This randomized clinical trial examines the efficacy of an immediate and sustained release oral ondansetron tablet in preventing vomiting for up to 24 hours in adolescents and adults with gastroenteritis.
Key Points
Question
Can a single bimodal immediate and sustained release oral ondansetron tablet prevent further vomiting for up to 24 hours among adolescents and adults with gastroenteritis-related emesis without intravenous hydration or rescue medication?
Findings
In this randomized clinical trial including 321 patients, the proportion of treatment success was 21% higher among patients who received bimodal release ondansetron than those who received the placebo, a statistically significant difference.
Meaning
These findings suggest that management of vomiting and dehydration from acute gastroenteritis without an intravenous line or rescue agents is attainable with an oral bimodal ondansetron tablet.
Abstract
Importance
Vomiting resulting from acute gastroenteritis is commonly treated with intravenous antiemetics in acute care settings. If oral treatment were beneficial, patients might not need intravenous administered hydration or medication. Furthermore, a long-acting treatment could provide sustained relief from nausea and vomiting.
Objective
To determine whether an experimental long-acting bimodal release ondansetron tablet decreases gastroenteritis-related vomiting and eliminates the need for intravenous therapy for 24 hours after administration.
Design, Setting, and Participants
This placebo-controlled, double-blind, randomized clinical trial included patients from 19 emergency departments and 2 urgent care centers in the United States from December 8, 2014, to February 17, 2017. Patients 12 years and older with at least 2 vomiting episodes from presumed gastroenteritis in the previous 4 hours and symptoms with less than 36 hours’ duration were randomized using a 3:2 active to placebo ratio. Analyses were performed on an intent-to-treat basis and conducted from June 1, 2017, to November 1, 2017.
Intervention
Bimodal release ondansetron tablet containing 6 mg of immediate release ondansetron and 18 mg of a 24-hour release matrix for a total of 24 mg of ondansetron.
Main Outcomes and Measures
Treatment success was defined as no further vomiting, no need for rescue medication, and no intravenous hydration for 24 hours after bimodal release ondansetron administration.
Results
Analysis included 321 patients (mean [SD] age, 29.0 [11.1] years; 195 [60.7%] women), with 192 patients in the bimodal release ondansetron group and 129 patients in the placebo group. Treatment successes were observed in 126 patients in the bimodal release ondansetron group (65.6%) compared with 70 patients in the placebo group (54.3%), with an 11.4% (95% CI, 0.3%-22.4%) absolute probability difference. The proportion of treatment success was 21% higher among patients who received bimodal release ondansetron compared with those who received a placebo (relative risk, 1.21; 95% CI, 1.00-1.46; P = .04). In an analysis including only patients with a discharge diagnosis of acute gastroenteritis and no major protocol violations, there were 123 treatment successes (69.5%) in the bimodal release ondansetron group compared with 67 treatment successes (54.9%) in the placebo group (relative risk, 1.27; 95% CI, 1.05-1.53; P = .01). Adverse effects were infrequent and similar to the known safety profile of ondansetron.
Conclusions and Relevance
This randomized clinical trial found that a long-acting bimodal release oral ondansetron tablet was an effective antiemetic among adolescents and adults with moderate to severe vomiting from acute gastroenteritis. The drug benefits extended to 24 hours after administration. Bimodal release ondansetron may decrease the need for intravenous access and emergency department care to manage acute gastroenteritis.
Trial Registration
ClinicalTrials.gov identifier: NCT02246439
Introduction
Acute gastroenteritis is a common illness, with approximately 179 million episodes occurring in the United States each year, resulting in 600 000 hospitalizations and 5000 deaths.1 Viruses are the most common identifiable cause,2 and disease occurs throughout the year, with outbreaks most frequent in the winter and spring.3 The disease is spread easily and clusters occur when people gather in close quarters or when food or water become contaminated with the virus.4,5,6
Nausea, vomiting, and diarrhea are typical symptoms of acute gastroenteritis, and other symptoms include fever, abdominal pain, and malaise.2 Dehydration is an important contributor to the morbidity associated with gastroenteritis, and fluid repletion is a mainstay of treatment. The illness is usually self-limited, and for most individuals, nausea and vomiting are the rate-limiting factors for successful fluid replacement. Patients unable to tolerate oral hydration may need intravenous fluids in the emergency department (ED) or other acute care settings.
