Abstract
The heterochronic gene Lin28 regulates diverse developmental processes. It was shown previously that global Lin28A overexpression during mouse embryogenesis results in perinatal lethality. However, the reason for this early lethality has not been elucidated. Here, we showed that Lin28A overexpression prevents normal lung development via the inhibition of the Let-7 micro RNAs, thus causing the perinatal lethality. We further found that Lin28A overexpression in lung mesenchymal cells, but not epithelial cells, is sufficient to recapitulate the lung phenotype. Moreover, we defined the specific time window wherein Lin28A expression exerts its effect. Deep characterization of the transgenic lungs suggests that the Lin28A–Let-7 pathway delays the transition from one developmental stage to another but does not completely abrogate the differentiation capacity of the lung progenitor cells. Finally, we suggested that the effect of Lin28A–Let-7 on embryonic lung development is mediated at least in part through the TGF-β1–signaling pathway. Altogether, these findings define for the first time the Lin28–Let-7 pathway as a critical heterochronic regulator of lung development.—Komarovsky Gulman, N., Armon, L., Shalit, T., Urbach, A. Heterochronic regulation of lung development via the Lin28–Let-7 pathway.
Keywords: heterochronic genes, miRNA, organogenesis
Lin28 is an RNA-binding protein, first identified as a gene that can alter developmental timing when dysregulated (a heterochronic gene) in Caenorhabditis elegans (1, 2). Lin28 regulates gene expression via 2 different mechanisms. The first mechanism is blocking the maturation of the Let-7 family of micro RNAs (miRNAs) (3–6), thus enabling the translation of genes that are suppressed by these miRNAs (6–8). The second mechanism is mediated by direct binding to mRNAs and modifying gene expression through a Let-7–independent pathway (7, 9, 10).
Lin28A and its paralog Lin28B are highly expressed in pluripotent stem cells (7, 8, 11), where they play an important role in survival and proliferation (9), and in the conversion of murine naive into primed pluripotent stem cells (12). Lin28A and B are also expressed in progenitor cells of diverse tissue types but are down-regulated upon further differentiation. For example, Lin28A and Lin28B play an important role in primordial germ cell development (13) and are also expressed in the early embryonic gut (14). In addition, Lin28A is highly expressed in kidney progenitor cells until embryonic day (E)13.5 (15, 16). Ectopic expression of Lin28A and B at later stages of germ cell, kidney, and gut development results in germ cell tumors (13), Wilms tumors (15), and intestinal and colorectal adenocarcinomas (14), respectively. These and other findings suggest that Lin28A and B expression in specific tissues and definite developmental stages is required for normal embryonic development. The fact that more than 90% of Lin28A knockout mice die within 1 d after birth (17) further demonstrates the critical role that Lin28A plays during normal embryonic development, although the reason for this early lethality is still unclear (17).
In previous attempts to study the effect of specific Lin28A overexpression in diverse tissues, we found that global constitutive expression of Lin28A in the embryo from E0 leads to perinatal lethality (15). Here, we showed that the perinatal lethality is caused as a result of abnormal embryonic lung development.
During mouse embryonic development, the primary lung buds are derived from the foregut endoderm and first appear around E9.5. The development of the lung continues through 4 main stages (18–21). Briefly, during the pseudoglandular stage (until ∼E16.5 in mice and 5–17 wk of gestation in humans), the primary buds undergo branching, which results in the generation of tree-like structures containing thousands of terminal tubules. These structures are essentially identical between different mouse strains (22). Next, the canalicular stage (E16.5–E17.5 in mice and 16–25 wk of gestation in humans) is characterized by the formation of the terminal bronchioles and the appearance of airway epithelium–specific cell types, such as basal, goblet, and ciliated cells. The early alveolar type (AT)1 and AT2 cells also emerge at this stage because they differentiate from common bipolar progenitor cells (23, 24). In addition, during this stage, the cell cycle activity decreases (25) and there is a massive increase in vascularization. The canalicular stage is followed by the saccular stage (E17.5 to approx. postnatal day (P)5 in mice and 24 to ∼36 wk of gestation in humans) in which the AT1 and AT2 cells continue their differentiation and maturation to generate functional alveolar cells responsible for gas exchange (AT1 cells) and surfactant secretion (AT2 cells). This stage is also characterized by the differentiation of the mesenchymal lung cells, rapid capillary growth in the mesenchymal tissue, and the development of the lung’s lymphatic network (21). Finally, the development of the lungs is completed at the alveolar stage (P5–P30 in mice and from the 36 wk of gestation and through the childhood period in humans) in which the majority of the gas exchange surface is generated.
In the current study, we showed that overexpression of Lin28A at a specific time interval during mouse embryogenesis leads to a significant delay in lung development. We further showed that restricted overexpression of Lin28A in the mesenchymal compartment of the embryonic lungs recapitulates the lung phenotype of global Lin28A overexpression. Finally, coexpression of Let-7 along with Lin28 results in normal lung development and proves that the effect of Lin28A overexpression is mediated via Let-7. Thus, our data demonstrated the essential role of the Let-7 miRNA family for lung organogenesis.
MATERIALS AND METHODS
Mice
All animal procedures were conducted according to animal care guidelines approved by the Institutional Animal Care and Use Committee at Bar-Ilan University. Lox-stop-Lox-TetOn-Lin28A mice were kindly provided by the laboratory of Dr. Daley (Boston Children’s Hospital; Boston, MA, USA) (currently available at The Jackson Laboratory, Bar Harbor, ME, USA; 023910). Let-7 mice were purchased from The Jackson Laboratory (023912).
The following Cre strains were purchased from The Jackson Laboratory: Vasa-Cre (006954), Wt1-Cre (010911), and Nkx2.1-Cre (008661). Cre strains were crossed to the Lox-stop-Lox-TetOn-Lin28A mice and 1 g/L doxycycline (Dox) was administered to the drinking water at different time points, as previously described, to induce Lin28A overexpression. To achieve cooverexpression of Lin28A and Let-7, we crossed Vasa-Cre mice with mice that express a Let-7g isoform resistant to Lin28A inhibition (i7s) and then crossed the Vasa-Cre;i7s females with Lox-stop-Lox-TetOn-Lin28A mice.
RNA extraction and quantitative RT-PCR analysis
Total RNA was isolated by Tri Reagent (RiboEx; GeneAll, Lisbon, Portugal) from embryonic lungs and reverse transcribed into cDNA by qScript microRNA cDNA Synthesis Kit (Quantabio, Beverly, MA, USA). The quantification of mature Let-7 miRNAs was performed by PerfeCTa SYBR Green SuperMix Reagent (Quantabio) using PerfeCTa miRNA Assay Primers. Data were analyzed using the ΔΔCt value method with U6 miRNA as a normalizer. Statistical significance was determined using a 2-way ANOVA with Tukey’s posttest.
