Abstract
Prenatal nicotine exposure (PNE) induces developmental toxicity in offspring. However, the long-term harmful effects on bone development and the intrauterine programming mechanism attributed to PNE remain unclear. In the present research, pregnant Wistar rats were injected subcutaneously with nicotine (2 mg/kg/d) to obtain and analyze bone samples from the fetal and adult offspring. Bone marrow mesenchymal stem cells (BMSCs) were treated with nicotine during osteogenic differentiation to clarify the related molecular mechanisms. The results indicated that PNE led to bone dysplasia in the fetuses and reduced bone mass in the adult offspring, which was mediated by the sustained activation of the local bone renin angiotensin system (RAS) and suppressed osteogenic differentiation before and after birth. In vitro, nicotine suppressed BMSCs’ osteogenic function through promoting angiotensin-converting enzyme (ACE) expression and activating RAS. Furthermore, nicotine induced histone acetylase p300 into the nuclei of the BMSCs by acting on the α4β2-nicotinic acetylcholine receptor (α4β2-nAChR), leading to the increased histone 3 lysine 9 acetylation level of ACE and RAS activation. Taken together, the sustained activation of local bone RAS mediated prenatal nicotine–induced osteopenia in adult offspring via the α4β2-nAChR–p300-ACE pathway.—Xiao, H., Wen, Y., Pan, Z., Shangguan, Y., Magdalou, J., Wang, H., Chen, L. Nicotine exposure during pregnancy programs osteopenia in male offspring rats via α4β2-nAChR–p300-ACE pathway.
Keywords: prenatal nicotine exposure, developmental toxicity, renin angiotensin system, intrauterine programming, histone acetylation
Smoking during pregnancy has become a global public health problem. According to statistics, the mean worldwide prevalence of smoking among pregnant women was 10–23%, even up to 59.3% in some regions (1, 2). Epidemiologic studies have shown that prenatal smoking increases the risk of complications such as intrauterine growth retardation, preterm delivery, and infant death (3, 4). Nicotine, as the main tobacco component, can cross the placenta, and when the mother smokes chronically, nicotine can reach even higher levels in the fetus than in the mother (5, 6). Animal experiments suggested that nicotine has reproductive and developmental toxicity and is the main exogenous factor for disrupting embryonic development (7). Our previous studies also found that prenatal nicotine exposure (PNE) resulted in low birth weight and retarded long bone development in fetal offspring rats (8, 9). However, the long-term harmful effects of PNE on bone mass accumulation in adult offspring and its intrauterine programming mechanism remain unclear.
Osteoblasts are derived from bone marrow mesenchymal stem cells (BMSCs), which determine bone mass formation (10, 11). Thus, the suppressed osteogenic differentiation of BMSCs induced by various factors could lead to decreased bone mass and susceptibility to osteoporosis in adulthood. The renin angiotensin system (RAS) is an important endocrine system regulating blood pressure balance in circulation (12). Angiotensin-converting enzyme (ACE) is a major component of RAS that promotes the expression of angiotensin II (Ang II), and the latter plays its role by binding to its receptors, Ang II type 1 receptor (AT1R) or Ang II type 2 receptor (AT2R) (13). To date, studies have found that the components of RAS are also expressed in various organs or tissues (including bone tissue) that are involved in regulating cell proliferation, differentiation, and apoptosis through the autocrine and paracrine pathways (14). These reports suggested that the local bone RAS activity affected by PNE may participate in developmental toxicity of bone.
Epigenetic regulation has been defined as the changes in gene expression and function that occur without a change in the DNA sequence (15). Histone modification is a common type of epigenetic form and includes histone acetylation, which is mediated by histone acetylase and can regulate the expression of target genes (16). Previous studies found that epigenetic modifications play important roles in the long-term developmental toxicity caused by an adverse environment during pregnancy (17, 18). In this research, pregnant Wistar rats were exposed to nicotine in the middle and late stages of pregnancy to elucidate the long-term effects of nicotine on the bone development in adult offspring, and the intrauterine programming mechanism about the activation of local bone RAS induced by PNE was further investigated. This study will provide a theoretical and experimental basis for defining the long-term harmful effects of prenatal nicotine–induced developmental toxicity of bone in offspring and for exploring its early prevention and treatment targets.
MATERIALS AND METHODS
Chemicals and reagents
Nicotine, captopril as an ACE inhibitor, and C646 as a p300 inhibitor were purchased from MilliporeSigma (Burlington, MA, USA). Dihydro-β-erythroidine (DH-β-E), a selective inhibitor of α4β2-nicotinic acetylcholine receptor (α4β2-nAChR), was purchased from Santa Cruz Biotechnology (Dallas, TX, USA). An Ang II ELISA Kit was obtained from Jiancheng Bioengineering Institute (Nanjing, China). Antibodies for ACE (ab11734), AT1R (ab9391), and AT2R (ab92445) were purchased from Abcam (Cambridge, United Kingdom). The antibodies for histone 3 lysine 9 acetylation (H3K9ac; A7255), histone 3 lysine 14 acetylation (H3K14ac; A7254), and histone 3 lysine 27 acetylation (H3K27ac; A7253) were purchased from ABclonal (Woburn, MA, USA).
Animals and treatment
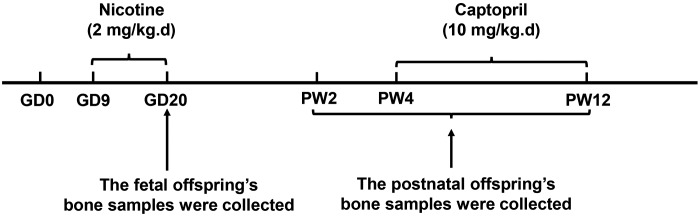
Specific pathogen–free Wistar rats weighing 209 ± 12 g (females) and 258 ± 17 g (males) were purchased from the Experimental Center of the Hubei Medical Scientific Academy (2012-2014, certification: 42000600002258; Hubei, China). The animal experiment was performed at the Center for Animal Experiments of Wuhan University (Wuhan, China). The animal experiments complied with the protocol approved by the Committee on the Ethics of Animal Experiments of the Wuhan University School of Medicine (Permit 201709) and abided by the Regulations for the Administration of Affairs Concerning Experimental Animals (Chinese Animal Welfare Committee). All of the rats were housed in a temperature-controlled room (temperature: 18–22°C; humidity: 40–60%; light cycle: 12-h light/dark cycle) and allowed free access to water and food. To reduce bias in the animal experiments, the rats were housed and treated by a technician, whereas different coauthors were in charge of the bone sample harvesting and data analysis. The experimental procedures in this study are described in Fig. 1.
Figure 1.
The animal experimental procedures.
