Abstract
Src homology domain 2–containing protein tyrosine phosphatase 2 (SHP2) participates in multiple cell functions including cell shape, movement, and differentiation. Therefore, we investigated the potential role of SHP2 in eosinophil recruitment into lungs in allergic airway inflammation and explored the underlying mechanism. Both SHP2 and Ras homolog family member A (RhoA) kinase were robustly activated in the airway eosinophils of children with allergic asthma and of a mouse model with allergic airway inflammation. Moreover, inhibition of SHP2 activity by its specific inhibitors reverses the dephosphorylation of p190-A Rho GTPase-activating protein and in turn attenuates RhoA/Rho-associated protein kinase (ROCK) signaling, resulting in the attenuation of eosinophil migration in response to platelet-activating factor stimulation. Specifically, SHP2 deletion in myeloid cells did not affect the number and classification of circulating leukocytes but significantly attenuated the allergen-induced inflammatory cell, especially eosinophil, infiltration into lungs, and airway hyperreactivity. Notably, genetic interaction between RhoA and SHP2 indicated that RhoA inactivation and SHP2 deletion synergistically attenuated the allergen-induced eosinophil infiltration into lungs and airway hyperreactivity, whereas overexpression of active RhoA robustly restored the SHP2 deletion–resultant attenuation of allergen-induced eosinophil recruitment into lungs and airway hyperreactivity as well. Thus, this study demonstrates that SHP2 via RhoA/ROCK signaling regulates eosinophil recruitment in allergic airway inflammation and possibly in allergic asthma.—Xu, C., Wu, X., Lu, M., Tang, L., Yao, H., Wang, J., Ji, X., Hussain, M., Wu, J., Wu, X. Protein tyrosine phosphatase 11 acts through RhoA/ROCK to regulate eosinophil accumulation in the allergic airway.
Keywords: SHP2, asthma, cell migration, airway inflammation
Allergic asthma is a chronic inflammatory disease characterized by the significant infiltration of eosinophils into the airway (1, 2). Mounting evidence has demonstrated the critical role of eosinophils in allergic asthma pathogenesis, including airway hyperreactivity, excessive mucus production, and airway remodeling (1, 2). Animal studies have demonstrated that eosinophil-lineage deficiency protected the mice against the cardinal features of asthma (3, 4), and clinical studies have also indicated an important role of eosinophils in asthma exacerbation (5, 6). In response to allergen challenge, eosinophils develop from the committed bone marrow progenitors, exit the bone marrow to migrate into the blood, and subsequently accumulate in peripheral tissues, where they survive for up to 2 wk and reside under homeostatic conditions (7). The mobilization of eosinophils from bone marrow to peripheral blood and the subsequent migration of eosinophils toward inflammatory sites are the hallmarks of asthma (2, 7); thus, blocking the recruitment of eosinophils into lungs and impairing the survival of mature eosinophils are rationally considered as 2 major approaches to drug development for eosinophil-associated diseases (2, 7, 8).
Like neutrophil migration, eosinophil migration is a tightly controlled process that depends on the cytoskeletal rearrangements (9). The Rho small GTPases, including Rac, cell division cycle 42, and Ras homolog family member A (RhoA), act as molecular switches that cycle between inactive guanosine triphosphate (GTP)-bound and active GTP-bound forms and regulate the cytoskeletal rearrangements and migration. The activation state of Rho small GTPases is controlled by regulatory proteins, guanine exchange factors, which catalyze the exchange of GDP for GTP, thereby activating Rho, and GTPase-activating proteins (GAPs), which increase the Rho-GTP hydration and hence inactivate Rho (10, 11). Leukocyte migration requires protrusion at a leading edge, contraction of cell body, and detachment of adhesions at cell rear (10, 11). Rac1 and cell division cycle 42 are believed to control cell protrusion at a leading edge, whereas RhoA is thought to regulate rear detachment through effector proteins such as Rho-associated protein kinase (ROCK) (10, 11). In addition to the importance of RhoA in inflammatory cell recruitment, RhoA plays critical roles in the pathogenesis of asthma. Inhibition of RhoA/ROCK signaling attenuates smooth muscle contraction, remodeling, and hyperreactivity in the airway (12, 13); thus, targeting the RhoA/ROCK signaling is a promising approach for the therapeutic intervention of allergic asthma (14).
Src homology domain 2–containing protein tyrosine phosphatase 2 (SHP2) is an intracellular protein tyrosine phosphatase that dephosphorylates multiple kinase substrates in regulation of cell functions (15–19). The abundant and universal expression of SHP2 in lungs implies its potential importance in pulmonary diseases, including asthma, chronic obstructive pulmonary disease, and pulmonary fibrosis (15–19). SHP2-mediated up-regulation of TGF-β1 production in airway epithelia contributes to asthmatic airway remodeling, SHP2 promotes the eosinophil development from the committed bone marrow progenitors, and modulation of eosinophil survival and activation by intercellular adhesion molecule 1 and granulocyte-macrophage colony-stimulating factor receptor is SHP2-dependent (16, 19, 20). In addition, SHP2 has been believed to regulate the cytoskeleton by controlling focal adhesion kinase, guanine nucleotide exchange factor Vav2, RhoGAP-p190, ROCK, and β-catenin (21–25).
In the present study, we have uncovered that SHP2 and RhoA/ROCK exhibit high activities in pulmonary eosinophils of both asthmatic children and mouse models with allergic airway inflammation and that SHP2 signals through RhoA/ROCK to regulate eosinophil recruitment into lungs, which is the hallmark of asthma.
MATERIALS AND METHODS
Materials
Sephadex G-200 and Percoll were purchased from Pharmacia (Pfizer, New York, NY, USA), and calcium-free HBSS (HBSS-C) and calcium containing HBSS (HBSS + C) were obtained from Thermo Fisher Scientific (Waltham, MA, USA). Ovalbumin (OVA), methacholine, specific SHP2 inhibitor phenylhydrazonopyrazolone sulfonate 1 (PHPS1; 314291-83-3), and specific ROCK inhibitor Y27632 (129830-38-2) were obtained from MilliporeSigma (Burlington, MA, USA). RMC4550 (2172651-73-7) was obtained from Selleck Chemicals (Houston, TX, USA). Platelet-activating factor (PAF; 74389-68-7) was purchased from Tocris Bioscience (Bristol, United Kingdom). Active RhoA Pull-Down and Detection Kit was obtained from Thermo Fisher Scientific. Mouse eosinophil cationic protein (ECP), C-C motif chemokine (CCL)11 (eotaxin-1), and CCL24 (eotaxin-2) ELISA kits were obtained from Cusabio (Houston, TX, USA). Bradford Protein Assay Kit was obtained from Beyotime (Haimen, China). Antibodies against phosphorylated (p-)Y580-SHP2, p-Y542-SHP2, phosphorylated (p)-myosin phosphatase target subunit 1 (MYPT1) (S695), phosphorylated (p)-myosin light chain 2 (MLC2) (S19), MLC2, phosphorylated ERK1/2 (p-ERK1/2), and ERK1/2 were obtained from Cell Signaling Technology (Danvers, MA, USA). Antibodies against RhoGAP-p190A and SHP2 were purchased from Santa Cruz Biotechnology (Dallas, TX, USA). Antibodies against C-C chemokine receptor type 3 (CCR3) and phosphorylated Rho-associated protein kinase 2 (p-S1366-ROCK2) were obtained from Abcam (Cambridge, MA, USA). Antibody against phosphotyrosine was obtained from Merck (Darmstadt, Germany). The IRDye 680 and 800s antibodies were obtained from Li-Cor Biosciences (Lincoln, NE, USA). Annexin V-FITC/PI Apoptosis Assay Kit was purchased from Thermo Fisher Scientific, and guinea pig ECP ELISA Kit was obtained from Gentaur Molecular Products (Kampenhout, Belgium).
Children with foreign body aspiration or allergic asthma
Children with the suspected diagnoses of foreign body aspiration (FBA), tuberculosis, or bronchomalacia received diagnostic fiberoptic bronchoscopy with bronchoalveolar lavage at the Zhejiang University School of Medicine Children’s Hospital. The remnant bronchoalveolar lavage fluids (BALFs) from children with discharged diagnoses of FBA (n = 4) or allergic asthma (n = 7) were used in the current study. The clinical characteristics of children with asthma or FBA were described in detail as previously described in Xu et al. (26). The BALFs were subjected to preparation of the cytospins and supernatants. The cytospins were further subjected to immunofluorescent staining for CCR3, p-Y542-SHP2, or p-S1366-ROCK2.
Animal strains and care
Fifteen-week-old male or female Sprague-Dawley rats and 8-wk-old male or female C57BL/6J mice were purchased from Shanghai Laboratory Animal Center (Shanghai, China). Male or female Dunkin-Hartley guinea pigs (300–350 g) were obtained from Vital River Laboratory Animal Technology (Beijing, China). Lysozyme M-Cre (LysM-Cre) mice with the genetic background of C57BL/6J were obtained from the Model Animal Research Center of Nanjing University (Nanjing, China). SHP2flox/flox (SHP2f/f) mice with the genetic background of C57BL/6J were a gift from Dr. Gen-Sheng Feng (University of California–San Diego, La Jolla, CA, USA) (17, 18). The conditional dominant-negative RhoA (dnRhoA; T19N) knock-in mouse strain CAT-dnRhoA+/− (dnRhoA+/−), was a gift from Dr. Kazuto Kobayashi (Fukushima Medical University, Fukushima, Japan) and generated as previously described in Kobayashi et al. (27). The conditional constitutively active form of RhoA (caRhoA; G14V) was cloned into the downstream of a constitutive EF1-α promoter followed by a transcriptional loxP-STOP-loxP cassette (pRP.ExBi-EF1-α-Loxp-stop-Loxp-caRhoA), and a conditional caRhoA knock-in mouse strain (caRhoA+/−) was generated by Cyagen Biosciences (Santa Clara, CA, USA). The description of genetically modified mice is summarized in Table 1. SHP2 deletion in myeloid cell lineage was achieved by crossing the SHP2f/f mice with mice carrying the LysM-Cre. To investigate the role of dnRhoA, LysM-Cre;SHP2f/+ mice were crossed with dnRhoA+/− mice to generate the LysM-Cre;SHP2f/+;dnRhoA+/− mice. To investigate the role of caRhoA, LysM-Cre;SHP2f/+ mice were crossed with SHP2f/+;caRhoA+/− mice to generate the LysM-Cre;SHP2f/f;caRhoA+/− mice. All animals were housed at the Zhejiang University Animal Care Facility according to the Institutional Guidelines for Laboratory Animals, and the animal protocol was approved by the Zhejiang University Institutional Animal Care and Use Committee.
