Abstract
The protection of constantly proliferating gut epithelia and hematopoietic tissues from cytotoxicity could improve conventional chemotherapy efficacy and widen its therapeutic window. Previously, we reported that, in mouse models, pretreatment of recombinant human IL-1 receptor antagonist (rhIL-1Ra) protected both types of vulnerable tissues from chemotherapeutics. Here, we showed that rhIL-1Ra treatment up-regulated the protein levels of phosphorylated p38, p53, and p21 and induced transient hematopoietic stem/progenitor cell (HS/PC) quiescence. Knockout of IL-1 receptor I (IL-1RI), p53, or p21 alleles and pharmacological inactivation of p38 mapped the rhIL-1Ra pathway in the induction of HS/PC quiescence. Therefore, rhIL-1Ra administration before but not after chemotherapy alleviated 5-fluorouracil–induced neutropenia. In addition, in vivo and in vitro cell proliferation assays revealed that the rhIL-1Ra treatment did not affect cancer cell proliferation or chemosensitivity. Lastly, we propose an IL-1/IL-1Ra pathway (IL-1RI → p38 → p53 → p21), which regulates HS/PC quiescence. The rhIL-1Ra may provide a new route for p53-based cyclotherapy, which spares normal cells but kills cancer cells during chemotherapy.—Ye, H., Qian, L., Zhu, S., Deng, S., Wang, X., Zhu, J., Chan, G. L., Yu, Y., Han, W. IL-1Ra protects hematopoietic cells from chemotoxicity through p53-induced quiescence.
Keywords: rhIL-1Ra, chemotherapy, neutropenia, cell cycle arrest, hematopoietic progenitor cell
Chemotherapeutic targets occur mainly in both normal and cancer cells. Cytotoxicity to normal cells is responsible for the major dose-limiting side effects of chemotherapy. Hematopoietic and intestinal epithelial cells are constantly regenerating and thus are more sensitive to chemotoxicity than other tissues. Inducing transient cell cycle arrest in hematopoietic and intestinal epithelial cells but not in tumor cells may reduce side effects without loss of chemotherapy efficacy. This approach is known as cyclotherapy (1) and was first proposed in 1992 (2). It is a promising effort to improve cancer treatment efficacy (3).
We previously reported mouse chemotherapy models in which recombinant human IL-1 receptor antagonist (rhIL-1Ra) prevented intestinal mucositis by inducing cell cycle arrest in cryptic epithelia but not in tumor cells (4). IL-1Ra is a natural IL-1 antagonist that uses IL-1 receptor I (IL-1RI) as a signaling receptor (5). Blocking the IL-1 pathway up-regulated the expression of cyclin-dependent kinase (CDK) inhibitors p21 and p27 by activating p53 (4, 6), which is the most frequently muted product of the tumor suppressor gene tumor protein p53(TP53) (7).
Neutropenia is another common side effect of chemotherapy. We previously showed in mouse chemotherapy models that rhIL-1Ra successfully alleviated neutropenia (8, 9). However, the mechanism by which rhIL-1Ra protects hematopoietic bone marrow (BM) cells from chemotherapy toxicity is unknown. Moreover, its role in leukemia progression within the context of cyclotherapy is also unclear.
Here, we reported that rhIL-1Ra up-regulated p53 and its downstream target p21 by activating p38 MAPK to induce cell cycle arrest in hematopoietic stem/progenitor cells (HS/PCs). rhIL-1Ra specifically protected BM hematopoietic cells but not leukemic cells from chemotoxicity. Our findings suggest that rhIL-1Ra may serve as a potent cyclotherapy agent in preventing chemotherapeutic BM damage. The components of the IL-1Ra signaling pathway that induce HS/PC quiescence are potential targets for the identification of other cyclotherapy drugs.
MATERIALS AND METHODS
Animals, cell lines, and rhIL-1Ra
Wild-type (WT) C57BL/6 and BALB/c mice (male, 8-wk-old) were purchased from Shanghai Laboratory Animal Center (SLAC; Shanghai, China). FVB/NJ mice were purchased from Vital River Laboratory Animal Technology (Beijing, China). The IL-1RI, p53, and p21 knockout mice in C57BL/6 background were acquired as previously described by Wang et al. (4). All mice were housed in a ventilated, pathogen-free caging system. All animal experiments were performed under the authorization of the Shanghai Jiao Tong University Animal Ethics Committee.
CT26.WT, K-562, and HL-60 cells were obtained from American Type Culture Collection (ATCC; Manassas, VA, USA) and cultured in Roswell Park Memorial Institute (RPMI) 1640 medium (Thermo Fisher Scientific, Waltham, MA, USA) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin. The human myeloid-related protein 8-promyelocytic leukemia/retinoic acid receptor-α (hMRP8-PML/RAR-α)–harboring acute promyelocytic leukemia (APL) cells stably expressing green fluorescent protein (GFP) (10) were kindly provided by Prof. Jiang Zhu (Rui-jin Hospital, Shanghai Jiaotong University, Shanghai, China) and passaged in vivo among syngeneic FVB/NJ mice.
The rhIL-1Ra protein was a Good Manufacturing Practice product approved for clinical trials and procured from General Regeneratives Ltd. (Shanghai, China).
In vivo p38 MAPK inhibition
All BALB/c mice were divided into rhIL-1Ra, rhIL-1Ra + SB203580, and saline treatment groups. The rhIL-1Ra (2 mg/kg) was subcutaneously injected daily for 5 d. Meanwhile, SB203580 (25 mg/kg; MilliporeSigma, Burlington, MA, USA), a specific inhibitor of p38 MAPK, was intraperitoneally injected daily for 5 d.
Establishment of a murine colon cancer allograft model
The tumor model was established by subcutaneous injection of the murine tumor cells CT26.WT as previously described by Zhang et al. (11). Tumor growth was measured by vernier caliper every other day. Tumor volume (mm3) = (length × width2) / 2. Experiments were initiated when the tumor volumes were 300–500 mm3.
Western blot analysis
Total murine BM cells (BMCs) were harvested at the indicated time points and incubated with lysate buffers containing proteinase and phosphatase inhibitors for protein extraction (Roche, Basel, Switzerland). Protein extracts from whole-cell lysates were separated on polyacrylamide gel as previously described (9). The primary antibodies were anti–IL-1Ra and anti–IL-1β (Abcam, Cambridge, United Kingdom); anti-p53, anti–phosphorylated p38, and anti-p38 (Cell Signaling Technology, Danvers, MA, USA); anti-p21 (BD Biosciences, San Jose, CA, USA); and anti–β-actin (Cell Signaling Technology). After incubation with the corresponding secondary antibodies, their conjugated horseradish peroxidase activity was detected using the ECL detection system (Thermo Fisher Scientific). The protein bands were analyzed with ImageJ (National Institutes of Health, Bethesda, MD, USA).
Peripheral blood cell and BMC analysis
Retro-orbital blood samples were obtained from mice for the blood cell count. White blood cells (WBCs) were counted with the Hematology Analyzer MEK-6318K (Nihon Kohden, Tokyo, Japan) according to the manufacturer’s instructions. Differential WBC, BMC, and total BM nucleated cells were counted as previously described (9).