Histology and immunofluorescent staining
Samples were fixed with 4% paraformaldehyde diluted in PBS, dehydrated in 75% EtOH, and embedded in paraffin wax. Sections were cut 7 μm thick and dried overnight at 37°C. The slides were dewaxed with xylene and rehydrated through a series of washes with decreasing percentages of ethanol. Antigen retrieval was performed in 10 mM sodium citrate buffer (pH 6.0) by placing it in a pressure cooker for 15 min on high temperature. Sections were blocked in 10% serum and incubated overnight at 4°C with the following primary antibodies: hamster anti-podoplanin (PDPN) antibody (clone 8.1.1; 1:150; Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA), rabbit anti-prosurfactant protein C (SFTPC) (ab3786; 1:200; MilliporeSigma, Burlington, MA, USA), and rabbit anti-Sox9 antibody (ab5535; 1:1000; MilliporeSigma). Tissue sections were washed with PBS and incubated for 1 h at room temperature with the following secondary antibodies: Alexa Fluor 594 goat anti-hamster IgG (A21113; 1:500; Thermo Fisher Scientific, Waltham, MA, USA) and Alexa Fluor 647–labeled goat anti-rabbit IgG (ab150079; 1:500; Abcam, Cambridge, MA, USA). Sections were treated with DAPI before mounting with mounting solution (Fluoromount-G; Thermo Fisher Scientific). Digital images were taken with an inverted Leica DMi8 scanning confocal microscope (Leica Microsystems, Buffalo Grove, IL, USA), driven by the LAS X software (Leica Microsystems). Excitation by means of a tunable white light laser was 405 nm, 594 nm, and 647 nm.
For hematoxylin and eosin (H&E) staining, sections were paraffin embedded. Next, the sections were stained with Harris hematoxylin solution (Kaltek, Padua, Italy) for 1 min and with eosin (MilliporeSigma) for 15 s. The tissue sections were examined under a light microscope (TS100; Nikon, Tokyo, Japan) after mounting with DPX Mountant for histology (MilliporeSigma).
Library construction and sequencing
Five hundred nanograms of total RNA for each sample was processed using an in-house poly-A–based RNA sequencing (RNAseq) protocol (INCPM mRNA-Seq). Libraries were evaluated by Qubit and TapeStation (both from Agilent Technologies, Santa Clara, CA, USA). Sequencing libraries were constructed with bar codes to allow multiplexing of the samples on 4 lanes of an Illumina HiSeq machine (Illumina, San Diego, CA, USA) using the Single-Read 60 protocol (v.4). The output was ∼19 × 106 reads per sample. Fastq files for each sample were generated by using bcl2fastq v.2.17.1.14 (Illumina).
Sequence data analysis
Poly-A/T stretches and Illumina adapters were trimmed from the reads using cutadapt (26). Resulting reads shorter than 30 bp were discarded. Reads of each sample were aligned independently using Spliced Transcript Alignment to a Reference (STAR; v.2.4.2a; https://www.encodeproject.org/software/star/) (27) (with EndToEnd) against the Mus musculus genome (GRCh38). Expression levels of each gene were quantified using htseq-count (v.0.6.1p1) (28), and gene annotations were downloaded from Ensembl (release 92; http://www.ensembl.org/). Only uniquely mapped reads were used to determine the number of reads for each gene (intersection-strict mode). Differential analysis was performed using the DESeq2 package (v.1.10.1) (29) with the betaPrior, cooksCutoff, and independentFiltering parameters set to false. Raw P values were adjusted for multiple testing using the procedure of Benjamini and Hochberg. Differentially expressed genes were determined by an adjusted value of P < 0.05, absolute fold changes >2, and maximum raw counts >30. Additional time-course analysis was performed using MaSigPro (30). Differentially expressed genes were determined by an adjusted value of P < 0.05, a ratio between maximum to minimum row counts >2, maximum raw counts >30, and r2 > 0.7.
Bioinformatics analysis
Principal component analysis (PCA) and hierarchical clustering analysis (distance: Pearson’s dissimilarity; method: Ward.d) were performed based on the 1000 most-variable genes. Unsupervised analysis was executed to explore a pattern of gene expression by clustering the genes based on 2833 genes (for this specific analysis, one of the control E14.5 samples was excluded because we found out that this sample was contaminated with thymus tissue, which affected the gene pattern analysis). These genes were determined as differentially expressed genes by both tools (intersection of the DESeq2 and the MaSigPro analysis). K-Means clustering using Pearson’s dissimilarity was performed. Standardized, log2–normalized counts were used for the clustering analysis. Clustering analysis was performed with Rstudio v.3.2.1 (https://www.rstudio.com/). Differentially expressed genes were analyzed through the use of MouseMine (www.mousemine.org) to determine the most significantly relevant biologic functions and pathways. Upstream regulators were identified by the Ingenuity Pathway Analysis (IPA) tool (Qiagen, Germantown, MD, USA).
RESULTS
Lin28A expression during embryogenesis prevents normal lung development
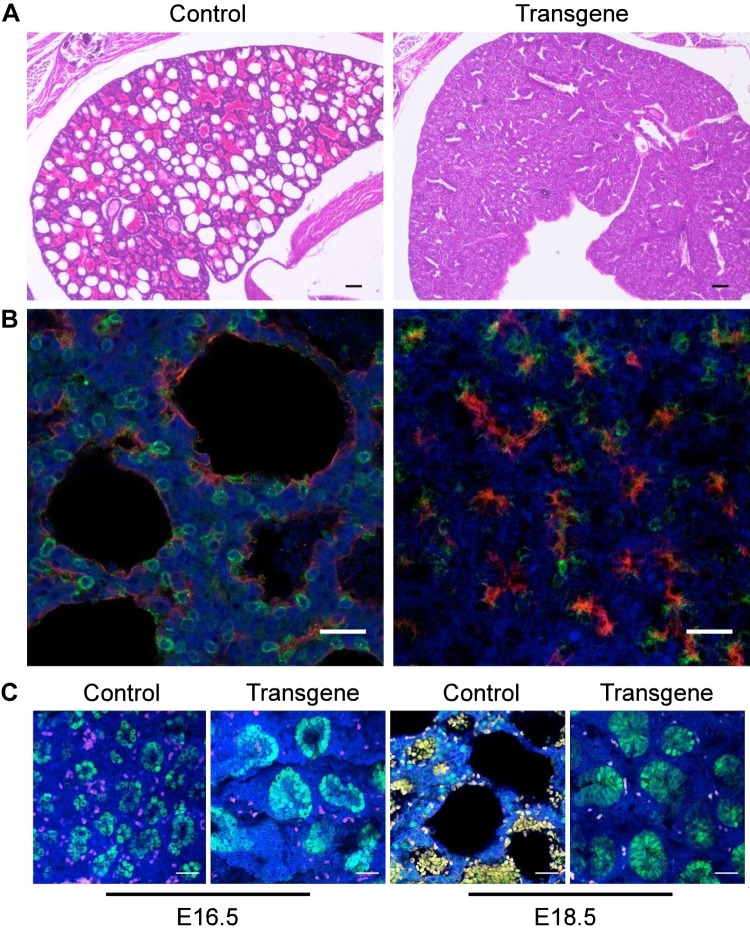
To study the effect of Lin28A overexpression (and thus Let-7 down-regulation) on mouse embryonic development, we used a transgenic mouse strain that combines a Cre-Lox system and a Tet-On system (herein, Lox-stop-Lox-TetOn-Lin28A mice) and enables spatial and temporal control of transgenic Lin28A overexpression as previously described in refs. 15 and 31. Crossing Lox-stop-Lox-TetOn-Lin28A males with females expressing Cre recombinase under the control of the Vasa promoter (Vasa-Cre) leads to Cre excision of the Lox-stop-Lox cassette in the oocytes (32) and to global Lin28A overexpression in the whole embryo upon Dox induction. Using this crossing, we have previously shown that global Lin28A overexpression from E0 results in perinatal lethality (15). Here, we aimed to study the reason for this early lethality. For this purpose, we performed Caesarean sections at E18.5 and tracked the embryos immediately upon their removal from the uterus. The E18.5 mice embryos were born alive and tried to breathe but died within 10–20 min. This observation suggests that Lin28A expression affects an organ or tissue that is not required during embryogenesis but becomes vital immediately after birth. Therefore, we hypothesized that Lin28A overexpression affects lung development. Indeed, H&E staining of the lungs derived from the E18.5 transgenic embryos showed abnormal lung histology and revealed the reason for the early lethality (Fig. 1A).
