescence >150 times stronger than FLuc. In addition, the NLuc exhibits high stability in various conditions and a longer half-life. Therefore, it has quickly brightened up the bioluminescent research field by increasing the scope of biologic processes that may now be studied (12). By using NLuc, a SLCA called NanoLuc Binary Technology (NanoBiT) was recently developed to monitor protein-protein interactions (13). In this NanoBiT SLCA, a 17.6 kDa large binary technology (LgBiT; large fragment of N-terminal NLuc) is complemented by small binary technology (SmBiT; 11 aa of C-terminal NLuc). Because of their bright bioluminescence, high stability, wide dynamic range, and low background (low-affinity LgBiT:SmBiT binding; KD = 190 μM), this breakthrough technology will undoubtedly have a great impact on the study of protein function and signaling in cells and animal models. In this study, using advanced NanoBiT technology we created a new ultra-bright NanoLuc LATS bioluminescent BS, which has a stronger signal and higher stability than the original FLuc-LATS-BS. We validated the BS using purified BS proteins in vitro as well as in vivo in both living cells and animal models. To identify novel kinases regulating the Hippo pathway in tumorigenesis, we also successfully used this BS to perform a kinome-wide inhibitor screen and identified several kinases that regulate the Hippo pathway. Most significantly, we validated receptor tyrosine kinase (RTK) anaplastic lymphoma kinase (ALK) as a novel regulator of the Hippo pathway in regulating tumorigenesis and immune evasion.
MATERIALS AND METHODS
Plasmid construction
LATS-FLuc-BS was made as previously described (9). To generate LATS-NBiT-BS [LgBiT-YAP15-S127 (wild type [WT])/YAP15-S127A (mutant ([Mut]) and 14-3-3-SmBiT] in pcDNA3.1/hygro vector, PCR was performed using pBiT1.1-N [thymidine kinase (TK)/LgBiT] vector (Promega, Madison, WI, USA) and full-length cDNA of human 14-3-3τ (accession no. NM_006826) as template. The BS was cloned into the BamHI/NotI sites of pcDNA3.1/hygro(+). In order to make LATS-NBiT-BS in TK vector, oligos coding for YAP15-S127 [WT] and YAP15-S127A [Mut] were annealed and directly cloned into the pBiT1.1-N [TK/LgBiT] vector (Promega), and full-length 14-3-3 was amplified by PCR and cloned into pBiT2.1-C [TK/SmBiT] vectors (Promega) with the EcoRI/Bglll sites.
To make His-tagged fusion proteins, LATS-NBiT-BS in TK vector was used as template, and amplified LgBiT-YAP15 S127 [WT]/YAP15-S127A [Mut] and 14-3-3-SmBiT were cloned into the NdeI/BamHI sites of pET16b. Full-length cDNAs of human ALK (accession number NM_004304.4) was subcloned into pcDNA3.1/hygro(+)-myc vector. For lentivirus production, ALK cDNA was cloned into pTRIPZ lentiviral vector. For echinoderm microtubule-associated protein like 4 (EML4)-ALK cloning, cDNA was synthesized by reverse transcription from H3122 cells. EML4-ALK cDNA was amplified by PCR and cloned into pTRIPZ lentiviral vector. The following primers were used for cloning:
LATS-NBiT-BS in pcDNA3.1/hygro vector
BamHI-kozak-LgBiT-YAP15-sense, 5′-CCGCGGATCCGCCGCCACCATGGTCTTCACACTCGAAGAT-3′; Not1-LgBiT-YAP15- antisense, 3′-CAAAGGAAGCGGCCGCTTACAACTGCAGAGAAGCTGGAGA-5′; BamH1-kozak-14-3-3-SmBiT-sense, 5′-CCGCGGATCCGCCGCCACCATGGAGAAGACTGAGCTGATC-3′; Not1-14-3-3-SmBiT-antisense, 3′-CAAAGGAAGCGGCCGCTTACAGAATCTCCTCGAACAGCCG-5′.
LATS-NBiT-BS in NanoBiT-TK vector
EcoR1-YAP-S127-BglII-sense, 5′-AATTCACCACAGCATGTTCGAGCTCATTCCTCTCCAGCTTCTCTGCAGTTGTGAA-3′; ECoR1-YAP-S127-BglII-antisense, 3′-ATCTTCACAACTGCAGAGAAGCTGGAGAGGAATGAGCTCGAACATGCTGTGGTG-5′; EcoR1-YAP-A127-BglII-sense, 5′-AATTCACCACAGCATGTTCGAGCTCATGCGTCTCCAGCTTCTCTGCAGTTGTGAA-3′; ECoR1-YAP-A127-BglII-antisense, 3′-ATCTTCACAACTGCAGAGAAGCTGGAGACGCATGAGCTCGAACATGCTGTGGTG-5′; BglII-14-3-3-sense, 5′-GGAAGATCTAATGGAGAAGACTGAGCTGATC-3′; ECoR1-14-3-3-antisense, 3′-CGCCGGAATTCCCGTTTTCAGCCCCTTCTGCC GC-5′.
LATS-NBiT-BS in pET16b vector
NdeI-LgBiT-YAP15-sense, 5′-GGAATTCATATGATGGTCTTCACACTCGAAGATTTCGT-3′; BamHI-LgBiT-YAP15-antisense, (3′-CGCGGGATCCTTACAACTGCAGAGAAGCTGGAGAGGAATG-5′); BamHI-LgBiT-YAP15A127-antisense, (3′-CGCGGGATCCTTACAACTGCAGAGAAGCTGGAGACGCATGAGC-5′); NdeI-14-3-3-SmBiT-sense, (5′-GGAATTCATATGATGGAGAAGACTGAGCTGATCCAGAA-3′); BamHI-14-3-3-SmBiT-antisense, (3′-CGCGGGATCCTTACAGAATCTCCTCGAACAGCCGGTAGCC-5′).
ALK and EML4-ALK in pTRIPZ vector
Age1-kozak-ALk-sense, 5′-ACCACCGGTGCCGCCACCATGGGAGCCATCGGGCTCCTG-3′; MLU1-ALK-FLAG-antisense, 3′-ACACGATACGCGTTCACTTGTCGTCATCGTCTTTGTAGTCGGGCCCAGGCTGGTTCATG-5′. Age1-kozak-FLAG-BgLII-EML4-ALK-sense, 5′-AACCACCGGTGCCGCCACCATGGACTACAAAGACGATGACGACAAGGGAAGATCTATGGACGGTTTCGCCGGCAGT-3′; MLU1-N1-EML4-ALK-antisense, 5′ CGACGCGTATAAGAATGCGGCCGCTCAGGGCCCAGGCTGGTTCATGCT-3′.
Cell culture
Human embryonic kidney (HEK)293AD, HEK293T, and Hs578T (human breast carcinoma cells) were cultured in DMEM (D6429; MilliporeSigma, Burlington, MA, USA). H1299 (human non-small cell lung carcinoma cells) and HCC1143 (human breast carcinoma) cells were cultured in Roswell Park Memorial Institute 1640 (8758; MilliporeSigma) supplemented with 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, 1 mM sodium pyruvate, and 2.5 mg/ml glucose. All abovementioned cells were cultured in medium supplemented with 10% fetal bovine serum, and 1% penicillin/streptomycin (Thermo Fisher Scientific, Waltham, MA, USA) as antibiotics. MCF10A cells were cultured in DMEM/F12 medium (D6421; MilliporeSigma) containing 5% horse serum (H1270; MilliporeSigma), 0.25 mM l-glutamine (G7513; MilliporeSigma), 10 μg/ml insulin (I6634; MilliporeSigma), 20 ng/ml human epidermal growth factor (E4269; MilliporeSigma), 100 ng/ml cholera toxin (C8052; MilliporeSigma), 0.5 μg/ml hydrocortisone (H4001; MilliporeSigma), and 1% penicillin/streptomycin. Cells were grown in a humidified CO2 (5%) atmosphere at 37°C. All experiments were conducted using cells with a passage number <25.
In vitro luciferase assay and NanoLuc luciferase assay
HEK293T cells (3 × 105 cells) were plated in triplicate in a 12-well plate 24 h before transfection. Two hundred nanograms of BS alone or alongside 300 ng LATS or 200 ng MST2, or both, was transfected using PolyJet transfection reagent (SignaGen Laboratories, Rockville, MD, USA). After 48 h, cells were collected and lysed using passive lysis buffer, and finally luciferase or NanoLuc luciferase assay was performed using a Luciferase Assay Kit (Promega) for LATS-FLuc-BS and a NanoLuc Luciferase Assay Kit (Nano-Glo Live Cell Reagent; Promega) for LATS-NBiT-BS. Turner Biosystems 20/20 Luminometer or GloMax Navigator Microplate Luminometer (Promega) were used, depending on the type of experiment. Results are presented as relative luminescence units or fold change compared with control. The data presented are the mean of 3 independent experiments. For live cell luciferase assay or NanoLuc luciferase assay, BSs were transfected into HEK293T cells. After 24 h, cells were trypsinized and different numbers of the cells were seeded in a 96-well plate. After 24 h, d-luciferin (150 μg/ml, d-luciferin, potassium salt, GoldBio UCK-250) or Nano-Glo Live Cell Reagent, which provides the cell-permeable furimazine substrate, was added to each well and immediately luminescence was measured.
