Abstract
Diabetes significantly affects the life quality and length of patients with diabetes, and almost half of the 4 million people who die from diabetes are under the age of 60. Because of the increasing number of patients with diabetes and the side effects of antidiabetic drugs, the search for new dietary supplementation from natural resources, especially functional oligosaccharides, has attracted much attention among scientific researchers. Functional oligosaccharides are potential antidiabetic treatments because of their nondigestible, low-calorie, and probiotic features. The antidiabetic activity of multiple functional oligosaccharides such as fructo-oligosaccharides, galacto-oligosaccharides, and xylo-oligosaccharides has been reviewed in this paper. Molecular mechanisms involved in the antidiabetic activity of oligosaccharides have been systematically discussed from multiple perspectives, including the improvement of pancreas function, α-glucosidase inhibition, the relief of insulin and leptin resistance, anti-inflammatory effects, regulation of gut microbiota and hormones, and the intervention of diabetic risk factors. In addition, the antidiabetic effects of functional oligosaccharides through the complex gut-brain-liver axis are summarized. The concepts addressed in this review have important clinical implications, although more works are needed to confirm the antidiabetic mechanisms of functional oligosaccharides, standardize safe dose levels, and clarify their metabolism in the human body.—Zhu, D., Yan, Q., Liu, J., Wu, X., Jiang, Z. Can functional oligosaccharides reduce the risk of diabetes mellitus?
Keywords: dietary supplementation, antidiabetic activity, risk factors, molecular mechanism
Diabetes mellitus (DM) is a chronic metabolic disease caused by the inadequate production of insulin or inefficient utilization of insulin by the body. According to the seventh edition of the International Diabetes Federation Diabetes Atlas in 2017, 425 million people are suffering from diabetes globally, and this number is predicted to increase to 629 million by 2045. In 2017, there were 4 million deaths caused by diabetes (1). Diabetes and its complications impair life quality and are associated with a substantial socioeconomic burden. DM can be divided into type 1 DM (T1DM; a deficiency in β-cell function leading to insulin deficiency), type 2 DM (T2DM; characterized by insulin resistance leading to a relative deficiency in insulin secretion), gestational DM (GDM; pregnant women without a previous history of diabetes developing high glucose levels), and prediabetes (impaired glucose tolerance and fasting glycemia) (2). For T1DM, exogenous insulin supplementation is the only effective treatment to date. Researchers continue discovering advanced therapy medicinal products and auxiliary treatments for T1DM, such as immune modulators and islet β-cell protectors (3, 4). T2DM makes up 90–95% of all diagnosed diabetes cases in high-income countries and may represent a higher proportion in low- and middle-income countries. Multiple underlying risk factors for T2DM have been identified, including hereditary diabetes, obesity, unhealthy dietary habits, physical exercise, hypertension, aging, and poor physical and nutritional state during pregnancy. People with diabetes have a greater chance of developing other serious cardiovascular complications such as chronic kidney failure, diabetic foot ulcers, diabetic retinopathy, and other chronic metabolic diseases (5). According to the World Health Organization (WHO), prediabetes is an intermediate hyperglycemic condition with 2 characteristic factors: damaged glucose tolerance, which is defined as a fasting plasma glucose (FPG) concentration of <7.0 mM and a 2-h post-load plasma glucose concentration of 7.8–11.0 mM (140–200 mg/dl); and impaired fasting glucose, in which FPG concentrations are 6.1–6.9 mM (110–125 mg/dl). In addition, GDM is a condition in which women without diabetes develop high blood glucose levels (5.1–6.9 mM) during pregnancy. Prediabetes and GDM increase the risk of T2DM development.
Drug therapies are the major methods of the prevention and management of DM. There are multiple oral and injectable medications, including carbohydrate modulators, insulin secretagogues, insulin sensitizers, incretin potentiators, and amylin analogs (6). Because of the side effects of the common antidiabetic drugs, changes in dietary pattern could be more suitable for long-term diabetes treatments. A major goal for diabetes nutritional care is to improve glycemic control by regulating carbohydrate intake. The WHO guidelines state that free sugar intake should not exceed 10% of the total energy intake for all age groups (7). Sugars, including mono- and disaccharides, are naturally found in syrups, honey, fruit juices, concentrates, and commercially available foods and beverages. These sugars are readily absorbed into the bloodstream, thereby increasing blood sugar levels and the burden of insulin secretion in patients with diabetes. Nondigestible dietary supplementation of carbohydrates, prebiotics of dietary fibers, and functional oligosaccharides have been recommended to improve glucose regulation. Functional oligosaccharides are attractive to patients with diabetes because of their low glycemic index. The exploration of metabolic mechanisms of functional oligosaccharides will aid our understanding of their antidiabetic activity and developing new antidiabetic bioactive supplements.
ROLE OF FUNCTIONAL OLIGOSACCHARIDES IN THE MANAGEMENT OF DIABETES
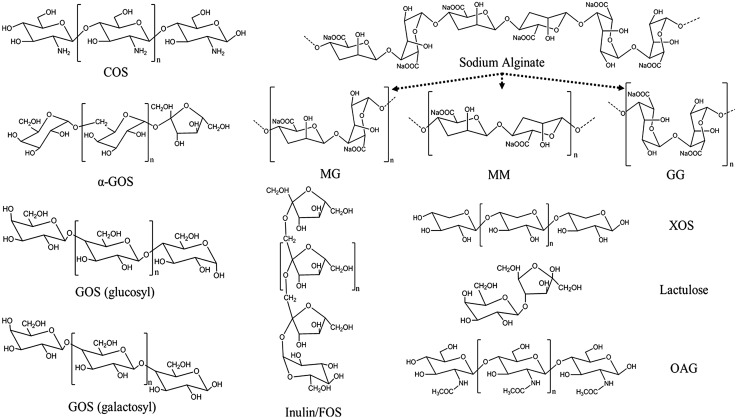
Oligosaccharides are low-MW polymers that consist of 2–10 monosaccharides linked by glycosidic bonds. Generally, oligosaccharides could be obtained through the degradation of carbohydrate polymers via chemical, physical, and biologic processes or synthesized from simple sugars through enzymatic transformation (8, 9). Functional oligosaccharides are nondigestible and nonsusceptible to human digestive enzymes because of the formation of osidic bonds of anomeric C atoms (either C1 or C2) of the monosaccharide units (10). This enables a wide range of modifications of the physicochemical properties of foods resulting in various pharmacological functions such as antimicrobial, antioxidant, antidiabetes, antihypertensive, anti-inflammatory, anticancer, antiobesity, and dental cavity prevention functions (11, 12). Currently, functional oligosaccharides are gaining more attention as prebiotics to be used in various forms (13). Structural features of functional oligosaccharides, including the nature of glycosidic bonds and degree of polymerization, are crucial for determining their bioactivities (Fig. 1). Functional oligosaccharides of fructo-oligosaccharides (FOSs), galacto-oligosaccharides (GOSs), mannanoligosaccharides (MOSs), xylo-oligosaccharides (XOS), neoagaro-oligosaccharides, isomalto-oligosaccharides, and chito-oligosaccharides (COSs) can improve glucose-lipid metabolic disorder and glycemic control to mitigate risks of DM (Tables 1 and 2). The following categories of typical functional oligosaccharides have been shown to exhibit antidiabetic activity.
Figure 1.
Molecular structures of some functional oligosaccharides. GG, polyguluronic acid; MG, heteropolymer of polymannuronic acid and polyguluronic acid; MM, polymannuronic acid; OAG, oligo-N-acetylglucosamine.
TABLE 1.
Effective intervention of functional oligosaccharides to delay or prevent diabetes and its risk factors
| Functional oligosaccharide | System | Design | Outcome | Reference |
|---|---|---|---|---|
| FOS + inulin | Female patients with T2D | Randomized, double-blind, placebo-controlled crossover. Total 27 subjects; 10 g/d for 8 wk | Improvements of glycemic indices, lipid profile, antioxidant status, and MDA; decreases in levels of IFN-γ and high-sensitivity CRP. | 116, 36 |
| FOS + probiotics | Patients with T2D | Randomized, double-blind, placebo-controlled crossover. Total 28 subjects; 100 mg/d FOS for 8 wk | Increase of serum calcium and a decreased serum ALT levels | 117 |
| GOS | Alloxan-induced diabetic rats | Rats fed GOS supplemented (10% w/w) diet for 42 d | Decreases of blood glucose, lipid profile, serum urea, serum creatinine, and increased level of antioxidative enzymes; increased lactobacillus count in the feces with a concomitant decrease in fecal coliform count | 23 |
| α-GOS | Women with overweight | Single-center, double-blinded, randomized, placebo-controlled trials. Total 0.88 subjects; 6–18 g/d for 14 d | Reduction in appetite, food intake, and the levels of plasma LPS and CRP | 64 |
| GOS | Women with overweight | Randomized double-blind, placebo-controlled crossover. Total 45 subjects; 5.5 g/d for 12 wk | Significant decreases in insulin levels, triglycerides, total cholesterol, HDL cholesterol, and total cholesterol:HDL cholesterol ratio | 85 |
ALT, alanine aminotransferase; MDA, malondialdehyde.
TABLE 2.
Effective intervention of functional oligosaccharides to delay or prevent diabetes and its risk factors
| Functional oligosaccharide | System | Design | Outcome | Reference |
|---|---|---|---|---|
| COS | db/db mice | Mice fed a COS-supplemented (4% w/w) diet for 42 d | Reduction in serum glucose and HbA1c levels, accompanied by inhibiting carbohydrate hydrolysis enzymes (sucrase, maltase, and SI complex) | 16 |
| Lactulose | Patients with obesity | 8.2 g lactulose/d for 2 d | Decreased mean daytime glucose and insulin | 118 |
| OAG | diabetic mice | Mice fed OAG 5–10 mg/kg/d for 21 d | Decreases of blood glucose and lipid profile, including TG, TC, LDL, and VLDL cholesterol | 15 |
| SAOS | Dahl salt-sensitive rats | Rats administered SAOS (60 mg/d using a continuous osmotic minipump) for 14 d. | Attenuation of systolic blood pressure and morphologic glomerular damage probably through a direct action on vascular vessels | 119 |
| XOS | Adults with prediabetes | Randomized, double-blind, placebo-controlled crossover. Total 18 subjects; 2.8 g/d for 8 wk | Attenuation in 0–2 h OGTT insulin response | 120 |
| XOS | Adults with prediabetes | Randomized, double-blind, placebo-controlled crossover. Total 34 subjects; 2 g/d for 8 wk | Decreases in abundance of Howardella, Enterorhabdus, Slackia, and OGTT 2-h insulin levels | 31 |
OAG, oligo-N-acetylglucosamine; SI, sucrose-isomaltase; TC, total cholesterol; TG, triglycerol; VLDL, very LDL.
