Abstract
Uremic pruritus with elevated levels of calcium phosphate (CaP) in skin is a common symptom in patients with chronic kidney disease (CKD). In this study, we demonstrate that intradermal injection of CaP into mice triggered scratching by up-regulating the IL-6 in skin and phosphorylation of ERKs in dorsal root ganglion (DRG) in a dose-dependent manner. IL-6 is essential because the CaP-induced up-regulation of phosphorylated (p)-ERK in DRG was considerably reduced in the IL-6 knockout mice. Microarray analysis in conjunction with real-time PCR revealed a higher mRNA expression of Bruton’s tyrosine kinase (BTK) gene in DRG after CaP injection. The inhibition of BTK by ibrutinib noticeably diminish the CaP-induced up-regulation of IL-6 and p-ERK in mice. A high amount of IL-6 was detected in itchy skin and blood of patients with CKD. The expressions of p-BTK and p-ERK in DRG primary cells reached maximum levels at 1 and 10 min, respectively, after treatment of recombinant IL-6 and were significantly reduced by treatment of IL-6 along with ibrutinib. The mechanism by which the CaP-induced pruritus mediated by the IL-6/p-BTK/p-ERK signaling was revealed.—Keshari, S., Sipayung, A. D., Hsieh, C.-C., Su, L.-J., Chiang, Y.-R., Chang, H.-C., Yang, W.-C., Chuang, T.-H., Chen, C.-L., Huang, C.-M. IL-6/p-BTK/p-ERK signaling mediates calcium phosphate–induced pruritus.
Keywords: Bruton’s tyrosine kinase, CaP, CKD
Pruritus manifests as an unpleasant impulse to scratch against internal or external stimuli (1). It can be broadly characterized as either histamine-dependent (histaminergic) or histamine-independent (nonhistaminergic) itch, both of which are relayed by subsets of C-fibers and by second-order gastrin-releasing peptide receptor–positive or spinothalamic tract neurons in the spinal cord of rodents. Nonhistaminergic pruritus is usually mediated by the signaling of protease-activated receptor or GPCR, including mas-related GPCR A3 (2).
Uremic pruritus is one of the most disturbing symptoms in patients with chronic kidney disease (CKD). Eighty-six percent of patients with CKD undergo hemodialysis. Patients with severe pruritus are more likely to be depressed and insomniac, with a high risk of mortality (3). CKD is a complex, detrimental alteration in physiologic and metabolic functions, causing endmost failure of kidney function and accumulation of uremic toxins, which lead to poor health outcomes or mortality (4–6). The major risk factor in patients with CKD is ectopic calcification owing to biomineralization in soft tissues. Ectopic calcification is associated with a systemic mineral imbalance, either resulting from hyperphosphatemia or from an increase in calcium phosphate (CaP) deposition (7). The Kidney Disease Outcomes and Quality Initiative guidelines on CKD indicated that patients with a decreased glomerular filtration rate have an elevated level of calcium and phosphate in sera (8). Studies associate a high level of CaP in sera with an increased relative risk (RR) of death. Patients with >72 of calcium and phosphate in sera have an RR of 1.34 (9). A spike in CaP levels in sera (>60 mg2/dl2) can also lead to aortic calcification in patients on hemodialysis (10). CaP deposition can drive the progression of inflammation (11), whereas CaP nanocrystals can lead to arterial calcification, triggering a proinflammatory response abetted by monocyte-macrophages. Dry skin (or xerosis) has been observed in nearly 85% of uremic patients undergoing hemodialysis or peritoneal dialysis. Dry skin promotes pruritus by lowering the itch threshold (12).
Previous studies revealed that renal injury can induce local and systemic elevation of IL-6 (13–15). Studies have also explored other possible causes of uremic pruritus (16–22). ERK plays a vital role in mediating renal responses to a diverse range of stimuli such as compensatory renal hypertrophy and glomerular and tubulointerstitial diseases. Robust ERK activation has been detected in the cyst epithelium of patients with polycystic kidney diseases. The cyst-induced gain in kidney mass was significantly reduced by the ERK pathway inhibition (23). Recent research found that ERK activation played a central role in histamine-dependent and dinitrofluorobenzene-induced spinal itch processing (22).
Bruton’s tyrosine kinase (BTK) is a member of the Tec protein tyrosine kinase family of nonreceptor kinases and is expressed by all cells belonging to the hematopoietic lineage, except NK, plasma, and T cells (24). BTK is important for B-cell development, FcR signaling, and macrophage polarization. Activation of BTK in B cells initiates a series of signaling events that include recruitment of BTK to the plasma membrane via its pleckstrin homology domain and autophosphorylation of Tyr223 in the SRC homology 3 domain (25). The expression is significantly up-regulated in patients with lupus nephritis (LN) (26). BTK may be a promising therapeutic target for the treatment of LN, as it affects the functions of both B cell and macrophage (27). A recent study illustrated that BTK suppression by ibrutinib, a selective inhibitor, ameliorated nephrotoxic nephritis-mediated kidney diseases (28). Furthermore, inhibition of BTK by ibrutinib reduced the levels of phosphorylated (p)-ERK, phosphorylated JNKs, and phosphorylated AKT, a PKB, in blood (29). The underlying mechanisms of pruritus induced by CaP deposition in the skin of patients with CKD are poorly understood. In this present study, we conduct both in vitro and in vivo experiments to validate the signaling of IL-6/ p-BTK/p-ERK in CaP deposition-induced pruritus.
MATERIALS AND METHODS
Ethics statement
This study was conducted in strict accordance with an approved Institutional Animal Care and Use Committee protocol of the National Central University. The Institute for Cancer Research (ICR) mice, C57BL/6 IL-6 knockout (KO) and C57BL/6 wild-type (WT) mice (8- to 9-wk-old female mice; National Laboratory Animal Center, Taipei, Taiwan) were sacrificed using dry ice in a closed box. The outbred strain of ICR mice were used because they exhibit genetic variability comparable with that naturally occurring in human populations (30). The Institutional Review Board at Landseed Hospital approved the consent procedure for sampling of skin and blood under an approved protocol (No. 16018C0, June 15, 2018). Skin biopsies and blood from the same patients were collected from healthy subjects (n = 4) and nonitchy (n = 4) and itchy (n = 4) skin of patients undergoing hemodialysis aged over 50 with CKD. The written consents from all participants were obtained before taking photos and conducting skin and blood sampling.
Dorsal root ganglia culture
Dorsal root ganglia (DRG) were collected using fine forceps (Dumont, Montignez, Switzerland) in DMEM (Thermo Fisher Scientific, Waltham, MA, USA) and digested with 1 mg/ml collagenase type IA (MilliporeSigma, Burlington, MA, USA) for 50 min followed by 0.25% trypsin EDTA for 10 min (MilliporeSigma) at 37°C in 5% CO2 and 95% air, respectively. Following trituration, DRG primary cells were resuspended in medium containing DMEM, 1% glutamine, 1% penicillin, and streptomycin (Thermo Fisher Scientific) and 10% fetal bovine serum (Thermo Fisher Scientific). The DRG primary cells were placed on a 30 × 10 mm tissue culture dish precoated with poly-l-lysine (SPL Life Sciences, Pocheon-si, South Korea) coated with poly-l-lysine (MilliporeSigma). Cells were then maintained at 37°C in 5% CO2 and 95% air. To examine the p-ERK activation, DRG primary cells were treated with 100 ng/ml of mouse recombinant IL-6 (Thermo Fisher Scientific), PBS, or ibrutinib (PCI-32765) (Selleckchem, Houston, TX, USA) dissolved in 0.1% DMSO (MilliporeSigma).
Chemical administration and behavioral analysis
The back nape skin of mice was shaved at least 2 h before injection. To determine the signaling of CaP-induced pruritus and inflammation, CaP (0.5 or 5 mg/ml; MilliporeSigma) was dissolved in double-distilled water (H2O) and intradermally injected into the dorsal skin of mice. Injection of H2O served as a control. Ibrutinib (50 µM) dissolved in 0.1% DMSO was intradermally administered into the dorsal skin 10 min prior to CaP injection. Multiple injections of CaP at 5 mg/ml were performed to induce chronic pruritus. Dorsal skin of mice was intradermally injected with CaP, H2O, or CaP with or without ibrutinib every day for 7 d. The response of pruritus to CaP was evaluated by counting the number of times that the hind paw scratched the injection site. Scratching behavior was observed for 2 h after CaP injection (31, 32).
