Abstract
Mesenchymal stem cells (MSCs) are multipotent progenitor cells capable of differentiating into adipocytic, osteogenic, chondrogenic, and myogenic lineages. There is growing evidence that MSCs home into the tumor microenvironment attracted by a variety of signals such as chemokines, growth factors, and cytokines. Tumor-homing stem cells may originate from bone marrow–derived MSCs (BMSCs) or adipose tissue-derived MSCs. Recent scientific data suggest that MSCs in combination with tumor cells can either promote or inhibit tumorigenic behavior. In head and neck squamous cell carcinoma (HNSCC), BMSCs are reported to be enriched with a potential negative role. Here, we evaluated the effect of BMSCs from 4 different donors in combination with 4 HNSCC cell lines in a 3-dimensional multicellular spheroid model. Heterogeneous combinations revealed an up-regulation of gene and protein expression of osteogenic markers runt-related transcription factor 2 (RUNX2) and alkaline phosphatase (ALP) together with a substantial secretion of matrix metalloproteinase 9. Moreover, heterogenous BMSC/tumor spheroids showed increased invasion compared with homogenous spheroids in a Boyden chamber invasion assay. Furthermore, inhibition of ALP resulted in a substantially decreased spreading of heterogeneous spheroids on laminin-rich matrix. In summary, our data suggest a prometastatic effect of BMSCs combined with HNSCC.—Wessely, A., Waltera, A., Reichert, T. E., Stöckl, S., Grässel, S., Bauer, R. J. Induction of ALP and MMP9 activity facilitates invasive behavior in heterogeneous human BMSC and HNSCC 3D-spheroids.
Keywords: mesenchymal stem cells, HNSCC, matrix-metalloproteinase, RUNX2, spheroid culture
Worldwide, head and neck squamous cell carcinoma (HNSCC) is the sixth most common malignancy, with an incidence of 644,000 new cases per year and with a strongly increasing incidence over the last 10 yr (1). Despite improved surgery, chemotherapy, and radiation therapy over the past years, the 5-yr survival rate is still only 50% (2). This is due to the fact that malignant oral keratinocytes show a fast invasion into cervical lymph nodes and spread to distant sites relatively quickly, which makes diagnosis difficult (3). Various publications report bone marrow–derived mesenchymal stem cells (BMSCs) in HNSCC tumor environment and impressively demonstrated the evidence for BMSCs in various other epithelial cancers (4–6). Under physiologic conditions, mesenchymal stem cells (MSCs) are involved in important processes such as wound healing and tissue regeneration. Recently, it has been demonstrated that MSCs can repair injured tissue through paracrine actions and direct differentiation into various mesodermal cell lineages (7, 8). BMSCs are able to differentiate into chondrogenic, osteogenic, and adipogenic lineages depending on their location and external stimuli. Besides fibroblasts and various inflammatory cell types, MSCs are recruited into the tumor microenvironment in response to multiple signals produced by cancer cells (9). Attracted by inflammatory cytokines and growth factors, circulating BMSCs home into the tumor stroma or are incorporated into the tumor mass itself (10). Various studies and clinical trials have reported opposite effects of transplanted MSCs on tumor metabolism. Although some studies show an inhibitory effect on cancer cell growth and metastasis (11, 12), the majority of studies demonstrate that MSCs migrate into tumor sites and exert stimulatory effects leading to tumor growth, metastasis, and transformation into cancer cells (13–16). It has also been observed that MSCs are capable of suppressing the immune response by inhibiting the maturation of dendritic cells and suppressing the function of T-lymphocytes (17). BMSCs were shown to be precursors of the stroma associated with HNSCC. There, they contribute to blood and lymph angiogenesis, modulate the immune system, and generate tumor-associated myofibroblasts (5). Here, we are aiming to understand the mechanisms of how BMSCs promote metastasis in HNSCC. We demonstrate a strong impact of BMSCs on gene and protein expression and tumor cell behavior by combining BMSCs with different HNSCC cell lines into heterogeneous BMSC or tumor spheroids. Heterogeneous spheroids exhibited gene and protein expression of osteogenic markers runt-related transcription factor 2 (RUNX2) and alkaline phosphatase (ALP) together with a strong expression and secretion of matrix metalloproteinase (MMP) 9. In highly metastatic HNSCC cells, RUNX2 was the most activated upstream regulator. Our data suggest a prometastatic and protumorigenic influence of BMSCs in the HNSCC tumor microenvironment. These data are valuable in assessing treatment strategies and help to design and improve therapeutic approaches.
MATERIALS AND METHODS
BMSC
Human BMSCs were supplied by the Department of Orthopedic Surgery (Experimental Orthopedics, Center of Medical Biotechnology, University Hospital Regensburg). Collection of human material was approved by the local ethics committee (Az: 14-101-0189; Ethikkommission, Universität Regensburg) and with patients’ written informed consent. All cell batches were derived from osteoarthritic patients undergoing hip prosthesis implantation. For experiments, we used 4 donors (Table 1). Bone marrow was collected from acetabulum and caput femoris; the cells were isolated by density gradient centrifugation with subsequent expansion of mononuclear cells until passage 3 in StemMACS MSC Expansion Medium (Miltenyi Biotec, Bergisch Gladbach, Germany). All cells were tested for human (h)MSC-associated markers CD73+, CD90+, CD105+, CD19−, and CD34− via flow cytometry.
TABLE 1.
BMSC donors
| Donor no. | Age (sex) | Localization |
|---|---|---|
| 706 |
67 (M) |
Acetabulum |
| 821 |
60 (F) |
Acetabulum |
| 822 |
84 (F) |
Acetabulum |
| 866 | 63 (M) | Caput femoris |
F, female; M, male.
HNSCC cell lines
Cell lines used for generation of multicellular 3-dimensional (3D) spheroids are originally derived from Pittsburgh Cancer Institute (PCI; Pittsburgh, PA, USA). Cells were isolated from oral squamous cell carcinoma (18, 19). Localization and tumor stage are listed in Table 2. All cell lines were tested by the Leibniz Institute German Collection of Microorganisms and Cell Cultures (DSMZ), Berlin, Germany; A1806475-1] via STR-DNA-typing in 2018.
TABLE 2.
OSCC cell lines
| Cell line | Localization | pTNM |
|---|---|---|
| PCI1 |
Larynx |
T2N0M0 |
| PCI9 |
Tongue base |
T4N3M0 |
| PCI13 |
Retromolar triangle |
T4N1M0 |
| PCI68 | Tongue | T4N0M0 |
pTNM, pathological tumor-node-metastasis.
3D cell culture
HNSCC cells and BMSC were cultivated as homogeneous spheroids (only HNSCCs or BMSCs) or heterogeneous spheroids (coculture of HNSCCs and BMSCs in 1 spheroid) in StemMACS MSC Expansion Medium. One spheroid contained 15,000 or 30,000 cells. After culture in 2D, cells were washed with PBS and detached with Accutase (Thermo Fisher Scientific, Waltham, MA, USA). After determination of the cell number, cells were centrifuged (1,200 rpm, 5 min) and the pellet resuspended in hanging drop medium (StemMACS MSC Expansion Medium, 0.16% MycoZap Plus, 0.24% methylcellulose) to a cell concentration of 750,000 cells/ml. For the generation of heterogenous spheroids, equal amounts of BMSCs and HNSCCs were mixed and centrifuged (1,200 rpm, 5 min), and the cell number was adjusted to 750,000 cells/ml. For cultivation via the hanging drop method, cells were pipetted onto the lid of a Petri dish (about 40 drops/Petri dish lid). Depending on the cell number, 20 or 40 µl drops with the cell suspension were pipetted. For humidification, the bottom of the Petri dish was filled with 5 ml PBS, the lid with drops was inverted, and the resulting hanging drops were cultivated for 3 d in 37°C, 5% CO2. During this time, multicellular spheroids were formed without plastic contact.
