Abstract
Nasopharyngeal carcinoma (NPC) is a malignant epithelial cancer of the head and neck with high prevalence in southern China, which is accompanied by notable invasiveness and metastasis. Long noncoding RNAs (lncRNAs) participate in the progression of various cancers including NPC. Microarray-based analysis identified highly expressed lncRNA mothers against decapentaplegic homolog 5 (SMAD5)-antisense RNA 1 (AS1) related to NPC. Interestingly, it is found that SMAD5-AS1 competitively bound to microRNA (miR)-106a-5p to regulate SMAD5. Herein, the study aimed to clarify the role of SMAD5-AS1/miR-106a-5p/SMAD5 axis in the process of epithelial mesenchymal transition (EMT) in NPC. SMAD5-AS1 was highly expressed and miR-106a-5p was poorly expressed in NPC tissues and cell lines. The NPC cells were treated with a series of small interfering RNAs, mimics, or inhibitors to explore the effects of SMAD5-AS1, SMAD5, and miR-106a-5p on EMT, cell proliferation, migration, and invasion in NPC. Of note, SMAD5-AS1 silencing or miR-106a-5p overexpression reduced expression of N-cadherin, matrix metallopeptidase 9, Snail, and Vimentin while elevating E-cadherin expression, thus inhibiting EMT, cell proliferation, migration, and invasion in NPC by down-regulation of SMAD5. Moreover, SMAD5 silencing could reduce the ability of EMT induced by SMAD5-AS1 up-regulation. SMAD5-AS1 silencing or miR-106a-5p elevation inhibited tumorigenesis in nude mice. Taken together, SMAD5-AS1 silencing suppressed EMT, cell proliferation, migration, and invasion in NPC by elevating miR-106a-5p to down-regulate SMAD5, which provided a novel therapeutic target for NPC treatment.—Zheng, Y.-J., Zhao, J.-Y., Liang, T.-S., Wang, P., Wang, J., Yang, D.-K., Liu, Z.-S. Long noncoding RNA SMAD5-AS1 acts as a microRNA-106a-5p sponge to promote epithelial mesenchymal transition in nasopharyngeal carcinoma.
Keywords: ceRNA, SMAD5, proliferation, migration, invasion
Nasopharyngeal carcinoma (NPC) is a type of nonlymphomatous, squamous cell cancer arising from the nasopharyngeal epithelial cell lining. NPC frequently occurs in southeast Asia with an incidence rate of 30–80 per 100,000 people every year (1). The development and progression of NPC have been found to accompany Epstein-Barr virus infections as well as environmental and genetic factors (2). Conventional fractionation radiotherapy in combination with adjuvant chemotherapy is the current mainstay treatment regimen for loco-regional NPC at advanced stages (3). However, despite the sensitivity of NPC to radiotherapy, local recurrence induced by radio-resistance typically impedes the successful treatment of NPC (4). Because of its highly metastatic and malignant characteristics, the treatment of NPC often fails because of distant metastasis (5). Recent studies have demonstrated that epithelial mesenchymal transition (EMT) has been implicated in drug resistance, tumor metastasis, and progression, which is also vital for the treatment of NPC (6, 7).
Increasing evidence has shown that some long noncoding RNAs (lncRNAs) may accelerate the occurrence and progression of NPC, such as lncRNA prostate cancer -associated transcript 7 (PCAT7), retinoid-related orphan receptor (ROR), and antisense noncoding RNA in the INK4 locus (ANRIL) (8–11). LncRNA small nucleolar RNA host gene 1 (SNHG1) has been reported to enhance invasion and EMT of NPC cells down-regulation of microRNA (miR)-145-5p and up-regulating (NUAK family kinase 1 (NUAK1) (12). Microarray dataset GSE64634 found that mothers against decapentaplegic homolog 5 (SMAD5)-antisense RNA 1 (AS1) was highly expressed in NPC. MiRs have been reported to be closely associated with progression of NPC (13). MiR-106a-5p is a critical member of the miR-106a-363 cluster from miR-17 family, and the aberrant expression of miR-106a-5p has been detected in various cancers (14). In addition, miR-106a-5p can also act as a competitor and compete for endogenous RNA, thereby affecting gastric cancer through regulation of lncRNA Fer-1–like family member 4 (FER1L4) (15). In our study, we found that miR-106a-5p could specifically bind to SMAD5. SMAD5 is a fundamental component of heterometric SMAD complex, which often localizes into the nucleus and regulates gene transcription (16). SMAD5 has been proved to be involved in the process of osteoblast proliferation and differentiation (17). Moreover, miR-27a and miR-23a contribute to enhanced apoptosis in human granulosa cells through regulating SMAD5 (18). Based on the above literature, we hypothesized that SMAD5-AS1 knockdown can inhibit EMT in NPC by regulating SMAD5 via miR-106a-5p. Currently, only a handful of studies have explored the roles and associated mechanisms of SMAD5-AS1 in NPC. Our study is therefore aimed at investigating the functional relevance of the SMAD5-AS1/miR-106a-5p/SMAD5 axis in EMT of NPC, in an attempt to provide new insights for understanding the mechanisms underlying NPC development.
MATERIALS AND METHODS
Ethics statement
The study was conducted under the approval of the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. All patients enrolled in the experiment signed informed written consents. All animal care and procedures performed in this study were conducted according to the Guidelines for Animal Experiments of the First Affiliated Hospital of Zhengzhou University.
Microarray data analysis
The NPC-related gene expression dataset was initially downloaded from the Gene expression Omnibus (GEO) database (https://www.ncbi.nlm.nih.gov/geo/). Standardized pretreatment was carried out for the gene expression datasets using Affy package in R language software (19). Limma package was employed to screen the differentially expressed lncRNAs (20). The corrected P value was expressed as adj.P.Val. The threshold of |log2FC| >1.5 and adj.P.Val <0.05 was applied to screen differentially expressed genes in order to plot a heat map.
Study subjects
A total of 50 patients with NPC who received treatment in the First Affiliated Hospital of Zhengzhou University from July 1, 2014, to December 30, 2016, were enrolled in the study. All patients were pathologically diagnosed as having NPC without distant metastasis after operative procedures. Among them, 26 cases were males and 24 cases were females. The average age was 58 ± 12 yr. Normal nasopharyngeal epithelial tissues were collected from 30 suspected patients and used as the control (21). The samples were collected immediately after the operation and stored at −80°C.
NPC cell lines CNE1, HONE1, C666-1, CNE2, and normal nasopharyngeal cell line NP69 (purchased from Biochemistry and Cell Biology Institute of Chinese Academy of Sciences, Shanghai, China) were cultured in Roswell Park Memorial Institute (RPMI) 1640 culture medium containing 10% fetal bovine serum in an incubator with 5% CO2 at 37°C. The culture medium was replaced every 2–3 d depending on the growth status. When the cell confluence reached 80–90%, the cells were passaged.
Cell treatment
The HONE1 cell line that exhibited the lowest expression of SMAD5-AS1 was therefore selected for overexpression treatment. The cells were grouped into the following groups: vector group (transfected with pc-DNA empty plasmid) and SMAD5-AS1 group (transfected with the pc-DNA SMAD5-AS1 overexpression plasmid). The CNE1 cell line with the highest expression of SMAD5-AS1 was selected for interfering treatment. The cells were assigned into negative control (NC) group [transfected with the small interfering RNA (siRNA)-NC plasmid], si-SMAD5-AS1-1 group (transfected with the si-SMAD5-AS1-1 plasmid), and si-SMAD5-AS1-2 group (transfected with the si-SMAD5-AS1-2 plasmid). The cells with the highest expression of miR-106a-5p were classified into NC group (transfected with empty vector), miR-106a-5p mimic group (transfected with the miR-106a-5p mimic), miR-106a-5p inhibitor group (transfected with miR-106a-5p inhibitor), si-NC group (transfected with negative interfering plasmid), siRNA-SMAD5 group (transfected with the SMAD5 interfering plasmid), and miR-106a-5p inhibitor + siRNA-SMAD5 group. The siRNA-SMAD5, miR-106a-5p mimic, and miR-106a-5p inhibitor were purchased from Ribobio (Guangzhou, China). Transfection protocol was performed according to the instructions of the Lipofectamine 2000 (Thermo Fisher ScientificY, Waltham, MA, USA).