ELISA assay
The dorsal skin of mice was removed 2 h after CaP injection. Skin was homogenized in a T-PER Tissue Protein Extraction Reagent (Thermo Fisher Scientific) supplemented with an EDTA-free protease inhibitor cocktail (MilliporeSigma). Human sera were collected from supernatants after centrifuging blood at 2800 g for 20 min. IL-6 concentration was determined using a mouse or human IL-6 ELISA assay kit (R&D Systems, Minneapolis, MN, USA) following the manufacturer’s instructions.
Western blotting
DRG was isolated 2 h after CaP injection. DRG primary cells were treated with or without mouse recombinant IL-6 (100 ng/ml) for 0, 0.5, 1, 5, 10, and 30 min in the presence or absence of ibrutinib. DRG tissue and primary cells were then lysed with RIPA buffer (Thermo Fisher Scientific). Cell lysates (30 µg) were subjected to 10% SDS-PAGE gel, which were then transferred to a PVDF membrane (MilliporeSigma) and blocked with 5% (w/v) nonfat milk before incubation overnight with primary antibodies to ERK (1:1000; Cusabio Technology, Houston, TX, USA), p-ERK (1:1,000; Cell Signaling Technology, Danvers, MA, USA), BTK (1:1,000; Cell Signaling Technology), p-BTK (1:2,000; Abcam, Cambridge, MA, USA), glyceraldehyde 3-phosphate dehydrogenase (GAPDH) (1:1,000; Thermo Fisher Scientific), or β-actin (1:1,000; Cell Signaling Technology). This was followed by treatment with horseradish peroxidase–conjugated secondary antibody [goat anti-rabbit or anti-mouse (1:5000); Thermo Fisher Scientific] for 1 h. Protein bands were detected with a chemiluminescent detection reagent (Thermo Fisher Scientific) and Omega Lum C Imaging System (Gel Co., San Francisco, CA, USA). Densitometric analysis of protein bands was conducted using ImageJ software (National Institutes of Health, Bethesda, MD, USA).
Immunostaining
DRG primary cells were starved in serum-free DMEM (Thermo Fisher Scientific) for 5 h, followed by treatment with 100 ng/ml of mouse recombinant IL-6 or PBS for 1 min. Cells were then fixed with 4% paraformaldehyde (MilliporeSigma) for 10 min, washed in PBS, permeabilized with PBS and 0.1% Triton X-100, and blocked in 8% goat sera (Abcam) for 1 h. Skin tissue slides prepared with skin samples from healthy subjects and patients undergoing hemodialysis (itchy and nonitchy) with CKD were blocked with blocking buffer (5% w/v bovine serum albumin in PBS). DRG primary cells were incubated with antibodies to p-BTK (1:500; Abcam). Skin tissue sections with antibodies to IL-6 (1:500) overnight at 4°C. Goat anti-rabbit DyLight 488 (1:200; Abcam) and goat anti-mouse IgG H&L Alexa Fluor 488 (1:200; Abcam) as second antibodies were used to incubate DRG primary cells and skin tissue sections, respectively, for 1 h. They were then stained with Hoechst nuclear stain (1:5000; Thermo Fisher Scientific) and mounted with Lab Vision PermaFluor Aqueous Mounting Medium (Thermo Fisher Scientific). Images were acquired with cellSens software connected to an Olympus BX63 microscope (Olympus, Tokyo, Japan).
Microarray analysis
DRG was isolated from ICR mice after 2 h with or without 5 mg/ml of CaP injection. Total RNA of DRG was extracted using a RiboPure RNA Purification Kit (Thermo Fisher Scientific). Concentration and purity of the extracted RNA were determined using Nanodrop 2000 (Thermo Fisher Scientific) with an optical density (OD)260/OD280 ratio ranging from 1.9 to 2.1. Gene expression was generated using a mouse Transcriptome Affymetrix Array 1.0 (Thermo Fisher Scientific). cDNA synthesis and labeling were carried out according to the protocol of Affymetrix GeneChip WT Plus Reagent. In brief, total RNA was first reverse transcribed to cDNA and then to cRNAs by in vitro transcription. The cRNA was purified and quantified. The second cycle of single-stranded cDNA synthesis was performed using purified cRNA as a template. After removing RNA, single-stranded cDNA was fragmented and hybridized to microarrays, which were scanned with Affymetrix GeneChip 3000 7G.
Analysis of gene expression profile in microarrays
Differential gene expression was selected using the GeneSpring software, version GX 7.3 (Agilent Technologies, Santa Clara, CA, USA). Log2-transformed expression intensities of single-stranded cDNA from 6 arrays (n = 3 in each group) were determined with Robust Multiarray Average normalization and principal component analysis so as to calculate the correlation coefficient in each cluster set; a heat map was constructed using TreeView software (https://treeview.co.uk/download-file/?v=2). The Student’s t test, P value, and fold-change for comparing the differential gene expression in the treatment and control group were calculated. A value of P < 0.05 and fold-change >1.1 were considered as differentially expressed. Overrepresented gene ontology (GO) terms (http://geneontology.org) were identified with Fisher’s exact test. A value of P < 0.001 was considered statistically significant.
Real-time PCR
RNA (1 µg) was converted into cDNA using an iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA, USA). All sets were designed using National Center for Biotechnology Information (NCBI) Primer-Blast (https://www.ncbi.nlm.nih.gov/tools/primer-blast/). The reaction was performed on StepOnePlus Real-Time PCR System (Thermo Fisher Scientific) using Power SYBR Green PCR Master Mix (Thermo Fisher Scientific). The reaction conditions for 40 cycles are as follows: 95°C for 10 min followed by 95°C for 15 s, 55°C for 60 s, and 72°C for 30 s. All reactions were carried out with 3 biological replicates, and each analysis consists of 3 technical replicates. The expression of GAPDH gene was used for normalization. The levels of relative expression levels were calculated using the cycle threshold (2−ΔΔCt) method. Primers used for BTK and GAPDH were 5′-GGCCATCAAGATGATCAGAGA-3′ (forward); 5′-GCTTCTCATGGGAAAGATTCA-3′ (reverse) and 5′-GACTTCAACAGCAACTCCCAC-3′ (forward); 5′-TCCACCACCCTGTTGCTGTA-3′ (reverse) respectively.
Statistical analysis
Data analysis was performed by an unpaired Student’s t test, or by 1-way ANOVA using Prism software (GraphPad Software, La Jolla, CA, USA). A value of P < 0.05 was considered significant. The mean ± se for at least 3 independent experiments was calculated.
RESULTS
CaP induces itch signaling by modulating IL-6 and p-ERK expression
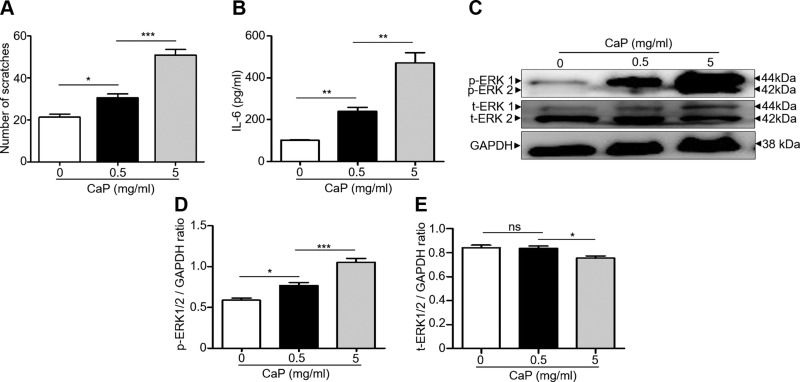
Disturbed CaP metabolism and pruritus are 2 of the major manifestations observed in patients with CKD who are undergoing dialysis (33). To mimic the CaP skin deposits in patients undergoing kidney dialysis, ICR mice were injected with or without CaP at 2 doses (5 and 0.5 mg/ml). We observed the behavior of hind paw–scratching for 2 h after injecting CaP (Supplemental Fig. S1). Injection of a lower dose of CaP at 0.5 mg/ml evoked a significant increase in the number of scratches. The higher dose of CaP at 5 mg/ml led to vigorous scratching, with a 3-fold increase in scratching numbers compared with those in mice injected without CaP (Fig. 1A). IL-6 activation has been reported to be a key phenomenon in skin inflammation (34). As shown in Fig. 1B, injection of CaP for 2 h has a dose-dependent effect on the up-regulated level of IL-6 in skin. Sensations like itch can deliver from skin to the DRG, and then to the spinal cord and brain (35). It has been documented that ERK activation played a vital role in spinal itch processing (22). Thus, the activation of p-ERK in DRG in CaP-injected mice was examined. Western blotting (Fig. 1C) and densitometric analysis of protein bands (Fig. 1D, E) of DRG isolated from mice injected with or without CaP demonstrated that the level of p-ERK, but not t-ERK, was markedly elevated with increasing doses of injected CaP. The results above indicate that CaP accumulation in skin up-regulates the IL-6 in skin and p-ERK in DRG.