Gelatin zymography
Gelatin zymography was performed as described in Bedal et al. (20). For the analysis of MMP activity, serum-free cell culture supernatants were used. Therefore, spheroids growing in hanging drops were collected after 2 d of cultivation, washed in 1 ml PBS, and seeded again for additional 24 h (each spheroid in one 20- or 40-µl drop, depending on the cell number) in serum-free medium using a sterile pipette whose tip had been cut off. Protein concentration of the supernatant was determined via Pierce Bicinchoninic Acid Protein Assay (Thermo Fisher Scientific). Two micrograms total protein was loaded per lane for zymography.
Transient small interfering RNA transfection
Two days prior to transfection, 6500 cells/cm2 were seeded. Cells were transfected in a dilution of 22.5 µl Lipofectamine RNAiMax with 375 µl Opti-MEM I Reduced Serum Medium (Thermo Fisher Scientific) and 6.25 µl small interfering RNA (siRNA) (On-TargetPlus Pool siRNA human MMP9; GE Healthcare, Waukesha, WI, USA) or nontargeting siRNA as control (also in 375 µl Opti-MEM Medium), respectively. Opti-MEM Medium and transfection reagent was added to the cells with subsequent cultivation for 24 h at 37°C, 5% CO2. For transfection control, Alexa Fluor Red Fluorescent Control (Thermo Fisher Scientific) was added. A total of 24 to 48 h after transfection, 10 spheroids with 30,000 cells each were seeded in hanging drops. Western blot analysis with protein lysates was performed to test knockdown efficiency, which was about 70–90%.
RNA isolation from multicellular spheroids
After 3 d of culture, spheroids were collected and washed twice with PBS, and 900 µl RNA lysis buffer (Qiagen, Germantown, MD, USA) were added. Cells were homogenized with grinding beads (RNase free Cerdur Ceramic Y-TZP 1.4–1.4 mm) via Precellys 24 Homogenizator (Bertin Technologies, Montigny-le-Bretonneux, France) at 6500 rpm, 3 × 30 s, 30-s pause. RNA was isolated with Qiagen RNeasy Mini Kit (Qiagen) according to the manufacturer’s protocol. RNA was stored at −80°C.
Immunoblot analysis
Lysates were prepared using RIPA buffer (MilliporeSigma, Burlington, MA, USA) containing protease inhibitor (Roche, Basel, Switzerland). The protein concentration of the cell lysates was determined by bicinchoninic acid assay (Pierce Bicinchoninic Acid Protein Assay Kit), and lysates were stored at −20°C. Thirty micrograms total protein per lane were separated by SDS-PAGE using a 10% resolving gel and subsequently transferred onto a PVDF membrane. Blots were blocked in 3% bovine serum albumin in PBS containing 0.1% Tween 20 (all from MilliporeSigma). The primary antibody was RUNX2 (1:500, ab192256; Abcam, Cambridge, MA, USA). The appropriate secondary antibody conjugated with horseradish peroxidase was purchased from Pierce (Rockford, IL, USA). For protein visualization, Roti Lumin kit (Roth, Karlsruhe, Germany) was used. Equal loading was verified with mouse antibody against β-actin (1∶10,000, ab8227; Abcam). All experiments were repeated at least 3 times. Semiquantitative analysis was performed using ImageJ (National Institutes of Health, Bethesda, MD, USA).
TaqMan real-time quantitative PCR
Quantitative PCR
Reverse transcription of 1 µg RNA to cDNA was performed using a Transcriptor High Fidelity cDNA Synthesis Kit (Roche) according to the manufacturer’s protocol. cDNA was amplified using the Brilliant III Ultra-Fast Quantitative PCR Master Mix (Stratagene; Agilent Technologies, Santa Clara, CA, USA) in combination with TaqMan UPL probes (Roche). Real-time PCR primers were obtained from Eurofins (Ebersberg, Germany). 18S mRNA was used for normalization. Primers were as follows: 18S, 5′-GCAATTATTCCCCATGAACG-3′ and 5′-GGGACTTAATCAACGCAAGC-3′, probe 48; MMP9, 5′-TTCGACGATGACGAGTTGTG-3′ and 5′-TCCAAACCGAGTTGGAACC-3′, probe 61; MMP2, 5′-TTGATGACGATGAGCTATGGA-3′ and 5′-ACTTGCAGTACTCCCCATCG-3′, probe 7; and ALP, biominerilization associated (ALPL), 5′-CCTCAAGGGTCAGCTCCAC-3′ and 5′-CCTGGGCATTGGTGTTGTA-3′, probe 64.
ALP activity assay
The ALP activity assay was performed using the QuantiChrom ALP Assay Kit (BioAssay Systems, Hayward, CA, USA) according to the manufacturer’s instruction. The assay is based on hydrolysis of para (p)-nitrophenylphosphate to p-nitrophenol (yellow product, absorption 405 nm) by ALP. The amount of ALP is proportional to p-nitrophenol. Spheroids were cultured for 3 d and washed with PBS. Depending on cell number, 10 spheroids were transferred in 150 and 300 µl of 0.2% Triton X-100/H2O, respectively. The samples were shaken for 20 min and homogenized with ultrasound (20 s, 2 cycles, 50%).
Invasion assay
To examine the invasive capabilities of the spheroids, a Boyden chamber invasion assay was performed with ThinCerts (Greiner Bio-One, Rainbach im Mühlkreis, Austria). The ThinCert compartment contains a membrane with 8-µM pores. Spheroids were cultured for 3 d, washed twice with PBS, and transferred to serum-free DMEM +2 mM Glutamine. The ThinCert was coated with 50 µl growth factor–reduced Matrigel Basement Membrane Matrix (1 mg/ml; Corning, Corning, NY, USA) and hung in 24-well plates. Five spheroids were transferred in 1 ThinCert. The lower 24-well compartment was filled with 500 µl MSC medium. Each condition was performed in triplicates. ThinCerts were incubated for 2 d at 37°C, 5% CO2. Subsequently, the content of the ThinCerts was removed with cotton swabs. Cells or spheroids that invaded to the other side of the membrane were stained via crystal violet assay. For the spreading of spheroids on matrigel, BMSC/tumor cell ratios were varied between 4000 BMSCs and 8000/13,000/18,000/23,000 tumor cells. Levamisole (ab141217; Abcam) was dissolved in water and stored in 100 mM stock solution at −20°C. Area size analysis was performed with ImageJ. Assays were repeated and performed in triplicates.
