Abstract
High-fat diet (HFD)-induced obesity is associated with accumulation of inflammatory cells predominantly in visceral adipose depots [visceral adipose tissue (VAT)] rather than in subcutaneous ones [subcutaneous adipose tissue (SAT)]. The cellular and molecular mechanisms responsible for this phenotypic difference remain poorly understood. Controversy also exists on the overall impact that adipose tissue inflammation has on metabolic health in diet-induced obesity. The endothelium of the microcirculation regulates both the transport of lipids and the trafficking of leukocytes into organ tissue. We hypothesized that the VAT and SAT microcirculations respond differently to postprandial processing of dietary fat. We also tested whether inhibition of endothelial postprandial responses to high-fat meals (HFMs) preserves metabolic health in chronic obesity. We demonstrate that administration of a single HFM or ad libitum access to a HFD for 24 h quickly induces a transient P-selectin–dependent inflammatory phenotype in the VAT but not the SAT microcirculation of lean wild-type mice. Studies in P-selectin–deficient mice confirmed a mechanistic role for P-selectin in the initiation of leukocyte trafficking, myeloperoxidase accumulation, and acute reduction in adiponectin mRNA expression by HFMs. Despite reduced VAT inflammation in response to HFMs, P-selectin–deficient mice still developed glucose intolerance and insulin resistance when chronically fed an HFD. Our data uncover a novel nutrient-sensing role of the vascular endothelium that instigates postprandial VAT inflammation. They also demonstrate that inhibition of this transient postprandial inflammatory response fails to correct metabolic dysfunction in diet-induced obesity.—Preston, K. J., Rom, I., Vrakas, C., Landesberg, G., Etwebe, Z., Muraoka, S., Autieri, M., Eguchi, S., Scalia, R. Postprandial activation of leukocyte-endothelium interaction by fatty acids in the visceral adipose tissue microcirculation.
Keywords: free fatty acids, inflammation, insulin resistance, adipocyte
Circulating leukocytes infiltrate the visceral adipose tissue (VAT) of the overweight/obese organism, a process that has been demonstrated to cause inflammatory VAT dysfunction, with subsequent cardiovascular and metabolic complications. Most studies are conducted in obese laboratory animals and humans, in which chronically dysfunctional adipocytes secrete a variety of inflammatory mediators able to sustain immune responses (1). Consequently, leukocyte recruitment in the inflamed adipose tissue of the obese organism is almost unanimously considered a phenomenon instigated by the dysfunctional adipocytes themselves (2). Recent research, however, poses a puzzling challenge to this widely accepted view. In fact, data in the literature demonstrate that high-fat meals (HFMs) and/or high-fat diets (HFDs) cause acute infiltration of leukocytes in the VAT of lean mice before overt weight gain and insulin resistance (3, 4).
Ingestion of HFMs causes alimentary lipemia (i.e., a transient postprandial elevation in triglyceride-rich lipoproteins). Postprandial lipemia is accompanied by transient activation of leukocytes in normal-weight, healthy humans (5) and exacerbation of inflammatory signs in patients with metabolic syndrome (6). Obviously, these transient postprandial inflammatory responses, especially those seen in the lean organism, cannot be explained by the theory of primary adipocyte dysfunction alone. Moreover, it remains unanswered whether these transient inflammatory postprandial responses to lipids affect the homeostasis of adipose depots that later experience chronic infiltration of immune cells.
Relevantly, the vascular endothelium of the microcirculation functions as the physiologic gatekeeper for leukocyte recruitment into target organs (7) and regulates the transport of triglycerides and free fatty acids (FFAs). Postprandial lipemia has been shown to associate with acute endothelial dysfunction (8). Dysfunctional endothelia tether circulating leukocytes by expressing endothelial cell adhesion molecules (eCAMs), which bind leukocytes in a process that activates and guides them to infiltrate the underlying tissue. P-selectin is an eCAM constitutively expressed in endothelial cells, where it is stored inactive in Webel-Palade bodies (9). Inflammatory stimuli quickly up-regulate P-selectin at the endothelial cell surface to initiate tethering of circulating leukocytes (10, 11). Interestingly, in humans, up-regulation of P-selectin occurs even after single intake of high-fat, hypercaloric meals (12, 13). Clinical studies also have detected significantly higher platelet and plasma levels of P-selectin in patients with dyslipidemia and insulin resistance (14–17).
Accordingly, we studied whether: 1) fatty meals initiate postprandial leukocyte trafficking in the adipose tissue of lean mice via up-regulation of P-selectin; 2) P-selectin’s response to HFMs can explain the different inflammatory phenotypes of VAT and subcutaneous adipose tissue (SAT); and 3) deletion of P-selectin prevents metabolic dysfunction in HFD-induced obesity.
MATERIALS AND METHODS
Animal models
All experiments were approved by the Institutional Animal Care and Use Committee of Temple University. Male C57BL/6J, P-selectin–deficient, and myeloperoxidase (MPO)-deficient mice (The Jackson Laboratory, Bar Harbor, ME, USA) with a mean body weight of 25 g were used. Mice were maintained on a 12-h light/dark cycle in the animal facility of Temple University.
Feeding protocols
Three different feeding protocols were used: 1) single HFM; 2) 24-h HFD; and 3) 10- or 16-wk HFD. For single-meal studies, mice were given isocaloric (1.5 kcal), low-fat (10% fat, D12450BL; Research Diets, New Brunswick, NJ, USA), or high-fat (60% fat; D12492L; Research Diets) liquid meals by oral gavage. Thus, the isocaloric low-fat and high-fat liquid meals differed only in fat composition. Mice were then studied at 1, 2, 3, and 4 h after oral gavage to establish a time-course response to the HFM. For 24-h, 10- and 16-wk continued-feeding studies, mice were given ad libitum access to either low-fat (10% fat, D12450B; Research Diets) or high-fat (60%, D12492; Research Diets) chow. Mice were then studied at the end of each feeding protocol. To dissect the contribution of the main saturated and unsaturated fatty acids contained in the HFM adopted in our protocol, we used the monoacid triglycerides tripalmitin and triolein (MilliporeSigma, Burlington, MA, USA). Thus, 2 additional groups of mice were gavaged with either 19 mg per mouse tripalmitin or 34 mg per mouse triolein. These concentrations were based on the amount of palmitate and oleate contained in the HFM.
Fat depots
Three fat depots were studied: 1) the mesenteric VAT because of its amenability to intravital microscopy (IVM) studies (18), its well-established role in the cardiovascular and metabolic complications of obesity (19), and its responsiveness to saturated and unsaturated FFAs (20); 2) the epididymal VAT because it provides a higher yield of tissue for biochemical analyses of proteins than the mesenteric VAT and because it develops an inflammatory phenotype similar to that of the mesenteric VAT in response to HFD (21); and 3) the subcutaneous adipose tissue (SAT) because of its noninflammatory phenotype in obesity (22).
Adipocyte size
Epididymal VAT and SAT were collected from each experimental group following 10 wk of ad libitum diet consumption. Fat pads were fixed in formalin, embedded in paraffin, and sliced in 5-µm sections. Sections were deparaffinized and rehydrated prior to staining and imaging. All images were visualized on an Olympus IX81 inverted microscope using an Olympus SC30 high-resolution camera and were acquired with Oylmpus CellSen Entry 1.11 software (Olympus, Tokyo, Japan). Images were loaded into ImageJ (National Institutes of Health, Bethesda, MD, USA] for analysis. To quantify adipocyte hypertrophy, sections were stained with hematoxylin and eosin. Adipocyte area was measured in 3 sections from each fat depot, and 30–40 adipocytes per section were quantified with ImageJ as previously described by Arnoldussen et al. (23).
Whole-body glucose metabolism
For glucose tolerance test, mice were unfed overnight. Tail tips were clipped, and blood glucose was measured using an Alpha Trak II glucose meter (Zoetis, Parsippany, NJ, USA). After baseline blood glucose was recorded, a bolus dose of dextrose (1 g/kg) was delivered by i.p. injection. Blood glucose was then measured at 15, 30, 45, 60, 90, and 120 min after glucose injection. For insulin tolerance test, mice were unfed for 4 h. After baseline blood glucose was recorded, a bolus dose of insulin (0.7 U/kg) was delivered by intraperitoneal injection. Blood glucose was then measured at 15, 30, 45, 60, 90, and 120 min after insulin injection.
Plasma measurement of FFA and TNF-α
Mouse whole-blood samples were collected in BD vacutainer test tubes (Becton Dickinson, Franklin Lakes, NJ, USA) containing EDTA 120 min after administration of the HFM. Blood samples were centrifuged at 600 g for 5 min to isolate plasma. Plasma samples were flash frozen in liquid nitrogen and stored at −80°C. Plasma FFA levels were then quantified by gas chromatography at the Vanderbilt University Mouse Metabolic Phenotyping Center (Nashville, TN, USA). Serum TNF-α analysis was performed by ELISA at Case Western University Mouse Metabolic Phenotyping Center (Cleveland, OH, USA).
Measurement of TNF-α, hypoxia inducible factor 1-α, and fibrosis
Epididymal VAT and SAT were collected from each experimental group following 10 wk of ad libitum HFD consumption, at which time point infiltration of TNF-α–producing macrophages has been reported (3, 24). Briefly, fat pads were fixed in formalin, embedded in paraffin, and sliced in 5-μm sections. Tissue levels of TNF-α and hypoxia inducible factor 1-α (HIF1-α) were detected using primary antibody against TNF-α (0.75 µg/ml; sc-52746; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and HIF1-α (0.75 µg/ml, NB100-479; Novus, Saint Charles, MO, USA), followed by biotinylated secondary antibodies, according to standard techniques (18). An equal concentration of isotype IgG was used side by side to assess staining specificity. To determine adipose tissue fibrosis, sections were stained in equal parts Weigert’s Iron Hematoxylin A and B (Electron Microscopy Sciences, Hatfield, PA, USA) and incubated with Sirius Red for 30 min at room temperature. Sections were then washed twice in 0.01 N HCI and then dehydrated and penetrated using ethanol and xylene, respectively. All images were visualized on an Olympus IX81 inverted microscope using an Olympus SC30 high-resolution camera and were acquired with Olympus CellSens Entry 1.11 software. Signal density was quantified using the ImageJ program.
Quantification of leukocyte-endothelium interactions in VAT and SAT
We used IVM of postcapillary venules to quantify leukocyte-endothelium interactions (LEIs) in all experimental groups of mice. Following anesthesia by i.p. administration of 80 mg/kg pentobarbital, mice were prepared for IVM studies as previously described by Stalker et al. (25). Mean arterial blood pressure, venular diameter, and wall shear rates were also measured to monitor main hemodynamic parameters in all groups of mice (25). In all adipose tissue preparations, a relatively straight, unbranched segment of postcapillary venule with a length >100 µm and a diameter between 25 and 40 µm was randomly studied in each mouse. Circulating leukocytes were stained with 1 mg/kg rhodamine 6G (MilliporeSigma), preparations were epi-illuminated with an intensified 75-W xenon arc lamp (Nikon, Tokyo, Japan), and fluorescence was assessed with an intensified charge-coupled device camera (QI Imaging; Diagnostic and Interventional Cardiology, Redwood City, CA, USA) using an appropriate dichroic filter. Leukocyte rolling is defined as the number of leukocytes rolling past a fixed point per minute; leukocyte adherence is defined as the number of leukocytes firmly adhered to a 100-µm length of endothelium for at least 30 s.
Endothelial function and vascular reactivity
Vascular reactivity was assessed as previously described by Pruefer et al. (26). Briefly, mice were euthanized by pentobarbatal overdose. Thoracic aorta was quickly removed and placed in ice-cold PBS. The aorta was cleaned from fat and connective tissue and subsequently cut into four 2.5-mm rings. Rings placed in an organ bath filled with Krebs-Henseleit buffer (mM: 118 NaCl, 4.75 KCl, 1.19 KH2PO4, 1.19 Mg2SO4, 2.54 CaCl2, 12.5 NaHCO3, and 10.0 glucose) and oxygenated with 95% O2 and 5% CO2 at 37°C. Stainless-steel wires (40 μm diameter) were threaded through rings and mounted on isometric force transducers connected to a graphic transducer (Radnoti Wire Myograph System; Radnoti, Monrovia, CA, USA). The rings were stretched to a tension of 0.5 g. To test ring viability and quality, KCl (100 mM) was added to the bath. After contractions reached a plateau, rings were washed with Krebs-Henseleit buffer. Rings were then precontracted with a half maximal effective concentration (EC50) norepinephrine, and endothelium-dependent relaxation to acetylcholine was assessed in the presence and absence of 500 µM palmitate.