Figure 1.
Morphologic characterization of E18.5 control and transgenic lungs. A) H&E staining of control (left panel) and transgenic (right panel) lungs. B) Micrographs of control (left panel) and transgenic (right panel) lungs costained with anti-PDPN (red) and anti-proSFTPC (green). C) Micrographs of E16.5 (left panels) and E18.5 (right panels) control and transgenic lungs stained with anti-Sox9 (green). In these panels, the red and yellow colors appear as a result of autofluorescence of red blood cells. Blue staining, DAPI. Scale bars, 25 μM.
To further examine the effect of Lin28A on the lung, we stained the E18.5 lungs with markers for AT1 cells (PDPN) and AT2 cells (SFTPC). The control lungs showed the expected pattern of AT1 lining the cavities of the alveolar structures and AT2 cells scattered around them (Fig. 1B). In contrast, the transgenic lungs did not show the typical alveolar structures and most of the SFTPC-positive cells were also positive for PDPN (Fig. 1B). The presence of these bipolar progenitor cells (positive for both AT1 and AT2 markers) and the nearly complete absence of mature AT2 cells are characteristics of developmental stages earlier than E18.5 (23, 24). Finally, the E18.5 transgenic lungs continued to undergo the branching morphogenesis process, which is normally terminated around E16.5 [by the end of the pseudoglandibular stage (18–21)], as indicated by Sox9 staining (Fig. 1C). Altogether, these observations indicate that the transgenic lungs fail to complete their differentiation as a result of Lin28A overexpression.
Lin28A expression affects lung developmental timing
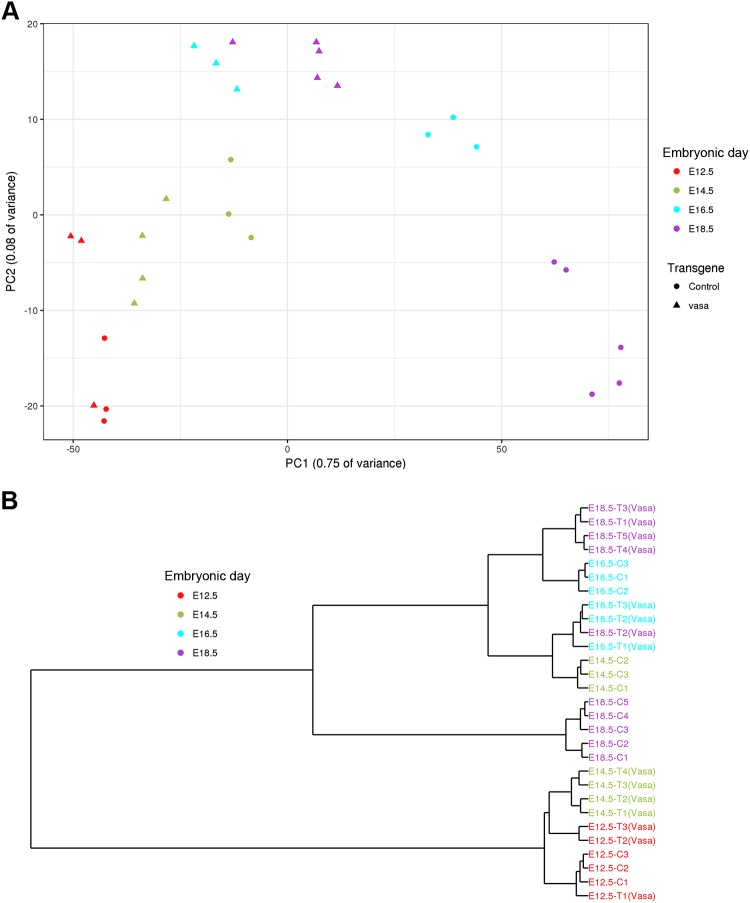
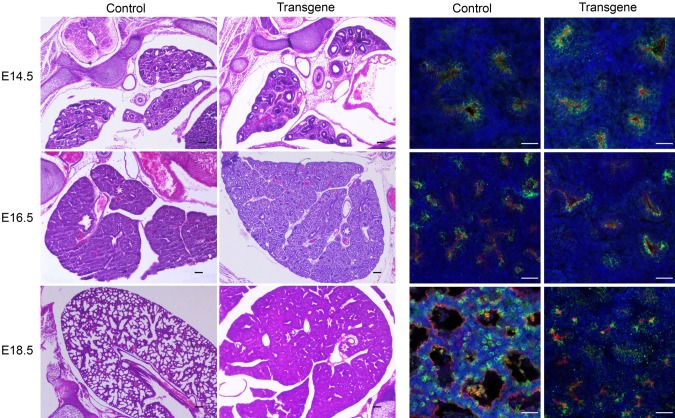
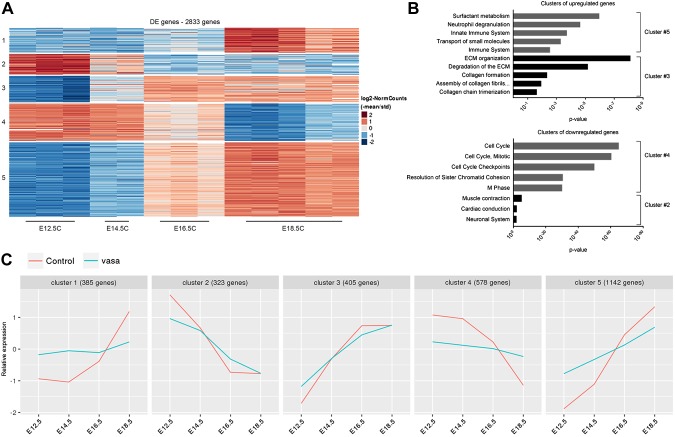
To further define the specific developmental stage affected by Lin28A overexpression, we performed an RNAseq analysis for transgenic and control lungs derived from E12.5, E14.5, E16.5, and E18.5 embryos. A global gene expression analysis by PCA (Fig. 2A) and a dendrogram hierarchical clustering analysis (Fig. 2B) showed that the transgenic and control lungs were still very similar at E12.5, but starting from E14.5, the developmental process of the transgenic lungs was significantly delayed compared with the control. Specifically, E14.5 transgenic lungs clustered together with E12.5 control lungs, E16.5 transgenic samples were very similar to E14.5 controls, and the transgenic E18.5 lungs clustered with the E16.5 controls. To verify these observations, we also compared the transgenic and control lungs at the morphologic level. Indeed, H&E and immunofluorescent staining showed similarities between E16.5 transgenic and E14.5 control lungs and between E18.5 transgenic and E16.5 control lungs (Fig. 3). Based on these results, we hypothesized that the control and transgenic lungs would show similar gene expression patterns in terms of up-regulation or down-regulation during development, whereas the rate of the change would be much slower in the transgenic lungs compared with control. To test this hypothesis, we first analyzed the RNAseq data of the control samples. We found 5 major clusters of differentially expressed genes (Fig. 4A). Clusters 1, 3, and 5 represent genes that underwent up-regulation during normal lung development. As expected, one of the most significantly enriched pathways in the clusters of the up-regulated genes was the surfactant metabolism pathway (Fig. 4B). One of the major functions of surfactant in the lung is related to its role in the lung innate immune system (33). Indeed, pathways related to the immune system were also enriched in cluster 5 (Fig. 4B). Additional pathways such as collagen formation and extracellular matrix organization, which are known to play an important role in embryonic lung development (18–20), were also found to be enriched in these clusters. The opposite clusters (2 and 4), which represent down-regulated genes, were enriched in pathways related to cell cycle and differentiation into other (nonlung) lineages (Fig. 4B). Next, we analyzed the expression pattern of the genes that generated each of the 5 above-mentioned clusters in the transgenic lungs (Supplemental Fig. S1). A comparison between the expression pattern of the control and transgene groups (Fig. 4C) supports our hypothesis regarding the directionality and rate of changes in gene expression.
