Abstract
Nonalcoholic fatty liver disease (NAFLD) is one of the most prevalent diseases worldwide. Exercise is a first-line therapy and an important preventive measure for patients with NAFLD, but the underlying mechanisms are not clear. C57BL/6 mice were fed a high-fat diet (HFD) and subjected to 12 wk swimming exercise. Exercise protected against hepatic lipid accumulation and alleviated hepatocyte damage in HFD mice. Tandem mass tag—based quantitative proteomic analyses and ingenuity pathway analysis revealed that exercise down-regulated fatty acid–binding protein (FABP)1 signaling pathway, which was most closely associated with lipid metabolism. Moreover, exercise significantly decreased FABP1 expression, and liver-specific overexpression of FABP1 abolished the protective effect of exercise in NAFLD mice. Specifically, exercise significantly increased autophagic flux via restoring lysosomal function, including lysosomal proteolysis and lysosomal acidification maintenance, contributing to enhancement in autophagic clearance and subsequently alleviation of hepatic steatosis. Conversely, Fabp1 overexpression in the mouse liver blocked the protective effect of exercise via inhibiting autophagy flux. The present study identified FABP1 inhibition–mediated replenishment of the autophagy-lysosomal machinery as a novel endogenous mechanism whereby long-term exercise improves lipid homeostasis and ameliorates hepatic steatosis in NAFLD.—Pi, H., Liu, M., Xi, Y., Chen, M., Tian, L., Xie, J., Chen, M., Wang, Z., Yang, M., Yu, Z., Zhou, Z., Gao, F. Long-term exercise prevents hepatic steatosis: a novel role of FABP1 in regulation of autophagy-lysosomal machinery.
Keywords: swimming, ingenuity pathway analysis, NAFLD, quantitative proteomic analysis
Nonalcoholic fatty liver disease (NAFLD) is characterized by the presence of hepatic steatosis in the absence of any secondary causes of excessive accumulation of fat, such as excessive alcohol drinking (1). The current prevalence of NAFLD is 80% in patients with obesity and 30% in healthy individuals in general Western populations, and it has become the leading causes of chronic liver damage in Western countries (2). Approximately 15–25% of patients with NAFLD develop nonalcoholic steatohepatitis, which may progress to more serious diseases such as liver cirrhosis and hepatocellular carcinoma (3, 4). The increasing prevalence of NAFLD presents an enormous public health challenge. Therefore, novel preventive and therapeutic strategies are required to alleviate NAFLD.
Exercise training is the most promising lifestyle change, and it has become a crucial strategy for the prevention and treatment of NAFLD (5). Approximately 50% of patients with NAFLD are not active, and nearly one-third of these patients do no physical exercise (6). Exercise training, such as swimming exercise (SE), attenuates hepatic steatosis, improves liver enzymes, and ameliorates glucose intolerance in rats fed a high-fat diet (HFD) (7–10). Therefore, identification of cellular and molecular signaling activated by physical activity may lead to the development of therapeutic approaches that can, to some extent, mimic effects of exercise for the prevention and treatment of NAFLD.
Fatty acid–binding protein (FABP)1 is a liver-specific FABP that plays important roles in intracellular lipid metabolism in the liver. Previous studies have shown that FABP1 overexpression significantly increased hepatocyte fatty acid uptake (11), whereas knockdown of FABP1 remarkably blocked lipid accumulation in hepatocytes (12). Interestingly, Martin et al. (13) identified a loss of FABP1-induced hepatic lipid accumulation in female mice. Considering some conflicting results regarding the role of FABP1 in the pathogenesis of NAFLD, further studies are needed to establish either a pro‐ or anti‐NAFLD role for FABP1.
Autophagy is a conserved cellular process by which cells “eat” their own components (organelles and proteins) to maintain cellular energetic balance for survival (14). Increasing evidence demonstrates that autophagy is supposed to break down intracellular lipids through a lysosomal degradation pathway, and deficiency in autophagy flux is implicated in the development of hepatic steatosis (15). Moreover, previous studies reported that the pharmacological or genetic activation of the autophagy-lysosomal pathway could enhance hepatic lipid droplet (LD) clearance, which highlights a therapeutic potential of autophagy in NAFLD prevention and treatment (16). Recent studies confirmed that exercise improved liver lipid profiles and maintained the liver metabolism and function via activating autophagy (17), but the underlying mechanisms by which exercise increases autophagy in the liver remain largely unexamined.
In the present study, tandem mass tag (TMT)-based quantitative proteomic analysis and ingenuity pathway analysis (IPA) were undertaken to comprehensively identify the proteins and regulatory pathways that may be involved in mediating the beneficial effects of exercise on NAFLD. Our findings may support the hypothesis that FABP1-autophagy system underscores long-term exercise-induced protective effects against NAFLD.
MATERIALS AND METHODS
Animals and experimental design
Male C57BL/6J mice (8 wk) were obtained from the animal center of the Third Military Medical University. Mice were maintained under pathogen-free conditions at a controlled temperature of 22 ± 2°C with a 12-h light/dark cycle. Food and water were offered ad libitum. Body weights were measured weekly (Mondays, 10:00 pm). All animals were euthanized under anesthetic conditions. All procedures were conducted according to Guide for the Care and Use of Laboratory Animals [National Institutes of Health (NIH), Bethesda, MD, USA] and the Care and Use of Laboratory Animals (Third Military Medical University).
Investigation of exercise inhibition of HFD-induced NAFLD in vivo
Eight-week-old mice were randomly placed into one of 3 diet-exercise groups for 12 wk: 1) standard diet (SD) group with 10% kcal from fat (D12450B; Research Diets, New Brunswick, NJ, USA; for more details, please see the website:https://researchdiets.com/formulas/d12450b) (n = 10/group); 2) HFD group with 60% kcal from fat (D12492; https://researchdiets. com/formulas/d12492) (n = 10/group); and 3) HFD + SE group (n = 10/group). The HFD contained 34.9% fat from lard and soybean oil fat (D12492). For comparison, the regular diet (D12450B) contained 4.3% total fat from lard and soybean oil fat. Mice swam voluntarily in pools (50 × 38 × 20 cm) that were filled to a depth of 10 cm with warm sterilized water. The water temperature was maintained 30 ± 1°C. The exercise protocol was modified from our previously published procedure (18–22). The SE protocol consisted of 12 wk of training. On the first and second days, the mice swam for 15 min/d. From the third to fourth days, the mice swam for 25 min/d. On the fifth day, the animals swam continuously for 30 min/d. The first week was an adaptation period, with a progressive increase of the time exercising (15 min/d until reaching 30 min/d, 5 times/wk) in the next 11 wk. This protocol was considered a moderate-intensity exercise model because lactate concentration in the blood was lower than 1 mM (23, 24). The blood level of lactate was 0.7 ± 0.03 mM during the last session of SE.
Investigation of the role of the FABP1 pathway in exercise prevention of HFD-induced NAFLD in vivo
Male C57BL/6J mice aged 7 wk in the HFD-induced NAFLD mouse model were randomly divided into 4 groups: SD + adeno-associated virus (AAV) 8-thyroid hormone-binding globulin (TBG) -Null group (n = 10/group); HFD + AAV8-TBG-Null group (n = 10/group); HFD + SE + AAV8-TGB-Null group (n = 10/group); and HFD + SE + AAV8-TGB-Fabp1 group (n = 10/group). For all AAV-TGB-Null groups, the mice were intravenously injected with 5 × 1011 viral genomes AAV-TGB-Null dissolved in 200 μl saline. After 1 wk, the AAV-TBG-Null mice were given SD feeding to establish SD + AAV8-TGB-Null group, or HFD feeding to establish HFD + AAV8-TBG-Null group, or HFD feeding and exercise to establish HFD + SE + AAV8-TGB-Null group. For the HFD +SE + AAV8-TGB-Fabp1 group, the mice received the same dose of AAV8-TGB-Fabp1 by injection. After 1 wk, the AAV–TGB-Fabp1 mice were given HFD feeding and exercise to establish HFD + SE + AAV8-TGB-Fabp1 group. One dose of AAV-TBG-Null or AAV8-TBG-Fabp1 was given 1 wk prior to food feeding and exercise for liver overexpression of FABP1 as the previous study (25).
Measurement of body weight, caloric intake, and blood lactate concentration
The body weights and food intake of mice were recorded and measured weekly. To estimate food consumption, food intake was assessed by weighing the food in each cage dispenser, including the food that was spilled on the floor of the cage. Energy intake was calculated on the basis of 5.21 kcal/g for the HFD and 3.82 kcal/g for the SD (caloric values obtained from Research Diets). In addition, at the end of the study period, animals’ serum was harvested for blood lactate analysis. Serum lactate concentration was analyzed with the Lactate ELISA Kit (CEV643Ge; USCN, Wuhan, China).
Histologic assessment
The liver samples were fixed immediately in 4% paraformaldehyde for at least 24 h. Fixed samples were embedded in paraffin wax and sectioned into 5-μm slices. The liver sections were subsequently deparaffinized and rehydrated through a xylene and alcohol series and stained with hematoxylin and eosin (H&E) using a standard procedure (26).
