Abstract
The major side effect of tacrolimus (Tac) is nephrotoxicity. We studied whether supplementation of coenzyme Q10, (CoQ10) a potent antioxidant, can reduce Tac-induced nephrotoxicity via improving mitochondrial function. In an in vitro study, CoQ10 reduced the production of Tac-induced mitochondrial reactive oxygen species and abolished the loss of mitochondrial membrane potential in proximal tubular cell line. Assessment of mitochondrial function revealed that CoQ10 decreased oxygen consumption and mitochondrial respiration rate increased by Tac, suggesting improvement of mitochondrial function to synthesize ATP with CoQ10 treatment. The effect of the CoQ10 in vitro study was observed in an experimental model of chronic Tac-induced nephropathy. CoQ10 attenuated Tac-induced oxidative stress and was accompanied by function and histologic improvement. On electron microscopy, addition of CoQ10 increased not only the number but also the volume of mitochondria compared with Tac treatment only. Our data indicate that CoQ10 improves Tac-induced mitochondrial dysfunction in kidney. Supplementary CoQ10 treatment may be a promising approach to reduce Tac-induced nephrotoxicity.—Yu, J. H., Lim, S. W., Luo, K., Cui, S., Quan, Y., Shin, Y. J., Lee, K. E., Kim, H. L., Ko, E. J., Chung, B. H., Kim, J. H., Chung, S. J., Yang, C. W. Coenzyme Q10 alleviates tacrolimus-induced mitochondrial dysfunction in kidney.
Keywords: 3D reconstruction, nephrotoxicity, reactive oxygen species
Tacrolimus (Tac) is a widely used immunosuppressive agent for the prevention of allograft rejection after renal transplantation. However, Tac is nephrotoxic, and its long-term administration causes progressive renal failure, which is accompanied by striped interstitial fibrosis, tubular atrophy, inflammatory cell infiltration, apoptosis, and hyalinosis of the afferent arterioles (1, 2). Chronic Tac-induced nephropathy involves a variety of mechanisms, with oxidative stress proposed as a common pathway for Tac-induced renal injury (3, 4).
Coenzyme Q10 (CoQ10) is an endogenous lipid-soluble benzoquinone compound that functions as a diffusible electron carrier in the mitochondrial respiratory chain. CoQ10 acts as a powerful antioxidant that scavenges free radicals, prevents lipid peroxidation in cellular biomembranes, and promotes α-tocophrol regeneration (5, 6). Previous studies confirmed the protective effects of CoQ10 in various models of oxidative injury (7–14).
Based on the anti-oxidative effect of CoQ10, in this study we investigated whether CoQ10 can protect against Tac-induced nephrotoxicity. We first evaluated the effect of CoQ10 on Tac-induced mitochondrial dysfunction in vitro, and then examined the effect of CoQ10 on mitochondrial morphology in a rat model of chronic Tac-induced nephropathy.
MATERIALS AND METHODS
Cell culture
Human kidney-2 cell lines (HK-2) immortalized human proximal tubular epithelial cells were grown in DMEM containing 10% fetal bovine serum supplemented with 100 U/ml penicillin and 100 µg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2. The cells were seeded in culture plates and treated with the Tac (50 µg/ml) and CoQ10 (1 ng/ml) for 12 h.
Cell viability assay
Cells were seeded in 96-well plates at a density of 2 × 104 cells/well for 24 h and then subjected to various treatments [Tac (50 µg/ml) and CoQ10 (1 pg/ml–10 μg/ml)] for the specified periods. Before the end of the treatments, Cell Counting Kit-8 solution (Dojindo Laboratories, Kumamoto, Japan) was added to each well for 2 h, and absorbance was measured at 450 nm on a VersaMax ELISA plate reader (Molecular Devices, San Jose, CA, USA).
Apoptosis
After treatment with the Tac (50 µg/ml) and CoQ10 (1 ng/ml) for 12 h, trypsinized cells were incubated with 5 µl of FITC-conjugated Annexin V (BD Biosciences, San Jose, CA, USA) in 1 time binding buffer (BD Biosciences) for 15 min at room temperature according to the manufacturer’s protocol. The stained cells were sorted on a FACS Calibur Flow Cytometer (BD Biosciences). Values are expressed as the percentage of fluorescent cells relative to total cell count.
Mitochondrial reactive oxygen species detection
After treatment with the Tac (50 µg/ml) and CoQ10 (1 ng/ml) for 12 h, mitochondrial reactive oxygen species (ROS) (superoxide anion, O2−) were detected using MitoSox Red (Thermo Fisher Scientific, Waltham, MA, USA) for 30 min at 37°C according to the manufacturer’s instructions and analyzed by flow cytometry. Forward and side scatter data were collected (10,000 events/sample).
5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide assay
The mitochondrial membrane potential (MMP) of HK-2 cells was measured using the fluorescent lipophilic cationic probe 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide (JC-1; Cayman Chemicals, Ann Arbor, MI, USA). In healthy cells, JC-1 fluorescence is observed in both green (FL-1) and red (FL-2) channels, with a loss of red fluorescence indicating depolarized mitochondria. After treatment with the Tac (50 µg/ml) and CoQ10 (1 ng/ml) for 12 h, cells were incubated with JC-1 solution for 20 min at 37°C according to the manufacturer’s instructions and then analyzed by flow cytometry.
Measurement of oxygen consumption rate
Cellular oxygen consumption rate (OCR) rate was assessed in real time with an XF24 Extracellular Flux Analyzer (Seahorse Bioscience, Billerica, MA, USA). After treatment with the Tac (50 µg/ml) and CoQ10 (1 ng/ml) for 12 h, the culture medium was changed to running medium [DMEM supplemented with 5.5 mM glucose, 1 mM sodium pyruvate, 4 mM l-glutamine (pH 7.4)] and the cells were incubated at 37°C in a non-CO2 incubator for 1 h. The mitochondrial inhibitors ATP synthase inhibitor oligomycin (1 µM), carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (0.5 µM), and the complex I and III inhibitor rotenone/antimycin A (0.5 µM) were used to evaluate mitochondrial function and bioenergetics, including basal respiration, ATP production, maximal respiration, and spare respiratory capacity, as previously described by Dott et al. (15). OCR values were normalized to the protein content of each sample. Three to four wells were analyzed for each experimental group.
Ethics statement
All procedures involving animals were performed in strict accordance with ethical guidelines for animal studies. Experimental animal care protocols were approved by the Animal Care and Use Committee of the Catholic University of Korea (CUMC-2015-0028-02). Animals were euthanized under xylazine/rompun anesthesia.
Experimental model of Tac-induced renal injury
Male Sprague-Dawley rats (Orient Bio, Seongnam, South Korea) were maintained on a 12-h light/dark cycle with free access to a 0.05% salt diet (Research Diets, New Brunswick, NJ, USA) and water. After acclimation for 1 wk, weight-matched rats were randomly assigned to 1 of 4 groups (n = 8/group) and subcutaneously injected with 1.5 mg/kg/d Tac (Prograft; Astellas Pharma, Tokyo, Japan) or 1 ml/kg/d vehicle (Vh; olive oil) (MilliporeSigma, Burlington, MA, USA) with or without CoQ10 diluted in olive oil (20 mg/kg/d by oral gavage; Chong Kun Dang Pharm, Seoul, South Korea) for 4 wk. Administration routes and drug doses were selected based on previous studies (16, 17).