Quantitative RT-PCR
The total RNA was extracted from the NPC tissues and cells. Reverse transcription was conducted to synthesize cDNA template according to the instructions provided by the Reverse Transcription Reagent Kit (TransGene Biotech, Beijing, China). The primers were designed and synthesized by Sangon Biotech (Shanghai, China) (Table 1). The reverse transcription reaction was performed in a PCR instrument (9700; Ding Guo Chang Sheng Biotech, Beijing, China), and the fluorescence quantitative PCR was conducted in a fluorescent quantitation PCR instrument (7500; ABI Co., Oyster Bay, NY, USA). U6 and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were used as internal references. The expression of genes was determined, and the ratio between the experimental group and the control group was calculated by 2−ΔΔCt; ΔΔCt = ΔCt experimental group − ΔCt control group (22). The experiment was repeated 3 times.
TABLE 1.
Primer sequences for qRT-PCR
| Gene | Primer sequence, 5′–3′ |
|
|---|---|---|
| Forward | Reverse | |
| SMAD5-AS1 | GACGCTGCTTTGGCATTCTC | TGCTTCTATGCTGGGTGACT |
| miR-106a-5p | GATGCTCAAAAAGTGCTTACAGTGCA | TATGGTTGTTCTGCTCTCTGTCTC |
| SMAD5 | CCAGCAGTAAAGCGATTGTTGG | GGGGTAAGCCTTTTCTGTGAG |
| E-cadherin | CGAGAGCTACACGTTCACGG | GGGTGTCGAGGGAAAAATAGG |
| N-cadherin | CATCCCTCCAATCAACTGC | ATGTGCCCTCAAATGAAACC |
| MMP-9 | TGTACCGCTATGGTTACACTCG | GGCAGGGACAGTTGCTTCT |
| Snail | CTTCCAGCAGCCCTACGACCA | GCCCAGGCTGAGGTACTCC |
| Vimentin | GACGCCATCAACACCGAGTT | CTTTGTCGTTGGTTAGCTGGT |
| U6 | AAAGCAAATCATCGGACGACC | GTACAACACATTGTTTCCTCGGA |
| GAPDH | TGTGGGCATCAATGGATTTGG | ACACCATGTATTCCGGGTCAAT |
Absolute quantification of quantitative RT-PCR
The absolute quantitative RT-PCR (qRT-PCR) method was employed to estimate the correct RNA copy numbers of SMAD5-AS1 and miR-106a-5p per cell (23). Using the vitro-transcribed full-length SMAD5-AS1 or reverse-transcribed miR-106a-5p cDNA used as the standard template, the standard curves were produced. Based on the MW of each molecule and cell count, the accurate copy number of SMAD5-AS1 or miR-106a-5p per cell was calculated.
RNA in situ hybridization
NPC tissue and normal nasopharyngeal epithelial tissue were embedded in paraffin and then were sliced into 4-μm-thick sections. The DNA fragments of SMAD5-AS1 were cloned into the pSPT19 vector (10999644001; MilliporeSigma, Burlington, MA, USA), and AS RNA probe was synthesized according to DIG RNA Labeling Kit (SP6/T7) (11175025910; MilliporeSigma). After dewaxing, the paraffin-embedded sections were subjected to proteinase K (Roche, Basel, Switzerland), followed by the hybridization with a 300-ng/ml probe at 65°C for 16 h. Next, the sections were incubated with antidigoxygenin–alkaline phosphatase (alkaline phosphatase–conjugated Fab fragments) (11 093 274 910, diluted at 1: 25; Roche) for 1 h at 25°C. Then, BM Purple (11 442 074 001; Roche) was adopted for coloration without exposure to light.Fluorescent in situ hybridization The expression of SMAD5-AS1 in NPC was determined using the Fluorescent In Situ Hybridization (FISH) Kit (C10910; Riobio, Guangzhou, China). The cell slides were initially placed at the bottom of the 24-well plate. Next, the CNE1 cells in the logarithmic growth phase were transferred onto the slides with a concentration of 6 × 104 cells/well. When the cell confluence reached 60–70%, the cells were washed with PBS for 5 min and fixed with 4% polyoxymethylene at room temperature for 10 min. After that, 1-ml precooled permeabilization solution was added into each well and then incubated at 4°C for 5 min. The permeabilization solution was then discarded followed by the addition of 200 μl prehybridization solution for sealing at 37°C for 30 min. At the same time, the hybridization solution was preheated at 37°C. Next, 2.5 μl of 20 μM FISH Probe Mix solution was added into the hybridization solution in the dark. The prehybridization solution was discarded, and the hybridization solution with the probes was added into each well for hybridization in the dark at 37°C overnight. The cells were washed in turn with lotion I 3 times (5 min each time). Cells were rinsed with lotion II and lotion III once at 42°C in the dark, and then washed with PBS at room temperature for 5 min. Following that, the cells were stained with the DAPI solution for 10 min in the dark and washed with PBS 3 (5 min each time). Finally, the cell slides were fixed with sealing solution for fluorescence detection. The specific probes of SMAD5-AS1 were synthesized by Riobio.
RNA pull-down assay
Cells were transfected with 50 nM biotin-labeled wild-type (WT)-bio-miR-106a-5p and mutant (Mut)-bio-miR-106a-5p. After 48 h, the cells were collected, washed with PBS, and incubated in a specific lysis buffer (Ambion, Austin, TX, USA) for 10 min. The lysate was incubated with M-280 streptavidin magnetic beads (S3762; MilliporeSigma) that were precoated with BSA without RNase and yeast tRNA (TRNABAK-RO; MilliporeSigma). The magnetic beads were incubated at 4°C for 3 h, washed with precooled lysis buffer twice, low-salt buffer 3 times, and high-salt buffer once. Finally, bound RNA was purified by Trizol and its content was determined.
RNA immunoprecipitation
The lysis buffer containing RNase inhibitor (RNasin) (Takara, Tokyo, Japan) and protease inhibitor mixture (B14001a; Roche) [25 mM Tris-HCl (pH 7.4), 150 mM NaCl, 0.5% NP-40, 2 mM EDTA, 1 mM NaF, and 0.5 mM DTT] were used for cell lysis. The lysate was centrifuged at 12,000 g for 30 min followed by a collection of the supernatant. Next, the magnetic beads precoated with either anti-Ago-2 or anti-IgG (BMFA-1; Biomarker Technologies, Beijing, China) were then added into the cell lysate, where the anti-IgG was set as the control group. After incubation at 4°C for 4 h, the magnetic beads were washed with washing buffer (50 mM Tris-HCl, 300 mM NaCl pH 7.4, 1 mM MgCl2 and 0.1% NP-40) 3 times. RNA was then finally extracted by Trizol and quantified.
Dual luciferase reporter gene assay
The binding sites between SMAD5-AS1 and miR-106a-5p or those between miR-106a-5p and SMAD5 were previously predicted by the bioinformatics website. Full-length sequences of SMAD5-AS1 and 3′ UTR of SMAD5 were amplified and cloned into pmirGLO luciferase vector (E1330; Promega, Madison, WI, USA). These were named as phosphorylated (p)SMAD5-AS1-WT and pSMAD5-WT, respectively. After that, site-directed mutation was conducted, followed by the construction of pSMAD5-AS1-Mut and pSMAD5-Mut vectors. pRL-TK vector (E2241; Promega) expressing Renilla luciferase was used as the internal reference. Afterward, miR-106a-5p mimic or miR-106a-5p NC was cotransfected with luciferase reporter vectors into the NPC cells. The fluorescence intensity was detected by a fluorescence detector (Glomax20/20; Promega).
