Abstract
α-Synuclein (α-syn) protein aggregation is associated with several neurodegenerative disorders collectively referred to as synucleinopathies, including Parkinson’s disease. We used protein misfolding cyclic amplification (PMCA) to study α-syn aggregation in brain homogenates of wild-type or transgenic mice expressing normal (D line) or A53T mutant (M83 line) human α-syn. We found that sonication-incubation cycles of M83 mouse brain gradually produce large quantities of SDS-resistant α-syn aggregates, involving both human and mouse proteins. These PMCA products, containing partially proteinase K–resistant α-syn species, are competent to accelerate the onset of neurologic symptoms after intracerebral inoculation to young M83 mice and to seed aggregate formation of α-syn following PMCA, including in D and wild-type mouse brain substrates. PMCA seeding activity in the M83 diseased brain correlates positively with regions mostly targeted by the α-syn pathology in this model. Our data indicate that similar to prions, PMCA can reproduce some characteristics of α-syn aggregation and seeded propagation in vitro in a complex milieu. This opens new opportunities for the molecular study of synucleinopathies.—Nicot, S., Verchère, J., Bélondrade, M., Mayran, C., Bétemps, D., Bougard, D., Baron, T. Seeded propagation of α-synuclein aggregation in mouse brain using protein misfolding cyclic amplification.
Keywords: prion, seeding, PMCA, α-syn
α-Synuclein (α-syn) is a neuronal protein critically involved in a group of human neurodegenerative diseases, called synucleinopathies, including Parkinson’s disease (PD), dementia with Lewy bodies, and multiple system atrophy (MSA). These diseases are characterized by α-syn intracellular inclusions in neurons in PD and dementia with Lewy bodies, presenting as Lewy bodies and Lewy neurites, or in oligodendrocytes in MSA, presenting as glial cytoplasmic inclusions. Most cases of these diseases are considered as sporadic, but some cases can be genetically triggered by duplication or triplication of the α-syn-encoding (SNCA) gene, leading to constitutive overexpression of the protein, or by mutations in the N-terminal region of the protein, which favor its oligomerization and aggregation (A30P, E46K, or A53T, for instance) (1). Evidence of somatic SNCA gains in brain, more commonly in nigral dopaminergic neurons of PD than in controls, and possibly commonest in some MSA cases, was also recently described by Mokretar et al. (2), suggesting that this may be a risk factor for sporadic synucleinopathies or, alternatively, a result of the disease process.
The detailed molecular pathways leading to α-syn aggregation still remain poorly understood. However, a primary event leading to α-syn oligomers would involve α-syn self-interactions in a rate-limiting step of α-syn dimers formation (3, 4). The initiation and amplification steps of α-syn aggregation can be dramatically changed by mutations associated with familial forms of PD (5), and the dimer formation was notably shown to be accelerated by the A30P and A53T mutations (3).
The pathogenesis of synucleinopathies is characterized by a progressive spreading of α-syn lesions in the patient body, and a number of recent studies suggested that it could involve molecular mechanisms similar to those occurring in prion diseases [for a recent review, see Steiner et al. (6)]. In this paradigm, pathologic α-syn species act as seeds that self-propagate at the expense of the normal protein and spread through interconnected brain regions. Previously, we and others have used a transgenic mouse model overexpressing the human mutated A53T α-syn on a nonknockout mouse α-syn background (called M83) (7) to show that a synucleinopathy can be experimentally accelerated in vivo following inoculation of both M83+/+ and M83+/− young mice with pathologic α-syn assemblies of different origin (from old M83 mice, humans with MSA, or recombinant fibrils) and inoculated via different routes, including intracerebral and peripheral (for example, intramuscular, intraperitoneal, intrasciatic) routes (8–19).
We recently showed that the spreading of pathologic α-syn in the M83 mouse brain can be monitored using an ELISA test, which specifically recognizes pathologic α-syn in some brain regions (mainly brain stem and midbrain) and in the spinal cords of sick M83 mice, which are characterized by neuronal α-syn inclusions (7, 11, 20). The precise molecular species recognized by this ELISA test remain to be determined, but immunoreactivity is observed with antibodies against epitopes in the C- or, to a lesser extent, N-terminal ends of the protein that are known to represent less-structured regions of the protein compared with the central region, which is involved in the formation of β-sheet structural motifs in amyloid fibrils (21).
Protein misfolding cyclic amplification (PMCA) provides faithful replication of mammalian prions in vitro. Presumably because it operates in a crude brain milieu containing potential cellular cofactors necessary for prions to multiply, PMCA has the unique ability to generate bona fide infectious prions (22). The method, conceptually based on the nucleation-dependent polymerization model of prion replication, allows conversion of the cellular prion protein from a normal brain substrate into an aggregated form using repeated cycles of sonication and incubation after seeding with a small quantity of a sample containing the pathologic prion protein (23). In the last few years, our laboratory has developed a highly efficient PMCA methodology, which allowed for the first time the preclinical detection of prions in the plasma of patients incubating variant Creutzfeldt-Jakob disease (24).
Regarding the α-syn protein, the use of PMCA has been reported on α-syn of recombinant origin (4, 25–29) but not, to our knowledge, on brain α-syn (30). In this study, we developed α-syn PMCA in mouse brain homogenates, including M83 (7) and D (31) transgenic mice expressing A53T-mutant and wild-type human α-syn, respectively, as well as C57Bl/6 wild-type mice. We demonstrate for the first time the in vitro generation and seeded propagation of α-syn aggregates in mouse brain tissue, with subsequent transmission of an experimental synucleinopathy in M83 mice by intracerebral inoculation.
MATERIALS AND METHODS
Brain samples
PMCA substrates were prepared by homogenization of whole brains using a mechanical homogenizer to obtain a 5–10% homogenate (w/v) in a conversion buffer (PBS pH = 7.2, 150 mM NaCl, 1% Triton X-100, and protease inhibitor cocktail) and then clarified at 2000 g for 20 s before freezing at −80°C in single-experiment aliquots. They were prepared from 2-mo-old transgenic mice bred in our laboratory, overexpressing either the human A53T-mutated α-syn under the prion promoter (M83 line) (7) or the normal human α-syn under the platelet-derived growth factor β promoter (D line) (31), and C57Bl/6 wild-type mice expressing (C57Bl/6-WT) or not (C57Bl/6-KO) mouse α-syn purchased from Janvier labs (Saint-Berthevin, France) and Envigo (Huntingdon, United Kingdom), respectively (32). M83 brain substrates were derived from homozygous (+/+) or hemizygous (+/−) mice obtained after breeding M83 homozygous mice with Bl/6; C3H mice, corresponding to the genetic background of the M83 mouse line (7). In some experiments, brain homogenates obtained in high-salt buffer (50 mM Tris-HCl pH 7.5, 750 mM NaCl, 5 mM EDTA, 1 mM DTT, 1% phosphatase and protease inhibitor cocktails) were used as seeds in PMCA reactions. These seeds were prepared from whole brains of healthy (≤6-mo-old) or sick M83 mice, or from dissected brain regions. Sick M83 mice had developed the typical M83 disease either after normal aging (≥1 yr old) (n = 3) or after intracerebral inoculations of brain homogenates from sick M83 mice or of fibrillar recombinant α-syn (33), as previously described (20). In one experiment, PMCA was performed using seeds prepared from dissected brain regions of sick M83 mice, as previously described (34).
PMCA
The PMCA procedure was previously described (24). Briefly, 100 µl of brain homogenates from healthy mice (M83+/+, M83+/−, D, C57Bl/6-WT, and C57Bl/6-KO) were used directly in PMCA reactions at 5 or 10% in comparison with shaking (1000 rpm at 37°C) and standing at 37°C. The same brain homogenates were also used as substrate either at 5% (M83+/−) or 10% (D, C57Bl/6-WT, and C57Bl/6-KO) after mixing with 10 µl of serially diluted brain homogenates (from 10−3 to 10−6) used as seeds. PMCA reactions were performed in PCR tubes containing 3 Teflon (Chemours, Wilmington, DE, USA) beads and submitted to amplification in a microplate sonicator (Q700; Qsonica, Newtown, CT, USA). Each cycle is composed of an incubation step (29 min 40 s at 37°C) and a sonication step (20 s at 140 W) for cycles ranging from 48 to 144 (1 round). For serial PMCA, amplified materials were diluted 1:10 in fresh PMCA substrate and submitted to a new PMCA round. To avoid any cross-contamination, experiments were carried out under strict quality-controlled PCR conditions.
