Abstract
Memory is a dynamic brain function that is continually processed after encoding. Although psychologic concepts of mental schema are now well established, they have rarely been considered in animal studies. We used a behavior paradigm of multiple flavor-place paired associates (PAs) and showed that memory schema facilitates fast acquisition of new PAs in a single trial. The hippocampus is necessary for the encoding of new PAs and for memory retrieval within a certain time window—24 h following new PA consolidation. Whereas the anterior cingulate cortex (ACC) plays a critical role for dynamic PA learning and consolidation during training sessions, ACC is essential in schema representation and activation. New myelin generation is essential for learning. Neural activity in the cortical regions impacts myelination by regulating oligodendrocyte (OL) proliferation, differentiation, and myelin formation. Here, we show that newly formed OL progenitor cells and mature OLs are increased following repeated PA learning and that establishment of the memory schema is associated with enhanced myelin strength in the ACC region. Furthermore, to ensure that myelination is necessary for the acquisition of paired-associate learning, ACC lysolecithin-induced demyelination revealed impaired PA learning associated with decrease in ACC θ band power and reduced spike-field coherence and phase-locking in ACC.—Hasan, M., Kanna, M. S., Jun, W., Ramkrishnan, A. S., Iqbal, Z., Lee, Y., Li, Y. Schema-like learning and memory consolidation acting through myelination.
Keywords: brain oscillations, anterior cingulate cortex, hippocampus, myelin plasticity, circuitry synchronization
Many lines of evidence have demonstrated that memory is a dynamic brain function that continues to be processed after encoding rather than being stored. Thus, learning occurs against the backdrop of a human’s cumulative experience, suggesting that the brain is not a simple stimulus-driven device but has the ability to dynamically create meaning by itself (1). Preston’s work on this topic provides a mechanistic account of how past and present experience dynamically interact (2). People learn new things easily by incorporating new information into meaningful and orderly patterns in preexisting knowledge structures called schemas (3–5). Although psychologic concepts of mental schema are now well established, they have rarely been considered in animal studies. This is due to the fact that most studies were conducted with inexperienced animals and that it is difficult to map the concept of mental schema precisely onto the existing neurobiological models, such as synaptic plasticity.
Hippocampal circuits are believed to be intensely involved in the early stage of the acquisition of events by providing the brain with various sensory, emotional, and cognitive information (6). Nevertheless, the hippocampus (HPC) shows only limited activation when a more permanent memory is stabilized in the neocortex. This well-accepted presumption excludes the chance of simultaneous encoding in the HPC and neocortex (7, 8). Previous studies in refs. 9, 10 have shown that rats can simultaneously learn multiple flavor-place paired associates (PAs) within a familiar testing environment called an event arena and create a cortex-dependent schema within that context. This memory schema, in the sense of Tse et al. (9), facilitates fast acquisition of new PAs in a single trial indicating that when systems memory consolidation happens in the presence of relevant previous knowledge (9, 10), the assimilation of new PA events into existing cortical schemas progresses very rapidly (7, 9, 10, 11). Notably, it has been shown that hippocampal lesions 48 h later not only failed to impair the recall of originally learned PAs but also failed to weaken the retrieval of the new PAs (9). These observations indicate that the 48 h following new PA acquisition is a critical time window for consolidation of new PAs and for expanding the existing memory schema in the cortex (12, 13).
To make progress in characterizing these interactions following previously published works (9, 10) we utilized a behavioral paradigm in which rats first learned multiple PAs over several weeks and were then expected to learn 2 new PAs, each in a single trial. Consistent with previous reports (9, 10), we showed that over the course of training the rats used spatial memory to find the correct flavored food location, indicating the development of an associative schema containing information about the locations of these flavored foods. Furthermore, the assimilation of new PAs into existing activated cortical schemas proceeded very rapidly, thus extending the existing knowledge base.
Myelination of certain brain areas coincides with cognitive and behavioral development over a lifetime (14). The axons continue to be myelinated into adulthood in humans, coinciding with learning (15, 16). Myelination is, therefore, a highly dynamic process that contributes to brain plasticity and learning (17). In the living human brain, using ultra-high-field MRI and image processing tools, researchers have identified a resemblance in intracortical myelin content correlated to resting-state functional connectivity (18).
Recent studies have revealed that experience, such as social isolation (19, 20) or early life stress (21), can causes myelination defects in this brain circuits leading to behavioral abnormalities. Animal studies in mice have shown that there are modifications in oligodendrocytes (OLs) and myelination related to changes to social and environmental conditions in learning a complex motor task (22). It is well known that white matter is essential for nerve impulse conduction beyond the synapse and transmits signals through neural networks that are critical for higher-level cognitive functions (23). Imaging studies have shown that different forms of learning correspond to structural changes in the white matter of the human brain (24). For example, professional musicians have been shown to have increased myelination in the white matter. Multiple ranges of evidence support the concept that excitatory neuronal activity initiates myelination, including OL progenitor cell (OPC) proliferation (25), differentiation, and myelin biosynthesis of nearby axons. On the other hand, blocking neuronal activity has been shown to decrease OPC proliferation (26, 27). However, myelin plasticity in certain brain areas related to prolonged cognitive performance has not been well demonstrated. In this study, we test our hypothesis that myelin is modifiable, and we report for the first time that prolonged multiple PA learning and memory retrieval performances are associated with the oligodendrogenesis and adaptive myelination and that enhanced myelination within the anterior cingulate cortex (ACC) network appears to be involved in dynamic schema-like memory consolidation and retrieval. Furthermore, we determine if ACC demyelination could prevent schema-like memory formation and retrieval. Finally, electrophysiological recording was performed to examine the role of myelination in ACC θ band power and spike-field coherence (SFC) and phase-locking.
MATERIALS AND METHODS
Animal use and care
Adult Sprague-Dawley male rats weighing 250–300 g were used in this experiment. The subjects were kept on a 12 h on–12 h off light schedule (lights on at 7:00 am). All experimental and surgical procedures were conducted according to the guidelines developed by the National Institutes of Health (NIH; Bethesda, MD, USA) for the care and use of animals for experimental procedures. The procedures were approved by the Committee on Use and Care of Animals at City University of Hong Kong, and the licensing to conduct experiments was granted by the Department of Health of Hong Kong (16–143; DH/HA&P/8/2/5 Pt.6). Rats were handled regularly by the experimenters in the laboratory room for at least 1 wk prior to starting the original experiment. Rats were housed 3–4 animals per cage during the handling period and then singly housed during the experiment period. The rats were provided with a restricted diet of 85% of their free-feeding weight. They had access to water ad libitum. A total of 140 rats were used in the full experiment.
Apparatus for PA behavioral task
All the behavioral experiments were conducted in an event arena where 6 PAs could be studied. The event arena and sand wells were constructed and set up as previously described by Tse et al. (9). The arrangement of the 6 sand wells used for the PAs was different in each experiment. During training sessions, dim light was used to illuminate the experimental room. A video recording camera was mounted on the ceiling to monitor the rats’ movement.
Paired-associate behavioral protocol
The paired-associate behavioral protocol developed by Tse et al. (9) was used in this study to assess learning and memory in rats. The entire procedure consists of habituation (session −8 to −3), pretraining (session −2 to −1), and training of 6 original PAs (OPAs; sessions 1–19). Three nonrewarded cued-recall probe tests (PTs) were conducted at sessions 3, 10, and 18 to test memory retrieval.
Habituation and pretraining
During habituation, rats were familiarized with the event arena. They learned to dig in a sand well located at the center of the arena to acquire 3 normal food pellets, bring them back to the starting box, and consume them. The pellets were initially hidden at the top of the sand well and were sequentially moved to the bottom as habituation progressed. During pretraining, rats were introduced to the 6 OPAs across 2 sessions.
Training protocol
To measure the pattern of memory acquisition, consolidation, and retrieval, rats were trained to learn 6 OPAs per session over 18 sessions. During the training session, 6 sand wells were opened, and only one of the sand wells contained 3 food pellets. At the beginning of each trial, the rat was given a flavored food pellet as cue (0.5 g) in a start box.
The door was then opened, and the rat entered the PA arena to find the sand well that was associated with the given flavor cue. Upon finding the correct sand well, the rat dug until it retrieved 3 cued food pellets one by one. Each time the rats brought the pellet back to the starting box and again ran to the cued location of the next food pellet. The door of the starting box was closed after the rat returned with the third pellet, and the rat was allowed some time to consume the pellet before being returned to the home cage. The sand used in the sand wells was mixed with ground-up food (25 g/2.5 kg sand) containing all 6 flavors before the start of each session. This was done to ensure that rats located the correct sand well by utilizing their memory of the flavor-place associations and not by the scent of the food pellets.
Because the rats were trained consecutively, the intertrial interval for an individual rat between successive flavor-place pairings was around 30 min, amounting to daily sessions of up to 6 h. Training sessions were conducted 3–4 times per week.
We also performed 1 PA training as a control experiment. The rats were trained to learn 1 PA over 18 sessions. During each training session, 6 sand wells were opened, and only one of the sand wells contained 3 food pellets. At the beginning of each trial, the rat was given the same flavored food pellet as cue (0.5 g) in a start box. The door was then opened, following which the rat entered the PA arena to find the sand well that was associated with the flavor cue. Upon finding the correct sand well, the rat dug until it retrieved 3 cued food pellets one by one.
New PA learning
Once the rats acquired the schema of the 6 OPAs, the rats were trained for 2 new PAs, PA7 and PA8 (which replaced PA1 and PA6, respectively), within a single session. A PT was conducted 24 h after the single training session, using one of the new PAs as the flavor cue. The performance was measured by the percentage of digging time at the cued new location with that of the percentage of digging time at the noncued new location and the noncued original locations. Similar new PA (PA7 and PA8) learning and new PA memory retrieval PT procedures were also conducted in rats that had learned 1 PA.
Performance index
During each session, the performance index (PI) was assessed as the percentage of incorrect sand well dug before digging the correct cued sand well. For the 6-PA task, this PI was calculated as (100% − mean number of errors × 20%).
PTs
To assess memory retrieval, 3 nonrewarded PTs (1–3) were conducted at various points during the training period. During the PT, the rats were cued with a single flavor in the starting box for 30 s, after which the start gate was opened, so that they moved freely in the arena for 120 s to find the correct sand well. During the PT, the correct sand well did not contain food rewards. The rats with good memory would dig the cued correct sand well for a longer time. If a rat did not dig during the 120 s, a further 60 s was given before ending the test. Memory recall performance was measured by calculating the percentage digging time at the cued location and the percentage digging time at the noncued locations. The rats were provided with 3 pellets of the cued flavor in the correct sand well after the PT to limit the extinction of memory.
