Abstract
Intercellular communication between lymphocytes plays a fundamental role in numerous immune responses. Previously, we demonstrated that hyperhomocysteinemia (HHcy) induced T cell intracellular glycolytic-lipogenic reprogramming and IFN-γ secretion via pyruvate kinase muscle isozyme 2 (PKM2) to accelerate atherosclerosis. Usually, B cells partially obtain help from T cells in antibody responses. However, whether PKM2 activation in T cells regulates B cell antibody production is unknown. Extracellular vesicles (EVs) are important cellular communication vehicles. Here, we found that PKM2 activator TEPP46-stimulated T-cell–derived EVs promoted B-cell IgG secretion. Conversely, EVs secreted from PKM2-null T cells were internalized into B cells and markedly inhibited B-cell mitochondrial programming, activation, and IgG production. Mechanistically, lipidomics analyses showed that increased ceramides in PKM2-activated T-cell EVs were mainly responsible for enhanced B cell IgG secretion induced by these EVs. Finally, quantum dots (QDs) were packaged with PKM2-null T cell EVs and anti-CD19 antibody to exert B-cell targeting and inhibit IgG production, eventually ameliorating HHcy-accelerated atherosclerosis in vivo. Thus, PKM2-mediated EV ceramides in T cells may be an important cargo for T-cell–regulated B cell IgG production, and QD-CD19-PKM2-null T cell EVs hold high potential to treat B cell overactivation-related diseases.—Yang, J., Dang, G., Lü, S., Liu, H., Ma, X., Han, L., Deng, J., Miao, Y., Li, X., Shao, F., Jiang, C., Xu, Q., Wang, X., Feng, J. T-cell–derived extracellular vesicles regulate B-cell IgG production via pyruvate kinase muscle isozyme 2.
Keywords: lymphocyte, antibody, ceramide
The crosstalk between T and B cells plays a key role in and most likely contributes to various diseases (1, 2). Over the last few years, a considerable amount of attention has focused on a new intercellular signaling pathway: extracellular vesicles (EVs). EVs, characterized by lipid bilayer–enclosed extracellular structures (3–5), can be isolated at high levels from all body fluids and various different cell types (6, 7). EVs can deliver their cargos, including donor cell–specific proteins, lipids, and nucleic acids, to target cells (8, 9). These molecules are natural carrier systems and might be important mediators of intercellular communication in various diseases. MicroRNA-loaded EVs secreted from T-cell lines or lymphoblasts are unidirectionally transferred to antigen-presenting cells including Raji B-cell lines (10). Additionally, CD19+ EVs from B cells impair CD8+ T-cell responses in chemotherapy-treated cancer cells (11). It is well known that activated B cells produce antibodies, and T cells provide assistance mainly by direct contact and cytokine secretion (12–15). The precise roles and potential regulatory mechanisms of T-cell–derived EVs (TEVs) in regulating B-cell IgG secretion remain unclear.
Intercrossing of pathways in the regulation of immune and metabolic systems and metabolic reprogramming is important for the functions of immunocytes, including macrophages, fibroblasts, and lymphocytes (16–19). Alterations in metabolism at both the cellular and tissue levels affect specific T-cell functions (19). Homocysteine (Hcy) is a sulfur-containing nonessential amino acid that forms as a key intermediate during methionine metabolism. Elevated level of plasma Hcy results in a condition known as hyperhomocysteinemia (HHcy), which has long been suggested as an independent risk factor for cardiovascular diseases (20, 21). We have reported that the T-cell intracellular glycolytic-lipogenic axis mediates HHcy-induced inflammatory activation via pyruvate kinase muscle isozyme 2 (PKM2), a key enzyme of glycolysis, to accelerate atherosclerosis by using T cell–specific PKM2 knockout (LckCrePKM2fl/fl) mice (22). We found significantly decreased plasma IgG in these LckCrePKM2fl/fl mice compared with that in PKM2fl/fl mice, which was accompanied by fewer EV-related gene expression in the PKM2-null T cells of these mice. Additionally, increased B cell IgG secretion was observed after coculture with EVs derived from PKM2-activated T cells. All of these preliminary data indicate that T-cell PKM2 not only regulates the intracellular glycolytic-lipogenic axis but also plays a secondary role to mediate T-cell–directed regulation of other cells, including B-cell activation, via EVs. A complex network of EV-mediated intercellular signaling and content exchange appears to exist between T and B cells. In the present study, we will further explore whether or not the large amounts of lipids that accumulate in PKM2-activated T cells can be transferred to recipient B cells in the form of EVs and subsequently affect B cell antibody secretion.
As native carriers of intercellular communication, EVs have also been widely used as pathologic diagnostic markers and therapeutic tools (23–25). A recent paper by Chen et al. (26) provided a rationale for the application of exosomal PD-L1 as an indicator and predictor of antitumor immunity. Gallet et al. (27) reported that exosomes secreted by cardiosphere-derived cells decreased scarring, halted adverse remodeling, and improved cardiac function in acute and chronic porcine myocardial infarction. On the other hand, nanocrystal quantum dots (QDs), a new class of fluorophores, have emerged as an indispensable tool in biomedical research, especially for biomolecular and cellular imaging as well as therapy (28, 29). In this study, we first identified the precise lipids of TEVs that inhibited B cells in a PKM2-dependent manner by HPLC tandem mass spectrometry (MS/MS)-based lipidomics and coculture experiments in vitro. Afterwards, a B-cell–targeted QD carrier was developed by combining QDs with PKM2-null TEVs and an anti-CD19 antibody. This carrier inhibited IgG production in response to (4-hydroxy-3-nitrophenyl) acetyl (NP)-LPS and NP-ovalbumin (OVA) antigen stimulation and, even, HHcy-accelerated atherosclerosis in vivo.
In the present study, we reported that PKM2-activated T cells up-regulated B-cell IgG production via secreting ceramide-filled EVs. EVs secreted from PKM2-null T cells were internalized into B cells and markedly inhibited B-cell mitochondrial programming, activation, and IgG production. As delivery platforms for direct and specific B-cell targeting, QDs conjugated with PKM2-null TEVs and anti-CD19 antibody could inhibit B-cell IgG production and therefore inhibit HHcy-accelerated atherosclerosis. These results suggest a drug-free immunotherapeutic strategy to treat B-cell overactivation-related diseases.
MATERIALS AND METHODS
Mice
Female C57BL/6J mice and ApoE−/− mice between 6 and 8 wk of age were housed in the Animal Center of Peking University Health Science Center. PKM2fl/fl mice (24048, on a B6129SF1/J background) and Lck-Cre transgenic mice (Jax-003803) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). LckCrePKM2fl/fl mice were generated by crossing PKM2fl/fl mice and Lck-Cre transgenic mice with help from the Shanghai Biomodel Organism Science and Technology Development (Shanghai, China) in our laboratory. For the atherosclerosis experiments, ApoE−/− mice were fed a normal mouse chow diet and were provided drinking water supplemented with or without 1.8 g/L DL-Hcy (H4628-25G; MilliporeSigma, Burlington, MA, USA) for 3 wk. All animal procedures were reviewed and approved by the Institutional Animal Care and Use Committee of Peking University Health Science Center in accordance with the U.S. Department of Agriculture, International Association for the Assessment and Accreditation of Laboratory Animal Care, and National Institutes of Health (NIH; Bethesda, MD, USA) guidelines.
Cell isolation and culture
Splenic B and T cells were isolated from mice and purified by positive immunomagnetic cell sorting with standard protocols and CD19 and CD90.2 microbeads (130-052-201, 130-049-101; Miltenyi Biotec, Bergisch Gladbach, Germany). Purified B and T cells were cultured in Roswell Park Memorial Institute (RPMI)1640 medium (c11875500BT; Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% (v/v) fetal bovine serum (900-108; Gemini Bio-Products, Sacramento, CA, USA) and maintained with LPS (0.1 μg/ml, as basic stimulation for B cells, L2880; MilliporeSigma) and anti-CD3 antibody [1 μg/ml, as basic stimulation for T cells, 53057, RRID: AB_394590; BD Biosciences, San Jose, CA, USA].
ELISA
Titers of IgG in serum and B-cell culture supernatants were determined by sandwich ELISAs using mouse-specific IgG ELISA kits (E101; Bethyl Laboratories, Montgomery, TX, USA) according to the manufacturer’s protocol.
Western blot analysis
Purified cell proteins or isolated EVs were used for SDS-PAGE and transferred onto nitrocellulose membranes by wet electrophoretic transfer. The membranes were then blocked and incubated overnight at 4°C with primary antibodies. Antibodies against phosphorylated (p)–mechanistic target of rapamycin (mTOR; Ser2448, 2971, RRID: AB_330970), mTOR (4517, RRID: AB_1904056), p-protein kinase B (Akt; Ser473, 4060, RRID: AB_2315049), Akt (2920, RRID: AB_1147620), CD9 (13403S), calnexin (2679S), and IFN regulatory factor 4 (IRF4; 4964S) were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies against CD63 (ab193349), TSG101 (ab125011, RRID: AB_10974262), Alix (aab117600), CD19 (ab134114), and B lymphocyte–induced maturation protein 1 (Blimp1; ab119401, RRID: AB_10899803) were purchased from Abcam (Cambridge, MA, USA). An antibody against eIF5 (Sc-282) was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). After the membranes were washed, they were incubated with DyLight 680-conjugated secondary antibodies (1:20,000, Kirkegaard & Perry Laboratories, 072-06-18-06) or DyLight 800-conjugated secondary antibodies (1:20,000, Kirkegaard & Perry Laboratories, 072-07-15-16) for 1 h at room temperature. The Odyssey infrared imaging system (Li-Cor Biosciences, Lincoln, NE, USA) was used to visualize the fluorescence signals.
Quantitative PCR measurement of mRNA levels
Total RNA was extracted using Trizol reagent (15596018; Thermo Fisher Scientific), and mRNA was isolated using the reverse transcription (RT) system 5X All-In-One RT MasterMix Kit (G490; Applied Biological Materials, Richmond, BC, Canada). Quantitative RT-PCR was then used to measure the cDNA content with an Mx3000 Multiplex Quantitative PCR (qPCR) System (Stratagene California, San Diego, CA, USA) and SYBR Green I fluorescence. The results were normalized to β-actin mRNA levels and analyzed using Stratagene Mx3000 software. The primer sequences used are listed as follows: activation-induced cytidine deaminase (Aicda) (forward 5′-AAGGGACGGCATGAGACCTA-3′; reverse 5′-GCCGAAGTTGTCTGGTTAGC-3′), μ CH region γ chain 1 (Iμ-Cγ1) (forward 5′-CTCTGGCCCTGCTTATTGTTG-3′; reverse 5′-GGATCCAGAGTTCCAGGTCACT-3′), Iμ-Cγ2b (forward 5′-CTCTGGCCCTGCTTATTGTTG-3′; reverse 5′-CACTGAGCTGCTCATAGTGTAGAGTC-3′), Iμ-Cγ3 (forward 5′-CTCTGGCCCTGCTTATTGTTG-3′; reverse 5′-CTCAGGGAAGTAGCCTTTGACA-3′), PR/SET Domain 1 (Prdm1) (forward 5′-CCCAAGAGCCTTACTGACCT-3′; reverse 5′-CAACCAACCAACCAACCACTG-3′), Irf4 (forward 5′-GGCAAGCAGGACTACAATCG-3′; reverse 5′-TGGGGTGGCATCATGTAGTT-3′), CD63 (forward 5′-GAAGCAGGCCATTACCCATGA-3′; reverse 5′-TGACTTCACCTGGTCTCTAAACA-3′), CD9 (forward 5′-TGGGAAGAGCTATTCAATGTTGG-3′; reverse 5′-AGAGGCCATATTGGGGATGATAA-3′), CD81 (forward 5′-GTGGAGGGCTGCACCAAAT-3′; reverse 5′-GACGCAACCACAGAGCTACA-3′), Ras-related protein Rab27a (Rab27a) (forward 5′-TCGGATGGAGATTACGATTACCT-3′; reverse 5′-TTTTCCCTGAAATCAATGCCCA-3′), Ras-related protein Rab27b (Rab27b) (forward 5′-CGTCAGGAAAAGCGTTTAAGGT-3′; reverse 5′-AGAAGCTCTGTTGACTGGTGA-3′), heat shock protein 70 (HSP70) (forward5′-GCCAAACGGTTCATCGGGA-3′; reverse 5′-AGGTGCTATTACCAGCAAGGT-3′), β-actin (forward 5′-GTGACGTTGACATCCGTAAAGA-3′; reverse 5′-GTGACGTTGACATCCGTAAAGA-3′), intercellular adhesion molecule 1 (Icam-1) (forward, 5′-AGCTCGGAGGATCACAAA-3′; reverse, 5′-TCTGCTGAGACCCCTCTTG-3′), vascular cell adhesion protein 1 (Vcam-1) (forward, 5′-CTGTTCCAGCGAGGGTCTA-3′; reverse, 5′-CACAGCCAATAGCAGCACA-3′), Ifn-γ (forward, 5′-TGGCTGTTTCTGGCTGTTAC-3′; reverse, 5′-TTCGCCTTGCTGTTGCTGAAG-3′), Tnf-α (forward, 5′-ACAGAAAGCATGATCCGCGAC-3′; reverse, 5′-CCGATCACCCCGAAGTTCAGTA-3′), C-C motif chemokine ligand 2 (CCL2) (forward, 5′-CAGATGCAGTTAACGCCC-3′; reverse, 5′-ATTCCTTCTTGGGGTCAGC-3′), and Il-2 (forward, 5′-CAGGAACCTGAAACTCCCCA-3′; reverse, 5′-AGAAAGTCCACCACAGTTGC-3′).
Extracellular flux analysis
Oxygen consumption rates (OCR) and extracellular acidification rates (ECAR) were measured on a Seahorse XF24 extracellular flux analyzer (Seahorse Bioscience, Billerica, MA, USA). B cells were collected 24 h postincubation with purified TEVs and seeded into a Seahorse 24-well plate coated with poly-l-lysine hydrobromide (P7890; MilliporeSigma). At the beginning of the assay, cells were incubated with Seahorse XF base medium supplemented with 10 mM glucose, 1 mM pyruvate, and 2 mM glutamine in a CO2-free incubator equilibrated for 1 h. During the course of the assay, 1 μM oligomycin, 1 μM carbonyl cyanide p-trifluoromethoxyphenylhydrazone (FCCP), 1 μM rotenone, and 1 μM antimycin A (all from MilliporeSigma) were added sequentially. The data were analyzed with Wave software and the XF Mito/Glycolysis Stress Test Report Generator (Seahorse Bioscience).