Antidiabetic activity of functional oligosaccharides in animal models
COSs and chitosan oligosaccharides (GO2Ka1s) are both linear polymers composed of d-glucosamine (deacetylated unit D) and β-(1→4)-linked N-acetyl-d-glucosamine (acetylated unit A), which can be prepared from the exoskeletons of arthropods and insects (14). COSs exhibited antidiabetic and oxidation resistance bioactivities in alloxan-induced type 1 diabetic mice (15). Long-term GO2KA1 supplementation for 41 d in db/db mice results in significant blood glucose and glycated hemoglobin (HbA1c) reduction to levels similar with those of acarbose (16).
FOSs contain 2-4 β (1, 2) or β-linked (2, 6) fructosyl units, which are produced from the transfructosylation of sucrose (17). Cani et al. (18) reported that FOS improved glucose tolerance, fasting blood glucose, glucose-stimulated insulin secretion, and insulin-sensitive hepatic glucose production but reduced body weight (BW) gain in high-fat diet (HFD)–induced diabetic mice.
XOSs are made up of xylose monomeric units with β (1–4) glycosidic bonds, which are commonly produced from xylan containing lignocellulosic materials through enzymatic hydrolysis or chemical fractionation of lignocellulosic materials (19). It has been found to relieve hyperglycemia, hyperlipidemia, and oxidative status in a randomized double-blind clinical trial (20). Furthermore, XOS supplementation reduced glucose, HbA1c, and fructosamine concentrations and regulated abnormal lipid metabolism in T2DM.
Typical GOSs [α-(1→4)-d-glucans] are composed of 2–10 molecules of galactose and 1 molecule of glucose (21). They are the best substitute for human milk oligosaccharides and are widely accepted as prebiotic supplements in infant formulas (22). GOS supplementation at a level of 10% has been found to have positive effects on blood glucose, lipid profiles, and level of antioxidative enzymes in alloxan-induced diabetic rats (23). It was also reported that fasting glycemia and BW management were improved by the treatment of α-GOS (at a physiologic dose of 2.2 g/kg BW/d) in HFD-induced diabetic mice (24).
MOSs consist of β-d-mannose and β-d-glucose residues joined by 1→4 linkages and are usually obtained from acid and enzymatic hydrolysis of mannan (25). In HFD-treated mice, MOSs are found to reduce serum insulin levels and Homeostatic Model Assessment for Insulin Resistance (26). Moreover, MOSs and metformin synergistically affect hypoglycemic activity by modulating gut microbiota (27).
Neoagaro-oligosaccharides (prepared by enzymatic hydrolysis of agar with β-agarase DagA from Streptomyces coelicolor) distinctly improve insulin resistance and glucose intolerance induced by HFD (28). It was reported that human milk oligosaccharides delayed and suppressed T1DM development in nonobese diabetic mice via the regulation of immune and gut microbiota development in early life (29).
Antidiabetic activity of functional oligosaccharides in clinical trials
Kim et al. (30) studied the effects of GO2KA1 (for 12 wk) on glucose control in people with prediabetes through placebo-controlled clinical trials. Serum glucose and HbA1c plasma levels, plasma adiponectin, and proinflammatory cytokines (IL-6 and TNF-α) levels were significantly lower in the GO2KA1 treatment group than the placebo group. Gut microbiota and insulin levels were modified by XOS supplementation (2 g/d) over 8 wk in subjects with prediabetes (31). Additionally, XOS (4 g/d) administered for 8 wk was found to relieve hyperglycemia, hyperlipidemia, and oxidative status in a randomized double-blind clinical trial of T2DM (20). Furthermore, XOS supplementation reduced glucose, HbA1c, and fructosamine concentrations and regulated abnormal lipid metabolism in T2DM. In comparison with baseline values (0 wk), trehalose (consumed 10 g/d with meals for 12 wk) significantly decreased blood glucose concentrations after a 2-h oral glucose tolerance test (OGTT) in applicants with metabolic syndrome [body mass index (BMI) >23] (32). FOS supplementation (21 g/d) over 12 wk decreased BW, suppressed ghrelin, enhanced peptide YY (PYY) levels, and increased glucose and insulin levels between initial and final tests in 48 adults with a BMI >25 (33). Hence, FOS could potentially promote weight loss and improve glucose regulation in overweight adults. However, 4 wk of 20-g FOS supplementation did not modify basal hepatic glucose production or FPG and insulin concentrations in volunteers with T2DM (n = 10; 6 men, 4 women) (19). Similarly, after GOS supplementation (15 g/d) over 12 wk, the abundance of fecal Bifidobacterium spp. increased but no significant alterations in peripheral tissue insulin sensitivity in men and women with prediabetes who were overweight or obese was observed (34). Thus, the regulative effects of functional oligosaccharides on diabetes need to be further explored, including their structure-activity relationship, effective intake dosages, and functional mechanisms.
Synergetic effect of functional oligosaccharides combinations on antidiabetic activity
Combinations of functional oligosaccharides could exhibit potentially antidiabetic efficacy depending on their synergetic effects. The supplementation of XOS and FOS [by 5% XOS and 5% FOS (w/w) in the basal diet] significantly reduced hyperglycemia in streptozocin-induced diabetic rats (35). Oligofructose-enriched inulin (consuming 10 g/d for 8 wk, Frutafit IQ; Sensus, Roosendaal, The Netherlands) induced a significant decline in the levels of fasting glucose, glycosylated hemoglobin, and inflammatory factors in women with T2DM (36). The combination of soybean oligosaccharides and insulin on GDM was tested in 46 pregnant women over 8 wk. Compared with the control group (only insulin), soybean oligosaccharides improved insulin resistance by enhancing antioxidant enzyme activity during pregnancy with GDM (37).
In addition, synbiotics, which contain functional oligosaccharide substrates and probiotic organisms, exhibited synergetic antidiabetic activity. Kassaian et al. (38) conducted a study on a group of subjects affected by prediabetes. They were administered either synbiotic capsules [Lactobacillus acidophilus, B. lactis, B. bifidum, and B. longum (1 × 109 for each)] and inulin-based prebiotics or placebo capsules. Results showed that the synbiotics group had significantly improved fasting blood glucose levels and insulin resistance compared with the placebo group. Similarly, the synbiotic capsules that contained probiotics (L. acidophilus, L. casei strain, and B. bifidum strain; 2 × 109 CFU/g each) and 800 mg inulin resulted in significantly lower FPG levels, serum insulin concentrations, and homeostasis model of assessment in the placebo group than patients with overweight and diabetes (39). However, synergetic antidiabetic effects of functional oligosaccharides and synbiotics remain poorly understood.
POTENTIAL MECHANISMS INVOLVED IN ANTIDIABETIC ACTION OF FUNCTIONAL OLIGOSACCHARIDES
Evidence suggests that functional oligosaccharides exerted antidiabetic activity involving multiple potential mechanisms, including the improvement of pancreas function, α-glucosidase inhibition, the relief of insulin and leptin resistance, anti-inflammatory effects, regulation of gut microbiota and hormones, and reduction of other diabetic risk factors.
Improvement of pancreas function
Both insulin deficiency and abnormality in insulin recognition are found in many patients with diabetes. The destruction of pancreatic β-cells induced by autoimmunity contribute to insulin deficiency (40). It is believed that the cease of autoimmune attacks on β cells and the decrease of the insulin requirement contributes to the recovery to normoglycemia in T1DM (41). FOSs inhibited lymphocytic infiltration into the pancreatic islets and increased insulin secretion, resulting in the improvement of insulin sensitivity and β-cell function in nonobese diabetic mice (42). Similarly, the direct effects of COSs on the propagation or neogenesis of pancreatic islet cells and secretory capacity of insulin from islet cells were mediated by their antioxidant capacity and the regulation of glucose transporter 2 mRNA expression (43–45). The induction of glucose-inducible insulin secretion and the prevention of β-cell attenuation in pancreatic islets of type 2 diabetic rats are believed to be the mechanisms of antidiabetic action adopted by COSs (45).
α-Glucosidase inhibition
In the small intestine of humans, α-glucosidase inhibitors can suppress the digestion of starch and disaccharides to monosaccharides, resulting in lower postprandial blood glucose levels and a retardation of carbohydrate digestion (46). In addition, α-glucosidase inhibitors reduce the onset of diabetic complications (47).
The antidiabetic effect of GO2KA1 is regulated by the inhibition of intestinal α-glucosidase, α-amylase, and glucose transporters (48). Kim et al. (30) reported that GO2Ka1 remarkably inhibited the expression of α-glucosidase, PPARγ, and glucose transporters in people with diabetes. Moreover, Radix Ophiopogonis oligosaccharides (extracted from Ophiopogon japonicus vinegar by alcoholic and acetic acid fermentation with water extracts from Radix Ophiopogon) inhibited α-glucosidase activity and reduced islet damage in alloxan-induced diabetic mice (49).
The relief of insulin resistance and leptin resistance
Abnormalities in insulin secretion, insulin transport, and other related processes may be induced by inflammation. In such instances, insulin-related actions fail to trigger a series of metabolic reactions in tissues, including the promotion of glucose uptake and usage efficiency, leading to insulin resistance. As a sine qua non of T2DM, insulin resistance is associated with many other clinical conditions such as obesity, lipodystrophy, coronary heart disease, sepsis, cancer, and neurodegenerative diseases (50).
