Abstract
Classic homocystinuria (HCU) is an inherited disorder characterized by elevated homocysteine (Hcy) in plasma and tissues resulting from cystathionine β-synthase (CBS) deficiency. There is no cure, and patients are predominantly managed by methionine-restricted diet (MRD) to limit the production of Hcy. In this study, we used the I278T mouse model of HCU to evaluate the long-term impact of a novel enzyme replacement therapy [truncated human CBS C15S mutant modified with linear 20-kDa N-hydroxysuccinimide ester polyethylene glycol (OT-58)] on clinical end points relevant to human patients with HCU. In addition, we compared its efficacy on a background of either MRD or normal methionine intake [regular diet (REG)] to that of MRD alone. We found that, compared with untreated I278T mice, OT-58 treatment of I278T mice fed with the REG diet resulted in a 90% decrease in plasma Hcy concentrations and correction of learning/cognition, endothelial dysfunction, hemostasis, bone mineralization, and body composition. On background of the MRD, OT-58 performed equally well with plasma Hcy entirely normalized. The MRD alone decreased plasma Hcy by 67% and corrected the HCU phenotype in I278T mice. However, the MRD increased anxiety and reduced bone mineral content in both I278T mice and wild-type controls. This study shows that OT-58 is a highly efficacious novel treatment for HCU on the background of either normal or restricted methionine intake.—Majtan, T., Park, I., Cox, A., Branchford, B. R., di Paola, J., Bublil, E. M., Kraus, J. P. Behavior, body composition, and vascular phenotype of homocystinuric mice on methionine-restricted diet or enzyme replacement therapy.
Keywords: cystathionine β-synthase, homocysteine, anxiety, endothelial dysfunction, thrombosis
Classic HCU, also known as CBS-deficient HCU, is a monogenic inborn error of sulfur amino acid metabolism primarily caused by the missense mutations in the CBS gene (1). CBS is a critical enzyme of the transsulfuration pathway, in which the essential amino acid methionine (Met) is converted to another sulfur amino acid cysteine (Cys). Hcy, an intermediate of this conversion, lies at a pivot point, where it can be either remethylated back to Met via ubiquitous Met synthase or liver-dependent betaine-Hcy methyltransferase or processed by CBS toward Cys synthesis (2). Human CBS is a pyridoxal-5′-phosphate–dependent hemoprotein with complex domain architecture and regulation, which catalyzes condensation of Hcy with serine to form cystathionine (Cth) and water (3). Lack of CBS activity in patients with HCU results in accumulation of Hcy and Met in plasma and tissues with multisystemic clinical complications, such as connective tissue defects, osteoporosis, dislocated optic lenses, learning difficulties, and developmental delay or thromboembolism (1).
The goal of treating patients with HCU is to decrease or normalize, if possible, plasma Hcy levels (4). Treatment options available are very limited and are based on severity of the disease. Mildly affected patients, such as those having panethnic I278T mutation, respond well to pyridoxine, a precursor of CBS cofactor pyridoxal-5′-phosphate, which can lead to plasma Hcy levels below 50 µM or occasionally within the normal range of 5–15 µM (5, 6). On the other hand, many affected patients, such as those carrying the Irish G307S or the Spanish T191M mutations, do not respond to therapeutic doses of pyridoxine, and currently the only way to lower their plasma Hcy levels is by limiting their Met/protein intake. It is important to note that the treatment aim is to keep plasma Hcy concentrations as close to normal as possible and that the recommended threshold values of 100–120 µM (4) reflect what is often achievable with the current therapeutic options. Dietary management of patients with HCU is difficult and complex, including Met-free formula, Cys supplementation, and adjuvant therapy with betaine (4, 7), so it often results in poor compliance and both progression and exacerbation of clinical symptoms (8). Therefore, there is an urgent need for novel therapeutic options for HCU among patients, their families, and their caregivers.
In the past years, we have developed and extensively studied an enzyme replacement therapy (ERT) for HCU using polyethylene glycol (PEG)-modified truncated human CBS (OT-58, previously also known as 20NHS PEG-CBS). We showed that OT-58 is consistently and reproducibly modified with PEG moieties and retains a full catalytic activity and in vivo efficacy of the unmodified enzyme (9). Furthermore, OT-58 showed remarkable efficacy in multiple mouse models of HCU, correcting biochemical as well as clinical symptoms of murine HCU, such as survival and liver disease (10), bone density, and body composition (11), or alopecia and ocular features (12). It is important to note that all these benefits of OT-58 were achieved on the background of normal Met intake with no adjuvant therapy. In addition, OT-58 was well tolerated in nonhuman primates (13).
In the present study, we evaluated benefits of a long-term treatment with OT-58 on the background of normal Met intake using a mouse model of severe HCU and compared it with Met restriction, the current standard of care for patients with HCU. In addition, we assessed, for the first time, the efficacy of OT-58 on the background of low Met intake. This has allowed us to estimate what to expect in the first in-human clinical trials, in which the enrolled patients will maintain their standard of care, primarily Met/protein restriction. We followed the relevant end points, which are similar or shared with clinical symptoms of patients with HCU, such as plasma metabolites, learning and cognitive capacity, endothelial dysfunction, thrombosis, bone mineralization, and body composition.
MATERIALS AND METHODS
Chemicals
Unless stated otherwise, all materials were purchased from MilliporeSigma (Burlington, MA, USA) or Thermo Fisher Scientific (Waltham, MA, USA). L-[U-14C]-serine was obtained from PerkinElmer Life Sciences (Waltham, MA, USA). Human truncated CBS carrying C15S mutation and PEGylated with linear 20-kDa N-hydroxysuccinimide ester PEG (OT-58) was prepared as previously described (9, 14).