Oil Red O staining
Liver sample cryosections (5 μm) were prepared in a cryostat (Leica Microsystems, Buffalo Grove, IL, USA). Hepatocytes were fixed in 4% neutral formalin for 30 min. Cryosections and fixed hepatocytes were stained with a prewarmed Oil Red O working solution for 30 min. Sections and cells were rinsed with distilled water 3 times, counterstained with hematoxylin for 3 min, and gently washed with distilled water. Three images per dish were captured at ×100 or 400 magnification using a light microscope (Leica Microsystems). Representative photomicrographs are shown.
Triglyceride and total cholesterol assay
Triglyceride (TG) and total cholesterol (TC) levels in liver samples were determined using an Enzymatic Assay Kit (Applygen Technologies, Shanghai, China). The results are expressed as micromole TG per milligram protein (μmol/mgprot) or micromole TC per milligram protein (μmol/mgprot) (27).
Biochemical parameters
Serum parameters, including TC, TG, LDL cholesterol, and alanine aminotransferase (ALT) were quantified using a biochemical analyzer (AU5400; Olympus, Tokyo, Japan).
TMT-based quantitative proteomic analysis
Briefly, total protein from each liver tissue sample was ground into a powder with liquid nitrogen and dissolved in lysis solution. The cell lysate was centrifuged at 12,000 rpm for 20 min, and the supernatant was collected. All extraction steps were performed at 4°C. Subsequently, 40-μl sequencing-grade trypsin solution (0.05 μg/μl) was added to the modified proteins, and the mixture was incubated at 37°C for 16 h. Supernatants were labeled using TMT reagent (Thermo Fisher Scientific, Waltham, MA, USA). Labeled peptides were divided into 10 fractions and fractionated using a High pH Reversed-Phase Fractionation Kit. The peptide mixture was loaded onto a Reversed-Phase Trap Column (Thermo Fisher Scientific) that was desalted with C18 spin tips. The mobile phase consisted of 0.1% (v/v) formic acid in water (eluent A) and water including 84% acetonitrile and 0.1% formic acid (eluent B). The flow rate was 300 nl/min (IntelliFlow, Kingston, United Kingdom), and the following gradient program was used: buffer solution B (0–55%) for 80 min; buffer solution B (55–100%) for 5 min; and buffer solution B (100%) for 5 min. Liquid chromatography with tandem mass spectrometry analyses (Aptbio, Shanghai, China) were performed for 90 min using a Q Exactive Mass Spectrometer (Thermo Fisher Scientific). Mass spectometry analysis used the positive ion mode, and the data were obtained using a superior data-dependent method (ranked in the top 10). The scan range was set as 300 m/z first mass and 1800 m/z last mass. The following program was set to guarantee that the targeted minimum percentage could be realized within the maximum fill time: the corresponding target of automatic gain control was set at 3e6, and the maximum injection time was set at 10 ms. The duration of dynamic exclusion was set at 40 s. The resolution for intact peptides was 70,000 at m/z 200. The resolution for ion fragments was set at 35,000 at m/z 200, and the width of isolation was 2 m/z. The normal energy owing to collision was 30 eV, and the underfill ratio was set at 0.1%. The tandem mass spectrometry spectra were filtered using a Mascot search engine (v.2.3.0), which was embedded in Proteome Discoverer 1.4 (v.1.3.0.339; Thermo Fisher Scientific). All identified proteins had an false discovery rate ≤1%, which was calculated at the peptide level. To be considered as being differentially expressed, proteins were required to have a value of P ≤ 0.05. Quantitative analyses were performed as described below. Results from Proteome Discoverer were exported. Unique peptides and their quantification values were appended to their corresponding protein accession number. A Student’s t test was performed on each quantification ratio using a 2-tailed, 1 sample Student’s t test. Data were considered statistically different when P < 0.05.
Proteomic bioinformatics analysis
The classification of differentially expressed proteins was performed based on the annotations obtained from the UniProt Knowledgebase (https://www.uniprot.org/), the terms of gene ontology, and the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway (https://www.genome.jp/kegg/pathway.html). IPA (Ingenuity Systems, Redwood City, CA, USA) was used to perform disease and functional analyses and to network with the lists of differentially expressed proteins. IPA uses right-tailed Fisher’s to calculate a P value for each network (the network score) and to determine which pathways are significantly linked to input data that are mapped to proteins in the whole Ingenuity Pathways Knowledge Base (Qiagen, Germantown, MD, USA). GeneMania (https://genemania.org/)was used for FABP1 and autophagy-related protein interaction networks construction.
Western blot analysis
Lysates were prepared in RIPA buffer. Samples (50 μg) were separated using SDS-PAGE and electrotransferred to PVDF membranes. Western blotting was performed following standard procedure. Supplemental Table S1 lists the primary antibodies. Western blot signals were captured with the ChemiDoc XRS + Imaging System (Bio-Rad, Hercules, CA, USA). Densitometry analyses were quantified using ImageJ software (NIH, Bethesda, MD, USA) (26).
Real-time quantitative PCR analysis
Real-time quantitative PCR was performed using the Real-Time PCR Detection System (iQ5; Bio-Rad) (28, 29). The perilipin 2 (Plin2) probes were (5′-ATGAACTTCTCCGGCAAGTACC-3′) and (5′-GGTCCTCGGGCAGACCTAT-3′); the Fabp1 probes were (5′-GAGGGAACGAGAAACCACAAT-3′) and (5′-AGTCTTGGTAGGAGGACATGA C-3′); and the Actb probes were (5′-TCTAGCTCCTATACCACCACCA-3′) and (5′-TCGTCTCCAACTTATCTCCTCC-3′). Relative genes expression was estimated using the 2−ΔΔCt method.
Immunoprecipitation
Two micrograms of sequestosome 1 (SQSTM1) antibody was incubated with the same amount of protein (500 μg) from each group. Then, protein G beads were used to capture the primary antibody overnight at 4°C according to the Beyotime Company’s instruction. The beads were washed 3 times and resuspended with 50 μl sample buffer (Cell Signaling Technology, Danvers, MA, USA). Denature was performed at 100°C for 30 min and analyzed by Western blotting.
Cathepsin B or cathepsin D activity assay
The catalytic activity of cathepsin B (CTSB) or cathepsin D (CTSD) was determined by CTSB or CTSD Activity Fluorometric Assay Kits (BioVision, Milpitas, CA, USA), as in our previous studies (30, 31).
Preparation of AAV8-TBG-FABP1
According to our previous studies (32–34), the AAV vectors containing the TBG promoter for liver-special overexpression of FABP1 was generated by OBio Technology (Shanghai, China).
Statistical analysis
Results are expressed as means ± sem. Differences were compared by ANOVA, followed by Bonferroni correction for post hoc t test, and P < 0.05 was regarded as significant. All of the statistical tests were performed with Prism software v.5.0 (GraphPad, La Jolla, CA, USA).
RESULTS
Exercise prevents HFD-induced hepatic steatosis and liver injury
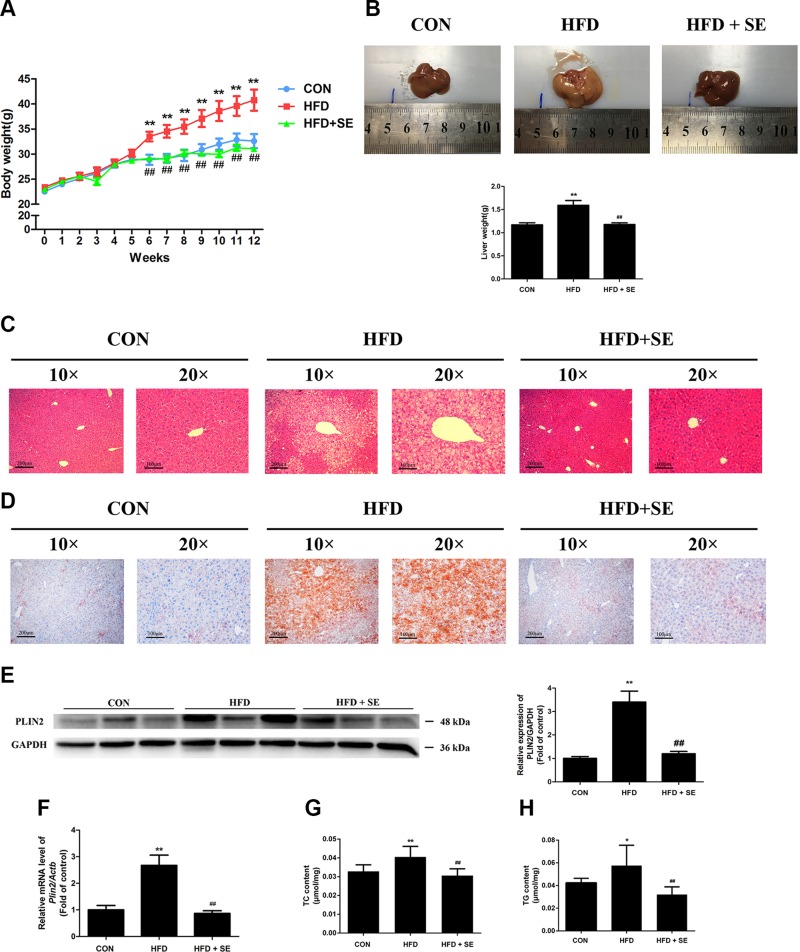
The body masses of the SD, HFD, and HFD + SE groups were 32.6 ± 4.42, 40.75 ± 5.98, and 31.04 ± 1.35 g, respectively, at the end of the experiment (Fig. 1A). Exercise efficiently prevented an increase in body weight in the HFD + SE group compared to the HFD group for 12 wk. The mean daily energy intake of HFD mice was significantly higher than that of SD mice during the feeding period; however, there were no significant differences in the mean daily energy intake between the HFD and HFD + SE groups (Supplemental Fig. S1). The livers of the HFD group were enlarged macroscopically and yellow in color (Fig. 1B). H&E and Oil Red O staining of liver tissues revealed larger areas of cytoplasmic LDs in hepatocytes (Fig. 1C, D). Growing evidence has shown that LD proteins such as PLIN2 play a role in the pathophysiology in fatty liver disease, which is characterized by hepatocytes containing LD with excessive neutral lipid (35). The expression of LD protein PLIN2 increased significantly by 2.7-fold in the HFD group (Fig. 1E, F). HFD mice exhibited elevated liver levels of TC and TG (Fig. 1G, H). Exercise significantly attenuated these changes (Fig. 1B, H), which indicates a protective effect of exercise against liver steatosis in HFD mice.