TABLE 1.
Genetically modified mice
| Genotype | Description |
|---|---|
| LysM-Cre;SHP2f/f | Two-copy deletion of SHP2 in myeloid cells |
| LysM-Cre;dnRhoA+/− | Overexpression of 1-copy dominant-negative form of RhoA in myeloid cells |
| LysM-Cre;SHP2flox/+;dnRhoA+/− | One-copy deletion of SHP2 and overexpression of 1-copy dominant-negative form of RhoA in myeloid cells |
| LysM-Cre;caRhoA+/− | Overexpression of 1-copy constitutively active form of RhoA in myeloid cells |
| LysM-Cre;SHP2f/f;caRhoA+/− | Two-copy deletion of SHP2 and overexpression of 1-copy constitutively active form of RhoA in myeloid cells |
Induction and isolation of eosinophils from peritoneum of rats and mice
Stock solutions of Sephadex G-200 and discontinuous density Percoll gradient were prepared for induction and isolation of rat or mouse peritoneal eosinophils as previously described in ref. 28. Briefly, rats or mice intravenously received serial 1 or 0.2 ml injection of Sephadex G-200 [4000 beads/ml normal saline (NS)], respectively, and injections were performed once every 2–3 d for a total of 4 to 5 injections. Twenty-four hours before harvesting and at least 24 h after the last Sephadex injection, the rats or mice received an intraperitoneal injection of 1.5 or 0.3 ml air, respectively. After the rats or mice were euthanized, the peritoneums were lavaged with 40 or 8 ml cold HBSS-C, respectively, and the cell suspensions were then centrifuged at 300 g for 10 min at 20°C. The cell pellets were resuspended in HBSS-C, pooled, and counted by hemocytometer. The cell suspensions were diluted in 5% rat or mouse serum to 1–2 × 107 cells/ml, and 2 ml was carefully layered over discontinuous Percoll density gradients. After centrifugation at 1000 g for 20 min at 20°C, the band at the interface of 1.075 and 1.085 g/ml was isolated and pooled in HBSS-C. The resultant cell suspension was washed and resuspended in HBSS + C, counted by hemocytometer, and subjected to CCR3 immunostaining and Trypan blue staining for cell purity assay, viability assay, and further experiments.
Eosinophil treatments
The isolated rat eosinophils were added into the HBSS + C solution at a density of 1 × 107 cells/ml and treated with PAF at the indicated concentrations for the indicated times or 30 min. Inhibitors including PHPS1 at the indicated concentration or 50 μM and Y27632 at 10 μM were administrated 2 h before and during PAF treatments. After treatments, eosinophils were harvested for protein extractions and subsequent Western blotting assays.
RhoA activation assays, immunoprecipitation, and Western blotting
Total protein extracts were prepared in a whole cell lysis buffer containing an inhibitor mixture. Protein concentrations were determined using a standard Bradford assay. RhoA activation assays were performed by an RhoA Activation Assay Kit, and immunoprecipitation was performed by using an antibody against RhoGAP-p190A as previously described in refs. 29 and 30. The protein lysates were subjected to SDS-PAGE followed by a transfer onto PVDF membranes (MilliporeSigma). Membranes were incubated overnight at 4°C with primary antibodies, and the IRDye 680 and 800 from Li-Cor Biosciences were used as second antibodies. The signals were visualized by an Odyssey Infrared Imaging System (Li-Cor Biosciences). Semiquantification of immunoblotting was performed by using ImageJ software (National Institutes of Health, Bethesda, MD, USA; http://rsb.info.nih.gov/ij/download.html). Total RhoA was used as an internal control for the active form of RhoA and phosphorylated proteins were normalized to total proteins, respectively. The mean values that arose from 3 independent experiments were presented under each band and the values of the first band were defined as 1.
Eosinophil transwell migration and chemotaxis assays
Eosinophil migration assays were performed by using a 24-well transwell insert (3 μm; MilliporeSigma) as previously described in refs. 29 and 30. Rat eosinophils at 4 × 105 cells were diluted in 100 μl HBSS + C medium containing 0, 10, or 50 μM PHPS1 and plated on top of the filter membrane in a transwell insert, and 600 μl HBSS + C medium containing 10 μM PAF was added into the bottom of the lower chamber in the 24-well plate. The migration toward PAF was allowed for 3 h and the migrated cells recovered from the bottom well were counted by hemocytometer; the effect of PHPS1 was assessed by the changes of PAF-induced eosinophil migration within 3 h. Eosinophil chemotaxis assays were performed by using a micropipette tip that released PAF to induce the eosinophil migration as previously described in ref. 31. Rat eosinophils at 1 × 105 cell/ml were plated into gelatin-coated chamber slides (Nalge Nunc International, Rochester, NY, USA) and allowed to settle for 30 min before the experiment. PHPS1 at 50 μM or Y27632 at 10 μM were treated 2 h before and during PAF treatments, and the micropipet loaded with PAF at 100 μM was then screwed in the plate to allow for the chemotaxis of eosinophils for 30 s. After that, cells were fixed and subjected to immunofluorescent staining.
Eosinophil apoptosis assays
Rat eosinophil apoptosis assays were performed by flow cytometry as previously described in Lee et al. Briefly, the isolated primary rat eosinophils were incubated with NS, PAF at 10 μM, PHPS1 at 10 and 50 μM, or RMC4550 at 0.5 and 1.0 μM for 4 h. After washing with PBS, cells were subjected to preparation of monoplast suspension and then stained by an Annexin V-FITC/PI Apoptosis Assay Kit per manufacturer’s instructions. Apoptosis was analyzed with a FACSCalibur Flow Cytometer (BD Biosciences, San Jose, CA, USA).
Immunofluorescence staining
The BALF cells or eosinophils were fixed in ice-cold methanol and permeabilized with 0.1% Triton X-100 in PBS (PBST). After rinsing with PBST, cells were incubated with blocking buffer for 30 min and further incubated with primary antibodies including CCR3, p-Y542-SHP2, RhoGAP-p190A, and p-1366-ROCK2 or PBS overnight at 4°C. After rinsing with PBST, cells were incubated with Alexa Fluor 488– or 555–conjugated secondary antibody (Thermo Fisher Scientific) or FITC-labeled phalloidin. The nuclei were counterstained with DAPI, and immunostaining was visualized by a confocal microscope. Quantification of p-Y542-SHP2 or p-S1366-ROCK2 in CCR3+ cells were performed as previously described in Xu et al. (26), and quantification of percentages for cells with long tails was performed by randomly counting 200 CCR3+ cells from different fields.
PAF-induced pulmonary eosinophil accumulation in guinea pigs
PAF-induced pulmonary eosinophil accumulation in guinea pigs was performed as previously described in Sanjar et al. (33). Briefly, guinea pigs were placed in a sealed acrylic case with a length, width, and height of 40 × 40 × 20 cm, which was connected to the outlet of a nebulizer. Aerosols were generated by use of compressed air (10 L/min). Guinea pigs were exposed for 15 min to aerosols of NS, 2 or 10 mg/ml PHPS1 dissolved in NS, or 10 or 50 μg/ml RMC4550 dissolved in NS. Two hours after exposure, guinea pigs were further aerosolized with NS or PAF at 10 μg/ml for 1 h. Forty-eight hours after last aerosolization, guinea pigs were subjected to preparation of BALFs for eosinophil counting, whereas lungs were used for ECP determination and hematoxylin and eosin (H&E) staining.
Mouse model with allergen-induced airway inflammation
Mice with different genotypes were sensitized and challenged with OVA as previously described in Tang et al. (34). Briefly, on d 0, mice were subcutaneously injected with 20 μg/mouse OVA emulsified in 4% aluminum hydroxide adjuvant at footpad, neck, back, and groin. On d 14, the mice were further intraperitoneally injected with 0.2 ml/mouse of 0.2% OVA. From d 21, OVA-sensitized mice were aerosolly challenged with 1% OVA or NS by a jet nebulizer (Pari Respiratory Equipment, Midlothian, VA, USA) for 30 min, once daily for 7 d. Twenty-four hours after the last OVA challenge, mice were subjected to examination of methacholine-provoked airway hyperreactivity and were then sacrificed to preparation of BALF cytospins, lung homogenates, and paraffin-embedded sections of lungs.
Measurement of airway resistance
The noninvasive lung function examination was performed by using a Buxco Enhanced Pause Measurement System (Data Sciences International, St. Paul, MN, USA) as previously described in Xia et al. (19). Briefly, 24 h after final OVA challenge, mice were placed into a whole-body plethysmograph and then aerosolly challenged with 10 μl of methacholine at concentrations of 0, 3.125, 6.25, 12.5, 25, and 50 mg/ml, respectively. The enhanced pause values were determined by the Buxco with the parameters as follows: aerosol volume, 10 μl; aerosol period, 30 s; determining duration, 2 min; and recovery duration, 3 min.
Preparation of BALFs and histologic examination
Preparation of BALFs and histologic examination were performed as previously described in Tang et al. (34). The BALFs of anesthetized mice or guinea pigs were obtained by washing the left lungs with 0.5 or 5 ml of sterilized NS containing 1% bovine serum albumin and 5000 IU/L heparin 3 times. BALFs were diluted with 1.5 ml HBSS containing 2% fetal calf serum and centrifuged at 500 g at 4°C for 10 min. The pellets were resuspended with HBSS for Wright-Giemsa staining and subsequent cell counting and classification. Two hundred cells of each cytospin were classified under light microscope. The results are expressed as the numbers of each type of cell population in 1 L of BALFs. Right lungs were infused via trachea with 1 ml of 10% neutral formalin and immersed in the same fixative for 7 d. After tissues were paraffinized, 5-μm sections were stained with H&E staining and CCR3 immunostaining for observation of inflammatory cell and eosinophil infiltration, respectively.
ELISA determination for ECP, CCL11, and CCL24 in lung homogenates
Mouse or guinea pig right lungs without lavage were homogenized and subsequently centrifuged. The supernatants were isolated for protein determination by standard Bradford protein assays and ELISA measurements of ECP, CCL11, or CCL24 by their respective kits according to the manufacturer’s instructions. ECP, CCL11, and CCL24 levels were normalized to the protein levels.
