Abstract
Bone morphogenetic protein (BMP)9 has been reported to be the most potent BMP to induce bone formation. However, the details of BMP9-transduced intracellular signaling remain ambiguous. Here, we have investigated signal transduction mechanisms of BMP9 in comparison to BMP2, another potent inducer of bone formation, in osteoblasts. In a mouse osteoblast cell line, BMP9 induced higher mRNA levels of alkaline phosphatase (ALP) and runt-related transcription factor 2 (Runx2) than BMP2 within 2 h. Unlike BMP2, BMP9 induced rapid phosphorylation of glycogen synthase kinase 3-β (GSK3-β) and protein kinase B (Akt) and increased the cellular protein content of β-catenin. BMP9 moderately increased mRNA levels of several canonical Wingless-related integration site to lower degrees than BMP2. Furthermore, BMP9-induced GSK3-β phosphorylation was not inhibited by pretreatment with actinomycin D, cycloheximide, or Brefeldin A, indicating it is independent of Wnt protein secretion. BMP9-induced GSK3-β phosphorylation was abrogated by Akt or class I PI3K-specific inhibitors. Moreover, inactivation of GSK3-β by LiCl did not further promote ALP and Runx2 mRNA induction by BMP9 as significantly as that by BMP2. Notably, BMP9-induced GSK3-β phosphorylation was inhibited by small interfering RNA against endoglin and GIPC PDZ domain–containing family, member 1. Taken together, our present findings have indicated that BMP9 directly activates GSK3β-β-catenin signaling pathway through class I PI3K-Akt Axis in osteoblasts, which may be essential for the potent osteoinductive activity of BMP9.—Eiraku, N., Chiba, N., Nakamura, T., Amir, M. S., Seong, C.-H., Ohnishi, T., Kusuyama, J., Noguchi, K., Matsuguchi, T. BMP9 directly induces rapid GSK3-β phosphorylation in a Wnt-independent manner through class I PI3K-Akt axis in osteoblasts.
Keywords: endoglin, osteoblast differentiation, GIPC1
Bone morphogenetic proteins (BMPs) are a protein family regulating skeletal development (1). To date, more than 20 BMPs have been identified. BMPs, with the exception of BMP-1, belong to the TGF-β super family of proteins and signal through a receptor complex consisting of serine/threonine kinases, including at least 2 BMP type I receptors (BMPRI) and 2 BMP type II receptors (BMPRII) (2). Upon ligand binding, oligomerization of these receptors induces the activation of BMPRI through cross-phosphorylation, subsequently initiating downstream signaling events. In the canonical BMP pathway, phosphorylation of 3 regulatory SMAD proteins, namely SMAD1, 5, and 8, are induced by most BMPs (3). These phosphorylated regulatory SMADs make complexes with SMAD4 (common SMAD), translocate to the nucleus as transcription factors, and induce target gene expressions. BMPs also induce SMAD-independent signals, known as the noncanonical pathway. It includes the activation of kinases such as ERKs, p38, JNKs, PI3Ks, PKA, and PKC as well as transcription factors such as NF-κB (3). However, detailed activation mechanisms of these signaling pathways have not been fully elucidated.
Although BMPs generally induce mesenchymal stem cells to differentiate into osteoblasts, this osteogenic potential varies among the members. BMP2 and BMP7 have been most comprehensively characterized for their osteogenic effects, partly because the human recombinant proteins became available earlier than other BMPs. BMP2 is especially considered as the gold standard for bone regeneration and has been extensively investigated in human clinical applications of bone defects, fracture healing, and spinal fusion (4, 5). Recent studies, however, have revealed that BMP9 induces osteogenesis better than BMP2 both in vivo and in vitro (6–8). In fact, BMP9 was demonstrated to be the most osteogenic BMP in several previous reports (9–11). Among the 14 BMP members (BMP2–15), BMP9 induced the highest level of alkaline phosphatase (ALP) expression in C2C12, an osteoblast progenitor cell line, in an in vitro assay, followed by BMP6, BMP2, BMP7, and BMP4 (10). In an orthotopic ossification animal model, forced expression of BMP9, as well as BMP6, induced strong ossification earlier than other BMPs, including BMP2 and BMP7 (10).
The mode of ligand-receptor interaction of BMP9 is unique among BMP family members. As for the type I receptors, BMP9 binds activin receptor–like kinase (ALK)1 with high affinity, whereas it shows poor affinity for BMPRIA (ALK3), the main type I receptor shared by other BMPs (12). Thus, BMP9 has been considered to transduce signals through receptor complexes of ALK1 [activin receptor type 2B (ActRIIB)] and BMPRII (12, 13). Another notable feature of BMP9 is that it binds endoglin, a coreceptor for BMPs, with high affinity (12, 14). Endoglin has been reported to induce intracellular signaling by interacting with PI3K p110-α and p85 subunits via GIPC PDZ domain–containing family member 1 (GIPC1) to activate protein kinase B (Akt) in endothelial cells (15). Interestingly, a recent study of endoglin crystal structure has indicated a receptor complex consisting of BMP9, ALK1, and endoglin but not type II receptor, suggesting a new unique aspect of BMP9 signaling mechanism (16). However, the detailed molecular basis of BMP9 signaling for its strong osteoinductive potential remains mostly enigmatic.
Here, we investigated characteristic signal transduction mechanisms of BMP9 in osteoblasts. We have found that BMP9 induces rapid phosphorylation of glycogen synthase kinase 3-β (GSK3-β), leading to the intracellular accumulation of β-catenin protein. This process appears to be independent of Wnt protein production and directly mediated by endoglin-PI3K-Akt signaling axis. Furthermore, our gene knockdown experiments have indicated the essential role of endoglin and GIPC1 in this process. These findings suggest that the mechanisms governing BMP9-mediated osteoinduction may differ from other osteogenic BMPs.
MATERIALS AND METHODS
Animals and cells
C57BL/6 mice were obtained from Clea Japan (Tokyo, Japan). Primary osteoblasts were isolated from newborn C57BL/6 mouse calvariae as previously described in Matsuguchi et al. (17). The animals were maintained in accordance with protocols approved by the Animal Care and Use Committee at Kagoshima University. MC3T3-E1 cells (RCB1126) were obtained from Riken Cell Bank (Tsukuba, Japan) and maintained in Eagle’s α-minimal essential medium (MilliporeSigma, Burlington, MA, USA) containing 10% fetal bovine serum, 10 mM 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (pH 7.2–7.5), 100 U/ml penicillin, and 100 μg/ml streptomycin.
Antibodies and reagents
Phosphorylation-specific antibodies against JNKs, ERKs, p38 kinases, GSK3-β (S9), and Akt (S473) as well as antibodies against JNKs, ERKs, GSK3-β, β-catenin, and Hes1 were all purchased from Cell Signaling Technology (Danvers, MA, USA). Antibodies against β-actin and p38 kinases were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). The anti-glyceraldehydes-3-phosphate dehydrogenase (GAPDH) antibody was purchased from Imgenex (San Diego, CA, USA). The anti-GIPC1 antibody was from Proteintech (Rosemont, IL, USA). The antibodies against endoglin and phosphorylated S6 were from R&D Systems (Minneapolis, MN, USA). Recombinant human BMP4 and BMP9 were purchased from Fujifilm Wako Pure Chemicals (Osaka, Japan). Recombinant human BMP2 was obtained from Prospec-Tany Technogene (Rehovot, Israel).
Alizarin red S staining
Cells were washed with Ca2+-free PBS 3 times and fixed in 10% formaldehyde/PBS for 20 min at 4°C. After 3 washes with distilled water, the cells were stained in 1% Alizarin Red S solution for 5 min to visualize matrix calcium deposition. The excess staining was removed by several washes with distilled water, and the stained matrix was photographed.
RNA interference
MC3T3-E1 cells and mouse primary osteoblasts were transfected with small interfering RNA (siRNA) duplexes specific for murine endoglin [ribonucleotide (r) (5′-GACAUGUGUGGGAGGCUGU-3′)dTdT and r(5′-ACAGCCUCCCACACAUGUC-3′)dTdT] and GIPC1 [r(5′-CCUGAGAGUUCAUACCUAU-3′)dTdT and r(5′-AUAGGUAUGAACUCUCAGG-3′)dTdT] obtained from MilliporeSigma or nontargeting control siRNA duplexes (control siRNA-A; Santa Cruz Biotechnology) using ScreenFect A Plus (Fujifilm Wako Pure Chemicals) according to the manufacturer’s instructions. For each transfection experiment, 40 pmol siRNA was used for 5 × 105 cells.
Western blot analysis
Cells were washed with PBS, and total cellular lysates were prepared in RIPA cell lysis buffer (150 mM NaCl, 5 mM EDTA, 1% deoxycholate, 0.1% SDS, 1% Triton-X) with the addition of 1 mM Na3VO4 and 1 time Proteinase Inhibitor Cocktail (Funakoshi, Tokyo, Japan). Cell lysates, containing 10 μg protein for each lane, were separated by SDS-PAGE. Immunoblotting was performed as previously described in Matsuguchi et al. (17). The antibody concentrations used for immunodetection were 0.2 μg/ml for both primary and horseradish peroxidase–conjugated secondary antibodies.