Fluorescence-activated cell sorting analysis of adipose tissue stromal vascular fraction
Infiltration of white blood cell populations in response to HFMs was monitored in the epididymal VAT and inguinal SAT of selected groups of mice using a standard flow cytometry protocol previously published (18). The epididymal fat was used in these studies because of its abundance and to avoid the potential contamination of blood cells trapped in the denser microcirculation of the mesenteric fat. In parallel experiments, the inguinal SAT was collected. All samples were finely minced and then further digested in a collagenase type 1 solution. The stromal vascular fraction (SVF) was isolated by centrifugation and fluorescently stained with antibodies to quantify CD11B+ [Becton Dickinson, San Diego, CA, USA] innate immune cells, CD4+ lymphocytes (Becton Dickinson), F4/80+ monocytes (Abcam, Cambridge, United Kingdom), and lymphocyte antigen 6 complex locus G6D (Ly6g)+ neutrophils (Becton Dickinson). Samples were loaded into a Becton Dickinson FACScan machine and quantified using Cell Quest Pro software (Becton Dickinson). Data analysis was performed using FlowJo 8.7 (Treestar, Ashland, OR, USA).
MPO abundance and activity
MPO abundance in the SVF of fat depots was measured by Western blot analysis using a pAb against the MPO dimer (Santa Cruz Biotechnology). Densitometry of MPO bands on X-ray films was determined using ImageJ software. MPO activity was measured according to a spectrophotometric assay previously described by Mullane et al. (27). Briefly, SVFs were suspended in a 10% (w/v) hexadecyltrimethylammonium bromide buffer. Samples were homogenized 4 times for 30 s and subjected to 3 freeze-thaw cycles using liquid nitrogen and a 37°C water bath. Homogenized samples were then centrifuged at 21,000 g for 30 min at 4°C. The supernatant, which contains the MPO enzyme, was collected. MPO activity was assayed by mixing 10 µl of sample with 190 µl of ODI solution [8.35 mg o-Dianisidine in 5.00 ml KH2PO4 buffer (50 mM, pH=6)] and 0.5% H2O2. The absorbance at 450 nm was measured every 15 s for 2 min with an ELx808 Spectrophotometer (BioSPX, Drogenbos, Belgium).
Quantification of cell surface expression of P-selectin
Cell surface expression of P-selectin in the endothelium of postcapillary venules was measured in whole-mount sections of VAT and SAT, as well as in cultured human coronary artery endothelial cells (HCAECs). VAT and SAT sections were excised, fixed, mounted onto gelatin-coated slides, and studied by immunofluorescence, as previously reported (18). Briefly, mounted tissue sample sections were then permeabilized with 0.25% saponin, 0.03 M sucrose, and 1% bovine serum albumin in PBS and incubated overnight at 37°C with an mAb that recognizes cell surface–expressed P-selectin (mAb PB1.3) (28). Slides were then incubated with Alexa Fluor 594–conjugated goat anti-mouse IgG1 in permeabilization buffer for 1 h at room temperature. Fluoromicrographs were captured with a Nikon Eclipse FN1 Physiostation Microscope at a ×200 magnification (Nikon). A mean of 3 postcapillary venules were studied in each mouse, and 3–4 mice were studied in each group. Fluorescence intensity along 100 μm of vessel wall was quantified by densitometry using ten 10-μm-diameter circular regions of interest along each side of the vessel. Twenty regions of interest were averaged for a single value of fluorescence intensity in each venule. Final measures were obtained by subtracting background and interstitium from vascular fluorescence values. Mean fluorescence intensity profiles (arbitrary units) were obtained by using ImageJ software.
HCAECs were serum starved for 24 h prior to treatment. Following stimulation with 1 μM histamine (positive control) or 600 μM palmitic acid for 1 h, cells were washed with PBS, fixed with paraformaldehyde for 15 min, and blocked with 5% goat serum for 30 min. Cells were then incubated at room temperature for 1 h with either 2.5 μg/ml rabbit monoclonal P-selectin antibody (3H20L10, lot number: RL239726; Thermo Fisher Scientific, Waltham, MA, USA) or control rabbit IgG (Peprotech, Rocky Hill, NJ, USA). An Alexa Fluor 488–conjugated goat anti-rabbit IgG was then used as secondary antibody (A-11070; Molecular Probes, Eugene, OR, USA) along with DAPI staining (26698W; Molecular Probes). Images were captured with an Olympus THX-100 microscope and photographed at ×40 magnification. Five random images were taken per treatment group at prefixed, consistent exposure times.
HFMs and adiponectin mRNA abundance
VAT and SAT samples were harvested and processed for RNA extraction according to previous methods by Shore et al. (29). Reverse transcription was performed, and the reverse transcription reactions were stored at −80°C. Quantitative real-time RT-PCR was performed with an iCycler iQ Real-Time Detection System and iQ Sybr Green Supermix (Bio-Rad, Hercules, CA, USA) in accordance with the manufacturer’s instructions. Primer sets and product sizes for adiponectin were forward 5′-TGTTGGAATGACAGGAGCTGAA-3′ and reverse 5′-CACACTGAACGCTGAGCGATAC-3′ (106 bp). Changes in VAT and SAT mRNA transcript copy number were assessed relative to changes in 18S rRNA transcript copy number.
Adiponectin production
The effect of MPO on adiponectin production was evaluated in 3T3-L1 cells derived from the mouse embryonic fibroblast cell line. 3T3-L1 cells were cultured in DMEM (MilliporeSigma) supplemented with 10% bovine serum (Thermo Fisher Scientific) and were differentiated as previously described by Student et al. (30). The cells were checked for the degree of differentiation microscopically. Well-differentiated (>90%) 3T3-L1 adipocytes were exposed to 50 nM MPO for 24 h. The culture medium was collected. The adiponectin concentration in each medium sample was measured using a mouse adiponectin ELISA kit (Abcam). The total quantity of production during 24 h was expressed as ng/ml per 24 hours.
A disintegrin and metalloproteinase 17/TNF-α converting enzyme activity assay
Differentiated 3T3-L1 cells were infected with adenovirus reporter construct encoding proTNF-α fused with alkaline phosphatase (ALP) (31), which generates secreted ALP activity in the medium upon a disintegrin and metalloproteinase 17 (ADAM17)-mediated cleavage. Medium ALP activity was measured as we previously reported (32).
Statistical analysis
All values are presented as means ± sem of n independent experiments. Data were compared by ANOVA using post hoc analysis with Fisher’s correct t test and/or by Student’s t test. Values of P ≤ 0.05 were considered statistically significant.
RESULTS
Elevation of plasma FFAs by HFM
To test whether the HFM composition used in this study resulted in significant postprandial lipemia, we measured total plasma FFAs. Two hours after administration of a liquid fat meal to lean wild-type (WT) mice, plasma FFAs increased from 138 ± 28 to 297 ± 36 µg/ml (P < 0.01; n = 6). Analyses of the most-common saturated and unsaturated FFAs found in dietary fat was also undertaken at the 2-h time point. Results revealed that palmitic and oleic acid reached the highest plasma levels, increasing from 37 ± 1 to 90 ± 4 µg/ml (n = 6; P < 0.01) and from 27 ± 2 to 72 ± 6 µg/ml (n = 6; P < 0.01), respectively. Taken together, these data demonstrate that postprandial lipemia with elevation in both saturated and unsaturated FFAs occurs in lean mice given an HFM.
HFMs acutely elevate LEIs in the VAT microcirculation
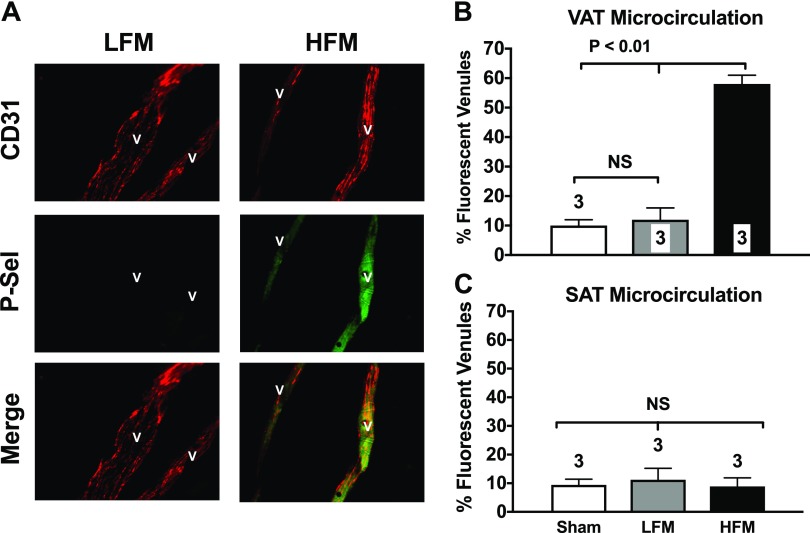
FFAs have been shown to acutely induce a proinflammatory, adhesive phenotype in cultured endothelial cells (33), whose consequences to adipose tissue microcirculation in vivo remains unknown. We measured endothelial cell surface expression of P-selectin. Different than other eCAMs that require de novo synthesis, P-selectin is constitutively expressed in endothelial cells, where it translocates to the luminal surface of the endothelium to initiate leukocyte trafficking within 15–20 min (34). Visceral mesenteric fat and subcutaneous fat samples were removed from WT mice 120 min after administration of an HFM. Data in Fig. 1 demonstrate that a single HFM causes a 6-fold increase in P-selectin cell surface expression in the postcapillary venules of the VAT microcirculation (Fig. 1A, B). In contrast, HFM failed to up-regulate P-selectin expression in the microcirculation of the SAT (Fig. 1C).
Figure 1.
A single HFM up-regulates P-selectin in the microcirculation of the mesenteric VAT. A) Photomicrographs show typical fluorescent staining for P-selectin (green staining; middle panel of right column) observed in the mesenteric VAT microcirculation of mice given a single HFM. Endothelial P-selectin was detected using a mAb that selectively recognizes surface-expressed P-selectin (green). Constitutively expressed platelet and endothelial cell adhesion molecule 1(PECAM-1) was also cross-linked (red stain) to confirm the vascular nature of the stained structures and to normalize expression levels of P-selectin. B, C) Specific secondary antibodies conjugated with fluorescein (P-selectin) and rhodamine (PECAM-1) were used. HFM acutely up-regulates P-selectin in the mesenteric VAT microcirculation (B) but not in the subcutaneous fat (SAT) microcirculation (C). Studies were performed 120 min after gavage administration of a liquid HFM to control C57BL/6J mice. Bar graphs show quantification of P-selectin staining in all groups of mice. Values are means ± se. Numbers at the bottom of bars indicate the number of mice studied in each group. Forty venules were counted in each mouse. NS, not significant. All images were obtained at ×200 magnification.