Isolation, purification, and characterization of EVs
EVs were purified by ultracentrifugation, as previously described (30–32). Spleen primary T cells (1 × 107/well) were grown in culture medium (which was depleted of endogenous EVs by ultracentrifugation at 120,000 g for 18 h) while seeded in 6-well plates and were treated with anti-CD3 antibody stimulation for 48 h. Plasma (∼2 ml) and T-cell culture supernatants (∼45 ml) were consecutively centrifuged at 350 g for 10 min at 4°C, 2000 g for 10 min at 4°C, and 12,000 g for 40 min at 4°C to remove cell debris and dead cells. The resulting supernatants were then filtered using a 0.2-μm filter (PN4612; Pall, Westborough, MA, USA) and ultracentrifugation (Beckman Coulter, Brea, CA, USA) at 120,000 g for 70 min at 4°C. The condensed pellet was washed with ice-cold PBS and centrifuged again at 120,000 g for another 70 min at 4°C. The resulting pelleted EVs were suspended in PBS, and concentrations of EV proteins were measured using the BCA Protein Assay Kit (Beyotime, Shanghai, China).
The size distribution of purified EVs was measured by nanoparticle tracking analysis (NTA) and a ζ potential distribution analyzer (Particle Metrix, Meerbusch, Germany). Morphology was visualized using a transmission electron microscope (TEM) (Tecnai G2 Spirit; Thermo Fisher Scientific). EV protein markers including CD63, TSG101, Alix, and CD9, the T-cell–specific marker CD3ε, and the EV-negative marker calnexin were measured by Western blot analysis.
Administration of B cells with EVs
To determine the effects of EVs on B cell activation and IgG antibody secretion, EVs (5 μg/ml) isolated from the plasma of LckCrePKM2fl/fl and PKM2fl/fl mice or T-cell culture supernatants were cocultured with primary splenic B cells (1 × 107 cells). B cells were treated with 0.1 μg/ml LPS as basic stimulation. After 24 h, B cell protein expression and gene levels were evaluated by Western blot analysis and qPCR, respectively. After 72 h, B cell IgG antibody secretion and mRNA expression for class switch recombination were measured by ELISA and qPCR.
Visualization of EV biodistribution in vitro and in vivo
EVs were labeled with PKH26 according to the manufacturer’s protocol. Briefly, 4 μl of PKH26 dye was added to 1 ml of Diluent C and incubated with the purified EVs for 4 min at room temperature. The reaction was inactivated by adding an equal volume of fetal bovine serum to bind the excess dye. Labeled EVs were washed, centrifuged at 120,000 g for 70 min at 4°C, and suspended in PBS for uptake experiments.
For the in vitro EV uptake study, B cells were treated with PKH26-labeled EVs (5 μg/ml) overnight and then fixed for confocal microscopy (Leica Microsystems, Buffalo Grove, IL, USA) after immunostaining for B cells with 647-conjugated B220 antibody (103226, RRID: AB_389330; BioLegend, San Diego, CA, USA).
For in vitro EV and QD biodistribution and specific B cell targeting studies, we fabricated bionanocomposite QD-EVs. First, QD nanocrystals (10 nM) with free carboxylic acid groups were modified with anti-CD63 monoclonal antibody (for ligation with EVs) and anti-CD19 antibody (for ligation with B cells). Second, CD19-QD-CD63 was packaged with PKH26-labeled EVs (5 μg/ml). Splenic cells were purified from adult C57BL/6 mice and seeded into 6-well plates at a density of 1 × 107 per well and then incubated with CD19-QD-CD63-EVs (targeted group) or QD-CD63-EVs (untargeted group) overnight. Cells were collected and fixed for 1 h in poly-l-lysine for confocal microscopy after immunostaining for B cells with 647-conjugated B220 antibody.
For in vivo EV and QD biodistribution and specific B cell targeting studies, adult C57BL/6 mice (aged 6–8 wk) were randomly assigned to different treatment groups via tail-vein injection: targeted group, CD19-QD-CD63-EVs; untargeted group, QD-CD63-EVs. After 24 h, images of the harvested spleen tissue were captured using the Cambridge Research and Instrumentation in vivo small animal living imaging system (Maestro2; Cambridge Research and Instrumentation, Hopkinton, MA, USA). Immunofluorescence staining of frozen spleen sections was visualized using confocal microscopy after immunostaining for splenic B cells with 647-conjugated B220 antibody.
Flow cytometry
To assess EV uptake, EVs from T-cell culture supernatants were labeled with PKH26 dye. The labeled EVs were washed in PBS, underwent ultracentrifugation at 120,000 g for 70 min at 4°C, and then were suspended in PBS. Primary B cells (1 × 106) were pretreated with Arg-Gly-Asp (RGD) peptide and/or Dynasore and then incubated with PKH26-labeled EVs. After 60 min, B cells were washed twice in PBS and analyzed with an ImageStreamX Flow Cytometer (Amnis, Seattle WA, USA) and FACSCalibur Flow Cytometer (BD Biosciences).
Metabolomics analysis
B cell water-soluble metabolite analysis was performed using liquid chromatography MS/MS (LC-MS/MS). B cells were washed with ice-cold PBS twice and lysed in 1 ml of 80% aqueous methanol at −80°C for 30 min. Then, they were centrifuged at 14,000 g for 5 min at 4°C. Cell supernatants containing metabolite extracts were collected and dried. Residues were dissolved in 120 μl of tridistilled water, and metabolites were quantified by LC-MS/MS using a QTRAP 6500 LC-MS/MS System (AB Sciex, Redwood City, CA, USA). Metabolite abundance was normalized to the cell number.
For EV ceramide metabolite analysis, EVs purified from cell culture supernatants were quantified by HPLC-MS/MS analysis. All species were verified with lipid standards. Metabolite abundance was normalized using EV concentrations. Integrated peak areas corresponding to metabolite concentrations were determined with MetaboAnalyst software (33).
Antigen-induced B-cell humoral immune response model and QD-EV administration
To test the possible inhibitory effect of PKM2-null TEVs on B-cell IgG secretion in vivo, we used classic NP-LPS and NP-OVA antigen-induced B-cell humoral immune response models and delivered different CD19-QD-CD63-EVs via tail-vein injection. Adult C57BL/6 mice (aged 6–8 wk) were randomly assigned to different treatment groups:
NP-LPS antigen treatment was as follows: NP-LPS group, intraperitoneal injection of 100 ng of NP-LPS in 100 μl of PBS; NP-LPS + PKM2fl/fl-TEV group, intraperitoneal injection of 100 ng of NP-LPS in 100 μl of PBS, after 30 min, tail-vein injection with CD19-QD-CD63-PKM2fl/fl-TEVs in 200 μl of PBS; NP-LPS + LckCrePKM2fl/fl-TEV group, intraperitoneal injection of 100 ng of NP-LPS in 100 μl of PBS, after 30 min, tail-vein injection with CD19-QD-CD63-LckCrePKM2fl/fl-TEVs in 200 μl of PBS; control group was administered PBS. Twenty-four hours after the injection, the mice were euthanized, and plasma was collected to assess IgG antibody levels by ELISA.
NP-OVA antigen treatment was as follows: NP-OVA group, intraperitoneal injection of 100 ng of NP-OVA in 100 μl of PBS; NP-OVA + PKM2fl/fl-TEV group, intraperitoneal injection of 100 ng of NP-OVA in 100 μl of PBS, after 1 d, tail-vein injection with CD19-QD-CD63-PKM2fl/fl-TEVs in 200 μl of PBS; NP-OVA + LckCrePKM2fl/fl-TEV group, intraperitoneal injection of 100 ng of NP-OVA in 100 μl of PBS, after 1 d, tail-vein injection with CD19-QD-CD63-LckCrePKM2fl/fl-TEVs in 200 μl of PBS; control group was administered PBS. Seven days after the injection, plasma was collected to assess plasma IgG antibody levels by ELISA.
Analysis of atherosclerotic lesions
Plaque extensions on tissue sections of the carotid artery were determined by measuring the area of Oil Red O staining. Heart and aortic roots were embedded in optimal cutting temperature compound (62534-10; Tissue-Tek, Sakura Finetek, Torrance, CA, USA), and the frozen aortic root sections were then cut into 5-μm frozen sections and stained with Oil Red O to visualize lesion areas. The lesion areas were determined with ImageJ software (NIH). The infiltration of inflammatory cells in lesions was detected by immunofluorescent staining, and the fluorescence signals were measured by confocal laser scanning microscopy (Leica Microsystems).
Statistical analysis
All other statistical analyses were performed using Prism v.7.0 (GraphPad Software, La Jolla, CA, USA). Data from ≥3 independent experiments are presented as the means ± sd. The significance of the difference between 2 independent samples was determined using unpaired Student’s t tests. A 1-way ANOVA with Tukey’s test was used to compare multiple groups. A value of P < 0.05 was considered statistically significant.
RESULTS
EVs secreted from PKM2-activated T cells promote B-cell IgG production
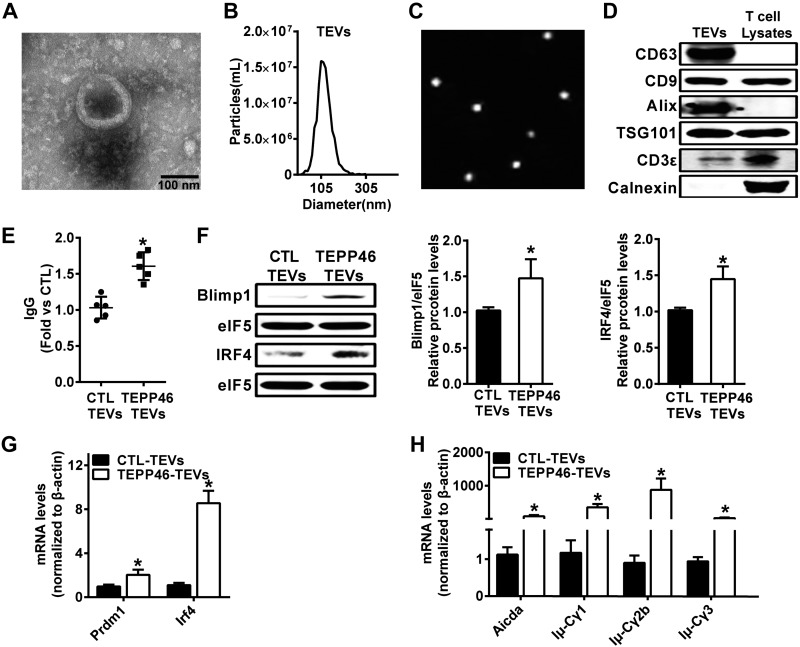
A sufficient high-affinity antibody response requires interaction between T and B cells (34). However, whether TEVs regulate B cell antibody production is unclear. First, TEVs were purified by ultracentrifugation from T-cell culture supernatants and further verified by TEM, NTA, and Western blot analysis. The results showed that most TEVs had a relatively uniform distribution, a classic cup shape and double membrane particle morphology (Fig. 1A); they were intact and had an average diameter of ∼113.4 nm (Fig. 1B, C). Western blot analyses confirmed the expression of the TEV markers CD63, CD9, TSG101, Alix, and the T-cell–specific marker CD3ε in these isolated EVs, whereas the TEV negative marker calnexin was not detected (Fig. 1D).
Figure 1.
EVs secreted from PKM2-activated T cells promote B-cell IgG production. TEVs were isolated by sequential centrifugation. A) Visualization of the EVs by TEM. B, C) Analysis of the size distribution of TEVs using the NAT and ζ potential distribution analyzer. D) The expression of EV-related protein markers CD63, CD9, Alix, and TSG101, the T cell marker CD3ε, and the EV-negative marker calnexin were measured by Western blot analysis. C57BL/6J mouse T cells were treated with TEPP46 (10 μM); after 48 h, TEVs were isolated and cocultured with normal B cells. E) The IgG level in the B cell culture supernatant was measured via ELISA. F) Blimp1 and IRF4 protein expression were analyzed via Western blot analysis. G) Gene expression of Prdm1 and Irf4 was measured via qPCR in B cells at 24 h. H) Gene expression of Aicda and postswitch transcripts (Iμ-Cγ1, Iμ-Cγ2b, and Iμ-Cγ3) was measured via qPCR in B cells at 72 h; n = 4, data are presented as the means ± sd. *P < 0.05 vs. Control (ctl)-TEVs group (1-way ANOVA followed by Tukey’s test for multiple comparisons).
Second, TEPP-46 is a small-molecule PKM2-specific activator (35). We found that TEPP-46 (10 μM, 48 h)-treated TEVs promoted B-cell IgG secretion by more than 49% (Fig. 1E). The terminal differentiation of B cells into antibody-secreting plasma cells depends on the expression of several transcription factors, the activation of Ig genes, and the generation of different antibody classes (36, 37). The protein levels of Blimp1 and IRF4, 2 key molecules of B cell activation, were markedly increased by TEPP-46–treated TEVs (Fig. 1F) and were accompanied by increased Prdm1 and Irf4 gene expression (Fig. 1G). qPCR analysis showed that EVs derived from TEPP46-treated T cells significantly increased gene expression levels of Aicda, which is critical for the initiation of class switch recombination (Fig. 1H). Additionally, the B-cell expression levels of postswitch transcripts, including Iμ-Cγ1, Iμ-Cγ2b, and Iμ-Cγ3, were increased by treatment with TEPP-46–activated TEVs (Fig. 1H). These results suggest that PKM2-stimulated TEVs can increase B-cell IgG production. This finding is consistent with a previous report that PKM2 may be associated with EV release under tumor conditions (38).
