Abstract
Currently, quantifying β-cell mass (BCM) requires harvesting the pancreas. In this study, we investigated a potential noninvasive method to quantify BCM changes longitudinally using [Lys12(111In-BnDTPA-Ahx)]exendin-4 ([111In]-Ex4) and single-photon emission computed tomography (SPECT). We used autoradiography and transgenic mice expressing green fluorescent protein under the control of mouse insulin 1 gene promotor to evaluate the specificity of [111In]-Ex4 toward β cells. Using nonobese diabetic (NOD) mice, we injected [111In]-Ex4 (3.0 MBq) intravenously and performed SPECT 30 min later, repeating this at a 2-wk interval. After the second scan, we harvested the pancreas and calculated BCM from immunohistochemically stained pancreatic sections. Specific accumulation of [111In]-Ex4 in β cells was confirmed by autoradiography, with a significant correlation (r = 0.94) between the fluorescent and radioactive signal intensities. The radioactive signal from the pancreas in the second SPECT scan significantly correlated (r = 0.89) with BCM calculated from the immunostained pancreatic sections. We developed a regression formula to estimate BCM from the radioactive signals from the pancreas in SPECT scans. BCM can be quantified longitudinally and noninvasively by SPECT imaging with [111In]-Ex4. This technique successfully demonstrated longitudinal changes in BCM in NOD mice before and after onset of hyperglycemia.—Fujita, N., Fujimoto, H., Hamamatsu, K., Murakami, T., Kimura, H., Toyoda, K., Saji, H., Inagaki, N. Noninvasive longitudinal quantification of β-cell mass with [111In]-labeled exendin-4.
Keywords: in vivo radioisotope, GLP-1 receptor, SPECT
Diabetes is a major concern, with increasing incidence worldwide and growing costs for care and the prevention of complications. The prevalence of diabetes in 2017 has been estimated at 425 million, with 4.0 million people dying from complications and a total of $727 billion spent on diabetes worldwide (1). In the United Kingdom Prospective Diabetes Study, the overall function of pancreatic β cells is reported to decrease with the disease progression (2), leading to a disturbance in glycemic control and the development of complications. β-cell mass (BCM) is also thought to decrease (3), with studies showing lower BCM in patients with diabetes than in normally glycemic subjects, both Western and Asian (4–8). However, clinical studies of BCM are currently limited to cross-sectional studies in which the pancreas is obtained from autopsy or operation. Although there are a few arguments regarding the feasibility of the clinical implementation on BCM quantification (9–13), the study of how BCM changes over time would provide a deeper understanding of the pathophysiology of diabetes and could be used to develop a scale to evaluate the efficacy of antidiabetic drugs in preserving or increasing BCM.
The most promising strategy for evaluating BCM changes longitudinally is to use a radioisotope-labeled probe that specifically accumulates in β cells in the pancreas; the radioactive signals from the probe could then be measured in vivo by positron emission tomography (PET) or single-photon emission computed tomography (SPECT) (14). Measuring the intensity of the signals would allow BCM to be estimated noninvasively. Probes currently considered promising include those targeting vesicular monoamine transporter 2 and glucagon-like peptide-1 receptor (GLP-1R) (15–20). We have previously reported the specific accumulation of GLP-1R–targeting probes in β cells (21–24). Brom et al. (25) conducted a clinical cross-sectional study using a GLP-1R–targeting probe and SPECT, which compared the pancreas signal intensity between patients with type 1 diabetes and healthy controls. There are a few longitudinal studies that aimed to measure BCM with bioluminescence imaging or MRI in basic research (26, 27). Souza et al. (28) measured pancreatic max standardized uptake values in their longitudinal study on biobreeding–diabetes-prone rats with a vesicular monoamine transporter 2–targeting probe and PET. However, as of yet there has been no longitudinal study of BCM measured noninvasively using SPECT and a GLP-1R–targeting probe, even in basic research.
In this study, we used a GLP-1R–targeting probe, exendin-4 labeled with [111In], to investigate whether the intensity of the radioactive signal from the pancreas acquired by SPECT scanning could be used to measure BCM noninvasively in vivo by comparing the signal intensity with BCM estimated from immunohistochemically stained pancreatic sections. We also applied the technique to evaluate longitudinal changes in BCM in a diabetes mouse model.
MATERIALS AND METHODS
Radiolabeling of exendin-4
All reagents, including 2-(N-morpholino)ethanesulfonic acid (MES) and polysorbate 80, were purchased from Nacalai Tesque (Kyoto, Japan), Wako Pure Chemical Industries (Tokyo, Japan), Merck GmbH (Darmstadt, Germany), and MilliporeSigma (Burlington, MA, USA). [111In]Cl3 was purchased from Nihon Medi-Physics (Tokyo, Japan). The precursor peptide [Lys12(BnDTPA-Ahx)]exendin-4 was synthesized by KNC Laboratories (Kobe, Japan), as previously described in Kimura et al. (23). In brief, [111In] labeling was achieved by adding 74 MBq of [111In]Cl3 to 100 pmol of [Lys12(BnDTPA-Ahx)]exendin-4 in a solution of 0.01 M MES buffer containing 0.1% polysorbate 80. The resulting [Lys12([111In]-BnDTPA-Ahx)]exendin-4 ([111In]-Ex4) was then purified with a Sep-Pak Light C18 Cartridge (Waters, Milford, MA, USA). A single peak of the [111In]-Ex4 was confirmed by HPLC. LC-20AD (Shimadzu, Tokyo, Japan) was used for the HPLC with an SPD-20A UV detector (Shimadzu) and a US-3000 radioisotope detector (Universal Giken, Odawara, Japan).