Biochemical analyses
Rats were pair fed, and their body weight was monitored daily. After the 4-wk treatments, animals were housed individually in metabolic cages (Tecniplast, Gazzada Schianno, Italy) for the measurement of water intake and urine volume over 24 h. The following day, animals were anesthetized, and blood samples and tissue specimens were obtained for further analysis. Serum creatinine (Scr) and blood urea nitrogen (BUN) were measured using an autoanalyzer (Coulter-STKS; Beckman Coulter Life Sciences, Indianapolis, IN, USA). Blood glutamic-pyruvate transaminase and glutamic-oxaloacetic transaminase levels were measured using automatic biochemical analyzer (Hitachi 7100; Hitachi, Tokyo, Japan). Creatinine clearance (ClCr) from 24-h urine and serum was calculated using a standard formula. Whole-blood Tac level was measured by liquid chromatography–tandem mass spectrometry (18) (Abbott Diagnostics, Chicago, IL, USA).
Measurement of CoQ10 level in plasma and tissue
Total CoQ10 level was measured using a competitive ELISA kit (Abx159767; Abbexa, Cambridge, United Kingdom). Briefly, 500 mg of kidney tissue was homogenized in 300 μl cold saline, the supernatant was used for ELISA according to the manufacturer’s instructions. Values on y axis are expressed as nanograms per milligram of tissue.
Histologic analysis of interstitial fibrosis
Tubulointerstitial fibrosis was evaluated by trichrome staining (3). Briefly, the tissue sections were refixed in Bouin’s solution for 1 h at 56°C to improve staining quality. Then they were stained in Weigert’s iron hematoxylin working solution for 10 min. After a wash in distilled water, the slides were soaked in the working solution of Biebrich scarlet-acid fuchsin and phosphomolybdicphosphotungstic acid solution 1 by 1. The slides were transferred directly (without rinsing) to aniline blue solution and stained for 5–10 min and then briefly rinsed in distilled water and 1% acetic acid solution. After dehydration through 95% ethyl alcohol, the slides were mounted with resinous mounting medium. The collagen fibers were stained in blue, the nuclei were stained in purple, and the background was stained in red. A finding of tubulointerstitial fibrosis was defined as a matrix-rich expansion of the interstitium with tubular dilatation, tubular atrophy, tubular cast formation, sloughing of tubular epithelial cells, or thickening of the tubular basement membrane. The extent of fibrosis was estimated by counting the percentage of injured area per field using polygon program. The extent of fibrosis was estimated in a minimum of 20 fields per section by measuring the injured area per field using TDI Scope Eye v.3.6 for Windows (TDI Systems, Seoul, South Korea). Histopathological analysis was performed in randomly selected cortical fields by a pathologist who was blinded to the identity of the treatment groups.
Periodic acid-Schiff staining
Glomerular injury was assessed using mesangial matrix area, podocyte number, and podocyte integrity. The mesangial matrix area was detected by periodic acid-Schiff staining (395B; MilliporeSigma) and was quantified for each glomerular cross section.
Detection of 8-hydroxy-2'-deoxyguanosine in urine
Oxidative DNA damage was evaluated based on the level of the DNA adduct 8-hydroxy-2'-deoxyguanosine (8-OHdG) in 24-h urine, which was measured using a competitive ELISA kit (Cell Biolabs, San Diego, CA, USA).
Immunohistochemistry
Dewaxed sections were incubated in retrieval solution (pH 6.0), methanolic H2O2, and 0.5% Triton X-100 and then washed in PBS. Nonspecific binding sites were blocked with 10% normal donkey serum (Jackson ImmunoResearch, West Grove, PA, USA). Sections were incubated overnight at 4°C with primary antibodies against 8-OHdG (MOG-100P), 4-hydroxy-hexenal (4-HHE; MHH-030n) (both from the Japan Institute for the Control of Aging, Shizuoka, Japan), and Wilms’ tumor1 (sc-192; Santa Cruz Biotechnology, Dallas, TX, USA), followed by peroxidase-conjugated secondary antibodies (Molecular Probes, Eugene, OR, USA) for 2 h at room temperature. Peroxidase activity was detected using 3,3′-diaminobenzidine (Vector Laboratories, Burlingame, CA, USA) as a chromogen. The stained sections were photographed with an Olympus photomicroscope equipped with differential-interference contras optics (BX 51; Olympus, Tokyo, Japan). Positive cells in ∼20 randomly selected areas for each animal in each group were counted, and quantitative analysis was performed by calculating the percent positive area with the same intensity by histogram equalization using TDI Scope Eye software.
TUNEL assay
Apoptotic cells in kidney tissue sections were detected by the TUNEL method using the In Situ Apoptosis Detection Kit (MilliporeSigma). TUNEL-positive cells were counted in ∼20 randomly selected nonoverlapping areas per animal in each group.
Transmission electron microscopy
Kidney tissue was processed for electron microscopy analysis as previously described by Lim et al. (19). Using an image analyzer, the number and area of mitochondria were measured in 20 random proximal tubular cells using TDI Scope Eye software.
Three-dimensional reconstruction of mitochondria
Long ribbons of up to ∼20 sections of kidney tissue were cut at a thickness of 70–90 nm on an ultramicrotome (Leica Microsystems, Buffalo Grove, IL, USA). The area of interest was selected, and consecutive serial sections were imaged under an electron microscope (Jem 1010; Jeol, Tokyo, Japan). The outline of individual mitochondria was manually traced with different colors through the image stacks using Photoshop software (Adobe, San Jose, CA, USA). Each section image was aligned and marked structures were masked and exported to the 3-dimensional (3D) modeling program Mimics v.19.0 (Materialise, Leuven, Belgium) along with information regarding slice thickness, actual pixel size, and image orientation. Mitochondrial volume in a stack consisting of equal numbers of sections was also calculated using Mimics v.19.0.
Statistical analysis
Data are expressed as means ± se of ≥4 independent experiments. Multiple comparisons between groups were performed by 1-way ANOVA with Bonferroni’s post hoc test using Prism v.7.03 for Windows (GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered significant.