Figure 2.
Global gene expression analysis of control and transgenic lungs during embryonic development. Analysis of lungs from transgenic mice and their littermate controls by PCA (A) and dendrogram clustering (B). The dendrogram analysis shows that E14.5 transgenic samples are clustered together with the E12.5 control samples, E16.5 transgenic samples are clustered with E14.5 controls, and E18.5 transgenic samples are in cluster with E16.5 controls.
Figure 3.
Morphologic characterization of control and transgenic lungs during embryonic development. H&E staining (left panels) and immunofluorescent staining (right panels) of control and transgenic lungs from E14.5, E16.5, and E18.5 embryos. Red, PDPN; green, proSFTPC; blue, DAPI. Scale bars, 25 μM.
Figure 4.
Gene expression patterns during normal and transgenic lung embryonic development. A) Clustering analysis of differentially expressed (DE) genes during normal lung embryonic development reveals 5 major clusters. The heatmap represents the relative expression level for each gene across the samples. B) Enriched pathways in each of the 5 clusters (based on the MouseMine tool). In cluster 4, only the top 5 pathways are presented. C) Comparison between the normal and transgenic clusters. Each horizontal line represents the median relative expression for all of the genes across the cluster. Note that the lines represent only relative expression levels but not absolute values. Therefore, only the dynamics of gene expression can be compared in this analysis. For a detailed description of the analysis, see Materials and Methods.
Altogether, these results suggest that Lin28A overexpression does not completely block lung development but rather delays their differentiation. Consequently, the lungs of the transgenic mice did not complete their maturation to a stage that enables self-breathing.
Lin28A expression during early stages of lung development is sufficient to cause the immature lung phenotype
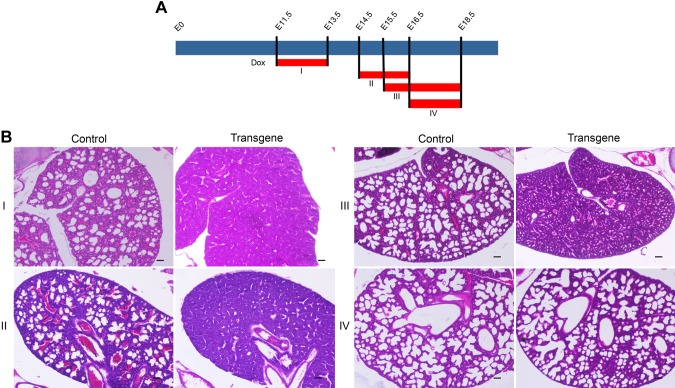
Next, we aimed to find the time window when Lin28A exerts its effect on lung development. For this purpose, we utilized the Tet-On system in our transgenic mice and induced Lin28A overexpression for short periods at several developmental time points (Fig. 5A). This experiment revealed that Lin28A induction from E11.5 to E13.5 (group I) or from E14.5 to E16.5 (group II) was sufficient to cause the same phenotype as the constitutive Lin28A overexpression (Fig. 5B). By contrast, when Lin28A was overexpressed from E16.5 (group IV) the embryos survived and had normal lungs (Fig. 5B). Interestingly, Lin28A overexpression from E15.5 (group III) led to a moderate phenotype as the lungs looked more mature than the transgenic lungs in groups I and II but still unlike normal E18.5 lungs (Fig. 5B). Overall, these results indicate that a short pulse of Lin28A as early as E11.5–E13.5 but no later than E16.5 is sufficient to affect the development of the lungs and that there is a correlation between the timing of Lin28A expression and the effect on lung development.
Figure 5.
Lin28A expression for specific time windows during embryonic lung development. A) A scheme showing the times of Dox (1 g/L) treatment. The red bars indicate the specific time of Dox treatment for each group (I–IV). B) H&E staining of control and transgenic lungs harvested from E18.5 embryos of groups I–IV. Scale bar, 25 μM.
Specific Lin28A expression in the lung mesothelial linage is sufficient to cause the immature lung phenotype
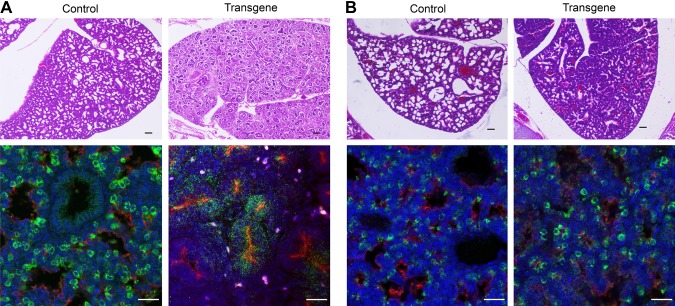
So far, we have shown that Lin28A delays the development of the lung when it is globally overexpressed in the entire embryo. However, lung embryonic development is a complex process that requires reciprocal interactions between the lung mesenchymal and epithelial cells (18, 19, 34). Therefore, we took the advantage of our Cre-Lox system to express Lin28A specifically in the lung epithelial cells or in the lung mesothelial linage. Nkx2.1 is expressed in the lung endoderm progenitors, which give rise to the different types of lung epithelial cells (18, 19). Thus, to express Lin28A in the lung epithelial cells, we crossed the Lox-stop-Lox-TetOn-Lin28A mice with an Nkx2.1-Cre strain (35). Wt1 is expressed in the mesothelial progenitors, which give rise to mesenchymal tissue of the lungs (36, 37). Therefore, we used a Wt1-Cre strain (35) to express Lin28A in the lung mesothelial cells. The Wt1-Cre–derived expression resulted in a similar phenotype as the expression in the entire embryo (Vasa-Cre). The neonates died immediately (10–20 min) after their birth and their lungs had exactly the same morphology as the lungs of the Vasa-Cre–derived embryos (Fig. 6A). Interestingly, Nkx2.1-Cre–derived Lin28A expression led to a completely different phenotype. In contrast with the Vasa-Cre– and the Wt1-Cre–derived embryos, the Nkx2.1-Cre–derived embryos survived for more than 2 h after birth. This relatively long survival suggests that the lungs were developed to a stage that enabled at least some of their functionality. Indeed, H&E staining revealed that the Nkx2.1-Cre–derived lungs developed to a more advanced developmental stage than the Vasa-Cre– and Wt1-Cre–derived lungs (Fig. 6B). Moreover, the pattern of SFTPC and PDPN staining of the Nkx2.1-Cre–derived lungs was almost similar to normal lungs (Fig. 6B). To confirm this observation, we analyzed gene expression of E18.5 lungs derived from the Wt1-Cre and the Nkx2.1-Cre embryos by RNAseq. A combined PCA and dendogram analysis of these samples together with the Vasa-Cre samples (Supplemental Fig. S2A, B) showed that E18.5 Wt1-Cre samples clustered with E18.5 Vasa-Cre samples, whereas E18.5 Nkx2.1-Cre samples clustered together with E18.5 control lungs. Importantly however, a gene ontology annotation of the differentially expressed genes between E18.5 control and Nkx2.1-Cre–derived lungs showed that Lin28A expression in the epithelial cells also affects the developmental process (Supplemental Table S1).