Statistics
Numerical data are presented as means ± sd. Statistical analyses were performed using SigmaStat software (v.2.0; IBM SPSS, Chicago, IL, USA), 1-way ANOVA and Student-Newman-Keuls multiple comparisons tests were used to calculate the difference. Significance was assessed at P < 0.05 and P < 0.01 levels. Experiments were repeated at least twice, and the results were qualitatively similar.
RESULTS
Enhancement of SHP2 and ROCK phosphorylation in BALF eosinophils of children with asthma and of mouse models with allergic airway inflammation.
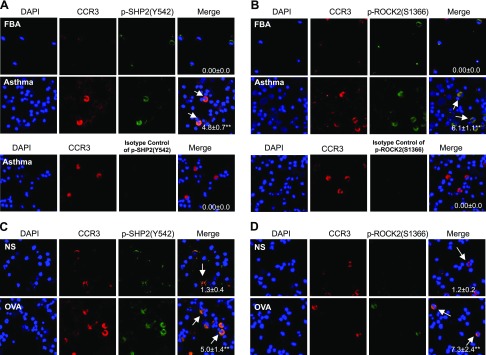
To determine the SHP2 and ROCK phosphorylation in eosinophils, BALFs from children with allergic asthma and from mice sensitized with OVA and challenged with either OVA or NS were prepared for the cytospins and immunofluorescent staining of p-Y542-SHP2 (an active form of SHP2), p-S1366-ROCK2 (an active form of ROCK2), and CCR3 (a marker of eosinophils). CCR3-derived immune signals were not detectable in the BALF cells of children with FBA, but p-Y542-SHP2- and p-S1366-ROCK2-derived immune signals were readily detectable in the CCR3-negative BALF cells of children with FBA (Fig. 1A, B). CCR3-derived immune signals were robustly detectable in the BALF cells of children with asthma, and apparent overlapping immune signals from either p-Y542-SHP2 and CCR3 or p-S1366-ROCK2 and CCR3 were readily observed in the BALF cells of children with asthma (Fig. 1A, B). Consistently, CCR3-positive cells were also robustly detectable in the BALF cells of OVA-challenged mice but not in those of NS-challenged mice, and p-Y542-SHP2– or p-S1366-ROCK2–derived immune signals were apparently colocalized with CCR3-derived immune signals in BALF cells of OVA-challenged mice but not of NS-challenged mice (Fig. 1C, D). Thus, the phosphorylation of both SHP2 and ROCK is enhanced in the lung eosinophils of not only a mouse model with allergic airway inflammation but also of children with allergic asthma.
Figure 1.
Phosphorylation levels of SHP2 and ROCK in eosinophils from BALFs of children with allergic asthma or FBA and of mice sensitized with OVA and challenged with OVA or NS. A, B) BALF cells from children with FBA or asthma were used for immunostaining of CCR3 and p-Y542-SHP2 and p-S1366-ROCK2 or isotype IgG of p-SHP2 (Y542), p-S1366-ROCK2, and DAPI, and subsequent quantification of p-Y542-SHP2 or p-S1366-ROCK2 in CCR3-positive cells. C, D) OVA-sensitized mice were aerosolized with 1% OVA or an equal volume of NS for 30 min once daily for 7 d. Twenty-four hours after the last OVA challenge, BALF cells were prepared for the immunostaining of CCR3, p-Y542-SHP2, p-S1366-ROCK2, and DAPI, and subsequent quantification of p-Y542-SHP2 or p-S1366-ROCK2 in CCR3-positive cells. White arrows indicate the positive p-Y542-SHP2 or p-S1366-ROCK2 staining in CCR3-stained cells. Children with FBA (n = 4); children with asthma (n = 7); mouse model, (n = 3). Representative results are shown. **P < 0.01 vs. children with FBA or mice challenged with NS.
Requirement of SHP2 activity in PAF-induced pulmonary eosinophil accumulation of guinea pigs
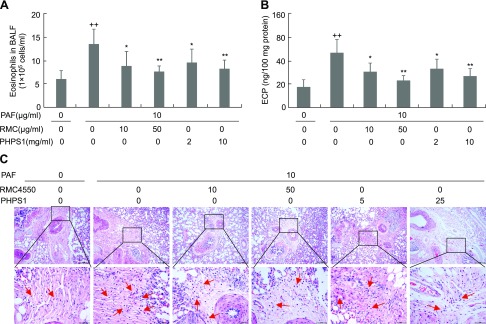
To determine the potential role of SHP2 in eosinophil migration, we used an established guinea pig model of eosinophil recruitment into lungs in response to PAF, a potent chemoattractant of eosinophils. Aerosolization with 10 μg/ml PAF increased the numbers of eosinophil in BALF and the ECP levels in lung by 1.9- and 2.0-fold, respectively, whereas aerosol administration of SHP2 inhibitors including RMC4550 at 10 and 50 μg/ml and PHPS1 at 2 and 10 mg/ml dose-dependently decreased both the numbers of eosinophil in BALF and ECP levels in lung by 42–58% (Fig. 2A, B). Consistently, histologic analyses of lung indicated that aerosolization with 10 μg/ml PAF led to a significant infiltration of inflammatory cells, especially eosinophils, into the peribronchiolar tissues in comparison with aerosolization with NS, whereas aerosol administration of either RMC4550 at 10 and 50 μg/ml or PHPS1 at 2 and 10 mg/ml markedly attenuated the PAF-induced infiltration of inflammatory cells, especially eosinophils, into the peribronchiolar tissues in comparison with aerosol administration of NS (Fig. 2C). Thus, SHP2 activity is essentially required for the recruitment of inflammatory cells, especially eosinophils, into lungs in response to PAF stimulation.
Figure 2.
Requirement of SHP2 activity in PAF-induced pulmonary eosinophil recruitment of guinea pigs. Guinea pigs were aerosolized for 15 min with NS, 2 or 10 mg/ml PHPS1 dissolved in NS, or 10 or 50 μg/ml RMC4550 dissolved in NS. After 2 h, guinea pigs were further aerosolized with NS or PAF at 10 μg/ml for 1 h. Forty-eight hours after last aerosolization, guinea pigs were subjected to preparation of BALFs for eosinophil counting (A), whereas lungs were used for ECP determination (B) and H&E staining and histologic evaluation (C). Data are presented as means ± sd (n = 5). ++P < 0.01 vs. without PAF, PHPS1, or RMC4550; **P < 0.01, *P < 0.05 vs. 10 μg/ml PAF alone.
Requirement of SHP2 activity in PAF-induced eosinophil migration in vitro
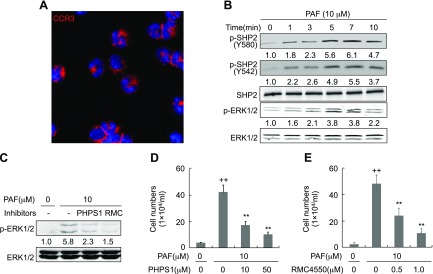
We next induced the peritoneal eosinophils in rats by Sephadex G-200 and performed CCR3 immunostaining and Trypan blue exclusion assays. The purity of the primary rat eosinophils ranged from 94 to 97% (Fig. 3A) and the viability of them was more than 92% (unpublished results). We then investigated the roles of SHP2 in eosinophil migration in response to PAF. PAF at 10 μM time-dependently induced the phosphorylation of SHP2 at both Y580 and Y542, the prerequisite for SHP2 activity, and the phosphorylation of ERK1/2, the downstream effector of SHP2 (16, 18, 19) within 10 min of stimulation, with maximal induction at 7 min for both p-SHP2 and p-ERK1/2 (Fig. 3B). To determine the specificity of SHP2 inhibitors including PHPS1 and RMC4550, we treated the primary rat eosinophils with PAF at 10 μM following the pretreatment with either PHPS1 at 50 μM or RMC4550 at 1.0 μM. PAF at 10 μM increased the p-ERK1/2 by 4.8-fold, whereas PHPS1 at 50 μM and RMC4550 at 1.0 μM negated PAF-induced p-ERK1/2 by 60 and 74%, respectively (Fig. 3C). To determine the role of SHP2 activity in eosinophil migration in response to PAF stimulation, we first validated the concentrations of PAF and SHP2 inhibitors including PHPS1 and RMC4550 by examining the apoptosis of eosinophils. Treatment with PAF at 10 μM, PHPS1 at 10 and 50 μM, or RMC4550 at 0.5 and 1.0 μM for 4 h did not apparently affect the apoptosis of primary rat eosinophils at all (Supplemental Fig. S1). However, transwell analyses indicated that inhibition of SHP2 by its specific inhibitors, PHPS1 and RMC4550, robustly attenuated PAF-induced eosinophil migration; PHPS1 at 10 and 50 μM reduced PAF-induced eosinophil transwell migration by ∼57 and 80%, respectively, whereas RMC4550 at 0.5 and 1.0 μM negated PAF-induced eosinophil transwell migration by ∼53 and 79%, respectively (Fig. 3D, E). Thus, SHP2 activity is required for eosinophil recruitment toward PAF.
Figure 3.
Involvement of SHP2 in PAF-induced eosinophil migration. A) Immunofluorescent staining of CCR3 in rat peritoneal eosinophils. B) Enhancement of the phosphorylation of SHP2 and ERK1/2 by PAF. Rat peritoneal eosinophils were treated with PAF at 10 μM for the indicated times and harvested for preparation of protein lysates and Western blotting. C) The specificity of SHP2 inhibitors. The primary rat eosinophils were treated with PAF at 10 μM for 7 min following the pretreatment with either PHPS1 at 50 μM or RMC4550 at 1.0 μM for 1 h. Cells were then harvested for preparation of protein lysates and Western blotting. D, E) Attenuation of eosinophil migration by SHP2 inhibitors PHPS1 and RMC4550. Rat peritoneal eosinophil migration toward 10 μM PAF was analyzed by transwell inserts in the presence or absence of the indicated concentration of PHPS1 or RMC4550 for 3 h. The results were expressed as the number of migrated cells in bottom well. Data are presented as means ± sd (n = 3). ++P < 0.01 vs. without PAF, PHPS1 and RMC4550; **P < 0.01 vs. 10 μM PAF alone.
