This cohort study examines patterns of individual pain trajectories after total knee arthroplasty (TKA) and assesses their independent associations with longer-term pain outcome after surgery.
Key Points
Question
Is knee pain recovery different between patient subgroups in the early postoperative period after total knee arthroplasty (TKA), and is it associated with intermediate-term TKA pain?
Findings
In this cohort study of 659 patients who underwent primary TKA, 2 pain trajectories (fast pain response and slow pain response) were evident at 8 weeks after the procedure. These trajectories were independently associated with clinically significant differences in pain outcome at 6 months between the 2 pain trajectories.
Meaning
Early identification of patients in the slow pain response trajectory at 8 weeks after TKA may offer an opportunity for interventions in the perioperative period to potentially improve the intermediate-term and long-term pain outcomes after TKA.
Abstract
Importance
Studies to date have not comprehensively examined pain experience after total knee arthroplasty (TKA). Discrete patterns of pain in this period might be associated with pain outcomes at 6 to 12 months after TKA.
Objectives
To examine patterns of individual post-TKA pain trajectories and to assess their independent associations with longer-term pain outcome after TKA.
Design, Setting, and Participants
This prospective cohort study combined data from a national US TKA cohort with ancillary pain severity data at 2 weeks and 8 weeks after the index TKA using a numeric rating scale. All participants received primary, unilateral TKA within the Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR) national network of community sites in 22 states or at the lead site (University of Massachusetts Medical School). Participants had a date of surgery between May 1, 2013, and December 1, 2014. The data analysis was performed between January 13, 2015, and July 5, 2016.
Exposures
Pain trajectories in the postoperative period (8 weeks).
Main Outcomes and Measures
Index knee pain at 6 months after TKA using the Knee Injury and Osteoarthritis Outcome Score (KOOS) pain scale. Group-based trajectory methods examined the presence of pain trajectories in the postoperative period (8 weeks) and assessed whether trajectories were independently associated with longer-term pain (6 months).
Results
The cohort included 659 patients who underwent primary TKA with complete data at 4 points (preoperative, 2 weeks, 8 weeks, and 26 weeks). Their mean (SD) age was 67.1 (8.0) years, 64.5% (425 of 659) were female, the mean (SD) body mass index (calculated as weight in kilograms divided by height in meters squared) was 30.77 (5.66), 94.5% (613 of 649) were white, and the mean (SD) preoperative 36-Item Short Form Health Survey physical component summary and mental component summary scores were 34.1 (8.2) and 53.8 (11.4), respectively. Two pain trajectory subgroups were identified at 8 weeks after TKA: patients who experienced fast pain relief in the first 8 weeks after TKA (fast pain responders, composing 72.4% [477 of 659] of the sample) and patients who did not (slow pain responders, composing 27.6% [182 of 659] of the sample). After adjusting for patient factors, the pain trajectory at 8 weeks after TKA was independently associated with the mean KOOS pain score at 6 months, with a between-trajectory difference of −11.3 (95% CI, −13.9 to −8.7).
Conclusions and Relevance
The trajectory among slow pain responders at 8 weeks after surgery was independently associated with improved but greater persistent index knee pain at 6 months after TKA compared with that among fast pain responders. Early identification of patients with a trajectory of slow pain response at 8 weeks after TKA may offer an opportunity for interventions in the perioperative period to potentially improve the long-term pain outcomes after TKA.
Introduction
Postoperative pain is commonly underestimated and undertreated.1,2,3 Undertreated postoperative pain not only leads to distress and poor patient satisfaction but also is associated with longer hospital stay, reluctance to engage in rehabilitation exercises, poorer health-related quality of life, and increased morbidity related to complications.4,5,6,7,8 Untreated postoperative pain is associated with persistent chronic pain after cardiac surgery.6,9
Total knee arthroplasty (TKA) is one of the most common elective surgical procedures performed in older patients to treat pain and functional limitation owing to refractory knee arthritis10 and is associated with optimal arthritis pain relief in most but not all patients. In the United States, 8% to 15% of patients who undergo TKA have residual moderate to severe index joint pain that persists 2 to 5 years after the procedure.11,12 A systematic review concluded that unfavorable long-term pain outcomes were seen in 10% to 34% of patients after knee arthroplasty.13 Few studies have examined intermediate-term postoperative pain and its association with long-term pain outcomes. In a single-center study14 of 116 patients, pain during the 1-year postoperative period generally improved, and 1-year pain status was associated with long-term pain outcomes. In 2 studies, researchers used preoperative and postoperative pain and function scores to assess pain outcome at 5 years after TKA15 or used preoperative scores to assess pain outcome at 3 months and 12 months after TKA16; these studies focused on preoperative pain and did not collect postoperative pain data (within 2 to 3 months of TKA).15,16 In addition, research to date is limited by single-center enrollment, small sample sizes with high dropout rates, and the lack of adjustment for common confounders (eg, race/ethnicity, body mass index [BMI; calculated as weight in kilograms divided by height in meters squared], and comorbidities).
To our knowledge, studies to date have not comprehensively examined pain experience in the post-TKA period or evaluated for the presence of discrete subgroups of individuals with different patterns of pain at 6 to 12 months after TKA. This factor is an important knowledge gap given (1) the co-occurrence of postoperative pain during post-TKA physical rehabilitation,17,18 (2) the treatment of early postoperative pain with opioids19,20,21 and the opioid epidemic in the United States,22,23,24,25 and (3) the significant prevalence of persistent pain after TKA.11,12,13 If early patterns of pain can be used to identify patients likely to experience prolonged pain, clinical treatment options could be developed and tailored to alter this outcome. The emerging tools for trajectory analysis shown to be associated with different care patterns and health care expenditures in patients with cancer26 offer the potential to provide new insights into patients’ pain experience and outcomes after TKA.
Our study objective was to examine whether discrete subgroups of patients undergoing TKA have different trajectories of pain in the period (8 weeks) after TKA in a nationally representative sample. We evaluated (1) whether the postoperative period pain trajectory is associated with the level of 6-month postarthroplasty pain outcome and (2) the patient characteristics associated with postarthroplasty pain trajectories.
Methods
Study Cohort and Survey
This research was an ancillary study of the Function and Outcomes Research for Comparative Effectiveness in Total Joint Replacement (FORCE-TJR) study,27 a nationally representative observational prospective cohort study that enrolled 28306 patients undergoing knee or hip arthroplasty (total or partial knee arthroplasty, total hip replacement, hip resurfacing, and knee or hip revision arthroplasty) across the United States, including more than 130 surgeons in academic and private centers. The FORCE-TJR study enrolled cases from April 11, 2011, to December 31, 2016. The study was approved by The University of Alabama at Birmingham and University of Massachusetts institutional review boards. Patient written informed consent was obtained before study enrollment and before any study activities were conducted. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
The FORCE-TJR cohort was based on total knee replacement surgical procedures referred by a stratified sample of surgeons from across the United States; 75% of surgeons were from community-based practices. Operating room schedulers at each site referred patients scheduled for primary, unilateral TKA to the University of Massachusetts Medical School (UMMS) data coordinating center for enrollment each day. A centralized UMMS research coordinator reviewed participation by telephone with all patients. Almost 95% (12 585 of 13 283) of TKA patients who returned the signed informed consent form completed the preoperative assessment surveys, including demographics, preoperative pain and function (36-Item Short Form Health Survey [SF-36] and Knee Injury and Osteoarthritis Outcome Score [KOOS] pain scale), and medical and musculoskeletal risk factors according to the FORCE-TJR protocol. Specific enrollment and measures have been described previously.27,28,29 All FORCE-TJR participants repeated these measures at 6 months after total joint replacement. In addition to the comprehensive preoperative and outcome data collected by FORCE-TJR, patients who enrolled in the FORCE-TJR substudy during the ancillary study period at a subset of sites completed a 2-week and 8-week assessment of pain severity and pain treatment strategies.