Figure 6.
Comparison between the effect of Lin28A overexpression in the mesenchymal and the epithelial cells of the lungs. A) Lungs of Wt1-Cre–derived transgenic embryos and their littermate controls stained with H&E (upper panel) or anti-PDPN (red) and anti-proSFTPC (green) (lower panel). B) Lungs of Nkx2.1-Cre–derived transgenic embryos and their littermate controls stained with H&E (upper panel) or anti-PDPN (red) and anti-proSFTPC (green) (lower panel). Blue, DAPI. Scale bars, 25 μM.
To conclude, these results show that Lin28A expression in the mesenchyme tissue is sufficient to mimic the effect of global Lin28A expression, whereas its expression in the epithelial cells results in a different and milder phenotype.
Lin28A exerts its effect on embryonic lung development via the Let-7 pathway
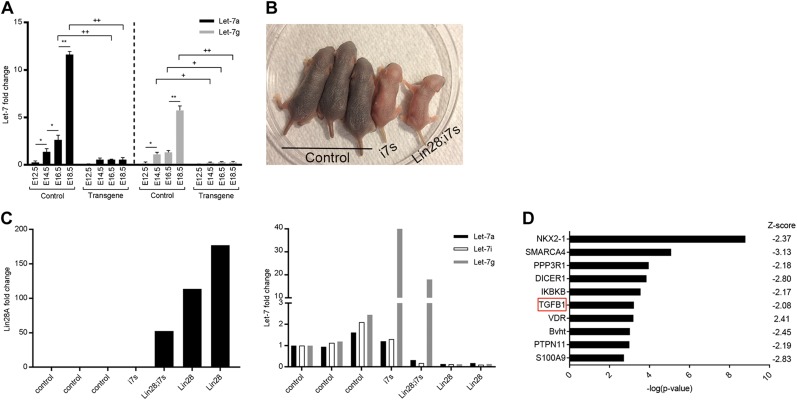
A previous analysis of miRNA expression revealed that all of the isoforms of the Let-7 miRNA family underwent significant up-regulation during mouse lung development (38, 39). Therefore, we presumed that the effect of Lin28A overexpression on lung organogenesis is mediated thorough inhibition of the Let-7 pathway. To test this assumption, we first compared mature Let-7 levels between control and transgenic (Vasa-Cre–derived) lungs along different stages of lung development. Indeed, the levels of mature Let-7s were significantly up-regulated during normal lung development but remained very low and constant in the transgenic lungs (Fig. 7A).
Figure 7.
Let-7–dependent mechanism of Lin28A overexpression. A) qRT-PCR analysis of mature Let-7a and Let-7g in embryonic lungs derived from E14.5, E16.5, and E18.5 embryos. Asterisks indicate comparisons between different embryonic days, and pluses indicate comparisons between transgene and control samples. */+P < 0.05, **/++P < 0.0001; n = 3. B) Five littermates, 2 d old, from the “rescue” experiment. i7s, mice overexpressing only i7s; Lin28A;i7s, mice overexpressing both Lin28A and i7s. Two additional embryos were found dead after delivery; therefore, they do not appear in the figure. As expected, these mice inherited only the Lin28A overexpression cassette and not the i7s isoform. C) qRT-PCR analysis of the 7 mice described above. Left panel, Lin28A expression levels. Right panel, mature Let7s levels. Note the high Let-7g levels in the Lin28;i7s mouse. The 2 last samples in each panel (“Lin28a”) are from the dead embryos that overexpressed only Lin28A and not i7s. D) Top 10 misregulated pathways in the E14.5 transgenic lungs, identified by IPA software.
Next, to verify that the lung phenotype is caused as a result of the Let-7 down-regulation, we crossed the Lox-stop-Lox-TetOn-Lin28A mice with i7s mice (15, 31, 40, 41). Two embryos of this crossing inherited only the Lin28A cassette. As expected, both of them died immediately after their birth. In contrast with these embryos, the pup that inherited both the Lin28A and the i7s cassettes breathed normally and survived (Fig. 7B). Note that this pup and another pup that inherited only the i7s cassette were much smaller than their littermate control, a phenomenon that had been reported previously for Let-7 overexpression (42). A quantitative RT-PCR (qRT-PCR) analysis (Fig. 7C) confirmed that the Lin28A;i7s pup indeed coexpressed both Lin28A and Let-7g, whereas, as expected, the other isotypes of Let-7 were down-regulated. These results showed that enforced expression of Let-7 prevented the effect of Lin28 overexpression and thus strongly supported our hypothesis that the effect of Lin28A on embryonic lung development was indeed mediated via the Let-7 pathway. Importantly, this is the first demonstration of the critical role of Let-7 in normal mouse embryonic lung development.
The Lin28A–Let-7 pathway regulates TGF-β signaling in the developing lung
To explore the molecular mechanism underlying the effect of Lin28A overexpression and Let-7 down-regulation, we chose to focus on E12.5 and E14.5 because at later developmental stages (E16.5 and E18.5), the transgenic lungs are highly distinct from their age-matched controls (see PCA analysis in Fig. 2 and Supplemental Table S2) and, therefore, the differentially expressed genes at these time points might reflect the result of the developmental failure rather than its molecular cause (for a complete list of the differentially expressed genes at E12.5 and E14.5 see Supplemental Table S3). An upstream regulator analysis of the E14.5 differentially expressed genes using the IPA software revealed a significant misregulation of the TGF-β1 pathway (Fig. 7D). Remarkably, it has been shown that the TGF-β pathway is a key player of the mesenchymal-epithelial interactions in the developing lung and that the expression of the 3 TGF-β isoforms (TGF-β1, TGF-β2, and TGF-β3) is tightly regulated during lung organogenesis (43–46). In addition, as we showed in Fig. 6, Lin28A overexpression in the mesenchymal tissue of the lung led to a similar lung phenotype as in global Lin28A overexpression. Taken together, these observations suggest that the Lin28–Let-7 pathway regulates the mesenchymal-epithelial interactions during embryonic lung development, at least in part, via the TGF-β pathway.
Notably, the IPA upstream regulator analysis identified also the NKX2.1 pathway, which is known to play a critical role in lung development (21). However, whereas NKX2.1 is specifically expressed in epithelial cells of the lung (21), the lung phenotype was caused by Lin28A overexpression in the mesenchymal cells. Therefore, the misregulation of the NKX2.1 pathway is probably not the cause but rather the result of the abnormal lung development.