Cell isolation and flow cytometry
Murine BMCs were isolated by flushing femurs and tibias with ice-cold 1640 medium containing 2% fetal bovine serum (Thermo Fisher Scientific). The total cell number was determined by manual counting with a hemocytometer. The BMCs were used for surface staining after red blood cell lysis. All antibodies and their matched isotype controls were acquired from BD Biosciences and Thermo Fisher Scientific. To identify Lin−Sca-1+c-Kit+ (LSK) cells by flow cytometry, BMCs were stained with antibodies against c-Kit (2B8)-phycoerythrin (PE) or -peridinin chlorophyll protein (PerCP)-eFluor 710 and stem cell antigen-1 (Sca-1) (D7)-Brilliant Blue 515 (BB515) or -PE and with an allophycocyanin (APC)-conjugated antibody cocktail against the following lineage markers: CD3e (145-2C11), CD11b (M1/70), B220 (RA3-6B2), Ter-119 (TER-119), and granulocyte-differentiation antigen (Gr-1) (RB6-8C5). To identify mature myeloid cells, BMCs were stained with an antibody against Gr-1-PE. To determine IL-1RI surface protein expression, BMCs were stained with an antibody against CD121a-PE. For the cell cycle analysis, BMCs were fixed, permeabilized, and stained with propidium iodide (MilliporeSigma). For Ki67 and Hoechst 33342 staining, BMCs were fixed, permeabilized, and stained with PE-cyanine 7–conjugated anti-Ki67 antibody and Hoechst 33342 (10 µg/ml; MilliporeSigma). Flow cytometry was performed with the LSRFortessa flow cytometer and FACSCalibur with Cell Quest (BD Biosciences). FlowJo v.7.6 (Treestar, Ashland, OR, USA) and Modfit LT v.3.1 (Verity Software House, Topsham, ME, USA) were used to analyze the data. The gating strategy for the flow cytometry analysis is shown in Supplemental Fig. S1.
Bromodeoxyuridine incorporation assay
For the bromodeoxyuridine (BrdU) incorporation assay, mice were intraperitoneally injected with BrdU (100 mg/kg; MilliporeSigma) before euthanasia (12, 13). The BrdU Staining Kit (Thermo Fisher Scientific) was used according to the manufacturer’s instructions.
In vitro cell proliferation assay
The in vitro cell proliferation assay was performed with the Cell Counting Kit-8 (Dojindo, Kumamoto, Japan) according to the manufacturer’s instructions. K-562 or HL-60 cells were seeded on a 96-well plate at a density of 5000 cells per well. After 24 h, the cells were incubated with 0.1–100 µg/ml rhIL-1Ra for 48 h. The Cell Counting Kit-8 solution was added and measured as described in the manufacturer’s instructions.
For the 5-fluorouracil (5-FU) treatment, cells were seeded as previously described. After 24 h, the cells were incubated with 0.1–100 µg/ml rhIL-1Ra for 24 h. The 5-FU (50 µg/ml; Xudong Haipu Pharmaceutical, Shanghai, China) was added and incubated for another 48 h. Cell viability was determined as previously described.
APL animal model
About 1 × 106 APL cells stably expressing GFP were intravenously injected into FVB/NJ mice. Then 20 µl retro-orbital blood was obtained from each mouse. After red blood cells lysis, the percentage of GFP+ cells in the mouse peripheral blood mononuclear cells was determined by flow cytometry at the indicated time points.
Statistical analysis
Data are means ± sd or means ± sem, as indicated in the figure legends, where appropriate. For the comparison between 2 groups in the Western blot experiments, flow cytometry and cell proliferation analysis, and determination of absolute neutrophil count (ANC) and BMC numbers, 2-tailed Student’s t tests were performed. The chemotherapy-induced neutropenia (CIN) incidences were compared by nonparametric Mann-Whitney U tests between 2 groups. Tumor volume between groups was tested using 1-way ANOVA. Survival analysis was determined by a log-rank test. Correlations were calculated by Pearson or Spearman correlation analysis, and the corresponding coefficients (r) were determined, which are shown in the figure legends, where appropriate. Values of P < 0.05 were considered statistically significant. Data were processed in SPSS v.18.0 (IBM SPSS, Chicago, IL, USA) or Prism 5 software (GraphPad Software, La Jolla, USA).
RESULTS
BM expression of IL-1β, IL-1Ra, and IL-1RI was associated with 5-FU–induced BM injury and postinjury recovery
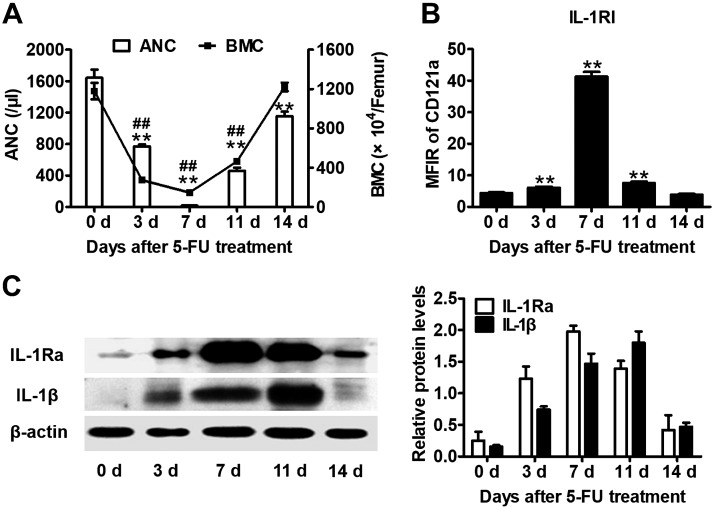
IL-1Ra competes with IL-1α and IL-1β for IL-1RI as an IL-1 signaling antagonist (5). Previously, we demonstrated that IL-1 and IL-1Ra are endogenous cytokines that participate in BM damage and postchemotherapy regeneration (8, 9). We characterized the entire hematopoietic process in a 5-FU chemotherapy mouse model. As shown in Fig. 1A, 5-FU induced a rapid decline in ANC and BM cellularity, both of which reached nadirs at d 7 and recovered by d 14 (Fig. 1A). On the other hand, IL-1RI and IL-1Ra protein expression reached their maximum levels by d 7 (Fig. 1B, C), whereas IL-1β expression was at its highest level by d 11 (Fig. 1C). Therefore, BM expression of IL-1 signaling molecules is negatively correlated with BM cellularity. This association indicates the potential roles of these molecules in postchemotherapy hematopoiesis.
Figure 1.
BM expression of IL-1β, IL-1Ra, and IL-1RI was associated with 5-FU–induced BM injury and postinjury recovery. Peripheral blood cells and BMCs from mice treated with 5-FU (100 mg/kg) were analyzed at the indicated time points. A) The bar graph indicates the mean ± sem of the ANC. The solid line represents the mean ± sem of the BMC count. **P < 0.01, ANC vs. baseline at d 0; ##P < 0.01, BMC vs. baseline at d 0 (n = 6). B) IL-1RI expression was determined by CD121a analysis in BMCs with flow cytometry. The bar graph indicates the mean ± sem of the mean fluorescence intensity ratio (MFIR) of CD121a to isotype control. **P < 0.01 vs. baseline at d 0 (n = 6). C) Western blot analysis of IL-1Ra and IL-1β proteins in BMCs at the indicated time points after 5-FU treatment. The bar graph on the right indicates the relative expression of IL-1Ra and IL-1β (n = 3/time point). The signal density was quantified after normalization to an internal β-actin signal.