Western blot analysis
The NPC cell lines in the logarithmic growth phase after transfection were collected. Total protein was extracted using the RIPA lysis buffer (Beyotime Biotechnology, Shanghai, China). Next, 10% separation gel and 5% stacking gel were then prepared according to instructions provided by the SDS-PAGE Kit. The proteins were separated by electrophoresis on polyacrylamide gel and transferred onto the nitrocellulose membrane by the wet transfer method. The membrane was then sealed by 5% BSA at room temperature for 1 h. Afterward, diluted primary antibodies, SMAD5 (ab40771, 1: 1000–1: 5000), E-cadherin (ab15148, 1: 500), N-cadherin (ab18203, 1: 1000), matrix metallopeptidase 9 (MMP-9) (ab73734, 1: 1000), Snail (ab82846, 1: 500), Vimentin (ab137321, 1: 2000), and GAPDH (ab37168, 1 µg/ml) were added to the membrane and left to sit at 4°C overnight. All antibodies were purchased from Abcam (Cambridge, MA, USA). On the following day, after being rinsed in Tris-buffered saline with Tween 20, the goat anti-rabbit pAb IgG (ab6721; Abcam) was diluted with 5% skim milk and incubated with the membrane at room temperature for 1 h. The membrane was then washed 3 times with Tris-buffered saline with Tween 20 (15 min each time), and proteins were then visualized with ECL solution followed by imaging using a Bio-Rad gel imaging system (MG8600; Thmorgan Biotechnology, Beijing, China). The gray value of each protein band was quantified by IPP7.0 (Media Cybernetics, Rockville, MD, USA), and the relative expression was expressed as the gray value ratio of SMAD5, E-cadherin, or N-cadherin to that of GAPDH.
5-Ethynyl-2’-deoxyuridine assay
The cells in logarithmic growth phase were seeded into a 96-well plate with a cell density of 4 × 103 cells per well. Next, 100 μl of 50 μM 5-ethynyl-2′-deoxyuridine (EdU) culture medium was then added into each well and incubated for 2 h. After that, the cells were incubated with 50 μl of stationary liquid (PBS containing 4% polyoxymethylene) at room temperature for 30 min, followed by incubation with 50 μl of 2-mg/ml glycine for 5 min and permeabilized with 100 μl of penetrating agent (PBS containing 0.5% TritonX-100) for 10 min. After being washed with PBS, the cells were treated with 100 μl of 1×Apollo staining reaction liquid (Guangzhou RiboBio, Guangzhou, Guangdong, China) for 30 min followed by the addition of 100 μl of penetrating agent and 100 μl of methanol for washing. Cells were subsequently washed with PBS and added with 100 μl of 1× Hoechst 33342 reaction reagent for 30 min.
Transwell assay
The cells were mixed with Matrigel (356234; BD Biosciences, San Jose, CA, USA) at 4°C overnight. On the next day, cells were diluted by serum-free culture medium at a ratio of 1:3 and added into the apical chamber of the Transwell chamber (50 μl/well). The chambers were then allowed to maintain balanced in an incubator for 30 min. Following that, the cells were detached, washed by serum-free medium, counted, and prepared into cell suspension. Matrigel was washed once with serum-free medium, and 1 × 105 cells/ml cells suspension was seeded in the apical chamber with the serum-free medium. Culture medium containing 10% fetal bovine serum was added into the basolateral chamber and left to incubate at 37°C for 24 h. The Transwell chamber was washed by PBS twice 5 min each time, fixed with 5% pentanediol at 4°C, stained by 0.1% crystal violet for 30 min, washed with PBS twice, and observed under a microscope. The numbers of cells that passed through the Matrigel were used as the index to evaluate how well the cells were able to invade. The experiment was repeated 3 times.
Scratch test
The cells were seeded into a 6-well plate (5 × 105 cells/well). When the cell confluence reached ∼90%, a sterile pipette head was used to gently scratch across the axis of each well. After removal of the floating cells by washing with PBS, serum-free culture medium was added to the cells for further incubation for 30 min to 1 h to allow cells to recover. After cell recovery, the cells were photographed at 0 and 24 h, respectively. The migration distance was calculated using an Image Pro Plus Analysis Software (Media Cybernetics). The migration distance rate = (migration distance/the width of scratch at 0 h) × 100%. The experiment was repeated 3 times.
Tumor xenograft in nude mice
Forty-eight clean grade male Kunming nude mice (3 mo old; weighing 20 ± 2 g) were purchased from the Animal Experimental Center of Southern Medical University (Guangdong, China). The single cell suspension was prepared. PBS and Matrigel were mixed at a ratio of 1:1 to resuspend the cells and obtain a final concentration of 1 × 106 cells/200 μl. Next, the nude mice were assigned into different groups: NC group, siRNA SMAD5-AS1 group, miR-106a-5p mimic group, and miR-106a-5p inhibitor group with 12 mice in each group. The mice were anesthetized with ether, and the previously prepared cell suspension was subcutaneously inoculated into the back of the right hind leg of mice in each group. The mice were fed in the same conditions and observed once every 3 d to measure tumor growth, volume, and weight as well as expression of SMAD5-AS1, miR-106a-5p and SMAD5, and lymph node metastasis.
Statistical analysis
All experimental data were analyzed by SPSS 21.0 statistical software (IBM, White Plains, NY, USA). The measurement data were expressed as means ± sd. Comparisons between NPC tissues and normal nasopharyngeal epithelial tissues were analyzed by a paired Student’s t test. The comparison between 2 groups was analyzed by a nonpaired Student’s t test. The comparison among multiple groups was analyzed by 1-way ANOVA, followed by Tukey’s post hoc test. Comparisons among multiple groups at different time points were analyzed via repeated measurement ANOVA, followed by Tukey’s post hoc test. The enumeration data were expressed as percentages, and the χ2 test was applied for comparing data between multiple groups. A value of P < 0.05 was considered to be statistically significant.
RESULTS
SMAD5-AS1 is highly expressed in NPC tissues and cells
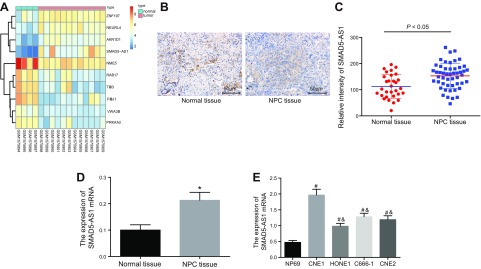
Microarray analysis of NPC-related gene expression dataset GSE64634 found that SMAD5-AS1 expression was elevated in NPC tissues (Fig. 1A). In order to investigate the correlation between SMAD5-AS1 expression and NPC, the expression of SMAD5-AS1 in the NPC tissues and the normal nasopharyngeal epithelial tissues was initially determined by FISH and qRT-PCR (Fig. 1B, C). Moreover, the expression of SMAD5-AS1 in the normal cell line and the NPC cell lines was determined by qRT-PCR (Fig. 1D). We found that SMAD5-AS1 was widely distributed in the NPC tissues and showed significantly higher expression levels compared with that in normal nasopharyngeal epithelial tissues (P < 0.05). Compared with the normal cell line, the NPC cell lines exhibited significantly higher SMAD5-AS1 expression levels, whereby the HONE1 cell line displayed the lowest level of SMAD5-AS1 and CNE1 exhibited the highest expression (Fig. 1E). Thus, HONE1 cell line was selected and treated with SMAD5-AS1 overexpression, whereas the CNE1 cell line was treated with si-SMAD5-AS1 in the following experiments. Taken together, we found that SMAD5-AS1 was highly expressed in the NPC tissues and cells.
Figure 1.
SMAD5-AS1 is highly expressed in NPC tissues and cells. A) The heatmap of NPC-related gene expression dataset GSE64634. B, C) Expression of SMAD5-AS1 in the NPC tissues (n = 50) and the normal nasopharyngeal epithelial tissues (n = 30) determined by RNA in situ hybridization assay. D) The expression of SMAD5-AS1 in the NPC tissues and the normal nasopharyngeal epithelial tissues determined by qRT-PCR. E) The expression of SMAD5-AS1 in the normal cell line and the NPC cell lines determined by qRT-PCR. Measurement data were expressed as means ± sd. Comparisons between NPC tissues and normal nasopharyngeal epithelial tissues were analyzed via Student’s t test and the comparisons among multiple groups were analyzed by 1-way ANOVA, followed by Tukey’s’s post hoc test. The experiment was repeated 3 times. *P < 0.05 vs. the normal nasopharyngeal epithelial tissues; #P < 0.05 vs. the NP69 cell line; &P < 0.05 vs. the CNE1 cell line ZNF197, zinc finger protein 197; NEURL4, neuralized homologue 4; AKR1D1, aldo-keto reductase 1D1; SMAD5-AS1, SMAD5 antisense RNA 1; NME5, non-metastatic cells 5; RAB17, ras-related protein 17; PRKAA2, activated protein kinase-alpha2.
