Abstract
Inflammatory processes have been shown to modify tryptophan (Trp) metabolism. Gut microbiota appears to play a significant role in the induction of peripheral and central inflammation. Ethanol (EtOH) exposure alters gut permeability, but its effects on Trp metabolism and the involvement of gut microbiota have not been studied. We analyzed several parameters of gut-barrier and of peripheral and central Trp metabolism following 2 different EtOH consumption patterns in mice, the binge model, drinking in the dark (DID), and the chronic intermittent (CI) consumption paradigm. Antibiotic treatment was used to evaluate gut microbiota involvement in the CI model. Mice exposed to CI EtOH intake, but not DID, show bacterial translocation and increased plasma LPS immediately after EtOH removal. Gut-barrier permeability to FITC-dextran is increased by CI, and, furthermore, intestinal epithelial tight-junction (TJ) disruption is observed (decreased expression of zonula occludens 1 and occludin) associated with increased matrix metalloproteinase (MMP)-9 activity and iNOS expression. CI EtOH, but not DID, increases kynurenine (Kyn) levels in plasma and limbic forebrain. Intestinal bacterial decontamination prevents the LPS increase but not the permeability to FITC-dextran, TJ disruption, or the increase in MMP-9 activity and iNOS expression. Although plasma Kyn levels are not affected by antibiotic treatment, the elevation of Kyn in brain is prevented, pointing to an involvement of microbiota in CI EtOH–induced changes in brain Trp metabolism. Additionally, CI EtOH produces depressive-like symptoms of anhedonia, which are prevented by the antibiotic treatment thus pointing to an association between anhedonia and the increase in brain Kyn and to the involvement of gut microbiota.—Giménez-Gómez, P., Pérez-Hernández, M., O’Shea, E., Caso, J. R., Martín-Hernández, D., Cervera, L. A., Centelles. M. L. G.-L., Gutiérrez-Lopez, M. D., Colado, M. I. Changes in brain kynurenine levels via gut microbiota and gut-barrier disruption induced by chronic ethanol exposure in mice.
Keywords: LPS, MMPs, iNOS
The gut microbiota includes a community of bacteria that play an integral part in nutrient metabolism and absorption (1). Over the last decade evidence has emerged indicating the existence of the microbiota-gut-brain axis, which could play an important role in neuroinflammation and neuropsychiatric pathologies (2), both of which are closely associated with alcohol abuse (3, 4). Intestinal microbiota participates in gut-brain axis modulation by regulating tryptophan (Trp) availability (5). Although Trp can generate serotonin (5-HT), the kynurenine (Kyn) pathway is the main route of Trp metabolism through which 95% of Trp is transformed to Kyn by the action of the enzymes indolamine 2,3-dioxygenase (IDO) and Trp 2,3-dioxygenase (TDO) (6). Approximately 40% of Kyn is formed in the CNS, whereas the rest is captured from the periphery (7) because Kyn easily crosses the blood-brain barrier to the brain. In recent years dysregulation of the Kyn pathway has been linked to neurodegenerative, psychiatric, and neurologic disorders (8, 9).
It is well established that the microbiota regulates the Kyn pathway via multiple mechanisms (5) and consequently can play a key role in several conditions, such as anxiety or depression (10). Furthermore, gut microbiota implication in Kyn pathway regulation could be strongly related to drug abuse because several recent reports demonstrate that pharmacological alterations in Kyn pathway modulate drug seeking and relapse (11, 12). Previous data from our laboratory indicate that an increase in the concentrations of Kyn and its metabolite, kynurenic acid, by administering Ro 61-8048, a Kyn-3-monooxygenase inhibitor, decreases binge ethanol (EtOH) consumption in rodents (13).
EtOH is the most widely consumed drug in the world (14), and it is one of the most important factors of risk for disease, disability, and death (15). Both acute and chronic EtOH consumption produce oxidative stress and inflammation, which contribute to tissue injury and the development of liver injury, cardiovascular disease, cognitive dysfunction, and neuropsychiatric disorders (16–19). Recently it has been described that EtOH exposure can affect the gut indigenous flora, which could, in turn, be associated with inflammation (20). Moreover, EtOH consumption could change intestinal permeability by affecting tight-junction (TJ) proteins such as zonula occludens 1 (ZO-1) and occludin (21). TJ disassembly might be mediated by matrix metalloproteinases (MMPs) (22), which might be involved in the increase of blood-brain barrier permeability in alcoholics (23) and are up-regulated in inflammatory bowel disease (24) by oxidative stress and inflammation. In particular, MMP-9 up-regulation, which can be mediated by both iNOS (24, 25) and cyclo-oxygenase 2 (COX-2) (26) during inflammation, is related to experimental colitis severity in mice (27). To our knowledge, there are no studies focused on the involvement of microbiota in EtOH-induced gut-barrier disruption, nor in Trp metabolism after different EtOH consumption patterns.
The present study evaluates the effects of 2 different patterns of voluntary EtOH consumption—a binge paradigm, drinking in the dark (DID), and a chronic model, chronic intermittent (CI)—on: 1) bacterial translocation [evaluating bacterial presence in mesenteric lymph nodes (MLNs)] and plasmatic LPS; 2) gut-barrier permeability and disruption (ZO-1 and occludin expression), MMP-2 and MMP-9 activities, and the expression of iNOS and COX-2 as markers of nitrosative stress and inflammation, respectively; 3) Kyn levels in plasma and limbic forebrain; and 4) the role of microbiota in the EtOH-induced changes. Finally, we evaluated 5) the effect of CI EtOH on the induction of anhedonic or anxious phenotypes and the role of microbiota in these behaviors.
MATERIALS AND METHODS
Mice
Adult male C57BL/6J mice (Envigo, East Millstone, NJ, USA) weighing 20–25 g (8 wk) were used. They were maintained in conditions of constant temperature (21 ± 2°C) and a 12-h reverse lighting cycle (lights on at 9:00 pm). Animals were housed in groups of 4–6 with ad libitum access to food and water during 10 d. Then, mice were individually housed, randomly allocated into water and EtOH groups, and habituated for 7 d to drinking water ad libitum from a 25 ml serological pipette fitted with a drinking spout. All experimental procedures were approved by the Animal Welfare Committee of the Universidad Complutense de Madrid (following European Union Directive 2010/63/EU).
Experimental design
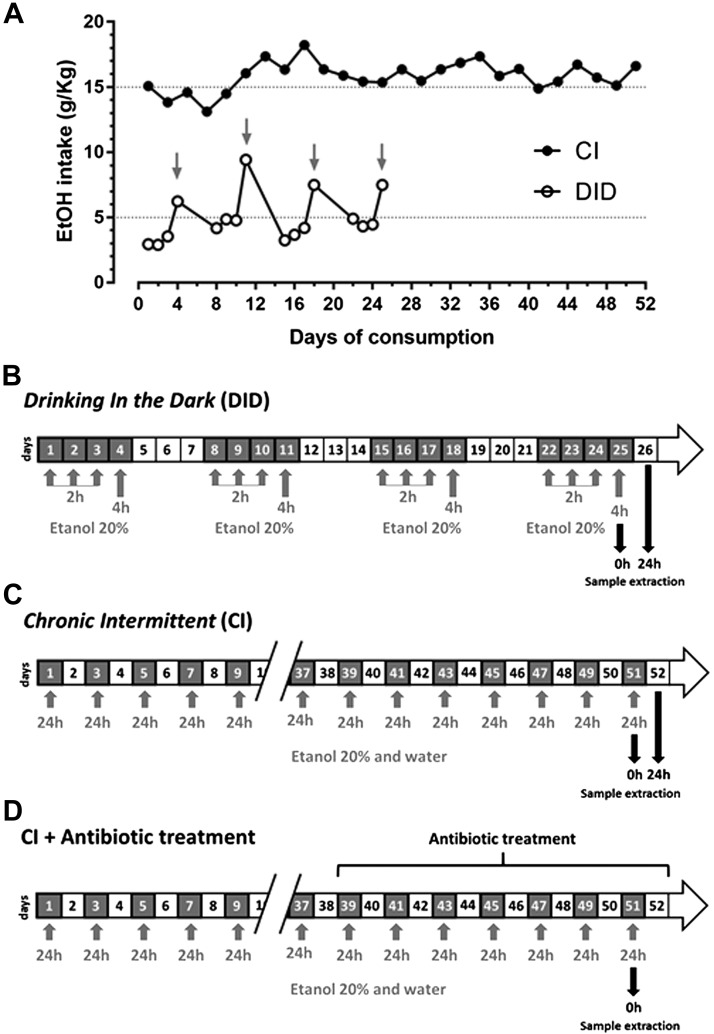
The paradigm DID was used as a model of binge drinking (13, 23, 28). For 4 consecutive days, starting 3 h after lights-off, water was replaced by EtOH 20% (v/v) for 2 h during the first 3 d and for 4 h on the fourth day (Fig. 1A, B). Following 3 EtOH-free days, the pattern of EtOH exposure was repeated for a total of 4 DID weeks to increase EtOH preference and consumption, as previously demonstrated in refs. 29, 30. The mean daily amount of EtOH consumed in 4 h on each binge day was 7.49 ± 1.3 g/kg, and the plasma EtOH concentration reached after each episode of consumption was 121 ± 22 mg/dl. In these conditions, DID produces cognitive deficits such as memory impairment (31) and disrupts the blood-brain barrier by a mechanism involving TLR4 signaling in the hippocampus (23). Mice were killed immediately or 24 h after the last EtOH exposure. Control mice were exposed to water at all times.
Figure 1.
A) Consumption of EtOH during the DID and CI EtOH paradigm. Arrows indicate the fourth day (binge) of every DID cycle. B) Time schedule of DID. Gray boxes, EtOH exposure; white boxes, water exposure. C, D) Chronograms of CI EtOH intake paradigm without (C) and with (D) antibiotic treatment. Gray boxes, EtOH plus water exposure. White boxes, water exposure. Gray arrows indicate EtOH exposure. Black arrows indicate day of death and sample extraction.
To study a different EtOH consumption behavior, the paradigm CI was used as a chronic model. It was based on a 2-bottle chronic model lasting 30 d (32), which was modified to produce an intermittent access to EtOH that contributes to higher voluntary EtOH intakes (33). Animals only had access to EtOH every other day but had access to water every day (Fig. 1A, C). The plasma EtOH concentration reached after each episode of consumption was 142 ± 15 mg/dl, and the daily amount of EtOH consumed in 24 h was 15.18 ± 1.1 g/kg. The CI study finished when total EtOH consumed was 400 ± 12 g/kg, as described in the original published study, which occurred after 26 d of EtOH drinking (i.e., a total of 52 d). We selected the CI EtOH paradigm because previous studies have shown an overlap in many of the genes expressed in prefrontal cortex of animals subjected to this paradigm of EtOH consumption with those given LPS, making CI a good model of the neuroimmune component of chronic EtOH consumption (32). Control mice were exposed to water at all times. Mice were killed immediately or 24 h after the last EtOH exposure. Body weights of mice and EtOH and water intake values were recorded weekly to calculate the self-administered EtOH dose (g/kg) in both models, and the relative preference for EtOH (EtOH:total fluid intake) (34) was also measured in the CI model.
Antibiotic treatment
Bacterial decontamination was performed by a modified protocol for rodents (35, 36). Animals in antibiotic groups were given drinking water ad libitum or EtOH 20% containing streptomycin sulfate (2 mg/ml; MilliporeSigma, Burlington, MA, USA) and penicillin G (1500 U/ml; MilliporeSigma) during the last 15 d of the CI model to reduce indigenous gastrointestinal microflora (Fig. 1D). We selected these antibiotics because of their limited oral absorption.
Sample extraction
Mice were killed by cervical dislocation. Brains were removed and limbic forebrain dissected out over ice using a mouse brain matrix for coronal slices (World Precision Instruments, Sarasota, FL, USA). After removal of the olfactory bulbs, brain tissue anterior to the optic chiasm was collected and referred to as “limbic forebrain” (37). This brain region was studied because we had previously demonstrated that a pharmacologically induced increase in Kyn reduced EtOH consumption in DID (13). An 8-cm portion of the distal colon was also collected. Cardiac blood samples were collected in K2-EDTA from animals anesthetized with sodium pentobarbital (120 mg/kg, i.p., Dolethal; Vetoquinol, Magny-Vernois, France) and immediately centrifuged at 1300 g for 10 min at 4°C. Plasma was then divided into separate tubes and frozen at −80°C until assayed.