In diabetic C57BL/KsJ-db/db mice, the oligomannuronate-chromium (III) complex (50 mg kg/ d for 42 d) improved insulin resistance, glycogen synthesis, gluconeogenesis, hyperglycemic symptoms, and lipid metabolism disorders via the AMPK pathway in the liver (51). Adipose tissue is also a key insulin-sensitive peripheral tissue. In 3T3-L1 cells, the oligomannuronate-chromium (III) complex up-regulated the AMPK-PGC1α pathway and down-regulated lipid accumulation by the improvement of fatty acid β-oxidation and adipose triacylglyceride lipase protein expression (52). In addition to the direct regulation of insulin metabolism, functional oligosaccharides also improved insulin resistance via intestinal gluconeogenesis (53) and endogenous glucagon-like peptide (GLP)1 secretion (18). Studies showed that GO2KA1 modified insulin resistance in the T2DM model of genetically obese (ob/ob) mice via enhanced levels of adiponectin and decreased levels of resistin, retinol binding protein 4, and inflammatory factors (54). In vitro, GO2KA1 regulated glucose uptake in C2C12 myotubes via signaling pathways of insulin-independent (insulin receptor substrate 1–PI3K-PKC) and insulin-dependent (AMPK–sirtuin 1 and mammalian target of rapamycin) pathways (55). Furthermore, treatment with rehmannia glutinosa oligosaccharides (ROSs) appeared to reverse insulin resistance as measured by surrogate markers of glucose transporter 4 translocation, possibly through the IRβ-PI3K-Akt pathway in L6 myocytes and 3T3-L1 preadipocytes (56).
In addition, the effects of leptin on energy balance is worth noting because it has the potential to influence nerve metabolism (57). Studies showed that the combination of green tea extract with isomalto-oligosaccharides–modulated HFD induced adiposity and lipid accumulation as well as fasting blood glucose, insulin, glucagon, and leptin levels (58). Similarly, FOSs have also been reported to improve insulin and leptin sensitivities (59).
Anti-inflammatory effects
Key inflammatory markers are correlated with obesity and complications such as prediabetes and insulin resistance. This suggests that a persistent, chronic, low-grade inflammatory reaction is an underlying qualifiable risk factor (60). Increased serum level LPS stimulates the TLR4 pathway in the liver, which increases hepatic lipogenesis due to insulin resistance (61, 62). It was hypothesized that short-chain fatty acids (SCFAs) resulting from nondigestible oligosaccharide fermentation in the gut could decrease intestinal permeability with a concomitant decline in the levels of inflammatory mediator circulation, especially that of bacterial LPS (63).
Functional oligosaccharides could reduce the circulating concentration of LPS, resulting in a low inflammatory state. The anti-inflammatory effects of FOSs and inulin were investigated through changes in inflammatory markers and endotoxemia. The results of in vivo studies suggested that FOSs and fructans affected inflammation by enhancing insulin levels. Furthermore, metabolic endotoxemia and FOS supplementation in mice increased the number of intestinal Bifidobacteria and reduced FPG, LPS, glycosylated hemoglobin, and TNF-α levels (36, 61). The effects of dose-dependent alginate-derived guluronate oligosaccharide consumption were investigated in 88 adults with overweight (64). The results demonstrated that alginate-derived guluronate oligosaccharide effectively reduced appetite, food intake, and plasma LPS and c-reactive protein (CRP) levels. In vitro, alginate-derived guluronate oligosaccharides were recognized by RAW264.7 macrophages and up-regulated TLR4. Following the endocytosis and increased expression of TLR4, the phosphorylation of the Akt, NF-κB pathway, mammalian target of rapamycin pathway, and immune system was subsequently activated (65). Here, alginate-derived guluronate oligosaccharides influenced the release of immunomodulatory cytokines. IL-15 treatment has been reported to improve glucose homeostasis and insulin sensitivity in obese mice (66). The proinflammatory cytokine IL-6 as well as immunomodulatory activities involved in liver and pancreatic β cell metabolism occur in diabetes and influence the onset and progression of diabetic vascular complications (67–69). Sucrose-derived oligosaccharides synthesized by a constitutive mutant L. mesenteroides dextransucrase were reported to exhibit anti-inflammatory activities in HFD mice by reducing serum glucose and insulin. They also decreased the levels of IL-6 and fetuin-A, which were responsible for inflammation (70). Similarly, GO2KA1 improved glucolipid metabolism disorders by suppressing the expression of proinflammatory cytokines (IL-6, MCP-1, and TNF-α) and up-regulating PPARγ expression (71). Therefore, the regulation of immunomodulatory cytokines and LPS by functional oligosaccharides contributes to their antidiabetic activity.
Regulation of gut microbiota and gut hormone
Functional oligosaccharides are fermented by intestinal bacterial species but not human intestinal hydrolases or digestive enzymes to produce colonic nutrients, including SCFAs (acetate, lactate, propionate, and butyrate) and gases (CO2, H2, and CH4) (72). Several studies showed that gut microbiota played an important role in the development of obesity, inflammation, insulin resistance, and T2DM (73). Hit Chip and quantitative PCR analyses showed that functional oligosaccharides increased the Bifidobacterium genus population in patients with prediabetes and obesity (74). The distinct alterations of gut microbiota composition have been identified by previous studies (75). The diversity of bacteria that participated in the fermentation of dietary fibers or synthesis of SCFAs were also significantly reduced in T1DM (76). Moreover, an interaction between intestinal microbes and the host’s innate immune system was discovered in T1DM (77). Changes in intestinal flora occurred in ob/ob mice, with the up-regulation of Firmicutes and down-regulation of Bacteroidetes (62, 78). Moreover, changes in patients with obesity and T2DM have been revealed in humans and other species. The Bifidobacterium abundance was higher in healthy adults than in patients with obesity and T2DM (79).
Studies have shown that the metabolic regulation of FOSs are closely related to the regulation of intestinal flora (80, 81). Compared with placebo groups, FOS supplementation down-regulated glycolipid metabolism related indicators such as plasma lactate, phosphatidyl choline ghrelin, glucose, insulin, and PYY (33, 82). In addition, FOSs regulated glucose and lipid metabolism via gut microbial communities in obese and diabetic mice (83). MOSs attenuated insulin resistance in HFD-induced mice by reversing the changes in the relative abundance of several species, including Akkermansia muciniphila, Bacteroides acidifaciens, L. gasseri, and B. pseudolongum (26). It was speculated that nondigestible MOSs might also alter the uptake of various minerals from the intestine and reduce fat uptake through the gastrointestinal (GI) transit. Yang et al. (31) randomly fed XOSs or a placebo to 18 adults with prediabetes or 16 healthy adults for 8 wk to analyze the effects of gut microbiota and glucose metabolism. XOSs selectively reduced the abundance of Enterorhabdus and Coriobacteria but stimulated the growth of Dialister in the human colon and lowered the 0–2 h OGTT insulin response of patients with prediabetes. In contrast, oligosaccharide-derived metabolites, especially SCFAs, significantly contributed to the enhancement of activity or growth of microbiota (84). It was also reported that XOSs lowered the abundance of gut microbiota, including Howardella, Enterorhabdus, and Slackia during the development of diabetes (31). The supplementation of inulin effectively reduced serum LPS and CRP, which was related with increased abundance of Bifidobacterium and Faecalibacterium prausnitzi communities. The increment in bacterial abundance was negatively correlated with serum LPS levels but suppressed the growth of B. intestinalis, B. vulgatus, and Propionibacterium (74). In both animal and human studies, GOSs have been shown to be the good moderators of gut microbiota. A 12-wk dietary supplementation of 5.5 g/d GOS in 45 obese women resulted in increased Bifidobacteria but decreased Bacteroides in levels in the gut. These results were correlated with a decreased inflammatory reaction caused by fecal calprotectin and plasma CRP. Significant decrements in insulin, triglyceride, total cholesterol, HDL cholesterol levels, and the ratio of total cholesterol to HDL cholesterol were also observed (85).
During the bacterial fermentation of functional oligosaccharides, SCFAs induced GLP-1 excretion from L cells of the enteroendocrine system and improved glucose tolerance (86). GLP-1 is also regarded as a biologic insulin secretagogue because it modifies both insulin sensitivity and glucose tolerance in T2DM (87). Oligofructose improved glucose and insulin tolerance, fasting blood glucose, and hepatic glucose production via increasing of GLP-1 secretion in HFD-induced diabetic mice (18).
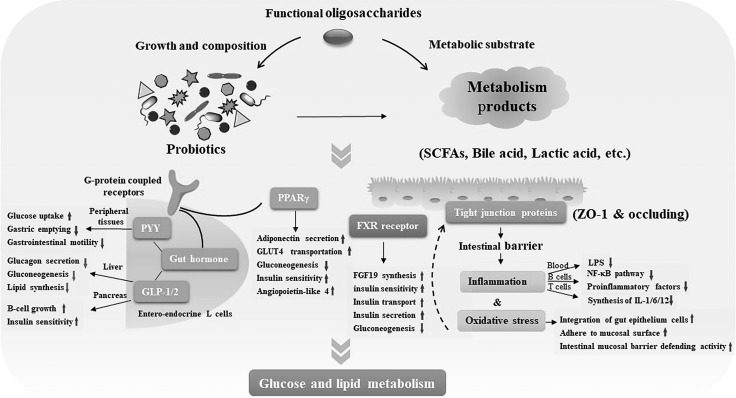
Prebiotics control glucose and lipid levels in the bloodstream and tissue by improving gut hormones, reducing oxidative stress and inflammation, producing SCFAs, and restoring intestinal permeability, and various other mechanisms (88, 89). Following bacterial fermentation, functional oligosaccharides drove qualitative and selective changes in the composition of gut microbiota (8, 90). The possible mechanisms are shown in Fig. 2. It was speculated that functional oligosaccharides regulated glucose and lipid homoeostasis and alleviated the risk of obesity and diabetes via complex gut microbiota.
