Abstract
It is recognized that stress can induce cardiac dysfunction, but the underlying mechanisms are not well understood. The present study aimed to test the hypothesis that chronic negative stress leads to alterations in DNA methylation of certain cardiac genes, which in turn contribute to pathologic remodeling of the heart. We found that mice that were exposed to chronic restraint stress (CRS) for 4 wk exhibited cardiac remodeling toward heart failure, as characterized by ventricular chamber dilatation, wall thinning, and decreased contractility. CRS also induced cardiac arrhythmias, including intermittent sinus tachycardia and bradycardia, frequent premature ventricular contraction, and sporadic atrioventricular conduction block. Circulating levels of stress hormones were elevated, and the cardiac expression of tyrosine hydroxylase, a marker of sympathetic innervation, was increased in CRS mice. Using reduced representation bisulfite sequencing, we found that although CRS did not lead to global changes in DNA methylation in the murine heart, it nevertheless altered methylation at specific genes that are associated with the dilated cardiomyopathy (DCM) (e.g., desmin) and adrenergic signaling of cardiomyocytes (ASPC) (e.g., adrenergic receptor-α1) pathways. We conclude that CRS induces cardiac remodeling and arrhythmias, potentially through altered methylation of myocardial genes associated with the DCM and ASPC pathways.—Zhang, P., Li, T., Liu, Y.-Q., Zhang, H., Xue, S.-M., Li, G., Cheng, H.-Y.M., Cao, J.-M. Contribution of DNA methylation in chronic stress–induced cardiac remodeling and arrhythmias in mice.
Keywords: chronic restraint stress, cardiovascular diseases, reduced representation bisulfite sequencing
Chronic stress is a critical factor in the pathogenesis and development of diseases, including cardiovascular diseases (CVDs). Many studies have focused on the connection between chronic stress and CVD, but there is still no clear evidence for a causal relationship between them. Epidemiologic studies have shown that chronic stress and CVD often accompany each other in patients, suggesting that chronic stress may cause cardiovascular dysfunction (1, 2), or vice versa (3, 4). The hypothalamic-pituitary-adrenal system, the sympathetic nervous system, and the immune system have long been considered to be the main pathways responsible for stress-induced diseases (5–7). However, in recent years, alternative pathways and mechanisms, including epigenetic control of gene expression, have been raised to explain the relationship between chronic stress and CVD (8, 9).
DNA methylation represents one form of epigenetic regulation and may be relevant to the development of CVD. For example, abnormal DNA methylation within the CpG islands (CGIs) of the promoter regions of voltage-gated potassium channel subfamily E genes has been linked to cardiac arrhythmias (10). In a recent genome-wide association study, 2 DNA methylation sites, cg07786668 and cg17218495, located within the genes zinc finger homeobox 3 and SWI/SNF related, matrix associated, actin dependent regulator of chromatin, subfamily a, member 4, were found to be significantly associated with myocardial infarction (11). Ecstasy, a psychoactive drug that can trigger heart attacks (12), has been shown to induce hypermethylation of clock genes (e.g., period 3 circadian clock 3, circadian locomoter output cycles kaput, aryl hydrocarbon receptor nuclear translocator-like, and neuronal PAS domain protein 2) in murine hearts and alter their expression. There is evidence that DNA methylation is also associated with cardiac autonomic regulation (13). Together, these studies indicate that cardiac DNA methylation is tightly associated with cardiac dysfunction. Despite this, it remains unclear whether altered DNA methylation in the heart plays a role in chronic stress–induced cardiac dysfunctions.
Exposing rodents to chronic restraint stress (CRS) for 4–6 wk has been shown to induce anhedonic-, depression-, and anxiety-like behaviors (14–19). However, murine CRS models have seldom been used to investigate the effects of acute negative stress on cardiac function (20). In the present study, we used a murine CRS model to investigate whether chronic stress would induce not only cardiac remodeling and arrhythmias but also changes in DNA methylation in cardiac tissues. We found that mice that were exposed to CRS exhibited multiple types of arrhythmias as well as cardiac remodeling toward heart failure. Although CRS did not induce global changes in DNA methylation in the heart, anywhere from 72 (q ≤ 0.001) to 3252 (q ≤ 0.05) regions of the genome were differentially methylated. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis on the 3252 differentially methylated regions (DMRs) revealed a significant enrichment of genes belonging to the dilated cardiomyopathy (DCM), hypertrophic cardiomyopathy, arrhythmogenic right ventricular cardiomyopathy, and adrenergic signaling in cardiomyocytes (ASPC) pathways. The alterations in site-specific DNA methylation were correlated with reduced expression at the mRNA level in the heart [e.g., adrenergic receptor-α1B (Adra1b); protein phosphatase 2, regulatory subunit A (Ppp2r1a); protein phosphatase 2, regulatory subunit B-γ (Ppp2r2c); desmin; Tgfb]. Interestingly, the expression of several of these genes (i.e., Adra1b, Ppp2r2c) was up-regulated in the hypothalamus, suggesting that CRS also induces gene expression changes in sites within the CNS that are important for stress response. Collectively, our results indicate that CRS alters cardiac DNA methylation in specific genes that are associated with the DCM and ASPC pathways and that the consequent changes in their expression may underlie the DCM-like cardiac remodeling and arrhythmias observed in CRS mice.
MATERIALS AND METHODS
Animals
Six-week-old male C57BL/6J mice were obtained from the Experimental Animal Center, Southwest Medical University (Luzhou, China). Mice were housed under a 12:12 h light/dark cycle (light intensity of 200 lux; lights on at 8:00 am, lights off at 8:00 pm) at an ambient temperature of 23 ± 2°C. Regular rodent chow and water were provided ad libitum. Animals were randomly assigned to the nonstress (NS) or CRS treatment group. All animal handling and experimental procedures were approved by the Life Ethics Committee of Southwest Medical University and were conducted in compliance with the Guidelines for the Care and Use of Laboratory Animals (National Institutes of Health, Bethesda, MD, USA).
Experimental design
A total of 56 mice were used in this study. Starting at 6 wk of age, mice were subjected to CRS daily for 4 consecutive weeks or were maintained under NS conditions for the same period (n = 28/treatment group). Behavioral tests and electrocardiogram (ECG) recordings were performed on a weekly basis during the 4-wk interval of CRS. At 10 wk of age, once all behavioral tests, ECG recording, and echocardiography were completed, mice were killed, and serum and heart tissues were harvested.
CRS paradigm
Starting at 6 wk of age, mice were subjected to CRS for 28 consecutive d. Mice were individually placed into plastic tubes (length 10 cm, inner diameter 3 cm) with holes that allowed for ample ventilation. Each day, mice in the CRS group received three 1-h sessions of restraint, administered at random, unpredictable times of the 24 h day. Body restraint did not lead to physical injury and can be considered as an emotional stress to the animal.
Forced swimming test and sucrose preference test
Forced swimming test (FST) was performed as previously described in ref. 21. Mice were placed individually into glass cylinders (height 25 cm, diameter 10 cm) filled to a depth of 15 cm with tap water (23–25°C), and observed for 6 min. The duration of immobile behavior in the final 4 min of the test was measured. Mice were judged to be immobile when they remained floating passively in the water.
Sucrose preference test was performed as previously described in Zhang et al. (22). Forty-eight hours prior to the test day, mice were habituated to the presence of 2 drinking bottles containing either 2% sucrose or water in their home cage. Following a 4-h deprivation of food and water on the test day, mice were presented with 2 identical bottles containing 2% sucrose or water for 1 h. Bottles were weighed before and after the 1-h interval to measure fluid consumption. Sucrose preference was calculated based on the following formula: sucrose preference (%) = weight of ingested sucrose solution ÷ (weight of ingested sucrose solution + weight of ingested water) × 100%.
ECG recording
To record the surface ECG in vivo, mice were anesthetized with an intraperitoneal injection of 1% (w/v) sodium pentobarbital (0.05 ml/10 g body weight). The recording electrodes were attached to the forelimbs, and ECG was recorded to monitor heart rate and rhythm with a computer assisted BL-420S system (Chengdu Software, Chengdu, China) with a sample rate of 1000 Hz. Each ECG recording lasted for 1 h between 9:00 and 10:00 am (zeitgeber time 1–2). Arrhythmias are compared with normal NS mice exhibiting regular sinus rhythms.
Echocardiography
After the final behavioral test and ECG recording at 10 wk of age, mice were anesthetized with isoflurane, and echocardiography was performed using a VisualSonics Vevo 3100 Echocardiographer (Fujifilm VisualSonics, Toronto, ON, Canada). Results from 2-dimensional M-mode analysis along the short and long axes [at the level of the largest left ventricular (LV) diameter] were used to determine the effects of CRS on cardiac structure and function. Parameters of echocardiographic data included LV systolic and diastolic dimensions and volumes, ejection fraction, fractional shortening, stroke volume, and cardiac output.