Kyn, Trp, and 5-HT measurements in plasma and limbic forebrain
Kyn, Trp, and 5-HT were purchased from MilliporeSigma. HPLC-grade acetonitrile and methanol were obtained from PanReac AppliChem (ITW, Glenview, IL, USA). Other chemicals were of analytical grade and obtained from MilliporeSigma.
Limbic forebrain samples were homogenized in 5 volumes of deionized water under sonication (Labsonic 2000U; B. Braun Melsungen, Melsungen, Germany). Samples were deproteinized by adding 25 μl of 6% perchloric acid per 100 µl of brain homogenate, or 12.5 μl of 6% perchloric acid and 187.5 μl of water to 50 μl plasma. After centrifugation, supernatants were frozen at −80°C until analysis.
Sixty microliters of supernatant was applied to a reversed-phase column (80 mm × 4.6 mm, 3 µm; HR-80; Thermo Fisher Scientific, Waltham, MA, USA), and Kyn was isocratically eluted using a mobile phase containing 0.1 M sodium acetate and 4% acetonitrile, pH 4.6, at a flow rate of 1 ml/min. Kyn was measured by UV detection (360 nm, 2487; Waters, Milford, MA, USA).
For 5-HT and Trp measurement, 20 µl of supernatant was applied to the same column. The mobile phase contained 0.5 M sodium acetate (adjusted to pH 6.2 with glacial acetic acid), 0.25 M zinc acetate, and 5% acetonitrile delivered at 1 ml/min. 5-HT and Trp were detected fluorometrically, using 290 and 337 nm for 5-HT and 270 and 360 nm for Trp as the excitation and emission wavelengths, respectively.
Blood EtOH measurements
Cardiac plasma samples were injected into an EtOH analyzer (AM1; Analox Instruments, Stourbridge, United Kingdom) (13) using EtOH 100 mg/dl as standard.
Plasma LPS levels
Plasma LPS levels were determined from cardiac blood using the Limulus Amebocyte Lysate Chromogenic Endpoint Assay following the manufacturer’s instructions (Hycult Biotech, Wayne, PA, USA) under sterile conditions.
Bacterial translocation studies
This procedure was performed as previously described in Martin-Hernández et al. (36). Briefly, following the induction of the terminal anesthesia, and after blood sampling, MLNs were removed under sterile conditions. Following weighing and homogenization in saline (0.9% NaCl), serial dilutions were plated onto agar plates for recovery of aerobic (Mueller-Hinton supplemented with 5% lysed sheep blood) and anaerobic (Brucella blood agar plates supplemented with vitamin K1 and hemin) bacteria. After 24 and 48 h of incubation at 37°C for aerobic and anaerobic cultures, respectively, colonies were counted and referred to milligrams of tissue. Any positive MLN culture was considered indicative of bacterial translocation from the intestinal lumen.
Intestinal permeability assay to FITC-dextran
Intestinal barrier integrity was assessed by permeability to a 4 kDa FITC-dextran (MilliporeSigma) as previously described in Volynets et al. (38). The experiment was carried out on the last day of exposure to EtOH. One hour before the end of the experiment (39), FITC-dextran was administered to mice by gavage at a dose of 600 mg/kg body weight, and mice were then returned to their boxes with access to drinking water or EtOH ad libitum to complete the CI cycle. Immediately after completing the CI cycle animals were anesthetized with sodium pentobarbital (120 mg/kg, i.p., Dolethal; Vetoquinol). Cardiac blood was obtained in heparinized, light-protected Eppendorf tubes and centrifuged (10 min, 12,000 g, 4°C). The recovered plasma was diluted with an equal volume of PBS (pH 7.4), and the concentration of FITC-dextran was then analyzed using a fluorescence spectrophotometer (Synergy H1; BioTek Instruments, Winooski, VT, USA) and Gen5.1 software at the excitation wavelength of 485 nm and the emission wavelength of 528 nm. A standard curve (range 0.312–50 µg/ml) was obtained by diluting serial concentrations of FITC-dextran in PBS.
Western blot
Colon samples were homogenized in lysis buffer [150 mM NaCl, 50 mM Trizma hydrochloride (Tris-HCl), 1% NP-40, pH 7.4] containing 5% protease and 1% phosphatase inhibitor cocktails (MilliporeSigma). Protein concentrations were measured with a DC Protein Assay kit (Bio-Rad, Hercules, CA, USA). Fifteen micrograms of protein were separated by 7% or 10% SDS-PAGE and transferred to PVDF membranes. Nonspecific binding was blocked by incubation in Tris-buffered saline buffer (0.5 M Tris-HCl, 1.5 M NaCl, pH7.5) containing 0.1% Tween-20 and 5% bovine serum albumin. After incubation with appropriate antibodies [anti-ZO-1 (61-7300; Thermo Fisher Scientific), anti-occludin (711500; Thermo Fisher Scientific), anti-iNOS (PA1-036; Thermo Fisher Scientific), anti–COX-2 (ab52237; Abcam, Cambridge, United Kingdom), anti–β-actin (A5441; MilliporeSigma), anti–MMP-9 (AB19016; MilliporeSigma)] followed by goat anti-rabbit IgG–horseradish peroxidase antibodies (Santa Cruz Biotechnology, Dallas, TX, USA), proteins were visualized by chemiluminescence using the ECL Plus Western Blotting Detection Kit (GE Healthcare, Waukesha, WI, USA) and captured using the Odyssey Fc Imaging System (Li-Cor Biosciences, Lincoln, NE, USA). β-Actin was visualized by fluorescence using an IRdye 680RD goat anti-mouse (926-68070; Li-Cor Biosciences). Band densities were quantified using the ImageJ Software v.1.42q (National Institutes of Health, Bethesda, MD, USA) and normalized by referring to the β-actin signal. Each sample was assayed 3 times on different days.
MMP-9 and MMP-2 zymography
MMP-9 and MMP-2 activities were assessed in the colon samples described above. Between 5 and 10 μg of protein in nonreducing Laemmli buffer was subjected to 9% SDS-PAGE containing 0.1% gelatin (MilliporeSigma). After a wash in enzymatic activation buffer (50 mM Tris-HCl, 6 mM CaCl2, 1.5 µM ZnCl2, pH 7.4) containing 2.5% Tween-20, gels were incubated at 37°C for 12 h (MMP-2) or 24 h (MMP-9) in enzymatic activation buffer. Gels were stained in Coomasie Brilliant Blue R-250 (Bio-Rad) and destained until bands were clear. Gels were digitized, and degraded bands were densitometrically analyzed. Each sample was assayed 3 times on different days.
Sucrose preference test and elevated plus maze
Both behavioral tests were carried out on the last day of the CI protocol, 4 h after the end of consumption when EtOH concentration in plasma dropped to 0 mg/dl.
Anhedonia was analyzed by means of a sucrose preference test as previously described in Vidal et al. (40). Briefly, the test is based on a 2-bottle free-choice regimen in which the mouse is given access for 8 h to 2 pipettes, one filled with a 1% sucrose solution and the other with water. The position of the bottles in the cage was alternated in order to prevent the possible effects of side-preference on drinking behavior. No food or water deprivation was applied before the test. The preference for sucrose was calculated as a percentage of sucrose solution intake per total liquid intake. A decrease in sucrose preference is widely accepted as a measure of anhedonia.
The elevated plus maze (EPM) test was used to evaluate levels of anxiety. The test was conducted as previously described in Ledesma et al. (41). The apparatus consisted of 2 open arms (30 × 5 cm) and 2 enclosed arms (30 × 5 cm) that form a central platform (5 × 5 cm) at the point of intersection. The floor of the maze was made of black Plexiglas and the walls of the enclosed arms of clear Plexiglas. The entire apparatus was elevated 45 cm above floor level. In order to facilitate adaptation, mice were transported to the dimly illuminated test room 1 h before the test. At the beginning of each session, the animal was placed on the central platform facing an open arm and was allowed to freely explore it for 5 min. The maze was cleaned thoroughly between trials with a 10% EtOH solution and dried with a cloth. The behavior displayed by the mice was video-recorded and later analyzed by a blinded observer using a computerized method. The percentage of time in the open arm was used because this parameter is commonly considered an indicator of anxiety in mice.
Statistical analyses
Data are presented as means ± sem. Outlier values were determined by the extreme studentized deviate method (Grubbs’ test) with significance level of α = 0.05. Statistical differences between >2 groups were studied by 1-way ANOVA followed by Tukey’s post hoc test. A 2-way ANOVA test followed by Bonferroni’s post hoc comparison was used to determine the effect of 2 independent factors. For analyses regarding bacterial translocation data, the Kruskal-Wallis test was used. All data was analyzed using Prism v.5.0 (GraphPad Software, La Jolla, CA, USA). Statistical significance was considered when P < 0.05.
RESULTS
CI EtOH, but not DID, induced bacterial translocation in MLNs and increased plasma LPS concentration
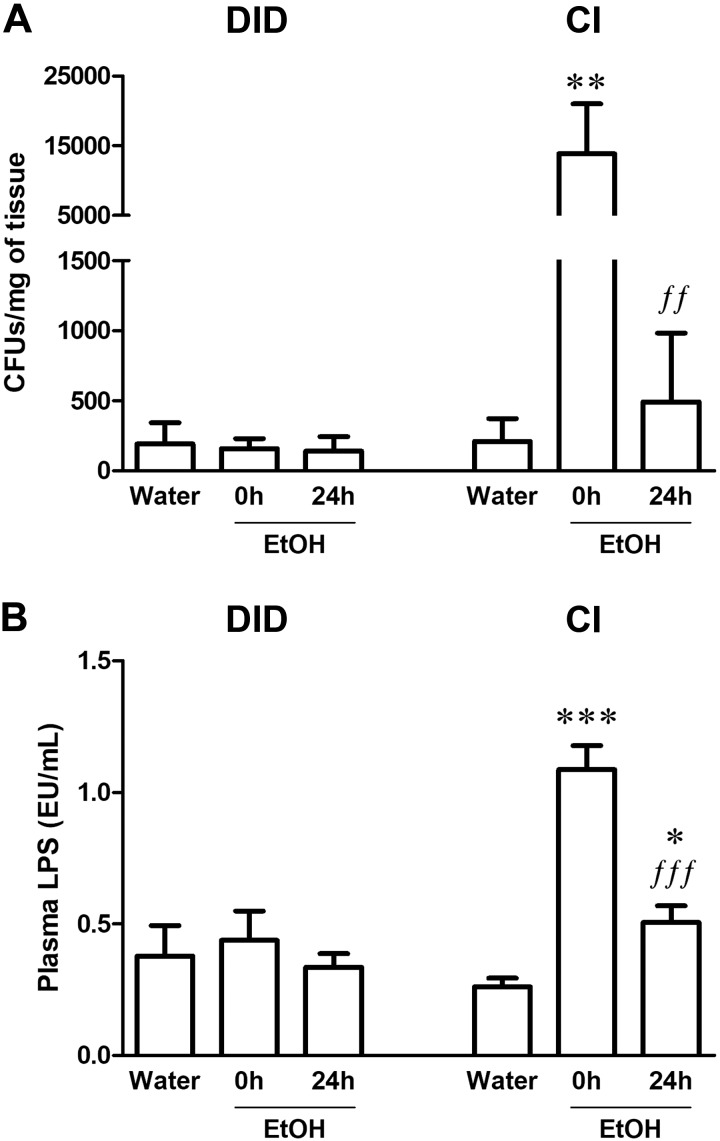
To study the effect of 2 different patterns of EtOH consumption, DID or CI EtOH intake, on gut microbiota translocation, we evaluated the presence of bacteria in MLNs (Fig. 2A) as well as the concentration of LPS in plasma (Fig. 2B).
Figure 2.
Bacterial translocation and increased plasma LPS concentrations after CI EtOH exposure and DID paradigms. A) CFUs of bacteria from MLNs after CI and DID EtOH exposure. B) Plasma LPS concentration measured in endotoxin units (EU) after CI and DID EtOH exposure. Results are shown as means ± sem; n = 6–12. *P < 0.05, **P < 0.01, ***P < 0.001 (different from water;. ffP < 0.01, fffP < 0.001 (different from EtOH 0 h).