To determine temperature stability of each BS, transfected cells were collected and lysed 48 h after transfection and after incubating the samples under different conditions; assays were performed using kits from Promega as previously described.
RNA extraction and quantitative RT-PCR
Total RNA was extracted using RNAzole (Molecular Research Center, Cincinnati, OH, USA) according to the manufacturer’s protocol. SuperScript III Platinum Sybr Green One-Step quantitative RT-PCR (qRT-PC) Kit (Thermo Fisher Scientific) was used for qRT-PCR by using 50 ng total RNA. For internal control, 18S rRNA was used. The following primers were used: rRNA, sense (5′-TCCCCATGAACGAGGAATTCC-3′), anti-sense (5′-AACCATCCAATCGGTAGTAGC-3′); Firefly, sense (5′-TCGAAATGTCCGTTCGGTTG-3′), antisense (5′-CGCAACTGCAACTCCGATAA-3′); and LgBiT, sense (5′-GTGTCCGTAACTCCGATCCA-3′), antisense (5′-TCATCCACAGGGTACACCAC-3′).
Western blotting and antibodies
The same samples used for measuring luminescence were used to perform immunoblotting. Proteins were heated in Laemmli sample buffer and subjected to SDS-PAGE. The proteins were transferred to nitrocellulose membranes using Mini Trans-Blot Cell (Bio-Rad, Hercules, CA, USA) and were immunoblotted using different primary antibodies. Antibodies for Western blot analysis were as follows: MST2, LATS1 (C66B5), ALK (C26G7) rabbit mAb, programmed death-ligand 1 [PD-L1 (E1L3N)], and pYAP (S127) from Cell Signaling Technology (Danvers, MA, USA); rabbit polyclonal anti-HA (H6908), mouse monoclonal anti-FLAG (F1804), and mouse monoclonal anti-β-actin (AC-15) from MilliporeSigma; mouse monoclonal anti-Myc (9E10) from Roche (Basel, Switzerland); mouse monoclonal anti-YAP (sc-101199) from Santa Cruz Biotechnology (Dallas, TX, USA; H125); and TAZ (M2-616) from BD Biosciences (San Jose, CA, USA).
Live cells and in vivo imaging
To determine if the LATS-NBiT-BS could be used to measure a response to different drug treatments and regulators of Hippo signaling, HEK293T cells were transfected with LATS-NBiT-BS and treated with 2-deoxy glucose (25 mM), ER-27-319 (10 μM), RO-318220 (10 μM), and axitinib (10 μM) for 4 h, or they were serum-starved overnight. Cells were then transferred to a black plate, and furimazine was mixed into each well for subsequent bioluminescent imaging (BLI). Imaging of cells was performed using a LightTools Research System (Synopsys, Mountain View, CA, USA) dark box and an Orca-Flash4.0 v.2 digital CMOS camera (Hamamatsu Photonics, Hamamatsu, Japan) over a course of 30 min to establish optimal peak luciferase activity. Pseudo-colored parametric overlays of BLI were dynamically constructed, and the BLI of the regions of interest was then analyzed for total emission flux using Image Pro Plus software (Media Cybernetics, Rockville, MD, USA). The BS activity in the live cells was measured using NanoLuc luciferase assay.
A xenograft mouse model was used to evaluate the sensitivity of the LATS-NBiT-BS in vivo. First, immunocompromised Rag2−/−;Il2rg−/− mice at least 12 wk in age were anesthetized and injected subcutaneously into the flank with increasing cell densities of HEK293T cells (1.0 × 103–1.0 × 106) expressing LATS-NBiT-BS mixed with 10 µg (≈0.5 mg/kg) furimazine in 200 µl sterile PBS. Next, 1.0 × 105 HEK293T cells transfected with BS alone (LATS-NBiT-BS), or with LATS (LATS-NBiT-BS+ LATS), were mixed with furimazine and implanted into opposing flanks for imaging. All images were taken with an IVIS Lumina Series III In Vivo Imaging System (PerkinElmer, Waltham, MA, USA) using a 30-s acquisition period. Bioluminescent quantification is represented as signal counts for the regions of interest designated by Living Image Software (PerkinElmer). All procedures and protocols were approved by the Queen’s University Animal Care Committee in accordance with the Canadian Council on Animal Care guidelines.
Fusion proteins
All proteins were purified as His-tagged fusion proteins from Escherichia coli according to the protocols with minimal changes from what was previously described (14, 15). Briefly, E. coli strain CodonPlusRIPL was transformed and used to purify the proteins. Fusion protein expression was induced with 0.3 mM isopropyl β-D-1-thiogalactopyranoside, when the optical density measured at a wavelength of 600 nm was between 0.6 and 0.8, for 16 h at 20°C in an environmental incubator shaker. After centrifugation and harvesting, cells were incubated at 4°C with protease inhibitor cocktail, DNase I (10 µg/ml) and lysozyme (10 µg/ml). Then, bacterial cells in buffer containing 30 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, 20 mM imidazole, and 2 mM β-mercaptoethanol were lysed by sonication. Bacterial lysates were centrifuged to collect soluble fractions, and tagged proteins were isolated from the supernatant via Ni-NTA affinity purification. In order to get rid of imidazole, proteins were subjected to dialysis overnight at 4°C using a standard buffer containing 30 mM Tris-HCl, pH 7.5, 150 mM NaCl, 5 mM MgCl2, and 3 mM DTT. All purified proteins were analyzed by SDS-PAGE and stored at −80°C.
In vitro kinase assay
For in vitro kinase assay, 100 ng of LgBiT-YAP15 WT (S127)/Mut (S127A) and 14-3-3-SmBiT fusion proteins were incubated with 25 ng of LATS fusion protein (SignalChem Life Sciences, Richmond, BC, Canada) at 30°C for different time points (1 min–20 h) in the presence of ATP and kinase buffer. Luminescence was measured for each sample, and samples were prepared for Western blot analysis as previously described. p-LgBiT-YAP15 was detected by anti–pYAP (S127) antibody.
Kinome-wide kinase inhibitor screen
High-throughput screens were performed in the Sinai-McLaughlin Assay and Robotic Technologies (S.M.A.R.T.) Laboratory for High-Throughput Screening Programs of the Lunenfeld-Tanenbaum Research Institute (Mount Sinai Hospital, Toronto, ON, Canada). Cells were seeded in 384-well plates in a total volume of 45 μl/well (2500 cells/well). On the following day, screening compounds, including 560 kinase inhibitors (1 mM in DMSO) were robotically pinned (200 nl) into assay wells to attain a final concentration of 4.4 μM. Equal amounts of DMSO (0.5%) were used as vehicle controls. After 24 h of incubation, 5 μl diluted furimazine (1:25 dilution) was added to each well and BS activity (luminescence signal) was measured by NanoLuc luciferase assay (reading time: 100 ms). Fold-change ratios were generated by comparing BS activity for each drug with that of DMSO-treated controls. All experiments were performed in duplicate.
Lentivirus production, infection, and establishment of stable cell lines
To produce lentiviruses, HEK293T cells were plated on a 150-mm plate. The next day, cells were transfected with 8 μg of ALK-FLAG-pTRIPZ or EML4-ALK-FLAG alongside 6 μg of psPAX2 (a packaging vector) and 2 μg of pMD2.G (an envelope vector) using PolyJet transfection reagent (SL100688; SignaGen Laboratories) according to the manufacturer’s instructions. The next day, the media were replaced with new media and NaButyrate with final concentration of 10 mM was added. Forty-eight hours after transfection, the virus-containing medium was collected and then concentrated using Lenti-X Concentrator (631231; Clontech Laboratories, Mountain View, CA, USA). To stably overexpress ALK-FLAG in MCF10A or EML4-ALK in H1299, HCC1143, and Hs578T, cells were infected with lentivirus with 8 μg/ml polybrene. Two days after infection, cells were selected with 1 μg/ml puromycin. Protein expression was assessed in cells selected for 1 wk by Western blot analysis, and immunofluorescence staining of MCF10A cells was performed as previously described (9).
Cell proliferation and colony assay
Cell proliferation and colony formation assay were performed as previously described (16).
Transient gene knockdown with small interfering RNA
Small interfering RNAs (siRNAs) targeting YAP and TAZ were purchased from Integrated DNA Technologies (Coralville, IA, USA) and transfected into cells using GenMute siRNA transfection reagent according to the manufacturer’s instructions. Final concentration of siRNAs was 50 nM. An siRNA with scrambled sequence (siCtrl) was used as a negative control. Forty-eight hours after transfection, knockdown efficiency was determined by Western blot analysis.