Figure 1.
Scratching and expression of IL-6 and p-ERK in mice injected with CaP. A) The number of hind paw scratches was counted for 2 h after intradermal injection of CaP (0, 0.5 and 5 mg/ml). B) The level of IL-6 in the dorsal skin of ICR mice injected with or without CaP was measured with ELISA. C) Western blot analysis of total ERK (t-ERK 1/2), p-ERK 1/2 and GAPDH in DRG of mice injected with/without CaP. D, E) The ratios of intensities of p-ERK 1/2 (D) and t-ERK 1/2 (E) relative to GAPDH in Western blot analysis were shown. The mean ± se for 3 independent experiments with 4 mice per group was calculated. Ns, not significant. *P < 0.05, **P < 0.01, ***P < 0.001.
CaP-induced itch signaling and p-ERK up-regulation are IL-6 dependent
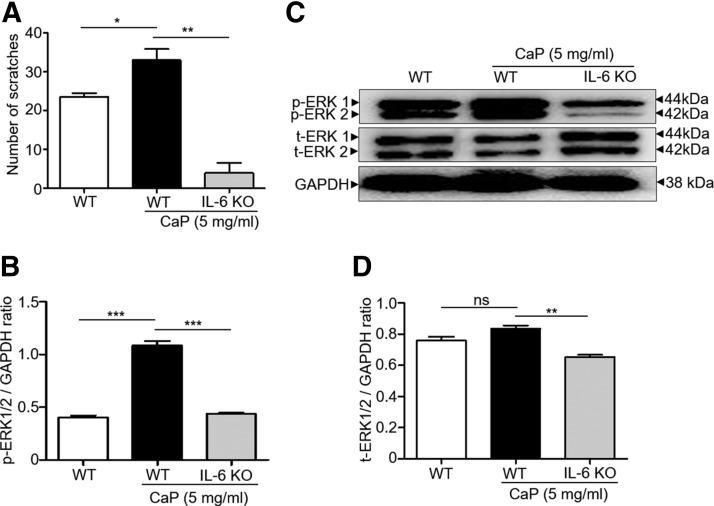
The IL-6 KO mice were used to investigate the essential role of IL-6 in the CaP-induced signaling of IL-6 and p-ERK up-regulation. CaP (5 mg/ml) or H2O was injected into the dorsal skin of C57BL/6 IL-6 KO and WT mice. Injection of CaP increased the number of skin scratches in WT mice in comparison with injection with H2O. The CaP-induced number of scratches was considerably reduced when CaP was injected into IL-6 KO mice (Fig. 2A). Consistent with the results in Fig. 1, injection of CaP (5 mg/ml) enhanced the expression of p-ERK, not t-ERK, in DRG cells (Fig. 2B–D). However, the enhancement of p-ERK expression by CaP was dramatically suppressed when CaP was injected onto IL-6 KO mice (Fig. 2B–D), suggesting that IL-6 is indispensable for CaP-induced up-regulation of p-ERK.
Figure 2.
The essential role of IL-6 in the CaP-induced elevation of p-ERK in DRG. A) Bar graphs show the number of scratches postinjection of CaP (0 or 5 mg/ml) in WT and IL-6 KO mice. B) Western blot analyses of p-ERK 1/2 and GAPDH in DRG of WT and IL-6 KO mice. C, D) The ratios of intensities of p-ERK 1/2 (C) and t-ERK 1/2 (D) relative to GAPDH were illustrated. Four mice per group were used for this experiment. The mean ± se for 3 separate experiments was calculated. *P < 0.05, **P < 0.01, ***P < 0.001.
CaP up-regulates BTK gene in DRG
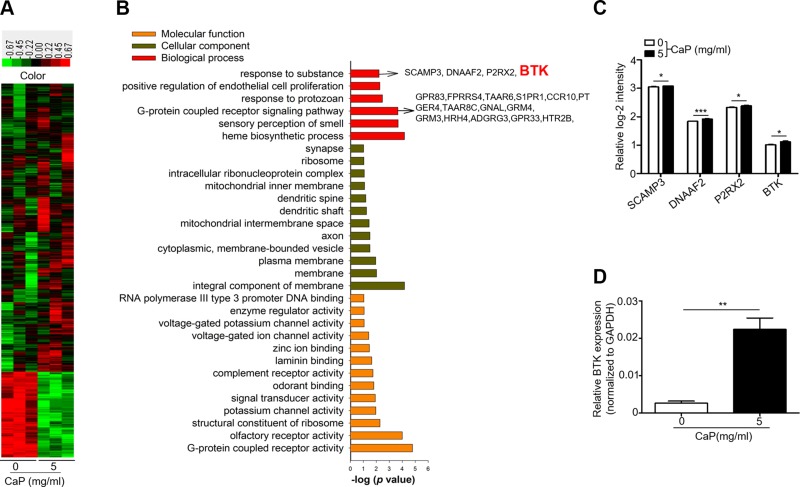
To determine which genes in DRG were regulated during CaP accumulation in skin, the mRNA expression in DRG of mice injected with or without CaP was analyzed by gene microarray. After normalization, 504 up-regulated and 86 down-regulated genes were identified out of the total 590 differentially expressed genes (DEGs) at the threshold of P < 0.05 and fold-change of >1.1. The heat map of the gene expression pattern in mice injected with or without CaP is shown in Fig. 3A. The DEGs were further mapped onto GO for cellular component, molecular function, and biologic process analyses. As shown in Fig. 3B, the following 6 out of 35 terms comprise the biologic process category: response to organic substance, positive regulation of endothelial cell proliferation, response to protozoan, GPCR signaling pathway, sensory perception of smell, and heme biosynthetic process. In the cellular component category, the following 12 out of 14 terms are included: integral component of membrane, membrane, plasma membrane, cytoplasmic, membrane-bounded vesicle, axon, mitochondrial intermembrane space, dendritic shaft, dendritic spine, mitochondrial inner membrane, intracellular RNP complex, ribosome, and synapse. In the molecular function category, 13 out of 13 terms are listed.
Figure 3.
Microarray analysis of mRNA expression in DRG of mice injected with CaP. A) Heat map comparison of the 1083 genes, including 590 DEGs in mice injected with (5 mg/ml) or without (0 mg/ml) CaP. Each column displays a profile of a specimen across genes, whereas each row shows a profile of a gene across specimens. Genes up- or down-regulated were denoted in red or green, respectively. B) GO analysis illustrates the molecular functions, cellular components, and biologic processes regulated by the genes. The respective genes of biologic processes of interest, including BTK (red), were indicated by arrows. C) A graph with relative log2 intensity reveals the levels of mRNA of SCAMP3, DNAAF2, P2RX2, and BTK genes in mice injected with or without CaP. D) The expression of the BTK gene relative to the GAPDH gene in mice injected with or without CaP was shown. The mean ± se was determined from experiments in triplicate. *P < 0.05, **P < 0.01, ***P < 0.001.
Among all the genes up-regulated by CaP injection, 4 genes (secretory carrier-associated membrane protein 3 (SCAMP3), DNAAF2, dynein axonemal assembly factor 2 (DNAAF2), P2X purinoceptor 2 (P2RX2), and BTK) were grouped under the, response to a substance, in biological response of GO category (Fig. 3C). BTK is a tyrosine kinase that is important for B-cell development, FcR signaling, and macrophage activation (24). Studies have shown that inhibition of BTK could be a good approach to treating LN prior to the end-stage renal disease (ESRD) (27). As shown in Fig. 3C, the BTK gene in the microarray analysis was up-regulated by 1.18-fold after CaP injection. The up-regulation of BTK gene by CaP injection was further normalized with GAPDH control gene (Fig. 3D). The result indicates that BTK is a target molecule in DRG that can be activated by CaP.