Tissue sampling
Following completion of the echocardiography, mice were terminally anesthetized with 1% (w/v) sodium pentobarbital (0.05 ml/10 g body weight), and blood samples were collected by cardiac puncture and stored at 4°C. Blood samples were centrifuged at 3000 rpm for 15 min to separate the serum, which was stored at −130°C until use. For extraction of genomic DNA, RNA, and proteins from the heart and hypothalamus, tissues were snap frozen in liquid nitrogen and stored at −130°C until extraction. For hematoxylin-eosin (H-E) and immunofluorescent staining, animals were perfused with 4% (w/v) paraformaldehyde in PBS and postfixed at 4°C overnight (for brain) or at room temperature for 15 min (for heart). Tissues were cryopreserved in 30% (w/v) sucrose in PBS at 4°C overnight prior to thin sectioning.
H-E and immunofluorescent staining
Serial frozen sections of whole heart (10 μm thick), LV tissues (10 μm thick), or whole brain (20 μm thick) were cut in coronal or horizontal directions and mounted on microscope slides. Routine H-E staining was performed to visualize the overall appearance of cardiac walls and chambers. Immunofluorescent staining was used to detect the expression of tyrosine hydroxylase (TH). For immunofluorescence, tissue sections were washed 5 times for 5 min each in PBS containing 0.1% Triton X-100. Sections were blocked with 10% horse serum in PBS containing 0.1% Triton X-100 at room temperature for 1 h and incubated overnight with the rabbit anti-TH antibody (1:500; sc-14007; Santa Cruz Biotechnology, Dallas, TX, USA) at 4°C. The next day, tissue sections were washed five times for 5 min each in PBS at room temperature. Tissue sections were then incubated with Alexa Fluor 594 donkey anti-rabbit IgG H&L (1:1000; ab98500; Abcam, Cambridge, MA, USA) in blocking solution, in the dark for 2 h at room temperature. Sections were washed 5 times for 5 min each in PBS, cover-slipped with antifade fluorescence mounting medium containing DAPI (HNFD-02; HelixGen; Guangzhou Haozhi Biotechnology, Hangzhou, China), and sealed with nail polish. Images were acquired on an Olympus BX63 fluorescence microscope (Olympus, Tokyo, Japan).
Western blotting
Heart and hypothalamic tissues were homogenized on ice in RIPA buffer containing protease inhibitors. Lysates were incubated on ice for 30 min, followed by centrifugation at 12,000 rpm for 20 min. Supernatants were collected and stored at −130°C until use. Protein concentration was measured using the Bradford assay. Protein lysates were mixed with SDS loading buffer to 1× concentration, heated to 95°C for 5 min, and centrifuged for 1 min at 12,000 rpm. Lysates (20 μg/well) were electrophoresed in a 10% SDS-polyacrylamide gel for ∼2 h at 100 V at room temperature, and proteins were electroblotted onto polyvinylidene fluoride membrane for 30 min at 25 V at room temperature. Membranes were blocked in 5% skim milk in Tris-buffered saline with 0.1% Triton X-100 for 1 h at room temperature, followed by incubation with the rabbit anti-TH antibody (1:500; sc-14007; Santa Cruz Biotechnology) in blocking solution at 4°C overnight. The next day, membranes were washed in Tris-buffered saline with 0.1% Triton X-100 and incubated for 2 h at room temperature with a horseradish peroxidase–conjugated secondary antibody in blocking solution. Blots were developed using an ECL Detection Kit (GE Healthcare, Waukesha, WI, USA), and images were captured using Universal Hood II (Bio-Rad, Hercules, CA, USA). Membranes were stripped, redeveloped with ECL to verify stripping efficiency, and reprobed with antibodies against β-actin (1:5000, 061M4808; MilliporeSigma, Burlington, MA, USA). Blots were quantified using the Analyze function in Quantity One v.4.4 (Bio-Rad). Values are presented as fold-change of the protein normalized to relative abundance of β-actin (n = 3 mice/group).
Reduced representation bisulfite sequencing library preparation and sequencing
For reduced representation bisulfite sequencing (RRBS), genomic DNA was extracted from the hearts of NS or CRS mice (n = 3/group), and 1 μg of DNA was resuspended in 200 μl nuclease-free water for each sample. Library construction and sequencing services were provided by RiboBio (Guangzhou, China). Briefly, the purified genomic DNA was digested overnight with MspI (New England Biolabs, Ipswich, MA, USA), and the sticky ends of the digested DNA fragments were filled with CG nucleotides followed by addition of 3′ A overhangs. Methylated Illumina sequencing adapters with 3′ T overhangs were ligated to the DNA fragments, which were subsequently purified. Fragments of 40–200 bp were selected and bisulfite converted using the EZ DNA Methylation Gold Kit (Zymo Research, Irvine, CA, USA). Libraries of 40–200 bp fragments were amplified by PCR, and the final concentration of the loaded sample was 8.75 pM. Libraries were sequenced (100 bp paired-end reads) on an Illumina HiSeq 2500 platform (Illumina, San Diego, CA, USA). The first 2 nt were trimmed from all of the second read sequences to blunt-end the MspI site. All reads were trimmed using Trim Galore v.0.4.0 software (Babraham Bioinformatics Group, Babraham Institute, Cambridge, United Kingdom; http://www.bioinformatics.babraham.ac.uk/projects/trim_galore/) and a minimum Phred quality score of 20. The adaptor pollution reads and multiple N reads (where N > 10% of 1 read) were removed to generate the clean reads. The quality control checks were performed by FastQC v.0.11.3 software (Babraham Bioinformatics Group). The clean reads were mapped to the Mus musculus reference genome (23) (GRCm37.p5, downloaded from Ensembl; http://asia.ensembl.org/Mus_musculus/Info/Index), and methylation calls were performed by Bismark v.0.14.5 (24) using default parameters. The bisulfite conversion rates were calculated as the number of covered CpGs that were unconverted, divided by the total number of covered CpGs (25). The bisulfite conversion efficiencies of our 6 libraries were 98.28% (CRS 1), 98.53% (CRS 2), 97.85% (CRS 3), 98.37% (NS 1), 98.49% (NS 2), and 98.37% (NS 3). The raw clean data have been deposited to Genbank (U.S. National Center for Biotechnology Information) under Sequence Read Archive accession number PRJNA495716.
Differential methylation analysis
The methylKit package for R (https://bioconductor.org/packages/release/bioc/html/methylKit.html) was used to identify differentially methylated positions (DMPs) between treatment groups (26). Based on the difference in methylation level, as well as the P and q values of relevant methylated positions, a total of 10,624,115 DMPs were identified. Using the eDMR software (https://code.google.com/p/edmr/), these DMPs were connected to a total of 13,172 DMRs in the genome. The total DMRs were further filtered to 3252 DMRs (distributed across 1854 genes) using the criteria of q value between positions of ≤0.05, and a difference in methylation level between positions of ≥25. Finally, the 3252 DMRs were stringently filtered to 72 DMRs (distributed across 48 genes) using a between-regions cutoff of q ≤ 0.001, and a difference in methylation level between regions of ≥25, and number of difference methylation on C bases ≥3. Supplemental Table S6 summarizes the results of the data filtering.
Quantitative RT-PCR
Total RNA was extracted from cardiac and hypothalamic tissues using Trizol reagent (Thermo Fisher Scientific, Waltham, MA, USA). The purity and concentration of RNA were determined using the NanoDrop 2000 (Thermo Fisher Scientific), and RNA integrity was confirmed by agarose gel electrophoresis. cDNA was synthesized from 2 μg of total RNA using the TransScript First-Strand cDNA Synthesis Super Mix Kit (Takara, Kyoto, Japan). Quantitative PCR reactions were set up using Sybr Premix Ex Taq (Takara) and were run on the CFX96 Quantitative RT-PCR (qRT-PCR) System (Bio-Rad). Supplemental Tables S1 and S2 provide the sequences of the quantitative PCR primers that were used to detect transcripts of genes in the signaling pathways of DCM and ASPC, respectively.
Methylation-specific PCR
Genomic DNA from cardiac tissues was modified by sodium bisulfite treatment using the EZ DNA methylation kit (Zymo Research) to convert unmethylated, but not methylated, cytosines to uracil. Following bisulfite removal and completion of the chemical conversion, the modified DNA was used as a template for PCR. For each DNA sample, 2 PCR reactions were performed: one was specific for DNA that was originally methylated (M-primers) at the desmin gene, and the other was specific for DNA that was originally unmethylated (U-primers) at the desmin gene. PCR reactions were set up using the EpiTect Methylation-Specific PCR (MSP) Kit (100) (Qiagen, Germantown, MD, USA) and were run on the Veriti 96-well thermal cycler (9902; Thermo Fisher Scientific). The PCR cycling conditions were 35 cycles of 30 s at 95°C (denaturation), 30 s at 57°C for M-primers or 55°C for U-primers (annealing), and 60 s at 72°C (elongation). Reaction products were electrophoresced on a 2% agarose gel. Universal methylated mouse DNA standard and positive control primers (corresponding to nucleotide positions 430–778 of mouse mutL homolog 1) were purchased from Zymo Research (D5012). Supplemental Table S3 provides the sequences of MSP primers that will amplify a region of the murine desmin gene corresponding to nucleotide positions chromosome 1, 75359874, to chromosome 1, 75360094.