Behavioral experimental design
Impact of intrahippocampal lidocaine on schematic learning and memory consolidation
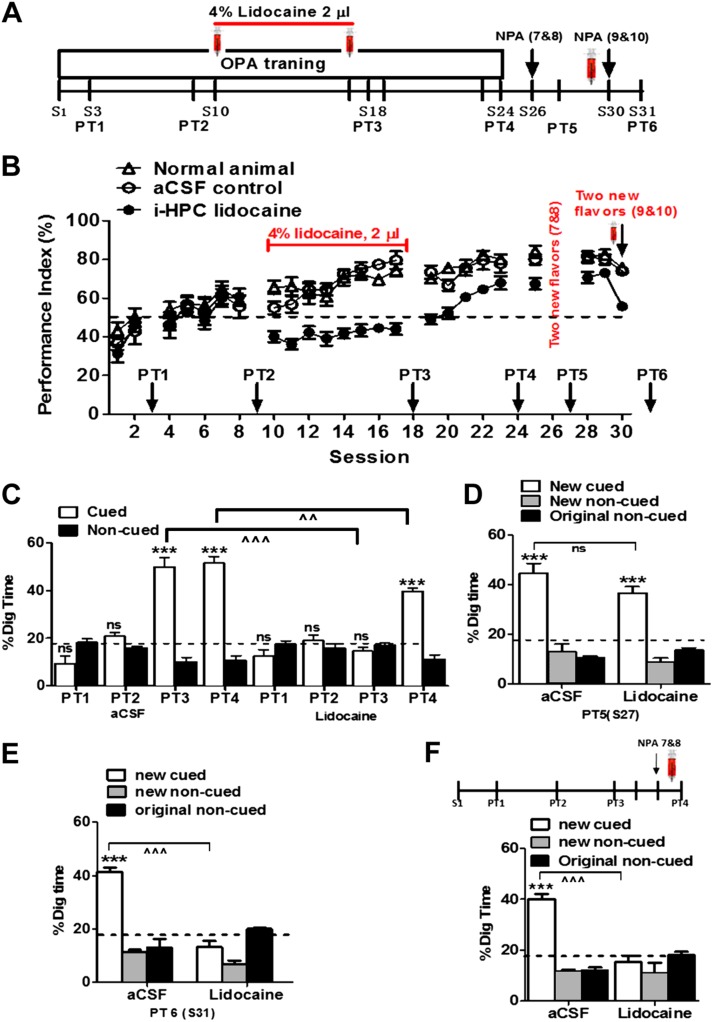
The aim of this experiment was to investigate the impact of hippocampal inactivation on schema learning and memory consolidation after a certain level of learning. The procedure for ACC cannulation surgery has described in our previous publication (28). To measure the impact of intrahippocampal lidocaine, from session 1 to 9, rats [lidocaine n = 6, artificial cerebrospinal fluid (aCSF) control n = 6, normal animals n = 6] were trained with 6 PAs per session on alternative days until they reached a certain level of learning (PI = 60–65%) of the 6 PAs (measured by PT1). Then, 4% lidocaine was injected bilaterally into the HPC 15 min before training from session 11 to 17 (Fig. 1A). PT2 was conducted at session 18 to measure memory retrieval (Fig. 1B). After that, we continued to train the rats with the 6 OPAs for 6 more sessions without drug treatment.
Figure 1.
Intrahippocampal lidocaine prevents schema formation, new learning, and memory retrieval. A) Experimental design showing timeline of schema training, new PA learning, and memory retrieval. Syringes indicate the time of bilateral intrahippocampal infusions performed 15 min before each session (S), S10–17. B) PI during the acquisition of the 6 OPAs (S1–24). Lidocaine was injected bilaterally into the HPC during S10–17. PI rose to around 70% at S9 and decreased to around 50% (chance level) after lidocaine infusions, whereas PI continued to increase steadily to 75–80% in the control animals (aCSF and normal) during S11–17. Note that PI of the lidocaine group gradually increased to 70% after stopping lidocaine infusion (S19–S23). C) Nonrewarded PTs for the acquisition of original schema across S3, S9, S18, and S24. The graph represents the percentage of dig time at the cued location (white bars) relative to the noncued locations (black bars). D) Memory recall in PT5 for new PA (white bar) in aCSF and lidocaine infusion group, 24 h after a single session of training with 2 new PAs. E) Impact of intrahippocampal lidocaine infusion on acquisition of new PAs in PT6 (lidocaine infusion before new PA acquisition). The graphs represent the percentage of dig time at the new cued location (white bars), the new noncued location (striped bars), and the old noncued locations (black bars) in the aCSF and lidocaine conditions. F) Impact of intrahippocampal lidocaine infusion on retrieval of new PAs in PT4 (lidocaine infusion before new PA PT). The graphs represent the percentage of dig time at the new cued location (white bars), the new noncued location (striped bars), and the old noncued locations (black bars) after aCSF and lidocaine infusion. i-HPC, intrahippocampal; NPA, new PA; ns, not significant. For all experiments shown, n = 6 rats/group. Results are expressed as means ± sem. ***P < 0.001 for Student’s t tests comparing the proportion of digging for the cued PAs with the chance level of 16.67%, ^^P < 0.01; ^^^P < 0.001 for Student’s t tests comparing the proportion of cued digging in lidocaine vs. aCSF infused rats.
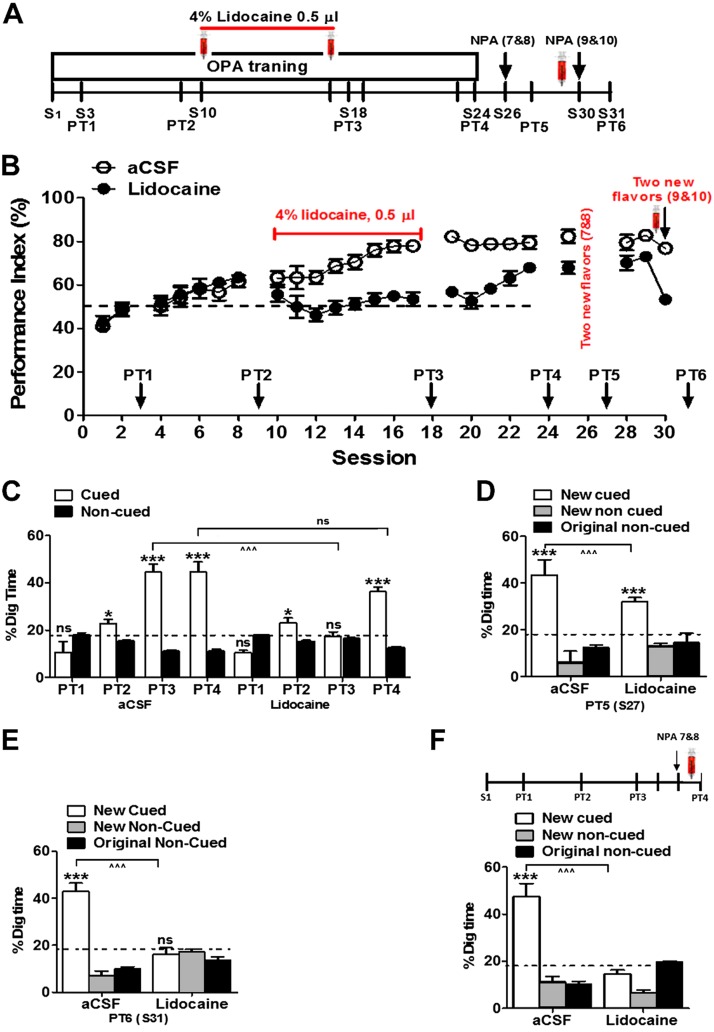
Impact of intra-ACC lidocaine on schematic learning and consolidation
The aim of this experiment was to examine the impact of ACC inactivation on schematic learning and memory consolidation. We hypothesized that after a certain level of paired-associate learning (HPC dependent), the ACC is no longer necessary to complete schema formation. To test this hypothesis, rats (n = 12) were trained to learn 6 OPAs from session 1 to 9. Graded learning of the 6 PAs was measured through 2 nonrewarded PTs (PT1 and PT2). After that, drugs were injected bilaterally into the ACC 15 min before training from session 11 to 17 (gray sessions in Fig. 1C). A third nonrewarded PT (PT3) was conducted at session 18 to measure memory consolidation and retrieval.
Impact of intra-ACC lidocaine on retrieval of new PAs
We have shown in our control experiment that new information can be rapidly assimilated after only a single training session and can be consolidated for long-term storage in the presence of preestablished original schema. We sought to investigate the impact of ACC inactivation on memory retrieval of new PAs after long schematic learning and memory consolidation. For this purpose, the rats (n = 11) were trained with 6 OPAs from session 1 to session 19. Cued-recall memory was measured through nonrewarded PTs (PT1, PT2, and PT3) in sessions 3, 9, and 18. These rats were then trained in a single session with 2 new PAs. Lidocaine and aCSF were injected 15 min before PT4, which was conducted 24 h after training with new PAs.
In vivo demyelination: lysolecithin infusion
Anesthetized rats were placed into a stereotactic frame using flat skull position. Lysolecithin (LPC; MilliporeSigma, Burlington, MA, USA) was dissolved in aCSF. Two microliters 1% LPC was administered bilaterally into the ACC (the coordinates were 2.4 mm anterior to bregma, 0.8 mm lateral, and 2.0 mm dorsal to ventral) using a Hamilton syringe (31-gauge Hamilton needle; Reno, NV, USA) connected with microinjector for a period of 5 min (19, 29) and aCSF as a control. The needle was retained in the brain for 5 min longer to complete diffusion of the liquid from the needle tip. LPC-treated rats experienced severe demyelination in the locally injected region in the first 7 d after injection; however, remyelination took place 4 wk later.
Immunohistochemistry and confocal microscopy
Immunohistochemistry
After completion of the PA behavior task, PA rats and control rats were acutely anesthetized with sodium pentobarbital and then perfused transcardially with 0.9% saline for 10 min, followed by perfusion with 0.1 M phosphate buffer (15 ml). Brains were postfixed in 4% paraformaldehyde overnight at 4°C. Tissue were cryoprotected with increasing concentration of sucrose [10, 20, 30% (w/v) sucrose] before freezing in optimum cutting temperature medium (Tissue-Tek; Sakura Finetek, Tokyo, Japan). Subsequently, brain tissues were embedded and fixed in optimum cutting temperature medium and sectioned in the coronal plane at 30 μm with a sliding microtome (HM450; Leica Microsystems, Wetzlar, Germany). For immunohistochemistry, sections were immunostained using the primary antibody followed by incubation in blocking solution (10% goat serum, 0.3% Triton X-100 in 0.01 M PBS) at room temperature for 1.5 h followed by incubation with secondary antibody.
The following primary antibodies were used: rabbit anti–myelin basic protein (MBP; a biomarker for myelinated fibers, 1:500), mouse anti–neural-glial antigen 2 (NG2; biomarker for OPCs; 1:500), mouse anti-CC1 (a biomarker for the mature OLs; 1:500 dilution), rabbit anti–OL transcription factor 2 (Olig2; a biomarker for OPCs; 1:500), and mouse anti-SMI31(a biomarker for intact axon) diluted in blocking solution (0.1% [v/v] Triton X-100 and 10% goat serum in 0.01 M PBS). Slices were incubated with primary antibodies overnight at 4°C, except with anti-CC1 antibody for which a 1-wk incubation was used. All antibodies have been validated in the literature for use in rat immunohistochemistry. After washing with PBS 3 times for 5 min, the slices were then incubated with secondary antibodies diluted in DAPI for 2 h. The following secondary antibodies were used: Alexa anti-rabbit 488, Alexa anti-rabbit 594, Alexa anti-mouse 488, and Alexa anti-mouse 594 (Thermo Fisher Scientific, Waltham, MA, USA) at dilution of 1:500. Finally, sections were rinsed 3 times for 5 min in PBS and mounted with Dako mounting medium (Agilent Technologies, Santa Clara, CA, USA) for further imaging.
In vivo 5-ethynyl-2′-deoxyuridine labeling
To estimate the overall OPC proliferation in response to PA training, 5-ethynyl-2′-deoxyuridine (EdU) was administered (25 mg/kg body weight, i.p.) (30) 30 min before session 1 of PA training. The rats were euthanized 3 h, 24 h, 7 d, and 18 d later, and brain samples containing ACC were sectioned and colabeled with monoclonal anti-CC1 and anti-NG2 antibodies along with EdU using the AlexaFluor-555 Click-It Detection Kit (Thermo Fisher Scientific).
Confocal imaging and quantification
Mounted slides were imaged using an inverted laser scanning confocal microscope (LSM 880; Carl Zeiss, Oberkochen, Germany). A total of 3–5 sections were scanned per rat, and 6–8 rats were studied per group. Confocal images obtained as stacks of 10 images collected at 1–2-μm steps. The MBP+ intensity was measured up to 1000 μm from the plial surface (midline) covering layers I–VI in the ACC region. The myelin MBP+ intensity was calculated using ImageJ (NIH). The MBP+ intensity was normalized with the control samples. The numbers of NG2+ cells and CC1 cells were also calculated in the field of view containing layers I–VI by using ImageJ software.