The rhIL-1Ra alleviated neutropenia by protecting hematopoietic cells from 5-FU cytotoxicity
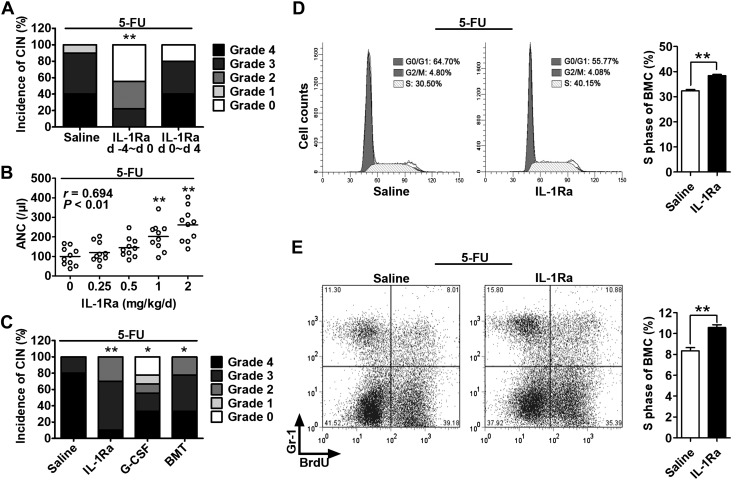
We examined the role of IL-1 signaling in CIN, which is a rapid index of BM damage. The rhIL-1Ra administration in normal mice before (−4 to 0 d) but not after (0–4 d) 5-FU treatment raised the ANC nadir from 206 ± 34 to 566 ± 119 (P < 0.01), decreased CIN morbidity from 100 to 56% (P < 0.01; Fig. 2A), and reduced CIN severity grades 3 + 4 from 90 to 22% (P < 0.01; Fig. 2A). The rhIL-1Ra pretreatment elevated the ANC nadirs in a dose-dependent manner (P < 0.01; Fig. 2B). In CT26 tumor-bearing mice, a syngeneic mouse transplantation model of colon cancer (11), the CIN preventive effect of rhIL-1Ra was comparable with that obtained after prophylactic administration of granulocyte colony-stimulating factor and BM transplantation (Fig. 2C). At d 7 after 5-FU, the percentage of BMCs in the S phase of the cell cycle and the number of newly produced granulocytes from the BM were significantly higher in the rhIL-1Ra pretreatment group than those in the saline control (P < 0.01; Fig. 2D, E). Taken together, our data suggest that rhIL-1Ra protects hematopoietic cells from 5-FU cytotoxicity. The cells in reserve account for the accelerated BM granulocyte production.
Figure 2.
rhIL-1Ra alleviated neutropenia by protecting hematopoietic cells from 5-FU cytotoxicity. A–C) Mice received 5-FU (100 mg/kg) at d 0. The ANC per microliter at d 7 after 5-FU (nadir point) was graded according to the Clinical Criteria for Adverse Events. CIN severity was rated as follows: 0, ≥600; 1, 450–600; 2, 300–450; 3, 150–300; 4, <150. A) Mice were treated with saline or rhIL-1Ra (2 mg/kg) daily for 5 d either before (−4 to 0 d) or after (0–4 d) 5-FU administration. CIN incidence and severity were plotted. **P < 0.01 (n = 9–10). B) The CT26 tumor-bearing mice received rhIL-1Ra before 5-FU. The correlation between ANC and rhIL-1Ra dose was plotted. Pearson correlation coefficient r = 0.694; P < 0.01 (n = 9–10). C) The CT26 tumor-bearing mice were treated with rhIL-1Ra (2 mg/kg/d; −4 to 0 d), recombinant human granulocyte colony-stimulating factor (G-CSF) (1 μg/d; 3–7 d), or BM transplantation (BMT) (4 × 106 BMCs; d 1). All mice received 5-FU at d 0. CIN incidence and severity were plotted. *P < 0.05, **P < 0.01 (n = 9–10). D) BMC cell cycle analysis at d 7 after 5-FU. The cells were analyzed by flow cytometry after propidium iodide staining. The bar graph on the right indicates the mean percentage ± sem of the BMCs in S phase in the saline or rhIL-1Ra pretreatment group. **P < 0.01 (n = 5). E) BrdU incorporation assay for newly BM-produced granulocytes. After in vivo BrdU labeling for 3 h on d 7 after 5-FU treatment, the BrdU-labeled BMCs were stained with Gr-1 antibody and analyzed by flow cytometry. The bar graph on the right indicates the mean percentage ± sem of BrdU+Gr-1+ cells in mouse BM derived from the saline or rhIL-1Ra pretreatment group. **P < 0.01 (n = 6).
The rhIL-1Ra suppressed the steady-state granulopoiesis
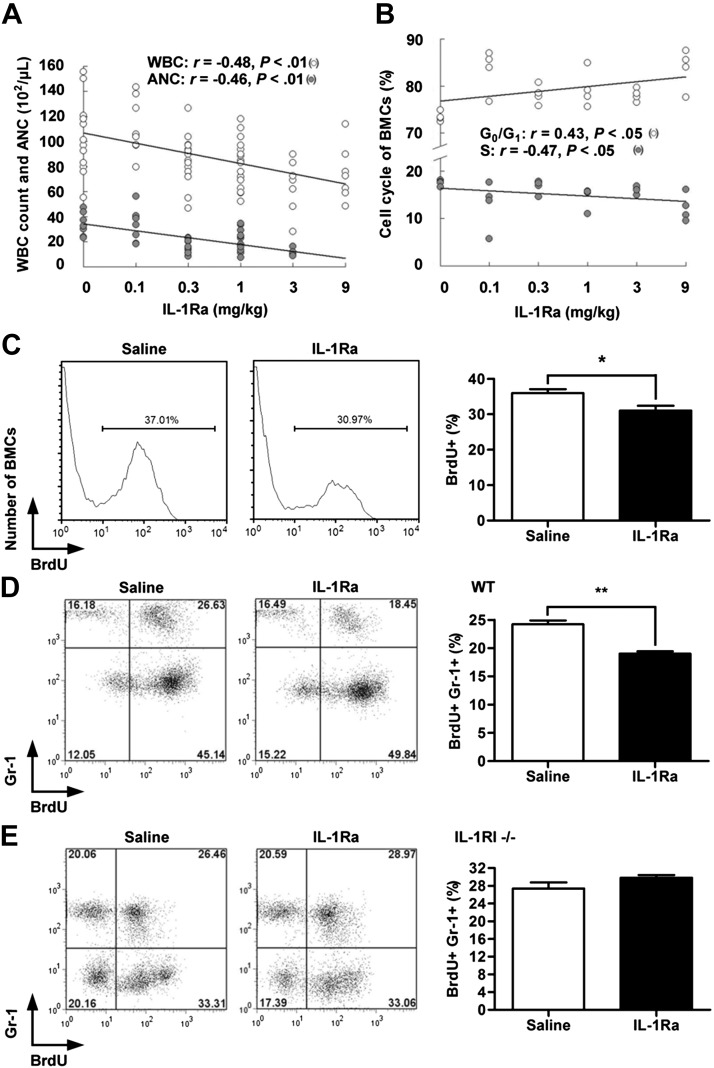
The uracil analog 5-FU has S phase–specific toxicity. Reduction of the toxicity of 5-FU to BM by rhIL-1Ra suggests that the latter inhibits BM hematopoiesis. Injection of rhIL-1Ra into mice decreased their WBC and neutrophil counts. There was a negative correlation between the rhIL-1Ra doses and the numbers of WBCs and neutrophils (P < 0.01; Fig. 3A) and the percentages of BMCs in the S phase of the cell cycle (P < 0.05; Fig. 3B). Furthermore, the percentages of proliferating BM (BrdU+) and newly produced granulocytes (BrdU+Gr-1+) decreased by 14% (P < 0.05; Fig. 3C) and 22% (P < 0.01; Fig. 3D), respectively, in mice treated with rhIL-1Ra. No reduction in granulopoiesis was observed in IL-1RI−/− mice (P > 0.05; Fig. 3E). Our data demonstrate that rhIL-1Ra inhibited steady-state granulopoiesis via IL-1RI. This finding supports the myeloid protective role of rhIL-1Ra in 5-FU chemotherapy.