Medications commonly used to treat nausea and vomiting in the acute care setting include ondansetron, metoclopramide, and prochlorperazine. Several pediatric studies, most involving children younger than 12 years, have demonstrated a reduction in the need for intravenous hydration and immediate hospitalization using oral or intravenous ondansetron to treat gastroenteritis-related emesis.7,8,9,10,11,12,13,14 In contrast, a Cochrane review15 indicated a paucity of studies evaluating adults treated in the ED and concluded there was no evidence to support the superiority of any antiemetic drug, oral or intravenous, over placebo in treating nausea and vomiting. Vomiting is a common complaint in the ED, and even without evidence-based guidance, these drugs are routinely given to adolescents and adults for a range of conditions, including acute gastroenteritis. Ondansetron is the most widely used antiemetic in the acute care setting, with over 21.7 million uses annually and is second only to sodium chloride as the most commonly prescribed drug in EDs in the United States.16
The bimodal release ondansetron tablet used in this study is an investigational 24 mg tablet that contains 6 mg of immediate release ondansetron and 18 mg of ondansetron in an extended release matrix. The drug was developed to provide therapeutic blood levels of ondansetron soon after administration and sustain therapeutic activity for up to 24 hours. Oral administration could potentially avoid the costs, discomfort, and resources associated with intravenous therapy and lead to fewer ED visits for acute gastroenteritis. Furthermore, this formulation could potentially avoid repeated dosing and reduce recurrent symptoms that occur with shorter acting agents.
In this multicenter randomized clinical trial (RCT), we tested the safety and efficacy of bimodal release ondansetron among adolescents and adults who presented to EDs or urgent care centers with nausea and vomiting due to acute gastroenteritis. The main goal of this study was to determine whether a bimodal release ondansetron tablet quickly relieves symptoms and prevents further vomiting for 24 hours without the need for rescue medication or intravenous fluid management.
Methods
Trial Design
This was a double-blind, placebo-controlled, parallel-group RCT evaluating the safety and efficacy of bimodal release ondansetron. The trial protocol and statistical analysis plan are presented in Supplement 1. The study was approved by the investigational review board for each participating institution. Written informed consent was obtained from participants 18 years and older, and study participants aged 12 to 17 years required both consent from a parent or guardian and assent from the participant. This study is reported following the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline.
Setting and Participants
Individuals 12 years and older who presented to 1 of 19 EDs or 2 urgent care centers in the United States with symptoms of acute gastroenteritis were screened for eligibility from December 8, 2014, to February 17, 2017. Those with 2 or more episodes of vomiting in the 4 hours prior to arrival and with duration of vomiting less than 36 hours were eligible for enrollment. Among the exclusion criteria were pregnancy, having recently undergone an abdominal surgical procedure, any history of bariatric surgical procedures or bowel obstruction, unhealthy alcohol use or illicit drug use, an electrocardiographic corrected QT interval greater than 450 ms on ED arrival, concomitant use of any drug known to prolong the QT interval, evidence of severe dehydration, or having serious medical comorbidities, including heart failure or severe renal or liver disease, or a presumed diagnosis other than acute gastroenteritis. Individuals with type 2 diabetes could be enrolled if they were not using insulin and their bedside glucose level at the time of enrollment was less than 200 mg/dL (to convert to millimoles per liter, multiply by 0.0555). Complete inclusion and exclusion criteria are presented in the eAppendix in Supplement 2.
Patients described current nausea using a 5-point Likert numeric rating scale that included written cues, with 0 indicating none (ie, no nausea) and 4 indicating the most severe symptoms (ie, nausea as bad as it can be). Initially, only patients 50 years and older were required to have data on baseline complete blood count, biochemical profile, and urinalysis results. A protocol amendment early in the study required all patients 40 years and older and those of any age with diabetes to have data from these baseline assessments. After approximately half of the required patients were enrolled, the protocol was modified at the request of the US Food and Drug Administration to include a baseline complete blood count and biochemical blood testing, including potassium and magnesium levels, and an electrocardiogram at baseline and 4 hours after dosing for all subjects.
Randomization and Study Procedures
A computer-generated allocation sequence was used for randomization, and a central call-in center directed stratification by age (<18 vs ≥18 years). The study drug was administered in a 3:2 active to placebo ratio. The experimental drug or a like-appearing placebo tablet was sealed in a box identified only by an assigned study number. After obtaining consent, the on-site clinical investigator contacted the central randomization call-in center and was directed to use the numbered sealed box corresponding to the randomized allocation. No information regarding the contents of the numbered sealed box was provided to the study investigator.