Protein extraction and Western blot analysis
Western blot analysis was executed from brain lysate collected from ACC, following our previous publication (31). Briefly, the brains were quickly detached after acute anesthesia with urethane (1.5 g/kg). Brains were sectioned on an anodized aluminum brain slicer (Braintree Scientific, Braintree, MA, USA). Bilateral ACCs were identified and dissected from sections and were collected and stored at −80°C until protein extraction and quantification. ACC samples were homogenized in ice-cold RIPA protein extraction buffer (50 mM Tris, 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS) containing 1 mM PMSF and protease inhibitor cocktail (Roche, Basel, Switzerland). Homogenates were protected on ice for 15 min and centrifuged at 15,000 g for 30 min at 4°C. Finally, the supernatant was extracted as total protein for Western immunoblot analysis. By using the Bio-Rad DC Protein Assay Kit, we determined the total protein content of each brain’s homogenate lysates and the synaptoneurosome fraction from all groups. By SDS-PAGE, 10 μg of proteins were separated and then transferred onto a polyvinylidene fluoride membrane (0.2 μm pore size; Bio-Rad, Hercules, CA, USA), then blocked in 5% nonfat milk in Tris-buffered saline (containing 0.1% Tween 20). The membrane was then incubated for overnight at 4°C with various primary antibodies diluted in blocking buffer. The next day, membranes were incubated with horseradish peroxidase–conjugated secondary antibodies diluted 1:10,000 in blocking buffer for 1 h at room temperature after being washed in Tris-buffered saline with 0.1% Tween 20. Western blots were treated with ECL Western Blotting Detection Reagent (Amersham; GE Healthcare, Waukesha, WI, USA) to visualize the protein bands. Images were obtained using a Gel Documentation system (Azure Biosystems, Dublin, CA, USA). The following commercial primary antibodies were used at the indicated dilutions: anti-MBP (1:5000), anti-NG2 (1:5000), anti-CC1 (1:5000, Abcam, Cambridge, MA, USA), and anti–β-actin (1:5000; ImmunoWay, Plano, TX, USA).
Electron microscopy
After 18 sessions of PA training, PA-trained rats and home cage control were perfused with Karnovsky’s fixative [2% glutaraldehyde [16000; Electron Microscopy Sciences (EMS), Hatfield, PA, USA] and 4% paraformaldehyde (15700; EMS) in 0.1 M sodium cacodylate (12300; EMS), pH 7.4] by transcardial perfusion. ACC was resected from brain (∼1 × 1 × 2 mm3) and postfixed in primary fixative followed by secondary fixative [2% osmium tetroxide (19100; EMS) in 0.1 M cacodylate buffer (pH 7.4) with 0.5% potassium ferrocyanide in the dark] for 2 h at room temperature. Samples were then washed with 0.1 M cacodylate buffer, followed by washing with double-distilled water. Samples were then dehydrated with increasing ethanol concentration, later replaced by acetone concentration. Next, samples were infiltrated with plastic EMbed-812 resin (14120; EMS). Finally, sections were taken between 75 and 90 nm on a Leica Ultracut S (Leica) and mounted on carbon-coated slot grids (FCF2010-Cu; EMS). Grids were contrast stained for 30 s in 3.5% uranyl acetate in 50% acetone, followed by staining in 0.2% lead citrate for 30 s. Samples were imaged using a Tecnai 12 transmission electron microscope (Thermo Fisher Scientific), and images were captured using a Gatan Bioscan digital camera (Gatan, Pleasanton, CA, USA) at low to higher magnification. The G ratios were measured as the ratio of diameter of axon to the diameter of axon with myelin sheath. For each group, 160 to 250 axons were measured. Myelinated fiber density was examined by measuring the number of myelinated axons per ×8000 electron micrograph. Approximately 20 such images were counted per animal, and the ordinary number of myelinated fibers per image was calculated for each animal.
Electrophysiology
Surgery for implanting multiple-channel electrodes
Eight out of 11 rats were randomly selected to implant multiple-channel electrodes for electrophysiological recordings before and after LPC induction. The detailed procedures of electrode implantation have been described in our previous publications (28, 31–36). After 2 wk of handling, rats were implanted with 16-channel microwire electrodes (polyimide-insulated platinum-iridium microwires with 20–50 kΩ impedance, electrode diameter = 25 μm, 4 × 4 arrangement with 250 μm electrode spacing) in the ACC (anterior-posterior 3.0–3.3 mm, medial-lateral 0.6–1.0 mm, dorsal-ventral 1.5–3.5 mm from dura). In this study, the ACC was defined as the cingulate cortex, area 2, and the prelimbic cortex with overlying cingulate cortex, area 1. A screw implanted in the occipital bone above the cerebellum was used as a reference. Then, the recording electrodes were advanced slowly into the brain using a micropositioner until the clear neuronal firings in most recording channels were observed online (OmniPlex System; Plexon, Dallas, TX, USA). Electrodes were secured to the rat’s skull using dental cement until clear spike signals could be observed in more than half of 16 channels.
Electrophysiological signal acquisition
After 6–8 d of recovery from implantation surgery of multiple-channel microwire electrode arrays, a 16-channel preamplifier head-stage (gain × 20) was connected to the implanted electrodes in the ACC. Both the local field potential (LFP) and extracellular spike activities were recorded using a multiple-channel neural data acquisition system (Omniplex D; Plexon). Spike signals were amplified (×1000), band-pass filtered (0.3–5 kHz, 4-pole Bessel), and sampled at 40 kHz. LFPs were amplified (×1000), band-pass filtered (0.05–200 Hz, 4-pole Bessel), and sampled at 1 kHz. Data was recorded for 2 min in each rat in the quiet waking state in their home cages at the following time points: before LPC infusion as baseline and 1, 5, 9, 14 d postinfusion.
Electrophysiological data analyses
Data analyses were performed using a combination of tools in MatLab (MathWorks, Natick, MA, USA), Neuroexplorer (Nex Technologies, Colorado Springs, CO, USA), and Offline Sorter (Plexon). Detailed procedures of multiple-channel data analyses have been described in our recent publications (28, 31–36).
Spectral analysis
To clarify alterations in the θ power spectra following LPC infusion, we calculated the ACC power spectral density (PSD). In order to achieve this, the raw LFPs of ACC were filtered between 1 and 20 Hz using noncausal 0-phase-shift filter (fourth-order Butterworth). The PSDs were characterized using multitaper estimates with a time-bandwidth product of 4 and 213 frequency bins (NeuroExplorer 5; Plexon). The overlapping percentage of each window is 50%. The power spectra were normalized so that the sum of all the spectrum values equaled the mean squared value of the signal. The PSD curve was smoothed with a gaussian filter (15 bins running average); θ band power was defined as the area under the curve of the frequencies between 4 and 10 Hz, and the band power from each animal was averaged over the 16 channels in the ACC (37).
Spike sorting
The single unit spike sorting was done using Offline Sorter software (v.4; Plexon). Automatic techniques were employed to separate waveforms into individual clusters using principal component analysis. The waveforms were also manually examined to confirm that the spike waveforms were consistent and that the cluster boundaries were clearly separated. A single unit was identified using the criterion of finding less than 3% of the spikes in the refractory period of 2 ms in the interspike interval histograms. The sorting quality of single units in each channel was assessed based on 2 statistical parameters: 1) multivariate ANOVA (probability threshold for significance was set to P < 0.01), which indicated that each of the unit clusters has a statistically different location in 3-dimensional space and that the clusters are statistically well separated, and 2) the nonparametric J3 statistic, which measured the ratio of between-cluster to within-cluster scatter. The per-unit sorting quality defined on a unit-by-unit basis was determined by the L-ratio and isolation distance as previously described (38). The L-ratio estimates the degree of noise contamination of a cluster; a smaller value represents a lower degree of noise contamination (38). The isolation distance estimates the mean distance expected between a cluster and an equal ensemble of spikes outside the cluster, and a larger value implies a well-isolated cluster.
SFC
An increasing body of study suggests that neuronal signal processes depend on the firing rates of neurons as well as on the timing of spikes relative to large-scale neuronal oscillations. The temporal coding is an important regulator in memory and perception. To measure the strength of synchrony between spike timing and phase of field potential oscillations, we quantified the SFC based on a previous publication (38).The SFC value varies from 0 to 100%, with higher values indicating a stronger strength of relationship between spike timing and LFP oscillation phase. To compute the SFC within the ACC, the spikes recorded in the ACC and the mean LFP from the ACC were used in the analyses. To ensure the validity of the statistical results, only neurons that had at least 50 spikes during the period analyzed were used for SFC analysis. For every spike, a segment of the LFP data centered on the spike ±480 ms was extracted. The spike-triggered average (STA) was calculated as the mean of all these sections. Then the spectrum of STA frequency (fSTA) was calculated using multitaper analysis in MatLab (39). The multitaper analysis applied in these analyses uses a series of discrete prolate spheroidal sequences (7 tapers) to give estimates of the PSD. The same method was then used to calculate the frequency spectra of each of the traces individually, then the mean of these individual frequency spectra results in the spike-triggered power (STP) as a function of fSTP(f). Finally, the SFC was calculated as the fSTA over the STP(f) as a percentage. SFC(f) = [fSTA(f) ÷ STP(f)] × 100%.
The phase-locking of single neuron to the θ oscillation
Furthermore, we plotted the phase distribution and analyzed Rayleigh’s test using custom-written Matlab scripts based on a previous publication (39), to study the angular distributions of spikes in relation to the ongoing θ oscillation and clarify the strength of phase-locking. To ensure the validity of the statistical results, only neurons with at least 50 spikes during the period analyzed were used for phase-locking estimation. First, 22 different frequencies ranging from 1.6 to 64 Hz were selected, such that f = 2x with x = (6/8, 8/8, 10/8, 12/8, …, 48/8). The LFP was then convolved with a series of Morlet wavelets centered about each of the selected frequencies, each with a length of 4 cycles. The wavelet transforms comprise a matrix of vectors whose absolute values (or length) and arguments (or angles) represent the amplitude and phase, respectively, of the LFP at frequency f and time t. The circular mean of the spike phases was calculated by taking the weighted sum of the cosine and sine of the angles at each spike location. Rayleigh’s test was used for identifying statistical significance to compare against uniformity by calculating the test statistic and a P value. A neuron was considered phase-locked in θ range if P < 0.0023, which is Bonferroni corrected (0.05) for multiple comparisons (0.05/22; 22 frequencies were tested).
Statistical analysis
All data were presented as means ± sem. Statistical comparisons were performed in SPSS v.19.0 (IBM SPSS, Chicago, IL, USA) or Prism v.7.0 (GraphPad Software, La Jolla, CA, USA). Comparisons were conducted with Student’s t test, factorial ANOVA, or nonparametric analyses where appropriate. Electrophysiological data were analyzed using a combination of tools in MatLab, Neuroexplorer, and Offline Sorter. PSD, SFC, spike phase-locking, and synchronization were analyzed as described in our recent publications (28, 31–34).