EVs secreted from PKM2-null T cells decrease B-cell IgG production
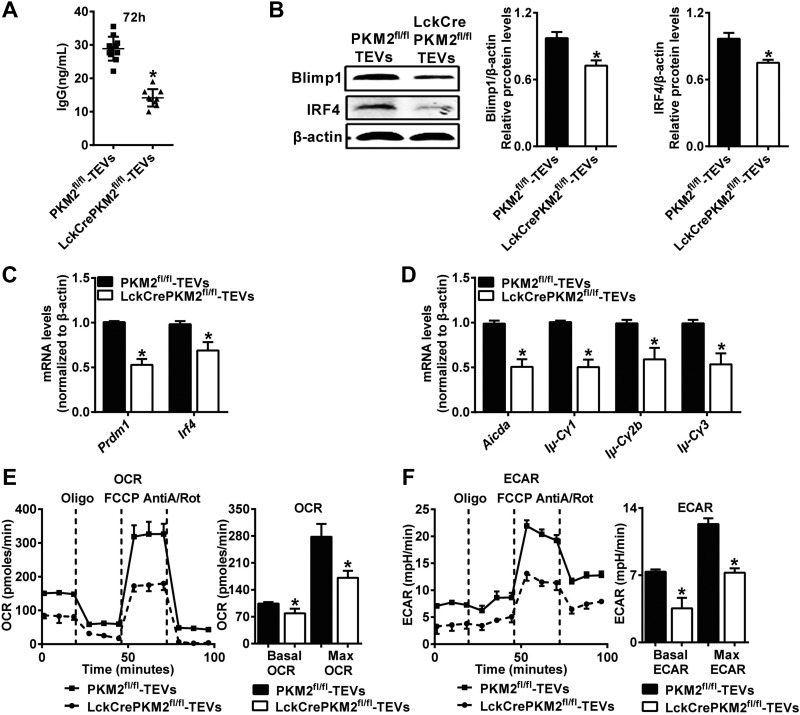
To further determine the effects of T-cell PKM2 on EV-mediated B-cell activation, we constructed PKM2fl/fl mice and crossed them with Lck-Cre mice to obtain T cell–specific PKM2 knockout (LckCrePKM2fl/fl) mice. TEVs were purified from PKM2fl/fl or LckCrePKM2fl/fl T-cell culture supernatants after anti-CD3 antibody (1 μg/ml) treatment for 48 h and then were cocultured with normal B cells with 0.1 μg/ml LPS stimulation for 24 or 72 h, in which anti-CD3 antibody and LPS were used as basal stimulation to maintain cell survival. A TEV concentration of 5 μg/ml (equal between PKM2fl/fl-TEVs and LckCrePKM2fl/fl-TEVs) was selected according to the data in Supplemental Fig. S1A–D. The IgG antibody secretion from B cells was inhibited by more than 53% (Fig. 2A) after treatment with TEVs purified from LckCrePKM2fl/fl T-cell supernatants compared with those from PKM2fl/fl T-cell supernatants. Protein levels of Blimp1 and IRF4 were markedly decreased by treatment with PKM2-null TEVs (Fig. 2B) and accompanied by decreased Prdm1 and Irf4 gene expression (Fig. 2C). qPCR analysis showed that PKM2-null TEVs significantly down-regulated gene expression levels of Aicda and various postswitch transcripts, including Iμ-Cγ1, Iμ-Cγ2b, and Iμ-Cγ3 (Fig. 2D). In addition, B-cell activation showed no significant difference between control (without EVs) and PKM2fl/fl-EVs treatments (Supplemental Fig. S1D).
Figure 2.
EVs secreted from PKM2-null T cells decrease B-cell IgG production. TEVs were isolated from T-cell culture supernatants and cultured (TEVs 5 μg/ml, equal concentration was used between PKM2fl/fl-TEVs and LckCrePKM2fl/fl-TEVs) with 1 × 107 primary splenic B cells purified from C57BL/6J mice seeded in a 6-well plate. A) B-cell culture supernatants at 72 h were assayed for the presence of IgG. B) Western blot analysis of B cells for Blimp1 and IRF4 protein expression. C) qPCR analysis of B-cell transcription factors at 24 h. D) Gene expression of Aicda and postswitch transcripts in B cells at 72 h. E, F) Metabolic parameters of TEV effect on B cells. OCR and ECAR were measured in real time after the addition of oligomycin (oligo), carbonyl cyanide p-trifluoromethoxyphenylhydrazone, antimycin A, and rotenone (AntiA/Rot); n = 4, data are presented as means ± sd. *P < 0.05 vs. the PKM2fl/fl-TEVs group (1-way ANOVA followed by Tukey’s test for multiple comparisons).
Upon activation, B cells program their metabolism to produce increased energy and biosynthetic substrates. Partly, mitochondrial metabolism may be used as an additional marker to indicate B-cell activation. Given the impact of the TEVs on the protein and mRNA levels related to B-cell activation, as shown above, we further conducted extracellular flux analysis to confirm whether PKM2-null TEVs affect the oxidative phosphorylation and glycolysis of B cells. As expected, B cells treated with PKM2-null TEVs exhibited a strikingly suppressed OCR (104.4 ± 3.71 pmol/min vs. 78.82 ± 12.16 pmol/min for basal OCR, 278 ± 33.39 pmol/min vs. 171.5 ± 17.85 pmol/min for maximal OCR) and ECAR (7.37 ± 0.23 mpH/min vs. 3.55 ± 1.09 mpH/min for basal ECAR, 12.33 ± 0.60 mpH/min vs. 7.26 ± 0.46 mpH/min for maximal ECAR) compared with those treated with PKM2fl/fl-TEVs (Fig. 2E, F). Therefore, B cells treated with PKM2-null T-cell EVs showed suppression of both glycolysis and oxidative phosphorylation, indicating the inhibition of B-cell activation and IgG production.
In parallel, a dramatic reduction in B-cell activation, including the level of plasma IgG secretion (Supplemental Fig. S2A) and protein and mRNA levels of the transcription factors Blimp1 (Prdm1) and IRF4 (Irf4) (Supplemental Fig. S2B, C), was observed in LckCrePKM2fl/fl mice relative to PKM2fl/fl mice. Furthermore, the PI3K/Akt/mTOR signaling pathway, which is essential for B-cell cycle progression and cell survival (39), was down-regulated as well (Supplemental Fig. S2B). qPCR analysis showed that the mRNA expression of Aicda and the postswitch transcripts Iμ-Cγ1, Iμ-Cγ2b, and Iμ-Cγ3 were markedly decreased in the splenic B cells of LckCrePKM2fl/fl mice (Supplemental Fig. S2D), and other antibody isotypes did not obviously change (unpublished results). Glycolysis activation mediates B-cell activation (40). Thus, we subsequently assessed the glycolytic metabolic profile in B cells from both LckCrePKM2fl/fl and PKM2fl/fl mice by LC-MS/MS. A principal component analysis (PCA) scatter plot showed that these 2 groups had a disparate metabolic profile (Supplemental Fig. S2E). Further heat map analysis showed global inhibition of water-soluble metabolites (Supplemental Fig. S2F), especially for the late glycolytic intermediates, lactic acid and acetyl-CoA, in B cells of LckCrePKM2fl/fl mice (Supplemental Fig. S2G). At the same time, the levels of EV-related genes were specifically down-regulated in T cells of LckCrePKM2fl/fl mice (Supplemental Fig. S3A) but not in B cells and macrophages (Supplemental Fig. S3B, C). These data above indicated that mice lacking PKM2 in T cells have lower basal B-cell activation, which may be associated with T-cell EVs. Additionally, the Cre protein was specifically detected in T cells of LckCrePKM2fl/fl mice, which excluded the off-target effect of the Cre-loxP system (Supplemental Fig. S4A). Various plasma inflammatory cytokines (Supplemental Fig. S4B) as well as macrophage activation were not affected (Supplemental Fig. S4C) in these mice, suggesting that plasma IgG was specifically decreased after T-cell–specific PKM2 knockout in vivo.
Secondary perturbation of lipid metabolites in EVs secreted from PKM2-null T cells
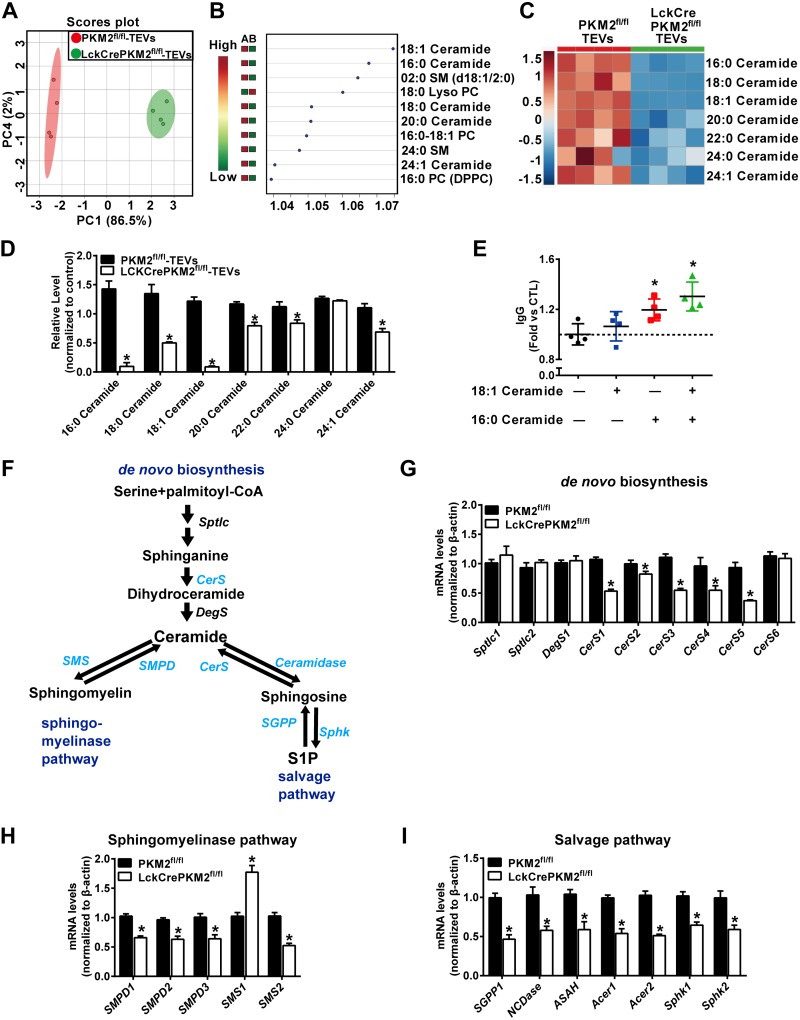
EVs, which contain functional biomacromolecules, including proteins, lipids, nucleic acids, and other metabolites from parental cells, have been described as a novel means of intercellular interaction (41). We have reported that PKM2-mediated intracellular lipid metabolites regulate T-cell function, especially cytokine secretion (22). However, whether T-cell PKM2 affects their secreted EV components is unclear. Lipid metabolites of EVs from T-cell culture supernatants were analyzed by HPLC-MS/MS. The PCA of lipidomics data displayed a different lipid metabolic profile in LckCrePKM2fl/fl-TEVs relative to PKM2fl/fl-TEVs (Fig. 3A). The most robust change, however, was the obviously decreased ceramides, in which 18:1 ceramide and 16:0 ceramide had higher variable importance in projection (VIP) values based on VIP scores (Fig. 3B). Because ceramides constitute one of the main cargos transported by EVs (42), we further specifically analyzed the levels of ceramides in TEVs. The heat map generated by hierarchical clustering of differentially abundant ceramide components showed marked global inhibition of 16:0, 18:0, 18:1, 20:0, 22:0, and 24:1 ceramides in LckCrePKM2fl/fl-TEVs relative to PKM2fl/fl-TEVs (Fig. 3C, D).
Figure 3.
Secondary perturbation of lipid metabolites in EVs secreted from PKM2-null T cells. HPLC-MS/MS analysis of lipid metabolites in EVs isolated from T-cell culture supernatants. A) PCA scatter plot of the lipid metabolites in TEVs. B) VIP scatter plot identified by PCA analysis showing the top 10 lipid metabolites in PKM2fl/fl-TEVs and LckCrePKM2fl/fl-TEVs. A: PKM2fl/fl-TEVs, B: LckCrePKM2fl/fl-TEVs. C) Heat map of changes in the TEV ceramide profile. D) The relative levels of ceramide metabolites, normalized to TEV protein levels, are presented. TEV protein levels were normalized to lysate total protein. E) IgG levels in the B-cell culture supernatants after 18:1 ceramide and 16:0 ceramide stimulation for 72 h. Ctl, control. F) Ceramide synthesis pathways: the de novo, sphingomyelin hydrolysis, and salvage pathways. G–I) The mRNA levels of enzymes required for de novo ceramide synthesis (G), sphingomyelin hydrolysis (H), and the salvage pathway that recreates ceramide from sphingosine (I) in T cells of PKM2fl/fl mice and LckCrePKM2fl/fl mice; n = 4, data are presented as means ± sd. *P < 0.05 vs. the PKM2fl/fl-TEVs group (D, G–I) or control group (E) (1-way ANOVA followed by Tukey’s test for multiple comparisons).
To further identify which component in PKM2-null TEVs was mainly responsible for B-cell inhibition, we selected the top 2 ceramides, 16:0 and 18:1 ceramide, based on the VIP score analysis from TEV lipidomics (Fig. 3B). The effects of individual or combined ceramides on IgG production were investigated in B cells. The results showed that 16:0 ceramide or the 16:0 and 18:1 ceramide combination, but not the 18:1 ceramide alone, significantly increased B cell IgG secretion (Fig. 3E), suggesting the 16:0 ceramide in EVs secreted from T cells with PKM2 expression is the major component that maintains normal B cell function. Conversely, decreased 16:0 ceramide levels may be responsible for PKM2-null TEV-induced B-cell inhibition.
Different contents of EVs can result from differences in their parental cells (43), thus indicating that the different ceramide contents in EV-produced T cells may affect EV ceramides. For ceramide production, 3 main metabolic pathways have been described: de novo pathway, sphingomyelinase pathway, and salvage pathway (44, 45). To further investigate the potential mechanisms by which PKM2 regulates T-cell ceramide metabolism, we examined the 3 metabolic pathway-related genes in T cells with or without PKM2. Our results showed that most ceramide synthesis-related genes were significantly reduced by PKM2 knockout, such as ceramide synthase (Cers)1, Cers2, Cers3, Cers4, and Cers5 in the de novo pathway; sphingomyelin phosphodiesterase (SMPD)1, SMPD2, and SMPD3 in the sphingomyelinase pathway; and sphingosine-1-phosphate phosphatase 1 (SGPP1) in the salvage pathway (Fig. 3F–I, in which blue font represents these significantly down-regulated genes by PKM2 knockout). On the other hand, we found decreased expression of ceramide catabolic enzymatic genes, including sphingomyelin synthase 2 (SMS2) (in the sphingomyelinase pathway) and neutral ceramidase (NCDase), acidic ceramidase (ASAH), alkaline ceramidase 1 (Acer1), Acer2, sphingosine kinase (Sphk)1, and Sphk2 (in the salvage pathway) in PKM2-deficient T cells (Fig. 3F–I, in which blue font represents these significantly down-regulated genes by PKM2 knockout). These enzymatic gene expression data suggested PKM2 knockout down-regulates T-cell ceramide metabolism, including synthesis and catabolism. Together with the ceramide metabolite contents tested by HPLC-MS/MS (Supplemental Fig. S5A–C), which showed decreased ceramides in PKM2-null T cells, these data indicated that the major metabolism affected by PKM2 knockout is decreased ceramide synthesis.