DISCUSSION
An organism’s development is based on precise spatial and temporal regulation of gene expression (47–49). Correct developmental timing is controlled by a wide array of processes such as differentiation, proliferation, and growth. In addition, a unique type of genes, called heterochronic genes, is directly responsible for the temporal regulation of the developmental process (48). Lin28A and B and their target, the Let-7s miRNA family, were discovered more than 3 decades ago as such heterochronic genes in C. elegans (1, 2, 50). In mammals, Lin28A and B are highly expressed in stem and progenitor cells of diverse tissues but undergo down-regulation, which is accompanied by up-regulation of the Let-7 miRNA family, during differentiation. In the last decade, Lin28 ectopic expression has been shown to play a key role in the formation and maintenance of numerous tumors (7, 11, 51, 52). Recently, ectopic expression of Lin28 in mammals has been also studied in the context of embryogenesis and shown to affect diverse developmental processes, such as nephrogenesis (15, 16), elongation of the caudal body axis (41), and brain development (53, 54). In each of these processes, Lin28 ectopic expression affects the progenitor stem cell’s fate and thus prevents normal development. Yet, the molecular and cellular mechanism whereby Lin28 affects the progenitor cells is unique for each process. In the kidney, Lin28 ectopic expression and the resulting Let-7 down-regulation delay the final differentiation of the nephron progenitor cells and thus prolong nephrogenesis (15, 16), probably via up-regulation of the Igf2–H19 pathway (16). Likewise, during posterior body development, ectopic expression of Lin28A and B dramatically increase the vertebrae number in the tail, whereas Let-7 overexpression results in a decreased number of caudal vertebrae and a short tail (41). The effect of Lin28A ectopic expression, in this case, is mediated at least in part by down-regulation of Sox2 and disruption of the balance between neuronal and mesodermal tissue specification of the progenitor cells (41). Finally, Lin28A ectopic expression during embryonic brain development (53) and postnatal neurogenesis (54) was shown to control the fate of the neuronal progenitor cells. Notably, in these 2 cases, the effect of Lin28 ectopic expression was attributed mainly to Let-7–independent pathways.
In contrast to the above-mentioned observations that were based on tissue-specific Lin28 ectopic expression, global Lin28A overexpression during mouse embryonic development leads to postnatal lethality several minutes after birth (15). Here, we show that this early lethality is caused by the failure of the lungs to complete their developmental process. This is evidenced by several observations: 1) The histology of transgenic lungs at E18.5 resembles this of normal lungs at an early developmental stage, 2) The branching process, which normally ceased at around E16.5, continues until birth upon Lin28A expression, and 3) E18.5 transgenic lungs contain mainly bipolar alveolar progenitor cells, whereas more mature AT1 and AT2 cells are almost completely absent from these lungs. Collectively, these results show that, as in the above-mentioned examples, Lin28A ectopic expression perturbs the differentiation of the early progenitor cells. This can be explained by 2 scenarios; one is that ectopic expression of Lin28A prevents a specific developmental stage during lung organogenesis. Alternatively, it is plausible that Lin28A functions as a heterochronic gene that regulates the timing of development rather than a specific process.
To distinguish between these 2 options, we characterized the transgenic lungs during several developmental stages (E12.5, E14.5, E16.6, and E18.5) both at the morphologic and at the gene expression levels. These analyses show that the transgenic lungs at each developmental stage are very similar to the control lungs of the previous stage. Moreover, the dynamics of gene expression in the transgenic lungs throughout development resemble those of control lungs but in a much slower rate. Overall, these results support the second scenario for the effect of Lin28 expression on lung development (heterochronic rather than specific differentiation effect).
Previous analysis of miRNA expression during lung organogenesis revealed a significant up-regulation of Let-7 isoforms upon the development of the lungs (38, 39). This observation led to the hypothesis that the Let-7 miRNA family is a heterochronic regulator of lung organogenesis (38, 39, 55). Yet, this assumption has never been proven before because it requires down-regulation of all 12 members of the Let-7 family. Here, by Lin28A ectopic expression, we were able to prevent the maturation of all the Let-7 isoforms at once and to show that the Let-7 miRNA family is indeed crucial for lung development. To further strengthen this conclusion, we coexpressed Let-7 (i7s) together with Lin28A and showed that the expression of Let-7 overcomes the effect of Lin28A and enables normal lung development. Therefore, our results reveal, for the first time, the Lin28A–Let-7 pathway as a heterochronic regulator of the lung. In contrast with other developmental processes such as kidney development and posterior vertebrae elongation (see above), Let-7 regulates the timing of lung development rather than a specific differentiation stage. Thus, it can be considered as a truly heterochronic regulator of the lung.
Remarkably, the fact that induction of Lin28A expression later than E16.5 does not appear to affect lung development indicates that there is a critical time window for Let-7 up-regulation during the early stages of embryonic lung development.
To identify the tissue within the lung wherein Lin28A overexpression and Let-7 down-regulation promote their effect, we expressed Lin28A in the lung’s mesenchymal (Wt1-Cre) or epithelial (Nkx2.1-Cre) cells. Because global Lin28A overexpression (Vasa-Cre) affects epithelial cell development, we hypothesized that Lin28A expression in these cells would result in the same effect. Unexpectedly, we found that although specific Lin28A expression in these cells led to abnormal lung development, the phenotype was different and much milder than the 1 conferred by global Lin28A expression. By contrast, Lin28A expression in the mesenchymal compartment of the lung resulted in a phenotype similar to the 1 derived by Vasa-Cre. These findings suggest that Let-7 miRNAs play a key role in the interactions between the mesenchymal and the epithelial cells. These mesenchymal-epithelial interactions were shown to be critical for normal embryonic lung development (34, 44, 56). In accordance, an upstream regulator IPA analysis of the RNAseq data revealed a significant misregulation of the TGF-β1 pathway, which is tightly regulated during normal development and is essential for the mesenchymal-epithelial interaction in the lung (43–46). Therefore, we conclude that the Let-7 miRNA family regulates embryonic lung development at least in part via the TGF-β1 pathway. Notably, however, Lin28A overexpression did not affect the expression levels of TGF-β1 itself or its receptors (TGF-βR1 and TGF-βR2). Thus, the Lin28–Let-7 pathway probably possesses another level of regulation on the downstream effectors of the TGF-β pathway.
Finally, published microarray data of miRNA expression during human lung development show that all members of the Let-7 family are dramatically up-regulated during the transition from the pseudoglandular stage to the canalicular stage of the human embryonic lungs [Gene Expression Omnibus Series (GSE) 76919 and 76920 (55)]. This observation suggests that the role of Let-7 in lung embryonic development is conserved in humans.
To summarize, our results provide the first evidence for the important heterochronic role of Let-7 during mouse lung development. In a more general perspective, this work, together with previously published studies, points to the general role of the Lin28–Let-7 pathway in the regulation of diverse mammalian developmental processes on the one hand and highlights the specificity and complexity of this regulation on the other hand.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Dr. Daley (Boston Children’s Hospital, Boston, MA, USA) for kindly providing the Lox-stop-Lox-TetOn-Lin28A mice, Dr. Avi Jacob and Dr. Irit Shoval (Scientific Equipment Center, Bar-Ilan University) for helping with the confocal microscopy analysis, and the staff at the Crown Genomics Institute of the Nancy and Stephen Grand Israel National Center for Personalized Medicine (Weizmann Institute of Science) for performing the RNA sequencing. This work was funded by Israel Science Foundation (ISF) Grants 1389/15 and 1204/17. The authors declare no conflicts of interest.