Before experimentation, all of the rats were acclimated for 1 wk, and 2 female rats were placed together in a cage with 1 male rat overnight for mating. The appearance of sperm in the vaginal smears confirmed mating, and the mating day was considered as gestational day (GD)0. The pregnant rats were injected subcutaneously with nicotine (2 mg/kg) or an equal volume of saline every morning (between 8:00 and 9:00 am) from GD9 to GD20. Some of the pregnant rats were euthanized after anesthesia with isoflurane, and 1 male fetus was randomly selected from each litter on GD20 (n = 8/group). The left femurs and tibias were collected for histologic and immunohistochemistry analysis, whereas the right femurs and tibias were frozen immediately for further analysis.
The rest of the pregnant rats in the control and PNE groups went into spontaneous labor to produce the postnatal offspring. The pregnant rats with litter sizes ≥10 were selected, and the litter size was normalized to 10; the male:female ratio was ∼1:1. The number of pregnant rats in each group was 8. Two subgroups of the male offspring were randomly selected and euthanized with isoflurane at postnatal week (PW)2 or PW12 (n = 8 from 8 different litters at each time point). The remaining offspring rats from the control and PNE groups were intragastrically administered with captopril at a single dose of 10 mg/kg or with distilled water as vehicle daily at PW4, and then these offspring were euthanized at PW12 (n = 8 from 8 different litters) (19). The left femurs were dissected for micro-computed tomography (micro-CT) analysis. The left tibias were used for histologic or immunohistochemistry analysis. The right femurs and tibias were frozen immediately for further analysis.
Histologic and immunohistochemistry analysis
The left femurs and tibias from the fetal offspring were fixed in 4% paraformaldehyde overnight and then processed and embedded in paraffin. Serial longitudinal sections (5 μm thick) were cut, and 2 of 6 sections were stained with von Kossa staining for quantification of the indices of bone development. For von Kossa staining, the slices were stained with 5% AgNO3 until they became dark brown (n = 8/group). The indices, including the lengths of the femurs and primary ossification centers, the mineralized area (Md.Ar), and bone perimeter (B.Pm), were measured on the von Kossa–stained sections at ×100 magnification using Image-Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA) as previously described in Dempster et al. (20). Alkaline phosphatase (ALP) staining for quantifying the osteoblasts in the primary ossification centers was performed using an ALP Staining Kit following the manufacturer’s protocols.
For the immunohistochemical analysis, the bone tissue slices were cut as previously mentioned at each time point (n = 8/group). The slices were then dewaxed and washed in PBS. After antigen retrieval, the bone tissue slices were blocked in 5% blocking serum at room temperature for 1 h and incubated with primary antibody for ACE (1:20 dilution) overnight at 4°C. After washing with PBS, the slices were incubated with a biotinylated secondary antibody and then with an avidin-biotinylated horseradish peroxidase complex solution according to the manufacturer’s directions. Finally, the peroxidase activity was determined with a diaminobenzidine staining kit. For negative immunohistochemistry controls, parallel slices were immunostained with nonimmune rabbit IgG as a primary antibody. The immunostaining intensity was determined by measuring the mean optical density in 6 sections from different samples using a photo imaging system (Nikon H550S; Nikon, Tokyo, Japan).
Micro-CT measurement
The bone mass was scanned and analyzed with a micro-CT system (VivaCT 40; Scanco Medical, Brüttisellen, Switzerland) as previously described in Mys et al. (21). To determine the bone mass indices, including the bone volume:total volume ratio (BV/TV), trabecular number (Tb.N), trabecular thickness (Tb.Th), and trabecular separation (Tb.Sp), 0.5–5.5 mm below the lowest point of the growth plate was selected as the region of interest, and cross-sectional images were scanned at 21 μm resolution. The parameters of the previously described trabecular bone microarchitecture were computed using 3-dimensional model-independent algorithms (n = 8/group).
Cell culture and treatment
BMSCs were isolated as previously described in Xu et al. (22). One-month-old male Wistar rats were euthanized and soaked in 75% ethanol for 10 min. Their femurs and tibias were separated under aseptic conditions. Both ends of the epiphyses were cut off in a sterile manner, and the bone marrow cavity was rinsed with a syringe with 5 ml of α-minimum essential medium. The evenly dispersed rinse was then filtered through a 200-mesh nylon filter, and the supernatant was centrifuged at room temperature at 1000 rpm for 10 min. The cells were then resuspended and plated at a density of 4 × 105 cells per well in 6-well plates with growth medium (α-minimum essential medium with 10% fetal bovine serum, 100 mg/ml streptomycin, and 100 U/ml penicillin). When the cells reached 80% confluence, the growth medium was switched to an osteogenic differentiation medium. The cells were then treated with various concentrations of nicotine or cotreated with nicotine and captopril, DH-β-E, or C646 for further analysis. The BMSCs’ mineralization function was determined using Alizarin Red S staining. Positive staining is represented as red color mineralization nodules.
ELISA for Ang II detection
To test the concentration of Ang II in the cell culture supernatant, the BMSCs were treated with nicotine to obtain supernatant from the cell culture medium, and the Ang II ELISA kit was used to detect the concentration of Ang II following the manufacturer’s protocols. To test the concentration of Ang II in the bone tissue, 1 ml of PBS was added to an equal weighting of bone tissue from each group for homogenate and centrifuged to collect the supernatant. The concentration of Ang II was then measured using the ELISA kit following the manufacturer’s protocols.
Total RNA extraction and quantitative RT-PCR
The total RNA was isolated from the bone tissue and BMSCs using Trizol Reagent following the manufacturer’s protocol. The total RNA was reverse transcribed using a First Strand cDNA Synthesis Kit. Quantitative RT-PCR (qRT-PCR) was then performed using a Sybr Green Quantitative PCR Master Mix Kit and ABI StepOnePlus Cycler (Thermo Fisher Scientific, Waltham, MA, USA) with 40 cycles. The relative gene expression was calculated for each gene using the 2−ΔΔCt method with glyceraldehyde 3-phosphate dehydrogenase for normalization. The rat primer sequences for the genes used in this study are shown in Supplemental Table S1.
Western blotting
The cells were washed twice with ice-cold PBS and lysed in 200 μl of RIPA lysis buffer and 1 mM of PMSF for 5 min on ice to extract the total protein. Cytoplasmic protein and nucleic protein were respectively extracted using the Nuclear and Cytoplasmic Protein Extraction Kit following the manufacturer’s protocols. Equal amounts of protein lysates (30 μg/lane) were resolved via SDS-PAGE on 10% polyacrylamide gels, transferred to PVDF membranes, and blotted with the primary antibodies for ACE (1:100 dilution), AT1R (1:500 dilution), AT2R (1:1000 dilution), or p300 (1:1000 dilution) at 4°C overnight. The band intensity was quantified by using Quantity One (Bio-Rad, Hercules, CA, USA).