The allocated study tablet was administered before any ED intervention. If the tablet was vomited within 15 minutes of administration, another tablet was given. If vomiting first occurred between 15 and 30 minutes after administration of the first tablet, another tablet was not given and the patient was permitted to continue the study.
Oral ingestion of fluids was encouraged during the ED stay to treat dehydration according to clinical status and the patient’s ability to tolerate oral hydration. Additional testing, including imaging or laboratory tests, could be performed any time at the discretion of the treating clinician. Intravenous preparations of other medications, such as antibiotics, were permitted as long as the infusion volume was less than 100 mL. At 4 hours after study drug ingestion, the patient was assessed for discharge, although the duration of ED treatment could exceed 4 hours if needed. Patients who were ED treatment successes were discharged with 3 additional study tablets to take 1 every 24 hours as needed for up to 3 days.
Antipyretic, antidiarrheal, proton pump inhibitor, and antacid medications were permitted at any point during the study. For patients whose treatment failed, metoclopramide was the suggested default rescue medication. Ondansetron and any other 5-HT3 antagonist were not permitted for at least 24 hours after study drug administration.
At discharge, patients were given diary cards to record any episodes of vomiting, bowel movements, and severity of nausea, as well as other symptoms of gastroenteritis, adverse events, and any medications taken, whether for gastroenteritis or other indications. Patients were contacted daily by telephone to retrieve the relevant information.
Primary End Point Definition
The primary end point was treatment success, defined as meeting all 3 of the following criteria from 30 minutes through 24 hours after the first dose of study medication: (1) without further vomiting, (2) without rescue medication for nausea or vomiting, and (3) no intravenous hydration.
Outcomes
The primary outcome was the frequency of treatment success at 24 hours after study tablet administration. The study population included all patients who received a tablet, even if the tablet was vomited soon after administration. Patients randomized but not treated because they were found ineligible before receiving the tablet (eg, because of positive results on a pregnancy test) were excluded from the study analysis. Therefore, the primary efficacy analysis was a modified intention to treat rather than a strict intention-to-treat analysis. Per-protocol secondary analyses determined treatment success among patients with a final discharge of acute gastroenteritis and no major inclusion violations. An additional efficacy outcome was the frequency of treatment success at 4 days. The safety population was defined as any patient given the study medication, including those who were unable to swallow or vomited soon after medication administration.
Statistical Analysis
It was estimated that treatment would fail for 40% of the placebo group during the 24 hours after initial dosing and that bimodal release ondansetron would reduce the frequency of treatment failure by approximately 50%, ie, to a rate of 20% in the active treatment group. To demonstrate a statistically significant decrease in the incidence of vomiting with a 2-tailed P value of .01 and power of 90%, 320 patients were needed, with plans for 192 patients randomized to the active drug and 128 patients randomized to placebo.
The primary result was expressed as the relative risk (RR) adjusted for age using the Mantel-Haenszel method. The P value was calculated using the Cochran-Mantel-Haenszel test. Patients who dropped out of the study before 24 hours were imputed as treatment failures. The responder analysis through 4 days reported statistics that mirrored the primary end point analysis. Data analyses were conducted using SAS statistical software version 9.4 (SAS Institute). For all analyses, a 2-tailed P value of less than .05 was considered statistically significant. Data were analyzed from June 1, 2017, to November 1, 2017.
Results
Demographic and Clinical Characteristics
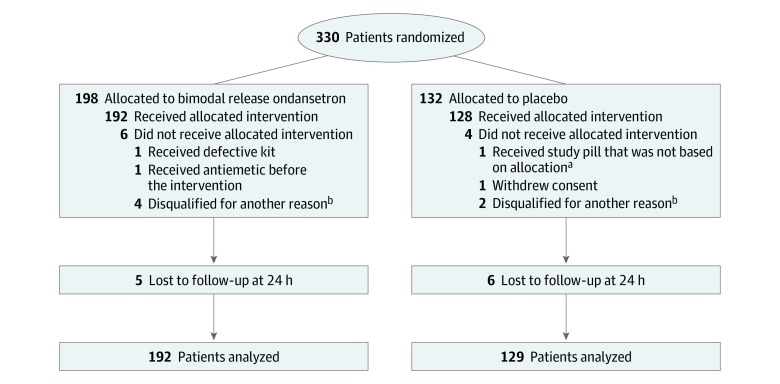
A total of 330 patients were randomized, and 321 were included in the final analysis (mean [SD] age, 29.0 [11.1] years; 195 [60.7%] women), with 192 patients randomized to the bimodal release ondansetron group and 129 patients randomized to the placebo group (Figure). Twenty-six patients (8%) were younger than 18 years. The bimodal release ondansetron and placebo groups appeared similar for age and sex at baseline (Table 1). Most patients had severe nausea at the time of drug administration, although the bimodal release ondansetron group had worse nausea at the time of randomization than the placebo group with 58 patients (30.2%) in the bimodal release ondansetron group and 27 patients (20.9%) in the placebo group reporting nausea was as bad as it could be. Other clinical variables were similar among the 2 treatment groups. All but 7 patients (5 in the bimodal release ondansetron group, 2 in the placebo group) had a final diagnosis of acute gastroenteritis.
