Abstract
Impaired glycolysis has pathologic effects on the occurrence and progression of liver diseases, and it appears that glycolysis is increased to different degrees in different liver diseases. As an important post-translational modification, reversible lysine acetylation regulates almost all cellular processes, including glycolysis. Lysine acetylation can occur enzymatically with acetyltransferases or nonenzymatically with acetyl–coenzyme A. Accompanied by the progression of liver diseases, there seems to be a temporal and spatial variation between enzymatic and nonenzymatic acetylations in the regulation of glycolysis. Here, we summarize the most recent findings on the functions and targets of acetylation in controlling glycolysis in the different stages of liver diseases. In addition, we discuss the differences and causes between enzymatic and nonenzymatic acetylations in regulating glycolysis throughout the progression of liver diseases. Then, we review these new discoveries to provide the potential implications of these findings for therapeutic interventions in liver diseases.—Li, J., Wang, T., Xia, J., Yao, W., Huang, F. Enzymatic and nonenzymatic protein acetylations control glycolysis process in liver diseases.
Keywords: acetyltransferase, acetyl-CoA, glucose metabolism, target, progression
Liver disease, a worldwide public health problem, has been increasing in prevalence, with tens of millions of patients been affected (1). It appears with a broad scope ranging from simple steatosis or nonalcoholic fatty liver disease (NAFLD) to nonalcoholic steatohepatitis (NASH), which is characterized by the recruitment of proinflammatory immune cells, to cirrhosis and liver cancer, such as hepatocellular carcinoma (HCC) (2–4). According to the statistics, ∼20–30% and 2–3% of the population in the west suffer from NAFLD and NASH, respectively (5, 6), and > 700,000 people die from HCC every year (7–9). As a core organ for the storage, synthesis, and metabolism of nutrients, the liver displays a remarkable capacity to maintain metabolic homeostasis (3, 10, 11). However, under various adverse conditions, the hepatic metabolic homeostasis is disrupted, especially the glucose and lipid metabolic functions such as glycolysis, gluconeogenesis, lipogenesis, and fatty acid oxidation. Recently, the disturbance of hepatic glycolysis has commanded growing attention because of its interaction with liver diseases (12, 13).
Glycolysis is a critical energy-generating process in almost all mammalian cells, during which glucose is catalyzed into pyruvate with a net gain of 2 ATP and 2 NADH molecules from 1 glucose molecule. Pyruvate can be oxidized into acetyl–coenzyme A (CoA) by pyruvate dehydrogenase (PDH) or be converted into oxaloacetate via pyruvate carboxylase (PC) in mitochondria. In the cytoplasm, under anaerobic conditions, pyruvate is reduced to lactate by lactate dehydrogenase (LDH) or decarboxylated into acetaldehyde by pyruvate decarboxylase and used in ethanol fermentation in yeast. Under physiologic conditions, pyruvate is decarboxylated to acetyl-CoA via PDH and processed in the Krebs cycle or used for de novo lipogenesis (3). Nevertheless, under the pathologic conditions of the liver, the growing evidence has demonstrated that there is an adaptive metabolic switch from producing energy preferentially from oxidative phosphorylation toward glycolysis in which pyruvate is partially converted to lactate (14–16). To support this, several studies have observed enhanced glycolytic activity and higher lactate levels in patients with NAFLD and NASH (15, 17). In addition, in hepatocellular cancer, the cancer cells preferably generate lactate through the glycolysis pathway (even in aerobic conditions) to meet their demands of rapid growth and proliferation, known as aerobic glycolysis (18). Indeed, metabolic reprogramming in liver diseases is associated with specific alterations of glycolytic enzyme activity as well as glycolysis-related genes.
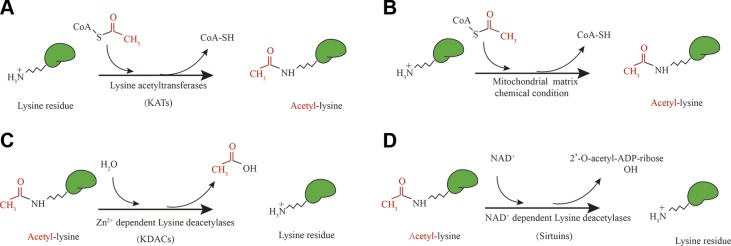
Increasing evidence has shown that glycolytic enzymes are subjected to post-translational modifications (PTMs), which influence their subcellular localization, stability, activity, and interaction with other proteins in different states of liver diseases (19–22). Among the most widely distributed PTMs, the reversible lysine acetylation that modifies not only histones but also nonhistone proteins has emerged as a major PTM, directing multitudinous epigenetic programs within the cell (23, 24). Lysine acetylation is defined as the addition of an acetyl group provided by acetyl-CoA to the ε-amino groups of lysine residues, which can occur enzymatically and nonenzymatically (25–27). On the one hand, the transfer of an acetyl group to the lysine residues is assisted by lysine acetyltransferases (KATs), known as enzymatic acetylation (Fig. 1A). On the other hand, in alkaline environments with high concentrations of acetyl-CoA such as the mitochondrial matrix, the lysine acetylation can occur spontaneously with no acetyltransferases needed (28) (Fig. 1B). Accordingly, as a crucial metabolic organ, the liver is exposed to lysine acetylation to a large extent, which takes place in a majority of the transcription factors and metabolic enzymes in hepatocytes in addition to the glycolytic enzymes (29, 30). However, how enzymatic acetylation and nonenzymatic acetylation coordinate to control glycolysis in liver diseases has not yet been reported. Here, we will primarily focus on the different mechanisms by which enzymatic and nonenzymatic protein acetylations control glycolysis process in liver diseases.
Figure 1.
The mechanisms of acetylation and deacetylation on the ε-amine group of lysine. A) Enzymatic acetylation catalyzed by KATs. B) Nonenzymatic acetylation with acetyl-CoA in mitochondrial matrix (pH 7.9–8.0 and acetyl-CoA 0.1–1.5 mM). C) Deacetylation mediated by Zn2+-dependent KDACs. D) Deacetylation mediated by NAD+-dependent SIRTs. SH, sulfydryl.
GLYCOLYTIC PROCESS IN LIVER DISEASE
As a universal metabolic pathway for the breakdown of glucose, glycolysis provides energy for vital movement and intermediate metabolites for other metabolic pathways, especially in the liver (31). Thus, abnormal glycolysis is assumed to have pathologic impacts on the onset and progression of liver diseases. A growing body of evidence has implicated impaired glycolysis as a particular concern in hepatic mitochondrial disorders such as oxidative stress and oxidative phosphorylation dysfunction, contributing to various liver diseases. Moreover, the extent of increased glycolysis deepens with the severity of liver diseases as a result of compensating for the ATP production defect in mitochondria.
The effect of glycolysis on the progression of liver diseases
Metabolic abnormalities in several glucose metabolic pathways, including glycolysis, make a core contribution to the occurrence and development of liver diseases. Some studies have revealed that the mechanisms by which altered glycolytic processes promote the progression of liver diseases from NAFLD to NASH and finally to cirrhosis and HCC appear to be related to the redox imbalance and mitochondrial disorder.
The up-regulation of glucose and lactate have been observed in NAFLD and NASH (32). NAFLD, associated with obesity and insulin resistance, takes the form of a deposition of fat in hepatocytes. Emerging molecular studies indicate that high glucose-induced oxidative stress represses the deacetylase sirtuin (SIRT)6 (33). Meanwhile, the specific disruption of SIRT6 in mice liver provokes hepatic steatosis as a result of increased glycolytic flux, with the significantly increased expression of glucokinase (GK), liver pyruvate kinase (PK), and glucose transporter 1 (GLUT1) and reduced mitochondrial respiration (34, 35). In addition, the high levels of glucose could activate carbohydrate-responsive element-binding protein, thus inducing the expression of the enzymes in lipogenesis such as acetyl-CoA carboxylase and fatty acid synthase with high expression of GK and PK in glycolysis (36). During a high-fat diet (HFD), the increased lactate uptake in hepatocytes could also activate sterol regulatory element-binding protein 1, a transcriptional factor involved in lipogenesis (37). These findings indicate that abnormal glycolysis plays an important role in the pathogenesis of fatty liver.
Pure steatosis is a benign condition. However, persistent steatosis can progress to NASH, which is characterized by steatosis, inflammation, and varying degrees of fibrosis. In the progression from NAFLD to NASH, the overproduction of reactive oxygen species (ROS) and cytokines have been indicated as significant events (36). Several studies have uncovered the importance of pyruvate decarboxylation by PDH and the subsequent oxidation in the Krebs cycle in removing NADPH-dependent peroxide. However, it has been demonstrated that HFD could result in the inhibition of PDH and could distinctly depress the capacity of hepatic mitochondria to remove peroxide in mice, resulting in oxidative stress and the exacerbation of NAFLD (38–40). Consistent with this, another study found a novel function of PDH kinase isoenzyme (PDK)4, an enzyme inhibiting the activity of PDH in NASH. The level of hepatic PDK4 is highly increased in NASH mice and human patients, which could aggravate hepatic steatosis. Conversely, its deficiency ameliorates the hepatic steatosis significantly in NASH mice (41). Both of the 2 studies indicate that the inhibition of PDH could impair the redox balance and promote the progression of liver diseases, and that the activation of PDH could ameliorate the redox imbalance and steatosis. In addition, enhanced glycolysis activity in monocytes and macrophages fuels the generation of mitochondrial ROS. Then, the enzyme PKM2, with higher sensitivity to redox imbalance, transfers into the nucleus, thus boosting the production of proinflammatory cytokines IL-6 and IL-1β and subsequent inflammation (42, 43). Consistent with this, it was reported that inflammation can be prevented in mice by blocking the glycolytic metabolic pathway in macrophages (44).
As a second hit in the pathogenesis of liver diseases, ROS attack mitochondria, causing the impairment of oxidative phosphorylation, the inability to maintain sufficient ATP concentrations, and a further enhancement of ROS production (45, 46). The vicious cycle of the mitochondrial dysfunction-ROS production axis caused by aberrant glycolysis could further worsen steatosis and initiate the progression to cirrhosis and HCC (46, 47). Increased aerobic glycolysis and elevated oxidative stress are 2 conspicuous hallmarks that can also be frequently observed in cancer cells (48, 49). The increased production of lactate protects cancer cells against apoptosis and contributes to the invasion and migration of cancer cells as well as the interaction between cancer cells (16, 50, 51). In addition, the elevated glycolysis could lead to the accumulation of intermediary glucose metabolites such as glucose-6-phosophate and glyceraldehyde-3-phosphate, which could be channeled into the pentose phosphate pathway and glycerol synthesis pathway respectively. It is observed that the enzymes in glycolysis and pentose phosphate pathway such as PK, LDH, and glucose-6-phosphate dehydrogenase are up-regulated simultaneously in tumors (52). Of note, the pentose phosphate pathway and glycerol synthesis both contribute to the accumulation of hepatic lipids, thus promoting the progression of liver diseases (52–55). Moreover, the pentose phosphate pathway facilitates the survival and proliferation of cancer cells by providing macromolecules and reduced environment (52, 53). These findings suggest that abnormal glycolysis contributes to the occurrence and development of liver diseases.
Enhanced glycolysis in liver diseases
Despite the contribution of glycolysis to the progression of liver diseases, in turn, the state of liver diseases can affect the glycolysis process itself at the same time. Under basic physiologic conditions, the rate of glycolysis in the cytoplasm is low, which generates 2 ATP molecules per glucose molecule. Its end-product pyruvate further undergoes oxidation via mitochondrial oxidative phosphorylation, producing energy efficiently. However, increased glycolysis activity has been observed in the different states of liver diseases. For example, the levels of glycolysis-related metabolites (glucose, lactate, and pyruvate) in the blood from the HFD-induced NAFLD group mice are significantly higher compared with those in the blood from the control group (15). Likewise, our recent study also showed an elevated level of glycolysis in LPS-induced liver inflammation (17). Furthermore, during acute liver failure, there is more lactate production observed in the liver as a result of accelerated glycolysis in comparison with that in the control group. Additionally, the up-regulated gene expression of glycolytic enzymes such as hexokinase (HK)2, aldolase A, and PKM2, is observed in precancerous cirrhotic livers, increasing the risk of developing into HCC (56). In HCC (the terminal liver disease), cancer cells display enhanced glycolysis as well and preferably metabolize glucose into lactate even under aerobic conditions (18). Together, these findings are relevant to a metabolic transition from oxidative phosphorylation to glycolysis. In NAFLD and NASH, the increased lactate is a sign of a degree of metabolic switch to aerobic glycolysis. In HCC, the degree of enhanced aerobic glycolysis is increased evidently to support cancer cells growth and proliferation.