Chromatin immunoprecipitation assay
Cell suspensions from the bone tissue and BMSCs were collected and fixed in 1% formaldehyde for chromatin cross-linking, and 125 mM of glycine was added to stop the reaction. The mixture was then centrifuged and resuspended in 0.5 ml of lysis buffer containing protease inhibitors. The cell lysates were sonicated to shear the DNA to lengths of ∼200 bp and transferred to a new tube with chromatin immunoprecipitation (ChIP) dilution buffer. Chromatin was incubated overnight at 4°C on a nutator-rocker with specific antibodies for H3K9ac (1:50 dilution), H3K9ac (1:50 dilution), or H3K27ac (1:50 dilution) and bovine serum albumin–treated protein G beads to reduce the nonspecific background binding. The immunoprecipitated DNA-protein complex with beads was collected and eluted. The samples were then placed in 65°C water baths overnight to reverse formaldehyde cross-linking and subsequently purified with DNA purification kits. The isolated DNA was assayed via qRT-PCR. The rat primer sequences of the ACE promoter region in this study were as follows: forward, 5′-TCCACAAACACAACAGCTCG-3′ and reverse, 5′-AGAGAGGAGGAAGGTGGCTA-3′.
Data and statistical analysis
SPSS 20.0 (IBM SPSS, Chicago, IL, USA) and Prism 8.0 (GraphPad Software, La Jolla, CA, USA) were used to analyze the experimental data. All data are expressed as means ± sem. A 2-tailed Student’s t test was performed on one factor of prenatal nicotine treatment (control or PNE). For the data from the in vitro study, a 1-way ANOVA followed by a post hoc Dunnett t test or a post hoc Bonferroni t test were used to conduct the multiple comparisons. A value of P < 0.05 was considered statistically significant.
RESULTS
PNE induced bone dysplasia and suppressed osteogenic differentiation in the male fetal offspring
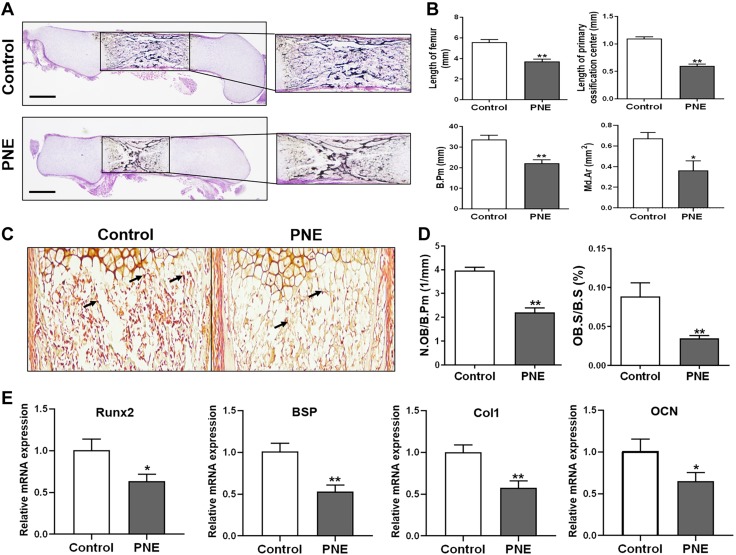
The effects of PNE on fetal bone development were first observed. The results showed that, compared with the control group, the bone development indices, including the lengths of the femurs and primary ossification centers, B.Pm, and Md.Ar, significantly decreased in the PNE group (P < 0.05, P < 0.01; Fig. 2A, B). This indicated that PNE caused bone development retardation in the fetal offspring rats. Furthermore, ALP staining and a quantitative analysis of the osteoblasts in the primary ossification center were carried out, and the relative ratio between the number of osteoblasts and B.Pm and the relative ratio between the osteoblast surface and bone surface were significantly reduced by PNE (P < 0.01; Fig. 2C, D). When detecting the marker genes of the osteogenic differentiation in the primary ossification centers, such as runt-related transcription factor 2 (Runx2), bone sialic acid protein (BSP), type I collagen (Col1), and osteocalcin (OCN), we also found that these marker genes significantly decreased in the PNE group (P < 0.05, P < 0.01; Fig. 2E). These results suggested that the inhibition of osteogenic differentiation contributed to the bone dysplasia induced by PNE in the male fetal offspring rats.
Figure 2.
Effects of PNE on bone development and osteogenic differentiation in the male fetal offspring. A) Representative von Kossa staining images of the full-length femurs from the control and PNE groups on GD20. Scale bar, 500 μm. B) Quantification of the length of the femurs and primary ossification centers, Md.Ar, and B.Pm. C) Representative ALP staining images from the control and PNE groups on GD20. D) Quantification of the relative ratio between the number of osteoblasts and B.Pm (N.OB/B.Pm) and the relative ratio between the osteoblast surface and bone surface (OB.S/B.S). E) qRT-PCR analysis of the gene expression of the osteogenic differentiation markers, including Runx2, BSP, Col1, and OCN, in the bone tissue on GD20; n = 8/group. Data are expressed as means ± sem. *P < 0.05, **P < 0.01 compared with the controls.
PNE reduced bone mass and inhibited osteogenic differentiation in the male adult offspring
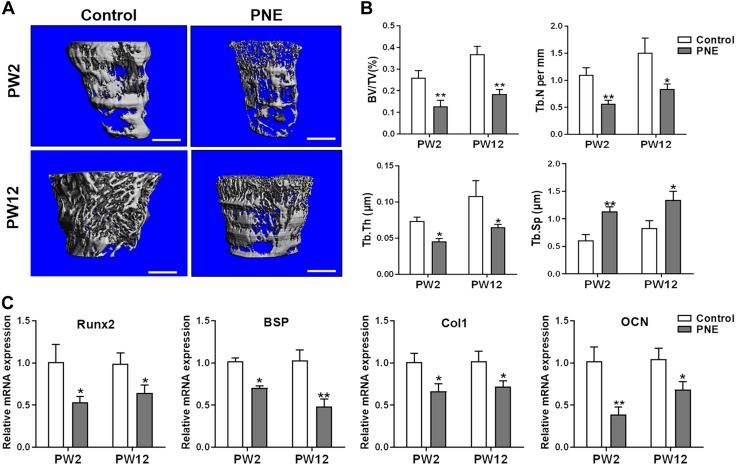
The bone mass was detected and quantitatively analyzed using micro-CT to explore the effects of PNE on bone mass accumulation in the postnatal offspring. The results indicated that PNE markedly reduced the bone mass in the male offspring at PW2 and PW12, which was manifested by the decrease in BV/TV, Tb.N, and Tb.Th but the increase in Tb.Sp (P < 0.05, P < 0.01; Fig. 3A, B). The osteogenic marker genes (Runx2, BSP, Col1, and OCN) were also significantly down-regulated by PNE (P < 0.05, P < 0.01; Fig. 3C). This suggested that the sustained inhibition of osteogenic differentiation mediated the reduced bone mass in response to PNE in the male adult offspring.
Figure 3.
Effects of PNE on bone mass and osteogenic differentiation in the adult male offspring. A) Representative micro-CT images of femurs from 2- or 12-wk-old offspring of the control and PNE groups. Scale bar, 500 μm. B) Quantitative micro-CT analysis of the trabecular bone microarchitecture. C) qRT-PCR analysis of the gene expression of osteogenic differentiation markers, including Runx2, BSP, Col1, and OCN, in the bone tissue from PW2 to PW12; n = 8/group. Data are expressed as means ± sem. *P < 0.05, **P < 0.01 compared with the controls.