Animals and study design
A breeding pair of heterozygous transgenic I278T mice on the C57BL6 background was provided by Dr. Warren Kruger (Fox Chase Cancer Center, Philadelphia, PA, USA). Mice were propagated and genotyped at our facility as previously described (15). Breeding pairs were maintained on extruded standard diet 2920X (Envigo, Huntingdon, United Kingdom) and water containing 25 mM ZnCl2 to induce transgene expression and thus rescue the homozygous I278T pups from neonatal death. After weaning at 21 d of age, homozygous I278T mice and their wild-type (WT) siblings were assigned into 1 of 8 groups. At 24 d of age, mice were switched to amino acid–defined diets with either normal (TD.170063, 4% Met; groups A, B, C, and D; Envigo) or restricted methionine content (TD.110591, 0.5% Met; groups E, F, G, and H; Envigo). In addition to a new diet at d 24, mice also received subcutaneously injected PBS vehicle (groups A, C, E, and G) or 10 mg/kg OT-58 (groups B, D, F, and H) 3 times a week (Monday, Wednesday, Friday) through wk 22 of age. Mice were weighed weekly, with weights used to calculate the OT-58 injection volumes for the week. Beginning at wk 6, blood samples were obtained every 4 wk to assess plasma sulfur metabolites. Samples were collected 24 h after the second injection in that week (i.e., on Thursdays). Beginning at wk 16, mice were subjected to the behavioral testing. They were subsequently analyzed by high-frequency ultrasound for noninvasive assessment of endothelium-dependent dilation at wk 19 of age. At wk 22, mice were subjected to dual-energy X-ray absorptiometry (DXA) scanning to assess bone density and body composition. Mice were allowed to recover for 1 d, and subsequently their in vivo thrombus formation time was determined using a ferric chloride–induced carotid artery chemical injury thrombosis model (CITM). Shortly thereafter, mice were exsanguinated and euthanized. Selected representative mice from each group were perfused with PBS, and their organs were harvested and archived for future analysis. Design of the study is summarized in Supplemental Fig. S1 and Supplemental Table S1. Procedures involving mice were performed at the University of Colorado Denver under Institutional Animal Care and Use Committee–approved protocol B-49414(03)1E. The University is an Assessment and Accreditation of Laboratory Animal Care–accredited (00235), Public Health Service–assured (A 3269-01), and United States Department of Agriculture–licensed (84-R-0059) institution.
Plasma collection and analysis
A single-use lancet for submandibular bleeding was used for blood collection into BD Microtainer Plasma Separation Tubes with lithium heparin (Becton Dickinson, San Diego, CA, USA). Tubes were then centrifuged at 10,000 g for 5 min, followed by transfer of plasma to 1.5-ml tubes and storage at −80°C. Plasma sulfur amino acid metabolites were determined by stable-isotope-dilution liquid chromatography tandem mass spectrometry as previously described by Arning and Bottiglieri (16).
Behavioral assessment
In all behavioral studies, the experimenter was blinded with regard to the genotype, diet, and treatment of the mice. The open field task examines aspects of anxiety-like behavior (17). Mice freely explored an open box (44 × 44 × 24 cm) while being tracked from above for a 10-min trial. Prior to starting the trial, the center and perimeter were delineated using the animal tracking software (Ethovision XT, Noldus, Wageningen, The Netherlands). The software was used to determine the amount of time spent in the center vs. periphery, as well as the distance traveled.
The puzzle box task was performed as previously described by Ben Abdallah et al. (18). Briefly, mice were given 3 min to enter a goal box (15 × 28 × 28 cm). If the mouse did not enter the goal box, it was encouraged and directed by the experimenter. The covered goal box was adjacent to a well-lit, uncovered arena (58 × 28 × 28 cm). The entry of the goal box began as a doorway (trial 1) and was then changed to an underpass (trials 2, 3, 4); the underpass was then filled with sawdust (trials 5, 6, 7), and then the filled underpass was covered with a small rectangular piece of cardboard (trials 8, 9). Each mouse underwent 9 trials over a period of 3 d. The first day consisted of trials 1–3, the second day consisted of trials 4–6, and trials 7–9 occurred on the third day.
Flow-mediated vasodilation
Endothelium-dependent flow-mediated vasodilation (FMD) was performed as previously described by Schuler et al. (19). Briefly, mice were anesthetized with isoflurane (3% induction and 1.5% maintenance). Body temperature was kept at 37 ± 1°C by using a heated examination table that was also equipped with electrocardiogram electrodes and monitored using rectal thermometer probe. Hind limbs were shaved and covered with warm ultrasound gel. The ultrasound probe was attached to a stereotactic holder and was manually aligned with the femoral vein visible at the upper inner thigh. We used the high-frequency, high-resolution Vevo 2100 imaging platform equipped with 30–70 MHz linear array microscan transducer (Fujifilm, Tokyo, Japan) to image the femoral arteries in mice. A vascular occluder (Docx, Ukiah, CA, USA) was placed around the lower limb to induce occlusion of the distal hind limb as an ischemic trigger. Once a clear image of the vessel wall was obtained and baseline readings were recorded, the experiment started by inflation of the vascular occluder for 5 min. Following hind limb ischemia, the cuff was deflated, and femoral artery–diameter measurements were continuously recorded for 5 min at 30-s intervals. Femoral artery diameter in acquired images was determined offline manually.
DXA
Bone density and body composition were assessed in anesthetized mice (combination of 60 mg/kg ketamine and 15 mg/kg xylazine in PBS injected intraperitoneally) using a GE Lunar PixiMus scanner (Lunar Corp., Madison, WI, USA). Mice were placed on a tray with their abdomen down and scanned from the neck down excluding the tail. After the scan, the mice were placed back into their cage to recover.
CITM
The measurement was performed as previously described by Marchi et al. (20). Mice were anesthetized with an intraperitoneal injection of a combination of ketamine (induction dose of 80–100 mg/kg, then 10–20 mg/kg/hr for maintenance) and xylazine (induction dose of 8–16 mg/kg, then 1–2 mg/kg/hr for maintenance), and body temperature was maintained at 36–38°C using a heating pad and monitored with a rectal thermometer probe. The right common carotid artery was exposed, and baseline flow was monitored with a 0.5 PBS Doppler flow probe (Transonic Systems, Ithaca, NY, USA). For the artery injury, a 1 × 1-mm Whatman filter paper soaked in 6% ferric chloride solution was placed on the carotid artery for 3 min. Blood flow in the vessel was monitored and recorded for up to 20 min using the ultrasound probe. The time to occlusion was defined as the time between FeCl3 administration and lack of flow for 1 min. Blood was drawn from the inferior vena cava into a syringe flushed with 3.8% sodium citrate and processed to platelet-poor plasma by centrifugation at 5,000 g for 10 min. At the end of the procedure, the mice were euthanized by cervical dislocation followed by bilateral thoracotomy.
Statistical analysis
Data are presented as means ± sem. Statistical analysis was conducted using ANOVA, followed by a Tukey’s multiple comparison test, and a value of P < 0.05 indicated statistical significance.