RESULTS
The HPC is required for PA schema memory assimilation and retrieval
To investigate the role of the HPC in schema memory assimilation and retrieval, lidocaine, a temporary signal blocker, was bilaterally infused into the HPC during the schema formation and memory consolidation after a certain level of learning. Rats (lidocaine n = 6, aCSF control n = 6, normal animals n = 6) were trained to acquire the original schema of 6 PAs, and memory retrieval was measured by a nonrewarded PT (Fig. 1A, B). During the drug treatment period, the PI of the lidocaine group decreased to chance level compared with that of controls, indicating that these rats were finding the correct cued location by chance and not by acquired memory. After withdrawal of lidocaine treatment at session 19, the PI increased gradually (Fig. 1A) but remained considerably lower than that of the control groups. To assess the memory retrieval of PAs, 3 nonrewarded PTs were conducted (Fig. 1A, B). The aCSF control group showed graded learning of the OPAs from session 1 to session 18 (Fig. 1B, F = 11.19, df = 2.61/10.47, P < 0.001), as evidenced by the increasing proportion of digging time at the cued location compared with the noncued location from PT3 (aCSF group, t = 8.476, df = 10, P < 0.0001). In contrast, the lidocaine-treated group showed no retrieval during the drug treatment period (Fig. 1C; PT3 at session 18; t = 1.264, df = 10, P = 0.235). The interaction between groups were significant (t = 8.271 df = 10, P < 0.0001). Lidocaine-treated rats showed significant memory retrieval in PT4 after several sessions of retraining without drug treatment (Fig. 1D, F = 2.94, t = 19.82, P < 0.001). Significant memory retrieval of new PAs was observed in both groups at PT5 24 h after training with 2 new PAs conducted at session 27 (Fig. 1D; aCSF group, F2, 15 = 42.73, P < 0.0001; lidocaine group, F2, 15 = 68.06, P < 0.0001) with no significant interaction in between groups (F2, 30 = 2.653, P = 0.086), and the digging time in cued location was better than chance (aCSF group, t = 7.14, df = 10, P < 0.0001; lidocaine group, t = 7.91, df = 10, P < 0.0001). Next, to investigate whether the HPC is required for new pair memory acquisition, we infused lidocaine 15 min prior to 2 new pair training and tested new pair memory 24 h later. PT6 showed that intrahippocampal lidocaine prevented the new PA learning (Fig. 1E; aCSF group, F2, 15 = 66.18, P < 0.0001; lidocaine group, F2, 15 = 16.89, P = 0.0869), and the interaction between groups was significant (F2, 30 = 46.22, P < 0.0001). Digging at the cued new location was significantly above chance only in the aCSF group (t = 15.29 df = 10, P < 0.0001) but lower in the lidocaine group (t = 1.490 df = 10, P = 0.1672). Then, using another group of rats, we studied whether the HPC is essential for new pair memory retrieval. After 25 sessions of training, we confirmed the rats have PI more than 70%, suggesting these rats had memory schema for PA. These rats were then trained with 2 new PAs, followed by infusion of lidocaine or aCSF in the HPC 24 h later, and then assessed for memory retrieval for new PAs in PT4 (Fig. 1F; see Supplemental Videos S1 and S2). PT4 showed that the lidocaine group had no significant change in the digging time of the new cued compared with noncued location (F2, 15 = 1.817, P = 0.1966), and the digging at the new cued location was below the chance level (t = 0.4841 df = 10, P = 0.638); however, the interaction in between groups was significant (F2, 27 = 26.32, P < 0.0001). Our results demonstrate that the HPC plays a key role in schematic learning, new memory acquisition, and new memory retrieval, which corroborates the findings in a previous report that had used hippocampal lesions (9).
PA schema is dependent on the ACC
Next, to further clarify the role of the ACC in PA schema formation, we performed bilateral infusion of lidocaine in ACC (Fig. 2A). In the first experiment, using a different group of rats (n = 12), we infused lidocaine or aCSF in ACC during sessions 10–17. The PI of the lidocaine infusion group dropped at the start of the treatment period compared with that of the aCSF infusion group (Fig. 2B). Memory retrieval was assessed through 3 nonrewarded PTs (PT1–PT3). The control group revealed a graded improvement in memory retrieval of the OPAs (Fig. 2C, F = 12.24, df = 3.64/18.23; P < 0.0001; n = 6 rats/group), whereas no memory retrieval was observed in the lidocaine group at PT3 (Fig. 2C, F = 0.33, P > 0.05; n = 6 rats per group), and the interaction was significant in between groups (t = 6.883, df = 10, P < 0.0001). Digging at the cued new location was significantly above chance only in the aCSF group (t = 15.29 df = 10, P < 0.0001) but lower in the lidocaine group (t = 1.490 df = 10, P = 0.1672). Lidocaine-treated rats showed significant memory retrieval in PT4 after several sessions of retraining without drug treatment (Fig. 2D; aCSF group, F2, 12 = 19.10, P = 0.0002; lidocaine group, F2, 12 = 16.86, P < 0.0003) with no significant interaction in between groups (F2, 24 = 3.27; P = 0.05), and the digging time in cued location was better than chance (aCSF group, t = 4.16, df = 8, P < 0.0031; lidocaine group, t = 9.26, df = 8, P < 0.0001). Next, we examined the role of ACC in new PA memory acquisition; lidocaine was infused 15 min prior to training on 2 new PAs. No significant difference of digging time of different cued holes was found in the lidocaine group in PT6 (Fig. 2E; aCSF group, F2, 15 = 74.83, P < 0.0001; lidocaine group, F2, 15 = 0.96, P = 0.40), and the interaction between groups was significant (F2, 30 = 43.27, P < 0.0001), indicating that ACC is essential for new PA memory acquisition. Then, to verify whether ACC is important for new pair memory retrieval, using another group of rats (PA = 6 rats and control = 5 rats), we infused lidocaine in ACC, 24 h after new PA training, and then tested new PA memory retrieval. Again, no significant change was found in the lidocaine group (Fig. 2F, t = 1.55, P < 0.05).
Figure 2.
Intra-ACC lidocaine infusions prevent memory consolidation, new learning, and memory retrieval. A) Experimental design showing timeline of schema training, new PA learning, and memory retrieval. Syringes indicate the time of bilateral intra-ACC infusions of 4% lidocaine [15 min before each session (S), S10–17]. B) PI during the acquisition of the OPAs and new PAs (S1–24). Lidocaine was injected bilaterally into the ACC during S10–17. PI rose to around 65% at S8 and decreased to around 50% (chance level) after lidocaine infusion (n = 6 rats/group). C) Nonrewarded cued-recall PTs for the acquisition of original schema across S3, S9, S18, and S24. The graph represents the percentage of dig time at the cued location (white bars) relative to that of the noncued locations (black bars). D) Memory recall in PT5 for the new PA (white bar) in aCSF and lidocaine infusion group, 24 h after a single session of training with 2 new PAs. E) Impact of intra-ACC lidocaine infusion on acquisition of new PAs in PT6 (lidocaine infusion 15 min before new PA acquisition; n = 6 rats/group) The graphs represents the percentage of dig time at the new cued location (white bars), the new noncued location (striped bars), and the old noncued locations (black bars) during PT6 after the aCSF and lidocaine infusion conditions. F) Impact of intra-ACC lidocaine infusion on retrieval of new PAs in PT4 by performing lidocaine or aCSF infusion 15 min before PT4 (lidocaine, n = 6 rats; aCSF, n = 5 rats). The graphs represent the percentage of dig time at the new cued location (white bars), the new noncued location (striped bars), and the old noncued locations (black bars) in the aCSF and lidocaine conditions. NPA, new PA; ns, not significant. Results are expressed as means ± sem. *P < 0.05; ***P < 0.001 for Student’s t tests comparing the proportion of digging for the cued PAs with the chance level of 16.67%, ^^^P < 0.001 for Student’s t tests comparing the proportion of cued digging in lidocaine vs. aCSF infused rats.
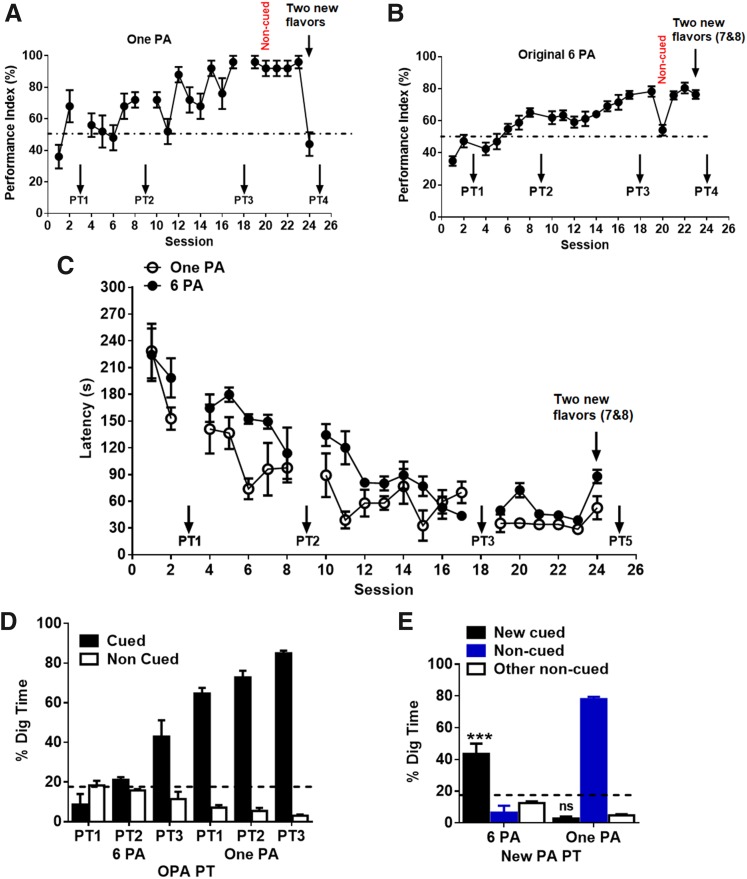
One PA training does not establish schema for rapid consolidation of new PAs
Rats trained with 1 PA training showed acquisition of 1 PA after sessions 19–23 (PI ≈ 85%, Fig. 3A) similar to that of the 6-PA training (PI ≈ 78%, Fig. 3B), and the latency to collect the food pellets was faster with training after sessions 19–23 (latency ≈ 30 s, Fig. 3C), similar to that of the 6-PA training (latency ≈ 40 s). PT1–3 revealed that rats can retrieve 1 PA memory (Fig. 3D). But after training of new PAs for a single session (session 24), new PA memory was not observed in PT4 in 1-PA trained rats (new cued digging time <16.6%, Supplemental Fig. S1E and Fig. 3E), whereas 6-PA trained rats showed effective memory retrieval.
Figure 3.
One PA training does not establish schema for rapid consolidation of new PAs. A) PI during the acquisition of the 1 PA (sessions 1–24). PI reached <70% by second session. Removing cue flavours from the start box on session 20 caused less effects on PI. Changing new PA at session 23 caused dropping of PI at chance level. B) PI during the acquisition of the 6 OPAs (sessions 1–24). PI increases gradually over the training. Removing cue flavors from the start box on session 20 caused in performance decreases and then returning to nearly 70% on the next normal session (session 21). Rats can learn 2 new PAs by a single session (session 23). C) Latency during the acquisition of OPAs and new PAs in the 6PA group and 1 PA group respectively (sessions 1–24). Latency improves with the training sessions in both 1 PA and 6 PA trained rats. D) Nonrewarded PTs for the acquisition of OPAs across sessions 3, 9, and 18. The graph represents the percentage of dig time at the cued location (white bars) relative to the noncued locations (black bars). E) Memory recall in PT4 for new PA (white bar) in 6 PA and one PA group, 24 h after a single session of training with new PA. Ns, not significant. Results are expressed as means ± sem. ***P < 0.001 (Student’s t test, percent digging time at the cued location vs. chance level of 16.67%).