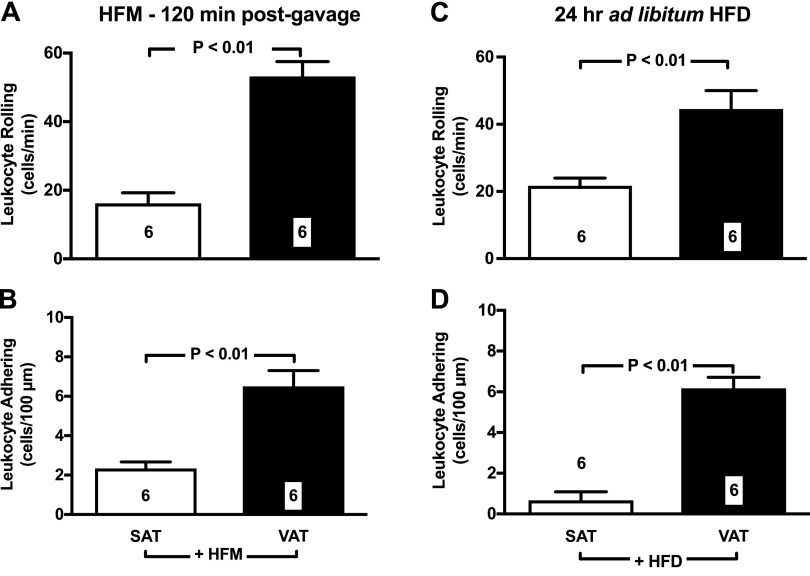
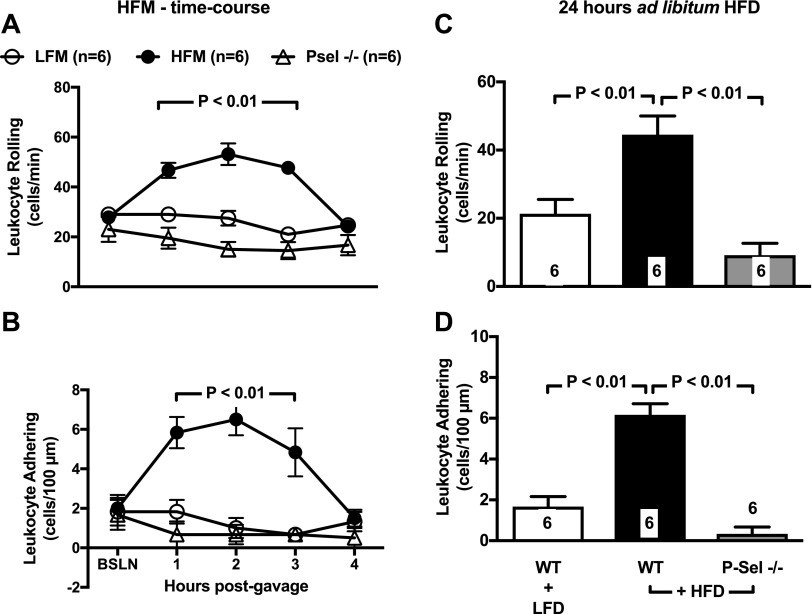
Accordingly, IVM studies demonstrated that HFMs acutely up-regulate leukocyte rolling (Fig. 2A) and adhesion (Fig. 2B) in the microcirculation of the VAT. Specifically, we found evidence of a 2-fold and 3-fold increase in leukocyte rolling and adhesion respectively (P < 0.001 vs. control), 120 min after administration of a single HFM. Detailed time-course studies demonstrated that this acute response to HFM is of transient duration, with LEIs returning to baseline values at 4-h postmeal (Fig. 3A, B). To further mimic obesogenic dietary habits of humans, we next studied LEIs in mice given 24-h ad libitum access to HFD. Data in Fig. 2C, D demonstrate that 24-h HFD caused a sustained increase in leukocyte rolling and adhesion values. Interestingly, no significant elevation in leukocyte rolling and adhesion was observed in the subcutaneous inguinal fat in response to both single HFM and 24-h ad libitum feeding protocols (Fig. 2).
Figure 2.
Dietary lipids increase leukocyte-endothelium in the microcirculation of the VAT. A, B) A single HFM increases rolling (A) and adhering (B) leukocytes in the microcirculation of the mesenteric fat. C, D) Ad libitum access to HFD for 24 h also caused sustained increase in leukocyte rolling (C) and adhesion (D). In contrast, both single HFM and 24-h HFD failed to induce leukocyte rolling and leukocyte adhesion in the SAT (A–D). Data are means ± sem. Numbers at the bottom of bars indicate the number of mice studied in each group.
Figure 3.
Genetic deletion of P-selectin abolishes LEIs induced by HFM. With administration of single HFMs (A, B) and 24-h HFD (C, D), WT mice experience a time-dependent increase in leukocyte rolling and adhesion in the VAT microcirculation of the mesentery. P-selectin–deficient mice are protected from inflammatory activation of the VAT microcirculation. Time-course studies also demonstrate that the inflammatory response to a single HFM peaks at 2 h and subsides at 4 h. Data are means ± sem. Numbers at the bottom of bars indicate the number of mice studied in each group. BSLN, baseline; LFD, low-fat diet; LFM, low-fat meal; Psel, P-selectin.
No significant changes in mean arterial blood pressure or other relevant rheological parameters were detected in all experimental groups of mice. Venular shear rates averaged between 2553 ± 191 s−1 and 2758 ± 135 s−1 among all experimental groups of mice (P > 0.05).
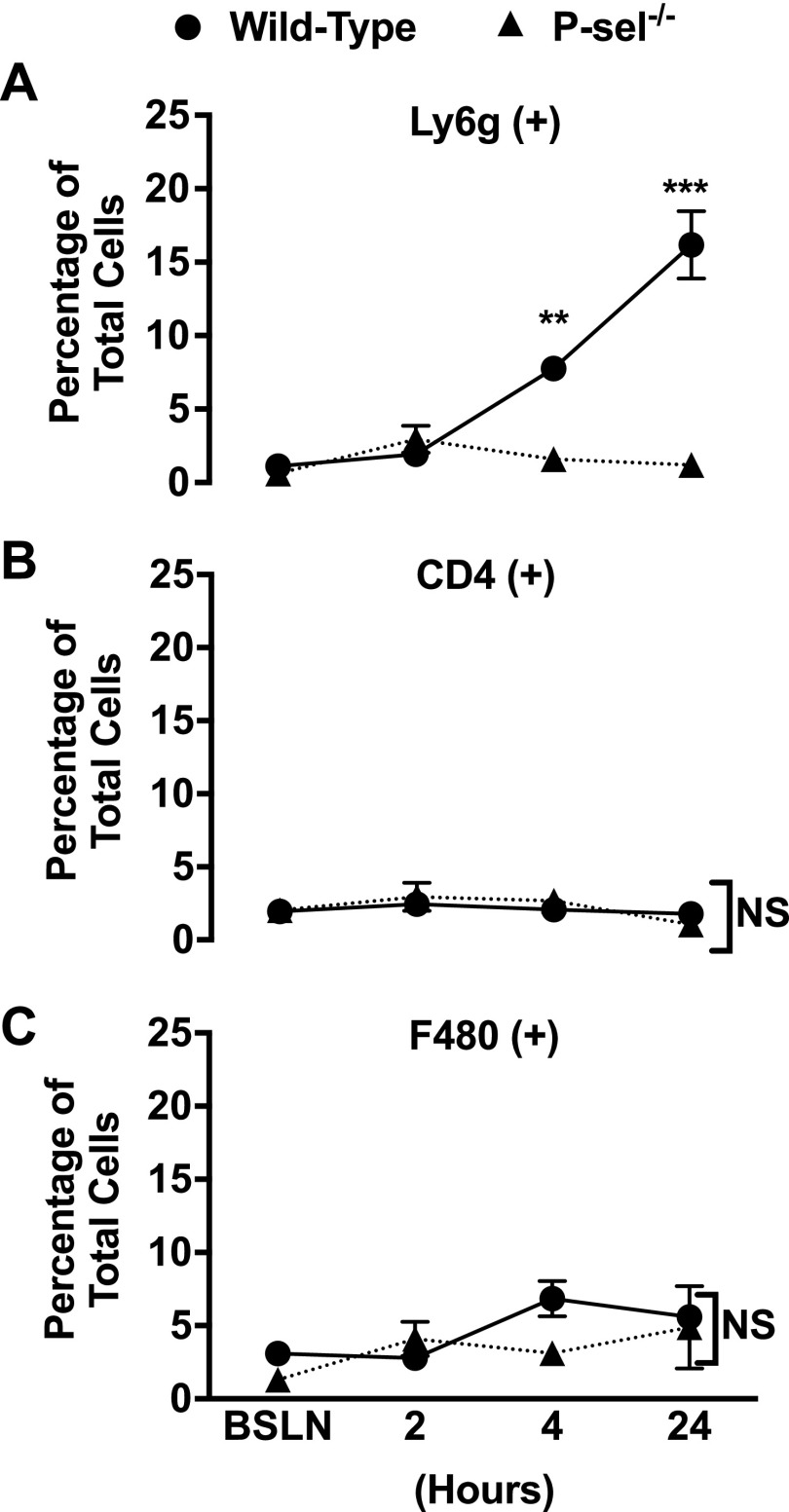
To determine whether circulating immune cells infiltrate adipose tissue in response to fat overload, epididymal visceral fat was collected from WT mice following either a single meal or 24-h ad libitum feeding. Flow cytometry analysis of white blood cell populations revealed that innate immune cells, especially neutrophils, quickly infiltrate the epididymal VAT following consumption of HFMs (Fig. 4).
Figure 4.
Neutrophils accumulate in the VAT following acute fat overload. Epididymal VAT samples were collected before high-fat feeding [baseline (BSLN)] and at 2 and 4 h following a single HFM, as well as after 24 h of ad libitum HFD feeding. SVFs were isolated and stained with antibodies for neutrophils [Ly6G (A)] lymphocytes [CD4 (B)] and monocytes [F480 (C)]. Neutrophils were found to be the main leukocyte subpopulation to postprandially infiltrate VAT following consumption of HFMs. Values are means ± sem from 3 mice in each experimental group. Three samples were obtained from each mouse. NS, not significant; P-sel, P-selectin.
To prove the mechanistic role of P-selectin in these studies, we investigated the effect of single HFM and 24-h HFD in the VAT microcirculation of lean P-selectin–deficient mice. Data shown in Fig. 3 clearly demonstrate that genetic deletion of P-selectin prevents leukocyte rolling and adhesion. Moreover, deletion of P-selectin prevented neutrophil infiltration (Fig. 4A).
Fatty acids composition
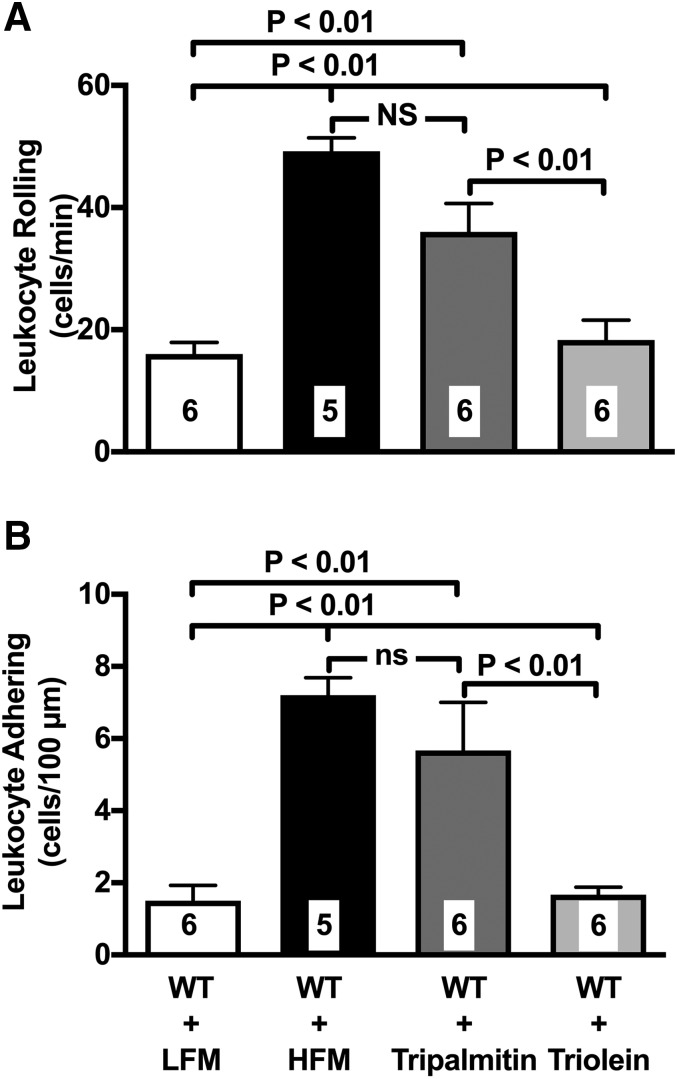
To differentiate the in vivo response to the saturated and unsaturated FFAs found elevated in the plasma of mice given the HFM, we used the monoacid triglycerides tripalmitin and triolein, which, once metabolized, only release palmitic and oleic acid, respectively. Mice were gavaged with a slurry of either tripalmitin or triolein, and LEIs in the VAT microcirculation were studied after 120 min. Data in Fig. 5 clearly demonstrate that tripalmitin but not triolein significantly up-regulates leukocyte rolling and leukocyte adherence in the VAT microcirculation. Additional studies demonstrated that palmitate causes acute endothelial dysfunction. Specifically, palmitate suppresses endothelial vasodilatory responses in isolated aortic rings (Supplemental Fig. S1). Furthermore, the mesenteries of unfed, lean WT mice experienced LEI following 60 min of local superfusion with palmitate (Supplemental Fig. S1).
Figure 5.
Palmitate-enriched triglycerides up-regulate LEIs in the VAT microcirculation. Gavage of a slurry of the modified triglycerides, tripalmitin and triolein, was used to isolate the effect of the relevant saturated FA palmitate and unsaturated FA oleate. Tripalmitin but not triolein up-regulates leukocyte rolling (A) and adhesion (B) in the VAT mesenteric postcapillary venules 90 min after gavage. Of note, the effect of triolein was comparable to that of the HFM used in this study. Values are means ± se. Numbers at the bottom of bars indicate the number of mice studied in each group. LFM, low-fat meal; ns, not sifnificant.