Figure 1.
Exercise inhibits HFD-induced hepatic steatosis. A, B) Weekly body weights and liver weights of mice measured over 12 wk. C, D) Histologic analyses of H&E staining and Oil Red O staining of liver sections. E) mRNA levels of Plin2 were quantified. F) Protein levels of PLIN2 were quantified. G, H) Liver TC (G) and TG (H) levels measured using corresponding assay kits. *P < 0.05, **P < 0.01 vs. control, ##P < 0.01 vs. HFD. (n = 10 for each group).
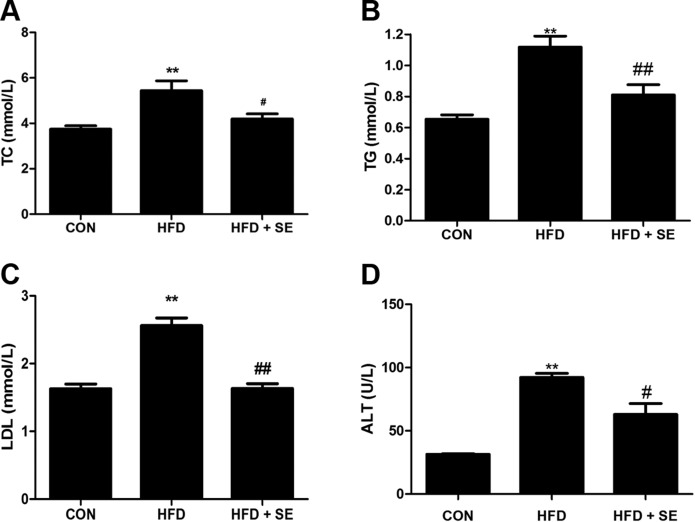
Measurement of serum lipid profiles and liver function of mice revealed that HFD induced notable changes in serum TC (Fig. 2A), TG (Fig. 2B), LDL (Fig. 2C), and ALT (Fig. 2D). Exercise significantly attenuated all of these changes (Fig. 2A–D), which indicates a protective effect of exercise against hepatic injury in HFD mice.
Figure 2.
Exercise improves serum lipid profiles and liver injury in HFD-induced NAFLD mice. Serum TC (A), TG (B), LDL (C), and ALT (D) levels measured using corresponding assay kits. *P < 0.05, **P < 0.01 vs. control, #P < 0.05, ##P < 0.01 vs. HFD. (n = 10 for each group).
Quantitative proteomic analysis of the liver between the HFD + SE group and the HFD group
Supplemental Figure S2A shows the identification and evaluation of the quantitative proteomics results. A total of 5542 proteins were identified and quantified using proteomic analysis. A total of 151 proteins exhibited statistically significant changes in expression in the HFD + SE group compared to the HFD group, and 61 of these proteins were increased >1.2-fold, and 90 proteins were decreased <0.8-fold. These differentially expressed proteins were involved in some critical biologic processes: 75% were associated with cellular process; 67% were associated with the metabolic process; and 49% were associated with the biologic regulation. The molecular function of these proteins was primarily involved in binding, catalytic activity, and molecular regulation. The cellular locations of these proteins were primarily in the cell, cell parts and organelles (Supplemental Fig. S2B). The pathway analysis using KEGG suggested that the significantly affected pathways were the peroxisome proliferator-activated receptor signaling pathway, retinol metabolism, inflammatory mediator regulation of transient receptor potential channels, chemical carcinogenesis, and steroid hormone biosynthesis (Supplemental Fig. S2C).
IPA identifies FABP1 is most closely associated with exercise-regulated lipid metabolism
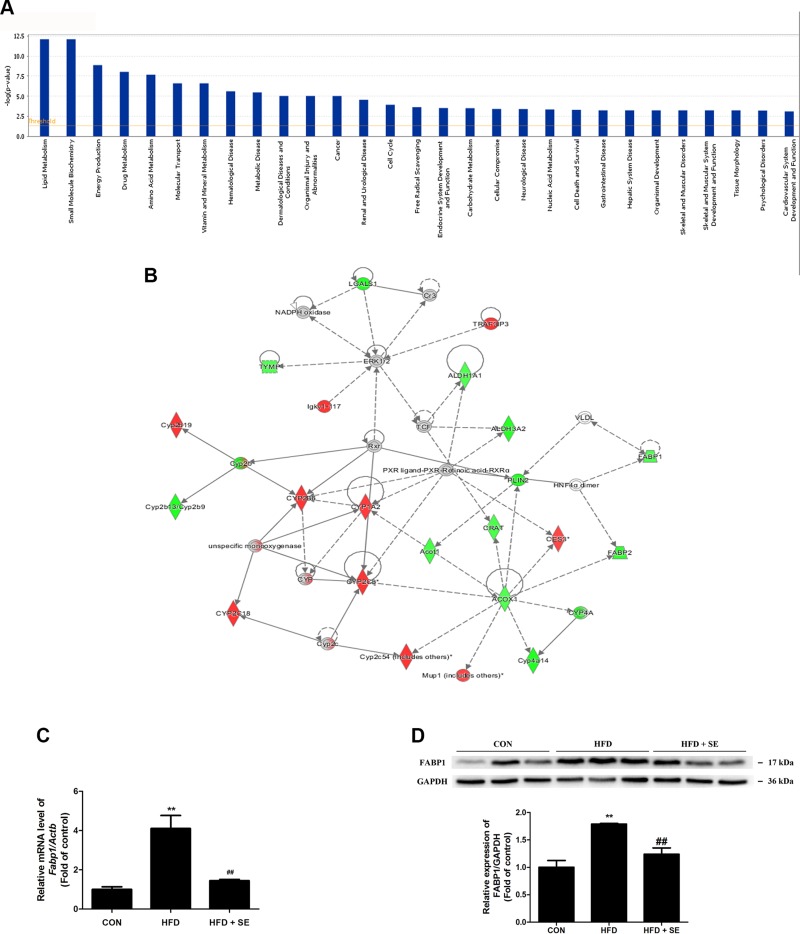
IPA revealed that these differentially expressed proteins were significantly related to many molecular and cellular functions, further enriched in the top 5 categories: lipid metabolism, small molecule biochemistry, energy production, drug metabolism, and amino acid metabolism (Fig. 3A). The top 1 protein integrated network was “lipid metabolism, small molecule biochemistry, and drug metabolism,” with a score of 43 and a total of 30 proteins involved in the network (Supplemental Table S2). Notably, a cluster of the exercise-regulated proteins constructed this most important signaling network, which strongly indicated the hub protein PLIN2, a target protein of FABP1 (Fig. 3B). The data afforded evidence to suggest that FABP1 might act as a critical factor in the exercise-regulated lipid metabolism.
Figure 3.
IPA identifies FABP1 as most closely associated with exercise-regulated lipid metabolism. A) The disease or function list and top canonical pathways of differentially expressed proteins. B) Network of target proteins affecting lipid metabolism as revealed by IPA. C) mRNA levels of Fabp1 were quantified. D) Protein levels of FABP1 were quantified. **P < 0.01 vs. control, ##P < 0.01 vs. HFD. (n = 10 for each group).
Exercise prevents HFD-induced NAFLD via inhibition of FABP
The IPA analysis study suggested that FABP1 was engaged in exercise-inhibited NAFLD. FABP1 expression increased in the HFD group compared to the SD group based on real-time PCR and Western blot analyses, and it was reduced in the HFD + SE group (Fig. 3C, D).