Prolonged PA training promotes adaptive myelination in ACC
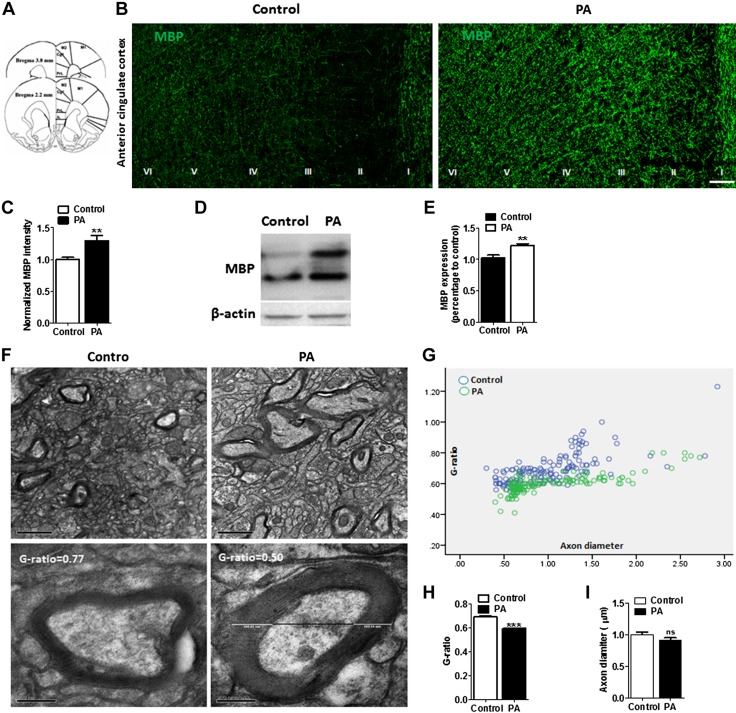
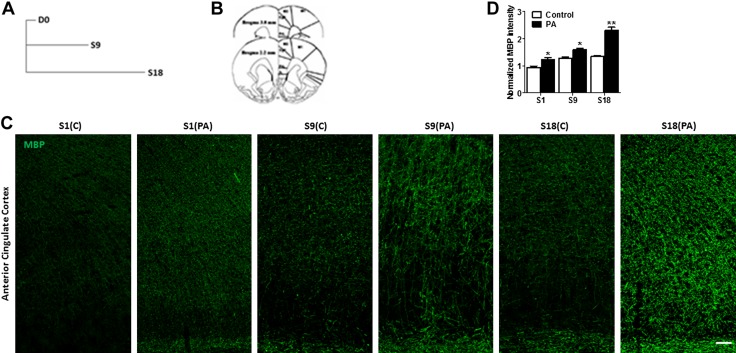
The expression of MBP, a biomarker for myelin fibers that encase axons, was examined in 2 groups of rats: 6 PAs and home cage control. Higher MBP+ expression was found in the ACC of the 6-PAs group compared with that of controls, and a greater proportion MBP+ immunofluorescence intensity was observed in ACC compared with that of controls (Fig. 4A, B). Quantitative analysis revealed a higher intensity of MBP+ fibers in the 6-PAs groups compared with the controls (Fig. 4C; control, 0.9970 ± 0.03487; PA, 1.293 ± 0.08792; n = 6 rats/group). Western blot analyses of protein extracted from the ACC also revealed higher MBP expression in PA rats compared with that of control (Fig. 4D, E; control, 1.028 ± 0.04246; PA, 1.220 ± 0.03235; n = 6 rats/group). Ultrastructural analysis performed using electron microscopy (EM) further showed that the myelin sheath thickness is increased in PA trained rats (Fig. 4F). Quantitative assessment of the G ratio of myelinated fibers revealed greater thickness of myelin sheath in PA rats than the control (Fig. 4G, H; control, 0.6447 ± 0.006932, n = 150; PA, 0.5803 ± 0.004616, n = 203; n = 3 rats/group; P = 0.0001), whereas the thickness of axons had not been altered (Fig. 4I; control, 1.005 ± 0.03556, N = 150; PA, 0.9435 ± 0.03540, n = 203; not significant, P = 0.23). Furthermore, the dynamic changes in myelination during the course PA training was studied. The rats were euthanized after sessions 1, 9, and 18 after PA training (Fig. 5A), and the MBP in the ACC region were analyzed (Fig. 5B). The PA training was found to gradually increase the myelination in the ACC region compared with that of untrained control rats (Fig. 5C, D).
Figure 4.
Prolonged PA training promotes adaptive myelination in rats. A) Schematic picture reconstructing the location of ACC (gray outlining). Cc, corpus callosum; Cg1, cingulate cortex, area 1; Cg2, cingulate cortex, area 2; PrL, prelimbic cortex. B) Representative confocal images of MBP in the ACC region show enhanced expression of MBP in PA trained rats compared with untrained controls. Scale bar, 100 µm. C) Relative intensity of MBP fluorescence in the ACC (layer I–VI), sections from 2.2 to 3.8 mm from the bregma. The MBP mean fluorescent intensity (n = 6) was increased after PA training (n = 6). D) Western blot images of MBP and β-actin in the ACC extracts from untrained control and PA trained rats using anti-MBP antibody. E) Mean MBP intensity analysis revealed that PA trained rats have higher myelin intensity compared with control rats (n = 6 rats/group). F) EM images of ACC showing myelinated axons in control and PA rats (n = 3 rats/group). Scale bar, 1 µm (upper panel). Lower panel shows representative EM pictures of myelinated axon at higher magnification (scale bar, 200 nm). G) Scatter plot of G-ratios in control rats (blue circle, n = 3 rats) and PA rats (green circle, n = 3 rats). H) Mean G ratio of myelinated axons in the ACC region in PA trained and untrained control rats. I) Axonal diameters do not change significantly between PA trained and control rats (n = 150–203 axons from 3 rats/group). Ns, not significant. Data are shown as means ± sem. **P < 0.01, ***P < 0.001 (unpaired Student’s t test).
Figure 5.
Dynamic changes of myelination in the rat ACC over the training. A) Experimental design showing timeline of MBP analysis at different time points over the training: day (D)0 [day before the start of training session (S)1], S9, S18. B) Schematic picture reconstructing the location of ACC (gray outlining). cc, corpus callosum; Cg1, cingulate cortex, area 1; Cg2, cingulate cortex, area 2; PrL, prelimbic cortex. C) Representative confocal images of MBP in the ACC region at different time points of PA training show that MBP expression was enhanced over the training in PA rats compared with home-cage control (C), which is from the same litter and of the same age as the PA rats. Scale bar, 50 µm. D) Relative intensity of MBP fluorescent in the ACC (layers I–VI), sections from 2.2 to 3.8 mm from the bregma. The MBP mean fluorescent intensity was increased after PA training. Data are shown as means ± sem (n = 6). *P < 0.05, **P < 0.01 (unpaired Student’s t test).
Prolonged PA training stimulates OPC proliferation and differentiation
To characterize the cells proliferating in response to PA training, we performed immunohistochemistry to determine the absolute number OPCs (NG2+ cells, a biomarker for OPC cells) and OLs (CC1+ cells, a biomarker for OLs) in the ACC of PA and control rats after intensive training (session 18) (Fig. 6A, B). We observed that the number of NG2+ OPCs was increased in PA rats compared with control (Fig. 6B, D; PA, 675.3 ± 3; control, 514.2 ± 15; P = 0.0069; n = 3/group). We further observed higher (13%) colabeling of NG2+ Olig2+ cells in PA rats than in controls, indicating reactive OPCs that had undergone differentiation (Fig. 6C, E). Again, we observed a significant increase in the number of mature OLs (Olig2+ CC1+) in PA rats than control (Fig. 6F–H; PA, 408.1 ± 15.32; control, 258.8 ± 24.41, P = 0.006; n = 3 rats/group). Furthermore, to study the effects of PA training on cell proliferation and differentiation, we administered thymidine analog EdU at the beginning of the PA training to label dividing cells and euthanized rats at different time points (3, 24 h, after sessions 9 and 18) (Fig. 6I). Three hours after the first session (session 1) of PA training, there was no significant increase in the density of NG2+ OPCs and the fraction of NG2+ OPCs that were EdU labeled (labeling index) in PA rats relative to control (Fig. 6J–L; P = 0.46 and 0.94, respectively; n = 3 rats/group). Twenty-four hours after session 1 of PA training there was significant increase (29.40%, P = 0.04, n = 3) in the fraction of NG2+ OPCs that were EdU labeled (labeling index) in PA rats relative to control (Fig. 6E, F). However, at sessions 9 and 18 there was a transient increase in the density of NG2+ OPCs (22.20%, P = 0.03; 23.48%, P = 0.007, respectively; n = 3 rats/group) and in the fraction of NG2+ OPCs that were EdU labeled (47.74%, P = 0.0005; 45.83%, P = 0.0007, respectively; n = 3 rats/group); this was also observed in PA rats relative to control.
Figure 6.
Prolonged PA training facilitates OPC proliferation and OLs production. A) Schematic figure showing the quantified area in the ACC (gray squares) for immunostained sections from 3.8 to 2.2 mm from the bregma. cc, corpus callosum; Cg1, cingulate cortex, area 1; Cg2, cingulate cortex, area 2; PrL, prelimbic cortex. B) Representative confocal imaging of OPCs (NG2+) cells in the ACC. Maximum intensity projection (from 10 successive confocal planes) showing double channel immunofluorescent labelling with NG2 (green) and the overlay with DAPI (blue). Scale bars: 20 μm. Data are shown as means ± sem (n = 3 rats/group). P > 0.05 C) Representative confocal micrograph of Olig2+ (red, a biomarker for oligodendrocyte transcription factor) colabelled with NG2+ (green) OPC cells in the ACC of PA and control rats. Scale bar, 20 μm. Inset showing Olig2+ (red) NG2+ OPC cells in higher magnification (scale bar, 2 μm). D) Total number of NG2+ OPC cells in the ACC of PA and control rats after S18. E) Total number of Olig2+ NG2+ OPC cells in the ACC of PA and control rats after S18. F) Representative confocal images of Olig2+ (red) CC1+ (green) OLs in the ACC of PA and control rats. Scale bars, 50 μm. Inset: EdU+ (red) NG2+ OPC cells in higher magnification (scale bar, 2 μm). G) Total number of OLig2+ CC1+ mature OLs cells in the ACC of PA and control rats after S18. H) Total number of Olig2+ CC1+ OLs cells in the ACC of PA and control rats after S18. I) Experimental timeline showing EdU protocol performed to assess OPC proliferation and OLs generation in ACC of PA and control rats. J) Representative confocal images showing colabelling of EdU (red) and NG2+ (green) OPC cells with an overlay of DAPI (blue) in the ACC of PA and control rats. Scale bars, 50 μm. Inset: EdU+ (red) NG2+ OPC cells at higher resolution (scale bar, 2 μm). K) Total number of NG2+ cells in the ACC of PA trained rats and untrained control rats at different time points of PA training. L) Total number of EdU+ NG2+ OPC cells in the ACC PA trained rats and untrained control rats at different time points of PA training. Prolonged PA training caused transient increase in the EdU labeling index of NG2+ OPCs in the ACC of PA rats after S9 and S18 of PA training. M) Representative confocal images showing colabelling of EdU (red) and CC1+ (green) Oligodedrocytes cells with an overlay of DAPI (blue) in the ACC of PA and control rats. Scale bars, 50 μm. Inset showing EdU+ (red) NG2+ OPC cells at higher resolution (scale bar, 2 μm). N) Total number of CC1+ oligodendrocyte (OL) cells in the ACC of PA trained rats and untrained control rats at different time points of PA training. O) Total number of EdU+ CC+ oligodendrocyte (OL) cells in the ACC PA trained rats and untrained control rats at different time points of PA training. Prolonged PA training caused transient increase in the EdU labeling index of CC1+ (OLs) in the ACC of PA rats after S9 and S18 of PA training. For all experiments shown, n = 3 rats/group at each time point. Results are expressed as means ± sem; n.s, not significant (P > 0.05), unpaired Student’s t test. *P < 0.05, **P < 0.01, ***P < 0.001.
To define the population dynamics and fate of OPC cells dividing in response to PA training, we repeated the above experiment but administered EdU for 7 consecutive days (session 9) intraperitoneally, trained rats for session 18 (Fig. 6I), and calculated the absolute CC1+ OL number and the fraction of CC1+ OLs that were EdU labeled. Three and 24 hours after 1 session, there was no significant increase in the density of CC1+ OLs (P = 0.70 and 0.90, respectively; n = 3 rats/group) or in the fraction of CC1+ OLs that were EdU labeled in PA rats relative to control (Fig. 6M; P = 0.43 and 0.45, respectively; n = 3 rats/group). However, a transient increase in the absolute number of CC1+ OLS (32.68%, P = 0.001; 36.58%, P = 0.006, respectively; n = 3 rats/group) and in the fraction of CC1+ OLs that were EdU labeled (41.21%, P = 0.001; 47.06%, P = 0.0002, respectively; n = 3 rats/group) was observed after sessions 9 and 18 of PA training in the ACC of PA rats relative to control (Fig. 6M–O; 41.31%, P = 0.001; 49.31%, P = 0.0002, respectively; n = 3 rats/group). Theoretically, enhanced myelination of the activated axons may be a result of myelin remodeling of existing axons supplemented by enhanced OL branching. The increase in EdU+ CC1+ cells observed in the PA group suggested that this effect may be mediated by the selective myelination of the activated axons by newly differentiated OLs (30).