RESULTS
Long-term treatment with OT-58 resulted in better plasma metabolic balance than Met restriction
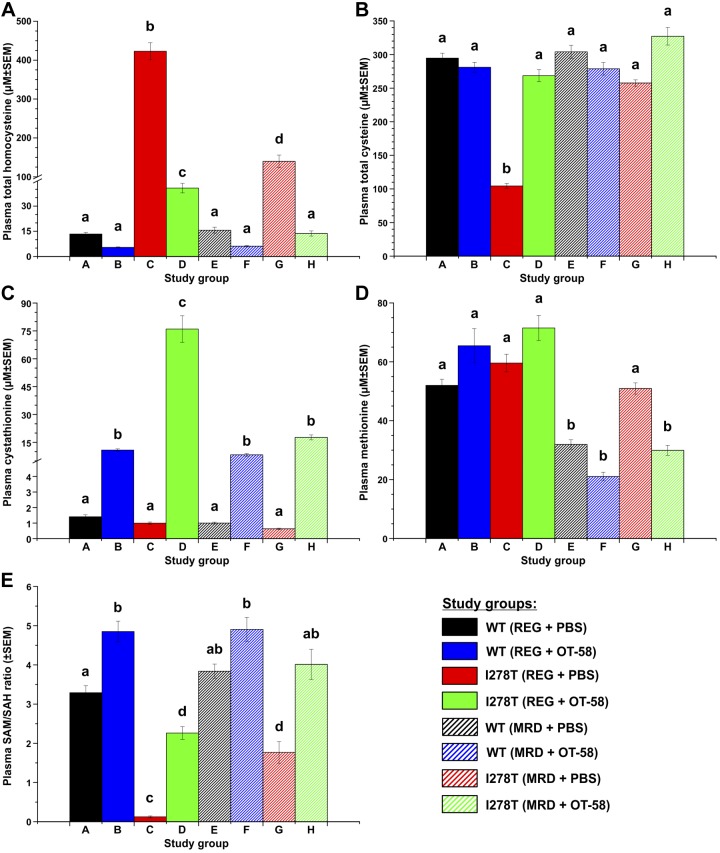
Dysregulation of sulfur amino acid metabolism is the key feature of HCU phenotype in both human patients and mouse models of the disease. Sustained improvement or restoration of metabolic control are the key aspects of successful treatment (4, 10, 12). As shown in Fig. 1A, plasma Hcy levels were in the normal healthy range (≤16 µM) in WT mice regardless of diet or treatment. However, in I278T mice fed a regular diet (REG) receiving vehicle, plasma Hcy levels were greatly elevated compared with the controls (423 µM, P < 0.001). For mice fed the methionine-restricted diet (MRD), plasma Hcy levels were substantially lower (140 µM, i.e., 67% decrease) but still remained markedly elevated compared with the controls (P < 0.001). The OT-58 treatment significantly decreased plasma Hcy levels in I278T mice fed the REG diet to 41 µM (90% decrease, P < 0.001) and entirely normalized at 14 µM in mice fed the MRD diet (P < 0.001) compared with vehicle-injected I278T mice. Plasma Cys levels (Fig. 1B) were almost 3-fold lower in I278T mice fed the REG diet and receiving vehicle compared with the WT controls (105 vs. 295 µM, P < 0.001) and were within the normal range in all the remaining groups. Consistent with its role as the product of the reaction catalyzed by OT-58, plasma Cth levels (Fig. 1C) were elevated in all mice receiving OT-58 treatment compared with their vehicle-injected counterparts. The elevation of plasma Cth depended on the concentration of Hcy, the limiting substrate, reaching 76 and 18 µM in I278T mice fed the REG and MRD diets, respectively (P < 0.001). OT-58 retained its efficacy even when substrate concentrations were very low, as indicated by ∼8-fold–higher plasma Cth levels in OT-58–treated controls compared with those receiving vehicle. Plasma Met levels (Fig. 1D) were higher in all groups fed the REG than those fed the MRD diet. However, plasma Met levels in I278T on the MRD were similar to the controls fed the REG diet (51 vs. 52 µM, P = ns) and were normalized by OT-58 treatment (30 µM, P < 0.001). The ratio of S-adenosylmethionine (SAM) to S-adenosylhomocysteine (SAH) (Fig. 1E), reflecting methylation capacity, was substantially reduced in I278T mice receiving the REG diet (0.12) and partially elevated in mice receiving MRD diet (1.77) compared with the respective controls (3.29 and 3.84 in WT mice receiving the REG and MRD diets, respectively; P < 0.001). With OT-58 treatment, the SAM/SAH ratios were significantly improved and fully normalized in I278T mice receiving the REG (2.26, P < 0.001) and MRD (4.01, P < 0.001), respectively. In general, differences among groups described herein for wk 18 time point, following the 12-wk treatment, were consistent with those observed during the entire course of the study, as indicated by full time course plots shown in Supplemental Fig. S2.
Figure 1.
Plasma sulfur metabolites at 18 wk of age. Representative snapshots at plasma concentrations of total Hcy (A), total Cys (B), Cth (C), methionine (D), and SAM/SAH ratio (E) in mice at 18 wk of age. There were 8 study groups, as detailed in Supplemental Table S1, following study design showed in Supplemental Fig. S1. Briefly, beginning from wk 4 of age, mice were maintained on diets with normal (REG) or restricted (MRD) methionine content and received subcutaneous injections of either PBS vehicle or 10 mg/kg OT-58 3 times a week until the study end at wk 22 of age. A time course for plasma metabolite profiles reflecting entire duration of the study can be found in Supplemental Fig. S2. Significance is designaed by letters (a–d) at the top of the error bars. Columns that are significantly different from each other (P < 0.05) are indicated with different letters. Two letters indicate that the column is not significantly different from the single letter–designated columns.
Both the MRD and OT-58 treatment of I278T mice improved their learning capability and cognition, but Met restriction increased anxiety
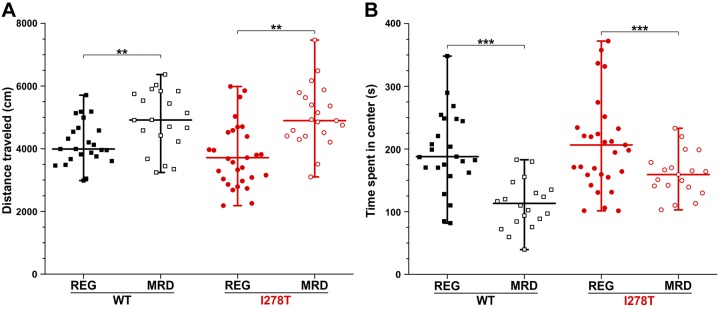
Developmental delay and various psychiatric symptoms are common among patients with HCU. First, we performed the open field test (Fig. 2) to assess anxiety level and locomotor activity in studied mice. We did not find any significant impact of OT-58 treatment on anxiety level or locomotion of both the control and I278T mice. However, the mice receiving the MRD, regardless of genotype, showed significantly increased distance traveled in the testing arena (P < 0.01, Fig. 2A) and were more anxious, as they spent less time in the center of the arena (P < 0.001, Fig. 2B) compared with the mice receiving the REG diet.