ELISA
Blood samples were collected from the tail veins of mice from both treatment groups at 6 and 10 wk of age at 4:00 pm (Zeitgeber time 8), and sera were separated by centrifugation. Commercial ELISA kits were used to measure the serum levels of stress hormones or cytokines, including corticosterone (CORT) (CEA540Ge 96 Test; Cloud-Clone, Houston, TX, USA), corticotropin-releasing hormone (CRH) (CEA835Mu 96 Test; Cloud-Clone), norepinephrine (NE) (CEA907Ge 96 Test; Cloud-Clone), NE transporter (SEC681Mu 96 Test; Cloud-Clone), and TGF-β1 (SEA124MU 96 Test; Cloud-Clone).
Statistical analyses
Statistical analyses were performed using Prism 7 (GraphPad Software, La Jolla, CA, USA). All data were expressed as means ± sd. Where appropriate, a Student’s t test or 2-way ANOVA was performed for comparison of group differences. Prior to the use of parametric statistics, we ensured that the data were normally distributed using the Shapiro-Wilk test. The χ2 test was used to analyze the frequency of arrhythmic events between treatment groups. Differences were considered statistically significant at P < 0.05.
RESULTS
CRS induces abnormal behaviors and elevates the circulatory stress hormones and the expression of TH in the paraventricular nucleus of mice
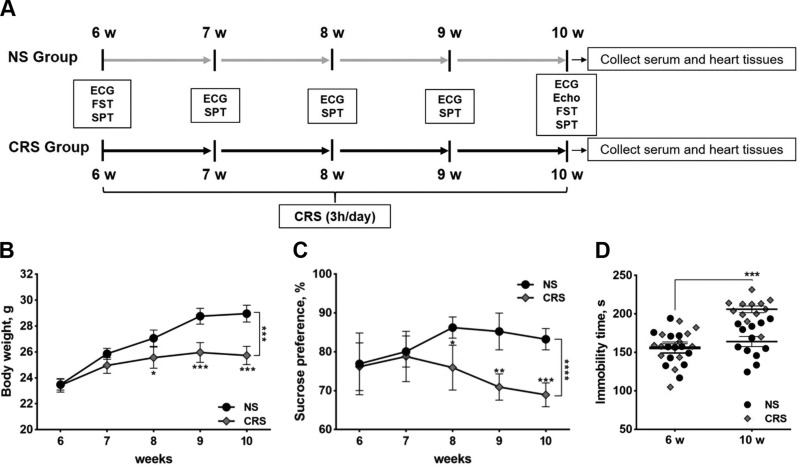
The experimental design, including the time course of stress exposure, ECG recording, echocardiography, and behavioral tests, is depicted in Fig. 1A. The CRS group was exposed to thrice daily physical restraint in a plastic tube for 4 consecutive weeks starting at 6 wk of age. Compared with the NS control group, CRS mice exhibited a significant reduction in body weight in the final 2 wk of stress exposure (Fig. 1B). Anhedonia- and depression-like behaviors were assessed by the sucrose preference test and FST, respectively. Sucrose preference was significantly reduced in CRS mice from 8 wk of age and onward relative to NS mice (Fig. 1C). After 4 wk of CRS treatment, mice exhibited prolonged immobility time in the FST compared with NS controls (Fig. 1D). Both CRS and NS groups had similar immobility times in the FST at 6 wk of age, prior to the onset of CRS exposure (Fig. 1D). As expected, CRS caused an elevation in circulating levels of stress hormones, including CORT, CRH, and NE (Supplemental Fig. S1A–C). Serum levels of NE transporter and TGF-β1 were decreased in CRS mice compared with NS controls (Supplemental Fig. S1D, E). Together, these results indicate that our CRS paradigm elicited signs of physiologic stress and induced abnormal mood-associated behaviors in mice.
Figure 1.
Experimental design and behavioral analyses of CRS and NS mice. A) Flow chart illustrating the experimental design, including time course of stress exposure, ECG recording, behavioral tests, echocardiography, and blood and tissue collection. Echo, echocardiography; SPT, sucrose preference test. B) Body weights of CRS and NS mice at 6, 7, 8, 9, and 10 wk of age. C) Sucrose preference of CRS and NS mice at 6, 7, 8, 9, and 10 wk of age. D) Immobility times in the FST of CRS and NS mice at 6 and 10 wk of age. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NS group (n = 13/group).
CRS induces cardiac remodeling toward heart failure
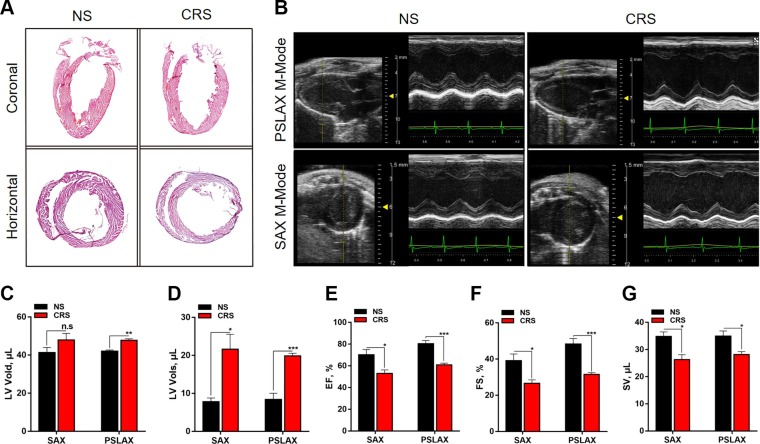
To investigate whether CRS could induce structural and functional remodeling of the heart and potentially heart failure, we performed H-E staining and cardiac echocardiography in both CRS and NS groups. H-E staining of coronal and horizontal sections of the hearts of CRS mice indicated significant LV chamber dilatation and wall thinning compared with NS controls (Fig. 2A). Representative M-mode echocardiographic parameters showed LV wall thinning, LV chamber dilatation, and reductions in ejection fraction, fractional shortening, and cardiac output in CRS mice relative to NS controls (Fig. 2B–G and Supplemental Table S4). These results demonstrate that CRS in mice induces pathologic cardiac remodeling that may lead to eventual heart failure.
Figure 2.
Results of cardiac H-E staining and echocardiography of 10-wk-old CRS and NS mice. A) H-E staining of whole hearts sectioned along the coronal or horizontal planes. Compared with NS controls, the hearts of CRS mice showed LV chamber dilatation and wall thinning, suggesting that CRS induced cardiac remodeling and heart failure. B) Representative echocardiographic images under parasternal long axis (PSLAX) and short axis (SAX) M-mode. The images show LV chamber dilatation and decline of LV contraction. C–G) Measurements of echocardiographic parameters in CRS and NS mice: LV diastolic volume (LV Vold), microliters (C); systolic volume (LV Vols), microliters (D); ejection fraction (EF), percentage (E); fractional shortening (FS), percentage (F); and stroke volume (SV), microliters (G). N.s., not significant. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NS group (n = 8 in NS group, n = 9 in CRS group).
CRS induces cardiac arrhythmias in mice
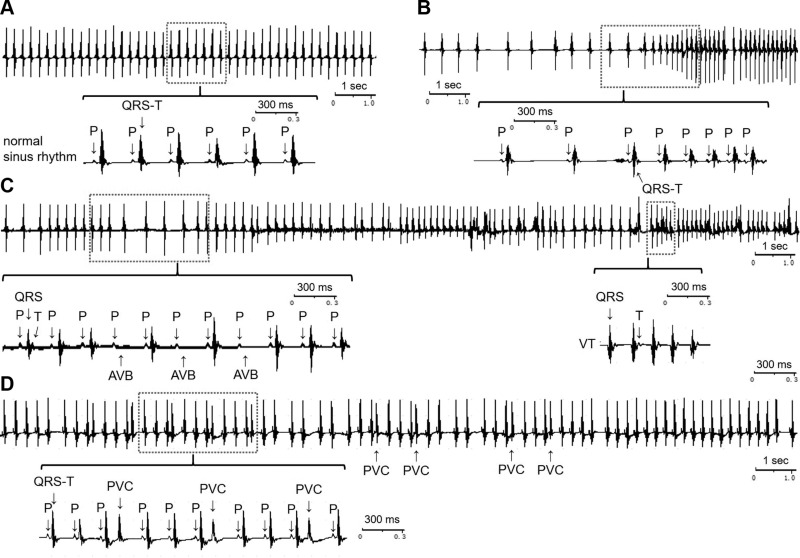
Surface ECGs were recorded in the early morning, between 1 and 2 h after light onset, with each recording lasting for 1 h. As expected, the ECGs of NS mice showed normal sinus rhythm (Fig. 3A). In contrast, CRS mice exhibited multiple phenotypes indicative of cardiac arrhythmias, including alternating sinus bradycardia and sinus tachycardia (Fig. 3B), sporadic atrioventricular conduction block (AVB), ventricular tachycardia (VT) (Fig. 3C), and frequent premature ventricular contractions (PVCs) (Fig. 3D). All of the CRS mice (28/28) displayed arrhythmias: 78.6% (22/28) showed both sinus arrhythmias and ectopic (nonsinus) arrhythmias, and the remaining 21.4% (6/28) showed sinus arrhythmias only (sinus tachycardia or sinus bradycardia). Supplemental Table S5 provides a statistical summary of arrhythmias in both CRS and NS groups. Various types of arrhythmias were significantly increased in the CRS mice compared with NS controls (Supplemental Table S5).
Figure 3.
