Abstract
Brain microvascular endothelial cells (BMECs) interact with astrocytes and pericytes to form the blood–brain barrier (BBB). Their compromised function alters the BBB integrity, which is associated with early events in the pathogenesis of cancer, neurodegenerative diseases, and epilepsy. Interestingly, these conditions also induce the expression of heat shock proteins (HSPs). Here we review the contribution of major HSP families to BMEC and BBB function. Although investigators mainly report protective effects of HSPs in brain, contrasted results were obtained in BMEC, which depend both on the HSP and on its location, intra- or extracellular. The therapeutic potential of HSPs must be scrupulously analyzed before targeting them in patients to reduce the progression of brain lesions and improve neurologic outcomes in the long term.—Thuringer, D., Garrido, C. Molecular chaperones in the brain endothelial barrier: neurotoxicity or neuroprotection?
Keywords: heat shock protein, brain injury, microvascular endothelial cells, extracellular vesicles
In response to various exogenous stresses, brain cells activate endogenous stress responses. The most general answer may be heat shock response (HSR). HSR is triggered by a wide variety of stress, including oxidative and metabolic stress, ischemia and reperfusion, gliomas, and neurodegenerative diseases. HSR is characterized by an almost exclusive synthesis of heat shock protein (HSP) families. HSPs are chaperones or cochaperones whose expression is intended to attenuate the accumulation of denatured cytoplasmic proteins known to form in response to the stressors listed above (1–4). The main families of chaperones are classified according to their MW: Hsp40 (DNAJA, B), Hsp60 (HSPD1), Hsp70 (HSPA1A, B), Hsp90α, β (HSP90AA1, HSP90B1), Hsp110 (HSPH1), and small HSPs to which belong Hsp27 (HSPB1) and αB-crystalline (HSPB5) (5, 6). Most chaperones require the help of cochaperones to increase protein association rates, ATPase activity, and nucleotide exchange. Hsp60, Hsp70, Hsp90, and Hsp110 each use ATP binding and hydrolysis to induce binding, folding and release cycles of a protein (Fig. 1A). The small HSPs, which are ATP-independent, work in refolding reactions by forming large oligomers capable of storing proteins in an aggregation independent state. Most HSPs are up-regulated under the same stress conditions but they perform different functions.
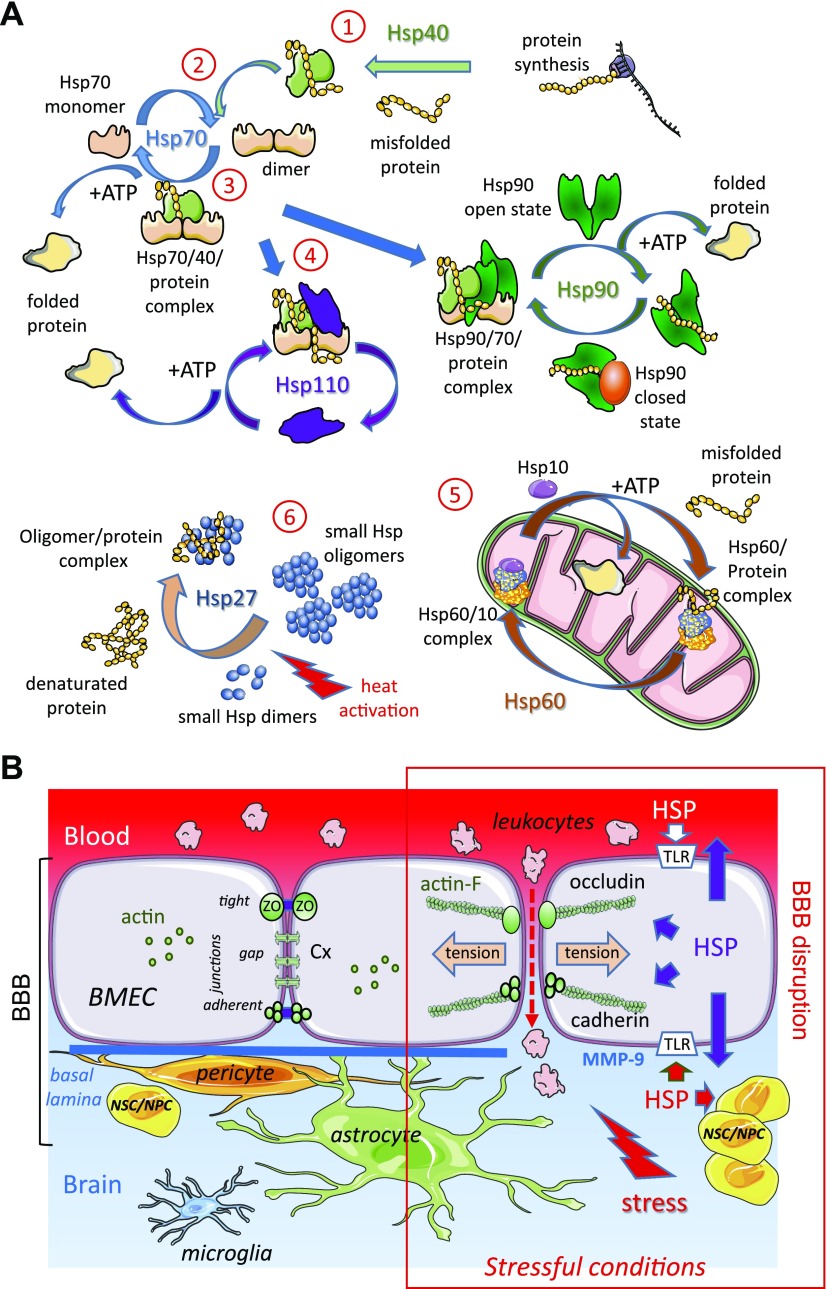
Figure 1.
The HSP chaperone system at the BBB level. A) Collaboration between HSPs in protein folding. Under nonstressful conditions, HSPs bind to newly synthesized polypeptides and facilitate their folding (folded protein), whereas under stressful conditions they recognize denatured proteins and prevent their premature degradation. 1) Hsp40 transiently associates with an altered or misfolded protein to deliver it to Hsp70. 2) Hsp40 binds to Hsp70 and promotes its ATPase activity, thereby generating ADP-linked Hsp70, which stably interacts with the misfolded protein. After nucleotide exchange (replacement of ADP with ATP), the protein is released from Hsp70 and leaves the chaperone system as a correctly folded protein. 3) During stress, Hsp70, in its ADP binding form, encompasses denatured protein. 4) The renaturation of the protein by Hsp70 requires the participation of cochaperones such as Hsp110 or Hsp90. In all cases, the replacement of ADP with ATP is necessary to release the correctly folded protein from these HSP complexes. 5) Some proteins require a subsequent folding step by a group of chaperones having a common structure, chaperonins: for example, Hsp60/Hsp10 present in mitochondria. 6) Under conditions where the system is saturated by a massive influx of proteins to replicate, a transient storage of these is carried out by oligomeric structures formed by Hsp27. These reservoirs of folding intermediates would then direct the proteins to the different protein steps of folding machinery. B) Interactions between astrocytes, pericytes, BMECs, and NSCs/NPCs ensure adequate cerebral blood flow. BMECs are tightly connected through transmembrane proteins present in the tight junctions (occludin), gap junctions (connexin, Cx), and adherens junctions (cadherin). These junction structures interact with cytosolic adaptor proteins that link junctions to the actin cytoskeleton, leading dynamic structures that respond to the local microenvironment. In stressful conditions, HSPs are overexpressed in and released by all cells in the brain. HSPs modulate the expression and/or activity of MMPs involved in basal lamina degradation; for example, MMP-9, which contributes to the disruption of BBB causing cerebral hemorrhage, is decreased by Hsp70 but increased by Hsp90α. Extracellular HSPs bind to TLRs and stimulate specific signaling pathways in the different cell types. In BMEC, these events result in actin polymerization (actin-F), actomyosin contraction (tension), junctional remodeling, and disruption of intercellular contacts, leading to BBB permeability increase. They also signal to adult niches of NSCs/NPCs to increase proliferation and differentiation in healthy or diseased cells. These processes allow transendothelial migration of leukocytes and can be induced by leukocyte secretions themselves. For example, Hsp70 released by M2 monocytes disrupts interendothelial communication by decreasing endothelial expression of Cx43 (67).
Defining the role of HSPs in the normal and pathologic brain is complicated by the diversity of both cell types and modes of cell-to-cell communication (7). For instance, overexpression of Hsp70 is generally associated with increased neuronal resistance to cerebral ischemia and better recovery from it (8). Endothelial cells, in particular, generate a stress response with induction of Hsp70 after cerebral ischemia (9). However, this Hsp70 response coincides with the death of endothelial cells and pericytes (10). More recently, extracellular vesicles (EVs) released from endothelial cells have been reported to contain Hsp70 and directly protect neurons from ischemia/reperfusion injury by activating their growth, migration, and invasion and by inhibiting their apoptosis (11). EVs carry not only HSPs but also small noncoding microRNAs (miRs) potentially related to the expression of hsp genes in the recipient cell (12–15). This review will attempt to answer the following questions: Can we define a specific role of each Hsp in the blood–brain barrier (BBB)? Are molecular chaperones toxic or protective?
HSP EXPRESSION AT THE BBB LEVEL
The BBB is a continuous monolayer of brain microvascular endothelial cells (BMECs) and is sheathed with pericytes and perivascular astrocytes end-feet (Fig. 1B). In addition to pericytes, undifferentiated or differentiated neural stem cells (NSCs)/neural progenitor cells (NPCs) also contribute to the induction and maintenance of the BBB (16). Transdifferentiation of NSCs/NPCs into BMEC has been described, and conversely, BMECs may generate NSCs/NPCs (17). The microvascular network is very close to each neuron (∼20 µm of a microvessel) promoting the transmission of BMEC secretory products to neurons and vice versa (18).