Figure. CONSORT Flow Diagram.
aA patient was assigned to receive placebo but erroneously received an unassigned study box that turned out to contain the active drug bimodal release ondansetron. All study boxes and content were of the same appearance and the blinding remained intact. Per intent-to-treat procedure, the data were analyzed by the assigned intervention (placebo group).
bDisqualifying information obtained after consent but before administration of the study drug included pregnancy, other abnormal blood test results, or abnormal electrocardiogram results.
Table 1. Patient Demographic Characteristics.
| Characteristics | Patients, No. (%) | |
|---|---|---|
| Bimodal Release Ondansetron Group (n = 192) | Placebo Group (n = 129) | |
| Age, y | ||
| Mean (SD) | 29.5 (11.9) | 28.4 (9.7) |
| <18 | 15 (7.8) | 11 (8.5) |
| ≥18 | 177 (92.2) | 118 (91.5) |
| Women | 122 (63.5) | 73 (56.6) |
| Weight, mean (SD), kg | 79.7 (21.9) | 76.0 (20.6) |
| Baseline nausea levela | ||
| None or mild | 26 (13.5) | 18 (14.0) |
| Moderate | 38 (19.8) | 40 (31.0) |
| Severe | 70 (36.5) | 44 (34.1) |
| Bad as it can be | 58 (30.2) | 27 (20.9) |
| Final clinical diagnosis gastroenteritis | 187 (97.4) | 127 (98.4) |
| History of type 2 diabetes | 4 (2.1) | 5 (3.9) |
| Abnormal serum level, No./total No. (%)b | ||
| Potassium | 12/101 (11.9) | 2/61 (3.3) |
| Sodium | 5/102 (4.9) | 2/61 (3.3) |
| Magnesium | 7/78 (9.0) | 5/52 (9.6) |
| Creatinine | 19/101 (18.8) | 7/61 (11.5) |
Assessed using a 5-point Likert numeric rating scale with 0 indicating none (ie, no nausea) and 4 indicating nausea as bad as it can be.
The abnormal designation is based on the laboratory parameters from each site and may represent an abnormally low or high value.
Primary Outcome
In the modified intention-to-treat analysis, a total of 126 treatment successes (65.6%) were observed in the bimodal release ondansetron group compared with 70 treatment successes (54.3%) in the placebo group. The absolute probability difference for treatment success between the 2 groups was 11.4% (95% CI, 0.3%-22.4%). The proportion of treatment success was 21% higher among patients who received bimodal release ondansetron compared with those who received the placebo (RR, 1.21; 95% CI, 1.00-1.46; P = .04) (Table 2).
Table 2. Treatment Success at 24 Hours.
| Analysis | Treatment Successes, No./Total No. (%)a | Relative Risk (95% CI) | P Value | Absolute Difference in Success Probability, % (95% CI) | |
|---|---|---|---|---|---|
| Bimodal Release Ondansetron | Placebo | ||||
| Modified intent to treat (n = 321)b | 126/192 (65.6) | 70/129 (54.3) | 1.21 (1.00-1.46) | .04 | 11.4 (0.3-22.4) |
| Per-protocol (n = 299)c | 123/177 (69.5) | 67/122 (54.9) | 1.27 (1.05-1.53) | .01 | 14.6 (3.0-25.8) |
Treatment success was defined as no further vomiting and no need for rescue medication or intravenous hydration from 30 minutes through 24 hours after the first dose of study medication.
Primary study analysis.
Determined treatment success among patients with a final discharge of acute gastroenteritis and no major inclusion violations.