Animals
Transgenic mice expressing green fluorescent protein (GFP) under the control of mouse insulin 1 gene promotor (MIP) were kindly provided by Dr. M. Hara (Chicago University, Chicago, IL, USA). The MIP-GFP mice were maintained on a C57BL/6 background. Female nonobese diabetic (NOD) mice were purchased from Clea Japan (Tokyo, Japan). These mice provide a model of type 1 diabetes, with insulitis occurring spontaneously, leading to destruction of β cells and a dramatic decrease in BCM. We chose the NOD mice because substantial alterations are expected to be seen in BCM. Moreover, it has been reported in refs. 29 and 30 that the insulitis can be induced earlier by administering cyclophosphamide, so we administered 8-wk-old female NOD mice with 200 mg/kg of cyclophosphamide intraperitoneally on 2 occasions, separated by an interval of 2 wk. All the mice were housed with free access to standard chow and water under a 12-h light/dark rhythm. Blood glucose levels were determined with a glucose analyzer (PocketChem BG PG-7320; Arkray Global Business, Kyoto, Japan) when the mice were 10 and 12 wk old. Serum C-peptide reactivity was also evaluated in the 12-wk-old mice using a Mouse C-peptide ELISA Kit (Morinaga Institute of Biological Science, Yokohama, Japan). Animal care and all the procedures were approved by the Animal Care Committee of Kyoto University.
Autoradiography and fluorescence image acquisition
[111In]-Ex4 (2.0 MBq) was injected into the tail veins of the MIP-GFP mice. After 60 min, the mouse was euthanized under anesthesia, and its pancreas was harvested. Pancreatic sections 10-µm thick were obtained. Fluorescence and radioactivity images of the pancreatic sections were acquired with an image analyzer (Typhoon 9410; GE Healthcare, Waukesha, WI, USA). The fluorescent and radioactive signal intensities of each pancreatic section were evaluated with ImageQuant TL software (GE Healthcare) from the respective images. The unit of these intensities was provided by the software and was arbitrary. Moreover, a blocking study was performed to confirm the specific accumulation of [111In]-Ex4 in islets. This involved injecting 1000 times the amount of nonradioactive exendin (9–39) 30 min before the injection of [111In]-Ex4 (2.0 MBq).
In addition, the correlation between the fluorescent and radioactive signal intensities were investigated on the same pancreatic sections (9 sections in total) to confirm the feasibility of estimating BCM in vivo using our [111In]-Ex4, based on the intensity of pancreatic accumulation of the radiotracer.
SPECT imaging and data acquisition
The SPECT/computed tomography (CT) scans were acquired on a Triumph LabPET12/SPECT4/CT scanner (TriFoil Imaging, Chatsworth, CA, USA), as previously reported (23). Ten NOD mice underwent 2 SPECT scans, both performed 30 min after the intravenous injection of [111In]-Ex4 (3.0 MBq). The first scans were performed on 10-wk-old euglycemic mice, and the second scans were performed on the same mice 2 wk later. The intensity of the radiation from the pancreas was evaluated as previously reported (31). In brief, to avoid the potentially huge influence of the kidneys on the pancreas signal intensity, an initial renal region of interest (ROI) was determined through comparison with the outline of the kidney on a CT image, and this ROI was then expanded by 2.7 mm in each direction and excluded from the analysis. A radiation intensity threshold was then established, using representative mice, to obtain a pancreatic ROI that was 75–80% of the whole pancreas. This evaluation was performed by an independent researcher who was not informed of the status of the mice. The threshold was then applied to establish the pancreatic ROIs for all the mice in both the first and second SPECT scans. The total intensity of radiation from the pancreatic ROIs was normalized by the injected dose of [111In]-Ex4.
After the second SPECT scan, the mice were euthanized under anesthesia, and their pancreases were harvested. Each pancreas was weighed, and the radioactivity from the pancreas was measured using a dose calibrator (IGC-7 curiemeter; Hitachi Aloka Medical, Tokyo, Japan). Pancreatic accumulation of [111In]-Ex4 was evaluated by dividing the measured radioactivity from the pancreas by that of the total injection of [111In]-Ex4. This was also normalized by dividing by the weight of the pancreas.
Other ex vivo and in vivo SPECT studies were performed to confirm the validity of the assumption that the second-strongest radioactive signal in the abdomen (other than the kidney) arises from the pancreas. A NOD mouse was euthanized under anesthesia, and its abdominal organs were harvested 30 min after the intravenous injection of [111In]-Ex4 (3.0 MBq). The harvested organs subsequently underwent SPECT scans (ex vivo SPECT study). In the next in vivo SPECT study, a preoperation SPECT scan was performed 30 min after the injection of [111In]-Ex4, and then pancreatectomy was performed on a NOD mouse under anesthesia. The harvested pancreas was put on the back of the mouse, and the postoperation SPECT scan was subsequently performed.
Six further NOD mice were simultaneously administered cyclophosphamide intraperitoneally 2 times, at 8 and 10 wk old. When aged 12 wk, they were injected with [111In]-Ex4, and their pancreases were harvested. Subsequently, each pancreas was weighed, and the radiation levels were measured using the same IGC-7 curiemeter (Hitachi Aloka Medical). Because of the limited number of SPECT scans performed in 1 d, 6 mice did not receive a SPECT scan; however, to increase the total number of mice investigated, the BCM calculation was performed for all 16 mice.
Estimating BCM from pancreatic sections
Ten pancreatic sections (4 µm thick) were evaluated for each mouse (n = 16) to provide an accurate calculation of BCM. Paraffin-embedded sections were stained with an anti-insulin antibody (H-86; Santa Cruz Biotechnology, Dallas, TX, USA), followed by overnight incubation. They were washed in PBS and incubated for 60 min with a secondary antibody (A11034; Thermo Fisher Scientific, Waltham, MA, USA). Adjacent sections were also stained with hematoxylin and eosin. Images of the entire pancreatic sections were scanned with an HS All-in-one Fluorescence Microscopy BZ-710 (Keyence, Osaka, Japan) with ×40 magnification. The images obtained were analyzed with BZ-II analyzer software (Keyence).
The total insulin-positive area was determined from pancreatic sections stained with the anti-insulin antibody and secondary antibody. The whole pancreatic area was determined from the adjacent hematoxylin and eosin–stained pancreatic sections. The relative β cell area was calculated by dividing the total insulin-positive area by the whole pancreatic area. The BCM was calculated by multiplying the relative β-cell area by the weight of the pancreas.
Sensitivity analysis
A sensitivity analysis was performed to assess the robustness of our method for analyzing the SPECT images. To do this, we investigated correlations between the radioactivity from the pancreas measured from the SPECT images and BCM calculated from immunohistochemically stained pancreatic sections, changing the radiation threshold used to set the pancreatic ROIs. The threshold was changed as follows +10, +5, +2.5, −2.5, −5, and −10%.