Figure 3.
rhIL-1Ra suppressed steady-state BM granulopoiesis. A, B) Blood differential counts (A) and BMC cycle analysis (B) in mice treated daily with rhIL-1Ra or saline for 5 d. A) The correlations between rhIL-1Ra dosage and WBC or ANC were plotted (r, Pearson correlation coefficient); P < 0.01 (n = 7–12). B) The correlations between rhIL-1Ra dosage and percentages of BMCs in the G0/G1 or S phase were plotted (r, Spearman correlation coefficient); P < 0.05 (n = 4). C) Mice received rhIL-1Ra (1 mg/kg/d) or saline for 5 d followed by BrdU injection (100 mg/kg). BMCs isolated 3 h later were analyzed by flow cytometry. The bar graph on the right indicates the mean percentages ± sem) of BrdU+ BMCs. *P < 0.05 (n = 6). D) Mice received rhIL-1Ra (1 mg/kg/d) or saline for 5 d and BrdU injection (100 mg/kg) before euthanasia. BMCs were analyzed for Gr-1 and BrdU by flow cytometry. The bar graph on the right indicates the mean percentage ± sem of BrdU+Gr-1+ cells. **P < 0.01 (n = 5). E) A similar approach was used in IL-1RI−/− mice; P > 0.05 (n = 5) (D).
The rhIL-1Ra induced transient and reversible HS/PC quiescence in vivo
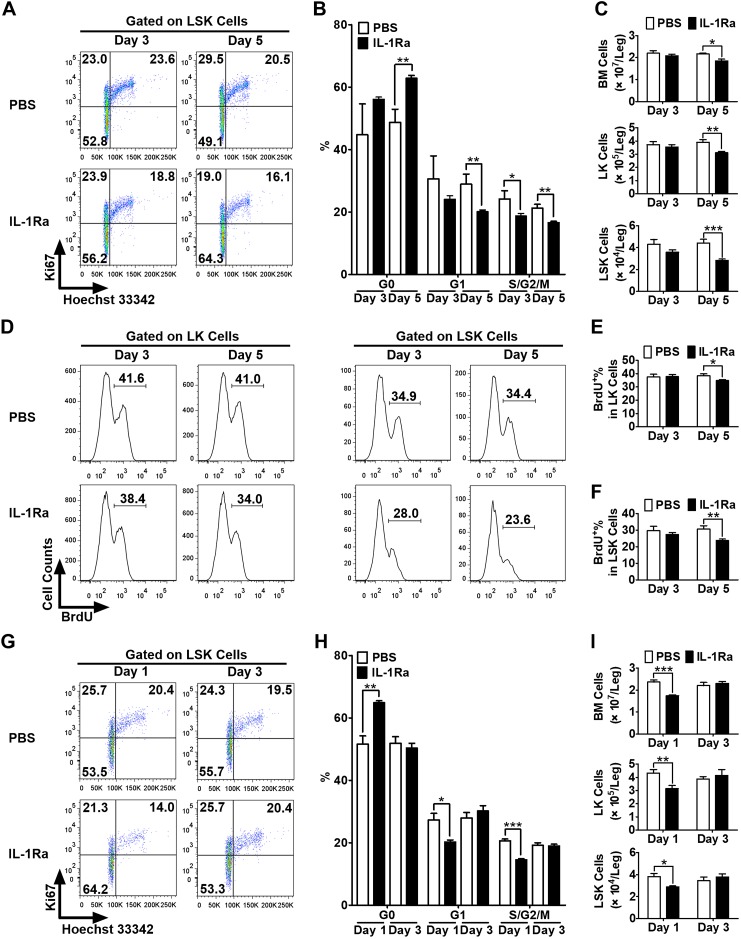
IL-1 stimulates hematopoietic cell proliferation synergistically with hematopoietic growth factor (14). We demonstrated that rhIL-1Ra suppressed steady-state BM granulopoiesis (Fig. 3). To determine whether rhIL-1Ra induces HS/PC quiescence, BALB/c mice received daily subcutaneous injections of rhIL-1Ra (3 mg/kg) or PBS for 3 or 5 d. After the 3- and 5-d treatments, HS/PC proliferation (LSK cells) and the proportion of S-, G2-, and M-phase cells markedly decreased (P < 0.05, P < 0.01, respectively), unlike in PBS-treated mice (Fig. 4A, B). After the 5-d treatment, a significant increase in the G0 phase (P < 0.01) and a significant decrease in the G1 phase (P < 0.01; Fig. 4A, B) of LSK cells were observed. Therefore, the total number of BM, Lin−Sca-1−c-Kit+ (LK) cells (myeloid progenitor cells), and LSK cells significantly decreased after 5 d of rhIL-1Ra treatment (Fig. 4C). Consistent with these findings, marked reductions in the proportions of LK and LSK cells in the S phase after 5 d of rhIL-1Ra treatment were evident, according to BrdU labeling (Fig. 4D–F). Our data suggest that the induction of HS/PC quiescence by rhIL-1Ra reduces the proliferation rate of hematopoietic cells and, therefore, their sensitivity to 5-FU cytotoxicity.
Figure 4.
rhIL-1Ra induced transient and reversible HS/PC quiescence in vivo. A–C) BALB/c mice received daily injections of either PBS or IL-1Ra (3 mg/kg) over 5 d. BMCs were harvested and analyzed at d 3 and 5. Levels of Ki67 and Hoechst 33342 staining were measured in LSK cells by flow cytometry. Representative dot plots (A) and mean percentages (B) of the LSK cells in G0, G1, and S/G2/M phases and the numbers of total BM, LK, and LSK cells per leg (C) are shown. D–F) The mice were treated with PBS or rhIL-1Ra as above and injected with BrdU (100 mg/kg) before euthanasia. BrdU incorporation was measured in LK and LSK cells by flow cytometry. Representative histograms (D) and mean percentages (E, F) of BrdU+ LK (E) and LSK (F) cells are shown. G–I) BALB/c mice received daily injections of either PBS or IL-1Ra (3 mg/kg) for 5 d. BMCs were harvested and analyzed at d 1 and 3 after the final injections (1 or 3 d recovery). Levels of Ki67 and Hoechst 33342 staining were measured in the LSK cells by flow cytometry. Representative dot plots (G) and mean percentages (H) of the LSK cells in G0, G1, and S/G2/M phases and the numbers of total BM, LK, and LSK cells per leg (I) are shown. All data are expressed as means ± sem (n = 3–6 mice/group at each time point). Statistical significance of the differences between groups was assessed with Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
The cell cycle arrest induced by IL-1Ra in HS/PCs was transient and reversible. In LSK cells, significant reductions in the G1 and S, G2, and M phases and an increase in the G0 phase were observed on the first day after the final rhIL-1Ra injection in the 5-d treatment schedule (Fig. 4G, H). However, the cell cycle status of the LSK cells and the total number of BMCs, myeloid progenitor cells, and HS/PCs returned to their normal levels 3 d after the final rhIL-1Ra injection (Fig. 4G–I). Therefore, transient and reversible BM suppression by rhIL-1Ra is essential for postchemotherapy BM recovery.
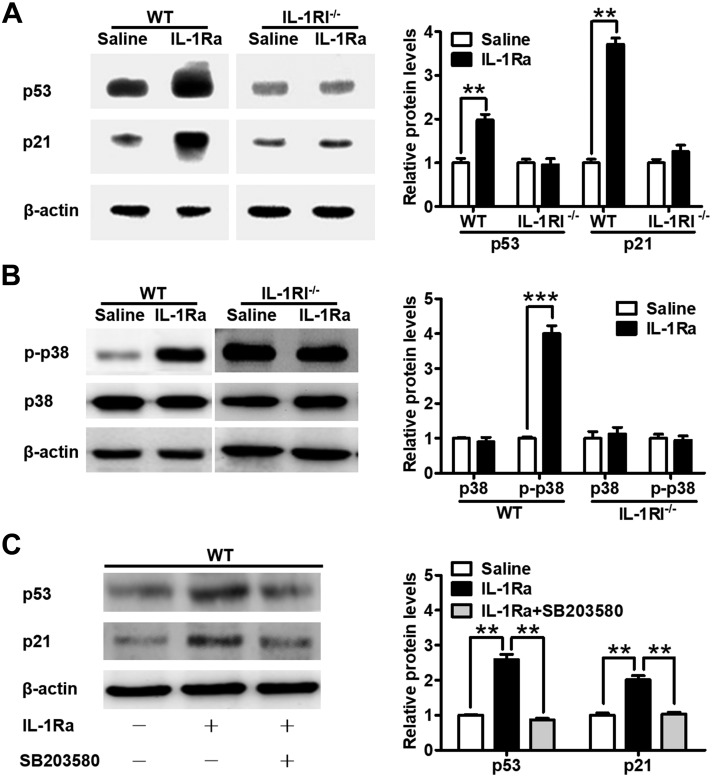
The rhIL-1Ra increased p53 and p21 protein levels by activating p38 MAPK in mouse BM
The p21 protein and its direct upstream transcriptional activator p53 are key cell cycle negative regulators of LSK-marked HS/PCs (15–17). Furthermore, p38 MAPK mediates IL-1 signaling in innate immunity and inflammation (5) and regulates cell cycle and p53 activity (18).