SMAD5-AS1 silencing suppresses NPC cell proliferation, migration, invasion, and EMT
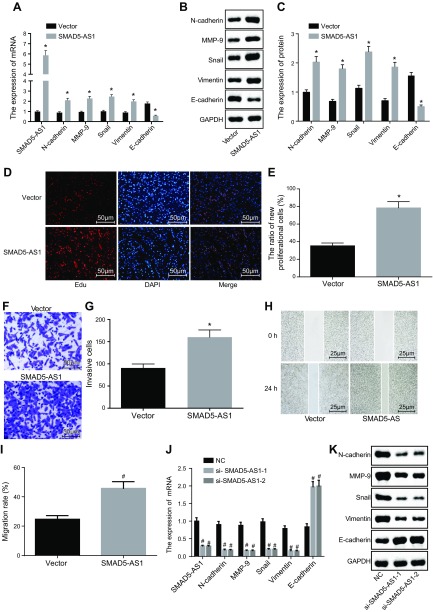
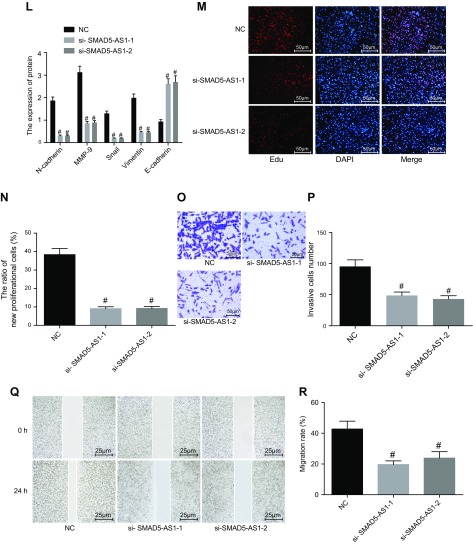
SMAD5-AS1 in the NPC cells was both overexpressed and silenced to explore its roles in cell proliferation, migration, invasion, and EMT (Fig. 2). The mRNA and protein expression of SMAD5-AS1, N-cadherin, MMP-9, Snail, and Vimentin was significantly higher, whereas that of E-cadherin was significantly lower in the SMAD5-AS1 group compared with the vector group (P < 0.05). Additionally, cell proliferation, invasion, and migration were significantly enhanced in the SMAD5-AS1 group compared with the vector group (P < 0.05). Compared with the NC group, the mRNA and protein expression of SMAD5-AS1, N-cadherin, MMP-9, Snail, and Vimentin was significantly lower, whereas that of E-cadherin was significantly elevated in the si-SMAD5-AS1-1 and si-SMAD5-AS1-2 groups. This was accompanied by an attenuation of cell proliferation, invasion, and migration (P < 0.05). Therefore, SMAD5-AS1 silencing inhibited cell proliferation, invasion migration, and EMT in NPC.
Figure 2.
SMAD5-AS1 silencing inhibits cell proliferation, invasion, migration, and EMT in NPC. A) The expression of SMAD5-AS1, E-cadherin, N-cadherin, MMP-9, Snail, and Vimentin in cells treated with pc-DNA SMAD5-AS1 determined by qRT-PCR. B) Gray value analysis of E-cadherin, N-cadherin, MMP-9, Snail, and Vimentin in cells treated with pc-DNA SMAD5-AS1. C) Protein expression of E-cadherin, N-cadherin, MMP-9, Snail, and Vimentin detected by Western blot analysis in cells treated with pc-DNA SMAD5-AS1. D, E) Cell proliferation of cells treated with pc-DNA SMAD5-AS1 measured by EdU assay. F, G) Cell invasion of cells treated with pc-DNA SMAD5-AS1 evaluated by Transwell assay. H, I) Cell migration of cells treated with pc-DNA SMAD5-AS1 measured by scratch test. J) The expression of SMAD5-AS1, E-cadherin, N-cadherin, MMP-9, Snail, and Vimentin in cells treated with empty vector, si-SMAD5-AS1-1, and si-SMAD5-AS1-2 determined by qRT-PCR. K) Gray value analysis of E-cadherin, N-cadherin, MMP-9, Snail, and Vimentin in cells treated with empty vector NC, si-SMAD5-AS1-1, and si-SMAD5-AS1-2. L) Protein expression of E-cadherin, N-cadherin, MMP-9, Snail, and Vimentin in cells treated with empty vector, si-SMAD5-AS1-1, and si-SMAD5-AS1-2 detected by Western blot analysis. M, N) Cell proliferation of cells treated with empty vector, si-SMAD5-AS1-1, and si-SMAD5-AS1-2 estimated by EdU assay. O, P) Cell invasion of cells treated with empty vector, si-SMAD5-AS1-1, and si-SMAD5-AS1-2 measured by Transwell assay. Q, R) Cell migration of cells treated with empty vector, si-SMAD5-AS1-1, and si-SMAD5-AS1-2 detected by scratch test. Measurement data were expressed as means ± SD. Comparison between 2 groups was analyzed by nonpaired Student's t test and comparisons among multiple groups were analyzed by 1-way ANOVA, followed by Tukey's post hoc test. The experiment was repeated 3 times. *P < 0.05 vs. the vector group; #P < 0.05 vs. the NC group.
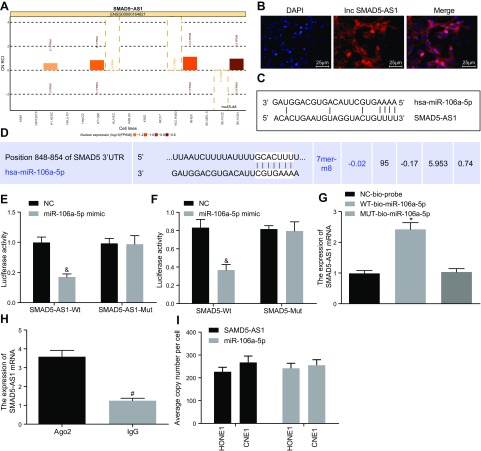
SMAD5-AS1 acts as a miR-106a-5p sponge to promote SMAD5 expression
The relationship between SMAD5-AS1, miR-106a-5p, and SMAD5 was evaluated. SMAD5-AS1 was initially predicted to function mainly in the cytoplasm of CNE1 cells, which was later confirmed by FISH (Fig. 3A, B). Dual luciferase reporter gene assay (Fig. 3C–F) demonstrated that the luciferase activity in SMAD5-AS1-WT 3′UTR group was greatly affected by miR-106a-5p (P < 0.05), whereas no obvious change was found in the SMAD5-AS1-Mut 3′UTR group, suggesting that there was a specific binding site between miR-106a-5p and SMAD5-AS1 3′UTR. Meanwhile, the luciferase activity in the SMAD5-WT group was inhibited by miR-106a-5p and produced no obvious changes in the luciferase activity in the SMAD5-Mut group, suggesting that miR-106a-5p could specifically bind to SMAD5.
Figure 3.
SMAD5-AS1 increases SMAD5 expression by competitively binding to miR-106a-5p. A) Subcellular location prediction of SMAD5-AS1. B) Subcellular location of SMAD5-AS1 detected by FISH assay, DAPI represented nuclear localization, Inc displayed localization of lncRNA SMAD5-AS1, and Merge represented the colocalization of nucleus and SMAD5-AS1. C) The relationship between SMAD5-AS1 and miR-106a-5p predicted by online website. D) The relationship between miR-106a-5p and SMAD5 predicted by online website. E) The relationship between SMAD5-AS1 and miR-106a-5p verified by dual luciferase reporter gene assay. F) The targeting relationship between miR-106a-5p and SMAD5 confirmed by dual luciferase reporter gene assay. G) The binding of SMAD5-AS1 and miR-106a-5p detected by RNA pull-down. H) The binding of SMAD5-AS1 and Ago2 was examined by RNA-IP. I) The absolute quantitation of SAMD5-AS1 and miR-106a-5p in HONE1 and CNE1 cell lines. Measurement data were expressed as means ± sd. Comparison between 2 groups was analyzed by nonpaired t test and comparisons among multiple groups were analyzed by 1-way ANOVA, followed by Tukey’s post hoc test. The experiment was repeated 3 times. &P < 0.05 vs. the NC group; *P < 0.05 vs. the NC-bio-probe group; #P < 0.05 vs. the Ago2 group FPKM, per kilobase of exon per million; hsa, human serum albumin.