During hyperthermia (higher than 40°C), the victims suffer from cognitive deficits and dementia, associated with BBB rupture, microhemorrhage, cerebral edema, and cell death (19) as also observed in Alzheimer’s and Parkinson’s diseases, schizophrenia, or ischemia-associated damage (20, 21). Very few reports have focused on BMEC’s HSR. Exposure of BMEC cultures to thermal shock results in both tight junction disassembly and overexpression of Hsp70, Hsp90, and Hsp110 (22). Recovery to 37°C induces overexpression of Hsp47 in the first hours. This latter expression was concomitant with the reassembly of tight junctions between BMEC in vitro. Such an increase was also reported in gliomas, during tumor angiogenesis in vivo, where the newly formed vessels have morphologic and functional abnormalities (leaks and anastomoses) related to the disassembly of tight junctions between BMECs. By expressing Hsp47, pericytes promote angiogenesis by producing the basal lamina of microvessels but do not induce re-expression of tight junction between BMEC and thus the establishment of the BBB (23, 24). Interestingly, sequential expression of HSPs has also been reported in primary vascular endothelial cell cultures exposed to microgravity as observed aboard an orbital station (25). The adaptive endothelial response resulted in early up-regulation of Hsp70, which was progressively replaced by the increase of other stress proteins, including Hsp27. The early increase in Hsp70 appeared crucial in this adaptation process because its inhibition (by small interfering RNA) induced cell death. Thus, the sequential expression of HSPs seems first to quickly adapt the cells to stress conditions, then to establish a new homeostatic status to preserve the viability and function of cells. It should be noted that a mild hyperthermia (38.5°C) increased NSCs/NPCs proliferation and neuronal differentiation through an initial and brief increase in Hsp70 followed by the more sustained increase in Hsp27, whereas no significant effect was reported on Hsp90 expression (26). In contrast, a mild hypothermia after a heat stress decreases Hsp70 expression in NPCs, as reported during the differentiation of NSCs/NPCs in brain cells (27).
Overexpression of Hsp27 in BMECs preserves the integrity of BBB by suppressing aberrant actin polymerization, stress fiber formation, and translocation of junctional proteins (28). Structural aberrations in BMECs have been described during ischemic stroke and would be responsible for the early rupture of BBB, occurring within 30–60 min following reperfusion after focal ischemia (29). It is worth noting that Hsp70 was overexpressed in a 300-μm-wide area surrounding the BBB disturbance zone. Matrix metalloproteinases (MMPs) involved in basal lamina degradation can lead to cerebral hemorrhage. Hsp70 expression decreases MMP expression and proteolytic activity in a traumatic brain injury model (30). As a result, cerebral hemorrhage decreases and neurologic function improves. Conversely, Hsp70 deficiency results in increased lesions, brain hemorrhages, and MMP activity. Studies on brain tissue and serum in patients with ischemic stroke demonstrate that MMP9 contributes to the disruption of BBB (31). Hsp90, the isoform α, is overexpressed and correlated with MMP9 levels in patients, and its inhibition by 17-dimethylaminoethylamino-17-demethoxygeldanamycin strongly attenuates the BBB disruption (32). The pharmacological inhibition of Hsp90 resulted in up-regulation of Hsp70 and Hsp40, which could control the expression of several synaptic proteins but could also drive the misfolded protein to proteasome degradation (33–36). Another chaperone involved is Hsp110, which interacts with Hsp70 and increases its ATPase activity (37, 38). Hsp110 is known to suppress the aggregation and folding of proteins and to protect them from the adverse effects of various stresses. Nevertheless, its physiologic function seems more complex. The absence of hsp110 gene in mice is associated with hyperphosphorylation of tau microtubule protein and accumulation of β-amyloid (Aβ) plaques, as described in Alzheimer’s disease (AD). The activity of Hsp110 is also present at the synaptic level and is recognized for its protection against the aggregation of α-synuclein in Parkinson’s disease (37). Thus, Hsp110 is critical in various neurodegenerative diseases called tauopathies (39). Unlike Hsp110, Hsp90 facilitates the aberrant aggregation of tau, and increases tau toxicity and Aβ metabolism (40). In addition, by modifying the structure of eNOS, Hsp90 affects the functions of eNOS in particular by changing its affinity to cofactors such as Ca2+ and l-arginine, and, subsequently, NO generation capacity (41). This results in phosphorylation of tau (p-tau) in neuronal tissue (42). Uncoupled eNOS in BMECs produce an excess of superoxides that react with NO produced by microglia or astrocytes to form peroxynitrite, in turn increasing the accumulation of p-tau in astrocytes (43). These Hsp90 mechanisms link endothelial dysfunction to the pathogenesis of AD. Hsp90 inhibitors have proven their efficiency in reducing tau pathology (40). However, these Hsp-modulating chemicals may affect cellular targets in cells other than the chaperones themselves, such as the many Hsp90 client proteins, for example. Some work reveals intriguing roles of Hsp90 in transcription regulation (44), but its contribution in tauopathies has proven to go beyond individual transcription factors (45).
When expressed on the endothelial cell surface, HSPs constitute possible autoantigens in the vessel wall. Such is the case of Hsp60. This chaperone participates in the folding of mitochondrial proteins and facilitates the proteolytic degradation of denatured proteins. Hsp60 is also detected on the membrane surface of stressed endothelial cells, and the antibody against Hsp60 induces endothelial cytotoxicity especially in cerebrovascular disease (46). Mutations in the Hsp60 gene have been associated with neurodegenerative disorders (47). An abnormal level of Hsp60 expression and subcellular localization was also detected in inflammatory diseases and gliomas (48). Moreover, extracellular Hsp60 increased secretion of proinflammatory factors by microglia, causing neuronal death (49–52). Concerning Hsp70, it has been reported to be overexpressed in primary glioblastoma (GBM) cells, whereas neighboring endothelial cells, isolated from GBM tissues, did not (53). More recently, Hsp90 has been reported to mediate the fusion of multivesicular bodies (MVBs) with the plasma membrane, leading to release of EVs (54). This activity requires an amphipathic α-helix that becomes exposed when the protein dimer is open. The membrane-binding amphipathic helix is a common motif encountered in various peptides and proteins such as Hsp90 (55, 56). Hence, by controlling the open vs. closed state of the Hsp90 dimer, cells can regulate MVB fate: open Hsp90 promotes the vesicular release, whereas closed Hsp90 blocks the process (54).
MODULATION OF BBB FUNCTION BY EXTRACELLULAR HSP
HSPs are released outside the cells where they display a function different from that of intracellular chaperone (Fig. 2A). Extracellular HSPs could be alert stress signals for other cells to prevent the spread of insult and promote repair (57, 58). Plasma membrane translocation, lipid vesicle–associated release, and passive postdeath release of necrotic cells have been proposed to explain the presence of HSPs in the extracellular medium (59). Extracellular HSPs take several forms: membrane-bound or not, associated with client protein or not (60). The diversity of their forms is probably associated with various interactions with cells, both in terms of target cell types and activated signaling pathways (59, 61, 62).
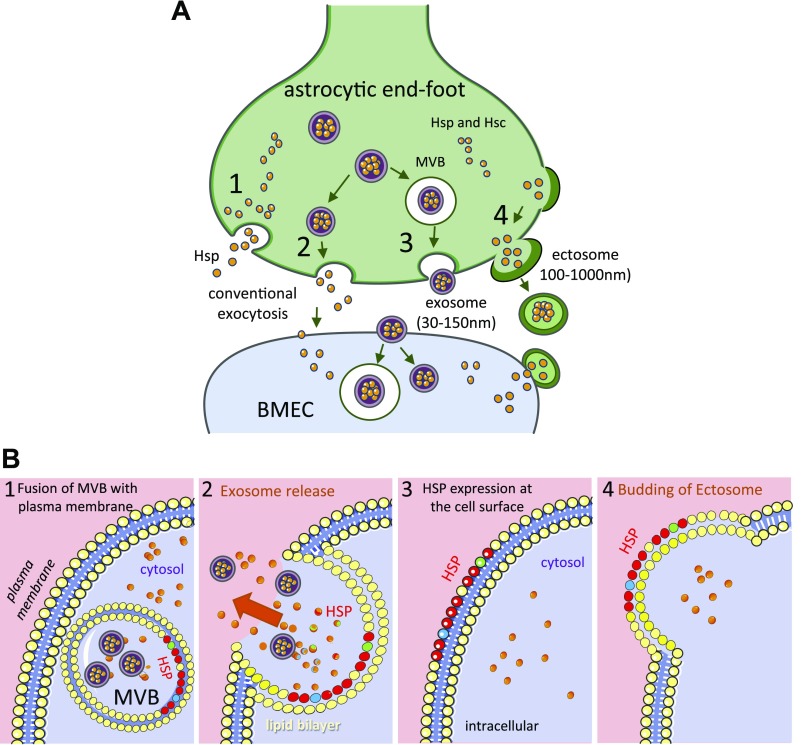
Figure 2.
Expression of HSPs outside the cell. A) Mechanisms for the cell release and uptake of HSPs. 1) Conventional exocytosis of Hsp (the stress-induced form) or heat-shock cognate (Hsc; the constitutively synthesized form) in the extracellular space, so that they are present in the extracellular fluid. 2) An unknown mechanism allows Hsp/c to concentrate in the small vesicles (exosome). 3) Exocytosis of the MVB (exosomes within a larger vesicle) resulting from autophagy. 4).Conventional apocrine secretion, in which small vesicles (ectosome) containing cytoplasmic constituents bud off the cell surface. The released vesicles (ectosome and exosome) can then interact with recipient cells (BMEC) in 2 hypothetical ways: either to fuse with the plasma membrane and release its contents or to be phagocytosed, forming another MVB. Soluble extracellular Hsp interacts with plasma membrane receptors or is internalized into the cytoplasm of the recipient cell by endocytosis, where it is free to interact with other cytoplasmic components. B) Hypothetical HSP expression model at the endothelial cell surface that constitute possible autoantigens in the vessel wall. Membrane associations and lipid interactions are reported for small HSPs, Hsp60, Hsp70, and Hsp90. 1) Excess HSPs are routed to MVBs where HSPs are linked to the inner leaflet of the vesicular membrane. The mechanisms of HSPs supply of the endolysosomal system are not known, but would involve mechanisms of autophagy and direct membrane crossing. 2) Fusion of MVB with the plasma membrane and release of exosomes containing HSPs. 3) HSPs are exposed to the cell surface (131). 4) By a conventional apocrine secretion, HSPs are found on the outer leaflet of ectosomes. Thus, HSPs could be expressed on the surface of the plasma membrane and ectosomes (104).