We examined the protein levels of p53 and p21 and their potential upstream activator p38 MAPK in BMCs isolated from mice treated with rhIL-1Ra. rhIL-1Ra significantly up-regulated p53, p21, and the phosphorylated form of p38 levels in WT mice but not in IL-1RI−/− mice (Fig. 5A, B). The level of phosphorylated p38 was increased in IL-1RI−/− mice when compared with WT mice (Fig. 5B). Inhibition of p38 activation with SB203580 completely abolished p53 and p21 up-regulation by rhIL-1Ra (Fig. 5C). Therefore, we identified for the first time that, via IL-1RI, rhIL-1Ra up-regulated p53, p21, and phosphorylated p38 protein levels in BMCs and that p38 MAPK activation was required for p53 and p21 up-regulation.
Figure 5.
rhIL-1Ra increased p53 and p21 protein levels by activating p38 MAPK in mouse BM. A) Western blot analysis of p53 and p21 in BM of WT and IL-1RI−/− mice after daily saline or rhIL-1Ra treatments for 5 d. The bar graph on the right indicates the means ± sd of the p53 and p21 levels. β-Actin was used as the housekeeping gene. The protein expression values were normalized to the saline control. **P < 0.01 (n = 3). B) Western blot analysis of phosphorylated p38 (p-p38) and p38 in BM of WT and IL-1RI−/−mice treated as above. β-Actin was used as the housekeeping gene. The protein expression values were normalized to the saline control. Data are means ± sd. ***P < 0.001 (n = 3). C) Western blot analysis of p53 and p21 in BM of WT mice treated daily with rhIL-1Ra (3 mg/kg) and the p38 inhibitor SB203580 (25 mg/kg) for 5 d. β-Actin was used as the housekeeping gene. The protein expression values were normalized to the saline controls. Results are means ± sd. **P < 0.01 (n = 3).
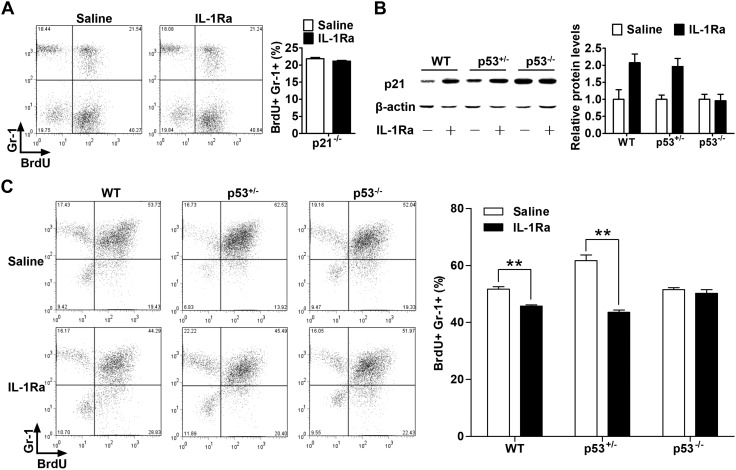
The rhIL-1Ra induced hematopoietic cell quiescence through p53-mediated up-regulation of p21
After demonstrating the associations among rhIL-1Ra-induced increases in p53 and p21 levels, cell cycle arrest, and decreased neutrophil production, we tested whether these relationships were causative by using p21−/− and p53−/− mice. The p21−/− mice were administered rhIL-1Ra, and their granulocyte proliferation levels were measured by a BrdU incorporation assay (12, 13). In contrast to the rhIL-1Ra-induced granulocyte reduction seen in WT mice (P < 0.01; Fig. 3D), p21−/− mice administered rhIL-1Ra did not show any significant change compared with the control mice, which received saline solution (P > 0.05; Fig. 6A). Because p21 keeps hematopoietic stem cells quiescent (17), the data show a causative association of p21 up-regulation, cell cycle arrest, and a decrease in blood cell production by rhIL-1Ra.
Figure 6.
rhIL-1Ra induced hematopoietic cell quiescence through p53-mediated up-regulation of p21. A) p21−/− mice received saline or rhIL-1Ra (2 mg/kg) daily for 5 d. BrdU (100 mg/kg) was injected daily for the last 2 d. BMCs were isolated 24 h after the final BrdU injection and analyzed by flow cytometry. Data are mean percentages ± sem (n = 5). B) Western blot analysis (left) and quantification (right) of p21 in BMCs extracts from WT, p53+/−, or p53−/− mice daily receiving rhIL-1Ra injections for 5 d. Data are means ± sd (n = 3). C) WT, p53+/−, or p53−/− mice were treated, labeled, and analyzed as in A. Data are mean percentages ± sem. **P < 0.01 (n = 4).
It is known that p53 is the major upstream activator of p21 (19, 20). To establish whether p53 is upstream of p21 and required for rhIL-1Ra-induced quiescence, p53−/− mice were analyzed after rhIL-1Ra treatment. Unlike in WT mice, p21 induction by rhIL-1Ra in p53−/− mice was not observed. Moreover, a single-allele deletion of the p53 gene appeared not to affect p21 induction (Fig. 6B). Similarly, the rhIL-1Ra-mediated granulocyte reduction seen in WT mice (P < 0.01) was absent in the p53 knockout mice (P > 0.05; Fig. 6C). Therefore, our data confirm that p53 is necessary for rhIL-1Ra-mediated p21 up-regulation and, by extension, the induction of hematopoietic cell quiescence.
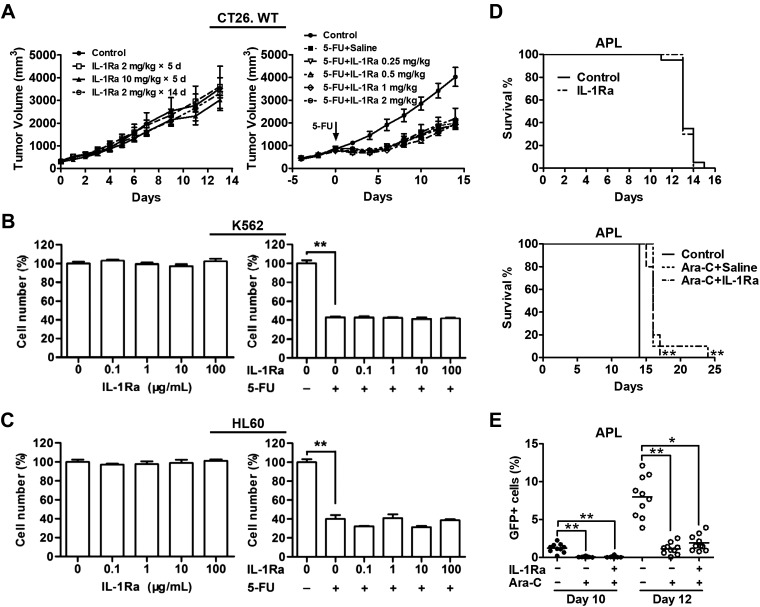
The rhIL-1Ra did not protect cancer cells from chemotoxicity
Our results demonstrate that rhIL-1Ra alleviated CIN by activating the p38-p53-p21 pathway which, in turn, induced HS/PC quiescence. In order for rhIL-1Ra to be efficacious in cyclotherapy (1, 2, 21), it should not protect tumor cells from chemotoxicity.