Figure 2.
Open field test. Although mouse gender and PBS vehicle or OT-58 injections did not show any significant impact on mouse behavior, the REG (filled symbols) and MRD (open symbols) diets resulted in changes in locomotion (A) and anxiety level (B) in both WT (black) and I278T (red) mice. Data points represent results for each individual mouse. Vertical lines delineate minimal and maximal values, whereas horizontal lines designate group mean values. **P < 0.01, ***P < 0.001.
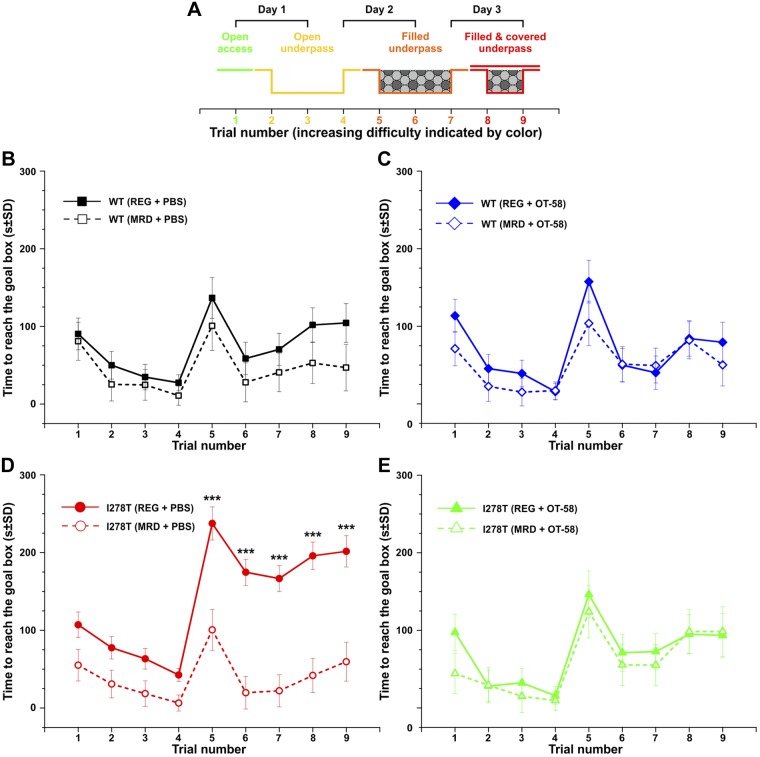
Second, we evaluated cognition/executive function of the studied mice in the puzzle box test (Fig. 3), in which mice must reach a dark goal box to escape a brightly lit arena. Access to the goal box is made more difficult by each trial, as indicated in Fig. 3A. This sequence enabled assessment of problem-solving abilities (trials 2, 5, and 8) and learning/short-term memory (trials 3, 6, and 9). In addition, repetition on the next day provided a measure of long-term memory (trials 4 and 7). Control mice receiving either diet and receiving vehicle or OT-58 performed similarly, with no signs of cognitive impairment (Fig. 3B, C). On the other hand, I278T mice fed the REG diet showed significantly poorer performance, indicating cognitive impairment, compared with those fed the MRD diet or WT controls (P < 0.001, Fig. 3D). With OT-58 treatment, the I278T mice performed as well as control mice fed both diets (Fig. 3E).
Figure 3.
Puzzle box test. A) Diagram of entry conditions in the puzzle box test for each trial during the 3-d testing period. B, C) Time to reach the goal box was similar in WT mice fed either diet and receiving vehicle (B) or OT-58 (C). D) I278T mice receiving vehicle and fed the REG diet were significantly cognitively impaired compared with the ones fed the MRD diet, as indicated by the longer time to reach goal box. E) However, I278T injected with OT-58 and fed the REG diet performed similarly as controls or I278T mice fed the MRD diet. Solid lines with filled symbols and dashed lines with open symbols designate mice fed the REG and MRD diets, respectively. Data points in all plots represent mean values, and error bars show sd. ***P < 0.001.
I278T mice suffered from endothelial dysfunction, which was normalized by either treatment
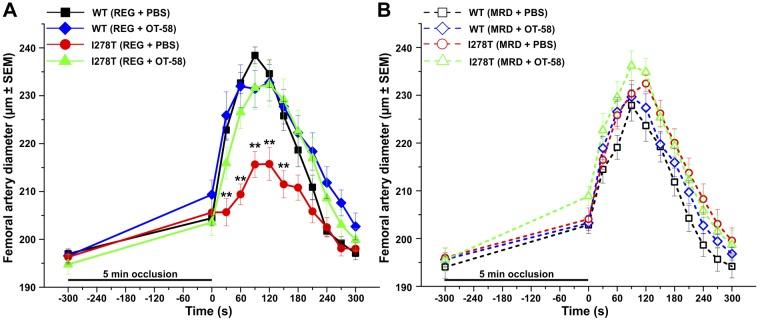
Thromboembolism is the major cause of morbidity and early death in patients with HCU (1). As the presence of endothelial dysfunction is closely associated with cardiovascular risk, we evaluated endothelium-dependent FMD in femoral artery of the studied mice (Fig. 4). We used a protocol with a 5-min period of total occlusion of the femoral artery to induce reactive hyperemia and monitor changes in femoral artery diameter. As shown in Fig. 4A, I278T mice fed the REG diet and receiving vehicle clearly showed endothelial dysfunction as indicated by significantly lower maximal dilation of the femoral artery at 90- and 120-s time points (both 216 µm) following 5 min ischemia than the controls (238 and 235 µm, respectively, P < 0.001), as well as I278T mice fed the same diet but receiving OT-58 treatment (both 232 µm, P < 0.01). In contrast, none of the mice fed the MRD diet showed any signs of impaired endothelial function at either time point and exhibited a normal vascular response to ischemia with and without OT-58 treatment (Fig. 4B).
Figure 4.
FMD. Femoral artery diameter of mice fed the REG (A) or MRD diet (B) was evaluated in 19-wk-old mice. After recording the baseline values, 5 min occlusion with no blood flow induced reactive hyperemia characterized by temporal increase of artery diameter before returning to baseline. I278T mice receiving vehicle and the REG diet showed significantly decreased response compared with all other groups. Solid lines with filled symbols and dashed lines with open symbols designate mice fed the REG and MRD diets, respectively. Data points in all plots represent mean values, and error bars show sem. **P < 0.01.