BTK was involved in CaP-induced scratching, activation of IL-6, and up-regulation of p-ERK
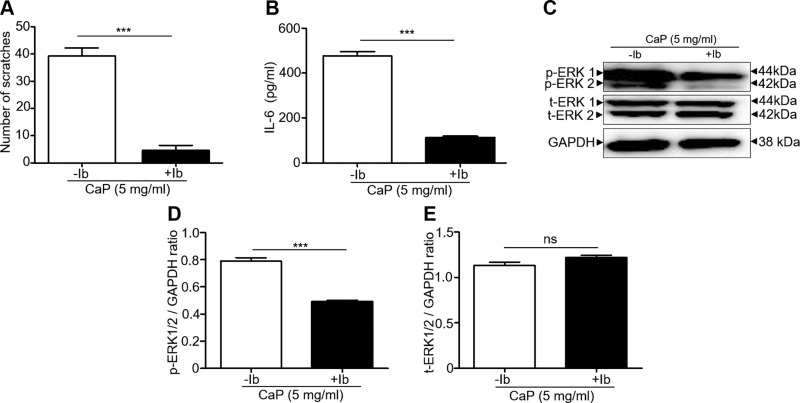
To examine the involvement of BTK in the CaP-induced signaling, the ibrutinib (50 µM in 0.1% DMSO), a selective BTK inhibitor, was intradermally administered into the dorsal skin 10 min prior to CaP injection. Injection of DMSO alone with ibrutinib served as a control. A significant decrease in the number of CaP-induced scratches was noted in the mice injected with ibrutinib compared to control mice (Fig. 4A). Coherently, the levels of IL-6 in skin (Fig. 4B) and p-ERK, but not t-ERK in DRG (Fig. 4C–E), were much lower in mice injected with CaP and ibrutinib than those in control mice. In addition, mice were intradermally injected with CaP every day for 7 d to mimic the chronic pruritus in patients undergoing hemodialysis with CKD. The number of scratches increased each day, and severe lesions caused by scratching were observed 5 d after CaP injection (Supplemental Fig. S2A, B). The vigorous scratching behavior, high levels of IL-6 in the back nape skin, and the up-regulation of p-BTK and p-ERK in DRG were detected 7 d after CaP injection. Injection of ibrutinib remarkably reduced the lesions and scratches and significantly suppressed the elevated IL-6, p-BTK, and p-ERK (Supplemental Fig. S2). Together with data in Figs. 1 and 4 and Supplemental Fig. S2, our results infer that the CaP-induced up-regulation of IL-6 and p-ERK can be remarkably diminished when BTK is inhibited by ibrutinib.
Figure 4.
Suppression of the CaP-induced scratching, IL-6 elevation, and p-ERK activation by BTK inhibition. A–C) The number of scratches (A), the levels of IL-6 (B), and protein expressions of p-ERK 1/2, t-ERK ½, and GAPDH in Western blot analysis (C) in mice injected with CaP (5 mg/ml) in the presence or absence of ibrutinib (Ib), a BTK inhibitor, were demonstrated. D, E) The ratios of intensities of p-ERK 1/2 (D) and t-ERK 1/2 (E) relative to GAPDH were illustrated. Four mice per group were used for this experiment. The mean ± se from 3 separate experiments was calculated. *P < 0.05, **P < 0.01, ***P < 0.001.
The higher levels of IL-6 in itchy skin and blood of patients undergoing hemodialysis
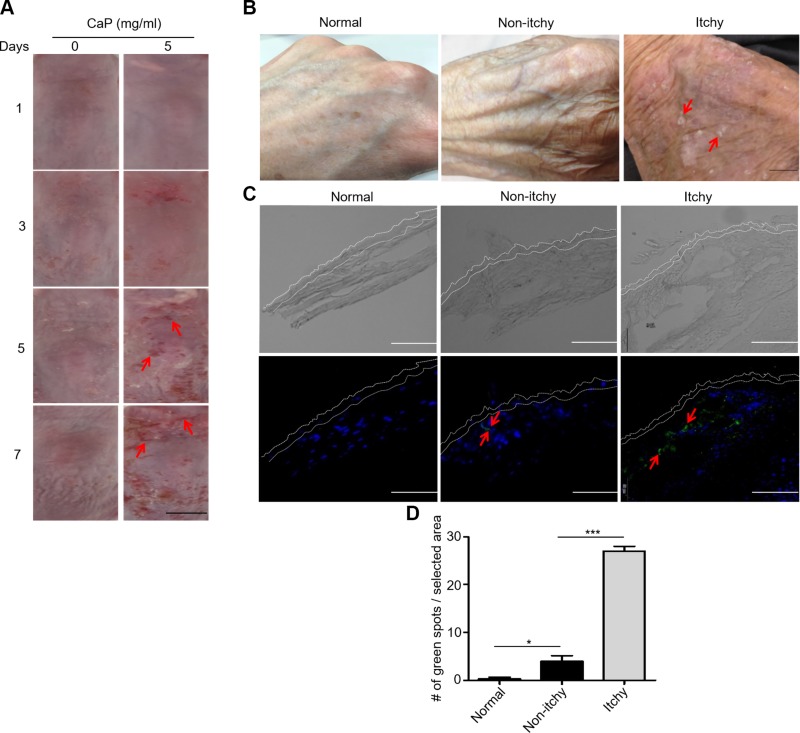
A number of etiologies for pruritus in CKD have been proposed, including xerosis, dysregulation, and deposition of CaP in skin (36, 37). In the current study, skin lesions in dorsal skin of ICR mice were observed 5 d after CaP injection (Fig. 5A). Further, the xerosis on the back of the hand was detected in the itchy skin of patients with CKD who were on hemodialysis (Fig. 5B). Figure 2 demonstrated the essential role of IL-6 in the CaP-induced scratching and up-regulation of p-ERK in mice. We next detected the levels of IL-6 in skin and blood of healthy subjects and patients with nonitchy and itchy skin of CKD who were on hemodialysis. Results from immunostaining in Fig. 5C revealed that the skin of healthy subjects has very little positive staining on IL-6. Quantification of IL-6–positive stains (Fig. 5D) showed a low level of IL-6 expression in nonitchy skin, but a high amount of IL-6 in itchy skin of patients with CKD. As shown in Supplemental Fig. S3, the level of IL-6 in blood in patients with nonitchy skin who were on hemodialysis was significantly higher than that in healthy subjects. Furthermore, the level (8.3 ± 0.6 pg/ml) of IL-6 in patients with itchy skin who were on hemodialysis was nearly 15 times higher than that (0.6 ± 0.1 pg/ml) in healthy subjects.
Figure 5.
Skin lesions in mouse skin injected with CaP and morphologies and IL-6 expression in the skin of healthy subjects and the nonitchy or itchy skin of patients with CKD. A) The skin morphologies of ICR mice after injection with H2O and CaP (5 mg/ml) for 7 d were displayed. Skin lesions were indicated by red arrows. Scale bar, 5 mm. B) Skin images of the back of the hand from healthy subjects (normal) and the nonitchy or itchy skin of patients with CKD were shown. Xerotic skin with white spots were pointed out with red arrows. Scale bar, 1 mm. C) Immunostaining of IL-6 (green dots and red arrows) in the skin of healthy subjects (normal) and in the nonitchy or itchy skin of patients with CKD. Nuclei were labeled with DAPI (blue dots). Dotted lines indicate the epidermal layers. Scale bars, 100 μm. D) Quantification of green dots in a selected area. The mean ± se was determined using at least 3 skin biopsies. *P < 0.05, ***P < 0.001.