Glossary
- AT
alveolar type
- Dox
doxycycline
- H&E
hematoxylin and eosin
- i7s
Let-7g isoform resistant to Lin28A inhibition
- IPA
Ingenuity Pathway Analysis
- miRNA
micro RNA
- PCA
principal component analysis
- PDPN
podoplanin
- qRT-PCR
quantitative RT-PCR
- RNAseq
RNA sequencing
- SFTPC
surfactant protein C
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
N. Komarovsky Gulman performed research and analyzed data; L. Armon analyzed data and wrote the paper; T. Shalit performed bioinformatic analysis; and A. Urbach designed research, analyzed data, and wrote the manuscript.
REFERENCES
- 1.Ambros V., Horvitz H. R. (1984) Heterochronic mutants of the nematode Caenorhabditis elegans. Science 226, 409–416 [DOI] [PubMed] [Google Scholar]
- 2.Moss E. G., Lee R. C., Ambros V. (1997) The cold shock domain protein LIN-28 controls developmental timing in C. elegans and is regulated by the lin-4 RNA. Cell 88, 637–646 [DOI] [PubMed] [Google Scholar]
- 3.Heo I., Joo C., Kim Y. K., Ha M., Yoon M. J., Cho J., Yeom K. H., Han J., Kim V. N. (2009) TUT4 in concert with Lin28 suppresses microRNA biogenesis through pre-microRNA uridylation. Cell 138, 696–708 [DOI] [PubMed] [Google Scholar]
- 4.Jiang S., Baltimore D. (2016) RNA-binding protein Lin28 in cancer and immunity. Cancer Lett. 375, 108–113 [DOI] [PubMed] [Google Scholar]
- 5.Nam Y., Chen C., Gregory R. I., Chou J. J., Sliz P. (2011) Molecular basis for interaction of let-7 microRNAs with Lin28. Cell 147, 1080–1091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Viswanathan S. R., Daley G. Q., Gregory R. I. (2008) Selective blockade of microRNA processing by Lin28. Science 320, 97–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shyh-Chang N., Daley G. Q. (2013) Lin28: primal regulator of growth and metabolism in stem cells. Cell Stem Cell 12, 395–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Viswanathan S. R., Daley G. Q. (2010) Lin28: a microRNA regulator with a macro role. Cell 140, 445–449 [DOI] [PubMed] [Google Scholar]
- 9.Peng S., Chen L. L., Lei X. X., Yang L., Lin H., Carmichael G. G., Huang Y. (2011) Genome-wide studies reveal that Lin28 enhances the translation of genes important for growth and survival of human embryonic stem cells. Stem Cells 29, 496–504 [DOI] [PubMed] [Google Scholar]
- 10.Wilbert M. L., Huelga S. C., Kapeli K., Stark T. J., Liang T. Y., Chen S. X., Yan B. Y., Nathanson J. L., Hutt K. R., Lovci M. T., Kazan H., Vu A. Q., Massirer K. B., Morris Q., Hoon S., Yeo G. W. (2012) LIN28 binds messenger RNAs at GGAGA motifs and regulates splicing factor abundance. Mol. Cell 48, 195–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Thornton J. E., Gregory R. I. (2012) How does Lin28 let-7 control development and disease? Trends Cell Biol. 22, 474–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J., Ratanasirintrawoot S., Chandrasekaran S., Wu Z., Ficarro S. B., Yu C., Ross C. A., Cacchiarelli D., Xia Q., Seligson M., Shinoda G., Xie W., Cahan P., Wang L., Ng S. C., Tintara S., Trapnell C., Onder T., Loh Y. H., Mikkelsen T., Sliz P., Teitell M. A., Asara J. M., Marto J. A., Li H., Collins J. J., Daley G. Q. (2016) LIN28 regulates stem cell metabolism and conversion to primed pluripotency. Cell Stem Cell 19, 66–80 [DOI] [PubMed] [Google Scholar]
- 13.West J. A., Viswanathan S. R., Yabuuchi A., Cunniff K., Takeuchi A., Park I. H., Sero J. E., Zhu H., Perez-Atayde A., Frazier A. L., Surani M. A., Daley G. Q. (2009) A role for Lin28 in primordial germ-cell development and germ-cell malignancy. Nature 460, 909–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tu H. C., Schwitalla S., Qian Z., LaPier G. S., Yermalovich A., Ku Y. C., Chen S. C., Viswanathan S. R., Zhu H., Nishihara R., Inamura K., Kim S. A., Morikawa T., Mima K., Sukawa Y., Yang J., Meredith G., Fuchs C. S., Ogino S., Daley G. Q. (2015) LIN28 cooperates with WNT signaling to drive invasive intestinal and colorectal adenocarcinoma in mice and humans. Genes Dev. 29, 1074–1086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Urbach A., Yermalovich A., Zhang J., Spina C. S., Zhu H., Perez-Atayde A. R., Shukrun R., Charlton J., Sebire N., Mifsud W., Dekel B., Pritchard-Jones K., Daley G. Q. (2014) Lin28 sustains early renal progenitors and induces Wilms tumor. Genes Dev. 28, 971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yermalovich A. V., Osborne J. K., Sousa P., Han A., Kinney M. A., Chen M. J., Robinton D. A., Montie H., Pearson D. S., Wilson S. B., Combes A. N., Little M. H., Daley G. Q. (2019) Lin28 and let-7 regulate the timing of cessation of murine nephrogenesis. Nat. Commun. 10, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shinoda G., Shyh-Chang N., Soysa T. Y., Zhu H., Seligson M. T., Shah S. P., Abo-Sido N., Yabuuchi A., Hagan J. P., Gregory R. I., Asara J. M., Cantley L. C., Moss E. G., Daley G. Q. (2013) Fetal deficiency of lin28 programs life-long aberrations in growth and glucose metabolism. Stem Cells 31, 1563–1573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cardoso W. V., Lü J. (2006) Regulation of early lung morphogenesis: questions, facts and controversies. Development 133, 1611–1624 [DOI] [PubMed] [Google Scholar]
- 19.Herriges M., Morrisey E. E. (2014) Lung development: orchestrating the generation and regeneration of a complex organ. Development 141, 502–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Morrisey E. E., Hogan B. L. M. (2010) Preparing for the first breath: genetic and cellular mechanisms in lung development. Dev. Cell 18, 8–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Warburton D., El-Hashash A., Carraro G., Tiozzo C., Sala F., Rogers O., De Langhe S., Kemp P. J., Riccardi D., Torday J., Bellusci S., Shi W., Lubkin S. R., Jesudason E. (2010) Lung organogenesis. Curr. Top. Dev. Biol. 90, 73–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Metzger R. J., Klein O. D., Martin G. R., Krasnow M. A. (2008) The branching programme of mouse lung development. Nature 453, 745–750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Desai T. J., Brownfield D. G., Krasnow M. A. (2014) Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature 507, 190–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Treutlein B., Brownfield D. G., Wu A. R., Neff N. F., Mantalas G. L., Espinoza F. H., Desai T. J., Krasnow M. A., Quake S. R. (2014) Reconstructing lineage hierarchies of the distal lung epithelium using single-cell RNA-seq. Nature 509, 371–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chen Y. W., Huang S. X., de Carvalho A. L. R. T., Ho S. H., Islam M. N., Volpi S., Notarangelo L. D., Ciancanelli M., Casanova J. L., Bhattacharya J., Liang A. F., Palermo L. M., Porotto M., Moscona A., Snoeck H. W. (2017) A three-dimensional model of human lung development and disease from pluripotent stem cells. Nat. Cell Biol. 19, 542–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martin M. (2011) Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J. 17, 10 [Google Scholar]