Receptor-mediated signaling by free extracellular HSP has been controversial because of the fact that recombinant preparations of HSP were contaminated with bacterial products (i.e., LPS, flagellins, lipoproteins, and DNA). These contaminants can activate cells via pattern recognition receptors, including TLRs, which result in activation of NF-κB and high production of various chemokines (63), similar to the response observed with HSP (64). Attention has been given to possible endotoxins and, more specifically, to LPS contamination, given the implications of TLR2 and/or TLR4 as suspected HSP receptors. The debate over possible endotoxin contamination is due to the ability of HSP to sequester LPS and protect it from the methods used for its detection or inactivation (i.e., proteinase or boiling). Nevertheless, the recent use of recombinant HSPs produced by mammalian cells has allowed the emergence of the notion that surface receptors are indeed capable of recognizing extracellular HSPs or even fragments of HSPs. The first receptor recognized is CD91 (or LDL receptor–related protein), which binds Hsp60, Hsp70, and gp96 (61, 65). Despite earlier controversy, TLR2 and TLR4 are still considered candidate receptors for Hsp60, Hsp70, Hsp90α, and gp96 (66–71). TLR3, which specifically recognizes double-strand RNA derived from virus and dead cells, is stimulated by Hsp27 (72, 73). These studies ascribed new functions to HSPs. For example, extracellular Hsp90α stimulates wound healing and metastasis of GBM cells (66). Extracellular Hsp27 also influences the behavior of surrounding cells (74). At the microvascular level, this extracellular chaperone promotes tumor angiogenesis by increasing VEGF in endothelial cells (72). Extracellular HSPs have been reported as pro- or anti-inflammatory (1). Surface-bound Hsp70 specifically activates NK cells, whereas Hsp70 when released into the extracellular medium exerts immunoregulatory effects, including up-regulation of adhesion molecules and release of cytokines and chemokines [a process called “chaperokine” activity of HSP (61, 75, 76)]. Hsp70, exogenously added or released by circulating monocytes, blocks the gap-junctional coupling between endothelial cells, reducing the transendothelial migration of monocytes (67). It is well known that the immune system contributes to neuronal cell death because of microglial activation, leukocyte recruitment, and cytokine secretion (77). The involvement of extracellular HSPs could be seen as an attempt to correct the inflammatory condition. Furthermore, extracellular Hsp70 interacts with Alzheimer’s Aβ peptides, arresting their oligomerization in fibers and plaques that are neurotoxic (36, 78–80). Thus, the final outcome seems to depend on the microenvironment.
HSPs are transferred from cell to cell via cell-derived EVs. EVs are released from all types of brain cells, healthy or diseased (81), and are bidirectionally transported through the BBB (7, 82–85). Depending on their subcellular origin, EVs are classified into 2 main categories: ectosomes (also called microparticles) that are released after budding from the plasma membrane as well as exosomes, which are produced inside the MVBs and released after fusion of the MVBs with the plasma membrane (86, 87). It should be noted that apoptotic bodies (∼800–5000 nm diameter) that are released from apoptotic cells exhibit ectosome characteristics but are rarely described in intracellular communication because they would be rapidly destroyed by phagocytes (87). Little is known about EVs derived from BMECs (12, 85, 88–98). Secreted by BMECs, EVs are heterogeneous in size and include ectosomes (∼150–1000 nm diameter) and exosomes (∼20–150 nm diameter) (99, 100). Their protein profiles reveal that ectosomes and exosomes share about one-third of their proteins (88). The remaining proteins are specific to the EV type (e.g., 544 proteins in ectosomes and 209 proteins in exosomes). HSPs are present in both types of EVs. Constitutively expressed heat shock members [heat-shock cognate (Hsc)] are present in almost all intracellular compartments. It is therefore not surprising to find them in both types of EVs. Inducible HSP forms (Hsp) are, however, described as a result of cellular stress so that their presence in EVs may reflect the state of stress of the secretory cell. It is easy to imagine that HSPs contribute to the correct folding and assembly of the polypeptide of newly synthesized proteins and are expected to play cytoprotective roles when transferred to the target cell. It has been reported that EVs isolated from BMEC under normal or ischemic conditions have diametrically opposite effects (101). Injected in a mouse model of transient cerebral ischemia, the normal EVs decreased the permeability of BBB by overexpressing tight junction proteins in BMEC, and improved local cerebral blood flow, reducing the volume of the infarcted area (101). In contrast, ischemic EVs favored the propagation of the insult. What would be the role of HSPs in this network of organelles? In the tumor context, Hsp60, Hsp70, and Hsp90 are secreted by healthy and cancerous cells via EVs but have opposite effects, suppressors or stimulators, on tumor cell growth (13, 102–104). Hsp27, αB-crystalline, and Hsp20 are also exported by exosomes with neuroprotective effects (105).
HSPs are also present in vesicular membranes (Fig. 2B). Their membrane location is difficult to interpret (106). For instance, Hsp60 is expressed on the surface of exosomes secreted by tumor cells and its depletion in in vivo models of GBM leads to the suppression of intracranial tumors (104). What are the interactive partners of HSPs at the membrane level? Rab GTP hydrolase [i.e., Rab1 involved in endoplasmic reticulum (ER)-to-Golgi and Rab3A transport involved in synaptic vesicle fusion] require Hsp90. This observation has led the authors to propose that Hsp90 could serve as a general regulator for the recycling of Rab GTP hydrolase in the exocytic and endocytic trafficking pathways involved in cell signaling and proliferation (107). The vesicle trafficking between Golgi stacks is dependent on the ATPase function of Hsp90 and can be inhibited by Hsp90-specific drugs (108). More recently, Hsp90 has been reported to release exosomes (54).
HSP OVEREXPRESSION: NEUROTOXIC OR NEUROPROTECTIVE?
Microvascular endothelial responses occurring after acute neurologic insults usually reduce pathogenic processes in the brain (28, 109, 110), although BMEC could also synthesize neurotoxic proteins, such as thrombin in AD (111). Are they related to specific HSP expression? Very few studies have analyzed the contribution of HSPs to cerebral endothelial functions. Nevertheless, we know that the suppression of some HSPs exacerbates neuronal cell death or, conversely, improves recovery (Table 1).
TABLE 1.
HSPs in CNS diseases
| Type | Function | Biologic activity | Pathologic | Preventive | AUC (au) | Reference |
|---|---|---|---|---|---|---|
| Hsp27 | Antiaggregation | Sustained angiogenesis (TLR3) VEGF release | GBM growth | 72, 147 | ||
| Antioxidative | Anti-inflammatory effect | Cortical spreading depression | 148, 149 | |||
| Antiapoptotic | BBB integrity (anti-actin polymerization) | I/R-induced neurovascular injury | 28 | |||
| Thermotolerance | Resistance to chemotherapeutics | GBM growth | 0.871 | 150, 151 | ||
| Prevent tau accumulation | Neurodegeneration (AD, PD, MS) | 152, 153 | ||||
| Neurite outgrowth, NSC/NPC differentiation | Neurodegeneration (AD, PD, MS) | 26, 153 | ||||
| Hsp40 | Protein folding | Hsp40/Hsp70 complex | Meningioma | 154 | ||
| Cochaperone of | Release of neurodegenerative proteins | AD, PD | 155, 156 | |||
| Hsp70 promoting | ||||||
| its ATPase activity | ||||||
| Hsp60 | Mitochondrial | Neuroinflammation (TLR4) | GBM, epilepsy, MS, ASD | 3, 157, 158 | ||
| Protein folding | Proliferation (mTOR pathway) | GBM | 158 | |||
| Hsp70 | Protein folding and | Neuro-inflammation (TLR4) | ASD | 0.987 | 159, 160 | |
| Membrane transport | Meningioma, GBM | 0.779 | 161 | |||
| Antiapoptotic | BBB disruption | ICH | 162 | |||
| Immunomodulatory | Transendothelial cell migration | ALS | 0.826 | 163 | ||
| Neuroinflammation (TLR2/TLR4) | Ischemic stroke | 68 | ||||
| Invasion, endocytosis | AD, glioma | 131 | ||||
| Anti-inflammatory and neuroprotective | AD, PD, HKD | 112, 115, 123 | ||||
| HSP90α | Folding of many | Cell proliferation | GBM | 164 | ||
| regulatory proteins | Migration (TLR4) | Cell invasion, metastasis | 66, 165 | |||
| Interaction with | Membrane deformation | Hyperthermia | 166 | |||
| signaling pathways | Fusion of MVBs with plasma membrane | Propagation (exosome) | 54 | |||
| Exosomal secretion | ||||||
| HSP110 | Protein | Reduce injury at the impact site | Traumatic brain injury | 167 | ||
| Disaggregation | Suppress cancer cell apoptosis | GBM growth | 169 | |||
| Stress tolerance | Prevent tau accumulation | Neurodegeneration (AD, PD, MS) | 168 | |||
| Increase levels of BDNF | Depression | 170 |
List of HSPs examined and their main chaperone functions, their biologic activity, including those interacting with TLRs, their involvement in CNS diseases (pathologic or preventive), and the AUC determined from biopsies fluid (plasma, cerebrospinal fluid, or both) that reflects the reliability of the HSP assay as biomarkers for early diagnosis in patients (3, 28, 54, 66, 68, 131, 147–170). ALS, amyotrophic lateral sclerosis; ASD, autism spectrum disorders; au., arbitrary units with values from 0 to 1; BDNF, brain-derived neurotrophic factor; HKD, Huntington’s and Kennedy’s diseases; ICH, intracerebral hemorrhage; IR, ischemia-reperfusion; MS, multiple sclerosis; PD, Parkinson’s disease.