Representative ECGs showing cardiac arrhythmias induced by CRS in mice in vivo. A) ECGs of NS mice showing normal sinus rhythm. B) Intermittent sinus bradycardia and sinus tachycardia in CRS mice. C) Sporadic AVB and unsustained VT in CRS mice. D) Frequent PVC in CRS mice. The type of arrhythmia could be clearly recognized from the expanded ECGs segments enclosed in the red boxes. n = 28/group. P, P wave; QRS, QRS complex; QRS-T, QRS complex and T wave (T).
CRS increases sympathetic activity on the heart
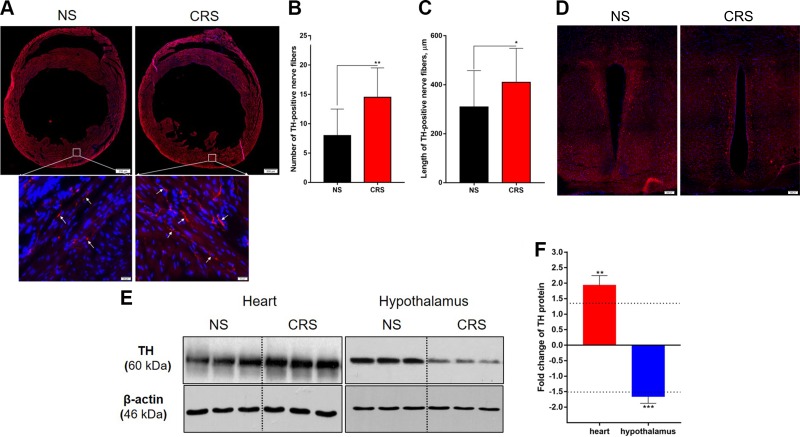
TH immunoreactivity (ir) has been shown to be a good indicator of sympathetic activity in the heart and other tissues or organs (27). Using TH as a marker of cardiac sympathetic nerves, we found that CRS increased TH-ir in the LV free wall of the heart relative to NS treatment (framed areas in Fig. 4A). The numbers and total length of TH-positive nerve fibers were significantly increased in CRS mice compared with the NS mice (Fig. 4B, C). In contrast, TH-ir was decreased in the paraventricular nucleus (PVN) of CRS mice compared with NS controls (Fig. 4D). These results were confirmed by Western blot analysis of TH expression in LV and hypothalamic tissues of CRS and NS mice. TH protein levels were increased by >1.5-fold in the left ventricule of CRS mice but were decreased by roughly the same factor in the hypothalamus (Fig. 4E, F). Together, these results suggest that CRS alters the TH-ir in the PVN and the heart and especially increases sympathetic innervation of (or sympathetic activity on) the heart, which may underlie the changes in cardiac structure and function following CRS.
Figure 4.
TH protein expression in the heart and hypothalamus of CRS and NS mice. A) Immunofluorescence detection of TH (red; blue indicates DAPI) in the heart. Note that the density of TH-positive nerves (arrow in the enlarged framed areas) in the LV free wall is greater in CRS mice compared with NS controls. Scale bars, 500 μm; inset: 20 μm. B) Statistical numbers of LV TH-positive nerve twigs per 1360 × 1024 pixels (n = 10 LV section images/group). C) Statistical length of LV TH-positive twigs counted by ImageJ (n = 50 nerve twigs/group). D) TH-ir in the PVN; note that TH-ir of CRS mice is less than that of NS mice. Scale bars, top: 200 μm. E) Representative electrophoretic bands of Western blot for TH expression in the left ventricle (heart) and hypothalamus of CRS and NS mice. F) Semiquantitative measurements (gray value) of TH expression levels. CRS mice showed higher expression of TH in the left ventricle (heart) but lower in the hypothalamus (including PVN) compared with NS controls. *P < 0.05, **P < 0.01, ***P < 0.001 vs. NS group (n = 3/group).
CRS alters gene-specific, but not global, DNA methylation levels in the heart
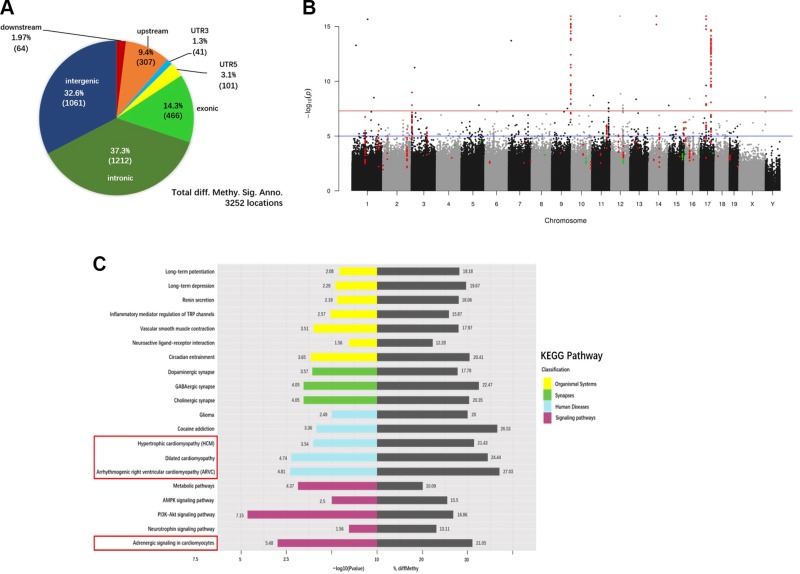
Epigenetic mechanisms, including DNA methylation, have been proposed to underlie pathologic remodeling of the failing heart (28, 29). To determine whether CRS alters DNA methylation patterns in the heart, we used RRBS to analyze the genome-wide methylation profiles of cardiac tissues from CRS and NS mice. Global DNA methylation levels were not altered by CRS (Supplemental Fig. S2). The percentage of methylcytosine events occurring in the contexts of CG, CHH, and CHG (H = C, T, or A) within CGIs and promoter regions were comparable between CRS and NS groups (Supplemental Fig. S2A). The distribution of methylation levels for CG, CHH, and CHG in CGIs (Supplemental Fig. S2B, top), promoters (Supplemental Fig. S2B, bottom), and the whole genome (Supplemental Fig. S2C) was likewise similar between both treatment groups. However, cytosine methylation levels were greater in the regions upstream of the transcription start site in CRS mice compared with NS controls (Supplemental Fig. S2D). Analysis of DMRs in the whole genome DNA identified a total of 3252 DMRs (cutoff of q ≤ 0.05) (Supplemental Table S7), with 37.3% being in intronic regions, 32.6% in intergenic regions, 14.3% in exonic regions, 9.4% in the 2-kb region upstream of the transcription start site, 3.1% in the 5′-UTR, 1.94% in the 2-kb region downstream of the transcription termination site, and 1.3% in the 3′-UTR (Fig. 5A).
Figure 5.
Differential DNA methylation of cardiac genes in CRS mice. A) Pie chart showing the distribution of the 3252 DMRs annotated to specific genomic regions. B) Manhattan plot of P values. Each point represents a CpG site (n = 10,624,115) with the chromosomal position along the x axis and the negative logarithm of the associated P value on the y axis. The blue and red lines represent P values of 1e−05 and 1e−08, respectively. The numbers of CpG sites that showed significantly greater or reduced methylation levels in CRS hearts compared with NS controls were 308 (red) and 35 (green), respectively. C) The top 20 significantly enriched KEGG pathways in CRS hearts, based on the analysis of the 3252 DMRs. Gray bars represent the percentage of differentially methylated genes (% diffMethy) relative to the total number of background genes in different KEGG pathways. Colored bars represent the P values of differentially methylated genes, expressed as −log10 values.
Figure 5B shows the Manhattan plot (30) of DMPs in the whole genome of CRS hearts. We identified 343 DMPs mapping onto 72 stringently filtered DMRs (cutoff of q ≤ 0.001) distributed across 48 genes. Of the 343 DMPs, 308 (89.8%) had elevated, and 35 (10.2%) had reduced, levels of methylation in CRS cardiac DNA samples compared with NS controls. The lists of 72 stringently filtered and 3252 filtered DMRs are provided in Supplemental Table S7.
To identify the biologic processes that may be perturbed by CRS treatment, we subjected the 3252 DMRs to KEGG pathway analysis. Among the top 20 most significantly enriched KEGG pathways were those associated with ASPC, arrhythmogenic right ventricular cardiomyopathy, hypertrophic cardiomyopathy, and DCM (Fig. 5C and Table 1). Collectively, these results are consistent with the cardiac remodeling and arrhythmias observed in CRS mice.
TABLE 1.
Summary of 4 significantly enriched KEGG pathways associated with cardiac physiology and diseases
| Term | KEGG pathway ID | Sample (n) | Background (n) | Corrected P |
|---|---|---|---|---|
| Adrenergic signaling in cardiomyocytes | mmu04261 | 32 | 152 | 3.28E-06 |
| ARVC | mmu05412 | 20 | 74 | 1.54E-05 |
| Dilated cardomyopathy | mmu05414 | 22 | 90 | 1.80E-05 |
| HCM | mmu05410 | 18 | 84 | 2.88E-04 |
ARVC, arrhythmogenic right ventricular cardiomyopathy; HCM, hypertrophic cardiomyopathy.