Flow cytometry
Flow cytometry was performed according to a general procedure for direct flow cytometry from Abcam (Cambridge, MA, USA) using a phycoerythrin-conjugated antibody for human PD-L1 (MIH1) from eBioscience (San Diego, CA, USA) and isotype control mouse IgG1κ from Thermo Fisher Scientific.
Jurkat T cell–tumor cells coculture and apoptosis assays
In order to do coculture, Jurkat T cells were stimulated with 1 µg/ml phytohemagglutinin and 50 ng/ml phorbol 12-myristate 13-acetate for 48 h before coculture to induce PD-1 expression. H1299, HCC1143, and Hs578T cells were transfected with siCtrl or siYAP/siTAZ and pretreated with doxycycline (Dox) for 48 h to induce EML4-ALK expression. To induce apoptosis in Jurkat T cells, cancer cell lines were coincubated with activated Jurkat cells for 16–24 h at a tumor:T cell ratio 10:1. Briefly, cancer cells were trypsinized using TrypLE Express reagent (Thermo Fisher Scientific). Cells were counted, and 5 × 105 cancer cells were plated into a 12-well plate and allowed to attach to the plate overnight. The following day, 5 × 104 activated Jurkat T cells were added to each well of the 12-well plate. Cells were allowed to interact for 16–24 h before collecting T cells. After collecting T cells, in order to get higher Jurkat cells purity and reduce cancer cell backgrounds, Jurkat cells were positively selected using EasySep Human PE Positive Selection Kit II (17664; Stem Cell Technologies, Vancouver, BC, Canada) according to the manufacturer’s instructions. After lysing cells in 1% NP-40 lysis buffer, T-cell apoptosis was measured using the Caspase-Glo 3/7 Assay from Promega as previously described (17).
Statistical analysis
Data shown in the graphs were performed in triplicate, except for qRT-PCR and T-cell apoptosis experiments, which were performed in duplicate. All data are the average of triplicate, or, where indicated, duplicate independent experiments and expressed as the mean ± sd. Student’s t test (2-tailed) was used for statistical analysis. A value of P < 0.05 was accepted as statistically significant.
RESULTS
Design of LATS-NBiT-BS
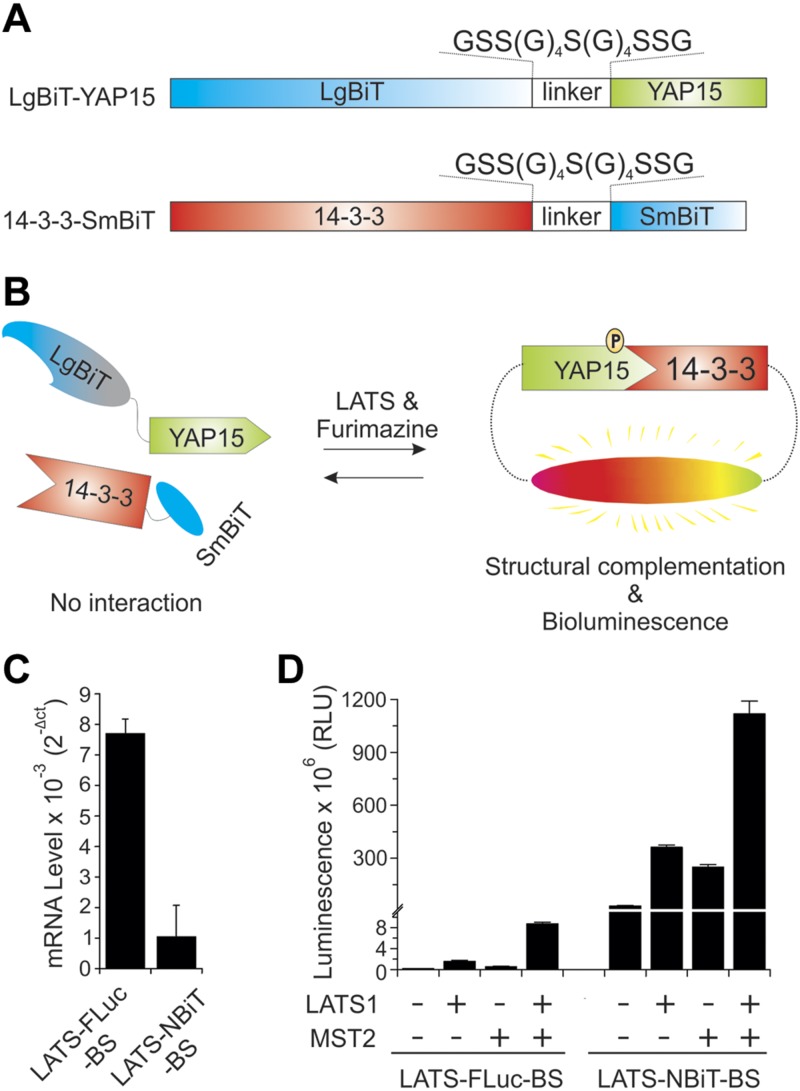
Our previously reported LATS-FLuc-BS was constructed by fusing Firefly N-terminal luciferase with 15 aa of YAP (YAP15) containing the LATS phosphorylation site at S127 and C-terminal luciferase with YAP-pS127–binding 14-3-3 (9). Active LATS increases YAP-pS127, which binds to 14-3-3, resulting in complementation of N-terminal and C-terminal luciferase. Using a similar strategy, we have designed a new LATS-NBiT-BS in which YAP15 is fused with LgBiT through a linker [GSS(G)4S(G)4SSG], whereas 14-3-3 is fused with SmBiT through the same linker (Fig. 1A). Activation of LATS kinase will cause increased levels of YAP-pS127, which binds to 14-3-3 to make structural complementation of LgBiT and SmBiT and emit bioluminescence in the presence of the NLuc substrate, furimazine (Fig. 1B).
Figure 1.
Hippo signaling pathway and establishment of LATS-NBiT-BS to quantify LATS activity. A) Domain organization of LATS-NBiT-BS. B) Overview of the mechanism of the action for the LATS-NBiT-BS. C) The relative RNA expression level of LATS-NBiT-BS and LATS-FLuc-BS. mRNA expression was determined by qRT-PCR (n = 2 technical replicates). D) Comparison of LATS-NBiT-BS and LATS-FLuc-BS activity. Both BSs were cloned in pcDNA3.1/hygro(+) and then transfected alone or with LATS1 and/or MST2 into HEK293T cells. Luminescence was measured 48 h after transfection. RLU values shown as bars clearly indicated that LATS-NBiT-BS is around 250× (on average) brighter than LATS-FLuc-BS. RLU, relative luminescence units. Data are expressed as means ± sd.
LATS-NBiT-BS is much brighter and has greater dynamic range, activity, sensitivity, and stability compared with LATS-FLuc-BS
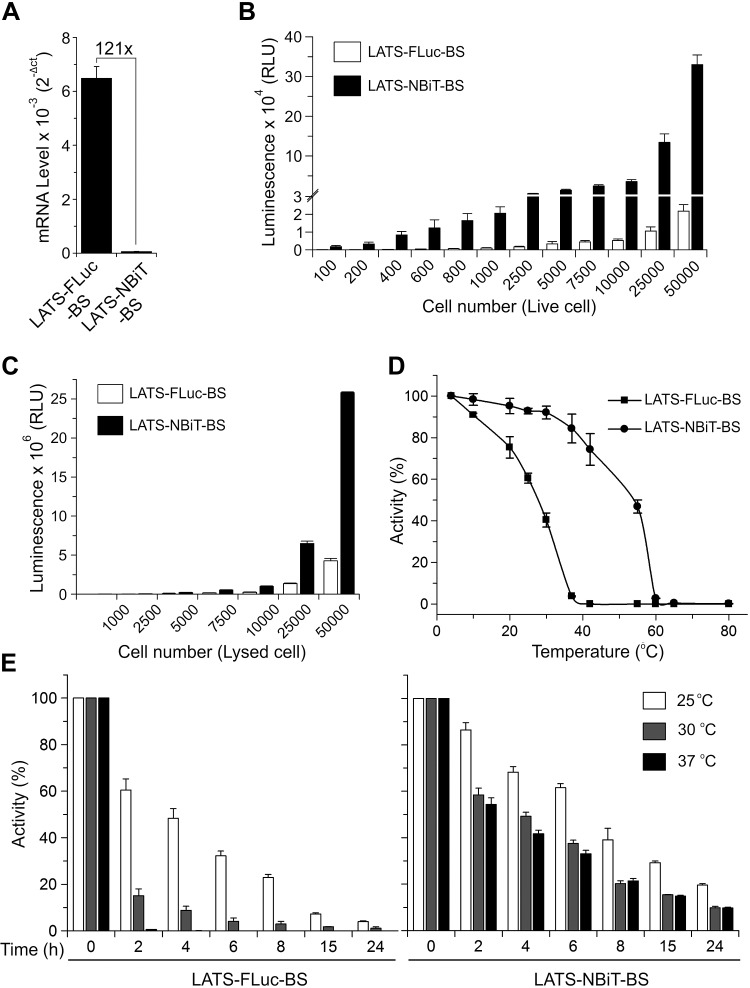
We next compared this new LATS-NBiT-BS with our previously constructed LATS-FLuc-BS (9). Although the mRNA levels of LATS-NBiT-BS are lower than those of LATS-FLuc-BS in cells when both BSs were cloned into cytomegalovirus (CMV) promoter-driven pcDNA3.1 vector (Fig. 1C), LATS-NBiT-BS is around 250 times (in average) brighter than LATS-FLuc-BS when they were expressed alone or together with LATS1 or its activator MST2 in cells (Fig. 1D). To reduce the potential buffering effect of the BS caused by its high levels of expression, which might outcompete the endogenous LATS kinase substrate YAP, we then cloned the LATS-NBiT-BS in a vector with the herpes simplex virus–TK promoter providing constitutive, low-level (>100-fold lower) expression in mammalian cells. Our qRT-PCR results show that the mRNA levels of LATS-NBiT-BS (TK) are 121× lower than those of LATS-FLuc-BS cloned in pcDNA3.1/CMV promoter (Fig. 2A). We next compared the sensitivity of our new LATS-NBiT-BS (TK promoter) with that of LATS-FLuc-BS (CMV promoter). Although 5000 cells are needed to detect LATS-FLuc-BS bioluminescent signal in live cells, LATS-NBiT-BS bioluminescence is detectable using only 200 cells in a 96-well plate (Fig. 2B). In addition, although the mRNA expression levels of LATS-NBiT-BS are much lower than those of LATS-FLuc-BS (Fig. 2A), the luminescence of LATS-NBiT-BS is significantly stronger than that of LATS-FLuc-BS (Fig. 2C). Moreover, compared with LATS-FLuc-BS, LATS-NBiT-BS expressed in cell lysate is more stable at 10–55°C for 30 min (Fig. 2D), stays active at 25, 30, and 37°C for several hours, and amazingly retains 10% activity even when incubated at 37°C for 24 h (Fig. 2E). In summary, our results strongly suggest that the newly developed LATS-NBiT-BS has higher activity, sensitivity, and stability compared with our previously reported LATS-FLuc-BS.