I278T mice showed delay in an experimental thrombosis model, which was normalized by either treatment
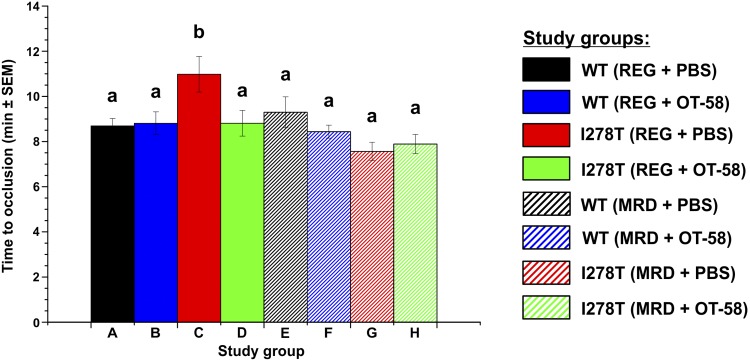
To assess whether I278T mice display prothrombotic phenotype and, if yes, how diet and ERT would affect it, we induced experimental thrombosis of the common carotid artery using 6% FeCl3. The vehicle-injected I278T mice fed the REG diet displayed substantially longer times to stable occlusion than did the other groups (10 min, 59 s, Fig. 5), indicating that, unlike patients with HCU, I278T mice do not show a prothrombotic phenotype and, moreover, showed significant delays in thrombosis compared with WT controls (8 min, 42 s, P < 0.05). Interestingly, treatment of I278T mice with OT-58 (8 min 49 s) or with the MRD diet (7 min 34 s) normalized the times for occlusion to values seen in controls (Fig. 5).
Figure 5.
CITM. Time to stable thrombotic occlusion in the carotid artery after chemical injury induced by 6% FeCl3 was determined in 22-wk-old mice. Only I278T mice fed the REG diet and receiving vehicle showed significantly prolonged time to vessel occlusion, whereas the MRD diet and/or the treatment with OT-58 normalized this parameter to control levels. Significance is designaed by letters (a, b) at the top of the error bars. Columns that are significantly different from each other (P < 0.05) are indicated with different letters (a, b).
Met restriction impaired bone mineralization and body composition, whereas OT-58 treatment normalized phenotype of I278T mice fed the REG diet
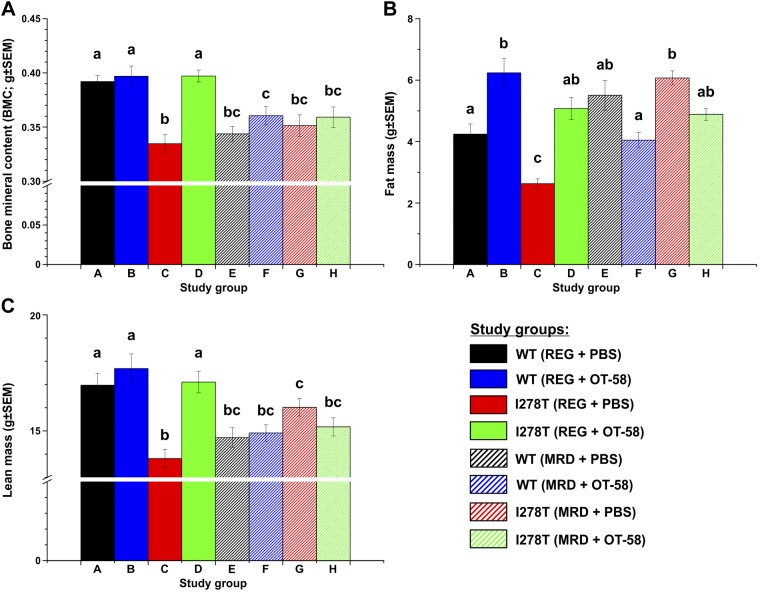
The clinical symptoms observed in patients with HCU include thinness, low body mass index, and osteoporosis (1). Therefore, we examined each group of mice using DXA to evaluate their bone mineral content (BMC) and body composition. For mice fed the REG diet, BMC (Fig. 6A) was significantly lower in vehicle-receiving I278T mice compared with the WT controls (0.33 vs. 0.39 g, P < 0.001), whereas treatment with OT-58 fully normalized it (0.4 vs. 0.4 g, P = ns). On the MRD diet, all groups showed equivalent BMC (0.34–0.36 g), which was significantly reduced compared with the controls fed the REG diet (P < 0.05) and similar to vehicle-injected I278T mice fed the REG diet. For I278T mice fed the REG diet, those receiving vehicle were significantly leaner than those in other groups [specifically, they contained less fat tissue (2.63 vs. 4.24 g, P < 0.01; Fig. 6B) and lean mass (13.81 vs. 16.97 g, P < 0.001; Fig. 6C) than controls]. Treatment with OT-58 normalized body composition of I278T mice fed the REG diet. On the MRD diet, amount of fat mass was comparable in all groups and similar to that of controls or OT-58–treated I278T mice fed the REG diet (Fig. 6B). Likewise, the amount of lean mass was similar in all groups fed the MRD diet (14.71–16.02 g) but significantly reduced compared with controls and OT-58–treated I278T mice fed the REG diet (P < 0.05, Fig. 6C).
Figure 6.
Bone mineralization and body composition assessed by DXA. The DXA scans were performed when the mice reached age of 22 wk. Individual panels show BMC (A), fat mass (B), and lean mass (C). Bars represent a mean value from individual mice, and error bars indicate sem. Significance is designated by letters (a–c) at the top of the error bars. Columns that are significantly different from each other (P < 0.05) are indicated with different letters. Two letters indicate that the column is not significantly different from the single letter–designated columns.
DISCUSSION
In the past years, we have been at the forefront of developing ERT for HCU (10, 12, 14), which entered the first in-human clinical trial at the beginning of 2019. Novel therapeutic options for HCU have been a pressing unmet need for decades for patients, caregivers, and their advocacy groups, as current management of the disease represents a significant compliance challenge, impacts quality of life, and ultimately yields unsatisfactory results leading to both progression and exacerbation of clinical complications (4). In the present study, we used an established murine model of HCU to evaluate benefits of ERT in conjunction with and in comparison to Met restriction, the standard of care. The main finding of this study is that ERT on the background of normal Met intake yielded equal or better results compared with Met restriction.