CRS alters the methylation of DCM-related genes and their respective mRNA levels
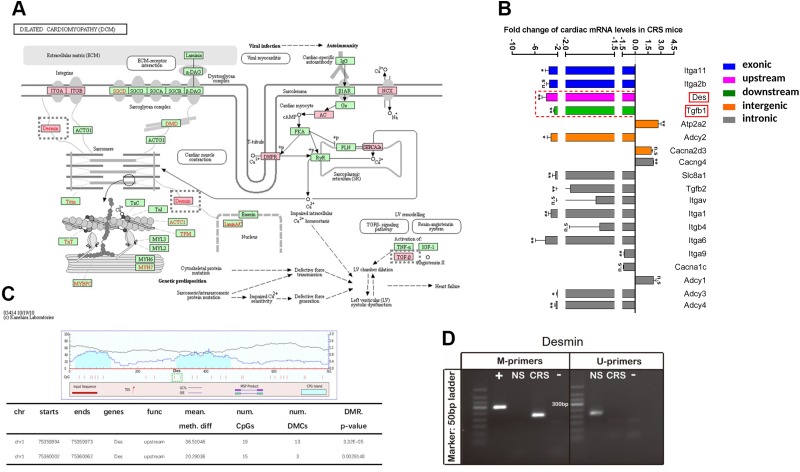
To assess whether the DCM-related genes that were differentially methylated in the hearts of CRS mice were altered in their expression, we used qRT-PCR to quantify transcript abundance (Fig. 6A, B). Thirty-one of the DMRs mapped to 19 DCM-related genes, all of which were examined by qRT-PCR. Eleven of the 19 genes were significantly down-regulated in the hearts of CRS mice, whereas 2 (ATPase, Ca++ transporting, cardiac muscle, slow twitch 2; calcium channel, voltage-dependent, γ-subunit 4) were significantly up-regulated (Fig. 6B). Eight of the 13 differentially expressed genes showed altered methylation within intronic genes (Fig. 6B). The remaining genes were differentially methylated within exonic (integrin-α11), upstream (desmin), downstream (Tgfb1), and intergenic (ATPase, Ca++ transporting, cardiac muscle, slow twitch 2; adenylate cyclase 2) regions (Fig. 6B). We turned our attention to desmin and Tgfb1, because our murine CRS model induced cardiac remodeling and arrhythmias similar to DCM, in which both genes have been implicated.
Figure 6.
Genes belonging to the DCM pathway are differentially methylated and expressed in the hearts of CRS mice. A) Schematic depiction of genes belonging to the DCM pathway. Genes with DMRs in CRS hearts are delineated by red boxes. Genes that are not differentially methylated between CRS and NS hearts are shown in green boxes. B) qRT-PCR analyses of differentially methylated genes associated with the DCM pathway. Expression was determined in cardiac tissues; n = 5/treatment group. C) Genomic locations of the 2 DMRs in the desmin gene as predicted by MethPrimer 2.0 (top). Note that both DMRs are located in CGIs. Locations of the 2 DMRs in the desmin gene as determined by RRBS (bottom). D) MSPs of the desmin gene. M-primers detect the methylated form, whereas U-primers detect the unmethylated form of desmin. +, positive control (Universal Methylated Mouse DNA Standard); −, negative control (water). The expected size of the desmin-specific PCR product is 304 bp. The CRS and NS lanes each represent a pool of 3 cardiac tissue samples.
Desmin encodes a muscle-specific class III intermediate filament that, along with actin and α- and β-tubulin, constitutes the cytoskeleton of cardiac myocytes (31). These filaments are also closely associated with DCM (12). Two of the stringently filtered DMRs (chromosome 1, 75359894–75359973, and chromosome 1, 75360002–75360062) were identified in the upstream region of the desmin gene in cardiac DNA samples from CRS mice (Fig. 6C, lower). The 2 DMRs were 80 and 61 bp in length and were separated by an interval of 28 bp. The software MethPrimer 2.0 (http://www.urogene.org/methprimer2/) predicted that both regions were located in CGIs (Fig. 6C, upper). MSP was used to examine the methylation status of desmin in cardiac DNA from CRS and NS mice (Fig. 6D). Only the methylated form (the product of M-primers) was detected in CRS cardiac samples, whereas only the unmethylated form (the product of U-primers) was detected in NS samples (Fig. 6D). Consequently, the reduction in desmin mRNA levels in CRS mice may be due to the increase in DNA methylation in the upstream region of the gene.
TGF-β1 has been linked to a variety of cardiomyopathies and is an important factor affecting cardiac remodeling (32). The Tgfb1 gene had 2 abnormal methylation locations in its downstream region. Both cardiac Tgfb1 mRNA levels (Fig. 6B, green) and serum TGF-β1 levels (Supplemental Fig. S1) were decreased in CRS mice compared with NS controls. Collectively, these data suggest that methylation of Tgfb1 and desmin may be partly responsible for the cardiac remodeling and arrhythmias observed in CRS mice.
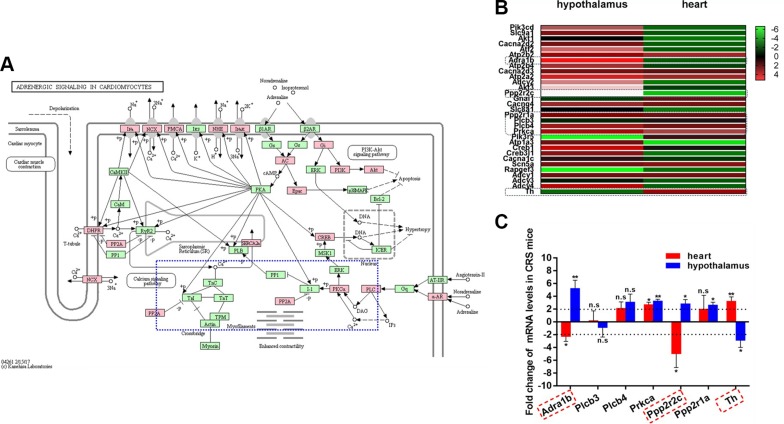
CRS affects DNA methylation of ASPC-related genes
So far, we have shown that CRS increases sympathetic activity on the heart, elevates serum NE levels, and induces cardiac remodeling and arrhythmias. Given the significant role that the sympathetic nervous system plays in cardiac remodeling and arrhythmias (33), we asked whether genes belonging to the ASPC pathway that were differentially methylated in CRS cardiac DNA samples were also altered at the mRNA level. There were 32 ASPC-related genes that were differentially methylated at a total of 56 genomic locations (Fig. 7A). Using qRT-PCR, we found that many of these genes were up-regulated in the hypothalamus but were down-regulated in the heart of CRS mice (Fig. 7B). We compared the expression of Adra1b, which encodes for the α-1B adrenergic receptor, along with genes encoding its downstream signaling effectors (Fig. 7C). Transcript levels of Adra1b and Ppp2r2c were decreased by >2-fold in the CRS heart but were increased in the hypothalamus (Fig. 7C). Prkca was up-regulated in both the heart and hypothalamus of CRS mice, whereas Ppp2r1a was selectively increased in the hypothalamus only (Fig. 7C). Similar to the protein data, Th transcript levels were elevated in the CRS heart but reduced in the hypothalamus (Fig. 7C). Together, these results show that CRS induces changes in the expression of genes that are important for ASPC.
Figure 7.
Genes belonging to the ASPC pathway are differentially methylated and expressed in the hearts of CRS mice. A) Schematic depiction of genes belonging to the ASPC pathway. Genes with DMRs in CRS hearts are delineated by red boxes. Genes that are not differentially methylated between CRS and NS hearts are shown in green boxes. The adrenergic α receptor signaling pathway is enclosed in the blue box (dashed blue lines). B) Heatmap depiction of qRT-PCR results of differentially methylated genes associated with the ASPC pathway. Data are expressed as relative difference in gene expression in the heart and hypothalamus of CRS mice compared with NS controls. C) Histogram of qRT-PCR results of select differentially methylated genes associated with the ASPC pathway. Data are expressed as fold-change in gene expression in CRS mice relative to NS controls. N.s., not significant. *P < 0.05, **P < 0.01 vs. NS group (n = 5/treatment group).
DISCUSSION
It is generally recognized that there is an association between stress and CVD. Chronic psychosocial stress, such as depression or anxiety, can induce or affect the outcome of CVDs. One hundred years ago, William Osler (34) first described a patient with mental disorder showing typical angina pectoris but with normal coronary arteries, suggesting that there may be a correlation between mental illness and coronary dysfunction. Since then, coronary heart disease and subsequent myocardial infarction have been considered to fall within the category of psychosomatic diseases (35–37). Numerous studies further found that anger, hostility to social isolation, anxiety, and depression contributed to the development of CVD and affected the prognostic outcomes (13, 38, 39). The combination of depression and arrhythmia is commonly found in the clinical setting (40, 41): patients with cardiac arrhythmia often also exhibit depression, anxiety, insomnia, tension, fear, or a combination of these. The mortality of patients with heart failure and depression is positively correlated with the severity of depression (42).