Secondary Outcomes
In the per-protocol analysis, a total of 123 treatment successes (69.5%) were observed in the bimodal release ondansetron group compared with 67 treatment successes (54.9%) in the placebo group, and the absolute probability difference for treatment success was 14.6% (95% CI, 3.0%-25.8%). The proportion of treatment success was 27% higher for patients who received bimodal release ondansetron compared with those who received the placebo (RR, 1.27; 95% CI, 1.05-1.53; P = .01) (Table 2).
Fifteen patients required repeated dosing because of vomiting in the first 15 minutes after tablet ingestion, including 8 patients (4.2%) in the bimodal release ondansetron group and 7 patients (5.4%) in the placebo group. Among these, 3 patients in the bimodal release ondansetron group were treatment successes and 1 patient in the placebo group was a treatment success.
Patients for whom treatment did not fail were given 3 additional tablets on discharge and directed to take 1 as needed every 24 hours. Most patients did not take additional doses after leaving the ED or urgent care center, with 20 of 196 discharged patients (10.2%) taking all 3 additional doses over the subsequent 3 days. Four days after initial ED discharge, treatment was successful for 114 patients (59.4%) in the bimodal release ondansetron group and 67 patients (51.9%) in the placebo group (RR, 1.14; 95% CI, 0.93-1.40; P = .19). A total of 4 patients (2.1%) in the bimodal release ondansetron group and 4 patients (3.1%) in the placebo treatment group returned to the ED for gastrointestinal symptoms within 4 days of initial dosing.
Fourteen patients were hospitalized during the study. Five patients (4 patients in the bimodal release ondansetron group and 1 patient in the placebo group) were hospitalized because of treatment failure. The remaining patients were hospitalized for reasons other than gastroenteritis. These hospitalizations were due to conditions not diagnosed until after the patient received study medication, including cholecystitis, bile duct stones, and small-bowel obstruction.
One or more episodes of diarrhea within 24 hours after the first dose of the study drug occurred in 35 patients (16.7%) in the bimodal release ondansetron group and 28 patients (21.7%) in the placebo group. Among patients with one or more episodes of diarrhea at 24 hours, the mean (SD) number was 2.2 (1.9) episodes in the bimodal release ondansetron group and 1.6 (1.1) episodes for the placebo group. Adverse events are presented in Table 3. Patients in the bimodal release ondansetron group, compared with the placebo group, more commonly reported constipation (9 patients [4.7%] vs 1 patients [0.8%]) and headache (22 patients [11.4%] vs 7 patients [5.5%]), as expected from the known adverse effect profile of bimodal release ondansetron.
Table 3. Treatment Emergent Adverse Events With Frequency of at Least 1%.
| System Involved | Patients, No. (%) | |
|---|---|---|
| Bimodal Release Ondansetron Group (n = 193) | Placebo Group (n = 128) | |
| ≥1 Treatment emergent adverse events | 66 (34.2) | 36 (28.1) |
| Abdominal | ||
| Distension | 3 (1.6) | 1 (0.8) |
| Pain | 3 (1.6) | 1 (0.8) |
| Constipation | 9 (4.7) | 1 (0.8) |
| Pyrexia | 3 (1.6) | 5 (3.9) |
| Chest pain | 2 (1.0) | 2 (1.6) |
| Dizziness | 2 (1.0) | 2 (1.6) |
| Headache | 22 (11.4) | 7 (5.5) |
Discussion
This RCT found showed that a single long-acting bimodal release ondansetron tablet prevented further vomiting for 24 hours after tablet ingestion among adolescents and adults presenting with symptoms of acute gastroenteritis. At study entry, all participants had vomited 2 or more times in the 4 hours prior to enrollment and most had moderate to very severe nausea on ED or urgent care arrival. Even with these acute symptoms, almost all patients tolerated the oral preparation, with fewer than 5% vomiting the medication within 15 minutes of ingestion. This demonstrates that oral antiemetic treatment is feasible for a substantial portion of symptomatic patients with acute gastroenteritis.
Ondansetron administration to treat vomiting is common practice in the acute care setting, although there are no published studies supporting this off-label use for gastroenteritis among adolescents and adults, to our knowledge. Of the few published clinical trials evaluating the efficacy of any antiemetic for undifferentiated vomiting compared with a placebo among an adult ED population, a study by Braudo et al17 found intravenous droperidol to decrease nausea at 30 minutes, while studies by Egerton-Warburton et al18 and Barrett et al19 found that ondansetron, metoclopramide and prochlorperazine were not effective. Findings in these trials were limited in that nausea, the primary study outcome, was not assessed beyond 30 minutes. A Cochrane review in 201515 concluded that among adults there is no definite evidence to support the superiority of any antiemetic drug over placebo in the acute care setting.
