Abstract
In advanced chronic liver disease (CLD), the translocation of intestinal bacteria and the resultant increase of proinflammatory cytokines in the splanchnic and systemic circulation may contribute to the progression of fibrosis. We therefore speculated that fibrosis and portal hypertension (PHT) would be attenuated in a mouse model of limited intestinal colonization with altered Schaedler flora (ASF) compared to a more complex colonization with specific pathogen-free (SPF) flora. We induced liver fibrosis in ASF and SPF mice by common bile duct ligation (BDL) or by carbon tetrachloride (CCl4) treatment. We then measured portal pressure (PP), portosystemic shunts (PSSs), and harvested tissues for further analyses. There were no differences in PP between sham-treated ASF or SPF mice. After BDL or CCl4 treatment, PP, PSSs, and hepatic collagen deposition increased in both groups. However, the increase in PP and the degree of fibrosis was significantly higher in ASF than SPF mice. Expression of fibrotic markers α-smooth muscle actin, desmin, and platelet-derived growth factor receptor β were significantly higher in ASF than SPF mice. This was associated with higher activation of hepatic immune cells (macrophages, neutrophils) and decreased expression of the intestinal epithelial tight junction proteins (claudin-1, occludin-1). In 2 models of advanced CLD, SPF mice presented significantly attenuated liver injury, fibrosis, and PHT compared to ASF mice. In contrast to our hypothesis, these findings suggest that a complex intestinal microbiota may play a “hepato-protective” role.—Moghadamrad, S., Hassan, M., McCoy, K. D., Kirundi, J., Kellmann, P., De Gottardi, A. Attenuated fibrosis in specific pathogen-free microbiota in experimental cholestasis- and toxin-induced liver injury.
Keywords: altered Schaedler flora, portal hypertension, bile duct ligation, α-SMA, carbon tetrachloride
Advanced chronic liver disease (CLD) is characterized by the persistent synthesis and deposition of extracellular matrix (ECM) proteins as a consequence of prolonged injury leading to tissue scarring (1). During this process, the functional viability of liver parenchymal cells is progressively lost, resulting in parenchymal extinction and deposition of fibrillar collagen mainly as a consequence of hepatic stellate cells (HSCs) activation. Following liver injury, HSCs acquire proliferative, contractile, proinflammatory, and profibrogenic properties and their expression of α-smooth muscle actin (α-SMA) increases. Activated HSCs can migrate and proliferate in different sites where liver injury occurs and secrete large amounts of ECM. Particularly in the space of Disse, ECM severely distorts liver architecture, increasing intrahepatic vascular resistance and portal pressure (PP) (2).
Under physiologic conditions, bacteria and bacterial-derived products are confined to the intestinal lumen by the mucosal and vascular intestinal barriers. While in CLD associated with portal hypertension (PHT), host immune homeostasis is disrupted; the liver function is compromised because of increased microbial burden and impaired intestinal permeability (3). Several studies have demonstrated that patients with advanced CLD are susceptible to develop endotoxemia as a result of increased gut mucosa permeability (tight junction and mucin disruption), bacterial overgrowth, alteration in the composition of intestinal flora, and host immune dysfunction (4–14). Therefore, translocation of bacteria and their products (LPS, peptidoglycan, bacterial DNA, and flagellin) into the mesenteric lymph nodes (MLNs), spleen, and liver across the gut-liver axis may mediate hepatic and systemic inflammatory responses through Toll-like receptor (TLR) activation. Among TLRs, TLR4 plays a central role in promoting liver fibrosis and inflammation through its activation on both parenchymal and nonparenchymal cells. Experimental evidence showed that activation of TLR4 signaling pathway on Kupffer cells and HSCs contributes to the production of TGF-β and platelet-derived growth factor (PDGF) (15–17). This, in turn, enhances fibrogenesis and disseminates inflammation by secreting proinflammatory cytokines (18), as demonstrated in several studies in which progression of liver fibrosis in TLR4 or TLR4 coreceptor–deficient mice was significantly reduced compared to wild-type mice. Furthermore, in advanced CLD, an accumulation and infiltration of neutrophils and macrophages (CD11b and osteopontin) has been demonstrated at the sites of inflammation, which contributes to liver injury (19–22).
However, recent data suggest that the presence of intestinal flora may not only be detrimental, but also protective during acute or chronic liver damage. Indeed, in different mouse models of liver injury, a significantly higher level of fibrosis has been observed in germ-free (GF) mice compared to conventionally raised (Conv-R) mice. The increased fibrosis in GF mice was associated with more toxin-induced oxidative stress and cell death (23). Moreover, in a mouse model of primary sclerosing cholangitis, fibrosis, ductopenia, and cholangiocyte senescence were significantly higher in GF mice than in Conv-R mice (24). Taken together, these findings suggest that both conditions, the colonization with a conventional flora, as well as absence of intestinal flora in GF conditions may contribute to enhance the damage during chronic liver injury.
However, the effect of limited colonization on liver fibrosis has not been studied so far. In this study, we investigated fibrosis and PHT in a gnotobiotic model of limited intestinal colonization with altered Schaedler flora (ASF) compared to a more complex colonization [specific pathogen-free (SPF) flora] in 2 different models of experimental fibrosis, bile duct ligation (BDL) and carbon tetrachloride (CCl4).
MATERIALS AND METHODS
Animals
Male C57BL/6 mice, aged 10–12 wk, were generated in the clean mouse facility of the University of Berne or purchased from Charles River Laboratories (Wilmington, MA, USA) and raised under ASF or SPF conditions. All animals were kept on a 12-h dark/light cycle. The ASF microbiota is a well-defined flora, which contains only 8 species, including Lactobacillus acidophilus, Lactobacillus murinus, Bacteroides distasonis, Mucispirillum schaedleri, Eubacterium plexicaudatum (a Fusiform-shaped bacterium), and 2 Clostridium species (25).
All experimental protocols were approved by the Research Animal Ethics Committee of Canton Bern (authorization 26/15) and were performed according to international guidelines concerning the conduct of animal experimentation.
Induction of fibrosis by common BDL
Before surgery, the mice received 60 µg/kg body weight of buprenorphine subcutaneously (0.3 mg/ml; Reckitt Benckiser, Slough, United Kingdom). BDL was performed as previously described by Georgiev et al. (26). Briefly, after induction of isoflurane anesthesia and midline laparotomy, the bile duct was isolated from the surrounding tissues, a single 7-0 silk ligature was tied around it proximally to the liver hilus, and a second and a third ligatures were placed around its distal end. Finally, the bile duct was resected between the proximal and the distal ligature. In sham-treated animals the bile duct was only isolated but not ligated.
Induction of fibrosis by intraperitoneal injection of CCL4
ASF or SPF mice received 60 µl of CCl4 (Merck GmbH, Darmstadt, Germany) (1:4 dissolved in olive oil; MilliporeSigma, Burlington, MA, USA) intraperitoneally, or olive oil as control, twice weekly for 10 wk. Experiments were performed 5 d after last injection.
Hemodynamic measurements
Hemodynamic measurements were performed 14 d after BDL or 10 wk after CCl4 treatment in groups of 10 animals. Under general anesthesia with isoflurane, laparotomy was performed, and the PP was measured by cannulating the ileocolic vein using a 26GA BD VasculonTM Plus cannula (Becton Dickinson, San Diego, CA, USA). The catheter was connected to a highly sensitive pressure transducer and the signal was recorded using a multichannel PowerLab instrumentation and LabChart-7 Software (AD Instruments, Sydney, NSW, Australia).