Statistical analysis
Continuous variables are reported as means ± sd unless otherwise stated. We used unpaired, 2-tailed Student’s t tests to compare continuous variables between 2 groups, and Pearson correlation analysis to investigate correlations between 2 continuous variables. All analyses were performed using Stata/SE v.11.2 (StataCorp, College Station, TX, USA). Values of P < 0.05 were considered statistically significant.
RESULTS
Radiolabeling of [111In]-DTPA12-exendin-4
The radiochemical purity and specific activity of the [111In]-Ex4 obtained were >95% and 700 GBq/μmol, respectively.
Autoradiography in the MIP-GFP mice
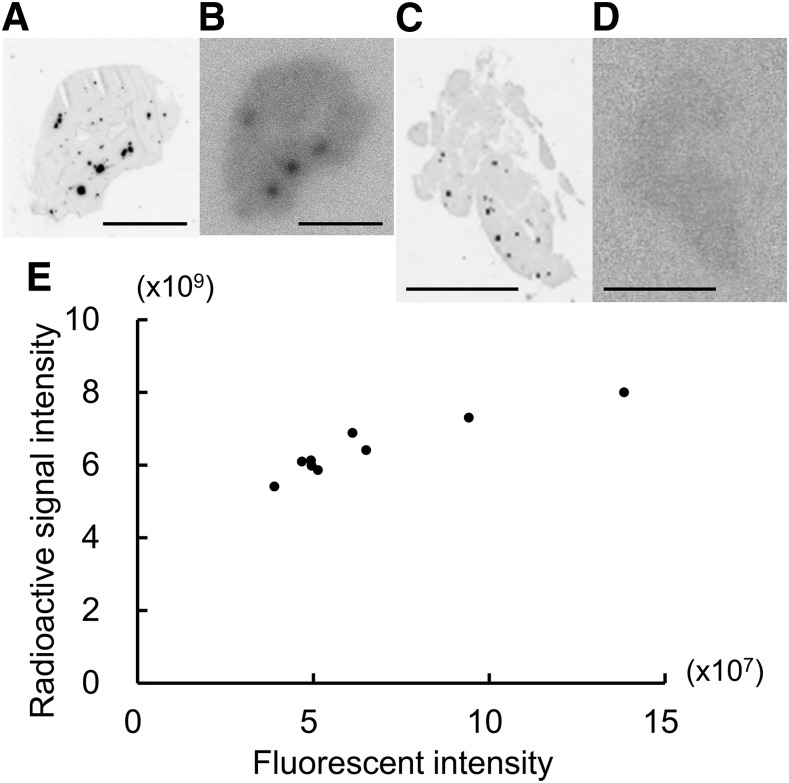
Fluorescence and radioactivity images of the pancreatic sections acquired with an image analyzer are shown in Fig. 1A–D. Because these pancreatic sections were obtained from MIP-GFP mice, black spots in the fluorescence images indicate β cells (i.e., islets). In the radioactivity images, hot spots indicate the accumulation of [111In]-Ex4. There was good colocalization of the islets and the accumulation of [111In]-Ex4.
Figure 1.
A–D) Fluorescence images (A, C) and radioactivity images (B, D) of pancreatic sections (10 µm thick) from an MIP-GFP mouse, acquired using an image analyzer. Images were obtained from a single pancreatic section after 1000 times the amount of nonradioactive exendin (9–39) was administered in advance to block the uptake of [111In]-Ex4 . The pancreas was harvested 60 min after the injection of [111In]-Ex4 (2.0 MBq). Scale bars, 5 mm. E) Fluorescent and radioactive signal intensities were evaluated from the respective images for 9 pancreatic sections. A strong correlation was observed between fluorescent and radioactive signal intensity (r = 0.94).
After blocking with a preinjection of an excess of nonradioactive exendin (9–39), no accumulation of [111In]-Ex4 was observed in the radioactivity images (Fig. 1D). This confirmed the islet-specific accumulation of [111In]-Ex4.
Fluorescent and radioactive signal intensities were evaluated from the respective images of 9 pancreatic sections obtained from the mouse. There was a strong correlation (r = 0.94) between the fluorescent and radioactive signal intensities (Fig. 1E).
NOD mice
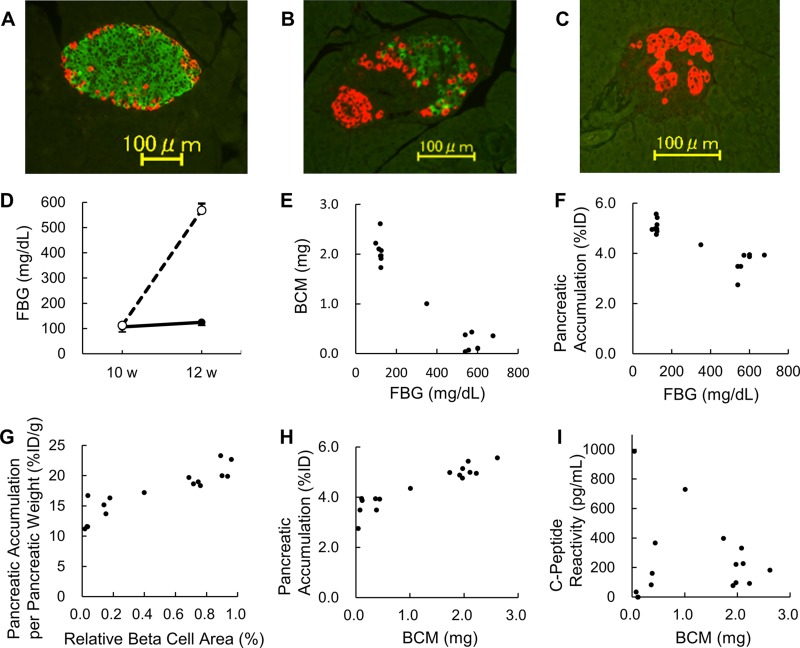
Ten NOD mice underwent 2 SPECT scans. All 10 were euglycemic at the beginning of the study (aged 8 wk) and at the time of the first SPECT scan (10 wk old). There was no significant difference in fasting blood glucose (FBG) levels at the time of the first SPECT scan, aged 10 wk, between the 2 groups (106 ± 20 vs. 113 ± 8 mg/dl, 0.59 ± 0.11 vs. 0.63 ± 0.05 mM, P = 0.58). Two weeks later, aged 12 wk, 4 of the 10 mice had become hyperglycemic, with a significant difference in FBG levels between the 6 euglycemic mice and the 4 hyperglycemic mice (124 ± 12 vs. 568 ± 27 mg/dl, 0.69 ± 0.07 vs. 3.16 ± 0.15 mM, P < 0.001). After the second SPECT scan, the pancreases were harvested and stained with anti-insulin and anti-glucagon antibodies; Fig. 2A shows representative sections from euglycemic mice, and Fig. 2B, C show representative sections from hyperglycemic mice. The course of FBG level is shown in Fig. 2D. Detailed data on FBG levels of the 10 mice are presented in Supplemental Table S1.