Figure 2.
LATS-NBiT-BS has greater sensitivity and stability compare with LATS-FLuc-BS. A) mRNA expression of LATS-NBiT-BS and LATS-FLuc-BS (qRT-PCR; means ± sd, n = 2 technical replicates). B) LATS-NBiT-BS and LATS-FLuc-BS activity measured in living cells (100–50,000 HEK293T cells), 48 h after transfection (means ± sd; n = 4). C) LATS-NBiT-BS and LATS-FLuc-BS activity of different cell lysates containing between 1000 and 50,000 cell equivalents (means ± sd; n = 4). D) Comparison of 2 different LATS BS activities at different temperature (10–80°C) after 30 min of incubation. E) Thermo stability of LATS-FLuc-BS (left panel) and LATS-NBiT-BS (right panel) at 25, 30, and 37°C at different time points. For D and E, HEK293T cell lysates after 48 h of transfection were used. For all the experiments, LATS-FLuc-BS cloned in pcDNA3.1/hygro (+) and LATS-NBiT-BS cloned in NanoBiT entry vectors—with the herpes simplex virus–TK promoter providing constitutive, low-level (>100-fold lower) expression in mammalian cells—were used.
Validation of LATS-NBiT-BS in vivo and in vitro
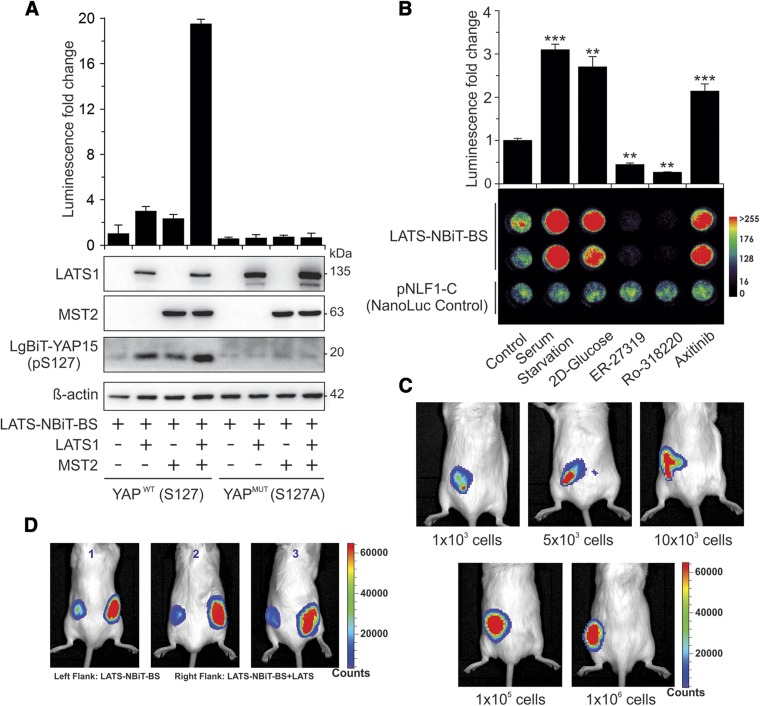
We further tested our LATS-NBiT-BS both in vitro using purified proteins and in vivo in living cells and mice. As shown in Fig. 3, cells transfected with LATS-NBiT-BS alone had low luciferase activity, which was correlated with a low degree of LgBiT-YAP15-S127 phosphorylation. Although cotransfection of LATS-NBiT-BS with LATS1 or MST2 alone increases both LgBiT-YAP15-S127 phosphorylation and luciferase activity, coexpression with both MST2 and LATS1 causes further increases in LgBiT-YAP15-S127 phosphorylation and luciferase activity (Fig. 3A). On the other hand, mutation of S127 phosphorylation on YAP15 into alanine (S127A) completely abolished LgBiT-YAP15-S127 phosphorylation and LATS-NBiT-BS activity (Fig. 3A). These data are consistent with the model that MST2 activates LATS1, which phosphorylates YAP-S127 and leads to binding of 14-3-3 and reconstitution of active luciferase (9).
Figure 3.
Validation of LATS-NBiT-BS activity. A) Phosphorylation-dependent LATS-NiT-BS. LATS-NBiT-BS in NanoBiT entry vector was transfected alone or with LATS1 and/or MST2 into HEK293T. BS activity, expression of LATS1, and MST2 and LgBiT-YAP15-S127 phosphorylation was determined 48 h after transfection. Obtained results showed that in the presence of LATS1 or MST2, LATS-NBiT-BS activity significantly increased. Mutation of serine in LgBiT-YAP15 abolishes LgBiT-YAP15 S127-phosphorylation and BS activation, which means the BS responds specifically to LATS kinase activity (means ± sd; n = 3). B) LATS-BS responds to different drug treatments and regulators of Hippo signaling. HEK293T cells were transfected with LATS-NBiT-BS (in NanoBiT entry vector) and treated with 2-deoxy glucose (2D-glucose; 25 mM), ER-27-319 (10 μM), RO-318220 (10 μM), and axitinib (10 μM) for 4 h, or serum-starved overnight. In this experiment, BS activity in live cells was measured using NanoLuc luciferase assay (upper panel) and BLI (lower panel) after adding furimazine (means ± sd; n = 3). **P < 0.01, ***P < 0.001. C, D) In vivo detection of LATS-NBiT activity. C) BLI of mice 30 s after flank implantation of furimazine (25 μM) preincubated HEK293T-LATS-NBiT-BS (different number of the cells). D) HEK293T cells were transfected with BS alone or with LATS. Flan- implanted cells (1 × 105) previously incubated with furimazine (25 μM) were imaged. A heat map of signal counts represents the relative comparison of BS activity with or without LATS. This experiment was performed in triplicate.
We further validated the LATS-NBiT-BS by transfecting it into cells and subsequently treating cells with agents regulating the Hippo pathway, followed by measuring BS activity in live cells using BLI and in cell lysates by in vitro NanoLuc luciferase assays. As depicted in Fig. 3B, the LATS-NBiT-BS faithfully reports LATS activity in response to positive [e.g., serum starvation, 2D-glucose, and axitinib (VEGF receptor inhibitor)] and negative [e.g., ER-27319 (SYK inhibitor) and Ro-318220 (broad kinase inhibitors)] regulators of the Hippo pathway (9). We also tested LATS activity in vivo by injecting HEK293 cells expressing LATS-NBiT-BS into mice and imaging the bioluminescence using an IVIS Spectrum In Vivo Imaging System. The bioluminescent signal increases with the increasing number of cells injected (Fig. 3C). Notably, the bioluminescent signal could be detected even when only 1000 HEK293-LATS-NBiT-BS cells were injected into the flank of each mouse (Fig. 3C). The signal is dependent upon LATS kinase activity because the bioluminescent signal of HEK293-LATS-NBiT-BS+LATS is dramatically higher than that of HEK293-LATS-NBiT-BS when they were injected into opposing flanks of the same mice (Fig. 3D).