No changes in colony-forming units (CFUs) in the MLNs were found following the DID paradigm at either time-point (0 or 24 h) after EtOH removal (Fig. 2A; Kruskal-Wallis test, K = 1.03, P = 0.60). In contrast, CI EtOH significantly increased the presence of CFUs in MLNs at 0 h by over 66 times (Fig. 2A; K = 11.16, P = 0.004), whereas at 24 h this effect returned to basal conditions. Similar to these results, 1-way ANOVA revealed changes in plasma LPS concentration after CI EtOH (Fig. 2B; F(2,24) = 53.40, P < 0.0001) but not after the DID paradigm (Fig. 2B; F(2,20) = 0.28, P = 0.76). Tukey’s posttest showed that CI EtOH increased plasma LPS levels at 0 h and concentrations were only partially restored after 24 h because LPS concentration at this time-point was still higher than the concentration detected in water-consuming mice.
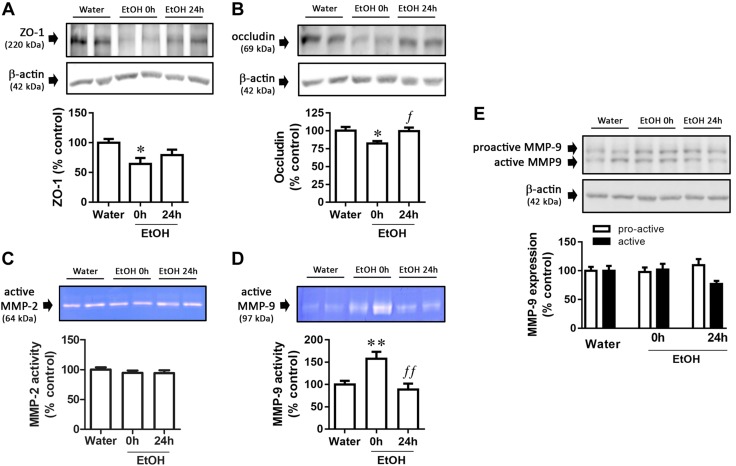
CI EtOH, but not DID, decreased ZO-1 and occludin expression and increased MMP-9 activity in colon
TJ protein expression was measured to determine if EtOH intake could alter the integrity of the gut barrier. CI EtOH decreased ZO-1 (Fig. 3A; F(2,18) = 4.17, P = 0.033) and occludin (Fig. 3B; F(2,20) = 4.44, P = 0.025) expression at 0 h, the latter being restored to basal levels at 24 h. Because this decrease in TJ protein expression could be the result of the action of MMPs, we focused our study on them. CI EtOH did not alter MMP-2 activity (Fig. 3C; F(2,23) = 0.63, P = 0.54) but increased MMP-9 activity at 0 h (Fig. 3D; F(2,20) = 8.60, P = 0.002), which returned to basal values after 24 h. To further evaluate the effect of CI EtOH on MMP-9, we analyzed the expression of both active and proactive forms by Western blot. No statistically significant changes were detected in proactive (Fig. 3E; F(2,21) = 0.54, P = 0.59) or active MMP-9 expression (Fig. 3E; F(2,20) = 2.65, P = 0.10).
Figure 3.
Gut-barrier alterations after CI EtOH exposure. A, B) Graph and representative immunoblots of CI EtOH effects on ZO-1 (A) and occludin (B) expression. C, D) Gelatinolytic bands in zymograms corresponding to the active MMP-2 (64 kDa) (C) and MMP-9 (97 kDa) (D) enzymes. E) MMP-9 expression data and representative immunoblot. Results are shown as means ± sem; n = 6–8. *P < 0.05, **P < 0.01 (different from water); fP < 0.05, ffP < 0.01 (different from EtOH 0 h).
In contrast to CI EtOH, the DID paradigm did not alter gut-barrier integrity (Supplemental Table S1), because no changes were detected either in occludin (F(2,47) = 0.34, P = 0.715) or in ZO-1 (F(2,47) = 1.28, P = 0.29) expression. MMP-2 activity was unchanged as occurred following CI EtOH (F(2,23) = 0.48, P = 0.63). DID did not modify MMP-9 activity (F(2,20) = 2.53, P = 0.11) although it decreased expression of the active form at 24 h compared with EtOH-consuming mice killed at 0 h (F(2,22) = 4.46, P = 0.03). No effect of the DID paradigm was observed on proactive MMP-9 expression (F(2,22) = 2.44, P = 0.11).
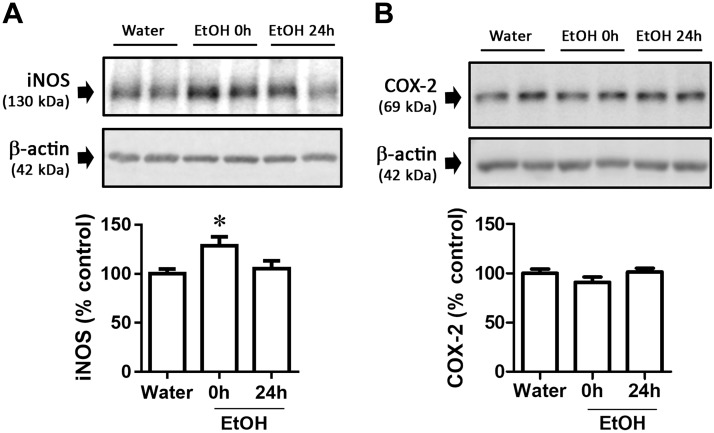
CI and DID EtOH intake effects on iNOS and COX-2 expression in colon
We also evaluated the effect of EtOH consumption on nitrosative stress and inflammation in colon using the common markers iNOS and COX-2, because these are 2 processes that are known to increase the activity of MMP-9 (24–26). CI EtOH intake increased iNOS expression at 0 h (Fig. 4A; F(2,21) = 3.95, P = 0.04), but did not modify COX-2 expression (Fig. 4B; F(2,23) = 1.62, P = 0.22). In contrast, DID did not modify iNOS (F(2,47) = 0.39, P = 0.68) or COX-2 (F(2,23) = 0.83, P = 0.45) expression (Supplemental Table S1).
Figure 4.
iNOS and COX-2 altered expression in colon from mice after CI EtOH exposure. Graph and representative immunoblots of CI EtOH effects on iNOS (A) and COX-2 (B) expression. Results are shown as means ± sem; n = 7–8. *P < 0.05 (different from water).
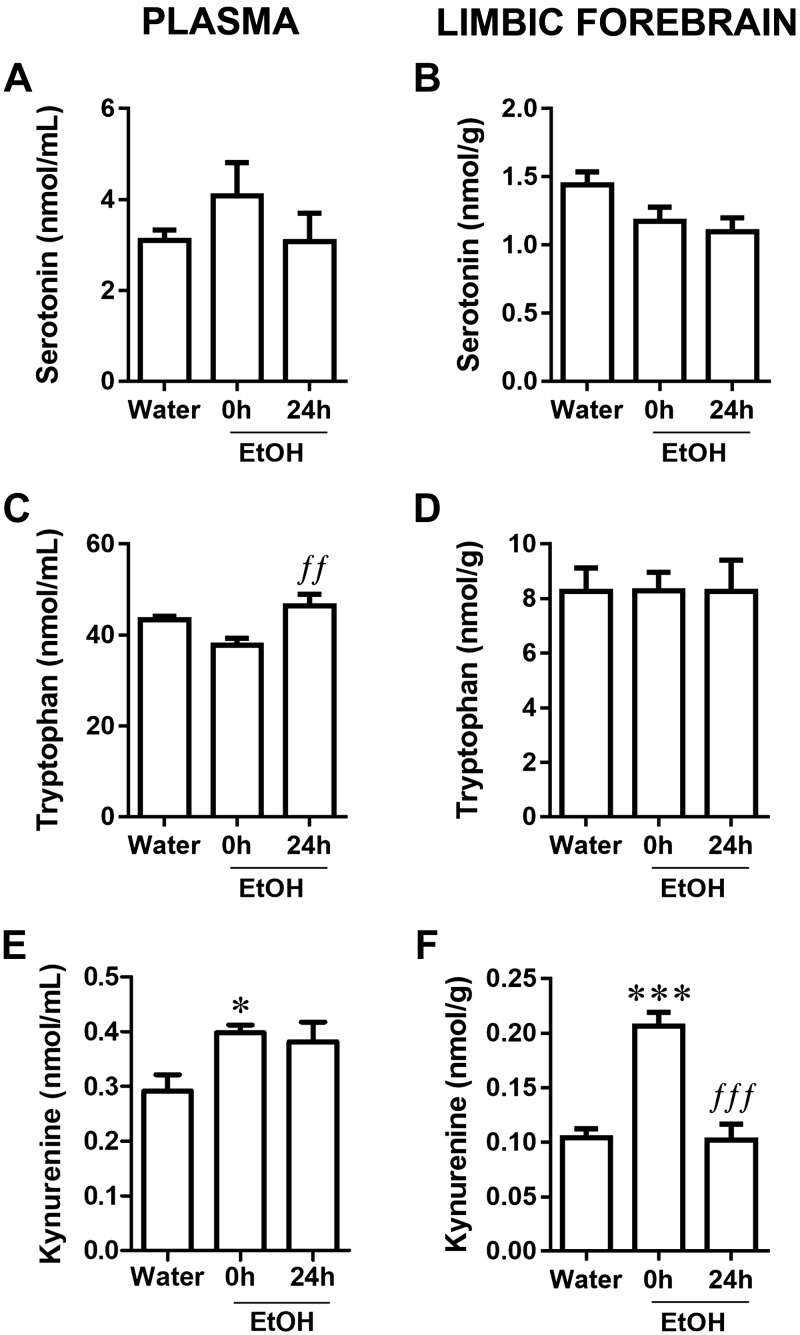
CI EtOH, but not DID, modified plasma and limbic forebrain concentrations of Trp and Kyn
To determine the effect of EtOH intake on Trp metabolism, we measured 5-HT, Trp, and Kyn in plasma and limbic forebrain because it has been demonstrated that translocation of microbial products like LPS into the circulation can cause release of proinflammatory cytokines that, along with LPS, relay to brain regions, where they induce a neuroinflammatory response (42–45). CI EtOH did not modify 5-HT concentrations either in plasma (Fig. 5A; F(2,17) = 0.91, P = 0.42) or limbic forebrain (Fig. 5B; F(2,18) = 3.10, P = 0.07). Higher Trp concentrations were found in plasma at 24 h compared with 0 h (Fig. 5C; F(2,21) = 5.53, P = 0.01), but no alteration was observed in limbic forebrain (Fig. 5D; F(2,20) = 0.0003, P = 1.000). However, CI EtOH increased plasma Kyn at 0 h but not at 24 h (Fig. 5E; F(2,22) = 4.15, P = 0.03) even though its concentration appeared to remain elevated. In limbic brain, CI EtOH increased Kyn concentration at 0 h but returned to basal concentration at 24 h (Fig. 5F; F(2,20) = 24.02, P < 0.0001).
Figure 5.
Effect of CI EtOH exposure on 5-HT concentrations in the plasma (A) and limbic forebrain (B), Trp concentrations in the plasma (C) and limbic forebrain (D), and Kyn concentrations in the plasma (E) and limbic forebrain (F). Results are shown as means ± sem; n = 6–8. *P < 0.05, ***P < 0.001 (different from water); ffP < 0.01, fffP < 0.001 (different from EtOH 0h).
The effects of binge EtOH consumption using the DID paradigm on Trp metabolism were also evaluated (Supplemental Table S1). Plasma 5-HT concentration decreased at 24 h compared with 0 h (F(2,21) = 6.04, P = 0.01; water group: 3.35 ± 0.89 μM; EtOH 0 h: 3.80 ± 0.11 μM, EtOH 24 h: 2.23 ± 0.46 μM). No changes were detected in limbic forebrain (F(2,18) = 0.57, P = 0.58). Trp levels were not modified by DID either in plasma (F(2,23) = 0.26, P = 0.78) or in limbic forebrain (F(2,18) = 0.64, P = 0.54). Plasma Kyn concentration (F(2,18) = 14.85, P = 0.02) was only increased at 24 h relative to both water and EtOH 0 h groups (EtOH 24 h: 0.50 ± 0.01 μM vs. water: 0.42 ± 0.02 μM, P < 0.05; and vs. EtOH 0 h: 0.36 ± 0.03 μM, P < 0.01). No changes in Kyn levels were detected in limbic forebrain (F(2,17) = 1.00, P = 0.39).