Figure 2.
A) Representative immunohistochemically stained pancreatic sections (4 μm) of a mouse that remained euglycemic. B, C) Representative immunohistochemically stained pancreatic sections of a mouse that became hyperglycemic. The pancreases were harvested and stained with anti-insulin and anti-glucagon antibodies after the second SPECT scan at the age of 12 wk. The insulin-stained and glucagon-stained areas are expressed with green and red, respectively. D) Course of FBG level in NOD mice. All 10 mice were euglycemic at 10 wk old. Four mice became hyperglycemic at 12 wk old, and the other 6 remained euglycemic. There was a significant difference in FBG levels at 12 wk old between the 2 groups (P < 0.001). The euglycemic and hyperglycemic mice are indicated by solid and broken lines, respectively. E) Correlation between FBG levels and BCM in female NOD mice aged 12 wk (n = 16). F) Correlation between FBG levels and the pancreatic accumulation of [111In]-Ex4. The pancreases were harvested 90 min after the injection of the [111In]-Ex4 (3.0 MBq). G) There was a strong correlation between the relative β cell area and the pancreatic accumulation of [111In]-Ex4 normalized by pancreatic weight (r = 0.90). H) A strong correlation was also observed between BCM and the pancreatic accumulation of [111In]-Ex4 (r = 0.93). I) There was no significant correlation between BCM and fasting serum C-peptide reactivity (r = 0.11).
BCM and pancreatic accumulation
Correlations between FBG levels and BCM or the pancreatic accumulation of [111In]-Ex4 are shown in Fig. 2E, F. These analyses included both the 10 mice scanned twice and the 6 additional mice. There was a strong correlation between the relative β-cell area and the pancreatic accumulation of [111In]-Ex4, normalized by pancreatic weight (r = 0.90; Fig. 2G). A strong correlation was also observed between BCM and the pancreatic accumulation of [111In]-Ex4 (r = 0.93; Fig. 2H). There was no significant correlation between BCM and serum C-peptide reactivity (r = 0.11; Fig. 2I).
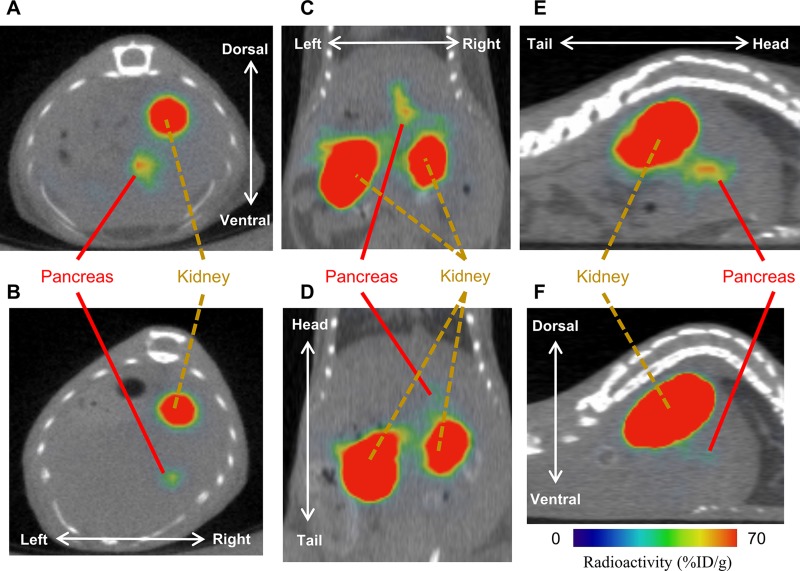
SPECT imaging and BCM
Representative SPECT images of euglycemic and hyperglycemic mice are shown in Fig. 3A–F. We confirmed the validity of the assumption that the second-strongest radioactive signal in the abdomen (other than the kidney) arose from the pancreas with the ex vivo and in vivo SPECT studies. In the ex vivo study, we were not able to find a radioactive signal from the liver, stomach, spleen, duodenum, small intestine, or colon. We detected a relatively strong radioactive signal from the pancreas (Supplemental Fig. S1A, B). In the in vivo pancreatomy study, the relatively strong radioactive signal in the abdomen disappeared in the postoperation scan, even though the radioactively strong signal other than that from the kidney had been detected in the preoperation scan. Instead, the relatively strong radioactive signal was confirmed to have arisen from the harvested pancreas located on the back of the mouse in the postoperation scan (Supplemental Fig. S2A–E).
Figure 3.
Representative SPECT images of euglycemic (A, C, E) and hyperglycemic (B, D, F) mice aged 12 wk. The SPECT imaging was acquired 30 min after injecting [111In]-Ex4 (3.0 MBq) intravenously. Transverse (A, B), coronal (C, D), and sagittal (E, F) images are shown.