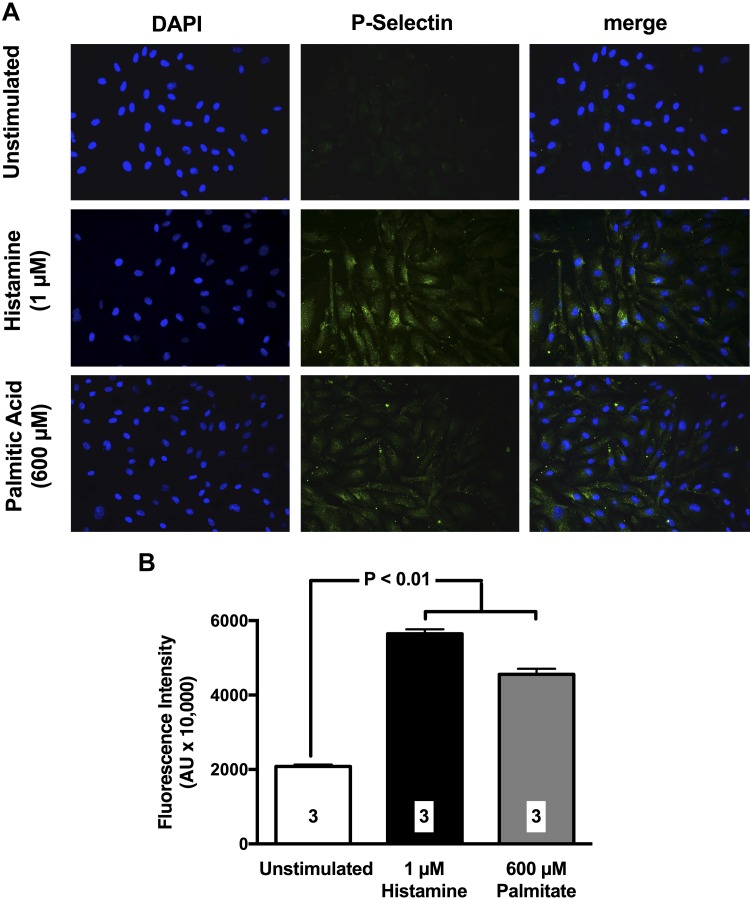
In parallel studies, we also investigated the effect of palmitate on P-selectin cell surface expression in cultured HCAECs. Data in Fig. 6 demonstrate that exposure of HCAECs to 600 µM palmitate for 60 min significantly up-regulates cell surface expression of P-selectin with values comparable to those observed with histamine, a well-established inflammatory activator of P-selectin (35). Thus, the saturated fatty acid (FA) palmitate quickly induces endothelial dysfunction, up-regulates P-selectin in cultured endothelial cells, and initiates a degree of leukocyte trafficking in the VAT microcirculation of live mice that is comparable in intensity to that induced by HFM.
Figure 6.
Palmitate causes cell surface expression of P-selectin in HCAECs. A) Representative photomicrographs illustrating the effect of palmitate on P-selectin cell surface expression are shown. Green fluorescence emission indicates positive staining. Histamine was used as a positive control because of its established ability of up-regulating P-selectin. B) Bar graphs show densitometry quantification of P-selectin staining in HCAECs. Values are means ± se. Three independent experiments were performed for each quantification (indicated by numbers at the bottom of bars). Images were obtained at ×40 magnification.
Lipid overload increases the accumulation and activity of the neutrophilic, oxidizing enzyme MPO
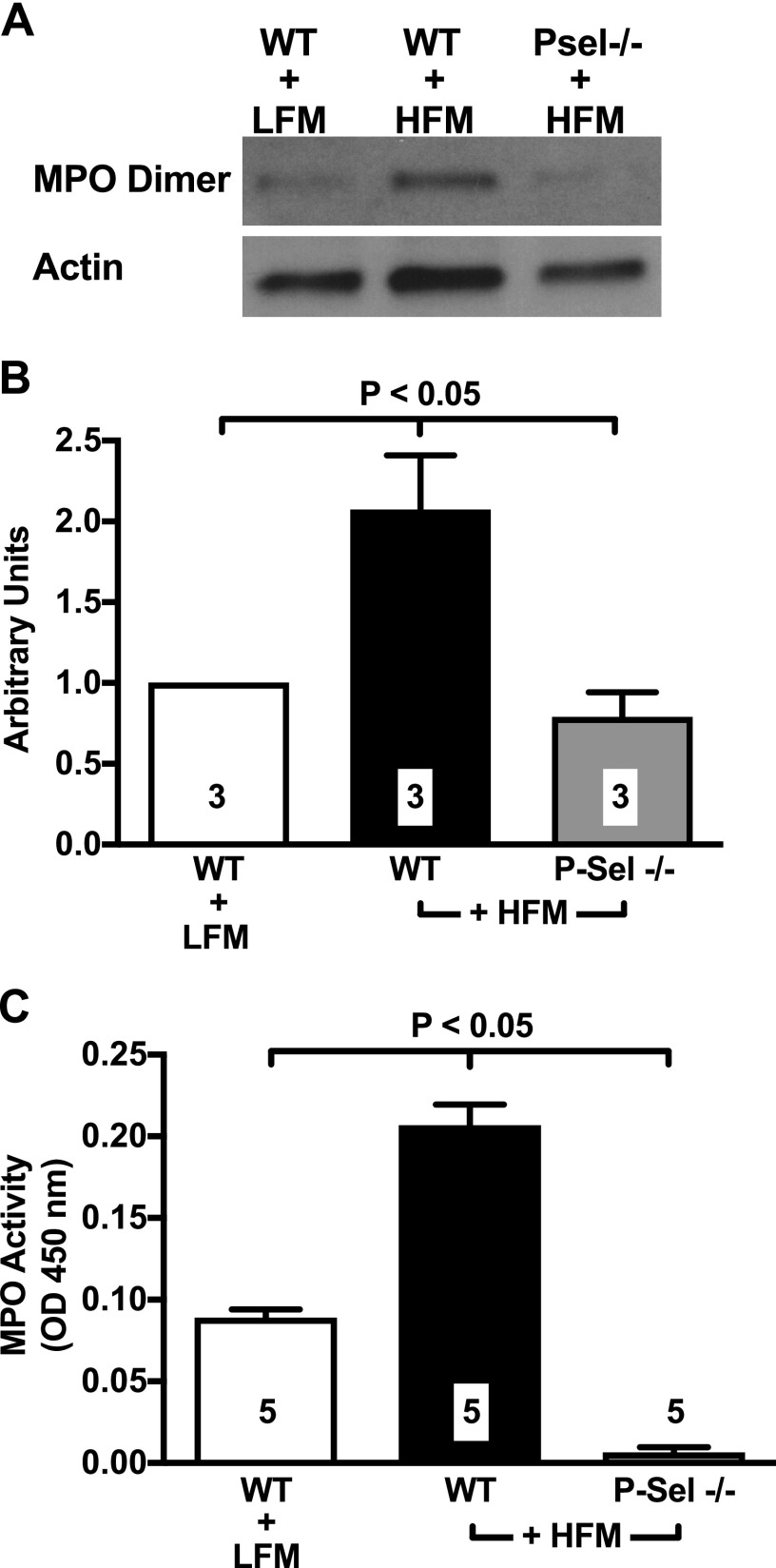
Neutrophils rolling on P-selectin become primed for degranulation (36). Recently, neutrophil-derived enzymes have been shown to induce insulin resistance and glucose intolerance in HFD-fed mice (37). In the present study, we focused on MPO, a neutrophilic oxidizing enzyme largely implicated in inflammation (38) and, more recently, in diet-induced insulin resistance of mice and humans (39, 40). Western blot analysis demonstrated that MPO abundance acutely and significantly increases in the VAT of WT mice given an HFM (Fig. 7A, B). We also measured the enzymatic activity of adipose tissue–bound MPO, which was found to be significantly increased 120 min after administration of the HFM (Fig. 7C; P < 0.01 vs. control). Importantly, P-selectin deletion prevented MPO accumulation and activity (Fig. 7). These data are consistent with the IVM results reported above, and they demonstrate that deletion of P-selectin prevents postprandial accumulation of neutrophil-derived MPO in VAT.
Figure 7.
HFMs cause accumulation and increased activity of MPO in VAT. A) Western blot analyses shows that, following ingestion of an HFM, MPO content increases in the epididymal VAT of WT mice, but not in that of P-selectin–deficient mice. B) Densitometry quantification of Western blot analysis confirmed that the HFM causes a 2-fold increase in MPO accumulation in the lean WT organism. Measurements were performed 120 min after administration of the HFM. C) MPO activity was measured by a spectrophotometric MPO activity assay. Consistent with Western blot data, HFM increased MPO activity in WT mice while failing to increase MPO activity in P-selectin–deficient mice. Data are means ± sem. Five independent experiments were performed for each quantification (indicated by numbers at the bottom of bars. LFM, low-fat meal; OD, optical density; P-sel, P-selectin.
MPO actions on adiponectin expression and TNF-α activity
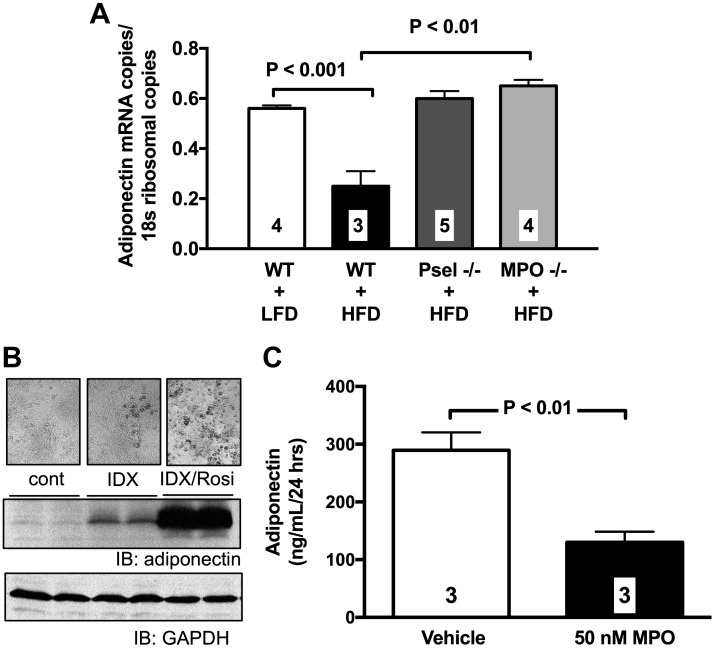
Studies have linked activation of leukocytes with acute reduction of adiponectin mRNA levels in adipose tissue (29). Accordingly, we found evidence of reduced adiponectin mRNA levels in the epididymal VAT of lean, WT mice exposed to HFD for 24 h (Fig. 8A). High-fat feeding for 24 h failed to reduce adiponectin mRNA levels in P-selectin– and MPO-deficient mice (Fig. 8A). Furthermore, incubation of differentiated 3T3-L1 adipocytes with 50 nM MPO significantly attenuated adiponectin production over a 24-h period (Fig. 8B, C).
Figure 8.
HFMs down-regulate adiponectin function via a P-selectin/MPO-mediated mechanism. A) HFD for 24 h reduces adiponectin mRNA copies content in the epididymal VAT of C57BL/6J mice but not in that of P-selectin and MPO-deficient mice. Values are means ± se. Numbers at the bottom of bars are the number of mice studied in each group. In parallel experiments, the effect of 50 nM MPO was studied in differentiated 3T3-L1 cells. B) Evidence of adiponectin expression in differentiated 3T3-L1 cells was first confirmed by Western blot analysis. C) Exposure of 3T3-L1 cells to 50 nM MPO significantly attenuated adiponectin production in the culture media over a 24-h period. cont, control; IB, immunoblot; IDX, isobutylmethylaxantine (I) + dexamethasone (DX); GAPDH, glyceraldehyde 3-phosphate dehydrogenase; LFD, low-fat diet; Psel, P-selectin; Rosi, rosiglitazone.