RESULTS
CoQ10 reduces Tac-induced apoptosis and mitochondrial ROS production in HK-2 cells
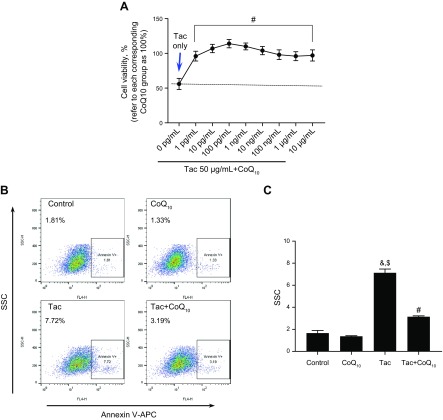
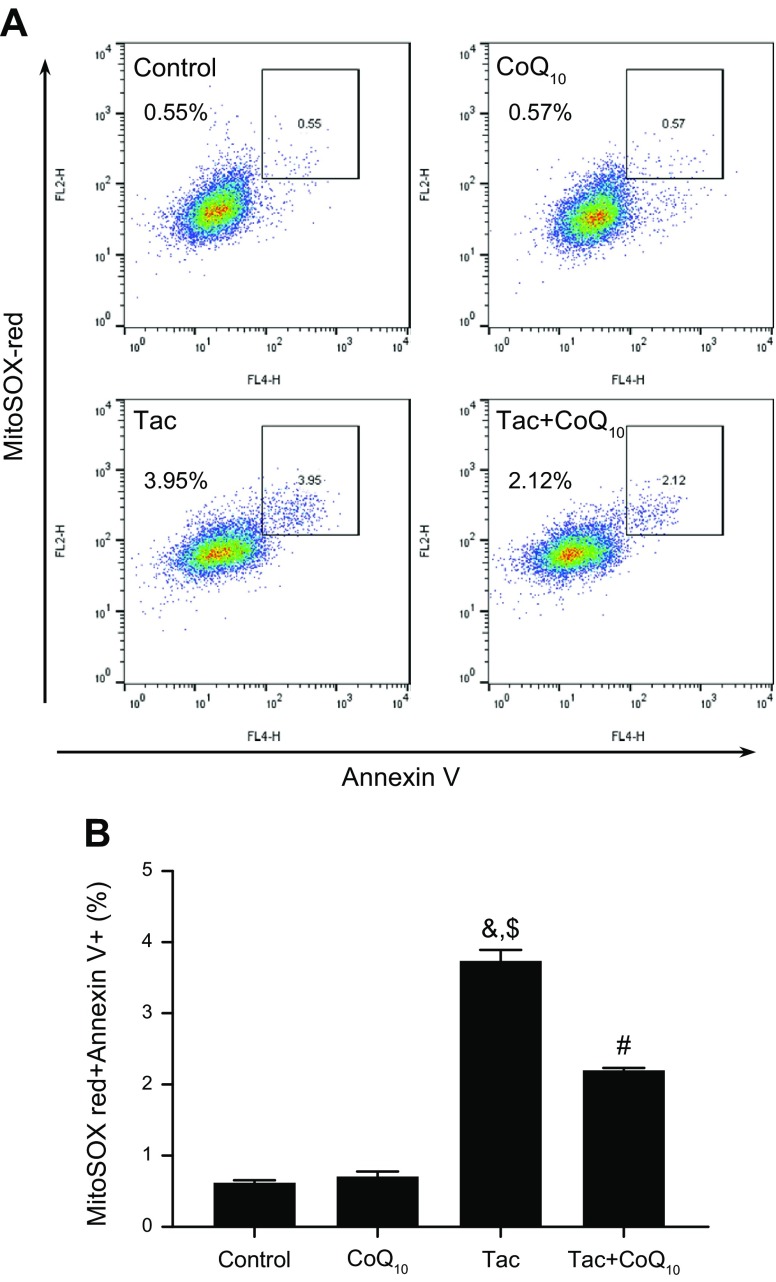
We evaluated the protective effect of CoQ10 on Tac-induced mitochondrial injury using HK-2 proximal tubule cells treated with different concentrations (1 pg/ml–10 μg/ml) of CoQ10 for 12 h during Tac-induced toxicity. CoQ10 enhanced HK-2 cell viability at all doses as compared to cells treated with Tac only (Fig. 1A). To examine whether CoQ10 inhibits mitochondrial pathways associated with apoptosis, cells stained with Annexin V were analyzed by flow cytometry. The Tac-induced increase in the number of Annexin V–positive cells was abrogated by CoQ10 treatment (Fig. 1B, C). We then examined whether CoQ10 reduced mitochondrial ROS-induced apoptosis during Tac-induced cell injury. Double staining with Annexin V and MitoSox Red staining for mitochondrial O2− in conjunction with flow cytometry revealed that CoQ10 reversed Tac-induced oxidative stress (Fig. 2A, B). Moreover, the Tac+CoQ10 treatment also reduced the number of Annexin V-positive cells in MitoSox Red–positive cells compared with the Tac group (Fig. 2C, D).
Figure 1.
Protective effects of CoQ10 against Tac-induced injury in HK-2 cells. Cells were seeded in plates at 90% confluence; the next day, they were treated with Tac (50 µg/ml) in the absence or presence of 1 pg/ml–10 μg/ml CoQ10 for 12 h. A) Cell Counting Kit-8 solution was added to each well for 2 h to evaluate cell viability. B, C) Annexin V solution was added to each well for 15 min to measure apoptosis, which was detected by flow cytometry. Data are presented as means ± se and are representative of ≥4 independent experiments. One-way ANOVA was used to analyze the data. &P < 0.05 vs. control group; $P < 0.05 vs. CoQ10 group; #P < 0.05 vs. Tac group.
Figure 2.
Effects of CoQ10 on mitochondrial ROS production during Tac-induced HK-2 cells injury. Cells were seeded in culture plates at 90% confluence; the next day, they were treated with Tac (50 µg/ml) in the absence or presence of 1 ng/ml CoQ10 for 12 h. Incidence of mitochondrial O2−-induced apoptotic cells was measured by double staining with MitoSox Red (A) and Annexin V using flow cytometry (B). Data are presented as means ± se and are representative of ≥4 independent experiments. One-way ANOVA was used to analyze the data. &P < 0.05 vs. control group; $P < 0.05 vs. CoQ10 group; #P < 0.05 vs. Tac group.
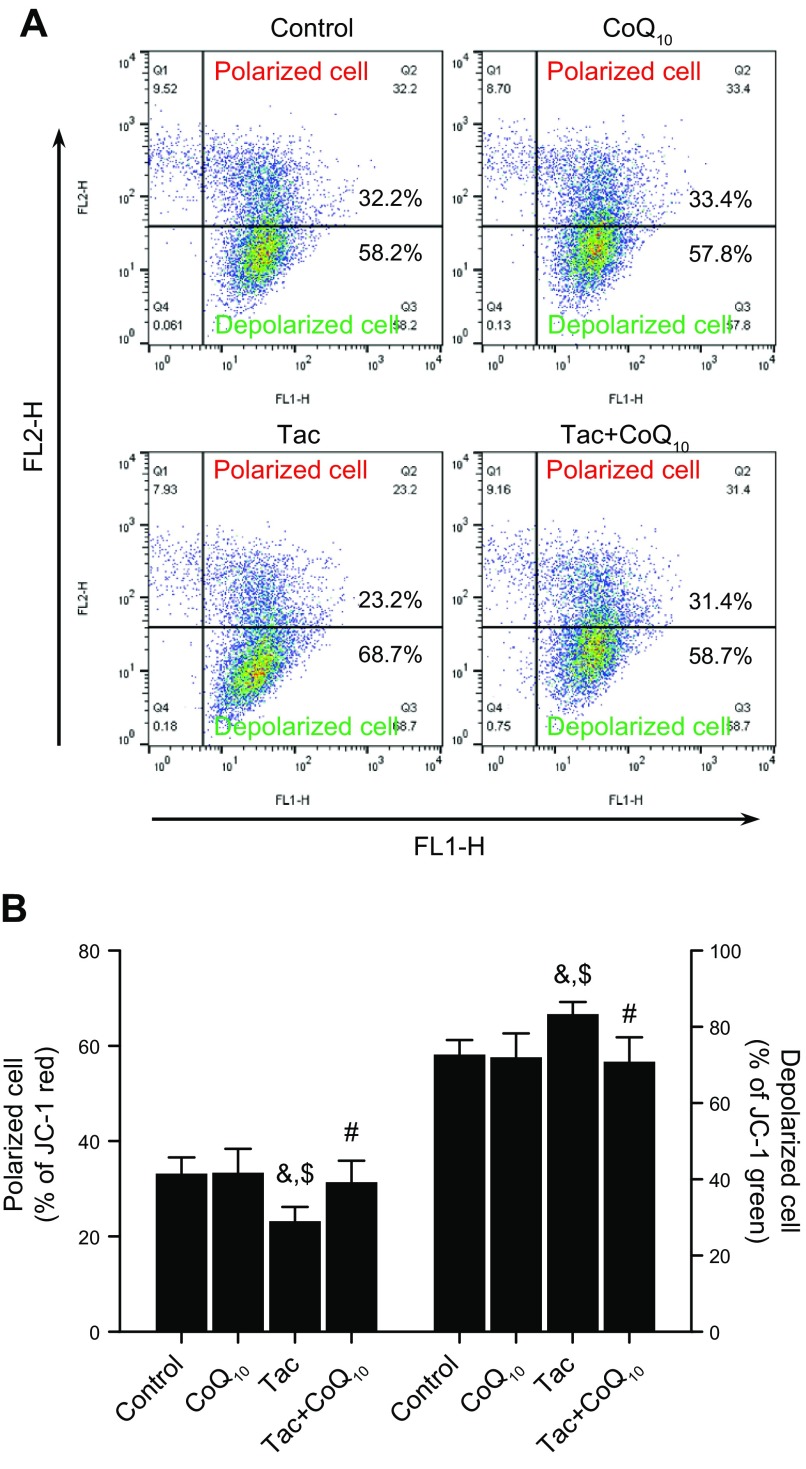
CoQ10 restores MMP in Tac-treated HK-2 cells
We evaluated the loss of MMP with the JC-1 assay. Tac treatment reduced MMP, as evidenced by a decrease in the number of polarized cells; this decrease was reversed by CoQ10 treatment. Conversely, the increase in the number of depolarized cells in the Tac group was abolished by coapplication of CoQ10 (Fig. 3).