Statistical analysis
Statistical analysis was performed using Prism 6 (GraphPad Software, La Jolla, CA, USA). Results are presented as means ± sd. Each assay was performed in replicates and repeated in 3 to 6 independent experiments. Comparisons between 2 groups were done using the unpaired Student’s t test. The influence of different categorical independent variables on 1 continuous dependent variable was analyzed using 2-way ANOVA. A value of P ≤ 0.05 was considered statistically significant.
RESULTS
Generation of homo- and heterogenous spheroids with BMSCs and HNSCCs and expression of BMSC differentiation markers
To emulate the interaction of BMSCs with tumor cells in vitro, we generated heterogeneous coculture spheroids with BMSCs and HNSCC. Originally, we were interested in a potential alteration of gene and protein expression of known BMSC differentiation markers after interaction of BMSC and HNSCC cells in 3D in each of the 2 cell types and the mixture thereof. The marker molecules investigated were SOX9, collagen II, collagen IX (chondrogenic), RUNX2, osteocalcin, tissue-nonspecific ALP (osteogenic), CCAAT/enhancer-binding protein-β (CEBPB), adiponectin, and peroxisome proliferator–activated receptor-γ (PPAR-γ) (adipogenic).
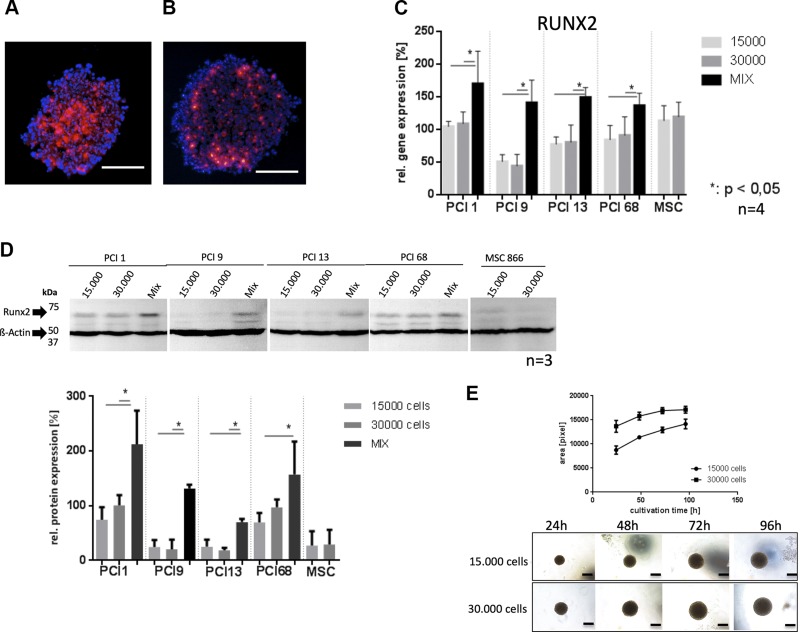
BMSCs from 4 donors were cocultured with 4 HNSCC cell lines (PCI1, PCI9, PCI13, and PCI68). Cryosections of homogeneous BMSC spheroids (30,000 cells) with cells stained with red fluorescent tracker dye exhibited substantial staining intensity in the spheroid core and decreasing intensity at the outer border. As the fluorescence intensity decreases proportionally to cell division, this suggests an increasing cell proliferation toward the spheroid border (Fig. 1A). Interestingly, BMSC/HNSCC coculture spheroids with 15,000 BMSCs (stained red) and 15,000 HNSCCs showed strong fluorescence intensity at the spheroid border and weak staining in the core, suggesting higher proliferation activity of BMSCs in the core of heterogeneous spheroids (Fig. 1B).
Figure 1.
A, B) 15,000 BMSCs were tracked with red fluorescent cell tracker (A) and mixed in equal amount with 15,000 HNSCC cells (B); scale bars, 500 µM. C) TaqMan quantitative PCR of RUNX2 gene expression in homogeneous tumor spheroids with 15,000 cells, 30,000 cells, and heterogeneous mixes with BMSCs of 4 donors (2 × 15,000 cells), respectively. All heterogeneous spheroids showed significantly increased RUNX2 gene expression compared with homogeneous BMSC and tumor spheroids. D) Western blot analysis of RUNX2 expression and semiquantitative evaluation. One representative blot is shown. Western blot analysis confirmed quantitative PCR results. E) Growth kinetics of pure HNSCC spheroids containing 15,000 and 30,000 cells, respectively. The images show an example for PCI 1. Scale bars, 300 µM.
Up-regulation of RUNX2 gene and protein expression in heterogenous BMSC/HNSCC spheroids
3D coculture of BMSCs (4 donors) with HNSCC cells (4 HNSCC cell lines) revealed a significant up-regulation (P < 0.05) of RUNX2 gene expression detected via TaqMan quantitative RT-PCR in heterogeneous BMSC/HNSCC spheroids (depicted as Mix, Fig. 1C) compared with homogenous HNSCC spheroids. Additionally, Western blot analysis showed a significant (P < 0.05) up-regulation of RUNX2 protein in heterogeneous spheroids. Semiquantitative evaluation corroborates these findings (Fig. 1D). Homogenous spheroid growth curves show constant growth with 15,000 and 30,000 cells per spheroid within the time of seeding and harvesting (72h) (Fig. 1E). We did not observe any changes in chondrogenic or adipogenic gene and protein expression after BMSC/HNSCC spheroid coculture (see Supplemental Data).
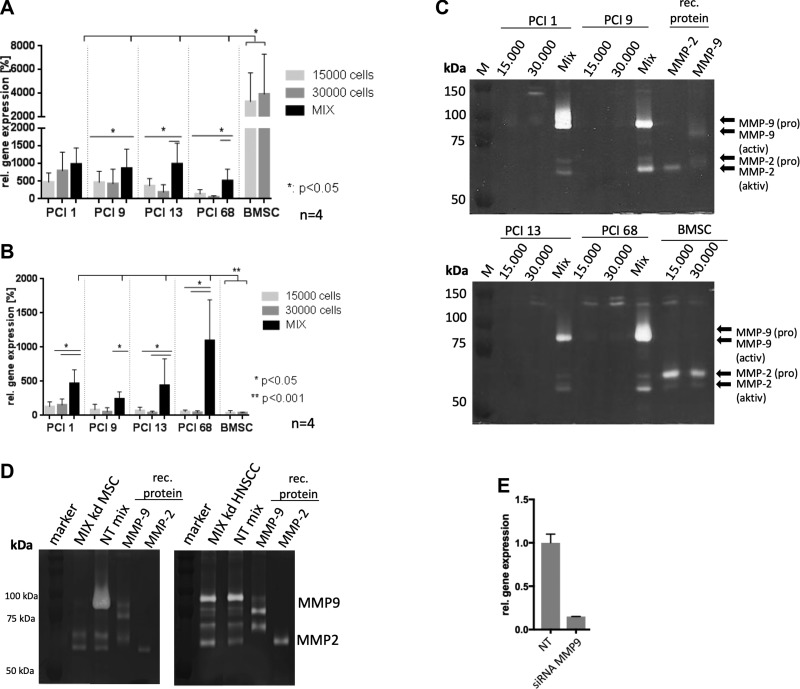
Because MMPs are transcriptional targets of RUNX2 (21, 22), we analyzed gene expression of MMP2 and MMP9 via TaqMan quantitative RT-PCR. Homogeneous BMSC spheroids showed a markedly high MMP2 expression relative to homogenous HNSCC spheroids (P < 0.05) (Fig. 2A). Moreover, 3 out of 4 heterogeneous BMSC/HNSCC spheroids exhibited higher MMP2 gene expression compared with the homogenous HNSCC spheroids and lower expression levels compared with homogenous BMSC spheroids, which most likely results from the mixture of the cells (P < 0.05; Fig. 2A).