BTK mediates IL-6–induced the up-regulation of p-ERK in DRG cells
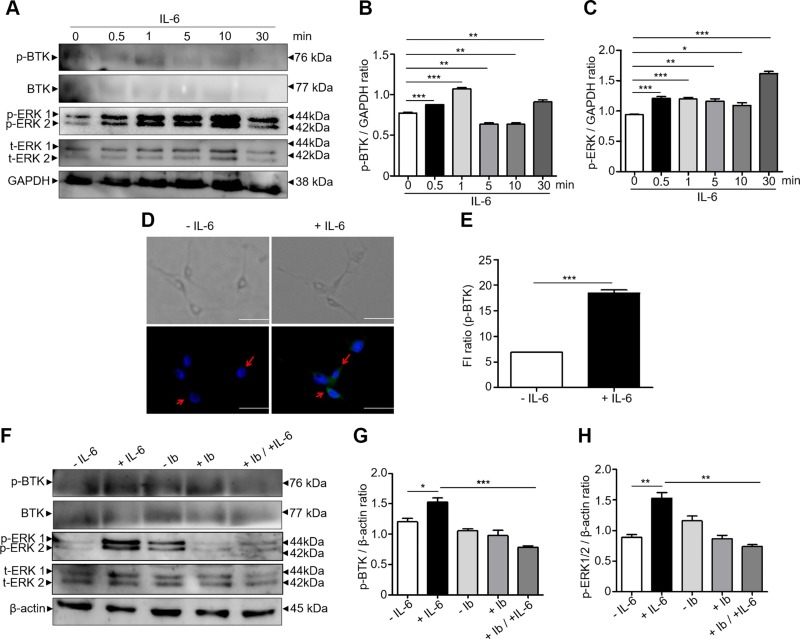
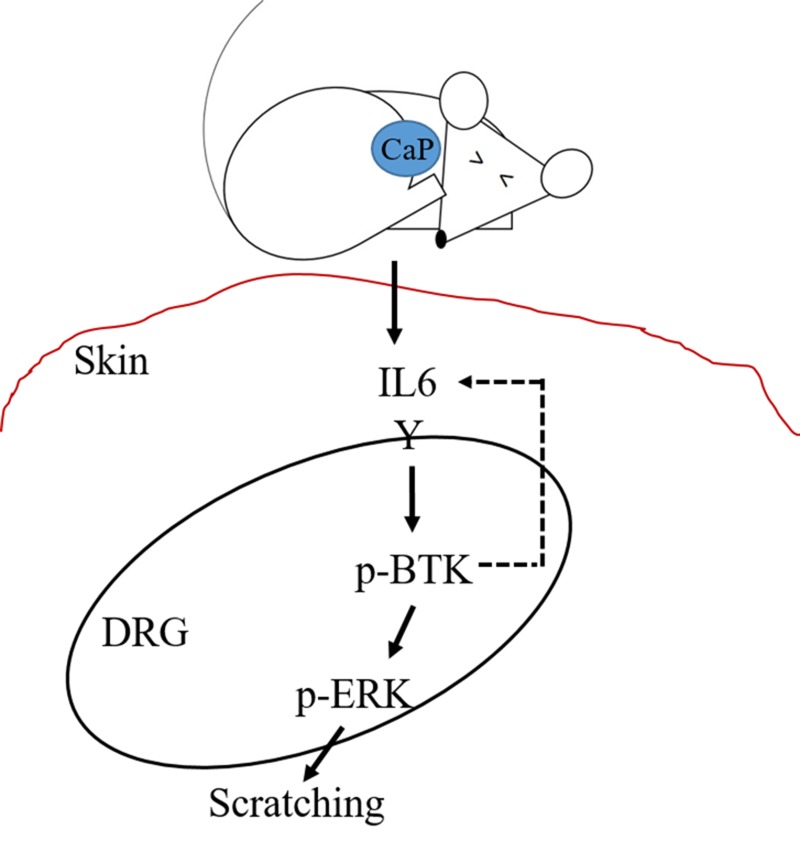
Activation of BTK in B cells involves the translocation of BTK to the plasma membrane via its pleckstrin homology domain and autophosphorylation of Tyr223 in the SRC homology 3 domain (38). Additionally, autophosphorylation of BTK was detectable in response to IL-3 or IL-6 cytokine in HepG2 cells (39). We used DRG cells to examine the role of p-BTK in regulating IL-6 and p-ERK in vitro. DRG cells were starved for 5 h in a serum-free condition, followed by a time-dependent treatment with mouse recombinant IL-6 (100 ng/ml) for 0–30 min. The expressions of p-ERK and p-BTK in DRG cells were examined by Western blotting. IL-6 treatment promoted the expressions of both p-BTK and p-ERK in a time-dependent manner. The expression of p-BTK and p-ERK reached the maximum level within 1 and 10 min, respectively (Fig. 6A–C). No changes in the levels of t-BTK (Supplemental Fig. S4A) and t-ERK (Supplemental Fig. S4B) by IL-6 treatment were detected. The immunostaining for p-BTK showed a strong signal that localized within DRG cells (Fig. 6D) after treatment of IL-6 for 1 min. The number of p-BTK–positive fluorescent cells treated with IL-6 is 3 times higher than that in control cells treated without IL-6 (Fig. 6E). To determine whether IL-6 induced up-regulation of p-ERK is mediated by p-BTK, DRG cells were treated with or without IL-6 in the presence or absence of ibrutinib (Fig. 6F–H). The IL-6–induced up-regulation of p-ERK was significantly reduced (Fig. 6H) when p-BTK was inhibited by ibrutinib (Fig. 6G), with no changes in the levels of t-BTK (Supplemental Fig. S4C) and t-ERK (Supplemental Fig. S4D), suggesting that p-BTK is an upstream molecule that triggers p-ERK in DRG cells in response to IL-6. Taken together, our data above suggest that CaP accumulation in skin induces the secretion of IL-6 that may bind to its receptor in DRG cells to activate the p-BTK. A positive feedback loop may occur between IL-6 and p-BTK (Fig. 7). The activation of p-BTK subsequently up-regulates p-ERK in DRG cells to trigger pruritus.
Figure 6.
Activation of p-BTK and p-ERK by IL-6. A) Western blot analysis of protein expressions of p-BTK, BTK, p-ERK 1/2, t-ERK 1/2, and GAPDH in DRG cells treated with mouse recombinant IL-6 (100 ng/ml) for 0, 0.5, 1, 5, 10, and 30 min. B, C) The ratios of intensities of p-BTK (B) and p-ERK 1/2 (C) relative to GAPDH were illustrated. D, E) Immunostaining (D) of p-BTK (green dots; arrows) and nuclei (DAPI blue dots) and quantification (E) of fluorescent intensities of p-BTK in DRG cells treated with or without IL-6 for 1 min. Scale bars, 100 μm. F) Western blot analysis of the expressions of p-BTK, BTK, p-ERK 1/2, t-ERK 1/2, and β-actin in DRG cells treated with or without IL-6 in the presence or absence of ibrutinib (Ib). G, H) The ratios of intensities of p-BTK (G) and p-ERK 1/2 (H) relative to β-actin were displayed. The mean ± se from 3 separate experiments was calculated (ns, not significant). ***P < 0.001.
Figure 7.
The signaling pathway of CaP-induced scratching, elevation of IL-6, activation of p-BTK and p-ERK, and a possible positive feedback loop between IL-6 and p-BTK is proposed.
DISCUSSION
In the present study, we investigated that CaP-induced up-regulation of IL-6 is a common risk factor in chronic pruritus in patients with CKD. CaP-induced prolonged inflammation mediates p-ERK activation in DRG depicting that pruritus may have its origin in skin that transmits to the nervous system via neurogenic mediators in DRG. Furthermore, inhibition of IL-6 in skin by blockade of BTK activity in the neuron, showing a positive feedback mechanism of regulation of BTK to control the chronic inflammation process during pruritus. The IL-6/p-BTK/p-ERK pathway could be applied for investigation of contact dermatitis which may be partially mediated by IL-6 (40–42). Disorders of mineral metabolism, such as abnormalities in calcium, phosphate, and parathyroid hormone, are the cardiovascular risk factors unique to patients with CKD (9). The levels of CaP product above 72 mg2/dl2 have been observed in 20% of patients and were associated with a significant increase in the RR of death in patients undergoing chronic hemodialysis (43). The Kidney Disease Outcomes and Quality Initiative guidelines recommend an absolute maximum elemental calcium load of 2000 mg/d, including calcium-containing medication and a maximum dialysate calcium concentration of 1.25 mM; however, it may not be ideal for every patient (44). A recent study on uremic pruritus demonstrated that the calcium concentration in the deeper layer (stratum basale and stratum spinosum) of the epidermis was significantly higher in the pruritus group than the nonpruritus group, and the calcium concentration in the normal skin increases toward the outer epidermis, forming a calcium gradient within the epidermis (37, 45). Here, we intradermally inject the CaP to mice mimic the CaP deposits in skin of patients with CKD who are undergoing dialysis.