PNE led to the sustained activation of local bone RAS in the male offspring
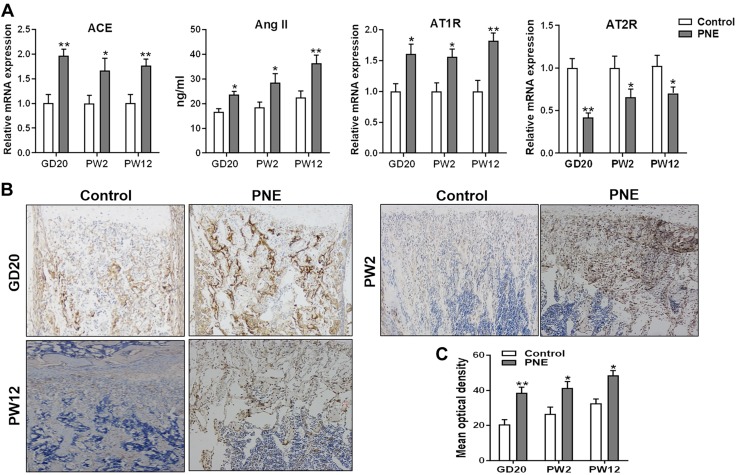
To investigate the potential mechanism of the PNE-induced inhibition of osteoblastic differentiation in the male offspring, we detected the expression of major components of local bone RAS in the prenatal and postnatal offspring. The results showed that the mRNA expression levels of ACE and AT1R and the Ang II content in the bone tissue significantly increased because of PNE before and after birth, whereas the expression level of AT2R mRNA decreased because of PNE (P < 0.05, P < 0.01; Fig. 4A). The immunohistochemical results also showed that the ACE protein levels in the bone tissue were up-regulated by PNE from GD20 to PW12 (P < 0.05, P < 0.01; Fig. 4B, C). These results demonstrated that PNE continuously activated the local bone RAS by promoting the expression of ACE.
Figure 4.
Effects of PNE on the expression of the main components of the local bone RAS in the male offspring. A) qRT-PCR analysis of the gene expression of RAS, including ACE and angiotensin receptors, and ELISA analysis of Ang II production in the bone tissue from GD20 to PW12. B) Representative immunostaining images of ACE in the bone tissue from GD20 to PW12. C) Quantitative immunostaining analysis of the mean optical density of ACE from GD20 to PW12; n = 8/group. Data are expressed as means ± sem. *P < 0.05, **P < 0.01 compared with the controls.
The intrauterine programming mechanism of the sustained activation of local bone RAS caused by PNE
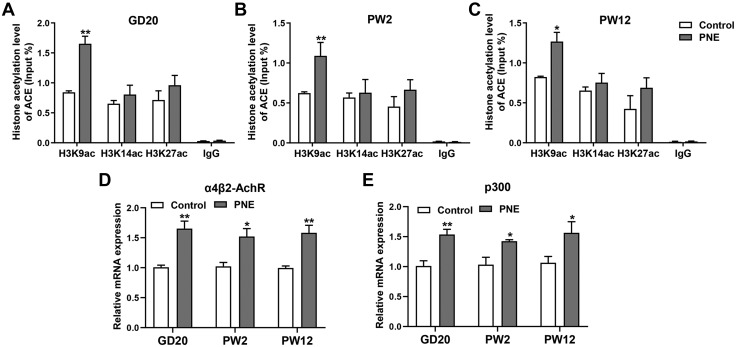
Exploring the intrauterine programming mechanism of the continued activation of local bone RAS induced by PNE demonstrated that the H3K9ac level in the ACE promoter increased because of PNE on GD20 (P < 0.01; Fig. 5A). These changes were also observed at PW2 and PW12 (P < 0.05, P < 0.01; Fig. 5B, C). Nicotine mainly regulates the histone modification of the target genes through the nicotine receptor (8). Thus, we detected the expression changes of the main nicotine receptors in the fetal bone tissue. PNE significantly increased α4β2-nAChR expression in the bone tissue from GD20 to PW12 (P < 0.05, P < 0.01; Fig. 5D). We also found that the expression level of histone acetylase p300 in the PNE group was significantly higher than in the control group from GD20 to PW12 (P < 0.05, P < 0.01; Fig. 5E). This suggested that nicotine may increase p300 expression by acting on the nicotine receptor α4β2-nAChR, leading to increased H3K9ac levels in the ACE promoter region, which then contributed to the sustained activation of RAS.
Figure 5.
Effects of PNE on the histone acetylation level of ACE and its intrauterine programming mechanism in the male offspring. A) ChIP assay of H3K9ac, H3K14ac, and H3K27ac levels in the ACE promoter region on GD20. B) ChIP assay of the H3K9ac, H3K14ac, and H3K27ac levels in the ACE promoter region at PW2. C) ChIP assay of the H3K9ac, H3K14ac, and H3K27ac levels in the ACE promoter region at PW12. D) qRT-PCR analysis of the nicotine receptor expression in the fetal and adult bone tissue from the control and PNE groups. E) qRT-PCR analysis of the gene expression of p300 in the fetal and adult bone tissue from the control and PNE groups; n = 8/group. Data are expressed as means ± sem. *P < 0.05, **P < 0.01 compared with the controls.
Nicotine inhibited the BMSCs’ osteogenic differentiation by activating RAS
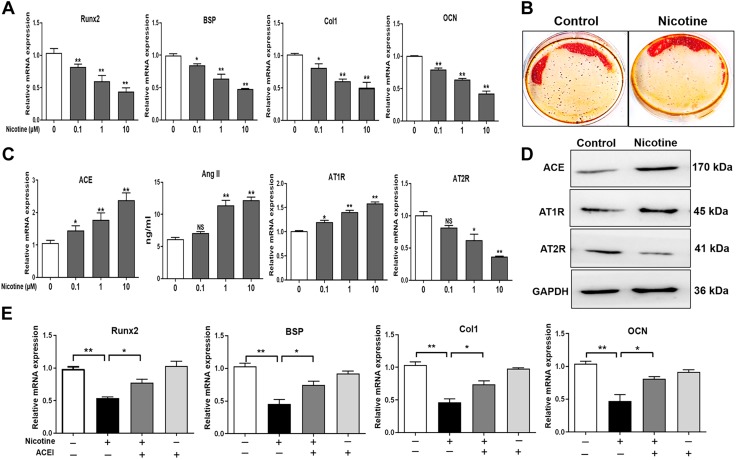
To determine the effect of nicotine on the BMSCs’ osteogenic differentiation in vitro, we treated the BMSCs with different concentrations of nicotine (0.1, 1, and 100 μM). By detecting the expression of the osteogenic marker genes, such as Runx2, BSP, Col1, and OCN, we found that nicotine down-regulated the expression of these marker genes with concentration-dependent changes (P < 0.05, P < 0.01; Fig. 6A). The Alizarin Red S staining results showed that a high concentration of nicotine significantly inhibited the mineralization function of the BMSCs (Fig. 6B). We further found that nicotine significantly promoted the expression levels of ACE mRNA and AT1R mRNA and decreased the expression level of AT2R mRNA. The Ang II content was also significantly increased by nicotine (P < 0.05, P < 0.01; Fig. 6C). The protein level changes were consistent with the mRNA expression changes (Fig. 6D). Moreover, when treating the BMSCs with nicotine or combined with the ACE inhibitor captopril in the process of osteogenic differentiation, we found that the ACE inhibitor partly reversed the inhibitory effect of nicotine on the BMSCs’ osteogenic differentiation (P < 0.05, P < 0.01; Fig. 6E). Collectively, this indicated that nicotine suppressed the BMSCs’ osteogenic differentiation by promoting ACE expression and RAS activation.