Determination of the extent of portal systemic collateral formation
The extent of portosystemic shunts (PSSs) was assessed by injecting 3 × 104 51Cr-labeled microspheres into the ileo-colic vein. Animals were then euthanized, and the liver and lungs were removed and weighed. The radioactivity of the organs was determined in a Canberra Packard Cobra II Auto-γ Counter (Canberra Packard, Concord, ON, Canada). PSSs were quantified for the ratio of radioactivity in the liver and lungs using the equation PSSs (%) = [pulmonary radioactivity/(pulmonary radioactivity + liver radioactivity)] × 100 (27).
Assessment of bacterial translocation
MLNs, spleen and liver were dissected aseptically and weighed. The cecum was then opened and an aliquot of cecal content was collected and weighed. Organs were homogenized in 0.5% Tergitol/PBS using a Tissuelyser (Qiagen, Hilden, Germany) and sterile stainless-steel ball bearings. Cecal contents and organ suspensions were then plated on Luria Bertani (LB) agar (MilliporeSigma) and blood agar (BdA) (anaerobe agar and sheep blood defibrinated, Wilkins-Chalgren; Oxoid, Hampshire, United Kingdom) plates for aerobic and anaerobic culture, respectively, and incubated for 48 h at 37°C for colony forming unit (CFU) quantification. Bacterial colonies were counted and normalized for milligrams of tissue or cecal content.
Identification of bacterial colony using 16S rRNA-PCR
Single bacterial colonies were isolated from LB or BdA plates and suspended in 20 µl of autoclaved Milli-Q water (EMD Millipore, Burlington, MA, USA). Five microliters of bacterial suspension was amplified in a 45 µl reaction by using a PCR kit (TaqDNA Polymerase, dNTPack; Roche, Basel, Switzerland). The lyophilized primers were purchased from Microsynth (Balgach, Switzerland) (f-D1: 5′-AGAGTTTGATCCTGGCTCAG-3′, f-D2: 5′-AGAGTTTGATCATGGCTCAG-3′ and r-P1: 5′-ACGGTTACCTTGTTACGACTT-3′). PCR conditions consisted of 94°C (5 min), 35 cycles of 94°C (2 min), 34°C (1 min), 72°C (2 min) and for final step 72°C (7 min), 10°C. After PCR, 1% agarose gel (running at 120 V) was used to check the quality of the PCR reactions. PCR reactions were purified using QiaQuick PCR Purification Kit (28106; Qiagen). DNA concentration was determined by NanoDrop (Thermo Fisher Scientific). Afterwards, a mixture of DNA (18 ng/100 bases) and 1 µl of forward-D1 primer was used for sequencing by Microsynth. The Basic Local Alignment Search Tool (BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi) was applied for identification (determination/comparison) of DNA sequencing.
Liver histology
Segments of liver were either snap frozen or fixed in 10% buffered formalin and embedded in paraffin blocks, and tissue slides were prepared for standard histology hematoxylin and eosin (H&E), Sirius red staining, or immunohistochemical (IHC) analysis. Morphometric quantification of Sirius red staining was performed using MetaMorph imaging software (NX software 64-bite, v.7.8.12.0; Molecular Devices, San Jose, CA, USA). The results are presented as a ratio of red-stained area per total area, using 3 randomly chosen field areas.
Total bile acids measurement in liver
The concentration of total bile acids (TBAs) was measured in liver tissues homogenates of BDL or CCl4 mice according to the manufacturer’s instructions (Mouse Total Bile Acids Assay Kit, 80470; Crystal Chem, Elk Grove Village, IL, USA).
IHC of α-SMA, osteopontin, and CD11b
Paraffin sections were deparaffinized in xylol and rehydrated in graded alcohol series. Endogenous peroxidase was blocked with 0.4% H2O2 in methanol for 10 min. Antigen retrieval was performed by boiling the sections in citrate buffer/Tris EDTA (pH 9.0) for 15 min. Endogenous blocking was performed with TCA (Tris-buffered saline–Casein, NaN3 0.1%) for 1 h at room temperature. Liver sections were incubated with the mouse anti–α-SMA (1:1000; MilliporeSigma), goat polyclonal osteopontin (15 µg/ml; R&D Systems, Minneapolis, MN, USA), CD11b (1:500; Thermo Fisher Scientific, Waltham, MA, USA) overnight at 4°C. Following overnight incubation, the slides were then incubated with the biotinylated goat anti-mouse secondary antibody (1:400; Agilent Technologies, Santa Clara, CA, USA), biotinylated rabbit anti-goat secondary antibody (1:150; Agilent Technologies), biotinylated rabbit anti-rat secondary antibody (1:200; Agilent Technologies), respectively, for 30 min at room temperature. After washing, the slides were treated with streptavidin, horseradish peroxidase (Vector Laboratories, Burlingame, CA, USA) for 20 min. The detection was performed using either 3,3-diaminobenzidine tetrahydrochloride substrate (Vector Laboratories), or AEC solution (Vector Laboratories), followed by hematoxylin counterstaining.
Western blot analysis
Samples of snap-frozen livers were homogenized in a buffer (pH 7.4) containing 250 mM sucrose, 50 mM Tris base, 5 mM magnesium chloride, and protease inhibitor. Determinations of proteins in the homogenates were performed with a Bicinchoninic Acid Assay Kit (Pierce, Rockford, IL, USA). Fifty micrograms of protein per lane was subjected to SDS-PAGE (12% gel) for α-SMA, desmin, and PDGF receptor β (PDGFR-β). Proteins were blotted on Protran nitrocellulose transfer membranes (Whatman GmbH, Dassel, Germany), charged with methanol. Ponceau-S staining 0.5% was performed to ensure equal protein loading. The membranes were blocked with 5% skim milk, incubated with primary antibodies overnight at 4°C (α-SMA, 1:1000; MilliporeSigma), desmin (1:1000), and PDGFR-β (1:1000; BD Biosciences, San Jose, CA, USA). Thereafter, membranes were incubated with corresponding secondary horseradish peroxidase–coupled antibodies. Blots were developed with ECL substrate Western Lightning Plus-ECL Kit (PerkinElmer, Waltham, MA, USA). Intensities of the resulting bands on each blot were compared densitometrically with Fusioncapt advance software. For quantification analysis, all proteins of interest were normalized to vinculin (1 µg/ml; MilliporeSigma). Representative blots are taken after 3 independent experiments.
Real-time quantitative PCR
Total RNA was extracted from 30 to 50 mg of liver or intestine using the RNeasy Plus Mini Kit (Qiagen). Reverse transcription was performed with M-MLV Reverse Transcriptase (Thermo Fisher Scientific) and a random hexamer mix. Probes and primers for the detection of mouse claudin-1, occludin-1, mucin-1 (Muc-1), secreted phosphoprotein-1 (Spp1), and integrin αM (Itgam or CD11b) were purchased from Thermo Fisher Scientific. Quantitative PCR was performed using an ABI TaqMan 7500 Sequence Detection System and TaqMan universal PCR Master Mix (Thermo Fisher Scientific) according to standard protocols. Each reaction was carried out in triplicate. All transcripts were normalized using glyceraldehyde 3-phosphate dehydrogenase as a housekeeping gene. All data are represented by fold change (2−ΔΔCt).
Statistical analysis
Statistical analyses were performed using Prism software 5 (GraphPad Software, La Jolla, CA, USA). Data are expressed as means ± sd. Values of P < 0.05 were considered statistically significant. Comparisons between 2 groups were performed using the Mann-Whitney U test.