Real-time PCR analyses
Total RNA was isolated from cells using Isogen II (Nippon Gene, Tokyo, Japan), and reverse transcription was performed using RiverTra Ace (Toyobo, Tokyo, Japan) according to the manufacturers’ instructions. Real-time PCR was conducted using CFX Connect (Bio-Rad, Hercules, CA, USA). Briefly, the cDNA synthesized from 0.05 μg total RNA was amplified in a volume of 20 μl with 0.11 × SYBR Green I (Cambrex, Rockland, ME, USA), 0.2 mM/each deoxynucleotide triphosphates, 0.5 μM/each pair of primers, and 0.5 U Ex Taq DNA polymerase (Takara Bio, Kyoto, Japan) under the following conditions: 95°C for 5 min, followed by 55 PCR cycles at 95°C for 30 s, 60°C for 20 s, and 72°C for 40 s. Fluorescent signals were measured in real time, and then each sample was quantified according to the manufacturer’s instructions. The primer sequences used in this study are listed in Table 1. In order to normalize the differences in the amount of total RNA added to each reaction, ribosomal protein L13a (Rpl13a) was used as the endogenous control. An arbitrary unit was determined by dividing the concentration of each PCR product by the concentration of the Rpl13a PCR product. Each real-time PCR analysis was independently done 6 times and statistically analyzed by Mann-Whitney U test.
TABLE 1.
Primer sequences used in this study
| Gene | Primer sequence, 5′–3′ |
Product size (bp) | |
|---|---|---|---|
| Forward | Reverse | ||
| Alpl | GGCTTCTTCTTGCTGGTGGAA | CCTGGTCCATCTCCACTGCT | 97 |
| Bglap | CTCACAGATGCCAAGCCCA | CCAAGGTAGCGCCGGAGTCT | 98 |
| Eng | GCCAAAGTGTGGCAATCAGG | TGGTCGTCAGTGTCTTCAGC | 126 |
| Gipc1 | GGAGTTTCGAGAGGACCGAG | TTTCTTCCTTCGCCCCAGTC | 112 |
| Hey1 | GCCTTTGAGAAGCAGGGATCT | GGCATTCCCGAAACCCCAAA | 157 |
| Rpl13a | GCTTACCTGGGGCGTCTG | ACATTCTTTTCTGCCTGTTTCC | 149 |
| Runx2 | ATCCCCATCCATCCACTCCA | AGTTCTGAAGCACCTGCCTG | 168 |
| Smad7 | TTTCTCAAACCAACTGCAGGC | GACACAGTAGAGCCTCCCCA | 186 |
| Wnt1 | CGATGGTGGGGCATCGTGAA | GGGTTCTGTCGGATCAGTCG | 131 |
| Wnt2 | TCAGCTCTTCATGGTGGTACAT | AAAGGCCGATTCCCGACTAC | 263 |
| Wnt3a | CTCTCGGGAGTTTGCCGATG | GTGGCATTTGACACTTGAGGT | 124 |
| Wnt5a | CAACTCCTCTGCCCGAGTC | AGCCACTCCCGGGCTTAATA | 233 |
| Wnt8a | TAACCGGTCCCAAGGCCTATC | AGAGCGGATGGCATGAATGA | 191 |
| Wnt10a | AGATCGCCATCCATGAGTGC | ACTCTCTCGAAAACCTCGGC | 122 |
Alpl, alkaline phosphatase; Eng, endogline.
RESULTS
BMP2 and BMP9 induce similar but distinct gene expression profiles in osteoblasts
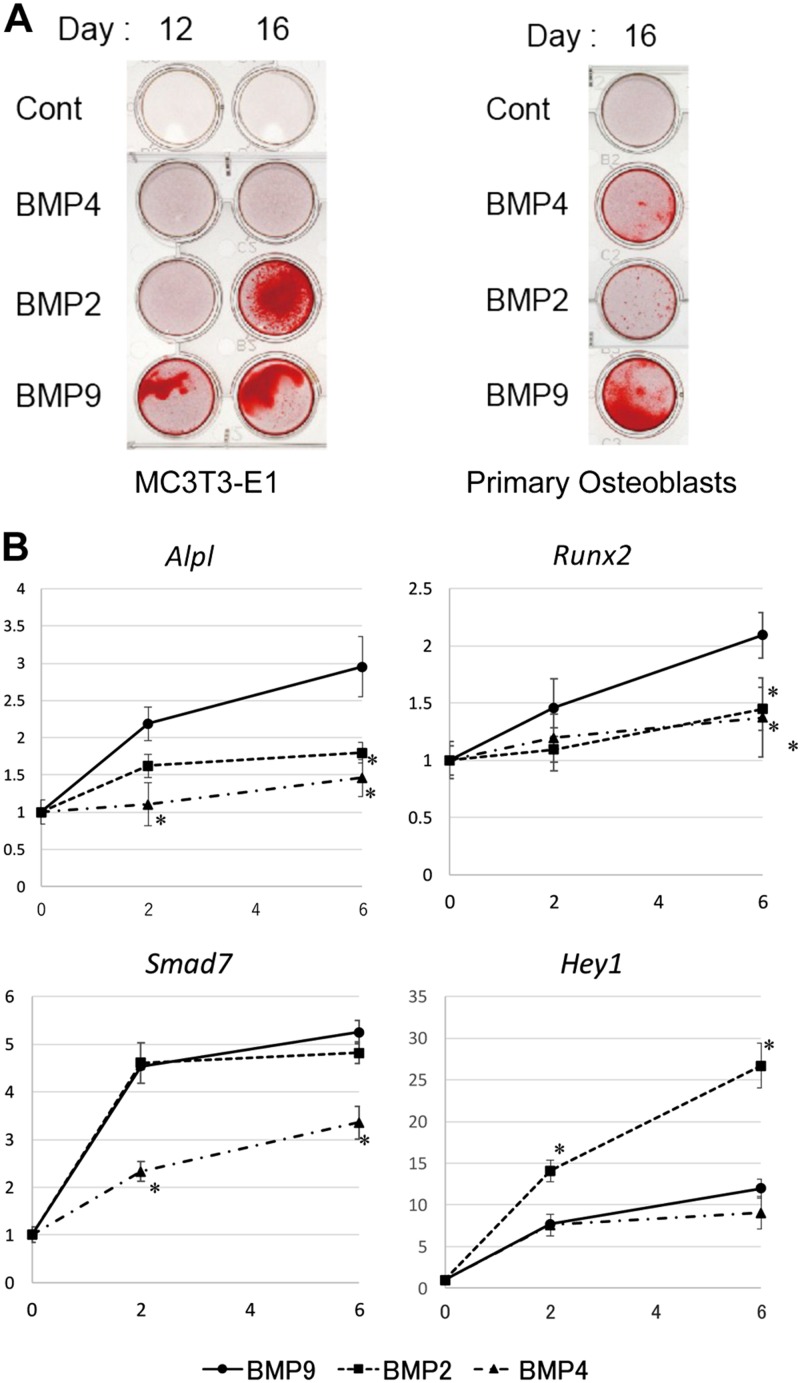
We first compared the osteogenic potentials of 3 BMPs (2, 4, 9) using MC3T3-E1, a commonly used mouse osteoblast cell line, and primary osteoblasts derived from newborn mouse calvaria. Calcium deposits, a well-known indicator of osteoblast differentiation, were clearly induced by BMP9 (50 ng/ml) within 12 d, whereas they were much slower with BMP2 (50 ng/ml) and BMP4 (50 ng/ml) for MC3T3-E1 cells (Fig. 1A). Similarly, significantly more calcium deposits were induced with 16-d treatment by BMP9 than those by BMP2 and BMP4 (Fig. 1A). We confirmed that increased amounts of BMP2 or BMP4 up to 100 ng/ml did not produce any further calcification (unpublished results), indicating the strong osteogenic potential of BMP9.
Figure 1.
Osteoblast differentiation induced by BMPs. A) Matrix mineralization of MC3T3-E1 cells and mouse primary osteoblasts stimulated with or without BMP2, BMP4, or BMP9. Cells were induced to differentiate with or without 50 ng/ml BMP2, BMP4, or BMP9 in combination with 10 mM β-glycerophosphate for the indicated days. Cells were fixed and stained with 1% Alizarin Red S as described in Materials and Methods. B) Transcriptional activation of ALP, Hey1, Runx2, and Smad7 by BMP2, BMP4, and BMP9. After serum starvation for 4 h, MC3T3-E1 cells were stimulated with BMP2, BMP4, or BMP9 (50 ng/ml) for the indicated hours. Total RNA was isolated and reverse transcribed. Quantitative real-time PCR analyses were performed in triplicate using the specific primers for ALP (Alpl), Hey1, Runx2, and Smad7. Rpl13a was used as the endogenous control for normalization. Fold increase represents an experimental value divided by the 0 h control (untreated) value. Data represent means ± sd of triplicate samples. Each experiment was repeated at least 3 times with similar results. *P < 0.05 (significant difference from the same hour value of the BMP9 stimulation group by Mann-Whitney U test; n = 6).