Liver-specific overexpression of FABP1 abolishes the protective effect of exercise in the NAFLD model
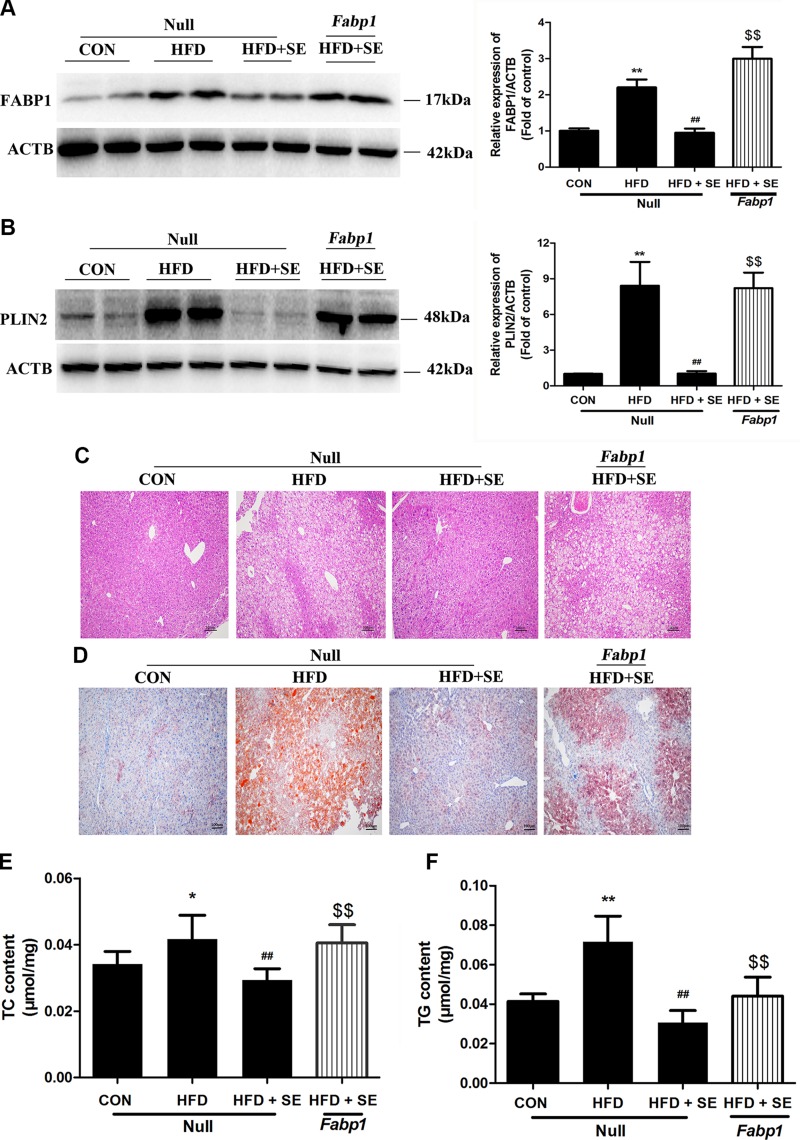
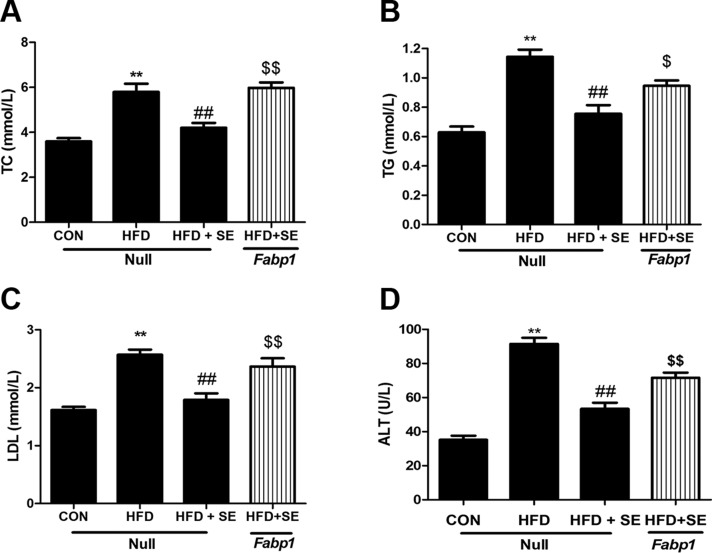
We used AAV8-TGB-Fabp1 to overexpress FABP1 in the mouse liver and confirm the role of FABP1 in the exercise-induced modulation of HFD-induced NAFLD. Fabp1 overexpression reversed the protective effects of exercise on HFD-induced accumulation of LDs, PLIN2 changes, and liver TG and TC increases (Fig. 4A–F). The exercise-induced decreases in serum TC, TG, LDL, and ALT expression were significantly attenuated using AAV8-TGB-Fabp1 in the HFD + SE group (Fig. 5A–D).
Figure 4.
Protective effect of exercise in HFD-induced hepatic steatosis is abolished via liver-special overexpression of FABP1. A) Protein levels of FABP1 were quantified. B) Histologic analysis of HE staining of liver sections. C) Histologic analysis of liver sections with Oil Red O staining. D) mRNA levels of Plin2 were quantified. E) Protein levels of PLIN2 were quantified. F, G) Liver TC (F) and TG (G) levels measured using corresponding assay kits. *P < 0.05, **P < 0.01 vs. control, ##P < 0.01 vs. HFD, $$P < 0.01 vs. HFD + SE. (n = 10 for each group).
Figure 5.
Protective effect of exercise in HFD-induced serum lipid profiles changes and liver injury is abolished by liver-special overexpression of FABP1. Serum TC (A), TG (B), LDL (C), and ALT (D) levels measured using corresponding assay kits. *P < 0.05, **P < 0.01 vs. control, #P < 0.05, ##P < 0.01 vs. HFD, $P < 0.05, $$P < 0.01 vs. HFD + SE. (n = 10 for each group).
Exercise prevents HFD-induced hepatic steatosis through activating autophagy
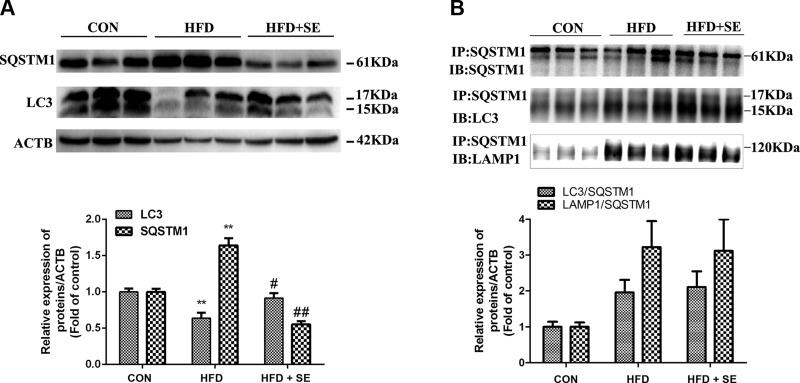
Microtubule-associated protein 1 light chain 3β (LC3) plays an important role in double-membrane autophagosome formation, and SQSTM1 protein is an important autophagy receptor for cargo and is efficiently degraded by autophagy (36). HFD impaired autophagy in the liver, as evidence by inhibiting LC3 expression but increasing P62/SQSTM1 protein expression, whereas exercise was able to recover autophagy deregulation (Fig. 6A).
Figure 6.
Exercise prevents HFD-induced NAFLD via increasing autophagy. A) Protein levels of LC3 and SQSTM1 were quantified via normalization of its density to ACTB. B) Antibodies against SQSTM1 were added to immunoprecipitate the SQSTM1-containing complexes and then immunoblotted for SQSTM1, LC3, or LAMP1. **P < 0.01 vs. control, #P < 0.05, ##P < 0.01 vs. HFD. (n = 10 for each group).
SQSTM1 cargo incorporation with LC3occurs concurrent with autophagosome maturation (37). The results of the immunoprecipitation assay indicate that HFD treatment had no significant effect on the interaction of SQSTM1 and LC3 (Fig. 6B). Inhibiting autophagosome-lysosome fusion also blocks autophagic flux (38). Interestingly, SQSTM1 and lysosomal marker lysosomal-associated membrane protein (LAMP)1 colocalization had no significant changes compared with the control group (Fig. 6B). Taken together, these results suggested that HFD did not block the cargo incorporation process associated with autophagosome maturation and autophagosome-lysosome fusion.