The protective effects of brain HSPs have been reported in various models of brain diseases (2, 28, 112–114). For example, overexpression of Hsp70 appears to be anti-inflammatory and to play a neuroprotective role in neurodegenerative diseases (such as AD and Parkinson’s disease) (112, 115). Its beneficial effects may be due to both its chaperone role and its ability to protect neuronal cells against various types of toxic factors (116). Brain ischemia can be modeled in vitro by exposing cell cultures to oxygen and glucose deprivation or excitotoxin exposure (117). Cultured hippocampal cortical neurons from transgenic mice overexpressing Hsp70 were protected from excitotoxin exposure and oxygen and glucose deprivation (118). Isolated glial cultures of this same mouse strain were also resistant. Conversely, the down-regulation of Hsp70 with an antisense oligonucleotide aggravated the damage following thermal shock. Although Hsp70 transgenic mice are protected against myocardial ischemia, this is not always the case for cerebral ischemia (119). The reasons for these discrepancies may be due to differences in transgene expression in different organs, or to the varying susceptibilities of brain regions. Some groups have studied the permanent arterial occlusion in various strains of Hsp70 transgenic mice with contradictory results. These different results may be due to transgenic animal limitations, such as alterations in the development of other biochemical systems caused by overexpression of transgenes. The extent of Hsp70 expression could also be different between transgenic strains because different promoters have been used (120–122). After occlusion of the cerebral artery, overexpression of Hsp70 improves the survival of the striatal neurons only during the first 2 h following the aggression. Nevertheless, these observations confirm that HSPs protect neuronal cells by their chaperone function, possibly by preventing protein aggregation. This is also the case in polyglutamine diseases (Huntington’s and Kennedy’s disease, certain spinocerebellar ataxic disorders) (123). This heterogeneous group of neurodegenerative diseases is characterized by a cellular accumulation of glutamine aggregates, resulting in cell death (124). Overexpression of Hsp70 in spinocerebellar ataxia models has been reported to significantly reduce protein aggregation and cytotoxicity as well as to improve behavioral test scores (125).
However, overexpression of Hsp70 is not protective in all cases. If overexpression of Hsp70 protects hippocampal and cortical neurons against shock or thermal ischemia, it does not protect against the toxicity of glutamate or 3-nitropropionic acid (126) or apoptotic stimuli (127). Glial cultures derived from Hsp70-overexpressing transgenic mouse brains are resistant to hydrogen peroxide damage but not to other stresses (128). Interestingly, hippocampal neurons are more resistant to stress than cortical neurons. Thus, Hsp70 protects against certain types of brain lesions but not all, and its protective effects may be related to the nature and severity of the attack. HSPs can be pathogenic factors (3). Associated with cancer, there are “chaperonopathies by collaborationism” in which the involved chaperone improves the survival and growth of tumor cells by inhibiting apoptosis and the anti-tumor immune response or by promoting neoangiogenesis. There is evidence of a positive correlation between HSP levels and the progression of gliomas, suggesting the use of anti-HSP antibodies in anti-tumor vaccines. Nevertheless, studies involving a large number of patients are needed to clearly define the relationship between HSPs and the aggressiveness of tumors. The inhibition of Hsp90 offers a dual therapeutic approach in aging and neurodegenerative diseases (129): on the one hand, it increases the protein hyperphosphorylation and their subsequent aggregation by reducing the activity of aberrant proteins; on the other hand, it can protect against the toxicity of proteins via the induction of Hsp70 and other chaperones.
Some HSPs are used as biomarkers of brain-related diseases [see area under the curve (AUC) in Table 1], including early diagnosis of brain tumors (88, 130, 131). Cotransported with neurotoxic or misfolded proteins in EVs, these chaperones contribute to the spread of their neurotoxicity and to the pathogenesis of chronic neurodegenerative diseases (2, 14). There are concepts about the role of chaperones in the organelle network and their regulation by miRs (8). Indeed, EVs also contain small, noncoding miRs, which regulate many aspects of BMEC biology, such as angiogenesis, inflammation, and BBB function, with clinical consequences (132–138). Interestingly, endothelial EVs contain neprilysin, a zinc-dependent metalloprotease, also called Calla for Common Acute Lymphoblastic Leukemia Antigen (139). This enzyme cleaves peptide hormones, such as enkephalins, substance P, and neurotensin, and also degrades Aβ peptide (140). This emphasizes the therapeutic potential of endothelial EVs in BBB.
CONCLUDING REMARKS
The cerebral microenvironment includes many types of resident or infiltrated cells. Resident cells have received the most attention so far. Nevertheless, the influence of their microenvironment in the progression of cerebral pathologies remains poorly understood. An imbalance in the cellular production of HSPs contributes to various neuropathies. Whether the chaperone system is directly or indirectly involved in these pathogenic processes must be determined. There is sufficient evidence to encourage the development of therapeutic strategies targeting HSPs, either to block their activity if they promote the progression of the disease, or to improve their performance when they are protective (6, 116, 141). However, the design of this type of drug is hampered by the fact that HSPs are actually more than cytosolic chaperones and should be considered as important regulators of signaling pathways. Their membrane localization and the interactions they maintain with lipids are associated with the facilitation of endocytosis or the release of exosomes and antiapoptotic mechanisms in cancers, for example (81). Thus, the membrane-HSP mechanistic interactions must be decrypted to reduce the toxicity of drugs. Future studies should also take into account the neurogenic niches closely linked to BMECs. In the adult brain, NSCs/NPCs contribute, directly or as a source of proangiogenic factors, to the re-endothelialization of the BBB during brain injury (17, 142). The metabolic activity and function of these neural cells are tightly regulated by many systemic and local factors, such as as HSPs, and can generate cancerous endothelial cells or metastasis, spreading glioma (17, 143). Given the importance of the sequential expression of HSPs in stressed BMECs and in the differentiation of NSCs/NPCs, it will be essential to show which HSPs contribute to functional neurons, astrocytes, or oligodendrocytes, capable of repairing lesions in the adult brain or of inducing cerebral angiogenesis (144). The answer to these questions will be beneficial for the implementation of new therapeutic concepts based on biomaterials and the use of HSPs in the treatment of cerebrovascular diseases, brain trauma, neurodegeneration, brain tumors, or neuroinfection (15, 145, 146).
ACKNOWLEDGMENTS
The authors thank Ligue Nationale contre le Cancer (Laboratory of Excellence) and Centre National de la Recherche Scientifique (CNRS). This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no conflicts of interest.
Glossary
- Aβ
β amyloid
- AD
Alzheimer’s disease
- BBB
blood–brain barrier
- BMEC
brain microvascular endothelial cell
- ER
endoplasmic reticulum
- EV
extracellular vesicle
- GBM
glioblastoma
- HSP
heat shock protein
- HSR
heat shock response
- miR
microRNA
- MMP
matrix metalloproteinase
- MVB
multivesicular body
- NPC
neural progenitor cell
- NSC
neural stem cell
AUTHOR CONTRIBUTIONS
D. Thuringer conceived, drafted, and revised the manuscript and figures; and C. Garrido revised the manuscript.
REFERENCES
- 1.Calderwood S. K., Gong J., Murshid A. (2016) Extracellular HSPs: the complicated roles of extracellular HSPs in immunity. Front. Immunol. 7, 159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Campanella C., Pace A., Caruso Bavisotto C., Marzullo P., Marino Gammazza A., Buscemi S., Palumbo Piccionello A. (2018) Heat shock proteins in Alzheimer’s disease: role and targeting. Int. J. Mol. Sci. 19, E2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graziano F., Bavisotto C. C., Gammazza A. M., Rappa F., de Macario E. C., Macario A. J. L., Cappello F., Campanella C., Maugeri R., Iacopino D. G. (2018) Chaperonology: the third eye on brain gliomas. Brain Sci. 8, E110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rajesh Y., Biswas A., Mandal M. (2017) Glioma progression through the prism of heat shock protein mediated extracellular matrix remodeling and epithelial to mesenchymal transition. Exp. Cell Res. 359, 299–311 [DOI] [PubMed] [Google Scholar]
- 5.Jee H. (2016) Size dependent classification of heat shock proteins: a mini-review. J. Exerc. Rehabil. 12, 255–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miller D. J., Fort P. E. (2018) Heat shock proteins regulatory role in neurodevelopment. Front. Neurosci. 12, 821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Broekman M. L., Maas S. L. N., Abels E. R., Mempel T. R., Krichevsky A. M., Breakefield X. O. (2018) Multidimensional communication in the microenvirons of glioblastoma. Nat. Rev. Neurol. 14, 482–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouyang Y. B., Giffard R. G. (2013) MicroRNAs regulate the chaperone network in cerebral ischemia. Transl. Stroke Res. 4, 693–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kafel J., Baldinger L., Chabla J. M., Hallas B. H., Horowitz J. M., Torres G. (2006) Blood content modulates the induction of heat shock proteins in the neurovascular network. Brain Res. Bull. 70, 304–311 [DOI] [PubMed] [Google Scholar]