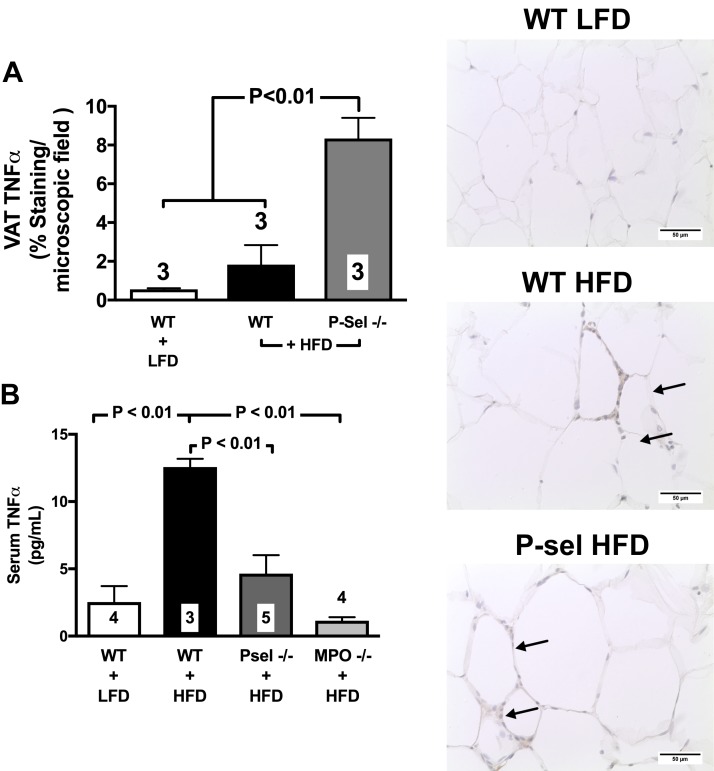
It has been demonstrated that MPO and its chlorinating byproducts increase the activity of the MMPs via the cysteine switch conserved among the MMPs and ADAMs (41). ADAM17, also known as TNF-α converting enzyme, is the major protease to produce mature forms of TNF-α (42), a cytokine up-regulated in mouse adipose tissue following 10 wk of HFD (3, 24) and also implicated in inflammatory complications of obesity (43). Accordingly, we found evidence that MPO acutely increases ADAM17/TNF-α converting enzyme activity in 3T3-L1 adipocytes, as detected by a 40 ± 3.8% increase in proTNF-α fused with ALP (P < 0.05 vs. control). Moreover, in WT mice given an HFD for 10 or 16 wk, TNF-α levels increased in the VAT (Fig. 9A and photomicrographs of VAT immunohistochemistry) and plasma (Fig. 9C), respectively, but not in the subcutaneous fat depots (Fig. 9B). Genetic deletion of P-selectin failed to attenuate TNF-α levels in the VAT of mice given HFD for 10 consecutive weeks (Fig. 9A) while preventing plasma TNF-α elevation even after 16 wk of feeding (Fig. 9B).
Figure 9.
Deletion of P-selectin fails to attenuate TNF-α up-regulation in the VAT while preventing TNF-α in the plasma. WT mice given an HFD for 10 consecutive weeks experienced elevated levels of TNF-α in the epidydimal fat, which were not prevented by deletion of P-selectin [photomicrographs and bar graph (A); arrows indicate positive brown peroxidase reaction for TNF-α staining]. With longer 16-wk feeding, TNF-α levels increased in the plasma of WT mice but not in that of P-selectin–deficient mice [bar graph (B)]. Values are means ± sem. Numbers at the bottom of bars are the number of mice studied in each group. In total, 2–5 tissue sections were studied in the VAT isolated from each mouse. LFD, low-fat diet; Psel, P-selectin
These data demonstrate that activation of endothelial-expressed P-selectin in the VAT microcirculation contributes to postprandial down-regulation of adiponectin, probably by instigating release of MPO from circulating leukocytes. They also demonstrate that, with prolonged consumption of HFD, up-regulation of TNF-α activity in VAT occurs independently of LEIs and that TNF-α plasma levels do not reflect TNF-α tissue levels in diet-induced obesity.
P-selectin deletion, adipose tissue remodeling, and glucose homeostasis
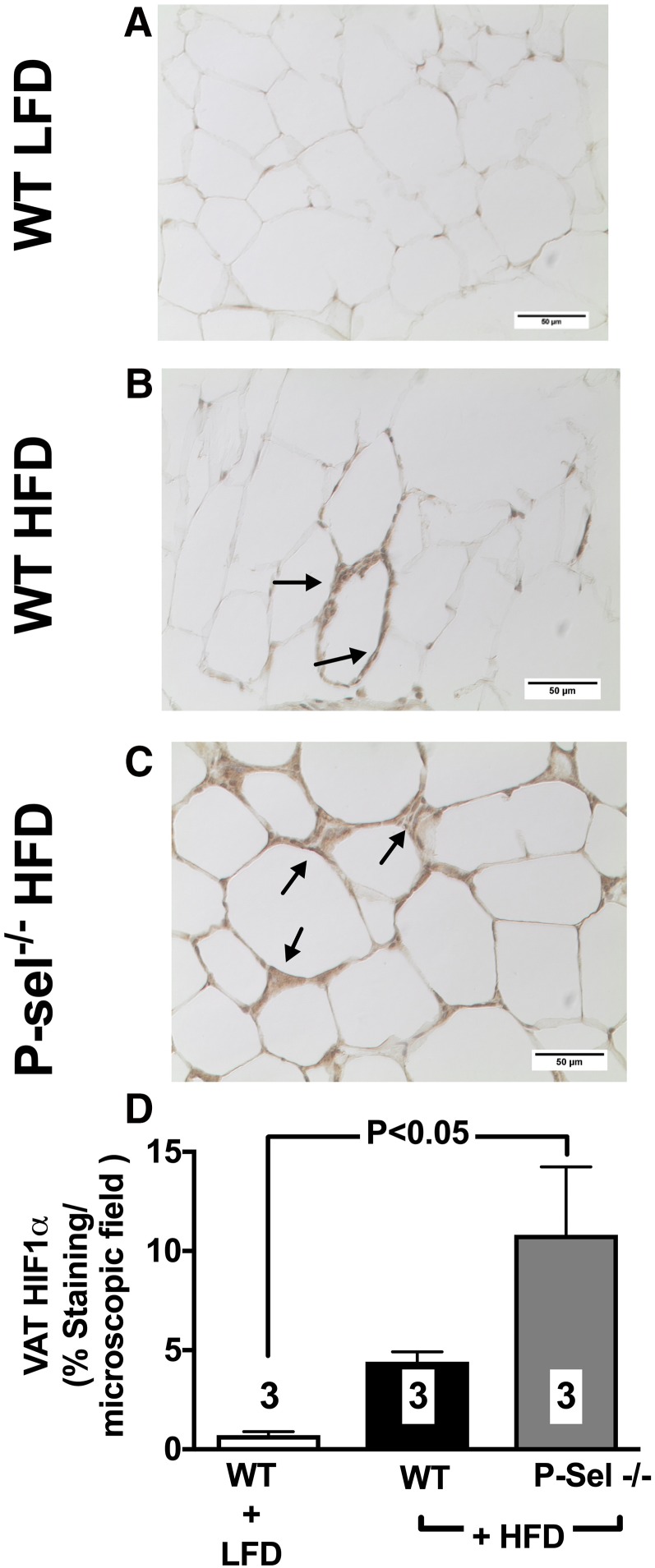
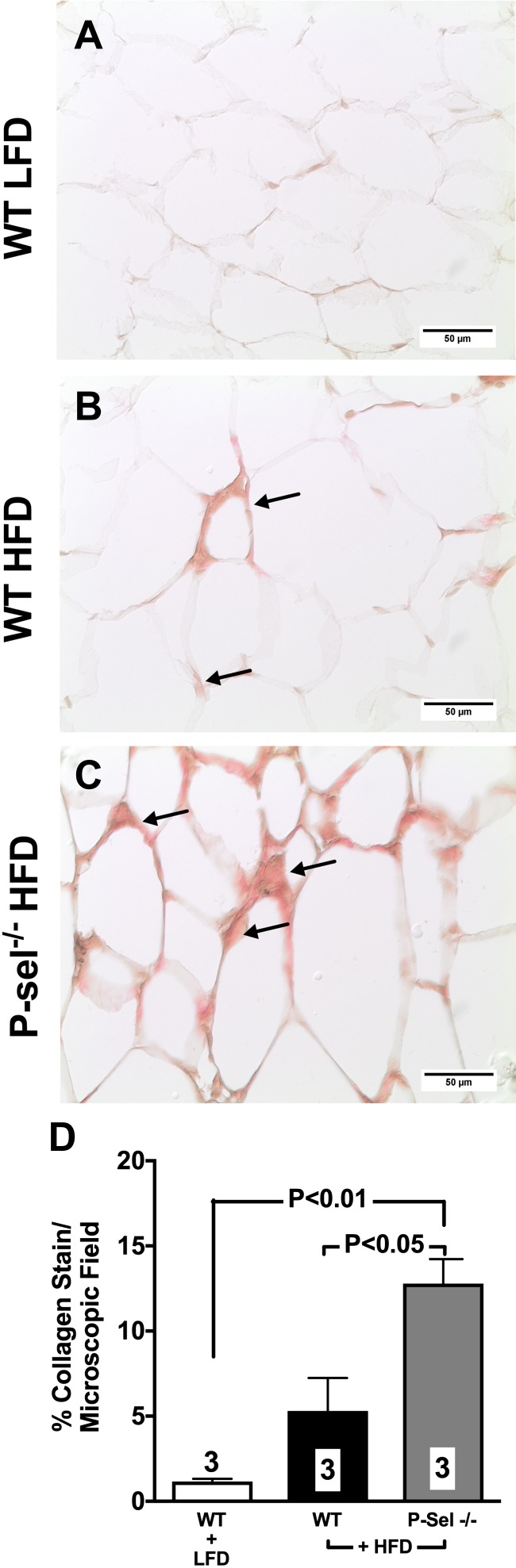
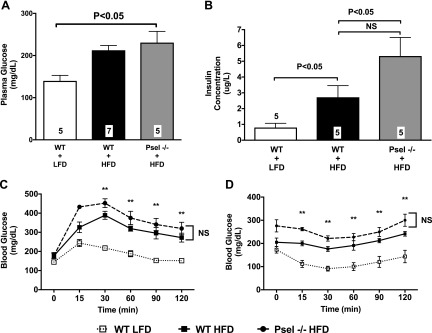
To further understand the role that P-selectin plays in the setting of chronic obesity, we studied changes in adipose tissue and whole-body metabolic health in WT and P-selectin–deficient mice placed on an HFD for either 10 or 16 consecutive weeks. Both HFD-fed groups exhibited a comparable degree of weight gain, which was significantly different from mice given a control diet (Supplemental Fig. S2). After 10 wk of high-fat feeding, adipocyte size increased in both WT and P-selectin–deficient mice, with a higher degree of adipocyte hypertrophy observed in VAT over SAT (Supplemental Fig. S3). On the HFD, P-selectin–deficient mice experienced a trend toward a higher level of HIF1-α (Fig. 10) expression and significant increase in collagen deposition (Fig. 11) in VAT when compared with WT mice. At the end of 16 wk of feeding, HFD WT mice exhibited significantly increased fasting plasma glucose (Fig. 12A) and insulin levels (Fig. 12B) with deterioration of glucose metabolism, as measured by glucose (Fig. 12C) and insulin (Fig. 12D) tolerance tests. Furthermore, deletion of P-selectin failed to correct glucose metabolism and insulin sensitivity (Fig. 12).
Figure 10.
A–C) Deletion of P-selectin exacerbates HIF1-α abundance in diet-induced obesity. Compared to WT mice fed a low fat diet (A), mice given an HFD for 10 consecutive weeks experienced up-regulation of HIF1-α (B), which was further exacerbated by deletion of P-selectin (C) (arrows indicate positive brown peroxidase reaction for HIF1-α staining). D) Bar graph shows quantification of data from all experimental groups of mice. Values are means ± sem. Numbers at the bottom of bars are the number of mice studied in each group. In total, 2–5 tissue sections were studied in the VAT isolated from each mouse. LFD, low-fat diet; P-sel, P-selectin.
Figure 11.
Deletion of P-selectin exacerbates visceral fat fibrosis in diet-induced obesity. A–C) Compared to WT mice fed a low fat diet (A), mice given an HFD for 10 consecutive weeks experienced collagen deposition in the epididymal fat (B), which was further exacerbated by deletion of P-selectin (C) (arrows indicate positive Sirius Red collagen staining). D) Bar graph ishows quantification of data from all experimental groups of mice. Values are means ± sem. Numbers at the bottom of bars are the number of mice studied in each group. In total, 2–5 tissue sections were studied in the VAT isolated from each mouse. LFD, low-fat diet; P-Sel, P-selectin.