The present study focused on understanding the impact of chronic stress on heart function and the potential underlying epigenetic mechanisms that link them using a murine CRS model. CRS has been used to establish depression- or anxiety-like behaviors in rodents (14–17), although in some cases mice habituate to the same stressor. As a result, chronic unpredictable stress or social defeat stress has also been used recently to induce depression or anxiety in rodents (43). We show that mice that experienced CRS at unpredictable times of day exhibited typical neurohumoral responses to stress, including elevations of serum CRH, CORT, and NE. More importantly, our CRS paradigm induced pathologic cardiac remodeling toward heart failure. To our knowledge, ours is the first study to show this striking impact of chronic negative stress on murine hearts. The cardiac remodeling and heart failure were reflected in a variety of histochemical and echocardiographic results, including LV chamber dilatation, wall thinning, and decline of cardiac performance.
CRS mice exhibited multiple cardiac arrhythmic phenotypes, including intermittent sinus bradycardia and sinus tachycardia, sporadic AVB, frequent PVCs, and unsustained VT. Most of the arrhythmias were apparent in the third and fourth wk after the start of CRS, although PVCs emerged as early as the second wk. Our observation that CRS induced both bradyarrhythmias and tachyarrhythmias cannot be explained by elevated sympathetic activity alone. A more plausible explanation may lie in the antagonism between the sympathetic and parasympathetic (vagal in this study) systems in our CRS model. When sympathetic nerve activity dominates, cardiac rhythms exhibit tachyarrhythmias, such as sinus tachycardia, PVCs, and VT. In the case in which vagal nerve activity dominates, cardiac rhythms exhibit bradyarrhythmias, such as sinus bradycardia and AVB. Interestingly, we have observed similar phenomena in a canine model of myocardial infarction and sudden cardiac death (44), in which short periods of VT were elicited when sympathetic hyperactivity occurred (as indicated by shortened P-P intervals), and the VT terminated when vagal nerve activity became dominant (indicated by prolonged P-P intervals). It would appear that maintaining proper balance between the sympathetic and parasympathetic branches of the cardiac nervous system is vital for normal autonomic regulation of cardiac function and that their imbalance is one way by which emotional stress may adversely affect the cardiovascular system.
Our results suggest that our CRS model induces sympathetic hyperactivity and possibly greater sympathetic innervation of the heart, as demonstrated by the elevated expression of TH in the myocardium. In addition to changes in cardiac TH, qRT-PCR results showed that the expression of other genes belonging to the adrenergic signaling pathway was up-regulated in the hearts of CRS mice. Changes in sympathetic innervation and adrenergic signaling may underlie the sympathetic hyperactivity in response to chronic negative stress exhibited by CRS mice. However, CRS not only triggered changes in the heart and peripheral nervous system; it also resulted in remodeling of central sites. The PVN is one of the most important brain centers that regulate sympathetic tone (45), stress response (46, 47), and cardiac function (48). The PVN and hypothalamus of CRS mice exhibited down-regulated expression of TH as well as adrenergic signaling–related genes. Based on these findings, we propose that the cardiac dysfunction and arrhythmias induced by chronic psychosomatic stress may be partly due to differential sympathetic innervation of the myocardium and functional changes to the hypothalamus.
In the present study, we were particularly interested in determining whether or not there may be an epigenetic mechanism underlying chronic stress–induced cardiac remodeling and arrhythmias. Alterations in DNA methylation have been reported in human patients with CVD. For example, patients with end-stage heart failure exhibit hypomethylated DNA in their heart tissues compared with healthy subjects (49–51). In contrast to results from these human studies, we found that CRS did not significantly affect global levels of DNA methylation in murine hearts, but differentially altered methylation at specific sites within the genome. Many of the DMRs mapped to genes that were related to specific signaling pathways involved in cardiac functions and diseases, including the DCM and ASPC pathways. The DCM-related gene, desmin, which is important for mechanical strength of the myocardium and is closely related to myocardial contractibility (52), was methylated at 2 upstream regions in CRS, but not NS, mice. This change in DNA methylation correlated with a down-regulation in desmin mRNA abundance in CRS hearts. Tgfb1, which is associated with LV chamber dilatation and systolic dysfunction, was shown to be methylated in the downstream region in the hearts of CRS mice. As expected, Tgfb1 transcripts in the heart and serum TGF-β1 were reduced in CRS mice compared with NS controls.
The ASPC pathway, which contributes to the responsiveness of the heart to sympathetic neurotransmitters and catecholamines, also showed site-specific alterations in DNA methylation levels, including sites within Adra1b, Ppp2r2c, Ppp2r1a, and Prkca. At the mRNA level, there was significant down-regulation of Adra1b and Ppp2r2c and up-regulation of Ppp2r1a and Prkca in the hearts of CRS mice. Adra1b, Ppp2r2c, Ppp2r1a, and Prkca all participate in α1-adrenoceptor signaling and are involved in the regulation of various cardiomyopathies, including hypertrophic cardiomyopathy and DCM (53, 54).
DNA methylation is highly cell type specific (55, 56). Thus, it is possible that the changes in DNA methylation that we observed are due to a change in cell-type composition in the CRS hearts rather than an alteration in DNA methylation in cardiomyocytes (57). However, in the case of desmin and Adra1b, the change in methylation is likely specific to cardiomyocytes and not caused by recruitment of noncardiomyocyte cells such as fibroblasts and endothelial cells, because desmin is a muscle-specific gene (58) and Adra1b is predominantly expressed in cardiomyocytes (59). Whether altered methylation of desmin or Adra1b, and the consequent change in their expression, is causal of, rather than correlational to, the LV chamber dilatation that we observed in CRS mice is unclear. However, it is interesting to note that loss of desmin has recently been shown to precede heart failure due to chronic volume overload (60). The authors of that study raised the possibility that disruption of desmin may play a causal role in LV dilatation and systolic dysfunction in volume overload. Moreover, a mutation in desmoplakin, a key protein that links desmin to the desmosome, was identified that caused arrhythmogenic LV cardiomyopathy (61), again suggesting the importance of desmin filaments and associated proteins to the development of cardiomyopathies.
In summary, our study demonstrates that CRS induces cardiac abnormalities in mice. CRS-induced cardiac remodeling and arrhythmias may arise from altered DNA methylation at specific genes, particularly those associated with DCM and ASPC, as well as changes in sympathetic tone. Our findings may provide new insights on the psychosomatic aspects of CVDs.
ACKNOWLEDGMENTS
This work was supported by the Sichuan Science and Technology Program (2019YJ0472 to P.Z. and 2019YJ0409 to T.L.), the Luzhou Science and Technology Program (2018LZXNYD-ZK44 to P.Z.), the Collaborative Innovation Center for Prevention and Treatment of Cardiovascular Disease of Sichuan Province, Southwest Medical University (xtcx-2016-11 to P.Z.), a fund for Shanxi “1331 Project” Key Subjects Construction (1331KSC), and a National Natural Science Foundation of China (NSFC) grant (81670313 to J.-M.C.). H.-Y.M.C. was supported by operating grants from the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Councils of Canada (NSERC). The authors declare no conflicts of interest.