- 10.DeGracia D. J., Kreipke C. W., Kayali F. M., Rafols J. A. (2007) Brain endothelial HSP-70 stress response coincides with endothelial and pericyte death after brain trauma. Neurol. Res. 29, 356–361 [DOI] [PubMed] [Google Scholar]
- 11.Xiao B., Chai Y., Lv S., Ye M., Wu M., Xie L., Fan Y., Zhu X., Gao Z. (2017) Endothelial cell-derived exosomes protect SH-SY5Y nerve cells against ischemia/reperfusion injury. Int. J. Mol. Med. 40, 1201–1209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.András I. E., Toborek M. (2015) Extracellular vesicles of the blood-brain barrier. Tissue Barriers 4, e1131804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caruso Bavisotto C., Graziano F., Rappa F., Marino Gammazza A., Logozzi M., Fais S., Maugeri R., Bucchieri F., Conway de Macario E., Macario A. J. L., Cappello F., Iacopino D. G., Campanella C. (2018) Exosomal chaperones and miRNAs in gliomagenesis: state-of-art and theranostics perspectives. Int. J. Mol. Sci. 19, E2626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciregia F., Urbani A., Palmisano G. (2017) Extracellular vesicles in brain tumors and neurodegenerative diseases. Front. Mol. Neurosci. 10, 276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campanella C., Caruso Bavisotto C., Logozzi M., Marino Gammazza A., Mizzoni D., Cappello F., Fais S. (2019) On the choice of the extracellular vesicles for therapeutic purposes. Int. J. Mol. Sci. 20, E236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lippmann E. S., Al-Ahmad A., Palecek S. P., Shusta E. V. (2013) Modeling the blood-brain barrier using stem cell sources. Fluids Barriers CNS 10, 2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guelfi S., Duffau H., Bauchet L., Rothhut B., Hugnot J. P. (2016) Vascular transdifferentiation in the CNS: a focus on neural and glioblastoma stem-like cells. Stem Cells Int. 2016, 2759403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pardridge W. M. (2002) Targeting neurotherapeutic agents through the blood-brain barrier. Arch. Neurol. 59, 35–40 [DOI] [PubMed] [Google Scholar]
- 19.Sharma H. S., Sharma A., Mössler H., Muresanu D. F. (2012) Neuroprotective effects of cerebrolysin, a combination of different active fragments of neurotrophic factors and peptides on the whole body hyperthermia-induced neurotoxicity: modulatory roles of co-morbidity factors and nanoparticle intoxication. Int. Rev. Neurobiol. 102, 249–276 [DOI] [PubMed] [Google Scholar]
- 20.Abdullahi W., Tripathi D., Ronaldson P. T. (2018) Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. Am. J. Physiol. Cell Physiol. 315, C343–C356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sweeney M. D., Kisler K., Montagne A., Toga A. W., Zlokovic B. V. (2018) The role of brain vasculature in neurodegenerative disorders. Nat. Neurosci. 21, 1318–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shivers R. R., Pollock M., Bowman P. D., Atkinson B. G. (1988) The effect of heat shock on primary cultures of brain capillary endothelium: inhibition of assembly of zonulae occludentes and the synthesis of heat-shock proteins. Eur. J. Cell Biol. 46, 181–195 [PubMed] [Google Scholar]
- 23.Hosono J., Morikawa S., Ezaki T., Kawamata T., Okada Y. (2017) Pericytes promote abnormal tumor angiogenesis in a rat RG2 glioma model. Brain Tumor Pathol. 34, 120–129 [DOI] [PubMed] [Google Scholar]
- 24.Jiang X., Zhou T., Wang Z., Qi B., Xia H. (2017) HSP47 promotes glioblastoma stemlike cell survival by modulating tumor microenvironment extracellular matrix through TGF-β pathway. ACS Chem. Neurosci. 8, 128–134 [DOI] [PubMed] [Google Scholar]
- 25.Cazzaniga A., Locatelli L., Castiglioni S., Maier J. A. M. (2019) The dynamic adaptation of primary human endothelial cells to simulated microgravity. FASEB J. 33, 5957–5966 [DOI] [PubMed] [Google Scholar]
- 26.Hossain M. E., Matsuzaki K., Katakura M., Sugimoto N., Mamun A. A., Islam R., Hashimoto M., Shido O. (2017) Direct exposure to mild heat promotes proliferation and neuronal differentiation of neural stem/progenitor cells in vitro. PLoS One 12, e0190356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vishwakarma S. K., Bardia A., Fathima N., Chandrakala L., Rahamathulla S., Raju N., Srinivas G., Raj A., Sandhya A., Satti V., Tiwari S. K., Paspala S. A. B., Khan A. A. (2017) Protective role of hypothermia against heat stress in differentiated and undifferentiated human neural precursor cells: a differential approach for the treatment of traumatic brain injury. Basic Clin. Neurosci. 8, 453–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shi Y., Jiang X., Zhang L., Pu H., Hu X., Zhang W., Cai W., Gao Y., Leak R. K., Keep R. F., Bennett M. V., Chen J. (2017) Endothelium-targeted overexpression of heat shock protein 27 ameliorates blood-brain barrier disruption after ischemic brain injury. Proc. Natl. Acad. Sci. USA 114, E1243–E1252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Obermeier B., Verma A., Ransohoff R. M. (2016) The blood-brain barrier. Handb. Clin. Neurol. 133, 39–59 [DOI] [PubMed] [Google Scholar]
- 30.Kim J. Y., Kim N., Zheng Z., Lee J. E., Yenari M. A. (2013) The 70 kDa heat shock protein protects against experimental traumatic brain injury. Neurobiol. Dis. 58, 289–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucivero V., Prontera M., Mezzapesa D. M., Petruzzellis M., Sancilio M., Tinelli A., Di Noia D., Ruggieri M., Federico F. (2007) Different roles of matrix metalloproteinases-2 and -9 after human ischaemic stroke. Neurol. Sci. 28, 165–170 [DOI] [PubMed] [Google Scholar]
- 32.Qi J., Liu Y., Yang P., Chen T., Liu X. Z., Yin Y., Zhang J., Wang F. (2015) Heat shock protein 90 inhibition by 17-Dimethylaminoethylamino-17-demethoxygeldanamycin protects blood-brain barrier integrity in cerebral ischemic stroke. Am. J. Transl. Res. 7, 1826–1837 [PMC free article] [PubMed] [Google Scholar]
- 33.Luo W., Dou F., Rodina A., Chip S., Kim J., Zhao Q., Moulick K., Aguirre J., Wu N., Greengard P., Chiosis G. (2007) Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc. Natl. Acad. Sci. USA 104, 9511–9516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen Y., Wang B., Liu D., Li J. J., Xue Y., Sakata K., Zhu L. Q., Heldt S. A., Xu H., Liao F. F. (2014) Hsp90 chaperone inhibitor 17-AAG attenuates Aβ-induced synaptic toxicity and memory impairment. J. Neurosci. 34, 2464–2470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang B., Liu Y., Huang L., Chen J., Li J. J., Wang R., Kim E., Chen Y., Justicia C., Sakata K., Chen H., Planas A., Ostrom R. S., Li W., Yang G., McDonald M. P., Chen R., Heck D. H., Liao F. F. (2017) A CNS-permeable Hsp90 inhibitor rescues synaptic dysfunction and memory loss in APP-overexpressing Alzheimer’s mouse model via an HSF1-mediated mechanism. Mol. Psychiatry 22, 990–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lackie R. E., Maciejewski A., Ostapchenko V. G., Marques-Lopes J., Choy W. Y., Duennwald M. L., Prado V. F., Prado M. A. M. (2017) The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases. Front. Neurosci. 11, 254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gorenberg E. L., Chandra S. S. (2017) The role of co-chaperones in synaptic proteostasis and neurodegenerative disease. Front. Neurosci. 11, 248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rampelt H., Mayer M. P., Bukau B. (2018) Nucleotide exchange factors for Hsp70 chaperones. Methods Mol. Biol. 1709, 179–188 [DOI] [PubMed] [Google Scholar]
- 39.Shorter J. (2017) Designer protein disaggregases to counter neurodegenerative disease. Curr. Opin. Genet. Dev. 44, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blair L. J., Sabbagh J. J., Dickey C. A. (2014) Targeting Hsp90 and its co-chaperones to treat Alzheimer’s disease. Expert Opin. Ther. Targets 18, 1219–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen Y., Jiang B., Zhuang Y., Peng H., Chen W. (2017) Differential effects of heat shock protein 90 and serine 1179 phosphorylation on endothelial nitric oxide synthase activity and on its cofactors. PLoS One 12, e0179978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Austin S. A., Katusic Z. S. (2016) Loss of endothelial nitric oxide synthase promotes p25 generation and tau phosphorylation in a murine model of Alzheimer’s disease. Circ. Res. 119, 1128–1134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shin N., Kim H. G., Shin H. J., Kim S., Kwon H. H., Baek H., Yi M. H., Zhang E., Kim J. J., Hong J., Lee S. Y., Lee W., Triantafillu U. L., Kim C. S., Kim Y., Kim D. W. (2019) Uncoupled endothelial nitric oxide synthase enhances p-tau in chronic traumatic encephalopathy mouse model. Antioxid. Redox Signal. 30, 1601–1620 [DOI] [PubMed] [Google Scholar]
- 44.Calderwood S. K., Neckers L. (2016) Hsp90 in cancer: transcriptional roles in the nucleus. Adv. Cancer Res. 129, 89–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sawarkar R., Sievers C., Paro R. (2012) Hsp90 globally targets paused RNA polymerase to regulate gene expression in response to environmental stimuli. Cell 149, 807–818 [DOI] [PubMed] [Google Scholar]
- 46.Kimura A., Sakurai T., Yamada M., Koumura A., Hayashi Y., Tanaka Y., Hozumi I., Takemura M., Seishima M., Inuzuka T. (2012) Elevated anti-heat shock protein 60 antibody titer is related to white matter hyperintensities. J. Stroke Cerebrovasc. Dis. 21, 305–309 [DOI] [PubMed] [Google Scholar]
- 47.Bross P., Fernandez-Guerra P. (2016) Disease-associated mutations in the HSPD1 gene encoding the large subunit of the mitochondrial HSP60/HSP10 chaperonin complex. Front. Mol. Biosci. 3, 49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meng Q., Li B. X., Xiao X. (2018) Toward developing chemical modulators of Hsp60 as potential therapeutics. Front. Mol. Biosci. 5, 35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lehnardt S., Schott E., Trimbuch T., Laubisch D., Krueger C., Wulczyn G., Nitsch R., Weber J. R. (2008) A vicious cycle involving release of heat shock protein 60 from injured cells and activation of toll-like receptor 4 mediates neurodegeneration in the CNS. J. Neurosci. 28, 2320–2331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang D., Sun L., Zhu H., Wang L., Wu W., Xie J., Gu J. (2012) Microglial LOX-1 reacts with extracellular HSP60 to bridge neuroinflammation and neurotoxicity. Neurochem. Int. 61, 1021–1035 [DOI] [PubMed] [Google Scholar]