RESULTS
PP after BDL or CCl4 treatment
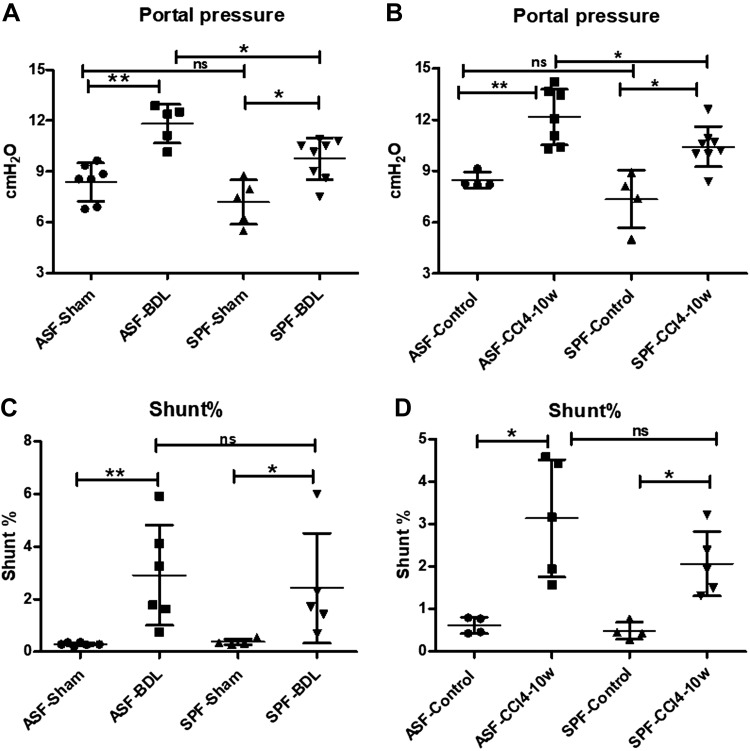
We performed BDL to verify whether the complexity of intestinal flora could affect splanchnic hemodynamic alteration in a cholestatic model of fibrosis. This surgical intervention induced fibrosis, with a significant intrahepatic increase of PP in ASF-BDL as well as in SPF-BDL mice, as measured 14 d after surgery. However, the increase in PP in ASF-BDL mice (11.8 ± 1.1 cmH2O) was significantly higher than in SPF-BDL mice (9.75 ± 1.2 cmH2O, P = 0.018). In sham-treated mice there was no significant difference in PP between ASF-sham–treated and SPF-sham–treated mice (ASF-Sham 8.34 ± 1.1 cmH2O vs. SPF-Sham 7.18 ± 1.3 cmH2O, P = 0.15, n = 5–8/group; Fig. 1A).
Figure 1.
PP and PSSs measurements in BDL- or CCl4-treated mice. PPs (cmH2O). A) ASF-BDL and SPF-BDL mice 14 d after BDL. B) ASF-CCl4 and SPF-CCl4 mice 10 wk after CCl4 immunoprecipitation (IP) injection. C) PSSs (shunt percentage) ASF-BDL and SPF-BDL mice 14 d after BDL. D) ASF-CCl4 and SPF-CCl4 mice 10 wk after CCl4 IP injection. ns, not significant. Data are expressed as means ± sd; n = 4–8/group. *P < 0.05, **P < 0.005, ***P < 0.0005.
These findings were confirmed in a toxin model of fibrosis induced by CCl4. PP increased significantly in ASF-CCl4 as well as in SPF-CCl4. When compared to control groups, ASF-CCl4 (12.15 ± 1.6 cmH2O) exhibited significantly higher PP as compared to SPF-CCl4 (10.43 ± 1.2 cmH2O, P = 0.04) n = 4–8/group. In control mice there was no significant difference in PP between ASF-control and SPF-control mice (ASF-control 8.46 ± 0.05 cmH2O vs. SPF-control 7.35 ± 1.7 cmH2O, P = 0.2) (Fig. 1B).
Development of portosystemic collaterals
We evaluated the extent of PSSs in ASF and SPF mice after BDL or CCl4 treatment. Our data showed a significant increase in PSSs in ASF-BDL and SPF-BDL mice as compared to sham-treated mice. The difference between ASF-sham (0.29 ± 0.06%) vs. SPF-sham (0.38 ± 0.11%, P = 0.239) and ASF-BDL (2.91 ± 1.9%) vs. SPF-BDL (2.42 ± 2%) was not significant (P = 0.662), n = 4–6/group (Fig. 1C).
The extent of PSSs in CCl4-treated mice was significantly increased in ASF-CCl4 (3.13 ± 1.4%) as well as SPF-CCl4 (2.07 ± 0.8%) as compared to controls. The differences between ASF-control (0.62 ± 0.2%) vs. SPF-control (0.49 ± 0.2%) or ASF-CCl4 and SPF-CCl4 were not significant, n = 4–6/group (Fig. 1D).
Bacterial translocation after BDL or CCl4 treatment
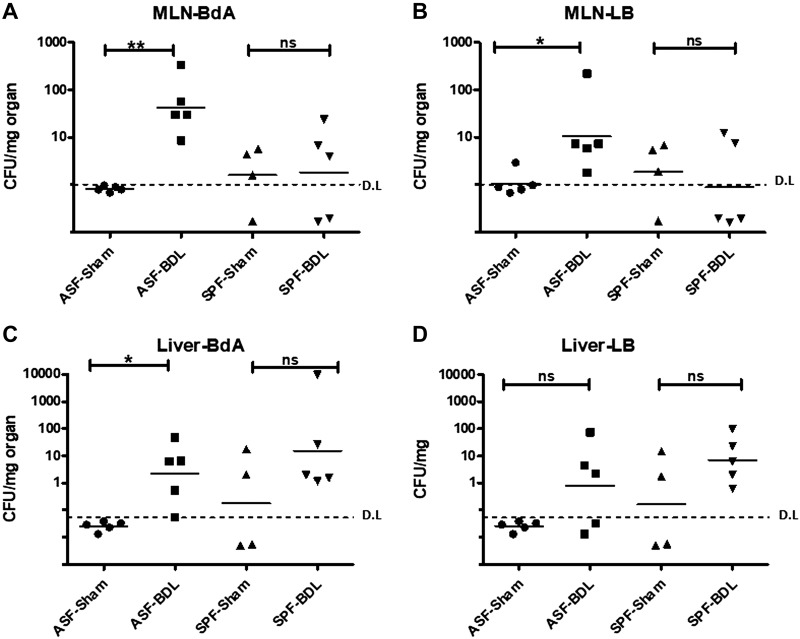
To detect bacterial translocation, we harvested MLNs and liver tissue under sterile conditions and plated the homogenized organs on LB agar for aerobic bacteria or BdA plates for anaerobic bacterial growth at 37°C for 48 h. Bacterial colonies were counted and normalized for milligrams of tissue. We observed a significant bacterial translocation to the MLN in only ASF-BDL mice on BdA and LB agar plates (Fig. 2A, B). In addition, the anaerobic culture of liver homogenate (BdA plates) was positive indicating a significant bacterial translocation in ASF-BDL (Fig. 2C). In the SPF-BDL mice, no significant bacterial translocation was detected (n = 4–5/group) (Fig. 2C, D). We also plated the cecal content to evaluate the abundance of intestinal bacteria and detected no difference in the cultures of cecal content in both animal models (Supplemental Fig. S1).
Figure 2.
Bacterial translocation (CFU) after BDL. A, B) CFU in MLNs of ASF-BDL and SPF-BDL mice 14 d after BDL, MLN-BdA (A) and MLN-LB (B). C, D) CFU in liver of ASF-BDL and SPF-BDL mice 14 d after (BDL). Geometric mean was calculated. Ns, not significant. Data are expressed as means ± sd; n = 4–5/group. *P < 0.05, **P < 0.005, ***P < 0.0005.
Next, bacterial species isolated from the MLNs and liver of ASF-BDL were analyzed using 16S rRNA-PCR. We observed translocation of only L.murinus to MLNs and liver of ASF-BDL mice among the 8 ASF bacteria species. In CCl4-treated mice, bacterial translocation was not detected in any of the cultured organs (Supplemental Fig. S2).