Western blot
PMCA samples (10 µl) were mixed with 2 µl of TD4215 denaturing buffer (final concentration: 4% SDS, 2% 2-ME, 192 mM glycine, 25 mM Tris, 5% sucrose). Proteins were separated after heat denaturation (5 min at 100°C) on 12% TGX Stain-Free FastCast acrylamide gels (1610185; Bio-Rad, Hercules, CA, USA) loaded with 2 µl of the denatured PMCA samples per lane, then blotted onto PVDF membranes (Immobilon-P; MilliporeSigma, Burlington, MA, USA). After migration, gels were activated for 1 min on Chemidoc MP apparatus (Bio-Rad), and image analysis was done using ImageLab software (Bio-Rad) to control the protein loads per lane, which are ∼25 µg proteins per lane in the case of 10% brain homogenates. α-Syn was cross-linked to membranes using 0.4% paraformaldehyde diluted in PBS for 30 min, and unspecific binding sites were saturated by adding 5% milk for 1 h. α-Syn was detected by either α-syn antibody (C-20R; 1:10,000) (sc-7011-R; Santa Cruz Biotechnology, Dallas, TX, USA) rabbit pAb, recombinant monoclonal α-syn antibody (MJFR1; 1:1000) rabbit mAb (ab138501; Abcam, Cambridge, United Kingdom), or D37A6 (1:1000) rabbit mAb (4179; Cell Signaling Technology, Danvers, MA, USA), diluted in PBS with 0.1% Tween overnight at +4°C. Membranes were then incubated with stabilized goat anti-rabbit horseradish peroxidase (1:1000) (32460; Interchim, Montluçon, France) for 1 h at room temperature. The signal was then revealed with chemiluminescent substrate (34076, Supersignal WestDura; Thermo Fisher Scientific, Waltham, MA, USA) and analyzed using the ChemiDoc system and ImageLab software. For some experiments, we performed proteinase K (PK) digestion on PMCA samples, with PK concentration from 0.5 to 10 µg/ml (final concentration). In the same manner, prior to migration on 12% TGX Stain-Free FastCast acrylamide gels (Bio-Rad), PMCA-digested products were mixed with 2 µl of TD4215 denaturing buffer.
ELISA
For the analyses of the PMCA samples, 200 µl of Superblock T20 (Thermo Fisher Scientific) was loaded in each well of MaxiSorp plates (Thermo Scientific Nunc; Thermo Fisher Scientific) for 1 h at 25°C under agitation at 150 rpm. After rinsing 5 times with PBS with 0.05% Tween (PBST), the PMCA samples [2 µl per well, adjusted to 100 µl in PBST + 1% bovine serum albumin (BSA)] were then loaded and incubated for 2 h at 25°C under agitation at 150 rpm. The plates were washed 5 times with PBST, and primary antibodies C-20R (1:10,000)/MJFR1 (1:1000)/D37A6 (1:1000) in PBST + 1% BSA (15260037; Thermo Fisher Scientific) were then incubated for 1 h at 25°C under agitation at 150 rpm. The plates were washed another 5 times with PBST, and anti-rabbit IgG horseradish peroxidase conjugate (4010-05; Southern Biotech, Birmingham, AL, USA) was added (1:2000 dilution) in PBST + 1% BSA for 1 h at 25°C. After washing the plates 5 times with PBST, 100 μl of 3,3′,5,5′-tetramethylbenzidine solution (T0440; MilliporeSigma) was added to each well, and plates were incubated for 15 min with shaking. The enzymatic reaction was stopped by adding 100 µl of 1 M HCl. Optical densities (ODs) were read at 450 nm by a microplate reader (Clariostar; BMG Labtech, Ortenberg, Germany). OD values of blank wells, performed in parallel but without adding brain homogenates, were subtracted from OD values for each well. Following bioassays, the disease of M83 mice was confirmed by ELISA detection of Ser129 phosphorylated α-syn as previously described by Sargent et al. (11) after α-syn capture with Syn303 antibody (1 µg/ml) (824301; BioLegend, San Diego, CA, USA) and detection with the rabbit pAb against α-syn phosphorylated at serine 129 α-syn (1/3000) (ab59264; Abcam).
Bioassays
Bioassays were performed by stereotaxic injection into the left striatum (anteroposterior: +0.14, mediolateral: +2, dorsoventral: −2.75) of 2 µl of inoculum in 2-mo-old M83+/+ mice. When M83 mice are not inoculated, they develop characteristic motor symptoms between 8 and 16 mo of life, beginning with reduced ambulation, balance disorders, and partial paralysis of a hind leg and then progressing to prostration, difficulty in feeding, weight loss, hunched back, and general paralysis (7). In our experiments, the inoculated M83 mice were housed per group in enriched cages in a temperature-controlled room on a 12-h light/dark cycle and received water and food ad libitum in our approved facilities (C69 387 0801) in accordance with European Economic Community (EEC) Directive 86/609/EEC and French Decree 2013-118. The experimental protocol was authorized (16-039) by the Comité d’éthique ComEth French Agency for Food, Environmental and Occupational Health and and Safety (ANSES)/National Veterinary School of Alfort (ENVA)/Université Paris-Est Créteil (UPEC). Daily monitoring of the mice took place to detect any symptom of the M83 disease as previously described by Sargent et al. (11), including slow ambulation, balance disorders, and partial paralysis of a hind limb. When mice develop these first symptoms or any intercurrent disease not associated with M83 disease, we proceed to euthanasia by lethal intraperitoneal injection of Dolethal (Alcyon, Miribel, France). In the 3 experiments in which mice did not show clinical signs before the age of 9 mo, corresponding to the inocula prepared from either unsonicated M83+/+ brain or from sonicated C57Bl/6-KO brain, surviving healthy mice were euthanized at the age of ∼14 mo old. For the statistical analysis of the data, time from inoculation until the onset of clinical signs was modeled with a Cox model, and a pairwise comparison of survival times between experiments was performed with a Tukey’s procedure to control the type I error rate.
RESULTS
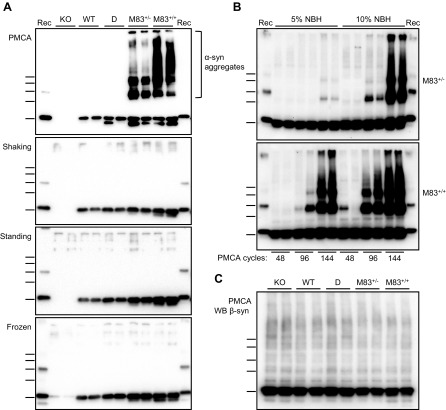
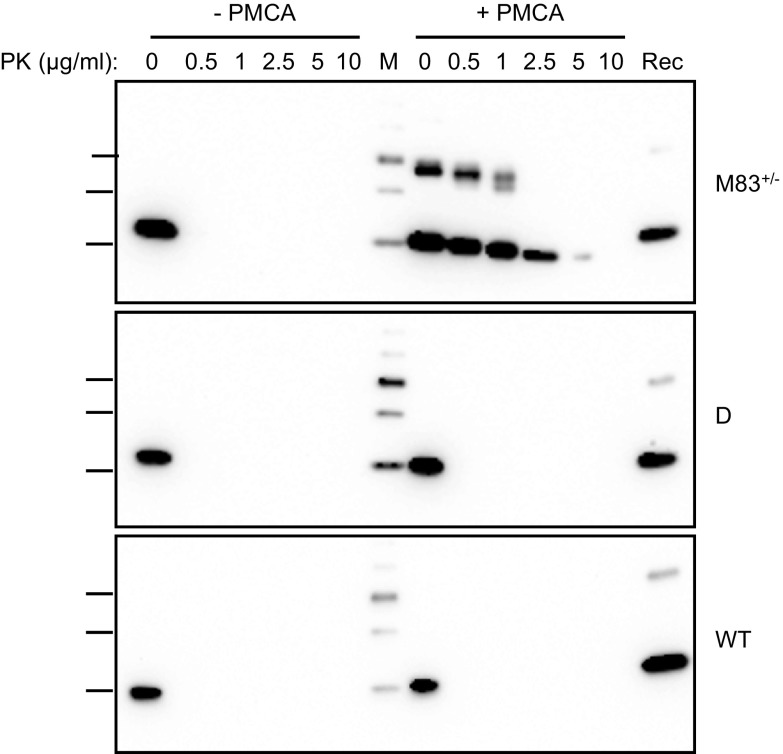
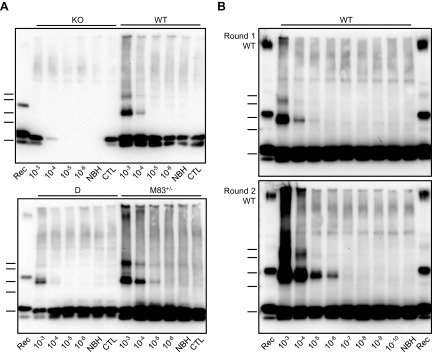
To determine the effects of PMCA on the α-syn protein in mouse brain, we prepared brain homogenates (10%, w/v) in conversion buffer from different mouse lines, including: 1) M83 mice expressing A53T-mutated human α-syn [homozygous (M83+/+) or hemizygous (M83+/−) for the transgene], 2) D+/+ mice expressing human wild-type α-syn, 3) C57Bl/6-WT mice, and 4) C57Bl/6-KO mice (32). These brain homogenates were submitted to a 72-h (144 cycles) PMCA procedure using a microplate sonicator (Fig. 1A). In the meantime, they were either only incubated at 37°C (standing condition) or under constant shaking at 1000 rpm (shaking condition). Unexpectedly, after 144 cycles of sonication-incubation, we observed the abundant formation of, at least partially, SDS-resistant α-syn aggregates, specifically in brain homogenates from M83 mice, as shown by Western blot detection using the C-20R antibody (Fig. 1A). This showed bands consistent with α-syn dimers and trimers, as well as higher MW species, some of which were retained in the stacking of the gels. The de novo aggregation of α-syn from M83 brain required the sonication process, as only monomeric α-syn was detected in the standing and shaking conditions and in the initial brain samples (frozen condition) (Fig. 1A). The sonication-induced formation of α-syn aggregates was enhanced by increased α-syn levels, as shown by comparisons of brain homogenates from M83+/+ and M83+/− mice, as well as of 10 vs. 5% (w/v) concentrations (Fig. 1B). This also showed an increase of α-syn aggregation with increasing numbers of cycles of sonication-incubation, and this required the addition of Teflon beads into the test tube (unpublished results). We did not observe α-syn aggregation from M83+/− 5% (w/v) homogenates after up to 96 cycles of sonication-incubation, which enabled us to apply these experimental conditions in the following experiments when using the M83+/− mouse brain as a substrate in seeding experiments. Using an antibody specifically recognizing β-syn did not reveal any aggregation of this protein in the 4 mouse brain substrates following PMCA (Fig. 1C). To further characterize α-syn species in the PMCA products, we performed limited in vitro proteolysis of the samples using PK digestion (Fig. 2). After PMCA, α-syn from M83+/− brain resists PK treatment for up to 5 µg/ml for 30 min, whereas without PMCA, it is fully degraded even at a 0.5 µg/ml PK concentration. In D and C57Bl/6-WT brain homogenates, either before or after PMCA, α-syn is similarly fully degraded at 0.5 µg/ml PK concentration. Thus, repeated cycles of sonication-incubation specifically induce the formation of partially PK-resistant α-syn aggregates in M83 mouse brain.