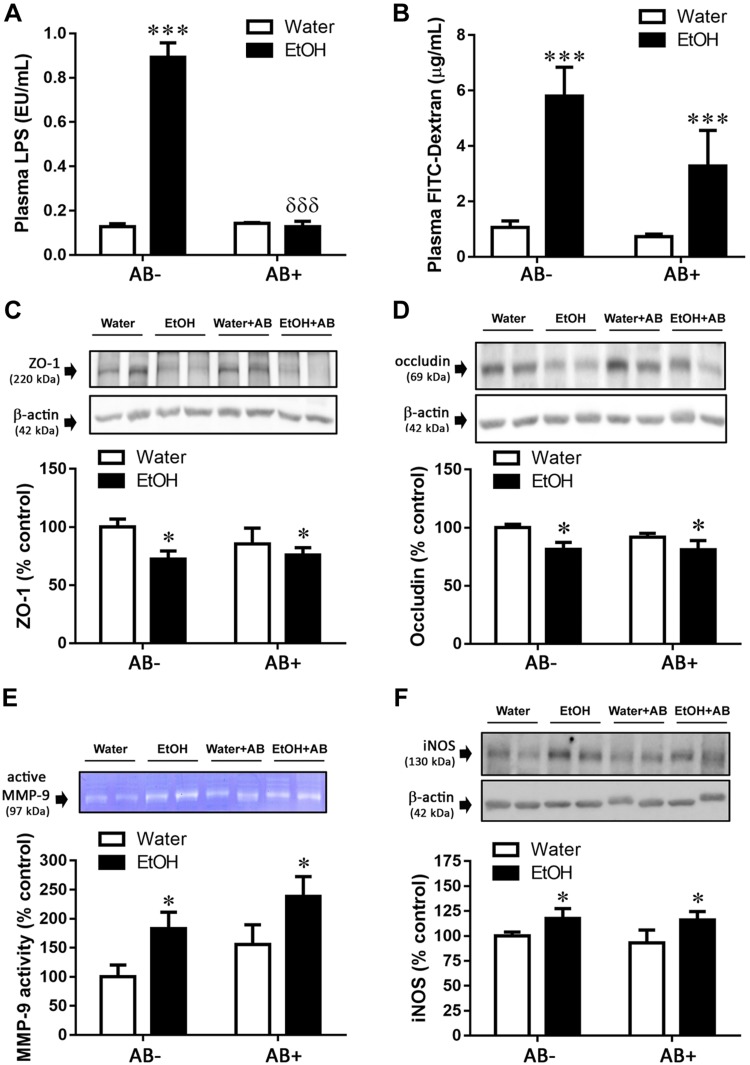
Antibiotic treatment prevented CI effects on plasma LPS concentration but not on colonic permeability, TJ protein expression, MMP-9 activity, or iNOS expression
In order to evaluate the role of gut bacteria on the changes detected in colon following CI EtOH, we administered oral antibiotic to both water and EtOH groups. All data were analyzed by 2-way ANOVA considering the factors EtOH and antibiotic treatment.
The plasmatic LPS increment produced immediately after CI EtOH was prevented by antibiotic treatment (Fig. 6A). A 2-way ANOVA revealed an effect of both factors (antibiotic: F(1,24) = 79.35, P < 0.0001; EtOH: F(1,24) = 79.41, P < 0.0001) as well as an interaction between them (EtOH × antibiotic: F(1,24) = 86.43, P < 0.0001), and the Bonferroni posttest indicated a significant effect of antibiotic in EtOH-consuming mice.
Figure 6.
Antibiotic (AB) treatment effects on CI EtOH induced parameters of colonic permeability immediately after EtOH removal. AB treatment prevented the CI EtOH–induced increase in LPS [endotoxin units (EU)] (A) but not the changes induced in permeability to FITC-dextran (B), ZO-1 (C), occludin (D), MMP-9 activity (E), and iNOS expression (F). Results are shown as means ± sem; n = 5–8. *P < 0.05, ***P < 0.001 (different from water group); δδδP < 0.001 (different from the EtOH-AB− group).
As suggested by the increase in circulating LPS after CI EtOH, intestinal permeability was confirmed by means of a functional test in which gavage-administered FITC-dextran was increased in plasma by EtOH treatment (Fig. 6B; EtOH: F(1,25) = 20.25, P < 0.0001), with no significant effect of antibiotic (antibiotic: F(1,25) = 3.12, P = 0.089) nor an interaction between factors (EtOH × antibiotic: F(1,25) = 1.84, P = 0.187). In line with this, antibiotic treatment did not modify the decrease in TJ protein expression induced by CI EtOH. Two-way ANOVA revealed only an effect of CI EtOH on both ZO-1 (Fig. 6C; antibiotic: F(1,23) = 0.46, P = 0.50; EtOH: F(1,23) = 5.28, P = 0.03; EtOH × antibiotic: F(1,23) = 1.17, P = 0.29) and occludin (Fig. 6D; antibiotic: F(1,20) = 0.48, P = 0.50; EtOH: F(1,20) = 5.55, P = 0.03; EtOH × antibiotic: F(1,20) = 0.37, P = 0.55).
With regard to MMP-9, its activity was only increased by EtOH treatment (Fig. 6E; EtOH: F(1,20) = 7.12, P = 0.02), because there was no significant effect of antibiotic administration (antibiotic: F(1,20) = 3.20, P = 0.09) nor an interaction between factors (EtOH × antibiotic: F(1,20) = 0.00007, P = 1.00).
Similarly, the effect of CI EtOH on iNOS expression was independent of antibiotic treatment (Fig. 6F; antibiotic: F(1,24) = 0.20, P = 0.66; EtOH: F(1,24) = 5.00, P = 0.04; EtOH × antibiotic: F(1,24) = 0.09, P = 0.77).
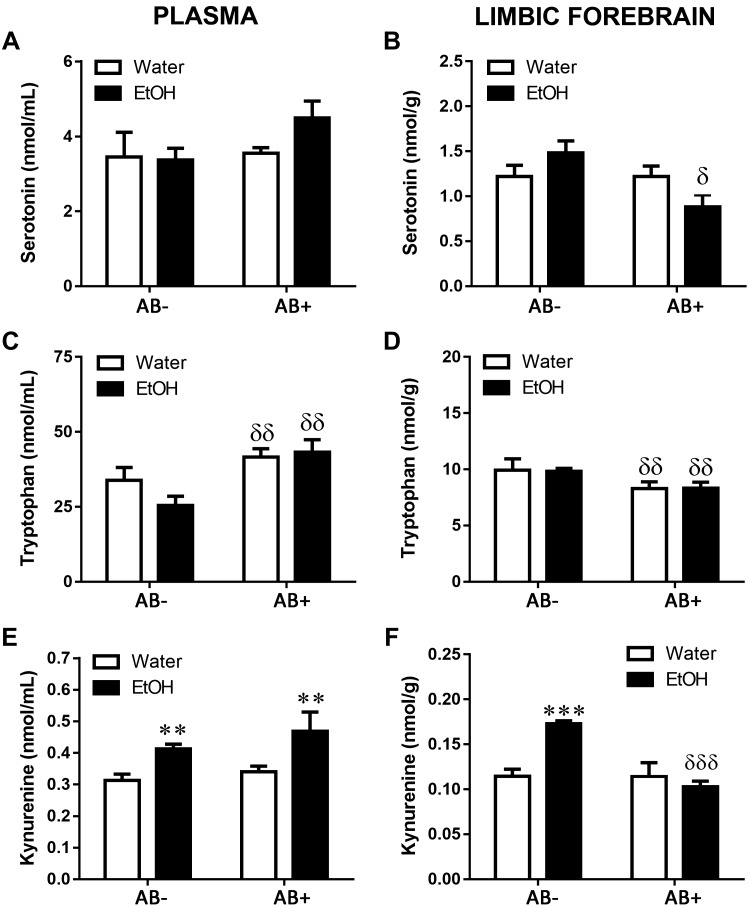
Antibiotic treatment prevented the changes induced by CI EtOH on Trp metabolism, except for the Kyn increase in plasma
With the aim of studying the involvement of gut microbiota in the dysregulation of Trp metabolism induced by CI EtOH, we determined 5-HT, Trp, and Kyn concentrations in mice with and without antibiotic treatment. No modifications were found in plasmatic 5-HT concentration (Fig. 7A; antibiotic: F(1,21) = 1.47, P = 0.24; EtOH: F(1,21) = 0.72, P = 0.40; EtOH × antibiotic: F(1,21) = 1.00, P = 0.33). In limbic forebrain, 2-way ANOVA revealed a significant effect of antibiotic treatment and interaction between the factors, but not of EtOH exposure (Fig. 7B; antibiotic: F(1,19) = 5.22, P = 0.03; EtOH: F(1,19) = 0.08, P = 0.78.; EtOH × antibiotic: F(1,19) = 5.20, P = 0.03). Changes in Trp concentration were produced only by antibiotic treatment, because no significant effect of EtOH was found nor an interaction between the factors, for both plasma (Fig. 7C; antibiotic: F(1,22) = 11.22, P = 0.003; EtOH: F(1,22) = 0.77, P = 0.39; EtOH × antibiotic: F(1,22) = 1.72, P = 0.20) and limbic forebrain (Fig. 7D; antibiotic: F(1,21) = 6.20, P = 0.02; EtOH: F(1,21) = 0.001, P = 0.94; EtOH × antibiotic: F(1,21) = 0.01, P = 0.93).
Figure 7.
Effects of antibiotic treatment (AB) on changes induced immediately after CI EtOH exposure on 5-HT concentration in the plasma (A) and limbic brain (B), Trp concentration in the plasma (C) and limbic brain (D), and Kyn concentration in the plasma (E) and limbic brain (F). Results are shown as means ± sem; n = 5–8. **P < 0.01, ***P < 0.001 (different from the corresponding water group); δP < 0.05, δδP < 0.01, δδδP < 0.001 (different from the corresponding group not treated with antibiotic).
Regarding Kyn, no significant effect of antibiotic treatment was observed in plasma concentration (Fig. 7E; antibiotic: F(1,22) = 1.22, P = 0.28; EtOH: F(1,22) = 9.41, P = 0.006; EtOH × antibiotic: F(1,22) = 0.14, P = 0.71), but in contrast, limbic forebrain Kyn changes were regulated by both EtOH and antibiotic treatment, which interacted with each other (Fig. 7F; antibiotic: F(1,23) = 18.49, P = 0.0003; EtOH: F(1,23) = 8.03, P = 0.009; EtOH × antibiotic: F(1,23) = 18.04, P = 0.0003) such that antibiotic treatment reduced the Kyn increase caused by EtOH.
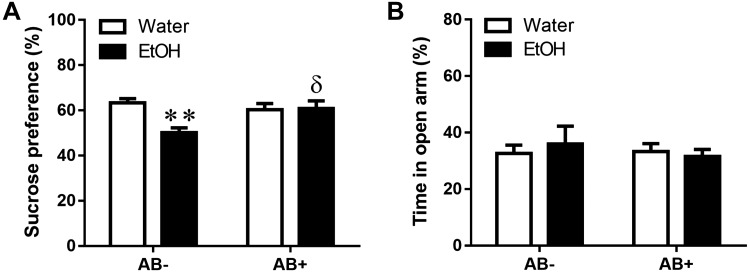
Sucrose preference decrease by CI EtOH was prevented by antibiotic treatment, but no changes were detected in the EPM test
To determine the role of the intestinal microbiota in the behavioral consequences of CI EtOH, the effect of antibiotic treatment was studied using 2 different tests, the sucrose preference test, which provides a measure of anhedonia as a symptom of depression, and the EPM, which provides a measure of anxiety-like behavior, both of which are reported to be associated with alcohol consumption. Two-way ANOVA analysis of sucrose preference (Fig. 8A) reveals a significant effect of CI EtOH (EtOH: F(1,27) = 6.3, P = 0.0183), as well as an interaction (EtOH × antibiotic: F(1,27) = 7.41, P = 0.011), but no effect of antibiotic treatment (F(1,27) = 2.34, P = 0.13), indicating that CI EtOH–induced anhedonia can be prevented by antibiotic administration. On the other hand, no differences were found in the time spent in the open arm between any of the groups studied, suggesting that neither CI EtOH nor antibiotic treatment produces anxiety (Fig. 8B; EtOH: F(1,27) = 0.04, P = 0.83; antibiotic: F(1,27) = 0.22, P = 0.63; EtOH × antibiotic: F(1,27) = 0.39, P = 0.53).
Figure 8.