Evaluation of liver histology after BDL
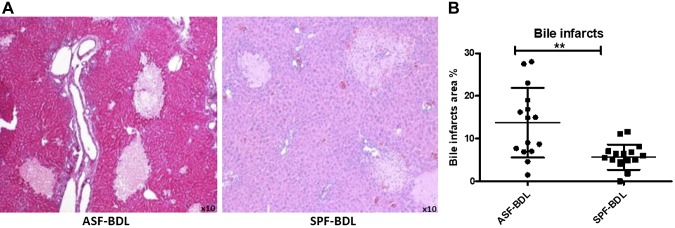
To examine the extent of liver damage after BDL, liver sections of ASF and SPF mice were stained with H&E (Fig. 3A). The ratio of bile infarction lesions was significantly higher in ASF-BDL (13.5 ± 4%) as compared to SPF-BDL (4.8 ± 3%) mice (P = 0.0015, n = 5/group) (Fig. 3B).
Figure 3.
Evaluation of liver histology and bile infarcts area quantification. Histopathological examination of liver specimens and quantification demonstrated numerous bile infarcts in ASF-BDL and SPF-BDL mice. Determination of bile infarcts in the liver were assessed by conventional H&E staining (A) in ASF-BDL and SPF-BDL mice 14 d after BDL, then quantified (bile infarcts area percentage) using digital image analysis, MetaMorph (B) ASF-BDL and SPF-BDL mice 14 d after BDL. Data are expressed as means ± sd; n = 5 mice/group. *P < 0.05, **P < 0.005.
Determination of collagen deposition and fibrosis within the liver sections in BDL- or CCl4-treated mice
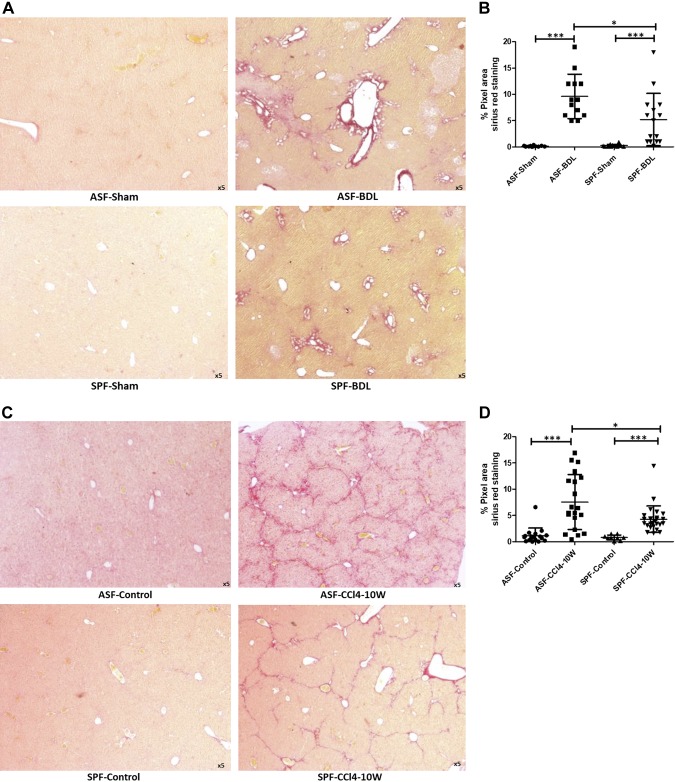
Sirius red staining was performed to assess localization and distribution of collagen fibers in liver tissues. The ratio of collagen deposition was determined from 3 different sections of each sample of the liver in ASF and SPF mice. In BDL mice, deposition of collagen fibers was predominantly around the area of bile duct proliferation (Fig. 4A), which increased remarkably after BDL in ASF or SPF mice. However, the amount of collagen deposition within the liver tissue was significantly higher in ASF-BDL (9.8 ± 2%) compared to SPF-BDL (5.2 ± 4%) mice (P = 0.014) (Fig. 4B). In CCl4-treated mice, collagen deposition was distributed around the fibrous septa (Fig. 4C). The ratio of collagen deposition was significantly higher in ASF-CCl4 (7.6 ± 5%) as compared to SPF-CCl4 mice (4.3 ± 2%) (P = 0.02), n = 4–5/group (Fig. 4D).
Figure 4.
Determination of fibrosis by collagen deposition within the liver sections in BDL- and CCl4-treated mice. Representative histologic images of livers stained with Sirius red to examine liver fibrosis ASF-sham vs. ASF-BDL (upper panel) and SPF-Sham vs. SPF-BDL (lower panel) mice 14 d after BDL (A), and quantification (percentage pixel area) ASF and SPF mice 14 d after BDL (B), representative histologic images of livers stained with Sirius red in ASF-Control vs. ASF-CCl4 or SPF-Control vs. SPF-CCl4 mice 10 wk after CCl4 treatment (C), and quantification (percentage pixel area) ASF and SPF mice 10 wk after CCl4 treatment (D). Data are expressed as means ± sd; n = 5/group. *P < 0.05, **P < 0.005, ***P < 0.0005.
Evaluation of TBAs in the liver
The concentration of TBA was increased after BDL as compared to sham in both groups ASF mice (528%) (P = 0.004) and SPF mice (444%) (P = 0.06). The TBA concentration in ASF-BDL mice was higher than SPF-BDL (n = 5) (Supplemental Fig. S3).
Evaluation of HSCs activation by IHC
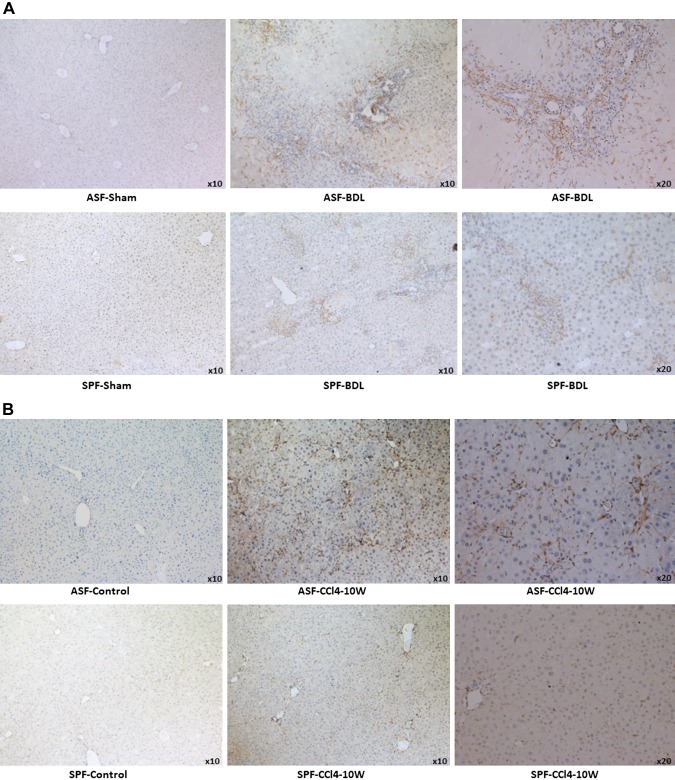
The results from IHC of α-SMA staining revealed activation of HSCs in ASF-BDL vs. SPF-BDL within portal, centri-lobular, and necrotic regions (Fig. 5A). Similarly, we observed more activated HSCs in ASF-CCl4 than SPF-CCl4 mouse livers n = 5–6/group (Fig. 5B).
Figure 5.