Accumulating evidence indicates that the pathogenesis of liver diseases is closely connected with mitochondrial dysfunction (46, 57–59). As mentioned, oxidative phosphorylation is the major energy-producing process. Thus, ATP production is diminished in liver diseases with impaired oxidative phosphorylation. To address the imbalance of ATP required for macromolecule synthesis and reduced energy production due to impaired oxidative phosphorylation in mitochondria, increased glycolysis may compensate. With high levels of glucose, the relatively higher rate of glycolysis is able to produce more energy than oxidative phosphorylation (15). It has been demonstrated that the dysfunction of mitochondria through the drug-induced inhibition of the respiratory chain or through the mutation of mitochondrial enzymes leads to the activation of glycolysis (60, 61). In liver cancer, the rapid growth of which often exceeds the blood and hence oxygen supply, increased glycolysis protects cancer cells against apoptosis in hypoxic environments by producing lactate via LDH-A (50, 51). The greater the demand for ATP from glycolysis to support macromolecule synthesis, the higher the amount of lactate accumulated in the cell. Moreover, lactate could inhibit histone deacetylase (HDAC) activity, acting as a transcriptional regulator (62). It has also been proposed that the elevated lactate is conducive for cancer cells to escape immune surveillance and provides an acidic tumor microenvironment for cancer metastasis (63–65).
Generally, increased glycolysis in different liver diseases is associated with mitochondrial dysfunction and contributes to compensating for the defect in energy production due to impaired oxidative phosphorylation. In the case of enhanced glycolytic activity, the key enzymes of glycolysis are more or less regulated in different pathways including lysine acetylation.
ENZYMATIC AND NONENZYMATIC LYSINE ACETYLATIONS
Lysine acetylation has been emerging as an important PTM, which was first discovered on histones half a century ago (66). Subsequently, with the development of affinity enrichment techniques and mass spectrometry, a comprehensive understanding of the acetylome was provided, and the spectrum and regulation of nonhistone acetylation were unmasked (67). The lysine acetylation of histones is involved in the regulation of gene transcription. The acetylation of nonhistones plays an important role in the regulation of cell signal transduction and metabolism processes (68). Lysine acetylation can occur when an acetyl group is transferred to the ε-amine group of lysine enzymatically with acetyltransferases or by nonenzymatic chemical modifications with acetyl-CoA (Fig. 1). The process is affected by the cellular level of acetyl-CoA and the activity of acetyltransferases. Interestingly, enzymatic acetylation mainly occurs in the nucleus and cytoplasm; this is in contrast to nonenzymatic acetylation, which principally takes place in mitochondria according to the location of acetyltransferases, the level of acetyl-CoA, and the physiologic conditions of the different subcellular compartments. In this section, we will introduce the 2 different mechanisms of lysine acetylation.
Enzymatic acetylation mainly occurs in the nucleus and cytoplasm
Enzymatic acetylation is characterized by the addition of an acetyl group to lysine residues with KATs, which are composed of the p300/cAMP response element binding protein binding protein (CBP) family, the general control of nucleotidesynthesis 5 (GCN5)-related N-acetyltransferase (GNAT) family, and the MYST family [including monocytic leukemia zine finger protein (MOZ), something about silencing 3 (YBF2/SAS3), something about silencing 2 (SAS2) and tat interacting protein (Tip60)] (25, 69–72). All KATs have a structurally similar recognition domain of acetyl-CoA (23, 72). Existing data suggest that KATs collectively adopt a direct attack mechanism for lysine acetylation (73). In the acetylation events catalyzed by the GNAT and MYST families, the positively charged ε-amine group of lysine is deprotonated by the active-site glutamate/aspartate on GNAT and MYST KATs. Thus, the nucleophilic ε-amine group of lysine attacks via the electrophilic carbonyl carbon on acetyl-CoA, causing the transfer of the acetyl group (74–76). Although no active-site base has been reported in p300/CBP, kinetic studies indicate that it uses a direct attack mechanism as well (77, 78). These results provide strong evidence for a unified catalytic mechanism of KATs.
It is worth noting that KATs are predominantly located in the nucleus and cytoplasm. To date, few details about the KATs in mitochondria are available in mammals. Within the KATs, the GNAT family, including GCN5 and p300/CBP-associated factor (PCAF), is located in the nucleus with functions in the acetylate histone (69, 79, 80). A study showed that under the circumstance of deletion of GCN5/PCAF in cells, the level of histone H3K9 acetylation is decreased remarkably and specifically (81). In addition to histones, PCAF can mediate the acetylation of nonhistones. The p300/CBP family located in the nucleus and cytoplasm could acetylate histones and several nuclear proteins, thus acting as a transcriptional coactivator. Meanwhile, p300 itself can also be autoacetylated (82). In comparison with the above 2 families, the MYST family, mainly located in the nucleus, is larger and more diverse and plays distinct roles in various cellular processes. Among the MYST family, Tip60 is the most studied KAT and is involved in DNA repair as well as transcription regulation (72). In contrast, there are only 2 potential acetyltransferases (GCN5L1 and acetyl-CoA acetyltransferase 1) reported in mitochondria, suggesting that enzymatic acetylation mostly occurs in nucleus and cytoplasm rather than in mitochondria.
Enzymatic lysine acetylation predominantly takes an important part in the regulation of the cytoplasmic metabolism process and gene transcription. Accordingly, aberrant acetylation could provoke cellular dysfunction and is associated with a broad spectrum of diseases. For example, the acetylation of p53 at K120 by Tip60 plays a key role in p53-dependent apoptosis (83), and the abnormal regulation of its target proteins contributes to the progression of Alzheimer’s disease (84). In addition, in the case of PKM2, decreased activity has been observed in tumors, resulting in the accumulation of glycolytic intermediates that are used by the tumor to meet the demand for rapid growth (85).
Nonenzymatic acetylation mainly occurs in mitochondria
In addition to the established enzyme-catalyzed acetylation, historical and recent evidence has shown that protein acetylation can occur nonenzymatically under physiologic conditions, especially in alkaline environments (28, 79, 86, 87) (Fig. 1B). In eukaryotic cells, the main acetyl donor for nonenzymatic acetylation is from acetyl-CoA, which is composed of an acetyl group connected to CoA, through a thioester bond (88). Several studies have demonstrated that under alkaline conditions, acetyl-CoA incubation with inactivated GCN5 acetyltransferase or without any enzymes still results in lysine acetylation (28). Actually, the notion of nonenzymatic acetylation is highlighted for its possible contribution to the landscape of protein acetylation. The mechanism proposed for nonenzymatic acetylation involves the initial transfer of the acetyl group to the amino group on proteins. Because the thioester bond is reactive and lysine can be deprotonated naturally at an alkaline pH, acetyl-CoA could spontaneously transfer the acetyl moiety to the amino groups on proteins so that no enzyme is required. Thus, nonenzymatic acetylation is favored by the abundance of acetyl-CoA and an alkaline pH. Moreover, the reactivity of nucleophilic acetyl-CoA to electrophilic lysine residues increases with the elevated pH by aggrandizing the proportion of deprotonated lysines.
What draws our attention is that although only 2 acetyltransferases have been identified in mitochondria, acetylation is highly abundant, with thousands of acetylation sites identified on mitochondrial proteins (89–91). Increasing evidence has demonstrated that acetylations in mitochondria are predominantly derived from nonenzymatic mechanisms through exposure to acetyl-CoA (79, 87, 89). The chemical environment in mitochondria where the concentration of acetyl-CoA is high (0.1–1.5 mM) and where the pH is 7.9–8.0 makes nonenzymatic acetylation favorable (72). Further supporting this idea, studies in various mouse tissues have indicated that the stoichiometry of protein acetylation in mitochondria is very low in comparison with that of phosphorylation. These findings support the idea that a large proportion of mitochondrial protein acetylations occur by nonenzymatic modification due to the alkaline pH and the high level of acetyl-CoA. Mitochondrial acetyl-CoA is derived from major carbon catabolic pathways and fuels metabolism, including the decarboxylation of the glycolysis-product pyruvate, fatty acid β-oxidation, and the catabolism of branched amino acids (23). In addition, ethanol can be converted into acetyl-CoA in hepatocytes, which may account for the hyperacetylation of mitochondrial proteins preferentially in the liver caused by ethanol ingestion (92, 93). In a study on calorie-restricted mice, the increased acetylation of overall mitochondrial proteins was observed because the lipolysis in adipose tissue leads to the release of fatty acids into hepatic mitochondria and increases the β-oxidation (94, 95). Interestingly, it has been demonstrated that the protein acetylation in mitochondria is also elevated in mice fed an HFD (96). These findings may be interpreted by the fact that the concentration of mitochondrial acetyl-CoA is increased in both fasting and feeding state, and the level of concentration-dependent nonenzymatic acetylation in mitochondria is elevated accordingly (97).
Although lysine acetylation could activate, inhibit, or stabilize proteins or even have immeasurable effects outside mitochondria, the acetylation in mitochondria mainly exerts inhibitory effects on its target proteins. Chemical acetylation (nonenzymatic acetylation) in mitochondria is emerging as a form of carbon stress and negatively regulates protein function, disturbing mitochondrial homeostasis. Aberrant acetylation in mitochondria has been implicated in the pathophysiology of metabolic syndrome and diabetes as well as cancer (28). For example, the activity of mitochondrial superoxide dismutase is restricted by the increased acetylation in mitochondria, pushing the accumulation of ROS and the redox imbalance (77). Likewise, elevated acetylation inhibits the activity of PDH, favoring lactate generation from glycolysis (17). In addition to nonenzymatic acetylation, mitochondria are also major sites of other nonenzymatic acylation events such as crotonylation and succinylation (98). Crotonylated proteins are mainly enriched at active gene promoters and potential enhancers involved in chromosome organization, nucleic acid metabolism, and RNA processing (99). In addition, the hypersuccinylation in mitochondria is reported to cause decreased fatty acid oxidation and hyperammonemia (100).
Compared with that in mitochondria, little information is available about the nonenzymatic acetylation in the nucleus and cytoplasm, which maintains a pH of 7.2 and lower levels of acetyl-CoA. However, a study in vitro indicated that nonenzymatic acetylation could also occur in the cytoplasm and nucleus when some acetylation sites were identified in vivo.
Lysine deacetylases
The reversible lysine acetylation is controlled by the contrary effects of acetyltransferases and deacetylases. The deacetylases mediate the removal of the acetyl group, including 4 classes. Class I, II, and IV lysine deacetylases (KDACs) are Zn2+ dependent, referred to as the classic KDAC family (Fig. 1C), and class III KDAC is NAD+ dependent, and is also called the SIRT family (25, 67) (Fig. 1D). The classic KDAC family is predominately present in the cytoplasm and nucleus, whereas SIRTs are localized in mitochondria additionally. Generally, class I (HDAC1, -2, -3, and -8) and II (HDAC4, -5, -6, -7, -9, and 10) display important regulating roles in transcription factors and histones. However, the target proteins of class II members HDAC6 and HDAC10 are additionally in the cytoplasm. So far, the only role of class IV (HDAC11) mainly located in the nucleus is implicated in the regulation of IL-10 expression and immune tolerance (72, 101). The SIRTs include 7 members, of which SIRT1 and SIRT6 are located in the nucleus and SIRT2 is found in the cytoplasm. SIRT7 is located in the nucleolus (24, 101). The deacetylations of mitochondrial proteins are mediated by SIRT3, SIRT4, and SIRT5, especially SIRT3, which exerts a crucial function on the activity of mitochondrial enzymes and the maintaining of mitochondrial homeostasis (96, 102, 103). The SIRTs present in the nucleus are in charge of nuclear transcription factors and histones, whereas SIRTs located in cytoplasm and mitochondria have pivotal roles in the deacetylation of key enzymes involved in various metabolic pathways such as glycolysis, the tricarboxylic acid (TCA) cycle, and fatty acid oxidation (104). In most cases, the deacylation mediated by SIRTs is propitious to maintain normal cellular function (105), and a series of diseases could be triggered or exacerbated under the circumstance of SIRT knockdown or ablation (106). Additionally, SIRTs could mediate other deacylation events. It is reported that SIRT5 mediates the desuccinylation of lysine (107), and SIRT1, SIRT2, and SIRT3 regulate histone decrotonylation (108).