Figure 3.
Effect of CoQ10 on MMP of HK-2 cells with Tac-induced injury. Cells were seeded in culture plates at 90% confluence; the next day, they were treated with Tac (50 µg/ml) in the absence or presence of 1 ng/ml CoQ10 for 12 h, then labeled with JC-1 and then analyzed by flow cytometry to evaluate MMP. Flow cytometry plots (A) and quantitation of JC-1 labeling (B). Data are presented as means ± se and are representative of ≥4 independent experiments. One-way ANOVA was used to analyze the data. &P < 0.05 vs. control group; $P < 0.05 vs. CoQ10 group; #P < 0.05 vs. Tac group.
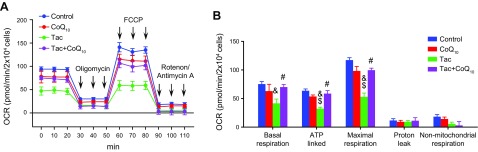
CoQ10 abrogates the Tac-induced reduction in mitochondrial respiration in HK-2 cells
Mitochondrial bioenergetics were evaluated by measuring oxygen consumption by cells following treatment with inhibitors of mitochondrial function (Fig. 4A). Cells treated with CoQ10 had higher rates of basal mitochondrial respiration than those treated with Tac only, indicating an increase in the number or activity of mitochondria (Fig. 4B). Compared to cells treated with Tac alone, those in the Tac+CoQ10 group showed higher rates of ATP-associated and total respiration. These results indicate that CoQ10 preserves the ability of mitochondria to synthesize ATP via ATP synthase after Tac treatment.
Figure 4.
Effect of CoQ10 on mitochondrial function in HK-2 cells with Tac-induced injury. Cells were seeded in culture plates at 90% confluence; the next day, they were treated with Tac (50 µg/ml) in the absence or presence of 1 ng/ml CoQ10 for 12 h, then cultured in a non-CO2 incubator for 1 h. The ATP synthase inhibitor oligomycin, respiratory chain uncoupler carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone, or respiratory chain complex I and III inhibitor rotenone/antimycin A were added to the culture medium as indicated. A) Areas under the curve for basal respiration. B) Basal respiration, ATP production, maximal respiration, proton leakage, and nonmitochondrial respiration were calculated from the OCR. Data represent means ± se and are representative of ≥4 independent experiments. One-way ANOVA was used to analyze the data. &P < 0.05 vs. control group; $P < 0.05 vs. CoQ10 group; #P < 0.05 vs. Tac group.
CoQ10 alleviates Tac-induced renal dysfunction and interstitial fibrosis in a rat model
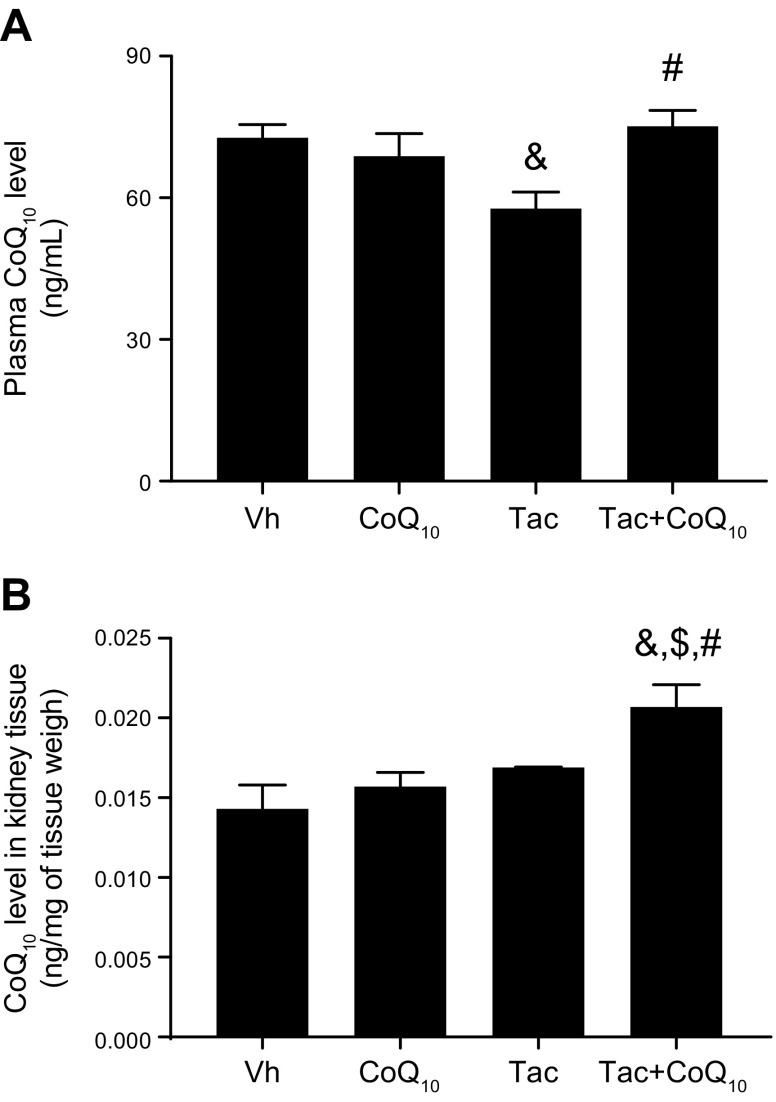
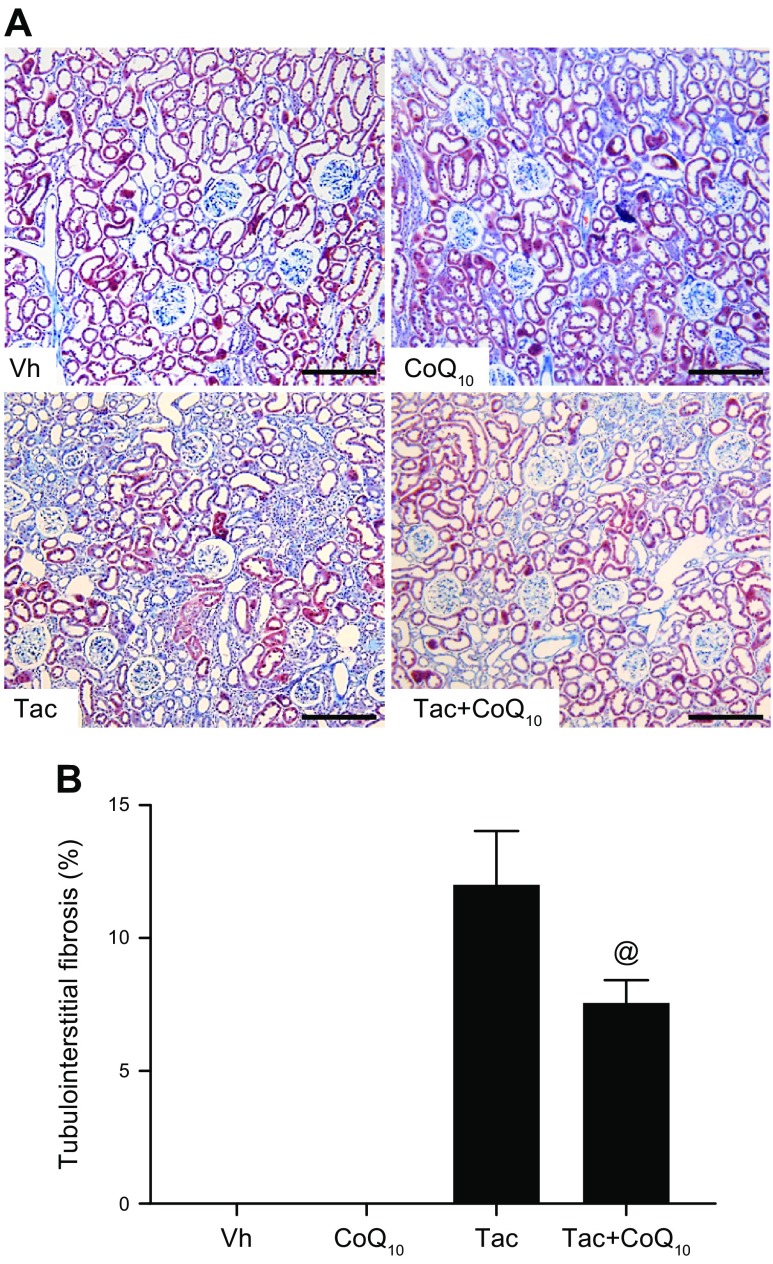
Table 1 shows changes in the biochemical parameters in rats after Tac and CoQ10 treatment for 4 wk. The Tac+CoQ10 group showed a lower level of water intake, urine volume, Scr, BUN, and ClCr than the Tac group. CoQ10 coadministration did not affect the trough level of Tac in whole blood, indicating that there was no drug interaction at the tested doses. In addition, liver function assessed by glutamic-pyruvate transaminase and glutamic-oxaloacetic transaminase level did not differ between Tac and Tac+CoQ10 groups (Supplemental Fig. S1). Cotreatment with CoQ10 increased total CoQ10 in the plasma and kidney tissue of rats treated with Tac (Fig. 5). The Tac group showed extensive tubulointerstitial fibrosis, which was decreased in the Tac+CoQ10 group (Fig. 6).