Figure 1.
PMCA triggers de novo α-syn aggregation in the M83 mouse brain. A) Immunoblots of α-syn (C-20R antibody) in 10% brain homogenates from C57Bl/6-KO, C57Bl/6-WT, D, M83+/−, and M83+/+ mice, obtained after treatment by 144 PMCA cycles or maintained in shaking and standing conditions, compared with the initial frozen brain homogenates. Abundant α-syn aggregates are specifically detected after PMCA in the M83 substrate. B) PMCA α-syn aggregation kinetics in M83+/− and M83+/+ substrates at 2 different NBH concentrations (5 vs. 10%) and as a function of sonication-incubation cycle number (48, 96, 144). C) Immunoblot of β-synuclein (Ab7611 antibody) in 10% brain homogenates from C57Bl/6-KO, C57Bl/6-WT, D, M83+/−, and M83+/+ mice after treatment by 144 PMCA cycles. Bars to the left of each panel indicate the ∼20-, 30-, 40-, 50-, and 60-kDa MW markers. KO, knockout; Rec, human recombinant α-syn control; WT, wild type.
Figure 2.
PMCA products from M83 mouse brain contain partially PK-resistant α-syn species. Immunoblots of α-syn (C-20R antibody) in 10% brain homogenates from M83+/−, D, and C57Bl/6-WT mice treated or not by 144 PMCA cycles after PK digestion for 30 min at 0-, 0.5-, 1-, 2.5-, 5-, and 10-μg/ml final concentrations. Bars to the left of each panel indicate the ∼20-, 30-, and 40-kDa MW markers. M, MW marker; Rec, human recombinant α-syn control; WT, wild type.
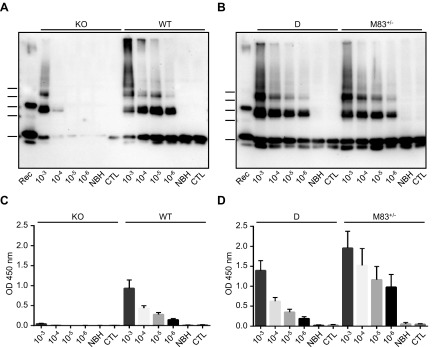
We next investigated whether the de novo PMCA-generated α-syn aggregates from M83 mice would act as seeds for the aggregation of α-syn when introduced in PMCA reactions (Fig. 3). To test this hypothesis, we serially diluted 10-fold (from 10−3 to 10−6) a 144-cycle PMCA product from M83+/− mice harboring abundant α-syn aggregates. These dilutions were then used to seed M83+/−, D, C57Bl/6-WT, and C57Bl/6-KO brain substrates, and the resulting mixtures were subjected to PMCA. As shown in the C57Bl/6-KO substrate, α-syn aggregates attributable to the seed were detected in the 10−3 and, to a lesser extent, in the 10−4 dilutions after PMCA. However, in all other substrates, abundant α-syn aggregates were still detected at the 10−6 dilution, whereas no signal was observed in unseeded PMCA reactions [normal brain homogenate (NBH) lanes] or in control PMCA reactions seeded with a 10−3 dilution from a M83+/+ brain that was not previously subjected to PMCA (control lanes) (Fig. 3A, B). To rule out the possibility that this seeding effect could be attributable to factors other than aggregated α-syn itself, some of which could have been generated during the process of sonication-incubation of brain homogenates to generate the seed, we similarly seeded PMCA reactions with the products of 144 PMCA cycles from D, C57Bl/6-WT, or C57Bl/6-KO brain substrates that were previously shown to be free of detectable α-syn aggregates. We were unable to detect any α-syn aggregation in M83+/− substrate following PMCA after seeding with these samples (unpublished results). Altogether, these experiments clearly show that the addition of α-syn aggregates produced by PMCA from M83 brain was able to seed α-syn aggregation, including in D and C57Bl/6-WT brain substrates, which otherwise do not show detectable de novo α-syn aggregation in the absence of seeding. Next, we examined the PMCA samples using an ELISA test, as previously described in refs. 11 and 20, for the specific identification of the pathologic α-syn in sick M83 mice, using the same C-20R antibody for α-syn detection (Fig. 3C, D). Overall, we found a good correlation between ELISA and Western blot results consistent with the detection of PMCA-generated α-syn aggregates. These results emphasize the fact that this ELISA test likely identifies, at least in part, aggregated α-syn species in the mouse brain, offering an additional and convenient method for measuring α-syn aggregates in a PMCA sample.
Figure 3.
De novo PMCA-generated α-syn aggregates from M83 mice seed the aggregation of α-syn in PMCA reactions. A, B) Immunoblots of α-syn (C-20R antibody) in PMCA samples after seeding with serial 10-fold dilutions (10−3 to 10−6) of a 144-cycle PMCA product from M83+/− mice containing de novo α-syn aggregates in comparison with unseeded NBH substrate treated by PMCA (NBH) or control after seeding with unsonicated M83+/+ brain at dilution 10−3 (CTL). In total, 144 PMCA cycles in 10% brain homogenates were performed for the C57Bl/6-KO, C57Bl/6-WT, and D substrates, or 96 PMCA cycles in 5% brain homogenates for the M83+/− substrate. Bars to the left of each panel indicate the ∼20-, 30-, 40-, 50-, and 60-kDa MW markers. Rec, human recombinant α-syn control. C, D) ELISA immunoreactivity after loading of the same PMCA samples on MaxiSorp plates, followed by detection of captured α-syn using C-20R antibody. Three repetitions of the ELISA test were performed. KO, knockout; WT, wild type.
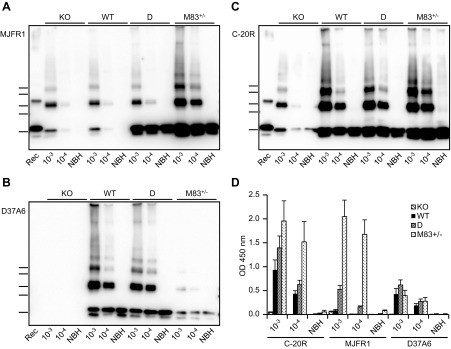
To examine the respective contributions of human and mouse α-syn proteins in the formation of the α-syn aggregates after seeding with de novo M83 PMCA products, we performed Western blot analyses using antibodies specific to either human (MJFR1) or mouse (D37A6) α-syn (Fig. 4A–C). We found a major difference in the composition of α-syn aggregates in D and M83+/− substrates. In the D substrate, detection of human α-syn with the MJFR1 antibody showed a strong labeling for the α-syn monomer, whereas α-syn dimers and trimers were labeled as in C57Bl/6-KO and C57Bl/6-WT substrate, which correspond to the human α-syn seed input from the PMCA-treated brain homogenate of M83 mice. Using the D37A6 antibody against mouse α-syn showed similar results in C57Bl/6-WT and D substrates, whereas no signal was detected in C57Bl/6-KO brain substrate. In the M83+/− substrate, both human α-syn aggregates and monomers were strongly labeled by the MJFR1 antibody; mouse α-syn detection with D37A6 was instead very low in comparison to C57Bl/6-WT and D substrates and, unexpectedly, also for the mouse α-syn monomer. In untreated brain homogenates, similar amounts of mouse α-syn monomer were detected with D37A6 in M83+/−, D, and C57Bl/6-WT brain substrates (unpublished results); thus, although still unexplained, decreased detection of mouse α-syn by Western blot is the result of PMCA treatment specifically in the M83 brain substrate. The same PMCA samples were also examined by ELISA with α-syn detection using MJFR1, D37A6, or C-20R antibodies (Fig. 4D). The results showed that the human protein is recognized much better from the M83 than from the D brain substrate, although increased immunoreactivity is clearly detected in the D substrate, but only after seeding; in contrast, the levels of mouse α-syn species recognized by D37A6 are roughly comparable in the 3 brain substrates (M83, D, and C57Bl/6-WT), including in the M83 samples that show decreased detection in Western blot after the PMCA reaction. Immunoreactivity is extremely low in samples that do not show α-syn aggregates by Western blot (NBH). Altogether, these data suggest a higher aggregation of human α-syn after seeding in the M83 brain substrate compared with the D brain substrate.