Figure 12.
A, B) Deletion of P-selectin fails to improve glucose metabolism in HFD. Fasting blood glucose (A) and serum insulin (B) levels were measured at the end of the 16-wk feeding regimen. Serum insulin levels were measured by ELISA, and tail vein blood glucose was measured by glucometer. C, D) Intraperitoneal glucose tolerance test (C) and insulin tolerance test (D) were performed in mice after 16 wk of feeding, and blood glucose measurements were measured at the indicated time points. LFD, low-fat diet; NS, not significant; Psel, P-selectin. All data represent the means ± sem; n = 5–7. **P < 0.01 vs. control.
Taken together, these data suggest that inhibition of transient endothelial-driven postprandial inflammatory responses fails to prevent adipose tissue hypoxia, remodeling, and fibrosis as well as alteration in whole-body glucose homeostasis in chronic obesity.
DISCUSSION
It is well established that leukocytes play a major pathogenic role in the adipocyte dysfunction and insulin resistance of overnutrition and obesity (44). To infiltrate most organ tissues, circulating leukocytes must bind to eCAMs expressed on the endothelium of postcapillary venules of the microcirculation (45). Thus, the endothelium is the physiologic gatekeeper for immune cells in inflamed tissues. In addition to this important immune function, endothelial cells are obligatory regulators of oxygen and nutrient transport because of their anatomic position. Specifically, fatty acids (FAs) are transported across blood vessels supplying metabolically active tissues, such as adipose tissue, in a process that relies on lipoprotein lipase docked on the luminal surface of capillary endothelium to release FA from the triglyceride-rich lipoproteins (46), that postprandially become elevated in the blood. Recently, Dr. Sessa’s laboratory (47) was the first to demonstrate that during postprandial processing of FA, endothelial cells protect themselves from lipotoxicity by transiently storing FFAs in lipid droplets. This study first demonstrated that the ability of endothelial cells to form lipid droplets is dependent on the FA saturation, with palmitic acid being a much weaker inducer of lipid droplet formation than oleic acid. Interestingly, in vitro studies have demonstrated that the inefficiency of palmitate to be incorporated in lipid droplets results in lipotoxicity via up-regulation of inflammatory and apoptotic pathways (48). In this study, we sought to investigate how postprandial processing of palmitate and oleate impacts LEIs, a critical immune function of the vascular endothelium both in cardiovascular disease and metabolic disorders. Based on data demonstrating the rapid and transient nature of FA transport and accumulation in endothelial cells after fat-meal gavage (47), we focused on the eCAM P-selectin because P-selectin is constitutively expressed in the vascular endothelium. Moreover, human studies demonstrate that elevations in plasma triglycerides and FFAs by lipid infusion acutely up-regulate P-selectin (49). Endothelia expressing P-selectin engage circulating leukocytes by ligating the leukocyte adhesive receptor P-selectin glycoprotein ligand-1 (PSGL-1) (50) to initiate a process that ultimately leads to leukocyte firm adhesion and extravasation (51). Interestingly, 2 independent laboratories have demonstrated that deletion of the leukocyte-expressed counter-ligand for P-selectin, PSGL-1, attenuates leukocyte infiltration and overall inflammation in the VAT mice fed HFDs (52, 53). More recently, the interaction between PSGL-1 and P-selectin has been proposed as a therapeutic target for the treatment of the metabolic syndrome (54). Our data also involve P-selectin in the inflammatory responses of the VAT, at least in the prandial processing of obesogenic HFDs. We did not find evidence, though, of improved metabolic health in diet-induced obese P-selectin–deficient mice. This discrepancy can be probably explained by the fact that leukocyte-expressed PSGL-1 also binds to other selectins such as L- and E-selectin (55, 56), which could have become also up-regulated with chronic feeding. Further studies are needed to fully understand how acute and chronic exposure of the vascular endothelium to FFA affects lipid droplet formation and the inflammatory activity of selectins.
Another interesting result of our study is the different inflammatory response to palmitate of the VAT and SAT endothelia. The enriched content in lipoprotein lipases of the VAT microcirculation (57) might help explain why the VAT is more responsive than the SAT to lipemia. Data in the literature show that, expressed relative to the same mass of adipose tissue, meal FFA uptake is greater in intra-abdominal fat than subcutaneous abdominal fat in both sexes (58). Thus, the higher lipoprotein lipase activity of VAT may expose the endothelium of the VAT microcirculation to a higher postprandial FFA flux, which in turn causes acute endothelial dysfunction with P-selectin up-regulation. Obviously, interpretation of in vitro data, as well as of data obtained in genetically manipulated mice, must consider the phenotypic differences existing between endothelia of different metabolic organs and/or fat depots.
Leukocytes that adhere to endothelia expressing P-selectin are activated and primed for degranulation of their enzymatic content (36). Accordingly, we found evidence of P-selectin–dependent accumulation of leukocyte MPO in mice given HFMs. MPO, a heme protein abundantly expressed in neutrophils and monocytes, is acutely released by activated leukocytes, especially neutrophils (59). Primarily characterized as a bactericidal enzyme, MPO levels have been shown to increase in the plasma of overweight humans (60) and in the epididymal VAT of mice fed an HFD (39). We found evidence that MPO acutely down-regulates adiponectin mRNA levels in the VAT in addition to inhibiting adiponectin secretion from 3T3-L1 adipocytes. Adiponectin is an adipokine with protective vascular and metabolic actions. Loss of adiponectin function in obesity is associated with cardiovascular and metabolic complications (61). Moreover, infiltrating leukocytes are considered causative of insulin resistance (37, 4). Surprisingly, P-selectin deletion failed to protect mice from glucose metabolic dysfunction induced by 16-wk high-fat feeding. These data agree with studies demonstrating reduced glucose tolerance in response to prolonged high-fat feeding in mice expressing a dominant-negative version of TNF-α (62). It has been recently hypothesized that acute inflammatory reactions, such as the postprandial responses here discussed, are important for adipose tissue functionality. In fact, proinflammatory responses in adipose tissue appear to be essential for both proper adipose remodeling and angiogenesis, 2 processes known to facilitate adipogenesis and secure adequate oxygenation to the expanding adipose depots in vivo (63, 64). We demonstrate that obese P-selectin–deficient mice experience a higher degree of HIF1-α up-regulation compared with obese WT mice. There is now substantial evidence, both in animal models (65, 66) and humans (67–69), indicating that white adipose tissue depots become hypoxic as tissue mass expands. Hypoxia induces the synthesis of several angiogenic factors by adipocytes, reflecting the classic response to low O2 tension, with the intent of growing the capillary network and restoring adequate blood delivery and tissue oxygenation. Accordingly, transcription factors such as HIF1-α stimulate expression of many proinflammatory molecules, leading to adipose inflammation. HIF1-α activation was reported in the adipose tissue of obese mice (65, 70). Expression and activation of this important transcription factor is increased in adipose tissue of patients with obesity, and its expression was reduced after surgery-induced weight loss (71, 72). Overall, prevailing theories contend that angiogenesis in adipose tissue is driven by and invariably associated with inflammation (73), and the inflammatory response was obviously hampered in P-selectin–deficient mice because of their inefficient ability to recruit circulating leukocytes in the expanding VAT. One limitation of our study is the lack of data on the potential leukocyte populations infiltrated in the adipose tissue of chronically fed P-selectin–deficient mice. It has been reported that, as result of removal deficiency, P-selectin–deficient mice experience reduced macrophage extravasation (74). Because macrophages are an important source of TNF-α in obesity, the source of TNF-α observed in the VAT of P-selectin–deficient mice fed for 16 wk remains to be determined. Indeed, the vast majority of adipose tissue volume is composed of lipid-laden adipocytes, and both isolated or differentiated adipocytes can produce TNF-α (75). Thus, further studies should be undertaken to fully understand how recruitment of inflammatory cells via endothelial-expressed eCAMs impacts cytokine production by expanding adipocytes.
TNF-α is one of the most implicated factors causing a reduction of adiponectin expression in high-fat feeding (76). ADAM17, also known as TNF-α converting enzyme, is the major protease to produce mature forms of TNF-α (42, 77). We found evidence that MPO increases ADAM17 activity in 3T3-L1 adipocytes. Highly relevant here, ADAM17 is expressed in normal adipocytes, and its expression is enhanced by obesity (78). In humans, single nucleotide polymorphisms of the ADAM17 gene have been recently associated with obesity and abnormal dietary FA metabolism (79). Enhanced ADAM17 expression and activity in adipose tissue of obese individuals leads to enhanced production of TNF-α, adipose tissue inflammation, and systemic insulin resistance (79). Moreover, reduction of adiponectin levels by high-fat feeding is markedly attenuated in ADAM17 heterozygous mice (80). We found evidence that 24-h consumption of high-fat food causes infiltration of neutrophils in VAT and acute reduction of adiponectin mRNA in WT mice but not in P-selectin null mice in which neutrophils infiltration is prevented. This observation is consistent with previous research demonstrating that granulocyte-mediated inflammatory responses cause an acute 30% reduction in serum adiponectin levels and a corresponding decrease in adipose tissue adiponectin mRNA expression (29). Surprisingly, though, insulin sensitivity and glucose tolerance were not preserved in P-selectin null mice fed a HFD for 16 wk, in which VAT TNF-α levels were elevated even in the face of reduced serum TNF-α levels. Several lines of evidence can help explain this apparent discrepancy. First, adiponectin circulates in the bloodstream on multimers (Admer), among which high MW forms are the most active (81). Alterations of Admer distribution have been described in murine models and patients with obesity, in which a change in Admer distribution in favor of the less-active forms has been reported (82). Interestingly, TNF-α impairs adiponectin multimerization by altering disulfide bond modification in the endoplasmic reticulum (83). Thus, preserved adiponectin mRNA expression levels might not be predictive of preserved adiponectin metabolic action. Second, it has been shown that in adipose tissue of obese mice, TNF-α occurs predominantly in the transmembrane form, resulting in elevated membrane-associated TNF-α in adipose tissue in obesity (84). Interestingly, we found evidence of increased TNF-α staining in VAT of P-selectin mice given a HFD for 10 wk (Fig. 9A). Paracrine TNF-α activity could account, therefore, for the impaired insulin signaling and reduced glucose tolerance in P-selectin–deficient mice. Third, HFD–fed P-selectin null mice experienced increased collagen deposition in VAT, a hallmark of fibrosis development in adipose tissue (85). Fibrosis and related hypoxia lead to a further reduction in beneficial adipokines, such as adiponectin, with an ensuing secondary impact on insulin sensitivity (85, 69). Obviously, further studies should be undertaken to fully understand how local vs. systemic TNF-α production along with modulation of the inflammatory function of the vascular endothelium impact the overall inflammatory dysmetabolic phenotype of adipose depots.
Our time-course studies on the effect of HFM on LEIs (Fig. 3) may have implications for dietary guidelines and may help explain recent data demonstrating that the interval of time between meals influences inflammatory and metabolic outcomes in humans and mice (86, 87). In fact, the observation that condensing food consumption to a limited amount of time, without restricting overall daily caloric intake, protects HFD-fed mice from metabolic dysfunction and inflammation (88) could be explained by the 4-h period required for returning the postprandial immune activation of the VAT to baseline conditions.
In summary, our study identifies novel integrative mechanisms of postprandial adipose tissue dysfunction that could provide insight into the relationship between inflammation and diet-induced obesity.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
Serum TNF-α analysis and plasma insulin levels were performed by ELISA at Case Western University Mouse Metabolic Phenotyping Center (Cleveland, OH, USA). Free fatty acid (FFA) analysis was performed at the Vanderbilt University Mouse Metabolic Phenotyping Center (Nashville, TN, USA), which is supported by U.S. National Institutes of Health (NIH) Grant DK59637. This study was supported by NIH National Institute of Diabetes and Digestive and Kidney Diseases Grants DK096521 (to R.S.), DK111042 (to R.S. and S.E.), NIH National Heart, Lung, and Blood Institute Grant HL133248 (to S.E.), and American Diabetes Association (ADA) Grant 1-12-BS-180 (to R.S.). The authors declare no conflicts of interest.