- 51.Ding F., Li F., Li Y., Hou X., Ma Y., Zhang N., Ma J., Zhang R., Lang B., Wang H., Wang Y. (2016) HSP60 mediates the neuroprotective effects of curcumin by suppressing microglial activation. Exp. Ther. Med. 12, 823–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cheng W., Li Y., Hou X., Zhang N., Ma J., Ding F., Li F., Miao Z., Zhang Y., Qi Q., Li G., Shen Y., Liu J., Huang W., Wang Y. (2014) HSP60 is involved in the neuroprotective effects of naloxone. Mol. Med. Rep. 10, 2172–2176 [DOI] [PubMed] [Google Scholar]
- 53.Thorsteinsdottir J., Stangl S., Fu P., Guo K., Albrecht V., Eigenbrod S., Erl J., Gehrmann M., Tonn J. C., Multhoff G., Schichor C. (2017) Overexpression of cytosolic, plasma membrane bound and extracellular heat shock protein 70 (Hsp70) in primary glioblastomas. J. Neurooncol. 135, 443–452 [DOI] [PubMed] [Google Scholar]
- 54.Lauwers E., Wang Y. C., Gallardo R., Van der Kant R., Michiels E., Swerts J., Baatsen P., Zaiter S. S., McAlpine S. R., Gounko N. V., Rousseau F., Schymkowitz J., Verstreken P. (2018) Hsp90 mediates membrane deformation and exosome release. Mol. Cell 71, 689–702.e9 [DOI] [PubMed] [Google Scholar]
- 55.Harris S. F., Shiau A. K., Agard D. A. (2004) The crystal structure of the carboxy-terminal dimerization domain of htpG, the Escherichia coli Hsp90, reveals a potential substrate binding site. Structure 12, 1087–1097 [DOI] [PubMed] [Google Scholar]
- 56.Renard H. F., Johannes L., Morsomme P. (2018) Increasing diversity of biological membrane fission mechanisms. Trends Cell Biol. 28, 274–286 [DOI] [PubMed] [Google Scholar]
- 57.Armijo G., Okerblom J., Cauvi D. M., Lopez V., Schlamadinger D. E., Kim J., Arispe N., De Maio A. (2014) Interaction of heat shock protein 70 with membranes depends on the lipid environment. Cell Stress Chaperones 19, 877–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.De Maio A. (2011) Extracellular heat shock proteins, cellular export vesicles, and the stress observation system: a form of communication during injury, infection, and cell damage. It is never known how far a controversial finding will go! Dedicated to Ferruccio Ritossa. Cell Stress Chaperones 16, 235–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Maio A., Vazquez D. (2013) Extracellular heat shock proteins: a new location, a new function. Shock 40, 239–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Maio A. (2014) Extracellular Hsp70: export and function. Curr. Protein Pept. Sci. 15, 225–231 [DOI] [PubMed] [Google Scholar]
- 61.Calderwood S. K., Mambula S. S., Gray P. J., Jr., Theriault J. R. (2007) Extracellular heat shock proteins in cell signaling. FEBS Lett. 581, 3689–3694 [DOI] [PubMed] [Google Scholar]
- 62.Calderwood S. K., Murshid A. (2017) Molecular chaperone accumulation in cancer and decrease in Alzheimer’s disease: the potential roles of HSF1. Front. Neurosci. 11, 192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lawrence T. (2009) The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect. Biol. 1, a001651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Asea A. (2008) Heat shock proteins and toll-like receptors. Handb. Exp. Pharmacol. 2008, 111–127 [DOI] [PubMed] [Google Scholar]
- 65.Binder R. J., Srivastava P. K. (2004) Essential role of CD91 in re-presentation of gp96-chaperoned peptides. Proc. Natl. Acad. Sci. USA 101, 6128–6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Thuringer D., Hammann A., Benikhlef N., Fourmaux E., Bouchot A., Wettstein G., Solary E., Garrido C. (2011) Transactivation of the epidermal growth factor receptor by heat shock protein 90 via Toll-like receptor 4 contributes to the migration of glioblastoma cells. J. Biol. Chem. 286, 3418–3428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thuringer D., Berthenet K., Cronier L., Jego G., Solary E., Garrido C. (2015) Oncogenic extracellular HSP70 disrupts the gap-junctional coupling between capillary cells. Oncotarget 6, 10267–10283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brea D., Blanco M., Ramos-Cabrer P., Moldes O., Arias S., Pérez-Mato M., Leira R., Sobrino T., Castillo J. (2011) Toll-like receptors 2 and 4 in ischemic stroke: outcome and therapeutic values. J. Cereb. Blood Flow Metab. 31, 1424–1431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsan M. F., Gao B. (2004) Cytokine function of heat shock proteins. Am. J. Physiol. Cell Physiol. 286, C739–C744 [DOI] [PubMed] [Google Scholar]
- 70.Tsan M. F., Gao B. (2009) Heat shock proteins and immune system. J. Leukoc. Biol. 85, 905–910 [DOI] [PubMed] [Google Scholar]
- 71.Boudesco C., Cause S., Jego G., Garrido C. (2018) Hsp70: a cancer target inside and outside the cell. Methods Mol. Biol. 1709, 371–396 [DOI] [PubMed] [Google Scholar]
- 72.Thuringer D., Jego G., Wettstein G., Terrier O., Cronier L., Yousfi N., Hébrard S., Bouchot A., Hazoumé A., Joly A. L., Gleave M., Rosa-Calatrava M., Solary E., Garrido C. (2013) Extracellular HSP27 mediates angiogenesis through Toll-like receptor 3. FASEB J. 27, 4169–4183 [DOI] [PubMed] [Google Scholar]
- 73.Wang P. F., Fang H., Chen J., Lin S., Liu Y., Xiong X. Y., Wang Y. C., Xiong R. P., Lv F. L., Wang J., Yang Q. W. (2014) Polyinosinic-polycytidylic acid has therapeutic effects against cerebral ischemia/reperfusion injury through the downregulation of TLR4 signaling via TLR3. J. Immunol. 192, 4783–4794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Batulan Z., Pulakazhi Venu V. K., Li Y., Koumbadinga G., Alvarez-Olmedo D. G., Shi C., O’Brien E. R. (2016) Extracellular release and signaling by heat shock protein 27: role in modifying vascular inflammation. Front. Immunol. 7, 285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asea A. (2008) Hsp70: a chaperokine. Novartis Found. Symp. 291, 173–179; discussion 179–183, 221–174 [DOI] [PubMed] [Google Scholar]
- 76.Asea A. (2003) Chaperokine-induced signal transduction pathways. Exerc. Immunol. Rev. 9, 25–33 [PMC free article] [PubMed] [Google Scholar]
- 77.Sochocka M., Diniz B. S., Leszek J. (2017) Inflammatory response in the CNS: friend or foe? Mol. Neurobiol. 54, 8071–8089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Arispe N., De Maio A. (2018) Memory loss and the onset of Alzheimer’s disease could be under the control of extracellular heat shock proteins. J. Alzheimers Dis. 63, 927–934 [DOI] [PubMed] [Google Scholar]
- 79.Rivera I., Capone R., Cauvi D. M., Arispe N., De Maio A. (2018) Modulation of Alzheimer’s amyloid β peptide oligomerization and toxicity by extracellular Hsp70. Cell Stress Chaperones 23, 269–279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Evans C. G., Wisén S., Gestwicki J. E. (2006) Heat shock proteins 70 and 90 inhibit early stages of amyloid beta-(1-42) aggregation in vitro. J. Biol. Chem. 281, 33182–33191 [DOI] [PubMed] [Google Scholar]
- 81.Caruso Bavisotto C., Scalia F., Marino Gammazza A., Carlisi D., Bucchieri F., Conway de Macario E., Macario A. J. L., Cappello F., Campanella C. (2019) Extracellular vesicle-mediated cell−cell communication in the nervous system: focus on neurological diseases. Int. J. Mol. Sci. 20, E434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Balusu S., Van Wonterghem E., De Rycke R., Raemdonck K., Stremersch S., Gevaert K., Brkic M., Demeestere D., Vanhooren V., Hendrix A., Libert C., Vandenbroucke R. E. (2016) Identification of a novel mechanism of blood-brain communication during peripheral inflammation via choroid plexus-derived extracellular vesicles. EMBO Mol. Med. 8, 1162–1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holm M. M., Kaiser J., Schwab M. E. (2018) Extracellular vesicles: multimodal envoys in neural maintenance and repair. Trends Neurosci. 41, 360–372 [DOI] [PubMed] [Google Scholar]
- 84.Paolicelli R. C., Bergamini G., Rajendran L. (2019) Cell-to-cell communication by extracellular vesicles: focus on microglia. Neuroscience 405, 148–157 [DOI] [PubMed] [Google Scholar]
- 85.Ramirez S. H., Andrews A. M., Paul D., Pachter J. S. (2018) Extracellular vesicles: mediators and biomarkers of pathology along CNS barriers. Fluids Barriers CNS 15, 19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tkach M., Théry C. (2016) Communication by extracellular vesicles: where we are and where we need to go. Cell 164, 1226–1232 [DOI] [PubMed] [Google Scholar]
- 87.Budnik V., Ruiz-Cañada C., Wendler F. (2016) Extracellular vesicles round off communication in the nervous system. Nat. Rev. Neurosci. 17, 160–172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dozio V., Sanchez J. C. (2017) Characterisation of extracellular vesicle-subsets derived from brain endothelial cells and analysis of their protein cargo modulation after TNF exposure. J. Extracell. Vesicles 6, 1302705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Osawa S., Kurachi M., Yamamoto H., Yoshimoto Y., Ishizaki Y. (2017) Fibronectin on extracellular vesicles from microvascular endothelial cells is involved in the vesicle uptake into oligodendrocyte precursor cells. Biochem. Biophys. Res. Commun. 488, 232–238 [DOI] [PubMed] [Google Scholar]