Figure 4.
Distinct aggregation behavior of human and mouse α-syn proteins after seeded PMCA in the different brain substrates. A–C) Immunoblots of α-syn in PMCA samples obtained after seeding the 4 mouse brain substrates with a PMCA product (dilutions 10−3 and 10−4) produced in M83+/− brain, as shown in Fig. 1A. In total, 144 PMCA cycles in 10% brain homogenates were performed for the C57Bl/6-KO, C57Bl/6-WT, and D substrates, or 96 PMCA cycles in 5% brain homogenates for the M83+/− substrate. The immunoblot membranes were revealed by MJFR1 or D37A6 antibodies specific for the human or mouse α-syn, respectively, or by C-20R antibody, which recognizes both human and mouse α-syn. Bars to the left of each panel indicate the ∼20-, 30-, 40-, 50-, and 60-kDa MW markers. Rec, human recombinant α-syn control. D) PMCA products were loaded on MaxiSorp plates, and captured α-syn was detected using C-20-R, MJFR1, or D37A6 antibodies. Three repetitions of the ELISA test were performed. KO, knockout; WT, wild type.
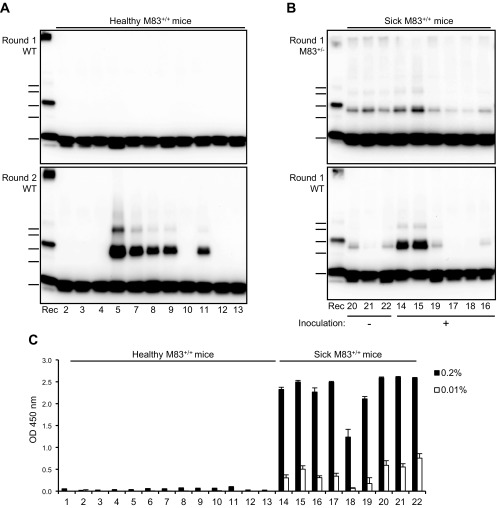
Having shown that PMCA can propagate α-syn aggregation after in vitro seeding of mouse brain substrates, including that of C57Bl/6-WT mice, we examined the seeding capacities of M83 mouse brain following development of the characteristic M83 clinical disease. Serial 10-fold dilutions of a 10% whole-brain homogenate were prepared from a sick M83+/+ mouse at the terminal stage of the disease before seeding in the different substrates (M83+/−, D, C57Bl/6-WT, and C57Bl/6-KO) (Fig. 5A). Following PMCA, SDS-resistant α-syn dimers and trimers were detected by Western blot in all substrates except for the C57Bl/6-KO control, which shows that these α-syn species are derived from the substrate and not from the seed. However, compared with previous experiments using a de novo PMCA-generated seed from M83+/− substrate, the detection limit achieved with this endogenous seed from sick M83 brain was lower. To increase this detection limit, we performed a second PMCA round in the nontransgenic C57Bl/6-WT substrate, a procedure generally used in PMCA studies for prions that can achieve a very high level of sensitivity. Aliquots of each dilution from the first PMCA round were mixed with fresh substrate and submitted to a new PMCA round. As shown in Fig. 5B, we could detect a seeding activity up to the 10−4 and 10−6 dilutions at the first and second PMCA round, respectively, from a sick M83+/+ mouse brain homogenate. Importantly, no α-syn aggregates were detected after 2 PMCA rounds of 144 cycles in either the unseeded C57Bl/6-WT brain substrate (Fig. 5B) or in the C57Bl/6-KO brain substrate seeded by M83 brain tissue (unpublished results).
Figure 5.
Detection of α-syn PMCA seeding activity in the brain of sick M83 mice. A) Western blot analysis of PMCA products obtained after seeding with serial 10-fold dilutions (10−3 to 10−6) of a brain homogenate from a sick M83+/+ mouse in comparison with unseeded NBH substrate treated by PMCA (NBH) or after seeding with unsonicated healthy M83+/+ brain at dilution 10−3 (CTL). In total, 144 PMCA cycles in 10% brain homogenates were performed for the C57Bl/6-KO, C57Bl/6-WT, and D substrates, or 96 PMCA cycles in 5% brain homogenates for the M83+/− substrate. The sick M83 mouse was a 170-d-old mouse that had been intracerebrally inoculated at 8 wk old with brain homogenate of sick M83 mouse, and the healthy mouse was a 60-d-old unchallenged mouse. B) Immunoblots of α-syn (C-20R antibody) in PMCA samples obtained after seeding the C57Bl/6-WT brain substrate (10%, 144 cycles) with a whole-brain homogenate (dilutions 10−3 to 10−10) from a sick M83 mouse that died with typical clinical signs of the disease in comparison with unseeded NBH. One or 2 PMCA rounds were performed. Bars to the left of each panel indicate the ∼20-, 30-, 40-, 50-, and 60-kDa MW markers. KO, knockout; Rec, human recombinant α-syn control; WT, wild type.
We next tested the capacity of this PMCA assay to distinguish between healthy and sick M83 mice in a panel of samples from healthy (uninoculated ≤180 d old) (n = 13) or sick (n = 9) M83+/+ mice. The different origins, clinical and pathologic status, and ages of these mice are summarized in Table 1. These experiments, performed from whole-brain homogenates at dilutions 10−3 and 10−4 and using M83+/− or C57Bl/6-WT brain substrates, readily discriminated sick and healthy M83 mice (Fig. 6A, B and Table 1). All the brains of the sick mice were shown to contain high levels of Ser129 phosphorylated α-syn by ELISA (Fig. 6C). From unchallenged and healthy M83 mice below the age of 6 mo, no α-syn aggregates were observed after a single PMCA round on either brain substrate; however, some of these samples (from mice 110 to 146 d old) showed a seeding activity that was identified by a second PMCA round performed with the C57Bl/6-WT brain substrate (Fig. 6A and Table 1). Very low levels of immunoreactivity were detected by ELISA with the antibody against Ser129 phosphorylated α-syn in some of these mice and at the lower dilution tested (0.2% brain homogenate) (Fig. 6C).
TABLE 1.
α-Syn PMCA seeding activities in a panel of brain samples from sick or healthy M83 mice
| Reference | Inoculation | Status | Age at death (day) | α-Syn aggregate detection by Western blot |
|
|---|---|---|---|---|---|
| M83+/− substrate (1 round) | C57Bl/6-WT substrate (round 1/2) | ||||
| 1 | — | Healthy | 60 | − | −/− |
| 2 | — | Healthy | 90 | − | −/− |
| 3 | — | Healthy | 90 | − | −/− |
| 4 | — | Healthy | 90 | − | −/− |
| 5 | — | Healthy | 110 | − | −/+ |
| 6 | — | Healthy | 110 | − | −/+ |
| 7 | — | Healthy | 110 | − | −/+ |
| 8 | — | Healthy | 146 | − | −/+ |
| 9 | — | Healthy | 146 | − | −/+ |
| 10 | — | Healthy | 146 | − | −/− |
| 11 | — | Healthy | 146 | − | −/+ |
| 12 | — | Healthy | 180 | − | −/− |
| 13 | — | Healthy | 180 | − | −/− |
| 14 | Fibrils | Sick | 139 | + | +/+ |
| 15 | Fibrils | Sick | 139 | + | +/+ |
| 16 | Sick M83 brain (1%) | Sick | 141 | + | +/+ |
| 17 | Sick M83 brain (5%) | Sick | 142 | + | −/+ |
| 18 | Sick M83 brain (5%) | Sick | 161 | + | −/+ |
| 19 | Fibrils | Sick | 182 | + | +/+ |
| 20 | — | Sick | 365 | + | +/+ |
| 21 | — | Sick | 451 | + | +/+ |
| 22 | — | Sick | 457 | + | +/+ |
One or 2 PMCA rounds were performed in M83+/− (5%, 96 cycles) or C57Bl/6-WT (10%, 144 cycles) brain substrate after seeding with whole-brain homogenates of M83+/+ mice diluted at 10−4 or 10−3 for sick or healthy mice, respectively. The sick M83+/+ mice were either old unchallenged mice (≥1 yr old) or 3–6-mo-old mice after intracerebral inoculations of brain extracts of sick M83+/+ mice or of recombinant fibrils. The Western blot detection results represent the presence (+) or absence (−) of SDS-resistant α-syn aggregates detected with C-20R antibody, as shown in Fig. 6.