Figure 2.
A) TaqMan quantitative PCR of MMP2 gene expression in 4 HNSCC spheroid cell lines with 15,000 cells, 30,000 cells, and BMSC/HNSCC mixes with 15,000 cells each (4 BMSC donors). MSCs revealed a significantly higher basal MMP2 expression compared with HNSCC spheroids. The mixes contained significantly decreased expression levels compared with the BMSC expression and an increased expression compared with individual HNSCC spheroid cultures. B) TaqMan quantitative PCR of MMP9 gene expression. A total of 3 out of 4 mixes showed significantly higher amounts of MMP9 gene expression. Individual BMSC spheroids yielded only basal amounts of MMP9 gene expression. The experiment was performed with 3 BMSC donors in 3D coculture with 4 HNSCC cell lines. C) MMP activity in serum-free supernatants of spheroids with 15,000 and 30,000 HNSCC cells and mixed coculture spheroids of 15,000 HNSCC and BMSCs. Mixed spheroids show markedly increased MMP9 proform as well as MMP2 active and proforms compared with individual HNSCC and BMSC spheroids. M, protein marker. D) Exemplified gelatin zymography of BMSC/HNSCC mixes after knockdown (kd) of MMP9 in BMSCs and HNSCCs via siRNA. Heterogeneous spheroids do not secrete MMP9 in BMSC kd spheroids. However, MMP9 kd in HNSCC cells still results in secretion of MMP9 in heterogeneous spheroids. E) Quantitative RT-PCR experiment depicts a relative MMP9 kd efficiency of about 80% in 3D spheroids of HNSCCs. NT, nontargeting control siRNA.
Up-regulation of MMP9 gene expression and activity in heterogenous BMSC/HNSCC spheroids
MMP9 gene expression was slightly lower in homogenous BMSC spheroids compared with homogenous HNSCC spheroids. However, there was a significant increase of MMP9 gene expression in all heterogeneous spheroid mixes of the 4 HNSCC cell lines and 4 BMSC donors (P < 0.001) (Fig. 2B). This result was verified via gelatin zymography, showing a marked increase of pro-MMP9 secretion in supernatants of heterogeneous BMSC/HNSCC spheroids compared with all homogenous HNSCC and BMSC spheroids (Fig. 2C). Additionally, we observed an increase of the active form of MMP2 in heterogeneous spheroids and almost no MMP2 secretion in homogenous HNSCC spheroids. In accordance with gene expression, homogenous BMSC spheroids secreted increased amounts pro-MMP2 (Fig. 2C).
To analyze if secreted MMP9 observed in heterogeneous spheroids is derived from BMSCs or HNSCC cells, we performed a siRNA knockdown of MMP9 in BMSCs and HNSCC, respectively. After the generation of heterogeneous BMSC/HNSCC spheroids (2 × 15,000), the supernatant was analyzed for gelatinase (MMP9) secretion. The left panel in Fig. 2D shows a representative gelatin zymogram of heterogeneous spheroids with MMP9 knockdown in BMSCs. Mixtures with HNSCC did not secret MMP9. However, MMP9 knockdown in HNSCC cells did exhibit MMP9 secretion in Mix spheroids, indicating that MMP9 secretion in heterogeneous spheroids is derived from BMSCs on contact with HNSCC cells.
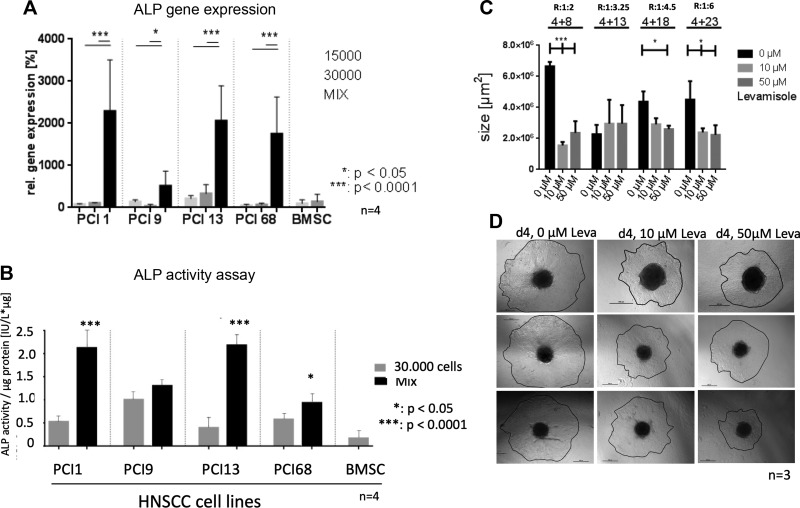
Up-regulation of ALPL gene expression and ALP activity in heterogenous BMSC/HNSCC spheroids
Furthermore, quantitative TaqMan PCR demonstrates a significant up-regulation of ALPL gene expression in heterogeneous BMSC/HNSCC spheroids compared with homogenous HNSCC and BMSC spheroids (Fig. 3A). ALP activity assay verified these results, showing significantly increased ALP activity in 3 out of 4 heterogeneous BMSC/HNSCC spheroids compared with homogenous HNSCC and BMSC spheroids with 30,000 cells (Fig. 3B).
Figure 3.
A) ALPL gene expression via TaqMan quantitative PCR of individual spheroids of 4 HNSCC cell lines (15,000 and 30,000 cells) and mixes of 15,000 BMSCs of 4 donors and 15,000 HNSCCs. All heterogeneous spheroids revealed an up-regulation of ALPL gene expression compared with homogeneous HNSCC and BMSCs spheroids. B) ALP activity assay revealed a significant increase of ALP activity in heterogeneous HNSCC/BMSC spheroids compared with homogeneous HNSCC spheroids. C) ALP inhibitor Levamisole (0, 10, 50 µM) inhibited spreading of heterogeneous tumor spheroids with different BMSC/tumor cell ratios (R) placed on Matrigel (cell number labeling , ×1000; exemplified ratios 1:2, 1:3.25, 1: 4.5, 1:6). D) Exemplified bright field microscopy images of spreading PCI1 tumor spheroid placed on Matrigel after d 4 with different concentrations of Levamisole. Scale bars, 500 µM. *P = 0.05, ***P = 0.001, ****P = 0.0001.
ALP is responsible for spheroid spreading on Matrigel
To analyze a potential functional impact of increased ALP activity on invasive HNSCC tumor cells, PCI cells were placed on Matrigel and treated with the ALP inhibitor Levamisole (0, 10, 50 µM). BMSCs and HNSCC cells were cocultured in different ratios with increasing HNSCC cell numbers of 4000/8000, 4000/13,000, 4000/18,000, and 4000/23,000 cells. The spheroid area was measured on 4 consecutive days. The most conspicuous difference was visible after d 4 (Fig. 3C, D). Inhibition of ALP activity in HNSCC tumor cells led to significantly diminished spreading on Matrigel depending on the BMSC/tumor cell ratio in the spheroids. (Fig. 3C, D).