Figure 2.
The regulation of glucose and lipid homoeostasis via gut microbiota. Functional oligosaccharides promote the growth of probiotics and reverse diversified composition of microbial flora. They also enhance the production of beneficial metabolites such as SCFAs, bile acids, and lactic acid. Subsequently, probiotics and their metabolism products affect glucose and lipid metabolism via numerous metabolic mechanisms. SCFAs bound to GPCRs GPR41 or GPR43 promote the expression of gut hormones such as PYY, GLP-1, and GLP-2. In peripheral tissues, insulin and glucose sensitivity are improved and gluconeogenesis is suppressed. This activation of GPCR influences the balance of lipid synthesis and lipolysis, peroxisome proliferator-activated receptor γ (PPARγ)–related differentiation, and the expression of angiopoietin-like 4. Bile acids control the production of fibroblast growth factor (FGF) 19 and GLP-1 and influence glucose homeostasis through the activation of the nuclear farnesoid X receptor and membrane-bound GPCR in the ileum. Probiotics and their metabolic products influence inflammation, oxidative stress, and intestinal permeability. Intestinal permeability is improved by the integration of gut epithelial cells, adherence to the mucosal surface, and the intestinal mucosal barrier. ZO-1, zonula occludens-1.
Intervention of other diabetic risk factors
The Global Noncommunicable Disease Network of the WHO focuses on 4 biologic risk factors associated with diabetes: the elevation of blood pressure, cholesterol, and blood glucose and a high BMI (7).
FOSs and XOSs have been repeatedly shown to have effects on glucose and lipid metabolism (18, 20). Today, various other oligosaccharides have been frequently studied to regulate glucolipid metabolic disorders. COSs and GO2Ka1s have become the new choice for antidiabetic drugs (91). The effects of long-term dietary supplementation with low-MW GO2KA1 were tested in a T2DM mouse model. It significantly lowered serum glucose and HbA1c levels and inhibited of carbohydrate hydrolysis enzyme activity, including that of sucrase, maltase, and the sucrose-isomaltase complex (16). Further studies on GO2KA1 highlighted its protective regulation in T2DM by ameliorating insulin resistance, blood glucose and pressure levels, oxidative stress, and lipid metabolism disorders (55, 71). Oligo-N-acetylglucosamine had significant effects on the reduction of lipid profile, including TG, TC, LDL, and VLDL (92). The metabolic syndrome and high-level chronic stress are often associated with T2DM. The link between functional changes in the hypothalamic-pituitary-adrenal axis and glucose absorption was studied in chronically stressed rats fed with oligosaccharides obtained from traditional Chinese aromatic plant ROS for 5 wk (93). ROS mainly consists of 60% stachyose, which has already been reported to have the ability to lower glucose levels in diabetic rats. It significantly increased plasma leptin levels, organ weights, and liver and muscle glycogen content in HFD-induced rats. It also significantly reduced glucogenesis, plasma fatty acids, plasma triglycerides, total cholesterol, and plasma corticosterone levels. A possible mechanism involved in these changes was the neuroendocrine immune-modulation network. ROS possibly influenced glucose homeostasis through multilinks and targeted the neuroendocrine immunomodulation network (93).
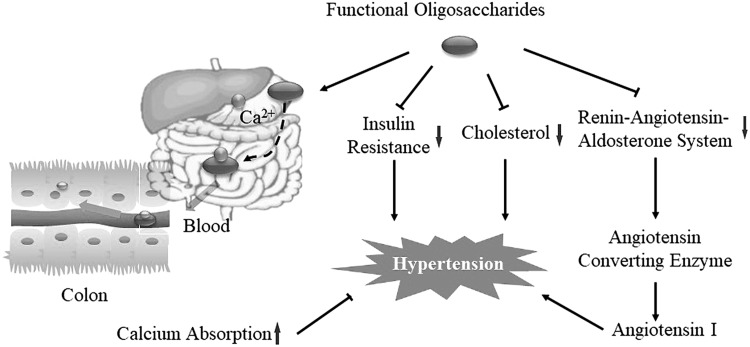
Various mechanisms are believed to be involved in the antihypertensive activities of prebiotics (94, 95). Sodium alginate oligosaccharides (SAOSs) attenuated systolic blood pressure and morphologic glomerular damage in hypertensive rats after a 7-wk (feeding) treatment period (96). Blood pressure in humans is regulated by the renin-angiotensin-aldosterone system. The angiotensin-converting enzyme (ACE) adjusts blood pressure via the regulation of angiotensin I, angiotensin II, and vasodilator bradykinin (97–99). GO2KA1s were found to be a natural ACE inhibitor by possessing positive charges and a close integration with ACE functional domains (100, 101). Its possible antihypertensive mechanisms might be related to its down-regulation of blood lipids and cholesterol, blood pressure and insulin resistance, or improvement of mineral absorption (102). Prebiotics promoted the assimilation of minerals, especially calcium, and thus played a role in the control of high blood pressure (103). Hence, we speculated that functional oligosaccharides decreased the uptake of calcium in the upper GI tract by binding ionic minerals to the functional oligosaccharides and then releasing the sequestered calcium in the colon. When the sequestered calcium reached the colon, it was released from the oligosaccharides and absorbed to elevate calcium concentration and thereby decrease the risk of hypertension (Fig. 3).
Figure 3.
Effect of functional oligosaccharides on high blood pressure. There are 4 possible mechanisms that have been postulated to explain the ability of functional oligosaccharides to reduce the risk of hypertension, including the regulation of the ACE regulation mechanism, cholesterol metabolism, insulin resistance, and mineral absorption. Among these, functional oligosaccharides decrease the uptake of calcium in the upper GI tract by binding ionic minerals to the functional oligosaccharides and then releasing the sequestered calcium in the colon.
THE ANTIDIABETIC ROLE OF FUNCTIONAL OLIGOSACCHARIDES VIA THE GUT-BRAIN-LIVER AXIS
In the pathogenesis of diabetes, the increase in hepatic glucose output and decrease in glucose uptake of insulin-sensitive peripheral tissues play a critical role in the development of T2DM (73, 74). Nonalcoholic fatty liver disease is a noninfectious chronic liver disease that has correlations with other extrahepatic diseases (104). The normal flora in the intestine influences metabolic processes by improving the energy yield from food, and an imbalance in the intestinal flora contributes to obesity and its comorbidities such as hepatic steatosis, insulin resistance, diabetes, and cardiovascular disease (105, 106). The transplanted microbiota from obese mice down-regulated AMPK phosphorylation and its downstream key enzymes of fatty acid oxidation (107). Currently, the metabolic systems of the liver and gut, the so-called gut-liver axis, has been the potential antidiabetic therapeutic target of functional oligosaccharides. Functional oligosaccharides and their derived metabolites (especially SCFAs) have been shown to improve gut microbiota in the cases of obesity and T2DM (108).
Gut microbiota also influences metabolism and behavior through the gut-brain axis. Through the regulation of gut microbiota Bifidobacterium, functional oligosaccharides have the potential to indirectly control the key factors of neural regulation such as amyloid β (Aβ) aggregates [interfere with insulin receptor signaling, leading to Alzheimer’s disease (Type 3 diabetes)] (109), arachidonic acid, docosahexaenoic acid (influences neurogenesis, neurotransmission, and neuroprotection) (110), and brain-derived neurotrophic factor (BDNF) (affects anxiety, fear, and appetite) (111). More importantly, decreased tropomyosin receptor kinase B–BDNF signaling was usually linked to an energy imbalance and orexigenic signaling in humans and rodents (112). In vivo studies showed that the down-regulation of tropomyosin receptor kinase B and BDNF resulted in excessive feeding and BW gain (113). Functional oligosaccharides influenced appetite at the level of the hypothalamus by affecting Bifidobacteria abundance and SCFA levels, leading to the regulation of energy loss and weight gain (9, 114). Research suggested that alginate oligosaccharides had the potential to reverse Aβ-induced neural damage and improve neurodegenerative disorders. Results showed that alginate oligosaccharides regulated apoptotic cell death induced by the endoplasmic reticulum and mitochondrial apoptotic cell death in H2O2-induced neuron-like PC-12 cells (115).
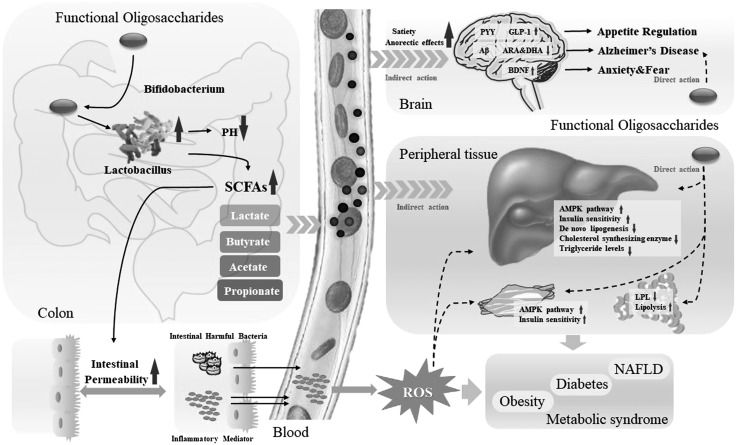
Thus, functional oligosaccharides improve the environment of intestinal flora and increase the generation and secretion of SCFAs. They limit the growth of harmful intestinal bacteria and the inflammatory response. They play a direct role in neuroprotection and insulin sensitivity and sequentially improve the progress of the metabolic syndrome. In addition, functional oligosaccharides indirectly improve glucose metabolism via the regulation of SCFAs and oxidative stress in the liver, muscle, fat cells, and other peripheral tissues. Therefore, they may regulate glucose homeostasis through the gut-brain-liver axis (Fig. 4).
Figure 4.