Glossary
- ADAM17
a disintegrin and metalloproteinase 17
- ALP
alkaline phosphatase
- eCAM
endothelial cell adhesion molecule
- FA
fatty acid
- FFA
free fatty acid
- HCAEC
human coronary artery endothelial cell
- HFD
high-fat diet
- HFM
high-fat meal
- IVM
intravital microscopy
- LEI
leukocyte-endothelium interaction
- MPO
myeloperoxidase
- PSGL-1
P-selectin glycoprotein ligand-1
- SAT
subcutaneous adipose tissue
- SVF
stromal vascular fraction
- VAT
visceral adipose tissue
- HIF1-α
hypoxia inducible factor 1-α
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHORS CONTRIBUTION
K. J. Preston performed research, analyzed data, and wrote the paper; I. Rom performed research and analyzed data; C. Vrakas performed research and analyzed data; G. Landesberg performed research and analyzed data; Z. Etwebi performed research and analyzed data; S. Muraoka performed research and analyzed data; M. Autieri analyzed data; S. Eguchi performed research and analyzed data; and R. Scalia designed research, analyzed data, and wrote the manuscript.
REFERENCES
- 1.Hotamisligil G. S., Shargill N. S., Spiegelman B. M. (1993) Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 [DOI] [PubMed] [Google Scholar]
- 2.Sell H., Habich C., Eckel J. (2012) Adaptive immunity in obesity and insulin resistance. Nat. Rev. Endocrinol. 8, 709–716 [DOI] [PubMed] [Google Scholar]
- 3.Elgazar-Carmon V., Rudich A., Hadad N., Levy R. (2008) Neutrophils transiently infiltrate intra-abdominal fat early in the course of high-fat feeding. J. Lipid Res. 49, 1894–1903 [DOI] [PubMed] [Google Scholar]
- 4.Talukdar S., Oh D. Y., Bandyopadhyay G., Li D., Xu J., McNelis J., Lu M., Li P., Yan Q., Zhu Y., Ofrecio J., Lin M., Brenner M. B., Olefsky J. M. (2012) Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nat. Med. 18, 1407–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Van Oostrom A. J., Sijmonsma T. P., Verseyden C., Jansen E. H., de Koning E. J., Rabelink T. J., Castro Cabezas M. (2003) Postprandial recruitment of neutrophils may contribute to endothelial dysfunction. J. Lipid Res. 44, 576–583 [DOI] [PubMed] [Google Scholar]
- 6.Perez-Martinez P., Alcala-Diaz J. F., Delgado-Lista J., Garcia-Rios A., Gomez-Delgado F., Marin-Hinojosa C., Rodriguez-Cantalejo F., Delgado-Casado N., Perez-Caballero A. I., Fuentes-Jimenez F. J., Camargo A., Tinahones F. J., Ordovas J. M., Perez-Jimenez F., Lopez-Miranda J. (2014) Metabolic phenotypes of obesity influence triglyceride and inflammation homoeostasis. Eur. J. Clin. Invest. 44, 1053–1064 [DOI] [PubMed] [Google Scholar]
- 7.Rao R. M., Yang L., Garcia-Cardena G., Luscinskas F. W. (2007) Endothelial-dependent mechanisms of leukocyte recruitment to the vascular wall. Circ. Res. 101, 234–247 [DOI] [PubMed] [Google Scholar]
- 8.Nappo F., Esposito K., Cioffi M., Giugliano G., Molinari A. M., Paolisso G., Marfella R., Giugliano D. (2002) Postprandial endothelial activation in healthy subjects and in type 2 diabetic patients: role of fat and carbohydrate meals. J. Am. Coll. Cardiol. 39, 1145–1150 [DOI] [PubMed] [Google Scholar]
- 9.Johnston G. I., Cook R. G., McEver R. P. (1989) Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell 56, 1033–1044 [DOI] [PubMed] [Google Scholar]
- 10.Ichimura H., Parthasarathi K., Quadri S., Issekutz A. C., Bhattacharya J. (2003) Mechano-oxidative coupling by mitochondria induces proinflammatory responses in lung venular capillaries. J. Clin. Invest. 111, 691–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takano M., Meneshian A., Sheikh E., Yamakawa Y., Wilkins K. B., Hopkins E. A., Bulkley G. B. (2002) Rapid upregulation of endothelial P-selectin expression via reactive oxygen species generation. Am. J. Physiol. Heart Circ. Physiol. 283, H2054–H2061 [DOI] [PubMed] [Google Scholar]
- 12.Bröijersén A., Karpe F., Hamsten A., Goodall A. H., Hjemdahl P. (1998) Alimentary lipemia enhances the membrane expression of platelet P-selectin without affecting other markers of platelet activation. Atherosclerosis 137, 107–113 [DOI] [PubMed] [Google Scholar]
- 13.Kälsch T., Elmas E., Nguyen X. D., Kralev S., Leweling H., Klüter H., Dempfle C. E., Borggrefe M. (2007) Effects of alimentary lipemia and inflammation on platelet CD40-ligand. Thromb. Res. 120, 703–708 [DOI] [PubMed] [Google Scholar]
- 14.Schneider D. J., Hardison R. M., Lopes N., Sobel B. E., Brooks M. M.; Pro-Thrombosis Ancillary Study Group (2009) Association between increased platelet P-selectin expression and obesity in patients with type 2 diabetes: a BARI 2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) substudy. Diabetes Care 32, 944–949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Pergola G., Pannacciulli N., Coviello M., Scarangella A., Di Roma P., Caringella M., Venneri M. T., Quaranta M., Giorgino R. (2008) sP-selectin plasma levels in obesity: association with insulin resistance and related metabolic and prothrombotic factors. Nutr. Metab. Cardiovasc. Dis. 18, 227–232 [DOI] [PubMed] [Google Scholar]
- 16.Gallistl S., Sudi K. M., Borkenstein M., Weinhandl G., Zotter H., Muntean W. (2000) Correlation between cholesterol, soluble P-selectin, and D-dimer in obese children and adolescents. Blood Coagul. Fibrinolysis 11, 755–760 [DOI] [PubMed] [Google Scholar]
- 17.Kato H., Kashiwagi H., Shiraga M., Tadokoro S., Kamae T., Ujiie H., Honda S., Miyata S., Ijiri Y., Yamamoto J., Maeda N., Funahashi T., Kurata Y., Shimomura I., Tomiyama Y., Kanakura Y. (2006) Adiponectin acts as an endogenous antithrombotic factor. Arterioscler. Thromb. Vasc. Biol. 26, 224–230 [DOI] [PubMed] [Google Scholar]
- 18.Scalia R., Gong Y., Berzins B., Freund B., Feather D., Landesberg G., Mishra G. (2011) A novel role for calpain in the endothelial dysfunction induced by activation of angiotensin II type 1 receptor signaling. Circ. Res. 108, 1102–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kranendonk M. E., van Herwaarden J. A., Stupkova T., de Jager W., Vink A., Moll F. L., Kalkhoven E., Visseren F. L. (2015) Inflammatory characteristics of distinct abdominal adipose tissue depots relate differently to metabolic risk factors for cardiovascular disease: distinct fat depots and vascular risk factors. Atherosclerosis 239, 419–427 [DOI] [PubMed] [Google Scholar]
- 20.Graf D., Barth S. W., Bub A., Narr J., Rüfer C. E., Watzl B., Seifert S. (2014) Dietary fat quality in regular fat diets has minor effects on biomarkers of inflammation in obese Zucker rats. Eur. J. Nutr. 53, 211–219 [DOI] [PubMed] [Google Scholar]
- 21.Kwon E. Y., Shin S. K., Cho Y. Y., Jung U. J., Kim E., Park T., Park J. H., Yun J. W., McGregor R. A., Park Y. B., Choi M. S. (2012) Time-course microarrays reveal early activation of the immune transcriptome and adipokine dysregulation leads to fibrosis in visceral adipose depots during diet-induced obesity. BMC Genomics 13, 450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee M.-J., Wu Y., Fried S. K. (2013) Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol. Aspects Med. 34, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnoldussen I. A. C., Zerbi V., Wiesmann M., Noordman R. H. J., Bolijn S., Mutsaers M. P. C., Dederen P. J. W. C., Kleemann R., Kooistra T., van Tol E. A. F., Gross G., Schoemaker M. H., Heerschap A., Wielinga P. Y., Kiliaan A. J. (2016) Early intake of long-chain polyunsaturated fatty acids preserves brain structure and function in diet-induced obesity. J. Nutr. Biochem. 30, 177–188 [DOI] [PubMed] [Google Scholar]
- 24.Weisberg S. P., McCann D., Desai M., Rosenbaum M., Leibel R. L., Ferrante A. W., Jr (2003) Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 112, 1796–1808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stalker T. J., Gong Y., Scalia R. (2005) The calcium-dependent protease calpain causes endothelial dysfunction in type 2 diabetes. Diabetes 54, 1132–1140 [DOI] [PubMed] [Google Scholar]
- 26.Pruefer D., Scalia R., Lefer A. M. (1999) Homocysteine provokes leukocyte-endothelium interaction by downregulation of nitric oxide. Gen. Pharmacol. 33, 487–498 [DOI] [PubMed] [Google Scholar]
- 27.Mullane K. M., Kraemer R., Smith B. (1985) Myeloperoxidase activity as a quantitative assessment of neutrophil infiltration into ischemic myocardium. J. Pharmacol. Methods 14, 157–167 [DOI] [PubMed] [Google Scholar]
- 28.Scalia R., Gefen J., Petasis N. A., Serhan C. N., Lefer A. M. (1997) Lipoxin A4 stable analogs inhibit leukocyte rolling and adherence in the rat mesenteric microvasculature: role of P-selectin. Proc. Natl. Acad. Sci. USA 94, 9967–9972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shore S. A., Terry R. D., Flynt L., Xu A., Hug C. (2006) Adiponectin attenuates allergen-induced airway inflammation and hyperresponsiveness in mice. J. Allergy Clin. Immunol. 118, 389–395 [DOI] [PubMed] [Google Scholar]
- 30.Student A. K., Hsu R. Y., Lane M. D. (1980) Induction of fatty acid synthetase synthesis in differentiating 3T3-L1 preadipocytes. J. Biol. Chem. 255, 4745–4750 [PubMed] [Google Scholar]
- 31.Zheng Y., Schlondorff J., Blobel C. P. (2002) Evidence for regulation of the tumor necrosis factor alpha-convertase (TACE) by protein-tyrosine phosphatase PTPH1. J. Biol. Chem. 277, 42463–42470 [DOI] [PubMed] [Google Scholar]
- 32.Ohtsu H., Dempsey P. J., Frank G. D., Brailoiu E., Higuchi S., Suzuki H., Nakashima H., Eguchi K., Eguchi S. (2006) ADAM17 mediates epidermal growth factor receptor transactivation and vascular smooth muscle cell hypertrophy induced by angiotensin II. Arterioscler. Thromb. Vasc. Biol. 26, e133–e137 [DOI] [PubMed] [Google Scholar]
- 33.Wang L., Gill R., Pedersen T. L., Higgins L. J., Newman J. W., Rutledge J. C. (2009) Triglyceride-rich lipoprotein lipolysis releases neutral and oxidized FFAs that induce endothelial cell inflammation. J. Lipid Res. 50, 204–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kansas G. S. (1996) Selectins and their ligands: current concepts and controversies. Blood 88, 3259–3287 [PubMed] [Google Scholar]
- 35.Geng J. G., Bevilacqua M. P., Moore K. L., McIntyre T. M., Prescott S. M., Kim J. M., Bliss G. A., Zimmerman G. A., McEver R. P. (1990) Rapid neutrophil adhesion to activated endothelium mediated by GMP-140. Nature 343, 757–760 [DOI] [PubMed] [Google Scholar]
- 36.Lorant D. E., Topham M. K., Whatley R. E., McEver R. P., McIntyre T. M., Prescott S. M., Zimmerman G. A. (1993) Inflammatory roles of P-selectin. J. Clin. Invest. 92, 559–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mansuy-Aubert V., Zhou Q. L., Xie X., Gong Z., Huang J. Y., Khan A. R., Aubert G., Candelaria K., Thomas S., Shin D. J., Booth S., Baig S. M., Bilal A., Hwang D., Zhang H., Lovell-Badge R., Smith S. R., Awan F. R., Jiang Z. Y. (2013) Imbalance between neutrophil elastase and its inhibitor α1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metab. 17, 534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Strzepa A., Pritchard K. A., Dittel B. N. (2017) Myeloperoxidase: a new player in autoimmunity. Cell. Immunol. 317, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Q., Xie Z., Zhang W., Zhou J., Wu Y., Zhang M., Zhu H., Zou M. H. (2014) Myeloperoxidase deletion prevents high-fat diet-induced obesity and insulin resistance. Diabetes 63, 4172–4185 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Olza J., Aguilera C. M., Gil-Campos M., Leis R., Bueno G., Martínez-Jiménez M. D., Valle M., Cañete R., Tojo R., Moreno L. A., Gil A. (2012) Myeloperoxidase is an early biomarker of inflammation and cardiovascular risk in prepubertal obese children. Diabetes Care 35, 2373–2376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fu X., Kassim S. Y., Parks W. C., Heinecke J. W. (2003) Hypochlorous acid generated by myeloperoxidase modifies adjacent tryptophan and glycine residues in the catalytic domain of matrix metalloproteinase-7 (matrilysin): an oxidative mechanism for restraining proteolytic activity during inflammation. J. Biol. Chem. 278, 28403–28409 [DOI] [PubMed] [Google Scholar]