- 90.Kurachi M., Mikuni M., Ishizaki Y. (2016) Extracellular vesicles from vascular endothelial cells promote survival, proliferation and motility of oligodendrocyte precursor cells. PLoS One 11, e0159158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lamichhane T. N., Leung C. A., Douti L. Y., Jay S. M. (2017) Ethanol induces enhanced vascularization bioactivity of endothelial cell-derived extracellular vesicles via regulation of microRNAs and long non-coding RNAs. Sci. Rep. 7, 13794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramakrishnan D. P., Hajj-Ali R. A., Chen Y., Silverstein R. L. (2016) Extracellular vesicles activate a CD36-dependent signaling pathway to inhibit microvascular endothelial cell migration and tube formation. Arterioscler. Thromb. Vasc. Biol. 36, 534–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Andrews A. M., Lutton E. M., Merkel S. F., Razmpour R., Ramirez S. H. (2016) Mechanical injury induces brain endothelial-derived microvesicle release: implications for cerebral vascular injury during traumatic brain injury. Front. Cell. Neurosci. 10, 43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Andrews A. M., Muzorewa T. T., Zaccheo K. A., Buerk D. G., Jaron D., Barbee K. A. (2017) Cholesterol enrichment impairs capacitative calcium entry, eNOS phosphorylation & shear stress-induced NO production. Cell. Mol. Bioeng. 10, 30–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jansen F., Li Q., Pfeifer A., Werner N. (2017) Endothelial- and immune cell-derived extracellular vesicles in the regulation of cardiovascular health and disease. JACC Basic Transl. Sci. 2, 790–807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Xu Z. H., Miao Z. W., Jiang Q. Z., Gan D. X., Wei X. G., Xue X. Z., Li J. Q., Zheng F., Qin X. X., Fang W. G., Chen Y. H., Li B. (2019) Brain microvascular endothelial cell exosome-mediated S100A16 up-regulation confers small-cell lung cancer cell survival in brain. FASEB J. 33, 1742–1757 [DOI] [PubMed] [Google Scholar]
- 97.Katt M. E., Linville R. M., Mayo L. N., Xu Z. S., Searson P. C. (2018) Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: the role of matrix composition on monolayer formation. Fluids Barriers CNS 15, 7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Couch Y., Akbar N., Roodselaar J., Evans M. C., Gardiner C., Sargent I., Romero I. A., Bristow A., Buchan A. M., Haughey N., Anthony D. C. (2017) Circulating endothelial cell-derived extracellular vesicles mediate the acute phase response and sickness behaviour associated with CNS inflammation. Sci. Rep. 7, 9574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Meldolesi J. (2018) Exosomes and ectosomes in intercellular communication. Curr. Biol. 28, R435–R444 [DOI] [PubMed] [Google Scholar]
- 100.Choi D. S., Kim D. K., Kim Y. K., Gho Y. S. (2015) Proteomics of extracellular vesicles: exosomes and ectosomes. Mass Spectrom. Rev. 34, 474–490 [DOI] [PubMed] [Google Scholar]
- 101.Pan Q., He C., Liu H., Liao X., Dai B., Chen Y., Yang Y., Zhao B., Bihl J., Ma X. (2016) Microvascular endothelial cells-derived microvesicles imply in ischemic stroke by modulating astrocyte and blood brain barrier function and cerebral blood flow. Mol. Brain 9, 63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhang Z. G., Chopp M. (2016) Exosomes in stroke pathogenesis and therapy. J. Clin. Invest. 126, 1190–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Cappello F., Logozzi M., Campanella C., Bavisotto C. C., Marcilla A., Properzi F., Fais S. (2017) Exosome levels in human body fluids: a tumor marker by themselves? Eur. J. Pharm. Sci. 96, 93–98 [DOI] [PubMed] [Google Scholar]
- 104.Caruso Bavisotto C., Cappello F., Macario A. J. L., Conway de Macario E., Logozzi M., Fais S., Campanella C. (2017) Exosomal HSP60: a potentially useful biomarker for diagnosis, assessing prognosis, and monitoring response to treatment. Expert Rev. Mol. Diagn. 17, 815–822 [DOI] [PubMed] [Google Scholar]
- 105.Reddy V. S., Madala S. K., Trinath J., Reddy G. B. (2018) Extracellular small heat shock proteins: exosomal biogenesis and function. Cell Stress Chaperones 23, 441–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Horváth I., Multhoff G., Sonnleitner A., Vígh L. (2008) Membrane-associated stress proteins: more than simply chaperones. Biochim. Biophys. Acta 1778, 1653–1664 [DOI] [PubMed] [Google Scholar]
- 107.Chen C. Y., Balch W. E. (2006) The Hsp90 chaperone complex regulates GDI-dependent Rab recycling. Mol. Biol. Cell 17, 3494–3507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lotz G. P., Brychzy A., Heinz S., Obermann W. M. (2008) A novel HSP90 chaperone complex regulates intracellular vesicle transport. J. Cell Sci. 121, 717–723 [DOI] [PubMed] [Google Scholar]
- 109.Yu Q. J., Tao H., Wang X., Li M. C. (2015) Targeting brain microvascular endothelial cells: a therapeutic approach to neuroprotection against stroke. Neural Regen. Res. 10, 1882–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hua Q., Qing X., Li P., Li W., Hou J., Hu J., Hong Q., Sun P., Zhu X. (2010) Brain microvascular endothelial cells mediate neuroprotective effects on ischemia/reperfusion neurons. J. Ethnopharmacol. 129, 306–313 [DOI] [PubMed] [Google Scholar]
- 111.Yin X., Wright J., Wall T., Grammas P. (2010) Brain endothelial cells synthesize neurotoxic thrombin in Alzheimer’s disease. Am. J. Pathol. 176, 1600–1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aridon P., Geraci F., Turturici G., D’Amelio M., Savettieri G., Sconzo G. (2011) Protective role of heat shock proteins in Parkinson’s disease. Neurodegener. Dis. 8, 155–168 [DOI] [PubMed] [Google Scholar]
- 113.Sun Y., Zhang J. R., Chen S. (2017) Suppression of Alzheimer’s disease-related phenotypes by the heat shock protein 70 inducer, geranylgeranylacetone, in APP/PS1 transgenic mice via the ERK/p38 MAPK signaling pathway. Exp. Ther. Med. 14, 5267–5274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Turturici G., Tinnirello R., Sconzo G., Asea A., Savettieri G., Ragonese P., Geraci F. (2014) Positive or negative involvement of heat shock proteins in multiple sclerosis pathogenesis: an overview. J. Neuropathol. Exp. Neurol. 73, 1092–1106 [DOI] [PubMed] [Google Scholar]
- 115.Turturici G., Sconzo G., Geraci F. (2011) Hsp70 and its molecular role in nervous system diseases. Biochem. Res. Int. 2011, 618127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kim J. Y., Han Y., Lee J. E., Yenari M. A. (2018) The 70-kDa heat shock protein (Hsp70) as a therapeutic target for stroke. Expert Opin. Ther. Targets 22, 191–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shevtsov M. A., Nikolaev B. P., Yakovleva L. Y., Dobrodumov A. V., Dayneko A. S., Shmonin A. A., Vlasov T. D., Melnikova E. V., Vilisov A. D., Guzhova I. V., Ischenko A. M., Mikhrina A. L., Galibin O. V., Yakovenko I. V., Margulis B. A. (2014) Neurotherapeutic activity of the recombinant heat shock protein Hsp70 in a model of focal cerebral ischemia in rats. Drug Des. Devel. Ther. 8, 639–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Van der Weerd L., Lythgoe M. F., Badin R. A., Valentim L. M., Akbar M. T., de Belleroche J. S., Latchman D. S., Gadian D. G. (2005) Neuroprotective effects of HSP70 overexpression after cerebral ischaemia--an MRI study. Exp. Neurol. 195, 257–266 [DOI] [PubMed] [Google Scholar]
- 119.Zhan X., Ander B. P., Liao I. H., Hansen J. E., Kim C., Clements D., Weisbart R. H., Nishimura R. N., Sharp F. R. (2010) Recombinant Fv-Hsp70 protein mediates neuroprotection after focal cerebral ischemia in rats. Stroke 41, 538–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Yenari M. A. (2002) Heat shock proteins and neuroprotection. Adv. Exp. Med. Biol. 513, 281–299 [DOI] [PubMed] [Google Scholar]
- 121.Brown I. R. (2007) Heat shock proteins and protection of the nervous system. Ann. N. Y. Acad. Sci. 1113, 147–158 [DOI] [PubMed] [Google Scholar]
- 122.Tirapelli D. P., Carlotti C. G., Jr., Leite J. P., Tirapelli L. F., Colli B. O. (2010) Expression of HSP70 in cerebral ischemia and neuroprotetive action of hypothermia and ketoprofen. Arq. Neuropsiquiatr. 68, 592–596 [DOI] [PubMed] [Google Scholar]
- 123.Wacker J. L., Huang S. Y., Steele A. D., Aron R., Lotz G. P., Nguyen Q., Giorgini F., Roberson E. D., Lindquist S., Masliah E., Muchowski P. J. (2009) Loss of Hsp70 exacerbates pathogenesis but not levels of fibrillar aggregates in a mouse model of Huntington’s disease. J. Neurosci. 29, 9104–9114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramani B., Panwar B., Moore L. R., Wang B., Huang R., Guan Y., Paulson H. L. (2017) Comparison of spinocerebellar ataxia type 3 mouse models identifies early gain-of-function, cell-autonomous transcriptional changes in oligodendrocytes. Hum. Mol. Genet. 26, 3362–3374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Nakazono A., Adachi N., Takahashi H., Seki T., Hamada D., Ueyama T., Sakai N., Saito N. (2018) Pharmacological induction of heat shock proteins ameliorates toxicity of mutant PKCγ in spinocerebellar ataxia type 14. J. Biol. Chem. 293, 14758–14774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fink S. L., Chang L. K., Ho D. Y., Sapolsky R. M. (1997) Defective herpes simplex virus vectors expressing the rat brain stress-inducible heat shock protein 72 protect cultured neurons from severe heat shock. J. Neurochem. 68, 961–969 [DOI] [PubMed] [Google Scholar]
- 127.Wagstaff M. J., Collaço-Moraes Y., Aspey B. S., Coffin R. S., Harrison M. J., Latchman D. S., de Belleroche J. S. (1996) Focal cerebral ischaemia increases the levels of several classes of heat shock proteins and their corresponding mRNAs. Brain Res. Mol. Brain Res. 42, 236–244 [DOI] [PubMed] [Google Scholar]
- 128.Kiyatkin E. A., Sharma H. S. (2011) Expression of heat shock protein (HSP 72 kDa) during acute methamphetamine intoxication depends on brain hyperthermia: neurotoxicity or neuroprotection? J. Neural Transm. (Vienna) 118, 47–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Shelton L. B., Koren J., III, Blair L. J. (2017) Imbalances in the Hsp90 chaperone machinery: implications for tauopathies. Front. Neurosci. 11, 724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zagrean A. M., Hermann D. M., Opris I., Zagrean L., Popa-Wagner A. (2018) Multicellular crosstalk between exosomes and the neurovascular unit after cerebral ischemia. Therapeutic implications. Front. Neurosci. 12, 811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Balogi Z., Multhoff G., Jensen T. K., Lloyd-Evans E., Yamashima T., Jäättelä M., Harwood J. L., Vígh L. (2019) Hsp70 interactions with membrane lipids regulate cellular functions in health and disease. Prog. Lipid Res. 74, 18–30 [DOI] [PubMed] [Google Scholar]