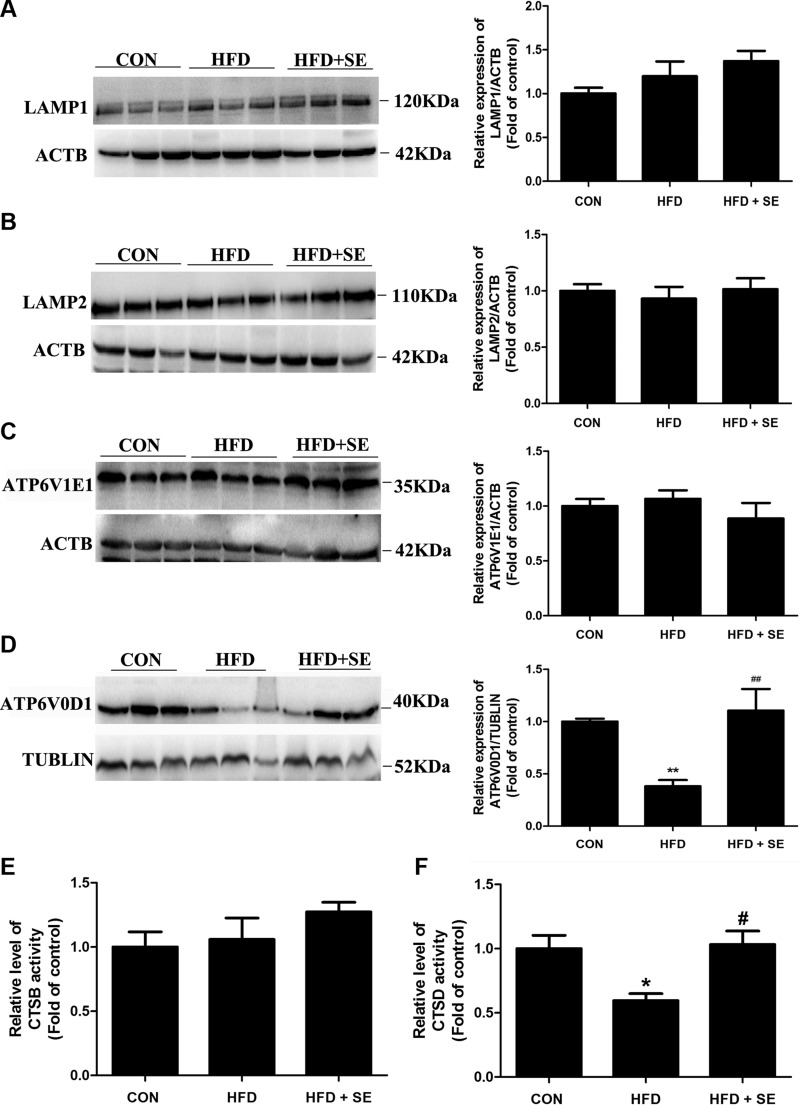
Proper lysosome degradation capacity is required to maintain the autophagic flux and necessary for recycling LDs (39). First, we assessed the levels of the lysosomal membrane proteins LAMP1 and LAMP2. However, LAMP1 and LAMP2 have no significant changes in the HFD group compared with the control group (Fig. 7A, B). The vacuolar ATPase complex is a key regulator of the acidification of endosomes, lysosomes, and the luminal compartments of several cell types, tissues, and organs (40), we examined whether HFD affects lysosomal acidification in the liver. HFD significantly reduces ATPase, H+ transporting, lysosomal V0 subunit D1 (ATP6V0D1) but not ATPase, H+ transporting, lysosomal V1 subunit E1 expression (Fig. 7C, D). Lysosomal protease activity is largely depended on lysosomal cathepsins, and thus we then determined whether HFD treatment alters the lysosomal cathepsins activity. CTSD activity was decreased by 40% upon HFD treatment, but there were no significant changes in CTSB activity (Fig. 7E, F). Exercise significantly attenuated these changes (Fig. 7), which indicates a protective effect of exercise against autophagic flux block via restoring lysosomal function.
Figure 7.
Exercise prevents HFD-induced NAFLD via increasing lysosomal function. A–D) Protein levels of LAMP1 (A), LAMP2 (B), ATPase, H+ transporting, lysosomal V1 subunit E1 (C), and ATP6V0D1 (D) were quantified. E, F) CTSB and CTSD activities were determined. *P < 0.05, **P < 0.01 vs. control, #P < 0.05, ##P < 0.01 vs. HFD. (n = 10 for each group).
Liver-specific overexpression of FABP1 abolishes the protective effect of exercise via inhibiting autophagy-lysosomal machinery in the NAFLD model
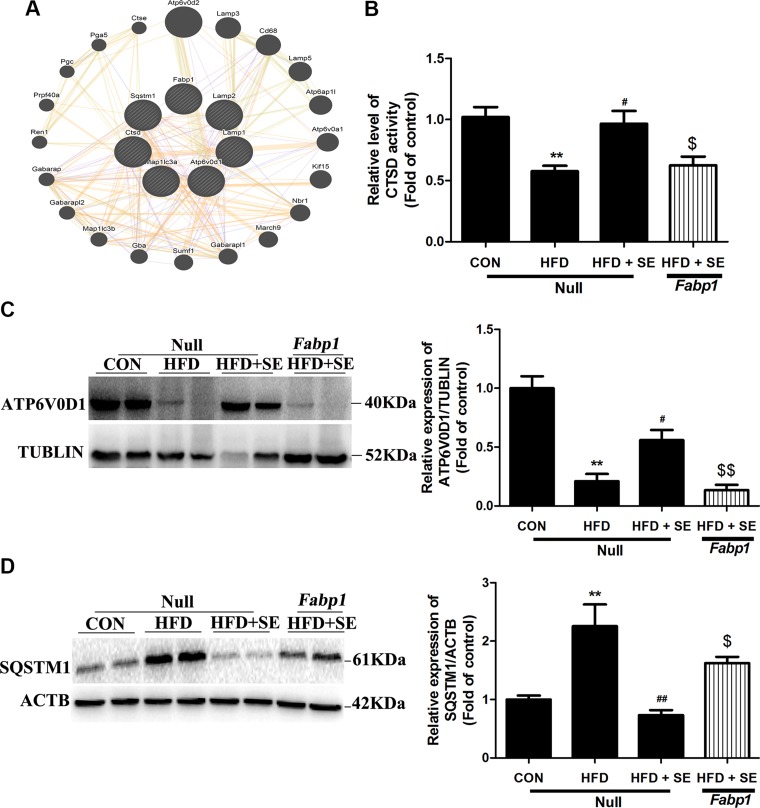
To study the effects of FABP1 on exercise-activated autopahgy, GeneMania network analysis was applied to determine the interrelationship among FABP1 and autophagy-related protein. Interestingly, the most relevant autophagy-related interactor predicted was LAMP2, a well-established lysosomal membrane protein, and this result showed that FABP1 might be important for lysosomal function (Fig. 8A). Moreover, Fabp1 overexpression antagonized the effects of exercise on HFD-induced ATP6V0D1 protein decreases and lysosomal protease activity reductions (Fig. 8B). Most importantly, exercise-induced down-regulation of SQSTM1 protein expression was significantly reversed via Fabp1 overexpression in the NAFLD model (Fig. 8C, D).
Figure 8.
Protective effect of exercise in HFD-induced autophagy inhibition is abolished by liver-special overexpression of FABP1. A) GeneMania was used for FABP1 and autophagy-related protein interaction networks construction. B) CTSD activity was determined. C, D) Protein levels of ATP6V0D1 (C) and SQSTM1 (D) were quantified. *P < 0.05, **P < 0.01 vs. control, #P < 0.05, ##P < 0.01 vs. HFD, $P < 0.05, $$P < 0.01 vs. HFD + SE. (n = 6 for each group).
DISCUSSION
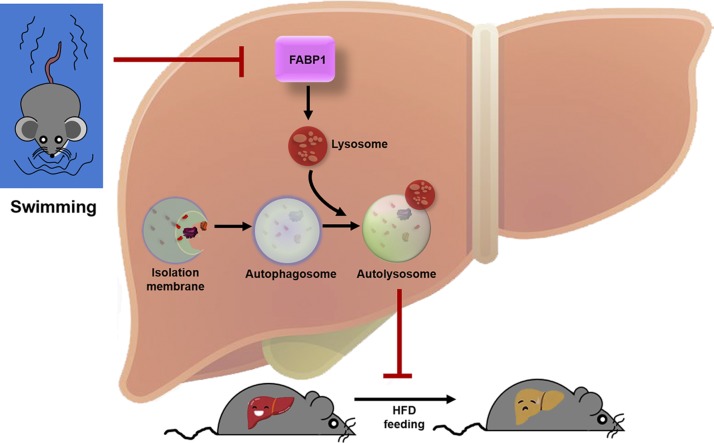
Exercise should be considered as a drug for intervention of NAFLD (41). Measurement of liver fat and physical activity in 169,347 participants from a recent study demonstrated a strong association between exercise and amelioration of hepatic steatosis in NAFLD (42). More notably, aerobic exercise provided a beneficial effect on liver function in NAFLD without weight loss (43). The biologic mechanisms for exercise-afforded beneficial effects against NAFLD are less well recognized or understood. Our current work, based on TMT-based quantitative proteomic analysis and IPA, is the first to demonstrate that FABP1 inhibition–mediated replenishment of the autophagy-lysosomal machinery as a novel endogenous mechanism whereby long-term exercise improves lipid homeostasis and ameliorates hepatic steatosis in NAFLD (Fig. 9).