In patients undergoing dialysis, the levels of soluble IL-6 receptor are increased, and both IL-6 and soluble IL-6 receptor are detected as independent predictors of mortality in patients with ESRD. Among cytokines, IL-6 has the highest predictive value for patient death, which establishes it as a potential uremic toxin (46, 47). IL-6 accelerates a number of pathways in the progression of CKD; for instance, it initiates the endothelial injury mainly via reducing eNOS and adiponectin (an antiatherogenic adipokine) expression (48). Increase in serum IL-6 is more likely to be associated with the severity of pruritus in pruritus nodularis, showing an immune-inflammatory activation during chronic itch (49). In the present study, we found that CaP deposition up-regulates IL-6 in the skin by inducing inflammation; the up-regulation of IL-6 was coherent with the CaP dose (Fig. 1). Several studies on abnormal nerve conduction in patients undergoing dialysis suggest that peripheral neuropathy or peripheral nervous system dysfunction may lead to uremic itching (50). Certain studies also highlighted the central role that p-ERK played in processing spinal itch in response to 2,4-dinitro-1-fluorobenzene (22). Additionally, IL-31 triggered p-ERK 1/2, the inhibition of which reduced the IL-31–induced scratching in vivo (51). We noted that injection of CaP induced a dose-dependent increase in p-ERK expression in DRG (Fig. 1D), although t-ERK expression remained unchanged; this describes that CaP induced p-ERK in DRG of the spinal cord in mice. Recovery from scratching and reduction in p-ERK expression were detected in IL-6 KO mice injected with CaP (Fig. 2). This finding verifies the essential role of IL-6 in the CaP-induced itch and signifies a neural ERK activation by IL-6 up-regulation in skin, ultimately leading to itching. Previous studies have reported IL-6 as a major biomarker and independent predictor of mortality in patients with ESRD (46). Consistently, we detected a higher level of IL-6 in skin and blood of the patients undergoing hemodialysis with CKD compared to healthy subjects (Fig. 5 and Supplemental Fig. S3).
A diverse group of GPCRs might be involved in the development of CaP-induced itch, which ultimately initiates the ERK cascade. Identification of ERK-dependent transcriptional events would be a necessary step to link extracellular stimuli to the expression of downstream effector genes that lead to itching. Microarray analysis of DRG from control and CaP-injected mice showed an up-regulation of 4 genes, namely SCAMP3, DNAAF2, P2RX2, and BTK, in response to a substance involved in the biologic process. BTK is a nonreceptor tyrosine kinase that plays an important role in signal transduction pathways regulating B-cell survival, activation, proliferation, and differentiation (52). Most importantly, there is substantial evidence from both preclinical and clinical studies showing that immune-mediated glomerulonephritis could be treated by targeting tyrosine kinase signaling. BTK is also implicated in the pathogenesis of LN, and inhibiting BTK was found to reduce the severity of renal diseases in experimental models of LN (53). Research showed that the irreversible binding of ibrutinib to BTK at the C481 residue can inhibit the kinase activity and phosphorylation of BTK (54). As shown in Fig. 4, we demonstrated that the scratching in the CaP-injected mice was ameliorated, and p-ERK in DRG was down-regulated upon BTK inhibition. Data from a mouse model of chronic pruritus demonstrated that inhibition of BTK noticeably attenuated CaP-induced scratching and up-regulation of IL-6 in skin and p-ERK in DRG (Supplemental Fig. S2), implying the active involvement of BTK in the progression and maintenance of CaP-induced itch.
Inhibition of BTK by ibrutinib reduced Fcγ receptor–induced IL-6 in primary monocytes, confirming that BTK plays an essential role in Fcγ receptor–mediated IL-6 production (55). Our result in Fig. 4 demonstrated that inhibition of BTK in DRG by ibrutinib significantly diminished the levels of IL-6 in skin. As shown in Fig. 3, post 2 h of intradermal injection of CaP into mouse skin up-regulated the mRNA expression of BTK in DRG. Treatment of DRG primary cells with recombinant IL-6 for 1 min increased the expression of p-BTK (Fig. 6), but not t-BTK (Supplemental Fig. S4A), indicating that IL-6 is an upstream regulator of the p-BTK and p-ERK signaling. A positive feedback loop is explained as a phenomenon where a positive gain exists within a loop of cause and effect. IL-6 can form a positive feedback loop with molecules such as aromatase (56), Wnt family member 5A (57), and apolipoprotein B MRNA editing enzyme catalytic subunit 3B (58). Continuous expression of IL-6 and these molecules promoted the prolonged inflammation or tumor progression. A positive feedback loop may exist between IL-6 and p-BTK (Fig. 7). It has been reported that inhibition of BTK in the brain reduced the level of proinflammatory IL-6 cytokine in the skin of murine lupus (59), suggesting that inhibition of BTK was a promising therapeutic option for cutaneous diseases. In the future, it is worth investigating whether CaP deposits in human skin elevate the expression of p-BTK or t-BTK in DRG and whether inhibition of BTK could effectively cure uremic pruritus.
CONCLUSIONS
Patients with CKD with CaP accumulation in skin suffer from mild to severe itching. Our study has uncovered that injection of CaP induced a secretion of IL-6 in skin. IL-6 is an essential molecule for CaP-induced pruritus in mice. Treatment of DRG cells with IL-6 triggered the p-BTK and subsequently activated p-ERK. The IL-6 and p-BTK may form a positive feedback to maintain their constitutive expressions, which led to a prolonged pruritus and inflammation in patients undergoing hemodialysis with CKD. The signaling of IL-6/p-BTK/p-ERK thus provides a novel target for development of therapeutic modalities for treatment of pruritus in patients with CKD.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
ACKNOWLEDGMENTS
The authors thank Wei-Hsin Sun [National Central University (NCU)], and postdoctoral scholar, Wei-Shin Chen (Academic Sinica, Taipei, Taiwan) for teaching isolation of DRG from mice and DRG primary cell culture. This work was supported by 106/107-Landseed Hospital-NCU joint grants, National Health Research Institutes (NHRI) Grant NHRI-EX106-10607SI, and Ministry of Science and Technology (MOST) Grants 107-2314-B-008 -001, 107-2622-B-008-002-CC1, and 106/107-2622-B-008-001-CC1. The authors declare no conflicts of interest.
Glossary
- BTK
Bruton’s tyrosine kinase
- CaP
calcium phosphate
- CKD
chronic kidney disease
- DEG
differentially expressed gene
- DNAAF2
Dynein axonemal assembly factor 2
- DRG
dorsal root ganglion
- ESRD
end-stage renal disease
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GO
gene ontology
- ICR
Institute for Cancer Research
- KO
knockout
- LN
lupus nephritis
- P2RX2
P2X purinoceptor 2
- RR
relative risk
- SCAMP3
secretory carrier-associated membrane protein 3
- WT
wild type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
C.-M. Huang conceived the study; C.-L. Chen and C.-M. Huang designed the experiments; C.-M. Huang prepared original draft; S. Keshari wrote the manuscript; S. Keshari, C.-L. Chen, and C.-M. Huang reviewed and edited the manuscript; S. Keshari performed most of the experiments; A. D. Sipayung, C.-C. Hsieh, L.-J. Su, Y.-R. Chiang, H.-C. Chang, W.-C. Yang, and T.-H. Chuang participated in some parts of the experiments; and all authors contributed substantially to this research and reviewed the final manuscript.