- 42.Blobel C. P. (2005) ADAMs: key components in EGFR signalling and development. Nat. Rev. Mol. Cell Biol. 6, 32–43 [DOI] [PubMed] [Google Scholar]
- 43.Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., Spiegelman B. M. (1995) Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 95, 2409–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gregor M. F., Hotamisligil G. S. (2011) Inflammatory mechanisms in obesity. Annu. Rev. Immunol. 29, 415–445 [DOI] [PubMed] [Google Scholar]
- 45.Kolaczkowska E., Kubes P. (2013) Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 13, 159–175 [DOI] [PubMed] [Google Scholar]
- 46.Davies B. S., Beigneux A. P., Barnes R. H., II, Tu Y., Gin P., Weinstein M. M., Nobumori C., Nyrén R., Goldberg I., Olivecrona G., Bensadoun A., Young S. G., Fong L. G. (2010) GPIHBP1 is responsible for the entry of lipoprotein lipase into capillaries. Cell Metab. 12, 42–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuo A., Lee M. Y., Sessa W. C. (2017) Lipid droplet biogenesis and function in the endothelium. Circ. Res. 120, 1289–1297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Listenberger L. L., Han X., Lewis S. E., Cases S., Farese R. V., Jr., Ory D. S., Schaffer J. E. (2003) Triglyceride accumulation protects against fatty acid-induced lipotoxicity. Proc. Natl. Acad. Sci. USA 100, 3077–3082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Aye M. M., Kilpatrick E. S., Aburima A., Wraith K. S., Magwenzi S., Spurgeon B., Rigby A. S., Sandeman D., Naseem K. M., Atkin S. L. (2014) Acute hypertriglyceridemia induces platelet hyperactivity that is not attenuated by insulin in polycystic ovary syndrome. J. Am. Heart Assoc. 3, e000706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sako D., Comess K. M., Barone K. M., Camphausen R. T., Cumming D. A., Shaw G. D. (1995) A sulfated peptide segment at the amino terminus of PSGL-1 is critical for P-selectin binding. Cell 83, 323–331 [DOI] [PubMed] [Google Scholar]
- 51.Lorant D. E., McEver R. P., McIntyre T. M., Moore K. L., Prescott S. M., Zimmerman G. A. (1995) Activation of polymorphonuclear leukocytes reduces their adhesion to P-selectin and causes redistribution of ligands for P-selectin on their surfaces. J. Clin. Invest. 96, 171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sato C., Shikata K., Hirota D., Sasaki M., Nishishita S., Miyamoto S., Kodera R., Ogawa D., Tone A., Kataoka H. U., Wada J., Kajitani N., Makino H. (2011) P-selectin glycoprotein ligand-1 deficiency is protective against obesity-related insulin resistance. Diabetes 60, 189–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Russo H. M., Wickenheiser K. J., Luo W., Ohman M. K., Franchi L., Wright A. P., Bodary P. F., Núñez G., Eitzman D. T. (2010) P-selectin glycoprotein ligand-1 regulates adhesive properties of the endothelium and leukocyte trafficking into adipose tissue. Circ. Res. 107, 388–397; erratum: e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Patel M. S., Miranda-Nieves D., Chen J., Haller C. A., Chaikof E. L. (2017) Targeting P-selectin glycoprotein ligand-1/P-selectin interactions as a novel therapy for metabolic syndrome. Transl. Res. 183, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hirata T., Merrill-Skoloff G., Aab M., Yang J., Furie B. C., Furie B. (2000) P-Selectin glycoprotein ligand 1 (PSGL-1) is a physiological ligand for E-selectin in mediating T helper 1 lymphocyte migration. J. Exp. Med. 192, 1669–1676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stadtmann A., Germena G., Block H., Boras M., Rossaint J., Sundd P., Lefort C., Fisher C. I., Buscher K., Gelschefarth B., Urzainqui A., Gerke V., Ley K., Zarbock A. (2013) The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. J. Exp. Med. 210, 2171–2180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scalia R. (2013) The microcirculation in adipose tissue inflammation. Rev. Endocr. Metab. Disord. 14, 69–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koutsari C., Ali A. H., Mundi M. S., Jensen M. D. (2011) Storage of circulating free fatty acid in adipose tissue of postabsorptive humans: quantitative measures and implications for body fat distribution. Diabetes 60, 2032–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lau D., Baldus S. (2006) Myeloperoxidase and its contributory role in inflammatory vascular disease. Pharmacol. Ther. 111, 16–26 [DOI] [PubMed] [Google Scholar]
- 60.Gómez García A., Rivera Rodríguez M., Gómez Alonso C., Rodríguez Ochoa D. Y., Alvarez Aguilar C. (2015) Myeloperoxidase is associated with insulin resistance and inflammation in overweight subjects with first-degree relatives with type 2 diabetes mellitus. Diabetes Metab. J. 39, 59–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yamauchi T., Kadowaki T. (2008) Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int. J. Obes. 32 (Suppl 7), S13–S18 [DOI] [PubMed] [Google Scholar]
- 62.Wernstedt Asterholm I., Tao C., Morley T. S., Wang Q. A., Delgado-Lopez F., Wang Z. V., Scherer P. E. (2014) Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 20, 103–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cao Y. (2007) Angiogenesis modulates adipogenesis and obesity. J. Clin. Invest. 117, 2362–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cristancho A. G., Lazar M. A. (2011) Forming functional fat: a growing understanding of adipocyte differentiation. Nat. Rev. Mol. Cell Biol. 12, 722–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ye J., Gao Z., Yin J., He Q. (2007) Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol. Endocrinol. Metab. 293, E1118–E1128 [DOI] [PubMed] [Google Scholar]
- 66.Hosogai N., Fukuhara A., Oshima K., Miyata Y., Tanaka S., Segawa K., Furukawa S., Tochino Y., Komuro R., Matsuda M., Shimomura I. (2007) Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes 56, 901–911 [DOI] [PubMed] [Google Scholar]
- 67.Blaak E. E., van Baak M. A., Kemerink G. J., Pakbiers M. T. W., Heidendal G. A. K., Saris W. H. M. (1995) Beta-adrenergic stimulation and abdominal subcutaneous fat blood flow in lean, obese, and reduced-obese subjects. Metabolism 44, 183–187 [DOI] [PubMed] [Google Scholar]
- 68.Kabon B., Nagele A., Reddy D., Eagon C., Fleshman J. W., Sessler D. I., Kurz A. (2004) Obesity decreases perioperative tissue oxygenation. Anesthesiology 100, 274–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pasarica M., Sereda O. R., Redman L. M., Albarado D. C., Hymel D. T., Roan L. E., Rood J. C., Burk D. H., Smith S. R. (2009) Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes 58, 718–725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ozeke O., Ozer C., Gungor M., Celenk M. K., Dincer H., Ilicin G. (2011) Chronic intermittent hypoxia caused by obstructive sleep apnea may play an important role in explaining the morbidity-mortality paradox of obesity. Med. Hypotheses 76, 61–63 [DOI] [PubMed] [Google Scholar]
- 71.Pasarica M., Rood J., Ravussin E., Schwarz J.-M., Smith S. R., Redman L. M. (2010) Reduced oxygenation in human obese adipose tissue is associated with impaired insulin suppression of lipolysis. J. Clin. Endocrinol. Metab. 95, 4052–4055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pasarica M., Gowronska-Kozak B., Burk D., Remedios I., Hymel D., Gimble J., Ravussin E., Bray G. A., Smith S. R. (2009) Adipose tissue collagen VI in obesity. J. Clin. Endocrinol. Metab. 94, 5155–5162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xu F., Burk D., Gao Z., Yin J., Zhang X., Weng J., Ye J. (2012) Angiogenic deficiency and adipose tissue dysfunction are associated with macrophage malfunction in SIRT1-/- mice. Endocrinology 153, 1706–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Johnson R. C., Mayadas T. N., Frenette P. S., Mebius R. E., Subramaniam M., Lacasce A., Hynes R. O., Wagner D. D. (1995) Blood cell dynamics in P-selectin-deficient mice. Blood 86, 1106–1114 [PubMed] [Google Scholar]
- 75.Cawthorn W. P., Sethi J. K. (2008) TNF-alpha and adipocyte biology. FEBS Lett. 582, 117–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nguyen M. T., Satoh H., Favelyukis S., Babendure J. L., Imamura T., Sbodio J. I., Zalevsky J., Dahiyat B. I., Chi N. W., Olefsky J. M. (2005) JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J. Biol. Chem. 280, 35361–35371 [DOI] [PubMed] [Google Scholar]
- 77.Seals D. F., Courtneidge S. A. (2003) The ADAMs family of metalloproteases: multidomain proteins with multiple functions. Genes Dev. 17, 7–30 [DOI] [PubMed] [Google Scholar]
- 78.Fiorentino L., Vivanti A., Cavalera M., Marzano V., Ronci M., Fabrizi M., Menini S., Pugliese G., Menghini R., Khokha R., Lauro R., Urbani A., Federici M. (2010) Increased tumor necrosis factor alpha-converting enzyme activity induces insulin resistance and hepatosteatosis in mice. Hepatology 51, 103–110 [DOI] [PubMed] [Google Scholar]
- 79.Junyent M., Parnell L. D., Lai C. Q., Arnett D. K., Tsai M. Y., Kabagambe E. K., Straka R. J., Province M., An P., Smith C. E., Lee Y. C., Borecki I., Ordovás J. M. (2010) ADAM17_i33708A>G polymorphism interacts with dietary n-6 polyunsaturated fatty acids to modulate obesity risk in the Genetics of Lipid Lowering Drugs and Diet Network study. Nutr. Metab. Cardiovasc. Dis. 20, 698–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Serino M., Menghini R., Fiorentino L., Amoruso R., Mauriello A., Lauro D., Sbraccia P., Hribal M. L., Lauro R., Federici M. (2007) Mice heterozygous for tumor necrosis factor-alpha converting enzyme are protected from obesity-induced insulin resistance and diabetes. Diabetes 56, 2541–2546 [DOI] [PubMed] [Google Scholar]
- 81.Lara-Castro C., Luo N., Wallace P., Klein R. L., Garvey W. T. (2006) Adiponectin multimeric complexes and the metabolic syndrome trait cluster. Diabetes 55, 249–259 [PubMed] [Google Scholar]
- 82.Liu M., Liu F. (2014) Regulation of adiponectin multimerization, signaling and function. Best Pract. Res. Clin. Endocrinol. Metab. 28, 25–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.He Y., Lu L., Wei X., Jin D., Qian T., Yu A., Sun J., Cui J., Yang Z. (2016) The multimerization and secretion of adiponectin are regulated by TNF-alpha. Endocrine 51, 456–468 [DOI] [PubMed] [Google Scholar]
- 84.Xu H., Uysal K. T., Becherer J. D., Arner P., Hotamisligil G. S. (2002) Altered tumor necrosis factor-alpha (TNF-alpha) processing in adipocytes and increased expression of transmembrane TNF-alpha in obesity. Diabetes 51, 1876–1883 [DOI] [PubMed] [Google Scholar]
- 85.Sun K., Tordjman J., Clément K., Scherer P. E. (2013) Fibrosis and adipose tissue dysfunction. Cell Metab. 18, 470–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Oike H., Oishi K., Kobori M. (2014) Nutrients, clock genes, and chrononutrition. Curr. Nutr. Rep. 3, 204–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Noyan H., El-Mounayri O., Isserlin R., Arab S., Momen A., Cheng H. S., Wu J., Afroze T., Li R. K., Fish J. E., Bader G. D., Husain M. (2015) Cardioprotective signature of short-term caloric restriction. PLoS One 10, e0130658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hatori M., Vollmers C., Zarrinpar A., DiTacchio L., Bushong E. A., Gill S., Leblanc M., Chaix A., Joens M., Fitzpatrick J. A. J., Ellisman M. H., Panda S. (2012) Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 15, 848–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.