To our knowledge, this is the first study to demonstrate that ondansetron is beneficial to treat vomiting in the adult and adolescent acute care population and the first to specifically evaluate acute gastroenteritis. Treatment with an oral agent has the advantage of avoiding intravenous drug administration and fluid replacement. A bimodal release antiemetic tablet has the dual benefit of rapid onset of efficacy and prolonged duration of activity compared with currently available oral preparations. The sustained therapeutic action of bimodal release ondansetron contrasts with shorter acting preparations whose effect may wear off before another dose is taken, risking early recurrence of nausea or vomiting.
Compared with shorter acting preparations, time-release formulations have the advantages of dosing convenience, less fluctuation in blood levels, and better health outcomes across a number of disease states.20,21 Although not studied in gastroenteritis to our knowledge, simplification of dosing regimens and reduction in tablet burden with a once daily administration has been shown to improve adherence compared with conventional formulations with short half-lives that must be given 3 or more times per day.22,23 Among the potential disadvantages of a longer acting preparation is the reduced potential for dosage adjustment.21 In our study, metoclopramide was given to those who failed study treatment, and additional study would be needed to determine optimal rescue strategies for those who do not respond to bimodal release ondansetron.
Similar to other studies evaluating antiemetics in adults who are acutely ill, we found a relatively high rate of successful outcomes in patients who received the placebo.17,18,19 Among the possible explanations are that some patients were enrolled toward the end of the active symptom period for a disease with a relatively short duration, contributing to a higher natural progression of improvement. Another explanation is an enhanced response among patients taking the inactive tablet leading to a larger than expected success rate. While an RCT is the standard for determining the efficacy of an intervention, the placebo effect and other influences in an RCT are difficult to estimate, and the assumption that a drug effect is simply the difference between the drug and placebo responses has been challenged.24,25,26 This can result in underestimated clinical trial drug effects, particularly in studies with large placebo responses.27
Another possible explanation for the placebo group response is that the protocol encouraged an oral hydration regimen soon after receiving the tablet. Past ED clinical trials suggest that intravenous hydration reduces nausea and potentially masks antiemetic effects of the study drugs.15,17,19 The prevention or treatment of dehydration has reduced nausea and vomiting in other conditions, such as postoperative nausea and vomiting syndrome.28,29,30 Our study was not designed to determine whether oral rehydration independently improves outcomes, which may have contributed to the higher than expected success rates in the placebo group, and this requires further research.
In practice, giving oral rather than intravenous therapy in the acute care setting could result in more rapid treatment and disposition using lower acuity treatment areas in which fewer personnel and resources would be needed. Treatment with bimodal release ondansetron presents other outpatient treatment options, including urgent care centers or office and clinic settings, with ED visits reserved for those who fail treatment. It may be feasible for treatment to be prescribed outside of a traditional clinical setting, such as by telephone consultation or in a telemedicine setting. Further study would be needed to confirm the safety and efficacy of providing antiemesis care in alternative treatment strategies.
Limitations
This study had limitations, including the relatively few adolescent patients enrolled; therefore, the comparison of treatment response among age subgroups was not feasible. Furthermore, most of the pediatric patients were enrolled at 1 site, which limits generalizability of the age-related results. Another limitation was the lack of confirmatory testing of the diagnosis of acute gastroenteritis. Stool sample diagnostics may not detect any pathogens, and results would not have been immediately available in the ED setting at the time of enrollment. Additionally, almost all patients had what clinicians determined to be acute gastroenteritis. Our inclusion criteria mimicked actual practice; therefore, results should be generalizable to patients treated in the acute care setting with similar symptoms.
Conclusions
In conclusion, this RCT found that a long-acting bimodal release oral ondansetron tablet was effective in treating nausea and vomiting from acute gastroenteritis. The drug appeared to work quickly, and benefits from the long acting component extended to 24 hours after tablet administration. If further studies find similar results, this drug may decrease the need for intravenous access and ED visits to manage acute gastroenteritis.