Figure 6.
PMCA seeding activity in a panel of brain samples from sick or healthy M83 mice. A) Immunoblots of α-syn (C-20R antibody) in PMCA products obtained after seeding C57Bl/6-WT (10%, 144 cycles) substrate with brain samples from healthy M83+/+ mice at dilution 10−3. One or 2 PMCA rounds were performed. B) Immunoblots of α-syn (C-20R antibody) in PMCA products obtained after seeding M83+/− (5%, 96 cycles) or C57Bl/6-WT (10%, 144 cycles) substrates with brain samples from sick M83+/+ mice at dilution 10−4. The figures below each of the panels indicate the reference of the mouse, as listed in Table 1. Bars to the left of each panel indicate the ∼20-, 30-, 40-, 50-, and 60-kDa MW markers. C) ELISA detection of Ser129 phosphorylated α-syn in the same panel of brain samples from healthy or sick M83+/+ mice. ELISA detection was performed using Syn303 as a capture antibody, and rabbit pAb against Ser129 phosphorylated α-syn for detection. Two quantities of brain homogenates were examined, including 0.2% [as described by Sargent et al. (11)] and 0.01% homogenates, this last concentration corresponding to a 10−4 dilution of seeds as examined in PMCA experiments. KO, knockout; Rec, human recombinant α-syn control; WT, wild type.
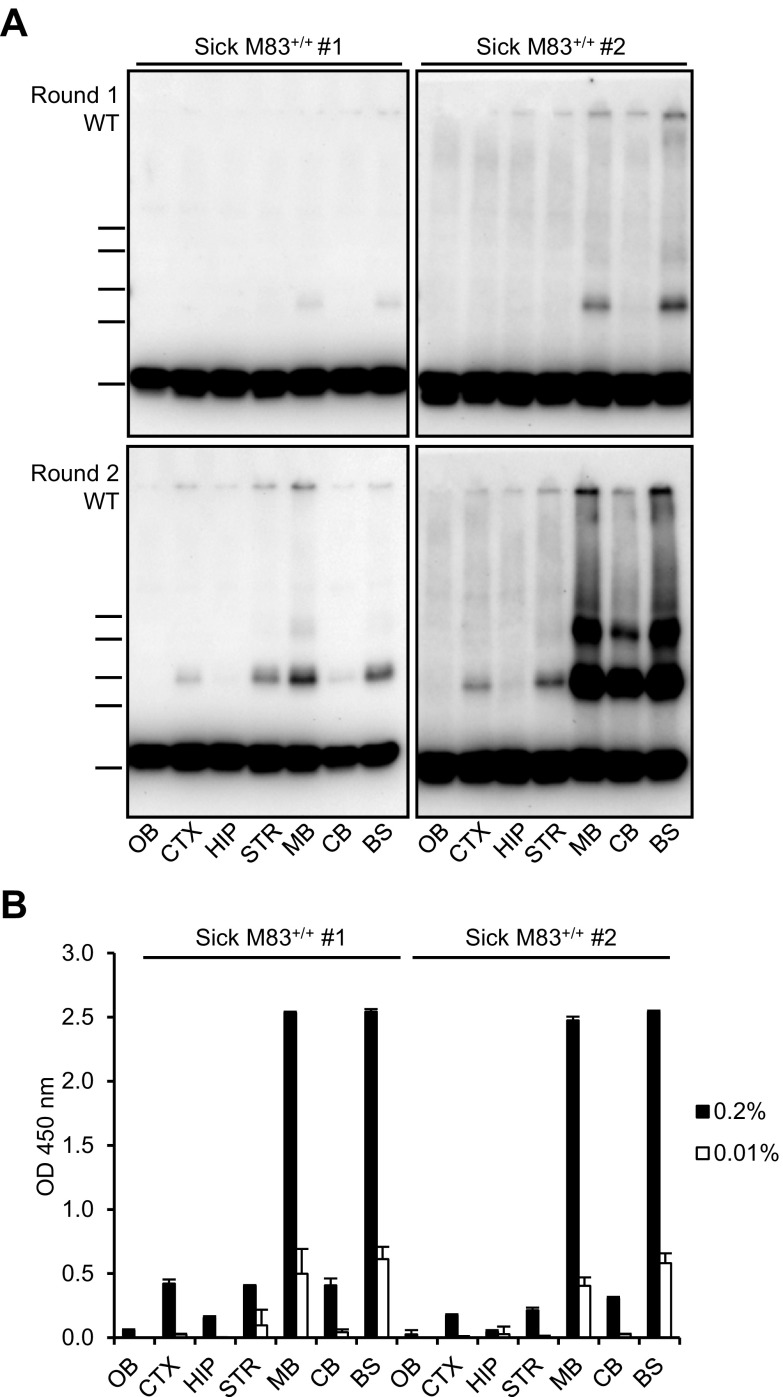
Because α-syn neuropathology is much more pronounced in the caudal brain regions (brain stem and midbrain) of sick M83 mice, we compared the PMCA seeding activities in different brain regions of 2 M83 mice at the stage of the disease (573 or 670 d old) (Fig. 7A). The highest PMCA seeding activities were detected in the brain stem and midbrain of these mice. Intermediate results were obtained in the cerebral cortex, striatum, and cerebellum, whereas the lowest seeding activities were found in the hippocampus and olfactory bulb, although α-syn aggregates could be detected after 2 PMCA rounds at the 10−3 dilution in these regions (unpublished results). These PMCA results are in good agreement with the results of direct detection of Ser129 phosphorylated α-syn by ELISA in these same brain regions, as illustrated in Fig. 7B. Thus, we found a positive correlation between the known degree of α-syn neuropathology and the PMCA seeding activity in different regions of the brain of sick M83 mice.
Figure 7.
PMCA seeding activity within the same M83 brain is higher in regions mostly targeted by the α-syn pathology. A) Comparison of PMCA seeding activities in different brain regions of 2 sick M83+/+ mice euthanized with typical clinical signs at the age of 573 (sick M83 #1) or 670 (sick M83 #2) days old. PMCA was performed using the C57Bl/6-WT brain substrate (10%, 144 cycles) after seeding with a 10−4 dilution of each of the following brain regions: olfactory bulb (OB), cerebral cortex (CTX), hippocampus (HIP), striatum (STR), midbrain (MB), cerebellum (CB), and brain stem (BS). Bars to the left of each panel indicate the ∼20-, 30-, 40-, 50-, and 60-kDa MW markers. B) ELISA detection of Ser129 phosphorylated α-syn in the same dissected brain regions from sick M83 #1 and #2 mice. Two quantities of brain homogenates were examined, including 0.2% [as described by Sargent et al. (11)] and 0.01% homogenates, this last concentration corresponding to the 10−4 dilution of seeds as examined by PMCA.
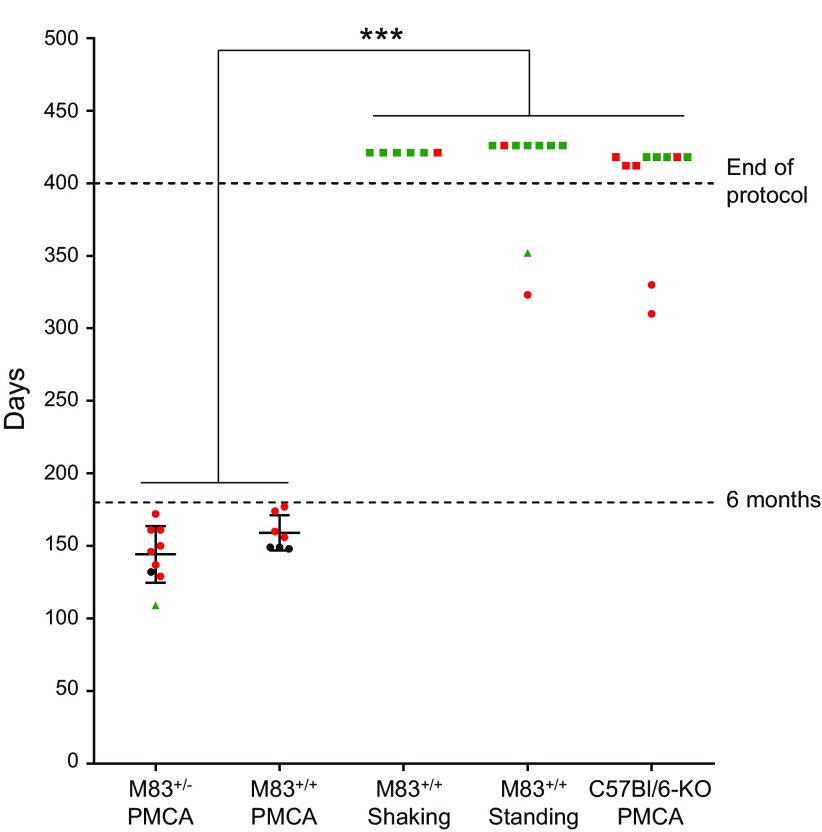
We finally addressed the question as to whether the PMCA products can seed α-syn pathology in vivo. For this, young M83+/+ mice were inoculated into the striatum (left side) with samples obtained after 144 PMCA cycles in the M83+/+, M83+/−, or C57Bl/6-KO (2 mo old) brain substrates. As additional controls, we inoculated the M83+/+ mouse brain substrate without sonication (i.e., standing or shaking conditions). Individual survival data during a 1-yr follow-up after stereotaxic intracerebral challenge are shown in Fig. 8. In M83 mice, the disease was strikingly accelerated by the inoculation of the sonicated M83+/+ or M83+/− brain samples. All the mice inoculated with these 2 M83 PMCA-treated inocula showed before the age of 6 mo the clinical signs of M83 mice, similarly to those previously described in this M83 mouse model (7, 11); typically, mice showed reduced spontaneous ambulation and hunched posture, with progressive paralysis beginning by the hind legs, rapidly worsening, and requiring euthanasia for ethical reasons within a few days. The disease was confirmed by ELISA and Western blot analysis and by immunohistochemistry showing detection of Ser129 phosphorylated pathologic α-syn in the brain of these mice (Supplemental Figs. S1 and S2). As previously described in refs. 11 and 20, pathologic α-syn is very abundant in the midbrain and brain stem, and at least in some of these mice, it was also found into the striatum and cerebral cortex in the left side (inoculated side) of the brain. In contrast, the inocula prepared from M83 brain in shaking or in standing conditions, or from sonicated C57Bl/6-KO brain homogenate, failed to accelerate the disease in M83 mice, which only began to develop the disease after the age of 9 mo, as has been previously described in refs. 7 and 8 for this model. Thus, the M83-derived PMCA samples that contained α-syn aggregates strikingly accelerated the M83 disease, as previously reported in refs. 8 and 9 with brain homogenates from sick M83 mice.