Effects of 3 BMPs (2, 4, 9) on BMP-responsive gene expressions were next examined by quantitative PCR in MC3T3-E1 (Fig. 1B). Briefly, serum-starved MC3T3-E1 cells were stimulated with 50 ng/ml of BMP2, BMP4, or BMP9 for up to 6 h, followed by total RNA preparation. Transcriptional activation of 4 BMP-responsive genes [Alp, runt-related transcription factor 2 (Runx2), Smad7, and Hey1] was analyzed by RT-PCR. At 50 ng/ml, BMP4 was the least potent inducer for each of the 4 gene expressions. BMP9 induced more Alp and Runx2 mRNAs but less Hey1 mRNA compared with BMP2. On the other hand, BMP2 and BMP9 were equally potent for the induction of Smad7 mRNA. These data have clearly indicated that osteogenic BMPs induce similar but distinct signaling pathways in osteoblasts.
BMP9 induces rapid phosphorylation of GSK3-β
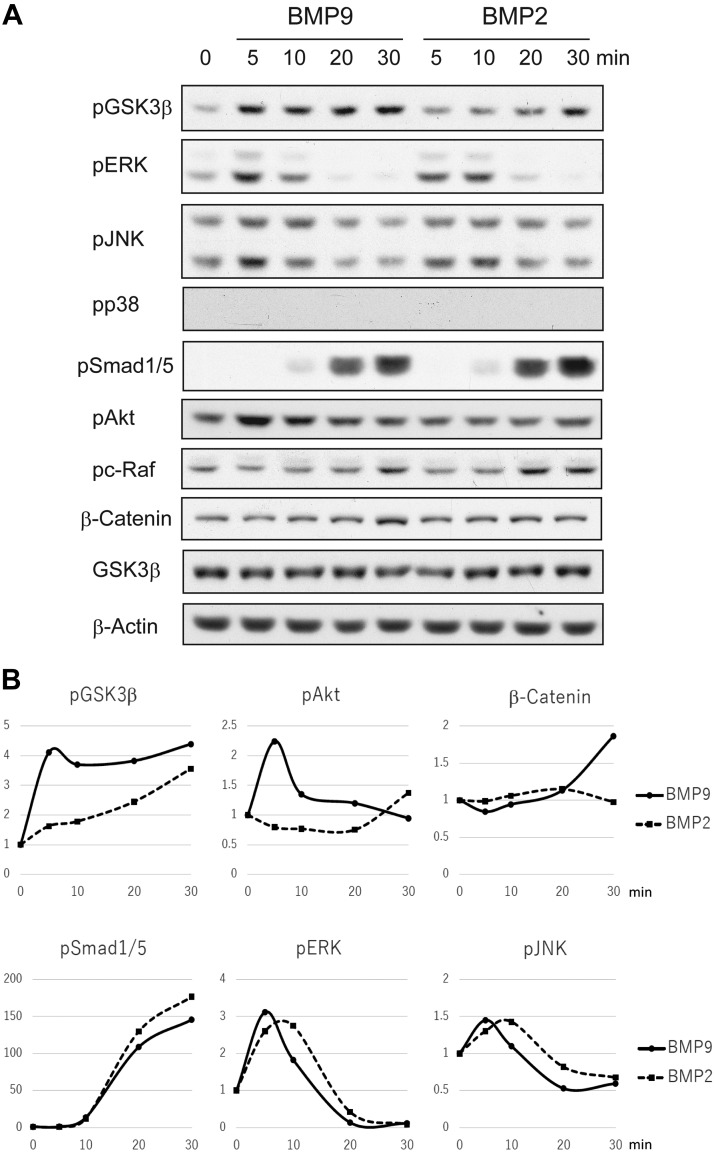
In order to compare the intracellular signal transduction mechanisms of BMP9 and BMP2, serum-starved MC3T3-E1 cells were stimulated with BMP9 or BMP2 for various time lengths, followed by cell lysate preparation and Western blotting analyses (Fig. 2A, B). Both BMP9 and BMP2 are known to activate the SMAD-mediated canonical signaling pathway and the noncanonical pathway characterized by MAPK phosphorylation. We found that phosphorylation of SMAD1/5 was similarly induced by BMP9 and BMP2 at 10 min, gradually reaching the maximum level at 30 min after stimulation. Among MAPKs, phosphorylation of ERKs and JNKs was induced relatively early, peaking at 5–10 min after stimulation by BMP9 and BMP2. Phosphorylation of p38 was only barely detected. We also found that BMP9 rapidly induced phosphorylation of GSK3-β, which could be easily detected at 5 min after stimulation. On the other hand, BMP2 induced GSK3-β phosphorylation with a significantly slower time course. It was also found that rapid phosphorylation of Akt, a known upstream kinase of GSK3-β, was observed with BMP9 stimulation, whereas it was not evident after BMP2 stimulation. As GSK3-β is a critical kinase regulating the accumulation of β-catenin protein, we also examined the protein expression level of β-catenin after BMP stimulation. Significant increase of β-catenin was detected at 30 min only after BMP9 stimulation. The accumulation of β-catenin in the nuclear fraction was also confirmed (unpublished results). We then examined primary osteoblasts derived from mouse calvaria for BMP signal transduction. Rapid phosphorylation of GSK3-β and Akt along with protein accumulation of β-catenin was observed in the primary osteoblasts after BMP9 stimulation, which was consistent with the findings in MC3T3-E1 cells (Fig. 3A, B).
Figure 2.
Intracellular signal transduction of BMP9 and BMP2 in MC3T3-E1 cells. A) After serum starvation for 4 h, MC3T3-E1 cells were stimulated with BMP2 or BMP9 (50 ng/ml) for the indicated minutes. Cell lysates were analyzed with the indicated antibodies by Western blotting. B) Protein phosphorylation ratios of GSK3-β (pGSK3-β/GSK3-β), Akt (pAkt/Akt), SMAD1/5 (pSMAD1/5 divided by the β-actin protein level), and ERK (pERK/ERK) were photographically measured. Fold induction of phosphorylation levels (the phosphorylation level of unstimulated cells was set as 1) calculated for BMP9 and BMP2 stimulation was shown as graphs. Additionally, the protein expression level of β-catenin was photographically measured (β-catenin/β-actin). Fold induction of β-catenin protein expression (the protein expression level of unstimulated cells was set as 1) calculated for BMP9 and BMP2 stimulations were shown as a graph. P, phosphorylated.
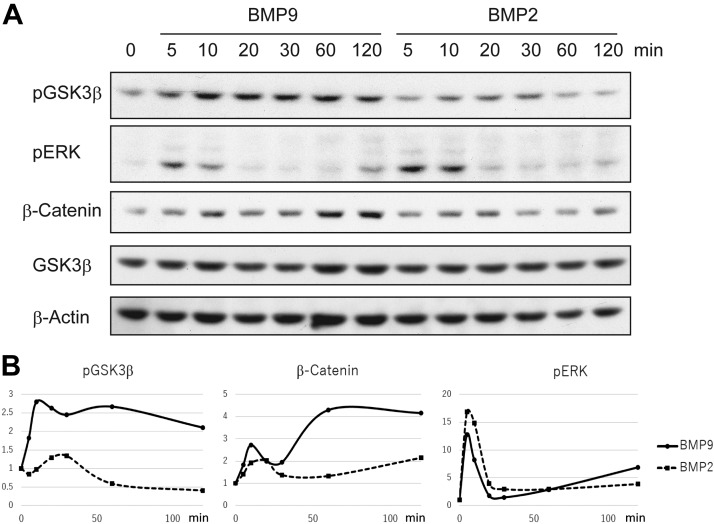
Figure 3.
Intracellular signal transduction of BMP9 and BMP2 in mouse primary osteoblasts. A) After serum starvation for 4 h, primary osteoblasts derived from newborn mouse calvariae were stimulated with BMP2 or BMP9 (50 ng/ml) for the indicated minutes. Cell lysates were analyzed with the indicated antibodies by Western blotting. B) Phosphorylation levels of GSK3-β and ERK, as well as the protein expression level of β-catenin, were photographically measured as in Fig. 2B. Fold inductions of phosphorylation levels and β-catenin protein expression for BMP9 and BMP2 stimulation were calculated and shown as graphs as in Fig. 2B. P, phosphorylated.