RNA pull-down assay showed that WT-miR-106a-5p bound more to SMAD5-AS1 than Mut-miR-106A-5p (P < 0.05), suggesting that miR-106a-5p could directly bind to SMAD5-AS1 (Fig. 3G). RNA-immunoprecipitation assay showed that the binding strength of SMAD5-AS1 to Ago2 was significantly increased compared with the binding to IgG (P < 0.05), suggesting that SMAD5-AS1 could bind to protein argonaute 2 (Ago2) (Fig. 3H). Additionally, the measurement of RNA copy number per cell for SMAD5-AS1 and miR-106a-5p displayed that the abundance of these 2 noncoding RNAs was comparable in HONE1 and CNE1 cells (Fig. 3I). Our data demonstrate that SMAD5-AS1 could competitively bind to miR-106a-5p and produce an elevation of SMAD5 expression.
MiR-106a-5p attenuates EMT by inhibiting SMAD5 expression
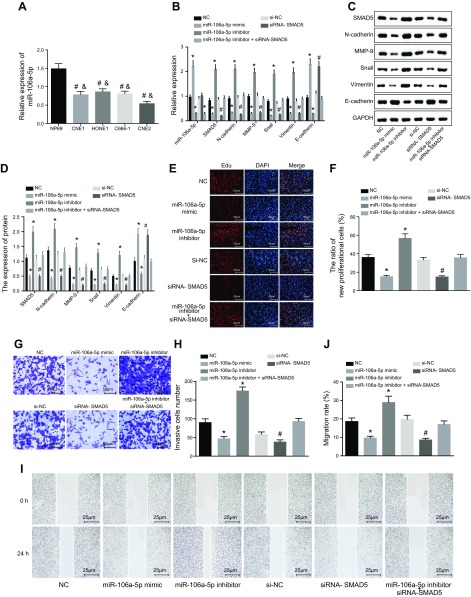
To clarify the effects of miR-106a-5p and SMAD5 on EMT, the miR-106a-5p expression in normal cell line NP69 and NPC cell lines CNE1, HONE1, C66-1, and CNE2 was analyzed by qRT-PCR. The results suggested that the miR-106a-5p expression was obviously lower in all NPC cells, whereby CNE2 cell exhibited the lowest expression compared with NP69 cells (Fig. 4A). Thus, CNE2 cell was employed for the following experiments.
Figure 4.
miR-106a-5p inhibits EMT in NPC by suppressing SMAD5 expression. The cells used for following assays were treated with empty vector, miR-106a-5p inhibitor, miR-106a-5p mimic, and siRNA-SMAD5 alone or in combination. A) Cell line that presented the lowest miR-106a-5p was screened. #P < 0.05 vs. normal cell line NP69; &P < 0.05 vs. NPC cell line CNE2. B) MiR-106a-5p expression and mRNA expression of SMAD5, E-cadherin, N-cadherin, MMP-9, Snail, and Vimentin determined by qRT-PCR. C) Gray value analysis of SMAD5, E-cadherin, N-cadherin, MMP-9, Snail, and Vimentin. D) Protein expression of SMAD5, E-cadherin, N-cadherin, MMP-9, Snail, and Vimentin detected by Western blot analysis. E, F) Cell proliferation detected by the EdU assay. G, H) Cell migration determined by the Transwell assay. I, J) The effect of miR-106a-5p on CNE2 cell migration detected by the scratch test. Measurement data were expressed as means ± sd. Comparisons among multiple groups were analyzed by 1-way ANOVA, followed by Tukey’s post hoc test. The experiment was repeated 3 times. *P < 0.05 vs. the NC group; #P < 0.05 vs. the miR-106a-5p inhibitor group.
Both qRT-PCR and Western blot analysis were carried out to determine mRNA and protein expression of miR-106a-5p, SMAD5, N-cadherin, MMP-9, Snail, Vimentin, and E-cadherin (Fig. 4B–D). The results showed that compared with the NC group, the miR-106a-5p expression was obviously lower, whereas SMAD5 expression was markedly higher in the miR-106a-5p inhibitor group. However, the miR-106a-5p expression was significantly higher, whereas SMAD5 expression was significantly lower in the miR-106a-5p mimic group (P < 0.05). We also found that miR-106a-5p expression exhibited no changes (P > 0.05), whereas SMAD5 expression was much lower in the siRNA-SMAD5 group (P < 0.05). The miR-106a-5p expression in the miR-106a-5p inhibitor + siRNA-SMAD5 group was significantly reduced (P < 0.05), whereas SMAD5 expression showed no obvious changes (P > 0.05). In addition, the mRNA and protein expression of N-cadherin, MMP-9, Snail, and Vimentin was lower, whereas that of E-cadherin was higher in the miR-106a-5p mimic and siRNA-SMAD5 groups when compared with the NC group (P < 0.05). However, mRNA and protein expression of N-cadherin, MMP-9, Snail, and Vimentin was enhanced, whereas that of E-cadherin was inhibited in the miR-106a-5p inhibitor group (P < 0.05).
Furthermore, cell proliferation, invasion, and migration were evaluated by the EdU assay (Fig. 4E, F), the Transwell assay (Fig. 4G, H), and the scratch test (Fig. 4I, J), respectively. The results showed that cell proliferation, invasion, and migration were all obviously enhanced in the miR-106a-5p inhibitor group compared with the NC group (P < 0.05). However, we found that this was significantly attenuated in the miR-106a-5p mimic and siRNA-SMAD5 groups (P < 0.05) No significant differences in cell proliferation, invasion, and migration were found in the miR-106a-5p inhibitor + siRNA-SMAD5 group relative to the NC group. Taken together, miR-106a-5p inhibited SMAD5 expression, thereby attenuating EMT in NPC.
SMAD5 silencing rescues SMAD5-AS1 overexpression-induced phenotypes and impedes cell proliferation, invasion, migration, and EMT in NPC
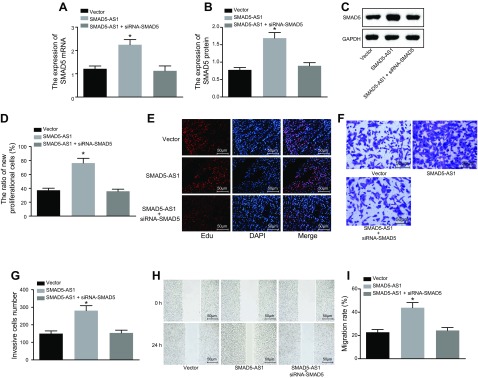
The phenotypic changes induced by SMAD5-AS1 were restored by rescue experiments. Experimental results (Fig. 5A–C) showed that the mRNA and protein expression of SMAD5 was significantly higher in the SMAD5-AS1 group (P < 0.05), whereas there was no obvious change in the SMAD5-AS1 + siRNA-SMAD5 group. From molecule expression, SMAD5 silencing restored SMAD5 expression induced by overexpressed SMAD5-AS1. Furthermore, EdU assay (Fig. 5D, E), Transwell assay (Fig. 5F, G), and scratch test (Fig. 5H, I) were all used to detect and distinguish the cell phenotypic differences in each group. Results highlighted that cell proliferation, invasion, and migration were all obviously enhanced in the SMAD5-AS1 group (P < 0.05), whereas cell proliferation, invasion, and migration showed no obvious change in the SMAD5-AS1 + siRNA-SMAD5 group when compared with the vector group (P > 0.05). Taken together, SMAD5 inhibition resulted in counteracted SMAD5-AS1 elevation-induced phenotypes, thereby suppressing cell proliferation, invasion, migration, and EMT of NPC cells.
Figure 5.
SMAD5 inhibition attenuates cell proliferation, invasion, migration, and EMT induced by SMAD5-AS1 elevation. The cells used for following assays were treated with pc-DNA, SMAD5-AS1, or siRNA-SMAD5. A) The mRNA expression of SMAD5. B, C) The protein expression of SMAD5. D, E) Cell proliferation detected by the EdU assay. F, G) Cell invasion tested by the Transwell assay. H, I) The effect of SMAD5 on CNE1 cell migration evaluated by the scratch test. Measurement data were expressed as means ± sd. Comparisons among multiple groups were analyzed by 1-way ANOVA, followed by Tukey’s post hoc test. The experiment was repeated 3 times. *P < 0.05 vs. the vector group.