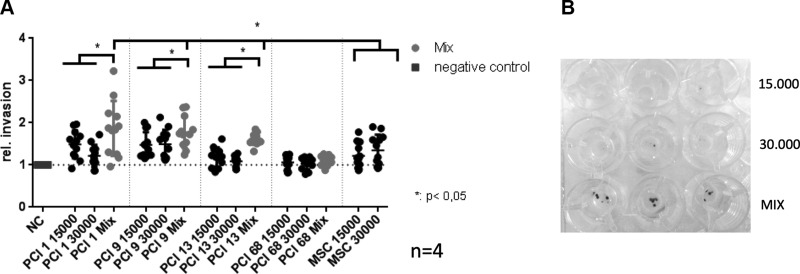
Increased invasion of heterogenous BMSC/HNSCC spheroids
Because tumor-derived ALP and MMP9 activity is highly associated with increased tumorigenic activity such as proliferation, metastasis, and invasion (21, 23), we analyzed the invasive capacity of heterogeneous BMSC/HNSCC spheroids (30,000 cells) compared with homogenous control spheroids (15,000 cells and 30,000 cells/spheroid). In Boyden chamber invasion assays, 3 out of 4 mixed spheroids with 4 BMSC donors each invaded significantly faster within 2 d through Matrigel (Fig. 4).
Figure 4.
A) Boyden chamber invasion assay of homo- and heterogeneous (Mix) HNSCC and BMSC spheroids with 15,000 and 30,000 cells, respectively. A total of 3 out of 4 mixed BMSC/HNSCC spheroids revealed a significant increase in invasion compared with both homogeneous HNSCC and BMSC spheroids. B) Exemplified version of invasion assay depicting the clear increase in invasion of heterogeneous BMSC/HNSCC spheroids (Mix) compared with homogeneous spheroids with 15,000 and 30,000 PCI1 HNSCC cells. Invasion assays with 4 HNSCC cell lines were performed with 4 BMSC donors each in triplicates. The y axis shows the invasion rate compared to the negative control set to 1. NC, negative control. *P < 0.05.
DISCUSSION
Since the first in vitro cultivation by Friedenstein et al. (24) in the 1960s, MSCs have been intensively studied in biomedical research. Because of their multipotency and self-renewal properties (25), this cell population soon became interesting for regenerative medicine and tissue engineering. MSCs are able to migrate into a variety of tissues to promote wound healing and tissue regeneration. Besides this, both local and distant MSCs are also able to invade tumors (25, 26), which brought MSCs into the focus of oncological research. Over the past few years, the functional role of MSCs in the stroma of several tumor types has been investigated, revealing both tumor-promoting and inhibiting properties or effects. Furthermore, irradiation of tumors increases the invasion of local as well as distant MSCs into the tumor stroma in vivo (27). Bearing this in mind and the fact that radiotherapy is still one of the most important therapy options for the treatment of advanced HNSCC, we aimed to elucidate the functional role of BMSCs in HNSCC and how BMSCs affect growth and invasion of HNSCC tumor cells.
To mimic the in vivo situation as closely as possible, we used a 3D coculture approach to study HNSCC cells and BMSCs in a 3D spheroid model and analyzed the gene and protein expression of several markers that are typically involved in chondro-, osteo-, and adipogenic differentiation of MSCs. Most of these markers are not only expressed in the 3 main lineages of mesenchymal differentiation but are also frequently dysregulated in a variety of cancer entities in which they significantly contribute to tumor growth and progression (28–33). We observed a clear up-regulation of the 2 osteogenic markers RUNX2 and ALPL in heterogeneous BMSC/HNSCC spheroids, suggesting that the direct interaction between HNSCC and BMSCs leads to a priming of BMSCs toward the osteogenic lineage.
The transcription factor RUNX2 regulates a variety of genes that are required for osteoblast differentiation and bone formation (34, 35). Besides its physiologic role as master regulator of osteogenic differentiation, several studies confirmed the tumor-promoting role of RUNX2 in different cancer entities, including HNSCC (22, 36–39). Ectopic RUNX2 expression leads to an increased parathyroid hormone–like hormone expression and promotes proliferation of HNSCC cells (40). Moreover, RUNX2 was up-regulated in HNSCC with lymph node metastasis and predicted poor prognosis in patients with HNSCC. RUNX2 expression significantly correlates with perineural invasion and marginally associates with lymph node status. Accordingly, RUNX2 mRNA expression levels appeared to be a poor prognostic marker in HNSCC (41). Besides HNSCC, aberrant RUNX2 expression is also associated with prostate cancer as well as advanced tumor progression and poor clinical outcome in epithelial ovarian cancer (39, 42).
Because RUNX2 was described as a transcriptional regulator of MMP9 and seems to be involved in epithelial-to-mesenchymal transition, invasion, and metastasis in various tumor entities (21, 22, 42, 43), we determined the MMP9 gene expression levels and enzyme activity by quantitative RT-PCR and zymography. MMP9 is a member of the large group of matrix-remodeling MMPs, which are the most prominent family of proteinases associated with tumorigenesis. Increased expression of MMP9 has been associated with the acquisition of an invasive phenotype in many tumors. In tongue squamous cell carcinoma, high MMP9 expression is observed in neoplastic cells at the invasion front (44), and MMP9 serves as a predictor of tumor recurrence when detected in histologically negative surgical margins of oral squamous cell cancer (OSCC) (45). Moreover, MMP9 inhibition mediated by RNA interference or antigelatinolytic peptides strongly reduced the invasive behavior of OSCC and ovarian cancer cells (46–48). We observed in our model system that heterogeneous BMSC/HNSCC spheroids exhibit a remarkably strong MMP9 gene expression and an increased secretion in contrast to both BMSC and HNSCC homogenous spheroids. It is noteworthy that MMP9 does not have to be produced necessarily by the tumor cells themselves to promote their invasive capacity. Van Kilsdonk et al. (49) revealed that MMP9 is activated early during melanoma progression and showed that it is not secreted by melanoma cells themselves but rather by adjacent keratinocytes. Interestingly, we observed a similar phenomenon in our 3D coculture system. Only transfection of MMP9-specific siRNAs and subsequent MMP9 inhibition in BMSCs but not in HNSCC tumor cells was sufficient to block the increased MMP9 secretion in heterogeneous spheroids. These data suggest that HNSCCs hijack and reprogram BMSCs for the production and secretion of MMP9 in order to degrade the extracellular matrix and facilitate the invasion of the tumor cells.
Compared with homogeneous HNSCC spheroids, most of the investigated heterogeneous BMSC/HNSCC spheroids also show increased secretion of MMP2, another gelatinase whose expression can be stimulated by RUNX2 as well (21). Increased activity of MMP9 and MMP2 has been described in higher grade OSCC at the invasion front (50). Interestingly, BMSCs require MMP2 for chemotaxis (51), and Ries et al. (52) demonstrated that MMP2 is essential for the invasive capacity of human MSCs. These findings suggest that MMP2 and MMP9 have a dual function in the tumor microenvironment. Extracellular matrix is degraded to enable invasion of both tumor cells and MSCs; furthermore, BMSCs are attracted and stimulated to increase the secretion of MMP2 and MMP9, which subsequently enhances matrix degradation and attracts more BMSCs, leading to the formation of a self-amplifying, tumor-promoting cycle. However, the increased MMP2 expression that we observed in our 3D model system in heterogeneous BMSC/HNSCC spheroids is most likely attributable to the highly abundant MMP2 expression and secretion of BMSCs because homogenous BMSC spheroids also expressed high levels of MMP2, indicating that this phenomenon is not the result of a direct interaction of tumor cells and BMSCs.