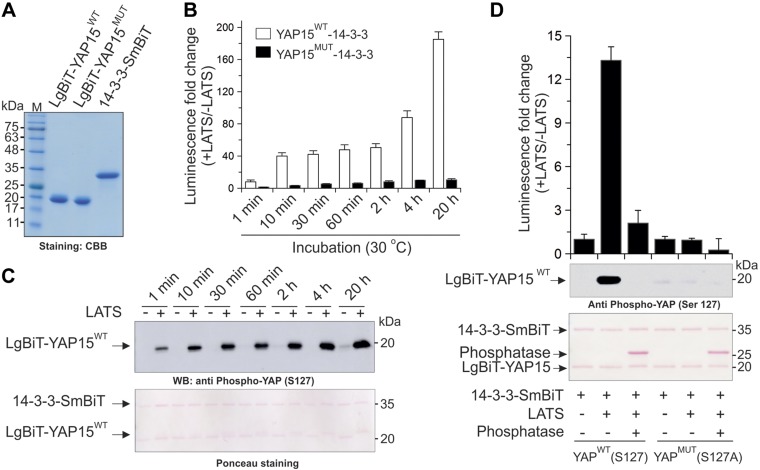
Finally, we were able to purify His-tagged LgBiT-YAP15 and SmBiT-14-3-3 in vitro (Fig. 4A) from bacteria. In vitro luciferase assays show that purified active LATS kinase increases LgBiT-YAP15-pS127 and luciferase activity of LATS-NBiT-BS [WT LgBiT-YAP15 (LgBiT-YAP15WT) + SmBiT-14-3-3] in a time-dependent manner in vitro (Fig. 4B, C), whereas mutation of LATS phosphorylation site S127 on YAP15 (LATS-NBiT-BS-Mut) or phosphatase treatment abolishes LgBiT-YAP15 S127 phosphorylation and BS activation by LATS (Fig. 4B, D).
Figure 4.
Characterization of LATS-NBiT-BS activity in vitro. A) Coomassie brilliant blue (CBB)-stained SDS-PAGE (15%) of purified proteins used in this study. B) In vitro kinase assay using 100 ng of LgBiT-YAP15 WT (S127)/MUT (S127A) and 14-3-3-SmBiT fusion proteins and in the presence of 25 ng LATS fusion protein at 30°C. Bars show the BS activity at different time points. C) Phosphorylation status of LgBiT-YAP15WT (S127) at the indicated times in the presence or absence of LATS, determined by Western blotting. This experiment was performed under the same condition as in B. D) In vitro kinase assay using 5 ng of fusion proteins, and in the presence or absence of λ-phosphatase. After 30-min incubation, BS activity and phosphorylation status were determined by NanoBiT luciferase assay and Western blotting, respectively.
Kinome-wide kinase inhibitor screen using the LATS-NBiT-BS
We next used our LATS-NBiT-BS to perform a kinome-wide high-throughput screen for kinases regulating Hippo signaling using a library containing inhibitors of 560 kinases (Supplemental Fig. S1A). Although 10 kinase inhibitors activate the BS [i.e., inhibitors of BRAF, TANK-binding kinase 1 (TBK1), etc.], 54 inhibitors reduce the BS signal ≥2-fold [i.e., inhibitors of ALK, Aurora A, phosphoinositide 3-kinase (PI3K), epidermal growth factor receptor (EGFR)/human epidermal growth factor receptor 2 (HER2), etc.] (Supplemental Fig. S1B and Supplemental Table S1). Several candidates of LATS regulators identified by our screen were already described in the literature (i.e., inhibitors of EGFR and PI3K) (18), whereas others [e.g., inhibitors of ALK, TBK1, and Janus kinase 2 (JAK2)] are novel. Among these candidate Hippo regulators identified, because ALK is a RTK that plays important roles in various biologic and pathologic processes (19–21), we further validated and characterized RTK ALK as a novel regulator of the Hippo pathway.
Validation of ALK as a novel upstream regulator of the Hippo pathway in tumorigenesis and immune evasion
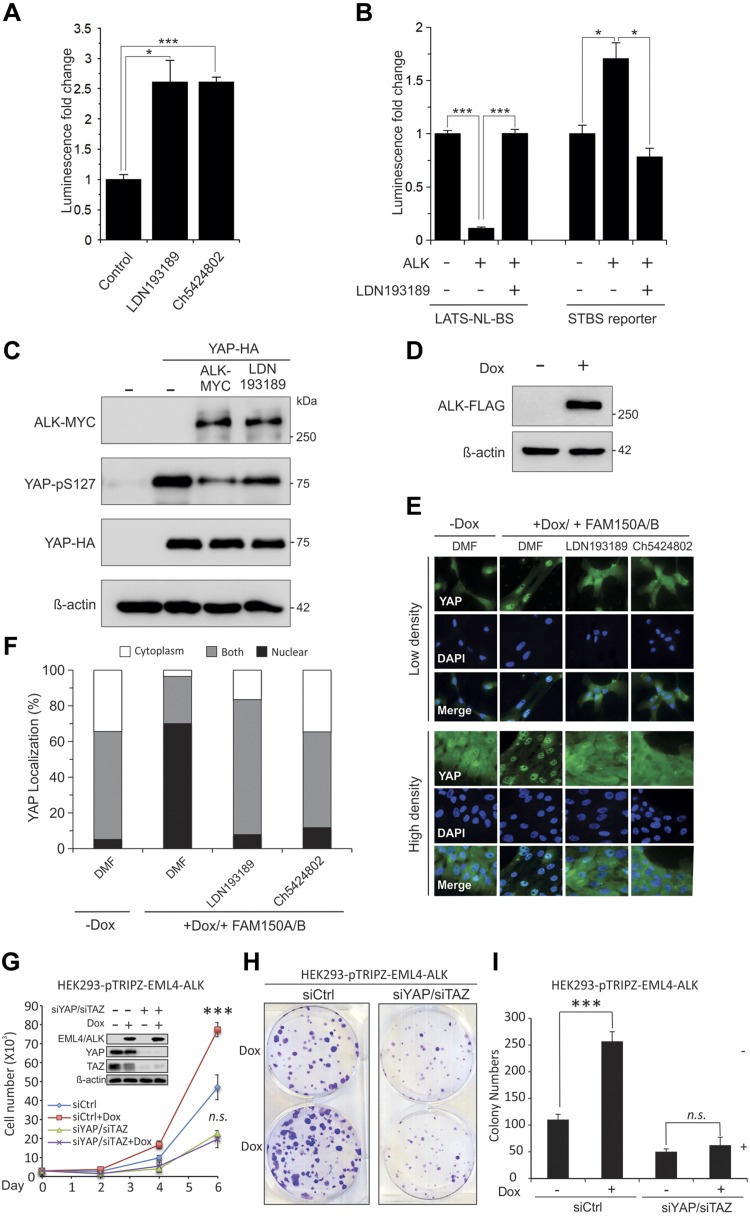
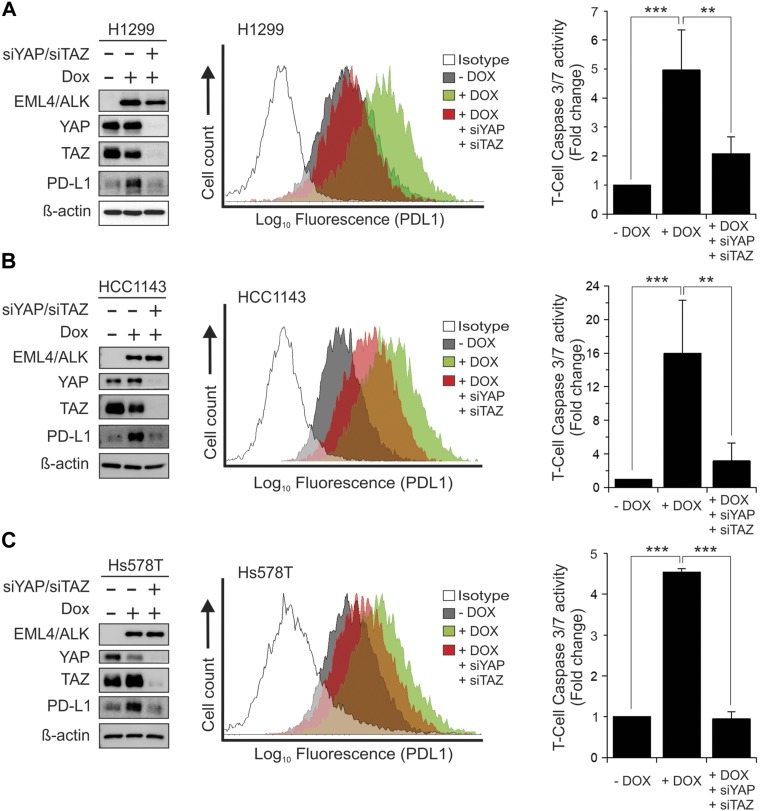
To further confirm the interaction between ALK and the Hippo pathway, we first tested whether the regulation of LATS activity by ALK inhibitor is specific. We show that the LATS-NBiT-BS activity significantly increases after 4 h of treatment of cells with 2 different ALK inhibitors (Fig. 5A). In addition, overexpression of ALK suppresses LATS-NBiT-BS but activates LATS downstream target YAP/TAZ (STBS-reporter containing 14x TEA Domain response sites) activities, which can be reversed by ALK inhibitor LDN193189 (Fig. 5B). Overexpression of ALK together with YAP in HEK293 cells inhibits YAP-S127 phosphorylation by LATS, which can be reversed by LDN193189 treatment (Fig. 5C). Finally, because LATS inhibits YAP by phosphorylating and sequestering YAP/TAZ in the cytoplasm, we established a Dox-inducible cell line that overexpressed ALK (Fig. 5D, upper panel) and then we examined the subcellular localization of YAP in the absence or presence of ALK and its ligands family with sequence similarity (FAM)150 (22). YAP is translocated into the nucleus after 45 min of FAM150 treatment, which is reversed by ALK inhibitors (Fig. 5D–F), suggesting that FAM150/ALK activates YAP/TAZ through enhancement of their nuclear localization by inhibiting LATS. Next, we further examined the functional significance of ALK-YAP/TAZ interaction. We show that Dox-induced overexpression of constitutively activated ELM4-ALK in HEK293 cells significantly enhances cell proliferation and colony formation, whereas knockdown of YAP/TAZ by siRNA that targets YAP and TAZ (siYAP/siTAZ) abolishes ELM4-ALK–induced tumorigenic phenotypes (Fig. 5G–I). In addition, since ALK induces PD-L1 (23) and our previous work reported that YAP/TAZ induces because evasion by transcriptionally activating PD-L1 (17), we further examined whether ALK enhances immune evasion via YAP/TAZ–PD-L1. Our results show that inducible expression of ELM4-ALK in H1299 lung and HCC1143 and Hs578T breast cancer cells increases total and membrane PD-L1, which can be reversed by YAP/TAZ knockdown (siYAP/siTAZ)(Fig. 6, left and middle panels). Significantly, YAP/TAZ knockdown completely blocks ELM4-ALK–induced T-cell killing (immune evasion) by multiple breast and lung cancer cell lines (Fig. 6, right panels), suggesting that ELM4-ALK causes PD-L1–mediated immune evasion by activating YAP/TAZ. Moreover, because our previous study shows that ALK can also up-regulate PD-L1 through activation of signal transducer and activator of transcription 3 (STAT3) (24), we also examined the levels of pSTAT3 after ALK overexpression in the absence or presence of YAP/TAZ knockdown. Our results show that ALK can indeed activate STAT3 by increasing pSTAT3 independent of YAP/TAZ levels in cells (Supplemental Fig. S2).
Figure 5.