Figure 9.
Schematic model of exercise prevents HFD-induced hepatic steatosis: regulation of autophagy-lysosomal machinery via inhibiting the FABP1 pathway. HFD exposure stimulates an increase in FABP1 in the lysosome, and FABP1 triggers lysosomal dysfunction and subsequently leads to autophagic flux impairment and hepatic steatosis. Moreover, FABP1 inhibition–mediated replenishment of the autophagy-lysosomal machinery represents a novel endogenous mechanism whereby long-term exercise improves lipid homeostasis and ameliorates hepatic steatosis in NAFLD.
Actually, the modalities of exercise (i.e., exercise intensity and exercise duration) should also be considered when evaluating its effects. To date, there are no standard criteria for the modalities of an exercise regimen. It was reported that 4 wk of training on a treadmill failed to reverse HFD-induced higher liver TG content and fatty liver deposits in rats (44). This is probably because the exercise duration was too short to compensate for the metabolic disorder induced by the HFD. However, a recent study demonstrated that a 12-wk moderate aerobic exercise program efficiently managed NAFLD progression in C57BL/6J mice (45). Consistent with the previous study, our result also showed that a long-term moderate-intensity exercise program was effective in preventing hepatic steatosis. Nevertheless, regarding SE, it is important to determine how much exercise the mice swam voluntarily, and quantifying exercise the mice swam voluntarily will provide more evidence of causality if a dose-response effect can be found in our future studies.
Previous studies indicated that the expression of several important proteins and the activities of some enzymes were significantly altered in response to exercise treatment in an NAFLD model based on immunoblotting analyses and biochemical tests (46). The present study used a highly sensitive proteomic tool to expand the list of proteins that played specific roles in exercise-induced effects. A total of 151 proteins exhibited a >1.2-fold change at the protein level between the HFD group and HFD + SE group. Gene ontology analysis demonstrated that a large number of proteins involved in the fatty acid process exhibited activated expression after exercise. The top 5 ranked “Disease and Biologic Functions” categories in response to exercise treatment in an NAFLD model were analyzed with IPA tool. We found that the “lipid metabolism” category was most closely linked to the annotation term “Diseases or Functions.” This result suggests that exercise exerts the greatest effect on hepatic steatosis. All common differentially regulated proteins were further analyzed using IPA to elucidate the possible functional interactions between proteins. PLIN2 is 1 hub in this network that directly or indirectly interacts with proteins involved in lipids, and this network demonstrated that FABP1 may act as a key factor in exercise-increased LD degradation.
FABPs are intracellular lipid chaperones (47). FABPs play important roles in lipid metabolism, including fatty acid uptake, trafficking, and lipid storage (48). FABP1 occurs at a very high concentration in the liver (makeup 5% of cytosolic proteins) and is involved in a large number of biologic processes in the liver (49). Silencing of FABP1 ameliorated oxidative stress, inflammation, and hepatic steatosis in mice with NAFLD disease (50–52). Hepatitis B virus X protein induced hepatic steatosis via increasing FABP1 expression (11). It has also been suggested that carnosic acid attenuated obesity-induced hepatic lipids accumulation and insulin resistance via inhibition of FABP1 in mice (53). Consistent with previous reports, our present work confirmed that HFD increased FABP1 expression in the liver, and FABP1 inhibited by exercise prevents the HFD-induced NAFLD. Notably, this study demonstrated that hepatoprotective effect of exercise reduced hepatic steatosis through inhibiting FABP1 signaling pathway. Importantly, this study now joins several recent studies that have unexpectedly demonstrated favorable results of FABP1 inhibition in NAFLD.
The mechanism by which FABP1 inhibition prevented hepatic steatosis via exercise has not been clearly elucidated. A previous study has confirmed that FABP1 inhibition reduced fatty acid uptake, decreased key rate‐limiting enzymes in fatty acid biosynthesis, and increased fatty acid β-oxidation, which may contribute to the amelioration of fatty liver (50). In our study, because the interactions between the significant autophagy-related genes and FABP1 were searched and predicted in GeneMania, we hypothesized that autophagy may be involved in FABP1-mediated hepatic steatosis. Autophagy is a highly conserved cellular homeostatic housekeeping process, and it plays a critical role in maintaining lipid metabolism (54, 55). Recent studies confirmed that exercise is beneficial in NAFLD due to increased autophagy (56–58). Aerobic endurance training improves NAFLD features via miR-33–dependent autophagy induction in HFD-fed mice (59). Moreover, irisin, muscle-derived myokines following exercise, stimulates AMPK-dependent autophagy in the liver (60). In the present study, Fabp1 overexpression antagonized the effects of exercise on HFD-induced SQSTM1 protein elevation, indicating that exercise-inhibited FABP1 expression has a beneficial effect in attenuating hepatic steatosis by activating autophagic flux. Autophagy targets intracellular lipids, but these lipid cargos are delivered to one organelle, the lysosome, for degradation. Lysosomal dysfunction should result in the perturbation of lipids degradation and autophagic flux (31). The results of the present study showed that exercise restored HFD-impaired lysosomal function by increasing lysosomal proteolysis and maintaining lysosomal acidification. More importantly, Fabp1 overexpression counteracted the effects of exercise on HFD-induced lysosomal protease activity decreases, lysosomal pH changes, suggesting a prominent role of FABP1 inhibition–mediated replenishment of the lysosomal function in exercise alleviating NAFLD.
In summary, our findings demonstrate that long-term exercise prevents HFD-induced NAFLD via FABP1-autophagy signaling. This provides an insight into the mechanisms of long-term exercise beneficial effects for the prevention of NAFLD via regulating lipid homeostasis. It should be noted that exercise without dietary change is effective for NAFLD prevention regardless of the preceding HFD period. These data also suggest that potent and selective FABP1 inhibitors could be a potential exercise mimetic or exercise pill for NAFLD prevention. Nevertheless, our study presents some limitations. Given the loss of nicotinamide nucleotide transhydrogenase function in the C57BL/6J stain, C57BL/6N mouse is the more logical and representative genetic background model for NAFLD studies. More importantly, translational process from experimental studies to clinical practice is intrinsically complex. The intensity of the exercise stands as an alternative to be further evaluated because the presented data are promising.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the National Postdoctoral Program for Innovative Talents (BX201700107 to H.P.) and the National Natural Science Foundation of China (81870273 to F.G.). The authors declare no conflicts of interest.
Glossary
- AAV
adeno-associated virus
- ALT
alanine aminotransferase
- ATP6V0D1
ATPase, H+ transporting, lysosomal V0 subunit D1
- CTSB
cathepsin B
- CTSD
cathepsin D
- FABP
fatty acid–binding protein
- H&E
hematoxylin and eosin
- HFD
high-fat diet
- IPA
ingenuity pathway analysis
- LAMP
lysosomal-associated membrane protein
- LC3
microtubule-associated protein 1 light chain 3β
- LD
lipid droplet
- NAFLD
nonalcoholic fatty liver disease
- PLIN2
perilipin 2
- SD
standard diet
- SE
swimming exercise
- SQSTM1
sequestosome 1
- TC
total cholesterol
- TBG
thyroid hormone-binding gobulin
- TG
triglyceride
- TMT
tandem mass tag
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Z. Zhou and F. Gao designed the experiments; animal studies were performed by H. Pi, M. Liu, and Y. Xi with help from M. Chen, L. Tian, J. Xie, and M. Chen; Z. Wang and M. Yang contributed new reagents or analytic tools; H. Pi, M. Liu, and Y. Xi analyzed data; and H. Pi, Z. Yu, Z. Zhou, and F. Gao wrote the manuscript.