In parallel, we quantified the above 3 ceramide metabolic pathway-related genes in classic PKM2 activator TEPP46-treated (10 μM) T cells. The results showed that various enzymatic mRNA levels involved in ceramide synthesis and hydrolysis in PKM2-activated T cells were increased markedly (Supplemental Fig. S6A–D). These enzymatic gene expression data suggested PKM2 activation up-regulates T-cell ceramide metabolism, including synthesis and catabolism. Together with the ceramide metabolite contents tested by HPLC-MS/MS, which showed increased ceramides in 10-μM TEPP46-treated T cells (Supplemental Fig. S7A–D), these data indicated that the major metabolic process affected by PKM2 activation is increased ceramide synthesis.
Collectively, TEV ceramide contents are mainly affected by the PKM2 level in T cells. PKM2 in T cells regulates the gene expression levels of metabolic enzymes, which subsequently determines T cells and their secreted EV lipid contents, specifically ceramides.
Adhesion and internalization are required for TEV-mediated B-cell IgG secretion
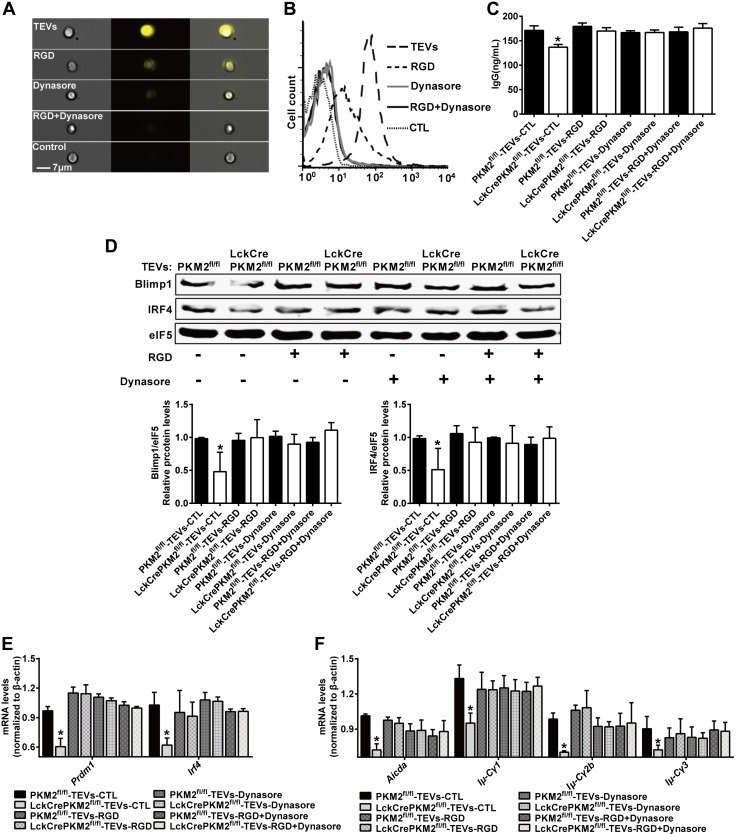
EVs have been identified as vehicles of a cell-to-cell signaling pathway to deliver bioactive molecules to recipient cells (46). The mechanisms associated with EV uptake by recipient cells have gradually been elucidated. In our experimental system, murine B cells pretreated with the fibronectin-integrin interaction-neutralizing RGD peptide and/or the dynamin-II inhibitor Dynasore, which blocks EV cell surface binding and internalization (47), were subsequently incubated with PKH26-labeled TEVs. Analysis by imaging flow cytometry revealed that the elevated fluorescence intensity of TEVs incorporated into B cells was partly inhibited by pretreatment with the RGD peptide and fully inhibited by Dynasore or RGD peptide + Dynasore pretreatment (Fig. 4A), suggesting that endocytosis mediated by cell surface binding and internalization into B cells may be required for TEVs to exert regulatory effects. The above results were further confirmed by flow cytometry analysis (Fig. 4B). The remarkable inhibitory effects of PKM2-null TEVs on B cell activation were completely diminished by the RGD peptide and/or Dynasore, including the level of IgG secretion (Fig. 4C), protein and mRNA levels of the transcription factors Blimp1 (Prdm1) and IRF4 (Irf4) (Fig. 4D, E), and mRNA expression of Aicda and the postswitch transcripts Iμ-Cγ1, Iμ-Cγ2b and Iμ-Cγ3 (Fig. 4F). In addition, fluorescence microscopy confirmed that PKH26-labeled TEVs were taken up by B cells after an overnight incubation (Supplemental Fig. S8). No significant difference was found in the fluorescence intensity of TEVs internalized into B cells between the LckCrePKM2fl/fl-TEV and PKM2fl/fl-TEV treatment groups (Supplemental Fig. S8). Therefore, B-cell inhibition by PKM2-null TEVs is mainly attributed to the different EV components rather than the EV quantity incorporated into recipient cells.
Figure 4.
Adhesion and internalization are required for TEV–mediated B-cell IgG secretion. Primary splenic B cells (1 × 107) pretreated with RGD peptide and/or Dynasore were treated with TEVs, and cells and cell lysates were used to evaluate EV-mediated signaling. A, B) ImageStream flow cytometry and flow cytometry were performed to evaluate the fluorescence intensity of TEVs internalized into cells. C) IgG levels in the B-cell culture supernatants at 72 h. D) Western blot analysis of B-cell protein levels. E) qPCR analysis of B-cell gene expression at 24 h. F) qPCR analysis of B cell gene expression at 72 h; n = 3–5, data are presented as means ± sd. *P < 0.05 vs. the PKM2fl/fl-TEV group (1-way ANOVA followed by Tukey’s test for multiple comparisons).
QDs packaged with PKM2-null TEVs specifically target and inhibit B-cell activation in vitro
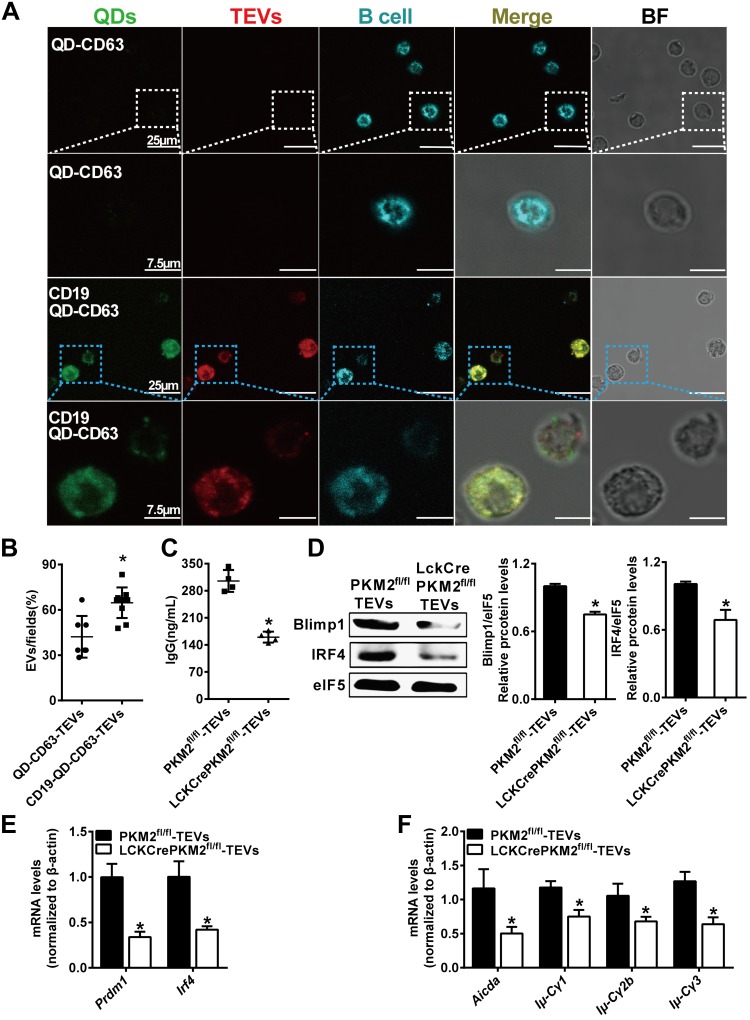
Specific drug delivery to defined organs or tissues, which will increase therapeutic efficacy, remains a major challenge (48). For treatment of various B-cell activation–related diseases, preferential delivery to B cells is very important for efficient therapeutics. A nanomaterial-based delivery system, CdSe/ZnS QD nanocrystals with free carboxylic acid groups as the vehicle, after modification with anti-CD63 monoclonal antibody (for ligation with TEVs) and anti-CD19 antibody (for ligation with B cells), should specially target B cells and deliver TEV-encapsulated components. First, QDs combined with PKH26-labeled TEVs were added into splenic cell culture medium. Green (QD) and red (TEV) fluorescence indicated costaining, suggesting effective packaging of QDs and TEVs (Fig. 5A). Second, compared with QD-CD63-TEVs (white box), the CD19-QD-CD63-TEVs preferentially entered B220-stained B cells (blue box) as indicated by the 3-color costaining of blue, green and red fluorescence in these cells, showing that CD19-QD-CD63-TEVs targeted B cells successfully (Fig. 5A, B). Furthermore, we proceeded to investigate the regulatory effects of the CD19-QD-CD63-TEVs (LckCrePKM2fl/fl-TEVs or PKM2fl/fl-TEVs) on B cell functions. As expected, incubation with CD19-QD-CD63-LckCrePKM2fl/fl-TEVs inhibited B-cell IgG antibody secretion by >46% relative to CD19-QD-CD63-PKM2fl/fl-TEV treatment (Fig. 5C). In addition, the protein expression levels of Blimp1 and IRF4 were substantially decreased in B cells, accompanied by inhibition of the mRNA levels of Prdm1 and Irf4 after CD19-QD-CD63-LckCrePKM2fl/fl-TEV treatment (Fig. 5D, E). Class switch recombination was also suppressed by CD19-QD-CD63-LckCrePKM2fl/fl-TEV treatment, along with a concomitant reduction in Aicda expression and Iμ-Cγ1, Iμ-Cγ2b, and Iμ-Cγ3 levels (Fig. 5F). In contrast, no changes were observed with QD-alone incubation (Supplemental Fig. S9A, B). Additionally, QDs alone and QDs modified with anti-CD19 antibody were cocultured with B cells for 24 or 72 h, respectively, and the data showed that CD19 combined with QDs did not activate B cells (Supplemental Fig. S10A–D). These results indicate that CD19-QD-CD63-TEVs can target B cells and therefore regulate B-cell activation in vitro.
Figure 5.
QDs packaged with PKM2-null TEVs specifically target and inhibit B-cell activation in vitro. QDs labeled with anti-CD63 and anti-CD19 monoclonal antibodies and combined with TEVs labeled using PKH26 membrane dye targeted B220-positive B cells. A) Confocal micrographs of increased entry of CD19-QD-CD63-TEVs into B cells (blue box) relative to QD-CD63-TEVs (white box). B) Quantitative analysis of the results of A. C–F) CD19-QD-CD63-TEVs (LckCrePKM2fl/fl-TEVs) targeted and impaired B-cell activation, including IgG levels in culture supernatants at 72 h (C), Blimp1 and IRF4 protein expression and quantification in B cells at 24 h (D), gene expression of Prdm1 and Irf4 in B cells at 24 h (E), and gene expression of Aicda and postswitch transcripts after treatment for 72 h (F). B cells stained by B220 in blue, TEVs stained by PKH26 in red, and QDs are shown in green; n = 3–5, data are presented as means ± sd. *P < 0.05 vs. the QD-CD63-TEV group (B) or PKM2fl/fl-TEV group (C–E) (1-way ANOVA followed by Tukey’s test for multiple comparisons).
Nanomaterial packaging with TEVs modulates B-cell IgG production in vivo
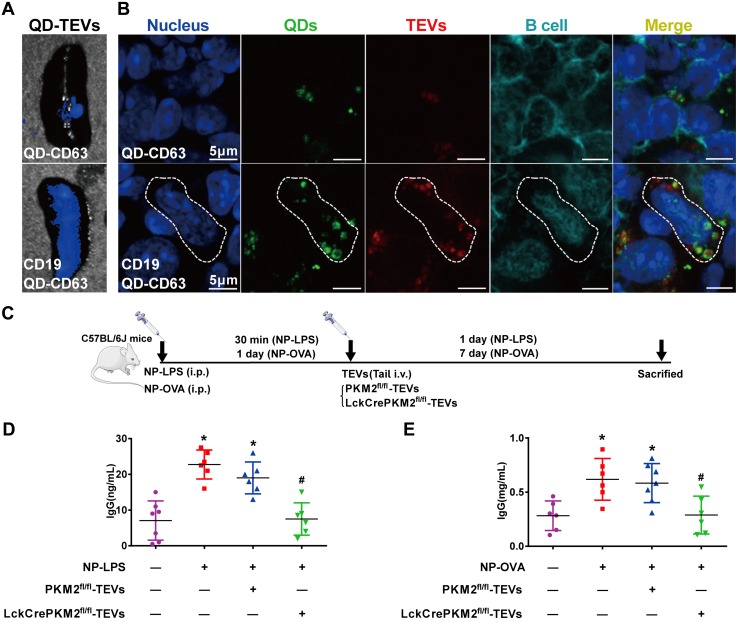
To determine whether the in vitro cell-specific targeting and regulatory effects on B cells were recapitulated in vivo, we observed an efficient and specific tissue uptake of different tail-vein injected QD-TEVs, as measured by the fluorescent appearance of PKH26-labeled TEVs (red), QDs (green), and B220-labeled B cells (blue) in murine spleens. C57BL/6J mice underwent tail-vein injection of bioconjugated QD-TEVs (normal TEVs), and their spleens were collected for detection with an in vivo imaging system (Fig. 6A) and confocal microscopy (Fig. 6B). The fluorescent signals in spleens (Fig. 6A) were significantly increased in the CD19-QD-CD63-TEV treatment group, while only slight fluorescence was detected in the B-cell untargeted QD-CD63-TEV control group. In the captured images of spleens, strong red (PKH26-labeled TEVs) and green (QDs) intracellular fluorescent signals were observed in splenic B cells (B220 staining positive) after injection of CD19-QD-CD63-TEVs (Fig. 6B, lower panel), but only slight signals were detected in the QD-CD63-TEV group (Fig. 6B, upper panel). Additionally, flow cytometry analysis showed specific targeting in splenic B cells (Supplemental Fig. S11A) but not T cells (Supplemental Fig. S11B). These results suggest that CD19-QD-CD63-EVs can be targeted and delivered to B cells in vivo.