Figure 8.
Survival of M83+/+ mice after intracerebral inoculation of PMCA products. The age at death is indicated by the vertical axis for the 5 experiments with inocula prepared from M83+/−, M83+/+, or C57Bl/6-KO brain homogenates (10%), which had been treated by PMCA for a single round (144 cycles) or, in the case of the M83+/+ brain substrate, incubated without sonication in shaking or in standing conditions. The mean ± sd of the ages at death are indicated within the graph for the 2 groups from which all the mice had died before the end of the protocol at 400 d. Time from inoculation until the onset of clinical signs was modeled with a Cox model, and a pairwise comparison of survival times between experiments was performed with a Tukey’s procedure to control the type I error rate. Mice with the typical clinical signs of M83 disease are illustrated as circles, mice that were found dead without previously observing clinical signs are illustrated as triangles, and mice that were euthanized after the end of the protocol without detectable clinical signs are shown as squares. Mice from which brain samples were found positive by ELISA for Ser129 phosphorylated α-syn are shown in red, and those that are negative are shown in green; those that could not be analyzed because of unavailable frozen samples are shown in black. ***P ≤ 0.01.
DISCUSSION
During the last decade, studies in cellular and animal experimental models have suggested that prion-like behavior of α-syn is involved in the pathogenesis of synucleinopathies (8–10, 19, 20, 35). In the present study, we tested the hypothesis that the sonication-based version of the PMCA technology, developed by Saborio et al. (23) in the early 2000s for the sensitive amplification of the prion protein aggregation, could be used to study α-syn prion-like properties. Our study demonstrates the in vitro generation and seeded propagation of α-syn aggregates in a biologic complex matrix of brain homogenate. This shows, for the first time, an efficient PMCA in a tissular substrate of a misfolded protein distinct from the prion protein, which further supports the idea that α-syn can act in the same way as a prion. Furthermore, our bioassay experiments revealed that the PMCA-generated α-syn aggregates were able to seed α-syn neuropathology in vivo.
Our study first revealed that PMCA provokes the spontaneous formation of partially PK-resistant α-syn aggregates in the M83 mouse brain substrate, identified by Western blotting in denaturing conditions. This PMCA-induced α-syn aggregation was not observed in the other D or C57Bl/6-WT substrates, suggesting the involvement of mutant A53T human α-syn expressed by M83 mice. Consistently, PMCA α-syn aggregation was correlated to the amount of A53T-mutated protein, as evidenced by the comparisons of M83+/+ and M83+/− substrates, and we confirmed the crucial effects of sonication in this phenomenon. Indeed, it is well known that, compared with its wild-type counterpart, human mutated A53T α-syn has an intrinsic tendency to aggregation (5, 36).
Strikingly, we next demonstrated a PMCA seed-dependent α-syn aggregation in both D and C57Bl/6-WT brain substrates expressing the normal human and/or mouse α-syn proteins, obtained with seeds either generated de novo after PMCA of M83 brain or derived from the brain of sick M83 mice. Immunoblot analysis using antibodies against human or mouse α-syn showed a preferential aggregation of human α-syn in the M83 brain substrate after PMCA, whereas human α-syn was poorly aggregated in the D brain substrate. Mouse α-syn, however, showed aggregation in the D and C57Bl/6-WT brain substrates after seeding. One explanation for the preferential aggregation of mouse α-syn over human α-syn in the D substrate would be that mouse α-syn has a threonine residue at position 53, making it a more aggregation-prone substrate, similar to mutations in familial PD. An in vitro study by Kang et al. (37) examining why the aggregation of human α-syn was remarkably much slower than that of mouse α-syn indeed suggested that the presence of the A53 sequence in humans was crucial.
We consistently detected robust seeding activity in brains of sick M83 mice compared with healthy M83 mice. Importantly, this was equally seen in spontaneous vs. accelerated illness of M83 mice, arguing against an age-dependent effect of brain material on its PMCA seeding activity. We also observed that the PMCA seeding activity within the same M83 brain is higher in regions mostly targeted by the α-syn pathology in this model (brain stem and midbrain). Our observations of α-syn aggregation after a sonication-based PMCA procedure are however not fully unprecedented (25–28, 30). Nevertheless, recombinant α-syn diluted in saline solution was constantly used as a PMCA substrate in those previous PMCA studies, and α-syn aggregation was generally monitored using thioflavine-T fluorescence. A recent study by Jung et al. (28) used Western blot detection and, interestingly, reported efficient seeding of PMCA reactions of recombinant α-syn with brain or spinal cord extracts from 2 other transgenic mouse models expressing A53T mutant human α-syn, concurring with our current observations with M83 mice and in brain substrates. The brain tissue milieu in our PMCA procedure may, however, provide a physiologically more relevant environment for modeling α-syn aggregation because of the presence of putative cofactors such as lipids and cell membranes that may be crucial to obtain relevant biologic features of misfolding and neurotoxicity of aggregated α-syn (38). Our study also shows that this PMCA-induced α-syn aggregation can be easily monitored and quantified using an ELISA test that we previously described for the specific detection of pathologic α-syn in M83 mice (11, 20). This was similarly found here from C57Bl/6-WT brain, but only after seeding with seeds containing aggregated α-syn from M83 mice.
Using serial PMCA rounds to increase our assay sensitivity led to the preclinical detection of α-syn seeding activity in some of the healthy (<6 mo old) M83 mouse brains analyzed. At these ages, our previous immunohistochemistry studies only showed cytoplasmic labeling of Ser129 phosphorylated α-syn in enteric neurons but not in the brain (39). It has been reported that all brain regions of M83 mice, including histologically unaffected regions, contained equivalent amounts of detergent-soluble SDS-resistant oligomers, formed at 4–6 mo, before the formation of α-syn inclusions and onset of symptoms (40), which may be consistent with our findings of a seeding activity in the brain of some of the healthy 3.5–5-mo-old M83 mice.
The bioassays for PMCA samples described here support the idea that de novo aggregated α-syn was a major contributor in the acceleration of the M83 disease after intracerebral inoculation. In control bioassays, we used inocula prepared from M83+/+ young brain substrate in standing and shaking conditions, as well as from PMCA-sonicated C57Bl/6-KO mouse brain substrate. We found no evidence of disease acceleration after intracerebral inoculation of these samples to young M83+/+ mice. In contrast, mice inoculated with PMCA-sonicated M83 samples containing de novo α-syn aggregates developed, after incubation periods of <120 d, the characteristic clinical signs of M83 mice quite similarly to those previously described in this mouse model following intracerebral inoculations of brain homogenates from sick M83 mice or aggregated recombinant α-syn (8, 9, 20). The distribution of disease-associated α-syn was also consistent with that previously described in M83 mice, including in recent studies after inoculation of either human MSA or preformed human recombinant α-syn fibrils (17, 41) and by different routes of inoculation (42). Interestingly, PMCA samples from hemizygous M83 mice also led to efficient transmission despite the fact that these mice are largely resistant to the development of spontaneous illness during normal aging (7, 10). Also of note, α-syn from PMCA-sonicated M83 inocula was partially resistant to PK digestion, reminding the tight association of protease resistance with infectivity, which is well known for the prion protein during prion diseases.