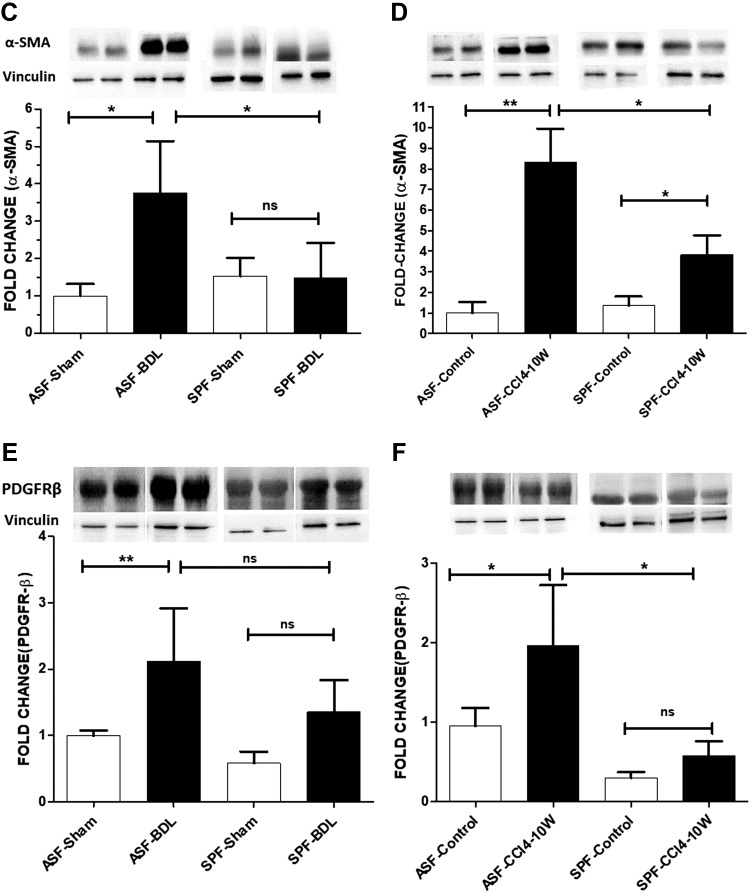
Evaluation of HSC activation by IHC of α-SMA, protein expression of α-SMA, and PDGFR-β. A) Representative images showing liver sections of ASF-sham vs. ASF-BDL (upper panel) and SPF-sham vs. SPF-BDL mice (lower panel), 14 d after BDL. B) Liver sections of ASF-Control vs. ASF-CCl4 (upper panel) and SPF-Control vs. SPF-CCl4 (lower panel), α-SMA positive staining (brown). C, D) Western blotting with protein quantification of α-SMA in livers of ASF-sham vs. ASF-BDL and SPF-sham vs. SPF-BDL mice (C), ASF-control vs. ASF-CCl4 or SPF-control vs. SPF-CCl4 mice (D). E, F) PDGFR-β protein expression with quantification from livers in BDL mice (E) or in CCl4-treated mice (F). The original blots are provided in Supplemental Fig. S7A, B. Ns, not significant. Data are expressed as means ± sd; n = 5–6/group. *P < 0.05, **P < 0.005, ***P < 0.0005.
Hepatic expression of profibrogenic markers by Western blotting
To confirm our data from Sirius red staining and IHC, protein expression of α-SMA in the liver was evaluated by Western blotting. A significant increase of α-SMA protein was observed in ASF-BDL (375%) and SPF-BDL mice (148%) as compared to sham. The increase was significantly higher in ASF-BDL (39%) as compared to SPF-BDL n = 5–6/group (Fig. 5C). Similarly, in CCl4-treated mice, the level of α-SMA protein expression was significantly increased in ASF-CCl4 (830%) and SPF-CCl4 (380%) as compared to controls, whereby this increase was significantly higher in ASF-CCl4 (45.7%) compared to SPF-CCl4 (n = 5–6/group) (Fig. 5D).
Furthermore, to compare the activation of HSCs (α-SMA expression) to the total number of HSCs, the expression of desmin was evaluated. We observed that the expression of desmin was also significantly higher in ASF-BDL (189%) mice and in SPF-BDL mice (118%) as compared to sham-treated animals. Consistently, an increase of 311% in ASF-CCl4 and 145% in SPF-CCl4 mice as compared to control was observed. The increase in desmin expression was higher in ASF-BDL (61.1%) and ASF-CCl4 (45%) as compared to SPF mice, respectively (n = 5–6/group) (Supplemental Fig. S4A, B).
We also evaluated the protein expression of profibrogenic mediator, PDGFR-β in liver tissue of ASF or SPF mice after BDL or CCl4 treatment. The expression of PDGFR-β protein was increased after BDL or CCl4. The increase in expression of PDGFR-β was higher in ASF-BDL (63.9% not statistically significant) or ASF-CCl4 (29.4% P = 0.002) as compared to SPF-BDL or SPF-CCl4 mice, respectively (Fig. 5E, F).
Evaluation of immune cell activation and alteration of intestinal tight junctions after liver injury
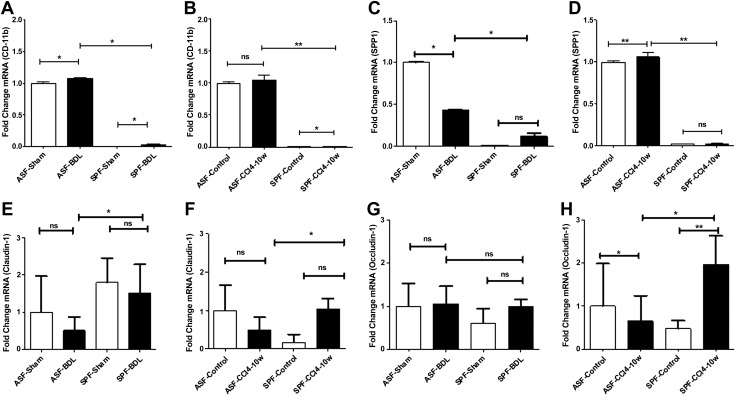
We further assessed the immune cell activation of CD11b and osteopontin under baseline and after liver injury in mice with different microbiota compositions by quantitative RT-PCR and IHC. We observed mRNA expression of CD11b (Itgam) was significantly higher after cholestatic liver injury in ASF or SPF-BDL mice as compared to sham. This increase was significantly higher in ASF-BDL vs. SPF-BDL mice (Fig. 6A). In CCl4-treated mice, the mRNA expression of CD11b was higher in ASF-CCl4 and SPF-CCl4 compared to control. Consistently the mRNA expression of CD11b was significantly higher in ASF-CCl4 compared to SPF-CCl4 (Fig. 6B).
Figure 6.
Evaluation of mRNA expression of hepatic immune cells activation and intestinal epithelial tight junctions in BDL or CCl4 mice. A, B) Level of hepatic mRNA expression of CD11b in BDL (A) and CCl4 (B) mice. C, D) Level of hepatic mRNA expression of SPP1 in BDL (C) and CCl4 (D) mice. E–H) Level of intestinal mRNA expression of claudin-1 in BDL (E) and CCl4 (F) mice or occludin-1 in BDL (G) and in CCl4-treated mice (H). Data are expressed as means ± sd; n = 5/group. Ns, not significant. *P < 0.05, **P < 0.005.
The expression of SPP1 was decreased in ASF-BDL mice but not remarkably changed in SPF-BDL compared to sham, respectively. However, we observed a significantly higher expression of SPP1 in ASF-BDL mice vs. SPF-BDL (n = 5/group) (Fig. 6C). We also found that the expression of SPP1 was increased after CCl4 in both groups but in ASF mice its expression was significantly higher before and after CCl4 treatment as compared to SPF mice n = 5/group (Fig. 6D). We also assessed by IHC and observed an increased expression of osteopontin and CD11b in ASF-BDL as well ASF-CCl4 as compared to their SPF counterparts, respectively (Supplemental Fig. S5A–D).