All patients receiving TKA within the FORCE-TJR national network of community sites in 22 states or at the lead site (UMMS) were invited to enroll in the ancillary pain study, as well as the parent study.27 Exposures were pain trajectories in the postoperative period (8 weeks). For the ancillary pain study from 24 practice sites, patients who underwent primary, unilateral TKA also completed a pain assessment at 2 weeks and 8 weeks after the index TKA using a numeric rating scale (range, 0-10; higher scores indicate worse pain). Of the 12 585 FORCE-TJR study participants, 2209 (17.6%) of those undergoing primary total knee replacement were enrolled from May 12, 2011, to March 4, 2015. The dates of surgery were between May 1, 2013, and December 1, 2014. The dates of analysis were January 13, 2015, to July 5, 2016. Pain study sites included all community-based orthopedics offices covered by the University of Massachusetts institutional review board. The FORCE-TJR patients agreed to complete baseline, 6-month, and 12-month surveys, and the FORCE-TJR study staff supported collection of these data. Ancillary study resources supported a dedicated study coordinator to perform pain assessments at 2 weeks and 8 weeks for a subsample enrolled into the pain study. The consent forms were given to the patients at a preoperative visit, and surgeons requested each patient to participate in the registry. Next, coordinators called patients within 3 days to review the consent form to confirm participation. By reviewing operating room records, we verified that 79.4% (13 283 of 16 721) of people with TKA invited to join this ancillary study of the FORCE-TJR registry participated in the study. Patients received a reminder telephone call if the 2-week or 8-week survey was not returned within 2 weeks of the target date. Patients or other members of the public were not involved in the development of the study protocol.
Pain Assessment Components
The 2-week and 8-week pain surveys contained the validated Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R),30 which includes pain severity (0-10 numeric rating scale; higher scores indicate worse pain),31 consequences of pain, common adverse effects of narcotics, and nonpharmacological strategies for managing postoperative pain. The pain numeric rating scale31 is valid, reliable, and feasible and has been used in multiple musculoskeletal conditions.32,33,34,35,36 We used the preoperative pain assessment along with 2-week and 8-week post-TKA pain severity to derive pain trajectories (detailed in the Statistical Analysis subsection).
Long-term Pain Outcomes and Covariates
Early post-TKA pain trajectories (3 assessments, as well as preoperative pain severity) were the primary variables of interest. The main study outcome was index knee pain at 6 months after TKA as assessed with the KOOS pain scale as a continuous variable.37,38 The KOOS39 is a 42-item, participant-administered, validated, knee-specific questionnaire for osteoarthritis commonly used in clinical trials, registries, and patient follow-up and has been used in patients aged 13 to 79 years. The KOOS is valid and reliable, and the thresholds for minimal clinically important change have been defined.40,41 The KOOS covers 5 dimensions, including pain (9 items), knee-specific symptoms (7 items), activities of daily living (ADLs) function (17 items), sport and recreation function (5 items), and knee-related quality of life (4 items). Each item is rated by the patient on a 5-point Likert-type scale ranging from no problems to extreme problems. Each of the 5 scores is calculated as the sum of the items included. Scores are transformed to a scale of 0 to 100, with 0 representing extreme knee problems and 100 representing no knee problems. A difference of 8 to 10 points represents a clinically meaningful difference for the KOOS pain scale.39,42
Covariates included factors previously shown to be associated with longer-term pain outcome up to 6 months after TKA, including sex, age, BMI, race, medical comorbidity assessed using the modified Charlson Comorbidity Index43 (a validated measure), and quality of life assessed using the SF-36.44,45 The SF-36 physical component summary (PCS) and mental component summary (MCS) scores were calculated using standard algorithms; both are norm based, with a mean (SD) of 50 (10). Back pain was measured using the Oswestry Disability Index, with scores ranging from 0 to 50, categorized as no (0-4), mild (5-14), moderate (15-24), severe (25-34), or complete (35-50) disability.46
Statistical Analysis
We performed all analyses using StataMP statistical software, version 13 (StataCorp LLC). We used a 2-sample Wilcoxon rank sum (Mann-Whitney) test (2 sided) for continuous variables and a Pearson χ2 test (1 sided) for categorical variables, with a significance level of .05 to examine whether there were any differences between the FORCE-TJR source cohort and the pain trajectory cohort. Descriptive statistics explored demographic characteristics, clinical variables, and pain scores before and after TKA. We used group-based trajectory models to assess trajectories of pain between pre-TKA and 8-week TKA scores.47,48 Specifically, we developed trajectories of the probability of having moderate to severe pain from before TKA to 8 weeks after TKA. We then examined factors associated with these trajectories and whether the trajectories could predict a pain outcome at 6 months. We used the trajectory program developed by Jones and Nagin.49
We assessed multicollinearity by examining the variance inflation factors for each of the variables in the model. A variable for which variance inflation factor values are greater than 10 may merit further investigation; the variance inflation factors were 1.08 or lower in our final linear regression model for 6-month outcome and in the logistic regression model for variables associated with trajectories. We examined the linearity assumption for variables that were continuous using the augmented partial residual plot, and no evidence of nonlinearity was found in our final linear regression model. The shape of the trajectories was forced to be linear owing to overfitting if quadratic or cubic terms were used with the limited number of time points that were available.
In sensitivity analyses, we evaluated the extent of missing surveys at 2 weeks and 8 weeks and whether any patterns of missingness altered trajectory assignment or outcome predictions. Missing pain values at 2 weeks or 8 weeks were imputed based on the predicted values from multilevel mixed-effects linear regression models.
Assessing the Trajectory Model
After basic exploratory data analysis has been performed, trajectory analysis requires assessment of (1) the number of trajectories, (2) the shape of each trajectory, and (3) the predictors of membership to each trajectory. We used several criteria to evaluate the group-based trajectory model to use. We assessed a priori 2 to 6 trajectory subgroups because models with more than 6 trajectory subgroups may be difficult to interpret clinically. We used the Bayesian information criterion to choose the optimal number of subgroups or trajectories.48 We also used the following criteria to assess model quality: (1) average posterior probability for membership in each trajectory of at least 0.70 and (2) nonoverlapping 95% CIs for the 2 trajectories.48 To avoid overfitting the data, we only examined linear trajectories for 3 points.50 We examined all combinations of linear trajectories and differing numbers of trajectories. Next, to create trajectory membership, all individuals were assigned to the trajectory to which they had the highest probability of belonging. This strategy was selected because our posterior probabilities of trajectory subgroup membership were high and using probabilistic membership in trajectories may not be useful in clinical practice. In other words, by using hard assignment to trajectories, we explicitly subgrouped patients according to pain scores.
Assessing the Factors Associated With Trajectory Membership
We used logistic regression to assess the factors associated with post-TKA pain trajectory membership, including patients who experienced pain relief in the postoperative period (fast pain responders) and patients who had less pain relief in the postoperative period (slow pain responders). Then all relevant factors associated with trajectory membership were screened for inclusion in the model. Those that were predictive (P < .10) were considered for inclusion in the final model. We calculated the area under the receiver operating characteristic curve to assess the ability of factors in the final model to explain the variability in the pain trajectory subgroups.51
Assessing the Predictive Ability of Trajectory Membership
We sought to assess whether the post-TKA pain trajectory was associated with pain severity at 6 months after TKA. We adjusted for preoperative covariates that are known factors associated with pain after TKA to assess the independent capability of trajectory subgroup assignment to predict pain. Model fit was examined using the Hosmer-Lemeshow test.52 We used adjusted R2 to assess the ability of trajectory subgroups to explain the variability in the KOOS pain score at 6 months (ie, 26 weeks) after TKA.51 Based on resources for this ancillary study and the timing of its funding, we aimed to enroll more than 500 FORCE-TJR patients without using formal sample size calculations.