- 27.Dobin A., Davis C. A., Schlesinger F., Drenkow J., Zaleski C., Jha S., Batut P., Chaisson M., Gingeras T. R. (2013) STAR: ultrafast universal RNA-seq aligner. Bioinformatics 29, 15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anders S., Pyl P. T., Huber W. (2015) HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics 31, 166–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Love M. I., Huber W., Anders S. (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Conesa A., Nueda M. J., Ferrer A., Talón M. (2006) maSigPro: a method to identify significantly differential expression profiles in time-course microarray experiments. Bioinformatics 22, 1096–1102 [DOI] [PubMed] [Google Scholar]
- 31.Zhu H., Shah S., Shyh-Chang N., Shinoda G., Einhorn W. S., Viswanathan S. R., Takeuchi A., Grasemann C., Rinn J. L., Lopez M. F., Hirschhorn J. N., Palmert M. R., Daley G. Q. (2010) Lin28a transgenic mice manifest size and puberty phenotypes identified in human genetic association studies. Nat. Genet. 42, 626–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallardo T., Shirley L., John G. B., Castrillon D. H. (2007) Generation of a germ cell-specific mouse transgenic Cre line, Vasa-Cre. Genesis 45, 413–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orgeig S., Morrison J. L., Daniels C. B. (2015) Evolution, development, and function of the pulmonary surfactant system in normal and perturbed environments. Compr. Physiol. 6, 363–422 [DOI] [PubMed] [Google Scholar]
- 34.Lüdtke T. H., Rudat C., Wojahn I., Weiss A. C., Kleppa M. J., Kurz J., Farin H. F., Moon A., Christoffels V. M., Kispert A. (2016) Tbx2 and Tbx3 act downstream of Shh to maintain canonical Wnt signaling during branching morphogenesis of the murine lung. Dev. Cell 39, 239–253 [DOI] [PubMed] [Google Scholar]
- 35.Rawlins E. L., Perl A. K. (2012) The a“MAZE”ing world of lung-specific transgenic mice. Am. J. Respir. Cell Mol. Biol. 46, 269–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dixit R., Ai X., Fine A. (2013) Derivation of lung mesenchymal lineages from the fetal mesothelium requires hedgehog signaling for mesothelial cell entry. Development 140, 4398–4406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morimoto M., Liu Z., Cheng H.-T., Winters N., Bader D., Kopan R. (2010) Canonical Notch signaling in the developing lung is required for determination of arterial smooth muscle cells and selection of Clara versus ciliated cell fate. J. Cell Sci. 123, 213–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y., Okubo T., Rawlins E., Hogan B. L. M. (2008) Epithelial progenitor cells of the embryonic lung and the role of microRNAs in their proliferation. Proc. Am. Thorac. Soc. 5, 300–304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lu Y., Thomson J. M., Wong H. Y. F., Hammond S. M., Hogan B. L. M. (2007) Transgenic over-expression of the microRNA miR-17-92 cluster promotes proliferation and inhibits differentiation of lung epithelial progenitor cells. Dev. Biol. 310, 442–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Piskounova E., Viswanathan S. R., Janas M., LaPierre R. J., Daley G. Q., Sliz P., Gregory R. I. (2008) Determinants of microRNA processing inhibition by the developmentally regulated RNA-binding protein Lin28. J. Biol. Chem. 283, 21310–21314 [DOI] [PubMed] [Google Scholar]
- 41.Robinton D. A., Chal J., Lummertz da Rocha E., Han A., Yermalovich A. V, Oginuma M., Schlaeger T. M., Sousa P., Rodriguez A., Urbach A., Pourquié O., Daley G. Q. (2019) The Lin28/let-7 pathway regulates the mammalian caudal body axis elongation program. Dev. Cell 48, 396–405.e3 [DOI] [PubMed] [Google Scholar]
- 42.Zhu H., Shyh-Chang N., Segrè A. V., Shinoda G., Shah S. P., Einhorn W. S., Takeuchi A., Engreitz J. M., Hagan J. P., Kharas M. G., Urbach A., Thornton J. E., Triboulet R., Gregory R. I., Altshuler D., Daley G. Q.; DIAGRAM Consortium ; MAGIC Investigators (2011) The Lin28/let-7 axis regulates glucose metabolism. Cell 147, 81–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartram U., Speer C. P. (2004) The role of transforming growth factor beta in lung development and disease. Chest 125, 754–765 [DOI] [PubMed] [Google Scholar]
- 44.Bragg A. D., Moses H. L., Serra R. (2001) Signaling to the epithelium is not sufficient to mediate all of the effects of transforming growth factor beta and bone morphogenetic protein 4 on murine embryonic lung development. Mech. Dev. 109, 13–26 [DOI] [PubMed] [Google Scholar]
- 45.Saito A., Horie M., Nagase T. (2018) TGF-β signaling in lung health and disease. Int. J. Mol. Sci. 19, 2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito A., Nagase T. (2015) Hippo and TGF-β interplay in the lung field. Am. J. Physiol. Lung Cell Mol. Physiol. 309, L756–L767 [DOI] [PubMed] [Google Scholar]
- 47.Keyte A. L., Smith K. K. (2014) Heterochrony and developmental timing mechanisms: changing ontogenies in evolution. Semin. Cell Dev. Biol. 34, 99–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Moss E. G. (2007) Heterochronic genes and the nature of developmental time. Curr. Biol. 17, R425–R434 [DOI] [PubMed] [Google Scholar]
- 49.Smith K. K. (2003) Time’s arrow: heterochrony and the evolution of development. Int. J. Dev. Biol. 47, 613–621 [PubMed] [Google Scholar]
- 50.Reinhart B. J., Slack F. J., Basson M., Pasquinelli A. E., Bettinger J. C., Rougvie A. E., Horvitz H. R., Ruvkun G. (2000) The 21-nucleotide let-7 RNA regulates developmental timing in Caenorhabditis elegans. Nature 403, 901–906 [DOI] [PubMed] [Google Scholar]
- 51.Carmel-Gross I., Bollag N., Armon L., Urbach A. (2016) LIN28: a stem cell factor with a key role in pediatric tumor formation. Stem Cells Dev. 25, 367–377 [DOI] [PubMed] [Google Scholar]
- 52.Balzeau J., Menezes M. R., Cao S., Hagan J. P. (2017) The LIN28/let-7 pathway in cancer. Front. Genet. 8, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang M., Yang S.-L., Herrlinger S., Liang C., Dzieciatkowska M., Hansen K. C., Desai R., Nagy A., Niswander L., Moss E. G., Chen J.-F. (2015) Lin28 promotes the proliferative capacity of neural progenitor cells in brain development. Development 142, 1616–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Romer-Seibert J. S., Hartman N. W., Moss E. G. (2019) The RNA-binding protein LIN28 controls progenitor and neuronal cell fate during postnatal neurogenesis. FASEB J. 33, 3291–3303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sanford E. L., Choy K. W., Donahoe P. K., Tracy A. A., Hila R., Loscertales M., Longoni M. (2016) MiR-449a affects epithelial proliferation during the pseudoglandular and canalicular phases of avian and mammal lung development. PLoS One 11, e0149425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McCulley D., Wienhold M., Sun X. (2015) The pulmonary mesenchyme directs lung development. Curr. Opin. Genet. Dev. 32, 98–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.