Nonenzymatic and enzymatic acetylations show specificity in different stages of liver disease
Nonenzymatic PTMs have been proposed as indicators of different cellular stresses (109, 110). Several studies have shown that chronic liver diseases with increased glycolysis appear to be an adverse outcome of hepatic mitochondria dysfunction. In addition, the alkaline pH (pH 7.9–8.0) and high concentration of acetyl-CoA (0.1–1.5 mM) in the mitochondrial matrix make nonenzymatic acetylation favorable (111). Predictably, nonenzymatic acetylation in mitochondria may play a crucial role in the increased glycolysis due to mitochondrial oxidative phosphorylation disorders during liver metabolic diseases, such as NAFLD and hepatic inflammation. Recently, our study found that the production of hepatic acetyl-CoA was increased remarkably in LPS-induced hepatic inflammation and led to the hyperacetylation of mitochondrial glutamate oxaloacetate transaminase 2 (GOT2) and malate dehydrogenase 2 (MDH2) and enhanced glycolysis. These findings suggest that elevated levels of mitochondrial acetyl-CoA could promote malate-aspartate shuttle activity and glycolysis activity via nonenzymatic acetylation during the inflammatory stress response (17). Nevertheless, increasing evidence demonstrates that in the case of cirrhosis and HCC, glycolysis is regulated mostly by PCAF and p300/CBP, which are known as 2 major KATs and have increased enzymatic activity (101, 112–114). Consequently, in this case, on some level it can be indicated that glycolysis process seems to be regulated by nonenzymatic acetylation in metabolic liver diseases, such as hepatic metabolic syndrome and inflammation, whereas in liver end-stage cirrhosis and tumors, it is controlled by enzymatic acetylation.
NONENZYMATIC ACETYLATION REGULATES GLYCOLYSIS IN HEPATIC METABOLIC SYNDROME AND INFLAMMATION
Nowadays, along with the changes of diet and lifestyle such as surplus energy intake, excess ethanol ingestion, and sedentary life habits, the number of people suffering from obesity, hyperlipidemia, and type 2 diabetes (referred to as metabolic syndrome) is rising dramatically. NAFLD is the hepatic performance of the metabolic syndrome, 20–30% of which could further progress into NASH (115–118).
In these chronic liver diseases, mitochondrial dysfunction is a common hallmark; chronic liver disease is actually a mitochondrial disease, with oxidative phosphorylation disorder, oxidative stress, and the dysregulation of metabolic enzymes (57, 59, 119). Accumulated studies uncovered that mitochondrial lysine acetylation is an important participator in mitochondrial dysfunction (120). As mentioned above, mitochondrial protein acetylation is predominantly nonenzymatic, with acetyl-CoA and SIRT3 (the only mitochondrial member with robust deacetylation activity), mainly reversing this process. The level of acetyl-CoA in mitochondria fluctuates with both extracellular and intracellular alterations. Several studies have shown that the level of hepatic mitochondrial protein acetylation increases remarkably in mice placed on an HFD, accompanied by the down-regulation of SIRT3. Moreover, HFD feeding in SIRT3 gene–deficient mice induces the aggravation of obesity, diabetes, and fatty liver in comparison with those of normal mice (96, 121). HFD-induced metabolic disturbances are closely relevant to the elevated abundance of acetyl-CoA in liver mitochondria as a result of the improperly increased lipolysis in peripheral adipocytes (122). For example, an elevated level of hepatic acetyl-CoA has been observed in type 1 diabetes resulting from the increased turnover of fatty acids (123). Another study showed that caloric restriction also induces overall hepatic mitochondrial hyperacetylation in mice for the accelerated fatty acid β oxidation within hepatic mitochondria to provide energy (94, 95). Consistent with this, it has been demonstrated that hepatic mitochondria hyperacetylation directly depends on the flux of fatty acid oxidation (124). During starvation, the mice with fatty acid oxidation defects show no increased acetylation in liver mitochondria, and the acetyl-CoA for mitochondrial acetylation is directly derived from the fatty acid oxidation through the use of radioactively labeled palmitate. Furthermore, ethanol intake also leads to mitochondrial protein hyperacetylation predominately in the liver because hepatocytes can convert ethanol into acetyl-CoA (93).
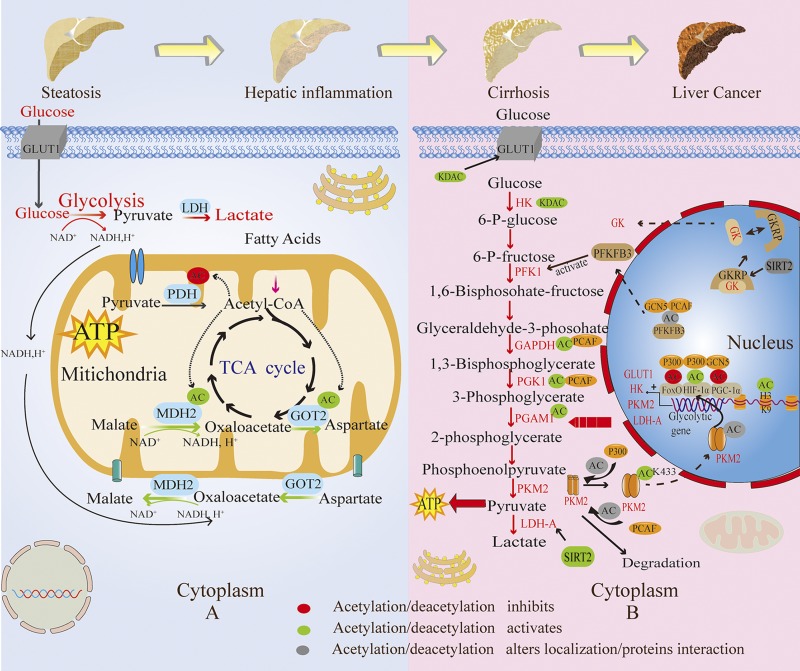
In the clinical context of hepatic metabolic syndrome and inflammation, how does the nonenzymatic acetylation regulate glycolysis? Hepatic metabolic syndrome is associated with inflammation because inflammation stimulates the reprogramming of nutrient metabolism and hepatic mitochondria-generated acetyl-CoA (17). Furthermore, in NAFLD, intrahepatic fat could induce mitochondrial β-oxidation and the generation of acetyl-CoA (125). In hepatic metabolic syndrome and inflammation in pigs injected with LPS, we found that inflammatory stress exacerbated the acetylation of mitochondrial proteins because of the accumulation of hepatic acetyl-CoA. Interestingly, the increased acetyl-CoA is mainly derived from fatty acid oxidation rather than pyruvate oxidative decarboxylation, leading to the hyperacetylation of MDH2 at K185, K301, K307, and K314 and of GOT2 at K159, K184, and K404 and their enhanced enzymatic activity. Thus, the activity of the malate-aspartate shuttle, which plays an indispensable role in the net transfer of NADH from the cytoplasm into mitochondria, was stimulated to maintain a high rate of glycolysis (126). Meanwhile, PDH, which transforms pyruvate into acetyl-CoA via pyruvate decarboxylation, was observed to be acetylated at K321 and thus its activity was inhibited by recruiting PDK. Its decreased activity indicates that pyruvate entering TCA through PDH to generate energy is restricted, and that increased glucose use mainly contributed to lactate production during inflammation. Of note, despite that the acetyl-CoA from pyruvate is reduced, the increased acetyl-CoA derived from fatty acid oxidation contributes to the activation of PC. The increased PC induction is both observed in HFD-induced fatty liver and LPS-induced hepatic inflammation, which could replenish the TCA cycle partially by converting pyruvate into oxaloacetate directly (127, 128). In contrast, deacetylation by SIRT3 at this site increases PDH activity. These observations suggest that acetyl-CoA generated from fatty acid oxidation promotes malate-aspartate shuttle activity and glycolysis via nonenzymatic acetylation during the inflammatory stress response (17). Likewise, a study found that in mice deficient in SIRT3 (which leads to protein hyperacetylation in mitochondria), the rates of glycolysis were increased (129) (Fig. 2A).
Figure 2.
The different regulations of glycolysis by enzymatic and nonenzymatic acetylations in different states of liver diseases. A) In hepatic metabolic syndrome and inflammation, glycolysis is regulated mainly by nonenzymatic acetylation in mitochondria. The acetylations of MDH2 and GOT2, which increase their enzymatic activity, promote the malate-aspartate shuttle and glycolytic activity. The acetylation of PDH, which inhibits its activity, leads to the glycolysis process toward lactate production. B) In cirrhosis and liver cancer, glycolysis is regulated by enzymatic acetylation largely in the nucleus and cytoplasm. Deacetylations enhance the glycolytic enzymatic activity of GLUT1, HK, and LDH-A. The deacetylation of GKRP by SIRT2 promotes its dissociation with GK, increasing glycolytic activity for cancer cell growth. PCAF and GCN5 acetylate PFKFB3, facilitating its accumulation in the cytoplasm and the activation of phosphofructokinase 1 (PFK1). The acetylations of GAPDH and PGK1 by PCAF and the acetylation of PGAM1 increase glycolytic activity, facilitating the rapid growth and tumorigenesis of hepatoma cells. The acetyltransferase p300 acetylates PKM2 at K433, promoting its nuclear import and protein kinase activity. The K305 acetylation of PKM2 by PCAF provokes its degradation, providing glycolytic intermediates for cancer cell growth. Meanwhile, the acetylations of transcription factors HIF-1α, PGC-1α, and FoxO and the histone H3K9 lead to the up-regulation of glycolytic genes like GLUT1, HK2, PKM2, and LDH-A. AC, acetyl group.
Although the influence of enzymatic acetylation on glycolytic enzymes cannot be formally excluded, the nonenzymatic acetylation in mitochondria plays a significant role in the regulation of glycolysis.
ENZYMATIC ACETYLATION REGULATES GLYCOLYSIS IN CIRRHOSIS AND LIVER CANCER
With the sustained invasion of risk factors, such as proinflammatory cytokines, ROS, and viruses, chronic liver diseases could further progress to cirrhosis (32, 130) and finally develop into HCC. Approximately 90% of patients with HCC progress from cirrhosis (7, 131, 132), which is the major type of primary hepatic cancer and has a high lethality rate (56). Metabolic reprogramming, especially the enhanced glycolysis, is a salient feature of cirrhosis and liver cancer. The highly increased glycolysis enables cancer cells to grow rapidly by providing metabolic intermediates for macromolecule biosynthesis. The glycolysis end-product pyruvate in cancer cells is largely transformed into lactate by LDH in the cytoplasm rather than oxidized via oxidative phosphorylation in mitochondria (133). Furthermore, the overproduction of lactate transported out of the cytoplasm may be beneficial to the interaction between cancer cells, consequently contributing to increased cancer cell invasion and migration (16).
The molecular pathogenesis of aerobic glycolysis in liver cancer is a complicated process which contains both the alterations of glycolytic enzyme activity in the cytoplasm and genetic changes in the nucleus such as DNA and histone modifications (132). Notably, the changes in the acetylation mechanism of DNA and histones promote the reversion of normal hepatocytes into progenitor and stem cells that can develop into cirrhosis and HCC (134). Thus, from simple steatosis and NASH to terminal cirrhosis and further liver cancer, it involves the extension of the lesion from mitochondria to the cytoplasm and nucleus (135). Predictably, in parallel, there may be a transition of the regulation mechanism of glycolysis from by nonenzymatic acetylation to by enzymatic acetylation accompanied by the development of liver disease from the early stage to the late stage. Consistent with this, in cirrhosis and HCC, glycolysis is largely regulated by acetylation with acetyltransferases including PCAF, p300/CBP, and GCN5. In addition, compared with the activity of acetyltransferases in normal liver cells, the activity of acetyltransferases in hepatoma cells is remarkably increased, and the rise in acetyltransferase activity has a significantly positive correlation with the increased growth rate of the tumors (136). In this section, we summarize the most recent findings on the targets of enzymatic acetylation in controlling glycolysis in cirrhosis and liver cancer (Fig. 2B).