REFERENCES
- 1.Ipsen D. H., Lykkesfeldt J., Tveden-Nyborg P. (2018) Molecular mechanisms of hepatic lipid accumulation in non-alcoholic fatty liver disease. Cell. Mol. Life Sci. 75, 3313–3327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asrani S. K., Devarbhavi H., Eaton J., Kamath P. S. (2019) Burden of liver diseases in the world. J. Hepatol. 70, 151–171 [DOI] [PubMed] [Google Scholar]
- 3.Stefan N., Häring H. U., Cusi K. (2019) Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 7, 313–324 [DOI] [PubMed] [Google Scholar]
- 4.Younossi Z. M. (2018) Patient-reported outcomes and the economic effects of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis: the value proposition. Hepatology 68, 2405–2412 [DOI] [PubMed] [Google Scholar]
- 5.Romero-Gómez M., Zelber-Sagi S., Trenell M. (2017) Treatment of NAFLD with diet, physical activity and exercise. J. Hepatol. 67, 829–846 [DOI] [PubMed] [Google Scholar]
- 6.Katsagoni C. N., Georgoulis M., Papatheodoridis G. V., Panagiotakos D. B., Kontogianni M. D. (2017) Effects of lifestyle interventions on clinical characteristics of patients with non-alcoholic fatty liver disease: a meta-analysis. Metabolism 68, 119–132 [DOI] [PubMed] [Google Scholar]
- 7.Sakr H. F., Abbas A. M., Haidara M. A. (2018) Swimming, but not vitamin E, ameliorates prothrombotic state and hypofibrinolysis in a rat model of nonalcoholic fatty liver disease. J. Basic Clin. Physiol. Pharmacol. 29, 61–71 [DOI] [PubMed] [Google Scholar]
- 8.Wu H., Jin M., Han D., Zhou M., Mei X., Guan Y., Liu C. (2015) Protective effects of aerobic swimming training on high-fat diet induced nonalcoholic fatty liver disease: regulation of lipid metabolism via PANDER-AKT pathway. Biochem. Biophys. Res. Commun. 458, 862–868 [DOI] [PubMed] [Google Scholar]
- 9.Botezelli J. D., Mora R. F., Dalia R. A., Moura L. P., Cambri L. T., Ghezzi A. C., Voltarelli F. A., Mello M. A. (2010) Exercise counteracts fatty liver disease in rats fed on fructose-rich diet. Lipids Health Dis. 9, 116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dallak M. A., Bin-Jaliah I., Albawardi A., Haidara M. A., Sakr H. F., Eid R. A., Hassan W. N., Al-Ani B. (2018) Swim exercise training ameliorates hepatocyte ultrastructural alterations in rats fed on a high fat and sugar diet. Ultrastruct. Pathol. 42, 155–161 [DOI] [PubMed] [Google Scholar]
- 11.Wu Y. L., Peng X. E., Zhu Y. B., Yan X. L., Chen W. N., Lin X. (2015) Hepatitis B virus X protein induces hepatic steatosis by enhancing the expression of liver fatty acid binding protein. J. Virol. 90, 1729–1740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfrum C., Buhlmann C., Rolf B., Börchers T., Spener F. (1999) Variation of liver-type fatty acid binding protein content in the human hepatoma cell line HepG2 by peroxisome proliferators and antisense RNA affects the rate of fatty acid uptake. Biochim. Biophys. Acta 1437, 194–201 [DOI] [PubMed] [Google Scholar]
- 13.Martin G. G., Atshaves B. P., Landrock K. K., Landrock D., Schroeder F., Kier A. B. (2015) Loss of L-FABP, SCP-2/SCP-x, or both induces hepatic lipid accumulation in female mice. Arch. Biochem. Biophys. 580, 41–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim H., Williams D., Qiu Y., Song Z., Yang Z., Kimler V., Goldberg A., Zhang R., Yang Z., Chen X., Wang L., Fang D., Lin J. D., Zhang K. (2019) Regulation of hepatic autophagy by stress-sensing transcription factor CREBH. FASEB J. 33, 7896–7914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang X., Zhang X., Chu E. S. H., Chen X., Kang W., Wu F., To K. F., Wong V. W. S., Chan H. L. Y., Chan M. T. V., Sung J. J. Y., Wu W. K. K., Yu J. (2018) Defective lysosomal clearance of autophagosomes and its clinical implications in nonalcoholic steatohepatitis. FASEB J. 32, 37–51 [DOI] [PubMed] [Google Scholar]
- 16.Ueno T., Komatsu M. (2017) Autophagy in the liver: functions in health and disease. Nat. Rev. Gastroenterol. Hepatol. 14, 170–184 [DOI] [PubMed] [Google Scholar]
- 17.Guo R., Liong E. C., So K. F., Fung M. L., Tipoe G. L. (2015) Beneficial mechanisms of aerobic exercise on hepatic lipid metabolism in non-alcoholic fatty liver disease. Hepatobiliary Pancreat. Dis. Int. 14, 139–144 [DOI] [PubMed] [Google Scholar]
- 18.Hou Z., Qin X., Hu Y., Zhang X., Li G., Wu J., Li J., Sha J., Chen J., Xia J., Wang L., Gao F. (2019) Longterm exercise-derived exosomal miR-342-5p. Circ. Res. 124, 1386–1400 [DOI] [PubMed] [Google Scholar]
- 19.Zhao D., Sun Y., Tan Y., Zhang Z., Hou Z., Gao C., Feng P., Zhang X., Yi W., Gao F. (2018) Short-duration swimming exercise after myocardial infarction attenuates cardiac dysfunction and regulates mitochondrial quality control in aged mice. Oxid. Med. Cell. Longev. 2018, 4079041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q. J., Li Q. X., Zhang H. F., Zhang K. R., Guo W. Y., Wang H. C., Zhou Z., Cheng H. P., Ren J., Gao F. (2007) Swim training sensitizes myocardial response to insulin: role of Akt-dependent eNOS activation. Cardiovasc. Res. 75, 369–380 [DOI] [PubMed] [Google Scholar]
- 21.Zhang K. R., Liu H. T., Zhang H. F., Zhang Q. J., Li Q. X., Yu Q. J., Guo W. Y., Wang H. C., Gao F. (2007) Long-term aerobic exercise protects the heart against ischemia/reperfusion injury via PI3 kinase-dependent and Akt-mediated mechanism. Apoptosis 12, 1579–1588 [DOI] [PubMed] [Google Scholar]
- 22.Xing W., Li Y., Zhang H., Mi C., Hou Z., Quon M. J., Gao F. (2013) Improvement of vascular insulin sensitivity by downregulation of GRK2 mediates exercise-induced alleviation of hypertension in spontaneously hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 305, H1111–H1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Martins D. F., Siteneski A., Ludtke D. D., Dal-Secco D., Santos A. R. S. (2017) High-intensity swimming exercise decreases glutamate-induced nociception by activation of G-protein-coupled receptors inhibiting phosphorylated protein kinase A. Mol. Neurobiol. 54, 5620–5631 [DOI] [PubMed] [Google Scholar]
- 24.Cardoso G. H., Petry D. M., Probst J. J., de Souza L. F., Ganguilhet G., Bobinski F., Santos A. R. S., Martins D. F., Bonorino K. C., Dafre A. L., Hizume Kunzler D. C. (2018) High-intensity exercise prevents disturbances in lung inflammatory cytokines and antioxidant defenses induced by lipopolysaccharide. Inflammation 41, 2060–2067 [DOI] [PubMed] [Google Scholar]
- 25.Vilà L., Elias I., Roca C., Ribera A., Ferré T., Casellas A., Lage R., Franckhauser S., Bosch F. (2014) AAV8-mediated Sirt1 gene transfer to the liver prevents high carbohydrate diet-induced nonalcoholic fatty liver disease. Mol. Ther. Methods Clin. Dev. 1, 14039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pi H., Xu S., Reiter R. J., Guo P., Zhang L., Li Y., Li M., Cao Z., Tian L., Xie J., Zhang R., He M., Lu Y., Liu C., Duan W., Yu Z., Zhou Z. (2015) SIRT3-SOD2-mROS-dependent autophagy in cadmium-induced hepatotoxicity and salvage by melatonin. Autophagy 11, 1037–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zeng X., Yang J., Hu O., Huang J., Ran L., Chen M., Zhang Y., Zhou X., Zhu J., Zhang Q., Yi L., Mi M. (2019) Dihydromyricetin ameliorates nonalcoholic fatty liver disease by improving mitochondrial respiratory capacity and redox homeostasis through modulation of SIRT3 signaling. Antioxid. Redox Signal. 30, 163–183 [DOI] [PubMed] [Google Scholar]
- 28.Fan T., Pi H., Li M., Ren Z., He Z., Zhu F., Tian L., Tu M., Xie J., Liu M., Li Y., Tan M., Li G., Qing W., Reiter R. J., Yu Z., Wu H., Zhou Z. (2018) Inhibiting MT2-TFE3-dependent autophagy enhances melatonin-induced apoptosis in tongue squamous cell carcinoma. J. Pineal Res. 64, e12457. [DOI] [PubMed] [Google Scholar]
- 29.Pi H., Xu S., Zhang L., Guo P., Li Y., Xie J., Tian L., He M., Lu Y., Li M., Zhang Y., Zhong M., Xiang Y., Deng L., Zhou Z., Yu Z. (2013) Dynamin 1-like-dependent mitochondrial fission initiates overactive mitophagy in the hepatotoxicity of cadmium. Autophagy 9, 1780–1800 [DOI] [PubMed] [Google Scholar]
- 30.Li M., Pi H., Yang Z., Reiter R. J., Xu S., Chen X., Chen C., Zhang L., Yang M., Li Y., Guo P., Li G., Tu M., Tian L., Xie J., He M., Lu Y., Zhong M., Zhang Y., Yu Z., Zhou Z. (2016) Melatonin antagonizes cadmium-induced neurotoxicity by activating the transcription factor EB-dependent autophagy-lysosome machinery in mouse neuroblastoma cells. J. Pineal Res. 61, 353–369 [DOI] [PubMed] [Google Scholar]