Wnt signaling is not involved in GSK3-β phosphorylation by BMP9
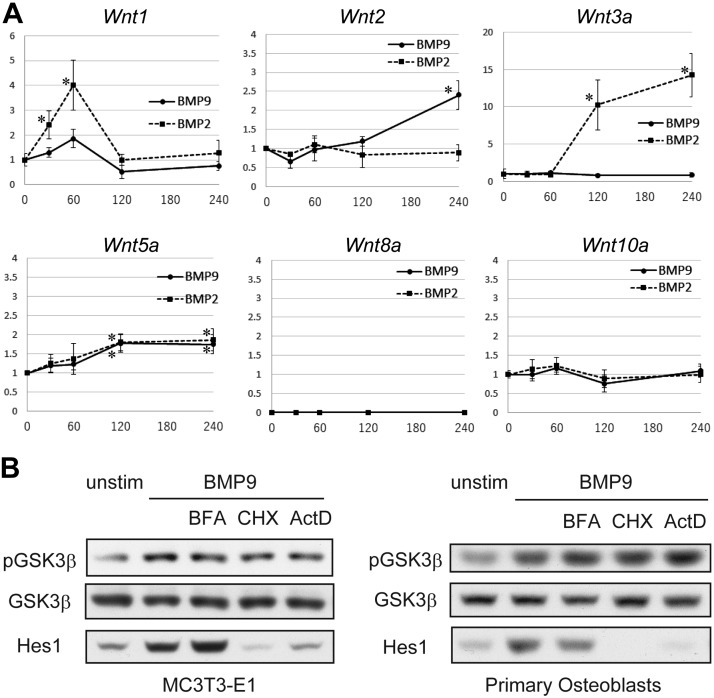
GSK3-β phosphorylation and consequent β-catenin accumulation are well established downstream events of canonical Wnt signaling pathway. As several BMPs are known to induce the secretion of canonical Wingless-related integration site (Wnts) from some cell types including osteoblasts, it is possible that the rapid phosphorylation of GSK3-β by BMP9 was mediated by Wnt production in an autocrine manner. To test the possibility, we examined mRNA levels of canonical Wnts (Wnt1, 2, 3a, 5a, 8a, and 10a) after stimulation by BMP2 or BMP9 (Fig. 4A). A significant mRNA increase of Wnt1 and Wnt3a was induced by BMP2, although the time course was relatively slower, peaking at 60 and 120 min, respectively. On the other hand, BMP9 induced mRNA expression of Wnt2 more significantly than BMP2. However, it was observed only after 120 min of BMP9 stimulation. These expression patterns of canonical Wnts have strongly suggested that the rapid phosphorylation of GSK3-β by BMP9 is independent of canonical Wnt production.
Figure 4.
BMP9 induces GSK3-β phosphorylation without the involvement of canonical Wnt production. A) Quantitative real-time RT-PCR analysis of canonical Wnt mRNA expression in MC3T3-E1 cell line. After 4 h serum starvation, cells were stimulated with BMP9 (50 ng/ml) or BMP2 (50 ng/ml) for the indicated minutes. Total RNAs were isolated, reverse transcribed, and analyzed by real-time PCR analyses. Relative Wnt mRNA expression levels were calculated based on the GAPDH mRNA level. Fold induction of mRNA in comparison with the unstimulated cell mRNA is shown. Data represent means ± sd. Each experiment was repeated at least 3 times with similar results. *P < 0.05 [significant difference from the unstimulated (0 min) value by Mann-Whitney U test; n = 6]. B) MC3T3-E1 cells and mouse primary osteoblasts were serum-starved for 4 h and then stimulated with 50 ng/ml BMP9 for 20 min with or without 30 min pretreatment by 5 μg/ml brefeldin A (BFA), 10 μg/ml cycloheximide (CHX), or 5 μg/ml actinomycin D (ActD). Cell lysates were prepared and separated by SDS-PAGE. Western blotting analyses were preformed, followed by detection with the indicated antibodies. P, phosphorylated.
In order to further rule out the involvement of indirect phosphorylation mechanisms of GSK3-β, we pretreated MC3T3-E1 cells with each of the 3 chemical inhibitors: brefeldin A (protein secretion inhibitor), cycloheximide (protein synthesis inhibitor), and actinomycin D (transcription inhibitor), followed by BMP9 stimulation. We found that BMP9-induced rapid GSK3-β phosphorylation was not significantly affected by any of the chemical inhibitors (Fig. 4B), indicating that it is a direct downstream event of BMP9 signaling. Similar results were obtained using primary osteoblasts (Fig. 4B), confirming that this is not a cell line–specific matter. As Wnt protein production was undetectable by shot stimulation by BMP9, successful inhibition of new protein production by cycloheximide and acinomycin D was confirmed by the abrogation of Hes1 protein induction (Fig. 4B), whereas successful inhibition of protein section by brefeldin A was confirmed by abrogation of Osteopontin secretion into culture supernatant (unpublished results).
Akt is an essential upstream mediator of GSK3-β phosphorylation by BMP9
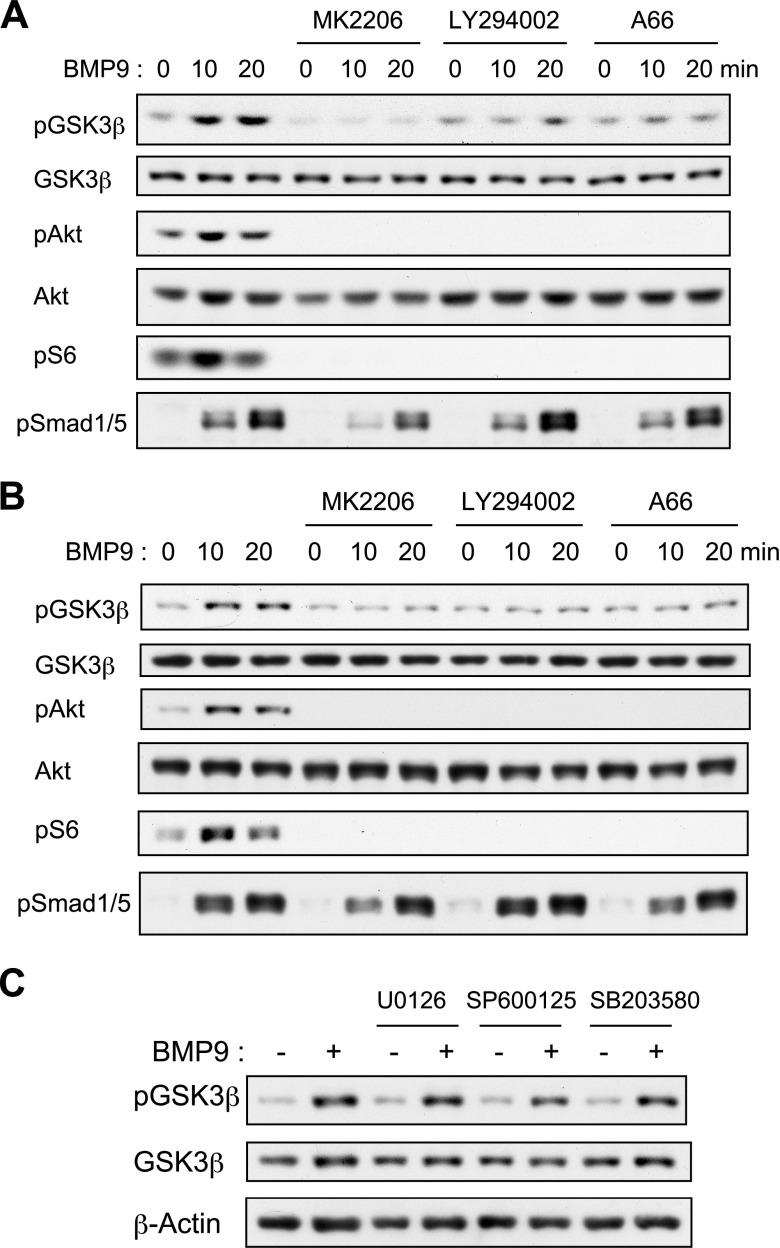
We then sought to examine the intracellular signaling mechanisms of BMP9 inducing GSK3-β phosphorylation in osteoblasts. As Akt is a known upstream kinase of GSK3-β and its phosphorylation was rapidly induced by BMP9 in osteoblasts similarly to that of GSK3-β, we examined the involvement of PI3K-Akt pathway using specific chemical inhibitors. As a result, BMP9-induced GSK3-β phosphorylation was significantly inhibited by MK2206 (a specific Akt inhibitor), LY294002 (a pan-PI3K inhibitor), and A66 (a specific inhibitor of class I PI3K) in both MC3T3-E1 (Fig. 5A) and primary mouse osteoblasts (Fig. 5B). Phosphorylation of S6, a downstream event of PI3K-Akt signaling, was abrogated, whereas phosphorylation of Smad1/5 was not significantly affected by each inhibitor in MC3T3-E1 and primary osteoblasts after BMP9 stimulation, confirming the specific effects of these 3 inhibitors. In contrast, pretreatment by neither MAPK inhibitors nor Raf inhibitors was effective (Fig. 5C and unpublished results). Thus, PI3K-Akt pathway is essential for the rapid GSK3-β phosphorylation by BMP9 in osteoblasts.
Figure 5.
Class I PI3K-Akt signaling pathway is essential for rapid GSK3-β phosphorylation induced by BMP9. A) MC3T3-E1 cells were serum starved for 4 h and then stimulated with 50 ng/ml BMP9 for 20 min with or without 30 min pretreatment by 5 μM MK2206, 10 μM LY294002, or 10 μM A66. Cell lysates were prepared and separated by SDS-PAGE. Western blotting analyses were performed, and protein bands were detected with the indicated antibodies. B) Primary osteoblasts derived from newborn mouse calvariae were treated and analyzed as in A. C) MC3T3-E1 cells were serum starved for 4 h and stimulated with 50 ng/ml BMP9 for 20 min with or without 30 min pretreatment by 10 μM U0126, 10 μM SP600125, or 10 μM SB203580. Cell lysates were prepared and separated by SDS-PAGE. Western blotting analyses were performed, and protein bands were detected with the indicated antibodies. P, phosphorylated.