Results
Cohort Characteristics and Assessment of Nonresponse Bias
In total, 2209 patients undergoing TKA returned either 2-week or 8-week surveys (or both) during the enrollment period. We identified 659 patients with primary TKA from the FORCE-TJR cohort who had complete preoperative, 2-week, 8-week, and 26-week pain data and constituted the study cohort. Details are shown in the patient flowchart (eFigure in the Supplement). The mean (SD) age was 67.1 (8.0) years, 64.5% (425 of 659) were female, the mean (SD) BMI was 30.77 (5.66), 94.5% (613 of 649) were white, 68.8% (440 of 640) had an educational level of high school graduate or greater, and 66.9% (355 of 531) had a median annual household income exceeding $45 000 (Table 1). In addition, 63.8% (415 of 650) had a Charlson Comorbidity Index of 0, and 31.8% (203 of 638) had undergone a previous joint replacement surgery; the mean (SD) preoperative SF-36 PCS score and MCS score were 34.1 (8.2) and 53.8 (11.4), respectively (Table 1).
Table 1. Demographic and Preoperative Characteristics of the Study Cohort.
| Variable | No./Total No. (%) (N = 659) |
|---|---|
| Sex | |
| Male | 234/659 (35.5) |
| Female | 425/659 (64.5) |
| Age, mean (SD), ya | 67.1 (8.0) |
| BMIb | |
| <25 | 90/640 (14.1) |
| 25-29.9 | 214/640 (33.4) |
| 30-34.9 | 202/640 (31.6) |
| 35-39.9 | 97/640 (15.2) |
| ≥40 | 37/640 (5.8) |
| Race | |
| White | 613/649 (94.5) |
| Nonwhite | 36/649 (5.5) |
| Marital status | |
| Married or living with someone as married | 467/642 (72.7) |
| Widowed, separated, or divorced | 142/642 (22.1) |
| Never married | 33/642 (5.1) |
| Adults living in household, No. | |
| 1 | 153/595 (25.7) |
| 2 | 355/595 (59.7) |
| 3 | 59/595 (9.9) |
| ≥4 | 28/595 (4.7) |
| Educational level | |
| Less than high school graduate | 183/640 (28.6) |
| High school graduate or greater | 440/640 (68.8) |
| Missing | 17/640 (2.7) |
| Insurance, plus secondaryc | |
| Medicare | 385/642 (60.0) |
| Private or HMO | 230/642 (35.8) |
| All othersd | 27/642 (4.2) |
| Annual household income, median, $e | |
| ≤45 000 | 176/531 (33.1) |
| >45 000 | 355/531 (66.9) |
| Charlson Comorbidity Index Score | |
| 0 | 415/650 (63.8) |
| 1 | 143/650 (22.0) |
| 2 | 56/650 (8.6) |
| ≥3 | 36/650 (5.5) |
| Previous joint replacement surgery | |
| No | 435/638 (68.2) |
| Yes | 203/638 (31.8) |
| Oswestry Disability Index low back pain | |
| None | 282/647 (43.6) |
| Mild | 191/647 (29.5) |
| Moderate | 126/647 (19.5) |
| Severe | 48/647 (7.4) |
| Nonsurgical joints with moderate to severe pain, No. | |
| 0 | 475/650 (73.1) |
| 1 | 142/650 (21.8) |
| 2 | 22/650 (3.4) |
| 3 | 11/650 (1.7) |
| Preoperative SF-36, mean (SD) | |
| PCS score | 34.1 (8.2) |
| MCS score | 53.8 (11.4) |
| Preoperative KOOS pain score for surgical knee, mean (SD)f | 48.4 (17.7) |
| Preoperative KOOS pain score for surgical kneef | |
| None | 3/659 (0.5) |
| Mild | 65/659 (9.9) |
| Moderate | 258/659 (39.2) |
| Severe | 333/659 (50.5) |
| Preoperative KOOS pain score for nonsurgical knee, mean (SD)f | 76.1 (23.3) |
| Preoperative KOOS pain score for nonsurgical kneef | |
| None | 246/646 (38.1) |
| Mild | 245/646 (37.9) |
| Moderate | 118/646 (18.3) |
| Severe | 37/646 (5.7) |
| Other preoperative KOOS scores for surgical knee, mean (SD)f | |
| Activities of daily living score | 55.4 (18.5) |
| Symptom score | 50.1 (20.1) |
| Sport score | 18.7 (19.2) |
| Quality of life score | 27.9 (17.7) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HMO, health maintenance organization; KOOS, Knee Injury and Osteoarthritis Outcome Score; MCS, mental component summary; PCS, physical component summary; SF-36, 36-Item Short Form Health Survey.
In total, 221 of 659 (33.5%) were younger than 65 years, and 438 of 659 (66.5%) were 65 years or older.
The mean (SD) BMI was 30.8 (5.7).
Medicaid as secondary insurance was an option for 12 of 385 (3.1%) with Medicare, 2 of 278 (0.7%) with private/HMO, and 5 of 27 (18.5%) with all others.
All others include uninsured and self-pay.
Income exceeding $45 000 was chosen for categorization based on the national median income.
The KOOS pain scale ranges from 0 to 100 (0 represents extreme knee problems, and 100 represents no knee problems). A preoperative score of 50 to less than 70 indicates moderate pain, and 0 to less than 50 indicates severe pain. The KOOS items are rated by the patient on a 5-point Likert-type scale (range, 0-10; 0 represents no problems, and 10 represents extreme problems), with each of the 5 scores calculated as the sum of the items included; using a Likert-type 2-week and 8-week pain scale, a preoperative score of 5 to 6 indicates moderate pain, and 7 to 10 indicates severe pain.
In a comparison of patients in the ancillary pain study (patients responding to the 2-week and 8-week pain surveys) with all patients in the main FORCE-TJR cohort (the parent study), no meaningful clinical differences were identified despite some statistically significant differences in the preoperative variables (including sex, BMI, emotional health, and comorbidities). Within the pain survey cohort, 1655 of 3614 (45.8%) TJR patients (total knee replacement plus total hip replacement) completed both 2-week and 8-week surveys. No clinically meaningful differences in preoperative factors were found between pain survey respondents (at both 2 weeks and 8 weeks) and all FORCE-TJR participants with the exception of a difference in percentage of nonwhite patients (5.5% [36 of 659] in the pain cohort vs 10.3% [2145 of 20 783] in the FORCE-TJR cohort). eTable 1 in the Supplement summarizes the KOOS pain scores, and eTable 2 in the Supplement compares other patient characteristics.
Factors Associated With Post-TKA Pain Trajectories
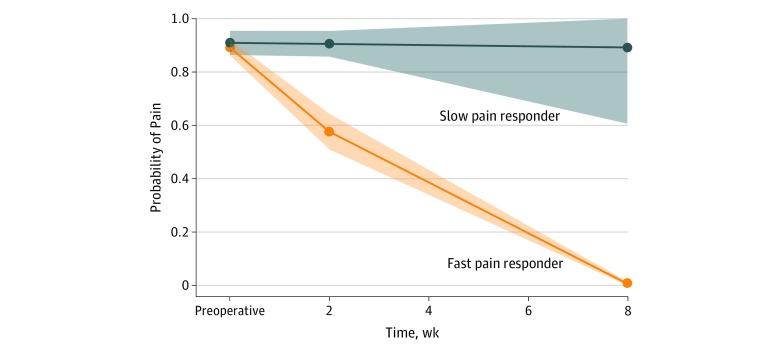
Two pain trajectory subgroups were identified at 8 weeks: fast pain relief in the first 8 weeks after TKA (fast pain response; 72.4% [477 of 659] of the sample) and no fast pain relief (slow pain response; 27.6% [182 of 659] of the sample) (Figure). The mean (SD) KOOS pain score at 6 months was 87.3 (13.4) in fast pain responders vs 74.5 (19.4) in slow pain responders.