Figure 6.
Effects of nicotine on osteogenic differentiation and the RAS in the BMSCs. A) qRT-PCR analysis of the gene expression of osteogenic differentiation markers, including Runx2, BSP, Col1, and OCN, in the BMSCs cultured in the osteogenic medium and treated with different concentrations of nicotine (0.1, 1, and 10 μM) for 14 d. B) Alizarin Red S staining of the mineralization nodules after treating the BMSCs with a high concentration of nicotine (10 μM) during the process of osteogenic differentiation. C) qRT-PCR analysis of the gene expression of RAS, including ACE and angiotensin receptors, and ELISA analysis of Ang II production in the BMSCs cultured in the osteogenic medium and treated with different concentrations of nicotine for 14 d. D) Western blotting assay of the ACE, AT1R, and AT2R protein levels in the BMSCs after treating with 10 μM nicotine; glyceraldehyde 3-phosphate dehydrogenase (GAPDH) used for normalization. E) qRT-PCR analysis of the gene expression of Runx2, BSP, Col1, and OCN after cotreating the BMSCs with nicotine and ACE inhibitor (ACEI). All of the experiments were performed at least 3 times; data are expressed as means ± sem. NS, not significant. *P < 0.05, **P < 0.01 compared with the untreated cells.
Nicotine increased the H3K9ac level in the ACE promoter region and RAS activation via α4β2-nAChR–p300 signaling
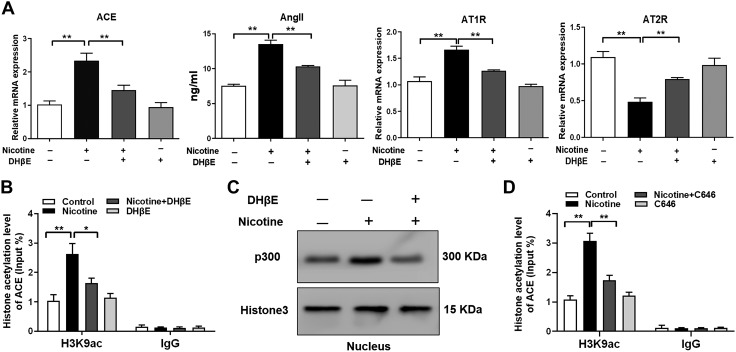
To elucidate the molecular mechanism regarding how nicotine promotes ACE expression and RAS activation, we treated the BMSCs with a high concentration of nicotine or combined with DH-β-E, a selective inhibitor of α4β2-nAChR, and found that DH-β-E partially reversed the nicotine-induced ACE hyperexpression and RAS activation (P < 0.01; Fig. 7A). Nicotine also increased the H3K9ac level in the ACE promoter region in vitro, whereas the α4β2-nAChR inhibitor DH-β-E partially reversed these effects (P < 0.05, P < 0.01; Fig. 7B). This indicated that nicotine could increase the H3K9ac and expression level of ACE through α4β2-nAChR, thus leading to RAS activation. We also found that nicotine induced an elevated level of p300 in the BMSCs’ nuclei, and the α4β2-nAChR inhibitor DH-β-E partially alleviated this change (Fig. 7C). Moreover, the p300 inhibitor C646 partially reversed the increased level of H3K9ac in the ACE promoter region caused by a high concentration of nicotine (P < 0.01; Fig. 7D). Taken together, nicotine promoted p300 into the BMSCs’ nuclei by acting on α4β2-nAChR and increased the H3K9ac level in the ACE promoter region, leading to the increased ACE expression and RAS activation.
Figure 7.
The molecular mechanism of the activated RAS induced by nicotine in the BMSCs. A) qRT-PCR analysis of the gene expression of RAS, including ACE, angiotensin receptors, and ELISA analysis of Ang II production in the BMSCs cotreated with nicotine and DH-β-E. B) ChIP assay of H3K9ac levels in the ACE promoter region in the BMSCs treated with nicotine or cotreated with nicotine and DH-β-E. C) Western blotting assay of the p300 protein levels in the nucleus after treating the BMSCs with nicotine or cotreating the BMSCs with nicotine and DH-β-E. D) ChIP assay of the H3K9ac levels in the ACE promoter region after cotreating the BMSCs with nicotine and p300 inhibitor C646. All of the experiments were conducted at least 3 times. Data are expressed as means ± sem. *P < 0 0.05, **P < 0.01 compared with the untreated cells.
The ACE inhibitor captopril blocked the effect of osteopenia induced by PNE in the adult offspring
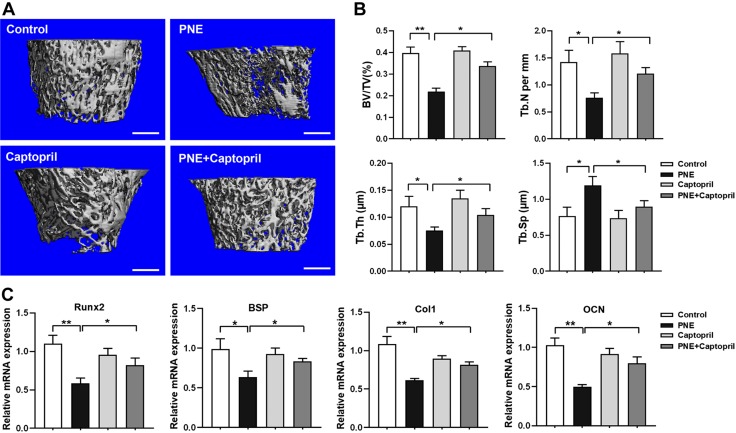
We then investigated whether captopril, an ACE inhibitor, could rescue the reduction in bone mass caused by PNE in the adult offspring. By detecting the bone mass using micro-CT, we found that PNE reduced the bone mass in the male offspring at PW12, which was manifested by the decrease in BV/TV, Tb.N, and Tb.Th and the increase in Tb.Sp (P < 0.05, P < 0.01; Fig. 8A, B), whereas captopril partly reversed the decrease in bone mass induced by PNE. Moreover, the expression changes of the osteogenic marker genes (Runx2, BSP, Col1, and OCN) were consistent with the changes in the bone mass (P < 0.05, P < 0.01; Fig. 8C). These results demonstrated that the ACE inhibitor captopril blocked the effect of osteopenia induced by PNE in the adult offspring.