Akt-GSK3-β signaling is essential for ALP and Runx2 gene expressions and osteoblast differentiation induced by BMP9
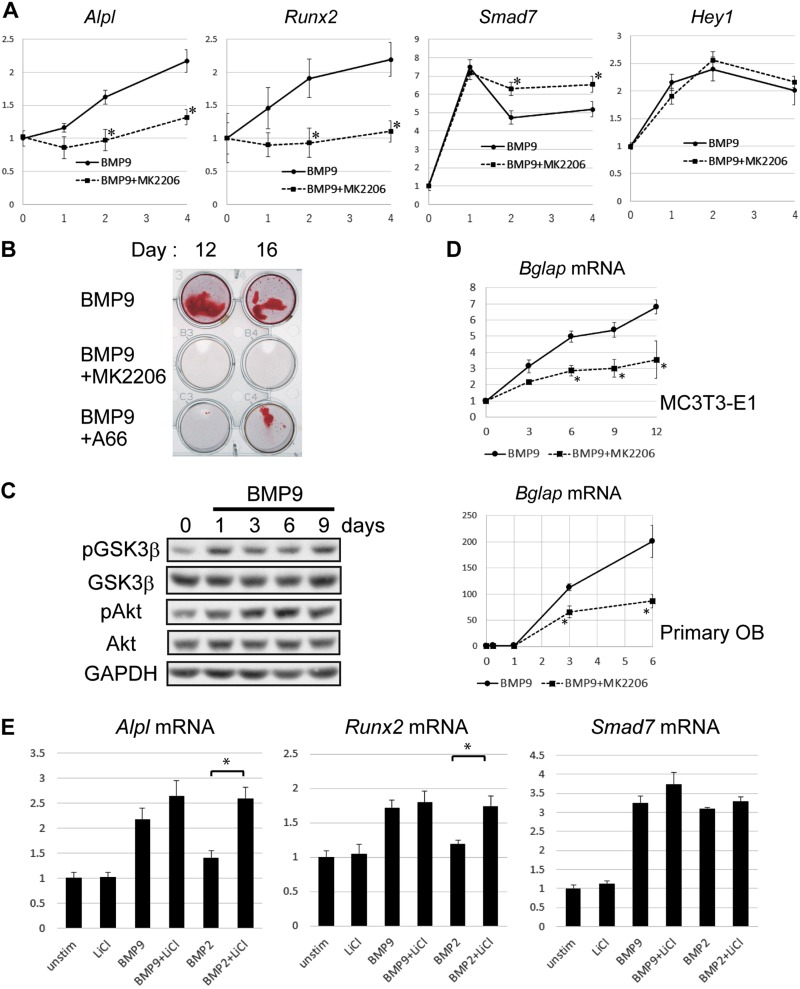
For the purpose of examining the roles of Akt activation in BMP9-mediated transcriptional activation, we analyzed the aforementioned 4 BMP-responsive mRNA expressions in MC3T3-E1 cells treated with BMP9 or BMP2 with or without MK2206, a specific inhibitor of Akt (Fig. 6A). Pretreatment with MK2206 significantly inhibited mRNA of ALP and Runx2 expression induced by BMP9, indicating that Akt-GSK3-β signaling plays an important role in the early induction of osteoblast differentiation. We also examined the effect of MK2206 on BMP9-induced mRNA expression of Hey1 and Smad7, which were more or equally responsive to BMP2 in comparison with BMP9, respectively (Fig. 1B). It was found that Hey1 mRNA induction was not significantly altered, whereas Smad7 mRNA induction by BMP9 became rather prolonged by MK2206 treatment, indicating that Akt-GSK3-β signaling is not essential for the induction of these BMP-responsive gene expressions.
Figure 6.
Akt-GSK3-β signaling pathway is important for the osteogenic potential of BMP9. A) MC3T3-E1 cells were serum starved for 4 h and stimulated with 50 ng/ml BMP9 for the indicated hours with or without 30 min pretreatment by 5 μM MK2206. Total RNAs were isolated, reverse transcribed, and analyzed by real-time PCR analyses. Relative mRNA expression levels of ALP, Runx2, Hey1, and Smad7 were calculated based on the GAPDH mRNA level. Fold induction of mRNA compared with the mRNA level of the unstimulated cells is shown. Data represent means ± sd of triplicate samples. *P < 0.05 [significant difference from the same day value of the BMP9 stimulation (without inhibitor) group by Mann-Whitney U test; n = 6]. B) MC3T3-E1 cells were induced to differentiate with 50 ng/ml BMP9/10 mM β-glycerophosphate for the indicated days in the absence or presence of MK2206 (5 μM) or A66 (10 μM). Cells were fixed and stained with 1% Alizarin Red S as described in Materials and Methods. C) MC3T3-E1 cells were induced to differentiate with 50 ng/ml BMP9/10 mM β-glycerophosphate for the indicated days. Cell lysates (10 μg total protein for each lane) were analyzed with the indicated antibodies by Western blotting. D) MC3T3-E1 cells and mouse primary osteoblasts were induced to differentiate with 50 ng/ml BMP9/10 mM β-glycerophosphate for the indicated days in the absence of presence of MK2206 (5 mM). Total RNAs were isolated, reverse transcribed, and analyzed by real-time PCR analyses. Relative mRNA expression levels of Osteocalcin (Bglap) were calculated based on the Rpl13a mRNA level. *P < 0.05 [significant difference from the same day value of the BMP9 stimulation (without inhibitor) group by Mann-Whitney U test; n = 6]. E) MC3T3-E1 cells were serum starved for 4 h, followed by stimulation with 50 ng/ml BMP9 for 6 h with or without 30 min pretreatment by 5 mM LiCl. Total RNAs were isolated, reverse transcribed, and analyzed by real-time PCR analyses. Relative mRNA expression levels of the indicated genes were calculated based on the Rpl13a mRNA level. Fold mRNA induction in comparison with unstimulated control is shown. P, phosphorylated. Data represent means ± sd. *P < 0.05 (significant difference by Mann-Whitney U test; n = 6).
We next examined the long-term effects of Akt-GSK3-β signal inhibition on osteoblast differentiation and found that both MK2206 and A66 treatments significantly inhibited calcium deposits of MC3T3-E1 cells induced by BMP9 (Fig. 6B), whereas these inhibitors did not decrease cell viability (unpublished results). We then found that phosphorylation levels of Akt and GSK3-β were modestly increased during the early differentiation process induced by BMP9 (Fig. 6C). It was also found that MK2206 significantly inhibited BMP9-mediated mRNA increase of osteocalcin [bone γ-carboxyglutamate protein (Bglap)], an established marker of late osteoblast differentiation, in both MC3T3-E1 and primary osteoblasts (Fig. 6D). Therefore, activation of Akt-GSK3-β signaling pathway appears essential for the osteogenic differentiation induced by BMP9.
We furthermore examined the effect of LiCl, a commonly used inhibitor of GSK3-β, on ALP and Runx2 mRNA expression in MC3T3-E1 cells stimulated with BMP9 and BMP2. Treatment by 5 mM LiCl only modestly increased the ratio of ALP and Runx2 mRNA induction by BMP9, whereas it enhanced the mRNA induction by BMP2 more robustly (Fig. 6E). In contrast, LiCl did not significantly enhance Smad7 mRNA induction by BMP2 or BMP9 (Fig. 6E), confirming the specific effect of LiCl treatment. These results have indicated that the rapid activation of Akt-GSK3-β signaling pathway is the essential mechanism in BMP9 inducing mRNA expression of ALP and Runx2, but not Hey1 or Smad7, more efficiently than BMP2.