Effect of CI EtOH exposure and antibiotic (AB) administration on anhedonia, in the sucrose preference test and on anxiety-related behaviors in the EPM. A) Preference for sucrose was calculated as a percentage of sucrose solution intake per total liquid intake. B) Graph showing the percentage of time spent in the open arm of overall test. Results are shown as means ± sem, n = 7–8. **P < 0.01 (different from the corresponding water group); δP < 0.05 (different from the corresponding group not treated with antibiotic).
Antibiotic treatment did not modify EtOH consumption, preference, or plasma EtOH concentration produced by CI EtOH
In order to demonstrate that the effects of antibiotic treatment cannot be attributed to changes in EtOH consumption, we compared both water and EtOH intake between groups during the period in which antibiotic was administered. Antibiotic treatment did not modify EtOH consumption (Supplemental Fig. S1A; antibiotic: F(1,93) = 0.08, P = 0.79; days of treatment: F(6,93) = 0.94, P = 0.47; EtOH × antibiotic: F(6,93) = 1.44, P = 0.21) or water intake (Supplemental Fig. S1B; antibiotic: F(1,91) = 0.15, P = 0.70; days of treatment: F(6,91) = 1.74, P = 0.12; EtOH × antibiotic: F(6,91) = 0.47, P = 0.93) at any of evaluated time-points. No changes in plasma EtOH concentration as a consequence of antibiotic treatment were found (Supplemental Fig. S1C; t(14) = 0.43, P = 0.68). Antibiotic treatment did not modify the preference for EtOH (Supplemental Fig. S1D).
DISCUSSION
In this study, we provide evidence that different alcohol consumption patterns can produce different effects because CI EtOH intake but not DID allows gut microbial translocation and an increase in LPS presence in blood, as a result of gut permeability as indicated by FITC-dextran extravasation into blood, and is associated with a decrease in TJ protein expression. Concurrently, we found an increase in colonic MMP-9 activity and iNOS expression. None of these factors (i.e., gut permeability, TJ protein decrease, MMP-9 induced activity, and iNOS expression, was affected by the administration of antibiotic. In addition, we describe for the first time an increase in the levels of Kyn following CI EtOH consumption in both periphery and brain and suggest that the gut microbiota is only involved in the increase that occurs in brain). Finally, we found that CI EtOH intake altered animal behavior, leading to a reduced preference for sucrose, a measure of anhedonia, which could be prevented with antibiotic treatment.
Bacterial translocation includes toxins, antigens, or other microbial products from the gut lumen into the circulation leading to systemic inflammation and various diseases (46). The current study shows that CI EtOH, but not DID, allows bacterial translocation immediately after EtOH removal as reflected by colony counting in MLNs and plasmatic LPS, which could be indicative of altered intestinal permeability. Our results agree with previous studies showing that chronic EtOH increased gut permeability to macromolecules, LPS, or both in animals (47–51) and subjects with alcohol use disorder (52–56). The fact that the DID model did not produce translocation might be due to the lower intensity of the model either in terms of daily or overall consumption of EtOH or in terms of duration of exposure, although the latter appears less probable because forced EtOH exposure by gavage administration produce a rapid and transient LPS increase in rats (57), an effect that is also observed after 1 binge drinking episode in humans (58).
FITC-dextran is considered as a marker of altered intestinal permeability that, in general, corresponds to impaired intestinal integrity, as has been demonstrated in rodent models of colitis (59) or inflammatory bowel disease (60). CI EtOH increases gut-barrier permeability because we detected FITC-dextran in plasma after oral administration. An increase in intestinal permeability to FITC-dextran by EtOH has been observed by others in ex vivo (61), in vitro (22), and in vivo (44) studies. Additionally, alcohol-dependent patients have been shown to have greater gut permeability than control subjects to tracers such as polyethylene glycol (55) or 51Cr-EDTA (62), and in the latter case the permeability is decreased during withdrawal.
Central to intestinal integrity are the TJ complexes that include the proteins ZO-1 and occludin (46, 63–66). Here we demonstrate that concurrent with bacterial and FITC-dextran translocation, CI EtOH induces a transient decrease in the expression of occludin and ZO-1 indicating that gut-barrier integrity is disrupted. These effects were not evident in mice exposed to DID along with the absence of bacteria in MLNs or LPS in plasma. Our data agree with studies showing reduced TJ protein expression after alcohol exposure in animal models and in colon biopsies from alcohol-dependent subjects (67, 68). Several mechanisms may contribute to reductions in TJ protein expression and the promotion of leakiness following alcohol such as redistribution of occludin and ZO-1 from the epithelial junctions (69), EtOH induction of microRNA 212 affecting ZO-1 translation (68), defective microtubule assembly caused by nitration and oxidation of tubulin (70), or changes in kinases that regulate epithelial contractility (71). Also, increased MMP-9 activity has been found in colonic tissue of a distal colitis model (72). In addition, its exogenous administration produces an increase in dextran flux in mice (27), and, by contrast, its targeted deletion attenuates experimental colitis (73). Interestingly, we found enhanced MMP-9 proteolytic activity in colon of mice following CI EtOH. Thus, it appears reasonable to propose that the increase in MMP-9 activity that we observe in the CI model might be involved in the reduction of TJ protein expression and contribute to the changes in gut-barrier permeability. Moreover, in the DID model we observed no change in MMP-9 activation consistent with unaltered gut TJ protein expression and absence of bacterial or LPS translocation. Interestingly, we recently described increased brain MMP-9 activity in human postmortem tissue of alcoholics along with disrupted blood-brain barrier (23).
MMP-9 expression and activity are tightly controlled by several factors including proinflammatory cytokines (74), reactive oxygen species (75), and NO (24, 25). Our study identifies an increase in colonic iNOS expression following CI EtOH that could reflect a nitrosative stress pathway activation in the intestine similar to that which occurs in inflammatory intestinal diseases (76, 77) and that could be the responsible for MMP-9 activation in our study. In fact, it has been demonstrated that alcohol-induced gut leakiness is iNOS dependent, and a relationship between nitrosative stress, intestinal hyperpermeability, and LPS presence in blood has been established (21, 50, 70, 78). That pro- and active MMP-9 protein expression were not modified suggests that the nitrosative pathway exerts a regulation on MMP-9 activity rather than at the transcriptional level. Although an inflammatory response has been described in alcohol dependence (79, 80), we have not observed modifications in colonic COX-2 expression after CI EtOH in spite of its known involvement in intestinal inflammation and changes in permeability (81, 82).
Recent studies have established the importance of microbiota in physiologic homeostasis (83) and in the regulation of metabolism of the amino acid Trp, which has a central role in physiology and pathology. Thus, altered Trp metabolism, driving it toward the Kyn pathway, may be of relevance in brain because it has been proposed to participate in neuroinflammation (84) and neuropsychiatric pathologies such as major depressive disorder and anxiety (85, 86).
Previous studies have shown altered peripheral Trp metabolism at various stages of alcohol intake, withdrawal, and sobriety in animal models and subjects with alcohol use disorder (87–91). Our study supports the EtOH-induced regulation of Trp metabolism, showing an increase in peripheral Kyn after CI EtOH. Interestingly, we also found increased limbic forebrain Kyn concentrations. To our knowledge, this study provides the first evidence of altered brain Kyn concentrations following chronic EtOH intake. Furthermore, we previously described no changes in plasma or brain Kyn concentrations immediately following DID (13), and the present data confirm and extend this observation to 24 h.
It has been demonstrated that translocation of microbial products like LPS into the circulation can cause release of proinflammatory cytokines that, along with LPS, relay to brain regions, where they induce a neuroinflammatory response (42–45). Chronic alcohol is a potent modulator of the innate immune system, leading to a proinflammatory response in the periphery as well as in the CNS (92), and both TLR4 activation and a proinflammatory cytokine profile have been found in animal models of EtOH consumption and in postmortem human alcoholic brain (93). In addition, TLR4 deficiency protects against neuroinflammation induced by chronic EtOH treatment (94). In order to determine the involvement of the plasma LPS found in our study in the changes after CI intake, we performed intestinal decontamination using an effective antibiotic combination (95), which we showed had no effects on EtOH or water intake, EtOH preference, or plasmatic EtOH concentration. Antibiotic treatment did not modify FITC-dextran extravasation into blood or the changes in TJ protein, EtOH-induced MMP-9 activation, or iNOS expression in colon. To our knowledge, this is the first study showing that MMP-9 may be involved in the gut leakiness induced by EtOH and, moreover, suggesting that these effects might be independent of microbiota because proteolytic activity induced by gut bacteria does not appear to contribute to the disruption of the intestinal barrier as has been suggested for other gastrointestinal disorders (96).
Focusing on Kyn levels, antibiotic treatment abolished only the increment found in brain after the CI paradigm, indicating that the mechanism through which EtOH increases Kyn concentration in brain involves the microbiota. Kyn synthesis is mediated by both TDO and IDO with different relative contributions of the enzymes depending on the tissue and presence of stimulating factors. Both enzymes show low basal activity in the brain, but IDO is under proinflammatory regulation (43, 97, 98) and can be substantially increased following stimulation and thus play a relevant role in brain (99). Based on previous suggestions that chronic excessive alcohol consumption leads to activation of the immune system (92) involving TLR4 (100), it is tempting to suggest that the LPS translocated from the gut following CI EtOH could produce a neuroinflammatory response, leading to the induction of IDO activity, which results in the observed increase in brain Kyn levels. Supporting this, preclinical studies showed that peripheral LPS injection produces an increase in central proinflammatory cytokines and IDO expression (101–103) as well as in Kyn concentration in brain tissue (104).
On the other hand, several studies in mice have shown that bacterial inoculation (101–103) or LPS administration (101, 104) produce depressive and anxiety-like behaviors specifically linked to brain IDO activation. Likewise, IDO antagonism by 1-methyltryptophan prevents that symptomatology (45). Dysregulation of the Kyn pathway has been described in depression (45, 105) and anxiety (44), both of which are associated with chronic EtOH consumption. Furthermore, gut bacteria have been implicated in alcohol withdrawal–induced anxiety (106), and intestinal permeability is positively associated with the persistence of depression, anxiety, and craving in alcohol-dependent subjects after detoxification (58). Similarly, antibiotic treatment reverses both the plasma LPS increase and the depressive-like phenotype caused by chronic mild stress, a well-established murine model of depression (36). In line with such evidence, we observed that the CI EtOH paradigm produced anhedonia that correlates with depressive-like symptoms in rodent models (107, 108) and in which the microbiota appears to play a role. This anhedonia may be related to the elevation in brain Kyn because, just as antibiotic treatment prevented the CI EtOH–induced elevation of brain Kyn, it also returned the sucrose preference to control values. In order to study another behavioral symptom associated to EtOH consumption, we assessed the effect of CI EtOH on anxiety-like behavior. Because we did not observe any effect after EtOH consumption when measured 4 h after EtOH exposure, our model does not allow us make any positive association between anxiety-like behaviors and the gut microbiota or brain Kyn concentrations.
In contrast to the changes in brain Kyn, the increase in plasma Kyn produced by the CI paradigm was not modified by bacterial decontamination during the last 15 d of CI, suggesting that EtOH induces the peripheral effect through a mechanism in which the microbiota might not be involved, although we cannot completely rule out its participation. Differences in Kyn concentration regulation between brain and periphery could also be the result of tissue-dependent alterations in the activity of Kyn-degrading enzymes by EtOH and antibiotic treatment because the balance of downstream Kyn metabolism and thus the Kyn concentration is a tightly regulated brain region–dependent process (102).
In summary, voluntary CI EtOH modulates Kyn levels, causing an increment both in brain and periphery. These effects are differently regulated because in brain, but not periphery, the microbiota seems to play an important role. Further research is required to evaluate the contribution of IDO, TDO, and the Kyn-degrading enzymes to the observed changes in Trp metabolism.
Plasmatic Trp changes occurred independently of EtOH but were modified following antibiotic treatment as expected considering that microbiota is a main factor contributing to Trp availability. In fact, studies in microbiota-deficient animals showed increased plasma Trp concentration that can be normalized by gut colonization (109). In contrast, we found a slight decrease in Trp concentration in brain caused by the microbiota decontamination both in control and EtOH-consuming mice. Changes in peripheral Trp without modifications in Kyn after an antibiotic treatment have previously been reported in the literature (see Hoban et al. 110). In this latter case, the reduced Trp levels along with the absence of a possible inflammatory activation of IDO might be contributing to prevent the Kyn increase induced by the chronic EtOH consumption.
Finally, no modification in brain or plasma 5-HT concentration was found after voluntary CI. In brain, bacterial decontamination produces a slight decrease of 5-HT in EtOH-consuming mice that could be related to the reduced availability of brain Trp because depletion of the amino acid impairs 5-HT brain synthesis (111, 112).