Interestingly, we also observed increased gene expression levels of the RUNX2 target gene ALPL (53) as well as an increased ALP activity in heterogeneous BMSC/HNSCC spheroids. Our findings are in line with data showing that serum ALP levels in patients with OSCC at advanced stages were significantly higher than in early stages. Moreover, the serum ALP level in OSCC patients with bone involvement was significantly higher than without bone involvement (54). ALP has been investigated mainly as a potential biomarker for several cancer entities as [i.e., colorectal carcinoma (55), osteosarcoma (56), and the presence of bone metastases (57)]. Apart from this, ALP also directly contributes to cancer progression. Tumor-derived ALP regulates tumor growth and epithelial plasticity in prostate cancer cells, and a reduction of tumor-derived ALP expression increased cell death and mesenchymal-to-epithelial transition and reduced migration (23). Similar to these findings, we observed a functional impact of the increased ALP activity on the invasiveness of the tumor cells in our 3D in vitro model. To study the functional role of ALP, heterogeneous spheroids with a different ratio of BMSC/HNSCC cells were treated with the ALP-specific inhibitor Levamisole. This treatment significantly decreased the spreading of the cells on a laminin-rich matrix, suggesting that ALP directly affects the migratory behavior of the cells and contributes to migration in HNSCC. The invasive capacity of the heterogenous mixture is drastically reduced in 1/2 BMSC/HNSCC ratio (~70%). However, this effect weakens to 30–40% with the increasing amount of HNSCC cells (see 1/4.5 and 1/6 ratio). These results suggest that ALP is produced by BMSCs, and the impact of Levamisole decreases with higher HNSCC cell numbers. Altogether, these data indicate that ALP and MMP9 might have an additive effect on the migratory and invasive behavior of tumor cells in our model system.
We also analyzed the gene expression levels of 6 chondrogenic (SOX10, collagen II, collagen IX) and adipogenic markers (CEBPB, PPAR-γ, adiponectin) in homogeneous and heterogeneous spheroids but observed no significant differences that implied up- or down-regulation as a result of heterogenous interaction. Adiponectin, collagen II, and collagen type IX α3 (Col9a3) gene expression levels were beyond a Ct of 35, which was considered not detectable (see Supplemental Data). CEBPB, SOX9, and bone γ-carboxyglutamate protein (BGLAP) gene expression levels were principally low (Ct 30–34). PPAR-γ gene expression is relatively high in homogenous HNSCC spheroids compared with homogenous BMSC spheroids with no expression. The mixture of both cell types, showing about 50% of HNSCC PPAR-γ expression, reflects the expression levels of the combined cell types. A very faint protein detection was only possible for PPAR-γ and SOX9. All other examined proteins could not be detected. Because of the low basal gene expression level in HNSCC and BMSC spheroids, generated proteins might be rapidly degraded and below the immunodetection threshold. Our results indicate that the direct interaction of HNSCC and BMSCs exclusively stimulates the expression of osteogenic markers as RUNX2 and its target genes ALPL, MMP2, and MMP9.
In summary, we postulate that the direct interaction of HNSCC and BMSCs promotes tumorigenic properties of BMSCs by up-regulating marker genes of the osteogenic lineage, leading to an increased invasive behavior of the tumor cells. These effects are most probably mediated by an enhanced activity of extracellular MMP9 or ALP, emphasizing the potential negative role of BMSCs being recruited to the head and neck tumor site. Therefore, the interaction of BMSCs and HNSCC cells could negatively influence the metastatic process and thereby accelerate HNSCC progression. Our findings provide new insights into the complex interaction between HNSCC and its tumor stroma and might help to develop novel treatment options to prevent HNSCC disease progression.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Brigitta Hauer (Department of Oral and Maxillofacial Surgery, University Hospital Regensburg, Regensburg, Germany) for excellent technical assistance. This work was supported by a German Research Foundation (DFG) grant to S.G. (GR1301/18-1). The authors declare no conflicts of interest.
Glossary
- 3D
3-dimensional
- ALP
alkaline phosphatase
- ALPL
ALP, biominerization associated
- BMSC
bone marrow–derived mesenchymal stem cell
- CEBPB
CCAAT/enhancer-binding protein-β
- HNSCC
head and neck squamous cell carcinoma
- MMP
matrix metalloproteinase
- MSC
mesenchymal stem cell
- OSCC
oral squamous cell cancer
- PCI
Pittsburgh Cancer Institute
- PPAR-γ
peroxisome proliferator–activated receptor-γ
- RUNX2
runt-related transcription factor 2
- siRNA
small interfering RNA
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Stöckl and R. J. Bauer developed concept and designed the study; R. J. Bauer designed the experiments; A. Wessely and A. Waltera performed experiments; S. Grässel provided BMSCs; and T. E. Reichert discussed data and is responsible for project management.