TABLE 1.
Effect of administration of CoQ10 on basic parameters
| Variable | Vh (n = 8) | CoQ10 (n = 8) | Tac (n = 8) | Tac+CoQ10 (n = 8) |
|---|---|---|---|---|
| Body weight (g) | 333 ± 5 | 322 ± 7 | 290 ± 4*,** | 316 ± 3† |
| Water intake (ml/d) | 29 ± 2 | 21 ± 1* | 38 ± 3*,** | 26 ± 2† |
| Urine volume (ml/d) | 15 ± 3 | 11 ± 1* | 31 ± 3*,** | 21 ± 2† |
| Scr (mg/dl) | 0.36 ± 0.01 | 0.33 ± 0.01 | 0.62 ± 0.03*,** | 0.52 ± 0.03† |
| BUN (mg/dl) | 13 ± 0.5 | 13 ± 0.6 | 58 ± 3.2*,** | 48 ± 4.3† |
| ClCr (ml/min/100 g) | 0.60 ± 0.05 | 0.58 ± 0.04 | 0.31 ± 0.02*,** | 0.37 ± 0.02† |
| Trough Tac (ng/ml) | — | — | 10.2 ± 1.3 | 10.8 ± 0.8 |
The values shown are means ± se (n = 8). *P < 0.05 vs. Vh; **P < 0.05 vs. CoQ10; †P < 0.05 vs. Tac.
Figure 5.
Effect of CoQ10 on a rat model of Tac-induced renal injury. CoQ10 concentration was determined in the plasma (A) and renal cortex (B) of rats treated with Tac or CoQ10 by oral administration after 4 wk. Total CoQ10 levels in both plasma and kidney tissue was higher in the Tac + CoQ10 than in the Tac group. Data represent means ± se (n = 8). One-way ANOVA was used to analyze the data. &P < 0.05 vs. Vh group; $P < 0.05 vs. CoQ10 group; #P < 0.05 vs. Tac group.
Figure 6.
Effect of CoQ10 on Tac-induced renal tubulointerstitial fibrosis in a rat model. Histologic analysis of renal cortex tissue from rats treated with Tac for 4 wk (A); striped tubulointerstitial fibrosis, mononuclear cell infiltration, and tubular atrophy were observed (B). Renal tissue damage was reduced by CoQ10 treatment. Scale bars, 100 µm. Data represent means ± se (n = 8). One-way ANOVA was used to analyze the data. @P < 0.05 vs. Tac group.
CoQ10 reduces Tac-induced oxidative stress and apoptosis in a rat model
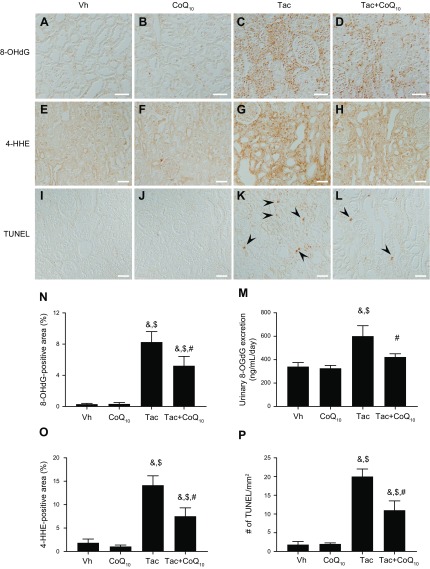
8-OHdG (Fig. 7A–D) and 4-HHE (Fig. 7E–H)—a marker of oxidative DNA or lipid damage—expression in kidney tissue was detected by immunohistochemistry. We also evaluated whether CoQ10 protects against Tac-induced apoptosis, an important cell death mechanism in Tac-induced nephrotoxicity (Fig. 7I–L). Urine 8-OHdG concentration and 8-OHdG immunoreactivity in tissue were evaluated by ELISA and increased in the Tac group, an effect that was reversed by CoQ10 treatment (Fig. 7M and N). Semiquantitative analysis of 4-HHE immunoreactivity in tissue was also attenuated in the Tac+CoQ10 group compared with the Tac group (Fig. 7O). The number of TUNEL-positive cells in tissue sections was higher in the Tac than in the Vh group, which was reversed in the presence of CoQ10 (Fig. 7P).
Figure 7.