Endoglin and GIPC1 are essential for GSK3-β phosphorylation but dispensable for SMAD1/5 phosphorylation by BMP9
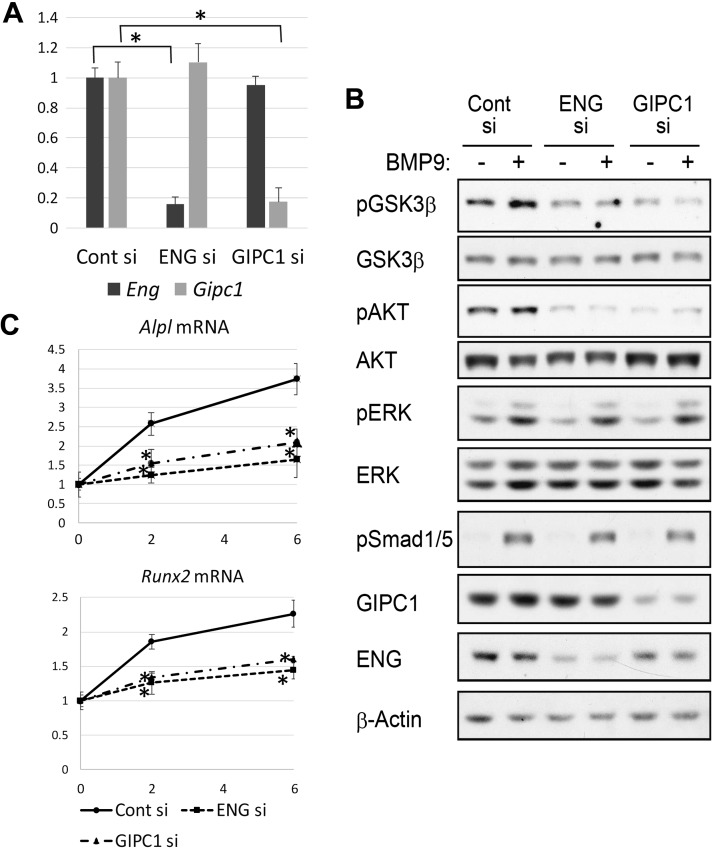
We next sought to examine the rapid activation mechanism of Akt-GSK3-β in BMP9-stimulated osteoblasts. It has previously been reported that endoglin, a TGF-β coreceptor, is essential for the activation of PI3K-Akt by BMP9 through the interaction with GIPC PDZ domain-containing family, member 1 (GIPC1) (15). The mRNA and protein expressions of both endoglin and GIPC1 were easily detected in MC3T3-E1 cells (Fig. 7A, B) and primary osteoblasts (unpublished results). We thus tested how BMP9 signals were affected by siRNA-mediated gene knockdown of endoglin and GIPC1. Introduction of specific siRNAs into MC3T3-E1 cells significantly decreased mRNA/protein expression of their targets (Fig. 7A, B). Notably, knockdown of either endoglin or GIPC1 significantly inhibited rapid phosphorylation of Akt and GSK3-β after stimulation with BMP9 (Fig. 7B). In contrast, BMP9-induced phosphorylation of neither SMAD1/5 nor ERKs was significantly affected (Fig. 7B), indicating both endoglin and GIPC1 are essential signaling mediators of BMP9, both of which specifically induce Akt-GSK3-β signaling. Consistent with this, siRNA-mediated knockdown of either endoglin or GIPC1 significantly suppressed ALP and Runx2 mRNA induction by BMP9 in a similar manner as Akt inhibitor (Fig. 7C). Interestingly, knockdown of GIPC1 constantly decreased endoglin protein expression, whereas it did not significantly affect endoglin mRNA expression, indicating that GIPC1 protein expression may be essential for the proper protein expression of endoglin in osteoblasts (Fig. 7A, B).
Figure 7.
Essential roles of endoglin and GIPC1 in Akt-GSK3-β signaling pathway and ALP and Runx2 mRNA induction by BMP9. MC3T3-E1 cells were transfected with control siRNA, endoglin siRNA, or GIPC1 siRNA, followed by 48 h incubation at 37°C. A) Total RNA was isolated, reverse transcribed, and analyzed by real-time PCR analyses for endoglin and GIPC1 mRNA expressions. Relative mRNA expression levels were calculated based on the GAPDH mRNA level. Fold induction in comparison with the control siRNA-transfected cell mRNA is shown. Data represent means ± sd. *P < 0.05 (significant difference by Mann-Whitney U test; n = 6). B) Cells were serum starved for 4 h, followed by stimulation with 50 ng/ml BMP9 for the indicated minutes. Western blotting analyses were performed using the indicated antibodies. C) Cells were serum starved for 4 h and stimulated with 50 ng/ml BMP9 for the indicated hours. Total RNAs were isolated, reverse transcribed, and analyzed by real-time PCR analyses. Relative ALP and Runx2 mRNA expression levels were calculated based on the Rpl13a mRNA level. Fold mRNA induction in comparison with unstimulated control is shown. P, phosphorylated. Data represent means ± sd. *P < 0.05 [significant difference from the same day value of the control (Cont) siRNA group by Mann-Whitney U test; n = 6].
DISCUSSION
Although previous studies have demonstrated the strong osteogenic potential of BMP9 (9–11), its characteristic signaling mechanisms compared with other BMPs have not been fully elucidated. Several studies, however, have indicated the unique osteogenic signal of BMP9 compared with other BMPs. For example, BMP3, a negative regulator of bone formation, failed to inhibit BMP9-induced ossification, whereas it effectively suppressed ossification by the other BMPs, including BMP2, BMP6, and BMP7 (10). Also, Noggin, a well-known BMP antagonist, failed to inhibit intracellular signaling of BMP9 (18). Although the underlining mechanisms of these differences have not been fully revealed, these previous findings strongly indicate the uniqueness of BMP9 signaling among the BMP family members.
In our current analysis comparing BMP-induced gene expressions in osteoblasts, mRNA induction patterns were apparently different among BMPs (Fig. 1B). BMP9 increased ALP and Runx2 mRNA expression more efficiently than BMP2, which is consistent with a previous report (10). In contrast, Hey1 mRNA induction was more evident for BMP2, whereas Smad7 mRNA induction was similar between BMP2 and BMP9. Although ALP, Runx2, and Hey1 are all known to be efficiently induced by BMPs, their roles in osteogenesis are somewhat different. In contrast to the undisputedly promotive role of ALP and Runx2 in osteogenesis, the role of Hey1 in osteogenesis appears to be more complex. Hey1 is a transcription factor of the HES protein family, a well-known target of Notch signaling (19). Although loss of Hey1 resulted in modest osteopenia in an animal model, the overexpression of Hey1 resulted in progressively distinct osteopenia in vivo and inhibition of osteoblasts ex vivo (20). Additionally, SMAD7 is known as an inhibitory SMAD and has been reported to inhibit osteoblastic differentiation of MC3T3-E1 cells (21), indicating that its induction is a negative feedback mechanism to suppress excessive osteogenesis by BMPs. Therefore, it is reasonable to presume that the more selective induction of ALP and Runx2 by BMP9 may contribute the distinctly strong ossification activity of BMP9 (10). However, molecular mechanisms leading to selective target gene induction by different BMPs have not been fully elucidated.
All BMPs, with the exception of BMP-1, are members of the TGF-β super family, and their signals are transduced though a heterodimeric receptor complex of serine/threonine kinases, BMPRI, and BMPRII (2). Ligand binding induces constitutively active BMPRII to cross-phosphorylate BMPRIs, which subsequently initiates downstream signaling events. Among 7 different BMPRIs identified so far, BMP9 shows poor affinity for BMPR-IA, a type 1 receptor that is generally responsible for the downstream signals of other BMPs, indicating unique downstream signals for BMP9 (12). Instead, 2 type 1 receptors, ALK1 and ALK2, directly bind BMP9 and are responsible for its osteogenic activity (22). As for type 2 receptors, it has previously been reported that BMP9 has a significant preference for ActRIIB and BMPRII (23).
Another notable feature of BMP9 receptor complex is the involvement of endoglin. Endoglin, abundantly expressed in vascular endothelium, was initially identified as a coreceptor for TGF-β1 and TGF-β3, associating with their type 1 and type 2 receptors (24). Notably, it was later found that endoglin binds to BMP9 with much higher affinity than TGF-β, indicating a possibility that endoglin may play an important role in BMP9 signaling (25). In contrast to BMP9, BMP2 failed to bind endoglin with a high affinity in an in vitro binding assay (14), indicating a possibility that the involvement of endoglin may contribute to the different signaling profiles of BMP2 and BMP9. Notably, a recent report demonstrated the crystal structures of the endoglin ectodomain and its complex with BMP9 (16). According to this report, the BMP9-endoglin complex is compatible with binding to ALK1 but not ActRIIB, indicating a possible existence of distinct BMP9-ALK1-endoglin receptor complex excluding type 2 receptor. Consistent with this signaling model, a previous report has demonstrated that endoglin interacts with PI3K p110-α and p85 subunits via GIPC1 to activate Akt in endothelial cells (15). Interestingly, the researchers have also found that BMP9 enhances endoglin-mediated Akt activation to induce capillary formation, whereas TGF-β1 attenuates it. This finding indicates that the involvement of endoglin in downstream signals varies among TGF-β family members.