Figure 8.
Effects of captopril on the decreased bone mass and inhibited osteogenic differentiation induced by PNE in the adult male offspring. A) Representative micro-CT images of the femurs of 12-wk-old offspring. Scale bars, 500 μm. B) Quantitative micro-CT analysis of the trabecular bone microarchitecture. C) qRT-PCR analysis of the gene expression of the osteogenic differentiation markers, including Runx2, BSP, Col1, and OCN in the bone tissue; n = 8/group. Data are expressed as means ± sem. *P < 0.05, **P < 0.01 compared with the controls.
DISCUSSION
Osteopenia caused by PNE originated from intrauterine development
Prenatal smoking causes an adverse intrauterine environment and has become a serious public health problem (23). Nicotine is one of the main components in tobacco. It has a high lipid solubility and can enter the fetal blood circulation through the placenta, thus affecting fetal development (24). Previous animal experiments suggested that nicotine exposure during pregnancy could lead to low birth weight and cause developmental toxicity of multiple organs in offspring (25–27). It was also found that maternal nicotine exposure during pregnancy caused bone dysplasia during the embryonic period in offspring rats (28). Other research showed that nicotine treatment resulted in femur hypoplasia in chicken embryos, which was mainly manifested as shortened femur lengths (29). Our previous studies also indicated that PNE caused endochondral ossification retardation in fetal rats (9, 30). However, the effects of nicotine on bone mass formation in adult offspring have not been reported. The present study confirmed that PNE could cause osteopenia in adult offspring. The PNE offspring also showed reduced bone mass in utero and during the early postnatal period. This indicated that PNE could decrease bone mass in adult offspring, which originated during intrauterine development. Cigarette smoke is a complex mixture of chemicals, which contains over 7000 chemicals (31). Although we have demonstrated in this research that nicotine exposure during pregnancy caused bone dysplasia and reduced bone mass in offspring, the harmful effects of cigarette smoke on bone development still cannot be fully clarified. Thus, the use of pure nicotine as a model of smoking has certain limitations.
The sustained activation of local RAS induced by nicotine led to the inhibition of osteogenic differentiation and decreased bone mass
BMSCs have the potential to differentiate into osteoblasts, chondrocytes, and adipocytes (32). In the process of osteogenic differentiation, BMSCs first differentiate into osteoblast progenitor cells and then migrate to the bone surface for further differentiation and maturation into osteoblasts (33). The osteogenic differentiation potential of BMSCs plays a crucial role in the process of endochondral osteogenesis and is one of the most important factors for bone mass formation (34). When exploring the mechanism of PNE on the reduced bone mass in the offspring before and after birth, we found that PNE led to a decrease in the number of osteoblasts and osteogenic marker gene expression in the ossification centers. This indicated that the sustained inhibition of osteogenic differentiation mediated the decrease in bone mass in the offspring induced by PNE. The differentiation and maturation of osteoblasts are regulated by a variety of factors that can lead to reduced bone mass and osteoporosis. Previous studies indicated that local RAS is present in various tissues (including bone tissues) and can regulate cell differentiation (35, 36).
In this study, we detected the main components of local RAS in bone tissue before and after the birth and found that PNE led to the sustained activation of local bone RAS, mainly manifested as the increased expression of ACE. This suggested that PNE may lead to the sustained inhibition of osteogenic differentiation by activating local bone RAS. We also demonstrated at the cellular level that the high concentration of nicotine significantly activated RAS and inhibited BMSCs differentiating into the osteoblasts. After inhibiting RAS activity with the ACE inhibitors, it was found that the inhibitory effect of nicotine on BMSCs osteogenic differentiation could be significantly reversed. Moreover, the ACE inhibitor captopril partly reversed the decrease in bone mass induced by PNE. Therefore, the present study confirmed that the sustained activation of local bone RAS mediates the inhibition of osteogenic differentiation and the reduction of bone mass in offspring induced by PNE, and ACE may be an early treatment target.
Nicotine induced the elevated histone acetylation level of ACE through α4β2-nAChR–p300 signaling
Adverse environment during pregnancy, such as exogenous exposure, continue to influence the phenotype of postnatal offspring through epigenetic regulation. Nicotine caused abnormal phenotypes and functions of multiorgan by inducing epigenetic modification of functional genes. For example, nicotine inhibited the expression of the fetal adrenosteroid synthase system (including steroidogenic acute regulatory protein or cytochrome P450 side-chain cleavage) and fetal adrenal function by reducing the histone deacetylation of steroidogenic factor 1 (37). Histone deacetylation of the yin yang 1 gene was also involved in the decreased expression of the fetal adrenal steroidogenic acute regulatory gene induced by nicotine (38). In this study, we found that PNE induced the continuous increase of the H3K9ac level in the ACE promoter region before and after birth. In vitro, nicotine increased the H3K9ac level in the ACE promoter region, whereas the α4β2-nAChR inhibitor DH-β-E partially reversed these effects. This indicated that nicotine increased the H3K9ac and expression levels of ACE through α4β2-nAChR, leading to RAS activation. We also found that nicotine caused an elevated level of p300 in the BMSCs’ nuclei by acting on α4β2-nAChR. Moreover, the p300 inhibitor C646 partially reversed the increased level of H3K9ac in the ACE promoter region caused by a high concentration of nicotine. Taken together, nicotine up-regulated the expression level of p300 and promoted it into the nuclei of BMSCs via acting on α4β2-nAChR, leading to the increased H3K9ac and expression levels of ACE. Therefore, these components of this pathway, such as α4β2-nAChR and p300, were also regulated by nicotine and might be one of the targets of osteopenia induced by prenatal nicotine treatment. However, considering ACE as the main effector molecule of this pathway, we focused on elucidating the target role of ACE in prenatal nicotine–induced osteopenia in the present study.
CONCLUSIONS
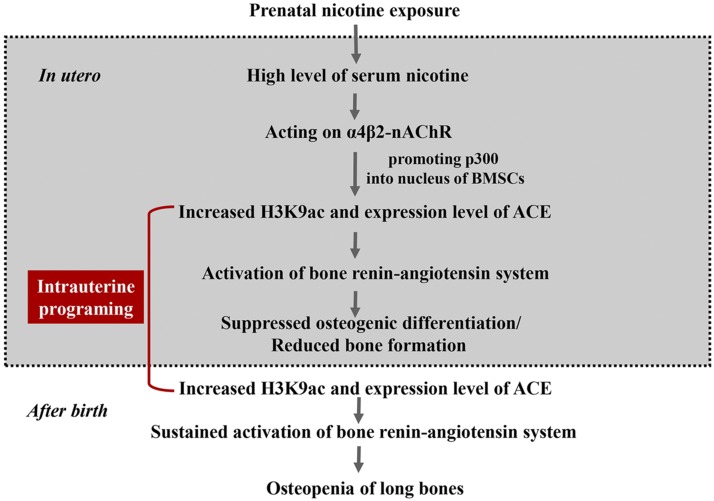
This study confirmed that PNE caused osteopenia in adult offspring. Its mechanism was that nicotine increased the expression level of p300 and promoted it into the nuclei of BMSCs via acting on α4β2-nAChR, which then contributed to the increased H3K9ac level in the ACE promoter region and the local bone RAS activation. The increased H3K9ac level of ACE continued from fetuses to adults, leading to the sustained ctivation of local bone RAS and inhibiting the BMSCs’ osteogenic differentiation (as shown in Fig. 9). This study clarified the long-term harmful effects of nicotine on bone development in offspring and its intrauterine programming mechanism, which elucidates the early prevention and treatment targets to provide a theoretical and experimental basis for the theory of the developmental origins of health and disease.