ALK is an upstream regulator of Hippo signaling. A) Activation of LATS-NBiT-BS by different ALK inhibitors. HEK293AD cells were transfected with LATS-NBiT-BS, treated with ALK inhibitors for 4 h, followed by luciferase assay (means ± sd; n = 3). *P < 0.05, ***P < 0.001. B) Treating HEK293AD for 4 h with ALK inhibitor (LDN193189) and perform Luciferase assay using STBS-luciferase reporter. C) ALK reduces YAP-S127 phosphorylation in HEK293AD. D) Western blot analysis of ALK-FLAG expression in MCF10A-ALK-FLAG-pTRIPZ Dox inducible cell line in the presence of Dox (1 µg/ml) after 24 h. E) Subcellular localization YAP after treatment of MCF10A cells with FAM150, the ligand of ALK, and/or ALK inhibitors (LDN193189 and CH5424802). ALK plus ligand treatments increase YAP/TAZ nuclear localization in MCF10A-ALK-FLAG-pTRIPZ, and inhibitor treatments reversed the observed phenotype. All of the samples were serum-starved for 24 h. For some conditions, cells were stimulated 45 min with ligand or were treated with the inhibitors for 4 h before treatment and during treatment. F) YAP subcellular localization was quantified in 3 separate experiments (high cell density) in which ≥150 cells were examined. G–I) Knockdown of YAP/TAZ by siRNA blocks ELM4-ALK–induced increased cell proliferation (G) and colony formation (H, I). HEK293 cells stably expressing inducible EML4-ALK in pTRIPZ lentiviral vector were transfected with siRNA against siCtrl or YAP and TAZ (siYAP/siTAZ). Transfected cells were subjected to cell proliferation (G) and colony formation analysis (H, I).
Figure 6.
Knockdown of YAP/TAZ by siRNA blocks ELM4-ALK–induced up-regulation of PD-L1 and immune evasion. H1299 lung (A), or HCC1143 (B), and Hs578T (C) breast cancer cells expressing inducible ELM4-ALK were transfected with siRNAs against siCtrl or YAP and TAZ (siYAP/siTAZ). One day after transfection, the transfected cells were untreated (−Dox) or treated (+Dox) with Dox for another day. Cells were subjected to Western blot analysis of EML4-ALK, YAP, TAZ, and PD-L1 (left panels). β-Actin was used as an internal control. Transfected cells were also subjected to FACS analysis of membrane PD-L1 levels (middle panels) and Caspase3/7 activity analysis of T-cell apoptosis (right panels).
DISCUSSION
In this work, we designed and developed the first ultra-bright NanoBiT LATS bioluminescent BS that monitors LATS kinase activity in the Hippo signaling pathway both in vitro and in vivo. We demonstrate that this new BS has a much higher sensitivity than our previously designed FLuc LATS BS (9). Compared with LATS-FLuc-BS, much stronger signals are detected for LATS-NBiT-BS, even when it is expressed 100 times lower than LATS-FLuc-BS. This feature with enhanced signal/BS ratio significantly reduces the side effect of overexpressed BS on the function of endogenous proteins (the buffering effect of the BS on endogenous YAP) in cells. In addition, we also show that this new LATS-NBiT-BS has higher stability than LATS-FLuc-BS at higher temperatures. The high sensitivity and stability of this BS will have a wider span of applications and significant implications for studying the roles of the Hippo pathway in various biologic processes. For example, it can be used to monitor the dynamics of LATS activity in live cells at physiologically relevant levels, which is not possible using FLuc BSs.
Moreover, we analyzed LATS kinase activity in vitro by luciferase assays using purified LATS-NBiT-BS (Fig. 4). Previously, kinase activity was mostly measured in vitro using [32P] radioisotope, purified protein as a substrate, and kinase immunoprecipitated from large amounts of protein lysates (25). Our ultra-sensitive BS luciferase assay is the first assay developed so far using NanoLuc luciferase to analyze kinase activity in vitro, which will significantly simplify the traditional in vitro immunoprecipitated kinase assay procedures and sensitivity. Therefore, this BS has the potential to test Hippo signaling intensity by measuring the LATS kinase activity using protein lysates extracted from cells or clinical patient tissues. It will have significant implication in clinical diagnosis. In addition, because small molecule inhibitors or activators of LATS kinase is still unavailable, this in vitro luciferase LATS kinase assay can be also used for screening new small molecules directly activating or inhibiting LATS kinase in vitro at ambient room temperature or 30°C, which is not possible for LATS-FLuc-BS.
Our in vivo work further demonstrates that the ultra-bright LATS-NBiT-BS is very sensitive in monitoring LATS activity in a xenograft mouse model in vivo. Significantly, only 1000 cells are required to detect LATS activity in tumor cells in a murine mammary xenograft model, which makes it much easier to perform in vivo preclinical screen studies on the effects of chemotherapeutic drugs, small molecules, or antibody inhibitors on LATS activity.
Protein kinases are important in relaying signaling in cells in almost all biologic processes. Dysregulation of protein kinases contributes to the development of many diseases, such as cancer and cardiovascular disease. Therefore, they are important targets for the treatment of various diseases (26–33). Although the roles of individual kinases in various biologic and pathologic processes have been extensively studied in the past, few kinome-wide studies have been performed. Recently, siRNA or short hairpin RNA and clustered regularly interspaced short palindromic repeats functional genomic screens were used for a kinome-wide screen of kinases regulating biologic functions, such as cell proliferation and drug resistance (34–38). However, there are currently few kinome-wide screenings for kinases affecting the activity of a specific protein. By using our newly developed LATS BS, in this study we performed the first kinome-wide screen for kinases positively or negatively regulating LATS kinase activity using 560 kinase inhibitors and identified many novel lead targets. The bioluminescent BS screen will provide another useful tool, other than siRNA and clustered regularly interspaced short palindromic repeats, with which to screen kinase regulators of any protein.