In summary, we have shown that a sonication-based PMCA procedure can be used as a new in vitro model of α-syn aggregation and seeded propagation in a tissular matrix (Fig. 9). A practical benefit of this system is the possibility to generate high amounts of brain-derived aggregated α-syn rapidly, and more generally, PMCA may offer unique opportunities of research, as in the field of prion diseases. Recent studies have shown that another version of PMCA without sonication (called real-time quaking-induced conversion) was promising for the diagnosis of synucleinopathies in humans (26, 27, 30), but it still suffers from limitations according to the form of human disease or tissue considered (43, 44). It remains to be seen whether our approach in tissular substrates may be used for the study of human diseases and/or other experimental models of synucleinopathies. However, our results importantly demonstrate for the first time that pathogenic properties can be induced after an in vitro amplification from pathologic α-syn seeds, which can now be studied in other experimental models, including nontransgenic animals (45). The sonication-based PMCA approach in a brain substrate may help in deciphering the molecular basis of α-syn aggregation in a physiologically relevant environment, including the assessment of aggregation-inhibitory molecules with potential therapeutic applications (46) or, more basically, to better understand unresolved questions in the field such as the possible existence of α-syn strains (10, 17, 47, 48).
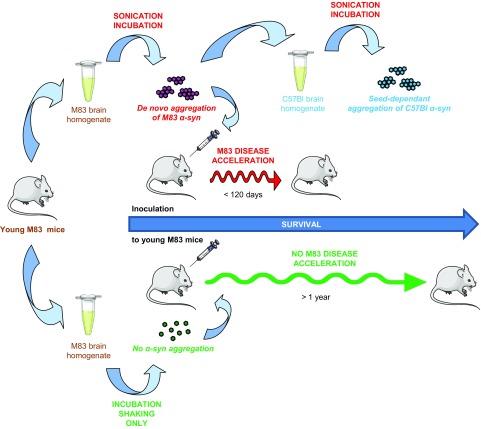
Figure 9.
Schematic representation of PMCA generation and seeded propagation of lethal α-syn aggregates. PMCA provokes spontaneous, sonication-dependant α-syn aggregation from young M83 brain homogenate. De novo–generated α-syn aggregation from M83 brain propagates in a wild-type mouse brain substrate after seeded PMCA and accelerates α-syn neuropathology and disease after intracerebral inoculation to young M83 mice. These findings corroborate the prion-like character of aggregated α-syn. PMCA emerges as a potential in vitro model of α-syn aggregation phenomenon in a biologic complex matrix.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Eric Morignat [Agency for Food, Environmental and Occupational Health and Safety (ANSES), Buenos Aires, Argentina] for statistical analyses, Habiba Tlili and Jean-Noël Arsac (both from ANSES) for immunohistochemical analyses, and Emilie Antier, Rabah Belkheir, Damien Gaillard, Latifa Lakhdar (all from ANSES), and Coralie Pulido [Plateforme d’Expérimentation Animale (PFEA)] for animal experiments. The authors declare no conflicts of interest.
Glossary
- α-syn
α-synuclein
- BSA
bovine serum albumin
- MSA
multiple system atrophy
- NBH
normal brain homogenate
- OD
optical density
- PBST
PBS with 0.05% Tween
- PD
Parkinson’s disease
- PK
proteinase K
- PMCA
protein misfolding cyclic amplification
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Nicot, J. Verchère, M. Bélondrade, D. Bougard, and T. Baron designed research; S. Nicot, J. Verchère, M. Bélondrade, C. Mayran, and D. Bétemps performed the experiments; S. Nicot, J. Verchère, M. Bélondrade, C. Mayran, D. Bétemps, D. Bougard, and T. Baron analyzed data; and S. Nicot and T. Baron wrote the manuscript.
REFERENCES
- 1.Lázaro D. F., Rodrigues E. F., Langohr R., Shahpasandzadeh H., Ribeiro T., Guerreiro P., Gerhardt E., Kröhnert K., Klucken J., Pereira M. D., Popova B., Kruse N., Mollenhauer B., Rizzoli S. O., Braus G. H., Danzer K. M., Outeiro T. F. (2014) Systematic comparison of the effects of alpha-synuclein mutations on its oligomerization and aggregation. PLoS Genet. 10, e1004741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mokretar K., Pease D., Taanman J. W., Soenmez A., Ejaz A., Lashley T., Ling H., Gentleman S., Houlden H., Holton J. L., Schapira A. H. V., Nacheva E., Proukakis C. (2018) Somatic copy number gains of α-synuclein (SNCA) in Parkinson’s disease and multiple system atrophy brains. Brain 141, 2419–2431 [DOI] [PubMed] [Google Scholar]
- 3.Krishnan S., Chi E. Y., Wood S. J., Kendrick B. S., Li C., Garzon-Rodriguez W., Wypych J., Randolph T. W., Narhi L. O., Biere A. L., Citron M., Carpenter J. F. (2003) Oxidative dimer formation is the critical rate-limiting step for Parkinson’s disease alpha-synuclein fibrillogenesis. Biochemistry 42, 829–837 [DOI] [PubMed] [Google Scholar]
- 4.Roostaee A., Beaudoin S., Staskevicius A., Roucou X. (2013) Aggregation and neurotoxicity of recombinant α-synuclein aggregates initiated by dimerization. Mol. Neurodegener. 8, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flagmeier P., Meisl G., Vendruscolo M., Knowles T. P., Dobson C. M., Buell A. K., Galvagnion C. (2016) Mutations associated with familial Parkinson’s disease alter the initiation and amplification steps of α-synuclein aggregation. Proc. Natl. Acad. Sci. USA 113, 10328–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Steiner J. A., Quansah E., Brundin P. (2018) The concept of alpha-synuclein as a prion-like protein: ten years after. Cell Tissue Res. 373, 161–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giasson B. I., Duda J. E., Quinn S. M., Zhang B., Trojanowski J. Q., Lee V. M. (2002) Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron 34, 521–533 [DOI] [PubMed] [Google Scholar]
- 8.Mougenot A. L., Nicot S., Bencsik A., Morignat E., Verchère J., Lakhdar L., Legastelois S., Baron T. (2012) Prion-like acceleration of a synucleinopathy in a transgenic mouse model. Neurobiol. Aging 33, 2225–2228 [DOI] [PubMed] [Google Scholar]
- 9.Luk K. C., Kehm V. M., Zhang B., O’Brien P., Trojanowski J. Q., Lee V. M. (2012) Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 209, 975–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prusiner S. B., Woerman A. L., Mordes D. A., Watts J. C., Rampersaud R., Berry D. B., Patel S., Oehler A., Lowe J. K., Kravitz S. N., Geschwind D. H., Glidden D. V., Halliday G. M., Middleton L. T., Gentleman S. M., Grinberg L. T., Giles K. (2015) Evidence for α-synuclein prions causing multiple system atrophy in humans with parkinsonism. Proc. Natl. Acad. Sci. USA 112, E5308–E5317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sargent D., Verchère J., Lazizzera C., Gaillard D., Lakhdar L., Streichenberger N., Morignat E., Bétemps D., Baron T. (2017) ‘Prion-like’ propagation of the synucleinopathy of M83 transgenic mice depends on the mouse genotype and type of inoculum. J. Neurochem. 143, 126–135 [DOI] [PubMed] [Google Scholar]
- 12.Woerman A. L., Kazmi S. A., Patel S., Freyman Y., Oehler A., Aoyagi A., Mordes D. A., Halliday G. M., Middleton L. T., Gentleman S. M., Olson S. H., Prusiner S. B. (2018) MSA prions exhibit remarkable stability and resistance to inactivation. Acta Neuropathol. 135, 49–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ayers J. I., Brooks M. M., Rutherford N. J., Howard J. K., Sorrentino Z. A., Riffe C. J., Giasson B. I. (2017) Robust central nervous system pathology in transgenic mice following peripheral injection of α-synuclein fibrils. J. Virol. 91, e02095-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breid S., Bernis M. E., Babila J. T., Garza M. C., Wille H., Tamgüney G. (2016) Neuroinvasion of α-synuclein prionoids after intraperitoneal and intraglossal inoculation. J. Virol. 90, 9182–9193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rutherford N. J., Dhillon J. S., Riffe C. J., Howard J. K., Brooks M., Giasson B. I. (2017) Comparison of the in vivo induction and transmission of α-synuclein pathology by mutant α-synuclein fibril seeds in transgenic mice. Hum. Mol. Genet. 26, 4906–4915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sacino A. N., Brooks M., Thomas M. A., McKinney A. B., Lee S., Regenhardt R. W., McGarvey N. H., Ayers J. I., Notterpek L., Borchelt D. R., Golde T. E., Giasson B. I. (2014) Intramuscular injection of α-synuclein induces CNS α-synuclein pathology and a rapid-onset motor phenotype in transgenic mice. Proc. Natl. Acad. Sci. USA 111, 10732–10737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Watts J. C., Giles K., Oehler A., Middleton L., Dexter D. T., Gentleman S. M., DeArmond S. J., Prusiner S. B. (2013) Transmission of multiple system atrophy prions to transgenic mice. Proc. Natl. Acad. Sci. USA 110, 19555–19560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sacino A. N., Brooks M., Thomas M. A., McKinney A. B., McGarvey N. H., Rutherford N. J., Ceballos-Diaz C., Robertson J., Golde T. E., Giasson B. I. (2014) Amyloidogenic α-synuclein seeds do not invariably induce rapid, widespread pathology in mice. Acta Neuropathol. 127, 645–665; erratum: 128, 897–898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ayers J. I., Riffe C. J., Sorrentino Z. A., Diamond J., Fagerli E., Brooks M., Galaleldeen A., Hart P. J., Giasson B. I. (2018) Localized induction of wild-type and mutant alpha-synuclein aggregation reveals propagation along neuroanatomical tracts. J. Virol. 92, e00586-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bétemps D., Verchère J., Brot S., Morignat E., Bousset L., Gaillard D., Lakhdar L., Melki R., Baron T. (2014) Alpha-synuclein spreading in M83 mice brain revealed by detection of pathological α-synuclein by enhanced ELISA. Acta Neuropathol. Commun. 2, 29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Riek R., Eisenberg D. S. (2016) The activities of amyloids from a structural perspective. Nature 539, 227–235 [DOI] [PubMed] [Google Scholar]