Figure 9.
Nicotine exposure during pregnancy caused osteopenia in the male offspring rats via the α4β2-nAChR–p300-ACE pathway.
ACKNOWLEDGMENTS
This work was supported by grants from the National Natural Science Foundation of China (81673490, 81430089, and 81673524) and the Hubei Province Health and Family Planning Scientific Research Project (WJ2017C0003). The authors declare no conflicts of interest.
Glossary
- ACE
angiotensin-converting enzyme
- ALP
alkaline phosphatase
- Ang II
angiotensin II
- AT1R
Ang II type 1 receptor
- AT2R
Ang II type 2 receptor
- B.Pm
bone perimeter
- BMSC
bone marrow mesenchymal stem cell
- BSP
bone sialic acid protein
- BV/TV
bone volume:total volume ratio
- ChIP
chromatin immunoprecipitation
- Col1
type I collagen
- DH-β-E
dihydro-β-erythroidine
- GD
gestational day
- H3K9ac
histone 3 lysine 9 acetylation
- H3K14ac
histone 3 lysine 14 acetylation
- H3K27ac
histone 3 lysine 27 acetylation
- Md.Ar
mineralized area
- micro-CT
micro-computed tomography
- nAChR
nicotinic acetylcholine receptor
- OCN
osteocalcin
- PNE
prenatal nicotine exposure
- PW
postnatal week
- qRT-PCR
quantitative RT-PCR
- RAS
renin angiotensin system
- Runx2
runt-related transcription factor 2
- Tb.N
trabecular number
- Tb.Sp
trabecular separation
- Tb.Th
trabecular thickness
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
H. Xiao, H. Wang, and L. Chen designed this study; H. Xiao conducted the experiments; H. Xiao, Y. Wen, Z. Pan, Y. Shangguan, H. Wang, and L. Chen analyzed the data; H. Xiao, J. Magdalou, H. Wang, and L. Chen wrote and revised the paper; and all of the authors approved the final manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Tong V. T., Dietz P. M., Morrow B., D’Angelo D. V., Farr S. L., Rockhill K. M., England L. J.; Centers for Disease Control and Prevention (CDC) (2013) Trends in smoking before, during, and after pregnancy--pregnancy risk assessment monitoring system, United States, 40 sites, 2000-2010. MMWR Surveill. Summ. 62, 1–19 [PubMed] [Google Scholar]
- 2.Cui Y., Shooshtari S., Forget E. L., Clara I., Cheung K. F. (2014) Smoking during pregnancy: findings from the 2009-2010 Canadian Community Health Survey. PLoS One 9, e84640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Behrooz L., Balekian D. S., Faridi M. K., Espinola J. A., Townley L. P., Camargo C. A., Jr (2018) Prenatal and postnatal tobacco smoke exposure and risk of severe bronchiolitis during infancy. Respir. Med. 140, 21–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Talati A., Wickramaratne P. J., Wesselhoeft R., Weissman M. M. (2017) Prenatal tobacco exposure, birthweight, and offspring psychopathology. Psychiatry Res. 252, 346–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlström A., Lundell B., Curvall M., Thapper L. (1990) Nicotine and cotinine concentrations in the nursing mother and her infant. Acta Paediatr. Scand. 79, 142–147 [DOI] [PubMed] [Google Scholar]
- 6.Rogers J. M. (2009) Tobacco and pregnancy. Reprod. Toxicol. 28, 152–160 [DOI] [PubMed] [Google Scholar]
- 7.Huang L. Z., Hsiao S. H., Trzeciakowski J., Frye G. D., Winzer-Serhan U. H. (2006) Chronic nicotine induces growth retardation in neonatal rat pups. Life Sci. 78, 1483–1493 [DOI] [PubMed] [Google Scholar]
- 8.Zhou J., Liu F., Yu L., Xu D., Li B., Zhang G., Huang W., Li L., Zhang Y., Zhang W., Wang H. (2018) nAChRs-ERK1/2-Egr-1 signaling participates in the developmental toxicity of nicotine by epigenetically down-regulating placental 11β-HSD2. Toxicol. Appl. Pharmacol. 344, 1–12 [DOI] [PubMed] [Google Scholar]
- 9.Hu H., Zhao X., Ma J., Shangguan Y., Pan Z., Chen L., Zhang X., Wang H. (2018) Prenatal nicotine exposure retards osteoclastogenesis and endochondral ossification in fetal long bones in rats. Toxicol. Lett. 295, 249–255 [DOI] [PubMed] [Google Scholar]
- 10.Wu C. T., Lu T. Y., Chan D. C., Tsai K. S., Yang R. S., Liu S. H. (2014) Effects of arsenic on osteoblast differentiation in vitro and on bone mineral density and microstructure in rats. Environ. Health Perspect. 122, 559–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P., Deng Q., Liu J., Yan J., Wei Z., Zhang Z., Liu H., Li B. (2019) Roles for HB-EGF in mesenchymal stromal cell proliferation and differentiation during skeletal growth. J. Bone Miner. Res. 34, 295–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sparks M. A., Crowley S. D., Gurley S. B., Mirotsou M., Coffman T. M. (2014) Classical renin-angiotensin system in kidney physiology. Compr. Physiol. 4, 1201–1228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farag E., Sessler D. I., Ebrahim Z., Kurz A., Morgan J., Ahuja S., Maheshwari K., John Doyle D. (2017) The renin angiotensin system and the brain: new developments. J. Clin. Neurosci. 46, 1–8 [DOI] [PubMed] [Google Scholar]
- 14.Paul M., Poyan Mehr A., Kreutz R. (2006) Physiology of local renin-angiotensin systems. Physiol. Rev. 86, 747–803 [DOI] [PubMed] [Google Scholar]
- 15.Ling C., Rönn T. (2019) Epigenetics in human obesity and type 2 diabetes. Cell Metab. 29, 1028–1044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shafabakhsh R., Aghadavod E., Ghayour-Mobarhan M., Ferns G., Asemi Z. (2019) Role of histone modification and DNA methylation in signaling pathways involved in diabetic retinopathy. J. Cell. Physiol. 234, 7839–7846 [DOI] [PubMed] [Google Scholar]
- 17.Fowden A. L., Giussani D. A., Forhead A. J. (2006) Intrauterine programming of physiological systems: causes and consequences. Physiology (Bethesda) 21, 29–37 [DOI] [PubMed] [Google Scholar]