Figure. Pain Trajectory in Fast Pain Responders and Slow Pain Responders at 8 Weeks After Total Knee Arthroplasty.
Probability of moderate to severe pain is scaled 0 to 1 (lower indicates less probability, while higher indicates greater probability). The shaded areas are 95% CIs. Using the Knee Injury and Osteoarthritis Outcome Score (KOOS) pain scale (range, 0-100; 0 represents extreme knee problems, and 100 represents no knee problems), a preoperative score of 50 to less than 70 indicates moderate pain, and 0 to less than 50 indicates severe pain. The KOOS items are rated by the patient on a 5-point Likert-type scale (range, 0-10; 0 represents no problems, and 10 represents extreme problems), with each of the 5 scores calculated as the sum of the items included. Using the Likert-type 2-week and 8-week pain scale, a preoperative score of 5 to 6 indicates moderate pain, and 7 to 10 indicates severe pain.
Significant factors associated with the slow pain responder trajectory in univariate analyses were lower (worse) preoperative SF-36 MCS scores (51.5 vs 54.6; P = .002), more pain before surgery in the surgical knee (46.4 vs 49.2; P = .04), and worse preoperative KOOS ADL scores in the surgical knee (51.2 vs 57.0; P < .001) (eTable 3 in the Supplement). Sex, age, BMI, marital status, educational level, insurance status, Charlson Comorbidity Index, and previous joint replacement surgery were not significantly associated with the pain trajectory (eTable 3 in the Supplement). In multivariable-adjusted analyses, preoperative SF-36 MCS scores and preoperative KOOS ADL scores in the surgical knee were associated with the pain trajectory (Table 2). Each unit increase in SF-36 MCS and KOOS ADL score in surgical knee scores was associated with odds ratios of 0.98 (95% CI, 0.96-1.00; P = .02) and 0.97 (95% CI, 0.95-0.99; P = .007) for the slow pain responder trajectory, respectively.
Table 2. Multivariable-Adjusted Logistic Regression Model Assessing Pre–Total Knee Arthroplasty (TKA) Factors Associated With the Slow Pain Responder Trajectorya.
| Variable | Full Modelb | |
|---|---|---|
| OR (95% CI) | P Value | |
| Preoperative SF-36 MCS score, with 1-unit increase | 0.98 (0.96-1.00) | .02 |
| Preoperative KOOS activities of daily living score for surgical knee, with 1-unit increase | 0.97 (0.95-0.99) | .007 |
| Sex | ||
| Male | 1 [Reference] | NA |
| Female | 1.13 (0.73-1.73) | .58 |
| Age, y | ||
| <65 | 1 [Reference] | NA |
| ≥65 | 0.97 (0.63-1.49) | .21 |
| BMI | ||
| <25 | ||
| 25-29.9 | 1.97 (1.00-3.84) | .05 |
| 30-34.9 | 1.12 (0.56-2.24) | .74 |
| ≥35 | 1.43 (0.69-2.95) | .34 |
| Race | ||
| White | 1 [Reference] | NA |
| Nonwhite | 1.56 (0.70-3.49) | NA |
| Charlson Comorbidity Index Score | ||
| 0 | 1 [Reference] | NA |
| 1 | 1.25 (0.79-2.00) | .34 |
| 2 | 1.20 (0.60-2.40) | .42 |
| ≥3 | 0.78 (0.32-1.91) | .35 |
| Previous joint replacement surgery | 0.82 (0.52-1.27) | .37 |
| No | 1 [Reference] | NA |
| Oswestry Disability Index low back pain | ||
| None | 1 [Reference] | NA |
| Mild | 0.93 (0.58-1.49) | .76 |
| Moderate | 0.92 (0.53-1.58) | .76 |
| Severe | 0.87 (0.40-1.90) | .72 |
| Nonsurgical joints with moderate to severe pain, No. | ||
| 0 | 1 [Reference] | NA |
| 1 | 0.91 (0.54-1.51) | .70 |
| 2 | 1.17 (0.43-3.15) | .76 |
| 3 | 0.73 (0.17-3.07) | .67 |
| Preoperative SF-36 PCS score, with 1-unit increase | 1.00 (0.97-1.03) | .95 |
| Preoperative KOOS pain score for surgical knee, with 1-unit increase | 1.02 (1.00-1.04) | .06 |
| Area under the receiver operating characteristic curve | 0.64 | NA |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); KOOS, Knee Injury and Osteoarthritis Outcome Score; MCS, mental component summary; NA, not applicable; OR, odds ratio; PCS, physical component summary; SF-36, 36-Item Short Form Health Survey.
No evidence of multicollinearity was found because the variance inflation factors for each of the variables were 1.08 or lower (as a rule of thumb, a variable whose variance inflation factor values are greater than 10 may merit further investigation). Multivariable-adjusted logistic regression included the following factors: sex, age, BMI, race, preoperative Charlson Comorbidity Index score, previous joint replacement surgery, preoperative Oswestry Disability Index low back pain, preoperative number of nonsurgical joints with moderate to severe pain, preoperative SF-36 PCS score and MCS score, preoperative KOOS pain score for surgical knee, and KOOS activities of daily living score.
Only significant variables are listed in the table (significance level, P ≤ .05).
Unadjusted and Multivariable-Adjusted Association of Pain Trajectories With 6-Month Pain
In univariate analyses, pain trajectories at 8 weeks were statistically significant and clinically meaningfully associated with the KOOS pain score at 6 months after TKA. Race, Charlson Comorbidity Index, and preoperative SF-36 MCS scores were also statistically significantly associated with the KOOS pain score at 6 months after TKA (Table 3). Nonwhite race was associated with a 9-point-lower KOOS pain score (75.4 vs 84.3, P = .01), Charlson Comorbidity Index of at least 3 with a 10-point-lower score compared with a score of 0 (74.7 vs 85.4, overall P < .001), and preoperative MCS score of at least 45 with a 10-point-higher score (85.7 vs 75.9, P < .001).
Table 3. Factors Associated With 6-Month Knee Injury and Osteoarthritis Outcome Score (KOOS) Pain Scores in Univariate Analysesa.
| Variable | KOOS Pain Score, Mean (SD) | P Value |
|---|---|---|
| Pain trajectory 8 wk after total knee arthroplasty | ||
| Fast pain respondersb | 87.3 (13.4) | <.001 |
| Slow pain respondersc | 74.5 (19.4) | |
| Sex | ||
| Male | 84.4 (17.4) | .16 |
| Female | 83.4 (15.7) | |
| Age, y | ||
| <65 | 82.2 (18.8) | .43 |
| ≥65 | 84.5 (14.9) | |
| BMI | ||
| <25 | 85.9 (13.6) | .08 |
| 25-29.9 | 82.9 (17.2) | |
| 30-34.9 | 84.7 (16.4) | |
| 35-39.9 | 80.9 (16.9) | |
| ≥40 | 87.0 (14.3) | |
| Race | ||
| White | 84.3 (15.8) | .01 |
| Nonwhite | 75.4 (22.2) | |
| Annual household income, $ | ||
| ≤45 000 | 82.3 (18.5) | .17 |
| >45 000 | 85.3 (14.6) | |
| Charlson Comorbidity Index | ||
| 0 | 85.4 (15.5) | <.001 |
| 1 | 82.5 (17.3) | |
| 2 | 80.1 (17.5) | |
| ≥3 | 74.7 (16.3) | |
| Preoperative SF-36 MCS score | ||
| <45 | 75.9 (19.3) | <.001 |
| ≥45 | 85.7 (14.8) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); MCS, mental component summary; SF-36, 36-Item Short Form Health Survey.