SHP2 targeting RhoA/ROCK to regulate eosinophil migration
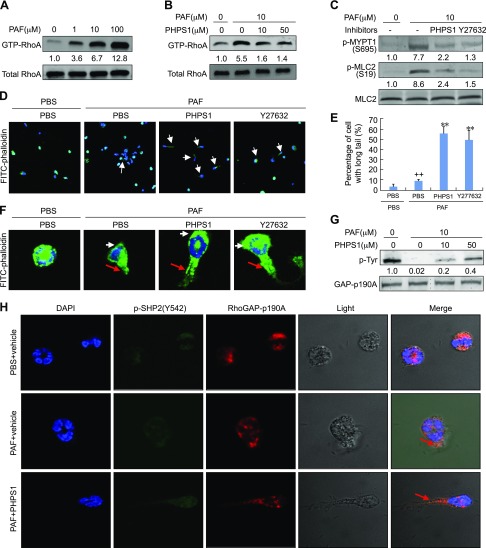
We next investigated the potential mechanism underlying SHP2-mediated eosinophil migration in response to PAF stimulation and emphasized on the RhoA/ROCK signaling that was critical for cell movement and migration. PAF ranging from 0 to 100 μM dose-dependently activated RhoA, and 100 μM of PAF increased the active form of RhoA (GTP-RhoA) by ∼12-fold over the control at 5 min poststimulation (average fold change at 100 μM: 12.8 ± 0.8; n = 3) (Fig. 4A). To examine whether SHP2 participates in RhoA/ROCK signaling, eosinophils were treated with PHPS1 and assayed for their responses to PAF in activation of RhoA. The RhoA activation by PAF was dose-dependently diminished by PHPS1, and 50 μM of PHPS1 reduced PAF-induced RhoA activation by 75% (Fig. 4B). The role of SHP2 in activation of RhoA/ROCK signaling in response to PAF was further evaluated by Western analyses of p-MYPT1 and p-MLC2, the downstream effectors of RhoA/ROCK. In line with activation of RhoA, PAF at 10 μM increased p-MYPT1 (S695) and p-MLC2 (T118) by 6.7- and 7.6-fold, respectively, whereas either SHP2 inhibitor PHPS1 at 50 μM or ROCK inhibitor Y27632 at 20 μM significantly attenuated p-MYPT1 (S695) (by 71 or 83%) and p-MLC2 (T118) (by 72 or 83%) in response to PAF stimulation at 10 μM (Fig. 4C).
Figure 4.
Participation of RhoA/ROCK signaling in SHP2-mediated eosinophil migration. A) RhoA activation by PAF. Rat eosinophils were treated with the indicated concentrations of PAF for 5 min. Cells were then harvested for RhoA pull-down assays and Western blotting. GTP-RhoA was normalized to total RhoA. B, C) Inhibition of RhoA/ROCK signaling by PHPS1. Rat eosinophils were treated with 10 μM PAF for 5 min in the presence or absence of 50 μM or indicated concentrations of PHPS1 or 10 μM Y27632. Cells were then harvested for RhoA pull-down assays and Western blotting. Phosphorylated protein was normalized to total protein. D–F, H) Eosinophil chemotaxis toward PAF. Rat eosinophils were plated into gelatin-coated chamber slides and pretreated with 50 μm PHPS1 or 10 μm Y27632 for 30 min. The micropipette loaded with 100 μM PAF was then screwed in the plate to allow the chemotaxis of eosinophils for 30 s. Cells were then fixed and subjected to immunostaining of FITC-labeled phalloidin (D, F) and quantification (E) of p-Y542-SHP2, RhoGAP-p190A, and DAPI (H). F) Representative cells from D. White arrow, membrane protrusions at the front of eosinophil; red arrow, retracting fiber at the rear of eosinophil. G) Rat eosinophils were treated with 0 or 10 μM PAF in the presence or absence of the indicated concentrations of PHPS1 for 5 min and cells were then harvested for immunoprecipitation by a RhoGAP-p190A antibody. Western blotting was performed to detect the levels of phosphotyrosine and RhoGAPp190A in the immunoprecipitated complex. Data are presented as means ± sd (n = 3). ++P < 0.01 vs. PBS and PBS; **P < 0.01 vs. PAF and PBS.
Leukocyte migration requires protrusion at a leading edge, contraction of cell body, and detachment of adhesions at cell rear, and RhoA/ROCK signaling is important for the organization of stress fibers and also in the regulation of actomyosin contractility (10, 11). We immunostained the adherent (gelatin-coated surface) eosinophils by FITC-labeled phalloidin and observed the rear detachment by a confocal microscope. PAF treatment induced a successful cell polarization and made membrane protrusions at the front of eosinophils and retracting fiber formation at the rear of eosinophils; however, a striking delay in uropod release was observed in the migrating eosinophils treated with either PHPS1 at 10 μM or Y27632 at 20 μM, and the inhibition of uropod release by PHPS1 was in a manner similar to Y27632 (Fig. 4D–F). Thus, SHP2 behaves essentially in the same manner as RhoA/ROCK in rear detachment during eosinophil migration. Because RhoGAP-p190 served as a GAP to inactive RhoA, we next immunoprecipitated RhoGAP-p190A from eosinophils treated with PAF in the presence or absence of PHPS1 and performed Western analyses of phosphotyrosine levels of RhoGAP-p190A. PAF at 10 μM diminished the phosphotyrosine levels of RhoGAP-p190A by 99.8%, whereas PHPS1 at 10 and 50 μM restored PAF-negated phosphotyrosine levels of RhoGAP-p190A by ∼20 and 40%, respectively (Fig. 4G). To confirm the importance of SHP2 and RhoGAP-p190A in rear detachment of eosinophils in response to PAF, we performed the immunostaining. Consistent with the result that SHP2 dephosphorylates RhoGAP-p190A, p-Y542-SHP2 was well colocalized with RhoGAP-p190A in the intact eosinophils, and an apparent colocalization in uropods was observed upon PAF stimulation at 10 μM in either the presence or absence of PHPS1 (Fig. 4H). Thus, SHP2 dephosphorylates RhoGAP-p190A to activate RhoA during eosinophil migration.
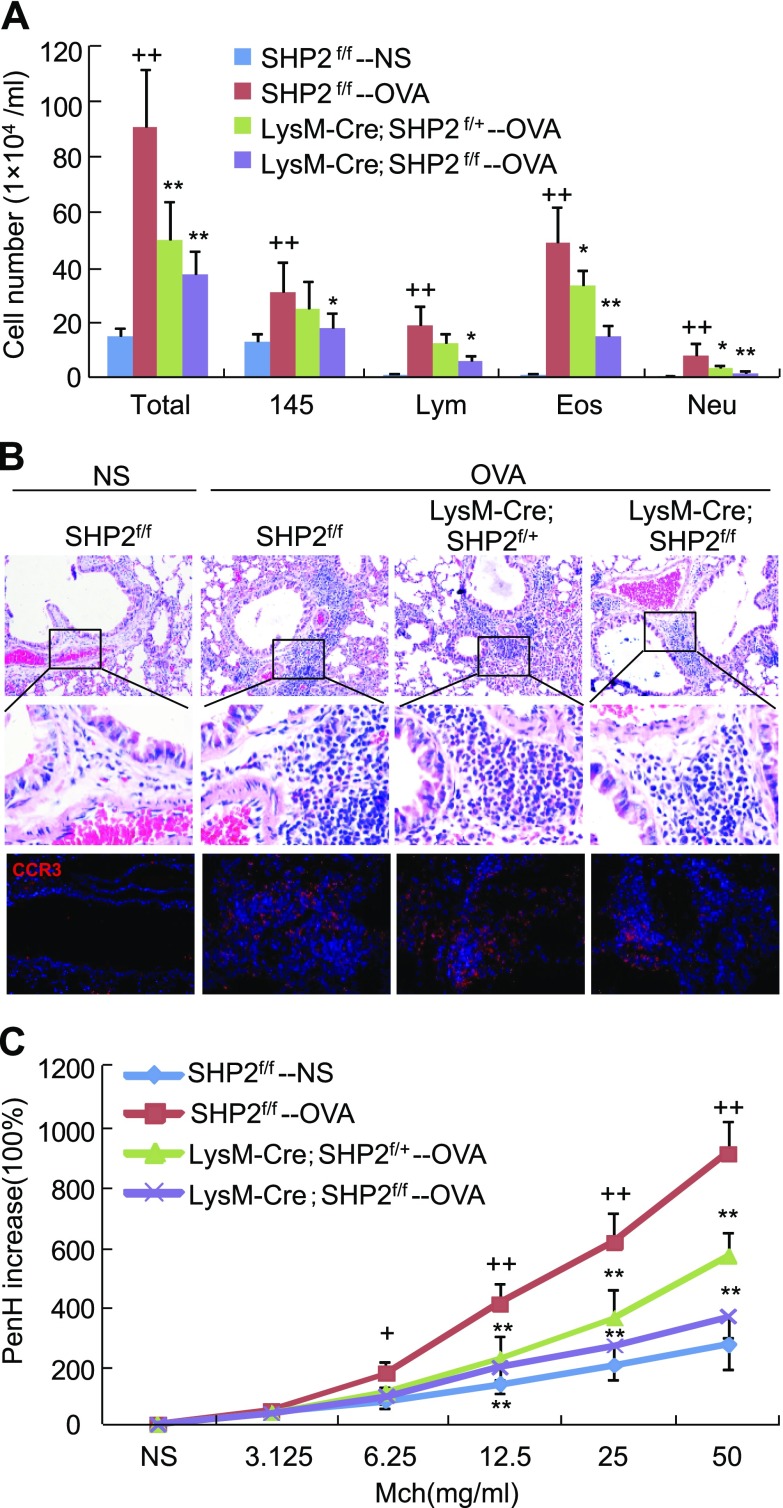
Attenuation of eosinophil infiltration into allergic lungs by SHP2 deletion
To investigate the impact of SHP2 on eosinophil recruitment in vivo, we genetically removed SHP2 from the myeloid cells by the Cre-loxP technique and generated age- and sex-matched SHP2f/f, LysM-Cre;SHP2f/+, and LysM-Cre;SHP2f/f mice for OVA sensitization and subsequent challenge. Peritoneal eosinophils of SHP2f/f, LysM-Cre;SHP2f/+, and LysM-Cre;SHP2f/f mice were induced by Sephadex G-200, and the deletion efficiency of SHP2 was examined by Western analyses. Peritoneal eosinophils of LysM-Cre;SHP2f/+ and LysM-Cre;SHP2f/f mice exhibited 30 and 60% decreases in SHP2 expression, respectively, compared with those of SHP2f/f mice (Supplemental Fig. S2A). In intact mice, the number and classification of circulating leukocytes in the LysM-Cre;SHP2f/f mice have no significant difference with those in the SHP2f/f mice (Supplemental Fig. S2B). In OVA-challenged mice, the numbers of circulating eosinophils were significantly increased in LysM-Cre;SHP2f/f, LysM-Cre;SHP2f/+, and SHP2f/f mice, with no significant difference (Supplemental Fig. S2C).