Myelination is essential for ACC-dependent cognitive function
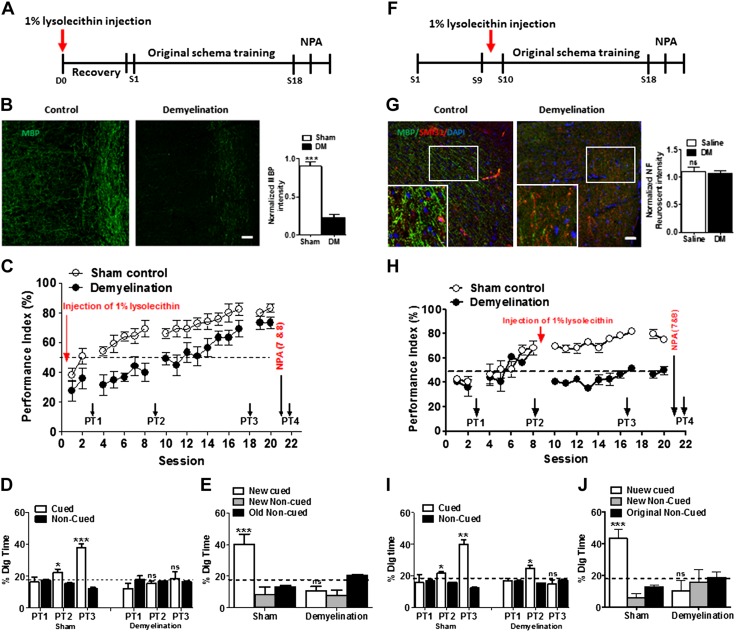
To verify the role of myelination in PA schema learning, an ACC-dependent cognitive function, we conducted LPC-induced focal demyelination restricted to the ACC before PA training (Fig. 7A). Seven days after focal injection into ACC, rats displayed severe demyelination in the region of ACC (Fig. 7B; F = 5.48, df = 2.11/6.34, n = 4; P < 0.035). Although LPC causes severe demyelination, no axonal damage was observed in the ACC (Fig. 7G; n = 3, P < 0.05). The PI of LPC group was lower than that of the sham-treated group, and the LPC group had no significant change in digging time during PT1, 2, 3, 4 (Fig. 7C–E; n = 6). Next, to determine whether myelination is necessary to conserve learned information, using a new group of rats, we induced focal demyelination in the ACC after session 10 of PA training (Fig. 7F; F = 3.39, df = 2.84/8.54, n = 4; P < 0.07). PTs revealed severe impairment in memory retrieval after demyelination, whereas control rats demonstrated effective memory retrieval. Furthermore, the demyelinated rats could not learn and retrieve the memory of new PAs (Fig. 7H–J).
Figure 7.
The effect of LPC-induced demyelination in the ACC on schema formation and memory retrieval. A) Experimental timeline showing intra-ACC LPC injection to cause focal demyelination followed by PA training. For control rats, saline was injected instead of LPC. B) Representative confocal images showing MBP+ myelinated fibers (green, upper panel) in the ACC region of demyelinated and control rats. Scale bar, 50 µm. The MBP mean fluorescent intensity was decreased in demyelinated rats (upper right) (n = 3 rats/group; unpaired Student’s t test). C) Focal ACC demyelination (n = 4 rats/group) in rats led to impairment in schema formation assessed by the PI [below the chance level until session (S)14]. D) Preferential digging during nonrewarded PTs (PT1–3) in demyelinated and control sham-treated rats (n = 4 rats/group). E) Preferential digging during nonrewarded PT for new PA (PT4) in demyelinated and control sham-treated rats (n = 4 rats/group). F) Experimental timeline showing intra-ACC LPC injection in S9 after PA training and followed by further training sessions after recovery. G) Representative confocal images showing MBP+ and SMI31+ (marker for axonal fiber) in the ACC region in demyelinated and control rats. Scale bar, 50 µm (n = 3 rats/group; unpaired Student’s t test). H) Focal ACC demyelination show drop in PI below the chance level after demyelination at S9 and causes impairment in schema formation (n = 4 rats/group; P < 0.001). I) Preferential digging during nonrewarded PTs (PT1–3) in demyelinated and control sham-treated rats. Nonrewarded cued-recall PTs for the acquisition of original schema across S9, S10, and S19 (n = 4 rats/group; unpaired Student’s t tests comparing the proportion of digging for the cued PAs with the chance level of 16.67%). J) Preferential digging during nonrewarded PT for new PA (PT4) in demyelinated and control sham-treated rats (n = 4 rats/group; Student’s t tests comparing the proportion of digging for the cued PAs with the chance level of 16.67%). DM, demyelination; NPA, new PA; ns, not significant (P > 0.05). Results are expressed as means ± sem. *P < 0.05, **P < 0.01, ***P < 0.001.
Furthermore, to look into whether this focal demyelination induces a general disruption of ACC function that is not specific to learning, we measured visceromotor reflex recordings to incremental pressures of colorectal distension (20, 40, 60, 80 mmHg) in control and demyelination rats. Measurement of visceral sensitivity in animals is mainly based on brain stem reflexes (29, 32, 33). Two-way ANOVA revealed that there was no difference in the factor of demyelination, indicating that demyelination into the ACC does not affect visceral pain sensation.
Electrophysiological characterization of LPC-induced ACC focal demyelination
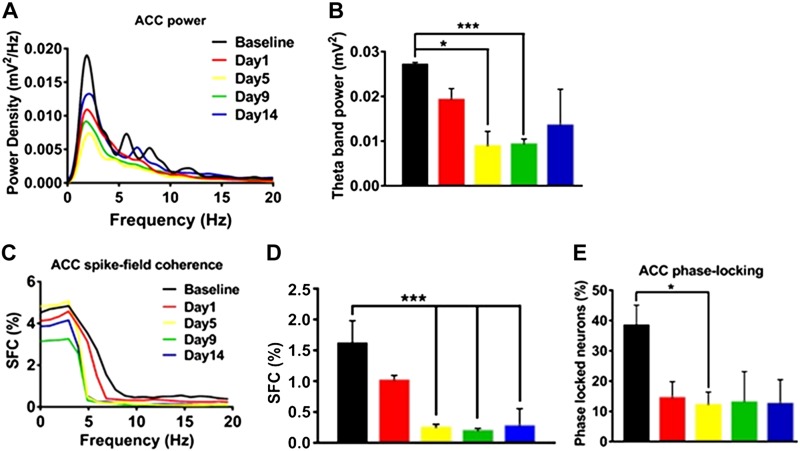
To study the involvement of demyelination in θ rhythm activity and the spike-timing synchrony of ACC single units, we recorded neuronal spikes and LFP simultaneously and analyzed SFC and phase-locking between ACC spikes and LFP. PSD analysis was performed during 120 s quiet waking state in LPC-infused rats having focal demyelination in the ACC region. The PSD in the frequency range 1–20 Hz and distributions of θ band power (4–10 Hz) in the ACC region 1, 5, 9, 14 d after LPC infusion (n = 4) are shown in Fig. 8A, B. Significant alterations of θ band power were detected from d 5 (Fig. 8B; 2-way ANOVA; P < 0.05). The mean θ band power was reduced on d 5 and d 9 after LPC infusion to 0.00885 ± 0.00135 and 0.00932 ± 0.00045, respectively, compared with the baseline θ band power of 0.02734 ± 0.00028 evaluated before LPC infusion.
Figure 8.
Decrease in ACC θ band power and reduction of SFC and phase-locking (between ACC spike and θ phase) following LPC-induced demyelination. A) PSD (including δ, θ, and β bands) of ACC LFP before LPC injection (n = 4 rats) (black color; baseline) and on d 1, 5, 9, and 14 following local LPC injection into ACC region. B) Mean ACC θ band power was decreased at d 5 and 9 after LPC-induced demyelination. C) The SFC distribution of ACC single units (n = 32 neurons and 4 rats) as a function of frequencies recorded at baseline and on d 1, 5, 9, and 14 following local LPC injection into the ACC region. D) Mean SFC values in θ band show reduction of θ band SFC in rats (n = 4) after LPC treatment. E) Mean percentages of phase-locked neurons show a reduction of ACC single-unit spikes phase-locking to the local θ band field potential after LPC treatment (n = 4). *P < 0.05, ***P < 0.001.
LPC infusion–associated demyelination in rats revealed a decrease in SFC values in the low frequency range compared with that of baseline (n = 4 rats; n = 15 neurons for baseline, n = 25 neurons for d 1, 5, and 9, and n = 18 neurons for d 14; Fig. 8C, D). The mean SFC values in θ range (4–10 Hz) were significantly decreased from 1.625 ± 0.397% to <0.248 ± 0.067% in d 5 after LPC infusion (t = 3.422, P = 0.0078, Student’s 2-tailed unpaired t test, Fig. 8D), and this reduction in SFC in the ACC region was maintained until d 14 after LPC infusion. In addition to that, the percentage of phase-locked neurons in the θ phase decreased from 38.75 ± 6.203% during baseline to 12.25% ± 6.232% in the infraorbital nerve (IoN)-CCI rats (P < 0.05, 1-way ANOVA; Fig. 8E). Altogether, our findings indicate that myelin plasticity is important to facilitate and shape learning and memory behavior, such as the quick assimilation of new information into existing schemas.
DISCUSSION
The concept that new learning interacts with existing memories is by no means new (2). It has been established that mental schemas, referred to as cognitive structures, are developed on the basis of several encounters with similar context (9, 40). The medial prefrontal cortex is thought to play a vital role for processing schema-related mnemonic function (41). This is also validated by lesion studies in both rodents (4, 42) and humans (41, 43). The mechanisms of the hippocampal-ACC interactions are essential to determine how decisions are guided by experience.
To start out, our data are consistent with previous findings (11, 40) that over the course of training, rats use spatial memory to find the correct flavored food location (Figs. 1 and 2). It has earlier been well demonstrated that the HPC is crucial for the learning of the PA schema (9). However, it is still unclear whether the HPC is necessary to completely establish the schema after a certain level of PA learning and consolidation. Here, we found that infusion of lidocaine, a temporal blocker, into the HPC from session 11 to 17 blocked the continuation of improvement in PA learning (Fig. 1A, B), indicating that the HPC is crucial for the full establishment of schema learning and consolidation. Furthermore, we observed by restarting PA training in session 19, the animals were able to meet the maximum PI by session 24, suggesting that the inhibitory effects of lidocaine in the HPC are fully reversible. To identify if the rats with hippocampal inactivation were still able to show a congruency benefit by learning new information, in this study, the lidocaine was administrated into the HPC 15 min before the new PA session. No memory retrieval was found during the PT, suggesting that the HPC is still necessary for the encoding of new PAs in the animals that had established schema (Fig. 1D). In a separate study, intrahippocampal lidocaine that was infused in the rats 24 h after learning new PA information was found to impair new PA memory retrieval (Fig. 1E). Considering previous reports that hippocampal lesion suppressed new PA memory retrieval (9), our data further point to the HPC’s role in memory acquisition and retrieval within the time window of 24 h following new PA consolidation.
To determine whether the ACC is essential for schema creation and activating appropriate schemas regardless of memory demands, we showed that lidocaine infusion into the ACC can affect PA learning and consolidation during training sessions (Fig. 1A, B) suggesting a critical role for the ACC in schema representation and activation. These results are also consistent with the clinical observation that patients with damaged medial prefrontal cortex who confabulate have difficulty activating task-relevant schemas and would make more errors during retrieval, perhaps reflecting an inability to utilize schema (43).
Our data are consistent with previous findings of HPC-prefrontal interaction during memory tasks (44); we showed a single trial of training is sufficient to develop memory of the new PAs (Figs. 1 and 2) (9, 10, 13). We showed that lidocaine infused into the ACC before a new PA PT impaired new memory retrieval 24 h later (Fig. 1E–H), suggesting that the assimilation of new PA memories into existing activated cortical schemas requires simultaneous cortical encoding and retrieval.