- 22.Castilla J., Saá P., Hetz C., Soto C. (2005) In vitro generation of infectious scrapie prions. Cell 121, 195–206 [DOI] [PubMed] [Google Scholar]
- 23.Saborio G. P., Permanne B., Soto C. (2001) Sensitive detection of pathological prion protein by cyclic amplification of protein misfolding. Nature 411, 810–813 [DOI] [PubMed] [Google Scholar]
- 24.Bougard D., Brandel J. P., Bélondrade M., Béringue V., Segarra C., Fleury H., Laplanche J. L., Mayran C., Nicot S., Green A., Welaratne A., Narbey D., Fournier-Wirth C., Knight R., Will R., Tiberghien P., Haïk S., Coste J. (2016) Detection of prions in the plasma of presymptomatic and symptomatic patients with variant Creutzfeldt-Jakob disease. Sci. Transl. Med. 8, 370ra182 [DOI] [PubMed] [Google Scholar]
- 25.Herva M. E., Zibaee S., Fraser G., Barker R. A., Goedert M., Spillantini M. G. (2014) Anti-amyloid compounds inhibit α-synuclein aggregation induced by protein misfolding cyclic amplification (PMCA). J. Biol. Chem. 289, 11897–11905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fairfoul G., McGuire L. I., Pal S., Ironside J. W., Neumann J., Christie S., Joachim C., Esiri M., Evetts S. G., Rolinski M., Baig F., Ruffmann C., Wade-Martins R., Hu M. T., Parkkinen L., Green A. J. (2016) Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 3, 812–818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shahnawaz M., Tokuda T., Waragai M., Mendez N., Ishii R., Trenkwalder C., Mollenhauer B., Soto C. (2017) Development of a biochemical diagnosis of Parkinson disease by detection of α-synuclein misfolded aggregates in cerebrospinal fluid. JAMA Neurol. 74, 163–172 [DOI] [PubMed] [Google Scholar]
- 28.Jung B. C., Lim Y. J., Bae E. J., Lee J. S., Choi M. S., Lee M. K., Lee H. J., Kim Y. S., Lee S. J. (2017) Amplification of distinct α-synuclein fibril conformers through protein misfolding cyclic amplification. Exp. Mol. Med. 49, e314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Becker K., Wang X., Vander Stel K., Chu Y., Kordower J., Ma J. (2018) Detecting alpha synuclein seeding activity in formaldehyde-fixed MSA patient tissue by PMCA. Mol. Neurobiol. 55, 8728–8737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Paciotti S., Bellomo G., Gatticchi L., Parnetti L. (2018) Are we ready for detecting α-synuclein prone to aggregation in patients? The case of “protein-misfolding cyclic amplification” and “real-time quaking-induced conversion” as diagnostic tools. Front. Neurol. 9, 415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masliah E., Rockenstein E., Veinbergs I., Mallory M., Hashimoto M., Takeda A., Sagara Y., Sisk A., Mucke L. (2000) Dopaminergic loss and inclusion body formation in alpha-synuclein mice: implications for neurodegenerative disorders. Science 287, 1265–1269 [DOI] [PubMed] [Google Scholar]
- 32.Specht C. G., Schoepfer R. (2001) Deletion of the alpha-synuclein locus in a subpopulation of C57BL/6J inbred mice. BMC Neurosci. 2, 11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bousset L., Pieri L., Ruiz-Arlandis G., Gath J., Jensen P. H., Habenstein B., Madiona K., Olieric V., Böckmann A., Meier B. H., Melki R. (2013) Structural and functional characterization of two alpha-synuclein strains. Nat. Commun. 4, 2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bétemps D., Verchère J., Mougenot A. L., Lachmann I., Morignat E., Antier E., Lakhdar L., Legastelois S., Baron T. (2015) Detection of disease-associated α-synuclein by enhanced ELISA in the brain of transgenic mice overexpressing human A53T mutated α-synuclein. J. Vis. Exp. e52752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luk K. C., Song C., O’Brien P., Stieber A., Branch J. R., Brunden K. R., Trojanowski J. Q., Lee V. M. (2009) Exogenous alpha-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA 106, 20051–20056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Conway K. A., Harper J. D., Lansbury P. T. (1998) Accelerated in vitro fibril formation by a mutant alpha-synuclein linked to early-onset Parkinson disease. Nat. Med. 4, 1318–1320 [DOI] [PubMed] [Google Scholar]
- 37.Kang L., Wu K. P., Vendruscolo M., Baum J. (2011) The A53T mutation is key in defining the differences in the aggregation kinetics of human and mouse α-synuclein. J. Am. Chem. Soc. 133, 13465–13470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ugalde C. L., Lawson V. A., Finkelstein D. I., Hill A. F. (2019) The role of lipids in α-synuclein misfolding and neurotoxicity. J. Biol. Chem. 294, 9016–9028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bencsik A., Muselli L., Leboidre M., Lakhdar L., Baron T. (2014) Early and persistent expression of phosphorylated α-synuclein in the enteric nervous system of A53T mutant human α-synuclein transgenic mice. J. Neuropathol. Exp. Neurol. 73, 1144–1151 [DOI] [PubMed] [Google Scholar]
- 40.Tsika E., Moysidou M., Guo J., Cushman M., Gannon P., Sandaltzopoulos R., Giasson B. I., Krainc D., Ischiropoulos H., Mazzulli J. R. (2010) Distinct region-specific alpha-synuclein oligomers in A53T transgenic mice: implications for neurodegeneration. J. Neurosci. 30, 3409–3418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dhillon J. S., Trejo-Lopez J. A., Riffe C., Levites Y., Sacino A. N., Borchelt D. R., Yachnis A. Y., Giasson B. I. (2019) Comparative analyses of the in vivo induction and transmission of α-synuclein pathology in transgenic mice by MSA brain lysate and recombinant α-synuclein fibrils. Acta Neuropathol. Commun. 7, 80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lohmann S., Bernis M. E., Tachu B. J., Ziemski A., Grigoletto J., Tamgüney G. (2019) Oral and intravenous transmission of α-synuclein fibrils to mice. [E-pub ahead of print] Acta Neuropathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Candelise N., Schmitz M., Llorens F., Villar-Piqué A., Cramm M., Thom T., da Silva Correia S. M., da Cunha J. E. G., Möbius W., Outeiro T. F., Álvarez V. G., Banchelli M., D’Andrea C., de Angelis M., Zafar S., Rabano A., Matteini P., Zerr I. (2019) Seeding variability of different alpha synuclein strains in synucleinopathies. Ann. Neurol. 85, 691–703 [DOI] [PubMed] [Google Scholar]
- 44.Fenyi A., Leclair-Visonneau L., Clairembault T., Coron E., Neunlist M., Melki R., Derkinderen P., Bousset L. (2019) Detection of alpha-synuclein aggregates in gastrointestinal biopsies by protein misfolding cyclic amplification. Neurobiol. Dis. 129, 38–43 [DOI] [PubMed] [Google Scholar]
- 45.Froula J. M., Castellana-Cruz M., Anabtawi N. M., Camino J. D., Chen S. W., Thrasher D. R., Freire J., Yazdi A. A., Fleming S., Dobson C. M., Kumita J. R., Cremades N., Volpicelli-Daley L. A. (2019) Defining α-synuclein species responsible for Parkinson’s disease phenotypes in mice. J. Biol. Chem. 294, 10392–10406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.do Carmo Ferreira N., Caughey B. (2018) Cell-free prion protein conversion assays in screening for anti-prion drug candidates. Curr. Opin. Pharmacol. 44, 1–7 [DOI] [PubMed] [Google Scholar]
- 47.Peng C., Gathagan R. J., Covell D. J., Medellin C., Stieber A., Robinson J. L., Zhang B., Pitkin R. M., Olufemi M. F., Luk K. C., Trojanowski J. Q., Lee V. M. (2018) Cellular milieu imparts distinct pathological α-synuclein strains in α-synucleinopathies. Nature 557, 558–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peelaerts W., Bousset L., Van der Perren A., Moskalyuk A., Pulizzi R., Giugliano M., Van den Haute C., Melki R., Baekelandt V. (2015) α-Synuclein strains cause distinct synucleinopathies after local and systemic administration. Nature 522, 340–344 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.