Upon OVA challenge, SHP2f/f mice exhibited a significant increase in the numbers of total inflammatory cell, macrophage, lymphocyte, eosinophil, and neutrophil in the BALFs. LysM-Cre;SHP2f/+ mice exhibited a significant decrease in the numbers of BALF total inflammatory cells, eosinophils, and neutrophils, whereas LysM-Cre;SHP2f/f mice exhibited a significant decrease in the numbers of BALF total inflammatory cells, macrophages, lymphocytes, eosinophils, and neutrophils compared with SHP2f/f mice (Fig. 5A). In line with the changes of eosinophil numbers, both LysM-Cre;SHP2f/+ and LysM-Cre;SHP2f/f mice exhibited a significant decrease in ECP levels in the lungs compared with SHP2f/f mice, upon OVA challenge (Supplemental Fig. S2D). OVA challenge caused significant increases in lung levels of CCL11 and CCL24, critical chemokines for eosinophil chemotaxis, in the SHP2f/f mice, whereas OVA challenge resulted in a similar increase in lung levels of CCL11 and CCL24 in either the LysM-Cre;SHP2f/+ or LysM-Cre;SHP2f/f mice (Supplemental Fig. S2E, F).
Figure 5.
Effects of SHP2 deletion in myeloid cells on the inflammatory cell infiltration into lungs and airway hyperreactivity. Mice with the indicated genotypes were sensitized and subsequently challenged with OVA; 24 h after the final OVA challenge, mice were subjected to preparation of BALFs for inflammatory cell counting and classification (A), paraffin-embedded sections, H&E staining, and CCR3 immunostaining (B), and examination of methacholine-provoked airway hyperrreactivity (C) (n = 6). +P < 0.05; ++P < 0.01 vs. SHP2f/f mice challenged with NS; *P < 0.05; **P < 0.01 vs. SHP2f/f mice challenged with OVA. Square frames define the magnified regions.
Histopathological analyses indicated that OVA challenge caused a significant infiltration of inflammatory cells, especially CCR3+ eosinophils, into the peribronchiolar and perivascular tissues in the SHP2f/f mice in comparison to NS challenge, whereas LysM-Cre;SHP2f/+ or LysM-Cre;SHP2f/f mice exhibited moderate or severe decreases in OVA-induced CCR3+ leukocyte infiltration, in comparison with SHP2f/f mice, respectively (Fig. 5B). Finally, OVA-challenged SHP2f/f mice exhibited a significant increase in airway hyperreactivity in response to nebulized methacholine ranging from 6.25 to 50 mg/ml, whereas OVA-challenged LysM-Cre;SHP2f/+ and LysM-Cre;SHP2f/f mice both exhibited a robust decrease in airway hyperreactivity in response to nebulized methacholine ranging from 12.5 to 50 mg/ml compared with OVA-challenged SHP2f/f mice (Fig. 5C). Thus, specific deletion of SHP2 in myeloid cells diminished not only the allergen-induced inflammatory cell, especially CCR3+ eosinophil, infiltration into lungs but also the airway hyperreactivity.
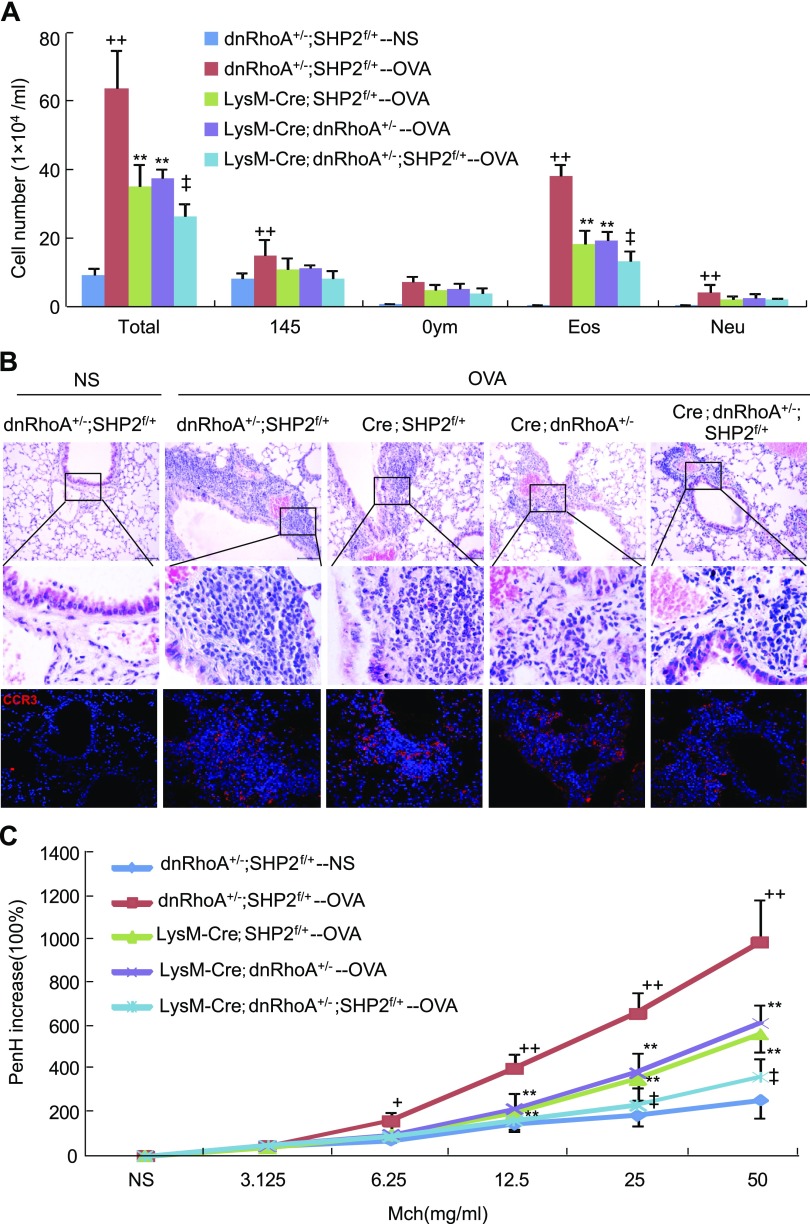
Genetic interaction between RhoA and SHP2 in eosinophil infiltration into lungs
To investigate the potential role of RhoA in SHP2-mediated eosinophil recruitment into lungs, we genetically removed 1 copy of SHP2 and overexpressed 1 copy of dnRhoA in the myeloid cells by the Cre-loxP technique and generated age- and sex-matched dnRhoA+/−;SHP2f/f, LysM-Cre;SHP2f/+, and LysM-Cre;dnRhoA+/−;SHP2f/+ mice for OVA sensitization and subsequent challenge. Peritoneal eosinophils of dnRhoA+/− and LysM-Cre;dnRhoA+/− mice were induced by Sephadex G-200, and inactivation of RhoA/ROCK signaling by dnRhoA in eosinophils was examined by Western analyses. Peritoneal eosinophils of LysM-Cre;dnRhoA+/− mice exhibited a 50% decrease in ROCK2 activity compared with those of dnRhoA+/− mice (Supplemental Fig. S3A). Removal of 1 copy of SHP2 and overexpression of 1 copy of dnRhoA in the myeloid cells did not significantly affect the number and classification of circulating leukocytes in LysM-Cre;dnRhoA+/−;SHP2f/+ mice in comparison with dnRhoA+/−;SHP2f/+ mice (Supplemental Fig. S3B). Upon OVA challenge, the numbers of circulating eosinophils were significantly increased in mice with different genotypes, whereas the number of circulating eosinophils in LysM-Cre;dnRhoA+/− or LysM-Cre;dnRhoA+/−;SHP2f/+ mice was increased less than that in dnRhoA+/−;SHP2f/+ or LysM-Cre;SHP2f/+ mice, respectively (Supplemental Fig. S3C).
OVA challenge led to a significant increase in the numbers of total inflammatory cells, macrophages, lymphocytes, eosinophils, and neutrophils in the BALF of dnRhoA+/−;SHP2f/+ mice in comparison with NS challenge. Either LysM-Cre;dnRhoA+/− or LysM-Cre;SHP2f/+ mice significantly attenuated the OVA-induced increase in the numbers of total inflammatory cells and eosinophils compared with dnRhoA+/−;SHP2f/+ mice, whereas LysM-Cre;dnRhoA+/−;SHP2f/+ mice synergistically reduced the OVA-induced increases in the numbers of total inflammatory cell and eosinophil compared with LysM-Cre;dnRhoA+/− or LysM-Cre;SHP2f/+ mice (Fig. 6A). In line with the changes of eosinophil numbers, both LysM-Cre;SHP2f/+ and LysM-Cre;dnRhoA+/− mice exhibited a significant decrease in lung ECP levels compared with dnRhoA+/−;SHP2f/+ mice, whereas LysM-Cre;dnRhoA+/−;SHP2f/+ mice exhibited a further decrease in lung ECP levels in comparison with either LysM-Cre;SHP2f/+ or LysM-Cre;dnRhoA+/− mice upon OVA challenge (Supplemental Fig. S3D). OVA challenge caused a significant increase in lung levels of CCL11 and CCL24 in the dnRhoA+/−;SHP2f/+ mice, whereas OVA challenge resulted in a similar increase in lung levels of CCL11 and CCL24 in the LysM-Cre;SHP2f/+, LysM-Cre;dnRhoA+/−, or LysM-Cre;dnRhoA+/−;SHP2f/+ mice (Supplemental Fig. S3E, F).
Figure 6.
Effects of overexpression of dnRhoA on SHP2 deletion–resultant inflammatory cell infiltration into lungs and airway hyperreactivity. Mice with the indicated genotypes were sensitized and subsequently challenged with OVA; 24 h after the last OVA challenge, mice were subjected to preparation of BALFs for inflammatory cell counting and classification (A), paraffin-embedded sections, H&E staining, and CCR3 immunostaining (B), examination of methacholine-provoked airway hyperreactivity (C) (n = 6). Square frames define the magnified regions. +P < 0.05; ++P < 0.01 vs. dnRhoA+/−;SHP2f/+ mice challenged with NS; **P < 0.01 vs. dnRhoA+/−;SHP2f/+ mice challenged with OVA; ‡P < 0.01 vs. LysM-Cre;dnRhoA+/− or LysM-Cre;SHP2f/+ mice challenged with OVA.