Kruskal-Wallis test was used for the categorical variables in the table.
Patients who experienced pain relief in the immediate postoperative period are referred to as fast pain responders. The mean (SD) preoperative KOOS pain score for this subgroup was 49.2 (17.5), which was not different from the 46.4 (18.0) of slow pain responders. The improvement from preoperative to 6-month KOOS pain scores was 38.1 (19.2) in fast pain responders vs 28.1 (21.0) in slow pain responders.
Patients who experienced minimal pain relief in the immediate postoperative period are referred to as slow pain responders.
The pain trajectory at 8 weeks was significantly and clinically meaningfully associated with higher KOOS pain scores at 6 months after TKA in both the full model and reduced multivariable models. These results are summarized in Table 4. After adjusting for patient factors, the pain trajectory at 8 weeks after TKA was independently associated with the mean KOOS pain score at 6 months, with a between-trajectory difference of −11.3 (95% CI, −13.9 to −8.7; P < .001).
Table 4. Assessing the Association of 8-Week Pain Trajectory With 6-Month Knee Injury and Osteoarthritis Outcome Score (KOOS) Pain Scoresa.
| Variable | Final Model 6-mo KOOS Pain Score (95% CI)b | P Value |
|---|---|---|
| Pain trajectory 8 wk after total knee arthroplasty | ||
| Fast pain responders | 1 [Reference] | NA |
| Slow pain responders | –11.3 (–13.9 to –8.7) | <.001 |
| Race | ||
| White | 1 [Reference] | NA |
| Nonwhite | –6.6 (–11.6 to –1.5) | .01 |
| Charlson Comorbidity Index Score | ||
| 0 | 1 [Reference] | NA |
| 1 | –1.3 (–4.1 to 1.5) | .36 |
| 2 | –1.7 (–5.9 to 2.5) | .42 |
| ≥3 | –6.8 (–11.8 to –1.8) | .008 |
| Preoperative SF-36, with 1-unit increase | ||
| PCS score | 0.3 (0.1 to 0.4) | <.001 |
| MCS score | 0.3 (0.2 to 0.4) | <.001 |
Abbreviations: MCS, mental component summary; NA, not applicable; PCS, physical component summary; SF-36, 36-Item Short Form Health Survey.
The reduced 6-month model included pain trajectory, race, Charlson Comorbidity Index, and preoperative SF-36 PCS score and MCS score.
Because the R2 value for the reduced model was the same as for the full model that included all the factors based on the principle of parsimony, reduced models were more desirable. No evidence of multicollinearity was found because the variance inflation factors for each of the variables were 1.08 or lower (a variable for which variance inflation factor values are greater than 10 may merit further investigation). The adjusted R2 was 0.2
We performed sensitivity analyses by imputing pain scores for those missing them at 2 weeks or 8 weeks. The same variables that were significant in the main analyses were significant in the sensitivity analyses, with minimal attenuation of coefficients.
Discussion
In this prospective cohort study of a representative US cohort, we combined data from the FORCE-TJR national cohort of primary TJR outcomes with ancillary pain survey data at 2 weeks and 8 weeks after TKA. We examined the patterns and characteristics of discrete posttrajectory subgroups in the first 8 weeks after surgery and how these trajectories predicted longer-term pain outcome after primary TKA. The 659 patients with preoperative, 2-week, and 8-week pain trajectory data were similar in patient profiles and comorbidities to the FORCE-TJR cohort. Two pain trajectory subgroups were identified at 8 weeks: two-thirds of patients who experienced fast pain relief in the postoperative period (fast pain responders) and one-third of patients who did not (slow pain responders). After adjusting for patient factors, the pain trajectory subgroup at 8 weeks after TKA was independently associated with the mean KOOS pain score at 6 months, indicating relevance to intermediate-term and long-term TKA outcomes.
Total knee arthroplasty is the most common and costly joint surgery, with approximately 700 000 TKA procedures being performed annually, making it a major public health burden.53 To our knowledge, the present work is the first study of a multisite, representative cohort of patients undergoing primary TKA in the United States that has examined the trajectory of pain after primary TKA and its association with pain outcomes at 6 months after surgery. Although previous studies14,15,16,54 examined preoperative pain (and in rare cases postoperative pain) and longer-term postoperative pain, to our knowledge, no systematic evaluation was done to assess pain trajectories and their association with longer-term outcomes (eg, patient pain experience). These retrospective studies also did not control for important confounders and were single-center studies, reducing confidence in the study findings and raising questions about generalizability. In the period after primary TKA, we found that 72.4% (477 of 659) of patients were fast pain responders and 27.6% (182 of 659) of patients were slow pain responders in the first 8 weeks. Evidence of the 2 pain trajectories existed as early as 8 weeks after the primary TKA and was possibly present even earlier. This observation was surprising and important because pain during this period includes postsurgical acute pain.55,56 Previous attempts at performing an analysis of pain trajectory were limited because they assessed preoperative factors only and did not investigate pain in the postoperative period.15,16 The findings of an association between preoperative variables and post-TKA pain in those single-center studies were similar to the results of other observational studies.11,57,58,59,60,61 Our study highlights the need to assess pain in patients during the period after primary TKA.
We also found that the postoperative pain trajectory was independently associated with longer-term post-TKA pain outcome. Specifically, although patients in the slow pain responder subgroup at 2 weeks and 8 weeks after surgery had significantly improved pain, they reported greater persistent index knee pain at 6 months after TKA compared with the fast pain responder subgroup. This finding has potential implications for patient care after primary TKA. Early identification of patients in the slow pain responder trajectory at 8 weeks after TKA (ie, for the subgroup continuing to experience moderate to severe pain) may offer an opportunity to target potentially modifiable factors for interventions in the perioperative period to possibly improve the long-term pain outcomes in patients who have undergone primary TKA. Persistent pain after TKA can contribute to long-term opioid use,62,63 which is a major public health crisis.64,65 Comprehensive pain management programs (eg, opioid prescribing guidelines66) and other behavioral interventions should be tested for this subgroup of patients in the postoperative period after TKA, with a goal to improve outcomes. Although the mean pain scores in the slow pain responder subgroup improved by 6 months, these patients did not reach the same status of the fast pain responder subgroup.
In the present study, preoperative physical health, general emotional and mental health, ADLs, and quality of life were associated with pain trajectory subgroups, whereas the other demographic or clinical characteristics were not. However, we did not assess immediate postoperative events and factors (ie, during the hospital stay and the first week after discharge to home) and thus are unable to provide additional insights regarding other postoperative variables amenable to intervention to improve outcomes. Future studies should examine whether the in-hospital, post-TKA period includes any modifiable factors associated with the 2-week to 8-week pain trajectory so that interventions targeting these factors may be developed and implemented.
The recent institution of bundled payments by the US Centers for Medicare & Medicaid Services (CMS) represents a major shift in the reimbursement of inpatient procedures, including TKA,67 but does not explicitly address patient-reported outcomes. According to this newly enacted policy, the CMS will pay for 90 days of care (ie, inpatient stay for TKA plus the first 90 days after discharge) rather than for each of the individual services to avoid fragmented care. This payment change by the CMS, which pays for most TKA procedures in the United States (totaling $7 billion in 2014 for knee and hip replacements68), will have major consequences on TKA reimbursement. A key change in adapting to the new payment system will be more explicit shared decision-making and a focus on improving patient-reported outcomes, as well as prevention of medical and surgical complications that lead to rehospitalization and increased cost of care.