The growth and chemosensitivity of cancer cell lines in culture and tumor-bearing mice were evaluated in the presence of high rhIL-1Ra doses. In CT26 tumor-bearing mice (11), rhIL-1Ra had no effect on colon cancer cell growth or 5-FU sensitivity (Fig. 7A), but it alleviated neutropenia (Fig. 3C). Because rhIL-1Ra protected normal BMCs, we also examined its effects on leukemia. No apparent effects of rhIL-1Ra were detected on the proliferation or 5-FU sensitivity of the erythroleukemia line K-562 derived from patients with chronic myelogenous leukemia (22) or the neutrophilic promyelocyte line HL-60 derived from patients with APL (23) (Fig. 7B, C).
Figure 7.
rhIL-1Ra did not protect cancer cells from chemotoxicity. A) Left: the CT26 tumor-bearing mice received either rhIL-1Ra as indicated or saline in the control group starting on d 0. Data are means ± sem of tumor volumes measured at the indicated time points; P > 0.05 by 1-way ANOVA among 4 groups (n = 7–8). Right: the CT26 tumor-bearing mice received either rhIL-1Ra or saline in the control group daily for 5 d followed by 5-FU (100 mg/kg) treatment in the IL-1Ra groups at d 0. Data are means ± sem of tumor volumes measured at the indicated time points; P > 0.05 by 1-way ANOVA among the 5 5-FU-treated groups (n = 10). B, C) Two human myeloid leukemia cell lines K-562 (B) and HL-60 (C) were cultured with increasing rhIL-1Ra concentrations (left) and with or without 5-FU (right) as indicated. Data are means ± sem of the percentages of cell numbers without rhIL-1Ra (left) or without rhIL-1Ra and 5-FU (right). Experiments were independently repeated 3 times. **P < 0.01 (n = 3). D) Top: after intravenous injection of murine leukemia APL-GFP+ cells, syngeneic FVB/NJ mice received either saline or rhIL-1Ra (2 mg/kg) daily for 13 d. Animal survival curves were plotted; P > 0.05 by log-rank test (n = 20). D) Bottom: after intravenous injection of APL-GFP+ cells, FVB/NJ mice received either saline or rhIL-1Ra (2 mg/kg) for 5 d followed by Ara-C (400 mg/kg). Animal survival curves were plotted. **P < 0.01 vs. control; P > 0.05 between 2 Ara-C arms by log-rank test (n = 10). E) Mice were treated as in D (bottom). APL-GFP+ cells in PB at d 10 and 12 after leukemic cell transplantation were measured by flow cytometry. Data are means ± sem of percentages of GFP+ cells in PB. *P < 0.05, **P < 0.01 by 1-way ANOVA (n = 10).
We established a syngeneic mouse leukemia model by transplanting a murine APL cell line carrying a GFP reporter (10). The rhIL-1Ra treatment had no effect on tumor-associated mouse mortality (Fig. 7D, top), the efficacy of cytarabine arabinoside (Ara-C) in prolonging mouse life span (Fig. 7D, bottom), or on the tumor killing by Ara-C (Fig. 7E). Therefore, our data demonstrate that rhIL-1Ra has no effect on tumor growth or sensitivity to cytotoxic drugs both in cell culture and in animal models and that rhIL-1Ra can be effective in cyclotherapy.
DISCUSSION
The rhIL-1Ra pretreatment reduced the incidence and severity of 5-FU–induced neutropenia (Fig. 2) without affecting chemotherapy efficacy against colon cancer and myeloid leukemia (Fig. 7). The effective dosage was 1–3 mg/kg for 5 d before chemotherapy. We previously reported similar chemoprotective effects of rhIL-1Ra on intestinal crypt epithelial cells and thymus (4, 24). The multiple organ protection of rhIL-1Ra may be explained by the wide expression of IL-1RI in nucleated cells and their common IL-1 signaling activity (25). Our data from the IL-1RI−/− studies showing IL-1RI dependency validated the function of IL-1Ra through this receptor (Fig. 3E). We demonstrated that rhIL-1Ra protected multiple organs against chemotoxicity. rhIL-1Ra has already been approved as a drug (25), so its clinical development in cyclotherapy should be facilitated.
The underlying mechanism of rhIL-1Ra as a BM chemoprotectant was first proposed by a time-effect study in which rhIL-1Ra alleviated neutropenia only before but not after 5-FU administration (Fig. 2A). The cell cycle analysis showed that LSK cells remain in G0 phase after rhIL-1Ra treatment in vivo (Fig. 4). These cells are susceptible to BM injury and are also responsible for BM repair (26). G1 and G2 arrests protect against S and M phase–specific chemotherapy drugs (27). However, quiescence induction is consistent with the observed broad protection provided by rhIL-1Ra for BM and intestinal epithelia against both cell cycle–dependent (e.g., 5-FU) and –independent (e.g., cyclophosphamide) chemotherapy drugs (4, 9, 28). Our results also corroborate recent findings that the induction of HS/PC quiescence before chemotherapy promotes hematopoietic recovery and improves animal survival in lethal-dose chemotherapy (29–31).
Another potent cell proliferation inhibitor is TGF-β (32). This cytokine protected mice from chemotoxicity by arresting normal epithelial proliferation in the late G1 phase (32, 33). Because it may promote tumorigenesis and metastasis (34), it was administered as a topical mouthwash formulation. Unfortunately, TGF-β failed to prevent chemotherapy-induced oral mucositis in late-stage clinical trials (35). In contrast, rhIL-1Ra was well-tolerated by humans, with a wide therapeutic window (5). IL-1β inhibition with the IL-1β mAb canakinumab reduced lung cancer incidence and mortality in a clinical trial (36). Therefore, human safety data encourage the clinical development of rhIL-1Ra for cyclotherapy.
The mechanism by which a cell exits the G0 phase of the cell cycle has already been extensively studied (37). However, investigations into the process whereby the cell enters G0 have only recently been reported (38). The CDK inhibitor p21 determines whether a cell enters quiescence or continues to proliferate (15). Cells derived from a cycling parental cell and with comparatively high p21 levels would enter a transient G0 state. In vitro study results align with the in vivo observation that mice without p21 had relatively higher percentages of hematopoietic stem cells in the active cell cycle (17). The p53−/− mice had the same phenotype as that of the p21−/− mice (16), as p21 is the only CDK inhibitor directly regulated by p53 (39). Additionally, the BM in the p38−/− mice contained hyperproliferative hematopoietic cells (40). Therefore, p38 MAPK is an upstream p53 activator. Our knockout mouse studies causatively correlated these molecules with the quiescent state of the HS/PCs after rhIL-1Ra treatment (Figs. 4–6). Carey et al. (41) reported that IL-1, dependent on p38, inhibited the in vitro colony formation of CD34+ cells from healthy donors. Our observation of rhIL-1Ra suppression of hematopoietic progenitor cell cycling in mice through p38 activation indicates that IL-1 promotes hematopoiesis in vivo. These differential results might be attributed to distinct hematopoietic progenitor cell environments in vitro and in vivo. Consistent with our in vivo results, p38−/− mice contained hyperproliferative hematopoietic cells in BM (40).
We report here that rhIL-1Ra pretreatment alleviated myelosuppression of 5-FU through the pathway IL-1RI → p38 → p53 → p21, which induced transient HS/PC quiescence. Both cell cycle negative regulators, such as p53 tumor suppresser, and activators, such Myc oncogene, regulate the restriction points of cell cycle (42). In normal cells, the accumulation of p21 leads cells to the transient G0 phase of cell cycle (15). However, the hallmark of cancer cells is mitogen-independent proliferation due to loss of cell cycle restriction points and DNA damage checkpoints (1). Therefore, unlike normal cells, cancer cells may not respond to rhIL-1Ra, because of null mutations of tumor suppressor genes and/or oncogenes that override the restriction points of this pathway.