The regulation of glucose homoeostasis via the gut-brain-liver axis. The regulation of glucose homoeostasis is a complex metabolic process and involves multiple organs. Metabolic disorders caused by HFD or high-fat and high-fructose diet (HFFD) may cause the alteration of colonic pH, intestinal bacteria, and intestinal permeability, accompanied by systemic inflammation. Functional oligosaccharides stimulate the growth of probiotics such as Bifidobacterium and Lactobacillus and down-regulate the pH. They also improve the secretion of SCFAs, including lactate, butyrate, acetate, and propionate, which suppress increases in intestinal permeability and the secretion of harmful intestinal bacteria and inflammatory mediators. SCFAs are transported to the brain, liver, and other tissues and impact glucolipid metabolism. In the brain, SCFAs up-regulate the expression of PYY, GLP-1, and BDNF and down-regulate the expression of Aβ, arachidonic acid (ARA), and docosahexaenoic acid (DHA), which control appetite, anxiety, and fear and promote the development of Alzheimer’s disease. In the liver, muscle, fat, and other peripheral tissues, functional oligosaccharides play indirect or direct roles in improving glucolipid metabolism via AMPK and insulin signal pathways, lipid synthesis, and lipidolysis. Thus, functional oligosaccharides may regulate glucolipid metabolism, gut microbiota, and inflammatory responses via the gut-brain-liver axis, thereby affecting the development of nonalcoholic fatty liver disease (NAFLD), diabetes, obesity, and other metabolic syndromes. LPL, lipoptotein lipase.
CONCLUSIONS AND PERSPECTIVES
To date, there is no complete diabetic treatment available for controlling blood glucose. Dietary supplementation, particularly functional oligosaccharides, have become the new research focus for the treatment of diabetics. Understanding their regulatory effects on blood glucose and the mechanisms involved is important for the development of new antidiabetic bioactive substances with functional oligosaccharides. Here, we reviewed the known effects and the current understanding of functional oligosaccharide intervention in the pathogenesis of diabetes. In particular, studies emphasized that functional oligosaccharides play a positive role in the prevention of diabetes, including improvement of pancreas function, α-glucosidase inhibition, the relief of insulin and leptin resistance, anti-inflammatory effects, regulation of gut microbiota and gut hormone, and intervention of diabetic risk factors. Considering the prebiotic effects of functional oligosaccharides on gut microflora, it was assumed that the antidiabetic and some other conducive effects of functional oligosaccharides could depend upon the gut-brain-liver axis to maintain glucose homoeostasis. However, the relationship between chemical structures of functional oligosaccharides and their various beneficial effects remain largely unclear, which limits their potential use. More in-depth studies are necessary to confirm the multiple potential targets of functional oligosaccharides, standardize safe dosages, and clarify their metabolism in the human body. Future studies on screening of highly potent and safe functional oligosaccharides will contribute to the development of dietary supplementation for treatment and prevention options for diabetes and its complications.
ACKNOWLEDGMENTS
This work was supported by the National Key Research and Development Program of China (Grant 2017YFD0400204), and the National Postdoctoral Program for Innovative Talents (BX201700285). The authors declare no conflicts of interest.
Glossary
- Aβ
amyloid β
- ACE
angiotensin-converting enzyme
- BDNF
brain-derived neurotrophic factor
- BMI
body mass index
- BW
body weight
- COS
chito-oligosaccharide
- CRP
c-reactive protein
- DM
diabetes mellitus
- FOS
fructo-oligosaccharide
- FPG
fasting plasma glucose
- GDM
gestational DM
- GI
gastrointestinal
- GLP
glucagon-like peptide
- GO2KA1
chitosan oligosacharide
- GOS
galacto-oligosaccharide
- HbA1c
glycated hemoglobin
- HFD
high-fat diet
- MOS
mannan-oligosaccharide
- OGTT
oral glucose tolerance test
- PYY
peptide YY
- ROS
rehmannia glutinosa oligosaccharide
- SAOS
sodium alginate oligosaccharide
- SCFA
short-chain fatty acid
- T1DM
type 1 DM
- T2DM
type 2 DM
- WHO
World Health Organization
- XOS
xylo-oligosaccharide
AUTHOR CONTRIBUTIONS
D. Zhu, J. Liu, and X. Wu wrote the manuscript and contributed to discussion; and Q. Yan and Z. Jiang instructed and reviewed the manuscript.
REFERENCES
- 1.Aldworth J., Patterson C., Jacobs E., Misra A., Tamayo T., Snouffer E. B., Piemonte L., Savuleac R., Yanez Jimenez B., Sartiaux D., Dupont S., Makaroff L., Sadikot S., Robert D., Pathan S., Sung E., Rivas Gonzales M., Ibrahim A., L’Heveder R., Hassanein M., Ohlrogge A. W. (2017) IDF Diabetes Atlas, 8th ed., International Diabetes Federation Centre for Disease Control (CDC), Brussels, Belgium [Google Scholar]
- 2.Solis-Herrera C., Triplitt C., Reasner C., DeFronzo R. A., Cersosimo E. (2000) Classification of diabetes mellitus. Endotext [Internet]. MDText.com, Inc., South Dartmouth, MA, USA [Google Scholar]
- 3.Cañibano-Hernández A., Sáenz Del Burgo L., Espona-Noguera A., Ciriza J., Pedraz J. L. (2018) Current advanced therapy cell-based medicinal products for type-1-diabetes treatment. Int. J. Pharm. 543, 107–120 [DOI] [PubMed] [Google Scholar]
- 4.Ni Q., Pham N. B., Meng W. S., Zhu G., Chen X. (2018) Advances in immunotherapy of type I diabetes. [E-pub ahead of print] Adv. Drug Deliv. Rev. 10.1016/j.addr.2018.12.003 [DOI] [PubMed] [Google Scholar]
- 5.Gregg E. W., Sattar N., Ali M. K. (2016) The changing face of diabetes complications. Lancet Diabetes Endocrinol. 4, 537–547 [DOI] [PubMed] [Google Scholar]
- 6.Mohler M. L., He Y., Wu Z., Hwang D. J., Miller D. D. (2009) Recent and emerging anti-diabetes targets. Med. Res. Rev. 29, 125–195 [DOI] [PubMed] [Google Scholar]
- 7.Ma J. X., Li J. H., Ge Z., Wu Y. F. (2016) Surveillance of non-communicable disease risk factors. In Urbanization and Public Health in China (Li M. and Wu Y. F., eds.), pp. 69–86, Imperial College Press, London [Google Scholar]
- 8.Roberfroid M., Slavin J. (2000) Nondigestible oligosaccharides. Crit. Rev. Food Sci. Nutr. 40, 461–480 [DOI] [PubMed] [Google Scholar]
- 9.Rastall R. A., Gibson G. R. (2015) Recent developments in prebiotics to selectively impact beneficial microbes and promote intestinal health. Curr. Opin. Biotechnol. 32, 42–46 [DOI] [PubMed] [Google Scholar]
- 10.Swennen K., Courtin C. M., Delcour J. A. (2006) Non-digestible oligosaccharides with prebiotic properties. Crit. Rev. Food Sci. Nutr. 46, 459–471 [DOI] [PubMed] [Google Scholar]
- 11.Nakakuki T. (2002) Present status and future of functional oligosaccharide development in Japan. Pure Appl. Chem. 74, 1245–1251 [Google Scholar]
- 12.Kothari D., Patel S., Goyal A. (2014) Therapeutic spectrum of nondigestible oligosaccharides: overview of current state and prospect. J. Food Sci. 79, R1491–R1498 [DOI] [PubMed] [Google Scholar]
- 13.Sarao L. K., Arora M. (2017) Probiotics, prebiotics, and microencapsulation: a review. Crit. Rev. Food Sci. Nutr. 57, 344–371 [DOI] [PubMed] [Google Scholar]
- 14.Muanprasat C., Chatsudthipong V. (2017) Chitosan oligosaccharide: biological activities and potential therapeutic applications. Pharmacol. Ther. 170, 80–97 [DOI] [PubMed] [Google Scholar]
- 15.Katiyar D., Singh B., Lall A. M., Haldar C. (2011) Efficacy of chitooligosaccharides for the management of diabetes in alloxan induced mice: a correlative study with antihyperlipidemic and antioxidative activity. Eur. J. Pharm. Sci. 44, 534–543 [DOI] [PubMed] [Google Scholar]
- 16.Kim J. G., Jo S. H., Ha K. S., Kim S. C., Kim Y. C., Apostolidis E., Kwon Y. I. (2014) Effect of long-term supplementation of low molecular weight chitosan oligosaccharide (GO2KA1) on fasting blood glucose and HbA1c in db/db mice model and elucidation of mechanism of action. BMC Complement. Altern. Med. 14, 272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh S. P., Jadaun J. S., Narnoliya L. K., Pandey A. (2017) Prebiotic oligosaccharides: special focus on fructooligosaccharides, its biosynthesis and bioactivity. Appl. Biochem. Biotechnol. 183, 613–635 [DOI] [PubMed] [Google Scholar]
- 18.Cani P. D., Knauf C., Iglesias M. A., Drucker D. J., Delzenne N. M., Burcelin R. (2006) Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide 1 receptor. Diabetes 55, 1484–1490 [DOI] [PubMed] [Google Scholar]
- 19.Al-Sheraji S. H., Ismail A., Manap M. Y., Mustafa S., Yusof R. M., Hassan F. A. (2013) Prebiotics as functional foods: a review. J. Funct. Foods 5, 1542–1553 [Google Scholar]