Figure 6.
Nanomaterial packaging with TEVs modulates B-cell IgG production in vivo. A, B) Animals administered tail-vein injections of the nanocomposites CD19-QD-CD63-TEVs or QD-CD63-TEVs (50 μg of TEVs/mouse) in C57BL/6J mice. Representative micrographs of the specificity of the CD19-QD-CD63-TEVs (TEVs labeled with PKH26) in mouse spleen tissues (A) and splenic B220-positive B cells (B). C) Schematic flowchart of C57BL/6J mice that were injected intraperitoneally with 100 ng of NP-LPS or 100 ng of NP-OVA antigen; after 30 min or 1 d, mice were given tail-vein injections of CD19-QD-CD63-TEVs (LckCrePKM2fl/fl-TEVs vs. PKM2fl/fl-TEVs). D) Plasma IgG levels induced by NP-LPS antigen were measured via ELISA. E) Plasma IgG levels induced by NP-OVA antigen were measured via ELISA; n ≥ 5, data are presented as means ± sd. *P < 0.05 vs. the control (CTL) group, #P < 0.05 vs. the NP-LPS + PKM2fl/fl-TEVs group (D) or NP-OVA + PKM2fl/fl-TEV group (E) (1-way ANOVA followed by Tukey’s test for multiple comparisons).
Upon antigen stimulation, B cells undergo maturation and differentiation into plasma cells and produce IgG antibody (49). We next evaluated the specific regulatory efficacy for IgG production of the CD19-QD-CD63-TEV targeting delivery nanosystem in vivo. C57BL/6J mice were injected intraperitoneally with 100 ng of (4-hydroxy-3-nitrophenyl) acetyl-LPS (NP-LPS) [T-cell–independent antigen] or 100 ng of (4-hydroxy-3-nitrophenyl) acetyl-OVA (NP-OVA) [T-cell–dependent antigen]. Thirty minutes after the injection of NP-LPS or 1 d after the injection of NP-OVA, mice were administered tail-vein injections of different CD19-QD-CD63-TEVs (LckCrePKM2fl/fl-TEVs or PKM2fl/fl-TEVs, TEVs 50 μg per mouse) (Fig. 6C). As expected, a dramatic increase in IgG antibody titers was observed in the NP-LPS (from 7.07 ± 5.46 to 22.75 ± 4.06 ng/ml) and NP-LPS plus with PKM2fl/fl-TEV treatment groups (from 7.07 ± 5.46 to 19 ± 4.47 ng/ml) relative to the group without NP-LPS treatment. In addition, the promoted IgG levels stimulated by NP-LPS was reversed by the administration of PKM2-null TEVs (Fig. 6D). Similar results were also obtained after NP-OVA antigen administration: the IgG levels increased in the NP-OVA group (from 0.28 ± 0.13 to 0.62 ± 0.19 mg/ml) and the NP-OVA plus with PKM2fl/fl-TEV treatment group (from 0.28 ± 0.13 to 0.58 ± 0.18 mg/ml) but decreased with the administration of PKM2-null TEVs (Fig. 6E). QDs alone did not show any significant effects in vivo (Supplemental Fig. S12A–D). Therefore, these results clearly provide evidence for the biomedical function of bioconjugated QD-PKM2-null TEVs that specifically target and inhibit IgG production in vivo.
PKM2-null TEVs capsulated in QDs inhibit HHcy-induced B-cell overactivation
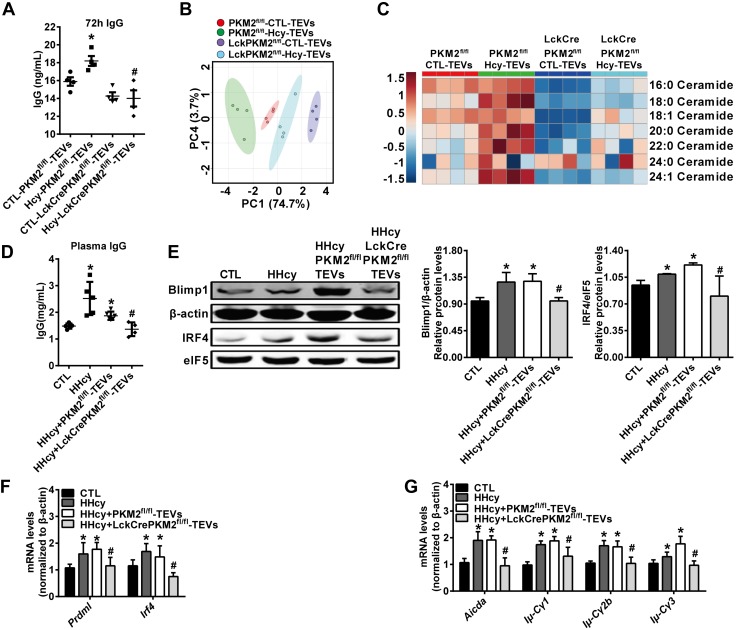
T-cell PKM2 can be up-regulated by Hcy, as reported in our recent work (22), indicating Hcy could be an additional PKM2 stimulator. As expected, EVs derived from Hcy (100 μM, 48 h)-activated T cells could increase B-cell IgG antibody secretion, which was decreased by LckCrePKM2fl/fl-TEV treatment (Fig. 7A). Then, we determined the lipid metabolites in LckCrePKM2fl/fl-TEVs and PKM2fl/fl-TEVs with or without Hcy treatment, to explore whether T-cell PKM2 mediated Hcy-stimulated TEV metabolic composition. From the parallel analysis of ceramide composition in TEVs, the PCA scatter plot displayed a disparate lipid metabolic profile among the 4 groups, PKM2fl/fl-TEVs and LckCrePKM2fl/fl-TEVs with or without Hcy stimulation (Fig. 7B). The heat map showed significantly increased amounts of various ceramides in Hcy-treated PKM2fl/fl-TEVs (Fig. 7C), which was almost abrogated in Hcy-stimulated LckCrePKM2fl/fl-TEVs. Such data demonstrate that PKM2 mediates Hcy-increased TEV metabolic composition, indicating LckCrePKM2fl/fl-TEVs may be potential intervening targets to inhibit Hcy-induced pathologic effects.
Figure 7.
PKM2-null TEVs capsulated in QDs inhibit HHcy-induced B cell overactivation. T cells from PKM2fl/fl or LckCrePKM2fl/fl mice were isolated and then treated with Hcy (100 μM) in vitro. After 48 h, TEVs were purified and subsequently cocultured with equal concentrations of PKM2fl/fl-TEVs or LckCre PKM2fl/fl-TEVs with normal B cells for an additional 72 h. A) B cell IgG production. Metabolites in these purified TEVs were analyzed using an HPLC-MS/MS system. B) PCA scatter plot of the metabolites in TEVs. C) Heat map of changes in the ceramide metabolites from TEVs given via tail-vein injection of the nanocomposites CD19-QD-CD63-TEVs or QD-CD63-TEVs (TEVs 50 μg/mouse) in C57BL/6J mice. D–G) TEVs isolated from LckCrePKM2fl/fl T-cell culture supernatants combined with QDs and CD19 mAb showed targeted inhibition of the HHcy-mediated increase in B cell IgG secretion (D), Blimp1 and IRF4 protein levels (E), Prdm1 and Irf4 gene expression levels (F), and Aicda, Iμ-Cγ1, Iμ-Cγ2b, and Iμ-Cγ3 gene expression levels (G). Ctl, control; n = 4, data are presented as means ± sd. *P < 0.05 vs. ctl-PKM2fl/fl-TEVs (A) or ctl group (D–G), #P < 0.05 vs. Hcy-PKM2fl/fl-TEVs group (A) or HHcy + PKM2fl/fl-TEV group (1-way ANOVA followed by Tukey’s test for multiple comparisons).
We next evaluated the in vivo specific regulatory efficacy of the CD19-QD-CD63-TEV targeting delivery nanosystem for HHcy-activated B cells. HHcy-ApoE−/− animals were further treated with or without QD19-QD-CD63-TEVs, in which TEVs were isolated from either LckCrePKM2fl/fl or PKM2fl/fl mice. As shown in Fig. 7, HHcy significantly increased B cell activation, including IgG production (Fig. 7D), Blimp1 (Prdm1) and IRF4 (Irf4) protein and mRNA levels (Fig. 7E, F), and gene expression of Aicda and postswitch transcripts (Fig. 7G). Compared with the QDs with PKM2fl/fl-TEVs and anti-CD19 antibody, administration of QDs with PKM2-null TEVs and anti-CD19 antibody significantly decreased (by 38%) plasma IgG levels induced by HHcy (Fig. 7D). In addition, protein levels of Blimp1 and IRF4 (Fig. 7E), mRNA levels of Prdm1 and Irf4 (Fig. 7F), mRNA expression of Aicda, and postswitch transcripts including Iμ-Cγ1, Iμ-Cγ2b, Iμ-Cγ3 (Fig. 7G), all induced by HHcy, were all successfully diminished in B cells by the administration of QDs with PKM2-null TEVs. These results are consistent with the impaired in vitro B-cell functions after treatment with PKM2-null TEVs.
Nanomaterial packaging with PKM2-null TEVs inhibits HHcy-accelerated atherosclerosis
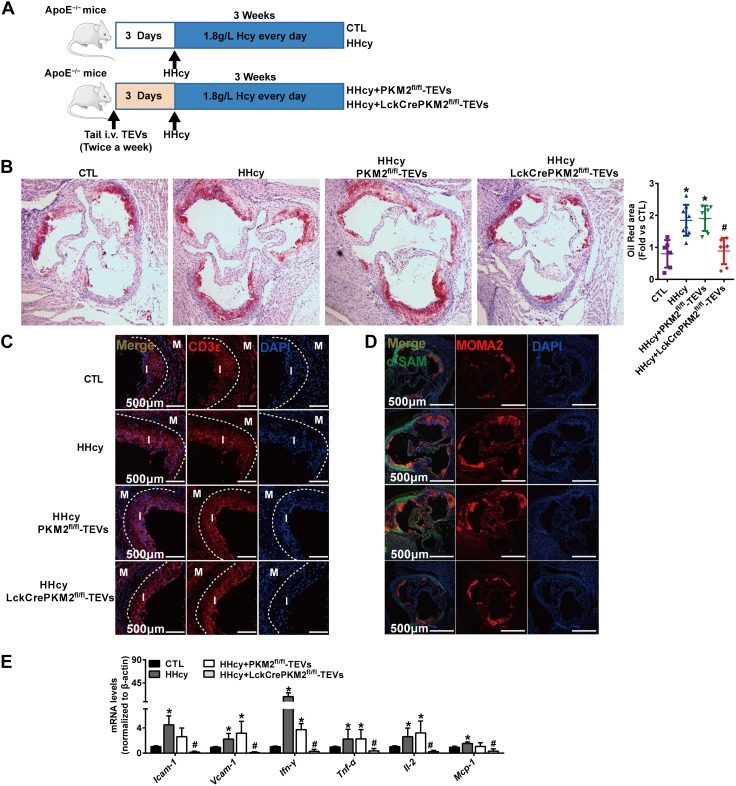
B-cell differentiation into IgG-producing plasma cells accelerated the development of atherosclerosis (50). Our previous research verified that HHcy, which has long been suggested as an independent risk factor for atherosclerosis, induces an early onset of atherosclerosis partly due to B-cell activation in ApoE−/− mice (40). Thus, targeting B cells and pathogenic IgG may provide possible strategies to prevent HHcy-accelerated atherosclerosis. To test this hypothesis, we attempted to further validate the feasibility of specific targeting and therapeutic properties of CD19-QD-CD63-TEVs. As shown in Fig. 8A, ApoE−/−mice were given tail-vein injections of different CD19-QD-CD63-TEVs twice a week. Three days after the first injection, mice were given drinking water supplemented with 1.8 g/L D.L.-Hcy for 3 consecutive weeks to initiate early atherosclerosis as previously described (51, 52). Compared with control, Oil Red O staining of dissected aortic roots showed markedly increased plaque areas in the HHcy and HHcy + PKM2fl/fl-TEVs groups, which were significantly reversed by HHcy + LckCrePKM2fl/fl-TEV treatment (Fig. 8B). Inflammatory cell infiltration is an early event in atherosclerotic development. HHcy increased the infiltration of CD3+ T cells and monocytes/macrophages (MOMA-2 staining positive) in the aortic roots, which were all effectively attenuated in the B cell–targeted PKM2-null TEV therapy group in ApoE−/− mice (Fig. 8C, D). Meanwhile, no obvious changes were observed in the α-SMA-stained area (Fig. 8D). Furthermore, B-cell–targeted specific delivery of PKM2-null TEVs markedly reduced the gene expression of vascular local adherent molecules including ICAM-1 and VCAM-1, inflammatory cytokines including IFN-γ, Tnf-α, Il-2, and chemotactic factors such as CCL2 in the aortas (Fig. 8E). Plasma inflammatory cytokine levels, such as those of TNF-α, IL-6, IL-12p70, and CCL2 (Supplemental Table S1), induced by HHcy were decreased by PKM2-null TEV treatment as well. These data clearly showed that QDs packaged with PKM2-null TEVs and anti-CD19 antibody were able to target B cells, downregulate their activation, and subsequently attenuate the development of atherosclerosis induced by HHcy, whereas there were no changes in the group injected with QDs alone (Supplemental Fig. S13A–C).
Figure 8.
Nanomaterial packaging with PKM2-null TEVs inhibits HHcy-accelerated atherosclerosis. A) Schematic flowchart of ApoE−/−mice tail-vein injected with CD19-QD-CD63-TEVs (LckCrePKM2fl/fl-TEVs vs. PKM2fl/fl-TEVs) twice per week; 3 d after the first injection, HHcy-accelerated atherosclerosis was induced by giving mice drinking water supplemented with 1.8 g/L Hcy. B) Oil Red O staining of aortic roots for quantitative lesion size (left panel). Quantification of the mean atherosclerotic lesion area is shown (right panel). C) Representative confocal images of T-cell infiltration (positive CD3 staining). D) Representative confocal micrographs of the infiltration of macrophages/macrophage [macrophages/monocytes antibody (MOMA2) staining positive] in lesion areas; there were no obvious changes in the α-SMA-stained area. E) The gene expression of Icam-1, Vcam-1, Ifn-γ, Tnf-α, Il-2, and CCL2 in thoracic aortas isolated from mice was measured via qPCR; n = 5–8, data are presented as means ± sd. *P < 0.05 vs. control (ctl) group;. #P < 0.05 vs. HHcy-PKM2fl/fl-TEVs group (1-way ANOVA followed by Tukey’s test for multiple comparisons).