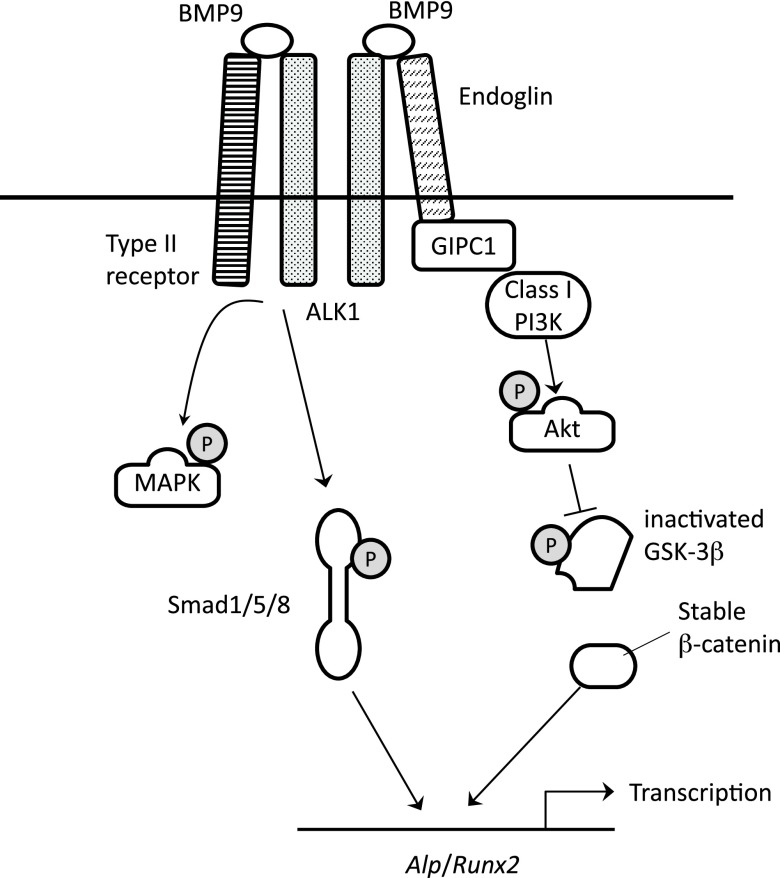
Our current data have demonstrated that BMP9 induces phosphorylation of Akt and GSK3-β within 5 min in osteoblasts (Fig. 2A). This rapid phosphorylation is absent in BMP2-stimulated cells, in which increase of Akt phosphorylation was detected at 30 min. On the other hand, BMP2 and BMP9 induce rapid phosphorylation of SMAD1/5, ERK, and JNK in MC3T3-E1 cells with similar time courses (Fig. 2A). A66, a specific inhibitor of class I PI3K, as well as LY294002, a pan PI3K inhibitor, potently inhibited the rapid phosphorylation of Akt and GSK3-β by BMP9 (Fig. 5A, B). Most notably, siRNA-mediated knockdown of either endoglin or GIPC1 expression significantly inhibited the rapid phosphorylation of Akt and GSK3-β (Fig. 7). As it has been well established that Akt directly phosphorylates and inactivates GSK3-β in response to various growth factor stimulations (26), it seems reasonable to presume that Akt directly inactivates GSK3-β in the downstream signal of BMP9. Our current result that pretreatment by MK2206, an Akt-specific inhibitor, efficiently inhibits BMP9-induced phosphorylation of GSK3-β strongly supports this idea. We also found that phosphorylation levels of Akt and GSK3-β were elevated during the early osteoblast differentiation induced by BMP9 (Fig. 6C). Furthermore, MK2206 and A66 potently inhibited the BMP9-induced mineral deposition of osteoblasts (Fig. 6B). It is therefore strongly suggested that the PI3K-Akt-GSK3-β axis is an essential noncanonical signaling pathway of BMP9 for its osteogenic potential. Taken together, these data are consistent with a previously demonstrated BMP9 signaling model in endothelial cells, indicating that the endoglin-GIPC1 signaling axis also plays an essential role in BMP9 signaling in osteoblasts, rapidly inducing the PI3K-Akt-GSK3-β signaling axis (Fig. 8).
Figure 8.
Schematic presentation of the BMP9 signaling model. Endoglin plays an important role in inducing class I PI3K-Akt-GSK3-β signaling axis. The resulted good β-catenin accumulation contributes to efficient osteogenic potential of BMP9.
Our data showed that ALP and Runx2 mRNA expression was induced by BMP9 more efficiently than by BMP2 (Fig. 1B). This seems to be caused by better β-catenin induction by BMP9 because LiCl treatment increased ALP and Runx2 mRNA induction by BMP2 more than that by BMP9 (Fig. 6E). LiCl is a commonly used inactivator of GSK3-β, mimicking its inactivation by phosphorylation and inducing increased accumulation of β-catenin. Thus, our LiCl data indicate that insufficient β-catenin induction by BMP2 is responsible for its poorer inducing potential of ALP and Runx2 compared with that of BMP9. ALP is a hallmark protein of early osteoblast differentiation and is responsible for matrix mineralization (27). It has previously been reported that ALP expression is induced by BMP2 in a manner dependent on distal-less homeobox 5, a homeobox transcription factor (28). Previous reports have also indicated that the gene expression of ALP is a target of Wnt/β-catenin signaling pathway (29, 30). The ALP gene promoter contains a functional T-cell factor/lymphoid enhancer-binding factor (TCF/LEF) binding site, which responds to β-catenin induction (31). Similar to ALP, it has been reported that Runx2 is transcriptionally activated by β-catenin signaling pathway (32). These previous reports are consistent with our current results, supporting the idea that efficient induction of β-catenin is important for the strong osteogenic potential of BMP9 (Fig. 8).
In summary, we have found that BMP9 induces rapid phosphorylation of GSK3-β, leading to the intracellular accumulation of β-catenin protein through the PI3K-Akt activation pathway. Our findings may contribute to the development of better osteoinduction protocols using osteoblast differentiation factors, including BMP family members.
ACKNOWLEDGMENTS
This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan. The authors declare no conflicts of interest.
Glossary
- ActRIIB
activin receptor type 2B
- Akt
protein kinase B
- ALK
activin receptor–like kinase
- ALP
alkaline phosphatase
- Bglap
bone γ-carboxyglutamate protein
- BMP
bone morphogenetic protein
- BMPRI
BMP receptor type I
- BMPRII
BMP receptor type II
- GAPDH
glyceraldehydes-3-phosphate dehydrogenase
- GIPC1
GIPC PDZ domain–containing family, member 1
- GSK3-β
glycogen synthase kinase 3-β
- Hey1
Hairy/enhancer-of-split related with YRPW motif protein 1
- Rpl13a
ribosomal protein L13a
- Runx2
runt-related transcription factor 2
- siRNA
small interfering RNA
- Wnts
Wingless-related integration site
AUTHOR CONTRIBUTIONS
N. Eiraku, N. Chiba, and T. Matsuguchi designed research; N. Eiraku, N. Chiba, T. Nakamura, M. S. Amir, C.-H. Seong, T. Ohnishi, J. Kusuyama, K. Noguchi, and T. Matsuguchi analyzed data; N. Eiraku, N. Chiba, and T. Matsuguchi performed research; and N. Eiraku, N. Chiba, and T. Matsuguchi wrote the paper.