- 20.Sheu W. H., Lee I. T., Chen W., Chan Y. C. (2008) Effects of xylooligosaccharides in type 2 diabetes mellitus. J. Nutr. Sci. Vitaminol. (Tokyo) 54, 396–401 [DOI] [PubMed] [Google Scholar]
- 21.Marín-Manzano M. C., Abecia L., Hernández-Hernández O., Sanz M. L., Montilla A., Olano A., Rubio L. A., Moreno F. J., Clemente A. (2013) Galacto-oligosaccharides derived from lactulose exert a selective stimulation on the growth of Bifidobacterium animalis in the large intestine of growing rats. J. Agric. Food Chem. 61, 7560–7567 [DOI] [PubMed] [Google Scholar]
- 22.Vera C., Córdova A., Aburto C., Guerrero C., Suárez S., Illanes A. (2016) Synthesis and purification of galacto-oligosaccharides: state of the art. World J. Microbiol. Biotechnol. 32, 197 [DOI] [PubMed] [Google Scholar]
- 23.Sangwan V., Tomar S. K., Ali B., Singh R. R., Singh A. K. (2015) Hypoglycaemic effect of galactooligosaccharides in alloxan-induced diabetic rats. J. Dairy Res. 82, 70–77 [DOI] [PubMed] [Google Scholar]
- 24.Chappuis E., Morel-Depeisse F., Bariohay B., Roux J. (2017) Alpha-galacto-oligosaccharides at low dose improve liver steatosis in a high-fat diet mouse model. Molecules 22, E1725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kato K., Watanabe T., Matsuda K. (1970) Studies on the chemical structure of konjac mannan. Part II. Isolation and characterization of oligosaccharides from the enzymatic hydrolyzate of the mannan. Agric. Biol. Chem. 34, 532–539 [Google Scholar]
- 26.Wang H., Zhang X., Wang S., Li H., Lu Z., Shi J., Xu Z. (2018) Mannan-oligosaccharide modulates the obesity and gut microbiota in high-fat diet-fed mice. Food Funct. 9, 3916–3929 [DOI] [PubMed] [Google Scholar]
- 27.Zheng J., Li H., Zhang X., Jiang M., Luo C., Lu Z., Xu Z., Shi J. (2018) Prebiotic mannan-oligosaccharides augment the hypoglycemic effects of metformin in correlation with modulating gut microbiota. J. Agric. Food Chem. 66, 5821–5831 [DOI] [PubMed] [Google Scholar]
- 28.Hong S. J., Lee J. H., Kim E. J., Yang H. J., Park J. S., Hong S. K. (2017) Anti-obesity and anti-diabetic effect of neoagarooligosaccharides on high-fat diet-induced obesity in mice. Mar. Drugs 15, E90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiao L., Van’t Land B., Engen P. A., Naqib A., Green S. J., Nato A., Leusink-Muis T., Garssen J., Keshavarzian A., Stahl B., Folkerts G. (2018) Human milk oligosaccharides protect against the development of autoimmune diabetes in NOD-mice. Sci. Rep. 8, 3829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim H. J., Ahn H. Y., Kwak J. H., Shin D. Y., Kwon Y. I., Oh C. G., Lee J. H. (2014) The effects of chitosan oligosaccharide (GO2KA1) supplementation on glucose control in subjects with prediabetes. Food Funct. 5, 2662–2669 [DOI] [PubMed] [Google Scholar]
- 31.Yang J., Summanen P. H., Henning S. M., Hsu M., Lam H., Huang J., Tseng C. H., Dowd S. E., Finegold S. M., Heber D., Li Z. (2015) Xylooligosaccharide supplementation alters gut bacteria in both healthy and prediabetic adults: a pilot study. Front. Physiol. 6, 216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mizote A., Yamada M., Yoshizane C., Arai N., Maruta K., Arai S., Endo S., Ogawa R., Mitsuzumi H., Ariyasu T., Fukuda S. (2016) Daily intake of trehalose is effective in the prevention of lifestyle-related diseases in individuals with risk factors for metabolic syndrome. J. Nutr. Sci. Vitaminol. (Tokyo) 62, 380–387 [DOI] [PubMed] [Google Scholar]
- 33.Parnell J. A., Reimer R. A. (2009) Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am. J. Clin. Nutr. 89, 1751–1759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Canfora E. E., van der Beek C. M., Hermes G. D. A., Goossens G. H., Jocken J. W. E., Holst J. J., van Eijk H. M., Venema K., Smidt H., Zoetendal E. G., Dejong C. H. C., Lenaerts K., Blaak E. E. (2017) Supplementation of diet with galacto-oligosaccharides increases bifidobacteria, but not insulin sensitivity, in obese prediabetic individuals. Gastroenterology 153, 87–97.e3 [DOI] [PubMed] [Google Scholar]
- 35.Gobinath D., Madhu A. N., Prashant G., Srinivasan K., Prapulla S. G. (2010) Beneficial effect of xylo-oligosaccharides and fructo-oligosaccharides in streptozotocin-induced diabetic rats. Br. J. Nutr. 104, 40–47 [DOI] [PubMed] [Google Scholar]
- 36.Dehghan P., Pourghassem Gargari B., Asghari Jafar-abadi M. (2014) Oligofructose-enriched inulin improves some inflammatory markers and metabolic endotoxemia in women with type 2 diabetes mellitus: a randomized controlled clinical trial. Nutrition 30, 418–423 [DOI] [PubMed] [Google Scholar]
- 37.Fei B. B., Ling L., Hua C., Ren S. Y. (2014) Effects of soybean oligosaccharides on antioxidant enzyme activities and insulin resistance in pregnant women with gestational diabetes mellitus. Food Chem. 158, 429–432 [DOI] [PubMed] [Google Scholar]
- 38.Kassaian N., Feizi A., Aminorroaya A., Jafari P., Ebrahimi M. T., Amini M. (2018) The effects of probiotics and synbiotic supplementation on glucose and insulin metabolism in adults with prediabetes: a double-blind randomized clinical trial. Acta Diabetol. 55, 1019–1028 [DOI] [PubMed] [Google Scholar]
- 39.Tajabadi-Ebrahimi M., Sharifi N., Farrokhian A., Raygan F., Karamali F., Razzaghi R., Taheri S., Asemi Z. (2017) A randomized controlled clinical trial investigating the effect of synbiotic administration on markers of insulin metabolism and lipid profiles in overweight type 2 diabetic patients with coronary heart disease. Exp. Clin. Endocrinol. Diabetes 125, 21–27 [DOI] [PubMed] [Google Scholar]
- 40.American Diabetes Association (2010) Diagnosis and classification of diabetes mellitus. Diabetes Care 33(Suppl 1), S62–S69; erratum: e57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Santamaria P. (2010) The long and winding road to understanding and conquering type 1 diabetes. Immunity 32, 437–445 [DOI] [PubMed] [Google Scholar]
- 42.Chan C., Hyslop C. M., Shrivastava V., Ochoa A., Reimer R. A., Huang C. (2016) Oligofructose as an adjunct in treatment of diabetes in NOD mice. Sci. Rep. 6, 37627; erratum: 7, 45800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liu B., Liu W. S., Han B. Q., Sun Y. Y. (2007) Antidiabetic effects of chitooligosaccharides on pancreatic islet cells in streptozotocin-induced diabetic rats. World J. Gastroenterol. 13, 725–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang C. M., Yu S. H., Zhang L. S., Zhao Z. Y., Dong L. L. (2014) Effects of several acetylated chitooligosaccharides on antioxidation, antiglycation and NO generation in erythrocyte. Bioorg. Med. Chem. Lett. 24, 4053–4057 [DOI] [PubMed] [Google Scholar]
- 45.Ju C., Yue W., Yang Z., Zhang Q., Yang X., Liu Z., Zhang F. (2010) Antidiabetic effect and mechanism of chitooligosaccharides. Biol. Pharm. Bull. 33, 1511–1516 [DOI] [PubMed] [Google Scholar]
- 46.Derosa G., Maffioli P. (2012) α-Glucosidase inhibitors and their use in clinical practice. Arch. Med. Sci. 8, 899–906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bischoff H. (1994) Pharmacology of alpha-glucosidase inhibition. Eur. J. Clin. Invest. 24 (Suppl3), 3–10 [PubMed] [Google Scholar]
- 48.Yu S. Y., Kwon Y. I., Lee C., Apostolidis E., Kim Y. C. (2017) Antidiabetic effect of chitosan oligosaccharide (GO2KA1) is mediated via inhibition of intestinal alpha-glucosidase and glucose transporters and PPARγ expression. Biofactors 43, 90–99 [DOI] [PubMed] [Google Scholar]
- 49.Lin W. L., Su W. W., Cai X. Y., Luo L. K., Li P. B., Wang Y. G. (2011) Fermentation effects of oligosaccharides of Radix Ophiopogonis on alloxan-induced diabetes in mice. Int. J. Biol. Macromol. 49, 194–200 [DOI] [PubMed] [Google Scholar]
- 50.Kang S., Tsai L. T., Rosen E. D. (2016) Nuclear mechanisms of insulin resistance. Trends Cell Biol. 26, 341–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hao C., Hao J., Wang W., Li G., Zeng Y., Wang P., Zhao X., Yu G. (2011) Oligomannuronate-chromium (III) complex ameliorates insulin resistance in C57BL/KsJ-db/db mice. J. Ocean Univ. China 10, 336–342 [Google Scholar]
- 52.Hao J., Hao C., Zhang L., Liu X., Zhou X., Dun Y., Li H., Li G., Zhao X., An Y., Liu J., Yu G. (2015) OM2, a novel oligomannuronate-chromium(III) complex, promotes mitochondrial biogenesis and lipid metabolism in 3T3-L1 adipocytes via the AMPK-PGC1α pathway. PLoS One 10, e0131930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.De Vadder F., Kovatcheva-Datchary P., Goncalves D., Vinera J., Zitoun C., Duchampt A., Bäckhed F., Mithieux G. (2014) Microbiota-generated metabolites promote metabolic benefits via gut-brain neural circuits. Cell 156, 84–96 [DOI] [PubMed] [Google Scholar]
- 54.Kumar S. G., Rahman M. A., Lee S. H., Hwang H. S., Kim H. A., Yun J. W. (2009) Plasma proteome analysis for anti-obesity and anti-diabetic potentials of chitosan oligosaccharides in ob/ob mice. Proteomics 9, 2149–2162 [DOI] [PubMed] [Google Scholar]