SMAD5-AS1 silencing or miR-106a-5p elevation inhibits tumorigenesis in NPC
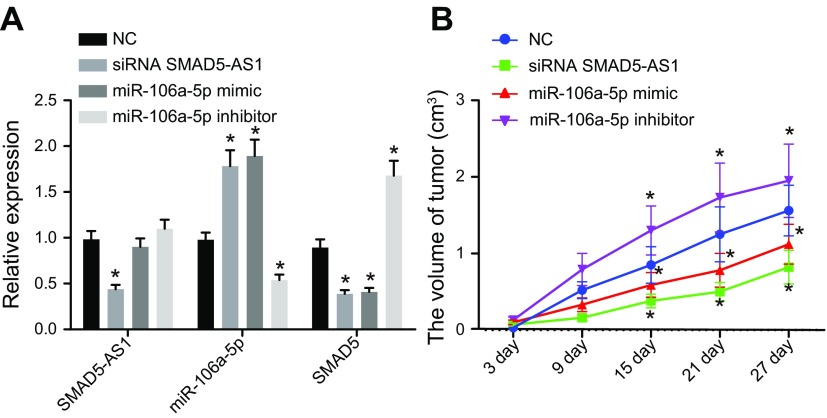
The tumor xenograft assay in nude mice was conducted to clarify the role of SMAD5-AS1 in tumorigenesis of NPC. The results (Fig. 6 and Table 2) displayed that compared with the NC group, the expression of SMAD5-AS1 and SMAD5 was significantly lower, whereas miR-106a-5p expression was obviously higher in the siRNA SMAD5-AS1 group. SMAD5 expression was markedly decreased and miR-106a-5p expression was significantly increased, whereas SMAD5-AS1 expression exhibited no significant changes in the miR-106a-5p mimic group. The expression of SMAD5-AS1 displayed no obvious change, whereas the SMAD5 expression was markedly increased and the miR-106a-5p expression was obviously decreased in the miR-106a-5p inhibitor group (P < 0.05).
Figure 6.
SMAD5-AS1 inhibition and miR-106a-5p overexpression can both attenuate NPC tumorigenesis. The nude mice used for following assays were injected with cells after treatment of empty vector, miR-106a-5p inhibitor, siRNA SMAD5-AS1, or miR-106a-5p mimic. A) Expression of SMAD5-AS1, miR-106a-5p, and SMAD5 in nude mice after different treatments. B) Tumor growth curve of nude mice after different treatments. Measurement data were expressed as means ± sd. Comparisons among multiple groups were analyzed by 1-way ANOVA, followed by Tukey’s post hoc test. Comparisons among multiple groups at different time points were analyzed via repeated measurement ANOVA, followed by Tukey’s post hoc test; n = 12. *P < 0.05 vs. the NC group.
TABLE 2.
Tumor volume, weight, and lymph node metastasis rate
| Group | n | Weight (g) | Volume (cm3) | LNM rate |
|---|---|---|---|---|
| NC | 12 | 2.72 ± 0.73 | 4.126 ± 0.330 | 60% |
| siRNA SMAD5-AS1 | 12 | 1.72 ± 0.66* | 3.882 ± 0.543* | 22.2%* |
| miR-106a-5p mimic | 12 | 1.74 ± 0.66* | 3.881 ± 0.543* | 22.2%* |
| miR-106a-5p inhibitor | 12 | 2.96 ± 0.83* | 4.923 ± 0.895* | 83.3%* |
The measurement data were expressed as means ± sd, and comparisons among multiple groups were analyzed by 1-way ANOVA, followed by Tukey’s post hoc test. The count data were expressed as a percentage, and a χ2 test was applied for comparisons among groups; the experiment was repeated 3 times; n = 12. LNM, lymph node metastasis.
P < 0.05 vs. the NC group.
The tumor growth curve, tumor volume, body weight, and lymph node metastasis rate were measured and recorded in the last part of this investigation. We found that tumor growth curve, tumor volume, body weight, and lymph node metastasis rate all increased in the miR-106a-5p inhibitor group, whereas those in the siRNA SMAD5-AS1 and miR-106a-5p mimic group all significantly decreased, compared with the NC group. This goes to show that tumorigenesis was inhibited through SMAD5-AS1 knockdown or miR-106a-5p up-regulation in NPC.
DISCUSSION
NPC is a type of squamous cell carcinoma arising from epithelial cells of the nasopharynx that is extremely malignant with distant metastasis at the early stage and local invasiveness (24). LncRNAs have been reported to be aberrantly expressed in multiple types of malignant tumors and have also been found to be involved in the pathogenesis of NPC (25). Therefore, our study was aimed at investigating the role of SMAD5-AS1 in EMT of NPC. Collectively, the data obtained from our study revealed that SMAD5-AS1 functions as an miR-106a-5p sponge to accelerate EMT progression in NPC via elevation of SMAD5 expression.
We first found that SMAD5-AS1 was highly expressed, whereas miR-106a-5p expression was reduced in the NPC tissues and cells. Based on the target prediction program and the dual luciferase activity determination, SMAD5-AS1 could competitively bind to miR-106a-5p and up-regulate SMAD5 expression. A previous report shows that an lncRNA known as AFAP1-AS1 is also highly expressed in NPC, and its overexpression contributes to poor prognosis and metastasis of NPC (26). Moreover, the expression of lncRNA ANRIL is also increased in NPC and is correlated with the acceleration of NPC tumorigenesis (27). MiR-106a-5p is expressed in low levels in osteosarcomas, and its low expression has been linked to enhanced osteosarcoma cell invasion, migration, and proliferation (28). MiR-320a has been revealed to exhibit reduced expression in NPC cells and tissues, which promotes cell invasion, migration, and tumor growth in NPC (29). MiR-21 has been revealed to modulate periodontal ligament stem cell osteogenic differentiation through regulating SMAD5 (30). In addition, lncRNA syntaxin 12 (STX12) has been reported to modulate the activation of pancreatic stellate cells through working with miR-148a and SMAD5 (31). The above findings are consistent with our result that SMAD5-AS1 is overexpressed in NPC and competitively bound to miR-106a-5p to enhance SMAD5 expression.
In the second part of our investigation, we demonstrated that miR-106a-5p overexpression or SMAD5-AS1 silencing led to decreased expression of N-cadherin, MMP-9, Snail, and Vimentin, whereas that of E-cadherin was elevated in NPC. Cadherins are implicated in tissue morphogenesis through regulating cell differentiation, migration, and adhesion (32). N-cadherin serves as a biomarker of EMT, whereby elevated nuclear N-cadherin expression has been associated with poor prognosis and poor overall survival for patients with NPC at advanced stages (33, 34). Decreased E-cadherin expression and increased Vimentin expression have also been found to be present in NPC tissues and cells as highlighted by another study (35). Snail functions as a critical inducer in the development of EMT (36). Moreover, a study suggests that the increase of Vimentin, N-cadherin, and Snail along with a reduction in E-cadherin leads to accelerated NPC cell migration, proliferation, and EMT by RAS protein activator like 2 (RASAL2) silencing (37). Besides, increased E-cadherin expression and decreased MMP-9 expression contribute to attenuation of cell proliferation migration and invasion in NPC (38). LncRNA myocardial infarction-associated transcript (MIAT) enhanced cell proliferation and metastasis of non-small cell lung cancer by up-regulating MMP (39). Additionally, silencing of lncRNA urothelial cancer-associated 1 (UCA1) contributes to elevated expression of E-cadherin but reduces the expression of N-cadherin, Vimentin, and Snail in breast cancer cells, which is in line with our findings (40).
In the last part of our investigation, we found that SMAD5-AS1 knockdown inhibited EMT, cell proliferation, migration, and invasion in NPC by up-regulating miR-106a-5p and down-regulating SMAD5. Previous evidence reveals that some lncRNAs act as sponges and titrate miRs, thereby preventing them from binding to mRNAs (41). LncRNA H19 has been reported to serve as an miR sponge to enhance EMT in colorectal cancer (42). A recent study illustrates that lncRNA nuclear paraspeckle assembly transcript 1 (NEAT1) silencing attenuates proliferation while enhancing NPC cell apoptosis by elevating miR-124 expression (43). Moreover, miR-106a-5p has been demonstrated to suppress renal cell carcinoma invasion and migration (44). By acting as a tumor suppressor, miR-145 was able to suppress NPC cell invasion and metastasis via reduction of SMAD3 (45). Another study has reported that SMAD2 silencing alleviates EMT of NPC through N-myc downstream-regulated gene 1 (46). Therefore, our results obtained in this investigation help support the findings that knockdown of SMAD5-AS1 was able to repress EMT, cell proliferation, migration, and invasion in NPC by increasing miR-106a-5p and decreasing SMAD5.