As we all know, the glycolytic enzymes are located in the cytoplasm. Thus, the enzymes could preferentially undergo acetylation with acetyltransferase. The aberrant activity and expression of HK, the first rate-limiting enzyme in glycolysis, makes a significant contribution to the elevated glucose uptake and increased glycolysis that enable cancer cells to proliferate and metastasize (137–139). The activity of glycolysis is increased significantly and is related to elevated HK2 activity and GLUT1 expression in HCC, resulting in altered glucose use (140–142). How are the HK activity and expression of GLUT1 regulated by enzymatic acetylation? Although the acetyltransferases participating in HK and GLUTI are unclear, broad-spectrum KDAC inhibition both down-regulates the expression of GLUT1 and decreases HK enzymatic activity. This suggests that KDAC-mediated deacetylation may play a crucial role in the activation of glucose transport and is the first step in glycolysis for rapid tumor growth and survival (143). Of note, the enzymatic activity of GK, an isozyme of HK known only in the liver, is affected by binding the GK regulatory protein (GKRP), leading to the incapability of GK transport from the nucleus to the cytoplasm. The acetylation of GKRP at K5 by the acetyltransferase p300 allows GKRP to resist degradation through the ubiquitin-proteasome system. Thus, the acetylated GKRP prevents its dissociation with GK by increasing its stability, causing GK retention in the nucleus. Inversely, SIRT2 mediates the deacetylation of GKRP and enhances the glycolytic activity in cancer cells (144).
The 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3) molecule is a strong allosteric activator of phosphofructokinase 1, catalyzing the production of fructose-2,6-bisphosphate. PFKFB3 displays an essential part in facilitating the rapid growth of cancer cells. The activation of PFKFB3 favors the increased activity of glycolysis, enabling cancer cells to metastasize rapidly and to resist the chemotherapy-induced apoptosis. A study revealed that cisplatin, a widely used chemical drug in various cancer therapies (including liver cancer), can induce the acetylation of PFKFB3 at K472. Acetylated PFKFB3 leads to the impaired activity of the nuclear localization signal and facilitates its accumulation and thus phosphorylation in the cytoplasm, resulting in the enhancement of PFKFB3 activity and glycolysis ability. The acetylation of PFKFB3 is mainly mediated by PCAF and GCN5, and SIRT1 is the major deacetylase for PFKFB3. As a result, acetylated PFKFB3 could protect cancer cells against apoptosis induced by the drug cisplatin (145).
The acetylation of the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH), catalyzing the formation of 1,3-bisphosphoglycerate, has become an important contributor to liver tumor growth. A recent study demonstrated that GAPDH is acetylated at K254 by the acetyltransferase PCAF at high levels of glucose, leading to the increased activity of GAPDH. In addition, glucose deprivation triggers the deacetylation of GAPDH mediated by HDAC5 and thus causes a decrease in GAPDH activity (19, 101, 112). Controversially, HDAC5 belongs to the class IIa HDACs, which are catalytically inactive against acetyl-lysine. Thus, HDAC5-mediated deacetylation of GAPDH remains uncertain. GAPDH is also an enzyme in gluconeogenesis. It is demonstrated that acetylated GAPDH contributes to increased glycolysis activity and deacetylated GAPDH favors glucose production by gluconeogenesis (30, 146). These results suggest that the acetylation of GAPDH at K254 by PCAF has a positive impact on liver cancer growth, and proliferation and could be a potential therapeutic target for liver cancer (93, 101).
Phosphoglycerate kinase 1 (PGK1), which catalyzes the reversible conversion of 1,3-bisphosphoglycerate to 3-phosphoglycerate in glycolysis and gluconeogenesis, was observed to be significantly overexpressed in liver cancer, and a positive correlation is shown between PGK1 enzymatic activity and hepatoma cell growth (113). It has been demonstrated that the proliferation and tumorigenesis of hepatoma cells are significantly weakened when the PGK1 gene is depleted, suggesting that PGK1 plays an oncogenic role in liver HCC progression. In liver cancer, PCAF mediates the K323 acetylation of PGK1, leading to the enhanced enzymatic activity of PGK1 and its carcinogenic effect as well as increased glycolysis. However, the effect of PCAF-mediated acetylation of PGK1 in gluconeogenesis has not been specifically addressed. The deacetylase SIRT7 was identified and validated to induce the deacetylation of PGK1 at K323. Accordingly, it can be concluded that the PCAF-mediated acetylation of PGK1 at K323 increases its activity and thus facilitates the rapid growth and tumorigenesis of hepatoma cells (113).
Phosphoglycerate mutase (PGAM)1, a key enzyme in glycolysis and gluconeogenesis, is deacetylated by SIRT1, whose expression is increased significantly in glucose restriction. The deacetylation of PGAM reduces its enzymatic activity and restricts the glycolysis process (147). Therefore, gluconeogenesis might be stimulated as a result of SIRT1-mediated deacetylation of PGAM under glucose restriction. However, the increased or decreased glucose production regulated by SIRT1 have both been reported (148–150). Thus, the role of deacetylated PGAM by SIRT1 in gluconeogenesis remains unclear. In contrast, the acetylation of PGAM1 enhances its enzymatic activity and glycolysis activity. K251, K253, and K254 are the major acetylation sites (147). Predictably, in liver cancer cells where the glucose level is high, the acetylation of PGAM1 is conducive to cancer cell growth.
PK, the third rate-limiting enzyme in glycolysis, catalyzes the production of pyruvate. There are 4 isoforms of PK: PKM1, PKM2, PKML, and PKMR. Interestingly, PKM2 is predominately expressed during the fetal period of development and transformed cancer cells (114, 151). Several studies found that the acetylation of K433 of PKM2 by p300 drives the switch of PKM2 from a tetramer to a dimer and its nuclear import in cancer cells, promoting the protein kinase activity of PKM2. Thus, hypoxia-inducible transcription factor 1α (HIF-1α) is activated, increasing the expression of glycolytic genes (152). In cancer, this contributes to the proliferation and tumorigenesis of cancer cells. Both HDACs and SIRTs are implicated in the deacetylation of PKM2 at K433 (153). Additionally, the acetylation of PKM2 can also occur at K305 by PCAF in cancer cells responding to high glucose levels, which reduces its activity and provokes its autophagic degradation. As a result, the increased amounts of glycolytic intermediates resulting from PKM2 degradation allow cancer cells to grow and proliferate rapidly (154). Conversely, SIRT2 deacetylates this residue (155).
LDH-A is regarded as a potential target in cancer treatments because of its pivotal role in maintaining an elevated glycolysis rate. It has been observed that the expression of LDH-A is generally up-regulated in a range of cancers. The acetylation of LDH-A at K5, which leads to the inhibition of its enzyme activity and its subsequent lysosomal degradation, is reduced in cancers. SIRT2 mediates the deacetylation of LDH-A, enhancing its protein level and enzyme activity (16).
High nuclear acetylation levels have been observed in cancer cells resulting from the increased activity of the acetyltransferase and the ectopic synthesis of acetyl-CoA in the nucleus (156–158). Accordingly, in addition to the regulation of glycolytic enzyme activity, acetylation can also control the expression of glycolysis-associated genes in the nucleus through the modification of histones and transcription factors (101). For instance, the increased glycolytic flux is associated with HIF-1α, which modulates the gene expression involved in glycolysis like GLUT1, HK2, PKM, and LDH-A (159). Likewise, the up-regulation of PDK1 is also controlled directly by HIF-1α, blocking the entry of pyruvate into the TCA cycle (160, 161). HIF-1α overexpression can be found in many tumors, including liver cancer, and HIF-1α is involved in tumor growth, infiltration, and metastasis (162). It has been observed that HIF-1α was acetylated by p300 at K709, which increases protein stability and HIF-1α activity (161); this regulation can be opposed by HDAC1 (161). However, other studies demonstrated that SIRT1 (162) and SIRT2 (163) could also deacetylate HIF-1α and reduce its enzyme activity to control glycolytic and inflammatory gene expression. The peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α) and forkhead box O (FoxO) are also regulated by acetylation to control the expression of glycolytic genes. In hepatocytes, GCN5 acetyltransferase specifically acetylates PGC-1α and inhibits its transcriptional activity, leading to the inactivation of gluconeogenic enzymes (164). The deacetylation of PGC-1α by SIRT1 appears to display an important role in modulating the balance of gluconeogenic and glycolytic genesis (165). The transcription factor FoxO has been implicated in the up-regulation of gluconeogenic genes and the down-regulation of glycolytic genes, playing a crucial role in tumor suppression (166, 167). Recent studies have disclosed the important role of FoxO acetylation in liver cancer treatment. The acetyltransferase p300/CBP mediates FoxO acetylation, inhibiting its transcriptional and biologic activities. In this regard, the acetylation of FoxO could heighten glycolysis activity and promote cancer cell growth and cisplatin resistance (168). Beyond these, compared with normal liver and hepatic cirrhosis, the acetylation levels of histone H3K9 and H4K8 in the tissues of HCC were remarkably higher. Interestingly, the up-regulation of both histone acetyltransferases and class II KDACs was observed. However, histone acetyltransferases outnumber the class II KDACs, leading to [3H] and H4 hyperacetylation (134, 169, 170). Consequently, H3K9 hyperacetylation activates the expression of a range of glycolytic genes that are dependent on HIF-1α. SIRT6 was also demonstrated to deacetylate H3K9 (101).
CONCLUSIONS AND FUTURE PERSPECTIVES
To date, ∼30 KATs have been identified in the human proteome and are predominantly located in the nuclear and cytoplasm, wherein enzymatic acetylation largely occurs. However, in mitochondria, rare acetyltransferases and high levels of acetyl-CoA and alkaline pH make nonenzymatic acetylation favorable. Studies have shown that primary liver diseases such as NAFLD and NASH are closely linked to mitochondrial disorders such as redox imbalance, aberrant oxidative phosphorylation, and insufficient supply of energy together with increased acetyl-CoA, giving rise to mitochondrial hyperacetylation. With the progression of liver diseases, the disorder could gradually extend from mitochondria to the nucleus and cytoplasm, including DNA damage, altered histones, transcription factors, and the abnormal activity of cytoplasmic proteins along with the dysregulation of acetylation. We propose that glycolysis is controlled mainly by mitochondrial nonenzymatic acetylation in NAFLD and NASH, whereas in terminal diseases such as liver cirrhosis and cancer, glycolysis is regulated mostly by enzymatic acetylation in the cytoplasm and nucleus. This review sheds light on the roles of nonenzymatic acetylation and enzymatic acetylation in glycolysis regulation in different liver diseases. In NAFLD and NASH, glycolysis is regulated indirectly by nonenzymatic acetylation in mitochondria. For example, in hepatic inflammation, the acetylation of MDH2 and GOT2 as well as PDH improve glycolysis activity and induce the glycolysis pathway to generate lactate. When these liver diseases develop into hepatic cirrhosis and HCC, the phenomenon we observed is the direct enzymatic acetylation of glycolytic enzymes as well as transcription factors and histones that regulate the glycolytic genes such as HIF-1α, FoxO, PGC-1α, and H3K9. Acetylation with PCAF, p300/CBP, and GCN5 enhances the activity of glycolysis, contributing to hepatic cancer cell growth, infiltration, and metastasis (Table 1). Of note, although the regulation of enzymatic lysine acetylation considerably differs from that of nonenzymatic lysine acetylation, they are not mutually exclusive and could occur simultaneously (171). However, identifying a direct method to distinguish nonenzymatic acetylation from enzyme-dependent acetylation still remains an analytical challenge. Meanwhile, it is known that glycolysis and gluconeogenesis regulate blood glucose levels by consuming glucose and production glucose, respectively. They share several reversible enzymes such as GAPDH, PGK1, and PGAM1. Therefore, the acetylation of these reversible enzymes not only affects the glycolysis process but also influences the pathway of gluconeogenesis. For example, the acetylation of GAPDH promotes glycolysis and its deacetylation favors gluconeogenesis.
TABLE 1.