Effect of CoQ10 on Tac-induced oxidative stress and apoptosis in a rat model. Representative images and quantification of immunopositivity for 8-OHdG (A–D, N) and 4-HHE (E–H, O) and results of the TUNEL assay (I–L, P) in rat kidney tissue. The high 8-OHdG and 4-HHE immunoreactivity induced by Tac was decreased by coadministration of CoQ10. M) Daily urinary 8-OHdG excretion. Tac-induced 8-OHdG excretion was reduced by CoQ10 coadministration. Arrowheads indicate TUNEL-positive cells. Scale bars, 50 µm. Data represent means ± se (n = 8). One-way ANOVA was used to analyze the data. &P < 0.05 vs. Vh group; $P < 0.05 vs. CoQ10 groups; #P < 0.05 vs. Tac group.
CoQ10 increases mitochondrial area and number in proximal tubular cells
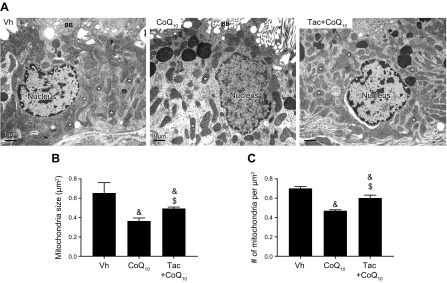
To determine whether the improvement of mitochondrial function by CoQ10 was associated with morphologic recovery, the size and number of mitochondria and their ultrastructure were examined by electron microscopy. In proximal tubular cells, Tac treatment reduced both mean mitochondrial size (0.37 ± 0.03 μm2 vs. 0.65 ± 0.12 μm2, P < 0.05 vs. Vh group) and number (0.47 ± 0.01 vs. 0.70 ± 0.2 μm2, P < 0.05 vs. Vh group), but CoQ10 reversed these changes (size, 0.49 ± 0.01 μm2 and number, 0.60 ± 0.03 μm2; P < 0.05, Tac+CoQ10 group vs. Tac group) (Fig. 8).
Figure 8.
Transmission electron microscopy analysis of the proximal tubule in rat kidneys. A) Representative transmission electron micrographs of mitochondrial ultrastructure in proximal tubules. Asterisks indicate mitochondria. Scale bars, 1 µm. B, C) Quantitative analysis of mitochondrial area and number of mitochondria. BB, brush border. Data represent means ± se. &P < 0.05 vs. Vh group; $P < 0.05 vs. Tac groups.
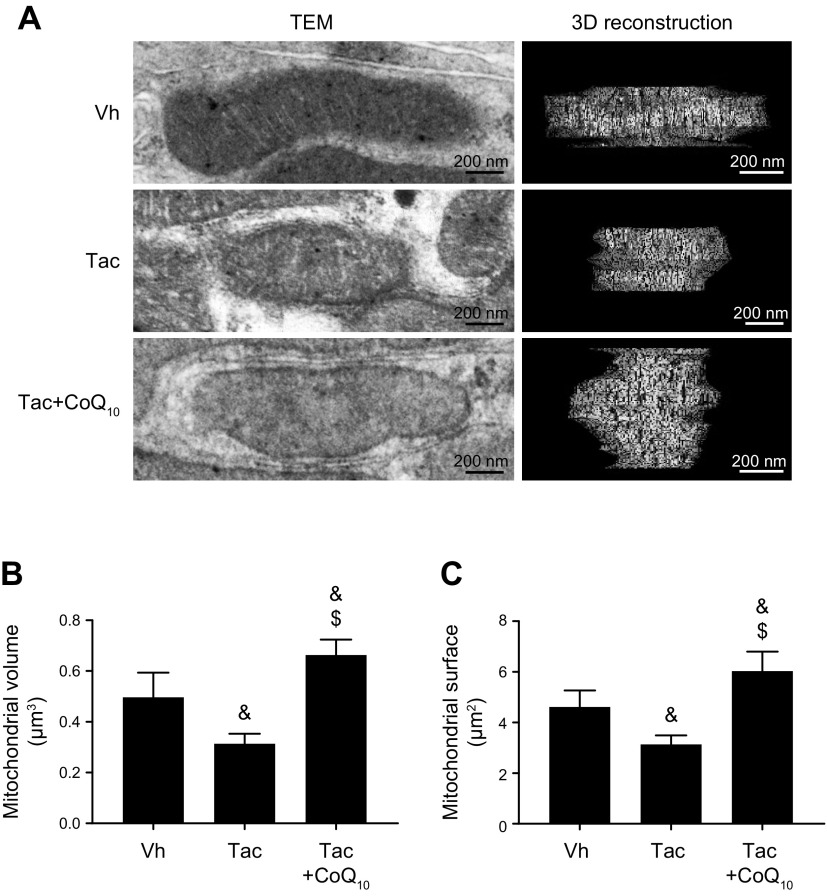
CoQ10 restores mitochondrial volume and surface area in proximal tubular cells
To evaluate the ultrastructure of mitochondria, we carried out 3D reconstruction of consecutive sections of a single mitochondrion and measured the mean volume and surface area. We found that cotreatment with CoQ10 on Tac increased mitochondrial volume (0.66 ± 0.06 vs. 0.31 ± 0.04 μm3, P < 0.05 vs. Tac group) and surface area (6.0 ± 0.8 vs. 3.1 ± 0.4 μm3, P < 0.05 vs. Tac group) relative to Tac treatment only (Fig. 9).
Figure 9.
3D reconstruction of mitochondria in proximal tubules. A) Representative transmission electron micrographs and 3D reconstruction of mitochondria, revealing mitochondrial morphology. The reconstruction was based on a series of about 20 sections cut using ultramicrotome. Scale bars, 200 nm. B, C) Quantitative analysis of mitochondrial volume (B) and surface area (C). SA, surface; V, volume. Data represent means ± se. &P < 0.05 vs. Vh group; $P < 0.05 vs. Tac groups.
DISCUSSION
The results of our in vitro and in vivo experiments demonstrate that CoQ10 attenuates Tac-induced renal dysfunction and interstitial fibrosis, which was associated with a decrease in Tac-induced oxidative stress and reversal of mitochondrial dysfunction. Thus, CoQ10 has beneficial effects in reducing Tac-induced mitochondrial injury via its anti-oxidative properties, providing a rationale for supplementary use of CoQ10 with Tac in clinical practice.
The main role of CoQ10 in cells is participation in the electron transport chain of the inner mitochondrial membrane (20). CoQ10 contributes to the control of cell redox status via modulation of ROS generation. Indeed, CoQ10 supplementation has been prescribed for oxidative stress-related diseases such as aging and metabolic disorders (21). The present study focused on the effects of CoQ10 on mitochondrial function and morphology in models of Tac-induced nephrotoxicity.
We focused on the renal tubular cell injury to evaluate the effect of CoQ10 on Tac-induced oxidative stress not on the glomerulus cells because there were no significant changes in the glomerular morphology and number of podocytes under current experimental condition (Supplemental Fig. S2). Moreover, previous literature revealed that tubular cells, especially proximal tubule cells, are most vulnerable to Tac-induced renal injury (22).
Our results clearly demonstrate that CoQ10 abrogated the Tac-induced accumulation of mitochondrial ROS (O2−) and restored MMP as well as basal, ATP-linked, and maximal respiration. Because mitochondrial dysfunction leads to apoptosis, we examined the mitochondrial cell death pathway in HK-2 cells and found that coapplication of CoQ10 reduced the number of Annexin V–positive cell in MitoSox Red–positive cells compared to cells treated with Tac only. The data indicate that CoQ10 preserves the ability of mitochondria to synthesize ATP via ATP synthase in Tac-induced renal cell injury and thereby prevents apoptotic cell death.