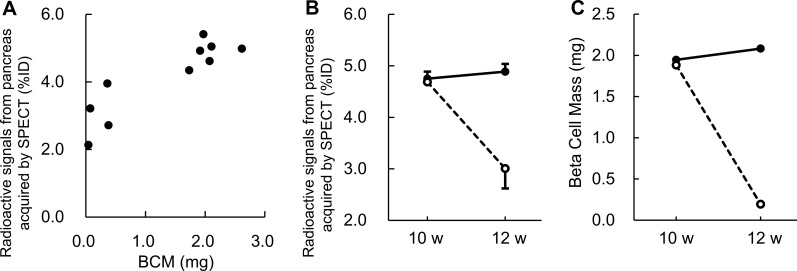
There was a good correlation between the radiation intensity from the pancreas measured from the second SPECT scan and BCM calculated from the immunostained pancreatic sections (r = 0.89; Fig. 4A). The associated regression formula for estimating BCM from the radioactivity level measured by SPECT imaging was as follows: BCM (mg) = (Y − 2.81) / 0.997, where Y is the ratio of pancreatic radioactivity measured from SPECT scan to percent injection dose (%ID). The volume of each pancreas was calculated from its weight based on a reported density of 1.08 g/cm3 (32). The proportion of the pancreas analyzed was 76.0% (range 43.7–94.4%) of the whole pancreas by volume.
Figure 4.
A) There was a good correlation (r = 0.89) between the radioactivity levels in the pancreas measured by SPECT imaging and β cell mass calculated from immunohistochemically stained pancreatic sections from 10 NOD mice aged 12 wk. The SPECT imaging was acquired 30 min after an intravenous injection of [111In]-Ex4 (3.0 MBq). B) Two SPECT scans were performed on the same 10 NOD mice at ages 10 and 12 wk, 30 min after the intravenous injection of [111In]-Ex4 (3.0 MBq). Radioactivity levels in the pancreases were measured in vivo. C) Changes in BCM estimated from radioactivity levels in the pancreas by applying the regression formula obtained in this study: BCM (mg) = [the ratio of pancreatic radioactivity measured from SPECT scan to %ID − 2.81] / 0.997. There was no significant difference (P = 0.73) between the 2 groups at the time of the first scan (10 wk old), but the difference was significant (P < 0.001) at the time of the second scan (12 wk old). The euglycemic and hyperglycemic mice are indicated by solid and broken lines, respectively.
Changes in pancreatic radioactivity levels between the first and second SPECT scans in the mice that became hyperglycemic and those that remained euglycemic are shown in Fig. 4B. The associated changes in BCM were calculated using the regression formula previously described (Fig. 4C). The mean pancreatic radioactivity levels changed from 4.69 to 3.01%ID and from 4.75 to 4.89%ID in the hyperglycemic mice and euglycemic mice, respectively. From this, changes in the mean BCM were estimated to be from 1.88 to 0.19 mg and from 1.95 to 2.08 mg in the hyperglycemic and euglycemic mice, respectively. There was no significant difference (P = 0.73) between the 2 groups at the time of the first scan (when all the mice were euglycemic), but the difference was significant (P < 0.001) at the time of the second scan.
Sensitivity analysis
Even when the threshold used to set the pancreatic ROI for analysis of the SPECT images was changed (by +10, +5, +2.5, −2.5, −5, and −10%), good correlations were observed between the pancreatic radioactivity levels measured by SPECT imaging and BCM calculated from immunostained pancreatic sections (Table 1).
TABLE 1.
Sensitivity analysis
| Threshold of pancreatic ROI (%) | Correlation between the radioactive signals from pancreas on SPECT images and BCM (r), P < 0.001 | Proportional volume of pancreas analyzed with SPECT (%) |
|---|---|---|
| +10.0 | 0.884 | 61.3 |
| +5.0 | 0.895 | 67.5 |
| +2.5 | 0.895 | 71.1 |
| 0 | 0.893 | 76.0 |
| −2.5 | 0.900 | 80.0 |
| −5.0 | 0.898 | 84.1 |
| −10.0 | 0.888 | 94.2 |
A threshold adopted to set pancreatic ROI for analysis on SPECT images was changed. BCM was calculated from immune-stained pancreatic sections.
DISCUSSION
The establishment of a noninvasive method to quantify BCM longitudinally will enable a deeper understanding of the pathophysiology of diabetes and the evaluation of the effectiveness of therapy in terms of BCM. It may also help identify approaching impaired glucose tolerance in advance by detecting changes in BCM before the postprandial glucose level becomes elevated. It could also facilitate personalized medicine based on each individual’s BCM.
In this study, we investigated a noninvasive method, using GLP-1R–targeting exendin-4 labeled with [111In], to longitudinally quantify BCM in mice. We showed a strong correlation between fluorescent and radioactive signal intensity in pancreatic sections from MIP-GFP mice injected with [111In]-Ex4. Because the intensity of fluorescence in pancreatic sections of MIP-GFP mice is considered to be an indicator of BCM (21), this finding confirmed that BCM could be quantified ex vivo from [111In]-Ex4 levels in sections from harvested mouse pancreases. We also found strong correlations between the pancreatic accumulation of [111In]-Ex4 in harvested pancreases and BCM calculated from immunostained pancreatic sections and between the radioactivity from the pancreas measured in vivo by SPECT scanning and BCM calculated from the pancreatic sections. We therefore succeeded in capturing longitudinal changes in BCM through SPECT scans of the same mice after injections of [111In]-Ex4. In the current study, BCM was measured at just 2 time points. However, the BCM measurement with SPECT scans did not require pancreas harvesting, and therefore, this procedure can be performed at more than 2 time points. Quantifying BCM at multiple time points can provide a deeper understanding of diabetic pathophysiology and could be used to develop a scale to evaluate the efficacy of antidiabetic therapies in preserving or increasing BCM.
Brom et al. (25) administered [111In]-[Lys40(DTPA)]exendin-4 to healthy subjects and patients with type 1 diabetes in a cross-sectional study. They also used [111In]-[Lys40(DTPA)]exendin-3 in rats, reporting a good correlation (r = 0.83) between BCM and the uptake of the [111In]-[Lys40(DTPA)]exendin-3 in harvested pancreases measured by ex vivo SPECT imaging in a cross-sectional study. In their ex vivo SPECT imaging on harvested organs of rats, the uptake in the stomach and duodenum as well as the pancreas was clearly demonstrated. The result was compatible with a biodistribution study performed using rats; the uptake of the [111In]-[Lys40(DTPA)]exendin-3 in the pancreas, stomach, and duodenum was 0.29%ID/g, 0.33%ID/g, 0.44%ID/g, respectively. On the contrary, in our ex vivo SPECT imaging on the harvested organs of mice, only the uptake in pancreas was apparently shown (Supplemental Fig. S1). The uptake of our probe ([111In]-Ex4) in the pancreas, stomach, and small intestine in a biodistribution study performed using mice was 21.5, 4.29, and 3.54% ID/g, respectively (23). Considering the difference in the uptake between pancreas and 2 other organs, it is also natural that the uptake of our probe has clearly been demonstrated only in the pancreas in the ex vivo SPECT scans in the current study, different from the study by Brom et al. (25). These differences may come from the difference in the animals (rats and mice) and the tracers ([111In]-[Lys40(DTPA)]exendin-3 and [111In]-Ex4).