Overview of the regulation of different targets related to glycolysis via enzymatic and nonenzymatic acetylation in different liver diseases
| Disease | Target | Acetylation/ deacetylation | KAT | KDAC | Effect | Metabolic response | Reference |
|---|---|---|---|---|---|---|---|
| Hepatic metabolic syndrome and inflammation | MDH2 | Acetylation | Nonenzymatic | — | Activation | Malate-aspartate shuttle activity↑ glycolysis↑ | 17 |
| GOT2 | Acetylation | Nonenzymatic | — | Activation | Malate-aspartate shuttle activity↑glycolysis↑ | 17, 126 | |
| PDH | Acetylation | Nonenzymatic | — | Inhibition | lactate production↑ | 17 | |
| Cirrhosis and liver cancer | GLUT1 | Deacetylation | — | — | Activation | Glycolysis↑ tumor growth and survival↑ | 143 |
| HK | Deacetylation | — | — | Activation | Glycolysis↑ tumor growth and survival↑ | 143 | |
| GKRP | Decetylation | p300 | SIRT2 | Stability↓ Releasing GK to cytoplasm | Glycolysis↑ | 144 | |
| PFKFB3 | Acetylation | GCN5 PCAF | SIRT1 | Activation Entering cytoplasm | Glycolysis↑ tumor cell growth↑ | 145 | |
| GAPDH | Acetylation | PCAF | HDAC5 | Activation | Glycolysis↑ liver tumor growth↑ | 101, 112 | |
| PGK1 | Acetylation | PCAF | SIRT7 | Activation | Glycolysis↑ cancer cell proliferation and tumorigenesis↑ | 113 | |
| PGAM1 | Acetylation | — | SIRT1 | Activation | Glycolysis↑ | 147 | |
| PKM2 | Acetylation | p300 | SIRT6 | Nuclear accumulation Protein kinase activity ↑ | Glycolysis↑ cell proliferation and tumorigenesis↑ | 152, 153 | |
| Acetylation | PCAF | SIRT2 | Degradation | Tumor growth and cell proliferation↑ | 154, 155 | ||
| LDH-A | Deacetylation | — | SIRT2 | Activation | Lactate production↑ | 16 | |
| HIF-1α | Acetylation | p300 | HDAC1, SIRT1/2 | Stability and activity↑ | Glycolysis↑ umor growth and metastasis↑ | 161–163 | |
| PGC-1α | Acetylation | GCN5 | SIRT1 | Inhibition | Glycolysis↑ | 164, 165 | |
| FoxO | Acetylation | p300 | — | Inhibition | Glycolysis↑ cancer cell growth↑ | 168 | |
| H3K9 | Acetylation | — | SIRT6 | Activation | Glycolytic genes↑ | 101, 169 |
In summary, during chronic liver diseases, glycolysis is largely controlled by nonenzymatic acetylation, whereas in liver cirrhosis and cancer, glycolysis is mainly regulated by enzymatic acetylation. This review provides new insights into the acetylation mechanisms of glycolysis regulation in liver diseases and new understandings that may unmask potential therapeutic treatments. It also raised several important questions that may be crucial for a more comprehensive molecular understanding of how nonenzymatic and enzymatic acetylation control glycolysis in different liver diseases:
In liver cirrhosis and cancer, what are the molecular mechanisms that control acetyltransferases activity in hepatocytes?
How does the interaction between cytoplasmic enzymatic acetylation and nuclear enzymatic acetylation during liver cirrhosis and cancer work, and which of them plays a dominant role in the regulation of glycolysis?
Could the nonenzymatic and enzymatic acetylations influence other PTMs such as succinylation and malonylation that affect the rate of glycolysis?
In NAFLD and NASH, is glycolysis regulated by the coordination of mitochondrial nonenzymatic acetylation and cytoplasmic enzymatic acetylation?
Are there specific sites on glycolytic enzymes that are targeted by both enzymatic acetylation and nonenzymatic acetylation?
Does nonenzymatic lysine acetylation partially compensate for dysfunctions of some enzyme-mediated processes?
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant 31572409), the Hubei Provincial Natural Science Foundation of China (Grant 2018CFA071), and the National Key Research and Development Program (Grant 2018YFD0500600). The authors declare no conflicts of interest.
Glossary
- CBP
cAMP response element binding protein
- CoA
coenzyme A
- FoxO
forkhead box O
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
- GCN5
general control of nucleotidesynthesis 5
- GK
glucokinase
- GKRP
GK regulatory protein
- GLUT1
glucose transporter 1
- GNAT
GCN5-related N-acetyltransferase
- GOT2
glutamate oxaloacetate transaminase 2
- HCC
hepatocellular carcinoma
- HDAC
histone deacetylase
- HFD
high-fat diet
- HIF-1α
hypoxia-inducible transcription factor 1α
- HK
hexokinase
- KAT
lysine acetyltransferase
- KDAC
lysine deacetylase
- LDH
lactate dehydrogenase
- MDH2
malate dehydrogenase 2
- MYST, MOZ, YBF2/SAS3, SAS2 NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PC
pyruvate carboxylase
- PCAF
p300/CBP-associated factor
- PDH
pyruvate dehydrogenase
- PDK
PDH kinase isoenzyme
- PFKFB3
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- PGAM
phosphoglycerate mutase
- PGC-1α
peroxisome proliferator-activated receptor γ coactivator 1α
- PGK1
phosphoglycerate kinase 1
- PK
pyruvate kinase
- PTM
post-translational modification
- ROS
reactive oxygen species
- SIRT
sirtuin
- TCA
tricarboxylic acid
- Tip60
tat interacting protein
AUTHOR CONTRIBUTIONS
All authors contributed to the concept, design, and writing of this article and experimental work described in the text.
REFERENCES
- 1.Yu Y., Wang X., Nyberg S. L. (2014) Application of induced pluripotent stem cells in liver diseases. Cell Med. 7, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Agrawal S., Duseja A. (2014) Nonalcoholic fatty liver disease--the clinician’s perspective. Trop. Gastroenterol. 35, 212–221 [DOI] [PubMed] [Google Scholar]
- 3.Bechmann L. P., Hannivoort R. A., Gerken G., Hotamisligil G. S., Trauner M., Canbay A. (2012) The interaction of hepatic lipid and glucose metabolism in liver diseases. J. Hepatol. 56, 952–964 [DOI] [PubMed] [Google Scholar]
- 4.Cohen J. C., Horton J. D., Hobbs H. H. (2011) Human fatty liver disease: old questions and new insights. Science 332, 1519–1523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellentani S., Scaglioni F., Marino M., Bedogni G. (2010) Epidemiology of non-alcoholic fatty liver disease. Dig. Dis. 28, 155–161 [DOI] [PubMed] [Google Scholar]
- 6.Milić S., Stimac D. (2012) Nonalcoholic fatty liver disease/steatohepatitis: epidemiology, pathogenesis, clinical presentation and treatment. Dig. Dis. 30, 158–162 [DOI] [PubMed] [Google Scholar]
- 7.El-Serag H. B. (2011) Hepatocellular carcinoma. N. Engl. J. Med. 365, 1118–1127 [DOI] [PubMed] [Google Scholar]
- 8.Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D. (2011) Global cancer statistics. CA Cancer J. Clin. 61, 69–90 [DOI] [PubMed] [Google Scholar]
- 9.Marrero J. A. (2006) Hepatocellular carcinoma. Curr. Opin. Gastroenterol. 22, 248–253 [DOI] [PubMed] [Google Scholar]
- 10.Petersen M. C., Vatner D. F., Shulman G. I. (2017) Regulation of hepatic glucose metabolism in health and disease. Nat. Rev. Endocrinol. 13, 572–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rui L. (2014) Energy metabolism in the liver. Compr. Physiol. 4, 177–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somvanshi P. R., Patel A. K., Bhartiya S., Venkatesh K. V. (2016) Influence of plasma macronutrient levels on hepatic metabolism: role of regulatory networks in homeostasis and disease states. RSC Adv. 6, 14344–14371 [Google Scholar]
- 13.Yao W., Wang T., Huang F. (2018) p300/CBP as a key nutritional sensor for hepatic energy homeostasis and liver fibrosis. BioMed Res. Int. 2018, 8168791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nishikawa T., Bellance N., Damm A., Bing H., Zhu Z., Handa K., Yovchev M. I., Sehgal V., Moss T. J., Oertel M., Ram P. T., Pipinos I. I., Soto-Gutierrez A., Fox I. J., Nagrath D. (2014) A switch in the source of ATP production and a loss in capacity to perform glycolysis are hallmarks of hepatocyte failure in advance liver disease. J. Hepatol. 60, 1203–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye J. H., Chao J., Chang M. L., Peng W. H., Cheng H. Y., Liao J. W., Pao L. H. (2016) Pentoxifylline ameliorates non-alcoholic fatty liver disease in hyperglycaemic and dyslipidaemic mice by upregulating fatty acid β-oxidation. Sci. Rep. 6, 33102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhao D., Xiong Y., Lei Q. Y., Guan K. L. (2013) LDH-A acetylation: implication in cancer. Oncotarget 4, 802–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang T., Yao W., Shao Y., Zheng R., Huang F. (2018) PCAF fine-tunes hepatic metabolic syndrome, inflammatory disease, and cancer. J. Cell. Mol. Med. 22, 5787–5800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weinhouse S. (1956) On respiratory impairment in cancer cells. Science 124, 267–269 [DOI] [PubMed] [Google Scholar]
- 19.Bond S. T., Howlett K. F., Kowalski G. M., Mason S., Connor T., Cooper A., Streltsov V., Bruce C. R., Walder K. R., McGee S. L. (2017) Lysine post-translational modification of glyceraldehyde-3-phosphate dehydrogenase regulates hepatic and systemic metabolism. FASEB J. 31, 2592–2602 [DOI] [PubMed] [Google Scholar]
- 20.Prakasam G., Iqbal M. A., Bamezai R. N. K., Mazurek S. (2018) Posttranslational modifications of pyruvate kinase M2: tweaks that benefit cancer. Front. Oncol. 8, 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hitosugi T., Chen J. (2014) Post-translational modifications and the Warburg effect. Oncogene 33, 4279–4285 [DOI] [PubMed] [Google Scholar]
- 22.Li L., Zhang P., Bao Z., Wang T., Liu S., Huang F. (2016) PGC-1α promotes ureagenesis in mouse periportal hepatocytes through SIRT3 and SIRT5 in response to glucagon. Sci. Rep. 6, 24156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Choudhary C., Weinert B. T., Nishida Y., Verdin E., Mann M. (2014) The growing landscape of lysine acetylation links metabolism and cell signalling. Nat. Rev. Mol. Cell Biol. 15, 536–550 [DOI] [PubMed] [Google Scholar]
- 24.Kaypee S., Sudarshan D., Shanmugam M. K., Mukherjee D., Sethi G., Kundu T. K. (2016) Aberrant lysine acetylation in tumorigenesis: implications in the development of therapeutics. Pharmacol. Ther. 162, 98–119 [DOI] [PubMed] [Google Scholar]
- 25.Drazic A., Myklebust L. M., Ree R., Arnesen T. (2016) The world of protein acetylation. Biochim. Biophys. Acta 1864, 1372–1401 [DOI] [PubMed] [Google Scholar]
- 26.Olia A. S., Barker K., McCullough C. E., Tang H. Y., Speicher D. W., Qiu J., LaBaer J., Marmorstein R. (2015) Nonenzymatic protein acetylation detected by NAPPA protein arrays. ACS Chem. Biol. 10, 2034–2047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baeza J., Smallegan M. J., Denu J. M. (2015) Site-specific reactivity of nonenzymatic lysine acetylation. ACS Chem. Biol. 10, 122–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner G. R., Hirschey M. D. (2014) Nonenzymatic protein acylation as a carbon stress regulated by sirtuin deacylases. Mol. Cell 54, 5–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhao S., Xu W., Jiang W., Yu W., Lin Y., Zhang T., Yao J., Zhou L., Zeng Y., Li H., Li Y., Shi J., An W., Hancock S. M., He F., Qin L., Chin J., Yang P., Chen X., Lei Q., Xiong Y., Guan K. L. (2010) Regulation of cellular metabolism by protein lysine acetylation. Science 327, 1000–1004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q., Zhang Y., Yang C., Xiong H., Lin Y., Yao J., Li H., Xie L., Zhao W., Yao Y., Ning Z. B., Zeng R., Xiong Y., Guan K. L., Zhao S., Zhao G. P. (2010) Acetylation of metabolic enzymes coordinates carbon source utilization and metabolic flux. Science 327, 1004–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jeong D. W., Kim T. S., Cho I. T., Kim I. Y. (2004) Modification of glycolysis affects cell sensitivity to apoptosis induced by oxidative stress and mediated by mitochondria. Biochem. Biophys. Res. Commun. 313, 984–991 [DOI] [PubMed] [Google Scholar]