ALK is an RTK activated by translocation into the locus of other oncogenes, such as ELM4 in breast, lung, and other cancers (19, 21, 39). ALK inhibitors have been clinically used for the treatment of these cancers, including lung cancer (40). Although previous studies identified downstream genes, such as PI3K and Stat3, as mediators of ALK-induced tumorigenesis (24), to our knowledge, after extensive review of the literature, there are no published reports on the interaction between ALK and the Hippo pathway. In this study, we validated ALK and its ligand FAM150 as a bona fide regulator of LATS-YAP/TAZ and the Hippo pathway. We also show that YAP/TAZ are critical downstream genes mediating ALK-induced increased cell proliferation and colony formation. Most recently, ALK was reported as a novel regulator of PD-L1 (23, 24), a checkpoint gene that is overexpressed in various cancers enhancing their tumorigenic functions by either evading immune surveillance (immune evasion) via PD-1–mediated T-cell inactivation (41, 42) or activating the intrinsic oncogenic pathway (43, 44). In this study, we further show that YAP/TAZ are critical in mediating ALK-induced up-regulation of PD-L1 in multiple cancer cell lines. Significantly, we show that knockdown of YAP/TAZ blocks ALK-induced immune evasion. The molecular mechanism underlying ALK-induced activation of YAP/TAZ is currently unclear. However, our recent studies indicate another RTK VEGF receptor activates YAP/TAZ through activation of MAPK and PI3K signaling pathways (9). Therefore, it is possible that ALK may activate YAP/TAZ through a similar mechanism. In addition, we have shown that ALK can induce PD-L1 and activate STAT3 independent of YAP/TAZ (Fig. 6 and Supplemental Fig. S2). Because JAK is the tyrosine kinase phosphorylating STAT3 (23) and because JAK was identified as a novel regulator of LATS in our kinome screen (Supplemental Table S1), it is also possible that ALK causes enhanced immune evasion and tumorigenesis through a JAK-STAT3-LATS-YAP/TAZ-PD-L1 signaling pathway. Further characterization of this newly discovered signaling axis during immune response and tumorigenesis will shed light on the molecular mechanism underlying the ALK-Hippo pathway involved in cancer progression and therapy.
Beside ALK, our kinome-wide inhibitor screen also identifies many regulators of LATS and YAP/TAZ. Some kinase regulators were already reported as upstream regulators of LATS or YAP/TAZ (e.g., Src, ATR, FAK, Syk, PAK, Aurora A, BRAF, PERK, p38) (9, 45, 46), whereas many positive (e.g., eEF2K, IRE1, SMG1, PIM, TrkA) or negative kinase (e.g., TBK1, γ-secretase, JAK2, PLK) regulators are novel. Further characterization of these kinases and their ligands in regulation of the Hippo pathway will shed a light on new roles that the Hippo pathway may play in regulating various biologic processes.
CONCLUSIONS
In this study, we developed the first ultra-bright NLuc LATS BS that has extremely high sensitivity and stability. Therefore, it will have wide applications for monitoring the dynamics and activity of LATS kinase and the Hippo pathway in many biologic and biochemical analyses in the future. Moreover, we identify YAP/TAZ-PD-L1 as a novel downstream signaling axis mediating ALK function in immune evasion. Our findings offer other scientists and clinicians a novel strategy of targeting Hippo component YAP/TAZ alone or together with ALK (combined therapy) for more effective treatment of ALK-involved or ALK inhibitor–resistant cancers.
ACKNOWLEDGMENTS
The authors thank Yawei Hao (Queen’s University) for performing the quantitative RT-PCR and technical support. The authors also thank Dr. Ruth Palmer (Gothenburg University, Gothenburg, Sweden) and Dr. Jikui Guan (Gothenburg University) for providing the FAM150 plasmid, and Dr. Alessandro Datti (S.M.A.R.T. Laboratory for the High-Throughput Screening Programs of the Lunenfeld-Tanenbaum Research Institute, Mount Sinai Hospital, Toronto, ON, Canada) for high-throughput screen for kinases regulating large tumor suppressor kinase 1. E.L. was supported by the Queen’s University Terry Fox Research Institute Transdisciplinary Training Program in Cancer Research Fellowship and the Dr. Robert John Wilson Graduate Fellowship. This work was supported by grants from Canadian Institute of Health Research (CIHR; 119325 and 148629 to X.Y.), the Canadian Breast Cancer Foundation/Canadian Cancer Society (CBCF/CRS; to X.Y. and C.J.N.), and the Breast Cancer Action Kingston and Kingston General Hospital Foundation (to C.J.N.). T.A. was supported by the Vanier Canada Graduate Scholarship and Ontario International Graduate Scholarship. The authors declare no conflicts of interest.
Glossary
- ALK
anaplastic lymphoma kinase
- BLI
bioluminescent imaging
- BS
biosensor
- CMV
cytomegalovirus
- Dox
doxycycline
- EML4
echinoderm microtubule-associated protein like 4
- FAM150
family with sequence similarity 150
- FLuc
Firefly luciferase
- HEK
human embryonic kidney
- LATS
large tumor suppressor kinase 1
- LgBiT
large binary technology
- MST
mammalian STE20-like
- Mut
mutant
- NanoBiT
NanoLuc Binary Technology
- NLuc
NanoLuc luciferase
- PD-L1
programmed death-ligand 1
- PI3K
phosphoinositide 3-kinase
- qRT-PCR
quantitative RT-PCR
- RTK
receptor tyrosine kinase
- siCtrl
siRNA with scrambled sequence
- si
small interfering
- SLCA
split luciferase complementation assay
- SmBiT
small binary technology
- STAT3
signal transducer and activator of transcription 3
- TAZ
transcriptional co-activator with PDZ- binding motif
- TBK1
TANK-binding kinase 1
- TK
thymidine kinase
- WT
wild type
- YAP
yes-associated protein
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
K. Nouri, T. Azad, and E. Lightbody analyzed the data; K. Nouri, T. Azad, E. Lightbody, and P. Khanal performed the research; K. Nouri, T. Azad, C. J. Nicol, and X. Yang designed the research; and K. Nouri and X. Yang wrote the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Yang X., Xu T. (2011) Molecular mechanism of size control in development and human diseases. Cell Res. 21, 715–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fu V., Plouffe S. W., Guan K. L. (2017) The Hippo pathway in organ development, homeostasis, and regeneration. Curr. Opin. Cell Biol. 49, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hansen C. G., Moroishi T., Guan K. L. (2015) YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 25, 499–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taha Z., Janse van Rensburg H. J., Yang X. (2018) The Hippo pathway: immunity and cancer. Cancers (Basel) 10, E94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Janse van Rensburg H. J., Yang X. (2016) The roles of the Hippo pathway in cancer metastasis. Cell. Signal. 28, 1761–1772 [DOI] [PubMed] [Google Scholar]
- 6.Zhao Y., Yang X. (2018) Targeting the Hippo pathway to improve response to chemotherapy. In Targeting Cell Survival Pathways to Enhance Response to Chemotherapy (Johnson D., ed.), pp. 165–189, Academic Press (Elsevier), Cambridge, MA; , USA [Google Scholar]
- 7.Zhao Y., Yang X. (2015) The Hippo pathway in chemotherapeutic drug resistance. Int. J. Cancer 137, 2767–2773 [DOI] [PubMed] [Google Scholar]
- 8.Zanconato F., Cordenonsi M., Piccolo S. (2016) YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Azad T., Janse van Rensburg H. J., Lightbody E. D., Neveu B., Champagne A., Ghaffari A., Kay V. R., Hao Y., Shen H., Yeung B., Croy B. A., Guan K. L., Pouliot F., Zhang J., Nicol C. J. B., Yang X. (2018) A LATS biosensor screen identifies VEGFR as a regulator of the Hippo pathway in angiogenesis. Nat. Commun. 9, 1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Azad T., Nouri K., Janse van Rensburg H. J., Hao Y., Yang X. (2018) Monitoring Hippo signaling pathway activity using a luciferase-based large tumor suppressor (LATS) biosensor. J. Vis. Exp. 139, E58416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall M. P., Unch J., Binkowski B. F., Valley M. P., Butler B. L., Wood M. G., Otto P., Zimmerman K., Vidugiris G., Machleidt T., Robers M. B., Benink H. A., Eggers C. T., Slater M. R., Meisenheimer P. L., Klaubert D. H., Fan F., Encell L. P., Wood K. V. (2012) Engineered luciferase reporter from a deep sea shrimp utilizing a novel imidazopyrazinone substrate. ACS Chem. Biol. 7, 1848–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.England C. G., Ehlerding E. B., Cai W. (2016) NanoLuc: a small luciferase is brightening up the field of bioluminescence. Bioconjug. Chem. 27, 1175–1187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dixon A. S., Schwinn M. K., Hall M. P., Zimmerman K., Otto P., Lubben T. H., Butler B. L., Binkowski B. F., Machleidt T., Kirkland T. A., Wood M. G., Eggers C. T., Encell L. P., Wood K. V. (2016) NanoLuc complementation reporter optimized for accurate measurement of protein interactions in cells. ACS Chem. Biol. 11, 400–408 [DOI] [PubMed] [Google Scholar]