- 55.Ha B. G., Park J. E., Shon Y. H. (2016) Stimulatory effect of balanced deep-sea water containing chitosan oligosaccharides on glucose uptake in C2C12 myotubes. Mar. Biotechnol. (NY) 18, 475–484 [DOI] [PubMed] [Google Scholar]
- 56.Muthukumaran P., Thiyagarajan G., Arun Babu R., Lakshmi B. S. (2018) Raffinose from Costus speciosus attenuates lipid synthesis through modulation of PPARs/SREBP1c and improves insulin sensitivity through PI3K/AKT. Chem. Biol. Interact. 284, 80–89 [DOI] [PubMed] [Google Scholar]
- 57.Zeng W., Pirzgalska R. M., Pereira M. M., Kubasova N., Barateiro A., Seixas E., Lu Y. H., Kozlova A., Voss H., Martins G. G., Friedman J. M., Domingos A. I. (2015) Sympathetic neuro-adipose connections mediate leptin-driven lipolysis. Cell 163, 84–94 [DOI] [PubMed] [Google Scholar]
- 58.Singh D. P., Singh J., Boparai R. K., Zhu J., Mantri S., Khare P., Khardori R., Kondepudi K. K., Chopra K., Bishnoi M. (2017) Isomalto-oligosaccharides, a prebiotic, functionally augment green tea effects against high fat diet-induced metabolic alterations via preventing gut dysbacteriosis in mice. Pharmacol. Res. 123, 103–113 [DOI] [PubMed] [Google Scholar]
- 59.Shinoki A., Hara H. (2011) Dietary fructo-oligosaccharides improve insulin sensitivity along with the suppression of adipocytokine secretion from mesenteric fat cells in rats. Br. J. Nutr. 106, 1190–1197 [DOI] [PubMed] [Google Scholar]
- 60.Cox A. J., West N. P., Cripps A. W. (2015) Obesity, inflammation, and the gut microbiota. Lancet Diabetes Endocrinol. 3, 207–215 [DOI] [PubMed] [Google Scholar]
- 61.Cani P. D., Amar J., Iglesias M. A., Poggi M., Knauf C., Bastelica D., Neyrinck A. M., Fava F., Tuohy K. M., Chabo C., Waget A., Delmée E., Cousin B., Sulpice T., Chamontin B., Ferrières J., Tanti J. F., Gibson G. R., Casteilla L., Delzenne N. M., Alessi M. C., Burcelin R. (2007) Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 [DOI] [PubMed] [Google Scholar]
- 62.Pejnovic N. N., Pantic J. M., Jovanovic I. P., Radosavljevic G. D., Milovanovic M. Z., Nikolic I. G., Zdravkovic N. S., Djukic A. L., Arsenijevic N. N., Lukic M. L. (2013) Galectin-3 deficiency accelerates high-fat diet-induced obesity and amplifies inflammation in adipose tissue and pancreatic islets. Diabetes 62, 1932–1944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu T., Li J., Liu Y., Xiao N., Suo H., Xie K., Yang C., Wu C. (2012) Short-chain fatty acids suppress lipopolysaccharide-induced production of nitric oxide and proinflammatory cytokines through inhibition of NF-κB pathway in RAW264.7 cells. Inflammation 35, 1676–1684 [DOI] [PubMed] [Google Scholar]
- 64.Morel F. B., Dai Q., Ni J., Thomas D., Parnet P., Fança-Berthon P. (2015) α-Galacto-oligosaccharides dose-dependently reduce appetite and decrease inflammation in overweight adults. J. Nutr. 145, 2052–2059 [DOI] [PubMed] [Google Scholar]
- 65.Fang W., Bi D., Zheng R., Cai N., Xu H., Zhou R., Lu J., Wan M., Xu X. (2017) Identification and activation of TLR4-mediated signalling pathways by alginate-derived guluronate oligosaccharide in RAW264.7 macrophages. Sci. Rep. 7, 1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barra N. G., Chew M. V., Holloway A. C., Ashkar A. A. (2012) Interleukin-15 treatment improves glucose homeostasis and insulin sensitivity in obese mice. Diabetes Obes. Metab. 14, 190–193 [DOI] [PubMed] [Google Scholar]
- 67.Rosa J. S., Oliver S. R., Mitsuhashi M., Flores R. L., Pontello A. M., Zaldivar F. P., Galassetti P. R. (2008) Altered kinetics of interleukin-6 and other inflammatory mediators during exercise in children with type 1 diabetes. J. Investig. Med. 56, 701–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cernea S., Herold K. C. (2006) Drug insight: new immunomodulatory therapies in type 1 diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2, 89–98 [DOI] [PubMed] [Google Scholar]
- 69.Sheng X., Wang M., Lu M., Xi B., Sheng H., Zang Y. Q. (2011) Rhein ameliorates fatty liver disease through negative energy balance, hepatic lipogenic regulation, and immunomodulation in diet-induced obese mice. Am. J. Physiol. Endocrinol. Metab. 300, E886–E893 [DOI] [PubMed] [Google Scholar]
- 70.Lindsay R. S., Funahashi T., Hanson R. L., Matsuzawa Y., Tanaka S., Tataranni P. A., Knowler W. C., Krakoff J. (2002) Adiponectin and development of type 2 diabetes in the Pima Indian population. Lancet 360, 57–58 [DOI] [PubMed] [Google Scholar]
- 71.Bai Y., Zheng J., Yuan X., Jiao S., Feng C., Du Y., Liu H., Zheng L. (2018) Chitosan oligosaccharides improve glucolipid metabolism disorder in liver by suppression of obesity-related inflammation and restoration of peroxisome proliferator-activated receptor gamma (PPARγ). Mar. Drugs 16, E455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Delzenne N. M. (2003) Oligosaccharides: state of the art. Proc. Nutr. Soc. 62, 177–182 [DOI] [PubMed] [Google Scholar]
- 73.Diamant M., Blaak E. E., de Vos W. M. (2011) Do nutrient-gut-microbiota interactions play a role in human obesity, insulin resistance and type 2 diabetes? Obes. Rev. 12, 272–281 [DOI] [PubMed] [Google Scholar]
- 74.Dewulf E. M., Cani P. D., Claus S. P., Fuentes S., Puylaert P. G., Neyrinck A. M., Bindels L. B., de Vos W. M., Gibson G. R., Thissen J. P., Delzenne N. M. (2013) Insight into the prebiotic concept: lessons from an exploratory, double blind intervention study with inulin-type fructans in obese women. Gut 62, 1112–1121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murri M., Leiva I., Gomez-Zumaquero J. M., Tinahones F. J., Cardona F., Soriguer F., Queipo-Ortuño M. I. (2013) Gut microbiota in children with type 1 diabetes differs from that in healthy children: a case-control study. BMC Med. 11, 46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.De Goffau M. C., Luopajärvi K., Knip M., Ilonen J., Ruohtula T., Härkönen T., Orivuori L., Hakala S., Welling G. W., Harmsen H. J., Vaarala O. (2013) Fecal microbiota composition differs between children with β-cell autoimmunity and those without. Diabetes 62, 1238–1244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wen L., Ley R. E., Volchkov P. Y., Stranges P. B., Avanesyan L., Stonebraker A. C., Hu C., Wong F. S., Szot G. L., Bluestone J. A., Gordon J. I., Chervonsky A. V. (2008) Innate immunity and intestinal microbiota in the development of Type 1 diabetes. Nature 455, 1109–1113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hildebrandt M. A., Hoffmann C., Sherrill-Mix S. A., Keilbaugh S. A., Hamady M., Chen Y. Y., Knight R., Ahima R. S., Bushman F., Wu G. D. (2009) High-fat diet determines the composition of the murine gut microbiome independently of obesity. Gastroenterology 137, 1716–1724.e1-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cai L., Wu H., Li D., Zhou K., Zou F. (2015) Type 2 diabetes biomarkers of human gut microbiota selected via iterative sure independent screening method. PLoS One 10, e0140827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu F., Li P., Chen M., Luo Y., Prabhakar M., Zheng H., He Y., Qi Q., Long H., Zhang Y., Sheng H., Zhou H. (2017) Fructooligosaccharide (FOS) and galactooligosaccharide (GOS) increase Bifidobacterium but reduce butyrate producing bacteria with adverse glycemic metabolism in healthy young population. Sci. Rep. 7, 11789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ose R., Hirano K., Maeno S., Nakagawa J., Salminen S., Tochio T., Endo A. (2018) The ability of human intestinal anaerobes to metabolize different oligosaccharides: novel means for microbiota modulation? Anaerobe 51, 110–119 [DOI] [PubMed] [Google Scholar]
- 82.De Luis D. A., de la Fuente B., Izaola O., Conde R., Gutiérrez S., Morillo M., Teba Torres C. (2011) Double blind randomized clinical trial controlled by placebo with an alpha linoleic acid and prebiotic enriched cookie on risk cardiovascular factor in obese patients. Nutr. Hosp. 26, 827–833 [DOI] [PubMed] [Google Scholar]
- 83.Everard A., Lazarevic V., Derrien M., Girard M., Muccioli G. G., Neyrinck A. M., Possemiers S., Van Holle A., François P., de Vos W. M., Delzenne N. M., Schrenzel J., Cani P. D. (2011) Responses of gut microbiota and glucose and lipid metabolism to prebiotics in genetic obese and diet-induced leptin-resistant mice. Diabetes 60, 2775–2786; erratum: 3307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cummings J. H., Macfarlane G. T., Englyst H. N. (2001) Prebiotic digestion and fermentation. Am. J. Clin. Nutr. 73 (Suppl), 415S–420S [DOI] [PubMed] [Google Scholar]
- 85.Vulevic J., Juric A., Tzortzis G., Gibson G. R. (2013) A mixture of trans-galactooligosaccharides reduces markers of metabolic syndrome and modulates the fecal microbiota and immune function of overweight adults. J. Nutr. 143, 324–331 [DOI] [PubMed] [Google Scholar]