- 32.Beyoğlu D., Idle J. R. (2013) The metabolomic window into hepatobiliary disease. J. Hepatol. 59, 842–858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu J., Wu Y., Yang P. (2016) High glucose-induced oxidative stress represses sirtuin deacetylase expression and increases histone acetylation leading to neural tube defects. J. Neurochem. 137, 371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim H. S., Xiao C., Wang R. H., Lahusen T., Xu X., Vassilopoulos A., Vazquez-Ortiz G., Jeong W. I., Park O., Ki S. H., Gao B., Deng C. X. (2010) Hepatic-specific disruption of SIRT6 in mice results in fatty liver formation due to enhanced glycolysis and triglyceride synthesis. Cell Metab. 12, 224–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhong L., D’Urso A., Toiber D., Sebastian C., Henry R. E., Vadysirisack D. D., Guimaraes A., Marinelli B., Wikstrom J. D., Nir T., Clish C. B., Vaitheesvaran B., Iliopoulos O., Kurland I., Dor Y., Weissleder R., Shirihai O. S., Ellisen L. W., Espinosa J. M., Mostoslavsky R. (2010) The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell 140, 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Begriche K., Knockaert L., Massart J., Robin M. A., Fromenty B. (2012) Mitochondrial dysfunction in nonalcoholic steatohepatitis (NASH): are there drugs able to improve it? Drug Discov. Today Dis. Mech. 6, e11–e23 [Google Scholar]
- 37.Carneiro L., Asrih M., Repond C., Sempoux C., Stehle J. C., Leloup C., Jornayvaz F. R., Pellerin L. (2017) AMPK activation caused by reduced liver lactate metabolism protects against hepatic steatosis in MCT1 haploinsufficient mice. Mol. Metab. 6, 1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Go Y., Jeong J. Y., Jeoung N. H., Jeon J. H., Park B. Y., Kang H. J., Ha C. M., Choi Y. K., Lee S. J., Ham H. J., Kim B. G., Park K. G., Park S. Y., Lee C. H., Choi C. S., Park T. S., Lee W. N., Harris R. A., Lee I. K. (2016) Inhibition of pyruvate dehydrogenase kinase 2 protects against hepatic steatosis through modulation of tricarboxylic acid cycle anaplerosis and ketogenesis. Diabetes 65, 2876–2887 [DOI] [PubMed] [Google Scholar]
- 39.Hwang B., Jeoung N. H., Harris R. A. (2009) Pyruvate dehydrogenase kinase isoenzyme 4 (PDHK4) deficiency attenuates the long-term negative effects of a high-saturated fat diet. Biochem. J. 423, 243–252 [DOI] [PubMed] [Google Scholar]
- 40.Tso S. C., Qi X., Gui W. J., Wu C. Y., Chuang J. L., Wernstedt-Asterholm I., Morlock L. K., Owens K. R., Scherer P. E., Williams N. S., Tambar U. K., Wynn R. M., Chuang D. T. (2014) Structure-guided development of specific pyruvate dehydrogenase kinase inhibitors targeting the ATP-binding pocket. J. Biol. Chem. 289, 4432–4443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang M., Zhao Y., Li Z., Wang C. (2017) Pyruvate dehydrogenase kinase 4 mediates lipogenesis and contributes to the pathogenesis of nonalcoholic steatohepatitis. Biochem. Biophys. Res. Commun. 495, 582–586 [DOI] [PubMed] [Google Scholar]
- 42.Shirai T., Nazarewicz R. R., Wallis B. B., Yanes R. E., Watanabe R., Hilhorst M., Tian L., Harrison D. G., Giacomini J. C., Assimes T. L., Goronzy J. J., Weyand C. M. (2016) The glycolytic enzyme PKM2 bridges metabolic and inflammatory dysfunction in coronary artery disease. J. Exp. Med. 213, 337–354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palsson-McDermott E. M., Curtis A. M., Goel G., Lauterbach M. A., Sheedy F. J., Gleeson L. E., van den Bosch M. W., Quinn S. R., Domingo-Fernandez R., Johnston D. G., Jiang J. K., Israelsen W. J., Keane J., Thomas C., Clish C., Vander Heiden M., Xavier R. J., O’Neill L. A. (2015) Pyruvate kinase M2 regulates Hif-1α activity and IL-1β induction and is a critical determinant of the warburg effect in LPS-activated macrophages. Cell Metab. 21, 65–80; erratum: 347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brazil M. (2003) Promise for glycolysis inhibitor. Nat. Rev. Drug Discov. 2, 338 [Google Scholar]
- 45.Russell J. W., Golovoy D., Vincent A. M., Mahendru P., Olzmann J. A., Mentzer A., Feldman E. L. (2002) High glucose-induced oxidative stress and mitochondrial dysfunction in neurons. FASEB J. 16, 1738–1748 [DOI] [PubMed] [Google Scholar]
- 46.Mantena S. K., King A. L., Andringa K. K., Eccleston H. B., Bailey S. M. (2008) Mitochondrial dysfunction and oxidative stress in the pathogenesis of alcohol- and obesity-induced fatty liver diseases. Free Radic. Biol. Med. 44, 1259–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang C. H., Wu S. B., Wu Y. T., Wei Y. H. (2013) Oxidative stress response elicited by mitochondrial dysfunction: implication in the pathophysiology of aging. Exp. Biol. Med. (Maywood) 238, 450–460 [DOI] [PubMed] [Google Scholar]
- 48.Lu W., Hu Y., Chen G., Chen Z., Zhang H., Wang F., Feng L., Pelicano H., Wang H., Keating M. J., Liu J., McKeehan W., Wang H., Luo Y., Huang P. (2012) Novel role of NOX in supporting aerobic glycolysis in cancer cells with mitochondrial dysfunction and as a potential target for cancer therapy. PLoS Biol. 10, e1001326; erratum: 15, e1002616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Trachootham D., Alexandre J., Huang P. (2009) Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat. Rev. Drug Discov. 8, 579–591 [DOI] [PubMed] [Google Scholar]
- 50.Gatenby R. A., Gillies R. J. (2004) Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 4, 891–899 [DOI] [PubMed] [Google Scholar]
- 51.Lunt S. Y., Vander Heiden M. G. (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 [DOI] [PubMed] [Google Scholar]
- 52.Rahman M., Hasan M. R. (2014) Pentose phosphate pathway in disease and therapy. Adv. Mater. Res. 995, 1–27 [Google Scholar]
- 53.Kowalik M. A., Columbano A., Perra A. (2017) Emerging role of the pentose phosphate pathway in hepatocellular carcinoma. Front. Oncol. 7, 87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chaves V. E., Frasson D., Garófalo M. A. R., Navegantes L. C. C., Migliorini R. H., Kettelhut I. C. (2012) Increased glyceride-glycerol synthesis in liver and brown adipose tissue of rat: in-vivo contribution of glycolysis and glyceroneogenesis. Lipids 47, 773–780 [DOI] [PubMed] [Google Scholar]
- 55.Ashworth W. B., Davies N. A., Bogle I. D. L. (2016) A computational model of hepatic energy metabolism: understanding zonated damage and steatosis in NAFLD. PLOS Comput. Biol. 12, e1005105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee N. C. W., Carella M. A., Papa S., Bubici C. (2018) High expression of glycolytic genes in cirrhosis correlates with the risk of developing liver cancer. Front. Cell Dev. Biol. 6, 138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Paradies G., Paradies V., Ruggiero F. M., Petrosillo G. (2014) Oxidative stress, cardiolipin and mitochondrial dysfunction in nonalcoholic fatty liver disease. World J. Gastroenterol. 20, 14205–14218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.García-Ruiz C., Baulies A., Mari M., García-Rovés P. M., Fernandez-Checa J. C. (2013) Mitochondrial dysfunction in non-alcoholic fatty liver disease and insulin resistance: cause or consequence? Free Radic. Res. 47, 854–868 [DOI] [PubMed] [Google Scholar]
- 59.Begriche K., Igoudjil A., Pessayre D., Fromenty B. (2006) Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion 6, 1–28 [DOI] [PubMed] [Google Scholar]
- 60.Sciacovelli M., Gaude E., Hilvo M., Frezza C. (2014) The metabolic alterations of cancer cells. Methods Enzymol. 542, 1–23 [DOI] [PubMed] [Google Scholar]
- 61.Dickman K. G., Mandel L. J. (1990) Differential effects of respiratory inhibitors on glycolysis in proximal tubules. Am. J. Physiol. 258, F1608–F1615 [DOI] [PubMed] [Google Scholar]
- 62.Latham T., Mackay L., Sproul D., Karim M., Culley J., Harrison D. J., Hayward L., Langridge-Smith P., Gilbert N., Ramsahoye B. H. (2012) Lactate, a product of glycolytic metabolism, inhibits histone deacetylase activity and promotes changes in gene expression. Nucleic Acids Res. 40, 4794–4803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gillies R. J., Raghunand N., Karczmar G. S., Bhujwalla Z. M. (2002) MRI of the tumor microenvironment. J. Magn. Reson. Imaging 16, 430–450 [DOI] [PubMed] [Google Scholar]
- 64.Gottfried E., Kunz-Schughart L. A., Ebner S., Mueller-Klieser W., Hoves S., Andreesen R., Mackensen A., Kreutz M. (2006) Tumor-derived lactic acid modulates dendritic cell activation and antigen expression. Blood 107, 2013–2021 [DOI] [PubMed] [Google Scholar]
- 65.Fischer K., Hoffmann P., Voelkl S., Meidenbauer N., Ammer J., Edinger M., Gottfried E., Schwarz S., Rothe G., Hoves S., Renner K., Timischl B., Mackensen A., Kunz-Schughart L., Andreesen R., Krause S. W., Kreutz M. (2007) Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–3819 [DOI] [PubMed] [Google Scholar]
- 66.Turner B. M. (1991) Histone acetylation and control of gene expression. J. Cell Sci. 99, 13–20 [DOI] [PubMed] [Google Scholar]
- 67.Glozak M. A., Sengupta N., Zhang X., Seto E. (2005) Acetylation and deacetylation of non-histone proteins. Gene 363, 15–23 [DOI] [PubMed] [Google Scholar]
- 68.Lee C. F., Tian R. (2015) Mitochondrion as a target for heart failure therapy- role of protein lysine acetylation. Circ. J. 79, 1863–1870 [DOI] [PubMed] [Google Scholar]
- 69.Yang X. J. (2004) The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 32, 959–976 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Berndsen C. E., Denu J. M. (2008) Catalysis and substrate selection by histone/protein lysine acetyltransferases. Curr. Opin. Struct. Biol. 18, 682–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Allis C. D., Berger S. L., Cote J., Dent S., Jenuwien T., Kouzarides T., Pillus L., Reinberg D., Shi Y., Shiekhattar R., Shilatifard A., Workman J., Zhang Y. (2007) New nomenclature for chromatin-modifying enzymes. Cell 131, 633–636 [DOI] [PubMed] [Google Scholar]
- 72.Gil J., Ramírez-Torres A., Encarnación-Guevara S. (2017) Lysine acetylation and cancer: a proteomics perspective. J. Proteomics 150, 297–309 [DOI] [PubMed] [Google Scholar]
- 73.Albaugh B. N., Arnold K. M., Denu J. M. (2011) KAT(ching) metabolism by the tail: insight into the links between lysine acetyltransferases and metabolism. ChemBioChem 12, 290–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Berndsen C. E., Albaugh B. N., Tan S., Denu J. M. (2007) Catalytic mechanism of a MYST family histone acetyltransferase. Biochemistry 46, 623–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tanner K. G., Langer M. R., Denu J. M. (2000) Kinetic mechanism of human histone acetyltransferase P/CAF. Biochemistry 39, 11961–11969 [DOI] [PubMed] [Google Scholar]
- 76.Tanner K. G., Langer M. R., Kim Y., Denu J. M. (2000) Kinetic mechanism of the histone acetyltransferase GCN5 from yeast. J. Biol. Chem. 275, 22048–22055 [DOI] [PubMed] [Google Scholar]
- 77.Liu X., Wang L., Zhao K., Thompson P. R., Hwang Y., Marmorstein R., Cole P. A. (2008) The structural basis of protein acetylation by the p300/CBP transcriptional coactivator. Nature 451, 846–850 [DOI] [PubMed] [Google Scholar]
- 78.Albaugh B. N., Kolonko E. M., Denu J. M. (2010) Kinetic mechanism of the Rtt109-Vps75 histone acetyltransferase-chaperone complex. Biochemistry 49, 6375–6385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wagner G. R., Payne R. M. (2013) Widespread and enzyme-independent Nε-acetylation and Nε-succinylation of proteins in the chemical conditions of the mitochondrial matrix. J. Biol. Chem. 288, 29036–29045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vetting M. W., S de Carvalho L. P., Yu M., Hegde S. S., Magnet S., Roderick S. L., Blanchard J. S. (2005) Structure and functions of the GNAT superfamily of acetyltransferases. Arch. Biochem. Biophys. 433, 212–226 [DOI] [PubMed] [Google Scholar]