REFERENCES
- 1.Ferlay J., Shin H. R., Bray F., Forman D., Mathers C., Parkin D. M. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 127, 2893–2917 [DOI] [PubMed] [Google Scholar]
- 2.Argiris A., Karamouzis M. V., Raben D., Ferris R. L. (2008) Head and neck cancer. Lancet 371, 1695–1709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baatenburg de Jong R. J., Hermans J., Molenaar J., Briaire J. J., le Cessie S. (2001) Prediction of survival in patients with head and neck cancer. Head Neck 23, 718–724 [DOI] [PubMed] [Google Scholar]
- 4.Liotta F., Querci V., Mannelli G., Santarlasci V., Maggi L., Capone M., Rossi M. C., Mazzoni A., Cosmi L., Romagnani S., Maggi E., Gallo O., Annunziato F. (2015) Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br. J. Cancer 112, 745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Boeck A., Narine K., De Neve W., Mareel M., Bracke M., De Wever O. (2010) Resident and bone marrow-derived mesenchymal stem cells in head and neck squamous cell carcinoma. Oral Oncol. 46, 336–342 [DOI] [PubMed] [Google Scholar]
- 6.Li H.-C., Stoicov C., Rogers A. B., Houghton J. (2006) Stem cells and cancer: evidence for bone marrow stem cells in epithelial cancers. World J. Gastroenterol. 12, 363–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vodyanik M. A., Yu J., Zhang X., Tian S., Stewart R., Thomson J. A., Slukvin I. I. (2010) A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell 7, 718–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Anthony D. F., Shiels P. G. (2013) Exploiting paracrine mechanisms of tissue regeneration to repair damaged organs. Transplant. Res. 2, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koontongkaew S. (2013) The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J. Cancer 4, 66–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutchinson L., Stenstrom B., Chen D., Piperdi B., Levey S., Lyle S., Wang T. C., Houghton J. (2011) Human Barrett’s adenocarcinoma of the esophagus, associated myofibroblasts, and endothelium can arise from bone marrow-derived cells after allogeneic stem cell transplant. Stem Cells Dev. 20, 11–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang L., Zhang Y., Cheng L., Yue D., Ma J., Zhao D., Hou X., Xiang R., Cheng P. (2016) Mesenchymal stem cells engineered to secrete pigment epithelium-derived factor inhibit tumor metastasis and the formation of malignant ascites in a murine colorectal peritoneal carcinomatosis model. Hum. Gene Ther. 27, 267–277 [DOI] [PubMed] [Google Scholar]
- 12.Cai C., Hou L., Zhang J., Zhao D., Wang Z., Hu H., He J., Guan W., Ma Y. (2017) The inhibitory effect of mesenchymal stem cells with rAd-NK4 on liver cancer. Appl. Biochem. Biotechnol. 183, 444–459; erratum: 185, 357 [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez M. E., Martin E. E., Anwar T., Arellano-Garcia C., Medhora N., Lama A., Chen Y.-C., Tanager K. S., Yoon E., Kidwell K. M., Ge C., Franceschi R. T., Kleer C. G. (2017) Mesenchymal stem cell-induced DDR2 mediates stromal-breast cancer interactions and metastasis growth. Cell Reports 18, 1215–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill B. S., Pelagalli A., Passaro N., Zannetti A. (2017) Tumor-educated mesenchymal stem cells promote pro-metastatic phenotype. Oncotarget 8, 73296–73311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kidd S., Spaeth E., Dembinski J. L., Dietrich M., Watson K., Klopp A., Battula V. L., Weil M., Andreeff M., Marini F. C. (2009) Direct evidence of mesenchymal stem cell tropism for tumor and wounding microenvironments using in vivo bioluminescent imaging. Stem Cells 27, 2614–2623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beckermann B. M., Kallifatidis G., Groth A., Frommhold D., Apel A., Mattern J., Salnikov A. V., Moldenhauer G., Wagner W., Diehlmann A., Saffrich R., Schubert M., Ho A. D., Giese N., Büchler M. W., Friess H., Büchler P., Herr I. (2008) VEGF expression by mesenchymal stem cells contributes to angiogenesis in pancreatic carcinoma. Br. J. Cancer 99, 622–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bassi E. J., Aita C. A. M., Câmara N. O. S. (2011) Immune regulatory properties of multipotent mesenchymal stromal cells: where do we stand? World J. Stem Cells 3, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heo D. S., Snyderman C., Gollin S. M., Pan S., Walker E., Deka R., Barnes E. L., Johnson J. T., Herberman R. B., Whiteside T. L. (1989) Biology, cytogenetics, and sensitivity to immunological effector cells of new head and neck squamous cell carcinoma lines. Cancer Res. 49, 5167–5175 [PubMed] [Google Scholar]
- 19.Lin C. J., Grandis J. R., Carey T. E., Gollin S. M., Whiteside T. L., Koch W. M., Ferris R. L., Lai S. Y. (2007) Head and neck squamous cell carcinoma cell lines: established models and rationale for selection. Head Neck 29, 163–188 [DOI] [PubMed] [Google Scholar]
- 20.Bedal K. B., Grässel S., Oefner P. J., Reinders J., Reichert T. E., Bauer R. (2014) Collagen XVI induces expression of MMP9 via modulation of AP-1 transcription factors and facilitates invasion of oral squamous cell carcinoma. PLoS One 9, e86777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pratap J., Javed A., Languino L. R., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2005) The Runx2 osteogenic transcription factor regulates matrix metalloproteinase 9 in bone metastatic cancer cells and controls cell invasion. Mol. Cell. Biol. 25, 8581–8591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Niu D.-F., Kondo T., Nakazawa T., Oishi N., Kawasaki T., Mochizuki K., Yamane T., Katoh R. (2012) Transcription factor Runx2 is a regulator of epithelial-mesenchymal transition and invasion in thyroid carcinomas. Lab. Invest. 92, 1181–1190 [DOI] [PubMed] [Google Scholar]
- 23.Rao S. R., Snaith A. E., Marino D., Cheng X., Lwin S. T., Orriss I. R., Hamdy F. C., Edwards C. M. (2017) Tumour-derived alkaline phosphatase regulates tumour growth, epithelial plasticity and disease-free survival in metastatic prostate cancer. Br. J. Cancer 116, 227–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Friedenstein A. J., Petrakova K. V., Kurolesova A. I., Frolova G. P. (1968) Heterotopic of bone marrow. Analysis of precursor cells for osteogenic and hematopoietic tissues. Transplantation 6, 230–247 [PubMed] [Google Scholar]
- 25.Cornelissen A. S., Maijenburg M. W., Nolte M. A., Voermans C. (2015) Organ-specific migration of mesenchymal stromal cells: who, when, where and why? Immunol. Lett. 168, 159–169 [DOI] [PubMed] [Google Scholar]
- 26.Stappenbeck T. S., Miyoshi H. (2009) The role of stromal stem cells in tissue regeneration and wound repair. Science 324, 1666–1669 [DOI] [PubMed] [Google Scholar]
- 27.Zielske S. P., Livant D. L., Lawrence T. S. (2009) Radiation increases invasion of gene-modified mesenchymal stem cells into tumors. Int. J. Radiat. Oncol. Biol. Phys. 75, 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Matheu A., Collado M., Wise C., Manterola L., Cekaite L., Tye A. J., Canamero M., Bujanda L., Schedl A., Cheah K. S. E., Skotheim R. I., Lothe R. A., López de Munain A., Briscoe J., Serrano M., Lovell-Badge R. (2012) Oncogenicity of the developmental transcription factor Sox9. Cancer Res. 72, 1301–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H., Leav I., Ibaragi S., Wegner M., Hu G. F., Lu M. L., Balk S. P., Yuan X. (2008) SOX9 is expressed in human fetal prostate epithelium and enhances prostate cancer invasion. Cancer Res. 68, 1625–1630 [DOI] [PubMed] [Google Scholar]