Trial Protocol
eAppendix. Trial Inclusion Criteria
Data Sharing Statement
References
- 1.Hall AJ, Rosenthal M, Gregoricus N, et al. Incidence of acute gastroenteritis and role of norovirus, Georgia, USA, 2004-2005. Emerg Infect Dis. 2011;17(8):-. doi: 10.3201/eid1708.101533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bresee JS, Marcus R, Venezia RA, et al. ; US Acute Gastroenteritis Etiology Study Team . The etiology of severe acute gastroenteritis among adults visiting emergency departments in the United States. J Infect Dis. 2012;205(9):1374-1381. doi: 10.1093/infdis/jis206 [DOI] [PubMed] [Google Scholar]
- 3.Mounts AW, Ando T, Koopmans M, Bresee JS, Noel J, Glass RI. Cold weather seasonality of gastroenteritis associated with Norwalk-like viruses. J Infect Dis. 2000;181(suppl 2):S284-S287. doi: 10.1086/315586 [DOI] [PubMed] [Google Scholar]
- 4.Kaplan JE, Feldman R, Campbell DS, Lookabaugh C, Gary GW. The frequency of a Norwalk-like pattern of illness in outbreaks of acute gastroenteritis. Am J Public Health. 1982;72(12):1329-1332. doi: 10.2105/AJPH.72.12.1329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fankhauser RL, Monroe SS, Noel JS, et al. Epidemiologic and molecular trends of “Norwalk-like viruses” associated with outbreaks of gastroenteritis in the United States. J Infect Dis. 2002;186(1):1-7. doi: 10.1086/341085 [DOI] [PubMed] [Google Scholar]
- 6.Kim SH, Cheon DS, Kim JH, et al. Outbreaks of gastroenteritis that occurred during school excursions in Korea were associated with several waterborne strains of norovirus. J Clin Microbiol. 2005;43(9):4836-4839. doi: 10.1128/JCM.43.9.4836-4839.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marchetti F, Bonati M, Maestro A, et al. ; SONDO (Study ONdansetron vs DOmperidone) Investigators . Oral Ondansetron versus Domperidone for acute gastroenteritis in pediatric emergency departments: multicenter double blind randomized controlled trial. PLoS One. 2016;11(11):e0165441. doi: 10.1371/journal.pone.0165441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stork CM, Brown KM, Reilly TH, Secreti L, Brown LH. Emergency department treatment of viral gastritis using intravenous ondansetron or dexamethasone in children. Acad Emerg Med. 2006;13(10):1027-1033. doi: 10.1197/j.aem.2006.05.018 [DOI] [PubMed] [Google Scholar]
- 9.Rerksuppaphol S, Rerksuppaphol L. Efficacy of intravenous ondansetron to prevent vomiting episodes in acute gastroenteritis: a randomized, double blind, and controlled trial. Pediatr Rep. 2010;2(2):e17. doi: 10.4081/pr.2010.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reeves JJ, Shannon MW, Fleisher GR. Ondansetron decreases vomiting associated with acute gastroenteritis: a randomized, controlled trial. Pediatrics. 2002;109(4):e62. doi: 10.1542/peds.109.4.e62 [DOI] [PubMed] [Google Scholar]
- 11.Ramsook C, Sahagun-Carreon I, Kozinetz CA, Moro-Sutherland D. A randomized clinical trial comparing oral ondansetron with placebo in children with vomiting from acute gastroenteritis. Ann Emerg Med. 2002;39(4):397-403. doi: 10.1067/mem.2002.122706 [DOI] [PubMed] [Google Scholar]
- 12.Roslund G, Hepps TS, McQuillen KK. The role of oral ondansetron in children with vomiting as a result of acute gastritis/gastroenteritis who have failed oral rehydration therapy: a randomized controlled trial. Ann Emerg Med. 2008;52(1):22-29.e6. doi: 10.1016/j.annemergmed.2007.09.010 [DOI] [PubMed] [Google Scholar]
- 13.Freedman SB, Adler M, Seshadri R, Powell EC. Oral ondansetron for gastroenteritis in a pediatric emergency department. N Engl J Med. 2006;354(16):1698-1705. doi: 10.1056/NEJMoa055119 [DOI] [PubMed] [Google Scholar]
- 14.Fedorowicz Z, Jagannath VA, Carter B. Antiemetics for reducing vomiting related to acute gastroenteritis in children and adolescents. Cochrane Database Syst Rev. 2011;7(9):CD005506. doi: 10.1002/14651858.CD005506.pub5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Furyk JS, Meek RA, Egerton-Warburton D. Drugs for the treatment of nausea and vomiting in adults in the emergency department setting. Cochrane Database Syst Rev. 2015;28(9):CD010106. doi: 10.1002/14651858.CD010106.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rui P, Kang K. National Hospital Ambulatory Medical Care Survey: 2015 emergency department summary tables. https://www.cdc.gov/nchs/data/nhamcs/web_tables/2015_ed_web_tables.pdf. Accessed September 30, 2019.