REFERENCES
- 1.Yosipovitch G., Greaves M. W., Schmelz M. (2003) Itch. Lancet 361, 690–694 [DOI] [PubMed] [Google Scholar]
- 2.Jeffry J., Kim S., Chen Z. F. (2011) Itch signaling in the nervous system. Physiology (Bethesda) 26, 286–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pisoni R. L., Wikström B., Elder S. J., Akizawa T., Asano Y., Keen M. L., Saran R., Mendelssohn D. C., Young E. W., Port F. K. (2006) Pruritus in haemodialysis patients: International results from the dialysis outcomes and practice patterns study (DOPPS). Nephrol. Dial. Transplant. 21, 3495–3505 [DOI] [PubMed] [Google Scholar]
- 4.Locatelli F., Pozzoni P., Tentori F., del Vecchio L. (2003) Epidemiology of cardiovascular risk in patients with chronic kidney disease. Nephrol. Dial. Transplant. 18 (Suppl7), vii2–vii9 [DOI] [PubMed] [Google Scholar]
- 5.Moe S., Drüeke T., Cunningham J., Goodman W., Martin K., Olgaard K., Ott S., Sprague S., Lameire N., Eknoyan G.; Kidney Disease: Improving Global Outcomes (KDIGO) (2006) Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 69, 1945–1953 [DOI] [PubMed] [Google Scholar]
- 6.Vaziri N. D. (2006) Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am. J. Physiol. Renal Physiol. 290, F262–F272 [DOI] [PubMed] [Google Scholar]
- 7.Brancaccio D., Tetta C., Gallieni M., Panichi V. (2002) Inflammation, CRP, calcium overload and a high calcium-phosphate product: a “liaison dangereuse”. Nephrol. Dial. Transplant. 17, 201–203 [DOI] [PubMed] [Google Scholar]
- 8.National Kidney Foundation (2002) K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am. J. Kidney Dis. 39 (2, Suppl 1), S1–S266 [PubMed] [Google Scholar]
- 9.Block G., Port F. K. (2003) Calcium phosphate metabolism and cardiovascular disease in patients with chronic kidney disease. Semin. Dial. 16, 140–147 [DOI] [PubMed] [Google Scholar]
- 10.Kimura K., Saika Y., Otani H., Fujii R., Mune M., Yukawa S. (1999) Factors associated with calcification of the abdominal aorta in hemodialysis patients. Kidney Int. Suppl. 71, S238–S241 [DOI] [PubMed] [Google Scholar]
- 11.Nadra I., Mason J. C., Philippidis P., Florey O., Smythe C. D., McCarthy G. M., Landis R. C., Haskard D. O. (2005) Proinflammatory activation of macrophages by basic calcium phosphate crystals via protein kinase C and MAP kinase pathways: a vicious cycle of inflammation and arterial calcification? Circ. Res. 96, 1248–1256 [DOI] [PubMed] [Google Scholar]
- 12.Morton C. A., Lafferty M., Hau C., Henderson I., Jones M., Lowe J. G. (1996) Pruritus and skin hydration during dialysis. Nephrol. Dial. Transplant. 11, 2031–2036 [DOI] [PubMed] [Google Scholar]
- 13.Nechemia-Arbely Y., Barkan D., Pizov G., Shriki A., Rose-John S., Galun E., Axelrod J. H. (2008) IL-6/IL-6R axis plays a critical role in acute kidney injury. J. Am. Soc. Nephrol. 19, 1106–1115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pecoits-Filho R., Lindholm B., Axelsson J., Stenvinkel P. (2003) Update on interleukin-6 and its role in chronic renal failure. Nephrol. Dial. Transplant. 18, 1042–1045 [DOI] [PubMed] [Google Scholar]
- 15.Zhang W., Wang W., Yu H., Zhang Y., Dai Y., Ning C., Tao L., Sun H., Kellems R. E., Blackburn M. R., Xia Y. (2012) Interleukin 6 underlies angiotensin II-induced hypertension and chronic renal damage. Hypertension 59, 136–144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun Y. G., Chen Z. F. (2007) A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature 448, 700–703 [DOI] [PubMed] [Google Scholar]
- 17.Sun Y.-G., Zhao Z.-Q., Meng X.-L., Yin J., Liu X.-Y., Chen Z.-F. (2009) Cellular basis of itch sensation. Science 325, 1531–1534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mishra S. K., Hoon M. A. (2013) The cells and circuitry for itch responses in mice. Science 340, 968–971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bautista D. M., Wilson S. R., Hoon M. A. (2014) Why we scratch an itch: the molecules, cells and circuits of itch. Nat. Neurosci. 17, 175–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaMotte R. H., Dong X., Ringkamp M. (2014) Sensory neurons and circuits mediating itch. Nat. Rev. Neurosci. 15, 19–31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu T., Berta T., Xu Z. Z., Park C. K., Zhang L., Lü N., Liu Q., Liu Y., Gao Y. J., Liu Y. C., Ma Q., Dong X., Ji R. R. (2012) TLR3 deficiency impairs spinal cord synaptic transmission, central sensitization, and pruritus in mice. J. Clin. Invest. 122, 2195–2207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang L., Jiang G. Y., Song N. J., Huang Y., Chen J. Y., Wang Q. X., Ding Y. Q. (2014) Extracellular signal-regulated kinase (ERK) activation is required for itch sensation in the spinal cord. Mol. Brain 7, 25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Omori S., Hida M., Fujita H., Takahashi H., Tanimura S., Kohno M., Awazu M. (2006) Extracellular signal-regulated kinase inhibition slows disease progression in mice with polycystic kidney disease. J. Am. Soc. Nephrol. 17, 1604–1614 [DOI] [PubMed] [Google Scholar]
- 24.Smith C. I., Baskin B., Humire-Greiff P., Zhou J. N., Olsson P. G., Maniar H. S., Kjellén P., Lambris J. D., Christensson B., Hammarström L., Bentley D., Vetrie D., Islam K. B., Vořechovský I., Sideras P. (1994) Expression of Bruton’s agammaglobulinemia tyrosine kinase gene, BTK, is selectively down-regulated in T lymphocytes and plasma cells. J. Immunol. 152, 557–565 [PubMed] [Google Scholar]
- 25.Park H., Wahl M. I., Afar D. E., Turck C. W., Rawlings D. J., Tam C., Scharenberg A. M., Kinet J. P., Witte O. N. (1996) Regulation of Btk function by a major autophosphorylation site within the SH3 domain. Immunity 4, 515–525 [DOI] [PubMed] [Google Scholar]
- 26.Mohan C., Putterman C. (2015) Genetics and pathogenesis of systemic lupus erythematosus and lupus nephritis. Nat. Rev. Nephrol. 11, 329–341 [DOI] [PubMed] [Google Scholar]
- 27.Kong W., Deng W., Sun Y., Huang S., Zhang Z., Shi B., Chen W., Tang X., Yao G., Feng X., Sun L. (2018) Increased expression of Bruton’s tyrosine kinase in peripheral blood is associated with lupus nephritis. Clin. Rheumatol. 37, 43–49 [DOI] [PubMed] [Google Scholar]
- 28.Chalmers S. A., Doerner J., Bosanac T., Khalil S., Smith D., Harcken C., Dimock J., Der E., Herlitz L., Webb D., Seccareccia E., Feng D., Fine J. S., Ramanujam M., Klein E., Putterman C. (2016) Therapeutic blockade of immune complex-mediated glomerulonephritis by highly selective inhibition of Bruton’s tyrosine kinase. Sci. Rep. 6, 26164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang B. Y., Huang M. M., Francesco M., Chen J., Sokolove J., Magadala P., Robinson W. H., Buggy J. J. (2011) The Bruton tyrosine kinase inhibitor PCI-32765 ameliorates autoimmune arthritis by inhibition of multiple effector cells. Arthritis Res. Ther. 13, R115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cui S., Chesson C., Hope R. (1993) Genetic variation within and between strains of outbred Swiss mice. Lab. Anim. 27, 116–123 [DOI] [PubMed] [Google Scholar]