CONCLUSIONS
Our results suggest that chronic EtOH intake induces nitrosative stress in the colon resulting in the activation of MMP-9, which may alter gut permeability by decreasing TJ expression, allowing bacterial traslocation, and increasing LPS in plasma. Furthermore, CI EtOH provokes an increase in brain Kyn concentration associated with the development of anhedonia, both of which appear to be dependent on the presence of the gut microbiota. This may be of particular importance because emergent data establish a relationship between brain Kyn dysregulation mediated by gut microbiota (5), on neuropsychiatric pathologies (113), both of which are altered by chronic EtOH consumption. The present study represents an interesting contribution to the establishment of a role of the microbiota-gut-brain axis in the consequences of EtOH abuse. These effects may depend on several differential factors of the models, such as the pattern of EtOH consumption and the duration of the exposure, because they were only apparent in mice subjected to CI EtOH model of voluntary EtOH exposure and not in those subjected to the DID model that resembles binge consumption.
ACKNOWLEDGMENTS
The authors thank Centro de Asistencia a Ia Investigación (CAI) Animalario, Universidad Complutense de Madrid (UCM) for the care and maintenance of the mice used in the study. This work was supported by Ministerio de Economia y Competitividad Grant SAF2016-78864-R, the Ministerio de Sanidad, Servicios Sociales e Igualdad [Plan Nacional Sobre Drogas (PNSD) 2014I015 and 2017I017)], Instituto de Salud Carlos III Redes Temáticas de Investigación Cooperativa en Salud-Red de Trastornos Adictivos (ISCII RETICS-RTA) Grants RD12/0028/0002 and RD16/0017/0021, and UCM Grant 910258. P.G.-G. is the recipient of a predoctoral fellowship (FPU) from the Ministerio de Educación, Cultura y Deporte. M.P.-H. is the recipient of a postdoctoral research contract (PNSD 2017I017); D.M.-H. is a postdoctoral fellow; J.R.C. is a Ramón y Cajal Fellow (Ministerio de Ciencia, Innovación y Universidades). The authors declare no conflicts of interest.
Glossary
- 5-HT
serotonin
- CFU
colony-forming unit
- CI
chronic intermittent
- COX-2
cyclo-oxygenase 2
- DID
drinking in the dark
- EPM
elevated plus maze
- EtOH
ethanol
- IDO
indolamine 2,3-dioxygenase
- Kyn
kynurenine
- MLN
mesenteric lymph node
- MMP
matrix metalloproteinase
- TDO
Trp 2,3-dioxygenase
- TJ
tight junction
- Tris-HCl
Trizma hydrochloride
- Trp
tryptophan
- ZO-1
zonula occludens 1
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
P. Giménez-Gómez, M. Pérez-Hernández, J. R. Caso, E. O’Shea, M. D. Gutiérrez-Lopez, and M. I. Colado designed research; P. Giménez-Gómez, M. Pérez-Hernández, D. Martín-Hernández, J. R. Caso, and L. A. Cervera performed research; P. Giménez-Gómez, M. Pérez-Hernández, E. O’Shea, L. A. Cervera, M. L. G.-L. Centelles, M. D. Gutiérrez-Lopez, and M. I. Colado analyzed data; and P. Giménez-Gómez, M. Pérez-Hernández, E. O’Shea, M. D. Gutiérrez-Lopez, and M. I. Colado wrote the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Luczynski P., McVey Neufeld K. A., Oriach C. S., Clarke G., Dinan T. G., Cryan J. F. (2016) Growing up in a bubble: using germ-free animals to assess the influence of the gut microbiota on brain and behavior. Int. J. Neuropsychopharmacol. 19, pyw020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rieder R., Wisniewski P. J., Alderman B. L., Campbell S. C. (2017) Microbes and mental health: a review. Brain Behav. Immun. 66, 9–17 [DOI] [PubMed] [Google Scholar]
- 3.Boden J. M., Fergusson D. M. (2011) Alcohol and depression. Addiction 106, 906–914 [DOI] [PubMed] [Google Scholar]
- 4.Morris E. P., Stewart S. H., Ham L. S. (2005) The relationship between social anxiety disorder and alcohol use disorders: a critical review. Clin. Psychol. Rev. 25, 734–760 [DOI] [PubMed] [Google Scholar]
- 5.Kennedy P. J., Cryan J. F., Dinan T. G., Clarke G. (2017) Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112 (Pt B), 399–412 [DOI] [PubMed] [Google Scholar]
- 6.Schwarcz R. (1993) Metabolism and function of brain kynurenines. Biochem. Soc. Trans. 21, 77–82 [DOI] [PubMed] [Google Scholar]
- 7.Gál E. M., Sherman A. D. (1978) Synthesis and metabolism of L-kynurenine in rat brain. J. Neurochem. 30, 607–613 [DOI] [PubMed] [Google Scholar]
- 8.Schwarcz R., Bruno J. P., Muchowski P. J., Wu H.-Q. (2012) Kynurenines in the mammalian brain: when physiology meets pathology. Nat. Rev. Neurosci. 13, 465–477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vécsei L., Szalárdy L., Fülöp F., Toldi J. (2013) Kynurenines in the CNS: recent advances and new questions. Nat. Rev. Drug Discov. 12, 64–82 [DOI] [PubMed] [Google Scholar]
- 10.Larroya-García A., Navas-Carrillo D., Orenes-Piñero E. (2018) Impact of gut microbiota on neurological diseases: diet composition and novel treatments. Crit. Rev. Food Sci. Nutr. 5, 1–15 [DOI] [PubMed] [Google Scholar]
- 11.Justinova Z., Mascia P., Wu H.-Q., Secci M. E., Redhi G. H., Panlilio L. V., Scherma M., Barnes C., Parashos A., Zara T., Fratta W., Solinas M., Pistis M., Bergman J., Kangas B. D., Ferré S., Tanda G., Schwarcz R., Goldberg S. R. (2013) Reducing cannabinoid abuse and preventing relapse by enhancing endogenous brain levels of kynurenic acid. Nat. Neurosci. 16, 1652–1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vengeliene V., Cannella N., Takahashi T., Spanagel R. (2016) Metabolic shift of the kynurenine pathway impairs alcohol and cocaine seeking and relapse. Psychopharmacology (Berl.) 233, 3449–3459 [DOI] [PubMed] [Google Scholar]
- 13.Giménez-Gómez P., Pérez-Hernández M., Gutiérrez-López M. D., Vidal R., Abuin-Martínez C., O’Shea E., Colado M. I. (2018) Increasing kynurenine brain levels reduces ethanol consumption in mice by inhibiting dopamine release in nucleus accumbens. Neuropharmacology 135, 581–591 [DOI] [PubMed] [Google Scholar]
- 14.WHO (2016) Global Status Report on Alcohol and Health 2014. WHO, Geneva, Switzerland [Google Scholar]
- 15.Lim S. S., Vos T., Flaxman A. D., Danaei G., Shibuya K., Adair-Rohani H., Amann M., Anderson H. R., Andrews K. G., Aryee M., Atkinson C., Bacchus L. J., Bahalim A. N., Balakrishnan K., Balmes J., Barker-Collo S., Baxter A., Bell M. L., Blore J. D., Blyth F., Bonner C., Borges G., Bourne R., Boussinesq M., Brauer M., Brooks P., Bruce N. G., Brunekreef B., Bryan-Hancock C., Bucello C., Buchbinder R., Bull F., Burnett R. T., Byers T. E., Calabria B., Carapetis J., Carnahan E., Chafe Z., Charlson F., Chen H., Chen J. S., Cheng A. T.-A., Child J. C., Cohen A., Colson K. E., Cowie B. C., Darby S., Darling S., Davis A., Degenhardt L., Dentener F., Des Jarlais D. C., Devries K., Dherani M., Ding E. L., Dorsey E. R., Driscoll T., Edmond K., Ali S. E., Engell R. E., Erwin P. J., Fahimi S., Falder G., Farzadfar F., Ferrari A., Finucane M. M., Flaxman S., Fowkes F. G. R., Freedman G., Freeman M. K., Gakidou E., Ghosh S., Giovannucci E., Gmel G., Graham K., Grainger R., Grant B., Gunnell D., Gutierrez H. R., Hall W., Hoek H. W., Hogan A., Hosgood H. D., III, Hoy D., Hu H., Hubbell B. J., Hutchings S. J., Ibeanusi S. E., Jacklyn G. L., Jasrasaria R., Jonas J. B., Kan H., Kanis J. A., Kassebaum N., Kawakami N., Khang Y.-H., Khatibzadeh S., Khoo J.-P., Kok C., Laden F., Lalloo R., Lan Q., Lathlean T., Leasher J. L., Leigh J., Li Y., Lin J. K., Lipshultz S. E., London S., Lozano R., Lu Y., Mak J., Malekzadeh R., Mallinger L., Marcenes W., March L., Marks R., Martin R., McGale P., McGrath J., Mehta S., Mensah G. A., Merriman T. R., Micha R., Michaud C., Mishra V., Mohd Hanafiah K., Mokdad A. A., Morawska L., Mozaffarian D., Murphy T., Naghavi M., Neal B., Nelson P. K., Nolla J. M., Norman R., Olives C., Omer S. B., Orchard J., Osborne R., Ostro B., Page A., Pandey K. D., Parry C. D. H., Passmore E., Patra J., Pearce N., Pelizzari P. M., Petzold M., Phillips M. R., Pope D., Pope C. A., III, Powles J., Rao M., Razavi H., Rehfuess E. A., Rehm J. T., Ritz B., Rivara F. P., Roberts T., Robinson C., Rodriguez-Portales J. A., Romieu I., Room R., Rosenfeld L. C., Roy A., Rushton L., Salomon J. A., Sampson U., Sanchez-Riera L., Sanman E., Sapkota A., Seedat S., Shi P., Shield K., Shivakoti R., Singh G. M., Sleet D. A., Smith E., Smith K. R., Stapelberg N. J. C., Steenland K., Stöckl H., Stovner L. J., Straif K., Straney L., Thurston G. D., Tran J. H., Van Dingenen R., van Donkelaar A., Veerman J. L., Vijayakumar L., Weintraub R., Weissman M. M., White R. A., Whiteford H., Wiersma S. T., Wilkinson J. D., Williams H. C., Williams W., Wilson N., Woolf A. D., Yip P., Zielinski J. M., Lopez A. D., Murray C. J. L., Ezzati M., AlMazroa M. A., Memish Z. A. (2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380, 2224–2260; erratum: 381, 1276; 628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghosh Dastidar S., Warner J. B., Warner D. R., McClain C. J., Kirpich I. A. (2018) Rodent models of alcoholic liver disease: role of binge ethanol administration. Biomolecules 8, E3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rossi M., Jahanzaib Anwar M., Usman A., Keshavarzian A., Bishehsari F. (2018) Colorectal cancer and alcohol consumption-populations to molecules. Cancers (Basel) 10, 172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Obad A., Peeran A., Little J. I., Haddad G. E., Tarzami S. T. (2018) Alcohol-mediated organ damages: heart and brain. Front. Pharmacol. 9, 81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Osna N. A., Donohue T. M., Jr., Kharbanda K. K. (2017) Alcoholic liver disease: pathogenesis and current management. Alcohol Res. 38, 147–161 [PMC free article] [PubMed] [Google Scholar]
- 20.Peterson V. L., Jury N. J., Cabrera-Rubio R., Draper L. A., Crispie F., Cotter P. D., Dinan T. G., Holmes A., Cryan J. F. (2017) Drunk bugs: chronic vapour alcohol exposure induces marked changes in the gut microbiome in mice. Behav. Brain Res. 323, 172–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forsyth C. B., Tang Y., Shaikh M., Zhang L., Keshavarzian A. (2011) Role of snail activation in alcohol-induced iNOS-mediated disruption of intestinal epithelial cell permeability. Alcohol. Clin. Exp. Res. 35, 1635–1643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosell A., Ortega-Aznar A., Alvarez-Sabín J., Fernández-Cadenas I., Ribó M., Molina C. A., Lo E. H., Montaner J. (2006) Increased brain expression of matrix metalloproteinase-9 after ischemic and hemorrhagic human stroke. Stroke 37, 1399–1406 [DOI] [PubMed] [Google Scholar]
- 23.Rubio-Araiz A., Porcu F., Pérez-Hernández M., García-Gutiérrez M. S., Aracil-Fernández M. A., Gutierrez-López M. D., Guerri C., Manzanares J., O’Shea E., Colado M. I. (2017) Disruption of blood-brain barrier integrity in postmortem alcoholic brain: preclinical evidence of TLR4 involvement from a binge-like drinking model. Addict. Biol. 22, 1103–1116 [DOI] [PubMed] [Google Scholar]