- 81.Jin Q., Yu L. R., Wang L., Zhang Z., Kasper L. H., Lee J. E., Wang C., Brindle P. K., Dent S. Y. R., Ge K. (2011) Distinct roles of GCN5/PCAF-mediated H3K9ac and CBP/p300-mediated H3K18/27ac in nuclear receptor transactivation. EMBO J. 30, 249–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Thompson P. R., Wang D., Wang L., Fulco M., Pediconi N., Zhang D., An W., Ge Q., Roeder R. G., Wong J., Levrero M., Sartorelli V., Cotter R. J., Cole P. A. (2004) Regulation of the p300 HAT domain via a novel activation loop. Nat. Struct. Mol. Biol. 11, 308–315 [DOI] [PubMed] [Google Scholar]
- 83.Tang Y., Luo J., Zhang W., Gu W. (2006) Tip60-dependent acetylation of p53 modulates the decision between cell-cycle arrest and apoptosis. Mol. Cell 24, 827–839 [DOI] [PubMed] [Google Scholar]
- 84.Pirooznia S. K., Sarthi J., Johnson A. A., Toth M. S., Chiu K., Koduri S., Elefant F. (2012) Tip60 HAT activity mediates APP induced lethality and apoptotic cell death in the CNS of a Drosophila Alzheimer’s disease model. PLoS One 7, e41776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hosp F., Lassowskat I., Santoro V., De Vleesschauwer D., Fliegner D., Redestig H., Mann M., Christian S., Hannah M. A., Finkemeier I. (2017) Lysine acetylation in mitochondria: from inventory to function. Mitochondrion 33, 58–71 [DOI] [PubMed] [Google Scholar]
- 86.Paik W. K., Pearson D., Lee H. W., Kim S. (1970) Nonenzymatic acetylation of histones with acetyl-CoA. Biochim. Biophys. Acta 213, 513–522 [DOI] [PubMed] [Google Scholar]
- 87.Weinert B. T., Iesmantavicius V., Moustafa T., Schölz C., Wagner S. A., Magnes C., Zechner R., Choudhary C. (2014) Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol. Syst. Biol. 10, 716; erratum: 11, 833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi L., Tu B. P. (2015) Acetyl-CoA and the regulation of metabolism: mechanisms and consequences. Curr. Opin. Cell Biol. 33, 125–131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Weinert B. T., Moustafa T., Iesmantavicius V., Zechner R., Choudhary C. (2015) Analysis of acetylation stoichiometry suggests that SIRT3 repairs nonenzymatic acetylation lesions. EMBO J. 34, 2620–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Scott I., Webster B. R., Li J. H., Sack M. N. (2012) Identification of a molecular component of the mitochondrial acetyltransferase programme: a novel role for GCN5L1. Biochem. J. 443, 655–661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fan J., Shan C., Kang H. B., Elf S., Xie J., Tucker M., Gu T. L., Aguiar M., Lonning S., Chen H., Mohammadi M., Britton L. M., Garcia B. A., Alečković M., Kang Y., Kaluz S., Devi N., Van Meir E. G., Hitosugi T., Seo J. H., Lonial S., Gaddh M., Arellano M., Khoury H. J., Khuri F. R., Boggon T. J., Kang S., Chen J. (2014) Tyr phosphorylation of PDP1 toggles recruitment between ACAT1 and SIRT3 to regulate the pyruvate dehydrogenase complex. Mol. Cell 53, 534–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cederbaum A. I. (2012) Alcohol metabolism. Clin. Liver Dis. 16, 667–685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Fritz K. S., Green M. F., Petersen D. R., Hirschey M. D. (2013) Ethanol metabolism modifies hepatic protein acylation in mice. PLoS One 8, e75868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hebert A. S., Dittenhafer-Reed K. E., Yu W., Bailey D. J., Selen E. S., Boersma M. D., Carson J. J., Tonelli M., Balloon A. J., Higbee A. J., Westphall M. S., Pagliarini D. J., Prolla T. A., Assadi-Porter F., Roy S., Denu J. M., Coon J. J. (2013) Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol. Cell 49, 186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Browning J. D., Baxter J., Satapati S., Burgess S. C. (2012) The effect of short-term fasting on liver and skeletal muscle lipid, glucose, and energy metabolism in healthy women and men. J. Lipid Res. 53, 577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hirschey M. D., Shimazu T., Jing E., Grueter C. A., Collins A. M., Aouizerat B., Stančáková A., Goetzman E., Lam M. M., Schwer B., Stevens R. D., Muehlbauer M. J., Kakar S., Bass N. M., Kuusisto J., Laakso M., Alt F. W., Newgard C. B., Farese R. V., Jr., Kahn C. R., Verdin E. (2011) SIRT3 deficiency and mitochondrial protein hyperacetylation accelerate the development of the metabolic syndrome. Mol. Cell 44, 177–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Hirschey M. D., Shimazu T., Goetzman E., Jing E., Schwer B., Lombard D. B., Grueter C. A., Harris C., Biddinger S., Ilkayeva O. R., Stevens R. D., Li Y., Saha A. K., Ruderman N. B., Bain J. R., Newgard C. B., Farese R. V., Jr., Alt F. W., Kahn C. R., Verdin E. (2010) SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature 464, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Garland P. B., Shepherd D., Yates D. W. (1965) Steady-state concentrations of coenzyme A, acetyl-coenzyme A and long-chain fatty acyl-coenzyme A in rat-liver mitochondria oxidizing palmitate. Biochem. J. 97, 587–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wei W., Mao A., Tang B., Zeng Q., Gao S., Liu X., Lu L., Li W., Du J. X., Li J., Wong J., Liao L. (2017) Large-scale identification of protein crotonylation reveals its role in multiple cellular functions. J. Proteome Res. 16, 1743–1752 [DOI] [PubMed] [Google Scholar]
- 100.Rardin M. J., He W., Nishida Y., Newman J. C., Carrico C., Danielson S. R., Guo A., Gut P., Sahu A. K., Li B., Uppala R., Fitch M., Riiff T., Zhu L., Zhou J., Mulhern D., Stevens R. D., Ilkayeva O. R., Newgard C. B., Jacobson M. P., Hellerstein M., Goetzman E. S., Gibson B. W., Verdin E. (2013) SIRT5 regulates the mitochondrial lysine succinylome and metabolic networks. Cell Metab. 18, 920–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Shakespear M. R., Iyer A., Cheng C. Y., Das Gupta K., Singhal A., Fairlie D. P., Sweet M. J. (2018) Lysine deacetylases and regulated glycolysis in macrophages. Trends Immunol. 39, 473–488 [DOI] [PubMed] [Google Scholar]
- 102.Carrico C., Meyer J. G., He W., Gibson B. W., Verdin E. (2018) The mitochondrial acylome emerges: proteomics, regulation by sirtuins, and metabolic and disease implications. Cell Metab. 27, 497–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Verdin E., Hirschey M. D., Finley L. W. S., Haigis M. C. (2010) Sirtuin regulation of mitochondria: energy production, apoptosis, and signaling. Trends Biochem. Sci. 35, 669–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Michan S., Sinclair D. (2007) Sirtuins in mammals: insights into their biological function. Biochem. J. 404, 1–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Hall J. A., Dominy J. E., Lee Y., Puigserver P. (2013) The sirtuin family’s role in aging and age-associated pathologies. J. Clin. Invest. 123, 973–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Finkel T., Deng C. X., Mostoslavsky R. (2009) Recent progress in the biology and physiology of sirtuins. Nature 460, 587–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Park J., Chen Y., Tishkoff D. X., Peng C., Tan M., Dai L., Xie Z., Zhang Y., Zwaans B. M., Skinner M. E., Lombard D. B., Zhao Y. (2013) SIRT5-mediated lysine desuccinylation impacts diverse metabolic pathways. Mol. Cell 50, 919–930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Feldman J. L., Dittenhafer-Reed K. E., Kudo N., Thelen J. N., Ito A., Yoshida M., Denu J. M. (2015) Kinetic and structural basis for acyl-group selectivity and NAD(+) dependence in sirtuin-catalyzed deacylation. Biochemistry 54, 3037–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jaisson S., Gillery P. (2010) Evaluation of nonenzymatic posttranslational modification-derived products as biomarkers of molecular aging of proteins. Clin. Chem. 56, 1401–1412 [DOI] [PubMed] [Google Scholar]
- 110.Harmel R., Fiedler D. (2018) Features and regulation of non-enzymatic post-translational modifications. Nat. Chem. Biol. 14, 244–252 [DOI] [PubMed] [Google Scholar]
- 111.König A. C., Hartl M., Boersema P. J., Mann M., Finkemeier I. (2014) The mitochondrial lysine acetylome of Arabidopsis. Mitochondrion 19(Pt B), 252–260 [DOI] [PubMed] [Google Scholar]
- 112.Li T., Liu M., Feng X., Wang Z., Das I., Xu Y., Zhou X., Sun Y., Guan K. L., Xiong Y., Lei Q. Y. (2014) Glyceraldehyde-3-phosphate dehydrogenase is activated by lysine 254 acetylation in response to glucose signal. J. Biol. Chem. 289, 3775–3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Hu H., Zhu W., Qin J., Chen M., Gong L., Li L., Liu X., Tao Y., Yin H., Zhou H., Zhou L., Ye D., Ye Q., Gao D. (2017) Acetylation of PGK1 promotes liver cancer cell proliferation and tumorigenesis. Hepatology 65, 515–528 [DOI] [PubMed] [Google Scholar]
- 114.Christofk H. R., Vander Heiden M. G., Harris M. H., Ramanathan A., Gerszten R. E., Wei R., Fleming M. D., Schreiber S. L., Cantley L. C. (2008) The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 [DOI] [PubMed] [Google Scholar]
- 115.Marchesini G., Moscatiello S., Di Domizio S., Forlani G. (2008) Obesity-associated liver disease. J. Clin. Endocrinol. Metab. 93 (Suppl 1), S74–S80 [DOI] [PubMed] [Google Scholar]
- 116.Czyzyk A. (1973) [Diabetes and chronic liver diseases]. Pol. Arch. Med. Wewn. 50, 947–957 [PubMed] [Google Scholar]
- 117.Porepa L., Ray J. G., Sanchez-Romeu P., Booth G. L. (2010) Newly diagnosed diabetes mellitus as a risk factor for serious liver disease. CMAJ 182, E526–E531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fabbrini E., Magkos F. (2015) Hepatic steatosis as a marker of metabolic dysfunction. Nutrients 7, 4995–5019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bellanti F., Villani R., Tamborra R., Blonda M., Iannelli G., di Bello G., Facciorusso A., Poli G., Iuliano L., Avolio C., Vendemiale G., Serviddio G. (2018) Synergistic interaction of fatty acids and oxysterols impairs mitochondrial function and limits liver adaptation during nafld progression. Redox Biol. 15, 86–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kendrick A. A., Choudhury M., Rahman S. M., McCurdy C. E., Friederich M., Van Hove J. L., Watson P. A., Birdsey N., Bao J., Gius D., Sack M. N., Jing E., Kahn C. R., Friedman J. E., Jonscher K. R. (2011) Fatty liver is associated with reduced SIRT3 activity and mitochondrial protein hyperacetylation. Biochem. J. 433, 505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yoshino J., Imai S. (2011) Mitochondrial SIRT3: a new potential therapeutic target for metabolic syndrome. Mol. Cell 44, 170–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Perry R. J., Camporez J. G., Kursawe R., Titchenell P. M., Zhang D., Perry C. J., Jurczak M. J., Abudukadier A., Han M. S., Zhang X. M., Ruan H. B., Yang X., Caprio S., Kaech S. M., Sul H. S., Birnbaum M. J., Davis R. J., Cline G. W., Petersen K. F., Shulman G. I. (2015) Hepatic acetyl CoA links adipose tissue inflammation to hepatic insulin resistance and type 2 diabetes. Cell 160, 745–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Perry R. J., Zhang X. M., Zhang D., Kumashiro N., Camporez J. P., Cline G. W., Rothman D. L., Shulman G. I. (2014) Leptin reverses diabetes by suppression of the hypothalamic-pituitary-adrenal axis. Nat. Med. 20, 759–763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Pougovkina O., te Brinke H., Ofman R., van Cruchten A. G., Kulik W., Wanders R. J., Houten S. M., de Boer V. C. (2014) Mitochondrial protein acetylation is driven by acetyl-CoA from fatty acid oxidation. Hum. Mol. Genet. 23, 3513–3522 [DOI] [PubMed] [Google Scholar]