- 31.Liu B., Tai Y., Achanta S., Kaelberer M. M., Caceres A. I., Shao X., Fang J., Jordt S.-E. (2016) IL-33/ST2 signaling excites sensory neurons and mediates itch response in a mouse model of poison ivy contact allergy. Proc. Natl. Acad. Sci. USA 113, E7572–E7579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Akiyama T., Carstens M. I., Piecha D., Steppan S., Carstens E. (2015) Nalfurafine suppresses pruritogen- and touch-evoked scratching behavior in models of acute and chronic itch in mice. Acta Derm. Venereol. 95, 147–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eddington H., Heaf J. G. (2009) Clinical management of disturbances of calcium and phosphate metabolism in dialysis patients. NDT Plus 2, 267–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes T. C., Anderson M. E., Moots R. J. (2011) The many faces of interleukin-6: the role of IL-6 in inflammation, vasculopathy, and fibrosis in systemic sclerosis. Int. J. Rheumatol. 2011, 721608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y., Willcockson H. H., Valtschanoff J. G. (2009) Vanilloid receptor TRPV1-mediated phosphorylation of ERK in murine adjuvant arthritis. Osteoarthritis Cartilage 17, 244–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cordova K. B., Oberg T. J., Malik M., Robinson-Bostom L. (2009) Dermatologic conditions seen in end-stage renal disease. Semin. Dial. 22, 45–55 [DOI] [PubMed] [Google Scholar]
- 37.Momose A., Kudo S., Sato M., Saito H., Nagai K., Katabira Y., Funyu T. (2004) Calcium ions are abnormally distributed in the skin of haemodialysis patients with uraemic pruritus. Nephrol. Dial. Transplant. 19, 2061–2066 [DOI] [PubMed] [Google Scholar]
- 38.Mohamed A. J., Yu L., Bäckesjö C. M., Vargas L., Faryal R., Aints A., Christensson B., Berglöf A., Vihinen M., Nore B. F., Smith C. I. (2009) Bruton’s tyrosine kinase (Btk): function, regulation, and transformation with special emphasis on the PH domain. Immunol. Rev. 228, 58–73 [DOI] [PubMed] [Google Scholar]
- 39.Takahashi-Tezuka M., Hibi M., Fujitani Y., Fukada T., Yamaguchi T., Hirano T. (1997) Tec tyrosine kinase links the cytokine receptors to PI-3 kinase probably through JAK. Oncogene 14, 2273–2282 [DOI] [PubMed] [Google Scholar]
- 40.Oliveira F. A., Lima-Junior R. C., Cordeiro W. M., Vieira-Júnior G. M., Chaves M. H., Almeida F. R., Silva R. M., Santos F. A., Rao V. S. (2004) Pentacyclic triterpenoids, alpha,beta-amyrins, suppress the scratching behavior in a mouse model of pruritus. Pharmacol. Biochem. Behav. 78, 719–725 [DOI] [PubMed] [Google Scholar]
- 41.Thomsen J. S., Petersen M. B., Benfeldt E., Jensen S. B., Serup J. (2001) Scratch induction in the rat by intradermal serotonin: a model for pruritus. Acta Derm. Venereol. 81, 250–254 [DOI] [PubMed] [Google Scholar]
- 42.Yamaura K., Akiyama S., Oda M., Suwa E., Ueno K. (2011) Acetaminophen enhances pruritus in a mouse model of contact dermatitis induced by suboptimal concentration of hapten. J. Toxicol. Sci. 36, 669–674 [DOI] [PubMed] [Google Scholar]
- 43.Palmer S. C., Hayen A., Macaskill P., Pellegrini F., Craig J. C., Elder G. J., Strippoli G. F. (2011) Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA 305, 1119–1127 [DOI] [PubMed] [Google Scholar]
- 44.Hoenich N. A., Ronco C. (2007) Haemodialysis fluid: composition and clinical importance. Blood Purif. 25, 62–68 [DOI] [PubMed] [Google Scholar]
- 45.Menon G. K., Elias P. M. (1991) Ultrastructural localization of calcium in psoriatic and normal human epidermis. Arch. Dermatol. 127, 57–63 [PubMed] [Google Scholar]
- 46.Pecoits-Filho R., Bárány P., Lindholm B., Heimbürger O., Stenvinkel P. (2002) Interleukin-6 is an independent predictor of mortality in patients starting dialysis treatment. Nephrol. Dial. Transplant. 17, 1684–1688 [DOI] [PubMed] [Google Scholar]
- 47.Sun J., Axelsson J., Machowska A., Heimbürger O., Bárány P., Lindholm B., Lindström K., Stenvinkel P., Qureshi A. R. (2016) Biomarkers of cardiovascular disease and mortality risk in patients with advanced CKD. Clin. J. Am. Soc. Nephrol. 11, 1163–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bruun J. M., Lihn A. S., Verdich C., Pedersen S. B., Toubro S., Astrup A., Richelsen B. (2003) Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am. J. Physiol. Endocrinol. Metab. 285, E527–E533 [DOI] [PubMed] [Google Scholar]
- 49.Konda D., Chandrashekar L., Rajappa M., Kattimani S., Thappa D. M., Ananthanarayanan P. H. (2015) Serotonin and interleukin-6: association with pruritus severity, sleep quality and depression severity in prurigo nodularis. Asian J. Psychiatr. 17, 24–28 [DOI] [PubMed] [Google Scholar]
- 50.Zakrzewska-Pniewska B., Jedras M. (2001) Is pruritus in chronic uremic patients related to peripheral somatic and autonomic neuropathy? Study by R-R interval variation test (RRIV) and by sympathetic skin response (SSR). Neurophysiol. Clin. 31, 181–193 [DOI] [PubMed] [Google Scholar]
- 51.Cevikbas F., Wang X., Akiyama T., Kempkes C., Savinko T., Antal A., Kukova G., Buhl T., Ikoma A., Buddenkotte J., Soumelis V., Feld M., Alenius H., Dillon S. R., Carstens E., Homey B., Basbaum A., Steinhoff M. (2014) A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: involvement of TRPV1 and TRPA1. J. Allergy Clin. Immunol. 133, 448–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.López-Herrera G., Vargas-Hernández A., González-Serrano M. E., Berrón-Ruiz L., Rodríguez-Alba J. C., Espinosa-Rosales F., Santos-Argumedo L. (2014) Bruton’s tyrosine kinase--an integral protein of B cell development that also has an essential role in the innate immune system. J. Leukoc. Biol. 95, 243–250 [DOI] [PubMed] [Google Scholar]
- 53.Ma T. K., McAdoo S. P., Tam F. W. (2017) Targeting the tyrosine kinase signalling pathways for treatment of immune-mediated glomerulonephritis: from bench to bedside and beyond. Nephrol. Dial. Transplant. 32 (Suppl1), i129–i138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Woyach J. A., Furman R. R., Liu T.-M., Ozer H. G., Zapatka M., Ruppert A. S., Xue L., Li D. H.-H., Steggerda S. M., Versele M., Dave S. S., Zhang J., Yilmaz A. S., Jaglowski S. M., Blum K. A., Lozanski A., Lozanski G., James D. F., Barrientos J. C., Lichter P., Stilgenbauer S., Buggy J. J., Chang B. Y., Johnson A. J., Byrd J. C. (2014) Resistance mechanisms for the Bruton’s tyrosine kinase inhibitor ibrutinib. N. Engl. J. Med. 370, 2286–2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ren L., Campbell A., Fang H., Gautam S., Elavazhagan S., Fatehchand K., Mehta P., Stiff A., Reader B. F., Mo X., Byrd J. C., Carson W. E., III, Butchar J. P., Tridandapani S. (2016) Analysis of the effects of the Bruton’s tyrosine kinase (Btk) inhibitor ibrutinib on monocyte Fcγ receptor (FcγR) function. J. Biol. Chem. 291, 3043–3052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Che Q., Liu B. Y., Liao Y., Zhang H. J., Yang T. T., He Y. Y., Xia Y. H., Lu W., He X. Y., Chen Z., Wang F. Y., Wan X. P. (2014) Activation of a positive feedback loop involving IL-6 and aromatase promotes intratumoral 17β-estradiol biosynthesis in endometrial carcinoma microenvironment. Int. J. Cancer 135, 282–294 [DOI] [PubMed] [Google Scholar]
- 57.Linnskog R., Mohapatra P., Moradi F., Prasad C. P., Andersson T. (2016) Demonstration of a WNT5A-IL-6 positive feedback loop in melanoma cells: dual interference of this loop more effectively impairs melanoma cell invasion. Oncotarget 7, 37790–37802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Li S., Bao X., Wang D., You L., Li X., Yang H., Bian J., Wang Y., Yang Y. (2017) APOBEC3B and IL-6 form a positive feedback loop in hepatocellular carcinoma cells. Sci. China Life Sci. 60, 617–626 [DOI] [PubMed] [Google Scholar]
- 59.Chalmers S. A., Wen J., Doerner J., Stock A., Cuda C. M., Makinde H. M., Perlman H., Bosanac T., Webb D., Nabozny G., Fine J. S., Klein E., Ramanujam M., Putterman C. (2018) Highly selective inhibition of Bruton’s tyrosine kinase attenuates skin and brain disease in murine lupus. Arthritis Res. Ther. 20, 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.