- 24.O’Sullivan S., Gilmer J. F., Medina C. (2015) Matrix metalloproteinases in inflammatory bowel disease: an update. Mediators Inflamm. 2015, 964131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ridnour L. A., Windhausen A. N., Isenberg J. S., Yeung N., Thomas D. D., Vitek M. P., Roberts D. D., Wink D. A. (2007) Nitric oxide regulates matrix metalloproteinase-9 activity by guanylyl-cyclase-dependent and -independent pathways. Proc. Natl. Acad. Sci. USA 104, 16898–16903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khan K. M. F., Howe L. R., Falcone D. J. (2004) Extracellular matrix-induced cyclooxygenase-2 regulates macrophage proteinase expression. J. Biol. Chem. 279, 22039–22046 [DOI] [PubMed] [Google Scholar]
- 27.Nighot P., Al-Sadi R., Rawat M., Guo S., Watterson D. M., Ma T. (2015) Matrix metalloproteinase 9-induced increase in intestinal epithelial tight junction permeability contributes to the severity of experimental DSS colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 309, G988–G997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rhodes J. S., Best K., Belknap J. K., Finn D. A., Crabbe J. C. (2005) Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol. Behav. 84, 53–63 [DOI] [PubMed] [Google Scholar]
- 29.Cox B. R., Olney J. J., Lowery-Gionta E. G., Sprow G. M., Rinker J. A., Navarro M., Kash T. L., Thiele T. E. (2013) Repeated cycles of binge-like ethanol (EtOH)-drinking in male C57BL/6J mice augments subsequent voluntary EtOH intake but not other dependence-like phenotypes. Alcohol. Clin. Exp. Res. 37, 1688–1695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wilcox M. V., Cuzon Carlson V. C., Sherazee N., Sprow G. M., Bock R., Thiele T. E., Lovinger D. M., Alvarez V. A. (2014) Repeated binge-like ethanol drinking alters ethanol drinking patterns and depresses striatal GABAergic transmission. Neuropsychopharmacology 39, 579–594; erratum: 2039–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rico-Barrio I., Peñasco S., Puente N., Ramos A., Fontaine C. J., Reguero L., Giordano M. E., Buceta I., Terradillos I., Lekunberri L., Mendizabal-Zubiaga J., Rodríguez de Fonseca F., Gerrikagoitia I., Elezgarai I., Grandes P. (2018) Cognitive and neurobehavioral benefits of an enriched environment on young adult mice after chronic ethanol consumption during adolescence. [E-pub ahead of print] Addict. Biol. [DOI] [PubMed] [Google Scholar]
- 32.Osterndorff-Kahanek E., Ponomarev I., Blednov Y. A., Harris R. A. (2013) Gene expression in brain and liver produced by three different regimens of alcohol consumption in mice: comparison with immune activation. PLoS One 8, e59870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becker H. C., Lopez M. F. (2004) Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol. Clin. Exp. Res. 28, 1829–1838 [DOI] [PubMed] [Google Scholar]
- 34.Melendez R. I. (2011) Intermittent (every-other-day) drinking induces rapid escalation of ethanol intake and preference in adolescent and adult C57BL/6J mice. Alcohol. Clin. Exp. Res. 35, 652–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ando T., Brown R. F., Berg R. D., Dunn A. J. (2000) Bacterial translocation can increase plasma corticosterone and brain catecholamine and indoleamine metabolism. Am. J. Physiol. Regul. Integr. Comp. Physiol. 279, R2164–R2172 [DOI] [PubMed] [Google Scholar]
- 36.Martín-Hernández D., Caso J. R., Bris Á. G., Maus S. R., Madrigal J. L. M., García-Bueno B., MacDowell K. S., Alou L., Gómez-Lus M. L., Leza J. C. (2016) Bacterial translocation affects intracellular neuroinflammatory pathways in a depression-like model in rats. Neuropharmacology 103, 122–133 [DOI] [PubMed] [Google Scholar]
- 37.Wang L., Liu J., Harvey-White J., Zimmer A., Kunos G. (2003) Endocannabinoid signaling via cannabinoid receptor 1 is involved in ethanol preference and its age-dependent decline in mice. Proc. Natl. Acad. Sci. USA 100, 1393–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Volynets V., Reichold A., Bárdos G., Rings A., Bleich A., Bischoff S. C. (2016) Assessment of the intestinal barrier with five different permeability tests in healthy C57BL/6J and BALB/cJ mice. Dig. Dis. Sci. 61, 737–746 [DOI] [PubMed] [Google Scholar]
- 39.Cani P. D., Bibiloni R., Knauf C., Waget A., Neyrinck A. M., Delzenne N. M., Burcelin R. (2008) Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes 57, 1470–1481 [DOI] [PubMed] [Google Scholar]
- 40.Vidal R., Garro-Martínez E., Díaz Á., Castro E., Florensa-Zanuy E., Taketo M. M., Pazos Á., Pilar-Cuéllar F. (2019) Targeting β-catenin in GLAST-expressing cells: impact on anxiety and depression-related behavior and hippocampal proliferation. Mol. Neurobiol. 56, 553–566 [DOI] [PubMed] [Google Scholar]
- 41.Ledesma J. C., Aguilar M. A., Giménez-Gómez P., Miñarro J., Rodríguez-Arias M. (2017) Adolescent but not adult ethanol binge drinking modulates cocaine withdrawal symptoms in mice. PLoS One 12, e0172956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qin L., Wu X., Block M. L., Liu Y., Breese G. R., Hong J.-S., Knapp D. J., Crews F. T. (2007) Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 55, 453–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yoshida R., Hayaishi O. (1978) Induction of pulmonary indoleamine 2,3-dioxygenase by intraperitoneal injection of bacterial lipopolysaccharide. Proc. Natl. Acad. Sci. USA 75, 3998–4000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salazar A., Gonzalez-Rivera B. L., Redus L., Parrott J. M., O’Connor J. C. (2012) Indoleamine 2,3-dioxygenase mediates anhedonia and anxiety-like behaviors caused by peripheral lipopolysaccharide immune challenge. Horm. Behav. 62, 202–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O’Connor J. C., Lawson M. A., André C., Briley E. M., Szegedi S. S., Lestage J., Castanon N., Herkenham M., Dantzer R., Kelley K. W. (2009) Induction of IDO by bacille Calmette-Guérin is responsible for development of murine depressive-like behavior. J. Immunol. 182, 3202–3212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nagpal R., Yadav H. (2017) Bacterial translocation from the gut to the distant organs: an overview. Ann. Nutr. Metab. 71 (Suppl 1), 11–16 [DOI] [PubMed] [Google Scholar]
- 47.Bode C., Bode J. C. (2003) Effect of alcohol consumption on the gut. Best Pract. Res. Clin. Gastroenterol. 17, 575–592 [DOI] [PubMed] [Google Scholar]
- 48.Bode C., Vollmer E., Hug J., Bode J. C. (1991) Increased permeability of the gut to polyethylene glycol and dextran in rats fed alcohol. Ann. N. Y. Acad. Sci. 625, 837–840 [DOI] [PubMed] [Google Scholar]
- 49.Draper L. R., Gyure L. A., Hall J. G., Robertson D. (1983) Effect of alcohol on the integrity of the intestinal epithelium. Gut 24, 399–404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Keshavarzian A., Farhadi A., Forsyth C. B., Rangan J., Jakate S., Shaikh M., Banan A., Fields J. Z. (2009) Evidence that chronic alcohol exposure promotes intestinal oxidative stress, intestinal hyperpermeability and endotoxemia prior to development of alcoholic steatohepatitis in rats. J. Hepatol. 50, 538–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Donnadieu-Rigole H., Pansu N., Mura T., Pelletier S., Alarcon R., Gamon L., Perney P., Apparailly F., Lavigne J.-P., Dunyach-Remy C. (2018) Beneficial effect of alcohol withdrawal on gut permeability and microbial translocation in patients with alcohol use disorder. Alcohol. Clin. Exp. Res. 42, 32–40 [DOI] [PubMed] [Google Scholar]
- 52.Bjarnason I., Peters T. J., Wise R. J. (1984) The leaky gut of alcoholism: possible route of entry for toxic compounds. Lancet 1, 179–182 [DOI] [PubMed] [Google Scholar]
- 53.Bode C., Kugler V., Bode J. C. (1987) Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J. Hepatol. 4, 8–14 [DOI] [PubMed] [Google Scholar]
- 54.Keshavarzian A., Holmes E. W., Patel M., Iber F., Fields J. Z., Pethkar S. (1999) Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am. J. Gastroenterol. 94, 200–207 [DOI] [PubMed] [Google Scholar]
- 55.Parlesak A., Schäfer C., Schütz T., Bode J. C., Bode C. (2000) Increased intestinal permeability to macromolecules and endotoxemia in patients with chronic alcohol abuse in different stages of alcohol-induced liver disease. J. Hepatol. 32, 742–747 [DOI] [PubMed] [Google Scholar]
- 56.Leclercq S., Cani P. D., Neyrinck A. M., Stärkel P., Jamar F., Mikolajczak M., Delzenne N. M., de Timary P. (2012) Role of intestinal permeability and inflammation in the biological and behavioral control of alcohol-dependent subjects. Brain Behav. Immun. 26, 911–918 [DOI] [PubMed] [Google Scholar]
- 57.Rivera C. A., Bradford B. U., Seabra V., Thurman R. G. (1998) Role of endotoxin in the hypermetabolic state after acute ethanol exposure. Am. J. Physiol. 275, G1252–G1258 [DOI] [PubMed] [Google Scholar]
- 58.Bala S., Marcos M., Gattu A., Catalano D., Szabo G. (2014) Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One 9, e96864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lin J.-C., Wu J.-Q., Wang F., Tang F.-Y., Sun J., Xu B., Jiang M., Chu Y., Chen D., Li X., Su S., Zhang Y., Wu N., Yang S., Wu K., Liang J. (2019) QingBai decoction regulates intestinal permeability of dextran sulphate sodium-induced colitis through the modulation of notch and NF-κB signalling. Cell Prolif. 52, e12547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen X., Liu R., Liu X., Xu C., Wang X. (2018) Protective role of coxsackie-adenovirus receptor in the pathogenesis of inflammatory bowel diseases. Biomed. Res. Int. 2018, 7207268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wang H. Y., Chi C., Xu Y. Q., Wang C., Wang T. Y., Lv D., Li X. (2019) Occludin endocytosis is involved in disruption of the intestinal epithelial barrier in a mouse model of alcoholic steatohepatitis. [E-pub ahead of print] J. Dig. Dis. [DOI] [PubMed] [Google Scholar]
- 62.Leclercq S., Matamoros S., Cani P. D., Neyrinck A. M., Jamar F., Stärkel P., Windey K., Tremaroli V., Bäckhed F., Verbeke K., de Timary P., Delzenne N. M. (2014) Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc. Natl. Acad. Sci. USA 111, E4485–E4493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Turner J. R. (2009) Intestinal mucosal barrier function in health and disease. Nat. Rev. Immunol. 9, 799–809 [DOI] [PubMed] [Google Scholar]
- 64.Al-Sadi R., Khatib K., Guo S., Ye D., Youssef M., Ma T. (2011) Occludin regulates macromolecule flux across the intestinal epithelial tight junction barrier. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G1054–G1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.McCarthy K. M., Skare I. B., Stankewich M. C., Furuse M., Tsukita S., Rogers R. A., Lynch R. D., Schneeberger E. E. (1996) Occludin is a functional component of the tight junction. J. Cell Sci. 109, 2287–2298 [DOI] [PubMed] [Google Scholar]
- 66.Suzuki T. (2013) Regulation of intestinal epithelial permeability by tight junctions. Cell. Mol. Life Sci. 70, 631–659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mir H., Meena A. S., Chaudhry K. K., Shukla P. K., Gangwar R., Manda B., Padala M. K., Shen L., Turner J. R., Dietrich P., Dragatsis I., Rao R. (2016) Occludin deficiency promotes ethanol-induced disruption of colonic epithelial junctions, gut barrier dysfunction and liver damage in mice. Biochim. Biophys. Acta 1860, 765–774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tang Y., Banan A., Forsyth C. B., Fields J. Z., Lau C. K., Zhang L. J., Keshavarzian A. (2008) Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol. Clin. Exp. Res. 32, 355–364 [DOI] [PubMed] [Google Scholar]