- 31.Pi H., Li M., Tian L., Yang Z., Yu Z., Zhou Z. (2017) Enhancing lysosomal biogenesis and autophagic flux by activating the transcription factor EB protects against cadmium-induced neurotoxicity. Sci. Rep. 7, 43466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pi H., Li M., Zou L., Yang M., Deng P., Fan T., Liu M., Tian L., Tu M., Xie J., Chen M., Li H., Xi Y., Zhang L., He M., Lu Y., Chen C., Zhang T., Wang Z., Yu Z., Gao F., Zhou Z. (2019) AKT inhibition-mediated dephosphorylation of TFE3 promotes overactive autophagy independent of MTORC1 in cadmium-exposed bone mesenchymal stem cells. Autophagy 15, 565–582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang L., Bell P., Morizono H., He Z., Pumbo E., Yu H., White J., Batshaw M. L., Wilson J. M. (2017) AAV gene therapy corrects OTC deficiency and prevents liver fibrosis in aged OTC-knock out heterozygous mice. Mol. Genet. Metab. 120, 299–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Guo Y., Wang Z., Qin X., Xu J., Hou Z., Yang H., Mao X., Xing W., Li X., Zhang X., Gao F. (2018) Enhancing fatty acid utilization ameliorates mitochondrial fragmentation and cardiac dysfunction via rebalancing optic atrophy 1 processing in the failing heart. Cardiovasc. Res. 114, 979–991 [DOI] [PubMed] [Google Scholar]
- 35.Orlicky D. J., Libby A. E., Bales E. S., McMahan R. H., Monks J., La Rosa F. G., McManaman J. L. (2019) Perilipin-2 promotes obesity and progressive fatty liver disease in mice through mechanistically distinct hepatocyte and extra-hepatocyte actions. J. Physiol. 597, 1565–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pi H., Wang Z., Liu M., Deng P., Yu Z., Zhou Z., Gao F. (2019) SCD1 activation impedes foam cell formation by inducing lipophagy in oxLDL-treated human vascular smooth muscle cells. [E-pub ahead of print] J. Cell Mol. Med. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ganley I. G., Wong P. M., Gammoh N., Jiang X. (2011) Distinct autophagosomal-lysosomal fusion mechanism revealed by thapsigargin-induced autophagy arrest. Mol. Cell 42, 731–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi Y., Tan S. H., Ng S., Zhou J., Yang N. D., Koo G. B., McMahon K. A., Parton R. G., Hill M. M., Del Pozo M. A., Kim Y. S., Shen H. M. (2015) Critical role of CAV1/caveolin-1 in cell stress responses in human breast cancer cells via modulation of lysosomal function and autophagy. Autophagy 11, 769–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hung Y. H., Buhman K. K. (2019) DGAT1 deficiency disrupts lysosome function in enterocytes during dietary fat absorption. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 1864, 587–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Klein Z. A., Takahashi H., Ma M., Stagi M., Zhou M., Lam T. T., Strittmatter S. M. (2017) Loss of TMEM106B ameliorates lysosomal and frontotemporal dementia-related phenotypes in progranulin-deficient mice. Neuron 95, 281–296.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Younossi Z. M., Loomba R., Rinella M. E., Bugianesi E., Marchesini G., Neuschwander-Tetri B. A., Serfaty L., Negro F., Caldwell S. H., Ratziu V., Corey K. E., Friedman S. L., Abdelmalek M. F., Harrison S. A., Sanyal A. J., Lavine J. E., Mathurin P., Charlton M. R., Chalasani N. P., Anstee Q. M., Kowdley K. V., George J., Goodman Z. D., Lindor K. (2018) Current and future therapeutic regimens for nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Hepatology 68, 361–371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim H. S., Ike A., Mathew J. (2017) Effect of exercise on the development of new fatty liver and the resolution of existing fatty liver. J. Hepatol. 66, 664–665 [DOI] [PubMed] [Google Scholar]
- 43.Johnson N. A., Sachinwalla T., Walton D. W., Smith K., Armstrong A., Thompson M. W., George J. (2009) Aerobic exercise training reduces hepatic and visceral lipids in obese individuals without weight loss. Hepatology 50, 1105–1112 [DOI] [PubMed] [Google Scholar]
- 44.Straczkowski M., Kowalska I., Dzienis-Straczkowska S., Kinalski M., Górski J., Kinalska I. (2001) The effect of exercise training on glucose tolerance and skeletal muscle triacylglycerol content in rats fed with a high-fat diet. Diabetes Metab. 27, 19–23 [PubMed] [Google Scholar]
- 45.Zheng F., Cai Y. (2019) Concurrent exercise improves insulin resistance and nonalcoholic fatty liver disease by upregulating PPAR-γ and genes involved in the beta-oxidation of fatty acids in ApoE-KO mice fed a high-fat diet. Lipids Health Dis. 18, 6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hashida R., Kawaguchi T., Bekki M., Omoto M., Matsuse H., Nago T., Takano Y., Ueno T., Koga H., George J., Shiba N., Torimura T. (2017) Aerobic vs. resistance exercise in non-alcoholic fatty liver disease: a systematic review. J. Hepatol. 66, 142–152 [DOI] [PubMed] [Google Scholar]
- 47.Furuhashi M., Hotamisligil G. S. (2008) Fatty acid-binding proteins: role in metabolic diseases and potential as drug targets. Nat. Rev. Drug Discov. 7, 489–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smathers R. L., Petersen D. R. (2011) The human fatty acid-binding protein family: evolutionary divergences and functions. Hum. Genomics 5, 170–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang G., Bonkovsky H. L., de Lemos A., Burczynski F. J. (2015) Recent insights into the biological functions of liver fatty acid binding protein 1. J. Lipid Res. 56, 2238–2247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mukai T., Egawa M., Takeuchi T., Yamashita H., Kusudo T. (2017) Silencing of FABP1 ameliorates hepatic steatosis, inflammation, and oxidative stress in mice with nonalcoholic fatty liver disease. FEBS Open Bio 7, 1009–1016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen A., Tang Y., Davis V., Hsu F. F., Kennedy S. M., Song H., Turk J., Brunt E. M., Newberry E. P., Davidson N. O. (2013) Liver fatty acid binding protein (L-Fabp) modulates murine stellate cell activation and diet-induced nonalcoholic fatty liver disease. Hepatology 57, 2202–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wu Y. L., Zhu Y. B., Huang R. D., Peng X. E., Lin X. (2017) Multiple microRNAs ameliorate hepatocyte steatosis and injury by suppressing FABP1 expression. Cell. Physiol. Biochem. 44, 2243–2255 [DOI] [PubMed] [Google Scholar]
- 53.Park M. Y., Sung M. K. (2015) Carnosic acid attenuates obesity-induced glucose intolerance and hepatic fat accumulation by modulating genes of lipid metabolism in C57BL/6J-ob/ob mice. J. Sci. Food Agric. 95, 828–835 [DOI] [PubMed] [Google Scholar]
- 54.Farah B. L., Landau D. J., Sinha R. A., Brooks E. D., Wu Y., Fung S. Y. S., Tanaka T., Hirayama M., Bay B. H., Koeberl D. D., Yen P. M. (2016) Induction of autophagy improves hepatic lipid metabolism in glucose-6-phosphatase deficiency. J. Hepatol. 64, 370–379 [DOI] [PubMed] [Google Scholar]
- 55.Martinez-Lopez N., Singh R. (2015) Autophagy and lipid droplets in the liver. Annu. Rev. Nutr. 35, 215–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gonçalves I. O., Passos E., Diogo C. V., Rocha-Rodrigues S., Santos-Alves E., Oliveira P. J., Ascensão A., Magalhães J. (2016) Exercise mitigates mitochondrial permeability transition pore and quality control mechanisms alterations in nonalcoholic steatohepatitis. Appl. Physiol. Nutr. Metab. 41, 298–306 [DOI] [PubMed] [Google Scholar]
- 57.Rosa-Caldwell M. E., Lee D. E., Brown J. L., Brown L. A., Perry R. A., Jr., Greene E. S., Carvallo Chaigneau F. R., Washington T. A., Greene N. P. (2017) Moderate physical activity promotes basal hepatic autophagy in diet-induced obese mice. Appl. Physiol. Nutr. Metab. 42, 148–156 [DOI] [PubMed] [Google Scholar]
- 58.Wang B., Zeng J., Gu Q. (2017) Exercise restores bioavailability of hydrogen sulfide and promotes autophagy influx in livers of mice fed with high-fat diet. Can. J. Physiol. Pharmacol. 95, 667–674 [DOI] [PubMed] [Google Scholar]
- 59.Ghareghani P., Shanaki M., Ahmadi S., Khoshdel A. R., Rezvan N., Meshkani R., Delfan M., Gorgani-Firuzjaee S. (2018) Aerobic endurance training improves nonalcoholic fatty liver disease (NAFLD) features via miR-33 dependent autophagy induction in high fat diet fed mice. Obes. Res. Clin. Pract. 12 (Suppl 2), 80–89 [DOI] [PubMed] [Google Scholar]
- 60.Chun S. K., Lee S., Yang M. J., Leeuwenburgh C., Kim J. S. (2017) Exercise-induced autophagy in fatty liver disease. Exerc. Sport Sci. Rev. 45, 181–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.