The activation of p53 in cyclotherapy has been pursued because ∼50% of all cancer cases carry the TP53 mutation, whereas the other half often present with defects in the p53 pathway (43). Nutlin-3 represents a class of small molecules that activate p53. They are currently under testing in various clinical cancer trials (3). A proof-of-concept animal study with nutlin-3 demonstrated that mouse pretreatment with nutlin-3 reduced the neutropenia (44). However, because p53 activation has divergent effects on cell cycle arrest and apoptosis (45), the improper use of small molecules may actually exacerbate chemotherapy side effects. The main driver for the exploration of p53-activating drugs for cancer therapy is the apoptosis-induction mechanism of p53 (3). In contrast, rhIL-1Ra-induced p53 activation is IL-1RI dependent. Even rhIL-1Ra overdose would only diminish endogenous IL-1 activity and would not elicit any additional signals, because the binding of IL-1Ra to IL-1RI does not activate the receptor (46). IL-1Ra has an excellent record of clinical safety (14). Therefore, rhIL-1Ra-induced p53 activation is potentially safer than the p53-activating small molecules that are currently used and tested for cyclotherapy. In vivo rhIL-1Ra-mediated activation of the p38-p53-p21 pathway in HS/PCs may be a new direction toward p53-based cyclotherapy.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by grants from the National Science and Technology Major Project (2018ZX09733001-001-004), the National Natural Science Foundation of China (30901873), and the Shanghai Science and Technology Committee (111431921300). The authors declare no conflicts of interest.
Glossary
- 5-FU
5-fluorouracil
- ANC
absolute neutrophil count
- APL
acute promyelocytic leukemia
- Ara-C
cytarabine arabinoside
- BrdU
bromodeoxyuridine
- BM
bone marrow
- BMC
BM cell
- CDK
cyclin-dependent kinase
- CIN
chemotherapy-induced neutropenia
- GFP
green fluorescent protein
- Gr-1
granulocyte-differentiation antigen
- HS/PC
hematopoietic stem/progenitor cell
- IL-1RI
IL-1 receptor I
- LK
Lin−Sca-1−c-Kit+
- LSK
Lin−Sca-1+c-Kit+
- PE
phycoerythrin
- rhIL-1Ra
recombinant human IL-1 receptor antagonist
- WBC
white blood cell
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
H. Ye, L. Qian, S. Zhu, S. Deng, Y. Yu, and W. Han developed the hypothesis and wrote the manuscript; H. Ye, L. Qian, S. Zhu, S. Deng, and X. Wang designed and performed the experiments and analyzed the data; J. Zhu provided the GFP+ APL cells; and J. Zhu and G. L. Chan advised on the experimental design and manuscript preparation.
REFERENCES
- 1.Blagosklonny M. V., Darzynkiewicz Z. (2002) Cyclotherapy: protection of normal cells and unshielding of cancer cells. Cell Cycle 1, 375–382 [DOI] [PubMed] [Google Scholar]
- 2.Lane D. P. (1992) Cancer. p53, guardian of the genome. Nature 358, 15–16 [DOI] [PubMed] [Google Scholar]
- 3.Khoo K. H., Verma C. S., Lane D. P. (2014) Drugging the p53 pathway: understanding the route to clinical efficacy. Nat. Rev. Drug Discov. 13, 217–236; erratum: 314 [DOI] [PubMed] [Google Scholar]
- 4.Wang X., Zhu S., Qian L., Gao J., Wu M., Gao J., Zhang Y., Chan G. L., Yu Y., Han W. (2014) IL-1Ra selectively protects intestinal crypt epithelial cells, but not tumor cells, from chemotoxicity via p53-mediated upregulation of p21(WAF1) and p27(KIP1.). Pharmacol. Res. 82, 21–33 [DOI] [PubMed] [Google Scholar]
- 5.Dinarello C. A. (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu. Rev. Immunol. 27, 519–550 [DOI] [PubMed] [Google Scholar]
- 6.Cheung T. H., Rando T. A. (2013) Molecular regulation of stem cell quiescence. Nat. Rev. Mol. Cell Biol. 14, 329–340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao B., Lain S., Thompson A. M. (2013) p53-based cyclotherapy: exploiting the ‘guardian of the genome’ to protect normal cells from cytotoxic therapy. Br. J. Cancer 109, 2954–2958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang J., Xiang D., Zhu S., Mao W., Lu H., Wu M., Wang Q., Yu Y., Herbst K. D., Han W. (2009) Interleukin 1 receptor antagonist inhibits normal hematopoiesis and reduces lethality and bone marrow toxicity of 5-fluouracil in mouse. Biomed. Pharmacother. 63, 501–508 [DOI] [PubMed] [Google Scholar]
- 9.Qian L., Xiang D., Zhang J., Zhu S., Gao J., Wang X., Gao J., Zhang Y., Shen J., Yu Y., Han W., Wu M. (2013) Recombinant human interleukin-1 receptor antagonist reduces acute lethal toxicity and protects hematopoiesis from chemotoxicity in vivo. Biomed. Pharmacother. 67, 108–115 [DOI] [PubMed] [Google Scholar]
- 10.Jiang L. J., Zhang N. N., Ding F., Li X. Y., Chen L., Zhang H. X., Zhang W., Chen S. J., Wang Z. G., Li J. M., Chen Z., Zhu J. (2011) RA-inducible gene-I induction augments STAT1 activation to inhibit leukemia cell proliferation. Proc. Natl. Acad. Sci. USA 108, 1897–1902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y., Gao J., Wang X., Deng S., Ye H., Guan W., Wu M., Zhu S., Yu Y., Han W. (2015) CXCL4 mediates tumor regrowth after chemotherapy by suppression of antitumor immunity. Cancer Biol. Ther. 16, 1775–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olson T. S., Caselli A., Otsuru S., Hofmann T. J., Williams R., Paolucci P., Dominici M., Horwitz E. M. (2013) Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood 121, 5238–5249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Passegué E., Wagers A. J., Giuriato S., Anderson W. C., Weissman I. L. (2005) Global analysis of proliferation and cell cycle gene expression in the regulation of hematopoietic stem and progenitor cell fates. J. Exp. Med. 202, 1599–1611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dinarello C. A. (1996) Biologic basis for interleukin-1 in disease. Blood 87, 2095–2147 [PubMed] [Google Scholar]
- 15.Spencer S. L., Cappell S. D., Tsai F. C., Overton K. W., Wang C. L., Meyer T. (2013) The proliferation-quiescence decision is controlled by a bifurcation in CDK2 activity at mitotic exit. Cell 155, 369–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Y., Elf S. E., Miyata Y., Sashida G., Liu Y., Huang G., Di Giandomenico S., Lee J. M., Deblasio A., Menendez S., Antipin J., Reva B., Koff A., Nimer S. D. (2009) p53 regulates hematopoietic stem cell quiescence. Cell Stem Cell 4, 37–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cheng T., Rodrigues N., Shen H., Yang Y., Dombkowski D., Sykes M., Scadden D. T. (2000) Hematopoietic stem cell quiescence maintained by p21cip1/waf1. Science 287, 1804–1808 [DOI] [PubMed] [Google Scholar]
- 18.Cuadrado A., Nebreda A. R. (2010) Mechanisms and functions of p38 MAPK signalling. Biochem. J. 429, 403–417 [DOI] [PubMed] [Google Scholar]
- 19.El-Deiry W. S., Tokino T., Velculescu V. E., Levy D. B., Parsons R., Trent J. M., Lin D., Mercer W. E., Kinzler K. W., Vogelstein B. (1993) WAF1, a potential mediator of p53 tumor suppression. Cell 75, 817–825 [DOI] [PubMed] [Google Scholar]