- 86.Tolhurst G., Heffron H., Lam Y. S., Parker H. E., Habib A. M., Diakogiannaki E., Cameron J., Grosse J., Reimann F., Gribble F. M. (2012) Short-chain fatty acids stimulate glucagon-like peptide-1 secretion via the G-protein-coupled receptor FFAR2. Diabetes 61, 364–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nurunnabi M., Lee S. A., Revuri V., Hwang Y. H., Kang S. H., Lee M., Cho S., Cho K. J., Byun Y., Bae Y. H., Lee D. Y., Lee Y. K. (2017) Oral delivery of a therapeutic gene encoding glucagon-like peptide 1 to treat high fat diet-induced diabetes. J. Control Release 268, 305–313 [DOI] [PubMed] [Google Scholar]
- 88.Nieuwdorp M., Gilijamse P. W., Pai N., Kaplan L. M. (2014) Role of the microbiome in energy regulation and metabolism. Gastroenterology 146, 1525–1533 [DOI] [PubMed] [Google Scholar]
- 89.Nath A., Molnár M. A., Csighy A., Kőszegi K., Galambos I., Huszár K. P., Koris A., Vatai G. (2018) Biological activities of lactose-based prebiotics and symbiosis with probiotics on controlling osteoporosis, blood-lipid and glucose levels. Medicina 54, 98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Delzenne N. M., Cani P. D., Everard A., Neyrinck A. M., Bindels L. B. (2015) Gut microorganisms as promising targets for the management of type 2 diabetes. Diabetologia 58, 2206–2217 [DOI] [PubMed] [Google Scholar]
- 91.Wu X., Wang J., Shi Y., Chen S., Yan Q., Jiang Z., Jing H. (2017) N-Acetyl-chitobiose ameliorates metabolism dysfunction through Erk/p38 MAPK and histone H3 phosphorylation in type 2 diabetes mice. J. Funct. Foods 28, 96–105 [Google Scholar]
- 92.Ohashi N., Morino K., Ida S., Sekine O., Lemecha M., Kume S., Park S. Y., Choi C. S., Ugi S., Maegawa H. (2017) Pivotal role of o-glcnac modification in cold-induced thermogenesis by brown adipose tissue through mitochondrial biogenesis. Diabetes 66, 2351–2362 [DOI] [PubMed] [Google Scholar]
- 93.Zhang R., Zhou J., Li M., Ma H., Qiu J., Luo X., Jia Z. (2014) Ameliorating effect and potential mechanism of Rehmannia glutinosa oligosaccharides on the impaired glucose metabolism in chronic stress rats fed with high-fat diet. Phytomedicine 21, 607–614 [DOI] [PubMed] [Google Scholar]
- 94.Ueno M., Tamura Y., Toda N., Yoshinaga M., Terakado S., Otsuka K., Numabe A., Kawabata Y., Murota I., Sato N., Uehara Y. (2012) Sodium alginate oligosaccharides attenuate hypertension in spontaneously hypertensive rats fed a low-salt diet. Clin. Exp. Hypertens. 34, 305–310 [DOI] [PubMed] [Google Scholar]
- 95.Hoshino-Takao I., Fujii S., Ishii A., Han L. K., Okuda H., Kumao T. (2008) Effects of mannooligosaccharides from coffee mannan on blood pressure in Dahl salt-sensitive rats. J. Nutr. Sci. Vitaminol. (Tokyo) 54, 181–184 [DOI] [PubMed] [Google Scholar]
- 96.Terakado S., Ueno M., Tamura Y., Toda N., Yoshinaga M., Otsuka K., Numabe A., Kawabata Y., Murota I., Sato N., Uehara Y. (2012) Sodium alginate oligosaccharides attenuate hypertension and associated kidney damage in Dahl salt-sensitive rats fed a high-salt diet. Clin. Exp. Hypertens. 34, 99–106 [DOI] [PubMed] [Google Scholar]
- 97.Chu K. Y., Leung P. S. (2009) Angiotensin II in type 2 diabetes mellitus. Curr. Protein Pept. Sci. 10, 75–84 [DOI] [PubMed] [Google Scholar]
- 98.Hussain M., Abbas S., Bilal A., Aslam M. N., Awan F. R. (2019) Association of ACE I/D polymorphism with risk of diabetes in cardiovascular disease patients. [E-pub ahead of print] Exp. Clin. Endocrinol. Diabetes [DOI] [PubMed] [Google Scholar]
- 99.Hussain M., Awan F. R. (2018) Hypertension regulating angiotensin peptides in the pathobiology of cardiovascular disease. Clin. Exp. Hypertens. 40, 344–352 [DOI] [PubMed] [Google Scholar]
- 100.Hong S. P., Kim M. H., Oh S. W., Han C. K., Kim Y. H. (1998) ACE inhibitory and antihypertensive effect of chitosan oligosaccharides in SHR. Korean J. Food Sci. Technol. 30, 1476–1479 [Google Scholar]
- 101.Wijesekara I., Kim S. K. (2010) Angiotensin-I-converting enzyme (ACE) inhibitors from marine resources: prospects in the pharmaceutical industry. Mar. Drugs 8, 1080–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yeo S. K., Ooi L. G., Lim T. J., Liong M. T. (2009) Antihypertensive properties of plant-based prebiotics. Int. J. Mol. Sci. 10, 3517–3530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Naveed M., Phil L., Sohail M., Hasnat M., Baig M. M. F. A., Ihsan A. U., Shumzaid M., Kakar M. U., Mehmood Khan T., Akabar M. D., Husain M. I., Zhou Q. G. (2019) Chitosan oligosaccharide (COS): an overview. Int. J. Biol. Macromol. 129, 827–843 [DOI] [PubMed] [Google Scholar]
- 104.Halmos T., Suba I. (2017) [Non-alcoholic fatty liver disease, as a component of the metabolic syndrome, and its causal correlations with other extrahepatic diseases [Article in Hungarian]]. Orv. Hetil. 158, 2051–2061 [DOI] [PubMed] [Google Scholar]
- 105.Tremaroli V., Bäckhed F. (2012) Functional interactions between the gut microbiota and host metabolism. Nature 489, 242–249 [DOI] [PubMed] [Google Scholar]
- 106.Zhao L., Zhang F., Ding X., Wu G., Lam Y. Y., Wang X., Fu H., Xue X., Lu C., Ma J., Yu L., Xu C., Ren Z., Xu Y., Xu S., Shen H., Zhu X., Shi Y., Shen Q., Dong W., Liu R., Ling Y., Zeng Y., Wang X., Zhang Q., Wang J., Wang L., Wu Y., Zeng B., Wei H., Zhang M., Peng Y., Zhang C. (2018) Gut bacteria selectively promoted by dietary fibers alleviate type 2 diabetes. Science 359, 1151–1156 [DOI] [PubMed] [Google Scholar]
- 107.Bäckhed F., Manchester J. K., Semenkovich C. F., Gordon J. I. (2007) Mechanisms underlying the resistance to diet-induced obesity in germ-free mice. Proc. Natl. Acad. Sci. USA 104, 979–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kirpich I. A., Marsano L. S., McClain C. J. (2015) Gut-liver axis, nutrition, and non-alcoholic fatty liver disease. Clin. Biochem. 48, 923–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bekkering P., Jafri I., van Overveld F. J., Rijkers G. T. (2013) The intricate association between gut microbiota and development of type 1, type 2 and type 3 diabetes. Expert Rev. Clin. Immunol. 9, 1031–1041 [DOI] [PubMed] [Google Scholar]
- 110.Forsythe P., Kunze W. A., Bienenstock J. (2012) On communication between gut microbes and the brain. Curr. Opin. Gastroenterol. 28, 557–562 [DOI] [PubMed] [Google Scholar]
- 111.Sah P., Faber E. S., Lopez De Armentia M., Power J. (2003) The amygdaloid complex: anatomy and physiology. Physiol. Rev. 83, 803–834 [DOI] [PubMed] [Google Scholar]
- 112.Rios M. (2013) BDNF and the central control of feeding: accidental bystander or essential player? Trends Neurosci. 36, 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kernie S. G., Liebl D. J., Parada L. F. (2000) BDNF regulates eating behavior and locomotor activity in mice. EMBO J. 19, 1290–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Van de Wouw M., Boehme M., Lyte J. M., Wiley N., Strain C., O’Sullivan O., Clarke G., Stanton C., Dinan T. G., Cryan J. F. (2018) Short-chain fatty acids: microbial metabolites that alleviate stress-induced brain-gut axis alterations. J. Physiol. 596, 4923–4944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tusi S. K., Khalaj L., Ashabi G., Kiaei M., Khodagholi F. (2011) Alginate oligosaccharide protects against endoplasmic reticulum- and mitochondrial-mediated apoptotic cell death and oxidative stress. Biomaterials 32, 5438–5458 [DOI] [PubMed] [Google Scholar]
- 116.Aliasgharzadeh A., Khalili M., Mirtaheri E., Gargari B. P., Tavakoli F., Farhangi M.A., Babaei H., Dehghan P. (2015) A combination of prebiotic inulin and oligofructose improve some of cardiovascular disease risk factors in women with type 2 diabetes: a randomized controlled clinical trial. Adv. Pharm. Bull. 5, 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Asemi Z., Bahmani S., Shakeri H., Jamal A., Faraji A. M. (2015) Effect of multispecies probiotic supplements on serum minerals, liver enzymes and blood pressure in patients with type 2 diabetes. Int. J. Diabetes Dev. C. 35, 90–95 [Google Scholar]
- 118.Bianchi G., Ronchi M., Marchesini G. (2016) Effect of lactulose on carbohydrate metabolism and diabetes mellitus. Scand. J. Gastroentero. 222, 62–64 [DOI] [PubMed] [Google Scholar]
- 119.Moriya Y., Shida Yamane Y., Miyamoto Y., Kimura M., Huse N., Ebisawa K., Kameda Y., Nishi A., Du D. (2013) Subcutaneous administration of sodium alginate oligosaccharides prevents salt-induced hypertension in Dahl salt-sensitive rats. Clin. Exp. Hypertens. 35, 607–613 [DOI] [PubMed] [Google Scholar]
- 120.Li Z., Yang J., Carslon P., Henning S., Hsu M., Hsu M., Tseng C., Thames G., Finegold S., Heber D. (2015) Xylooligosaccharide induced changes in gut microbiota in healthy and prediabetic adults. FASEB J. 29 (Suppl 1), 924.28 (abstr.) [Google Scholar]