Guidelines for management of CBS-deficient HCU recommend keeping the plasma total Hcy concentration below 50 and 100 µM in patients who are fully responsive and nonresponsive to pyridoxine, respectively (4). In our study, I278T mice were unable to achieve this target level, even when maintained on severe Met restriction receiving 8-fold–less Met than the standard recommended intake for mice (0.5 and 4 g/kg Met in the MRD and REG diets, respectively). Except for the initial sample taken at wk 6 of age, plasma Hcy levels fluctuated between 121 and 195 µM in I278T mice fed the MRD diet during the study (Fig. 1 and Supplemental Fig. S2). Using the same mouse model, Gupta et al. (21) found that a similar MRD diet containing 0.5 g/kg Met decreased serum Hcy to 81 µM in 34-wk-old I278T mice. The difference could be attributed to the age of mice and length of their maintenance on the diet. In contrast, plasma Hcy levels of I278T mice fed the REG diet and treated with OT-58 varied in the 26–41 µM range, except the last sample taken at 22 wk of age. Moreover, administration of OT-58 to I278T mice fed the MRD diet completely normalized plasma Hcy below 15 µM. These results show that, unlike ERT, even severe Met restriction does not reduce plasma Hcy levels to the therapeutic target in HCU mice. If the full metabolic normalization is desired, a combination of ERT with some level of Met restriction would be needed to achieve this goal. Considering published data and other results mentioned in this study, it is apparent that full metabolic normalization is not required to achieve correction of HCU phenotype, and thus, there seems to exist a threshold effect when it comes to manifestation of HCU phenotype in mice. We and others showed that less severe mouse models of HCU with plasma Hcy levels between 170 and 300 µM had much less severe phenotype (22) or showed no clinical symptoms of HCU (11).
Many psychologic and psychiatric conditions are common in patients with HCU, such as psychosis, obsessive-compulsive disorder, depression, and behavior/personality disorders (23). In addition, individuals with HCU have many developmental and cognitive difficulties, with a significant number of cases having learning disability and lower IQs (24). Behavioral testing of our mice showed that this phenotype is indeed replicated in I278T mice showing cognitive impairment when maintained on the REG diet and receiving vehicle compared with controls and I278T mice maintained on the MRD or treated with OT-58 (Fig. 3). To our knowledge, this is the first report of such phenotype and its successful correction in any mouse model of CBS-deficient HCU. In addition, we observed that mice showed significantly increased activity and anxiety level when maintained on the MRD compared with those fed the REG diet (Fig. 2). These behavioral traits are likely unrelated to HCU, as it affected mice regardless of genotype and OT-58 treatment. The MRD and REG diets were isocaloric and isonitrogenous, and no differences in diet consumption were detected between the groups. Nevertheless, although highly unlikely, increased anxious behavior could also be attributed to hunger in mice fed the MRD caused by the methionine restriction. It has been shown that essential amino acid deprivation, including Met restriction, is sensed in the anterior piriform cortex, and subsequent glutamatergic signaling influences mouse behavior (25). Glutamate is the main excitatory neurotransmitter in the CNS, which could explain increased locomotion, restlessness, and anxiety level.
Thromboembolism is the major cause of morbidity and mortality in HCU (1, 4), yet I278T mice fed the REG diet and receiving placebo failed to exhibit the propensity for arterial thrombosis (Fig. 5), despite significant endothelial dysfunction (Fig. 4). A similar finding was reported earlier using the same I278T mouse model (26), as well as for total CBS knockout (KO) mouse (27). On the other hand, another transgenic mouse model of HCU, called HO, showed a hypercoagulative state, which was significantly ameliorated, but not normalized, by betaine treatment (28). Except for the diet-induced hyperhomocysteinemia in a mouse model (29), the HO model is so far the only genetic mouse model of HCU that recapitulates this major clinical symptom of HCU. Significantly lower plasma Hcy concentrations, markedly elevated plasma Cth levels, normal survival, unaffected bone mineralization, and body composition and lack of hepatopathy in HO mice compared with other mouse models of HCU could be responsible for prothrombotic phenotype (11, 28). It has been hypothesized that hepatopathy observed in I278T (15) or KO mice (10, 27, 30) may cause defects in liver-dependent production of coagulation factors, whose deficit in turn would mask prothrombotic effects of elevated Hcy (27). However, no changes in activated plasma prothrombin time or amount of plasma fibrinogen (26) or plasma levels of liver enzymes alanine aminotransferase, aspartate aminotransferase, and alkaline phosphatase (12) have been demonstrated in I278T mice, which would argue that liver is competent enough to maintain physiologic hemostasis. Importantly, both the endothelial dysfunction and paradoxically prolonged time to occlusion have been normalized in I278T mice fed the MRD diet (regardless of OT-58 coadministration) or those fed the REG diet but treated with OT-58. These results indicate that, despite lack of hypercoaguable state in I278T mice, Met restriction or administration of OT-58 may be effective in correcting pathologic hemostasis observed in other mouse models of HCU and, more importantly, in human patients.
Skeletal abnormalities and connective tissue defects are the most prominent clinical symptoms in patients with HCU (1). Osteoporosis, often associated with scoliosis, and low body mass index are typical findings in untreated or late-diagnosed patients, regardless of severity of the disease (1, 31). Decreased bone mineralization, low fat content, and low overall weight was found in the KO and I278T (10, 12, 22) but not the HO mouse model of HCU (11). As shown in Fig. 6, we confirmed these findings in I278T mice fed the REG diet and receiving vehicle as well as the efficacy of OT-58 in normalizing this phenotype (11, 12). On the other hand, severe Met restriction resulted in a decreased bone mineralization, lower amount of lean mass, and normal to increased amount of fat mass in all mice fed the MRD regardless of genotype and treatment compared with WT mice fed the REG diet. With exception of fat mass, MRD essentially yielded an overall picture very similar to untreated I278T mice fed the REG diet and receiving vehicle (Fig. 6). Previously, MRD diet was found very effective at correcting HCU phenotype in I278T mice, including bone mineralization (21, 32). Our data on mice indicate that Met restriction, the mainstay therapy for the disease, could lead to an unwanted outcome in patients with HCU. Indeed, the guidelines emphasize that Met restriction should not compromise growth and nutrition and should be determined individually by an experienced metabolic dietitian (4).