- 20.Gartel A. L., Tyner A. L. (1999) Transcriptional regulation of the p21((WAF1/CIP1)) gene. Exp. Cell Res. 246, 280–289 [DOI] [PubMed] [Google Scholar]
- 21.Van Leeuwen I. M. (2012) Cyclotherapy: opening a therapeutic window in cancer treatment. Oncotarget 3, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lozzio C. B., Lozzio B. B. (1975) Human chronic myelogenous leukemia cell-line with positive Philadelphia chromosome. Blood 45, 321–334 [PubMed] [Google Scholar]
- 23.Gallagher R., Collins S., Trujillo J., McCredie K., Ahearn M., Tsai S., Metzgar R., Aulakh G., Ting R., Ruscetti F., Gallo R. (1979) Characterization of the continuous, differentiating myeloid cell line (HL-60) from a patient with acute promyelocytic leukemia. Blood 54, 713–733 [PubMed] [Google Scholar]
- 24.Yu H., Wu M., Wen B., Sun N., Xiang D., Zhang J., Zhu S., Weng S., Yu Y., Han W. (2016) Antagonist effect of interleukin 1 receptor on normal thymopoiesis and thymus toxicity of 5-azacytidine in mouse. Am. J. Transl. Res. 8, 1237–1245 [PMC free article] [PubMed] [Google Scholar]
- 25.Dinarello C. A., Simon A., van der Meer J. W. (2012) Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat. Rev. Drug Discov. 11, 633–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilson A., Laurenti E., Oser G., van der Wath R. C., Blanco-Bose W., Jaworski M., Offner S., Dunant C. F., Eshkind L., Bockamp E., Lió P., Macdonald H. R., Trumpp A. (2008) Hematopoietic stem cells reversibly switch from dormancy to self-renewal during homeostasis and repair. Cell 135, 1118–1129 [DOI] [PubMed] [Google Scholar]
- 27.Blagosklonny M. V., Pardee A. B. (2001) Exploiting cancer cell cycling for selective protection of normal cells. Cancer Res. 61, 4301–4305 [PubMed] [Google Scholar]
- 28.Xiang D., Guo Y., Zhang J., Gao J., Lu H., Zhu S., Wu M., Yu Y., Han W. (2011) Interleukin-1 receptor antagonist attenuates cyclophosphamide-induced mucositis in a murine model. Cancer Chemother. Pharmacol. 67, 1445–1453 [DOI] [PubMed] [Google Scholar]
- 29.Velardi E., Tsai J. J., Radtke S., Cooper K., Argyropoulos K. V., Jae-Hung S., Young L. F., Lazrak A., Smith O. M., Lieberman S., Kreines F., Shono Y., Wertheimer T., Jenq R. R., Hanash A. M., Narayan P., Lei Z., Moore M. A., Kiem H. P., van den Brink M. R. M., Dudakov J. A. (2018) Suppression of luteinizing hormone enhances HSC recovery after hematopoietic injury. Nat. Med. 24, 239–246; erratum: 525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.He S., Roberts P. J., Sorrentino J. A., Bisi J. E., Storrie-White H., Tiessen R. G., Makhuli K. M., Wargin W. A., Tadema H., van Hoogdalem E. J., Strum J. C., Malik R., Sharpless N. E. (2017) Transient CDK4/6 inhibition protects hematopoietic stem cells from chemotherapy-induced exhaustion. Sci. Transl. Med. 9, eaal3986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taylor S. J., Duyvestyn J. M., Dagger S. A., Dishington E. J., Rinaldi C. A., Dovey O. M., Vassiliou G. S., Grove C. S., Langdon W. Y. (2017) Preventing chemotherapy-induced myelosuppression by repurposing the FLT3 inhibitor quizartinib. Sci. Transl. Med. 9, eaam8060 [DOI] [PubMed] [Google Scholar]
- 32.Massagué J., Blain S. W., Lo R. S. (2000) TGFbeta signaling in growth control, cancer, and heritable disorders. Cell 103, 295–309 [DOI] [PubMed] [Google Scholar]
- 33.Grzegorzewski K., Ruscetti F. W., Usui N., Damia G., Longo D. L., Carlino J. A., Keller J. R., Wiltrout R. H. (1994) Recombinant transforming growth factor beta 1 and beta 2 protect mice from acutely lethal doses of 5-fluorouracil and doxorubicin. J. Exp. Med. 180, 1047–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Massagué J. (2008) TGFbeta in cancer. Cell 134, 215–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Khemissa F., Mineur L., Amsellem C., Assenat E., Ramdani M., Bachmann P., Janiszewski C., Cristiani I., Collin F., Courraud J., de Forges H., Dechelotte P., Senesse P. (2016) A phase III study evaluating oral glutamine and transforming growth factor-beta 2 on chemotherapy-induced toxicity in patients with digestive neoplasm. Dig. Liver Dis. 48, 327–332 [DOI] [PubMed] [Google Scholar]
- 36.Ridker P. M., MacFadyen J. G., Thuren T., Everett B. M., Libby P., Glynn R. J.; CANTOS Trial Group (2017) Effect of interleukin-1β inhibition with canakinumab on incident lung cancer in patients with atherosclerosis: exploratory results from a randomised, double-blind, placebo-controlled trial. Lancet 390, 1833–1842 [DOI] [PubMed] [Google Scholar]
- 37.Johnson A., Skotheim J. M. (2013) Start and the restriction point. Curr. Opin. Cell Biol. 25, 717–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rumman M., Dhawan J., Kassem M. (2015) Concise review: quiescence in adult stem cells: biological significance and relevance to tissue regeneration. Stem Cells 33, 2903–2912 [DOI] [PubMed] [Google Scholar]
- 39.Karimian A., Ahmadi Y., Yousefi B. (2016) Multiple functions of p21 in cell cycle, apoptosis and transcriptional regulation after DNA damage. DNA Repair (Amst.) 42, 63–71 [DOI] [PubMed] [Google Scholar]
- 40.Hui L., Bakiri L., Mairhorfer A., Schweifer N., Haslinger C., Kenner L., Komnenovic V., Scheuch H., Beug H., Wagner E. F. (2007) p38alpha suppresses normal and cancer cell proliferation by antagonizing the JNK-c-Jun pathway. Nat. Genet. 39, 741–749 [DOI] [PubMed] [Google Scholar]
- 41.Carey A., Edwards D. K., V, Eide C. A., Newell L., Traer E., Medeiros B. C., Pollyea D. A., Deininger M. W., Collins R. H., Tyner J. W., Druker B. J., Bagby G. C., McWeeney S. K., Agarwal A. (2017) Identification of interleukin-1 by functional screening as a key mediator of cellular expansion and disease progression in acute myeloid leukemia. Cell Rep. 18, 3204–3218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blagosklonny M. V., Pardee A. B. (2002) The restriction point of the cell cycle. Cell Cycle 1, 103–110 [PubMed] [Google Scholar]
- 43.Cheok C. F., Verma C. S., Baselga J., Lane D. P. (2011) Translating p53 into the clinic. Nat. Rev. Clin. Oncol. 8, 25–37 [DOI] [PubMed] [Google Scholar]
- 44.Sur S., Pagliarini R., Bunz F., Rago C., Diaz L. A., Jr., Kinzler K. W., Vogelstein B., Papadopoulos N. (2009) A panel of isogenic human cancer cells suggests a therapeutic approach for cancers with inactivated p53. Proc. Natl. Acad. Sci. USA 106, 3964–3969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Batchelor E., Loewer A., Lahav G. (2009) The ups and downs of p53: understanding protein dynamics in single cells. Nat. Rev. Cancer 9, 371–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Boraschi D., Tagliabue A. (2013) The interleukin-1 receptor family. Semin. Immunol. 25, 394–407 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.