To further investigate whether expression of intestinal tight junctions was altered under baseline and after liver injury in mice with different microbiota compositions, the mRNA expression of claudin-1, occludin-1, and Muc-1 were determined by quantitative RT-PCR. The expression of claudin-1 was significantly decreased (200% P = 0.031, n = 5/group) after BDL in ASF than SPF mice and the level of expression was less in ASF before and after BDL as compared to SPF (Fig. 6E). In CCl4 group mice, mRNA expression of claudin-1 was significantly lower (212% P = 0.038) in ASF-CCl4 than SPF-CCl4 (Fig. 6F). There were no significant differences in expression of claudin-1 between sham and CCl4 in any of the groups. Additionally, we did not observe significant differences in the expression pattern of occludin-1 in BDL of both groups (Fig. 6G). However, in ASF-CCl4 the expression of occludin-1 was reduced significantly (300%, P = 0.001) compared to SPF-CCl4 (n = 5/group) (Fig. 6H). Furthermore, we observed a trend toward decreased expression of Muc-1 in ASF-BDL compared to sham (not statistically significant), and this expression was significantly increased in SPF-BDL as compared to sham. Nevertheless, the expression of Muc-1 was significantly less in ASF mice after BDL (192% P = 0.031) as compared to SPF-BDL mice (Supplemental Fig. S6A). In CCl4-treated mice, we observed similar results as BDL. The expression of Muc-1 was significantly reduced in ASF mice after CCl4 (260% P = 0.028) as compared to SPF mice (n = 5/group) (Supplemental Fig. S6B).
DISCUSSION
In this study, we aimed to investigate whether a different composition of intestinal flora could have any effects on liver fibrosis and PHT. We hypothesized that fibrosis and PHT would be attenuated in the presence of a limited number of species in intestinal flora (ASF) compared to a more complex composition (SPF). In contrast with our initial hypothesis, we observed that liver fibrosis and PHT after BDL or CCl4 were attenuated in SPF mice compared to ASF mice. After evaluating the extent of fibrosis, we found significantly less collagen deposition in SPF mice. These results were consistent with the expression of fibrotic markers such as α-SMA or desmin, which were significantly lower in SPF than in ASF mice. We also observed a lower expression of major profibrogenic and inflammatory mediator (PDGFR-β) in SPF than in ASF mice. These findings suggest that a complex bacterial composition of the intestinal flora may exert a protective role in cholestatis- and toxin-induced liver injury. In a recent study, Mazagova et al. (23) reported that GF mice were more susceptible to fibrosis in different experimental models (CCl4, thioacetamide, or MyD88/Trif-deficient mice), mainly because the oxidative stress in GF mice was significantly higher than in Conv-R mice. In addition, low hepatic expression of P450 enzymes (xenobiotic metabolizing enzymes) in GF mice led to an accumulation of toxic products and thus increased oxidative stress (increased hepatotoxicity). In a mouse model of primary sclerosing cholangitis (mdr2−/−), Tabibian et al. (24) observed that fibrosis, ductopenia, and cholangiocyte senescence were significantly higher in GF-mdr2−/− than in Conv-R-mdr2−/− mice. In this study, the absence of secondary bile acids in GF mice promoted cholangiocyte senescence, and this was reversed by ursodeoxycholic acid treatment. Taken together, these findings indicate that the absence of intestinal flora is associated to a more severe liver injury.
Additionally, Rosshart et al. (28) reported that upon different disease challenges, natural microbiota balances systemic and local inflammatory responses. In this study, the authors compared wild rodent and laboratory mice harboring different microbial compositions and found that mice with natural microbiota showed less proinflammatory and increased anti-inflammatory cytokines. These mice also presented immune system resistance and higher survival rate to different diseases such as influenza infections and colon tumorigenesis. This supports the assumption that the complex microbiota is essential for host physiology and immunity promoting host resistance to diseases.
Immune cell activation is one of the central pathogenic features in advanced chronic liver injury (29–31). The infiltrated immune cells function as profibrogenic mediators during liver injury by activating Kupffer cells and HSCs (32). To confirm the data from previous investigators, whether the differences in fibrosis were associated with activation of circulating neutrophils and macrophages (19, 20, 22, 30, 33) in our experimental models, we evaluated the gene expression levels of hepatic immune cells implicated in fibrosis. We observed a significantly increased expression of CD11b (neutrophil chemoattractant) in ASF cholestatic (BDL) and ASF-CCl4 group as compared to SPF group, respectively. Additionally, the expression of osteopontin (chemoattractant for macrophages and neutrophils) was up-regulated in ASF-BDL as well as ASF-CCl4 mice compared to SPF group, respectively. These data suggest that a limited intestinal bacterial colonization could be correlated with increased fibrosis associated with immune cell activation in our study (28).
It is well known that in the absence of intestinal flora (GF), mice present morphologic and immunologic intestinal alterations such as reduced intestinal mass, irregular villi with thinner mucosa, shorter crypts, decreased IgA, and T-cell response (34). Therefore, the diversity and complexity of intestinal microorganism is crucial to shape intestinal barrier systems (28). In order to investigate whether the different composition of intestinal microbial colonization could alter intestinal tight junctions and could promote liver fibrosis, we evaluated the gene expression of intestinal epithelial tight junctions. We observed that the expression of claudin-1 was significantly less in ASF mice after BDL or CCl4 as compared to SPF groups, whereas the expression of occludin-1 was altered in ASF mice after CCl4 but not in BDL as compared to SPF groups. The increased expression of occludin-1 or claudin-1 in SPF mice may correlate to the protective role of microbiota in modulating the intestinal epithelial barrier system, making ASF mice more susceptible to liver injury. In contrast, the function of intestinal epithelial barrier system in SPF mice is more sophisticated and provides more protection to the host (28, 35). Furthermore, in a recent study, Li et al. (36) measured the mucus thickness in GF, ASF, monocolonized (with Escherichia coli or Bacteroides thetaiotaomicron) and SPF mice. They observed significant differences in the mucus thickness between ASF and SPF mice. We further investigated the expression of intestinal Muc-1, which has been shown to play an important role in mucosal barrier against infection (13). Besides its role as a part of static as well as dynamic component of mucosal barrier, it is regulated by both innate and adaptive immunity (13). The mucins are known to protect the underlying epithelium against pathogen penetration (14). Moreover, lack of Muc-1 in mice showed more epithelial damage following bacterial infection. Therefore, Muc-1 has been recognized as a crucial element of mucosal defense against pathogen infection and protects epithelial cells from bacterial toxins (13, 37). We observed a significant reduction in the expression of Muc-1 in both ASF-BDL and ASF-CCl4 models with a subsequent bacterial translocation in ASF-BDL mice.
In addition to bacterial translocation, increased levels of microbiota-derived factors in portal and systemic circulation have been associated with systemic inflammation, which worsens the course of liver disease in experimental models of fibrosis and in patients with cirrhosis (16, 38). Recently, Schierwagen et al. (39) found a patient-specific correlation between circulatory compartment-specific microbial genera and systemic inflammatory markers in blood samples of patients with cirrhosis taken from different compartments. This data may provide novel insights into the role of other microbial-mediated mechanisms contributing to the severity of liver disease. Whether animal models with distinct microbial communities differ in the mechanisms of liver disease progression is currently a matter of debate.