Histopathological analyses indicated that OVA challenge caused a significant infiltration of inflammatory cells, especially CCR3+ eosinophils, into the peribronchiolar and perivascular tissues in comparison with NS challenge in the dnRhoA+/−;SHP2f/+ mice. Either LysM-Cre;dnRhoA+/− or LysM-Cre;SHP2f/+ mice significantly attenuated the OVA-induced infiltration of CCR3+ eosinophils into the peribronchiolar and perivascular tissues, whereas LysM-Cre;dnRhoA+/−;SHP2f/+ mice synergistically reduced the OVA-induced infiltration of CCR3+ eosinophils (Fig. 6B). Finally, OVA-challenged dnRhoA+/−;SHP2f/+ mice exhibited a significant increase in airway hyperreactivity in response to nebulized methacholine ranging from 6.25 to 50 mg/ml compared with NS-challenged dnRhoA+/−;SHP2f/+ mice. OVA-challenged LysM-Cre;dnRhoA+/− or LysM-Cre;SHP2f/+ mice exhibited a significant decrease in airway hyperreactivity in response to nebulized methacholine ranging from 12.5 to 50 mg/ml compared with OVA-challenged dnRhoA+/−;SHP2f/+ mice, whereas OVA-challenged LysM-Cre;dnRhoA+/−;SHP2f/+ mice exhibited a further significant decrease in airway hyperreactivity in response to nebulizes methacholine ranging from 25 to 50 mg/ml compared with OVA-challenged LysM-Cre;dnRhoA+/− or LysM-Cre;SHP2f/+ mice, respectively (Fig. 6C). Thus, removal of 1 copy of SHP2 and overexpression of 1 copy of dnRhoA in myeloid cells synergistically diminished not only the allergen-induced inflammatory cell, especially CCR3+ eosinophil, recruitment into lungs but also the airway hyperreactivity.
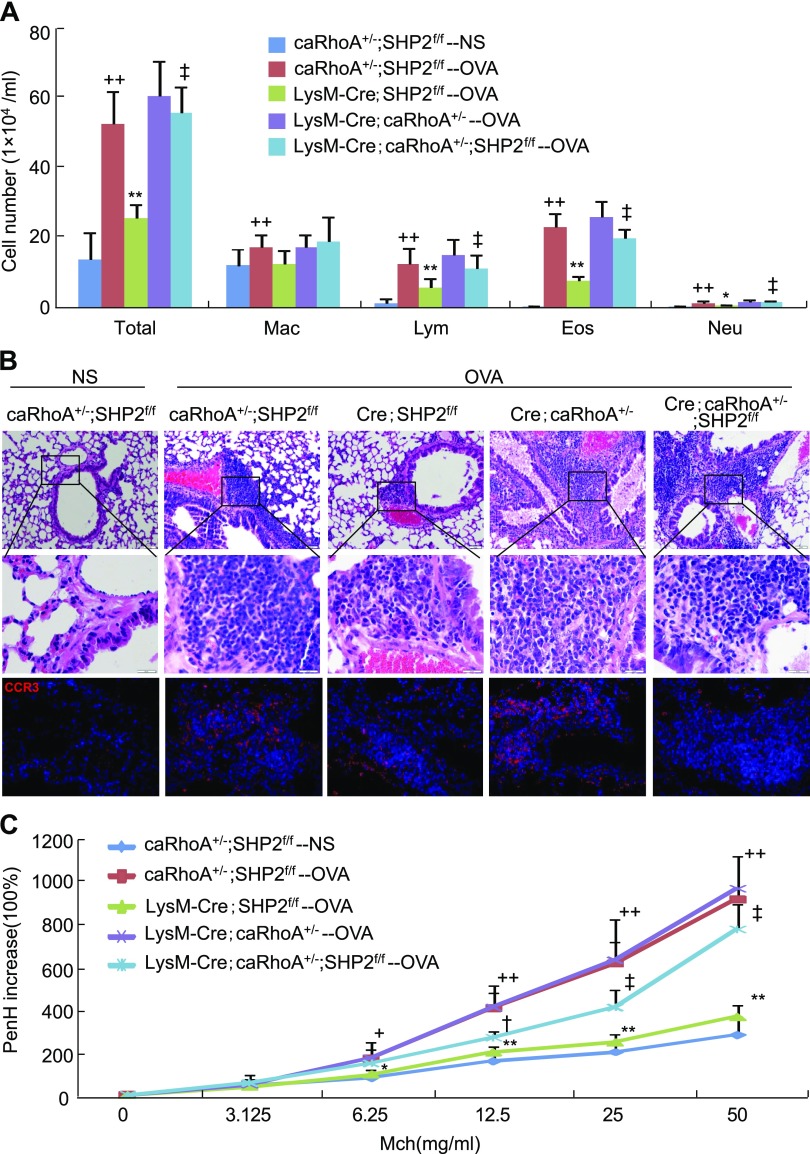
Restoration of the effects of SHP2 deletion on eosinophil recruitment into lungs by constitutive activation of RhoA
To determine whether activation of RhoA is sufficient to restore the attenuation of eosinophil recruitment into lungs caused by SHP2 deletion, we genetically removed 2 copies of SHP2 and overexpressed 1 copy of caRhoA in the myeloid cells by Cre-loxP technique and generated age- and sex-matched caRhoA+/−;SHP2f/f, LysM-Cre;SHP2f/f, and LysM-Cre;caRhoA+/−;SHP2f/f mice for OVA sensitization and subsequent challenge. Peritoneal eosinophils of caRhoA+/− and LysM-Cre;caRhoA+/− mice were induced by Sephadex G-200, and activation of RhoA/ROCK signaling by caRhoA in eosinophils was examined by Western analyses. Peritoneal eosinophils of LysM-Cre;caRhoA+/− mice exhibited a 2.3-fold increase in ROCK2 activity compared with those of caRhoA+/− mice (Supplemental Fig. S4A). Deletion of SHP2 and overexpression of caRhoA in the myeloid cells did not significantly affect the number and classification of circulating leukocytes in LysM-Cre;caRhoA+/−;SHP2f/f mice in comparison with caRhoA+/−;SHP2f/f mice (Supplemental Fig. S4B). Upon OVA challenge, the numbers of circulating eosinophils were significantly increased in mice with different genotypes, whereas the numbers of circulating eosinophils in LysM-Cre;caRhoA+/− or LysM-Cre;caRhoA+/−;SHP2f/f mice were increased significantly more than those in caRhoA+/−;SHP2f/f or LysM-Cre;SHP2f/f mice, respectively (Supplemental Fig. S4C).
OVA challenge led to a significant increase in the numbers of BALF total inflammatory cells, macrophages, lymphocytes, eosinophils, and neutrophils in caRhoA+/−;SHP2f/f mice in comparison with NS challenge. Upon OVA challenge, SHP2 deletion significantly attenuated the numbers of BALF total inflammatory cells, lymphocytes, eosinophils, and neutrophils in LysM-Cre;SHP2f/f mice, whereas activation of RhoA did not significantly affect the numbers of these BALF inflammatory cells in LysM-Cre;caRhoA+/− mice compared with caRhoA+/−;SHP2f/f mice. However, upon OVA challenge, activation of RhoA significantly restored the SHP2 deletion-negated numbers of BALF total inflammatory cells, lymphocytes, eosinophils, and neutrophils in LysM-Cre;caRhoA+/−;SHP2f/f mice compared with LysM-Cre;SHP2f/f mice (Fig. 7A). In line with the changes of eosinophil numbers, upon OVA challenge, LysM-Cre;SHP2f/f mice exhibited a significant decrease in lung ECP levels and LysM-Cre;caRhoA+/− mice had no significant effect on lung ECP levels compared with caRhoA+/−;SHP2f/f mice, whereas LysM-Cre;caRhoA+/−;SHP2f/f mice exhibited restored lung ECP levels in comparison with LysM-Cre;SHP2f/f mice (Supplemental Fig. S4D). OVA challenge caused significant increases in lung CCL11 and CCL24 levels of caRhoA+/−;SHP2f/+ mice, whereas OVA challenge resulted in a similar increase in lung CCL11 and CCL24 levels of LysM-Cre;SHP2f/f, LysM-Cre;caRhoA+/−, or LysM-Cre;caRhoA+/−;SHP2f/f mice (Supplemental Fig. S4E, F).
Figure 7.
Effects of overexpression of caRhoA on SHP2 deletion–resultant inflammatory cell infiltration into lungs and airway hyperreactivity. Mice with the indicated genotypes were sensitized and subsequently challenged with OVA; 24 h after the last OVA challenge, mice were subjected to preparation of BALFs for inflammatory cell counting and classification (A), paraffin-embedded sections, H&E staining, and CCR3 immunostaining (B), examination of methacholine-provoked airway hyperreactivity (C) (n = 6). Square frames define the magnifiesd regions. +P < 0.05; ++P < 0.01 vs. caRhoA+/−;SHP2f/f mice challenged with NS; *P < 0.05; **P < 0.01 vs. caRhoA+/−;SHP2f/f mice challenged with OVA; †P < 0.05; ‡P < 0.01 vs. LysM-Cre;SHP2f/f mice challenged with OVA.
Histopathological analyses indicated that OVA challenge caused a significant infiltration of inflammatory cells, especially CCR3+ eosinophils, into the peribronchiolar and perivascular tissues in comparison with NS challenge in caRhoA+/−;SHP2f/f mice. Upon OVA challenge, LysM-Cre;SHP2f/f mice markedly attenuated the OVA-induced infiltration of inflammatory cells, especially CCR3+ eosinophils, and LysM-Cre;caRhoA+/− mice did not significantly affect the infiltration of inflammatory cells, especially CCR3+ eosinophils compared with caRhoA+/−;SHP2f/f mice, whereas LysM-Cre;caRhoA+/−;SHP2f/f mice apparently restored the infiltration of inflammatory cells, especially CCR3+ eosinophil, into the peribronchiolar and perivascular tissues in comparison with LysM-Cre;SHP2f/f mice (Fig. 7B). Finally, OVA-challenged caRhoA+/−;SHP2f/f mice exhibited a significant increase in airway hyperreactivity in response to nebulized methacholine ranging from 6.25 to 50 mg/ml compared with NS-challenged caRhoA+/−;SHP2f/f mice. OVA-challenged LysM-Cre;SHP2f/f mice exhibited a significant decrease in airway hyperreactivity in response to nebulized methacholine ranging from 6.25 to 50 mg/ml and OVA-challenged LysM-Cre;caRhoA+/− exhibited no significant changes in airway hyperreactivity in response to nebulized methacholine compared with OVA-challenged caRhoA+/−;SHP2f/f mice; however, OVA-challenged LysM-Cre;caRhoA+/−;SHP2f/f mice exhibited a significant restoration in airway hyperreactivity in response to nebulized methacholine ranging from 25 to 50 mg/ml compared with OVA-challenged LysM-Cre;SHP2f/f mice (Fig. 7C). Thus, overexpression of the active form of RhoA in myeloid cells rescues deletion-negated SHP2 not only the inflammatory cell, especially CCR3+ eosinophil, recruitment into lungs but also the airway hyperreactivity.