- 17.Braude D, Soliz T, Crandall C, Hendey G, Andrews J, Weichenthal L. Antiemetics in the ED: a randomized controlled trial comparing 3 common agents. Am J Emerg Med. 2006;24(2):177-182. doi: 10.1016/j.ajem.2005.08.017 [DOI] [PubMed] [Google Scholar]
- 18.Egerton-Warburton D, Meek R, Mee MJ, Braitberg G. Antiemetic use for nausea and vomiting in adult emergency department patients: randomized controlled trial comparing ondansetron, metoclopramide, and placebo. Ann Emerg Med. 2014;64(5):526-532.e1. doi: 10.1016/j.annemergmed.2014.03.017 [DOI] [PubMed] [Google Scholar]
- 19.Barrett TW, DiPersio DM, Jenkins CA, et al. A randomized, placebo-controlled trial of ondansetron, metoclopramide, and promethazine in adults. Am J Emerg Med. 2011;29(3):247-255. doi: 10.1016/j.ajem.2009.09.028 [DOI] [PubMed] [Google Scholar]
- 20.Richter A, Anton SF, Koch P, Dennett SL. The impact of reducing dose frequency on health outcomes. Clin Ther. 2003;25(8):2307-2335. doi: 10.1016/S0149-2918(03)80222-9 [DOI] [PubMed] [Google Scholar]
- 21.Jayanathi B, Manna PK, Madhusudhan S, Mohanta GP, Manavalan R. Per oral extended release products: an overview. J Appl Pharm Sci. 2011;1(2):50-55. [Google Scholar]
- 22.Falagas ME, Karagiannis AKA, Nakouti T, Tansarli GS. Compliance with once-daily versus twice or thrice-daily administration of antibiotic regimens: a meta-analysis of randomized controlled trials. PLoS One. 2015;10(1):e0116207. doi: 10.1371/journal.pone.0116207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Claxton AJ, Cramer J, Pierce C. A systematic review of the associations between dose regimens and medication compliance. Clin Ther. 2001;23(8):1296-1310. doi: 10.1016/S0149-2918(01)80109-0 [DOI] [PubMed] [Google Scholar]
- 24.Enck P, Klosterhalfen S, Weimer K, Horing B, Zipfel S. The placebo response in clinical trials: more questions than answers. Philos Trans R Soc Lond B Biol Sci. 2011;366(1572):1889-1895. doi: 10.1098/rstb.2010.0384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dutile S, Kaptchuk TJ, Wechsler ME. The placebo effect in asthma. Curr Allergy Asthma Rep. 2014;14(8):456. doi: 10.1007/s11882-014-0456-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Finniss DG, Kaptchuk TJ, Miller F, Benedetti F. Biological, clinical, and ethical advances of placebo effects. Lancet. 2010;375:686-695. doi: 10.1016/S0140-6736(09)61706-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lund K, Vase L, Petersen GL, Jensen TS, Finnerup NB. Randomised controlled trials may underestimate drug effects: balanced placebo trial design. PLoS One. 2014;9(1):e84104. doi: 10.1371/journal.pone.0084104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashok V, Bala I, Bharti N, Jain D, Samujh R. Effects of intraoperative liberal fluid therapy on postoperative nausea and vomiting in children-A randomized controlled trial. Paediatr Anaesth. 2017;27(8):810-815. doi: 10.1111/pan.13179 [DOI] [PubMed] [Google Scholar]
- 29.Voldby AW, Brandstrup B. Fluid therapy in the perioperative setting-a clinical review. J Intensive Care. 2016;4:27. doi: 10.1186/s40560-016-0154-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Magner JJ, McCaul C, Carton E, Gardiner J, Buggy D. Effect of intraoperative intravenous crystalloid infusion on postoperative nausea and vomiting after gynaecological laparoscopy: comparison of 30 and 10 ml kg(-1). Br J Anaesth. 2004;93(3):381-385. doi: 10.1093/bja/aeh219 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix. Trial Inclusion Criteria
Data Sharing Statement