Our study findings suggest that tailored pain treatment that targets the slow pain responders in the postoperative period should be tested for association with final pain outcomes. Taken together, the present study results and the new CMS payment initiative indicate the need for more research in this area and may ultimately result in improved patient pain outcomes and satisfaction. In addition, this study presents a new application of an emerging analytic method (trajectory analysis technique) to predict postoperative outcomes (using pain as an example). More research is needed in the arena of perioperative interventions, which have the potential to improve long-term post-TKA outcomes.69,70
Strengths and Limitations
The study has notable strengths, including a national, pragmatic sample of contemporary patients undergoing TKA; use of a replicable, simple pain numeric rating scale at 2 weeks and 8 weeks; application of emerging trajectory tools; robustness of our study results; and a comprehensive assessment that included potential confounders and covariates in the analyses.
This study has limitations. First, although the patient profiles were comparable between the FORCE-TJR national cohort and our ancillary pain trajectory study sample, fewer nonwhite patients and patients with less comorbidity were included in the latter because of the nature of patients treated at the community sites that participated in the trajectory study. Second, this study did not collect pain data during the inpatient stay and the first week after TKA. This information may augment future post-TKA trajectory analyses. We included patients with complete data at 4 time points (preoperative, 2 weeks, 8 weeks, and 26 weeks) in these analyses. To ensure that patients with complete data were similar to the total FORCE-TJR cohort, we also performed sensitivity analyses, and the analytic subgroup did not meaningfully differ from the cohort.
Conclusions
We identified 2 postoperative pain trajectories after primary TKA. The 2 trajectories were evident as early as 2 weeks after TKA and suggest that patients at risk of poor pain outcome may be identified after TKA. A simple pain numeric rating scale measure can be used after TKA in orthopedic practices to identify these pain trajectories. The wide availability of cell phones with texting ability and the use of electronic health records in orthopedic surgeon offices in the United States make this possible and feasible. We also found that the postoperative pain trajectory was independently associated with longer-term pain outcome at 6 months after TKA. Preoperative emotional and mental health status and ability to perform ADLs were associated with the pain trajectory and may guide tailored pain management. More research is needed to elucidate modifiable contributors to slow pain responder status in the postoperative period. Interventions targeting the postoperative pain trajectory and its correlates have the possibility to improve primary TKA outcomes.
eFigure. Patient Flow Chart
eTable 1. Preoperative and Postoperative KOOS Pain Characteristics of FORCE-TJR Cohort, Patients at Eligible Site During Our Pain Study Enrollment, and Those Participating in Our Pain Study
eTable 2. Comparison of Patient and Clinical Characteristics Between the FORCE-TJR Cohort and the Ancillary Pain Study Cohort
eTable 3. Unadjusted Correlates of Pain Trajectories
References
- 1.Chou R, Gordon DB, de Leon-Casasola OA, et al. . Management of postoperative pain: a clinical practice guideline from the American Pain Society, the American Society of Regional Anesthesia and Pain Medicine, and the American Society of Anesthesiologists’ Committee on Regional Anesthesia, Executive Committee, and Administrative Council [published dosage correction appears in J Pain. 2016;17(4):508-510]. J Pain. 2016;17(2):-. doi: 10.1016/j.jpain.2015.12.008 [DOI] [PubMed] [Google Scholar]
- 2.Aubrun F, Nouette-Gaulain K, Fletcher D, et al. . Revision of expert panel’s guidelines on postoperative pain management. Anaesth Crit Care Pain Med. 2019;38(4):405-411. doi: 10.1016/j.accpm.2019.02.011 [DOI] [PubMed] [Google Scholar]
- 3.Cooney MF. Postoperative pain management: clinical practice guidelines. J Perianesth Nurs. 2016;31(5):445-451. doi: 10.1016/j.jopan.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 4.Rosenberg J, Kehlet H. Does effective postoperative pain management influence surgical morbidity? Eur Surg Res. 1999;31(2):133-137. doi: 10.1159/000008631 [DOI] [PubMed] [Google Scholar]
- 5.Peters CL, Shirley B, Erickson J. The effect of a new multimodal perioperative anesthetic regimen on postoperative pain, side effects, rehabilitation, and length of hospital stay after total joint arthroplasty. J Arthroplasty. 2006;21(6)(suppl 2):132-138. doi: 10.1016/j.arth.2006.04.017 [DOI] [PubMed] [Google Scholar]
- 6.Guimarães-Pereira L, Farinha F, Azevedo L, Abelha F, Castro-Lopes J. Persistent postoperative pain after cardiac surgery: incidence, characterization, associated factors and its impact in quality of life. Eur J Pain. 2016;20(9):1433-1442. doi: 10.1002/ejp.866 [DOI] [PubMed] [Google Scholar]
- 7.Shyu YI, Chen ML, Chen MC, Wu CC, Su JY. Postoperative pain and its impact on quality of life for hip-fractured older people over 12 months after hospital discharge. J Clin Nurs. 2009;18(5):755-764. doi: 10.1111/j.1365-2702.2008.02611.x [DOI] [PubMed] [Google Scholar]
- 8.Wu CL, Naqibuddin M, Rowlingson AJ, Lietman SA, Jermyn RM, Fleisher LA. The effect of pain on health-related quality of life in the immediate postoperative period. Anesth Analg. 2003;97(4):1078-1085, table of contents. doi: 10.1213/01.ANE.0000081722.09164.D5 [DOI] [PubMed] [Google Scholar]
- 9.Choinière M, Watt-Watson J, Victor JC, et al. . Prevalence of and risk factors for persistent postoperative nonanginal pain after cardiac surgery: a 2-year prospective multicentre study. CMAJ. 2014;186(7):E213-E223. doi: 10.1503/cmaj.131012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ethgen O, Bruyère O, Richy F, Dardennes C, Reginster JY. Health-related quality of life in total hip and total knee arthroplasty: a qualitative and systematic review of the literature. J Bone Joint Surg Am. 2004;86(5):963-974. doi: 10.2106/00004623-200405000-00012 [DOI] [PubMed] [Google Scholar]
- 11.Singh JA, Gabriel S, Lewallen D. The impact of gender, age, and preoperative pain severity on pain after TKA. Clin Orthop Relat Res. 2008;466(11):2717-2723. doi: 10.1007/s11999-008-0399-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wylde V, Hewlett S, Learmonth ID, Dieppe P. Persistent pain after joint replacement: prevalence, sensory qualities, and postoperative determinants. Pain. 2011;152(3):566-572. doi: 10.1016/j.pain.2010.11.023 [DOI] [PubMed] [Google Scholar]
- 13.Beswick AD, Wylde V, Gooberman-Hill R, Blom A, Dieppe P. What proportion of patients report long-term pain after total hip or knee replacement for osteoarthritis? a systematic review of prospective studies in unselected patients. BMJ Open. 2012;2(1):e000435. doi: 10.1136/bmjopen-2011-000435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brander VA, Stulberg SD, Adams AD, et al. . Predicting total knee replacement pain: a prospective, observational study. Clin Orthop Relat Res. 2003;(416):27-36. doi: 10.1097/01.blo.0000092983.12414.e9 [DOI] [PubMed] [Google Scholar]
- 15.Dowsey MM, Smith AJ, Choong PFM. Latent class growth analysis predicts long term pain and function trajectories in total knee arthroplasty: a study of 689 patients. Osteoarthritis Cartilage. 2015;23(12):2141-2149. doi: 10.1016/j.joca.2015.07.005 [DOI] [PubMed] [Google Scholar]
- 16.Lenguerrand E, Wylde V, Gooberman-Hill R, et al. . Trajectories of pain and function after primary hip and knee arthroplasty: the ADAPT cohort study. PLoS One. 2016;11(2):e0149306. doi: 10.1371/journal.pone.0149306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singelyn FJ, Deyaert M, Joris D, Pendeville E, Gouverneur JM. Effects of intravenous patient-controlled analgesia with morphine, continuous epidural analgesia, and continuous three-in-one block on postoperative pain and knee rehabilitation after unilateral total knee arthroplasty. Anesth Analg. 1998;87(1):88-92. doi: 10.1097/00000539-199807000-00019 [DOI] [PubMed] [Google Scholar]