- 69.Cho Y.-E., Song B.-J. (2018) Pomegranate prevents binge alcohol-induced gut leakiness and hepatic inflammation by suppressing oxidative and nitrative stress. Redox Biol. 18, 266–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Banan A., Fields J. Z., Decker H., Zhang Y., Keshavarzian A. (2000) Nitric oxide and its metabolites mediate ethanol-induced microtubule disruption and intestinal barrier dysfunction. J. Pharmacol. Exp. Ther. 294, 997–1008 [PubMed] [Google Scholar]
- 71.Samak G., Gangwar R., Meena A. S., Rao R. G., Shukla P. K., Manda B., Narayanan D., Jaggar J. H., Rao R. (2016) Calcium channels and oxidative stress mediate a synergistic disruption of tight junctions by ethanol and acetaldehyde in Caco-2 cell monolayers. Sci. Rep. 6, 38899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Medina C., Videla S., Radomski A., Radomski M. W., Antolín M., Guarner F., Vilaseca J., Salas A., Malagelada J.-R. (2003) Increased activity and expression of matrix metalloproteinase-9 in a rat model of distal colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 284, G116–G122 [DOI] [PubMed] [Google Scholar]
- 73.Castaneda F. E., Walia B., Vijay-Kumar M., Patel N. R., Roser S., Kolachala V. L., Rojas M., Wang L., Oprea G., Garg P., Gewirtz A. T., Roman J., Merlin D., Sitaraman S. V. (2005) Targeted deletion of metalloproteinase 9 attenuates experimental colitis in mice: central role of epithelial-derived MMP. Gastroenterology 129, 1991–2008 [DOI] [PubMed] [Google Scholar]
- 74.Vecil G. G., Larsen P. H., Corley S. M., Herx L. M., Besson A., Goodyer C. G., Yong V. W. (2000) Interleukin-1 is a key regulator of matrix metalloproteinase-9 expression in human neurons in culture and following mouse brain trauma in vivo. J. Neurosci. Res. 61, 212–224 [DOI] [PubMed] [Google Scholar]
- 75.Katsu M., Niizuma K., Yoshioka H., Okami N., Sakata H., Chan P. H. (2010) Hemoglobin-induced oxidative stress contributes to matrix metalloproteinase activation and blood-brain barrier dysfunction in vivo. J. Cereb. Blood Flow Metab. 30, 1939–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Singer I. I., Kawka D. W., Scott S., Weidner J. R., Mumford R. A., Riehl T. E., Stenson W. F. (1996) Expression of inducible nitric oxide synthase and nitrotyrosine in colonic epithelium in inflammatory bowel disease. Gastroenterology 111, 871–885 [DOI] [PubMed] [Google Scholar]
- 77.Cross R. K., Wilson K. T. (2003) Nitric oxide in inflammatory bowel disease. Inflamm. Bowel Dis. 9, 179–189 [DOI] [PubMed] [Google Scholar]
- 78.Tang Y., Forsyth C. B., Farhadi A., Rangan J., Jakate S., Shaikh M., Banan A., Fields J. Z., Keshavarzian A. (2009) Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol. Clin. Exp. Res. 33, 1220–1230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Crews F. T., Bechara R., Brown L. A., Guidot D. M., Mandrekar P., Oak S., Qin L., Szabo G., Wheeler M., Zou J. (2006) Cytokines and alcohol. Alcohol. Clin. Exp. Res. 30, 720–730 [DOI] [PubMed] [Google Scholar]
- 80.Achur R. N., Freeman W. M., Vrana K. E. (2010) Circulating cytokines as biomarkers of alcohol abuse and alcoholism. J. Neuroimmune Pharmacol. 5, 83–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang D., Dubois R. N. (2010) The role of COX-2 in intestinal inflammation and colorectal cancer. Oncogene 29, 781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fredenburgh L. E., Velandia M. M., Ma J., Olszak T., Cernadas M., Englert J. A., Chung S. W., Liu X., Begay C., Padera R. F., Blumberg R. S., Walsh S. R., Baron R. M., Perrella M. A. (2011) Cyclooxygenase-2 deficiency leads to intestinal barrier dysfunction and increased mortality during polymicrobial sepsis. J. Immunol. 187, 5255–5267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sommer F., Bäckhed F. (2013) The gut microbiota--masters of host development and physiology. Nat. Rev. Microbiol. 11, 227–238 [DOI] [PubMed] [Google Scholar]
- 84.Badawy A. A.-B., Bano S., Steptoe A. (2011) Tryptophan in alcoholism treatment I: kynurenine metabolites inhibit the rat liver mitochondrial low Km aldehyde dehydrogenase activity, elevate blood acetaldehyde concentration and induce aversion to alcohol. Alcohol Alcohol. 46, 651–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Badawy A. A.-B. (2017) Kynurenine pathway of tryptophan metabolism: regulatory and functional aspects. Int. J. Tryptophan Res. 10, 1178646917691938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Agus A., Planchais J., Sokol H. (2018) Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23, 716–724 [DOI] [PubMed] [Google Scholar]
- 87.LeMarquand D., Pihl R. O., Benkelfat C. (1994) Serotonin and alcohol intake, abuse, and dependence: findings of animal studies. Biol. Psychiatry 36, 395–421 [DOI] [PubMed] [Google Scholar]
- 88.Neupane S. P., Lien L., Martinez P., Hestad K., Bramness J. G. (2015) The relationship of alcohol use disorders and depressive symptoms to tryptophan metabolism: cross-sectional data from a Nepalese alcohol treatment sample. Alcohol. Clin. Exp. Res. 39, 514–521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Gleissenthall G. V., Geisler S., Malik P., Kemmler G., Benicke H., Fuchs D., Mechtcheriakov S. (2014) Tryptophan metabolism in post-withdrawal alcohol-dependent patients. Alcohol Alcohol. 49, 251–255 [DOI] [PubMed] [Google Scholar]
- 90.Badawy A. A.-B. (2002) Tryptophan metabolism in alcoholism. Nutr. Res. Rev. 15, 123–152 [DOI] [PubMed] [Google Scholar]
- 91.Badawy A. A.-B., Doughrty D. M., Marsh-Richard D. M., Steptoe A. (2009) Activation of liver tryptophan pyrrolase mediates the decrease in tryptophan availability to the brain after acute alcohol consumption by normal subjects. Alcohol Alcohol. 44, 267–271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kelley K. W., Dantzer R. (2011) Alcoholism and inflammation: neuroimmunology of behavioral and mood disorders. Brain Behav. Immun. 25 (Suppl 1), S13–S20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Crews F. T., Sarkar D. K., Qin L., Zou J., Boyadjieva N., Vetreno R. P. (2015) Neuroimmune function and the consequences of alcohol exposure. Alcohol Res. 37, 331–341, 344–351 [PMC free article] [PubMed] [Google Scholar]
- 94.Alfonso-Loeches S., Pascual-Lucas M., Blanco A. M., Sanchez-Vera I., Guerri C. (2010) Pivotal role of TLR4 receptors in alcohol-induced neuroinflammation and brain damage. J. Neurosci. 30, 8285–8295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gárate I., García-Bueno B., Madrigal J. L., Bravo L., Berrocoso E., Caso J. R., Micó J. A., Leza J. C. (2011) Origin and consequences of brain Toll-like receptor 4 pathway stimulation in an experimental model of depression. J. Neuroinflammation 8, 151–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Steck N., Mueller K., Schemann M., Haller D. (2012) Bacterial proteases in IBD and IBS. Gut 61, 1610–1618 [DOI] [PubMed] [Google Scholar]
- 97.Campbell B. M., Charych E., Lee A. W., Möller T. (2014) Kynurenines in CNS disease: regulation by inflammatory cytokines. Front. Neurosci. 8, 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schröcksnadel K., Wirleitner B., Winkler C., Fuchs D. (2006) Monitoring tryptophan metabolism in chronic immune activation. Clin. Chim. Acta 364, 82–90 [DOI] [PubMed] [Google Scholar]
- 99.Kim H., Chen L., Lim G., Sung B., Wang S., McCabe M. F., Rusanescu G., Yang L., Tian Y., Mao J. (2012) Brain indoleamine 2,3-dioxygenase contributes to the comorbidity of pain and depression. J. Clin. Invest. 122, 2940–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pascual M., Baliño P., Aragón C. M. G., Guerri C. (2015) Cytokines and chemokines as biomarkers of ethanol-induced neuroinflammation and anxiety-related behavior: role of TLR4 and TLR2. Neuropharmacology 89, 352–359 [DOI] [PubMed] [Google Scholar]
- 101.Linderholm K. R., Alm M. T., Larsson M. K., Olsson S. K., Goiny M., Hajos M., Erhardt S., Engberg G. (2016) Inhibition of kynurenine aminotransferase II reduces activity of midbrain dopamine neurons. Neuropharmacology 102, 42–47 [DOI] [PubMed] [Google Scholar]
- 102.Parrott J. M., Redus L., O’Connor J. C. (2016) Kynurenine metabolic balance is disrupted in the hippocampus following peripheral lipopolysaccharide challenge. J. Neuroinflammation 13, 124–137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Connor T. J., Starr N., O’Sullivan J. B., Harkin A. (2008) Induction of indolamine 2,3-dioxygenase and kynurenine 3-monooxygenase in rat brain following a systemic inflammatory challenge: a role for IFN-γ? Neurosci. Lett. 441, 29–34 [DOI] [PubMed] [Google Scholar]
- 104.Larsson M. K., Faka A., Bhat M., Imbeault S., Goiny M., Orhan F., Oliveros A., Ståhl S., Liu X. C., Choi D. S., Sandberg K., Engberg G., Schwieler L., Erhardt S. (2016) Repeated LPS injection induces distinct changes in the kynurenine pathway in mice. Neurochem. Res. 41, 2243–2255 [DOI] [PubMed] [Google Scholar]
- 105.Moreau M., André C., O’Connor J. C., Dumich S. A., Woods J. A., Kelley K. W., Dantzer R., Lestage J., Castanon N. (2008) Inoculation of Bacillus Calmette-Guerin to mice induces an acute episode of sickness behavior followed by chronic depressive-like behavior. Brain Behav. Immun. 22, 1087–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Xiao H. W., Ge C., Feng G. X., Li Y., Luo D., Dong J. L., Li H., Wang H., Cui M., Fan S. J. (2018) Gut microbiota modulates alcohol withdrawal-induced anxiety in mice. Toxicol. Lett. 287, 23–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Katz R. J. (1982) Animal model of depression: pharmacological sensitivity of a hedonic deficit. Pharmacol. Biochem. Behav. 16, 965–968 [DOI] [PubMed] [Google Scholar]
- 108.Scheggi S., De Montis M. G., Gambarana C. (2018) Making sense of rodent models of anhedonia. Int. J. Neuropsychopharmacol. 21, 1049–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Clarke G., McKernan D. P., Gaszner G., Quigley E. M., Cryan J. F., Dinan T. G. (2012) A distinct profile of tryptophan metabolism along the kynurenine pathway downstream of toll-like receptor activation in irritable bowel syndrome. Front. Pharmacol. 3, 90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoban A. E., Moloney R. D., Golubeva A. V., McVey Neufeld K. A., O’Sullivan O., Patterson E., Stanton C., Dinan T. G., Clarke G., Cryan J. F. (2016) Behavioural and neurochemical consequences of chronic gut microbiota depletion during adulthood in the rat. Neuroscience 339, 463–477 [DOI] [PubMed] [Google Scholar]
- 111.Nishizawa S., Benkelfat C., Young S. N., Leyton M., Mzengeza S., de Montigny C., Blier P., Diksic M. (1997) Differences between males and females in rates of serotonin synthesis in human brain. Proc. Natl. Acad. Sci. USA 94, 5308–5313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lieben C. K. J., Blokland A., Westerink B., Deutz N. E. P. (2004) Acute tryptophan and serotonin depletion using an optimized tryptophan-free protein-carbohydrate mixture in the adult rat. Neurochem. Int. 44, 9–16 [DOI] [PubMed] [Google Scholar]
- 113.Cryan J. F., Dinan T. G. (2012) Mind-altering microorganisms: the impact of the gut microbiota on brain and behaviour. Nat. Rev. Neurosci. 13, 701–712 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.