What is the biologic basis of schema-like learning and memory consolidation in the HPC-ACC network? A model used for a memory mechanism of cellular consolidation research is synaptic tagging and capture, proposed by Frey and Morris (45), which stipulates that neural signaling targets a particular synapse for plasticity-related protein trafficking, resulting in synaptic long-term potentiation and stabilized memory traces within neurons (46). A similar model, called behavioral tagging, considers the learning process at a broader behavioral level (47). Although the standard model of memory consolidation asserts that systems level consolidation occurs after learning, the interaction of hippocampal and neocortical ensembles can sometimes be very rapid when consolidation involves the interaction with activated associative schemas stored in the neocortex (48). Brain activity at the systems level is mostly rhythmic, and the interaction between different brain regions is crucial for complex information processing. Precisely timed pre- and postsynaptic activity creates neural oscillations (49) that synchronize neurons, resulting in coherence and plasticity between different brain areas (50). Recent computational analysis suggests that conduction delays between coupled oscillators in the brain can be sensitive to minute changes in conduction speed that are a consequence of delicate changes in myelin (46). Myelination of certain brain areas coincides with the cognitive and behavioral development over a lifetime (14). A multitude of studies have shown that myelin is a highly dynamic structure; changes in myelination are important in contributing to brain plasticity and learning (17). Myelination increases the speed of nerve impulse propagation along the axon and thus facilitates long-range oscillations and synchrony of spike time arrival between neurons in different brain areas (16, 51).
Therefore, increased myelination may facilitate activated schema model of systems level consolidation. Recently, our electrophysiological and behavioral studies have shown that coherence and synchronization within the ACC and between the ACC and basolateral amygdala play a key role in the regulation of executive behavioral experiences, such as decision making in rats (28, 32–36). However, the molecular mechanisms through which experiences affect learning and memory in the brain is unclear. In this context, the axon is covered by myelin, a multilayer fatty structure developed from mature OLs originating from OPCs that facilitate fast propagation of action potentials and conduction velocity in axons, critical for higher-level cognitive functions. Repeated neuronal firing may stimulate myelination of axons, whereas myelin remodeling increases the efficiency of neuronal activation. MRI studies in humans showed that learning a new language (52), playing piano (53), and complex motor tasks in adults (54, 55) promote myelination. Evidence from rodent studies suggests that motor learning requires newly produced OLs (22). In rodents and zebrafish, electrically active neurons signal to the surrounding OPCs that trigger differentiation and promote myelination (25, 56, 57). Nevertheless, the mechanism and the extent to which behavioral actions and experiences affect the production of OLs and myelin sheath formation are relatively unknown (58). In this study, we test our hypotheses that prolonged schema-like learning and memory retrieval experience regulate both oligodendrogenesis and myelination, which promise that relevant new information can be quickly assimilated into the existing activated cortical schemas. We show that prolonged training of schema-like learning facilitates OPC proliferation and oligodendrogenesis (Fig. 6), OL differentiation, and maturation associated with hypermyelination (Fig. 5). In the present study, a significant increase in OPC proliferation was observed within the ACC in sessions 7 and 18 following consecutive PA training. A large proportion of OPCs differentiated into mature OLs, increasing the density of newly produced (EdU+ CC1+) OLs in the ACC by 49.31%. Although the density of OPCs and newly produced OLs was less after session 1 (d 1), the proliferation and differentiation of OPCs into mature OLs were more protracted after sessions 7 and 18 compared with control (Fig. 6). Furthermore, the proliferation and differentiation of OPCs into mature OLs were more protracted in session 18 than in session 7 (Fig. 6). Trained rats exhibited an ∼35.53% rise in EdU incorporation rate among OPCs and an ∼23.48% increase in OPC density at session 18. This particular finding reinforces the likelihood that prolonged PA training–induced surge in OPC mitosis is a requirement for OL production (59). Here we observed greater myelination and increased myelin thickness within this neuronal circuit (Figs. 4 and 5) in PA rats than in control, which was dynamic in nature and significantly changed over the training period (Figs. 4 and 5). These data are consistent with previous studies found that rats and rhesus monkeys raised in a consistently stimulating and enriched environment exhibited increase myelination, which was correlated with improved cognition (60).
In this experiment, we showed that significant dynamic changes in ACC myelination follow the prolonged PA training acquisition at different time points (Fig. 6), indicating the plastic nature of myelin, which changes dynamically during the adult period in response to intensive training. We performed other studies for training with only 1 PA in the event arena for 25 sessions, similar to 6-PA training. We observed that the group of rats that performed 1-PA training failed to establish a schema and were unable to create new pair learning (Fig. 3). To clarify that the OL lineage dynamics and myelin plasticity in our 6-PA learning and memory retrieval model are not due to other covariates, such as arousal from handling, running around the large enclosure, consuming palatable rewards, and uncertainty of digging for potentially hidden food rewards, further studies are needed with equivalent handling but that do not have training relevant to schema learning and consolidation, as described previously in Tse et al. (9).
Is myelin necessary for hippocampal-ACC–dependent learning and memory? Animal studies have shown that maternal separation impairs prefrontal cortical myelination and cognitive functions (21). Local hippocampal demyelination induced by LPC is associated with spatial learning disability (61). Until now it has not been clear whether schema-like learning and memory retrieval require myelin plasticity. Here, we used a PA behavior paradigm and showed that the ACC region was demyelinated. LPC is a detergent-like membrane solubilizing agent that disrupts myelin lipids (29). Plemel et al. (62) reported that LPC integrated into cellular membranes and rapidly induced cell membrane permeability, nonspecifically disrupting myelin lipids in mice. Now, we have shown that demyelination of the ACC impaired PA learning and memory retrieval (Fig. 7C, D).
Myelin produced by mature OLs facilitates rapid propagation of action potentials along axons. Investigators have reported that minor changes in myelin thickness produce significant alterations to conduction speed and neural circuit function (51). To clarify the mechanistic role of experience-induced adaptive myelination in modulating neural function, in this study we provide evidence that ACC LPC-induced demyelination (Fig. 8B, F) led to a decrease in ACC θ band power and reduced SFC and phase-locking (Fig. 8). These data suggest a key role of myelination for long-lasting oscillations and coherence of spike time arrival in the neurons. Thus, brain network synchronization requires active central myelination. These findings are consistent with our other recent findings in the rat with chronic visceral pain (31, 33) and the maternally separated rat (34), which show that a decision-making deficit is associated with disruption of SFC and desynchronized θ waves between the basolateral amygdala and ACC (33, 34). However, we realized that further studies are wanted to demonstrate the direct effects of behaviorally relevant brain neuronal activity on myelin plasticity in vivo. Using a drug that depletes myelination seems likely to be a very general kind of disruption that may simply damage the function of the ACC in general. Therefore, we have performed colorectal distention to measure visceromotor reflex (28, 31, 32) after focal demyelination into the ACC and found no changes compared with saline control rats (unpublished results) indicating that focal demyelination could not deplete ACC function in general; rather it specifically blocks multiple PA learning and memory. Further study may be needed to determine if ACC demyelination is affected in a more general way when ACC-dependent learning is conducted.
Notice that our studying schemas in the sense of Tse et al. and others (4, 9, 42) does not bear on the large body of work regarding schemas in human cognitive psychology. Furthermore, with the present study, we may not be able to distinguish between different possibilities: that neuronal activity causes myelination of unmyelinated axons, that new OLs are responsible for the addition of new myelin into existing myelin sheaths, and whether new myelin formed by existing OLs increase the thickness of the myelin sheath. Platelet-derived growth factor receptor has been identified as a factor responsible for proliferation of astrocytes and OPCs (63). Further studies are wanted to address this question.
In summary, using a rat model to monitor PA learning and memory retrieval, we showed that the schema (in the sense of the previous publication, ref. 9) acquisition of 6 PAs was gradually established. Then, rats learned 2 new PAs that were encoded and assimilated into the existing schema rapidly. The roles of the HPC and ACC on different time points of encoding, consolidation, and memory recall were identified. Learning a complex PA behavior task is associated with an increase in newly formed OPCs and mature OLs as well as myelin plasticity in the ACC regions. ACC demyelination impaired PA learning, correlated with decrease in ACC θ band power and reduced SFC and phase-locking in ACC.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the Research Grants Council (RGC) of Hong Kong (11100914, 11166116, 11101315, and 160713), the National Natural Science Foundation of China (NSFC) and RGC Joint Research Scheme (N_CityU114/17 and 3171101014), the Innovation and Technology Support Programme (ITS/300/15), City University of Hong Kong Neuroscience Research Infrastructure (9610211), and Centre for Biosystems, Neuroscience, and Nanotechnology (9360148). The authors express their gratitude to Dr. Thomas A. Cleland and Dr. David Smith (Department of of Psychology, Cornell University, Ithaca, NY, USA) for their valuable comments during our preliminary data presentation. The authors declare no conflicts of interest.
Glossary
- ACC
anterior cingulate cortex
- aCSF
artificial cerebrospinal fluid
- EdU
5-ethynyl-2′-deoxyuridine
- EM
electron microscopy
- EMS
Electron Microscopy Sciences
- fSTA
STA frequency
- HPC
hippocampus
- LFP
local field potential
- LPC
lysolecithin
- MBP
myelin basic protein
- NG2
neural-glial antigen 2
- OL
oligodendrocyte
- Olig2
OL transcription factor 2
- OPA
original PA
- OPC
OL progenitor cell
- PA
flavor-place paired associate
- PI
performance index
- PSD
power spectral density
- PT
probe test
- SFC
spike-field coherence
- STA
spike-triggered average
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
Y. Li, acquired funding and conceived the study; M. Hasan, M. S. Kanna, W. Jun, A. S. Ramkrishnan, and Z. Iqbal, carried out experiments and analyzed data and prepared the draft of manuscript and the responses to reviewers; Y. Lee helped to analyze the immunohistochemistry data; and M. Hasan and Y. Li wrote the manuscript. All authors gave final approval of the manuscript as submitted.