Glossary
- Adra1b
adrenergic receptor-α1B
- ASPC
adrenergic signaling of cardiomyocytes
- AVB
atrioventricular conduction block
- CGI
CpG island
- CORT
corticosterone
- CRH
corticotropin-releasing hormone
- CRS
chronic restraint stress
- CVD
cardiovascular disease
- DCM
dilated cardiomyopathy
- DMP
differentially methylated position
- DMR
differentially methylated region
- ECG
electrocardiogram
- FST
forced swimming test
- H-E
hematoxylin-eosin
- ir
immunoreactivity
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- LV
left ventriclular
- MSP
methylation-specific PCR
- NE
norepinephrine
- NS
nonstress
- Ppp2r1a
protein phosphatase 2, regulatory subunit A
- Ppp2r2c
protein phosphatase 2, regulatory subunit B-γ
- PVC
premature ventricular contraction
- PVN
paraventricular nucleus
- qRT-PCR
quantitative RT-PCR
- RRBS
reduced representation bisulfite sequencing
- TH
tyrosine hydroxylase
- VT
ventricular tachycardia
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. Zhang conceived and designed the experiments; J.-M. Cao gave suggestions about the study design; H.-Y. M. Cheng gave suggestions about revising the experiments; P. Zhang, T. Li, Y.-Q. Liu, and S.-M. Xue performed experiments; P. Zhang and T. Li analyzed the data; G. Li performed analysis; P. Zhang, H.-Y. M. Cheng, and J.-M. Cao wrote and revised the manuscript; and all authors reviewed the final manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Nicholson A., Kuper H., Hemingway H. (2006) Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur. Heart J. 27, 2763–2774 [DOI] [PubMed] [Google Scholar]
- 2.Pan A., Sun Q., Okereke O. I., Rexrode K. M., Hu F. B. (2011) Depression and risk of stroke morbidity and mortality: a meta-analysis and systematic review. JAMA 306, 1241–1249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gottlieb S. S., Kop W. J., Ellis S. J., Binkley P., Howlett J., O’Connor C., Blumenthal J. A., Fletcher G., Swank A. M., Cooper L.; HF-ACTION Investigators (2009) Relation of depression to severity of illness in heart failure (from heart failure and a controlled trial investigating outcomes of exercise training [HF-ACTION]). Am. J. Cardiol. 103, 1285–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ruo B., Rumsfeld J. S., Hlatky M. A., Liu H., Browner W. S., Whooley M. A. (2003) Depressive symptoms and health-related quality of life: the Heart and Soul Study. JAMA 290, 215–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nemeroff C. B., Goldschmidt-Clermont P. J. (2012) Heartache and heartbreak--the link between depression and cardiovascular disease. Nat. Rev. Cardiol. 9, 526–539 [DOI] [PubMed] [Google Scholar]
- 6.Sanchez-Gonzalez M. A., May R. W., Koutnik A. P., Kabbaj M., Fincham F. D. (2013) Sympathetic vasomotor tone is associated with depressive symptoms in young females: a potential link between depression and cardiovascular disease. Am. J. Hypertens. 26, 1389–1397 [DOI] [PubMed] [Google Scholar]
- 7.Slavich G. M., Irwin M. R. (2014) From stress to inflammation and major depressive disorder: a social signal transduction theory of depression. Psychol. Bull. 140, 774–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li-Tempel T., Larra M. F., Sandt E., Mériaux S. B., Schote A. B., Schächinger H., Muller C. P., Turner J. D. (2016) The cardiovascular and hypothalamus-pituitary-adrenal axis response to stress is controlled by glucocorticoid receptor sequence variants and promoter methylation. Clin. Epigenetics 8, 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zapata-Martín Del Campo C. M., Martínez-Rosas M., Guarner-Lans V. (2018) Epigenetic programming of synthesis, release, and/or receptor expression of common mediators participating in the risk/resilience for comorbid stress-related disorders and coronary artery disease. Int. J. Mol. Sci. 19, E1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xiao J., Sluijter J. P., Das S., Yang Y., Shen Z. (2015) A snapshot of genetic and epigenetic basis of arrhythmia and heart failure. Front. Genet. 6, 74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakatochi M., Ichihara S., Yamamoto K., Naruse K., Yokota S., Asano H., Matsubara T., Yokota M. (2017) Epigenome-wide association of myocardial infarction with DNA methylation sites at loci related to cardiovascular disease. Clin. Epigenetics 9, 54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koczor C. A., Ludlow I., Hight R. S., II, Jiao Z., Fields E., Ludaway T., Russ R., Torres R. A., Lewis W. (2015) Ecstasy (MDMA) alters cardiac gene expression and DNA methylation: implications for circadian rhythm dysfunction in the heart. Toxicol. Sci. 148, 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Januzzi J. L., Jr., Stern T. A., Pasternak R. C., DeSanctis R. W. (2000) The influence of anxiety and depression on outcomes of patients with coronary artery disease. Arch. Intern. Med. 160, 1913–1921 [DOI] [PubMed] [Google Scholar]
- 14.Christiansen S. H., Olesen M. V., Wörtwein G., Woldbye D. P. (2011) Fluoxetine reverts chronic restraint stress-induced depression-like behaviour and increases neuropeptide Y and galanin expression in mice. Behav. Brain Res. 216, 585–591 [DOI] [PubMed] [Google Scholar]
- 15.Khalid A., Kim B. S., Seo B. A., Lee S. T., Jung K. H., Chu K., Lee S. K., Jeon D. (2016) Gamma oscillation in functional brain networks is involved in the spontaneous remission of depressive behavior induced by chronic restraint stress in mice. BMC Neurosci. 17, 4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naert G., Ixart G., Maurice T., Tapia-Arancibia L., Givalois L. (2011) Brain-derived neurotrophic factor and hypothalamic-pituitary-adrenal axis adaptation processes in a depressive-like state induced by chronic restraint stress. Mol. Cell. Neurosci. 46, 55–66 [DOI] [PubMed] [Google Scholar]
- 17.Zhao X., Seese R. R., Yun K., Peng T., Wang Z. (2013) The role of galanin system in modulating depression, anxiety, and addiction-like behaviors after chronic restraint stress. Neuroscience 246, 82–93 [DOI] [PubMed] [Google Scholar]
- 18.Chiba S., Numakawa T., Ninomiya M., Richards M. C., Wakabayashi C., Kunugi H. (2012) Chronic restraint stress causes anxiety- and depression-like behaviors, downregulates glucocorticoid receptor expression, and attenuates glutamate release induced by brain-derived neurotrophic factor in the prefrontal cortex. Prog. Neuropsychopharmacol. Biol. Psychiatry 39, 112–119 [DOI] [PubMed] [Google Scholar]
- 19.Lapmanee S., Charoenphandhu J., Charoenphandhu N. (2013) Beneficial effects of fluoxetine, reboxetine, venlafaxine, and voluntary running exercise in stressed male rats with anxiety- and depression-like behaviors. Behav. Brain Res. 250, 316–325 [DOI] [PubMed] [Google Scholar]
- 20.Xie Y. F., Jiao Q., Guo S., Wang F. Z., Cao J. M., Zhang Z. G. (2005) Role of parasympathetic overactivity in water immersion stress-induced gastric mucosal lesion in rat. J. Appl. Physiol. (1985) 99, 2416–2422 [DOI] [PubMed] [Google Scholar]
- 21.Porsolt R. D., Le Pichon M., Jalfre M. (1977) Depression: a new animal model sensitive to antidepressant treatments. Nature 266, 730–732 [DOI] [PubMed] [Google Scholar]
- 22.Zhang P., Li G., Li H., Tan X., Cheng H. M. (2017) Environmental perturbation of the circadian clock during pregnancy leads to transgenerational mood disorder-like behaviors in mice. Sci. Rep. 7, 12641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Groenen M. A., Archibald A. L., Uenishi H., Tuggle C. K., Takeuchi Y., Rothschild M. F., Rogel-Gaillard C., Park C., Milan D., Megens H. J., Li S., Larkin D. M., Kim H., Frantz L. A., Caccamo M., Ahn H., Aken B. L., Anselmo A., Anthon C., Auvil L., Badaoui B., Beattie C. W., Bendixen C., Berman D., Blecha F., Blomberg J., Bolund L., Bosse M., Botti S., Bujie Z., Bystrom M., Capitanu B., Carvalho-Silva D., Chardon P., Chen C., Cheng R., Choi S. H., Chow W., Clark R. C., Clee C., Crooijmans R. P., Dawson H. D., Dehais P., De Sapio F., Dibbits B., Drou N., Du Z. Q., Eversole K., Fadista J., Fairley S., Faraut T., Faulkner G. J., Fowler K. E., Fredholm M., Fritz E., Gilbert J. G., Giuffra E., Gorodkin J., Griffin D. K., Harrow J. L., Hayward A., Howe K., Hu Z. L., Humphray S. J., Hunt T., Hornshøj H., Jeon J. T., Jern P., Jones M., Jurka J., Kanamori H., Kapetanovic R., Kim J., Kim J. H., Kim K. W., Kim T. H., Larson G., Lee K., Lee K. T., Leggett R., Lewin H. A., Li Y., Liu W., Loveland J. E., Lu Y., Lunney J. K., Ma J., Madsen O., Mann K., Matthews L., McLaren S., Morozumi T., Murtaugh M. P., Narayan J., Nguyen D. T., Ni P., Oh S. J., Onteru S., Panitz F., Park E. W., Park H. S., Pascal G., Paudel Y., Perez-Enciso M., Ramirez-Gonzalez R., Reecy J. M., Rodriguez-Zas S., Rohrer G. A., Rund L., Sang Y., Schachtschneider K., Schraiber J. G., Schwartz J., Scobie L., Scott C., Searle S., Servin B., Southey B. R., Sperber G., Stadler P., Sweedler J. V., Tafer H., Thomsen B., Wali R., Wang J., Wang J., White S., Xu X., Yerle M., Zhang G., Zhang J., Zhang J., Zhao S., Rogers J., Churcher C., Schook L. B. (2012) Analyses of pig genomes provide insight into porcine demography and evolution. Nature 491, 393–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Krueger F., Andrews S. R. (2011) Bismark: a flexible aligner and methylation caller for Bisulfite-Seq applications. Bioinformatics 27, 1571–1572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu H., Bock C., Mikkelsen T. S., Jäger N., Smith Z. D., Tomazou E., Gnirke A., Lander E. S., Meissner A. (2010) Genome-scale DNA methylation mapping of clinical samples at single-nucleotide resolution. Nat. Methods 7, 133–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akalin A., Kormaksson M., Li S., Garrett-Bakelman F. E., Figueroa M. E., Melnick A., Mason C. E. (2012) methylKit: a comprehensive R package for the analysis of genome-wide DNA methylation profiles. Genome Biol. 13, R87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Burgi K., Cavalleri M. T., Alves A. S., Britto L. R., Antunes V. R., Michelini L. C. (2011) Tyrosine hydroxylase immunoreactivity as indicator of sympathetic activity: simultaneous evaluation in different tissues of hypertensive rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 300, R264–R271 [DOI] [PubMed] [Google Scholar]