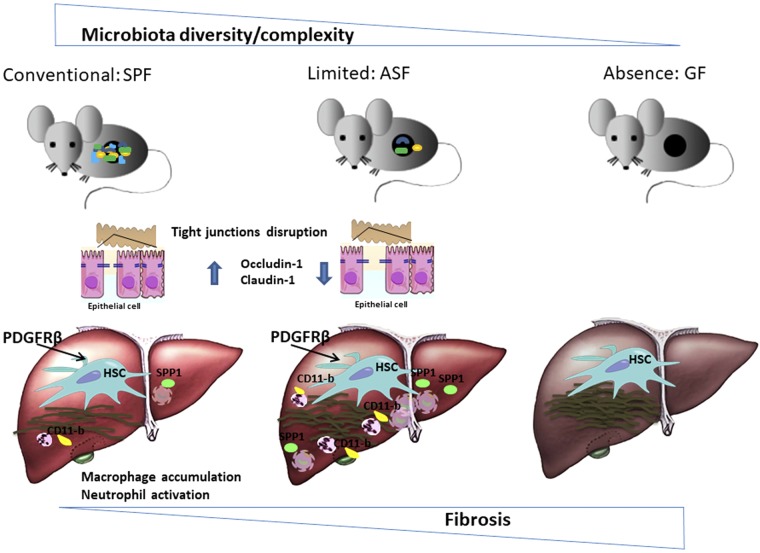
Our data provide evidence that a diverse microbiota is essential to regulate epithelial barrier function via immune mediated host defense response that further prevents disease progression. This may in part explain the underlying mechanism of different responses between ASF and SPF mice after chronic liver injury mainly due to the absence of a more diversified microbiota and, therefore, not fully developed mucosa in ASF mice (35). We demonstrate here that in the cholestatic and hepatotoxic models of fibrosis mice colonized with fewer intestinal microbiota species (ASF) had a more severe fibrotic response than mice with a complex microbiota (SPF). The protective role of microbiota and its role in the development of diseases are both part of the balance in keeping host homeostasis (the paradoxical effects of microbiota), but under which circumstances and how 1 part of balance weighs more is a fundamental question that requires more investigations. One possibility would be to colonize ASF mice with SPF flora or probiotics to recapitulate the SPF status of intestinal habitat in order to attenuate the degree of fibrosis and associated inflammation. Based on the data obtained in this work, we propose a model that recapitulates the protective role of a complex microbiota in attenuating liver injury (Fig. 7).
Figure 7.
Proposed schematic model showing complex microbiota plays a hepatoprotective role during liver injury. In the presence of a more complex microbiota community, fibrosis is attenuated as observed by less ECM deposition, accumulation of macrophages, neutrophil infiltration, and disruption of intestinal epithelial tight junctions in conventional mice (SPF) as compared to gnotobiotic mice (ASF). These findings suggest a “hepatoprotective role of intestinal microbiota” because it is also evidenced by Mazagova et al. (23) that in the absence of microbiota, GF mice showed more sever fibrosis.
ACKNOWLEDGMENTS
The authors thank Dr. Deborah Stroka and Felix Baier (Department for Biomedical Research, University of Berne), for help in total bile acids measurement, and the staff from the Clean Mouse Facility of the University of Bern for help during experiments with ASF mice. This work was supported by a grant from the Novartis Foundation for Medical-Biological Research (Project 10A19) and from the Swiss National Science Foundation (Grant 31003A_129842 to A.D.G.). The authors declare no conflicts of interest.
Glossary
- α-SMA
α-smooth muscle actin
- ASF
altered Schaedler flora
- BdA
blood agar
- BDL
bile duct ligation
- CCl4
carbon tetrachloride
- CFU
colony forming unit
- CLD
chronic liver disease
- Conv-R
conventionally raised
- ECM
extracellular matrix
- GF
germ free
- H&E
hematoxylin and eosin
- HSC
hepatic stellate cell
- IHC
immunohistochemical
- LB
Luria Bertani
- MLN
mesenteric lymph node
- Muc-1
mucin-1
- PDGF
platelet-derived growth factor
- PDGFR-β
platelet-derived growth factor receptor β
- PHT
portal hypertension
- PP
portal pressure
- PSS
portosystemic shunts
- SPF
specific pathogen free
- SPP1
secreted phosphoprotein-1
- TBA
total bile acid
- TLR
Toll-like receptor
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
AUTHOR CONTRIBUTIONS
S. Moghadamrad acquired data, analyzed and interpreted data, performed statistical analysis, and drafted the manuscript; M. Hassan provided technical support and critically revised the manuscript for important intellectual content; K. D. McCoy critically revised the manuscript for important intellectual content and supervised the study; J. Kirundi provided technical support and critically revised the manuscript for important intellectual content; P. Kellmann provided technical support and critically revised the manuscript for important intellectual content; and A. De Gottardi provided study concept and design, acquired data, analyzed and interpreted data, drafted the manuscript, provided statistical analysis, obtained funding, and supervised the study.
Supplementary Material
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Friedman S. L. (2008) Mechanisms of hepatic fibrogenesis. Gastroenterology 134, 1655–1669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bosch J., Groszmann R. J., Shah V. H. (2015) Evolution in the understanding of the pathophysiological basis of portal hypertension: how changes in paradigm are leading to successful new treatments. J. Hepatol. 62 (Suppl), S121–S130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albillos A., Lario M., Álvarez-Mon M. (2014) Cirrhosis-associated immune dysfunction: distinctive features and clinical relevance. J. Hepatol. 61, 1385–1396 [DOI] [PubMed] [Google Scholar]
- 4.Pinzone M. R., Celesia B. M., Di Rosa M., Cacopardo B., Nunnari G. (2012) Microbial translocation in chronic liver diseases. Int. J. Microbiol. 2012, 694629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Artis D. (2008) Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat. Rev. Immunol. 8, 411–420 [DOI] [PubMed] [Google Scholar]
- 6.Wiest R., Lawson M., Geuking M. (2014) Pathological bacterial translocation in liver cirrhosis. J. Hepatol. 60, 197–209 [DOI] [PubMed] [Google Scholar]
- 7.Lin R. S., Lee F. Y., Lee S. D., Tsai Y. T., Lin H. C., Lu R. H., Hsu W. C., Huang C. C., Wang S. S., Lo K. J. (1995) Endotoxemia in patients with chronic liver diseases: relationship to severity of liver diseases, presence of esophageal varices, and hyperdynamic circulation. J. Hepatol. 22, 165–172 [DOI] [PubMed] [Google Scholar]
- 8.Qin N., Yang F., Li A., Prifti E., Chen Y., Shao L., Guo J., Le Chatelier E., Yao J., Wu L., Zhou J., Ni S., Liu L., Pons N., Batto J. M., Kennedy S. P., Leonard P., Yuan C., Ding W., Chen Y., Hu X., Zheng B., Qian G., Xu W., Ehrlich S. D., Zheng S., Li L. (2014) Alterations of the human gut microbiome in liver cirrhosis. Nature 513, 59–64 [DOI] [PubMed] [Google Scholar]
- 9.Schnabl B., Brenner D. A. (2014) Interactions between the intestinal microbiome and liver diseases. Gastroenterology 146, 1513–1524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tilg H., Cani P. D., Mayer E. A. (2016) Gut microbiome and liver diseases. Gut 65, 2035–2044 [DOI] [PubMed] [Google Scholar]
- 11.Fouts D. E., Torralba M., Nelson K. E., Brenner D. A., Schnabl B. (2012) Bacterial translocation and changes in the intestinal microbiome in mouse models of liver disease. J. Hepatol. 56, 1283–1292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assimakopoulos S. F., Scopa C. D., Vagianos C. E. (2007) Pathophysiology of increased intestinal permeability in obstructive jaundice. World J. Gastroenterol. 13, 6458–6464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McAuley J. L., Linden S. K., Png C. W., King R. M., Pennington H. L., Gendler S. J., Florin T. H., Hill G. R., Korolik V., McGuckin M. A. (2007) MUC1 cell surface mucin is a critical element of the mucosal barrier to infection. J. Clin. Invest. 117, 2313–2324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McGuckin M. A., Every A. L., Skene C. D., Linden S. K., Chionh Y. T., Swierczak A., McAuley J., Harbour S., Kaparakis M., Ferrero R., Sutton P. (2007) Muc1 mucin limits both Helicobacter pylori colonization of the murine gastric mucosa and associated gastritis. Gastroenterology 133, 1210–1218 [DOI] [PubMed] [Google Scholar]