To summarize, the MRD diet leads to increased anxiety and reduced bone mineralization in all mice, whereas it corrects cognition and vascular phenotype in HCU mice in spite of failing to reduce plasma Hcy concentration below the recommended level. On the other hand, ERT with OT-58 decreased plasma Hcy concentrations below the suggested threshold of 100 µM and corrected all the monitored symptoms of HCU. Furthermore, OT-58 retains its efficacy under Met restriction, yielding fully normalized plasma biochemical profile. By extrapolating these data to human patients, our results argue that OT-58 as a single life-long therapy could be efficacious in prevention and correction of clinical symptoms of HCU. Because of the changes in size and rates of metabolism between mice and humans, we previously estimated that the dose of 0.66 mg/kg administered once a week would achieve the same benefit in human patients as dosing regimen used in mice (13). With OT-58 available in concentration of 25 mg/ml, this would correspond to a single, once-weekly subcutaneous injection of volume <2 ml to a <75-kg patient. In addition, treatment with OT-58 should allow for elimination of Met/diet restriction and thus, in turn, substantially improve quality of life of patients with HCU and their families.
ACKNOWLEDGMENTS
The authors thank Teodoro Bottiglieri (Institute of Metabolic Disease, Baylor Scott and White Research Institute, Dallas, TX, USA) for plasma metabolites measurements; Nicolas Busquet and Stacey Zander (University of Colorado) for help with behavioral assessment; Korey Haefner, Sara Wennersten, and Maria Cavasin (University of Colorado) for assistance with flow-mediated vasodilation (FMD) measurements; and Kimberley Bruce (University of Colorado Anschutz Medical Campus, Aurora, CO, USA) for access to the dual-energy X-ray absorptiometry (DXA) scanner. T.M. is a recipient of American Heart Association Scientist Development Grant 16SDG30040000. This study was supported by research grants from Orphan Technologies, Ltd., a private pharmaceutical company developing an enzyme replacement therapy for cystathionine β-synthase (CBS)-deficient homocystinuria (to J.P.K. and T.M.). Shared resources funded by Grant 1S10OD018156-01 from the U.S. National Institutes of Health, Office of the Director were used. The authors declare the following conflicts of interest: T.M., E.M.B., and J.P.K. are inventors on patents related to the processes and products referred here (U.S. patents 9034318 and 9243239). T.M. provides consulting services to Orphan Technologies, Ltd. E.M.B. is an employee of Orphan Technologies, Ltd.
Glossary
- BMC
bone mineral content
- CBS
cystathionine β-synthase
- CITM
carotid artery chemical injury thrombosis model
- Cth
cystathionine
- DXA
dual-energy X-ray absorptiometry
- ERT
enzyme replacement therapy
- FMD
flow-mediated vasodilation
- HCU
homocystinuria
- Hcy
homocysteine
- KO
knockout
- MRD
methionine-restricted diet
- ns
nonsignificant
- OT-58
truncated human CBS C15S mutant modified with linear 20-kDa N-hydroxysuccinimide ester polyethylene glycol
- PEG
polyethylene glycol
- REG
regular diet
- SAH
S-adenosylhomocysteine
- SAM
S-adenosylmethionine
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
T. Majtan designed and performed animal studies, analyzed data, prepared figures, wrote the initial draft, and coordinated the project and collaborations; I. Park took care of the mouse colony and assisted in execution of the studies; A. Cox, B. R. Branchford, and J. di Paola provided analytical tools, performed thrombosis model studies, and interpreted the results; E. M. Bublil provided reagents and assisted in data analyses; J. P. Kraus codesigned the studies, reviewed the data, and coordinated the project; and all authors reviewed the draft, contributed to its revisions, and approved the final form of the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Mudd S. H., Levy H. L., Kraus J. P. (2001) Disorders of transsulfuration. In The Metabolic and Molecular Bases of Inherited Disease (Scriver C. R., Beaudet A. L., Sly W. S., Valle D., Childs B., Kinzler K., Vogelstein B., eds.), pp. 2007–2056, McGraw-Hill, New York [Google Scholar]
- 2.Finkelstein J. D. (1990) Methionine metabolism in mammals. J. Nutr. Biochem. 1, 228–237 [DOI] [PubMed] [Google Scholar]
- 3.Majtan T., Pey A. L., Ereño-Orbea J., Martínez-Cruz L. A., Kraus J. P. (2016) Targeting cystathionine beta-synthase misfolding in homocystinuria by small ligands: state of the art and future directions. Curr. Drug Targets 17, 1455–1470 [DOI] [PubMed] [Google Scholar]
- 4.Morris A. A., Kožich V., Santra S., Andria G., Ben-Omran T. I., Chakrapani A. B., Crushell E., Henderson M. J., Hochuli M., Huemer M., Janssen M. C., Maillot F., Mayne P. D., McNulty J., Morrison T. M., Ogier H., O’Sullivan S., Pavlíková M., de Almeida I. T., Terry A., Yap S., Blom H. J., Chapman K. A. (2017) Guidelines for the diagnosis and management of cystathionine beta-synthase deficiency. J. Inherit. Metab. Dis. 40, 49–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mudd S. H., Edwards W. A., Loeb P. M., Brown M. S., Laster L. (1970) Homocystinuria due to cystathionine synthase deficiency: the effect of pyridoxine. J. Clin. Invest. 49, 1762–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kluijtmans L. A., Boers G. H., Kraus J. P., van den Heuvel L. P., Cruysberg J. R., Trijbels F. J., Blom H. J. (1999) The molecular basis of cystathionine beta-synthase deficiency in Dutch patients with homocystinuria: effect of CBS genotype on biochemical and clinical phenotype and on response to treatment. Am. J. Hum. Genet. 65, 59–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiff M., Blom H. J. (2012) Treatment of inherited homocystinurias. Neuropediatrics 43, 295–304 [DOI] [PubMed] [Google Scholar]
- 8.Walter J. H., Wraith J. E., White F. J., Bridge C., Till J. (1998) Strategies for the treatment of cystathionine beta-synthase deficiency: the experience of the Willink Biochemical Genetics Unit over the past 30 years. Eur. J. Pediatr. 157 (Suppl. 2), S71–S76 [DOI] [PubMed] [Google Scholar]
- 9.Majtan T., Park I., Carrillo R. S., Bublil E. M., Kraus J. P. (2017) Engineering and characterization of an enzyme replacement therapy for classical homocystinuria. Biomacromolecules 18, 1747–1761 [DOI] [PubMed] [Google Scholar]