REFERENCES
- 1.Engel A. K., Fries P., Singer W. (2001) Dynamic predictions: oscillations and synchrony in top-down processing. Nat. Rev. Neurosci. 2, 704–716 [DOI] [PubMed] [Google Scholar]
- 2.Schlichting M. L., Preston A. R. (2015) Memory integration: neural mechanisms and implications for behavior. Curr. Opin. Behav. Sci. 1, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maguire E. A., Frith C. D., Morris R. G. M. (1999) The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain 122, 1839–1850 [DOI] [PubMed] [Google Scholar]
- 4.Van Kesteren M. T. R., Ruiter D. J., Fernández G., Henson R. N. (2012) How schema and novelty augment memory formation. Trends Neurosci. 35, 211–219 [DOI] [PubMed] [Google Scholar]
- 5.Knierim J. J. (2015) The hippocampus. Curr. Biol. 25, R1116–R1121 [DOI] [PubMed] [Google Scholar]
- 6.Zeithamova D., Dominick A. L., Preston A. R. (2012) Hippocampal and ventral medial prefrontal activation during retrieval-mediated learning supports novel inference. Neuron 75, 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teixeira C. M., Pomedli S. R., Maei H. R., Kee N., Frankland P. W. (2006) Involvement of the anterior cingulate cortex in the expression of remote spatial memory. J. Neurosci. 26, 7555–7564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benchenane K., Peyrache A., Khamassi M., Tierney P. L., Gioanni Y., Battaglia F. P., Wiener S. I. (2010) Coherent theta oscillations and reorganization of spike timing in the hippocampal- prefrontal network upon learning. Neuron 66, 921–936 [DOI] [PubMed] [Google Scholar]
- 9.Tse D., Langston R. F., Kakeyama M., Bethus I., Spooner P. A., Wood E. R., Witter M. P., Morris R. G. M. (2007) Schemas and memory consolidation. Science 316, 76–82 [DOI] [PubMed] [Google Scholar]
- 10.Tse D., Takeuchi T., Kakeyama M., Kajii Y., Okuno H., Tohyama C., Bito H., Morris R. G. M. (2011) Schema-dependent gene activation and memory encoding in neocortex. Science 333, 891–895 [DOI] [PubMed] [Google Scholar]
- 11.Bethus I., Tse D., Morris R. G. M. (2010) Dopamine and memory: modulation of the persistence of memory for novel hippocampal NMDA receptor-dependent paired associates. J. Neurosci. 30, 1610–1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frankland P. W., Bontempi B. (2005) The organization of recent and remote memories. Nat. Rev. Neurosci. 6, 119–130 [DOI] [PubMed] [Google Scholar]
- 13.Wang S. H., Tse D., Morris R. G. M. (2012) Anterior cingulate cortex in schema assimilation and expression. Learn. Mem. 19, 315–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill R. A., Li A. M., Grutzendler J. (2018) Lifelong cortical myelin plasticity and age-related degeneration in the live mammalian brain. Nat. Neurosci. 21, 683–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fields R. D. (2010) Neuroscience. Change in the brain’s white matter. Science 330, 768–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fields R. D. (2015) A new mechanism of nervous system plasticity: activity-dependent myelination. Nat. Rev. Neurosci. 16, 756–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fields R. D. (2013) Neuroscience: map the other brain. Nature 501, 25–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huntenburg J. M., Bazin P. L., Goulas A., Tardif C. L., Villringer A., Margulies D. S. (2017) A systematic relationship between functional connectivity and intracortical myelin in the human cerebral cortex. Cereb. Cortex 27, 981–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu J., Dietz K., DeLoyht J. M., Pedre X., Kelkar D., Kaur J., Vialou V., Lobo M. K., Dietz D. M., Nestler E. J., Dupree J., Casaccia P. (2012) Impaired adult myelination in the prefrontal cortex of socially isolated mice. Nat. Neurosci. 15, 1621–1623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makinodan M., Rosen K. M., Ito S., Corfas G. (2012) A critical period for social experience-dependent oligodendrocyte maturation and myelination. Science 337, 1357–1360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Y., Cheng Z., Tang H., Jiao H., Sun X., Cui Q., Luo F., Pan H., Ma C., Li B. (2017) Neonatal maternal separation impairs prefrontal cortical myelination and cognitive functions in rats through activation of Wnt signaling. Cereb. Cortex 27, 2871–2884 [DOI] [PubMed] [Google Scholar]
- 22.McKenzie I. A., Ohayon D., Li H., de Faria J. P., Emery B., Tohyama K., Richardson W. D. (2014) Motor skill learning requires active central myelination. Science 346, 318–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sampaio-Baptista C., Johansen-Berg H. (2017) White matter plasticity in the adult brain. Neuron 96, 1239–1251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zatorre R. J., Fields R. D., Johansen-Berg H. (2012) Plasticity in gray and white: neuroimaging changes in brain structure during learning. Nat. Neurosci. 15, 528–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gibson E. M., Purger D., Mount C. W., Goldstein A. K., Lin G. L., Wood L. S., Inema I., Miller S. E., Bieri G., Zuchero J. B., Barres B. A., Woo P. J., Vogel H., Monje M. (2014) Neuronal activity promotes oligodendrogenesis and adaptive myelination in the mammalian brain. Science 344, 1252304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barres B. A., Raff M. C. (1993) Proliferation of oligodendrocyte precursor cells depends on electrical activity in axons. Nature 361, 258–260 [DOI] [PubMed] [Google Scholar]
- 27.Demerens C., Stankoff B., Logak M., Anglade P., Allinquant B., Couraud F., Zalc B., Lubetzki C. (1996) Induction of myelination in the central nervous system by electrical activity. Proc. Natl. Acad. Sci. USA 93, 9887–9892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J., Zhang X., Cao B., Liu J., Li Y. (2015) Facilitation of synaptic transmission in the anterior cingulate cortex in viscerally hypersensitive rats. Cereb. Cortex 25, 859–868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hall S. M. (1972) The effect of injections of lysophosphatidyl choline into white matter of the adult mouse spinal cord. J. Cell Sci. 10, 535–546 [DOI] [PubMed] [Google Scholar]
- 30.Xiao L., Ohayon D., McKenzie I. A., Sinclair-Wilson A., Wright J. L., Fudge A. D., Emery B., Li H., Richardson W. D. (2016) Rapid production of new oligodendrocytes is required in the earliest stages of motor-skill learning. Nat. Neurosci. 19, 1210–1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang J., Tu J., Cao B., Mu L., Yang X., Cong M., Ramkrishnan A. S., Chan R. H. M., Wang L., Li Y. (2017) Astrocytic l-lactate signaling facilitates amygdala-anterior cingulate cortex synchrony and decision making in rats. Cell Rep. 21, 2407–2418 [DOI] [PubMed] [Google Scholar]
- 32.Fan J., Wu X., Cao Z., Chen S., Owyang C., Li Y. (2009) Up-regulation of anterior cingulate cortex NR2B receptors contributes to visceral pain responses in rats. Gastroenterology 136, 1732–1740.e3 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 33.Cao B., Wang J., Mu L., Poon D. C. H., Li Y. (2016) Impairment of decision making associated with disruption of phase-locking in the anterior cingulate cortex in viscerally hypersensitive rats. Exp. Neurol. 286, 21–31 [DOI] [PubMed] [Google Scholar]
- 34.Cao B., Wang J., Zhang X., Yang X., Poon D. C. H., Jelfs B., Chan R. H. M., Wu J. C. Y., Li Y. (2016) Impairment of decision making and disruption of synchrony between basolateral amygdala and anterior cingulate cortex in the maternally separated rat. Neurobiol. Learn. Mem. 136, 74–85 [DOI] [PubMed] [Google Scholar]
- 35.Cao B., Wang J., Shahed M., Jelfs B., Chan R. H. M., Li Y. (2016) Vagus nerve stimulation alters phase synchrony of the anterior cingulate cortex and facilitates decision making in rats. Sci. Rep. 6, 35135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mu L., Wang J., Cao B., Jelfs B., Chan R. H., Xu X., Hasan M., Zhang X., Li Y. (2015) Impairment of cognitive function by chemotherapy: association with the disruption of phase-locking and synchronization in anterior cingulate cortex. Mol. Brain 8, 32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Von Nicolai C., Engler G., Sharott A., Engel A. K., Moll C. K., Siegel M. (2014) Corticostriatal coordination through coherent phase-amplitude coupling. J. Neurosci. 34, 5938–5948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lu Y., Zhong C., Wang L., Wei P., He W., Huang K., Zhang Y., Zhan Y., Feng G., Wang L. (2016) Optogenetic dissection of ictal propagation in the hippocampal-entorhinal cortex structures. Nat. Commun. 7, 10962; erratum: 12019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rutishauser U., Ross I. B., Mamelak A. N., Schuman E. M. (2010) Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 464, 903–907 [DOI] [PubMed] [Google Scholar]
- 40.Ghosh V. E., Moscovitch M., Melo Colella B., Gilboa A. (2014) Schema representation in patients with ventromedial PFC lesions. J. Neurosci. 34, 12057–12070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Van Kesteren M. T., Fernández G., Norris D. G., Hermans E. J. (2010) Persistent schema-dependent hippocampal-neocortical connectivity during memory encoding and postencoding rest in humans. Proc. Natl. Acad. Sci. USA 107, 7550–7555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Richards B. A., Xia F., Santoro A., Husse J., Woodin M. A., Josselyn S. A., Frankland P. W. (2014) Patterns across multiple memories are identified over time. Nat. Neurosci. 17, 981–986 [DOI] [PubMed] [Google Scholar]
- 43.Squire L. R. (2007) Neuroscience. Rapid consolidation. Science 316, 57–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takehara-Nishiuchi K., McNaughton B. L. (2008) Spontaneous changes of neocortical code for associative memory during consolidation. Science 322, 960–963 [DOI] [PubMed] [Google Scholar]
- 45.Frey U., Morris R. G. (1997) Synaptic tagging and long-term potentiation. Nature 385, 533–536 [DOI] [PubMed] [Google Scholar]
- 46.Barco A., Lopez de Armentia M., Alarcon J. M. (2008) Synapse-specific stabilization of plasticity processes: the synaptic tagging and capture hypothesis revisited 10 years later. Neurosci. Biobehav. Rev. 32, 831–851 [DOI] [PubMed] [Google Scholar]
- 47.Moncada D., Ballarini F., Viola H. (2015) Behavioral tagging: a translation of the synaptic tagging and capture hypothesis. Neural Plast. 2015, 650780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morris R. G. M. (2006) Elements of a neurobiological theory of hippocampal function: the role of synaptic plasticity, synaptic tagging and schemas. Eur. J. Neurosci. 23, 2829–2846 [DOI] [PubMed] [Google Scholar]
- 49.Markram H., Lübke J., Frotscher M., Sakmann B. (1997) Regulation of synaptic efficacy by coincidence of postsynaptic APs and EPSPs. Science 275, 213–215 [DOI] [PubMed] [Google Scholar]
- 50.Buzsáki G., Draguhn A. (2004) Neuronal oscillations in cortical networks. Science 304, 1926–1929 [DOI] [PubMed] [Google Scholar]
- 51.Pajevic S., Basser P. J., Fields R. D. (2014) Role of myelin plasticity in oscillations and synchrony of neuronal activity. Neuroscience 276, 135–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schlegel A. A., Rudelson J. J., Tse P. U. (2012) White matter structure changes as adults learn a second language. J. Cogn. Neurosci. 24, 1664–1670 [DOI] [PubMed] [Google Scholar]
- 53.Bengtsson S. L., Nagy Z., Skare S., Forsman L., Forssberg H., Ullén F. (2005) Extensive piano practicing has regionally specific effects on white matter development. Nat. Neurosci. 8, 1148–1150 [DOI] [PubMed] [Google Scholar]
- 54.Scholz J., Klein M. C., Behrens T. E. J., Johansen-Berg H. (2009) Training induces changes in white-matter architecture. Nat. Neurosci. 12, 1370–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sampaio-Baptista C., Khrapitchev A. A., Foxley S., Schlagheck T., Scholz J., Jbabdi S., DeLuca G. C., Miller K. L., Taylor A., Thomas N., Kleim J., Sibson N. R., Bannerman D., Johansen-Berg H. (2013) Motor skill learning induces changes in white matter microstructure and myelination. J. Neurosci. 33, 19499–19503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hines J. H., Ravanelli A. M., Schwindt R., Scott E. K., Appel B. (2015) Neuronal activity biases axon selection for myelination in vivo. Nat. Neurosci. 18, 683–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mensch S., Baraban M., Almeida R., Czopka T., Ausborn J., El Manira A., Lyons D. A. (2015) Synaptic vesicle release regulates myelin sheath number of individual oligodendrocytes in vivo. Nat. Neurosci. 18, 628–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tomlinson L., Leiton C. V., Colognato H. (2016) Behavioral experiences as drivers of oligodendrocyte lineage dynamics and myelin plasticity. Neuropharmacology 110, 548–562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mitew S., Gobius I., Fenlon L. R., McDougall S. J., Hawkes D., Xing Y. L., Bujalka H., Gundlach A. L., Richards L. J., Kilpatrick T. J., Merson T. D., Emery B. (2018) Pharmacogenetic stimulation of neuronal activity increases myelination in an axon-specific manner. Nat. Commun. 9, 306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sánchez M. M., Hearn E. F., Do D., Rilling J. K., Herndon J. G. (1998) Differential rearing affects corpus callosum size and cognitive function of rhesus monkeys. Brain Res. 812, 38–49 [DOI] [PubMed] [Google Scholar]
- 61.Mousavi Majd A., Ebrahim Tabar F., Afghani A., Ashrafpour S., Dehghan S., Gol M., Ashrafpour M., Pourabdolhossein F. (2018) Inhibition of GABA A receptor improved spatial memory impairment in the local model of demyelination in rat hippocampus. Behav. Brain Res. 336, 111–121; erratum: 361, 167 [DOI] [PubMed] [Google Scholar]
- 62.Plemel J. R., Michaels N. J., Weishaupt N., Caprariello A. V., Keough M. B., Rogers J. A., Yukseloglu A., Lim J., Patel V. V., Rawji K. S., Jensen S. K., Teo W., Heyne B., Whitehead S. N., Stys P. K., Yong V. W. (2018) Mechanisms of lysophosphatidylcholine-induced demyelination: a primary lipid disrupting myelinopathy. Glia 66, 327–347 [DOI] [PubMed] [Google Scholar]
- 63.Raff M. C., Lillien L. E., Richardson W. D., Burne J. F., Noble M. D. (1988) Platelet-derived growth factor from astrocytes drives the clock that times oligodendrocyte development in culture. Nature 333, 562–565 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.