- 18.Xie Z., Zhao Z., Yang X., Pei L., Luo H., Ni Q., Li B., Qi Y., Tie K., Magdalou J., Chen L., Wang H. (2018) Prenatal nicotine exposure intergenerationally programs imperfect articular cartilage via histone deacetylation through maternal lineage. Toxicol. Appl. Pharmacol. 352, 107–118 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Li X. L., Sha N. N., Shu B., Zhao Y. J., Wang X. L., Xiao H. H., Shi Q., Wong M. S., Wang Y. J. (2017) Differential response of bone and kidney to ACEI in db/db mice: a potential effect of captopril on accelerating bone loss. Bone 97, 222–232 [DOI] [PubMed] [Google Scholar]
- 20.Dempster D. W., Compston J. E., Drezner M. K., Glorieux F. H., Kanis J. A., Malluche H., Meunier P. J., Ott S. M., Recker R. R., Parfitt A. M. (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 28, 2–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mys K., Varga P., Gueorguiev B., Hemmatian H., Stockmans F., van Lenthe G. H. (2019) Correlation between cone-beam computed tomography and high-resolution peripheral computed tomography for assessment of wrist bone microstructure. J. Bone Miner. Res. 34, 867–874 [DOI] [PubMed] [Google Scholar]
- 22.Xu X., Li R., Zhou Y., Zou Q., Ding Q., Wang J., Jin W., Hua G., Gao J. (2017) Dysregulated systemic lymphocytes affect the balance of osteogenic/adipogenic differentiation of bone mesenchymal stem cells after local irradiation. Stem Cell Res. Ther. 8, 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Holbrook B. D. (2016) The effects of nicotine on human fetal development. Birth Defects Res. C Embryo Today 108, 181–192 [DOI] [PubMed] [Google Scholar]
- 24.Mitchell J. A., Hammer R. E. (1985) Effects of nicotine on oviducal blood flow and embryo development in the rat. J. Reprod. Fertil. 74, 71–76 [DOI] [PubMed] [Google Scholar]
- 25.Liu L., Liu F., Kou H., Zhang B. J., Xu D., Chen B., Chen L. B., Magdalou J., Wang H. (2012) Prenatal nicotine exposure induced a hypothalamic-pituitary-adrenal axis-associated neuroendocrine metabolic programmed alteration in intrauterine growth retardation offspring rats. Toxicol. Lett. 214, 307–313 [DOI] [PubMed] [Google Scholar]
- 26.McEvoy C. T., Spindel E. R. (2017) Pulmonary effects of maternal smoking on the fetus and child: effects on lung development, respiratory morbidities, and life long lung health. Paediatr. Respir. Rev. 21, 27–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou J., Zhu C., Luo H., Shen L., Gong J., Wu Y., Magdalou J., Chen L., Guo Y., Wang H. (2019) Two intrauterine programming mechanisms of adult hypercholesterolemia induced by prenatal nicotine exposure in male offspring rats. FASEB J. 33, 1110–1123 [DOI] [PubMed] [Google Scholar]
- 28.Carmines E. L., Gaworski C. L., Faqi A. S., Rajendran N. (2003) In utero exposure to 1R4F reference cigarette smoke: evaluation of developmental toxicity. Toxicol. Sci. 75, 134–147 [DOI] [PubMed] [Google Scholar]
- 29.Shan M., Qamar K., Iqbal I. (2015) Role of nicotine and camellia sinensis on the developing femur of chick. J. Pak. Med. Assoc. 65, 1094–1096 [PubMed] [Google Scholar]
- 30.Deng Y., Cao H., Cu F., Xu D., Lei Y., Tan Y., Magdalou J., Wang H., Chen L. (2013) Nicotine-induced retardation of chondrogenesis through down-regulation of IGF-1 signaling pathway to inhibit matrix synthesis of growth plate chondrocytes in fetal rats. Toxicol. Appl. Pharmacol. 269, 25–33 [DOI] [PubMed] [Google Scholar]
- 31.Colombo G., Dalle-Donne I., Orioli M., Giustarini D., Rossi R., Clerici M., Regazzoni L., Aldini G., Milzani A., Butterfield D. A., Gagliano N. (2012) Oxidative damage in human gingival fibroblasts exposed to cigarette smoke. Free Radic. Biol. Med. 52, 1584–1596 [DOI] [PubMed] [Google Scholar]
- 32.Chen X. D., Dusevich V., Feng J. Q., Manolagas S. C., Jilka R. L. (2007) Extracellular matrix made by bone marrow cells facilitates expansion of marrow-derived mesenchymal progenitor cells and prevents their differentiation into osteoblasts. J. Bone Miner. Res. 22, 1943–1956 [DOI] [PubMed] [Google Scholar]
- 33.Guan M., Yao W., Liu R., Lam K. S., Nolta J., Jia J., Panganiban B., Meng L., Zhou P., Shahnazari M., Ritchie R. O., Lane N. E. (2012) Directing mesenchymal stem cells to bone to augment bone formation and increase bone mass. Nat. Med. 18, 456–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gautam A. K., Bhargavan B., Tyagi A. M., Srivastava K., Yadav D. K., Kumar M., Singh A., Mishra J. S., Singh A. B., Sanyal S., Maurya R., Manickavasagam L., Singh S. P., Wahajuddin W., Jain G. K., Chattopadhyay N., Singh D. (2011) Differential effects of formononetin and cladrin on osteoblast function, peak bone mass achievement and bioavailability in rats. J. Nutr. Biochem. 22, 318–327 [DOI] [PubMed] [Google Scholar]
- 35.Sadik N. A., Metwally N. S., Shaker O. G., Soliman M. S., Mohamed A. A., Abdelmoaty M. M. (2017) Local renin-angiotensin system regulates the differentiation of mesenchymal stem cells into insulin-producing cells through angiotensin type 2 receptor. Biochimie 137, 132–138 [DOI] [PubMed] [Google Scholar]
- 36.Wen Y., Shangguan Y., Pan Z., Hu H., Magdalou J., Chen L., Wang H. (2019) Activation of local bone RAS by maternal excessive glucocorticoid participated in the fetal programing of adult osteopenia induced by prenatal caffeine exposure. Toxicol. Appl. Pharmacol. 363, 1–10 [DOI] [PubMed] [Google Scholar]
- 37.Yan Y. E., Liu L., Wang J. F., Liu F., Li X. H., Qin H. Q., Wang H. (2014) Prenatal nicotinic exposure suppresses fetal adrenal steroidogenesis via steroidogenic factor 1 (SF-1) deacetylation. Toxicol. Appl. Pharmacol. 277, 231–241 [DOI] [PubMed] [Google Scholar]
- 38.Liu L., Wang J. F., Fan J., Rao Y. S., Liu F., Yan Y. E., Wang H. (2016) Nicotine suppressed fetal adrenal StAR expression via YY1 mediated-histone deacetylation modification mechanism. Int. J. Mol. Sci. 17, E1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.