We next evaluated the effect of CoQ10 on Tac-induced oxidative stress in a rat model of Tac-induced nephropathy. Oxidative stress is a common pathway for Tac-induced renal injury (3, 16, 23), and we and others have reported that antioxidants can reduce Tac-induced nephrotoxicity (19, 24–26). Our current findings demonstrate that CoQ10 reduced oxidative stress in Tac-treated cells, as evidenced by the decreases in 8-OHdG and 4-HHE expression; this was accompanied by improved renal function and reduced interstitial fibrosis. These observations are consistent with previous reports that oxidative stress causes structural and functional damage to the kidney, and that CoQ10 functions as an ROS scavenger to improve kidney histology and function in renal injury models (27–29).
Mitochondria continuously alter their morphology by fusing or dividing to maintain mitochondrial homeostasis (30). Disruption of this process is manifested by reductions in mitochondrial size and volume (31). We examined the ultrastructure of mitochondria by transmission electron microscopy and found that Tac treatment reduced the number and size of mitochondria, changes that were reversed in the presence of CoQ10. Moreover, the 3D reconstruction revealed that CoQ10 improved mitochondrial ultrastructure, including volume and surface area.
Patients with CoQ10 deficiency exhibit nephrotic syndrome, along with proteinuria, and have podocytes that are enriched in mitochondria; oral CoQ10 supplementation at a dose of 30 mg/kg body weight/d improved renal function and reduced the level of proteinuria after 20 d (32). These authors suggested that CoQ10 nephropathy should be considered when mitochondrial abnormalities in podocytes are detected by electron microscopy. In this study, we measured CoQ10 deficiency in plasma because the current experimental model mainly produces renal tubular toxicity (33–35). Tac treatment reduced total CoQ10 level in plasma, but this was restored in the Tac+CoQ10 group. Based on these findings, we suggest that CoQ10 protects against mitochondria damage and renal injury caused by Tac.
Our results indicate that CoQ10 level in the CoQ10 treated group is similar to that in Vh group. We expected that CoQ10 treatment to increase plasma level of CoQ10; however, we did not observe such increase. Possible reasons for this observation can be as follows: First, endogenous plasma and tissue levels of CoQ10 are so high that small changes by exogenous CoQ10 treatment may not be detected. Second, CoQ10 shows poor availability. Thus, administration of CoQ10 in normal rats may not increase plasma CoQ10 levels. Third, we measured total CoQ10 in plasma and kidney using a competitive ELISA kit, which may be less sensitive than HPLC.
The major barrier to the clinical application of CoQ10 is poor bioavailability. A recent study reported that in rats, only 3% of orally administered CoQ10 was absorbed (36). Thus, strategies such as solubility enhancement, drug carriers, and self-emulsifying systems that enhance CoQ10 absorption are needed. Another issue for consideration is the optimal dose of CoQ10. In this study, we used 20 mg/kg for rats based on previous studies with rodents (37, 38), but this is several times higher than the dose recommended for healthy humans (1.2–3.0 mg/kg of CoQ10/d). Therefore, additional studies are needed to establish the clinically effective dose of CoQ10.
In summary, CoQ10 improves mitochondrial function and structure in Tac-induced renal injury. Thus, CoQ10 supplementation may be beneficial in patients prescribed Tac following renal transplantation.
ACKNOWLEDGMENTS
This work was supported by the Korean Health Technology Research and Development Project, Ministry for Health and Welfare (HI14C3417, HI16C1641), and the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Science, Information and Communications Technology (ICT) and Future Planning (NRF-2018R1D1A1A02043014). The authors declare no conflicts of interest.
Glossary
- 3D
3-dimensional
- 4-HHE
4-hydroxy-hexenal
- 8-OHdG
8-hydroxy-2'-deoxyguanosine
- BUN
blood urea nitrogen
- ClCr
creatinine clearance
- CoQ10
coenzyme Q10
- HK-2
human kidney-2 cell lines
- JC-1
5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolcarbocyanine iodide
- MMP
mitochondrial membrane potential
- OCR
oxygen consumption rate
- ROS
reactive oxygen species
- Scr
serum creatinine
- Tac
tacrolimus
- Vh
vehicle
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
J. H. Yu, S. W. Lim, and C. W. Yang designed the research and wrote the report; S. W. Lim, K. Luo, S. Cui, Y. Quan, K. E. Lee, and H. L. Kim conducted the animal experiments; J. H. Yu and S. W. Lim performed the cell culture studies; and E. J. Ko. B. H. Chung, J. H. Kim, and S. J. Chung analyzed the data and edited the manuscript.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Olyaei A. J., de Mattos A. M., Bennett W. M. (2001) Nephrotoxicity of immunosuppressive drugs: new insight and preventive strategies. Curr. Opin. Crit. Care 7, 384–389 [DOI] [PubMed] [Google Scholar]
- 2.Shihab F. S., Bennett W. M., Tanner A. M., Andoh T. F. (1997) Mechanism of fibrosis in experimental tacrolimus nephrotoxicity. Transplantation 64, 1829–1837 [DOI] [PubMed] [Google Scholar]
- 3.Lim S. W., Jin L., Piao S. G., Chung B. H., Yang C. W. (2015) Inhibition of dipeptidyl peptidase IV protects tacrolimus-induced kidney injury. Lab. Invest. 95, 1174–1185 [DOI] [PubMed] [Google Scholar]
- 4.Piao S. G., Lim S. W., Doh K. C., Jin L., Heo S. B., Zheng Y. F., Bae S. K., Chung B. H., Li C., Yang C. W. (2014) Combined treatment of tacrolimus and everolimus increases oxidative stress by pharmacological interactions. Transplantation 98, 22–28 [DOI] [PubMed] [Google Scholar]
- 5.Crane F. L. (2001) Biochemical functions of coenzyme Q10. J. Am. Coll. Nutr. 20, 591–598 [DOI] [PubMed] [Google Scholar]
- 6.Bentinger M., Dallner G., Chojnacki T., Swiezewska E. (2003) Distribution and breakdown of labeled coenzyme Q10 in rat. Free Radic. Biol. Med. 34, 563–575 [DOI] [PubMed] [Google Scholar]
- 7.Spindler M., Beal M. F., Henchcliffe C. (2009) Coenzyme Q10 effects in neurodegenerative disease. Neuropsychiatr. Dis. Treat. 5, 597–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sohet F. M., Neyrinck A. M., Pachikian B. D., de Backer F. C., Bindels L. B., Niklowitz P., Menke T., Cani P. D., Delzenne N. M. (2009) Coenzyme Q10 supplementation lowers hepatic oxidative stress and inflammation associated with diet-induced obesity in mice. Biochem. Pharmacol. 78, 1391–1400 [DOI] [PubMed] [Google Scholar]
- 9.Upaganlawar A., Farswan M., Rathod S., Balaraman R. (2006) Modification of biochemical parameters of gentamicin nephrotoxicity by coenzyme Q10 and green tea in rats. Indian J. Exp. Biol. 44, 416–418 [PubMed] [Google Scholar]