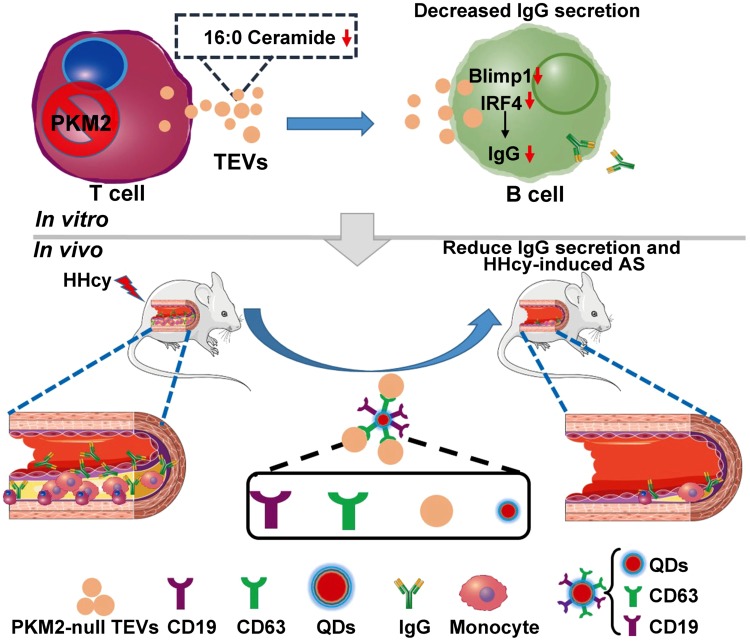
In summary, this study provides evidence showing that ceramide-filled EVs secreted from PKM2-activated T cells promote B-cell pathogenic IgG production via PKM2. Additionally, PKM2-null TEVs inhibit B cell activation and IgG antibody production. Nanomaterial QDs packaged with ceramide-less PKM2-null TEVs and anti-CD19 antibody specifically target B cells and inhibit HHcy-induced pathologic IgG secretion and ultimately ameliorate atherosclerosis (Fig. 9).
Figure 9.
Proposed model of the study. Schematic representation of the proposal that EVs secreted from PKM2-activated T cells promote B-cell pathogenic IgG production. EVs derived from PKM2-null T cells with low ceramide components, especially 16:0 ceramide, were internalized into B cells and then inhibited B-cell IgG antibody production. Antibodies against CD19 and CD63 were loaded onto QDs and combined with PKM2-null TEVs; we fabricated the bionanocomposite QD-EVs and demonstrated their prominent effects as B-cell–targeted nanovectors on HHcy-accelerated pathologic IgG production and atherosclerosis.
DISCUSSION
In addition to assisting B cells with producing high-affinity antibodies via direct contact and releasing various cytokines, the present study provided the first evidence from the cellular metabolism perspective that murine primary TEVs regulate B-cell IgG secretion via PKM2. PKM2-activated T cells transferred ceramide-filled EVs into B cells to promote their activation and IgG production, while PKM2-null TEVs inhibited these effects. More importantly, by use of a reported pathogenic IgG-mediated cardiovascular animal model, we found that bionanocomposites fabricated with PKM2-null TEVs and anti-CD19 antibody could exert B cell–specific targeting inhibition and eventually ameliorate HHcy-induced atherosclerosis.
PKM2 catalyzes the final rate-limiting reaction of the glycolytic pathway and functions as the central regulator of cancer metabolism and activated immune cells by elevating glucose uptake and oxygen-independent lactate fermentation, which is known as the Warburg effect (53, 54). We previously demonstrated that PKM2 mediated T-cell activation to secrete much more IFN-γ by reprogramming intracellular metabolism (22). In a previous study, LckCrePKM2fl/fl mice were created successfully. Further, we unexpectedly found lower B-cell activation in these LckCrePKM2fl/fl mice, indicating that T-cell PKM2 may regulate B-cell activation by unknown mechanisms.
As an important communication vehicle among cells, EVs can interact and directly affect recipient cell function by conveying their bioactive proteins and genetic materials (41, 55, 56). Herein, we first purified EVs by ultracentrifugation and identified these EV physical characteristics by TEM, NTA, and Western blot analyses for the EV-specific markers CD63, CD9, TSG101, and Alix, and the T cell marker CD3ε (Fig. 1A–D). Consistent with the published data (57), purified EVs in our experimental system showed a relatively uniform distribution, a classic cup shape and a double membrane morphology and were intact with an average size of ∼113.4 nm in diameter. EVs secreted from T cells activated by the PKM2-specific activator TEPP46 enhanced normal B-cell activation, IgG production, terminal differentiation, class switch recombination as well as mitochondrial reprogramming, while PKM2-null TEVs reversed all of these effects, suggesting T-cell PKM2 regulation of B-cell activation via EVs. Therefore, PKM2 in T cells exerts its regulatory effects in 2 ways. One is to affect T-cell intracellular glucose-lipid metabolism and in turn to regulate cytokine production as we reported before (22). The other is to mediate the communication between T and B cells via secreted EVs. Herein, we demonstrated that EVs derived from T cells regulated B-cell IgG secretion and the key role of PKM2 in this process, which extends the previous knowledge that PKM2 may be associated with EV release under tumor conditions with unknown targeting effects (38). However, the precise molecular association between PKM2 expression and EV production needs to be further explored.
EVs are loaded with a wide variety of contents from donor cells, as their protein composition depends on the original cells. Interestingly, PKM2 is also a package protein of EVs. Mathivanan et al. (58, 59) first identified PKM2 as 1 of 10 proteins that are often found in EVs and then confirmed this finding. Subsequently, Buschow et al. (60) provided evidence that PKM2 could be detected in B-cell EVs. Actually, the presence of PKM2 in EVs was also observed in our experimental system, and the PKM2 expression in these EVs was down-regulated with PKM2 knockdown in T cells (unpublished results). The regulatory effects of PKM2 on various cell functions contribute to its glycolytic-lipogenic metabolic enzymatic activity with the accumulation of metabolites as well as protein expression levels (40). Therefore, PKM2-mediated lipid metabolites may be the main functional cargos transported by EVs. Thus, lipidomics analysis was employed to analyze EV composition, and lipid profiling showed decreased accumulation of various ceramide metabolites in PKM2-null TEVs. To further determine which ceramides in EVs are primarily responsible for the B cell inhibitory effect, we used the VIP score plot of the metabolites and focused on the top 2 ceramides, 18:1 ceramide and 16:0 ceramide. After incubation with B cells, we observed that compared with the 18:1 ceramide alone, the 16:0 ceramide significantly increased B-cell IgG secretion (Fig. 3E). These results clearly indicate that T cells communicate with B cells by releasing a distinct class of EVs, which are loaded with ceramides and appear to be mediated by T-cell PKM2. Moreover, the EVs released by T cells may carry additional molecules, including RNAs and proteins, potentially affecting B-cell function. This issue should be further investigated in the future.
In addition, a dramatic reduction in the EV-related protein markers CD63 and CD9 was detected in plasma EVs isolated from LckCrePKM2fl/fl mice (unpublished results). This result indicated that there were fewer EVs in LckCrePKM2fl/fl mice than in PKM2fl/fl mice. The PKM2 expression level in T cells was associated with their secreted EV quantity, which was consistent with a study reporting that tumor cell exosome release was decreased with down-regulated PKM2 expression (38). On the other hand, a significant reduction in splenic T cell numbers was observed in LckCrePKM2fl/fl mice relative to PKM2fl/fl mice as well (unpublished results). Together with the decreased ceramides in PKM2-null TEVs, there are at least 3 factors contributing to lower B-cell activation in LckCrePKM2fl/fl mice, including decreased EV numbers, EV ceramide contents, and splenic T-cell numbers. In the present experimental system, equal concentrations of PKM2fl/fl-TEVs and LckCrePKM2fl/fl-TEVs were incubated with B cells in vitro, which indicated that B-cell inhibition by PKM2-null TEVs was mainly attributed to different EV components rather than quantity.
Once released, EVs carrying biologic information can interact with recipient cells and influence cellular functions by conveying their contents (9). In this study, both the fibronectin-integrin interaction-neutralizing RGD peptide and the dynamin-II inhibitor Dynasore were used in our experimental system. The RGD peptide partially suppressed, whereas Dynasore alone or combined with RGD peptide almost completely suppressed, TEVs–regulated B-cell function, suggesting that, compared with earlier adhesion, Dynasore-mediated internalization predominated among the EV-mediated effects. Therefore, uptake of T-cell EVs by B cells allows for the direct transfer of lipids, revealing a form of intercellular communication and nutrient exchange with important implications for HHcy-associated pathologies. EVs can also transport their cargos by other mechanisms, including ligand-receptor interactions and so on (5). Further investigations are currently underway to confirm other mechanisms by which B cells acquire information from TEVs.
As natural carriers, EVs have been identified as a diagnostic and therapeutic platform for several diseases. In clinical trials, EVs have been tested in patients with type 1 diabetes mellitus (clinical trial: NCT02138331), melanoma (61), and non-small cell lung cancers (62, 63), indicating that EVs may have potential therapeutic value in several diseases. B-cell activation is involved in many diseases. Systemic lupus erythematosus is an autoimmune disease with multiple associated autoantibodies from activated B cells (64). Tumor-infiltrating B cells are critical for tumor progression in certain solid cancers, including cervical and ovarian cancer, non–small-cell lung cancer, and pancreatic ductal adenocarcinoma (65, 66). For the role of B cells in cardiovascular disease, which is still the leading cause of mortality worldwide, typical human atherosclerotic lesions contain various cell types, including B cells. Additionally, we and other researchers have determined that B-cell secretion of pathogenic IgG antibodies is associated with atherosclerosis (40, 50). In terms of HHcy-accelerated atherosclerosis, activated T cells secrete inflammatory cytokines that participate in the atherosclerotic progress and activate B cells to produce IgG (22). The present study provided further evidence for EV-mediated communications between T and B cells, which is an alternative explanation for the HHcy-increased atherosclerosis in ApoE−/− mice from a new perspective. In addition, after B-cell–targeted PKM2-null TEV therapy, HHcy-induced macrophage polarization was reversed as well (unpublished results). Because these mice were treated with B-cell targeting intervention, the regulation of macrophage polarization may be secondary to the B-cell responses. More importantly, this study also provided a possible setup for an EV-based method to target B-cell function and, in turn, postpone atherosclerosis.
Currently, nanomaterials have been applied to meet the most important medical needs of biologic imaging, clinical diagnostics, and therapeutics (67). QDs are cadmium- and selenium-based semiconductor nanoparticles that are regarded as promising carriers in biomedical research, especially for targeted drug delivery, as well as long-term fluorescence imaging (29, 68). More importantly, QDs contain cell-specific proteins, such as antibodies of the B-cell marker CD19, on their outer surface, thereby enabling them to specifically target B cells. In this scenario, QD-EV–based delivery platforms were developed here by assembling anti-CD63 antibody and anti-CD19 antibody–modified QDs with EVs, which showed the best efficacy for highly specific and successful B-cell targeting in vivo and in vitro. The superior intervention efficiency of the packaged QDs was determined by validating the B-cell functional inhibition of PKM2-null TEVs after injection of bionanocomposite CD19-QD-CD63-LckCrePKM2fl/fl-TEVs into mice. Subsequently, by use of the well-established NP-LPS and NP-OVA antigen-induced antibody production animal models (69, 70), we showed that the QDs packaged with PKM2-null TEVs and anti-CD19 antibody could successfully decrease the plasma IgG levels. Additionally, these developed B-cell–targeted nanocarrier QDs with PKM2-null TEVs were also effective at ameliorating HHcy-accelerated atherosclerosis by suppressing vascular local and systemic inflammatory immune responses, representing drug-free therapeutics. With the growing interest in developing QDs for biomedical applications, their toxicity is being carefully investigated (71–74). In our developed EV-cell–targeting delivery system, water-soluble QDs were selected, and antibodies were cross-linked onto the surface of QDs, with a diameter of ∼12–18 nm. The applied concentration of the modified QDs was 10 nM, which did not cause toxicity (71, 72). In addition, no significant cell function change was found in our experimental system with these packaged QDs in vitro or in vivo (Supplemental Figs. S9 and S12).
In summary, this study leads to an emerging picture in which PKM2-activated T cells secrete ceramide-filled EVs that bypass the canonical pathway for lipid mobilization to promote B-cell IgG production via PKM2. EVs derived from PKM2-null T cells with low ceramide components, especially 16:0 ceramide, are internalized into B cells and then inhibit both basal and LPS-induced B-cell activation by programming mitochondrial metabolism, leading to decreased IgG production. By loading PKM2-null TEVs and anti-CD19 antibody onto QDs, the fabricated QD-EVs demonstrate their prominent effects as B-cell–targeted nanovectors to inhibit IgG secretion and eventually mitigate HHcy-induced atherosclerosis. Because of the fundamental importance of EV-mediated cellular communication in pathophysiology, as well as the role of B-cell overactivation in inflammatory-metabolism diseases, this study may shed light on treatments for many other related diseases.
ACKNOWLEDGMENTS
The authors are grateful to Prof. Wei Kong (Peking University) for the helpful advice. The authors thank Prof. Shiqiang Wang, Prof. Xuemei Hao, and Prof. Yingchun Hu (Peking University) for assistance with the extracellular vesicle transmission electron microscope assays. This work was supported by the National Natural Science Foundation of China (91739303 and 81770445). The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or writing of the article. The authors were not paid to write this manuscript by a pharmaceutical company or other agency. The authors declare no conflicts of interest.