- 10.Majtan T., Hůlková H., Park I., Krijt J., Kožich V., Bublil E. M., Kraus J. P. (2017) Enzyme replacement prevents neonatal death, liver damage, and osteoporosis in murine homocystinuria. FASEB J. 31, 5495–5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Majtan T., Park I., Bublil E. M., Kraus J. P. (2018) Enzyme replacement therapy prevents loss of bone and fat mass in murine homocystinuria. Hum. Mutat. 39, 210–218 [DOI] [PubMed] [Google Scholar]
- 12.Majtan T., Jones W., Jr., Krijt J., Park I., Kruger W. D., Kožich V., Bassnett S., Bublil E. M., Kraus J. P. (2018) Enzyme replacement therapy ameliorates multiple symptoms of murine homocystinuria. Mol. Ther. 26, 834–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Majtan T., Bublil E. M., Park I., Arning E., Bottiglieri T., Glavin F., Kraus J. P. (2018) Pharmacokinetics and pharmacodynamics of PEGylated truncated human cystathionine beta-synthase for treatment of homocystinuria. Life Sci. 200, 15–25 [DOI] [PubMed] [Google Scholar]
- 14.Bublil E. M., Majtan T., Park I., Carrillo R. S., Hůlková H., Krijt J., Kožich V., Kraus J. P. (2016) Enzyme replacement with PEGylated cystathionine β-synthase ameliorates homocystinuria in murine model. J. Clin. Invest. 126, 2372–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang L., Chen X., Tang B., Hua X., Klein-Szanto A., Kruger W. D. (2005) Expression of mutant human cystathionine beta-synthase rescues neonatal lethality but not homocystinuria in a mouse model. Hum. Mol. Genet. 14, 2201–2208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arning E., Bottiglieri T. (2016) Quantitation of S-adenosylmethionine and S-adenosylhomocysteine in plasma using liquid chromatography-electrospray tandem mass spectrometry. Methods Mol. Biol. 1378, 255–262 [DOI] [PubMed] [Google Scholar]
- 17.Prut L., Belzung C. (2003) The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur. J. Pharmacol. 463, 3–33 [DOI] [PubMed] [Google Scholar]
- 18.Ben Abdallah N. M., Fuss J., Trusel M., Galsworthy M. J., Bobsin K., Colacicco G., Deacon R. M., Riva M. A., Kellendonk C., Sprengel R., Lipp H. P., Gass P. (2011) The puzzle box as a simple and efficient behavioral test for exploring impairments of general cognition and executive functions in mouse models of schizophrenia. Exp. Neurol. 227, 42–52 [DOI] [PubMed] [Google Scholar]
- 19.Schuler D., Sansone R., Freudenberger T., Rodriguez-Mateos A., Weber G., Momma T. Y., Goy C., Altschmied J., Haendeler J., Fischer J. W., Kelm M., Heiss C. (2014) Measurement of endothelium-dependent vasodilation in mice--brief report. Arterioscler. Thromb. Vasc. Biol. 34, 2651–2657 [DOI] [PubMed] [Google Scholar]
- 20.Marchi R., Walton B. L., McGary C. S., Lin F. C., Ma A. D., Pawlinski R., Mackman N., Campbell R. A., Di Paola J., Wolberg A. S. (2012) Dysregulated coagulation associated with hypofibrinogenaemia and plasma hypercoagulability: implications for identifying coagulopathic mechanisms in humans. Thromb. Haemost. 108, 516–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gupta S., Melnyk S. B., Kruger W. D. (2014) Cystathionine β-synthase-deficient mice thrive on a low-methionine diet. FASEB J. 28, 781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gupta S., Kühnisch J., Mustafa A., Lhotak S., Schlachterman A., Slifker M. J., Klein-Szanto A., High K. A., Austin R. C., Kruger W. D. (2009) Mouse models of cystathionine beta-synthase deficiency reveal significant threshold effects of hyperhomocysteinemia. FASEB J. 23, 883–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abbott M. H., Folstein S. E., Abbey H., Pyeritz R. E. (1987) Psychiatric manifestations of homocystinuria due to cystathionine beta-synthase deficiency: prevalence, natural history, and relationship to neurologic impairment and vitamin B6-responsiveness. Am. J. Med. Genet. 26, 959–969 [DOI] [PubMed] [Google Scholar]
- 24.El Bashir H., Dekair L., Mahmoud Y., Ben-Omran T. (2015) Neurodevelopmental and cognitive outcomes of classical homocystinuria: experience from Qatar. JIMD Rep. 21, 89–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anthony T. G., Gietzen D. W. (2013) Detection of amino acid deprivation in the central nervous system. Curr. Opin. Clin. Nutr. Metab. Care 16, 96–101 [DOI] [PubMed] [Google Scholar]
- 26.Dayal S., Chauhan A. K., Jensen M., Leo L., Lynch C. M., Faraci F. M., Kruger W. D., Lentz S. R. (2012) Paradoxical absence of a prothrombotic phenotype in a mouse model of severe hyperhomocysteinemia. Blood 119, 3176–3183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maclean K. N., Sikora J., Kožich V., Jiang H., Greiner L. S., Kraus E., Krijt J., Crnic L. S., Allen R. H., Stabler S. P., Elleder M., Kraus J. P. (2010) Cystathionine beta-synthase null homocystinuric mice fail to exhibit altered hemostasis or lowering of plasma homocysteine in response to betaine treatment. Mol. Genet. Metab. 101, 163–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maclean K. N., Sikora J., Kožich V., Jiang H., Greiner L. S., Kraus E., Krijt J., Overdier K. H., Collard R., Brodsky G. L., Meltesen L., Crnic L. S., Allen R. H., Stabler S. P., Elleder M., Rozen R., Patterson D., Kraus J. P. (2010) A novel transgenic mouse model of CBS-deficient homocystinuria does not incur hepatic steatosis or fibrosis and exhibits a hypercoagulative phenotype that is ameliorated by betaine treatment. Mol. Genet. Metab. 101, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dayal S., Wilson K. M., Leo L., Arning E., Bottiglieri T., Lentz S. R. (2006) Enhanced susceptibility to arterial thrombosis in a murine model of hyperhomocysteinemia. Blood 108, 2237–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watanabe M., Osada J., Aratani Y., Kluckman K., Reddick R., Malinow M. R., Maeda N. (1995) Mice deficient in cystathionine β-synthase: animal models for mild and severe homocyst(e)inemia. Proc. Natl. Acad. Sci. USA 92, 1585–1589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Parrot F., Redonnet-Vernhet I., Lacombe D., Gin H. (2000) Osteoporosis in late-diagnosed adult homocystinuric patients. J. Inherit. Metab. Dis. 23, 338–340 [DOI] [PubMed] [Google Scholar]
- 32.Kruger W. D., Gupta S. (2016) The effect of dietary modulation of sulfur amino acids on cystathionine β synthase-deficient mice. Ann. N. Y. Acad. Sci. 1363, 80–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.