- 15.Seki E., Brenner D. A. (2008) Toll-like receptors and adaptor molecules in liver disease: update. Hepatology 48, 322–335 [DOI] [PubMed] [Google Scholar]
- 16.Seki E., De Minicis S., Osterreicher C. H., Kluwe J., Osawa Y., Brenner D. A., Schwabe R. F. (2007) TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nat. Med. 13, 1324–1332 [DOI] [PubMed] [Google Scholar]
- 17.Wang B., Trippler M., Pei R., Lu M., Broering R., Gerken G., Schlaak J. F. (2009) Toll-like receptor activated human and murine hepatic stellate cells are potent regulators of hepatitis C virus replication. J. Hepatol. 51, 1037–1045 [DOI] [PubMed] [Google Scholar]
- 18.Schon H. T., Weiskirchen R. (2014) Immunomodulatory effects of transforming growth factor-β in the liver. Hepatobiliary Surg. Nutr. 3, 386–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gujral J. S., Farhood A., Bajt M. L., Jaeschke H. (2003) Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology 38, 355–363 [DOI] [PubMed] [Google Scholar]
- 20.Yang M., Ramachandran A., Yan H. M., Woolbright B. L., Copple B. L., Fickert P., Trauner M., Jaeschke H. (2014) Osteopontin is an initial mediator of inflammation and liver injury during obstructive cholestasis after bile duct ligation in mice. Toxicol. Lett. 224, 186–195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fickert P., Thueringer A., Moustafa T., Silbert D., Gumhold J., Tsybrovskyy O., Lebofsky M., Jaeschke H., Denk H., Trauner M. (2010) The role of osteopontin and tumor necrosis factor alpha receptor-1 in xenobiotic-induced cholangitis and biliary fibrosis in mice. Lab. Invest. 90, 844–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xu R., Huang H., Zhang Z., Wang F. S. (2014) The role of neutrophils in the development of liver diseases. Cell. Mol. Immunol. 11, 224–231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mazagova M., Wang L., Anfora A. T., Wissmueller M., Lesley S. A., Miyamoto Y., Eckmann L., Dhungana S., Pathmasiri W., Sumner S., Westwater C., Brenner D. A., Schnabl B. (2015) Commensal microbiota is hepatoprotective and prevents liver fibrosis in mice. FASEB J. 29, 1043–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tabibian J. H., O’Hara S. P., Trussoni C. E., Tietz P. S., Splinter P. L., Mounajjed T., Hagey L. R., LaRusso N. F. (2016) Absence of the intestinal microbiota exacerbates hepatobiliary disease in a murine model of primary sclerosing cholangitis. Hepatology 63, 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schaedler R. W., Dubos R., Costello R. (1965) The development of the bacterial flora in the gastrointestinal tract of mice. J. Exp. Med. 122, 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Georgiev P., Jochum W., Heinrich S., Jang J. H., Nocito A., Dahm F., Clavien P. A. (2008) Characterization of time-related changes after experimental bile duct ligation. Br. J. Surg. 95, 646–656 [DOI] [PubMed] [Google Scholar]
- 27.Groszmann R. J., Vorobioff J., Riley E. (1982) Splanchnic hemodynamics in portal-hypertensive rats: measurement with gamma-labeled microspheres. Am. J. Physiol. 242, G156–G160 [DOI] [PubMed] [Google Scholar]
- 28.Rosshart S. P., Vassallo B. G., Angeletti D., Hutchinson D. S., Morgan A. P., Takeda K., Hickman H. D., McCulloch J. A., Badger J. H., Ajami N. J., Trinchieri G., Pardo-Manuel de Villena F., Yewdell J. W., Rehermann B. (2017) Wild mouse gut microbiota promotes host fitness and improves disease resistance. Cell 171, 1015–1028.e13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kawashima R., Mochida S., Matsui A., YouLuTuZ Y., Ishikawa K., Toshima K., Yamanobe F., Inao M., Ikeda H., Ohno A., Nagoshi S., Uede T., Fujiwara K. (1999) Expression of osteopontin in Kupffer cells and hepatic macrophages and stellate cells in rat liver after carbon tetrachloride intoxication: a possible factor for macrophage migration into hepatic necrotic areas. Biochem. Biophys. Res. Commun. 256, 527–531 [DOI] [PubMed] [Google Scholar]
- 30.Lorena D., Darby I. A., Gadeau A. P., Leen L. L., Rittling S., Porto L. C., Rosenbaum J., Desmoulière A. (2006) Osteopontin expression in normal and fibrotic liver. altered liver healing in osteopontin-deficient mice. J. Hepatol. 44, 383–390 [DOI] [PubMed] [Google Scholar]
- 31.Riva A., Mehta G. (2019) Regulation of monocyte-macrophage responses in cirrhosis-role of innate immune programming and checkpoint receptors. Front. Immunol. 10, 167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tacke F., Zimmermann H. W. (2014) Macrophage heterogeneity in liver injury and fibrosis. J. Hepatol. 60, 1090–1096 [DOI] [PubMed] [Google Scholar]
- 33.Sato A., Nakashima H., Nakashima M., Ikarashi M., Nishiyama K., Kinoshita M., Seki S. (2014) Involvement of the TNF and FasL produced by CD11b Kupffer cells/macrophages in CCl4-induced acute hepatic injury. PLoS One 9, e92515; erratum: e114065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith K., McCoy K. D., Macpherson A. J. (2007) Use of axenic animals in studying the adaptation of mammals to their commensal intestinal microbiota. Semin. Immunol. 19, 59–69 [DOI] [PubMed] [Google Scholar]
- 35.Macpherson A. J., Heikenwalder M., Ganal-Vonarburg S. C. (2016) The liver at the nexus of host-microbial interactions. Cell Host Microbe 20, 561–571 [DOI] [PubMed] [Google Scholar]
- 36.Li H., Limenitakis J. P., Fuhrer T., Geuking M. B., Lawson M. A., Wyss M., Brugiroux S., Keller I., Macpherson J. A., Rupp S., Stolp B., Stein J. V., Stecher B., Sauer U., McCoy K. D., Macpherson A. J. (2015) The outer mucus layer hosts a distinct intestinal microbial niche. Nat. Commun. 6, 8292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nishida A., Lau C. W., Zhang M., Andoh A., Shi H. N., Mizoguchi E., Mizoguchi A. (2012) The membrane-bound mucin Muc1 regulates T helper 17-cell responses and colitis in mice. Gastroenterology 142, 865–874.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wiest R., Albillos A., Trauner M., Bajaj J. S., Jalan R. (2017) Targeting the gut-liver axis in liver disease. J. Hepatol. 67, 1084–1103; erratum: 68, 1336 [DOI] [PubMed] [Google Scholar]
- 39.Schierwagen R., Alvarez-Silva C., Madsen M. S. A., Kolbe C. C., Meyer C., Thomas D., Uschner F. E., Magdaleno F., Jansen C., Pohlmann A., Praktiknjo M., Hischebeth G. T., Molitor E., Latz E., Lelouvier B., Trebicka J., Arumugam M. (2018) Circulating microbiome in blood of different circulatory compartments. [E-pub ahead of print] Gut [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.