Glossary
- Aicda
activation-induced cytidine deaminase
- Akt
protein kinase B
- Blimp1
B lymphocyte–induced maturation protein 1
- CCL2
C-C motif chemokine ligand 2
- Cers
ceramide synthase
- ECAR
extracellular acidification rate
- EV
extracellular vesicle
- Hcy
homocysteine
- HHcy
hyperhomocysteinemia
- Icam-1
intercellular adhesion molecule 1
- IRF4
IFN regulatory factor 4
- LC-MS/MS
liquid chromatography MS/MS
- LckCrePKM2fl/fl
T cell–specific PKM2 knockout
- MS/MS
tandem mass spectrometry
- mTOR
mechanistic target of rapamycin
- NP
(4-hydroxy-3-nitrophenyl) acetyl
- NTA
nanoparticle tracking analysis
- OCR
oxygen consumption rates
- OVA
ovalbumin
- PCA
principal component analysis
- PKM2
pyruvate kinase muscle isozyme 2
- Prdm1
PR/SET domain 1
- QD
quantum dot
- qPCR
quantitative PCR
- SMPD
sphingomyelin phosphodiesterase
- TEM
transmission electron microscope
- TEV
T-cell-derived EV
- Vcam-1
vascular cell adhesion protein 1
- VIP
variable importance in projection
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
X. Wang and J. Feng secured funding for the project; J. Yang, X. Wang, and J. Feng searched the literature, initiated the project, generated hypotheses, designed the experiments, and analyzed the data; J. Yang purified the EVs; J. Yang, G. Dang, S. Lü, Y. Miao, X. Li, and F. Shao contributed to sample preparation and data collection; H. Liu and C. Jiang helped perform the LC-MS/MS–based lipidomics analyses; X. Ma and L. Han assisted in breeding the mice; J. Deng and Q. Xu guided the project; J. Yang, X. Wang, and J. Feng wrote, reviewed, and edited the manuscript; and all authors reviewed and approved the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Lund F. E., Randall T. D. (2010) Effector and regulatory B cells: modulators of CD4+ T cell immunity. Nat. Rev. Immunol. 10, 236–247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitchison N. A. (2004) T-cell-B-cell cooperation. Nat. Rev. Immunol. 4, 308–312 [DOI] [PubMed] [Google Scholar]
- 3.Théry C., Zitvogel L., Amigorena S. (2002) Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2, 569–579 [DOI] [PubMed] [Google Scholar]
- 4.Colombo M., Raposo G., Théry C. (2014) Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu. Rev. Cell Dev. Biol. 30, 255–289 [DOI] [PubMed] [Google Scholar]
- 5.Boulanger C. M., Loyer X., Rautou P. E., Amabile N. (2017) Extracellular vesicles in coronary artery disease. Nat. Rev. Cardiol. 14, 259–272 [DOI] [PubMed] [Google Scholar]
- 6.Plebanek M. P., Angeloni N. L., Vinokour E., Li J., Henkin A., Martinez-Marin D., Filleur S., Bhowmick R., Henkin J., Miller S. D., Ifergan I., Lee Y., Osman I., Thaxton C. S., Volpert O. V. (2017) Pre-metastatic cancer exosomes induce immune surveillance by patrolling monocytes at the metastatic niche. Nat. Commun. 8, 1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Todorova D., Simoncini S., Lacroix R., Sabatier F., Dignat-George F. (2017) Extracellular vesicles in angiogenesis. Circ. Res. 120, 1658–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ibrahim A., Marbán E. (2016) Exosomes: fundamental biology and roles in cardiovascular physiology. Annu. Rev. Physiol. 78, 67–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathieu M., Martin-Jaular L., Lavieu G., Théry C. (2019) Specificities of secretion and uptake of exosomes and other extracellular vesicles for cell-to-cell communication. Nat. Cell Biol. 21, 9–17 [DOI] [PubMed] [Google Scholar]
- 10.Mittelbrunn M., Gutiérrez-Vázquez C., Villarroya-Beltri C., González S., Sánchez-Cabo F., González M. A., Bernad A., Sánchez-Madrid F. (2011) Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2, 282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F., Li R., Yang Y., Shi C., Shen Y., Lu C., Chen Y., Zhou W., Lin A., Yu L., Zhang W., Xue Z., Wang J., Cai Z. (2019) Specific decrease in B-cell-derived extracellular vesicles enhances post-chemotherapeutic CD8+ T cell responses. Immunity 50, 738–750.e7 [DOI] [PubMed] [Google Scholar]
- 12.Weinstein J. S., Herman E. I., Lainez B., Licona-Limón P., Esplugues E., Flavell R., Craft J. (2016) TFH cells progressively differentiate to regulate the germinal center response. Nat. Immunol. 17, 1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen C. D., Okada T., Cyster J. G. (2007) Germinal-center organization and cellular dynamics. Immunity 27, 190–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gaya M., Barral P., Burbage M., Aggarwal S., Montaner B., Warren Navia A., Aid M., Tsui C., Maldonado P., Nair U., Ghneim K., Fallon P. G., Sekaly R. P., Barouch D. H., Shalek A. K., Bruckbauer A., Strid J., Batista F. D. (2018) Initiation of antiviral B cell immunity relies on innate signals from spatially positioned NKT cells. Cell 172, 517–533.e20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hirohata S., Jelinek D. F., Lipsky P. E. (1988) T cell-dependent activation of B cell proliferation and differentiation by immobilized monoclonal antibodies to CD3. J. Immunol. 140, 3736–3744 [PubMed] [Google Scholar]
- 16.Cubuk C., Hidalgo M. R., Amadoz A., Pujana M. A., Mateo F., Herranz C., Carbonell-Caballero J., Dopazo J. (2018) Gene expression integration into pathway modules reveals a pan-cancer metabolic landscape. Cancer Res. 78, 6059–6072 [DOI] [PubMed] [Google Scholar]
- 17.Stienstra R., Netea-Maier R. T., Riksen N. P., Joosten L. A. B., Netea M. G. (2017) Specific and complex reprogramming of cellular metabolism in myeloid cells during innate immune responses. Cell Metab. 26, 142–156 [DOI] [PubMed] [Google Scholar]
- 18.Biswas S. K. (2015) Metabolic reprogramming of immune cells in cancer progression. Immunity 43, 435–449 [DOI] [PubMed] [Google Scholar]
- 19.MacIver N. J., Michalek R. D., Rathmell J. C. (2013) Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31, 259–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steed M. M., Tyagi S. C. (2011) Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid. Redox Signal. 15, 1927–1943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Herrmann M., Taban-Shomal O., Hübner U., Böhm M., Herrmann W. (2006) A review of homocysteine and heart failure. Eur. J. Heart Fail. 8, 571–576 [DOI] [PubMed] [Google Scholar]
- 22.Lü S., Deng J., Liu H., Liu B., Yang J., Miao Y., Li J., Wang N., Jiang C., Xu Q., Wang X., Feng J. (2018) PKM2-dependent metabolic reprogramming in CD4+ T cells is crucial for hyperhomocysteinemia-accelerated atherosclerosis. J. Mol. Med. (Berl.) 96, 585–600 [DOI] [PubMed] [Google Scholar]
- 23.Tang N., Sun B., Gupta A., Rempel H., Pulliam L. (2016) Monocyte exosomes induce adhesion molecules and cytokines via activation of NF-κB in endothelial cells. FASEB J. 30, 3097–3106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Manna I., Iaccino E., Dattilo V., Barone S., Vecchio E., Mimmi S., Filippelli E., Demonte G., Polidoro S., Granata A., Scannapieco S., Quinto I., Valentino P., Quattrone A. (2018) Exosome-associated miRNA profile as a prognostic tool for therapy response monitoring in multiple sclerosis patients. FASEB J. 32, 4241–4246 [DOI] [PubMed] [Google Scholar]
- 25.Shi C., Ulke-Lemée A., Deng J., Batulan Z., O’Brien E. R. (2019) Characterization of heat shock protein 27 in extracellular vesicles: a potential anti-inflammatory therapy. FASEB J. 33, 1617–1630 [DOI] [PubMed] [Google Scholar]
- 26.Chen G., Huang A. C., Zhang W., Zhang G., Wu M., Xu W., Yu Z., Yang J., Wang B., Sun H., Xia H., Man Q., Zhong W., Antelo L. F., Wu B., Xiong X., Liu X., Guan L., Li T., Liu S., Yang R., Lu Y., Dong L., McGettigan S., Somasundaram R., Radhakrishnan R., Mills G., Lu Y., Kim J., Chen Y. H., Dong H., Zhao Y., Karakousis G. C., Mitchell T. C., Schuchter L. M., Herlyn M., Wherry E. J., Xu X., Guo W. (2018) Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 560, 382–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gallet R., Dawkins J., Valle J., Simsolo E., de Couto G., Middleton R., Tseliou E., Luthringer D., Kreke M., Smith R. R., Marbán L., Ghaleh B., Marbán E. (2017) Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur. Heart J. 38, 201–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bahmani Jalali H., Mohammadi Aria M., Dikbas U. M., Sadeghi S., Ganesh Kumar B., Sahin M., Kavakli I. H., Ow-Yang C. W., Nizamoglu S. (2018) Effective neural photostimulation using indium-based type-II quantum dots. ACS Nano 12, 8104–8114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wegner K. D., Hildebrandt N. (2015) Quantum dots: bright and versatile in vitro and in vivo fluorescence imaging biosensors. Chem. Soc. Rev. 44, 4792–4834 [DOI] [PubMed] [Google Scholar]
- 30.Crewe C., Joffin N., Rutkowski J. M., Kim M., Zhang F., Towler D. A., Gordillo R., Scherer P. E. (2018) An endothelial-to-adipocyte extracellular vesicle axis governed by metabolic state. Cell 175, 695–708.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kamerkar S., LeBleu V. S., Sugimoto H., Yang S., Ruivo C. F., Melo S. A., Lee J. J., Kalluri R. (2017) Exosomes facilitate therapeutic targeting of oncogenic KRAS in pancreatic cancer. Nature 546, 498–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Théry C., Amigorena S., Raposo G., Clayton A. (2006) Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. Chapter 3, Unit 3.22 [DOI] [PubMed] [Google Scholar]
- 33.Xia J., Sinelnikov I. V., Han B., Wishart D. S. (2015) MetaboAnalyst 3.0–making metabolomics more meaningful. Nucleic Acids Res. 43, W251–W257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park C., Kim T. J. (2018) Expansion and sub-classification of T cell-dependent antibody responses to encompass the role of innate-like T cells in antibody responses. Immune Netw. 18, e34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anastasiou D., Yu Y., Israelsen W. J., Jiang J. K., Boxer M. B., Hong B. S., Tempel W., Dimov S., Shen M., Jha A., Yang H., Mattaini K. R., Metallo C. M., Fiske B. P., Courtney K. D., Malstrom S., Khan T. M., Kung C., Skoumbourdis A. P., Veith H., Southall N., Walsh M. J., Brimacombe K. R., Leister W., Lunt S. Y., Johnson Z. R., Yen K. E., Kunii K., Davidson S. M., Christofk H. R., Austin C. P., Inglese J., Harris M. H., Asara J. M., Stephanopoulos G., Salituro F. G., Jin S., Dang L., Auld D. S., Park H. W., Cantley L. C., Thomas C. J., Vander Heiden M. G. (2012) Pyruvate kinase M2 activators promote tetramer formation and suppress tumorigenesis. Nat. Chem. Biol. 8, 839–847; erratum: 1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shapiro-Shelef M., Calame K. (2005) Regulation of plasma-cell development. Nat. Rev. Immunol. 5, 230–242 [DOI] [PubMed] [Google Scholar]
- 37.Kallies A., Hasbold J., Fairfax K., Pridans C., Emslie D., McKenzie B. S., Lew A. M., Corcoran L. M., Hodgkin P. D., Tarlinton D. M., Nutt S. L. (2007) Initiation of plasma-cell differentiation is independent of the transcription factor Blimp-1. Immunity 26, 555–566 [DOI] [PubMed] [Google Scholar]
- 38.Wei Y., Wang D., Jin F., Bian Z., Li L., Liang H., Li M., Shi L., Pan C., Zhu D., Chen X., Hu G., Liu Y., Zhang C. Y., Zen K. (2017) Pyruvate kinase type M2 promotes tumour cell exosome release via phosphorylating synaptosome-associated protein 23. Nat. Commun. 8, 14041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dibble C. C., Cantley L. C. (2015) Regulation of mTORC1 by PI3K signaling. Trends Cell Biol. 25, 545–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Deng J., Lü S., Liu H., Liu B., Jiang C., Xu Q., Feng J., Wang X. (2017) Homocysteine activates B cells via regulating PKM2-dependent metabolic reprogramming. J. Immunol. 198, 170–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoshino D., Kirkbride K. C., Costello K., Clark E. S., Sinha S., Grega-Larson N., Tyska M. J., Weaver A. M. (2013) Exosome secretion is enhanced by invadopodia and drives invasive behavior. Cell Rep. 5, 1159–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Trajkovic K., Hsu C., Chiantia S., Rajendran L., Wenzel D., Wieland F., Schwille P., Brügger B., Simons M. (2008) Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 319, 1244–1247 [DOI] [PubMed] [Google Scholar]
- 43.Puhm F., Afonyushkin T., Resch U., Obermayer G., Rohde M., Penz T., Schuster M., Wagner G., Rendeiro A. F., Melki I., Kaun C., Wojta J., Bock C., Jilma B., Mackman N., Boilard E., Binder C. J. (2019) Mitochondria are a subset of extracellular vesicles released by activated monocytes and induce type I IFN and TNF responses in endothelial cells. Circ. Res. 125, 43–52 [DOI] [PubMed] [Google Scholar]
- 44.Jęśko H., Stępień A., Lukiw W. J., Strosznajder R. P. (2019) The cross-talk between sphingolipids and insulin-like growth factor signaling: significance for aging and neurodegeneration. Mol. Neurobiol. 56, 3501–3521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Régnier M., Polizzi A., Guillou H., Loiseau N. (2019) Sphingolipid metabolism in non-alcoholic fatty liver diseases. Biochimie 159, 9–22 [DOI] [PubMed] [Google Scholar]