- 30.Albergaria A., Resende C., Nobre A. R., Ribeiro A. S., Sousa B., Machado J. C., Seruca R., Paredes J., Schmitt F. (2013) CCAAT/enhancer binding protein β (C/EBPβ) isoforms as transcriptional regulators of the pro-invasive CDH3/P-cadherin gene in human breast cancer cells. PLoS One 8, e55749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee H.-P., Lin C.-Y., Shih J.-S., Fong Y.-C., Wang S.-W., Li T.-M., Tang C.-H. (2015) Adiponectin promotes VEGF-A-dependent angiogenesis in human chondrosarcoma through PI3K, Akt, mTOR, and HIF-α pathway. Oncotarget 6, 36746–36761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ganapathi M. K., Jones W. D., Sehouli J., Michener C. M., Braicu I. E., Norris E. J., Biscotti C. V., Vaziri S. A. J., Ganapathi R. N. (2016) Expression profile of COL2A1 and the pseudogene SLC6A10P predicts tumor recurrence in high-grade serous ovarian cancer. Int. J. Cancer 138, 679–688 [DOI] [PubMed] [Google Scholar]
- 33.Kawashima A., Ueda Y., Tsuchiya H., Tomita K., Nagai Y., Nakanishi I. (1993) Immunohistochemical localization of collagenous proteins in cartilaginous tumors: characteristic distribution of type IX collagen. J. Cancer Res. Clin. Oncol. 120, 35–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neve A., Corrado A., Cantatore F. P. (2011) Osteoblast physiology in normal and pathological conditions. Cell Tissue Res. 343, 289–302 [DOI] [PubMed] [Google Scholar]
- 35.Javed A., Gutierrez S., Montecino M., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (1999) Multiple Cbfa/AML sites in the rat osteocalcin promoter are required for basal and vitamin D-responsive transcription and contribute to chromatin organization. Mol. Cell. Biol. 19, 7491–7500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kayed H., Jiang X., Keleg S., Jesnowski R., Giese T., Berger M. R., Esposito I., Löhr M., Friess H., Kleeff J. (2007) Regulation and functional role of the Runt-related transcription factor-2 in pancreatic cancer. Br. J. Cancer 97, 1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mundy G. R. (2002) Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer 2, 584–593 [DOI] [PubMed] [Google Scholar]
- 38.Martin J. W., Zielenska M., Stein G. S., van Wijnen A. J., Squire J. A. (2011) The role of RUNX2 in osteosarcoma oncogenesis. Sarcoma 2011, 282745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Li W., Xu S., Lin S., Zhao W. (2012) Overexpression of runt-related transcription factor-2 is associated with advanced tumor progression and poor prognosis in epithelial ovarian cancer. J. Biomed. Biotechnol. 2012, 456534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chang W.-M., Lin Y.-F., Su C.-Y., Peng H.-Y., Chang Y.-C., Hsiao J.-R., Chen C.-L., Chang J.-Y., Shieh Y.-S., Hsiao M., Shiah S.-G. (2017) Parathyroid hormone-like hormone is a poor prognosis marker of head and neck cancer and promotes cell growth via RUNX2 regulation. Sci. Rep. 7, 41131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Aguirre-Gamboa R., Gomez-Rueda H., Martínez-Ledesma E., Martínez-Torteya A., Chacolla-Huaringa R., Rodriguez-Barrientos A., Tamez-Peña J. G., Treviño V. (2013) SurvExpress: an online biomarker validation tool and database for cancer gene expression data using survival analysis. PLoS One 8, e74250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Akech J., Wixted J. J., Bedard K., van der Deen M., Hussain S., Guise T. A., van Wijnen A. J., Stein J. L., Languino L. R., Altieri D. C., Pratap J., Keller E., Stein G. S., Lian J. B. (2010) Runx2 association with progression of prostate cancer in patients: mechanisms mediating bone osteolysis and osteoblastic metastatic lesions. Oncogene 29, 811–821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pratap J., Wixted J. J., Gaur T., Zaidi S. K., Dobson J., Gokul K. D., Hussain S., van Wijnen A. J., Stein J. L., Stein G. S., Lian J. B. (2008) Runx2 transcriptional activation of Indian Hedgehog and a downstream bone metastatic pathway in breast cancer cells. Cancer Res. 68, 7795–7802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Henriques Á. C. G., de Matos F. R., Galvão H. C., Freitas R. de A. (2012) Immunohistochemical expression of MMP-9 and VEGF in squamous cell carcinoma of the tongue. J. Oral Sci. 54, 105–111 [DOI] [PubMed] [Google Scholar]
- 45.Ogbureke K. U. E., Weinberger P. M., Looney S. W., Li L., Fisher L. W. (2012) Expressions of matrix metalloproteinase-9 (MMP-9), dentin sialophosphoprotein (DSPP), and osteopontin (OPN) at histologically negative surgical margins may predict recurrence of oral squamous cell carcinoma. Oncotarget 3, 286–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hu X., Li D., Zhang W., Zhou J., Tang B., Li L. (2012) Matrix metalloproteinase-9 expression correlates with prognosis and involved in ovarian cancer cell invasion. Arch. Gynecol. Obstet. 286, 1537–1543 [DOI] [PubMed] [Google Scholar]
- 47.Iwai S., Amekawa S., Yomogida K., Sumi T., Nakazawa M., Yura Y., Nishimune Y., Nozaki M. (2008) ESE-1 inhibits the invasion of oral squamous cell carcinoma in conjunction with MMP-9 suppression. Oral Dis. 14, 144–149 [DOI] [PubMed] [Google Scholar]
- 48.Heikkilä P., Suojanen J., Pirilä E., Väänänen A., Koivunen E., Sorsa T., Salo T. (2006) Human tongue carcinoma growth is inhibited by selective antigelatinolytic peptides. Int. J. Cancer 118, 2202–2209 [DOI] [PubMed] [Google Scholar]
- 49.Van Kilsdonk J. W. J., Bergers M., Van Kempen L. C. L. T., Schalkwijk J., Swart G. W. M. (2010) Keratinocytes drive melanoma invasion in a reconstructed skin model. Melanoma Res. 20, 372–380 [DOI] [PubMed] [Google Scholar]
- 50.Tamamura R., Nagatsuka H., Siar C. H., Katase N., Naito I., Sado Y., Nagai N. (2013) Comparative analysis of basal lamina type IV collagen α chains, matrix metalloproteinases-2 and -9 expressions in oral dysplasia and invasive carcinoma. Acta Histochem. 115, 113–119 [DOI] [PubMed] [Google Scholar]
- 51.Clutter S. D., Fortney J., Gibson L. F. (2005) MMP-2 is required for bone marrow stromal cell support of pro-B-cell chemotaxis. Exp. Hematol. 33, 1192–1200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ries C., Egea V., Karow M., Kolb H., Jochum M., Neth P. (2007) MMP-2, MT1-MMP, and TIMP-2 are essential for the invasive capacity of human mesenchymal stem cells: differential regulation by inflammatory cytokines. Blood 109, 4055–4063 [DOI] [PubMed] [Google Scholar]
- 53.Weng J.-J., Su Y. (2013) Nuclear matrix-targeting of the osteogenic factor Runx2 is essential for its recognition and activation of the alkaline phosphatase gene. Biochim. Biophys. Acta 1830, 2839–2852 [DOI] [PubMed] [Google Scholar]
- 54.Acharya S., Kale J., Rai P., Anehosur V., Hallikeri K. (2017) Serum alkaline phosphatase in oral squamous cell carcinoma and its association with clinicopathological characteristics. South Asian J. Cancer 6, 125–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wei J. S., Chung N. C., Wei L. L., Tzeng W. F., Liu T. Z., Wang J. Y. (1993) High-molecular-mass alkaline phosphatase as a tumor marker for colorectal cancer: comparison of two test methods. Clin. Chem. 39, 540–543 [PubMed] [Google Scholar]
- 56.Farley J. R., Hall S. L., Herring S., Tarbaux N. M., Matsuyama T., Wergedal J. E. (1991) Skeletal alkaline phosphatase specific activity is an index of the osteoblastic phenotype in subpopulations of the human osteosarcoma cell line SaOS-2. Metabolism 40, 664–671 [DOI] [PubMed] [Google Scholar]
- 57.Oremek G. M., Weis A., Sapoutzis N., Sauer-Eppel H. (2003) Diagnostic value of bone and tumour markers in patients with malignant diseases. Anticancer Res. 23(2A), 987–990 [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.