- 28.Kim S. Y., Morales C. R., Gillette T. G., Hill J. A. (2016) Epigenetic regulation in heart failure. Curr. Opin. Cardiol. 31, 255–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Vujic A., Robinson E. L., Ito M., Haider S., Ackers-Johnson M., See K., Methner C., Figg N., Brien P., Roderick H. L., Skepper J., A Ferguson-Smith, Foo R. S. (2015) Experimental heart failure modelled by the cardiomyocyte-specific loss of an epigenome modifier, DNMT3B. J. Mol. Cell. Cardiol. 82, 174–183 [DOI] [PubMed] [Google Scholar]
- 30.Gervin K., Andreassen B. K., Hjorthaug H. S., Carlsen K. C. L., Carlsen K. H., Undlien D. E., Lyle R., Munthe-Kaas M. C. (2016) Intra-individual changes in DNA methylation not mediated by cell-type composition are correlated with aging during childhood. Clin. Epigenetics 8, 110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hein S., Kostin S., Heling A., Maeno Y., Schaper J. (2000) The role of the cytoskeleton in heart failure. Cardiovasc. Res. 45, 273–278 [DOI] [PubMed] [Google Scholar]
- 32.Gardner R. T., Ripplinger C. M., Myles R. C., Habecker B. A. (2016) Molecular mechanisms of sympathetic remodeling and arrhythmias. Circ. Arrhythm Electrophysiol. 9, e001359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosenkranz S. (2004) TGF-beta1 and angiotensin networking in cardiac remodeling. Cardiovasc. Res. 63, 423–432 [DOI] [PubMed] [Google Scholar]
- 34.Osler W. (1910) The Lumleian Lectures on angina pectoris. Lancet 175, 973–977 [Google Scholar]
- 35.Bruhn J. G., Chandler B., Wolf S. (1969) A psychological study of survivors and nonsurvivors of myocardial infarction. Psychosom. Med. 31, 8–19 [DOI] [PubMed] [Google Scholar]
- 36.Bruhn J. G., Wolf S. (1970) Studies reporting “low rates” of ischemic heart disease: a critical review. Am. J. Public Health Nations Health 60, 1477–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weiss E., Dolin B., Rollin H. R., Fischer H. K., Bepler C. R. (1957) Emotional factors in coronary occlusion. I. Introduction and general summary. AMA Arch. Intern. Med. 99, 628–641 [DOI] [PubMed] [Google Scholar]
- 38.Powell R. C. (1977) Helen Flanders Dunbar (1902-1959) and a holistic approach to psychosomatic problems. I. The rise and fall of a medical philosophy. Psychiatr. Q. 49, 133–152 [DOI] [PubMed] [Google Scholar]
- 39.Powell R. C. (1978) Helen Flanders Dunbar (1902-1959) and a holistic approach to psychosomatic problems. II. The role of Dunbar’s nonmedical background. Psychiatr. Q. 50, 144–157 [DOI] [PubMed] [Google Scholar]
- 40.Frasure-Smith N., Lespérance F. (2005) Reflections on depression as a cardiac risk factor. Psychosom. Med. 67 (Suppl 1), S19–S25 [DOI] [PubMed] [Google Scholar]
- 41.Musselman D. L., Evans D. L., Nemeroff C. B. (1998) The relationship of depression to cardiovascular disease: epidemiology, biology, and treatment. Arch. Gen. Psychiatry 55, 580–592 [DOI] [PubMed] [Google Scholar]
- 42.Moraska A. R., Chamberlain A. M., Shah N. D., Vickers K. S., Rummans T. A., Dunlay S. M., Spertus J. A., Weston S. A., McNallan S. M., Redfield M. M., Roger V. L. (2013) Depression, healthcare utilization, and death in heart failure: a community study. Circ. Heart Fail 6, 387–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu S., Shi R., Wang J., Wang J. F., Li X. M. (2014) Unpredictable chronic mild stress not chronic restraint stress induces depressive behaviours in mice. Neuroreport 25, 1151–1155 [DOI] [PubMed] [Google Scholar]
- 44.Cao J. M., Chen L. S., KenKnight B. H., Ohara T., Lee M. H., Tsai J., Lai W. W., Karagueuzian H. S., Wolf P. L., Fishbein M. C., Chen P. S. (2000) Nerve sprouting and sudden cardiac death. Circ. Res. 86, 816–821 [DOI] [PubMed] [Google Scholar]
- 45.Pyner S. (2014) The paraventricular nucleus and heart failure. Exp. Physiol. 99, 332–339 [DOI] [PubMed] [Google Scholar]
- 46.Flak J. N., Myers B., Solomon M. B., McKlveen J. M., Krause E. G., Herman J. P. (2014) Role of paraventricular nucleus-projecting norepinephrine/epinephrine neurons in acute and chronic stress. Eur. J. Neurosci. 39, 1903–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Salter E. W., Sunstrum J. K., Matovic S., Inoue W. (2018) Chronic stress dampens excitatory synaptic gain in the paraventricular nucleus of the hypothalamus. J. Physiol. 596, 4157–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendonça M. M., Santana J. S., da Cruz K. R., Ianzer D., Ghedini P. C., Nalivaiko E., Fontes M. A. P., Ferreira R. N., Pedrino G. R., Colugnati D. B., Xavier C. H. (2018) Involvement of GABAergic and adrenergic neurotransmissions on paraventricular nucleus of hypothalamus in the control of cardiac function. Front. Physiol. 9, 670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Movassagh M., Choy M. K., Goddard M., Bennett M. R., Down T. A., Foo R. S. (2010) Differential DNA methylation correlates with differential expression of angiogenic factors in human heart failure. PLoS One 5, e8564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Movassagh M., Choy M. K., Knowles D. A., Cordeddu L., Haider S., Down T., Siggens L., Vujic A., Simeoni I., Penkett C., Goddard M., Lio P., Bennett M. R., Foo R. S. (2011) Distinct epigenomic features in end-stage failing human hearts. Circulation 124, 2411–2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Haider S., Cordeddu L., Robinson E., Movassagh M., Siggens L., Vujic A., Choy M. K., Goddard M., Lio P., Foo R. (2012) The landscape of DNA repeat elements in human heart failure. Genome Biol. 13, R90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Maloyan A., Osinska H., Lammerding J., Lee R. T., Cingolani O. H., Kass D. A., Lorenz J. N., Robbins J. (2009) Biochemical and mechanical dysfunction in a mouse model of desmin-related myopathy. Circ. Res. 104, 1021–1028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cotecchia S., Del Vescovo C. D., Colella M., Caso S., Diviani D. (2015) The alpha1-adrenergic receptors in cardiac hypertrophy: signaling mechanisms and functional implications. Cell. Signal. 27, 1984–1993 [DOI] [PubMed] [Google Scholar]
- 54.Kokoz Y. M., Evdokimovskii E. V., Maltsev A. V., Nenov M. N., Nakipova O. V., Averin A. S., Pimenov O. Y., Teplov I. Y., Berezhnov A. V., Reyes S., Alekseev A. E. (2016) Sarcolemmal α2-adrenoceptors control protective cardiomyocyte-delimited sympathoadrenal response. J. Mol. Cell. Cardiol. 100, 9–20 [DOI] [PubMed] [Google Scholar]
- 55.Chatterjee N., Yang J., Yoon D., Kim S., Joo S. W., Choi J. (2017) Differential crosstalk between global DNA methylation and metabolomics associated with cell type specific stress response by pristine and functionalized MWCNT. Biomaterials 115, 167–180 [DOI] [PubMed] [Google Scholar]
- 56.Xuan Lin Q. X., Sian S., An O., Thieffry D., Jha S., Benoukraf T. (2019) MethMotif: an integrative cell specific database of transcription factor binding motifs coupled with DNA methylation profiles. Nucleic Acids Res. 47, D145–D154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perrino C., Barabási A. L., Condorelli G., Davidson S. M., De Windt L., Dimmeler S., Engel F. B., Hausenloy D. J., Hill J. A., Van Laake L. W., Lecour S., Leor J., Madonna R., Mayr M., Prunier F., Sluijter J. P. G., Schulz R., Thum T., Ytrehus K., Ferdinandy P. (2017) Epigenomic and transcriptomic approaches in the post-genomic era: path to novel targets for diagnosis and therapy of the ischaemic heart? Position Paper of the European Society of Cardiology Working Group on Cellular Biology of the Heart. Cardiovasc. Res. 113, 725–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tsikitis M., Galata Z., Mavroidis M., Psarras S., Capetanaki Y. (2018) Intermediate filaments in cardiomyopathy. Biophys. Rev. 10, 1007–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sato M., Evans B. A., Sandström A. L., Chia L. Y., Mukaida S., Thai B. S., Nguyen A., Lim L., Tan C. Y. R., Baltos J. A., White P. J., May L. T., Hutchinson D. S., Summers R. J., Bengtsson T. (2018) α1A-Adrenoceptors activate mTOR signalling and glucose uptake in cardiomyocytes. Biochem. Pharmacol. 148, 27–40 [DOI] [PubMed] [Google Scholar]
- 60.Guichard J. L., Rogowski M., Agnetti G., Fu L., Powell P., Wei C. C., Collawn J., Dell’Italia L. J. (2017) Desmin loss and mitochondrial damage precede left ventricular systolic failure in volume overload heart failure. Am. J. Physiol. Heart Circ. Physiol. 313, H32–H45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Norman M., Simpson M., Mogensen J., Shaw A., Hughes S., Syrris P., Sen-Chowdhry S., Rowland E., Crosby A., McKenna W. J. (2005) Novel mutation in desmoplakin causes arrhythmogenic left ventricular cardiomyopathy. Circulation 112, 636–642 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.