As Mathijs et al. (33) noted, a problem when measuring radioactive signals from the pancreas in vivo is the huge influence of accumulation of the probe in the kidney. They therefore performed unilateral nephrectomy 4 wk prior to in vivo SPECT imaging to reduce the influence of the kidney. They also used [123I]-labeled l-phenylalanine to select the pancreatic area for analysis. They reported that the mean volume of pancreas selected for analysis in their study was 24.9 mm3. However, they did not investigate the correlation between BCM and the uptake of [111In]-[Lys40(DTPA)]exendin-3 in the pancreatic area selected for analysis by SPECT imaging. Van der Kroon et al. (34) used [99 mTc]-demobesin-4 to select the pancreatic ROI for the analysis of SPECT images. In rats, with resected left kidneys, they found a strong correlation (r = 0.92) between the pancreatic uptake of [111In]-[Lys40(DTPA)]exendin-3 measured in harvested pancreases and in vivo SPECT images (34). However, they did not investigate the relationship between BCM and [111In]-[Lys40(DTPA)]exendin-3 uptake in the pancreatic area that was identified in in vivo SPECT images.
In the current study, the correlation between radioactive signals from pancreas acquired by SPECT scan and BCM revealed that although 2 pairs of mice had similar BCM (near 0 mg and near 0.3 mg), the SPECT quantification appeared ∼50% higher in 1 mouse in each of the 2 sets (Fig. 4A). This discrepancy can be attributed to the 40% difference in the analyzed pancreatic volume in both sets. The difference might be due to the anatomic variation between the kidney and pancreas in each mouse. It can be assumed that in a mouse whose pancreas is anatomically close to the kidney, the pancreatic ROI may tend to be smaller because a larger volume of original pancreatic ROI would be excluded in the process of setting renal ROI. Moreover, Fig. 2H shows that there is some difference in the pancreatic accumulation of [111In]-Ex4 on harvested pancreas with almost no BCM. This indicates that there is some variation in the accumulation of [111In]-Ex4 into pancreatic non-β cells (such as exocrine cells). The reduced accumulation of [111In]-Ex4 into pancreatic non-β cells could lead to a smaller pancreatic ROI, especially in a mouse with smaller BCM. This is because we adopted the same threshold to establish the pancreatic ROI in all of the mice in order to exclude arbitrariness in the current study. The difference in the analyzed pancreatic volume due to these reasons could lead to a comparatively unstable estimation of BCM from the SPECT quantification, especially in a mouse with less BCM.
In the present study, we were able to substantially increase the pancreatic area for analysis using a previously reported improved method (31). This resulted in mean volumes of pancreas selected for analysis in the first and second SPECT scans of 182 and 173 mm3, respectively. We have previously reported that analyzing more than 40% of the volume of the whole pancreas is necessary for an accurate estimate of the radioactivity for the whole pancreas (31). The mean pancreatic area selected for the analysis of the second SPECT scans in the present study was 76.0% of the whole pancreas. At least 40% of the whole pancreas was included in the analysis area for all of the 10 mice. This may have contributed to the good results.
We successfully showed a good correlation between BCM and [111In]-Ex4 uptake in the pancreatic area selected for analysis on in vivo SPECT images. Furthermore, a sensitivity analysis confirmed the robustness of our analysis method: even after changing the threshold for establishing the pancreatic ROI, good correlations remained between the pancreatic radioactivity measured from SPECT imaging and BCM calculated by the immunohistochemical method.
This study had several limitations. The accuracy of the estimation of BCM from the radioactivity measured by SPECT imaging was fairly good (r = 0.89), but not completely accurate. Further improvement in SPECT/CT devices could be helpful to improving the accuracy. More accurate estimation might allow the detection of smaller and more subtle changes in BCM. It would also be worth trying to develop a probe that undergoes little renal accumulation. Because of the high accumulation of [111In]-Ex4 in the kidneys, parts of the pancreas had to be excluded from the analysis, with the mean area selected for analysis being around 75% of the whole pancreas, which was considerably <100%.
In this study, we successfully captured longitudinal changes in BCM in a type 1 diabetes model for the first time using [111In]-Ex4 and SPECT imaging. In future studies, it would be worth investigating the use of this method with a type 2 diabetes model in which smaller changes in BCM are expected. Although we need to pay enough attention and must try to minimize the renal radiation exposure because of its high renal excretion, our exendin probe also shows promise for use in clinical studies.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Asami Mori (Kyoto University) for technical support. This study was funded by the Translational Research Network Program and the Practical Research for Innovative Cancer Control from the Japan Agency for Medical Research and Development (AMED), the Center of Innovation Program from the Ministry of Education, Culture, Sports, Science, and Technology (MEXT), the Japan Science and Technology Agency (JST), and the Japan Society for the Promotion of Science (JSPS) Grants-in-Aid for Scientific Research (KAKENHI; JP18K08475); however, they played no role in the study design or conduct, data collection, analysis, or interpretation, or the preparation, review, or approval of the manuscript. H.S. received grants from Nihon Medi-Physics Co. Ltd., outside the submitted work. N.I. received grants from Sumitomo Dainippon Pharma Co. Ltd., and personal fees from MSD Co. Ltd., Mitsubishi Tanabe Pharma Corp., AstraZeneca Co. Ltd., Ono Pharmaceutical Co. Ltd., and Daiichi-Sankyo Co. Ltd., outside the submitted work. Moreover, H.K., H.S., and N.I. have a patent, WO2011071083A1, issued to Kyoto University and Arkray, Inc. All remaining authors declare no conflicts of interest.