REFERENCES
- 1.Brazil D. P., Church R. H., Surae S., Godson C., Martin F. (2015) BMP signalling: agony and antagony in the family. Trends Cell Biol. 25, 249–264 [DOI] [PubMed] [Google Scholar]
- 2.Poniatowski L. A., Wojdasiewicz P., Gasik R., Szukiewicz D. (2015) Transforming growth factor beta family: insight into the role of growth factors in regulation of fracture healing biology and potential clinical applications. Mediators Inflamm. 2015, 137823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bragdon B., Moseychuk O., Saldanha S., King D., Julian J., Nohe A. (2011) Bone morphogenetic proteins: a critical review. Cell. Signal. 23, 609–620 [DOI] [PubMed] [Google Scholar]
- 4.Lane J. M. (2005) Bone morphogenic protein science and studies. J. Orthop. Trauma 19, S17–S22 [DOI] [PubMed] [Google Scholar]
- 5.Bessa P. C., Casal M., Reis R. L. (2008) Bone morphogenetic proteins in tissue engineering: the road from laboratory to clinic, part II (BMP delivery). J. Tissue Eng. Regen. Med. 2, 81–96 [DOI] [PubMed] [Google Scholar]
- 6.Lamplot J. D., Qin J., Nan G., Wang J., Liu X., Yin L., Tomal J., Li R., Shui W., Zhang H., Kim S. H., Zhang W., Zhang J., Kong Y., Denduluri S., Rogers M. R., Pratt A., Haydon R. C., Luu H. H., Angeles J., Shi L. L., He T. C. (2013) BMP9 signaling in stem cell differentiation and osteogenesis. Am. J. Stem Cells 2, 1–21 [PMC free article] [PubMed] [Google Scholar]
- 7.Leblanc E., Trensz F., Haroun S., Drouin G., Bergeron E., Penton C. M., Montanaro F., Roux S., Faucheux N., Grenier G. (2011) BMP-9-induced muscle heterotopic ossification requires changes to the skeletal muscle microenvironment. J. Bone Miner. Res. 26, 1166–1177 [DOI] [PubMed] [Google Scholar]
- 8.Fujioka-Kobayashi M., Sawada K., Kobayashi E., Schaller B., Zhang Y., Miron R. J. (2017) Osteogenic potential of rhBMP9 combined with a bovine-derived natural bone mineral scaffold compared to rhBMP2. Clin. Oral Implants Res. 28, 381–387 [DOI] [PubMed] [Google Scholar]
- 9.Cheng H., Jiang W., Phillips F. M., Haydon R. C., Peng Y., Zhou L., Luu H. H., An N., Breyer B., Vanichakarn P., Szatkowski J. P., Park J. Y., He T. C. (2003) Osteogenic activity of the fourteen types of human bone morphogenetic proteins (BMPs). J. Bone Joint Surg. Am. 85, 1544–1552 [DOI] [PubMed] [Google Scholar]
- 10.Kang Q., Sun M. H., Cheng H., Peng Y., Montag A. G., Deyrup A. T., Jiang W., Luu H. H., Luo J., Szatkowski J. P., Vanichakarn P., Park J. Y., Li Y., Haydon R. C., He T. C. (2004) Characterization of the distinct orthotopic bone-forming activity of 14 BMPs using recombinant adenovirus-mediated gene delivery. Gene Ther. 11, 1312–1320 [DOI] [PubMed] [Google Scholar]
- 11.Kang Q., Song W. X., Luo Q., Tang N., Luo J., Luo X., Chen J., Bi Y., He B. C., Park J. K., Jiang W., Tang Y., Huang J., Su Y., Zhu G. H., He Y., Yin H., Hu Z., Wang Y., Chen L., Zuo G. W., Pan X., Shen J., Vokes T., Reid R. R., Haydon R. C., Luu H. H., He T. C. (2009) A comprehensive analysis of the dual roles of BMPs in regulating adipogenic and osteogenic differentiation of mesenchymal progenitor cells. Stem Cells Dev. 18, 545–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown M. A., Zhao Q., Baker K. A., Naik C., Chen C., Pukac L., Singh M., Tsareva T., Parice Y., Mahoney A., Roschke V., Sanyal I., Choe S. (2005) Crystal structure of BMP-9 and functional interactions with pro-region and receptors. J. Biol. Chem. 280, 25111–25118 [DOI] [PubMed] [Google Scholar]
- 13.Bidart M., Ricard N., Levet S., Samson M., Mallet C., David L., Subileau M., Tillet E., Feige J. J., Bailly S. (2012) BMP9 is produced by hepatocytes and circulates mainly in an active mature form complexed to its prodomain. Cell. Mol. Life Sci. 69, 313–324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Castonguay R., Werner E. D., Matthews R. G., Presman E., Mulivor A. W., Solban N., Sako D., Pearsall R. S., Underwood K. W., Seehra J., Kumar R., Grinberg A. V. (2011) Soluble endoglin specifically binds bone morphogenetic proteins 9 and 10 via its orphan domain, inhibits blood vessel formation, and suppresses tumor growth. J. Biol. Chem. 286, 30034–30046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee N. Y., Golzio C., Gatza C. E., Sharma A., Katsanis N., Blobe G. C. (2012) Endoglin regulates PI3-kinase/Akt trafficking and signaling to alter endothelial capillary stability during angiogenesis. Mol. Biol. Cell 23, 2412–2423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saito T., Bokhove M., Croci R., Zamora-Caballero S., Han L., Letarte M., de Sanctis D., Jovine L. (2017) Structural basis of the human endoglin-BMP9 interaction: insights into BMP signaling and HHT1. Cell Rep. 19, 1917–1928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsuguchi T., Chiba N., Bandow K., Kakimoto K., Masuda A., Ohnishi T. (2009) JNK activity is essential for Atf4 expression and late-stage osteoblast differentiation. J. Bone Miner. Res. 24, 398–410 [DOI] [PubMed] [Google Scholar]
- 18.Bergeron E., Senta H., Mailloux A., Park H., Lord E., Faucheux N. (2009) Murine preosteoblast differentiation induced by a peptide derived from bone morphogenetic proteins-9. Tissue Eng. Part A. 15, 3341–3349 [DOI] [PubMed] [Google Scholar]
- 19.Weber D., Wiese C., Gessler M. (2014) Hey bHLH transcription factors. Curr. Top. Dev. Biol. 110, 285–315 [DOI] [PubMed] [Google Scholar]
- 20.Salie R., Kneissel M., Vukevic M., Zamurovic N., Kramer I., Evans G., Gerwin N., Mueller M., Kinzel B., Susa M. (2010) Ubiquitous overexpression of Hey1 transcription factor leads to osteopenia and chondrocyte hypertrophy in bone. Bone 46, 680–694 [DOI] [PubMed] [Google Scholar]
- 21.Yano M., Inoue Y., Tobimatsu T., Hendy G., Canaff L., Sugimoto T., Seino S., Kaji H. (2012) Smad7 inhibits differentiation and mineralization of mouse osteoblastic cells. Endocr. J. 59, 653–662 [DOI] [PubMed] [Google Scholar]
- 22.Luo J., Tang M., Huang J., He B. C., Gao J. L., Chen L., Zuo G. W., Zhang W., Luo Q., Shi Q., Zhang B. Q., Bi Y., Luo X., Jiang W., Su Y., Shen J., Kim S. H., Huang E., Gao Y., Zhou J. Z., Yang K., Luu H. H., Pan X., Haydon R. C., Deng Z. L., He T. C. (2010) TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J. Biol. Chem. 285, 29588–29598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Townson S. A., Martinez-Hackert E., Greppi C., Lowden P., Sako D., Liu J., Ucran J. A., Liharska K., Underwood K. W., Seehra J., Kumar R., Grinberg A. V. (2012) Specificity and structure of a high affinity activin receptor-like kinase 1 (ALK1) signaling complex. J. Biol. Chem. 287, 27313–27325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheifetz S., Bellón T., Calés C., Vera S., Bernabeu C., Massagué J., Letarte M. (1992) Endoglin is a component of the transforming growth factor-beta receptor system in human endothelial cells. J. Biol. Chem. 267, 19027–19030 [PubMed] [Google Scholar]
- 25.Scharpfenecker M., van Dinther M., Liu Z., van Bezooijen R. L., Zhao Q., Pukac L., Löwik C. W., ten Dijke P. (2007) BMP-9 signals via ALK1 and inhibits bFGF-induced endothelial cell proliferation and VEGF-stimulated angiogenesis. J. Cell Sci. 120, 964–972 [DOI] [PubMed] [Google Scholar]
- 26.Cross D. A., Alessi D. R., Cohen P., Andjelkovich M., Hemmings B. A. (1995) Inhibition of glycogen synthase kinase-3 by insulin mediated by protein kinase B. Nature 378, 785–789 [DOI] [PubMed] [Google Scholar]
- 27.Whyte M. P. (1994) Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr. Rev. 15, 439–461 [DOI] [PubMed] [Google Scholar]
- 28.Kim Y. J., Lee M. H., Wozney J. M., Cho J. Y., Ryoo H. M. (2004) Bone morphogenetic protein-2-induced alkaline phosphatase expression is stimulated by Dlx5 and repressed by Msx2. J. Biol. Chem. 279, 50773–50780 [DOI] [PubMed] [Google Scholar]
- 29.Rawadi G., Vayssiere B., Dunn F., Baron R., Roman-Roman S. (2003) BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J. Bone Miner. Res. 18, 1842–1853 [DOI] [PubMed] [Google Scholar]
- 30.Gong Y., Slee R. B., Fukai N., Rawadi G., Roman-Roman S., Reginato A. M., Wang H., Cundy T., Glorieux F. H., Lev D., Zacharin M., Oexle K., Marcelino J., Suwairi W., Heeger S., Sabatakos G., Apte S., Adkins W. N., Allgrove J., Arslan-Kirchner M., Batch J. A., Beighton P., Black G. C., Boles R. G., Boon L. M., Borrone C., Brunner H. G., Carle G. F., Dallapiccola B., De Paepe A., Floege B., Halfhide M. L., Hall B., Hennekam R. C., Hirose T., Jans A., Jüppner H., Kim C. A., Keppler-Noreuil K., Kohlschuetter A., LaCombe D., Lambert M., Lemyre E., Letteboer T., Peltonen L., Ramesar R. S., Romanengo M., Somer H., Steichen-Gersdorf E., Steinmann B., Sullivan B., Superti-Furga A., Swoboda W., van den Boogaard M. J., Van Hul W., Vikkula M., Votruba M., Zabel B., Garcia T., Baron R., Olsen B. R., Warman M. L.; Osteoporosis-Pseudoglioma Syndrome Collaborative Group (2001) LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell 107, 513–523 [DOI] [PubMed] [Google Scholar]
- 31.Matsuzaki E., Takahashi-Yanaga F., Miwa Y., Hirata M., Watanabe Y., Sato N., Morimoto S., Hirofuji T., Maeda K., Sasaguri T. (2006) Differentiation-inducing factor-1 alters canonical Wnt signaling and suppresses alkaline phosphatase expression in osteoblast-like cell lines. J. Bone Miner. Res. 21, 1307–1316 [DOI] [PubMed] [Google Scholar]
- 32.Hamidouche Z., Haÿ E., Vaudin P., Charbord P., Schüle R., Marie P. J., Fromigué O. (2008) FHL2 mediates dexamethasone-induced mesenchymal cell differentiation into osteoblasts by activating Wnt/beta-catenin signaling-dependent Runx2 expression. FASEB J. 22, 3813–3822 [DOI] [PubMed] [Google Scholar]