- 46.Tassew N. G., Charish J., Shabanzadeh A. P., Luga V., Harada H., Farhani N., D’Onofrio P., Choi B., Ellabban A., Nickerson P. E. B., Wallace V. A., Koeberle P. D., Wrana J. L., Monnier P. P. (2017) Exosomes mediate mobilization of autocrine Wnt10b to promote axonal regeneration in the injured CNS. Cell Rep. 20, 99–111 [DOI] [PubMed] [Google Scholar]
- 47.Wang R., Ding Q., Yaqoob U., de Assuncao T. M., Verma V. K., Hirsova P., Cao S., Mukhopadhyay D., Huebert R. C., Shah V. H. (2015) Exosome adherence and internalization by hepatic stellate cells triggers sphingosine 1-phosphate-dependent migration. J. Biol. Chem. 290, 30684–30696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yun Y. H., Lee B. K., Park K. (2015) Controlled drug delivery: historical perspective for the next generation. J. Control Release 219, 2–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mesin L., Ersching J., Victora G. D. (2016) Germinal center B cell dynamics. Immunity 45, 471–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tay C., Liu Y. H., Kanellakis P., Kallies A., Li Y., Cao A., Hosseini H., Tipping P., Toh B. H., Bobik A., Kyaw T. (2018) Follicular B cells promote atherosclerosis via T cell-mediated differentiation into plasma cells and secreting pathogenic immunoglobulin G. Arterioscler. Thromb. Vasc. Biol. 38, e71–e84 [DOI] [PubMed] [Google Scholar]
- 51.Feng J., Zhang Z., Kong W., Liu B., Xu Q., Wang X. (2009) Regulatory T cells ameliorate hyperhomocysteinaemia-accelerated atherosclerosis in apoE-/- mice. Cardiovasc. Res. 84, 155–163 [DOI] [PubMed] [Google Scholar]
- 52.Feng J., Lü S., Ding Y., Zheng M., Wang X. (2016) Homocysteine activates T cells by enhancing endoplasmic reticulum-mitochondria coupling and increasing mitochondrial respiration. Protein Cell 7, 391–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang L., Xie M., Yang M., Yu Y., Zhu S., Hou W., Kang R., Lotze M. T., Billiar T. R., Wang H., Cao L., Tang D. (2014) PKM2 regulates the Warburg effect and promotes HMGB1 release in sepsis. Nat. Commun. 5, 4436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu F., Ma F., Wang Y., Hao L., Zeng H., Jia C., Wang Y., Liu P., Ong I. M., Li B., Chen G., Jiang J., Gong S., Li L., Xu W. (2017) PKM2 methylation by CARM1 activates aerobic glycolysis to promote tumorigenesis. Nat. Cell Biol. 19, 1358–1370; erratum: 1442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Van Niel G., D’Angelo G., Raposo G. (2018) Shedding light on the cell biology of extracellular vesicles. Nat. Rev. Mol. Cell Biol. 19, 213–228 [DOI] [PubMed] [Google Scholar]
- 56.Lindenbergh M. F. S., Stoorvogel W. (2018) Antigen presentation by extracellular vesicles from professional antigen-presenting cells. Annu. Rev. Immunol. 36, 435–459 [DOI] [PubMed] [Google Scholar]
- 57.Théry C., Witwer K. W., Aikawa E., Alcaraz M. J., Anderson J. D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G. K., Ayre D. C., Bach J. M., Bachurski D., Baharvand H., Balaj L., Baldacchino S., Bauer N. N., Baxter A. A., Bebawy M., Beckham C., Bedina Zavec A., Benmoussa A., Berardi A. C., Bergese P., Bielska E., Blenkiron C., Bobis-Wozowicz S., Boilard E., Boireau W., Bongiovanni A., Borràs F. E., Bosch S., Boulanger C. M., Breakefield X., Breglio A. M., Brennan M. A., Brigstock D. R., Brisson A., Broekman M. L., Bromberg J. F., Bryl-Górecka P., Buch S., Buck A. H., Burger D., Busatto S., Buschmann D., Bussolati B., Buzás E. I., Byrd J. B., Camussi G., Carter D. R., Caruso S., Chamley L. W., Chang Y. T., Chen C., Chen S., Cheng L., Chin A. R., Clayton A., Clerici S. P., Cocks A., Cocucci E., Coffey R. J., Cordeiro-da-Silva A., Couch Y., Coumans F. A., Coyle B., Crescitelli R., Criado M. F., D’Souza-Schorey C., Das S., Datta Chaudhuri A., de Candia P., De Santana E. F., De Wever O., Del Portillo H. A., Demaret T., Deville S., Devitt A., Dhondt B., Di Vizio D., Dieterich L. C., Dolo V., Dominguez Rubio A. P., Dominici M., Dourado M. R., Driedonks T. A., Duarte F. V., Duncan H. M., Eichenberger R. M., Ekström K., El Andaloussi S., Elie-Caille C., Erdbrügger U., Falcón-Pérez J. M., Fatima F., Fish J. E., Flores-Bellver M., Försönits A., Frelet-Barrand A., Fricke F., Fuhrmann G., Gabrielsson S., Gámez-Valero A., Gardiner C., Gärtner K., Gaudin R., Gho Y. S., Giebel B., Gilbert C., Gimona M., Giusti I., Goberdhan D. C., Görgens A., Gorski S. M., Greening D. W., Gross J. C., Gualerzi A., Gupta G. N., Gustafson D., Handberg A., Haraszti R. A., Harrison P., Hegyesi H., Hendrix A., Hill A. F., Hochberg F. H., Hoffmann K. F., Holder B., Holthofer H., Hosseinkhani B., Hu G., Huang Y., Huber V., Hunt S., Ibrahim A. G., Ikezu T., Inal J. M., Isin M., Ivanova A., Jackson H. K., Jacobsen S., Jay S. M., Jayachandran M., Jenster G., Jiang L., Johnson S. M., Jones J. C., Jong A., Jovanovic-Talisman T., Jung S., Kalluri R., Kano S. I., Kaur S., Kawamura Y., Keller E. T., Khamari D., Khomyakova E., Khvorova A., Kierulf P., Kim K. P., Kislinger T., Klingeborn M., Klinke D. J., II, Kornek M., Kosanović M. M., Kovács A. F., Krämer-Albers E. M., Krasemann S., Krause M., Kurochkin I. V., Kusuma G. D., Kuypers S., Laitinen S., Langevin S. M., Languino L. R., Lannigan J., Lässer C., Laurent L. C., Lavieu G., Lázaro-Ibáñez E., Le Lay S., Lee M. S., Lee Y. X. F., Lemos D. S., Lenassi M., Leszczynska A., Li I. T., Liao K., Libregts S. F., Ligeti E., Lim R., Lim S. K., Linē A., Linnemannstöns K., Llorente A., Lombard C. A., Lorenowicz M. J., Lörincz A. M., Lötvall J., Lovett J., Lowry M. C., Loyer X., Lu Q., Lukomska B., Lunavat T. R., Maas S. L., Malhi H., Marcilla A., Mariani J., Mariscal J., Martens-Uzunova E. S., Martin-Jaular L., Martinez M. C., Martins V. R., Mathieu M., Mathivanan S., Maugeri M., McGinnis L. K., McVey M. J., Meckes D. G., Jr., Meehan K. L., Mertens I., Minciacchi V. R., Möller A., Møller Jørgensen M., Morales-Kastresana A., Morhayim J., Mullier F., Muraca M., Musante L., Mussack V., Muth D. C., Myburgh K. H., Najrana T., Nawaz M., Nazarenko I., Nejsum P., Neri C., Neri T., Nieuwland R., Nimrichter L., Nolan J. P., Nolte-’t Hoen E. N., Noren Hooten N., O’Driscoll L., O’Grady T., O’Loghlen A., Ochiya T., Olivier M., Ortiz A., Ortiz L. A., Osteikoetxea X., Østergaard O., Ostrowski M., Park J., Pegtel D. M., Peinado H., Perut F., Pfaffl M. W., Phinney D. G., Pieters B. C., Pink R. C., Pisetsky D. S., Pogge von Strandmann E., Polakovicova I., Poon I. K., Powell B. H., Prada I., Pulliam L., Quesenberry P., Radeghieri A., Raffai R. L., Raimondo S., Rak J., Ramirez M. I., Raposo G., Rayyan M. S., Regev-Rudzki N., Ricklefs F. L., Robbins P. D., Roberts D. D., Rodrigues S. C., Rohde E., Rome S., Rouschop K. M., Rughetti A., Russell A. E., Saá P., Sahoo S., Salas-Huenuleo E., Sánchez C., Saugstad J. A., Saul M. J., Schiffelers R. M., Schneider R., Schøyen T. H., Scott A., Shahaj E., Sharma S., Shatnyeva O., Shekari F., Shelke G. V., Shetty A. K., Shiba K., Siljander P. R., Silva A. M., Skowronek A., Snyder O. L., II, Soares R. P., Sódar B. W., Soekmadji C., Sotillo J., Stahl P. D., Stoorvogel W., Stott S. L., Strasser E. F., Swift S., Tahara H., Tewari M., Timms K., Tiwari S., Tixeira R., Tkach M., Toh W. S., Tomasini R., Torrecilhas A. C., Tosar J. P., Toxavidis V., Urbanelli L., Vader P., van Balkom B. W., van der Grein S. G., Van Deun J., van Herwijnen M. J., Van Keuren-Jensen K., van Niel G., van Royen M. E., van Wijnen A. J., Vasconcelos M. H., Vechetti I. J., Jr., Veit T. D., Vella L. J., Velot É., Verweij F. J., Vestad B., Viñas J. L., Visnovitz T., Vukman K. V., Wahlgren J., Watson D. C., Wauben M. H., Weaver A., Webber J. P., Weber V., Wehman A. M., Weiss D. J., Welsh J. A., Wendt S., Wheelock A. M., Wiener Z., Witte L., Wolfram J., Xagorari A., Xander P., Xu J., Yan X., Yáñez-Mó M., Yin H., Yuana Y., Zappulli V., Zarubova J., Žėkas V., Zhang J. Y., Zhao Z., Zheng L., Zheutlin A. R., Zickler A. M., Zimmermann P., Zivkovic A. M., Zocco D., Zuba-Surma E. K. (2018) Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 7, 1535750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mathivanan S., Simpson R. J. (2009) ExoCarta: a compendium of exosomal proteins and RNA. Proteomics 9, 4997–5000 [DOI] [PubMed] [Google Scholar]
- 59.Mathivanan S., Ji H., Simpson R. J. (2010) Exosomes: extracellular organelles important in intercellular communication. J. Proteomics 73, 1907–1920 [DOI] [PubMed] [Google Scholar]
- 60.Buschow S. I., van Balkom B. W., Aalberts M., Heck A. J., Wauben M., Stoorvogel W. (2010) MHC class II-associated proteins in B-cell exosomes and potential functional implications for exosome biogenesis. Immunol. Cell Biol. 88, 851–856 [DOI] [PubMed] [Google Scholar]
- 61.Escudier B., Dorval T., Chaput N., André F., Caby M. P., Novault S., Flament C., Leboulaire C., Borg C., Amigorena S., Boccaccio C., Bonnerot C., Dhellin O., Movassagh M., Piperno S., Robert C., Serra V., Valente N., Le Pecq J. B., Spatz A., Lantz O., Tursz T., Angevin E., Zitvogel L. (2005) Vaccination of metastatic melanoma patients with autologous dendritic cell (DC) derived-exosomes: results of thefirst phase I clinical trial. J. Transl. Med. 3, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morse M. A., Garst J., Osada T., Khan S., Hobeika A., Clay T. M., Valente N., Shreeniwas R., Sutton M. A., Delcayre A., Hsu D. H., Le Pecq J. B., Lyerly H. K. (2005) A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. J. Transl. Med. 3, 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Besse B., Charrier M., Lapierre V., Dansin E., Lantz O., Planchard D., Le Chevalier T., Livartoski A., Barlesi F., Laplanche A., Ploix S., Vimond N., Peguillet I., Théry C., Lacroix L., Zoernig I., Dhodapkar K., Dhodapkar M., Viaud S., Soria J. C., Reiners K. S., Pogge von Strandmann E., Vély F., Rusakiewicz S., Eggermont A., Pitt J. M., Zitvogel L., Chaput N. (2015) Dendritic cell-derived exosomes as maintenance immunotherapy after first line chemotherapy in NSCLC. OncoImmunology 5, e1071008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jenks S. A., Cashman K. S., Zumaquero E., Marigorta U. M., Patel A. V., Wang X., Tomar D., Woodruff M. C., Simon Z., Bugrovsky R., Blalock E. L., Scharer C. D., Tipton C. M., Wei C., Lim S. S., Petri M., Niewold T. B., Anolik J. H., Gibson G., Lee F. E., Boss J. M., Lund F. E., Sanz I. (2018) Distinct effector B cells induced by unregulated toll-like receptor 7 contribute to pathogenic responses in systemic lupus erythematosus. Immunity 49, 725–739.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Burger J. A., Wiestner A. (2018) Targeting B cell receptor signalling in cancer: preclinical and clinical advances. Nat. Rev. Cancer 18, 148–167 [DOI] [PubMed] [Google Scholar]
- 66.Nelson B. H. (2010) CD20+ B cells: the other tumor-infiltrating lymphocytes. J. Immunol. 185, 4977–4982 [DOI] [PubMed] [Google Scholar]
- 67.Avitabile E., Bedognetti D., Ciofani G., Bianco A., Delogu L. G. (2018) How can nanotechnology help the fight against breast cancer? Nanoscale 10, 11719–11731 [DOI] [PubMed] [Google Scholar]
- 68.Lo Giudice M. C., Herda L. M., Polo E., Dawson K. A. (2016) In situ characterization of nanoparticle biomolecular interactions in complex biological media by flow cytometry. Nat. Commun. 7, 13475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sasaki Y., Iwai K. (2018) Crucial role of linear ubiquitin chain assembly complex-mediated inhibition of programmed cell death in TLR4-mediated B cell responses and B1b cell development. J. Immunol. 200, 3438–3449 [DOI] [PubMed] [Google Scholar]
- 70.Gao Y., Feng J., Ma K., Zhou Z., Zhu Y., Xu Q., Wang X. (2012) 8,9-Epoxyeicosatrienoic acid inhibits antibody production of B lymphocytes in mice. PLoS One 7, e40258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Derfus A. M., Chan W. C. W., Bhatia S. N. (2004) Probing the cytotoxicity of semiconductor quantum dots. Nano Lett. 4, 11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hauck T. S., Anderson R. E., Fischer H. C., Newbigging S., Chan W. C. (2010) In vivo quantum-dot toxicity assessment. Small 6, 138–144 [DOI] [PubMed] [Google Scholar]
- 73.Tsoi K. M., Dai Q., Alman B. A., Chan W. C. (2013) Are quantum dots toxic? Exploring the discrepancy between cell culture and animal studies. Acc. Chem. Res. 46, 662–671 [DOI] [PubMed] [Google Scholar]
- 74.Dubertret B., Skourides P., Norris D. J., Noireaux V., Brivanlou A. H., Libchaber A. (2002) In vivo imaging of quantum dots encapsulated in phospholipid micelles. Science 298, 1759–1762 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.