Glossary
- [111In]-Ex4
[Lys12([111In]-BnDTPA-Ahx)]exendin-4
- Ahx
aminohexanoic acid
- BCM
β-cell mass
- BnDTPA
isothiocyanate-benzyl-diethylenetriamine-pentaacetic acid
- CT
computed tomography
- DTPA
diethylenetriamine-pentaacetic acid
- FBG
fasting blood glucose
- GFP
green fluorescent protein
- GLP-1R
glucagon-like peptide-1 receptor
- %ID
percent injection dose
- MIP
mouse insulin 1 gene promotor
- NOD
nonobese diabetic
- PET
positron emission tomography
- ROI
region of interest
- SPECT
single-photon emission computed tomography
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
N. Fujita designed the experiments; acquired, analyzed, and interpreted the data; and drafted and critically revised the manuscript; H. Fujimoto provided a substantial contribution to the conception and design and the analysis and interpretation of data; K. Hamamatsu acquired the data and contributed to analysis and interpretation of the data; T. Murakami, H. Kimura, K. Toyoda, and H. Saji contributed to analysis and interpretation of the data; N. Inagaki provided a substantial contribution to the conception and design and the analysis and interpretation of data and critically revised the manuscript for important intellectual content; all authors reviewed the results and approved the final version of the manuscript; and N. Inagaki was responsible for the integrity of the work as a whole.
REFERENCES
- 1.International Diabetes Federation (2017) IDF Diabetes Atlas, 8th ed., International Diabetes Federation, Brussels, Belgium. Accessed January 3, 2019, at: http://www.idf.org/diabetesatlas [DOI] [PubMed]
- 2.U.K. Prospective Diabetes Study Group (1995) U.K. prospective diabetes study 16: Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes 44, 1249–1258 [PubMed] [Google Scholar]
- 3.Rhodes C. J. (2005) Type 2 diabetes-a matter of beta-cell life and death? Science 307, 380–384 [DOI] [PubMed] [Google Scholar]
- 4.Sakuraba H., Mizukami H., Yagihashi N., Wada R., Hanyu C., Yagihashi S. (2002) Reduced beta-cell mass and expression of oxidative stress-related DNA damage in the islet of Japanese Type II diabetic patients. Diabetologia 45, 85–96 [DOI] [PubMed] [Google Scholar]
- 5.Butler A. E., Janson J., Bonner-Weir S., Ritzel R., Rizza R. A., Butler P. C. (2003) Beta-cell deficit and increased beta-cell apoptosis in humans with type 2 diabetes. Diabetes 52, 102–110 [DOI] [PubMed] [Google Scholar]
- 6.Yoon K. H., Ko S. H., Cho J. H., Lee J. M., Ahn Y. B., Song K. H., Yoo S. J., Kang M. I., Cha B. Y., Lee K. W., Son H. Y., Kang S. K., Kim H. S., Lee I. K., Bonner-Weir S. (2003) Selective beta-cell loss and alpha-cell expansion in patients with type 2 diabetes mellitus in Korea. J. Clin. Endocrinol. Metab. 88, 2300–2308 [DOI] [PubMed] [Google Scholar]
- 7.Rahier J., Guiot Y., Goebbels R. M., Sempoux C., Henquin J. C. (2008) Pancreatic beta-cell mass in European subjects with type 2 diabetes. Diabetes Obes. Metab. 10 (Suppl 4), 32–42 [DOI] [PubMed] [Google Scholar]
- 8.Meier J. J., Menge B. A., Breuer T. G., Müller C. A., Tannapfel A., Uhl W., Schmidt W. E., Schrader H. (2009) Functional assessment of pancreatic beta-cell area in humans. Diabetes 58, 1595–1603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alavi A., Werner T. J. (2018) Futility of attempts to detect and quantify beta cells by PET imaging in the pancreas: why it is time to abandon the approach. Diabetologia 61, 2512–2515 [DOI] [PubMed] [Google Scholar]
- 10.Gotthardt M., Eizirik D. L., Aanstoot H. J., Korsgren O., Mul D., Martin F., Boss M., Jansen T. J. P., van Lith S. A. M., Buitinga M., Eriksson O., Cnop M., Brom M. (2018) Detection and quantification of beta cells by PET imaging: why clinical implementation has never been closer. Diabetologia 61, 2516–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cline G. W., Naganawa M., Chen L., Chidsey K., Carvajal-Gonzalez S., Pawlak S., Rossulek M., Zhang Y., Bini J., McCarthy T. J., Carson R. E., Calle R. A. (2018) Decreased VMAT2 in the pancreas of humans with type 2 diabetes mellitus measured in vivo by PET imaging. Diabetologia 61, 2598–2607 [DOI] [PubMed] [Google Scholar]
- 12.Cline G. W., McCarthy T. J., Carson R. E., Calle R. A. (2018) Clinical and scientific value in the pursuit of quantification of beta cells in the pancreas by PET imaging. Diabetologia 61, 2671–2673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eriksson O., Laughlin M., Brom M., Nuutila P., Roden M., Hwa A., Bonadonna R., Gotthardt M. (2016) In vivo imaging of beta cells with radiotracers: state of the art, prospects and recommendations for development and use. Diabetologia 59, 1340–1349 [DOI] [PubMed] [Google Scholar]
- 14.Jodal A., Schibli R., Béhé M. (2017) Targets and probes for non-invasive imaging of β-cells. Eur. J. Nucl. Med. Mol. Imaging 44, 712–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wild D., Béhé M., Wicki A., Storch D., Waser B., Gotthardt M., Keil B., Christofori G., Reubi J. C., Mäcke H. R. (2006) [Lys40(Ahx-DTPA-111In)NH2]exendin-4, a very promising ligand for glucagon-like peptide-1 (GLP-1) receptor targeting. J. Nucl. Med. 47, 2025–2033 [PubMed] [Google Scholar]
- 16.Brom M., Oyen W. J., Joosten L., Gotthardt M., Boerman O. C. (2010) 68Ga-labelled exendin-3, a new agent for the detection of insulinomas with PET. Eur. J. Nucl. Med. Mol. Imaging 37, 1345–1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Selvaraju R. K., Velikyan I., Johansson L., Wu Z., Todorov I., Shively J., Kandeel F., Korsgren O., Eriksson O. (2013) In vivo imaging of the glucagonlike peptide 1 receptor in the pancreas with 68Ga-labeled DO3A-exendin-4. J. Nucl. Med. 54, 1458–1463 [DOI] [PubMed] [Google Scholar]