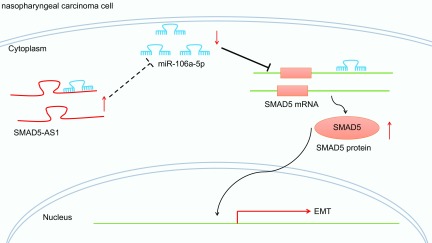
In summary, our data demonstrate that SMAD5-AS1 acts as an oncogene in NPC. In NPC cells, highly expressed SMAD5-AS1 could competitively bind to miR-106a-5p to up-regulate expression of SMAD5, which facilitated EMT in NPC. Meanwhile, SMAD5 expression is repressed when treated with si-SMAD5-AS1 or miR-106a-5p mimics, ultimately hindering the extent of EMT in NPC (Fig. 7). These findings may provide potential therapeutic targets for future treatment approaches for treating patients with NPC. The function of lncRNAs and their mechanism remain to be further defined and investigated. Therefore, future studies are warranted in order to elucidate the underlying mechanisms that govern lncRNA-miR interactions.
Figure 7.
Diagram showing the mechanisms that involve the regulatory role of SMAD5-AS1/miR-106a-5p/SMAD5 axis in the EMT process in NPC. In NPC cells, highly expressed SMAD5-AS1 could competitively bind to miR-106a-5p to increase SMAD5 expression, which promoted EMT in NPC.
ACKNOWLEDGMENTS
Y.-J.Z. and J.-Y.Z. are regarded as co-first authors. The authors declare no conflicts of interest.
Glossary
- Ago2
protein argonaute 2
- AS1
antisense RNA 1
- EdU
5-ethynyl-2′-deoxyuridine
- EMT
epithelial mesenchymal transition
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- FISH
fluorescent in situ hybridization
- lncRNA
long noncoding RNA
- miR
microRNA
- MMP-9
matrix metallopeptidase 9
- Mut
mutant
- NPC
nasopharyngeal carcinoma
- qRT-PCR
quantitative RT-PCR
- SMAD5
mothers against decapentaplegic homolog 5
- WT
wild type
AUTHOR CONTRIBUTIONS
Y.-J. Zheng conceived the study and together with P. Wang designed the study; J.-Y. Zhao and D.-K. Yang were involved in data collection; T.-S. Liang and Z.-S. Liu performed the statistical analysis and preparation of figures and tables; J. Wang drafted the paper; and all authors read and approved the final manuscript.
REFERENCES
- 1.Li T., Chen J. X., Fu X. P., Yang S., Zhang Z., Chen KhH., Li Y. (2011) microRNA expression profiling of nasopharyngeal carcinoma. Oncol. Rep. 25, 1353–1363 [DOI] [PubMed] [Google Scholar]
- 2.Qi G., Chen J., Shi C., Wang Y., Mi S., Shao W., Yu X., Ma Y., Ling J., Huang J. (2016) Cinnamic acid (CINN) induces apoptosis and proliferation in human nasopharyngeal carcinoma cells. Cell. Physiol. Biochem. 40, 589–596 [DOI] [PubMed] [Google Scholar]
- 3.Lee A. W., Tung S. Y., Ngan R. K., Chappell R., Chua D. T., Lu T. X., Siu L., Tan T., Chan L. K., Ng W. T., Leung T. W., Fu Y. T., Au G. K., Zhao C., O’Sullivan B., Tan E. H., Lau W. H. (2011) Factors contributing to the efficacy of concurrent-adjuvant chemotherapy for locoregionally advanced nasopharyngeal carcinoma: combined analyses of NPC-9901 and NPC-9902 Trials. Eur. J. Cancer 47, 656–666 [DOI] [PubMed] [Google Scholar]
- 4.Qu C., Liang Z., Huang J., Zhao R., Su C., Wang S., Wang X., Zhang R., Lee M. H., Yang H. (2012) MiR-205 determines the radioresistance of human nasopharyngeal carcinoma by directly targeting PTEN. Cell Cycle 11, 785–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X. J., Peng L. X., Shao J. Y., Lu W. H., Zhang J. X., Chen S., Chen Z. Y., Xiang Y. Q., Bao Y. N., Zheng F. J., Zeng M. S., Kang T. B., Zeng Y. X., Teh B. T., Qian C. N. (2012) As an independent unfavorable prognostic factor, IL-8 promotes metastasis of nasopharyngeal carcinoma through induction of epithelial-mesenchymal transition and activation of AKT signaling. Carcinogenesis 33, 1302–1309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Du B., Shim J. S. (2016) Targeting epithelial-mesenchymal transition (EMT) to overcome drug resistance in cancer. Molecules 21, E965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Feng S., Yang G., Yang H., Liang Z., Zhang R., Fan Y., Zhang G. (2017) NEDD4 is involved in acquisition of epithelial-mesenchymal transition in cisplatin-resistant nasopharyngeal carcinoma cells. Cell Cycle 16, 869–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.He R., Hu Z., Wang Q., Luo W., Li J., Duan L., Zhu Y. S., Luo D. X. (2017) The role of long non-coding RNAs in nasopharyngeal carcinoma: as systemic review. Oncotarget 8, 16075–16083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu Y., Tao Z., Qu J., Zhou X., Zhang C. (2017) Long non-coding RNA PCAT7 regulates ELF2 signaling through inhibition of miR-134-5p in nasopharyngeal carcinoma. Biochem. Biophys. Res. Commun. 491, 374–381 [DOI] [PubMed] [Google Scholar]
- 10.Wu J. H., Tang J. M., Li J., Li X. W. (2018) Upregulation of SOX2-activated lncRNA ANRIL promotes nasopharyngeal carcinoma cell growth. Sci. Rep. 8, 3333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li L., Gu M., You B., Shi S., Shan Y., Bao L., You Y. (2016) Long non-coding RNA ROR promotes proliferation, migration and chemoresistance of nasopharyngeal carcinoma. Cancer Sci. 107, 1215–1222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lan X., Liu X. (2019) LncRNA SNHG1 functions as a ceRNA to antagonize the effect of miR-145a-5p on the down-regulation of NUAK1 in nasopharyngeal carcinoma cell. J. Cell. Mol. Med. 23, 2351–2361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang F., Lu J., Peng X., Wang J., Liu X., Chen X., Jiang Y., Li X., Zhang B. (2016) Integrated analysis of microRNA regulatory network in nasopharyngeal carcinoma with deep sequencing. J. Exp. Clin. Cancer Res. 35, 17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhi F., Zhou G., Shao N., Xia X., Shi Y., Wang Q., Zhang Y., Wang R., Xue L., Wang S., Wu S., Peng Y., Yang Y. (2013) miR-106a-5p inhibits the proliferation and migration of astrocytoma cells and promotes apoptosis by targeting FASTK. PLoS One 8, e72390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yue B., Sun B., Liu C., Zhao S., Zhang D., Yu F., Yan D. (2015) Long non-coding RNA Fer-1-like protein 4 suppresses oncogenesis and exhibits prognostic value by associating with miR-106a-5p in colon cancer. Cancer Sci. 106, 1323–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cho J. H., Han M. Y., Cha S. H., Jung J. H., Yoon K. L. (2014) Genetic polymorphism of SMAD5 is associated with Kawasaki disease. Pediatr. Cardiol. 35, 601–607 [DOI] [PubMed] [Google Scholar]
- 17.Fang T., Wu Q., Mu S., Yang L., Liu S., Fu Q. (2016) Shikonin stimulates MC3T3-E1 cell proliferation and differentiation via the BMP-2/Smad5 signal transduction pathway. Mol. Med. Rep. 14, 1269–1274 [DOI] [PubMed] [Google Scholar]
- 18.Nie M., Yu S., Peng S., Fang Y., Wang H., Yang X. (2015) miR-23a and miR-27a promote human granulosa cell apoptosis by targeting SMAD5. Biol. Reprod. 93, 98 [DOI] [PubMed] [Google Scholar]
- 19.Gautier L., Cope L., Bolstad B. M., Irizarry R. A. (2004) affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20, 307–315 [DOI] [PubMed] [Google Scholar]
- 20.Smyth G. K. (2004) Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat. Appl. Genet. Mol. Biol. 3, Article3 [DOI] [PubMed] [Google Scholar]