- 10.Chen H., Tappel A. L. (1995) Protection of vitamin E, selenium, trolox C, ascorbic acid palmitate, acetylcysteine, coenzyme Q0, coenzyme Q10, beta-carotene, canthaxanthin, and (+)-catechin against oxidative damage to rat blood and tissues in vivo. Free Radic. Biol. Med. 18, 949–953 [DOI] [PubMed] [Google Scholar]
- 11.Chen H., Tappel A. L. (1995) Vitamin E, selenium, trolox C, ascorbic acid palmitate, acetylcysteine, coenzyme Q, beta-carotene, canthaxanthin, and (+)-catechin protect against oxidative damage to kidney, heart, lung and spleen. Free Radic. Res. 22, 177–186 [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa A., Homma Y. (2012) Beneficial effect of ubiquinol, the reduced form of coenzyme Q10, on cyclosporine nephrotoxicity. Int. Braz J Urol 38, 230–234, discussion 234 [DOI] [PubMed] [Google Scholar]
- 13.Venegoni W., Shen Q., Thimmesch A. R., Bell M., Hiebert J. B., Pierce J. D. (2017) The use of antioxidants in the treatment of traumatic brain injury. J. Adv. Nurs. 73, 1331–1338 [DOI] [PubMed] [Google Scholar]
- 14.Huo J., Xu Z., Hosoe K., Kubo H., Miyahara H., Dai J., Mori M., Sawashita J., Higuchi K. (2018) Coenzyme Q10 prevents senescence and dysfunction caused by oxidative stress in vascular endothelial cells. Oxid. Med. Cell. Longev. 2018, 3181759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dott W., Mistry P., Wright J., Cain K., Herbert K. E. (2014) Modulation of mitochondrial bioenergetics in a skeletal muscle cell line model of mitochondrial toxicity. Redox Biol. 2, 224–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jin J., Jin L., Luo K., Lim S. W., Chung B. H., Yang C. W. (2017) Effect of empagliflozin on tacrolimus-induced pancreas islet dysfunction and renal injury. Am. J. Transplant. 17, 2601–2616 [DOI] [PubMed] [Google Scholar]
- 17.Sun I. O., Jin L., Jin J., Lim S. W., Chung B. H., Yang C. W. (2019) The effects of addition of coenzyme Q10 to metformin on sirolimus-induced diabetes mellitus. Korean J. Intern. Med. 34, 365–374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piao S. G., Bae S. K., Lim S. W., Song J. H., Chung B. H., Choi B. S., Yang C. W. (2012) Drug interaction between cyclosporine and mTOR inhibitors in experimental model of chronic cyclosporine nephrotoxicity and pancreatic islet dysfunction. Transplantation 93, 383–389 [DOI] [PubMed] [Google Scholar]
- 19.Lim S. W., Jin L., Luo K., Jin J., Shin Y. J., Hong S. Y., Yang C. W. (2017) Klotho enhances FoxO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell Death Dis. 8, e2972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hernández-Camacho J. D., Bernier M., López-Lluch G., Navas P. (2018) Coenzyme Q10 supplementation in aging and disease. Front. Physiol. 9, 44 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Acosta M. J., Vazquez Fonseca L., Desbats M. A., Cerqua C., Zordan R., Trevisson E., Salviati L. (2016) Coenzyme Q biosynthesis in health and disease. Biochim. Biophys. Acta 1857, 1079–1085 [DOI] [PubMed] [Google Scholar]
- 22.Chevalier R. L. (2016) The proximal tubule is the primary target of injury and progression of kidney disease: role of the glomerulotubular junction. Am. J. Physiol. Renal Physiol. 311, F145–F161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoon H. E., Yang C. W. (2009) Established and newly proposed mechanisms of chronic cyclosporine nephropathy. Korean J. Intern. Med. (Korean. Assoc. Intern. Med.) 24, 81–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Piao S. G., Kang S. H., Lim S. W., Chung B. H., Doh K. C., Heo S. B., Jin L., Li C., Yang C. W. (2013) Influence of N-acetylcysteine on Klotho expression and its signaling pathway in experimental model of chronic cyclosporine nephropathy in mice. Transplantation 96, 146–153 [DOI] [PubMed] [Google Scholar]
- 25.Doh K. C., Lim S. W., Piao S. G., Jin L., Heo S. B., Zheng Y. F., Bae S. K., Hwang G. H., Min K. I., Chung B. H., Yang C. W. (2013) Ginseng treatment attenuates chronic cyclosporine nephropathy via reducing oxidative stress in an experimental mouse model. Am. J. Nephrol. 37, 421–433 [DOI] [PubMed] [Google Scholar]
- 26.Back J. H., Ryu H. H., Hong R., Han S. A., Yoon Y. M., Kim D. H., Hong S. J., Kim H. L., Chung J. H., Shin B. C., Kwon Y. E. (2015) Antiproteinuric effects of green tea extract on tacrolimus-induced nephrotoxicity in mice. Transplant. Proc. 47, 2032–2034 [DOI] [PubMed] [Google Scholar]
- 27.Fouad A. A., Al-Sultan A. I., Refaie S. M., Yacoubi M. T. (2010) Coenzyme Q10 treatment ameliorates acute cisplatin nephrotoxicity in mice. Toxicology 274, 49–56 [DOI] [PubMed] [Google Scholar]
- 28.Sato T., Ishikawa A., Homma Y. (2013) Effect of reduced form of coenzyme Q10 on cyclosporine nephrotoxicity. Exp. Clin. Transplant. 11, 17–20 [DOI] [PubMed] [Google Scholar]
- 29.Carrasco J., Anglada F. J., Campos J. P., Muntané J., Requena M. J., Padillo J. (2014) The protective role of coenzyme Q10 in renal injury associated with extracorporeal shockwave lithotripsy: a randomised, placebo-controlled clinical trial. BJU Int. 113, 942–950 [DOI] [PubMed] [Google Scholar]
- 30.Archer S. L. (2013) Mitochondrial dynamics--mitochondrial fission and fusion in human diseases. N. Engl. J. Med. 369, 2236–2251 [DOI] [PubMed] [Google Scholar]
- 31.Zorzano A., Sebastián D., Segalés J., Palacín M. (2009) The molecular machinery of mitochondrial fusion and fission: an opportunity for drug discovery? Curr. Opin. Drug Discov. Devel. 12, 597–606 [PubMed] [Google Scholar]
- 32.Montini G., Malaventura C., Salviati L. (2008) Early coenzyme Q10 supplementation in primary coenzyme Q10 deficiency. N. Engl. J. Med. 358, 2849–2850 [DOI] [PubMed] [Google Scholar]
- 33.Andoh T. F., Burdmann E. A., Lindsley J., Houghton D. C., Bennett W. M. (1995) Functional and structural characteristics of experimental FK 506 nephrotoxicity. Clin. Exp. Pharmacol. Physiol. 22, 646–654 [DOI] [PubMed] [Google Scholar]
- 34.Andoh T. F., Burdmann E. A., Lindsley J., Houghton D. C., Bennett W. M. (1994) Enhancement of FK506 nephrotoxicity by sodium depletion in an experimental rat model. Transplantation 57, 483–489 [PubMed] [Google Scholar]
- 35.Stillman I. E., Andoh T. F., Burdmann E. A., Bennett W. M., Rosen S. (1995) FK506 nephrotoxicity: morphologic and physiologic characterization of a rat model. Lab. Invest. 73, 794–803 [PubMed] [Google Scholar]
- 36.Bhagavan H. N., Chopra R. K. (2007) Plasma coenzyme Q10 response to oral ingestion of coenzyme Q10 formulations. Mitochondrion 7 (Suppl), S78–S88 [DOI] [PubMed] [Google Scholar]
- 37.Modi K., Santani D. D., Goyal R. K., Bhatt P. A. (2006) Effect of coenzyme Q10 on catalase activity and other antioxidant parameters in streptozotocin-induced diabetic rats. Biol. Trace Elem. Res. 109, 25–34 [DOI] [PubMed] [Google Scholar]
- 38.Moreira P. I., Santos M. S., Sena C., Nunes E., Seiça R., Oliveira C. R. (2005) CoQ10 therapy attenuates amyloid beta-peptide toxicity in brain mitochondria isolated from aged diabetic rats. Exp. Neurol. 196, 112–119 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.