- 18.Normandin M. D., Petersen K. F., Ding Y. S., Lin S. F., Naik S., Fowles K., Skovronsky D. M., Herold K. C., McCarthy T. J., Calle R. A., Carson R. E., Treadway J. L., Cline G. W. (2012) In vivo imaging of endogenous pancreatic β-cell mass in healthy and type 1 diabetic subjects using 18F-fluoropropyl-dihydrotetrabenazine and PET. J. Nucl. Med. 53, 908–916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Freeby M. J., Kringas P., Goland R. S., Leibel R. L., Maffei A., Divgi C., Ichise M., Harris P. E. (2016) Cross-sectional and test-retest characterization of PET with [(18)F]FP-(+)-DTBZ for β cell mass estimates in diabetes. Mol. Imaging Biol. 18, 292–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eriksson O., Jahan M., Johnström P., Korsgren O., Sundin A., Halldin C., Johansson L. (2010) In vivo and in vitro characterization of [18F]-FE-(+)-DTBZ as a tracer for beta-cell mass. Nucl. Med. Biol. 37, 357–363 [DOI] [PubMed] [Google Scholar]
- 21.Mukai E., Toyoda K., Kimura H., Kawashima H., Fujimoto H., Ueda M., Temma T., Hirao K., Nagakawa K., Saji H., Inagaki N. (2009) GLP-1 receptor antagonist as a potential probe for pancreatic β-cell imaging. Biochem. Biophys. Res. Commun. 389, 523–526 [DOI] [PubMed] [Google Scholar]
- 22.Kimura H., Matsuda H., Ogawa Y., Fujimoto H., Toyoda K., Fujita N., Arimitsu K., Hamamatsu K., Yagi Y., Ono M., Inagaki N., Saji H. (2017) Development of 111In-labeled exendin(9-39) derivatives for single-photon emission computed tomography imaging of insulinoma. Bioorg. Med. Chem. 25, 1406–1412 [DOI] [PubMed] [Google Scholar]
- 23.Kimura H., Fujita N., Kanbe K., Matsuda H., Watanabe H., Arimitsu K., Fujimoto H., Hamamatsu K., Yagi Y., Ono M., Inagaki N., Saji H. (2017) Synthesis and biological evaluation of an 111In-labeled exendin-4 derivative as a single-photon emission computed tomography probe for imaging pancreatic β-cells. Bioorg. Med. Chem. 25, 5772–5778 [DOI] [PubMed] [Google Scholar]
- 24.Kimura H., Ogawa Y., Fujimoto H., Mukai E., Kawashima H., Arimitsu K., Toyoda K., Fujita N., Yagi Y., Hamamatsu K., Murakami T., Murakami A., Ono M., Nakamoto Y., Togashi K., Inagaki N., Saji H. (2018) Evaluation of 18F-labeled exendin(9-39) derivatives targeting glucagon-like peptide-1 receptor for pancreatic β-cell imaging. Bioorg. Med. Chem. 26, 463–469 [DOI] [PubMed] [Google Scholar]
- 25.Brom M., Woliner-van der Weg W., Joosten L., Frielink C., Bouckenooghe T., Rijken P., Andralojc K., Göke B. J., de Jong M., Eizirik D. L., Béhé M., Lahoutte T., Oyen W. J., Tack C. J., Janssen M., Boerman O. C., Gotthardt M. (2014) Non-invasive quantification of the beta cell mass by SPECT with 111In-labelled exendin. Diabetologia 57, 950–959 [DOI] [PubMed] [Google Scholar]
- 26.Virostko J., Radhika A., Poffenberger G., Dula A. N., Moore D. J., Powers A. C. (2013) Bioluminescence imaging reveals dynamics of beta cell loss in the non-obese diabetic (NOD) mouse model. PLoS One 8, e57784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vinet L., Lamprianou S., Babič A., Lange N., Thorel F., Herrera P. L., Montet X., Meda P. (2015) Targeting GLP-1 receptors for repeated magnetic resonance imaging differentiates graded losses of pancreatic beta cells in mice. Diabetologia 58, 304–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Souza F., Simpson N., Raffo A., Saxena C., Maffei A., Hardy M., Kilbourn M., Goland R., Leibel R., Mann J. J., Van Heertum R., Harris P. E. (2006) Longitudinal noninvasive PET-based beta cell mass estimates in a spontaneous diabetes rat model. J. Clin. Invest. 116, 1506–1513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasunami R., Bach J. F. (1988) Anti-suppressor effect of cyclophosphamide on the development of spontaneous diabetes in NOD mice. Eur. J. Immunol. 18, 481–484 [DOI] [PubMed] [Google Scholar]
- 30.Harada M., Makino S. (1984) Promotion of spontaneous diabetes in non-obese diabetes-prone mice by cyclophosphamide. Diabetologia 27, 604–606 [DOI] [PubMed] [Google Scholar]
- 31.Hamamatsu K., Fujimoto H., Fujita N., Murakami T., Kimura H., Saji H., Inagaki N. (2018) Establishment of a method for in-vivo SPECT/CT imaging analysis of 111In-labeled exendin-4 pancreatic uptake in mice without the need for nephrectomy or a secondary probe. Nucl. Med. Biol. 64-65, 22–27 [DOI] [PubMed] [Google Scholar]
- 32.Bolender R. P. (1974) Stereological analysis of the guinea pig pancreas. I. Analytical model and quantitative description of nonstimulated pancreatic exocrine cells. J. Cell Biol. 61, 269–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mathijs I., Xavier C., Peleman C., Caveliers V., Brom M., Gotthardt M., Herrera P. L., Lahoutte T., Bouwens L. (2015) A standardized method for in vivo mouse pancreas imaging and semiquantitative β cell mass measurement by dual isotope SPECT. Mol. Imaging Biol. 17, 58–66 [DOI] [PubMed] [Google Scholar]
- 34.Van der Kroon I., Joosten L., Nock B. A., Maina T., Boerman O. C., Brom M., Gotthardt M. (2016) Improved quantification of the beta cell mass after pancreas visualization with 99mTc-demobesin-4 and beta cell imaging with 111In-exendin-3 in rodents. Mol. Pharm. 13, 3478–3483 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.