DISCUSSION
The present study, to our best knowledge, is the first to reveal that SHP2 acts through RhoA/ROCK signaling to regulate eosinophil recruitment into lungs in allergic airway inflammation and possibly in allergic asthma.
Concurrent activation of both SHP2 and RhoA/ROCK signaling in BALF eosinophils of children with allergic asthma and of a mouse model with allergic airway inflammation suggests the potential importance of them in allergic airway diseases and the potential interactions between SHP2 and RhoA/ROCK signaling in the eosinophil biology and pathogenesis of eosinophil-associated diseases. Consistent with this finding, the present study indicates that SHP2 acts through RhoA/ROCK signaling to regulate the isolated eosinophil migration in response to PAF stimulation and the eosinophil infiltration into lungs in response to allergen challenge. Previous study indicates that genetic deletion of SHP2 in myeloid cells fails to induce eosinophil infiltration into lungs as well as airway hyperresponsiveness in a mouse model with allergen-induced asthma (19), and mounting evidence demonstrates the importance of RhoA/ROCK signaling in the inflammatory cell recruitment and the pathogenesis of asthma in turn (10–14). In line with the previous finding that phosphotyrosyl p190 proteins serve as target substrates of SHP2 in serum- or platelet-derived growth factor–stimulated cells and SHP2 control RhoGAP-p190A phosphorylation levels and RhoA activation by angiotensin II type 1 receptor (35, 36), the present data indicate that SHP2 activates RhoA/ROCK signaling possibly through dephosphorylation of RhoGAP-p190A. Conflicting with these proposals, a previous study suggests that SHP2 mediates direct dephosphorylation of ROCK2 at Y722 to fine-tune RhoA-dependent activation of ROCK2 (25). The discrepancy between this and our present study could indicate that the role of SHP2 in ROCK activation depends on the duration, degree, and cellular context.
Although SHP2 gain-of-function mutations caused myeloid malignancy (37), in the intact mice, neither deletion of SHP2 nor overexpression of dnRhoA and caRhoA in the myeloid cells affects the numbers and populations of circulating leukocytes, this suggests that deletion of SHP2 and overexpression of dnRhoA and caRhoA in the myeloid cells affect neither the development and differentiation of myeloid progenitors nor the mobilization of myeloid cells from bone marrow to peripheral blood, and that these mouse strains are applicable for the study of allergen-induced airway inflammation. LysM-Cre mice allow for both specific and highly efficient Cre-mediated deletion of loxP-flanked target genes in myeloid cells, with a deletion efficiency of 83–98% in mature macrophages, near 100% in granulocytes, 16% in CD11C+ splenic dendritic cells, and no significant deletion in purified T and B cells (38). Given the fact that eosinophils are overwhelmingly increased in the mouse model with allergen-induced airway inflammation, the observed effects in the present study should effectively reflect the roles of SHP2 deletion and dnRhoA and caRhoA overexpression in eosinophil biologic behaviors.
In OVA-challenged mice, overexpression of caRhoA in myeloid cells affects the numbers of neither circulating nor lung eosinophils. Because RhoA is already activated in OVA-challenged eosinophils, it is rational that further overexpression of caRhoA in eosinophils is not able to potentiate the effect of OVA challenge on eosinophil recruitment into lungs. Because RhoA acts as a downstream effector of SHP2 signaling, it is rational that overexpression of caRhoA in eosinophils is able to rescue the effect of SHP2 deletion on eosinophil recruitment into lungs. In contrast, overexpression of dnRhoA in myeloid cells reduces the numbers of circulating eosinophils by 20% and of lung eosinophils by 50%. This suggests that inhibition of RhoA activity also effectively attenuates eosinophil recruitment into lungs and possibly attenuates the development of eosinophils from the committed bone marrow progenitors as well, in response to OVA challenge. Further experiments are needed to address this issue. Importantly, deletion of SHP2 in myeloid cells does not affect the number of circulating eosinophils but rather those of lung eosinophils. Because SHP2 knockdown does not affect the life span (apoptosis) of eosinophils (19), we suggest that SHP2 could regulate the migration of eosinophils toward inflammatory sites, the hallmark of asthma. Conflicting with our observations, previous study demonstrates that genetic deletion of SHP2 in myeloid cells fails to induce eosinophil infiltration into lungs because of the attenuation of eosinophil differentiation in the bone marrow of allergen-challenged mice (19). Although systemic administration of PHPS1 decreases the numbers of circulating eosinophils by 20% and of bone marrow eosinophil by 45%, this study has not shown the number of circulating eosinophils in LysM-Cre;SHP2f/f mice challenged with allergen.
In line with the in vitro data indicating that SHP2 regulates eosinophil migration through the activation of RhoA/ROCK signaling, genetic interaction between RhoA and SHP2 indicates overexpression of 1 copy of dnRhoA and deletion of 1 copy of SHP2 produce a synergistic effect on attenuation of eosinophil recruitment into lungs in response to OVA challenge. Consistently, overexpression of caRhoA in myeloid cells apparently rescues the SHP2 deletion–resultant attenuation of eosinophil recruitment into lungs, further suggesting that RhoA/ROCK lies on the downstream of SHP2 signaling in the regulation of eosinophil migration. However, whether SHP2 acts through RhoA/ROCK to regulate the eosinophil biology other than cell migration (such as cell adhesion) but closely relates to the allergen-induced airway inflammation is still unknown. In addition, because LysM-Cre mice delete the SHP2 or overexpress daRhoA and caRhoA in not only eosinophils but also in other myeloid cells, it is still possible that SHP2 acts through RhoA/ROCK signaling in myeloid cells other than eosinophils to attenuate the eosinophil recruitment.
Eosinophil recruitment into airway and resultant airway remodeling and hyperreactivity are the most striking characteristics in allergic airway diseases (39, 40). The activation of SHP2 and RhoA/ROCK in allergic eosinophils has a significant implication in the pathogenesis of allergic airway inflammation and possibly of asthma. Given the fact that inhibition of ROCK by its specific inhibitor is applauded for the therapy of allergic asthma (14), the blockage of the SHP2-signaling pathway is also a promising therapeutic intervention for allergic airway inflammation, the hallmark of asthma pathogenesis.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (81170016, 81170787, 81200022, 81200023, 81270067, 81470214, 31571493, 8157080078, and 81571928) and the Natural Science Foundation of Zhejiang Province (LY18H150002). The authors declare no conflicts of interest.
Glossary
- BALF
bronchoalveolar lavage fluid
- caRhoA
constitutively active form of RhoA
- caRhoA+/−
caRhoA knock-in mouse strain
- CCL
C-C motif chemokine
- CCR3
C-C chemokine receptor type 3
- dnRHoA
dominant-negative RhoA
- dnRhoA+/−
knock-in mouse strain
- ECP
eosinophil cationic protein
- FBA
foreign body aspiration
- H&E
hematoxylin and eosin
- HBSS + C
calcium containing HBSS
- HBSS-C
calcium-free HBSS
- LysM-Cre
lysozyme M-Cre
- MLC2
myosin light chain 2
- MYPT1
myosin phosphatase target subunit 1
- NS
normal saline
- OVA
ovalbumin
- PAF
platelet-activating factor
- PBST
Triton X-100 in PBS
- PHPS1
phenylhydrazonopyrazolone sulfonate 1
- RhoA
Ras homolog family member A
- RhoGAP
Rho GTPase-activating protein
- ROCK
Rho-associated protein kinase
- SHP2
Src homology domain 2–containing protein tyrosine phosphatase 2
- SHP2f/f
SHP2flox/flox
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
C. Xu, Xiling Wu, M. Lu, L. Tang, H. Yao, J. Wang, X. Ji, M. Hussain, J. Wu, and Ximei Wu were the main contributors in the conception, design, acquisition, and interpretation of the data and in writing the article; C. Xu, Xiling Wu, M. Lu, H. Yao, J. Wang, and M. Hussain performed experiments and data analysis; M. Lu, L. Tang, and J. Wang interpreted histopathology; X. Ji and M. Hussain were responsible for image analysis; and J. Wu and Ximei Wu wrote the manuscript.