- 132.Zhao Z., Zlokovic B. V. (2017) Remote control of BBB: a tale of exosomes and microRNA. Cell Res. 27, 849–850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Katakowski M., Buller B., Wang X., Rogers T., Chopp M. (2010) Functional microRNA is transferred between glioma cells. Cancer Res. 70, 8259–8263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Katakowski M., Chopp M. (2016) Exosomes as tools to suppress primary brain tumor. Cell. Mol. Neurobiol. 36, 343–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Dehghani R., Rahmani F., Rezaei N. (2018) MicroRNA in Alzheimer’s disease revisited: implications for major neuropathological mechanisms. Rev. Neurosci. 29, 161–182 [DOI] [PubMed] [Google Scholar]
- 136.Vijayan M., Kumar S., Yin X., Zafer D., Chanana V., Cengiz P., Reddy P. H. (2018) Identification of novel circulatory microRNA signatures linked to patients with ischemic stroke. Hum. Mol. Genet. 27, 2318–2329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim D. G., Kim K. H., Seo Y. J., Yang H., Marcusson E. G., Son E., Lee K., Sa J. K., Lee H. W., Nam D. H. (2016) Anti-miR delivery strategies to bypass the blood-brain barrier in glioblastoma therapy. Oncotarget 7, 29400–29411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhuang Y., Peng H., Mastej V., Chen W. (2016) MicroRNA regulation of endothelial junction proteins and clinical consequence. Mediators Inflamm. 2016, 5078627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sarko D. K., McKinney C. E. (2017) Exosomes: origins and therapeutic potential for neurodegenerative disease. Front. Neurosci. 11, 82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Madani R., Poirier R., Wolfer D. P., Welzl H., Groscurth P., Lipp H. P., Lu B., El Mouedden M., Mercken M., Nitsch R. M., Mohajeri M. H. (2006) Lack of neprilysin suffices to generate murine amyloid-like deposits in the brain and behavioral deficit in vivo. J. Neurosci. Res. 84, 1871–1878 [DOI] [PubMed] [Google Scholar]
- 141.Kourtis N., Tavernarakis N. (2018) Small heat shock proteins and neurodegeneration: recent developments. Biomol. Concepts 9, 94–102 [DOI] [PubMed] [Google Scholar]
- 142.Borovski T., Beke P., van Tellingen O., Rodermond H. M., Verhoeff J. J., Lascano V., Daalhuisen J. B., Medema J. P., Sprick M. R. (2013) Therapy-resistant tumor microvascular endothelial cells contribute to treatment failure in glioblastoma multiforme. Oncogene 32, 1539–1548 [DOI] [PubMed] [Google Scholar]
- 143.Mei X., Chen Y. S., Chen F. R., Xi S. Y., Chen Z. P. (2017) Glioblastoma stem cell differentiation into endothelial cells evidenced through live-cell imaging. Neuro-oncol. 19, 1109–1118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bustamante F. A., Miró M. P., VelÁsquez Z. D., Molina L., Ehrenfeld P., Rivera F. J., BÁtiz L. F. (2019) Role of adherens junctions and apical-basal polarity of neural stem/progenitor cells in the pathogenesis of neurodevelopmental disorders: a novel perspective on congenital Zika syndrome. Transl. Res. 210, 57–79 [DOI] [PubMed] [Google Scholar]
- 145.Dabrowski A., Robinson T. J., Felling R. J. (2019) Promoting brain repair and regeneration after stroke: a plea for cell-based therapies. Curr. Neurol. Neurosci. Rep. 19, 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Moriarty N., Parish C. L., Dowd E. (2019) Primary tissue for cellular brain repair in Parkinson’s disease: promise, problems and the potential of biomaterials. Eur. J. Neurosci. 49, 472–486 [DOI] [PubMed] [Google Scholar]
- 147.van Ommeren R., Staudt M. D., Xu H., Hebb M. O. (2016) Advances in HSP27 and HSP90-targeting strategies for glioblastoma. J. Neurooncol. 127, 209–219 [DOI] [PubMed] [Google Scholar]
- 148.Ghaemi A., Sajadian A., Khodaie B., Lotfinia A. A., Lotfinia M., Aghabarari A., Khaleghi Ghadiri M., Meuth S., Gorji A. (2016) Immunomodulatory effect of toll-like receptor-3 ligand poly I:C on cortical spreading depression. Mol. Neurobiol. 53, 143–154 [DOI] [PubMed] [Google Scholar]
- 149.Shi C., Ulke-Lemée A., Deng J., Batulan Z., O’Brien E. R. (2019) Characterization of heat shock protein 27 in extracellular vesicles: a potential anti-inflammatory therapy. FASEB J. 33, 1617–1630 [DOI] [PubMed] [Google Scholar]
- 150.Belkacemi L., Hebb M. O. (2014) HSP27 knockdown produces synergistic induction of apoptosis by HSP90 and kinase inhibitors in glioblastoma multiforme. Anticancer Res. 34, 4915–4927 [PubMed] [Google Scholar]
- 151.Cai H. Q., Wang P. F., Zhang H. P., Cheng Z. J., Li S. W., He J., Zhang Y., Hao J. J., Wang M. R., Yan C. X., Wan J. H. (2018) Phosphorylated Hsp27 is mutually exclusive with ATRX loss and the IDH1R132H mutation and may predict better prognosis among glioblastomas without the IDH1 mutation and ATRX loss. J. Clin. Pathol. 71, 702–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Abisambra J. F., Blair L. J., Hill S. E., Jones J. R., Kraft C., Rogers J., Koren J., III, Jinwal U. K., Lawson L., Johnson A. G., Wilcock D., O’Leary J. C., Jansen-West K., Muschol M., Golde T. E., Weeber E. J., Banko J., Dickey C. A. (2010) Phosphorylation dynamics regulate Hsp27-mediated rescue of neuronal plasticity deficits in tau transgenic mice. J. Neurosci. 30, 15374–15382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chen C. M., Chen W. L., Hung C. T., Lin T. H., Chao C. Y., Lin C. H., Wu Y. R., Chang K. H., Yao C. F., Lee-Chen G. J., Su M. T., Hsieh-Li H. M. (2018) The indole compound NC009-1 inhibits aggregation and promotes neurite outgrowth through enhancement of HSPB1 in SCA17 cells and ameliorates the behavioral deficits in SCA17 mice. Neurotoxicology 67, 259–269 [DOI] [PubMed] [Google Scholar]
- 154.Alexiou G. A., Karamoutsios A., Lallas G., Ragos V., Goussia A., Kyritsis A. P., Voulgaris S., Vartholomatos G. (2014) Expression of heat shock proteins in brain tumors. Turk Neurosurg. 24, 745–749 [DOI] [PubMed] [Google Scholar]
- 155.Hasegawa T., Yoshida S., Sugeno N., Kobayashi J., Aoki M. (2018) DnaJ/Hsp40 family and Parkinson’s disease. Front. Neurosci. 11, 743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Fontaine S. N., Zheng D., Sabbagh J. J., Martin M. D., Chaput D., Darling A., Trotter J. H., Stothert A. R., Nordhues B. A., Lussier A., Baker J., Shelton L., Kahn M., Blair L. J., Stevens S. M., Jr., Dickey C. A. (2016) DnaJ/Hsc70 chaperone complexes control the extracellular release of neurodegenerative-associated proteins. EMBO J. 35, 1537–1549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Deighton R. F., McGregor R., Kemp J., McCulloch J., Whittle I. R. (2010) Glioma pathophysiology: insights emerging from proteomics. Brain Pathol. 20, 691–703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Tang H., Li J., Liu X., Wang G., Luo M., Deng H. (2016) Down-regulation of HSP60 suppresses the proliferation of glioblastoma cells via the ROS/AMPK/mTOR pathway. Sci. Rep. 6, 28388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.El-Ansary A., Al-Ayadhi L. (2012) Neuroinflammation in autism spectrum disorders. J. Neuroinflammation 9, 265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abruzzo P. M., Ghezzo A., Bolotta A., Ferreri C., Minguzzi R., Vignini A., Visconti P., Marini M. (2015) Perspective biological markers for autism spectrum disorders: advantages of the use of receiver operating characteristic curves in evaluating marker sensitivity and specificity. Dis. Markers 2015, 329607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Reifenberger G., Wirsching H. G., Knobbe-Thomsen C. B., Weller M. (2017) Advances in the molecular genetics of gliomas - implications for classification and therapy. Nat. Rev. Clin. Oncol. 14, 434–452 [DOI] [PubMed] [Google Scholar]
- 162.Senn R., Elkind M. S., Montaner J., Christ-Crain M., Katan M. (2014) Potential role of blood biomarkers in the management of nontraumatic intracerebral hemorrhage. Cerebrovasc. Dis. 38, 395–409 [DOI] [PubMed] [Google Scholar]
- 163.Zucchi E., Bedin R., Fasano A., Fini N., Gessani A., Vinceti M., Mandrioli J. (2018) Cerebrospinal fluid neurofilaments may discriminate upper motor neuron syndromes: a pilot study. Neurodegener. Dis. 18, 255–261 [DOI] [PubMed] [Google Scholar]
- 164.Liu Z., Li H., He L., Xiang Y., Tian C., Li C., Tan P., Jing J., Tian Y., Du L., Huang Y., Han L., Li M., Zhou Y. (2019) Discovery of small-molecule inhibitors of the HSP90-calcineurin-NFAT pathway against glioblastoma. Cell Chem. Biol. 26, 352–365.e7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Binder D. C., Davis A. A., Wainwright D. A. (2015) Immunotherapy for cancer in the central nervous system: current and future directions. OncoImmunology 5, e1082027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Zhang M., Wang D., Li P., Sun C., Xu R., Geng Z., Xu W., Dai Z. (2018) Interaction of Hsp90 with phospholipid model membranes. Biochim. Biophys. Acta. Biomembr. 1860, 611–616 [DOI] [PubMed] [Google Scholar]
- 167.Eroglu B., Kimbler D. E., Pang J., Choi J., Moskophidis D., Yanasak N., Dhandapani K. M., Mivechi N. F. (2014) Therapeutic inducers of the HSP70/HSP110 protect mice against traumatic brain injury. J. Neurochem. 130, 626–641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Eroglu B., Moskophidis D., Mivechi N. F. (2010) Loss of Hsp110 leads to age-dependent tau hyperphosphorylation and early accumulation of insoluble amyloid beta. Mol. Cell. Biol. 30, 4626–4643 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yamagishi N., Ishihara K., Saito Y., Hatayama T. (2006) Hsp105 family proteins suppress staurosporine-induced apoptosis by inhibiting the translocation of Bax to mitochondria in HeLa cells. Exp. Cell Res. 312, 3215–3223 [DOI] [PubMed] [Google Scholar]
- 170.Hashikawa N., Utaka Y., Ogawa T., Tanoue R., Morita Y., Yamamoto S., Yamaguchi S., Kayano M., Zamami Y., Hashikawa-Hobara N. (2017) HSP105 prevents depression-like behavior by increasing hippocampal brain-derived neurotrophic factor levels in mice. Sci. Adv. 3, e1603014 [DOI] [PMC free article] [PubMed] [Google Scholar]