- 18.Munin MC, Rudy TE, Glynn NW, Crossett LS, Rubash HE. Early inpatient rehabilitation after elective hip and knee arthroplasty. JAMA. 1998;279(11):847-852. doi: 10.1001/jama.279.11.847 [DOI] [PubMed] [Google Scholar]
- 19.Goesling J, Moser SE, Zaidi B, et al. . Trends and predictors of opioid use after total knee and total hip arthroplasty. Pain. 2016;157(6):1259-1265. doi: 10.1097/j.pain.0000000000000516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patanwala AE, Jarzyna DL, Miller MD, Erstad BL. Comparison of opioid requirements and analgesic response in opioid-tolerant versus opioid-naïve patients after total knee arthroplasty. Pharmacotherapy. 2008;28(12):1453-1460. doi: 10.1592/phco.28.12.1453 [DOI] [PubMed] [Google Scholar]
- 21.Singh JA, Lewallen DG. Predictors of use of pain medications for persistent knee pain after primary total knee arthroplasty: a cohort study using an institutional joint registry. Arthritis Res Ther. 2012;14(6):R248. doi: 10.1186/ar4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manchikanti L, Helm S II, Fellows B, et al. . Opioid epidemic in the United States. Pain Physician. 2012;15(3)(suppl):ES9-ES38. [PubMed] [Google Scholar]
- 23.Sehgal N, Manchikanti L, Smith HS. Prescription opioid abuse in chronic pain: a review of opioid abuse predictors and strategies to curb opioid abuse. Pain Physician. 2012;15(3)(suppl):ES67-ES92. [PubMed] [Google Scholar]
- 24.Manchikanti L, Abdi S, Atluri S, et al. ; American Society of Interventional Pain Physicians . American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain: part 2–guidance. Pain Physician. 2012;15(3)(suppl):S67-S116. [PubMed] [Google Scholar]
- 25.Manchikanti L, Abdi S, Atluri S, et al. ; American Society of Interventional Pain Physicians . American Society of Interventional Pain Physicians (ASIPP) guidelines for responsible opioid prescribing in chronic non-cancer pain, part I: evidence assessment. Pain Physician. 2012;15(3)(suppl):S1-S65. [PubMed] [Google Scholar]
- 26.Downey L, Engelberg RA. Quality-of-life trajectories at the end of life: assessments over time by patients with and without cancer. J Am Geriatr Soc. 2010;58(3):472-479. doi: 10.1111/j.1532-5415.2010.02734.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Franklin PD, Allison JJ, Ayers DC. Beyond joint implant registries: a patient-centered research consortium for comparative effectiveness in total joint replacement. JAMA. 2012;308(12):1217-1218. doi: 10.1001/jama.2012.12568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ayers DC, Fehring TK, Odum SM, Franklin PD. Using joint registry data from FORCE-TJR to improve the accuracy of risk-adjustment prediction models for thirty-day readmission after total hip replacement and total knee replacement. J Bone Joint Surg Am. 2015;97(8):668-671. doi: 10.2106/JBJS.N.00889 [DOI] [PubMed] [Google Scholar]
- 29.Franklin PD, Lewallen D, Bozic K, Hallstrom B, Jiranek W, Ayers DC. Implementation of patient-reported outcome measures in U.S. total joint replacement registries: rationale, status, and plans. J Bone Joint Surg Am. 2014;96(suppl 1):104-109. doi: 10.2106/JBJS.N.00328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gordon DB, Polomano RC, Pellino TA, et al. . Revised American Pain Society Patient Outcome Questionnaire (APS-POQ-R) for quality improvement of pain management in hospitalized adults: preliminary psychometric evaluation. J Pain. 2010;11(11):1172-1186. doi: 10.1016/j.jpain.2010.02.012 [DOI] [PubMed] [Google Scholar]
- 31.McCaffery M, Beebe A. Pain: Clinical Manual for Nursing Practice. St Louis, MO: CV Mosby Co; 1989. [Google Scholar]
- 32.Eriksson K, Wikström L, Årestedt K, Fridlund B, Broström A. Numeric rating scale: patients’ perceptions of its use in postoperative pain assessments. Appl Nurs Res. 2014;27(1):41-46. doi: 10.1016/j.apnr.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 33.Michener LA, Snyder AR, Leggin BG. Responsiveness of the numeric pain rating scale in patients with shoulder pain and the effect of surgical status. J Sport Rehabil. 2011;20(1):115-128. doi: 10.1123/jsr.20.1.115 [DOI] [PubMed] [Google Scholar]
- 34.Childs JD, Piva SR, Fritz JM. Responsiveness of the numeric pain rating scale in patients with low back pain. Spine (Phila Pa 1976). 2005;30(11):1331-1334. doi: 10.1097/01.brs.0000164099.92112.29 [DOI] [PubMed] [Google Scholar]
- 35.Strout TD, Burton JH. Clinically significant change in physician-assigned numeric pain rating scale scores. Am J Emerg Med. 2004;22(3):243-245. doi: 10.1016/j.ajem.2004.02.039 [DOI] [PubMed] [Google Scholar]
- 36.Joos E, Peretz A, Beguin S, Famaey JP. Reliability and reproducibility of visual analogue scale and numeric rating scale for therapeutic evaluation of pain in rheumatic patients. J Rheumatol. 1991;18(8):1269-1270. [PubMed] [Google Scholar]
- 37.Roos EM, Roos HP, Lohmander LS, Ekdahl C, Beynnon BD. Knee Injury and Osteoarthritis Outcome Score (KOOS): development of a self-administered outcome measure. J Orthop Sports Phys Ther. 1998;28(2):88-96. doi: 10.2519/jospt.1998.28.2.88 [DOI] [PubMed] [Google Scholar]
- 38.Roos EM, Toksvig-Larsen S. Knee Injury and Osteoarthritis Outcome Score (KOOS): validation and comparison to the WOMAC in total knee replacement. Health Qual Life Outcomes. 2003;1:17. doi: 10.1186/1477-7525-1-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roos EM, Lohmander LS. The Knee Injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health Qual Life Outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Collins NJ, Misra D, Felson DT, Crossley KM, Roos EM. Measures of knee function: International Knee Documentation Committee (IKDC) Subjective Knee Evaluation Form, Knee Injury and Osteoarthritis Outcome Score (KOOS), Knee Injury and Osteoarthritis Outcome Score Physical Function Short Form (KOOS-PS), Knee Outcome Survey Activities of Daily Living Scale (KOS-ADL), Lysholm Knee Scoring Scale, Oxford Knee Score (OKS), Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC), Activity Rating Scale (ARS), and Tegner Activity Score (TAS). Arthritis Care Res (Hoboken). 2011;63(suppl 11):S208-S228. doi: 10.1002/acr.20632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Alviar MJ, Olver J, Brand C, Hale T, Khan F. Do patient-reported outcome measures used in assessing outcomes in rehabilitation after hip and knee arthroplasty capture issues relevant to patients? results of a systematic review and ICF linking process. J Rehabil Med. 2011;43(5):374-381. doi: 10.2340/16501977-0801 [DOI] [PubMed] [Google Scholar]
- 42.Ingelsrud LH, Terwee CB, Terluin B, et al. . Meaningful change scores in the Knee Injury and Osteoarthritis Outcome Score in patients undergoing anterior cruciate ligament reconstruction. Am J Sports Med. 2018;46(5):1120-1128. doi: 10.1177/0363546518759543 [DOI] [PubMed] [Google Scholar]
- 43.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373-383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- 44.Gandek B, Ware JE, Aaronson NK, et al. . Cross-validation of item selection and scoring for the SF-12 Health Survey in nine countries: results from the IQOLA Project: International Quality of Life Assessment. J Clin Epidemiol. 1998;51(11):1171-1178. doi: 10.1016/S0895-4356(98)00109-7 [DOI] [PubMed] [Google Scholar]
- 45.Ware J Jr, Kosinski M, Keller SDA. A 12-Item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 46.Fairbank JC, Pynsent PB. The Oswestry Disability Index. Spine (Phila Pa 1976). 2000;25(22):2940-2952. doi: 10.1097/00007632-200011150-00017 [DOI] [PubMed] [Google Scholar]
- 47.Nagin DS. Group-based trajectory modeling: an overview In: Piquero AR, Weisburd D, eds. Handbook of Quantitative Criminology. New York, NY: Springer New York; 2010:53-67. doi: 10.1007/978-0-387-77650-7_4 [DOI] [Google Scholar]
- 48.Nagin DS, Odgers CL. Group-based trajectory modeling in clinical research. Annu Rev Clin Psychol. 2010;6:109-138. doi: 10.1146/annurev.clinpsy.121208.131413 [DOI] [PubMed] [Google Scholar]
- 49.Jones BL, Nagin DS A Stata plugin for estimating group-based trajectory models. https://ssrc.indiana.edu/doc/wimdocs/2013-03-29_nagin_trajectory_stata-plugin-info.pdf. Published May 21, 2012. Accessed October 9, 2019.