REFERENCES
- 1.Julia V., Macia L., Dombrowicz D. (2015) The impact of diet on asthma and allergic diseases. Nat. Rev. Immunol. 15, 308–322 [DOI] [PubMed] [Google Scholar]
- 2.Landolina N. A. C., Levi-Schaffer F. (2014) Eosinophils as a pharmacological target for the treatment of allergic diseases. Curr. Opin. Pharmacol. 17, 71–80 [DOI] [PubMed] [Google Scholar]
- 3.Humbles A. A., Lloyd C. M., McMillan S. J., Friend D. S., Xanthou G., McKenna E. E., Ghiran S., Gerard N. P., Yu C., Orkin S. H., Gerard C. (2004) A critical role for eosinophils in allergic airways remodeling. Science 305, 1776–1779 [DOI] [PubMed] [Google Scholar]
- 4.Lee J. J., Dimina D., Macias M. P., Ochkur S. I., McGarry M. P., O’Neill K. R., Protheroe C., Pero R., Nguyen T., Cormier S. A., Lenkiewicz E., Colbert D., Rinaldi L., Ackerman S. J., Irvin C. G., Lee N. A. (2004) Defining a link with asthma in mice congenitally deficient in eosinophils. Science 305, 1773–1776 [DOI] [PubMed] [Google Scholar]
- 5.Haldar P., Brightling C. E., Hargadon B., Gupta S., Monteiro W., Sousa A., Marshall R. P., Bradding P., Green R. H., Wardlaw A. J., Pavord I. D. (2009) Mepolizumab and exacerbations of refractory eosinophilic asthma. N. Engl. J. Med. 360, 973–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro M., Mathur S., Hargreave F., Boulet L. P., Xie F., Young J., Wilkins H. J., Henkel T., Nair P.; Res-5-0010 Study Group (2011) Reslizumab for poorly controlled, eosinophilic asthma: a randomized, placebo-controlled study. Am. J. Respir. Crit. Care Med. 184, 1125–1132 [DOI] [PubMed] [Google Scholar]
- 7.Rosenberg H. F., Dyer K. D., Foster P. S. (2013) Eosinophils: changing perspectives in health and disease. Nat. Rev. Immunol. 13, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bel E. H., Ten Brinke A. (2017) New anti-eosinophil drugs for asthma and COPD: targeting the trait! Chest 152, 1276–1282 [DOI] [PubMed] [Google Scholar]
- 9.McBrien C. N., Menzies-Gow A. (2017) The biology of eosinophils and their role in asthma. Front. Med. (Lausanne) 4, 93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Biro M., Munoz M. A., Weninger W. (2014) Targeting Rho-GTPases in immune cell migration and inflammation. Br. J. Pharmacol. 171, 5491–5506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baker M. J., Pan D., Welch H. C. E. (2016) Small GTPases and their guanine-nucleotide exchange factors and GTPase-activating proteins in neutrophil recruitment. Curr. Opin. Hematol. 23, 44–54 [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya M., Sundaram A., Kudo M., Farmer J., Ganesan P., Khalifeh-Soltani A., Arjomandi M., Atabai K., Huang X., Sheppard D. (2014) IQGAP1-dependent scaffold suppresses RhoA and inhibits airway smooth muscle contraction. J. Clin. Invest. 124, 4895–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amison R. T., Momi S., Morris A., Manni G., Keir S., Gresele P., Page C. P., Pitchford S. C. (2015) RhoA signaling through platelet P2Y1 receptor controls leukocyte recruitment in allergic mice. J. Allergy Clin. Immunol. 135, 528–538 [DOI] [PubMed] [Google Scholar]
- 14.Kume H. (2008) RhoA/Rho-kinase as a therapeutic target in asthma. Curr. Med. Chem. 15, 2876–2885 [DOI] [PubMed] [Google Scholar]
- 15.Li F. F., Shen J., Shen H. J., Zhang X., Cao R., Zhang Y., Qui Q., Lin X. X., Xie Y. C., Zhang L. H., Jia Y. L., Dong X. W., Jiang J. X., Bao M. J., Zhang S., Ma W. J., Wu X. M., Shen H., Xie Q. M., Ke Y. (2012) Shp2 plays an important role in acute cigarette smoke-mediated lung inflammation. J. Immunol. 189, 3159–3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin X. J., Zhang G. S., Zhang X., Qiu Z. W., Wang P. L., Li Y. W., Li W., Xie Q. M., Ke Y. H., Lee J. J., Shen H. H. (2012) Protein tyrosine phosphatase SHP2 regulates TGF-β1 production in airway epithelia and asthmatic airway remodeling in mice. Allergy 67, 1547–1556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang X., Zhang Y., Tao B., Teng L., Li Y., Cao R., Gui Q., Ye M., Mou X., Cheng H., Hu H., Zhou R., Wu X., Xie Q., Ning W., Lai M., Shen H., Feng G. S., Ke Y. (2012) Loss of Shp2 in alveoli epithelia induces deregulated surfactant homeostasis, resulting in spontaneous pulmonary fibrosis. FASEB J. 26, 2338–2350 [DOI] [PubMed] [Google Scholar]
- 18.Tao B., Jin W., Xu J., Liang Z., Yao J., Zhang Y., Wang K., Cheng H., Zhang X., Ke Y. (2014) Myeloid-specific disruption of tyrosine phosphatase Shp2 promotes alternative activation of macrophages and predisposes mice to pulmonary fibrosis. J. Immunol. 193, 2801–2811 [DOI] [PubMed] [Google Scholar]
- 19.Xia L. X., Hua W., Jin Y., Tian B. P., Qiu Z. W., Zhang C., Che L. Q., Zhou H. B., Wu Y. F., Huang H. Q., Lan F., Ke Y. H., Lee J. J., Li W., Ying S. M., Chen Z. H., Shen H. H. (2016) Eosinophil differentiation in the bone marrow is promoted by protein tyrosine phosphatase SHP2. Cell Death Dis. 7, e2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pazdrak K., Young T. W., Stafford S., Olszewska-Pazdrak B., Straub C., Starosta V., Brasier A., Kurosky A. (2008) Cross-talk between ICAM-1 and granulocyte-macrophage colony-stimulating factor receptor signaling modulates eosinophil survival and activation. J. Immunol. 180, 4182–4190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kodama A., Matozaki T., Fukuhara A., Kikyo M., Ichihashi M., Takai Y. (2000) Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell scattering. Mol. Biol. Cell 11, 2565–2575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ukropec J. A., Hollinger M. K., Salva S. M., Woolkalis M. J. (2000) SHP2 association with VE-cadherin complexes in human endothelial cells is regulated by thrombin. J. Biol. Chem. 275, 5983–5986 [DOI] [PubMed] [Google Scholar]
- 23.Kontaridis M. I., Eminaga S., Fornaro M., Zito C. I., Sordella R., Settleman J., Bennett A. M. (2004) SHP-2 positively regulates myogenesis by coupling to the Rho GTPase signaling pathway. Mol. Cell. Biol. 24, 5340–5352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsutsumi R., Takahashi A., Azuma T., Higashi H., Hatakeyama M. (2006) Focal adhesion kinase is a substrate and downstream effector of SHP-2 complexed with Helicobacter pylori CagA. Mol. Cell. Biol. 26, 261–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee H. H., Chang Z. F. (2008) Regulation of RhoA-dependent ROCKII activation by Shp2. J. Cell Biol. 181, 999–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu C., Zou C., Hussain M., Shi W., Shao Y., Jiang Z., Wu X., Lu M., Wu J., Xie Q., Ke Y., Long F., Tang L., Wu X. (2018) High expression of Sonic hedgehog in allergic airway epithelia contributes to goblet cell metaplasia. Mucosal Immunol. 11, 1306–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi K., Takahashi M., Matsushita N., Miyazaki J., Koike M., Yaginuma H., Osumi N., Kaibuchi K., Kobayashi K. (2004) Survival of developing motor neurons mediated by Rho GTPase signaling pathway through Rho-kinase. J. Neurosci. 24, 3480–3488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cypcar D., Sorkness R., Sedgwick J., Nagata M., Clough J., Kaplan M., Lemanske R. F., Jr. (1996) Rat eosinophils: isolation and characterization of superoxide production. J. Leukoc. Biol. 60, 101–105 [DOI] [PubMed] [Google Scholar]
- 29.Yao H. Y., Chen L., Xu C., Wang J., Chen J., Xie Q. M., Wu X., Yan X. F. (2011) Inhibition of Rac activity alleviates lipopolysaccharide-induced acute pulmonary injury in mice. Biochim. Biophys. Acta 1810, 666–674 [DOI] [PubMed] [Google Scholar]
- 30.Yao H., Shi W., Wu J., Xu C., Wang J., Shao Y., Wu X., Zhang Z. (2015) Endothelial Rac1 is essential for hematogenous metastasis to the lung. Oncotarget 6, 17501–17513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mishra R. K., Scaife J. E., Harb Z., Gray B. C., Djukanovic R., Dent G. (2005) Differential dependence of eosinophil chemotactic responses on phosphoinositide 3-kinase (PI3K). Allergy 60, 1204–1207 [DOI] [PubMed] [Google Scholar]
- 32.Lee H. J., Lee E. K., Seo Y. E., Shin Y. H., Kim H. S., Chun Y. H., Yoon J. S., Kim H. H., Han M. Y., Kim C. K., Kim K. E., Koh Y. Y., Kim J. T. (2017) Roles of Bcl-2 and caspase-9 and -3 in CD30-induced human eosinophil apoptosis. J. Microbiol. Immunol. Infect. 50, 145–152 [DOI] [PubMed] [Google Scholar]
- 33.Sanjar S., Aoki S., Boubekeur K., Chapman I. D., Smith D., Kings M. A., Morley J. (1990) Eosinophil accumulation in pulmonary airways of Guinea-pigs induced by exposure to an aerosol of platelet-activating factor: effect of anti-asthma drugs. Br. J. Pharmacol. 99, 267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tang L. F., Xu Y. C., Wang Y. S., Wang C. F., Zhu G. H., Bao X. E., Lu M. P., Chen L. X., Chen Z. M. (2009) Airway foreign body removal by flexible bronchoscopy: experience with 1027 children during 2000-2008. World J. Pediatr. 5, 191–195 [DOI] [PubMed] [Google Scholar]
- 35.Agazie Y. M., Hayman M. J. (2003) Development of an efficient “substrate-trapping” mutant of Src homology phosphotyrosine phosphatase 2 and identification of the epidermal growth factor receptor, Gab1, and three other proteins as target substrates. J. Biol. Chem. 278, 13952–13958 [DOI] [PubMed] [Google Scholar]
- 36.Bregeon J., Loirand G., Pacaud P., Rolli-Derkinderen M. (2009) Angiotensin II induces RhoA activation through SHP2-dependent dephosphorylation of the RhoGAP p190A in vascular smooth muscle cells. Am. J. Physiol. Cell Physiol. 297, C1062–C1070 [DOI] [PubMed] [Google Scholar]
- 37.Xu D., Liu X., Yu W. M., Meyerson H. J., Guo C., Gerson S. L., Qu C. K. (2011) Non-lineage/stage-restricted effects of a gain-of-function mutation in tyrosine phosphatase Ptpn11 (Shp2) on malignant transformation of hematopoietic cells. J. Exp. Med. 208, 1977–1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Clausen B. E., Burkhardt C., Reith W., Renkawitz R., Förster I. (1999) Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 8, 265–277 [DOI] [PubMed] [Google Scholar]
- 39.Hussain M., Xu C., Ahmad M., Yang Y., Lu M., Wu X., Tang L., Wu X. (2017) Notch signaling: linking embryonic lung development and asthmatic airway remodeling. Mol. Pharmacol. 92, 676–693 [DOI] [PubMed] [Google Scholar]
- 40.Hussain M., Xu C., Lu M., Wu X., Tang L., Wu X. (2017) Wnt/β-catenin signaling links embryonic lung development and asthmatic airway remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 1863, 3226–3242 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.