- 125.Quercioli A., Montecucco F., Mach F. (2009) Update on the treatments of non-alcoholic fatty liver disease (NAFLD). Cardiovasc. Hematol. Disord. Drug Targets 9, 261–270 [DOI] [PubMed] [Google Scholar]
- 126.Yang H., Zhou L., Shi Q., Zhao Y., Lin H., Zhang M., Zhao S., Yang Y., Ling Z. Q., Guan K. L., Xiong Y., Ye D. (2015) SIRT3-dependent GOT2 acetylation status affects the malate-aspartate NADH shuttle activity and pancreatic tumor growth. EMBO J. 34, 1110–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lee P., Leong W., Tan T., Lim M., Han W., Radda G. K. (2013) In vivo hyperpolarized carbon-13 magnetic resonance spectroscopy reveals increased pyruvate carboxylase flux in an insulin-resistant mouse model. Hepatology 57, 515–524 [DOI] [PubMed] [Google Scholar]
- 128.Garcia M., Bequette B. J., Moyes K. M. (2015) Hepatic metabolic response of Holstein cows in early and mid lactation is altered by nutrient supply and lipopolysaccharide in vitro. J. Dairy Sci. 98, 7102–7114 [DOI] [PubMed] [Google Scholar]
- 129.Koentges C., Pfeil K., Schnick T., Wiese S., Dahlbock R., Cimolai M. C., Meyer-Steenbuck M., Cenkerova K., Hoffmann M. M., Jaeger C., Odening K. E., Kammerer B., Hein L., Bode C., Bugger H. (2015) SIRT3 deficiency impairs mitochondrial and contractile function in the heart. Basic Res. Cardiol. 110, 36 [DOI] [PubMed] [Google Scholar]
- 130.Constantinou M. A., Theocharis S. E., Mikros E. (2007) Application of metabonomics on an experimental model of fibrosis and cirrhosis induced by thioacetamide in rats. Toxicol. Appl. Pharmacol. 218, 11–19 [DOI] [PubMed] [Google Scholar]
- 131.Jemal A., Bray F., Center M. M., Ferlay J., Ward E., Forman D. (2011) Global cancer statistics. CA Cancer J. Clin. 61, 69–90; erratum: 134 [DOI] [PubMed] [Google Scholar]
- 132.Wong C. M., Ng I. O. L. (2008) Molecular pathogenesis of hepatocellular carcinoma. Liver Int. 28, 160–174 [DOI] [PubMed] [Google Scholar]
- 133.Massart J., Robin M. A., Noury F., Fautrel A., Lettéron P., Bado A., Eliat P. A., Fromenty B. (2012) Pentoxifylline aggravates fatty liver in obese and diabetic ob/ob mice by increasing intestinal glucose absorption and activating hepatic lipogenesis. Br. J. Pharmacol. 165, 1361–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.French S. W. (2013) Epigenetic events in liver cancer resulting from alcoholic liver disease. Alcohol Res. 35, 57–67 [PMC free article] [PubMed] [Google Scholar]
- 135.Iansante V., Choy P. M., Chokshi S., Williams R., Anders R. A., Bubici C., Papa S. (2015) P0315: increased aerobic glycolysis is associated with poor outcome and suppression of apoptosis in human liver cirrhosis and HCC. J. Hepatol. 62, S427 [Google Scholar]
- 136.Grunicke H. H., Yamada Y., Natsumeda Y., Helliger W., Puschendorf B., Weber G. (1989) Histone acetyltransferase activity in rat hepatomas. J. Cancer Res. Clin. Oncol. 115, 435–438 [DOI] [PubMed] [Google Scholar]
- 137.Roberts D. J., Miyamoto S. (2015) Hexokinase II integrates energy metabolism and cellular protection: akting on mitochondria and TORCing to autophagy. Cell Death Differ. 22, 248–257; erratum: 364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Wolf A., Agnihotri S., Micallef J., Mukherjee J., Sabha N., Cairns R., Hawkins C., Guha A. (2011) Hexokinase 2 is a key mediator of aerobic glycolysis and promotes tumor growth in human glioblastoma multiforme. J. Exp. Med. 208, 313–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Bustamante E., Morris H. P., Pedersen P. L. (1981) Energy metabolism of tumor cells. Requirement for a form of hexokinase with a propensity for mitochondrial binding. J. Biol. Chem. 256, 8699–8704 [PubMed] [Google Scholar]
- 140.Hay N. (2016) Reprogramming glucose metabolism in cancer: can it be exploited for cancer therapy? Nat. Rev. Cancer 16, 635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Amann T., Maegdefrau U., Hartmann A., Agaimy A., Marienhagen J., Weiss T. S., Stoeltzing O., Warnecke C., Schölmerich J., Oefner P. J., Kreutz M., Bosserhoff A. K., Hellerbrand C. (2009) GLUT1 expression is increased in hepatocellular carcinoma and promotes tumorigenesis. Am. J. Pathol. 174, 1544–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Amann T., Hellerbrand C. (2009) GLUT1 as a therapeutic target in hepatocellular carcinoma. Expert Opin. Ther. Targets 13, 1411–1427 [DOI] [PubMed] [Google Scholar]
- 143.Wardell S. E., Ilkayeva O. R., Wieman H. L., Frigo D. E., Rathmell J. C., Newgard C. B., McDonnell D. P. (2009) Glucose metabolism as a target of histone deacetylase inhibitors. Mol. Endocrinol. 23, 388–401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Park J. M., Kim T. H., Jo S. H., Kim M. Y., Ahn Y. H. (2015) Acetylation of glucokinase regulatory protein decreases glucose metabolism by suppressing glucokinase activity. Sci. Rep. 5, 17395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li F. L., Liu J. P., Bao R. X., Yan G., Feng X., Xu Y. P., Sun Y. P., Yan W., Ling Z. Q., Xiong Y., Guan K. L., Yuan H. X. (2018) Acetylation accumulates PFKFB3 in cytoplasm to promote glycolysis and protects cells from cisplatin-induced apoptosis. Nat. Commun. 9, 508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Howlett K. F., Bond S., Walder K. R., McGee S. L. (2013) Glucose homeostasis regulation: reversible lysine acetylation of GAPDH. Proceeding of the Australian Physiological Society. 44, 39P [Google Scholar]
- 147.Hallows W. C., Yu W., Denu J. M. (2012) Regulation of glycolytic enzyme phosphoglycerate mutase-1 by Sirt1 protein-mediated deacetylation. J. Biol. Chem. 287, 3850–3858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liang F., Kume S., Koya D. (2009) SIRT1 and insulin resistance. Nat. Rev. Endocrinol. 5, 367–373 [DOI] [PubMed] [Google Scholar]
- 149.Gillum M. P., Erion D. M., Shulman G. I. (2011) Sirtuin-1 regulation of mammalian metabolism. Trends Mol. Med. 17, 8–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Caton P. W., Nayuni N. K., Kieswich J., Khan N. Q., Yaqoob M. M., Corder R. (2010) Metformin suppresses hepatic gluconeogenesis through induction of SIRT1 and GCN5. J. Endocrinol. 205, 97–106 [DOI] [PubMed] [Google Scholar]
- 151.Jurica M. S., Mesecar A., Heath P. J., Shi W., Nowak T., Stoddard B. L. (1998) The allosteric regulation of pyruvate kinase by fructose-1,6-bisphosphate. Structure 6, 195–210 [DOI] [PubMed] [Google Scholar]
- 152.Lv L., Xu Y. P., Zhao D., Li F. L., Wang W., Sasaki N., Jiang Y., Zhou X., Li T. T., Guan K. L., Lei Q. Y., Xiong Y. (2013) Mitogenic and oncogenic stimulation of K433 acetylation promotes PKM2 protein kinase activity and nuclear localization. Mol. Cell 52, 340–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Bhardwaj A., Das S. (2016) SIRT6 deacetylates PKM2 to suppress its nuclear localization and oncogenic functions. Proc. Natl. Acad. Sci. USA 113, E538–E547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lv L., Li D., Zhao D., Lin R., Chu Y., Zhang H., Zha Z., Liu Y., Li Z., Xu Y., Wang G., Huang Y., Xiong Y., Guan K. L., Lei Q. Y. (2011) Acetylation targets the M2 isoform of pyruvate kinase for degradation through chaperone-mediated autophagy and promotes tumor growth. Mol. Cell 42, 719–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Park S. H., Ozden O., Liu G., Song H. Y., Zhu Y., Yan Y., Zou X., Kang H. J., Jiang H., Principe D. R., Cha Y. I., Roh M., Vassilopoulos A., Gius D. (2016) SIRT2-mediated deacetylation and tetramerization of pyruvate kinase directs glycolysis and tumor growth. Cancer Res. 76, 3802–3812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Wellen K. E., Hatzivassiliou G., Sachdeva U. M., Bui T. V., Cross J. R., Thompson C. B. (2009) ATP-citrate lyase links cellular metabolism to histone acetylation. Science 324, 1076–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Luo W., Semenza G. L. (2012) Emerging roles of PKM2 in cell metabolism and cancer progression. Trends Endocrinol. Metab. 23, 560–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kim J. W., Tchernyshyov I., Semenza G. L., Dang C. V. (2006) HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 3, 177–185 [DOI] [PubMed] [Google Scholar]
- 159.Papandreou I., Cairns R. A., Fontana L., Lim A. L., Denko N. C. (2006) HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 3, 187–197 [DOI] [PubMed] [Google Scholar]
- 160.Song W. W., Gui A. P., Li W., Chen H. S., Li J. M. (2017) Expressions of HIF-1α and KISS-1 in patients with liver cancer and correlation analysis. Eur. Rev. Med. Pharmacol. Sci. 21, 4058–4063 [PubMed] [Google Scholar]
- 161.Geng H., Liu Q., Xue C., David L. L., Beer T. M., Thomas G. V., Dai M. S., Qian D. Z. (2012) HIF1α protein stability is increased by acetylation at lysine 709. J. Biol. Chem. 287, 35496–35505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lim J. H., Lee Y. M., Chun Y. S., Chen J., Kim J. E., Park J. W. (2010) Sirtuin 1 modulates cellular responses to hypoxia by deacetylating hypoxia-inducible factor 1α. Mol. Cell 38, 864–878 [DOI] [PubMed] [Google Scholar]
- 163.Seo K. S., Park J. H., Heo J. Y., Jing K., Han J., Min K. N., Kim C., Koh G. Y., Lim K., Kang G. Y., Uee Lee J., Yim Y. H., Shong M., Kwak T. H., Kweon G. R. (2015) SIRT2 regulates tumour hypoxia response by promoting HIF-1α hydroxylation. Oncogene 34, 1354–1362 [DOI] [PubMed] [Google Scholar]
- 164.Lerin C., Rodgers J. T., Kalume D. E., Kim S. H., Pandey A., Puigserver P. (2006) GCN5 acetyltransferase complex controls glucose metabolism through transcriptional repression of PGC-1alpha. Cell Metab. 3, 429–438 [DOI] [PubMed] [Google Scholar]
- 165.Rodgers J. T., Lerin C., Haas W., Gygi S. P., Spiegelman B. M., Puigserver P. (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434, 113–118 [DOI] [PubMed] [Google Scholar]
- 166.Zhang W., Patil S., Chauhan B., Guo S., Powell D. R., Le J., Klotsas A., Matika R., Xiao X., Franks R., Heidenreich K. A., Sajan M. P., Farese R. V., Stolz D. B., Tso P., Koo S. H., Montminy M., Unterman T. G. (2006) FoxO1 regulates multiple metabolic pathways in the liver: effects on gluconeogenic, glycolytic, and lipogenic gene expression. J. Biol. Chem. 281, 10105–10117 [DOI] [PubMed] [Google Scholar]
- 167.Xiong X., Tao R., DePinho R. A., Dong X. C. (2013) Deletion of hepatic FoxO1/3/4 genes in mice significantly impacts on glucose metabolism through downregulation of gluconeogenesis and upregulation of glycolysis. PLoS One 8, e74340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Carbajo-Pescador S., Mauriz J. L., García-Palomo A., González-Gallego J. (2014) FoxO proteins: regulation and molecular targets in liver cancer. Curr. Med. Chem. 21, 1231–1246 [DOI] [PubMed] [Google Scholar]
- 169.Bai X., Wu L., Liang T., Liu Z., Li J., Li D., Xie H., Yin S., Yu J., Lin Q., Zheng S. (2008) Overexpression of myocyte enhancer factor 2 and histone hyperacetylation in hepatocellular carcinoma. J. Cancer Res. Clin. Oncol. 134, 83–91 [DOI] [PubMed] [Google Scholar]
- 170.Asano J., Kudo T., Shimizu T., Fan Y., Nanashima N., Yamana D., Miura T., Yamada T., Tsuchida S. (2010) Histone acetylation and steroid receptor coactivator expression during clofibrate-induced rat hepatocarcinogenesis. Cancer Sci. 101, 869–875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Keller M. A., Piedrafita G., Ralser M. (2015) The widespread role of non-enzymatic reactions in cellular metabolism. Curr. Opin. Biotechnol. 34, 153–161 [DOI] [PMC free article] [PubMed] [Google Scholar]