- 14.Nouri K., Fansa E. K., Amin E., Dvorsky R., Gremer L., Willbold D., Schmitt L., Timson D. J., Ahmadian M. R. (2016) IQGAP1 interaction with RHO family proteins revisited: kinetic and equilibrium evidence for multiple distinct binding sites. J. Biol. Chem. 291, 26364–26376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaiswal M., Dubey B. N., Koessmeier K. T., Gremer L., Ahmadian M. R. (2012) Biochemical assays to characterize Rho GTPases. Methods Mol. Biol. 827, 37–58 [DOI] [PubMed] [Google Scholar]
- 16.Ho K. C., Zhou Z., She Y. M., Chun A., Cyr T. D., Yang X. (2011) Itch E3 ubiquitin ligase regulates large tumor suppressor 1 stability [corrected]. Proc. Natl. Acad. Sci. USA 108, 4870–4875; erratum: 8914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Janse van Rensburg H. J., Azad T., Ling M., Hao Y., Snetsinger B., Khanal P., Minassian L. M., Graham C. H., Rauh M. J., Yang X. (2018) The Hippo pathway component TAZ promotes immune evasion in human cancer through PD-L1. Cancer Res. 78, 1457–1470 [DOI] [PubMed] [Google Scholar]
- 18.Zhao Y., Montminy T., Azad T., Lightbody E., Hao Y., SenGupta S., Asselin E., Nicol C., Yang X. (2018) PI3K positively regulates YAP and TAZ in mammary tumorigenesis through multiple signaling pathways. Mol. Cancer Res. 16, 1046–1058 [DOI] [PubMed] [Google Scholar]
- 19.Cao Z., Gao Q., Fu M., Ni N., Pei Y., Ou W. B. (2019) Anaplastic lymphoma kinase fusions: roles in cancer and therapeutic perspectives. Oncol. Lett. 17, 2020–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Della Corte C. M., Viscardi G., Di Liello R., Fasano M., Martinelli E., Troiani T., Ciardiello F., Morgillo F. (2018) Role and targeting of anaplastic lymphoma kinase in cancer. Mol. Cancer 17, 30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallberg B., Palmer R. H. (2016) The role of the ALK receptor in cancer biology. Ann. Oncol. 27 (Suppl3), iii4–iii15 [DOI] [PubMed] [Google Scholar]
- 22.Guan J., Umapathy G., Yamazaki Y., Wolfstetter G., Mendoza P., Pfeifer K., Mohammed A., Hugosson F., Zhang H., Hsu A. W., Halenbeck R., Hallberg B., Palmer R. H. (2015) FAM150A and FAM150B are activating ligands for anaplastic lymphoma kinase. eLife 4, e09811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ota K., Azuma K., Kawahara A., Hattori S., Iwama E., Tanizaki J., Harada T., Matsumoto K., Takayama K., Takamori S., Kage M., Hoshino T., Nakanishi Y., Okamoto I. (2015) Induction of PD-L1 expression by the EML4-ALK oncoprotein and downstream signaling pathways in non-small cell lung cancer. Clin. Cancer Res. 21, 4014–4021 [DOI] [PubMed] [Google Scholar]
- 24.Koh J., Jang J. Y., Keam B., Kim S., Kim M. Y., Go H., Kim T. M., Kim D. W., Kim C. W., Jeon Y. K., Chung D. H. (2015) EML4-ALK enhances programmed cell death-ligand 1 expression in pulmonary adenocarcinoma via hypoxia-inducible factor (HIF)-1α and STAT3. OncoImmunology 5, e1108514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hao Y., Chun A., Cheung K., Rashidi B., Yang X. (2008) Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 283, 5496–5509 [DOI] [PubMed] [Google Scholar]
- 26.Shin C. H., Choi D. S. (2019) Essential roles for the non-canonical IκB kinases in linking inflammation to cancer, obesity, and diabetes. Cells 8, E178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burke J. E. (2018) Structural basis for regulation of phosphoinositide kinases and their involvement in human disease. Mol. Cell 71, 653–673 [DOI] [PubMed] [Google Scholar]
- 28.Baltussen L. L., Rosianu F., Ultanir S. K. (2018) Kinases in synaptic development and neurological diseases. Prog. Neuropsychopharmacol. Biol. Psychiatry 84, 343–352 [DOI] [PubMed] [Google Scholar]
- 29.Nandipati K. C., Subramanian S., Agrawal D. K. (2017) Protein kinases: mechanisms and downstream targets in inflammation-mediated obesity and insulin resistance. Mol. Cell. Biochem. 426, 27–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yokota T., Wang Y. (2016) p38 MAP kinases in the heart. Gene 575, 369–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thompson B. J., Sahai E. (2015) MST kinases in development and disease. J. Cell Biol. 210, 871–882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Regad T. (2015) Targeting RTK signaling pathways in cancer. Cancers (Basel) 7, 1758–1784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loirand G. (2015) Rho kinases in health and disease: from basic science to translational research. Pharmacol. Rev. 67, 1074–1095 [DOI] [PubMed] [Google Scholar]
- 34.Wang L., Adamski C. J., Bondar V. V., Craigen E., Collette J. R., Pang K., Han K., Jain A., Y Jung S., Liu Z., Sifers R. N., Holder J. L., Jr., Zoghbi H. Y. (2019) A kinome-wide RNAi screen identifies ERK2 as a druggable regulator of Shank3 stability. [E-pub ahead of print] Mol. Psychiatry 10.1038/s41380-018-0325-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tzeng Y. T., Liu P. F., Li J. Y., Liu L. F., Kuo S. Y., Hsieh C. W., Lee C. H., Wu C. H., Hsiao M., Chang H. T., Shu C. W. (2018) Kinome-wide siRNA screening identifies Src-enhanced resistance of chemotherapeutic drugs in triple-negative breast cancer cells. Front. Pharmacol. 9, 1285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen J., Najafi S., Stäble S., Fabian J., Koeneke E., Kolbinger F. R., Wrobel J. K., Meder B., Distel M., Heimburg T., Sippl W., Jung M., Peterziel H., Kranz D., Boutros M., Westermann F., Witt O., Oehme I. (2018) A kinome-wide RNAi screen identifies ALK as a target to sensitize neuroblastoma cells for HDAC8-inhibitor treatment. Cell Death Differ. 25, 2053–2070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pavan S., Meyer-Schaller N., Diepenbruck M., Kalathur R. K. R., Saxena M., Christofori G. (2018) A kinome-wide high-content siRNA screen identifies MEK5-ERK5 signaling as critical for breast cancer cell EMT and metastasis. Oncogene 37, 4197–4213 [DOI] [PubMed] [Google Scholar]
- 38.Beauchamp R. L., James M. F., DeSouza P. A., Wagh V., Zhao W. N., Jordan J. T., Stemmer-Rachamimov A., Plotkin S. R., Gusella J. F., Haggarty S. J., Ramesh V. (2015) A high-throughput kinome screen reveals serum/glucocorticoid-regulated kinase 1 as a therapeutic target for NF2-deficient meningiomas. Oncotarget 6, 16981–16997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Siraj A. K., Beg S., Jehan Z., Prabhakaran S., Ahmed M., R Hussain A., Al-Dayel F., Tulbah A., Ajarim D., Al-Kuraya K. S. (2015) ALK alteration is a frequent event in aggressive breast cancers. Breast Cancer Res. 17, 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Golding B., Luu A., Jones R., Viloria-Petit A. M. (2018) The function and therapeutic targeting of anaplastic lymphoma kinase (ALK) in non-small cell lung cancer (NSCLC). Mol. Cancer 17, 52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim J. M., Chen D. S. (2016) Immune escape to PD-L1/PD-1 blockade: seven steps to success (or failure). Ann. Oncol. 27, 1492–1504 [DOI] [PubMed] [Google Scholar]
- 42.Salmaninejad A., Valilou S. F., Shabgah A. G., Aslani S., Alimardani M., Pasdar A., Sahebkar A. (2019) PD-1/PD-L1 pathway: basic biology and role in cancer immunotherapy. J. Cell. Physiol. 234, 16824–16837 [DOI] [PubMed] [Google Scholar]
- 43.Clark C. A., Gupta H. B., Curiel T. J. (2017) Tumor cell-intrinsic CD274/PD-L1: a novel metabolic balancing act with clinical potential. Autophagy 13, 987–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Clark C. A., Gupta H. B., Sareddy G., Pandeswara S., Lao S., Yuan B., Drerup J. M., Padron A., Conejo-Garcia J., Murthy K., Liu Y., Turk M. J., Thedieck K., Hurez V., Li R., Vadlamudi R., Curiel T. J. (2016) Tumor-intrinsic PD-L1 signals regulate cell growth, pathogenesis, and autophagy in ovarian cancer and melanoma. Cancer Res. 76, 6964–6974; erratum: 77, 2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Furth N., Aylon Y. (2017) The LATS1 and LATS2 tumor suppressors: beyond the Hippo pathway. Cell Death Differ. 24, 1488–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Reuven N., Shanzer M., Shaul Y. (2019) Hippo pathway regulation by tyrosine kinases. Methods Mol. Biol. 1893, 215–236 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.