- 50.Franklin JM, Shrank WH, Pakes J, et al. . Group-based trajectory models: a new approach to classifying and predicting long-term medication adherence. Med Care. 2013;51(9):789-796. doi: 10.1097/MLR.0b013e3182984c1f [DOI] [PubMed] [Google Scholar]
- 51.Pepe M, Longton G, Janes H. Estimation and comparison of receiver operating characteristic curves. Stata J. 2009;9(1):1-16. doi: 10.1177/1536867X0900900101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hosmer DW, Lemeshow S. Goodness of fit tests for the multiple logistic regression model. Commun Stat Theory Methods. 1980;9(10):1043-1069. doi: 10.1080/03610928008827941 [DOI] [Google Scholar]
- 53.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89(4):780-785. doi: 10.2106/JBJS.F.00222 [DOI] [PubMed] [Google Scholar]
- 54.Brander V, Gondek S, Martin E, Stulberg SD. Pain and depression influence outcome 5 years after knee replacement surgery. Clin Orthop Relat Res. 2007;464(464):21-26. doi: 10.1097/BLO.0b013e318126c032 [DOI] [PubMed] [Google Scholar]
- 55.Chan EY, Blyth FM, Cheow SL, Fransen M. Postoperative pain following hospital discharge after knee replacement surgery: a patient survey. Pain Manag. 2013;3(3):177-188. doi: 10.2217/pmt.13.14 [DOI] [PubMed] [Google Scholar]
- 56.Chan EY, Blyth FM, Nairn L, Fransen M. Acute postoperative pain following hospital discharge after total knee arthroplasty. Osteoarthritis Cartilage. 2013;21(9):1257-1263. doi: 10.1016/j.joca.2013.06.011 [DOI] [PubMed] [Google Scholar]
- 57.Burns LC, Ritvo SE, Ferguson MK, Clarke H, Seltzer Z, Katz J. Pain catastrophizing as a risk factor for chronic pain after total knee arthroplasty: a systematic review. J Pain Res. 2015;8:21-32. doi: 10.2147/JPR.S64730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hilton ME, Gioe T, Noorbaloochi S, Singh JA. Increasing comorbidity is associated with worsening physical function and pain after primary total knee arthroplasty. BMC Musculoskelet Disord. 2016;17(1):421. doi: 10.1186/s12891-016-1261-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pinto PR, McIntyre T, Ferrero R, Almeida A, Araújo-Soares V. Risk factors for moderate and severe persistent pain in patients undergoing total knee and hip arthroplasty: a prospective predictive study. PLoS One. 2013;8(9):e73917. doi: 10.1371/journal.pone.0073917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Singh JA, Lewallen DG. Better functional and similar pain outcomes in osteoarthritis compared to rheumatoid arthritis after primary total knee arthroplasty: a cohort study. Arthritis Care Res (Hoboken). 2013;65(12):1936-1941. doi: 10.1002/acr.22090 [DOI] [PubMed] [Google Scholar]
- 61.Singh JA, Lewallen DG. Ipsilateral lower extremity joint involvement increases the risk of poor pain and function outcomes after hip or knee arthroplasty. BMC Med. 2013;11:144. doi: 10.1186/1741-7015-11-144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bedard NA, DeMik DE, Dowdle SB, Callaghan JJ. Trends and risk factors for prolonged opioid use after unicompartmental knee arthroplasty. Bone Joint J. 2018;100-B(1)(suppl A):62-67. doi: 10.1302/0301-620X.100B1.BJJ-2017-0547.R1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Trasolini NA, McKnight BM, Dorr LD. The opioid crisis and the orthopedic surgeon. J Arthroplasty. 2018;33(11):3379-3382.e1. doi: 10.1016/j.arth.2018.07.002 [DOI] [PubMed] [Google Scholar]
- 64.Volkow ND, Collins FS. The role of science in addressing the opioid crisis. N Engl J Med. 2017;377(4):391-394. doi: 10.1056/NEJMsr1706626 [DOI] [PubMed] [Google Scholar]
- 65.Kolodny A, Courtwright DT, Hwang CS, et al. . The prescription opioid and heroin crisis: a public health approach to an epidemic of addiction. Annu Rev Public Health. 2015;36:559-574. doi: 10.1146/annurev-publhealth-031914-122957 [DOI] [PubMed] [Google Scholar]
- 66.Holte AJ, Carender CN, Noiseux NO, Otero JE, Brown TS. Restrictive opioid prescribing protocols following total hip arthroplasty and total knee arthroplasty are safe and effective. J Arthroplasty. 2019;34(7S):S135-S139. doi: 10.1016/j.arth.2019.02.022 [DOI] [PubMed] [Google Scholar]
- 67.Centers for Medicare & Medicaid Services. Bundled Payments for Care Improvement (BPCI) initiative: general information. https://innovation.cms.gov/initiatives/bundled-payments/. Updated July 1, 2016. Accessed October 6, 2019.
- 68.Centers for Medicare & Medicaid Services Comprehensive Care for Joint Replacement model. https://innovation.cms.gov/initiatives/CJR. Updated September 18, 2019. Accessed October 6, 2019.
- 69.Niraj G, Rowbotham DJ. Persistent postoperative pain: where are we now? Br J Anaesth. 2011;107(1):25-29. doi: 10.1093/bja/aer116 [DOI] [PubMed] [Google Scholar]
- 70.Mishriky BM, Waldron NH, Habib AS. Impact of pregabalin on acute and persistent postoperative pain: a systematic review and meta-analysis. Br J Anaesth. 2015;114(1):10-31. doi: 10.1093/bja/aeu293 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eFigure. Patient Flow Chart
eTable 1. Preoperative and Postoperative KOOS Pain Characteristics of FORCE-TJR Cohort, Patients at Eligible Site During Our Pain Study Enrollment, and Those Participating in Our Pain Study
eTable 2. Comparison of Patient and Clinical Characteristics Between the FORCE-TJR Cohort and the Ancillary Pain Study Cohort
eTable 3. Unadjusted Correlates of Pain Trajectories