- 21.Wang T., Liu H., Chen Y., Liu W., Yu J., Wu G. (2009) Methylation associated inactivation of RASSF1A and its synergistic effect with activated K-Ras in nasopharyngeal carcinoma. J. Exp. Clin. Cancer Res. 28, 160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu J., Lu M. X., Cui Y. D., Du Y. Z. (2017) Selection and evaluation of reference genes for expression analysis using qRT-PCR in Chilo suppressalis (Lepidoptera: Pyralidae). J. Econ. Entomol. 110, 683–691 [DOI] [PubMed] [Google Scholar]
- 23.Zheng J., Huang X., Tan W., Yu D., Du Z., Chang J., Wei L., Han Y., Wang C., Che X., Zhou Y., Miao X., Jiang G., Yu X., Yang X., Cao G., Zuo C., Li Z., Wang C., Cheung S. T., Jia Y., Zheng X., Shen H., Wu C., Lin D. (2016) Pancreatic cancer risk variant in LINC00673 creates a miR-1231 binding site and interferes with PTPN11 degradation. Nat. Genet. 48, 747–757 [DOI] [PubMed] [Google Scholar]
- 24.Lu J., He M. L., Wang L., Chen Y., Liu X., Dong Q., Chen Y. C., Peng Y., Yao K. T., Kung H. F., Li X. P. (2011) MiR-26a inhibits cell growth and tumorigenesis of nasopharyngeal carcinoma through repression of EZH2. Cancer Res. 71, 225–233 [DOI] [PubMed] [Google Scholar]
- 25.Wang Q., Fan H., Liu Y., Yin Z., Cai H., Liu J., Wang Z., Shao M., Sun X., Diao J., Liu Y., Tong L., Fan Q. (2014) Curcumin enhances the radiosensitivity in nasopharyngeal carcinoma cells involving the reversal of differentially expressed long non-coding RNAs. Int. J. Oncol. 44, 858–864 [DOI] [PubMed] [Google Scholar]
- 26.Bo H., Gong Z., Zhang W., Li X., Zeng Y., Liao Q., Chen P., Shi L., Lian Y., Jing Y., Tang K., Li Z., Zhou Y., Zhou M., Xiang B., Li X., Yang J., Xiong W., Li G., Zeng Z. (2015) Upregulated long non-coding RNA AFAP1-AS1 expression is associated with progression and poor prognosis of nasopharyngeal carcinoma. Oncotarget 6, 20404–20418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zou Z. W., Ma C., Medoro L., Chen L., Wang B., Gupta R., Liu T., Yang X. Z., Chen T. T., Wang R. Z., Zhang W. J., Li P. D. (2016) LncRNA ANRIL is up-regulated in nasopharyngeal carcinoma and promotes the cancer progression via increasing proliferation, reprograming cell glucose metabolism and inducing side-population stem-like cancer cells. Oncotarget 7, 61741–61754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.He Q. Y., Wang G. C., Zhang H., Tong D. K., Ding C., Liu K., Ji F., Zhu X., Yang S. (2016) miR-106a-5p suppresses the proliferation, migration, and invasion of osteosarcoma cells by targeting HMGA2. DNA Cell Biol. 35, 506–520 [DOI] [PubMed] [Google Scholar]
- 29.Qi X., Li J., Zhou C., Lv C., Tian M. (2014) MicroRNA-320a inhibits cell proliferation, migration and invasion by targeting BMI-1 in nasopharyngeal carcinoma. FEBS Lett. 588, 3732–3738 [DOI] [PubMed] [Google Scholar]
- 30.Wei F., Yang S., Guo Q., Zhang X., Ren D., Lv T., Xu X. (2017) MicroRNA-21 regulates osteogenic differentiation of periodontal ligament stem cells by targeting Smad5. Sci. Rep. 7, 16608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang H., Jiang Y., Lu M., Sun B., Qiao X., Xue D., Zhang W. (2017) STX12 lncRNA/miR-148a/SMAD5 participate in the regulation of pancreatic stellate cell activation through a mechanism involving competing endogenous RNA. Pancreatology 17, 237–246 [DOI] [PubMed] [Google Scholar]
- 32.Clendenon S. G., Sarmah S., Shah B., Liu Q., Marrs J. A. (2012) Zebrafish cadherin-11 participates in retinal differentiation and retinotectal axon projection during visual system development. Dev. Dyn. 241, 442–454 [DOI] [PubMed] [Google Scholar]
- 33.Luo W. R., Wu A. B., Fang W. Y., Li S. Y., Yao K. T. (2012) Nuclear expression of N-cadherin correlates with poor prognosis of nasopharyngeal carcinoma. Histopathology 61, 237–246 [DOI] [PubMed] [Google Scholar]
- 34.Sun H., Liu M., Wu X., Yang C., Zhang Y., Xu Z., Gao K., Wang F. (2017) Overexpression of N-cadherin and β-catenin correlates with poor prognosis in patients with nasopharyngeal carcinoma. Oncol. Lett. 13, 1725–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Su Z., Li G., Liu C., Ren S., Tian Y., Liu Y., Qiu Y. (2016) Ionizing radiation promotes advanced malignant traits in nasopharyngeal carcinoma via activation of epithelial-mesenchymal transition and the cancer stem cell phenotype. Oncol. Rep. 36, 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wu Y., Zhou B. P. (2010) Snail: more than EMT. Cell Adhes. Migr. 4, 199–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z., Wang J., Su Y., Zeng Z. (2015) RASAL2 inhibited the proliferation and metastasis capability of nasopharyngeal carcinoma. Int. J. Clin. Exp. Med. 8, 18765–18771 [PMC free article] [PubMed] [Google Scholar]
- 38.Sun X. J., Zhang P., Li H. H., Jiang Z. W., Jiang C. C., Liu H. (2014) Cisplatin combined with metformin inhibits migration and invasion of human nasopharyngeal carcinoma cells by regulating E-cadherin and MMP-9. Asian Pac. J. Cancer Prev. 15, 4019–4023 [DOI] [PubMed] [Google Scholar]
- 39.Lai I. L., Yang C. A., Lin P. C., Chan W. L., Lee Y. T., Yen J. C., Chang Y. S., Chang J. G. (2017) Long noncoding RNA MIAT promotes non-small cell lung cancer proliferation and metastasis through MMP9 activation. Oncotarget 8, 98148–98162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xiao C., Wu C. H., Hu H. Z. (2016) LncRNA UCA1 promotes epithelial-mesenchymal transition (EMT) of breast cancer cells via enhancing Wnt/beta-catenin signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 20, 2819–2824 [PubMed] [Google Scholar]
- 41.Song X., Cao G., Jing L., Lin S., Wang X., Zhang J., Wang M., Liu W., Lv C. (2014) Analysing the relationship between lncRNA and protein-coding gene and the role of lncRNA as ceRNA in pulmonary fibrosis. J. Cell. Mol. Med. 18, 991–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liang W. C., Fu W. M., Wong C. W., Wang Y., Wang W. M., Hu G. X., Zhang L., Xiao L. J., Wan D. C., Zhang J. F., Waye M. M. (2015) The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget 6, 22513–22525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng N., Guo Y. (2017) Long noncoding RNA NEAT1 promotes nasopharyngeal carcinoma progression through regulation of miR-124/NF-κB pathway. OncoTargets Ther. 10, 5843–5853 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Pan Y. J., Wei L. L., Wu X. J., Huo F. C., Mou J., Pei D. S. (2017) MiR-106a-5p inhibits the cell migration and invasion of renal cell carcinoma through targeting PAK5. Cell Death Dis. 8, e3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Huang H., Sun P., Lei Z., Li M., Wang Y., Zhang H. T., Liu J. (2015) miR-145 inhibits invasion and metastasis by directly targeting Smad3 in nasopharyngeal cancer. Tumour Biol. 36, 4123–4131 [DOI] [PubMed] [Google Scholar]
- 46.Hu Z. Y., Xie W. B., Yang F., Xiao L. W., Wang X. Y., Chen S. Y., Li Z. G. (2015) NDRG1 attenuates epithelial-mesenchymal transition of nasopharyngeal cancer cells via blocking Smad2 signaling. Biochim. Biophys. Acta 1852, 1876–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]